Note: Descriptions are shown in the official language in which they were submitted.
CA 02099869 2001-03-06
ENDOCYTOSABLE PHARMACEUTICAL PARTICLES
This invention relates to the use of particulate
agents in therapeutic or prophylactic treatments of
conditions involving cellular infection or malfunction.
Many human and animal diseases involve infection or
malfunction of the body's cells, eg. by virtue of viral,
bacterial (eg. mycobacterial) or protozoal infection or
cancerous malfunction. While the body's immune system
is generally able to cope with foreign matter, it is not
always able to recognise and destroy cells which have
become infected or have begun to malfunction. This is
particularly serious in the case of certain infections
where the pathogen may remain dormant within infected
cells for prolonged periods and also where, as for
instance is the case with HIV infection, by infecting
cells of the immune system the disease weakens the
body's ability to defend itself.
Besides viral infection where the parasite is
obliged to replicate within a host cell, the range of
known intracellular pathogens is wide and includes the
pathogens associated with tuberculosis, listeria,
Crohn's disease and leprosy as well as brucella,
rickettsias and legionella.
Much of the research effort directed at the
development of therapeutic agents for the treatment of
such diseases has been directed towards the production
of complex organic chemicals which on the whole are not
very efficiently delivered into the infected or at-risk
cells.
The present invention is based on the realisation
r.~'lrlt Pf fP!='f' 1 lIC /~al i wnrw ~F
__. _~ .... ~::vr:jui~lC ciirt.iVC aijC111_.5 Illdy
be achieved by administration of particulate agents
incorporating the active inorganic agent where the
particles are of a size that permits their endacytosis,
WO 92/11846 h PGTfEP92/0002~. '
a c~,,.~ ~ J
J 4 _ 2 _ V:--.,.
or more especially phagocytosis. This administration
route is especially attractive since, unlike complex and
chemically more delicate organic drugs, inorganic agents
and in particular metal cations and radionuclides will
generally not be particularly susceptible to loss of
activity as a result of exposure to the relatively harsh
chemical environments commonly experienced during or
following endocytotic particle uptake.
Thus viewed from one aspect the invention provides
the use of a physiologically tolerable, preferably
inorganic, material containing atoms or ions of a
therapeutically or prophylactically effective element
for the manufacture of a particulate agent containing
said element, said agent being for use in a therapeutic
or prophylactic treatment of the human or animal body
which involves delivery of said particles or fragments
thereof into cells of said body by endocytosis.
Viewed from a further aspect the invention provides
a method of prophylactic or therapeutic treatment of the
human ar animal, preferably mammalian, body said method
comprising administering into said body a particulate
agent comprising particles of a physiologically
tolerable, preferably inorganic, material containing
atoms or ions of a therapeutically or prophylactically
effective element whereby to deliver said particles or
fragments thereof into cells within said body by
endocytosis.
Viewed from a still further aspect, the invewtion
provides a pharmaceutical composition comprising
endocytosable particles of a physiologically tolerable,
preferably inorganic, material containing atoms or ions
of a therapeutically or praphylactically effective
element together with at least one pharmaceutical
carrier or exc; r"'_ent .
In order that delivery of the "active" element into
body cells by endocytosis may be achieved, the particles
which effectively serve as a carrier for the active
WO 92/118A6 ~ ~ ~ ~ ~ ~ ~ PCT/EP92/00021
_ 3 _
element will generally be substantially insoluble in the
body fluids that they are to encounter between
administration into the body and endocytosis by the
targeted cells. Some diminution in particle size can be
tolerated of course and if desired particles may of
course be provided with protective coatings or
disintegrants so as to ensure that particles of desired
sizes axe present at or throughout the body zone at
which endocytotic uptake is desired or so as to ensure
that endocytosable particles are present at such sites
for a prolonged period of time.
Administration may be by any route which delivers
the particles to body sites at which endocytosis by the
targeted cells may take place. This will of course
depend on the type of cell that is being targeted, which
in turn depends upon the condition that is being treated
or against which prophylactic treatment is being given.
However in general administration into the systemic
vasculature, into the musculature, topically or into
body cavities having external voidance ducts (in
particular into the vagina or, by inhalation, into the
lungs for access to pulmonary alveolar macrophages) will
be the preferred routes.
When particles are injected intravenously in humans
or animals they are swept from the blood stream
relatively rapidly, e.g. within a few hours, by
endocytosis by monocytes and macrophages or they are
endocytosed by circulating peripheral blood monocytes.
This has been demonstrated for,59Fe labelled particles of
mixed particle size (20-100 nm) injected into a rabbit.
Serial 1ml blood samples were taken from a vein in the
ear and as shown by the decreasing radiation count shown
in Figure 7 approximately 50% clearance was achieved
within 2 hours. PaYt l C'.1 P t~jt~'t'f~ka i n th i s f~cJhipn lay
spleen, liver, lymph nodes, lung, and by the Langerhans
cells, after topical application by vaginal macrophages
and by monocyte lineage cells in the marrow, by
WO 92/11846 n ~~~ PCT/EP92/00021 ~,
microglia of the central nervous system and by
gastrointestinal tract lymphoid tissue, is a well known
phenomenon and in certain cases has been utilised as a
means of improving image contrast for various organs in '
diagnostic imaging techniques by the administration of
particulate contrast agents, for example the
superparamagnetic ferrite particles used as Tz contrast
agents in magnetic resonance imaging of liver and spleen
tumours. In this regard particle distribution to and
slow breakdown in other types of macrophages and
monocytes has hitherto been viewed as an undesirable
aspect of the particles' biodistribution.
However in the case of diseases associated with
infection or malfunction of particle endocytosing cells,
this pattern of distribution can be seen to be most
fortuitous if the particles are used to deliver
therapeutically or praphylactically effective agents
into those cells.
This is of particular. importance in the cellular
level therapy or prophylaxis of diseases in which
macrophages are liable to infection, especially
mycobacterial and retroviral infections, as it is well
known that as macrophages succumb to infection, for
example HIV infection, they can still engage in the
"non-specific" phagocytosis of particles long after they
loose the ability to selectively ingest C3 or Fc bearing
structures.
Thus macrophages and monocytes, which are a
principal reservoir, incubation Site and pathogenic
target of diseases such ~as HIV, retain, even up to the
latest stages of pathological change, their ability to
ingest the particles used according to the invention and '
thus their ability to receive into the cell the
thPrapP,utirally or nroph~~lactic~ll ~t'~ , t
r- s r' u... i Jc cicu 2ii 5
contained in those particles.
The delivery of the particles used according to the
invention to their targeted cells may not only be
WO 92/11846 '~ ~ ~ ~ ~ ~~ ~ PCT/EP92/00021
E: ,':;i. _
.affected by the administration route but also by
modification of the medium in which the particles are
administered, the size, coating or packing of the
particles or by surface modification of the particles to
promote uptake by particular cell types.
Thus for example to gain access to CNS macrophages
for intravenously administered particles it may be
desirable to reduce the effectiveness of the blood brain
barrier, eg. by intravenous administration of an agent
such as mannitol either simultaneously with or,
preferably, in advance of administration of the
particles. To this end, one may conveniently administer
mannitol, eg. in 20o aqueous solutions, at a dose of
lg/kg bodyweight.
Similarly, cell adhesion molecules (CAMS) or
molecular fragments may be coupled to the particle
a
surface to promote uptake by the targeted cells. By
appropriate selection of the labelling CAM, or by
selection of a range of CAMS, one may ensure that
particles are preferentially endocytosed (phagocytosed)
by cells which are uninfected (but liable to infection)
or by infected cells at different stages of infection.
Since different active elements may exert different
cytotoxic or pathogen replication suppressing effects,
this may be used as a means to achieve a combined
therapeutic and prophylactic treatment or to effect a
therapeutic treatment against a combination of pathogens
or against one pathogen at several of its replicative
stages.
CAMS may typically be used in two ways to improve
the selectivity of particle uptake and so direct them to
the sub~populations of macrophages that are or are not
actually infested, If a mirt,.zrc of particl '
Cj 1S
administered, some conjugated with CD4 (or the gpl2o
binding site) while others are unconjugated or
conjugated with Fc the first set will tend to be
WO 92/t 1846 PCT/EP92/00~21 ~,
. ~a~ ~'~~ -
6 -
phagocytosed by HIV infected cells while the second will
tend to be taken up by uninfected cells. Alternatively
using smaller particles which are phagocytosed much more
slowly if at all, these may be conjugated with
immunoadhesins or anti-gp120 antibody and they will
enter infected cells via an immunologic route as well as
by phagocytosis. Additionally the gp'2° protein itself or
the gp120/gp41 HIV envelope complex with or without a
mutation in the fusion peptide of gp41 or the CD4
binding site of the gp120 protein alone may be
conjugated to the particles so that they will mimic the
selectivity of the HIV virus and so preferentially enter
CD4 positive cells.
Besides being endocytosable by macrophages and
monocytes, the particles used according to the invention
may be endocytosed by other infected or at-risk cells or
by cancerous (malfunctioning) cells. Particularly
interestingly it has been shown that therapeutically/
prophylactically effective particles may be taken up by
cells such as nerve cells, sarcoma cells and T-cells,
including J'urkat T-cells stably expressing the HIV-1 tat
gene.
The "active element" in the particles used
according to the invention will generally be a
radionuclide capable of exerting a cytotoxic effect on
particle/radiation emission or, more preferably, a metal
which in cationic form interferes with the initiation or
progression of the cellular infection of concern or with
the metabolism of a malfunctioning cell. In this latter
case of cation cell therapy, the efficacy of the
technique is closely linked to the difference between
the relatively effective mechanisms that the host
animal's (e. g. human's) cells have for either tolerating
or deactivating (e.g. by chelation or i~o1_ar,'_r_,.,) tho
therapeutic metal cations as compared with the
relatively poor ability of the infecting organism to
protect itself from the therapeutic cations. This
WO 92/118A6 '~ ~ ~ ~ ~~ ~ ~ Pf'I'/EP92/00021
A,
r;. .'
t' -...'..
efficacy also derives at least in part from differences
in the equilibrium or affinity constants of various
enzymes, proteins, and other biomolecules for the
therapeutic cations.
In the case of cellular infection, eg. retroviral
infection or mycobacterial infection, it is possible to
use particles according to the invention which on
intracellular breakdown release metal cations that
interfere with the metabolism or replication of the
infectious agent, eg. by interfering with the
functioning of the reverse transcriptase (RT) enzyme
that is vital to retroviral replication. The Mg2+
dependence of RT, the Mnz+/Mg2-" dependence of RNase H and
the conserved cysteine rich/CCHC "zinc finger" sites on
the gag protein and the cystein rich dimerization site
of the tat protein are thus all potential targets for ,
cation cell therapy to inhibit HIV infectivity.
The use of inorganic crystallites, e.g, ceramic
metal oxide crystal matrices, which breakdown gradually
in the chemical environment encountered within the
endocytosing cell allows gradual, slow intracellular
release of the physiologically active element by a
relatively non-toxic mechanism. Such a slow release
mechanism means that "drug" administration may be
relatively infrequent, e.g. weekly, fortnightly, or
monthly.
While cation cell therapy will be exemplified below
with particular reference to HIV therapy it should be
borne in mind that other intracellular pathogens are
also susceptible to such treatments and the choice of
appropriate catians, cation mixtures and cation loading
levels within the particles used according to the
invention may be made on the basis of relatively
straight-forward lahnra~or;T tAsts~ for examYlc i.. vitro
tests showing the response of the particular pathogenic
agent to the metal ions/metal ion mixtures in question.
Thus by way of example the response of retroviruses
WO 92/11846 PC1'/EP92/00021
~C.,'~J~~ ~ .
~~ v 8 - ,.
oth~r than just HIV, eg. SIV, MoMuLV and AMV, ma be
Y
monitored in vitro by an RT assay.
The range of metals that can be loaded into
endocytosable particles to effect cation cell therapy
according to the invention is extremely large, indeed
virtually any metal can be delivered this way. Metals
which occur naturally in the target cells may be
considered as well as metals which compete with
naturally occurring can ons or which effect a
suppressive effect on the pathogen in other ways. Thus
cation cell therapy may be used to create a broad range
of selectively altered intracellular cationic milieux,
e.g. by altering the concentration of a cation which is
normally present or by providing an alternative cation,
and investigations suggest that in many instances the
infective agent is more susceptible to such manipulation
than is the host cell.
The acquired immune deficiency syndrome (AIDS] is
caused by infection of certain human cell types by the
Human Immunodeficiency Virus (HIVj. A variety of drug
strategies have been developed which target various
aspects of the viral infection and pathogenic sequence;
however there are no effective treatments or cures for
AIDS infection. All those infected are expected to die
from the disease and currently that includes tens of
millions of people~throughout the world.
Most current, single agent treatments now under
investigation involve small molecules such as nucleoside
analogs or protein antibodies. However, the cells
particularly affected by HIV are naturally active in
attacking large particles, including particles similar
in size to the virus, and the anti-HIV treatment
particles of the invention take advantage of this
natural feature of t, hPCA r_.e1 1 s _ Fur ther , gi~:en the high
pathogenicity of HIV and its ability to evolve
relatively rapidly, attention should be directed towards
multiple lines of attack in a single treatment, and this
bV0 92/11846 ,~ ~, PCT/EP92/00021
_,
__ 9
invention provides further ways of combining multiple
points of attack in a single particulate agent. Indeed
in general the particles used according to the invention
may be provided with a coating which comprises one or
more organic drugs and so be used in a combined therapy
whereby the organic drug acts in concert or
synergistically with the active element contained in the
particle.
HIV is a retrovirus, meaning that its genetic
information is encoded in RNA rather than DNA. It has a
glycoprotein coat which is quite variable in structure.
However, one of its coat proteins called gp120 has an
invariable region which recognises and binds to an
antigen called CD4 and this appears to be a key to the
disease process.
CD4 occurs on T helper lymphocytes and on a variety
of related cells in the monocyte group. These monocytes
include blood monocytes, reticuloendathelial system
(RES) cells such as splenic macrophages, liver Kuppfer
cells, Iymph node dendritic cells, Langerhans cells in
various locations, alveolar macrophages in the lungs,
and microglia and related phagocytic cells in the
central nervous system arid together form a principal
location of the HIV infection.
When an HIV virus gpl2o coat protein comes in
contact with a CD4+ cell, it binds to it and so causes a
conformational change exposing the fusogenic domain of
the associated gp41 envelope glycoprotein which fuses
with the cell membrane of the human CD4+ cells, and so
introduces the virus into the interior of the cell.
There, the virus reverse transcriptase (RT) enzyme is
activated, a DNA copy of the viral RNA is made, and that
viral DNA is intercalated into the cell's normal DNA.
Once .i ntercalated, the viral Dr:r is r eud r epca tGCliy
under the transcriptional control of the viral "tat"
(transactivator of transcription) protein and'the viral
rev (regulator of expression of virion) protein to
WO 92/11846 PCT/~P92l00021 ,.
c~n~'~ - to - ~t'':~'.
J
rd
produce large quantities of viral protein transcript and
viral ANA. Via a series of protease and protein
modification steps, new viral particles are generated.
During this process, multiple copies of gp120
appear in the cell membrane and may bind to the CD4
antigen on uninfected macrophages. The infected cell
can then bind to and fuse with the uninfected cell and
so doom that cell as well. Syncytia of multiple
captured monocytes are formed in this fashion. Also,
during this process, CD4 bearing T-cells and other T-
cells may approach the,infected monocyte and so, by
contact, become infected by the virus.
To date, the only successful pharmaceutical
treatment for HIV infection is AZT which is an analog of
one of the constituent nucleosides of the viral genetic
material. This drug hinders the viral replication
process and so slows the progress of the disease.
