Note: Descriptions are shown in the official language in which they were submitted.
BIOCIDAL SELF-ADHESIVE AND PROCESS FOR PRODUCING THE SAME,
AS WELL AS SELF-ADHESIVE ARTICLE AND APPLICATION THEREOF
TECHNICAL FIELD
The present invention relates to a biocidal self-adhesive
comprising an adhesive containing an iso-thiocyanate efficiently held
therein to have bacteriostatic, bactericidal and antimicrobial
properties and the like, and a process for producing the same, as well
as a self-adhesive article made by efficiently applying such a self-
adhesive onto a support. Particularly, the present invention relates to
such a biocidal self-adhesive which can be put to uses widely intended
for the purpose of providing bactericidal, fungicidal and microbiocidal
effects in building and food-related fields and the like.
The present invention also relates to an iso-thiocyanate gradually-
releasing sheet, as an application of a self-adhesive article of the
type described above, made by efficiently holding an iso-thiocyanate
onto a support such as a paper, a non-woven fabric or the like through
the medium of an adhesive or a low volatile oily liquid, so that the
sheet has antibacterial, bactericidal and antimicrobial properties and
the like, and particularly,. such an iso-thiocyanate gradually-releasing
sheet which can be put to uses widely intended for the purpose of
providing bactericidal, fungicidal and microbiocidal effects in various
fields such as building, food-related and shoe insole fields.
Further, the present invention relates to a biocidal or freshness-
maintaining self-adhesive sheet, as another application of a self-
adhesive article of the type described above, having antibacterial and
bactericidal properties and made by efficiently applying an adhesive
containing an iso-thiocyanate held therein onto a support, and to a
- 1 -
sterilizing and freshness-maintaining method using such a sheet for the
purpose of providing bactericidal, fungicidal and microbiocidal effects
to a variety of materials to be enclosed by the sheet, including food
products such as vegetables and fruits, fresh foods, dried goods and
grains.
BACKGROUND ART
It is known that an iso-thiocyanate contained in a mustard or a
wasabi (i.e., Japanese horseradish) have bacteriostatic and
bactericidal actions, and any of synthetic and natural iso-thiocyanates
provides bacteriostatic and bactericidal effects even at an extremely
small concenetrat.ion of vapor thereof on the order of 10-odd ppm to
hundreds ppm. Before now after reporting of this fact, several attempts
have been made to adsorb the iso-thiocyanate to a porous carrier such
as clay minerals or to a surface of a film or a sheet to industrially
utilize the bacteriostatic and bactericidal actions thereof, and a
certain article made in such a manner, e.g., a freshness maintaining
material has been brought to market.
In the above prior art, however, it is impossible to utilize the
bacteriostatic and bactericidal actions of the iso-thiocyanate
profitably. This is because much of the iso-thiocyanate adsorbed in
these methods volatilizes off in the course of production of the
article or during storage of the article, because of its high
volatility, and consequently, an effective amount of iso-thiocyanate
cannot be supported on a carrier. Particularly, when the iso-
thiocyanate is adsorbed to a porous carrier, it is necessary to take a
measure that the iso-thiocyanate supported on a porous carrier is
placed into a sack having a gradully releasing property, because the
releasing rate largely depends upon the temperature. When the iso-
-2-
1
a ;
thiocyanate is adsorbed to a film or a sheet, there is encountered a
problem that it is difficult to impregnate the film or the sheet with
an amount of iso-thiocyanate enough to exhibit an efficacy, but also it
is difficult to control the gradually releasing.
The freshness maintaining means which has conventionally been used
is a vacuum pack, or Ageless (Trademark) which is a deoxygenator
consisting essentially of active iron oxide, for preventing the
oxidation and the propagation of bacteria. However, with the vacuum
pack, a special apparatus is required, and with the Ageless, it is
necessary to pack the Ageless together with a material to be packed.
Many food products such as breads, fishes, meats, box lunches, etc.,
are only packed with a film such as polyvinyliden chloride and
polyethylene (PE) films, or only placed into a container made of a
polyvinyliden chloride or a polyethylene. In this case, they are, of
course, not durable at room temperature and hence, it is necessary to
store them, for example, in a refrigerator.
Thereupon, it is conceived to utilize a freshness maintaining
material made using the iso-thiocyanate, but this is also accompanied
by the above-described problems, resulting in a difficulty to put it to
practical use.
DISCLOSURE OF THE INVENTION
The present inventors have repeated zealous studies and as a
result, it have been found that by using, as an adhesive, a polymer
soluble in or swellable by an iso-thiocyanate, the iso-thiocyanatethe
can be held in the polyer by impregnation, with physical properties of
the adhesive being maintained, thus overcoming the above-described
problems.
A biocidal self-adhesive according to the present invention
-3-
,! 4,.' "'~
comprises an adhesive impregnated with an iso-thiocyanate to hold the
latter therein, the adhesive consisting essentially of a polymer which
is soluble in or swellable by the iso-thiocyanate.
A process for producing a biocidal self-adhesive according to the
present invention comprises the steps of forming an adhesive layer from
an adhesive consisting essentially of a polymer which is soluble in or
swellable by an iso-thiocyanate, and impregnating the adhesive layer
with the iso-thiocyanate.
