Note: Descriptions are shown in the official language in which they were submitted.
` ` 2100~
O.Z. 0050/43411
Substituted acryl~tes and crop protection acents
containin~ them
The present invention relates to sub~tituted
acrylates and their use as crop protection agents, in
particular for controlling fungi, insects, nematodes and
spider mites.
It i8 known that acrylate~, for example methyl ~-
(2-hydroxyphenyl)-~-me~hoxyacrylate (EP 251 082, EP 178
826) can be used as fungicides. However, their fungicidal
action i~ unsatisfactory.
We have found, surprisingly, that sub~tituted
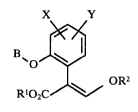
acrylates of the formula I
X Y
B~o~ I
Rl02C oR2
where
B is alkyl which is sub~tituted by 1-4 identical or
different sub~tituents R3, alkenyl which i8 substituted
by 1-4 identical or different ~ubstituents R4, alkynyl
which i8 ~ub~titu~ed by 1-4 identical or different
substit~ents R5, cycloalkyl which is sub6tituted by 1-4
identical or d:ifferent sub~tituents R6, cycloalkenyl which
i8 ~ubstituted by 1-4 identical or different substituents
R7, cycloalkynyl which is substituted by 1-4 identical or
different sub~tituents R8 or heterocyclyl which is
substituted by 1-4 identical or different substituents R9,
X and Y independently of one another ~re each hydrogen,
halogen, cyano, nitro, haloalkyl, alkyl, haloalkoxy,
alkenyl, alkynyl, cycloalkyl, aryl, hetaryl, heterocycl-
yl, cycloalkenyl, cycloal~ynyl, ~lkoxy, alkenyloxy,
alkynyloxy, cycloalkyloxy, aryloxy, hetarylo~y, hetero-
cyclyloxy, cycloalkenyloxy, cycloalkynyloxy, alkoximino,
21~0~46
- 2 - O.Z. 0050/43411
alkonyloximino, alkynyloximino, cycloalkyloximino,
cycloalkenyloximino, cycloalkynyloximino, aryloximino,
hetaryloximino, heterocyclyloximino, alkoxycarbonyl,
alkenyloxycarbonyl, alkynyloxycarbonyl, cycloalkoxy-
carbonyl aryloxycarbonyl, hetaryloxycarbonyl, hetero-
cyclyloxycarbonyl,cylcoalkenyloxycarbonyl,cycloalkynyl-
oxycarbonyl, alkylaminocarbonyl, dialkylaminocarbonyl,
alkenylaminocarbonyl, dialkenylaminocarbonyl, alkynyl-
aminocarbonyl, dialkynylaminocarbonyl, cycloalkylamino
carbonyl, cycloalkenylaminocarbonyl, cycloalkynylamino-
carbonyl, arylaminocarbonyl, hetarylaminocarbonyl,
heterocyclylaminocarbonyl, alkylthio, alkenylthio,
alkynylthio, cycloalkylthio, arylthio, hetarylthio,
heterocyclylthio, cycloalkenylthio, cycloalkynylthio,
alkylamino, alkenylamino, alkynylamino, cycloalkylamino,
arylamino, hetarylamino, heterocyclylamino, cycloalkenyl-
amino, cycloalkynylamino, alkylcarbonyl, alkenylcarbonyl,
alkynylcarbonyl, cycloalkylcarbonyl, arylcarbonyl, het-
arylcarbonyl,heterocyclylcarbonyl,cycloalkenylcarbonyl,
cycloalkynylcarbonyl, alkylsulfoxyl, alkenylsulfoxyl,
alkynylsulfoxyl, cycloalkylsulfoxyl, arylsulfoxyl,
hetarylsulfoxyl, heterocyclylsulfoxyl, cycloalkynyl-
.. sulfoxyl, cycloalkenylsulfoxyl, alkylsulfonyl, alkenyl-
sulfonyl, alkynylsulfonyl, cycloalkyl~ulfonyl, aryl-
sulfonyl, hetarylsulfonyl, heterocyclylsulfonyl, cyclo-
alkenylsulfonyl, cycloalkynylsulfonyl, alkylsulfinyl,
alkenylsulfinyl, alkynylsulfinyl, cycloalkylsulfinyl,
aryl~ulfinyl, hetarylsulfinyl, heterocyclylsulfinyl,
cycloalkenylsulfinyl, cycloalkynylsulfinyl, alkyl-
carbonyloxy, alkenylcarbonyloxy, alkynylcarbonyloxy,cycloalkylcarbonyloxy, cycloalkenylcarbonyloxy, cyclo-
alkynylcarbonyloxy, arylcarbonyloxy, hetarylcarbonyloxy,
h~terocyclylcarbonyloxy, alkylcarbonylamino, alkenyl-
csrbonylamino, alkynylcarbonylamino, cycloalkylcarbonyl-
amino, cycloalkenylcarbonyl~mino, cycloalkynylcarbonyl-
amino, arylcarbonylamino, hetarylcarbonylamino, hetero-
cyclylcarbonylamino, cycloalkylalkoxy, cycloalkenyl-
210~4~
- 3 - O.~. 0050/43411
alkoxy, cylcoalkynylalkoxy, arylalkoxy, hetarylalkoxy or
heterocyclylalkoxy,
if X and Y are on ad~acent carbon atoms, they may be
condensed to form an unsubstituted or substituted aroma-
tic or heteroaromatic, alicycli~ or heterocyclic, par-