However, it is not curative and its associated toxicity
has led to an intensive further search for drugs which
can act synergistically with it. Also, there have been
reports of new strains of HIV which are resistant to
AZT. There are a variety of other dideoxynucleoside
analogs which may be similarly efficacious and these are
being actively investigated.
There are three principal new drug strategies. one
involves some analogs of the benzadiazepine drug family
(HIPO) which have been discovered during massive
screening programs which have sought anti-viral activity
from huge numbers of compounds: The exact mechanism of
action is not clear, but, as with AZT, the formation of
viral DNA by reverse transcriptase seems to be the point
of action. These and related drugs are being actively
explored.
A second new str ateg;~ 1':a~ r ~~ul.'~..2d fr aiu the
discovery that sulfated dextran molecules may
irreversibly hind viral particles. This has led to
related discoveries of a number of sulfated polymer
WO 92/11846 PCTlEP92/OOQ21
~~9~8~~
,,..;
- 11 -
molecules which can adsorb viral particles. The best
way to use such agents is not yet clear; if they bind a
virus but are then swept into monocytes along with the
virus, they may actually enhance the infectivity of the
virus.
The third group of strategies involves immunology
and genetically engineered proteins focused on the CD4+
protein and its receptor on the gp120 molecule. The CD4
protein or relevant portions thereof will attach to HIV
arid it will attach to gp120 where it is presented on the
surface of infected cells. Simply administering soluble
CD4 should .block the gp120 recognition site and may thus
prevent cellular infection by individual viruses.
CD4 can also be used as a delivery vehicle to
deliver a cytotoxic agent selectively to infected cells.
Thus in one approach, recombinant CD4 is attached to
pseudomonas exotoxin so as to deliver this powerful
cellular toxin to infected cells. A similar approach
attaches CD4 to r uin, a poisonous plant leatin.~
Cenenteclu has proposed an 'immunoadhesin' molecule which
is a hybrid between a recognition/targeting portion
composed of the region of CD4 that binds gp120, and, a
cytotoxic portion from the human antibody Fc region -
the portion that triggers cytotoxic attack in e.g.
bacterial infection after antibody binding.
For all of these CD4 related strategies, there has
been the problem of providing sufficient cytotoxicity
with an administered toxin without harming the patient
too severely, or, in the case of the immunoadhesin, of
how to stimulate a vigorous attack on the infected
monocytes when the patient's Fc based cytotoxic system
is compromised - the very essence of AIDS.
While there are numerous drugs under investigation
for the tr.patmPnt of nlDs and c:hile thcr~ are a ~arieLy
of prior uses of particulate agents, eg. metal oxide
particles, there has hitherto been no suggestion of the
use of particles to achieve cellular level cation
WO l2/I 1846 PCT/EP92/OOe21
r
.:
12
therapy or short range radiotherapy by endocytotic
uptake: Thus for example, this is the first use of
metal oxide particles for the treatment of any viral
infection; it is also the first use of ~3-emitter
particles or of Auger electron radiation for the
treatment of an HIV infection; this is also the first
use of metal oxide ceramic particles as a means of
delivering slow release selective metal cation
inhibitors of reverse transcriptase; and further, it is
the first application of spinel mediated microwave
diathermy to the treatment of AIDS.
The particles used according to the invention may
be of virtually any physiologically tolerable material;
as mentioned above however they will generally be of a
material which is substantially insoluble in
extracellular body fluids, in particule serum or plasma.
Organic matrices (eg, natural or synthetic, essentially
inert, organic polymer particles such as dextran coated
microspheres or latex nanospheres) may be used but
particularly preferably the particles will be inorganic,
especially alloys and metal sulphides or oxides as these
may break down naturally within the cells to release the
metal can ons active in the cation cell therapy aspect
of the invention.
Many metal oxide structures may be utilized as the
inorganic particles, and spinets, garnets and
perovskites have been found to be particularly useful in
this regard. It should however be stressed that other
well known inert and preferably essentially water
insoluble metal compounds may be used, especially those
having lattices such as permit desired metal cations or
radioisotopes to be included. By alloys, mixed metals '
are of course included.
In a' referred cmbo~'
p- .,ia;,~:,t, ~he pal ticie5 comprise a
core metal oxide crystal, eg. of spinet or garnet
structure, if desired coated for example by dextran
carbohydrate, wherein the total size of the particle is
WO 92/I1~46 ,t) ~ ~ r~ I, PCT/EP92/00021
c.::~. v;l
- 13 -
5nm to 15~,m, preferably lOnm-5~.m, especially l0nm-lam,
particularly 10-100nm, more particularly 10 to 50
nanometers and most especially 20-30nm, and where a
targeting moiety (TM) is optionally bound to the coating
at a concentration of TM per particle which may for
example be as low as about 1:1. Where TM labelling is
used to achieve highly specific uptake of the particles
by particular cell types, by cells infected by
particular agents or by infected cells at particular
stages of infection, it may be desirable that the agent
be virtually free of particles lacking an active TM.
For particles smaller than 100 nm, the particle
compositions are preferably sterilized by 0.2 or 0.1
micron microfiltration after final synthesis, affinity
purification and concentration. Otherwise, heat
sterilization may be used prior to conjugation to any
targetting moiety, e.g. protein or protein fragment.
The uses of a given version of a particulate metal
oxide agent depend upon the elements and isotopes
(radionuclides) used in the initial precipitation step
in which the metal oxide crystal core is precipitated
and also upon the types of coating and targeting moiety
that may be used. For each use, the metals/nuclides,
coatings and targeting moieties may be selected to
benefit both from the general advantages of the
simplicities of the preparatory method and to take
advantage of the new types of pharmaceutical
distribution which can be achieved by materials prepared
in this way.
In cationic cell therapy of retroviral infection
according to the invention one can have an attack on the
Mgz+ dependence of RT on two parallel strategies - by
delivering a competing metal ion into the cytosol free
OY hound t0 a ph~Sphate mCLCw~' Of a Tli.iCi2Wl~e. SiUSt'
metal ions at physiological pH tend to cause hydrolysis
which results in their precipitation as polymeric
hydrous oxides. However, it has proven possible in
WO 92/11846
PCT/EP92/00021 .
~~~~">~~ . - _. I;
14
vitro to use scandium as a magnesium antagonist in
several enzyme systems. This trivalent first row
transition metal is effectively non-toxic although its
only known clinical use is as a bacteriostatic agent.
The trivalent lanthanide elements including
scandium (Sc), yttrium (Y), and lanthanum (La) in the
III13 group as well as the fourteen chemically similar
rare earths of the lanthanide series have been
extensively exploited over the past twenty years in
studies of the interactions of divalent canons with
their physiological enzyme substrates. Except for
scandium, most have ionic radii similar to that of
calcium and have been useful in studies of various
active sites since they provide a gradually progressive
array of charge/surface densities.
Scandium, however, in addition to being the least
toxic of the entire group, is similar to magnesium in
ionic radius, reduction potential, and
electronegativity, differing primarily in its stable 3+
oxidation state. Scandium has recently been exploited
because of its similarity to aluminium, and this work
has led to better appreciation of its solution
chemistry.
Replacement of Mg'* with Sc3* in muscle actin has
been demonstrated. However, the demonstration that. SC3*
acts as a competitive antagonist of Mg'-+ in one of the
two Mgz+ sites on the beta-adrenergic receptor-adenylate
cyclase complex of the murine S49 lymphoma cell is also
most interesting. Since sc'* and La3* have similar
affinities for ATP but different effects on the
adenylate cyclase, it was possible to rule out the
generation of non-productive SeATP- as the cause of the
inhibition. The effect at the Mg'+ site responsible for
activation but nat at the ~i.t~ respc:,sible for agon:i.st
selectivity also served to demonstrate that the
inhibition by Sc3+ was due to a specific interaction at
an Mg'* site on the enzyme rather than to some non-
WO 92/11846 ~ ~ ~ ~ ~ ~ ~ PCT/EP92/00021
~.- , r,
- 15 -
specific metal/protein interaction.
In some bacterial DNA polymerases, there are as
many as 21 sites for divalent rations per enzyme
molecule with the binding affinity and importance for
enzymatic function varying widely among the sites. The
number and function of RT ration sites for HIV or other
retroviruses are not completely understood, although it
is clear that there are differences among closely
related Group C retroviruses in ration preference and
that incomplete dimer structure of HIV R'f may result in
distinctive roles for such can ons. Possibilities for
ration dependency are further extended by the
demonstration of two manganese/magnesium ration sites in
the RNAse H portion of the HIV RT protein.
For these reasons, for ration cell therapy
according to the invention consideration may be given to
using Ec'+, Y'+, and La'+, as well as several other
heavier lanthanides and, because of the preference of
other polymerase enzymes for Mnz* rather than Mg2*, other
metal ions of the first~and second row transition
metals. The ions of interest include Coz+, Ni2*, CuZ*,
and Znz* and from the second row, Ru3*, and Pdz*. Lithium
and strontium also deserve consideration.
Accordingly in one embodiment of the present
invention there are used as particles for ration cell
therapy particles which on degradation will release a
retroviral replication suppressing, eg. RT activity
inhibiting, metal ion. Experiments on the effects of
various metal ions on RT activity have been carried out
and the majority of mono, di and trivalent metal rations
were found to influence RT activity. The influence is
dependent on the element and its concentration as well
as on the particular viral infection under study. For
HIV RT, diva.l_er,t ions appeared especially efficacious,
in particular Pd, Zn, Cu and Ni and of these most
especially Pd. While scandium and sixth series
trivalent metal ions exhibit RT activity inhibiting
WO 92/11846
PCTI E P92/00021
,~: ~ .
_ 1~ - .
effects with some viruses, they seem to be less
effective with HIV.
Cationic cell therapy may also be achieved by
interference with zinc-binding sites; thus panels of
metal ions (eg. Cd, Co, Cu, Fe, Hg, Mn, Zn) have been
explored previously by various researchers in attempts '
to characterise the function and structure of Cys-XZ-Cys-
X~-His-XQ-Cys (CCHC) and other cysteine-rich, putatively
metal binding conserved sequences occurring in several
HIV proteins. The functional importance of zinc binding
to some of these sites remains unclear.
Mutations in the CCHC sequence in the gag protein
result in the production of non-infectious viral
particles which do not carry genomic RNA. The enhancer
proteins HIV-EP1 and HIV-EP2 which cause viral
activation upon binding the NF-KB site enhancer on the
HIV-1 LTR both include classical Xenopus TFIIIA zinc ,
binding sites. The tat protein is dimerized by the
action of zinc or cadmium at its conserved cysteine rich
regzon and mutations in that region interfere with viral
replication. The inventors have also demonstrated that
tat function is altered when 3urkat-tat T cells
endocytose and digest particles carrying substitute
metal cations such as Pd'+. Because of this complex
multiple involvement of various sites which either
require zinc or which are conserved but may
physiologically or pathologically bind zinc, it is
necessary, having selected the target virus, to carry
out relatively straightforward in vitro tests to select
the appropriate concentration and mix of metal can ons
to optimise effective interference with the proteins and
the pathogenic effects of the infecting organism.
For this reason and others, the general cellular
respo.~.se to digested particles as a source of iW roduced '
can ons may be used to assess their effectiveness when
the digesting cells are infected by or are subsequently
exposed to a selected pathogenic organism. This is
WO 92/11846
P(.'T/EP92/00021
f.....,., . .
_ 17 _
conveniently achieved by involving the preparation of
unconjugated or CD4-conjugated particles for
phagocytosis by cultured human monocytes or cultured T-
cells which are then infected, e.g. with HIV; where the
cell line chosen is one susceptible to synctium
formation this may then be used as an assessment of
inf ectivity .
Among the primary candidates for competition are
those metal cations whose tetracoordinate complexes are
similar in ionic radius to tetracoordinated zinc. These
include Pdz+ and Li°~ which together provide a range of
ionic radii. The previous metal panels assessed
suggested that larger ions may be accommodated more
readily than ions which are too small.
Palladium has an ionic radius and coordinatian
chemistry quite similar to manganese and, when included
in an organic chelate complex, it has long been known to
inhibit retroviral reverse transcriptase. However,
there have been no clinical uses for palladium canons
because there has been no way to deliver such cations
selectively to infected cells in sufficient amounts
without causing very high systemic levels.
When palladium is included in particles
administered to HIV infected patients a very useful
distribution is achieved. Even with no specific
directing antigen, these particles will be swept up by
the macrophage system. Tn this fashion, uninfected
macrophages will be made to ingest and slowly to degrade
the particles. These cells can therefore be made to
have a long term steady state release of intracellular
palladium. This will confer protection effective
against retroviral infection because it will inhibit the
function of the reverse transcriptase of any entering
virus as wPl7. as haw.; various ether effects.
In this fashion, relatively high intracellular
levels of this protective element can be achieved in
those cells most subject to attack. In combined therapy
WO 92/31846 fCTlEP92/00021
~~~~,~>~
-
f . ..
.':~.: i
with ,Q-emitting particles, palladium is used in a
portion of the mix of particles conjugated with an
indifferent antigen or with Fc since it is helpful in
the uninfected cells of infected patients.
When various concentrations of active rations, eg.
active transition rations, are used in assays of reverse
transcriptase activity, three general patterns of
response are found: 1) the ration proves irrelevant to
the enzyme and does not activate, but also permits full .
activation by tYie subsequent addition of magnesium; z)
the ration fully ar partially activates the enzyme and
the subsequent addition of magnesium has either no or
little effect or actually decreases the enzyme activity;
or 3) the ration does not activate the enzyme and
subsequently prevents activation by magnesium. One
additional pattern is demonstrated by rations which 4)
activate the enzyme when present in low concentration
but actually inhibit it and prevent magnesium activation
when present in higher concentration. Similar
expectations apply for Ag2~ and various other metal
nations.
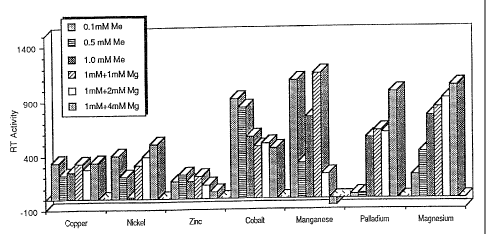
These four patterns are exhibited by various
rations, but the effect of any given ration on RT
activity is dependent on the strain and species of the
virus involved. The results for the retrovirus Moloney
Murine Leukemia Virus (MoMuLV) show pattern 1 for
lithium and strontium, pattern 2 for cobalt and
manganese, pattern 3 for copper, nickel, palladium,
scandium and the lanthanides, and pattern 4 for zinc.
A different array of activities is seen for Human
Tmmunodeficiency Virus. For HIV, aluminium shows
pattern 1, lithium and cobalt show pattern 2,.copper,
zinc, palladium, nickel and ruthenium show pattern 3,
and manganese shows pattern 4.
When dextran coated ration sources are crystallised
and prepared using Mnz*, Pd'+ and Niz+ as the divalent
ration in spinel crystals, important effects on viral
WO 92/1184b ~ ~ . ~ ~~ J ,f~ f~ PCT/EP92/00021
- 19 -
activity are seen. Concentrations of particles
equivalent to 1mg/ml of trivalent cation chloride
hydrate were exposed to cultured human macrophage cells
(THP-1 line) for periods of 1 or 2 hours to permit
particle uptake, after crhich the cells were washed and
placed in fresh medium. Twenty four hours later, a
small infectious inoculum of the RF strain of HIV was
introduced into the various culture wells and a variety
of uninfected controls were also observed. At intervals
of 3, 7, 11, and 14 days, cells were counted, viability
assessed with trypan blue, and cell free supernatants
were collected far reverse transcriptase assay.