Further, a self-adhesive article according to the present invention
is made by applying a biocidal self-adhesive of the type described
above onto at least one surface of a base material.
Examples of the adhesives consisting essentially of the polymer
soluble in or swellable by the iso-thiocyanate include acrylic
adhesives such as copolymers of one or more of acrylic and methacrylic
esters including n-butyl acrylate and methacrylate, hexyl acrylate and
methacrylate, iso-octyl acrylate and methacrylate, 2-methoxyethyl
acrylate and methacrylate, 2-ethylhexyl acrylate and methacrylate, decyl
acrylate and methacrylate, dodecyl acrylate and methacrylate, tridecyl
acrylate and methacrylate, with a functional monomer which is
copolymerizable with such esters, such as acrylic, methacrylic,
itaconic, malefic and anhydrous malefic acids, hydroxyethyl acrylate,
hydroxypropyl acrylate, acrylic amido, dimethylacrylic amido,
methylaminoethyl methacrylate, and methoxyethyl acrylate and
methacrylate7 vinyl polymer adhesives such as ethyl-vinyl ether,
propyl-vinyl ether, butyl-vinyl ether and 2-ethylhexyl-vinyl ether; and
rubber-based adhesives consisting essentially of natural rubbers and
synthetic rubbers such as stylene-isoprene-stylene block terpolymer,
stylene-butadiene copolymer, polybutene and butyl rubbers.
-
n~(!'~ .f. "i
1~ 'r) ~ .
The acrylic, rubber-based and vinyl polymer adhesives can be
selectively used, but it is preferred to selectively use any one of the
acrylic adhesives. A particulaly preferable adhesive is a copolymer of
an alkyl acrylate or methacrylate having 4 or more carbon atoms in an
alkyl group and a monomer which is copolymerizable with such ester
monomer, and a cross-linked copolymer is more preferable.
Optionally, other additives may be incorporated into the adhesive,
including tackifiers such as terpene and petroleum resins; softners such
as liquid paraffin, animal and vegetable oils, e.g., olive, soybean and
bovine oils and lard, polybutene, lower isoprene and wax; fillers such
as titanium oxide, zinc oxide, aluminum methasilicate, calcium sulfate
and calcium phosphate; water; emulsifiers such as sorbitan mono-oleate,
sodium lauryl sulfonate, emulsifier aids such as magnesium and aluminum
stealates. It should be noted that any of adhesives other than those
described herein can be used, if they are capable of being dissolved in
an iso-thiocyanate or impregnated with an iso-thiocyanates by swelling
to hold the iso-thiocyanate therein.
The iso-thiocyanates which can be used are vaxious esters
irrespective of aliphatic or aromatic type such as the allyl and alkyl
esters of iso-thiocyanic acid, but particularly when the self-adhesive
of the present invention is intended for food products, it is preferred
to use natural iso-thiocyanate contained in an extraction from a
mustard and the like.
The adhesive is preferably impregnated with an iso-thiocyanate to
hold the latter therein at a concentration within a range of 0.1 to 50
~ by weight based on the adhesive, and desirably to hold the iso-
thiocyanate therein in an amount of 0.1 g/mz or more. In impregnating
the adhesive with an isa-thiocyanate, the iso-thiocyanate may be mixed
-5-
..
~; .i t
with a solvent or fatty oil having a good compatibility with the
adhesive, and the adhesive may be then impregnated with the resulting
mixture. The method for impregnating the adhesive with the iso-
thiocyanate is not limited and may be a gravure coating and a Mayer bar-
coating, as well as a spray coating, a curtain coating, a stripe coating
from a nozzle, an immersion, etc. It is preferable that the
impregnating process is carried out at a temperature of an atmosphere
including the adhesive layer in a range of room temperature or less.
To produce the above-described biocidal self--adhesive, an adhesive
promptly soluble in or swellable by an iso-thiocyanaye because of its
compatibility with the latter is applied to a gas-barrierable film
capable of barriering the iso-thiocyanaye, such as an aluminum-
deposited polyethylene terephthalate film, thereby forming an adhesive
layer, and impregnating the adhesive layer with the iso-thiocyanate.
This process ensures that an amount of iso-thiacyanate which is not
available in the prior art process can be supported effectively. It
should be noted that the gradual releasing of the iso-thiocyanate can be
controlled by laminating a film such as polyethylene and polypropylene
films having a permeability to the iso-thiocyanate, to a surface of the
adhesive layer. In addition, the iso-thiocyanate can be held for a long
period of time by laminating a gas-barrierable film to the surface of
the adhesive layer.
The self-adhesive article is basically of such a configuration that
the biocidal self-adhesive is applied to at least one surface of a base
material, including the above-described configuration. More
specifically, the self--adhesive article comprises a biocidal self-
adhesive applied to the whole or a portion of one surface or opposite
surfaces of a base material such as a resinous film made of a
-6-
2~.~~ ~e~~
polyethylene terephthalate, a polypropylene, a polyvinyl chloride, a
ployethylene, a polycarbonate, a polyvinylidene chloride, an
polyacrylonitrile, an ethylene-vinyl alcohol copolymer, etc.; a paper;
a fabric; a synthetic paper; a metal foil or the like. Usually, a film
treated with a release agent and having a gas-barrierability is
previously temporarily laminated to the self-adhesive article in order
to protect the adhesive layer. Such a self-adhesive article is referred
to as an adhesive label or an adhesive sheet. It will be understood that
the article of the present invention includes the form of a so-called
adhesive tape made by applying an adhesive layer to one surface of a
base material, while treating the other surface with a release agent,
and rolling the resulting material.