The data obtained demonstrated that, relative to
controls, Fe/Fe particles (i.e. ferrites in which the
di- and trivalent metals are both iron) promote host
cell growth and viability but do not affect viral spread
or replication. Fe/Mn particles cause some impairment
of cell viability but completely block HIV infection.
Most usefully, the Fe/Ni and most strikingly the Fe/Pd
particles have minimal effect upon ar actually improve
cell viability but cause dramatic or near complete
suppression of HIV infectivity and so may be used
therapeutically or prophylactically.
Besides providing a route to cellular level cation
therapy of intracellular infection by HIV and the like
which proceeds via cation competition interference with
nucleic acid replication, the particulate endocytosis
route provides a means by which canons may be
administered into infected or infection susceptible
cells so as to interfere with any selected stage of
infection. Thus cations may bind directly to nucleic
acids, form abnormal substrates (eg. Me"+ ATP in place of
Mgz+ ATP, where Me"+ is the therapeutic or prophylactic
mete 7 c~,t ion ) or they may . interfer a u: i t:: the
polymerization of cyotoskeletal or nucleocapsid
proteins, change membrane characteristics, interfere
with protein/nucleic acid interactions or reduce the
WO 92/1 x846 P~'/EP92/00029
_ 20 _
.availability of reactants necessary for proliferation of
the pathogen.
Inorganic particles, eg. spinel particles, may be
made to behave as stable aqueous colloids by
precipitation from the metal salts in dextran solutions.
By sulfating the dextran after precipitation, or by
doing the precipitation with acrylic acid/polyvinyl
alcohol sulfate, the special HzV adhesion property of
these molecules can be conferred upon the particles.
When such particles, or similar particles bearing
conjugated CD4 or anti-gp120 are injected intravenously,
they will sweep up any free viruses in circulation and
carry them into already infected macrophages. As the
particles degrade, the coating material will continue to
have its inhibitory effect on viral replication within
the cell.
Besides single cell cation therapy the invention
also encompasses cellular level radiotherapy.
The intravenous administration of radiotherapeutic
agents generally speaking has hitherto been limited to
the use of small molecules. Where immune targeting is
wanted, current techniques have generally involved
coupling to an antibody a limited number of radioactive
atoms, normally no more than one or two. The present
invention however offers the possibility of synthesizing
the particulate agent, eg. a spinel, with a high
concentration of radiation emitting atoms.
By constructing large particles, eg. metal oxides
containing positron emitters such as spinet positron
emitters (SPE) or ,'electron emitters it is possible to
achieve great reduction of the range of the majority of
the emitted positrons or electrons. Most of the
cytotoxic ionizations will take place within 200-300
mi Vrn c of tile °~t:;ul ~; ~ ~ '-~ ~~- o. ,
w -"1~-- .-... p,~ru..~Cu.c l.GCur..~.GU 'vJiv.il :it'fo tdklllt~
place within less than 100 microns. Further, because of
the Bragg peak effect, there will be substantial
ionization yield even from those positrons which lose
WO 92/11846 PCTlEP92l00021
,.
the majority of their energy within the particle.
Thus, any viruses adherent to the positron emitter
containing particle will receive a very high dose rate
of ionizating radition and many will be destroyed in
this fashion. Once the particle is ingested by a
monocyte, many of those cells, already doomed by
infection, will die rapidly because of the radiation
effect. If the half-,life of the emitter is chosen to be
in the range of 2-4 hours, eg. Scandium43, then most of
the activity will be expended before the cell dies and
the particle is expelled for ingestion by another
phagocytic cell. It is also possible to use an a-
emitting nuclide such as Pt'86 for even greater toxicity
if necessary. The particular value of an a-emitter is
that it will be effective for only a very short range
around the particle even when very small, non-moderating
particles are synthesized. Finally, electron capture
nuclides such as Palladium'°3 can be used for their high ,
dose/decay, very short range Auger electron cytotoxic
effect. The decay characteristics of many radionuclides
are known and appropriate radionuclei may be selected
relatively easily - see for example "Periodic table of
the elements", compiled by Fluck and Heumann, VCH, 1986.
Although the treatment of HIV infection is of
tremendous concern, the methods described above as
earlier noted are also applicable to a wide variety of
other intracellular pathogens. Many of these pathogens
are of heightened importance in the setting of AIDS, so
that treatments which can simultaneously attack the AIDS
virus as well as the other pathogens will be
particularly useful. Intracellular pathogens of
increased importance in the compromised host include:
Histoplasmosis, Toxoplasmosis, Listeria, Candida, and
MvC_7nh_a,t_teripm ~:rl~m i;;t~r~Cll:;iure v.OiTipiGx. t7t'le't
intracellular pathogens amenable to treatment by
particles ingested by macrophages include Trypanosomes,
Neisseria, Mycobacterium tuberculosis, Leishmania,
r r v i is 1 1 O'Jp
pcri~r~z~oooz~
~~ ,~~~, ~ ..
U v. f r,"~a.
22 -
,Salmonella typhi, Legionella, Brucellosis, Ricketsia,
Cytomegalovirus, Chlamydia, Shigella, some
staphylococci, slow viruses or prions such as
Creutzfeld-Jacob, Mycoplasma, herpes simplex, EBV,
varicella zoster, or the papova virus associated with
~'C-type progressive multifocal levcoencephalopathy.
The inventors have for instance used cation source
particles to influence the course of infection of human
cells in culture by the HTLV-1 virus which causes both
hematological cancer and also a focal myelopathy.
For any intracellular pathogen, conventional
therapy is complicated by the relative inability of many
drugs to achieve adequate intracellular doses. Thus,
any pathogen which need not expose itself to the high
antibiotic concentrations in the extracellular fluid,
and which is also able to survive after ingestion by
macrophages poses a serious risk to the infected host.
Indeed many of the organisms mentioned in the previous
paragraph continue to be terrible scourges even in the
modern age ot~ antibiotics.
Attacks on these various intracellular organisms
have included the use of various drug carriers such as
liposomes. However, the hydrophobic coating of these
i
agents and the difficulties with targeting have limited
success so far. Among the problems is the tendency for
most particulate agents to be swept up by macrophages in
the spleen and liver and then to fail to reach other
macrophage compartments. The particles used according
to the invention may be of a size or series or range of
sizes and may carry targeting moieties such that more
general or more specific endocytosis occurs. Thus using
relatively small, eg. ca lo-50nm, particles optionally
provided with a hydrophilic coating, eg. a dextran
r_nat i ng ~ onwbt e~ ~«~ .t~ pZ.v~ ldc an ci i iClCi'~'i.
intracellular delivery vehicle for a broader range of
macrophage sites. Further, many of these organisms are
less able to tolerate abnormal concentrations of metal
WO 92/11846 ~ ~ ~ ,~ ~ ~) ~ pC;flEP92/00021
~:.~~.i
- 23 -
can ons than are the host cells. Thus many of the
considerations discussed above for cation therapy of HIV
apply equally for these organisms.
A further consideration is the potential for
including various antibiotic drugs in the particle
coating along with or in place of the dextran coating.
In AIDS patients, this permits cation cell therapy of
HIV to be effected simultaneously with administration of
high intracellular doses of specific antibiotics for
opportunistic coinfecting organisms. Further, where
various cations interfere with function of viral,
bacterial/yeast, and host functions, the addition of a
specific antibiotic agent or antiviral agent (eg. AZT)
can permit synergistic attack on the infecting organism
while sparing the host cell from full bimodal attack.
Ln the course of exploring the sensitivity of
control cell cultures to various mixed metal particle
formulations, the inventors have learned that some
formulations such as Fe/Mn mix~:d metal oxides tend to
inhibit host cell growth without reducing cell
viability. This sort of pharmacologic effect is the
desired function of chemotherapeutic agents for cancer
treatment. Since these particles have ready access to
bone marrow, they provide a ready means of achieving
intracellular therapy, both targeted and non-specific,
for a variety of cancers where adequate phagocytic
activity exists or can be induced. Experiments by the
inventors have confirmed this alteration in cell culture
growth kinetics in osteogenic sarcoma cells. Moreover,
using 5~'Fe labelled dextran-coated metal oxide particles
and electron microscopy it was confirmed that sarcoma
cells do ingest the particles. In cellular level cancer
therapy according to the invention, the clinical
Qf f iv~c~' i~ i 11. dGr 1 V C .1.11 lJal. t s:i uuu i.iie sensitivity to
ration substitution of the rapidly dividing cells and
their mitotic machinery. Additionally, since for many
cancers viruses and especially retroviruses play a
WO 92/11846 PCT/EP92/00021
~, ~,~i~i~
- 24 -
causative role (see zur Hausen in Science 254:1167-1173
(1991)), the efficacy of cation cell 'therapy according
to the invention will involve the effect of the
therapeutic cations on the various stages of viral
oncogenesis. Similar considerations apply in the
therapeutic or prophylactic treatment of other virally '
influenced diseases such as for example multiple
sclerosis.
Similar considerations also apply where there is an
intention to depress host immune function for
transplantation or to treat tissues to prevent graft
versus host disease. Particulate cation therapy
provides the opportunity to depress cell division for a
limited period time without excessive toxicity.
Consideration of applicability for use in 'treating
viral hepatitis involves differing strategies for the
various subtypes. In Hepatitis B, the liver injury is
due substantially to the inflammatory response to
antigens presented on the surface of otherwise
relatively healthy but infected hepatocytes. By
introducing cation sources into local macrophages and
other inflammatory cel2s, a local depression of the
inflammatory process may be achieved although inhibiting
the display of viral protein on the surface of such
liver cells could be particularly effective. In other
types of viral hepatitis such as C and D, the viral
infection is cytopathic for the hepatocytes and cell
surface receptors in a lipid coat for the cation source
will aid in directing the particles into the hepatocytes
rather than into macrophage cells.
Besides therapeutic and prophylactic uses, the
particles used according to the invention may be useful
in imaging procedures. Hitherto the imaging of HIV
l:lfC~tivii haS bccii 111Ti1tCia j.JLl.ltIc9L'l1x t.o studies or the ,
characteristic infections associated with AIDS.
However, since the particulate agents according to the
invention can be engineered to be visualizable as
WO 92/11846 ~ ~ C~ t~ ~ ~ ~ PCT/EP92/OOU21
",.
- 25 -
contrast agents upon X-ray computer tomographic (CT)
scanning or magnetic resonance imaging (MRI), and can be
detected upon magnetic resonance spectroscopy (MRS),
positron emission tomography (PET) or single photon
emission computer tomography (SPELT), it becomes
possible to follow their distribution quite closely.
For such uses, particles which are superparamagnetic or
which incorporate appropriate metal atoms or
radionuclides may conveniently be used. The opportunity
to image infection sites, or more accurately to enhance
image contrast in body zones in which the particles are
endocytosed is of interest not only in studying and
diagnosing the disease, but also in following the course
of therapy and in the actual development and
optimization of these agents.
There is a long history of use of magnetic
particles for biochemical separations. For in vitro
studies of the selectivity of the particles for infected
as opposed to uninfected cells, it will be possible to
prepare non-magnetic particles to be mixed with
magnetic/CD4 particles. The targeted cells that have
ingested the particles can then be separated from cells
which have not ingested particles and then be assayed
for presence of virus.
where it is necessary to use radioactivity for
various assays, MR spectroscopic properties of the
particles can be used for non-interfering assessment of
the particles themselves.
In vivo, it will be possible to use magnetized
catheters such as central catheters in the atrium, to
retrieve particles after their injection. In this
fashion, particles coated with free viral particles can
be removed from circulation as can any circulating
mOnC:.. E~'..n Ch auvv iia 2~tcu
~j't " 4:h3. :"'°"' ~ g "' t hE par tiClCS. ~I11S 1S
similar to a technique used for separating leukemic
cells but would be a new use for viral infection in
AIDS.
WO 92/11846 PCT/EP92/00021
cl ~~ ~'''y..
26
It has been known for some time that ferrimagnetic,
ferromagnetic or superparamagnetic particles, eg.
ferrite particles, can be synthesized so as to make them
increase in temperature in response to radiation of '
selected wavelengths. This method had been difficult to
apply for any actual medical uses because there has been
no consistent motive or means to achieve intracellular
distribution of the particles in cells which need to be
killed. If however such particles are targeted upon HIV
infected cells, eg. by surface labelling with CD9, the
distribution question is well solved. The particles are
given intravenously, and, because of CD4 conjugaticn,
are selectively phagocytosed by infected monocytes and
macrophages. After adequate distribution is achieved
(one to two hours after injection}, the patient can
receive whole body irradiation with microwave
frequencies tuned to activate just those particles with ,
CD4 label. These particles can b~ heated sufficiently
to have significant cytotoxic effect as well as
synergistic effect with other concurrent cytotoxic
treatments.
It will be appreciated that although the metals of
the metal oxide, sulphide or alloy matrices of the
inorganic particles of the invention may have naturally
occurring radioisotopes, the particles used according to
the invention for cellular level radiotherapy or for
combined therapy and scintigraphic or PET imaging (where
the radionuclide serves as a diagnostic marker for cells
which endocytose the particles} will have significantly
higher than natural abundance contents of the
radioisotopes, e.g. for positron emitters an average of
at least one, perhaps 10 or more atoms per 100 nm
crystal. The natural occurrence of many /3+ emitters is
l acg than 1 i n ~ nil) ~n~ ~.yn v:~~c cWi ttiiy atuiTt per
particle may suffice.
For other novel ",doped" particles according to the
invention, the therapeutically or prophylactively active
WO 92/11846 ~ ~ (, ~ PCT/EP92/00021
,.. ..,
- 27 -
or marker nuclei may be isotopes which occur naturally,
e.g. as impurities in naturally occurring oxides,
sulphides or alloys - in 'this case again the particles
according to the invention will generally be
distinguished by containing such atoms at higher than
natural values, e.g. a hundred or even more per 100 nm
particle.
The particles of the invention may be coated or
uncoated and may derive their physiological tolerability
at least in part from such a coating. They may moreover
be coupled to a biotargetting moiety, for example an
antibody, an antibody fragment, a CAM or a protein or
protein fragment of a target pathogen, eg. a coating
protein fragment such as from HIV's gp120.
The particles are preferably of a spinet or garnet
structure - the manufacture of particles of these types
is already well known and need not be described further
here. By way of interest however it may be noted that
superparamagnetic crystals of this type have been
proposed for use as MRI contrast agents in various
patent publications of Nycomed AS, Schering AG, Advanced
Magnetics Inc, etc (eg. US-A-4863715 (Jacobsen) and US-
A-4827945 (Groman}).
There are a wide variety of targeting moieties or
CAMS which can be used according to the invention.
These include antibodies, monoclonal antibodies,
antibody fragments, receptors, peptides such as
endorphins, steroid molecules, viral fragments or coat
proteins, cell surface antigens including various
carbohydrates, lectins, immunoadhesins, neurotransmitter
molecules, growth factors, immunomodulators,
prostacyclins, prostaglandins, interleukins,
leukotrienes, and proteins or other molecules which
nrn nte onri~yari-~~; ~ .. a. ~. a.,. ~>" L
r- T;?.... ", 1 .. y ..r up~a'l.me s.~y 'va.izcl iGilLCS u1 the
pharmaceutical agent by the target cells. The use of
uncoated particles, eg. palladium ferrites, without any
targeting moieties is nonetheless of very great
WO 92/11846 PC'f/EP92/00021
r,,.;,:.,
-
28 -
''"'i.nterest .
The synthesis of metal oxide crystals as
particulates in stable aqueous solution has been of
interest in crystalography and in the paint pigment
industry. However, many of the relevant advances have
grown out of studies of magnetism. '
Many of the agents described herein involve
speoially synthesized versions of magnetite (Fe304j. The
crystal structure of magnetite is based on a mineral
called spinet MgAtZo4. However, when specific
proportions of ferric and ferrous ions are used instead
of magnesium and aluminum as the metal ions in the
lattice: Fe(II)(Fe(III))204, a particular set of
electronic alignments and exchanges are produced which
result in spontaneous magnetization.
The basic structure of magnetite involves a ctose-
packed, face centred cubic crystal of oxygen atoms with .
metal ions placed at interstitial spaces in the crystal.
~'he interstices are divided into "A" sites and "B" sites
which have different interstitial locations relative to
the oxygen array and which therefore give rise to two
distinct sub-lattices within the crystal. In the
naturally occurring mineral "spinet" (MgA12o4) the A-
sites are filled by Mg(II) and the B sites by Al(ITI).
The assignment of atoms to sublattices is determined in
part by size. The A-sites allow atoms of 0.3 to 0.6
angstrom radius while the B-sites allow atoms of 0.6 to
1.0 angstroms. In a normal spinet crystal, the A-sites
are filled by divalent atoms while the B-sites are
filled by trivalent atoms.
Magnetite is an "inverse spinet" crystal because it
has trivalent iron in its A-sites, and a mix of divalent
and trivalent iron in its B-sites. Each crystal subunit
ha.a W vxyi'eiaS, f A-Si tS Fe ( III ) d (.UIItS, ~ B-Slte Fe ( L.11
atoms and 8 B-site Fe(II) atoms. The general formula
for spinet ferrites is Mt(II): (Fe(III))~(0)4, where Mt
can be any divalent transition metal or a charge
WO 92/11846 ~ ~ ~ i~ ~ r ~ pC~/Ep92100021
- 29 -
balanced mix of monovalent and trivalent metals of
appropriate ionic radius.
The Fe(III) atoms in the A sublattice are
positioned so as to oppose and cancel the spin
magnetization of the Fe(III) in the B sublattice.
However, after this cancellation, the 8 Fe(II) remaining
in the B sublattice have completely unopposed spin
magnetizations. For each Fe30~ formula unit, there is a
neL magnetization of 4 Bohr Magnetons due to the
unopposed Fe(II) atoms. Each crystal subunit therefore
has a magnetization of 32 Bohr Magnetons packed into a
cube with a face that is 837 pm in length.
The magnetization of a ferrite can be altered by
substituting different metals into the various
interstices. Far instance, Mn(II) has a magnetization
of 5 Bohr Magnetons, so creation of an inverse spinel
with the formula Mn(II) (Fe(III) )xOA should yield crystals ,
with 5 Bohr Magnetons per unit. The use of Zn(TI) has a
quite different effect. Tt has no unfilled d-orbitals
and so has zero magnetic moment. However, zinc tends to
enter A sites causing a normal spinet organization for
the crystal. Therefore, at each formula unit, a zero
moment zinc opposes an Fe(III) with a moment of 5 Bohr
Magnetons resulting in a net moment of 5 far the pair,
the remaining Fe(III) are also unopposed, so the net
moment is 10 Bohr Magnetons per formula unit (80 Bohr
Magnetons per crystal subunit}.
In actuality, this situation can prevail only for a
low percentage of the total number of sites in a larger
crystal. Zn(II) is actually too large for the A sites
(0.77 angstrom radius) sa that as the concentration of
zinc exceeds 50%, there is a transformation into inverse
spinet structure. In this arrangement, Fe(III) opposes
FCl TTZ r r~nol l ,~.-. ..1, ..t). a. ~ .~L
-~I__, a..........1...~ a..'...., " .,2r ~vW, auu me uuOj~Fiu~CG2
Zn(II) have no moment, so the ferrite has a net
magnetization of zero. This is sometimes useful in
applications such as the heteronuclear tracers described
WO 92!11846 PCT/EP92100021
- 30
n
-below in which magnetization is not necessarily
desirable.
In 1955 the term superparamagnetism was proposed to
describe the behaviour of extremely small magnetic '
particles. The fundamental idea is that there is
sufficient thermal agitation in a small particle that '
the tendency for the magnetic dipole axis to flip into
various orientations is greater than the tendency to
align as a coherent domain with a single fixed axis.
As the particle size increases above a critical
size in the range of 10° atoms, it becomes stable and
coherently aligned as a spontaneously magnetized single
domain. Below this critical size, the magnetic
susceptibility is temperature and size dependent.
Smaller particles at higher temperatures require
stronger external fields to become delectably
magnetized. Once magnetization is achieved, however,
the total magnetization is related directly to the size
of the particle.
'fhe behaviour of a superparamagnetic particle is
described by a relaxation rate which reflects the rate
at which local magnetic moments within the particle will
flip spontaneously. In order to flip, an energy barrier
which is proportional to the volume of the particle and
to the anisotropy of the material must be overcome. In
a domain sized particle, the magnetization settles along
one single axis because the energy barrier is too great
to permit flipping at the temperature of the experiment.
At sub-domain size, the energy barrier is low enough
that the flip rate becomes exceedingly rapid. The size
at which this transition occurs is temperature dependent
and also dependent on the composition of the particle.
(For present purposes the relevant temperature for
ri°-t'er:I;l.~.ing 'i::'iCtil2r yr nOt a SL-t't.'W'GdIIC:E.' tS
superparamagnetic is body temperature).
By the substitution of some metals such as cobalt
in place of some of the Fe(II) in the lattice, the
WO 92/1146 PCf/EP92/0002x
~~~~CJ~~
- 31 -
crystals become more anisotropic and this tends to slow
the rate of flipping and so lower the critical size for
a stable domain.
When larger ions are included in the crystal
matrix, the spinal structure cannot accommodate them.
This is particularly important for the use of elements
from the lanthanide series. However, lanthanides may be
accommodated by the garnet crystal structure. The
natural form of this crystal is Ca3A12(Sio4)~ or
3Cao.A1203.3SiOZ. An analogous structure is achieved with
the composition LnjFe,50~2, wherein Ln is a lanthanide
element. (A common example made using Yttrium is called
YIG or Yttrium-Iron-Garnet and is used for instance in
lasers). Although small amounts of the lanthanides are
accommodated within spinal crystals, stoichiometries
which favour garnet formation are more important as
larger percentages of lanthanides are included.
A novel type of spinal crystal uses scandium in
place of aluminium in the preparation of coated,
colloidal spinal crystals. The most stable of these are
Mg(II)(Sc(III)).,04 or magnesium scandites. These are
helpful vehicles in several of the applications
described herein. These crystals are not magnetic.
Scandium has stable trivalent chemistry but, unlike
yttrium and lanthanides, is similar in ionic size to the
remaining transition metals.
Methods for precipitating ferrites from metal salts
date back into the 1800~s and several investigators have
modified these methods in attempts to develop improved
ferrofluids. Elmore in Phys. Rev. 54: 309-320 (1938)
explored ammonia precipitation of ultrafine ferrite
particles in aqueous solutions and first demonstrated
that their aggregation increased when they approached an
atlnl iod m~nnot.'..C ~r . d,
fic~
A further step towards developing stable colloidal
ferrofluids'came in 2965 with the development of a
method for grinding magnetic materials into fine powders
WO 92!11846
PCT/EP92/00021
..
32 -
.and then suspending them in oleic acid by sonication
(see US-A-3215572). Takada and Kiyama in Proc. Int.
Conf. (ICF-1), U. Tokyo Press (Ed. Hoshino et al), p.69-
71 (1970) reexplored a variety of methods for
precipitating ultrafine crystals of magnetite and
developed a new oxidation method although this body of
work did not address the problem of keeping the
particles in suspension.
Reimers and Khalafalla in Bu Mines TPR 59:13 (1972)
used an ammonia peptization method to create aqueous
suspensions of ground particles. In their initial
method, an acid treatment followed by sonication is used
to induce interaction with solvent molecules to prevent
clumping of the particles and maintain suspension.
Subsequently, they developed a modification of Elmore°s
ammonia precipitation method to create more stable,
dilutable suspensions in which molecules of dodecanoic
acid are chemically adsorbed onto the surface of the
magnetite particle (see Khalafalla and Reimers in IEEE
Trans Mag 16: 178-183 (1980)). This yielded dilutian-
stable solutions of superparamagnetic particles.
Biologists became interested in small magnetic
particles as potential means of carrying out biochemical
separations and developed various means of incorporating
domain sized particles into beads. These did not need
to be soluble in the form initially used. FIowever,
building on methods used to create dense immunospecific
labels for electron microscopy, an aqueous technique
developed by Molday (see US-A-4452773 and J. Immunol.
Meth 52: 353-367 (1982)) opened the way to a variety of
biological applications.
The Molday method involves an ammonia precipitation
synthesis in which dextrans are used to coat the
~rrr,ct; te. ThiC- r ~" , t~
m~m C...l~ ,~ ~.:1 u;, uqucvuS ~u~p21°sjiviY ut
superparamagnetic particles which can be conjugated to a
wide variety of types of molecules including antibodies
and so used to carry out various types of separations.
1~'~ 92/11846 ~ ~~ ~ ~~ ~ ~i ~ PCT/~P921OU021
- 33 -
The advantage of the superparamagnetism of the Molday
particles is that they do not tend to aggregate
magnetically unless they are in an applied magnetic
field. This simplifies the preparation of more
elaborate compounds while permitting recovering of the
magnetic properties when they are wanted after the
synthesis is completed.
Whitehead et al (US-A-4554088) developed a silane
binding technique in which clusters of superparamagnetic
magnetite particles each about 30 nm in size are bound
in groups into larger particles about 5o0 nm in diameter
(now marketed as "AMI-25"), In the silane matrix, the
small particles are held apart from each other and so
retain their superparamagnetism. They therefore do not
aggregate and remain relatively soluble. However, the
total magnetic moment of the entire larger particle is
quite large so that biological separations can be
carried out.
Sub-micron coated Iran oxide particles have been
proposed far use as intravascular X-ray contrast agents
and a number of other medical uses have been described
for other superparamagnetic particles including magnetic
confinement for blockage of fistulas and thrombosis of
aneurysms, use in producing focal diathermy for
treatment of infection, selective removal of tumour
cells from bone marrow, and use as MRI contrast agents.
In the field of therapeutic/prophylactic
particulate agents, the current invention achieves
particular improved characteristics through the
discovery that the use of repeated purification steps
during the synthesis greatly improves the performance of
the particles as biochemical reagents. These
purifications remove dissolved metal ions as they appear
~?,_,r;nn the shnthesic ~i:.cc. t:ay can preu:ipii.ai:e as
hydrous oxides which impair the gel flow characteristics
of the preparation during the synthesis. In addition,
by using serial filtration steps after the initial
WO 92/11846
PET/E~'92100021 ,
t~ ~''v,O~°~°
~~e~~ .e3 " ::%;'..,'.
34 -
precipitation, particles may be selected whose sizes are
appropriate f.or endocytosis by the target cells, eg.
dextran-coated spinels less than 500 angstroms in size
(including the dextran coat). This helps assure the
flow characteristics of the particles through the
remainder of the synthesis and results in the production
of only 100-500 angstrom particles which have a number
of physiological advantages.
Finally, where a targeting moiety is used, when all
these measures are taken, it is possible to take
advantage of the versatility and convenience of re-
usable agarose based affinity chromatography media to
remove all particles which are not bound to a targeting
moiety as well as permitting the discard of all
particles whose bound targeting moiety has been
inactivated or otherwise lost its specificity during the
synthetic process. The potential to use these media is ,
quite important since this permits the preparation of
affinity media with a wide variety of ligands which can
be used to purify a correspondingly wide variety of
targeted particles.
The final result is an agent with very nearly one
active targeting moiety per particle with all particles
selectively active and small enough for effective use.
This can then be concentrated or formulated as desired
and filter sterilized in small volume if necessary. The
final sterilization can be with conventional 0.2 micron
filters for bacterial clearance or with 0.1 micron
filters to assure removal of small mycobacterial
contaminants.
An alternative method of obtaining high specific '
activity is to actually coat all of the particles in the
preparation with a large number of molecules ofi the
twr tin '~ d-.. L.
g.- 7 .~~:.~e~Y . Ts~is iia5 i.iie undesirable effects of
greatly increasing the expense of the product when the
targeting moiety is expensive to produce, increasing the
antigenicity of the particle, and in many cases,
WO 92/11846
PC'f/EP92/00021
E:,..-
- 35 -
altering the distribution of the particle in undesirable
ways. It is well known from work in affinity
chromatography on solid supports that spacing and
density cf affinity ligands are crucial determinants of
efficacy.
There has been considerable interest in the medical
uses of various types of particulate therapeutic or
diagnostic agents.
Thus Widder (US-A-4849210) and 3acobsen (US-A-
4863715) demonstrated the effectiveness of suspensions
of ferromagnetic particles as intravenous MRI contrast
agents with various methods of synthesis. Groman (US-A-
4827945) provided a number of additional methods of
synthesis of superparamagnetic particles and suggested
the MR intravascular use of a wide range of labelled
particles analogous to those disclosed for in vitro use
by Molday. Although the compounds they describe are ,
physically very similar to those disclosed by Malday
(US-A-4452773) they discuss sterilization techniques and
methods of use involving diagnostic MRT. However, the
particles produced by the;methods of Groman vary in size
from 100 to 5,000 Angstroms, cannot be filter sterilized
in concentrated final form, and cannot be effectively
purified by affinity chromatography since, like the
compounds of Molday, they contain many constituents
which will not pass readily through agarose based
affinity media late in the preparation. Because of the
need for autoclaving of the Groman products, the use of
delicate protein ligands is severely limited because
they cannot withstand autoclaving. Tt is possible to
carry out the synthesis of Groman using ultraclean
facilities so that final sterilization of the product is
less important but this adds considerably to the expense
~vf auuiiuf aC tore .
Other types of particulate agent have also
generated much interest, eg, microspheres and
nanospheres. The composition of such particles include
CA 02099869 2001-03-06
- 36 -
latex polymers from various methacrylates, polylactic
acid, protein/ albumin, lipids and various other
materials (see for example Proc. Soc. Exp. Biol. Med 58:
141-146 (1978), AJR 149: 839-843 (1987), J. Cell Biol.
64: 75-88 (1975), J. Microencaps 5: 147-157 (1988), Ann
NY Acad Sci 507: 141-154 (1987), Ann NY Acad Sci 507:
120-140 (1987), Ann NY Acad Sci 507: 104-119 (1987) and
Radiol. 163: 255-258 (1987)). These particles have been
used as organic drug delivery systems, imaging agents,
and for histological studies of axonal transport. They
offer unique patterns of metabolism and biodistribution
and continue to be the subject of intense investigation
by many groups. The use of such particles for in vivo
diagnostic imaging of axonal transport or as part of a
drug delivery system that employs an intraneural route
and axonal transport is also described in
PCT/EP91/01780.