It is as described above that the gradual releasing can be
controlled by changing the gas-barrierability of the base material. It
is optional that a deposited or coated layer may be provided on the
base material, if required.
With the biocidal self-adhesive and the self-adhesive article
impregnated with the iso-thiocyanate to hold the latter therein
according to the present invention, the bacteriostatic, bactericidal,
fungicidal, microbiocidal and freshness-maintaining effects possessed
by the iso-thiocyanate can be widely utilized in a variety of fields,
because the iso-thiocyanate can be held in the adhesive at a high
concentration and effectively, but also it is possible to utilize the
releasing of the vapor of the iso-thiocyanate without need for a
special apparatus by profitably using an advantage of the adhesive that
the article can be bonded to all materials. In addition, the use of the
process comprising the steps of forming the adhesive layer and then
impregnating the adhesive layer with the iso-thiocyanate ensures that
-7-
the iso-thiocyanate can be hold in the adhesive layer at a far higher
concentration, as compared with a process comprising the steps of
previously incorporating an iso-thiocyanate into an adhesive and then
forming an adhesive layer.
The present inventors have repeated zealous studies for an
application of the above-described self-adhesive article and as a
result, it has been found that 'the above-described problems are overcome
by a combination of an iso-thiocyanate-impermeable barrier sheet and/or
an iso-thiocyanate permeation controlling sheet with a self-adhesive
impregnated with an iso-thiocyanate to hold the latter therein, or an
iso-thiocyanate-containing layer formed from a paper or a non-woven
fabric impregnated with an iso-thiocyanate to hold the latter therein
through the meadium of a low volatile oily liquid.
More specifically, an iso-thiocyanate gradually-releasing sheet
according to the present invention comprises an iso-thiocyanate
supporting layer which is covered, on its opposite sides, with an iso-
thiocyanate-impermeable barrier sheet and/or an iso-thiocyanate
permeation controlling sheet.
In this case, when one side of the layer is covered with the
barrier sheet and the other side is covered with the controlling sheet,
or when both the sides are covered with the controlling sheet, the
gradual releasing of the iso-thiocyanate is controlled by the
controlling sheet. When both the sides are covered with the barrier
sheet, the gradual releasing of the iso-thiocyanate is controlled by
the exposed area of the end face of the iso-thiocyanate supporting
layer.
The iso-thiocyanate supporting layer is formed by impregnating an
adhesive with the iso-thiocyanate to hold the latter therein, or by
_8_
1 i!2 i.)
~ _,. J tiW:a ~~. .x
impregnating a support SLlCh as a paper and a non-woven fabric with the
iso-thiocyanate to hold the latter therein through the medium of a low
volatile oily liquid or the like.
In impregnating the adhesive with the iso-th:iocyanate to hold the
latter therein, the adhesive promptly soluble in or swellable by the
iso-thiocyanate because of its compartibility with the iso-thiocyanate
is applied to the gas-barrierable film such as an aluminum-deposited
polyethylene terephthalate for barriering the iso-thiocyanate, thereby
forming an adhesive layer, which is then impregnated with the iso-
thiocyanate, and a film permeable to the iso-thiocyanate, such as a
polyethylene film, a polypropylene film, an extremely thin polyethylene
terephthalate film, is laminated to adhesive layer, thereby forming an
iso-thiocyanate gradually-releasing sheet. This process ensures that an
amount of the iso-thiocyanate which is incapable of being supported in
the prior art can be supported effectively.
When the paper or the non-woven fabric is impregnated with the iso-
thiocyanate to hold the latter therein through the medium of a low
volatile oily liquid or the like, the low volatile oily liquid such as
a polybutene containing the iso-thiocyanate dissolved therein is applied
to a non-laminated surface of a paper or a non-woven fabric laminated,
on its one side, by an adhesive or by coextrusion, with a gas-
barrierable film for barriering the iso-thiocyanate, such as an
aluminum-deposited polyethylene terephthalate film, and such surface is
further laminated with a film permeable to the iso-thiocyanate, such as
a polyethylene film formed through an adhesive applying treatment,
thereby forming an iso-thiocyanate gradually-releasing sheet. This
process ensures that an amount of the iso-thiocyanate which is incapable
of being supported in the prior art can be supported effectively.
_g_
Examples o.f the low volatile oily liquids are a polybutene, acrylic
and metacrylic resins having a lower molecular weight, an epoxy resin,
mineral oils, animal and vegetable oils such as olive, soybean and
bovine oils, liquid paraffin, etc., but any liquids other than those
described herein by way of example can be used, if they have a
compartibility with the iso--thiocyanates, cannot react with the iso-
thiocyanates and have a fluidity at ambient temperature.