The particles used according to the invention
preferably comprise therapeutically or prophylacti~ally
loaded and optionally diagnostically marked inorganic
crystals, e.g. radionuclide containing metal oxides. It
will be recalled that in the methods of the invention
where the particles carry specific targetting moieties
radionuclides may perform a dual role: as cytotoxic
agents to kill off infected cells which en$ocytose the
particles, and as diagnostic markers to enable particle
distribution, and by implication disease distribution,
to be detected and possibly imaged. Suitable
radionuclides include a number of nuclides emitting
positron and electron (3-particles all of which can be
included in metal oxides, eg. spinels such as ferrites,
<L
~.lt:':Cr u.~ S::bStitilcTltS i1"~ ~ttc Cly5tal ldtt:tt:;e UL' i35
seeds, eg. Zr0" inside ferrite spinel shells. In one
set of embodiments, the positron emitting isotopes of
manganese (,SMn~-') , iron (,hFe''-) , cobalt (,,Co'5) , or rhodium
WO 92/11846 ~ ~ !~ (~ ~ ~ ~~ p~'/Ep9z/00021
- 37 -
(4aRh9v) are used in the synthesis of spinel particles,
eg. sub-domain sized ferrite particles. The inclusion
of cobalt or manganese in this type of ferrite has
previously been difficult to achieve efficiently, but
it is possible to reliably introduce cobalt, manganese,
or other metals in amounts up to lj3 of the number of
metal atoms per formula unit, e.g. with the remaining
2j3 being Fe(III} if the stoichiometry of the desired
crystal structure, e.g. garnet or spinel, is carefully
considered and factors such as pH, temperature, and
precursor metal salt and coating compound concentrations
and the duration of heat incubation after precipitation
are carefully controlled, preferably after optimization
by routine experimentation. Thus as an example, for
dextran coated particles it has generally been found
advantageous to precipitate out from a saturated dextran
solution. Thus all the divalent metal atoms may be
replaced as opposed to the 2 or fewer suggested by.
Groman in US-A-4827945.
These particles may be synthesized in such a way
that they are stably coated with dextran ar other
hydrophilic molecules and the coating may then if
desired be activated and bound covalently to antibodies
or any type of cell adhesion molecule which will promote
uptake of the particles by the cells which are to be
'treated according to the method of the invention:
Particles so fashioned will be detectable upon Positron
Emission Tomography (PET) as positron sources, and also
upon Magnetic Resonance Imaging (MRI) as
superparamagnetic particles. Some of these will alsa be
detectable upon Magnetic Resonance Spectroscopy (MRS) as
high receptivity nuclei at selected frequencies or on X-
ray CT scanning where the 2-number and particle
CvnCcittrativit 15 SL7~LIl:tCllL.
In positron ferrites made with ,SMn'- the emission
detection is based on the 0.511 MeV annihilation photons
due to positron decay (j3+ 2?,g%, 0.575 MeV, E.C. 72.1%)
WO 92/11~8~~ p~'/Ep92100021 ,
~~0~ ~ '~J
.,
.~ 4-.,., , ..
- 38 -
with a half life of 5.59 days and associated gamma
emissions of (1000, 1.434 MeV; 94.5%, 0.935 MeV; 90%,
0.744 MeV; 50, 1.33 MeV; 40, 1.25 MeV; 30, 0.85 MsV} to
zaCrsz which is stable. This is a decay half life which
is quite well suited to long nerve transports and to
full monoclonal antibody distribution for tumour studies
and to the assessment of particle distribution among the
various populations of CD4 positive cells. Further,
with a relatively low positron energy of just 0.575 MeV,
the spatial resolution is substantially better than any
positron emitter in active clinical use including 9F'R.
The high gamma emission may make zSMn== less attractive
for clinical use in some situations, but there are many
alternatives.
Positron ferrites can also be made with z6Fe5-' which
undergaes positron decay (/3+560, 0.804 MeV; EC 43.5%)
with a half life of 8.275 hours and associated gamma ~
emissions (99.2%, 0.169 MeV) to zSMnsz"' which is
metastable and decays with a half life of 21.1 minutes
by positron decay (~i+96.27%, 2.631 MeV; EC 1.530} and
associated gamma emission (97.80, 1.434 MeV) to stable
zaCrsz as well as by isomeric internal conversion (2.2%,
0.378 MeV) to zsMnSz.
This type of positron ferrite has the advantage of
a strong positron emission signal during the day of
injection with a fairly rapid decline towards the
continuing positron emission of the z5Mn5z with a 5.7 day
half life.
An intermediate half life can be provided by
positron ferrites made with z,eoss which undergoes
positron decay (~+ 77%, 1.54 MeV; EC 23%) with a half
life of 17.5 hours and associated gamma emissions (75%,
0.93 MeV; 16.50, 1.41 MeV; 20.3%, 0.477 MeV; 70, 1.32
M~ ~I. 3,00 -,-, SS
v , 1 . ~ v i~icv ) tv ,6Fe . '1'h1S nuCllCle O~ lrCn then
decays slowly by K-shell electron capture (0.006 MeV)
with a half life of 2.7 years to ,SMn55 which is stable.
Although the half life of this cobalt positron
WO 92/11846 ~ ~ ~ ~ ,~;1 ~ PCT/EP92/00021
- 39 -
emitter may be useful in same cases, its use is
inhibited by the decay pattern of Z6Fe55; the energy of
the photon is quite low, but the irradiation continues
for a long time and virtually all the energy is
deposited within tissue as non-penetrating radiation.
A fourth type of positron ferrite can be
synthesized with 4,SRh9y which undergoes positron decay
(1.03 MeV} with a half-life of 16.0 days and no
associated gamma emission to ~,Ruy~ which is stable. This
however is a longer half life than will generally be
needed.
The decay for ,~SC43 (~i+ 78%, 1.22 MeV; EC 22 0} and
associated gamma emission (22%, 0.373 MeV) with half
life of 3.9 hours to stable 2~Ca'3 make this very
promising. The substantial increase in ionic radius and
the tendency to change from trivalence to divalence upon
transition from Sc to Ca will be disruptive to the , '
spinet crystal, but this may aid in the more rapid
metabolism of the particles and thus the more rapid
release within the targeted cell of the therapeutically
or prophylactically effective element with which the
particle is loaded.
Except for calcium, all of these nuclides are
accommodated in the spinet ferrite crystal, although the
chromium decay products from z5Mns2 and ,6Fe"- will generate
some regions of spinet chromite (FeCr,oa) within the
inverse spinet ferrite (Mt[II]O: Fe[III],03) crystal.
Similarly, some regions of ilmenite, perovskite, and
titanium spinal will form in consequence of eg. ,;V°H
decay.
The optimal method for producing ~~Fe" with minimal
zeFe55 contamination is by the irradiation of ,aCrs°
enriched chromium with cyclotron generated 38MeV ,He4
bca~.m~ (zyr5u(t1,211}z6FC5~) with Subsequent acid extraction,
oxidation, evaporative drying, ether phase separation,
redrying and filtration for sterilization (see Zweit
Int. J. Radiat. Appl. Instrum. Part A, Appl. Radiat,
WO 92/11846 P~'T/EP92/U0021
~c y 40 - .
I~~ 39: 1197-1201 (1988)). Other reactions available
for the production of z6Fesz include zSMnss(p,4n)z6Fesz,
z°Cr""(a,xn)z6Fesz, z°Cr";n(zHe3fxn)z6Feszr
zRNi"."(p,spall)z6Fesz with
subsequent acid extraction and purification by anion
exchange chromatography, wherein z°Cr"" includes z°Crs°
(4.350) , z°Crsz (83.79 0) , z°Crs3 (9.50%) , and
z°Crs° (2.360) .
zsMnsz may also be synthesized by standard techniques
including zHe3 activation of Vanadium ,3Vs' (,He3, 2n) zsMnsz
(see Sastri Int. J. Appl. Rad. Isol. 32: 246-247 (1981))
or other cylcotron reactions including ,°Crsz (p, n) zsMns'-,
z°Crsz (d, 2n) zsMnsz. Methods for z,Coss include z6Fes° (d, n)
z~Coss,
56 Sp na1 3 55 $5
zsFe (pr2n)27C0 r ~6FE? ~ (,1'~e ,Xnp),7C0 r 25Mn (~11e3, 317)27COSSr
zsMnss (a r 4n) z7Coss r wherein z6Fe"°' is composed of z6Fes° (
5 . 82 0 ) ,
zeFesb(91.80) , z6Fes~(2.1%) , and z6Fes8(0.28%) .
Generator techniques in which a longer half-life
parent nuclide is synthesized and transported to the
clinical site with subsequent extraction of the
clinically useful daughter nuclide just prior to use can
be arranged for several useful metals. These include
°aPd~oo (4.Od K,'Y)~asRh~oo (ZOh ~i+) , ~°W~ax (69d (3-: 188m,
18m y)
~~sReEea ( 16 . 7h ~i-) , and ~60S~9a ( 6. Oy ~i-) ~~~Ir~9° ( 17 . 4h
(~-) .
A proposed cyclotron ,~Sc43 synthesis involves the
following scheme which would apply for alpha particle
bombardment of z~,Ca°o (thermal neutron cross section =
0.43 barns):
zoCa(a, zzTi3~~i+ (0.56s)~,iSca3
n)
zoCa( a z,
, Sc'
p
)
2oCao(a, ?~SCa'-~,~+(0.68s)~zo(.'a4'
d)
zoCao(a,2n)zzTiz~~i+(0.2s)~,~Sc~~i+(0.68s)~ZOCa'-'
zuCa( a "Tip
,
xn
)
z~,Ca~(ar3n)"Ti'~~~~i+(0.09s)~,~Sc'~,Q+(0.60s)~Z~Car
zoCa~(ar4n)(zzTi]
The calcium and scandium are readily separated
either by phase separation (see Hara in Int. J. Appl.
Rad. 24: 373-376 (1973)) or by chromatography (see
WO 92/11$46 ~ ~ ~ ~ ~ ~; ~ P(.T/EP92/00021
- 41 -
Kuroda in J. Chrom 22: 143-148 (1966)) which also
permits separation of any titanium.
These and other transition metal or lanthanide
nuclides can be used in the synthesis of radioactive
metal compounds (e.g. a metal oxide, metal sulphide or
alloy, such as a ferrite) for use in monoclonal antibody
based treatment of tumours by irradiation. Here again,
the biodistribution and clearance of the delivered
radionuclides is quite different from single atoms
chelated to the proteins. Intravascular injection of
Fe59 labelled particles of the type described
demonstrated a biphasic plasma half-life with: about ; of
the dose being cleared to spleen, liver; marrow, and
slightly to lung over 1-2 hours, but with a substantial
fraction of the dose demonstrating a quite prolonged
plasma half life of many hours. Each antibody molecule
can be used to deliver several hundred or several
thousand atoms of the desired nuclide so achieving a
high local dose. It should also be noted that binding
multiple emitter atoms to a single protein molecule has
been known to rapidly destroy the protein - this problem
is substantially alleviated by the particles according
to the invention because the emitting nuclei are for
example up to 100 angstroms distant from the targeting
moiety - thus the chance of any electron, positron, or
gamma-ray interacting with the targeting moiety is
reduced by several orders of magnitude. Methods
developed far antibody delivery of 3yYy° can be applied
with a far higher concentration of this nuclide included
in a conjugated ferrite.
Magnetic properties of the particles can also be
used to help control delivery.
Turning now to PET image resolution, one of the
1 imit ;tlC;l~ o:. scanuiay r c8viuti'vi~ i~ d 1 ~W 11 i. Ui Llle
distance travelled by the positron after the decay event
but before electron-positron annihilation. This
distance is dependent upon the energy of the
WO 92!11846 PCTlEP92l00021
,,~Vt'~>
".
~:;.;:..
42 -
characteristic (3 emission for a given nuclide. The
maximum range for an yFi" positron emitted at 0.64 MeV is
2.6 mm while the particles from 3.,Rbg' decay emitted at
3.35 MeV travel up to 16.5 mm before annihilation.
Along this path, the positron loses energy by
interacting with the electrons of atoms it passes,
causing a variety of ionizations and excitations. only
when most of the kinetic energy is expended does the
positron interact with an electron in a matter-
antimatter annihilation reaction generating two 0.511
MeV photons travelling approximately 180° away from each
other. The residual momentum of the positron at the
time of the annihilation imparts some translational
momentum to the emitted photons resulting in an angle
between the two which differs from 180°. Measurements
of this angle reflect the nuclide and the medium in
which the energy losses and subsequent annihilation take
place.
Tt has been known for some time that the distance
of travel. of the positron prior to annihilation is
proportional to the density of the medium. The density
of magnetite is 5,180 kg/m3, just over five times greater
than most animal tissues and, according to classical
calculations based on electron range measurements, this
potentially results in an 80% decrease in the maximum
distance travelled by a positron travelling in magnetite
as opposed to travelling in tissue. There is an
increase in Brehmsstrahlung braking radiation
proportional to the effective Z number of magnetite
(which = 52}, but this only accounts far 1% of energy
loss for a population of positrons.
The numbers stated above for travel of the positron
before annihilation reflect maxima. In fact during
r..:.s.i.trvu a~i~iasi0i~, t iG cicGay ~iiCYcJy 15 uiviueu iJecween
the positron and a neutrino and the division is
variable, thus resulting in a population of energies.
The mean energy of a positron from a given nuclide is
W~ 92/11$46 PCT/EP92/00021
- 43 -
about 1/3 of the maximum usually given as the particle
energy. The means positron energy from 25Mn5' is 0.19 MeV
and in magnetite this classically would result in a
range of about 20 microns if the travel were entirely in
magnetite.
However various elements have characteristic
positron affinities and these have profound impact an
positron lifetimes. Therefore, the classical view of
positron range in relation to a general density
measurement proves to be a substantial
oversimplification.
It can therefore be seen that by using high
affinity nuclides such as lithium in the /3-emitter
loaded particles, the positron range can be further
decreased.
In addition, it has been learned that defects in a
crystal can cause trapping of positrons. Defects in
YBaCuoX perovskite crystals are particularly effective at
positron trapping even when these materials are not in a
superconducting state, however, even mechanical stress
defects in metals are fairly effective. There are also
effects due to the magnetic field generated by a moving
positron and its interaction with the spontaneous field
of a material such as magnetite, as well as electron
interaction enhancement effects due to the number of
unpaired, anti-spin matched electrons from d or f
orbitals in the particular spinel used for the
particulate shield.
The consequence of these considerations is that it
is possible to begin with a crystal seed of a positron
emitting nuclide including several thousands atoms of
the emitter and then to precipitate a lithium or zinc
doped, defected, magnetite shield around the positron
Pmit.ti_nc-r ~n_ra Thig ghield ;;ill
a ~ - suusc a 'v2ry ial:Cje
fraction of the emitted positrons to undergo all of
their ionization producing collisional losses within the
particle and therefore to annihilate without ever
W~ 92/11846 PCT/E P92/00021
_ 4 4 _ . ,,
~~lEeav~ng) the particle. Those positrons that do emerge
from tl7e surface of the particle without being affected
by reflection or surface trapping effects will have a
greatly reduced energy distribution, travel far shorter
distances through tissue, and create far fewer
ionizations in tissue per decay event than standard
unshielded positron emitters.
The annihilation photons themselves are relatively
unaffected by the presence of ferrite as opposed to
tissue in their surroundings. Therefore, there will be
a very large decrease in tissue ionizations with only a
trivial decrease in photon emissions. Further, the
photon emissions will all take place far closer to the
location of the actual tracer atom, typically within
several microns rather than within millimetres and this
will result in an improvement in the spatial resolution
of the PET scan. Further, the annihilations, as a
population, will have lower momentum and this will shift
the population annihilation angle closer to 180°,
further improving the resolution of the scan.
This shielding effect can thus be used to achieve
extremely limited ranges of cytotoxic ionization injury.
An example of a use for such an ultra-short range ~3
emitting particle would be in the treatment of AIDS.