When the iso-thiocyanate supporting layer is formed of an adhesive,
it is desirable that the iso-thiocyanate is held in the adhesive by
impregnation at a concentration in a range of 0.1 to 50 $ by weight
based on the adhesive. Even if the iso-thiocyanate supporting layer is
formed of either an adhesive or the paper or the non-woven fabric, it is
desirable that the iso-thiocyanate is held in the adhesive or the paper
or the non-woven fabric by impregnation at an amount of 0.1 g/mz or
more.
However, the amount of the iso-thiocyanate is selected depending
upon individual uses and hence, may be out of the above-described range
in some cases.
Examples of the iso-thiocyanate-impermeable barrier sheets employed
in the present invention include resinous films such as polyvinylidene
chloride, polyacrylonitrile, ethylene-vinyl alcohol copolymer,
polyethylene terephthalate and polycarbonate films; metal foils and the
like. Examples of the iso-thiocyanate permeation controlling sheets
include resinous films such as polyethylene, polypropylene and
polyvinyl chloride films, papers, fabrics and synthetic papers. Even
with the barrier sheet, the permeability thereof can be varied by
reducing the thickness of a film and thus, such a barrier sheet can be
also used as a controlling sheet.
-10-
21~~~'~~~
The iso-thiocyanate-impermeable barrier sheet and the iso-
thiocyanate-permeation controlling sheet may be used as a single layer
structure or as a different sheet-laminated structure.
The iso-thiocyanates and the adhesives soluble in or swellable by
the iso-thiocyanates which can be used are those described above for
the adhesive article.
The iso-thiocyanate gradually-releasing sheet according to the
present invention is capable of holding the iso-thiocyanate therein at a
high concentration and effectively by the above-described
configuration, but also capable of controlling the releasing of the
vapor of the iso-thiocyanate by selection of the film.
Further, the present inventors have repeated zealous studies for
another application of the above-described adhesive article and as a
result, have overcome the problems in the prior art freshness
maintaining means by utilizing the above-described knowledge that by
using, as an adhesive, a polymer soluble in or swellable by an iso-
thiocyanate, the iso-thiocyanate can be held in the polymer by
impregnation, with physical properties of the adhesive being maintained.
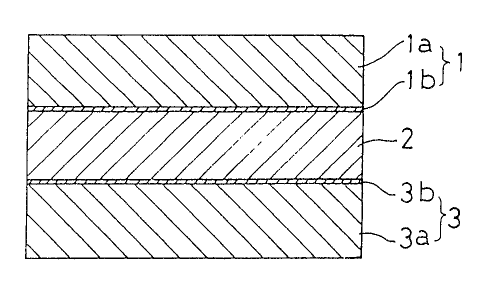
More specifically; a self-adhesive sheet according to the present
invention for sterilizing a material to be packed and maintaining the
freshness of the material comprises an adhesive layer 2 impregnated
with an iso-thiocyanate to hold the latter therein and mounted on a
base sheet 1 having a low permeability to the iso-thiocyanate, as
compared with a packing material for a material to be packed, as shown
in Fig. 1.
Tn the self-adhesive sheet shown in Fig.l, used as a base sheet 1
was a sheet which includes an aluminum deposited film 1b on one side of
a polyethylene terephthalate (PET) film 1a and which has little
- 1 1 -
~)~~~(~r~~~1
permeability to iso-thiocyanates. In Fig.l, reference numeral 3
designates an iso-thiocyanate-barrierable release sheet including a
silicone-treated release layer 3b on one side of a PET film 3a.
The self-adhesive sheet for sterilizing a material to be packed and
maintaining the freshness of the material is selected depending upon
the relative relationship with a packing material, but it is preferable
that a base material having a completely iso-thiocyanate-barriering
property is used. Such materials include polymers such as a polyethylene
terephthalate, a polycarbonate, a polyvinylidene chloride, a
polyacrylonitrile and an ethylene-vinyl alcohol copolymer, and metal
foils.
With the above-described sterilizing and freshness-maintaining
self-adhesive sheet, the types of the adhesive and the iso-thiocyanate,
the amount and method of. impregnation of the iso-thiocyanate in adhesive
and the self-adhesive sheet producing process are the same as those
described above for the self-adhesive article and hence, the
description thereof is not repeated here.
A method for sterilizing and freshness-maintaining a material to be
packed according to the present invention comprises affixing a self-
adhesive sheet of the type described above to an outer surface of a
packing material for a material to be packed.
Thus, it is possible to sterilize the material to be packed and to
maintain the freshness of such material, without destruction of the
packing material. The packing material is not limited in any way, and
any compositions can be used, if they have a lower barrierability to
iso-thiocyanates than that of the self-adhesive base material.
The extrernely appropriate sterilization and freshness-maintaining
can be performed by selecting the size of the self-adhesive sheet, the
-12-
affixing place, the affixing time and the amount of iso-thiocyanate held
by impregnation.