Here, a particle with antibadies or other adhesion
molecules targeted to infected CD4+ macrophages,
monocytes, and T-cells would be used to cause selective
endocytosis of the particles. Larger particles are
cleared from the blood stream over 1-2 hours by
monocytes and fixed RES~ cells in lymph nodes, spleen,
liver and lung, but coinjection with cold-indifferent
antibody particles allows selective uptake of ~i-emitting
particles targeted to gp-120 antigens (such as the CD4
b7.udiitg Si.tCj dCtCC:t.dIJl~ Ufl Lice ceii surLace of infected
CD4 cells. The (3-emitting particles provide the
cytotoxcity which the compromised patient is unable to
provide. Killing of infected monocytes will prevent
y
~h'O 92/11846 PCT/EP92/00021
U
- 45 -
their excessive uptake of circulating IL-2, prevent
their contact infection of approaching T-cells, and
prevent them undergoing destructive syncytial fusion
with uninfected macrophages. The rancJe of the emitted
~i-radiation can be designed to be not much greater than
the size of a single target cell, thus limiting
effective irradiation to only those cells that actually
ingest the particle and taking advantage of the terminal
Bragg peak effect which increases the ionization rate
for a low energy positron just before annihilation. A
short half life emitter could be used to minimize the
effect of increasing exposure range with digestion of
the coating (which may take days) and multiple
treatments could then be carried out. Larger particles
can be used without magnetic aggregation by composing
the shell of less magnetic nuclides.
The particles used according to the invention, e.g.,
mixed spinets, may be additionally be diagnostically
marked to enable their distribution to be detailed using
magnetic resonance spectroscopic tracing and
heteronuclear imaging methods. When large percentages
of 3Li , zvsc . z~Co ~ zsMn ~ zsCu ~ svPr ~ mLu ~ or ~SRe are
introduced into ferrite crystals these become vehicles
for delivering large groups of those atoms to a desired
site. These elements and their various isotopes have
high nuclear resonant receptivity when in the
appropriate oxidation state and electron/chemical
environment and sa the MR machine can be used as a
spectrometer to detect the presence of 'these crystals.
Any high receptivity metal in an oxidation state where
electrons do not produce confounding relaxation (e. g.
Mn'+, Co3+) or in which d-electron orbitals are entirely
empty (Sc3+) or full (Znz+) are particularly amenable.
Thv: .~.h2iviiCR. l CJL V 1~ o~iri~Giit i5 also important to minimize
the effects of quadrupolar relaxation for nuclei with I
>1/2.
Nuclei such as Fey and In'~5, ie. markers for Fey or
WO 92/11846
PCf/EP92/00021
ih.,;\..'(~'r".~'~1~
F"'..~'
,v'~;r z
- 46 -
.In"s MR imaging, can be included in compounds which can
then be included or embedded in latex, protein,
polylactic acid or other polymers and in the coating of
metal compound particles with a targeting moiety also
present in the coating.
Using particle types and delivery targeting systems
as described above, a different group of metals can be
used instead of the (3-emitters to achieve the very short
range radiotherapy effect. These are a variety of
nuclides in which decay is by K-shell capture. Although
decay in these nuclides involves collapse of an electron
into the nucleus, the resulting vacancy causes effects
among the remaining electrons which result in Auger and
Coster-Kronig electron emissions. These have extremely
low energies and resulting ranges of micron and sub-
micron distances, although several such electrons may be
emitted for each single decay event. An optimal nuclide
with this behaviour for HIV therapy is 46Pd~°' which is a
pure K-capture nuclide with a 17 day half life; ZqCrs' may
also advantageously be used.
The particles used generally should be metal
compounds capable of precipitation to a stable colloid
having a particle size suitable for cell uptake and
preferably having a surface capable of being coated with
or bound to biochemically useful materials, e.g.
carbohydrates or proteins.
The particles useful in the methods according to
the invention may be produced by relatively
straightforward methods - forming particles of a matrix
material comprising the therapeutically or
prophylactically active element and optionally a
diagnostic marker, for example by precipitation, e.g.
from an appropriately buffered solution; optionally
Sepurating vlit thClCftVlll IJdL'~:1C:.LE2S Of a desired size
range; optionally coating said particles with a
physiologically tolerable optionally biodegradable
coating material, e.g. a natural or synthetic polymer or
wo 9an asa6 ~ ~ t~ ~ ~ ~ ~ p~'/Ep92/00021
f..'::
.. - 47 -
derivative thereof such as latex, polylactic acid,
proteins, albumin, polysaccharides, starches, dextrans,
polymerized sugar alcohols, etc (see for example EP-A-
184899 (Jacobsen)}; and optionally conjugating said
particle (optionally via coupling to a said coating,
optionally after appropriate derivatization thereof e.g.
to provide a binding site or to block excess binding
sites) to a cell adhesion molecule, preferably with a
CAM: particle ratio of up to lo, especially up to 5 more
especially up to 2 and most preferably about 1;
optionally separating CAM-conjugated particles so formed
from unconjugated particles, preferably by size
separation, especially preferably by repeated size
separation followed by at least one affinity separation;
optionally sterilising the CAM-conjugated particles, if
desired after formulation thereof with a pharmaceutical
carrier and optionally with further conventional
pharmaceutical excipients, e.g, viscosity enhancing
agents, pH regulators, osmolality adjusting agents, etc.
As discussed above, the matrix material used may be
an inorganic matrix, e.g. a metal oxide, or an organic
matrix, e.g. a polymer such as a cross-linked starch or
dextran, which will serve as a carrier for the
therapeutically or prophylactically active element or
diagnostic marker.
Incorporation of the active element or marker
within a carrier matrix can be achieved by conventional
techniques, for example by co-precipitation, by steeping
a porous matrix material to impregnate it with the
desired agent or marker, by exposing the agent to
ultrasonically suspended, uncoated metal oxide
particles, or by means of the buffered precipitation
technique described herein.
Tha matrix pa.L Licies si~ouid desirably be rela~ivei.y
uniformly dimensioned, e.g. within the ranges discussed
above, and this may be achieved far example by
conventional screening or particle precipitation
WO 92!11846
~,,~.~~tl, i.;;O. PCT/EP92!00021 .
. "~
48
techniques. Monodisperse particles will be preferred.
Viewed from a further aspect the invention provides
a process for the preparation of a particulate
pharmaceutical agent according to the invention which
process comprises admixing endocytosable particles
comprising a therapeutically or prophylactically active
element with at least one pharmaceutical carrier or
excipient.
Viewed from a yet still further aspect the
invention also provides a process for the preparation of
the modified spinal, garnet and perovskite particles
according to the invention which process comprises
precipitating di and trivalent metal ions of ionic radii
such as to permit crystals of spinal, garnet or
perovskite structure to form, said precipitation being
from a solution containing an element having a desired
therapeutic or prophylactic activity, especially
palladium, and optionally also containing a further
element selected to modify the crystal structure of the
precipitated crystals to permit particle size control
and to adjust the rate of post-phagocytosis
intracellular breakdown of the particles; and optionally
conjugating resulting particles, optionally after size
separation and coating, with a cell adhesion molecule,
preferably gp120.
In the particle precipitation processes according
to the invention the active elements or markers to be
incorporated into the particles may themselves be in
solution or alternatively they may be in fine "seed"
crystals which become included in the precipitating
particles.
For administration in vivo, the dosages used will
clearly depend upon a wide range of factors such as the
pGit.l.etlt~~ lhfCl~iti., ~.I1C SpeCiIlClty of the 'fM Or CAM (fOr
TM ar CAM-conjugated agents), the nature of the active
element or diagnostic marker component of the
pharmaceutical agent, the nature extent or severity of
wo ~zm~46 ~ ~ ~ c~ ~ ~' ~ rcr!E~z!oooz~
49
the disease that is being treated, etc. The appropriate
dosage however can readily be determined taking these
factors into account. Generally doses may be expected
to be of the order of 50 micromoles of therapeutic
cation per kg bodyweight.
Endocytosable particles may also be used generally
as a research tool for investigation o.f cell function
where the particles are such as to release within the
cell cations that can be detected externally, e.g. due
to their radioactive decay or nuclear magnetic resonance
characteristics. Thus they may be used to substitute
externally detectable cations for undetectable
physiological cations in intact cells and in targetted
groups of cells in intact animals in order to mark metal
binding proteins and other biomolecules to study cation
metabolism and other activities of such proteins and
biomolecules. Viewed from this aspect the invention
provides a composition comprising particles capable of
being endocytosed and of subsequent intracellular
release of metal cations which compete with cations
native to the endocytosing cells and which are
detectable from outside the cells.
The palladium containing iron oxide particles
useful according to the invention are themselves novel
and viewed from a still further aspect the invention
provides a crystalline material comprising palladium .
disposed within an iron oxide matrix.
The invention is illustrated in more detail by the
following Example.
WO 92111846 PCT/E P92/~0021
_ 5 0 _ a.,.:..;
Example
Ferrite particle synthesis can be efficiently
carried out in less than 24 hours. The chloride salts
of the of 2+ and 3+ oxidation state metals, eg. FeC~.2,
FeClz, MtCl2, MtCl3 where Mt is a therapeutically or
prophylactically active metal or a diagnostic marker are
dissolved in a saturated or supersaturated solution of
1,500 to 10,000 MW dextran, preferably 10,000 MW in a
ratio near Mt(II)1.O:Fe(III} 2.0 at a concentration of
0.2 to 1.0 molar, and at a temperature of 0-60°C
depending upon the final particle size distribution
desired but preferably at 50°C and where Mt is the
divalent cation of a transition metal or of a mix of
transition metals. Typical starting amounts are 540 mg
FeCl3, 230 mg FeCl2, 3 gm Dextran lOK, in 4.5 ml of dH20.
The dextran solution should be heated only briefly to
avoid recrystalization or sludging. (It should be noted
however that for. palladium ferrites, dextran coating in
this fashion is not required for particle phagocytosis
and subsequent intracellular palladium release.)
Trivalent cations (such as Sc(III)) may be used in
low ratios if they are stoichiometrically balanced with
monovalent metal salts, preferably Li.CI. The ferrites
are precipitated by addition of 5 to 100, preferably
7.5% aqueous solution of NH3 to reach a pH of 9 to 12 and
preferably pH 11 (about l5 ml added to 7.5 ml of
dextran/metal salt solution). This solution can be
heated to 60°C prior to adding it to the metal/dextran
solution.
A variety of sizes of dextrans can be used, for
example ranging from 1.5K to 4oK MW although the loK
dextrans have proven most reliable in these syntheses.
CrlaT')~lPg in nytcr ~n~ty~~- - -r ;1.i0 effc.Ct t ie tut4tJl1tltj
behaviour of the particles and this can have an effect
on some resonant behaviour of the particles and on their
interactions with water molecules. It is also possible
WO 92/11$46 PC'f/EP92/00021
~~'~~~-~~~
..: .
- 51 -
'to coat the particles with non-metabolizing latex from
for example cyanoacrylate monomers to alter their rate
of processing through the cells. Other biodegradable
coatings such as polylactic acid or even protein/albumin
coats can be applied.-~ A shift in average crystal core
size towards smaller size can be produced by lowering
the temperature of the synthetic reaction or elevating
the pH. However, a variety of separation techniques may
then be required to trim the size distribution to select
the desired size range.
Additionally, the spinel crystal can be constituted
of mixed metals in various amounts in order to achieve
various specific optimizations. Mixed spinels including
various useful transition series metals, and even some
lanthanide metals can be made by adding the metal
chloride directly to the saturated dextran solution
prior to alkali precipitation.
The product of the reaction is centrifuged 2 times
at 1,0008 x 10 minutes and one time at 1,5008 x 10
minutes to remove particulates which are discarded in
the precipitate. The resulting suspension is passed
through a 2.5cm x 40 em column of Sephadex G-~25M/150
a (Pharmacia) equilibrated in O.1M NaAcetate buffer
pH6.5 in order to remove free metal ions, particulates,
ferrous hydrous oxides, chloride and ammonia.
The Sephadex eluant is then passed through
successively finer microfilters. Two passes through a
0.22 micron nylon filter are followed by two passes
through a 0.1 micron nylon filter. The third filtration
is slow but can be accomplished with 100mm or 4'7mm
diameter filters on a suction funnel using a 50 nm
filter such as Millipore ~ VMWP-04700 Cellulose MF
filters although nylon or polycarbonate filters are
prGS'CraLie. Tire speed and general success of this step
are highly dependent on the initial precipitation
conditions - being most efficacious with smaller
particle size distribution. (It should be noted that
WO 92/11846 PCT/E P92/00021
;~~u' ,:::
the ionic content of the water and the precipitation
medium and the manner of preparation and purification of
the dextran used can also affect particle size
distribution). These filtrations may also be
accomplished with centrifugal filters.
This is cleared, desalted, and size trimmed product
is then concentrated with a Centriprep-30 ~ (Amicon)
ultrafilter, at 1,500g for 45 minutes, to achieve a
final volume of five to seven ml. The sample is then
applied to a 2.5 cm x 25 cm column of Sephacryl-200
(Pharmacia) equilibrated with 0.1M NaAcetate buffer
pH6.5 with elution by the same buffer. This traps
dextran and small ferrous hydrous oxides while letting
the particles pass in the excluded, unfractionated
volume. The late tail of this fraction should be
discarded as it contains much of the hydrous oxide. The
resulting eluant is concentrated to 4 ml with a
Centriprep-30 concentrator (1,500g for 15 minutes) for
conjugation.
The particle sample in a volume of 4 ml is oxidized
adding slowly 1 ml of 20 mM NaIO~ at 23°C. This mixture
is reacted while stirring (non-magnetic stirring only)
for 6o minutes in the dark.
The period~tion reaction is halted by passing the
sample through two PD-10 Sephadex G-25M/150 columns
equilibrated with 20mM NaBorate buffer pH8.5,
concentrating with a Centriprep-3o ultrafilter to 1-2m1
then passing the sample through a third PD-10 column of
Sephadex G-25M/150 to completely remove any unreacted
periodate. The final volume is brought up to 4m1 with
borate buffer.
A protein solution is prepared having 2-lOmg of
antibody, lectin, growth factor, or other selective cell
adhesion molecuia uiss~iveu in i ml of 20mM Nat~orate
buffer, pH8.5. Where possible, blocking molecules to
protect the active/recognition site should be added at
this point if the blocker wi21 not be bound by the
WO 92/11846 ~ ~ ~ ~~~ y ~ a '~s E~'T/EP'92/00021
- 53 -
~periodate activated dextran. For example, adding 1 mid
CaCIzJMnClz helps protect the binding site on some
lectins. This solution is then added to the particle
solution, mixed, and allowed to incubate for 4 to 12
hours depending upon the molecule involved and the
number of adhesion molecules desired per particle. The
reaction is quenched by the addition of 200 microliters
of 0.5M glycine with an additional two hours of
incubation.
The covalent bonds are then reduced by the addition
of o.5 ml of 0.25M NaBH4 with allowance for the
generation of H, gas. After one hour of reaction, 'the
mixture is passed through three PD-l0 columns of ,
Sephadex G-25M/150 equilibrated with 20mM HEPES buffer
at a pH of 7.4 to remove glycine, NaBHa and HZ, then
concentrated to a 1-2m1 volume with a Centriprep-100
concentrator (500g for 60 minutes) to clear unbound
adhesion molecule and smaller, unconjugated particles.
This product is then applied to a 1.6 x 35 cm column of
Sephacryl 200 and eluted with 20mM HEPES buffer at pH
7.4. This column run further removes unbound targeting
molecules and traps any newly formed hydrous oxides.
The eluant is collected and concentrated with a
Centriprep-100 concentrator at 500g x 30 minutes to
achieve a final volume of 4m1.
The four ml of reaction product are then applied to
a 4m1 column of affinity ligand Sepharose 6B with
divinyl sulfone links (such as Sigma A2278 for some
lectins) equilibrated with 20mM I-IEPES buffer pH 7.4. 2t
is preferable to avoid conditions normally intended to
maximize binding as this may make it impossible to elute
the specific fraction. The column is then washed
extensively with four to five volumes of buffer and then
~2 :"1 ~olu~~~e of 1 ri~oini dLiinity eluant in the same
buffer is applied. This elutes the active fraction in a
fairly sharp band.