According to the 'present invention, the freshness--maintaining,
bactericidal and fungicidal effects and like for food products can be
widely utilized, because the above-described means makes it possible to
release the vapor of the iso-thiocyanate through the packing material to
the content in the packing material such as food products without need
for a special apparatus by profitably using an advantage of the adhesive
that the article and sheet can be bonded to all materials.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig.1 is a diagram illustrating a biocidal self-adhesive sheet for
sterilizing a material to be packed and maintaining the freshness of the
material according to the present invention;
Fig.2 is a graph illustrating the iso-thiocyanate releasing
characteristic of a biocidal self-adhesive according to the present
invention;
Fig.3 is a diagram illustrating one embodiment of an iso-
thiocyanate gradually-releasing sheet according to the present
invention;
Fig.4 is a diagram illustrating another embodiment of an iso-
thiocyanate gradually-releasing sheet according to the present
invention; and
Fig.5 is a diagram illustrating a further embodiment of an iso-
thiocyanate gradually-releasing sheet according to the present
invention.
THE BEST MODE FOR CARRYING 0'UT THE INVENTION
The present invention will now be described in detail by way of
particular examples, but is not limited to these examples in any way.
-13-
~f~ y'ii
Example 1
There was prepared an ethyl acetate solution containing 40 o by
weight of a copolymer formed of 97 ~ by weight of 2-ethylhexyl acrylate
and 3 o by weight of acrylic acid. An isocyanate-based cross-linking
agent was added in an amount of 1 o by weight to the solution, and the
mixture was applied onto an aluminum-deposited polyester film. The
resulting material was dried for 2 minutes at 100 °C , thus producing
an
self-adhesive sheet with its adhesive layer having a thickness of 30~ m.
Further, the adhesive layer was impreganted with a stock solution of an
allyl iso-thiocyanate at room temperature, thereby preparing a self-
adhesive sheet containing 10 ~ by weight of the allyl iso-thiocyanate
in the adhesive. An aluminum-deposited polyester film treated on one
side with a silicone was laminated to the self-adhesive sheet to provide
a self-adhesive article having a release film thereon.
Example 2
A self-adhesive article having a release film thereon was produced
in the same manner as in Example 1, except that 0.1 ~ by weight of the
allyl iso-thiocyanate was contained in the adhesive.
Example 3
A self-adhesive article having a release film thereon was produced
in the same manner as in Example 1, except that 25 ~ by weight of the
allyl iso-thiocyanate was contained in the adhesive.
Example 4
A self-adhesive article having a release film thereon was produced
in the same manner as in Example 1, except that 50 $ by weight of 'the
allyl iso-thiocyanate was contained in the adhesive.
Example 5
Dissolved in toluene were 100 parts of a stylene-isoprene-stylene
-l~-
CA 02100074 2001-12-13
block copolymer (Califlex*TR1107 made by Shell Chemistry Corp.), 60
parts of liquid paraffin and 150 parts of a tackifier (Alcori P100 made
by Arakawa Chemistry K.K.). The resulting solution was applied to an
aluminum-deposited polyester film, and dried for 2 minutes at 100°C ,
thereby producing a self-adhesive sheet with its adhesive layer having a
thickness of 30,~,1m. Further, the adhesive layer was impreganted with a
stock solution of an allyl iso-thiocyanate at room temperature, thereby
preparing a self-adhesive sheet containing 5 $ by weight of the allyl
iso-thiocyanate. An aluminum-deposited polyester film treated on one
side with a silicone was laminated to the self-adhesive sheet to
provide a self-adhesive article having a release film thereon.
Example 6
A solution of 2-ethylhexyl-acrylate acrylic acid copolymer in ethyl
acetate, prepared in the same manner as in Example 1, .was applied onto
a release surface of an aluminum-deposited polyester film having one
side treated with a release agent, and was then dried for 2 minutes at
100 °C , thereby producing a self-adhesive sheet with its adhesive
layer
having a thickness of 25,u m. This sheet was laminated to a non-woven
fabric. Further, an adhesive layer having a thickness of 25,~,~m was
separately formed on a release surface of an aluminum-deposited
polyester film treated on one side with a release agent, and was
impregnated with an allyl iso-thiocyanate at room temperature. The non-
woven fabric side of the self-adhesive sheet/non-woven fabric laminate
and the adhesive side of the self-adhesive sheet impregnated with the
allyl iso-thiocyanate were laminated to each other, thereby providing a
double-sided self-adhesive article containing 10 ~ by weight of the
allyl iso-thiocyanate.
Comparative Example 1
* Trademark
-15-
~~~ ~~~1 i
Using a silicone adhesive, an adhesive layer of 30 ~ m was formed
on an aluminum-deposited polyester film, and a stock solution of an
allyl iso-thiocyanate was applied to the adhesive layer to provide a
self-adhesive sheet. This sheet is laminated to an aluminum-deposited
film treated on one side with a fluorine-based release agent, thereby
providing a self-adhesive article having a release film laminated to the
aluminum-deposited film.
Comparative Example 2
A double sided self-adhesive article was produced in the same
manner as in Example 6, except that the adhesive layer was impregnated
with no allyl iso-thiocyanate.
Then, the adhesion (according to JIS 20237) was measured for the
self-adhesive articles produced in Examples 1 to 5 and Comparative
Example 1, thereby providing results as given in Table 1 below.
Table 1
Example Adhesion (g/25 mm, against SUS)
1 900
2 1,200
3 650
4 160
1,350
Comparative Example
1 0
As apparent from Table 1, it can be seen that each of the self-
adhesive articles produced in Examples 1 to 5 has an adhesion property,
even if the allyl iso-thiocyanate is contained therein at a high
concentration, and this enables the formation of a sheet from these
articles, the affixing thereof to an object and the lamination thereof.
-16-
On the contrast, it can be seen that when the silicone adhesive
insoluble in or inswellable by the allyl iso-thiocyanate is used as the
adhesive, the resulting article has no adhesion property, because the
allyl iso-thiocyanate remains as liquid on the surface of the adhesive
and the surface of the release film.
The ~ residue of the allyl iso-thiocyanate, when the self-adhesive
article having the release film and produced in Example 1 was left to
stand at room temperature, was measured to provide results as given in
Table 2.
Table 2
Residue ~
Lapse of time (day)
Example a ter ay a ter aye a ter aye a te~ays
1 99.3 99.0 98.2 96.7
As apparent from Table 2, it can be seen that even if the self-
adhesive article is left to stand at room temperature, the
volatilization of the allyl iso-thiocyanate can be reduced to the
minimum by the fact that the self-adhesive article assumes the form as
in Example 1.
Then, using the double-sided self-adhesive articles produced in
Example 6 and Comparative Example 2, two veneers each having a
thickness of 5 mm were laminated thereto. The resulting laminates were
left to stand in a 70 % Rh atmosphere at 25°C , and the propagation of
fungi thereon was observed with the lapse of time. As a result, when
the double sided self-adhesive article produced in Example 6 was used,
little generation of fangi was observed even after one month, whereas
when the double sided self-adhesive article produced in Comparative
Example 2 was used, fungi were generated on a portion of the laminate
after one week, and the fungi were spreaded over the substantially
-17-
CA 02100074 2001-12-13
entire surface of the laminate after two weeks.
Then, the release film of each of the self-adhesive articles having
the release film and produced in Examples 1, 3 and 4 was peeled off and
left to stand in a 500 cm~ stainless container, so that the adhesive
surface cannot come into contact with an inner wall of the container.
The concentration of the allyl iso-thiocyanate in the container was
measured with the lapse of time by a gas chromatography. The result
showed that each of the self-adhesive articles released the allyl iso-
thiocyanate rapidly, so that the concentration of the allyl iso-
thiocyanate in the sealed container was kept constant, as shown in
Fig.2.
Example 7
There was prepared an ethyl acetate solution containing 40 ~ by
weight of a copolymer formed of 97 $ by weight of 2-ethylhexyl acrylate
and 3 $ by weight of acrylic acid. An iso-cyanate-based cross-linking
agent was added in an amount of 1 $ by weight to the solution. The
resulting mixture was applied to a polyester film having a thickness of
38,~ m and dried for one minute at 100 °C to form an adhesive layer of
30
,~t m. Then, an allyl iso-thiocyanate was applied to the adhesive layer in
a room temperature condition by a gravure coating (using a gravure
mesh: Lattice 120 Mesh made by Asahi Roll, Corp.), so that the allyl
iso-thiocyanate was contained in the adhesive layer. The resulting film
was laminated to a polyethylene film having a thickness of 50u m to
produce an allyl iso-thiocyanate containing sheet.
Example 8
An acrylic adhesive layer of 30 a m was formed on a polyester film
having a thickness of 38 ,u m in the same manner as in Example 7. Then,
using a Mayer bar, the adhesive surface was coated with an allyl iso-
* Trademark
-18-
r~ ,~, i.s "~; -
n, .;_ ~,~ e.~ . ~ _ ~..:
thiocyanate, so that the allyl iso-thiocyanate was contained in the
adhesive layer. The resulting film was laminated to a polyester film
treated with a release agent and having a thickness of 38 ~ m to provide
a self-adhesive sheet containing the allyl iso-thiocyanate.
Comparative Example 3
An iso-cyanate-based cross-linking agant was added in an amount of
1 o by weight to an ethyl actate solution containing 40 $ by weight of a
copolymer formed of 97 ~ by weight of 2-ethylhexyl acrylate and 3 $ by
weight of acrylic acid, and an allyl iso-thiocyanate was added to the
solution in an amount of 20 parts by weight per 100 parts of the
solution. The resulting mixture was applied to a polyester film having
a thickness of 38 ,~ m and dried for 1 minute at 80°C to form an
adhesive
layer of 30,~ m. The resulting film was laminated to a polyethylene film
having a thickness of 50 ,~ m to provide an allyl iso-thiocyanate
containing sheet.
Comparative Example 4
Added to an emulsion solution containing 65 ~ by weight of a
copolymer formed of 97 ~ by weight of 2-ethylhexyl acrylate and 3 ~ by
weight of acrylic acid was an allyl iso-thiocyanate in an amount of
32.5 parts by weight per 100 parts by weight of the solution. The
resulting mixture was applied to a polyester :Film having a thickness of
38 ,~ m and dried for 1 minute at 80°C to form an adhesive layer of
30,~
m. The resulting film was laminated to a polyester film treated with a
release agent and having a thickness of 38 ~t m to provide an allyl iso-
thiocyanate containing sheet.
Then, the content of each of the allyl iso-thiocyanates in Examples
7 and 8 and Comparative Examples 3 and 4 was measured to provide
results as given in Table 3.