The specific fraction is collected and passes
WO 92/1146 pCf/EP92/00021
~ ._ 5 4 _
.through a PD-10 Sephadex G-25M/150 column to help clear
affinity eluant and then concentrated to 1mL with a
small volume Centricon-30 centrifugal concentrator
(1,50og x 20 minutes). This product is passed through a
second PD-10 column and the final output then
concentrated to a volume of 300 to 500 microliters with
a Centricon-30 concentrator (1,500g x 60 minutes). The
final product is then sterilized by 0.22 or 0.1 micron
filtration using a Costar 1 ml centrifugal microfilter
and stored for use.
The ferrite particles can be obtained by similar
procedures, e.g.:
(a) The ferrite particles are synthesized by a
modification of the method of Molday (J. Immunal. Meth.
52:353-367 (1982)) which can be efficiently carried out
in less than 24 hours. The chloride salts~of the metals
with the positron nuclide at specific activities of IO-
loOmCi/~CM (370 MBq-3.7GBq/~CM) of 2+ oxidation state
metal are dissolved in a supersaturated solution of
10,000 MW dextran in.a ratio near Mt(II)1.O:Fe(TII) 2.0
at a concentration of 0.5:1.0 molar and at a temperature
of 20-60°C depending upon the final particle size
distribution desired and the ferrites are precipitated
by addition of 8% aqueous solution of NH3 to reach a pH
of L1 (about 4m1 added to 2m1 of dextran/metal salt
solution), centrifuged at 1,OOOg to remove particulates,
separated and concentrated with a Centriprep-30 (Amicon)
concentrator at 2,OOOg,for collection of small particles
in the filtrate when desired.
The products of this concentration/separation step,
either filtrate (reconcentrated with Centriprep-10
concentrator) or retentate, are passed through a
prCp,:,ruti'v2 i.aiuTOl u1 JC~JIIdtl~X V-25M (15U) equ.lllbrdted
in O.1M NaAcetate buffer pH6.5 at least four times the
volume of the applied sample in order to remove free
metal ions, chloride and ammonia.
WO 92/11846 ~ ~ ~ ~ ~ ~ ~ p(,'T/EP92/00021
- 55 -
This desalted sample is again concentrated with a
Centripre,p-30 concentrator (2,5008 far one hour) to a 3-
4 ml volume then passed through a 2.5cm x 25cm column of
Sephacryl-300 (Pharmacia) equilibrated with O.1M
NaAcetate buffer pH6.5 with elution by O.1M
NaAcetate/0.15M NaCl buffer pH6.5 and 0.15M NaCl to
separate unbound dextran, and the resulting fraction
concentrated to 4m1 with a Centriprep-30 concentrator
(2,5008 for 15 minutes) and activated by reacting with 1
ml of 2omM NaI04 at 23°C while stirring (non-magnetic
stirring only) for 60 minutes in the dark.
The periodation reaction is halted by passing the
ferrite sample through a Sephadex G-25M (150) column
equilibrated with 20mM NaBorate buffer pH8.5,
concentrating with Centriprep-30 to 1-2ml then passing
the sample through a second column of Sephadex G-
25M(150) to completely remove any unreacted periodate.
The protein solution of 2-lOmg of antibody, lectin,
growth factor, or other selective cell adhesion~molecule
dissolved in 1 ml of 20mM NaBorate buffer pH8.5 is then
added to the ferrite solution, mixed, and allowed to
incubate for 4 to 12 hours depending upon the molecule
involved and the number of adhesion molecules desired
per ferrite particle. The reaction is quenched by the
addition of 200 microliters of 0.5M glycine with
additional two hours of incubation.
The covalent bonds are then reduced by the addition
of 0.5m1 of 0.25M NaBH~, with allowance for the generation
of HZ gas. After one hour of reaction, the mixture is
passed through a column of Sephadex G-25M(150)
equilibrated with 20mM HEPES buffer at a pH of 7.4 to
remove NaBH~ and H" concentrated to a 1-2m1 volume with
a Centriprep-30 concentrator (2,5008 for 30 minutes) and
apr.li~d to ~ l . SCU ;i flocs CGiuiun of Sej~ilaca~yi-3vu
equilibrated with 20mM HEPES buffer pH7.4 for subsequent
elution with 20mM HEPES/0.15M NaCl buffer pH7.4 in order
to remove unbound adhesion molecules and passaged into
WO 92/11846
pCT/EP92/OOU21
~ ~s
- 56 - ..
~~, \: '
,O.1M phosphate buffer pH7.4 via Sephadex G-25M for
administration.
The resulting fraction can then be concentrated to
a 1 ml volume with a Centriprep-30 concentrator for use
or further purified with affinity chromatography and
subsequent concentration when necessary. Reconstitution
after freeze drying can also be used if desired.
The product of the precipitation reaction may
alternatively be centrifuged 3 times at 1,0008 to remove
particulates which are discarded in the precipitate.
The resulting suspension is passed through a preparative
column of Sephadex G-25M/150 ~ (Pharmacia) equilibrated
in O.1M NaAcetate buffer pH6.5 at least five times 'the
volume of the applied sample in order to remove free
metal ions, chloride and ammonia.
This cleared and desalted product may then be
concentrated with a Centriprep-100 ~ (Amicon)
ultrafilter, at 1,5008 for two hours, resuspended and
again concentrated to a 4m1 volume. This yields good
clearance of particles below 5 nm and of unbound dextran
into the filtrate for discard and this is a preferred
method far the particulate agent.
When a range of particle sizes including smaller
particles are to be processed, this concentration step
is done with a Centriprep-30 concentrator. zn this
case, the unbound dextran will have to be removed by
applying the sample as a 3-4 ml volume to a 2.5cm x 25cm
column of Sephacryl-200 ~ (Pharmacia) equilibrated with
O.1M NaAcetate buffer pEI6.5 with elution by 0.1M
NaAcetate/0.15M NaCl buffer pH6.5 and 0.15M NaCI. The
resulting fraction concentrated to 4m1 with a
Centriprep-30 concentrator (2,5008 for 15 minutes) for
COn~l,tt~~i_ i an.
When.only very small particles are desired, the
initial concentration is done with a Centriprep-100
ultrafilter, but it is the filtrate which is then
WO 92/11846 ~ ~ ~ ~ 7 ~ ~ Pi:T/RP92/00021
- 57 -
processed further. This filtrate is reconcentrated
three times with a Centriprep-30 ultrafilter to clear
the dextran.
When primarily larger particles (in the 50 to 300
nm range) are desired, the desalted, ultrafiltered
sample is concentrated with a Centriprep-10o
concentrator (2,5008 for one hour) to a 4m1 volume and
then applied to a 2.5em x 25cm column of Sephacryl-40o R
(Pharmacia) equilibrated with 0.1M NaAcetate buffer
pH6.5 with elution by O.1M NaAcetate/0.15M NaCl buffer
pH6.5 and 0.15M NaCl. The resulting fraction
concentrated to 4m1 with a Centriprep-30 concentrator
(2,5008 for 15 minutes) for conjugation.
For some uses it is preferable for the particles to
be less than 50 nm in diameter. Therefore, the
Centriprep 100 product may be passed through first 0.2
micron and then 0.1 micron Nalgene ~ nylon microfilters.
The resulting product is then concentrated to a 2 ml
volume and applied to a 2.5cm x 50cm column of
Sephacryl-1000 ~ (Pharmacia) for size fractionation.
Particles in the later fractions are collected for
further processing.
The particle sample in a volume of 4m1 is oxidized
adding slowly l ml of 20mM Nalo' at 23°C. This mixture
is reacted while stirring (non-magnetic stirring only)
for 60 minutes in the dark.
The periodation reaction is halted by passing the
sample through a Sephadex G-25M (150) column
equilibrated with 20mM NaBorate buffer pH8.5,
concentrating with a Centriprep-30 ultrafilter to 1-2m1
'then passing the sample through a second column of
Sephadex G-25M(150) to completely remove any unreacted
periodate. The protein solution of 2-lOmg of antibody,
lectin, gr ;~.th factor, or ui.i~er selective adhesion
molecule dissolved in 1 ml of 20mM NaBorate buffer pH8.5
is then added to the particle solution, mixed, and
allowed to incubate for 4 to 12 hours depending upon the
;,
WO 92/11846 ' . PCT/EP92/00021
s.....: .
~~ ~1,~~ - 5 8 -
~olecule involved and the number of adhesion molecules
desired per particle. The reaction is quenched by the
addition of 200 microliters of 0.5M glycine with an
additional two hours of incubation.
The covalent bonds are then reduced by the addition
of 0.5m1 of 0.25M NaBH4 with allowance for the generation
of HZ gas. After one hour of reaction, the mixture is
passed through a column of Sephadex G-25M(150)
equilibrated with 20mM HEPES buffer at a pH of 7.4 to
remove NaBHa and H2, concentrated to a 1-2m1 volume with
a Centriprep-100 concentrator (1,5008 for 60 minutes) to
clear unbound adhesion molecule and smaller,
unconjugated particles. This product can then be .
passaged into O.1M phosphate buffer pH7.4 via Sephadex
G-25M for administration, or further purified by
affinity chromatography on non-porous beads ar Nalgene
affinity membranes.
The resulting fraction can then be diluted to 20m1
in sterile buffer and passed through a 0.2 micron or
preferably 0.1 micron microfilter to assure
sterilization. The final product is concentrated to a
lm1 volume with a Centriprep-100 concentrator for use.
Reconstitution after freeze drying can also be used to
achieve desired concentrations for some preparations.
Alternatively the product of the precipitation
reaction is centrifuged 2 times at 1,0008 x 10 minutes
and one time at 1,5008 x 10 minutes to remove
particulates which are discarded in the precipitate.
The resulting suspension is passed through a 2.5cm x 40
cm of Sephadex G-25M/150 ~ (Pharmacia) equilibrated in
O.1M NaAcetate buffer pH6.5 in order to remove free
metal ions, particulates, ferrous hydrous oxides,
chloride and ammonia. The Sephadex eluant is then
pu33cC~.a t hrOui~ h SL1~:CC531Z%Cly riner microfiiters. 'i'wo
passes through a 0.22 micron nylon filter are followed
by two passes through a 0.2 micron nylon filter. The
third filtration is slow but can be accomplished with
WO 92!11846 ~? ~ ~ ,~ PCTlEP92l00021
- 59 -
100mm or 47mm diameter filters on a suction funnel using
a 50 nm filter such as Millipore ~ VMWP-04700 Cellulose
MF filters.
This cleared, desalted, and size trimmed product is
then concentrated with a Centriprep-30 ~ (Amicon}
ultrafilter, at 1,5008 for 45 minutes, to achieve a
final volume of five to seven ml. The sample is then
applied to a 2.5em x 25 cm colum of Sephacryl-200 0
(Pharmacia) equilibrated with O.1M NaAcetate buffer
pH6.5 with elution by the same buffer. This traps
dextran and small ferrous hydrous oxides while letting
the particles pass in the excluded, unfractionated
volume. The late tail of this fraction should be
discarded as it contains much of the hydrous oxide. The
resulting eluant is concentrated to 4 ml with a
Centriprep-30 concentrator (1,5008 for 15 minutes) for
conjugation.
The particle sample in a volume of 4 ml is axidized
adding slowly 1 ml of 2omM NaIOA at 23"C. This mixture
is reacted while stirring (non-magnetic stirring only}
for 60 minutes in the dark.
The periodation reaction is halted by passing the
sample through two PD-10 Sephadex G-25M/150 columns
equilibrated with 20mM NaBorate buffer pH8.5,
concentrating with a Centriprep-30 ultrafilter to 1-2m1
then passing the sample through a third PD-10 column of
Sephadex G-25M/150 to completely remove any unreacted
periodate. The final volume is brought up to 4m:L with
borate buffer.
The protein solution of 2-lomg of antibody, lectin,
growth factor, or other selective cell adhesion molecule
dissolved in 1 ml of 20mM NaBorate buffer pH8.5. Where
possible, blocking molecules to protect the
active/ recog:,ition site sii~uiu be added at this point if
the blocker will not be bound by the periodate activated
dextran. For example, adding 1 mM CaCl,/MnCl, helps
protect the binding site on some lectins. This solution
WO 92/11846 ~s~ P~'/Ep92100021
- 60 -
is then added to the particle solution, mixed, and
allowed to incubate for 4 to 12 hours depending upon the
molecule involved and the number of adhesion molecules
desired per particle. The reaction is quenched by the
addition of 200 microliters of 0.5M glycine with an
additional two hours of incubation.
The covalent bonds are then reduced by the addition
of 0.5m1 of 0.25M NaBH4 with allowance for the generation
of H, gas. After one hour of reaction, the mixture is
passed through three PD-10 columns of Sephadex G-
125M/150 equilibrated with 20mM HEPES'buffer at a pH of
7.4 to remove glycine, NaBH~ and H" then concentrated to
a 1-2m1 volume with a Centriprep-100 concentrator (500g
for 60 minutes) to clear unbound adhesion molecule and
smaller, unconjugated particles. This product is then
applied to a 1.6 x 35 cm column of Sephacryl 200 and
eluted with 20mM HEPES buffer at pH 7.4. This column
run further removes unbound targeting molecules and
traps any newly formed hydrous oxides. The eluant is
collected and concentrated with a Centriprep-100
concentrator at 5008 x 30 minutes to achieve a final
volume of 4m1.
The four ml of reaction product are then applied to
a 4m1 column of affinity ligand Sepharose 6B with
divinyl sulfone links (such as Sigma A2278 for some
lectins) equilibrated with 20mM HEPES buffer pH7.4. It
is preferable to avoid conditions normally intended to
maximize binding as this may make it impossible to elute
the specific fraction. The column is then washed
extensively with four to five volumes of buffer and then
a 2 ml volume of Z molar affinity eluant in the same
buffer is applied. This elutes the active fraction in a
fairly sharp band.
Th° °p°ClflC fruCtivii is CV't'tCct~U dnd paSSeS
through a PD-10 Sephadex G-25M/150 column to help clear
affinity eluant and then concentrated to 1 ml with a
small volume Centricon-30 centrifugal concentrator
WO X2/11846 PC'T/EP92/00021
2~~~~~~~~~
c
- 61 -
~(1,50og x 2o minutes). This product is passed through a
second PD-10 column and the final output then
concentrated to a volume of 300 to 500 microliters with
a Centricon-30 concentrator (1,5008 x 60 minutes). The
final product is then sterilized by 0.22 or 0.1 micron
filtration using a Costar 1 ml centrifugal microfilter
and stored for use.
(b) Coated particles and particularly ferrite particles
have been prepared by a novel buffered precipitation
technique. This type of synthesis is particularly
helpful when a coating is required which includes
delicate molecules which cannot tolerate the strong
alkaline conditions used in other precipitation methods.
The chloride salts of the desired divalent and trivalent
metals, preferably Fe2+ and Fe3+ but also including a~
variety of other transition and other metals are
dissolved in H20 or in dextran/H20 solution or in a
saturated dextran/H,O solution at a metal concentration
of 0.2 to 1.0 molar as indicated by the dictates of
stoichiometry and of the requirements of the particular
application. Lower concentrations will tend to produce
particle size distributions including generally smaller
particles.
A precipitation bath is prepared as a strong buffer
solution in H20, for example 1 molar HEPES or Z molar
Tris. When only iron chlorides are used, the pH of the
solution may be as low as pH 6.0, although solutions at
a pH of 7.4 are effective for many rations. When the
intended coating molecule can tolerate higher pH, the
buffer solution may be prepared at the highest tolerable
pH, as for instance with many proteins which readily
tolerate pH of 8:0 or 8.5.