- 1 9 --
co '~ ,'~~ ''~ ''~ "1
i., ~'_. .. '..~ ~.~ I. ~:.9C
The content was determined by cutting a sample sheet into a piece
having a size of 20 cm x 10 cm, leaving the cut piece to stand in an
atmosphere at 40°C under 10 Torr for 48 hours using a vacuum drier,
removing the allyl iso-thiocyanate, and finding the content from the
resulting variation in weight and the amount of adhesive applied.
Table 3
Example Content of allyl iso-thiocyanate (o)
7 19.19
8 28.26
Comparative Example
3 0.11
4 0.23
Example 9
An acrylic adhesive layer 11 containing 20 ~ by weight of an allyl
iso-thiocyanate was formed on a deposited surface 10a of an aluminum-
deposited polyethylene terephthalate film 10 having a thickness of 25;~
m, as shown in Fig.3. The resulting film and a polyethylene film 12
having a thickness of 60,t~ m were laminated to each other to provide a
gradually releasing sheet.
The releasing of the allyl iso-thiocyanate was examined, and the
result showed that the releasing was sustained for about one hour at a
release rate of 10.6 g/m' ~ hr.
Example 10
A gradually releasing sheet was produced in the same manner as in
Example 9, except that the polyethylene in Example 9 was replaced by a
polyethylene terephthalate film having a thickness of 16,~ m.
The releasing of the allyl iso-thiocyanate was examined, and the
result showed that the releasing was sustained for about 36 hours at a
-20-
,.
N ~ ~l~a ~~~
release rate of 0.28 g/m' ~ hr.
Example 11
A gradually releasing sheet was produced in the same manner as in
Example 9, except that the polyethylene in Example 9 was replaced by a
polyethylene terephthalate film having a thickness of 50,u m.
The releasing of the allyl iso-thiocyanate was examined, and the
result showed that the releasing was sustained for about 105 hours at a
release rate of 0.09 g/m= ~ hr.
Example 12
A gradually releasing sheet was produced in the same manner as in
Example 9, except that the polyethylene in Example 9 was replaced by a
polypropylene film having a thickness of 60 ,~ m.
The releasing of the allyl iso-thiocyanate was examined, and the
result showed that the releasing was sustained fox about 12 hours at a
release rate of 0.83 g/m' ~ hr.
Example 13
A gradually releasing sheet was produced in the same manner as in
Example 9, except that the polyethylene in Example 9 was replaced by an
aluminum-deposited polyethylene terephthalate film having a thickness
of 25,u m.
The releasing of the allyl iso-thiocyanate was examined, and the
result showed that the releasing was sustained for about 2900 hours at a
release rate of 0.003 g/mz ~ hr.
Example 14
An acrylic adhesive layer 21 containing 20 $ by weight of an allyl
iso-thiocyanat2 was formed on a deposited surface 20a of an aluminum-
deposited polyethylene terephthalate film 20 having a thickness of 25,~
m, as shown in Fig.4. The film was laminated to a wood free paper 22
-21-
CA 02100074 2001-12-13
(Konywrap White 100 g/m2 made by Lintec Corp.). The adhesive surface of
a sheet comprising an acrylic adhesive layer 24 formed on a
polyethylene film 23 having a thickness of 60,~ m was laminated to the
paper surface of the resulting laminate to provide a gradulally
releasing sheet having a nerve.
The releasing of the allyl iso-thiocyanate was examined, and the
result showed that the releasing was sustained for about one hour at a
release rate of 9.5 g/m~ ~ hr.
Example 15
An acrylic adhesive layer 31 was formed on a deposited surface 30a
of an aluminum-deposited polyethylene terephthalate film 30 having a
thickness of 25u m, as shown in Fig.5. The film was laminated to a
paper 32 (Linter paper LCL-145 made by Taihei Paper Co., Ltd.) to
prepared a laminate 33.
Then, an allyl iso-thiocyanate was dissolved in a polybutene
(Nisseki*Polybutene HV-100 made by Nippon Oil Co., Ltd.), so that the
concentration of the allyl iso-thiocyanate was of 5 $ by weight. This
solution of allyl iso-thiocyanate in the polybutene was applied to the
paper surface of the laminate 33, and then, an adhesive surface of a
sheet having an acrylic adhesive layer 35 formed on a polyethylene
terephthalate film 34 having a thickness of 25 ,~ m was laminated to the
coated surface of the laminate 33 to provide a gradulally releasing
sheet.
The releasing of the allyl iso-thiocyanate was examined, and the
result showed that the releasing was sustained for about 60 hours at a
release rate of 0.14 g/m' ~ hr.
Example 16
A self-adhesive sheet was produced, in accordance with the
* Trademark
-22-
configuration shown in Fig.l, by permitting an allyl iso-thiocyanate to
be contained in an amount of 10 $ by weight in a self-adhesive article
which was made by adding an iso-cyanate-based cross-linking agent in an
amount of 1 ~ by weight to a copolymer acrylic adhesive formed of 97
by weight of 2-ethylhexyl acrylate and 3 ~ by weight of acrylic acid. A
loaf of bread and a boiled rice were placed into a polyethylene sack
(120 mm x 85 mm x 0.08 mm) which was then sealed. Subsequently, the
self-adhesive sheet was cut into a piece having a size of 2 cm x 2 cm,
and the release sheet was peeled off from the cut piece. The resulting
sheet was affixed to the polyethylene sack from the ouitside. This sack
was left to stand at room temperature. After two weeks, with regard to
the loaf of bread, no variation was observed, and with regard to the
boiled rice, a moisture condensation was only observed on an inner
surface of the sack.
As a control, a loaf of bread and a boiled rice were placed into a
polyethylene sack which was then sealed, in the same manner as in
Example 16. This sack was left to stand at room temperature without
affixing of the self-adhesive sheet thereto. After about 5 days, fungi
were developed on the loaf of bread, and after 10 days, these fungi
were spreaded over the substantially entire surface of the loaf of
bread. With regard to the boiled rice, on the other hand, fungi were
developed thereon after 4 days, and the rice was rotted to have no
trace of original form after 10 days.
Example 17
A self-adhesive sheet similar to that in Example 16 was cut into a
piece of 5 cm x 5 cm, and the release sheet was peeled off therefrom.
Then, the cut piece was affixed to a central portion of an outer surface
of a polyethylene package of each of three packs of boiled noodles. In
-23-
~w .~ ~' ~ ~' r~ ~~
addition, three packs of boiled noodles with no self-adhesive sheet
affixed thereto were prepared as a control. The six packs in total were
stored at room temperature, and after 7 days, variations in freshness
thereof were compared.
The result of observation was as given in Table 4. A distinct
freshness-maintaining effect due to the affixing of the a11y1 iso-
thiocyanate containing self-adhesive sheet was observed, as given in
Table 4.
Table 4
Sample Result of observation
c ange o co or Fou sme
A -ter days ayer 'Trays a ter ays a ter ays
group with 0/3 1/3 * 0/3 0/3
self-adhesive
sheet affixed
Control group 1/3 3/3 2/3 3/3
* change of color extremely in part
Example 18
Twelve small rice cake packs each placed into a polyethylene sack
were divided into four groups each comprising three rice cake packs.
One group was used as a control (untreated), and with regard t~ the
three other groups, an allyl iso-thiocyanate containing self-adhesive
sheet similar to that in Example 16 was affixed to a central portion of
a wrapping film of each group under conditions given in Table 5.
Table 5
Test No. Size of self-adhesive sheet (cm')
~(1) 3
(2) 5
(3) 7
(4) 0 (Control)
-24-
G f r-, r
~~ ~.I~a~ ~r;~
Each sample was stored for long time at room temperature, and the
test was carried out for the following items:
1) The condition of fungi developed: after 1 day, 3 days and 7 days from
the affixing
2) The remaining odor of mustard: after 1 day and 7 days from the
affixing
The test results were as given in Table 6.
Table 6
Test Test result
group Appearance Remaining mustar odor *
No. a ter ay a ter ays after ays a ter day a ter days
1 - - + + -
2 - - + + -
g - _ _ + -
4 - _+ +
(control)
* Judgement of the appearance is as follows:
+ Fungi were developed over the substantially entire region
~ Fungi were developed in part
- There was no change
** Criterion of judgment is as follow:
+ There was odor of mustard
+ There was a little odor of mustard
- There was no odor of mustard
In the group with the allyl iso-thiocyanate containing self-
adhesive sheet affixed, the development of fungi was inhibited in
proportion to the size of the sheet. On the other hand, the odor of
mustard was sensed in all of the groups (1) to (3) after 1 day, but was
reduced after 7 days to such an extent that it could not be already
sensed, which indicates the odor of this degree little exert an
-25-
2~~.~~~~
influence to the food taste.
Possibility of Industrial Application
With the biocidal self-adhesive and the process for producing the
same according to the present invention, the iso-thiocyanate can be
supported at a high concentration and moreover, the self-adhesive
article can be properly affixed to a desired place in any form such as
sheet, tape and label forms by utilization of the adhesion property of
the adhesive, thereby effectively utilizing the bacteriostatic,
bactericidal and microbiocidal properties of the iso-thiocyanate.
In the prior art methods for incorporating the iso-thiocyanate in
clathration to a cyclodextrin and for adsorbing the iso-thiocyanate to
a porous carrier such as a clay mineral, a slight amount of the iso-
thiocyanate is merely contained in the final form of the sheet, but also
the cost of the sheet is very high. On the contrast, with the iso-
thiocyanate gradually-releasing sheet of the present invention, a large
amount of the iso-thiocyanate can be contained in the sheet and
further, the gradual releasing of the iso-thiocyanate is possible. In
addition, the cost can be minimized, leading to an inexpensive article.
In the case of the prior art vacuum pack and Ageless (Trademark),
it is necesaary to conduct the packing in the packing line together
with the material to be packed, and the sealability and the air-
tightness are required for packing. On the contrast, with the self-
adhesive sheet for sterilizing the material to be packed and for
maintaining the freshness of the material to be packed according to the
present invention, even if a small amount of the iso-thiocyanate is
leaked, the iso-thiocyanate is replenished from the self-adhesive
sheet. Therefore, the sterilizing and freshness-maintaining effects can
be exhibited sufficiently, even if the air-tightness is small. Further,
-26-
the time for affixing of the sheet need not necessarily be in the
manufacture line, and even if the sheet is affixed after buying of a
food product by a general purchaser, the same effect is obtained.
-27-