The protein, pharmaceutical, targeting moiety, or
other biomolaaiiLe is dissolved in the buffer solution at
a concentration of 1 to 100 mg/ml or at higher or lower
concentrations optimized for the expense of the agent
and the efficiency of binding for a given type of
WO 92111$46 1 ~~ pCT/EP92/00021
~,~~~'~~
'~>
- 62 -
molecule. The precipitation bath may also include
bovine serum albumin, peptides, dextrans or other
molecules which can help to coat the particles along
with the pharmaceutically active agent. The bath is
heated to 37°C for many proteins, or may be used at
ambient temperature, or at 0-4°C, or at higher
temperatures up to 60°C as tolerated by the coating
molecule of choice.
The metal chloride solution mixture is then brought
to the chosen reaction temperature and added to the
buffered precipitation/coating bath in dropwise fashion
with continuous or intermittent mixing, preferably by a
non-magnetic mixing technique. The ionic strength of
the buffer bath will need to be two to ten, times greater
than the ionic strength of the metal solutions,
preferably four to six times greater. This will limit
the total volume as well as the total concentration of .
the metal solution, but in any case will result.in the
precipitation bath retaining its pH in the tolerable
buffered range throughout the process of adding the
metal solution.
For example, one ml of a solution of 0.33 M PdCl2
and 0.66 M of FeCl3 may be added to ten ml of a solution
of HEPES pH 7.8 1 Molar, with two mg per ml of CD4,
possibly blocked with gp120 fragments to protect the
binding site, and also 20 mg/ml of azidodideoxythymidine
(AZT).
The precipitation mixture is incubated for 20 to 40
minutes at the reaction.temperature, then centrifuged at
l,OOOg three times for 20 minutes each and the
supernatants saved while any precipitate is discarded.
The particle solution is then passed through a column of
Sephadex G-25M equilibrated in the final buffer of
VhVlI.C a~ fot~ example in. H~;r~;5 pH7..4 200 mMwhere the
volume of the column is five times the volume of the
reaction product. The excluded fraction is collected
then concentrated in Amicon Centriprep-30 ultrafilters
WO 92!11846 "' ~' U 11 ~ PCT/EP92/00021
- 63 -
~t 1,SOOg x 45 minutes, diluted in buffer to 24 ml
total, and then reconcentrated in Centriprep-100
ultrafilters to clear unbound coating molecules which
are small than MW 100,000.
This concentrate may either be applied to an
affinity column to selectively purify coated particles
with active targeting moiety (e.g. on immobilized gp-
220, or brought directly to filter sterilization in
200nm centrifugal microfilters where no targeting moiety
is used.
The efficacy of a range of different cationic cell
therapy particles in the treatment of intracellular '
infection was demonstrated using as a model an assay of
HIV-1 RT activity derived from the assay described by
Potts (in "Techniques in HIV research", Ed Aldovini et
al, Stockton, NY, 1990, pp 103-106) and also by
demonstrating particle phagocytosis by target c~lzs by
electron micragraphy. These investigations are
discussed below with reference to the accompanying
drawings, in which:
Figures 1 is a graph indicating the effects on HIV-
1 RT activity of a range of metal cations (Me) alone or
in combination with magnesium;
Figure 2 is an electron micrograph of a THP-1
(human macrophage) cell illustrating cellular uptake of
particles according to the invention;
Figures 3 and 4 are graphs showing the effects of
particles according to the invention on cell viability;
Figure 5 is a graph showing the effects on HIV
infection, as measured by HIV-1 RT activity, of cation
cell therapy using different cations;
Figure 6 is a graph illustrating the blood
clearance rate for intravenously injected particles;
F'i_nltra 7 i c a cn c..,ati~ dr,::~. ~ g f X1'1 dT ' iru5;
..... in o ,. a . aG v
and
Figures 8 to 14 illustrate schematically stages of
HIV infection and replication.
WO 92/11846
PCf/EP92/00(321
.'
.. '3 6 4
Before turning to the experimental details it may
be helpful to provide a brief description of the
structure and replication of the HIV virus. Thus in
Figure 7 there are illustrated the gp120/gp41 coat
glycoproteins (1} of HIV-1 virus disposed in the
external lipid bilayer (3) which~surrounds the viral
core outer shell (2) which itself comprises p18
subunits. Within outer shell (2) is the inner core (?)
which is made up of p24 subunits and within this the
nucleocapsid gag protein (4), the RT enzyme (5) and the
genomic RNA (6).
Figure 8 shows the viral gp120 protein bound to CD4
protein (8) in the lipid bilayer (9) of a macrophage
cell surface. The Figure also shows an intact palladium
ferrite dextrin coated particle (l0) with conjugated
gp120, being phagocytosed into an endosome, and a
partially digested ferrite particle (11) in:v the
macrophage. Figure 9 illustrates the process of
uncoating of the HIV viral genome inside a human
macrophage, the partially uncoated virus (12) having
lost the viral lipid coat which has fused with the
cellular lipid coat (13). The Figure also illustrates
an alternate entry path for virus after phagocytosis
with subsequent uncoating into internal endoplasmic
channels (14).
Figure 10 provides a schematic indication of the
role of metal rations in viral replication. Thus (15)
indicates a metal ration bound to reverse transcriptase
enzyme attached to genomic RNA strand (16). The
metal/RT enzyme remains during completion of the RNA-
dependent DNA polymerise step of reverse transcription
(2?) and remains attached to the DNA (18) during
completion of the RNase H step to degrade the RNA
tem 1 ato ~nr7 tY~~ f a_
or.,.atl~u of ~.ilG t,c~ukjle St4L'dnded viral
DNA (18) which is completed by DNA dependent DNA
polymerise function of the reverse transcriptase enzyme.
Figure 11 shows,the nuclear membrane (20) of the
i
CVO 92111846 ' ~ ~l~ PCT/EP92i00021 r
~~tJ~:.)~~
r:;r:~.
- 65 -
Host cell with integrase protein (21) establishing
provirus by inserting the viral DNA into the host DNA
genome. The CD4 protein (22) in cell surface lipid
bilayer is also shown. Figure 12 illustrates the trans-
activator of transcription (tat) protein (23) with metal
cation (15) at the dimerization site and shows
transcription of mRNA/genomic RNA (24) under control of
tat as well as showing the integrated proviral DNA (25).
Figure 13 shows how the nucleocapsid protein (4)
binds the viral RNA (6). A metal nation (15) maintains
the conformation at the nucleic acid binding site. In
Figure 14 it is shown how the nucleocapsid protein (4)
is bound into the forming p24 inner core (7). The two
copies of the genomic RNA (6) are attached to the capsid
by the nucleocapsid protein. A cation (15) is involved
at a dimerization site between the two RNA strands.
Reverse transcriptase (RT) assays with a wide ,
variety of metal cations can be conducted by modifying
the Potts (Supra) method to accommodate the special
requirements for solubility imposed by the chemistry of
the various elements. Dithiothreitol (DTT) or elevated
pH causes immediate precipitation of transition metals
so the suggestion of Temin et al (Nature 226: 1211-1213
(1970)) who showed good activity without DTT when assays
were run at 0-4°C were followed and a buffer pH of 7.3
as talerated by most of the cations and by the enzyme
was used. Copper cations precipitate in HEPES buffer
but not in Tris, Ferrous and ferric cations are
insoluble at pI-I greater,than 4.0 and so iron cannot be
used in these assays without a "metal buffer".chelator
at greatly reduced effective concentrations.
The inhibitory effects of copper, nickel, and zinc
were similar to those found with RT from MoMuLV (Moloney
M!~rl.n~-' Leuk°=n~.a ~,11rt1~) , 1',..Ti.lt tile paiiadiuiti
:ii'~'1111~1C.lOn l.n
MoMuLV was not reversed by magnesium. Also, the ability
of higher concentrations of manganese to inhibit the HIV
enzyme in the presence of magnesium was not seen in
WO 92/11846
P(.'ClEP92/00021
.-..
f-'~
:~ - 66 -
MoMuLV .
In dextran coated particles for cationic. cell
therapy, ferric iron can, be used to establish a spinel
structured ceramic oxide crystal ( [ Fay'' ], [Nlt~* ] O4} and the
divalent metal sites in the crystal fill entirely with
the selected divalent cation (Fe'*, Mn'*, Pd'*, or Ni2*) or
a stoichiometric mixture of elements. The crystal
structures of some compounds have not been established
but are probably either of garnet or perovskite type and
the synthesis described produces a low yield in the
desired size range. Each ferrite particle used in these
trials contains about 10s-106 metal atoms so the
concentration of 3 mM for the trivalent element used in
the THP-1 experiment reflects a concentration of
particles in the nanomolar range. Uptake studies with
s9Fe labelled particles show a rate of approximately 5 x
10~ particles per THP-1 cell per hour over 2 hours.
Since the particles are readily seen by electron
microscopy, it was ,possible to directly confirm their
presence both adherent to the cell surface and in
intracellular locations in amounts consistent with the
s9Fe results. Intravenous injection of a bolus of 50
~Moles/kg of the particles revealed four hour vascular
clearance in the rabbit primarily to spleen, marrow,
liver and lung of 75% of the injected dose, closely
reflecting human data for similar MR contrast agents
(see Ranney, Ann. NY Acad Sci 507: 104-119 (1987)).
Only the Fe/Mn particles significantly affected
THP-1 cell viability relative to either untreated,
uninfected controls or relative to untreated, infected
controls (see figures 3 and 4). Both Fe/Mn and Fe/Pd
particles effectively aborted the viral infection.
Cation source particles may be coated with dextran
t1-.nd CO!ljptatre~j +'~ tBrgCtin g prGteinS SiiC h aS fW lc~mLnL.S
of CD4 or of gp120. The dextran coat can be augmented
or replaced with coatings which include co-active
antiviral agents. However, there is a great potential
WO 92/11846 ~ ~ j ~ ~' ~;; ~~ PCT/EP92/00021
- 67 -
for the use of simple, untargeted, dextran coated
palladium ferrite particles for macrophage directed HIV
treatment and acute prophylaxis.
Figure 1 shows HIV-1 reverse transcriptase activity
with divalent transition metals. Assays for Fig. 1 ran
at 0-4°C with no DTT in 150mM HEPES, pH7.3 except Cu2+
which was run in 150mM Tris, pH 7.5. The various
complete buffers made in deionized, metal free water
with 75mM KC1, poly-rA 5~,gjml, oligo dT~,2_,g~ 5~,g/ml,
peroxide free NP-40 0.05%, and [3zP]dTTp S~.Ci/ml. The
various cocktails'were transferred to 96 well plates in
volumes of 100' ~.l/well, and the plates were then frozen.
Metal solutions were prepared as 250 mM in 0.1 N HC1 and
serial dilutions then made in O.1N HC1 to set up 96 well
plates containing the various metals in the various
final concentrations. The RT cocktail plates were then
thawed and 20 ~cl of HIV infected cell free supernatant
added to ane set of plates while a control set received
20 ~,1 of uninfected cell free supernatant from identical
cultures and media, and an internal control well for
each complete buffer channel received both 10 units of
purified MoMuLV RT (Pharmacia) and 20 ul of cell free,
uninfected supernatant. Reactions were started by
transferring the various metal cation dilutions and MgCl2
dilutions in 8 ~cl volumes to appropriate locations in
HIV, MoMuLV, and control wells for each
buffer/DTT/temperature condition using a multi-channel
pipettor. 5 ~l dot transfers to pre-numbered array
locations on DE81 Whatman paper at 2 hours (34°C plates)
or at 4 hours (0-4°C plates} after start of the reaction
were dried, washed, cut in squares arid (3-counted in 5 ml
of scintillant. For this graph, activity from each
control well is subtracted from the value for its
t'nrrn~pnnrling ~~~~~' vi.~..11, i~viiuLV ~.ntE11la1. CVI~II.LUl~..i dL~E.'
not shown, and only one magnesium value is shown for
each temperature condition.
In Figure 2 there is shown an electron micrograph
WO 92/11846 P(.'T/EP92/00021
~~,~ .1 - 68 -
T~
section (60,000x) of a THP-1 cell (see Int. J. Cancer
76: 171-176 (1980)) showing ingested and surface
adherent cation loaded particles. THP-1 cells were
incubated at 37°C for two hours with Fe/Pd dextran
- coated particles at a concentration equivalent to 3mM
Fe3+, then washed, fixed in glutaraldehyde/PBS, spun,
osmicated, embedded, and sectioned without staining.
For each type of particle, 0.33 mMole of trivalent metal
chloride hydrate and 0.1? mMole of divalent chloride
hydrate (except Fe'-+ added as 0.20 mMole to account fo.r
oxidation before precipitation) were added directly to
solutions made up by dissolving 500 mg 10,000 MW dextran
in 750 ~.1 of H,o. PdCl., hydrates slawly so requires 12
hours in 4N HC1 (e. g. 100 ~cl for 35 mg of the chloride)
to dissolve fully and is added as a solution to the
dextran. 7,50 NH3 solution is heated to 65°C and after
briefly heating each metal/dextran solution, 3m1 of the
hot NH3 solution added as six 500 ~1 aliquots for each
mix, with 4 ml required for the Fe/Pd preparation. The
precipitated, coated particles are incubated at 60°C for
one hour, during which the Fe/Pd solution gradually
clears and the resulting suspensions then spun at 1,OOOg
x 10 min twice to clear large particles. 1.5 ml of each
' solution is passed through a PD-IO Sephadex (Pharmacia)
column equilibrated in 50 mM HEPES pH 7.4 buffering, to
I
remove ammonia, and to clear unreacted ions. The 2.5 ml
eluants are diluted to a volume of 14 ml with 50 mM
HEPES pH 7,4 then concentrated in two steps in
Centriprep-100 (Amicon), ultrafilters at 500g x 30min,
and 50og x 1 hour to a final volume of 0.5 ml to clear
free dextran. These concentrates are then sterilized by
0.2 micron filtration with (Costar) 1m1 volume
centrifugal microfilters at 3,o00g x 1 hour. Final
products were used for THP--I ir.f~cti~~ity trials and fvr
EM studies (Fe/Fe and Fe/Pd). Similar Fe/Fe particles
including syFe and subject to 0.1 micron filtration were
used for THP-I uptake studies and intravenous
WO 92111846 '~ ~~ ~ '~ ~ ~~ ,~ PG'f/EP92/000~1 ,
- 69 -
distribution/clearance assessment.
Figure 3 shows the relative viability of cation
source particle treated, uninfected compared to
untreated, uninfected cells, and Figure 4 shows the
relative viability of treated, infected compared to
untreated, infected cells. Figure 5 shows RT assays (8
hours reaction time) from the various treatments. THP-1
cells were washed and incubated for two hours with one
of four cation source particle suspensions at a
concentration adjusted to be equivalent to 3mM for the
trivalent ferric cation (nanomolar particle
concentrations) after which the cells were washed then
seeded in 24 well microtitre plates at a concentration
of 50,000 cells per well, four identical wells per metal
and four control channels. After 24 hours, an aliquot
of cells was withdrawn from each well to check viability
and identical quantities of FiTV RF strain then
inoculated into two of each of the four wells in each .
group of cultures and incubated with regular medium
changes. Un days 3, 7, 11, and 14, aliquots of
supernatant were withdrawn and stored at -70°C for
subsequent Patts (Supra) RT assay (dot transfers for
counting were done after 2 hours and after 8 hours) and
at the same time aliquots of cells were removed for
total cell count and assessment of % viability by Trypan
Blue exclusion.