tially or completely hydrogenated ring,
Rl and R2 may be sub~tituted and independently of one
another are each alkyl, alkenyl, alkynyl, cycloalkyl or
cycloalkenyl, R3 and R4 may be substituted by 1-4 identi-
cal or different substituents Rl, and R3 and R~ are eachnitro, alkoxy, haloalkoxy, alkynyl, cycloalkyl, hetaryl,
heterocyclyl, cycloalkenyl, cycloalkynyl, alkenyloxy,
alkynyloxy, cycloalkoxy, hetaryloxy, het~rocyclyloxy,
cycloalkenyloxy, cycloalkynyloxy, alkoximino, alkenyl-
oxLmino, alkynyloximino, cycloalkoxLmino, cycloalkenyl-
oximino, cycloalkynyloximino, aryloximino, hetarylox-
imino, heterocyclyloximino, cycloalkoxycarbonyl
aryloxycarbonyl, hetaryloxycarbonyl, he~erocyclyloxy-
carbonyl, cycloalkenyloxycarbonyl, cycloalkynyloxy-
carbonyl, alkylaminocarbonyl, dialkylaminocarbonyl,alkenylaminocarbonyl, dialkenylaminocarbonyl, alkynyl-
aminocarbonyl, dialkynylaminocarbonyl, cycloalkyl2mino-
carbonyl, cycloalkenylaminocarbonyl, cycloalkynylamino-
carbonyl, arylaminocarbonyl, hetarylaminocarbonyl,
heterocyclylaminocarbonyl, alkyl~hio, alkenylthio,
alkynylthio, cycloalkylthio, arylthio, hetarylthio,
heterocyclylthio, cycloalkenylthio, cycloalkynylthio,
alkylamino, alkenylamino, al~ynylamino, cycloalkylamino,
arylamino, hetarylamino, heterocyclylamino, cycloalkenyl-
amino, cycloalkynylamino, alkylcarbonyl, alkenylcarbonyl,alkynylcarbonyl, cycloalkylcarbonyl, arylcarbonyl, het-
arylcarbonyl,heterocyclylcarbonyl,cycloalkenylcarbonyl,
cycloalkynylcarbonyl, alkylsulfoxyl, alkenylsulfoxyl,
cycloalkylsulfoxyl, aryl~ulfoxyl, hetarylsulfoxyl,
heterocyclylsulfoxyl,cycloalkynylsulfoxyl,cycloalkenyl-
sulfoxyl, alkylsulfonyl, alkenyl~ulfonyl, alkynyl-
sulfonyl, cycloalkylsulfonyl, arylsulfonyl, hetaryl-
2100~46
- 4 - O.Z. 0050/43411
sulfonyl, heterocyclylxulfonyl, cycloalkenylsulfonyl,
cycloalkynyl~ulfonyl, alkyl~ulfinyl, alkenylsulfinyl,
alkynyl~ulfinyl, cycloalkyl~ulfinyl, arylsulfinyl,
hetarylsulfinyl, heterocyclylsulfinyl, cyclsalkenyl-
sulfinyl, cycloalkynylsulfinyl, alkylcarbonyloxy,
alkenylcarbonyloxy,alkynylcarbonyloxy,cycloalkylcarbon-
yloxy, cycloalkenylcarbonyloxy, cycloalkynylcarbonyloxy,
arylcarbonyloxy, hetarylcarbonyloxy, heterocyclylcarbon-
yloxy, alkylcarbonylamino, alkenylcarbonylamino, alkynyl-
carbonylamino, cycloalkylcarbonylamino, cycloalkenyl-
carbonylamino, cycloalkynylcarbonylamino, arylcarbonyl-
amino, hetarylcarbonylamino, heterocyclylcarbonylamino,
cycloalkylalkoxy,cycloalkenylalkoxy,cycloalkynylalkoxy,
hetarylalkoxy, heterocyclylalkoxy, alkynylalkenyl, cyclo-
alkylalkenyl, cycloalkenylalkenyl, cycloalkynylalkenyl,
hetarylalkenyl, heterocyclylalkenyl, alkoximinoalkyl,
alkenyloxLminoalkyl, alkynyloxminoalkyl, cycloalkox-
iminoalkyl, cycloalkenyloximino~lkyl, cycloalkynylox-
iminoalkyl, aryloximinoalkyl, hetaryloxLminoalkyl,
heterocyclyloximinoalkyl, alkoximinoalkenyl, alkenylox-
iminoalkenyl, alkynyloxLminoalkenyl, cycloalkoximino-
alkenyl,cycloalkenyloximinoalkenyl,cycloalkynyloxLmino-
alkenyl, aryloximinoalkenyl, hetaryloximinoalkenyl or
heterocyclyloxLminoalkenyl,
R5, R6, R7 and R8 may be substituted by 1-4 identical or
different substituents Rl, and R5, R6, R7 and R8 are each
nitro, haloalkyl, alkyl, haloalkoxy, alkenyl, alkynyl,
cycloalkyl, sryl, hetaryl, heterocyclyl, cycloalkenyl,
cycloalkynyl, alkoxy, alkenyloxy, alkynyloxy, cyclo-
alkoxy, aryloxy, hetaryloxy, heterocyclyloxy, cyclo-
alkenyloxy, cycloalkynyloxy, alkoximino, alkenyloximino,
alkynyloximino, cycloalkoxLmino, cycloalkenyloximins,
cycloalkynyloximino, aryloximino, hetaryloximino, hetero-
cyclyloximino, cycloalkoxycarbonyl, aryloxycarbonyl, het-
aryloxycarbonyl,heterocyclyoxycarbonyl,cycloalkenyloxy-
carbonyl, cycloalkynyloxycarbonyl, alkylaminocarbonyl,
dialkylaminocarbonyl/ alkenylaminocarbonyl, dialkenyl-
2100.'J46
- 5 - O.Z. 0050/43411
aminocarbonyl, alkynylaminocarbonyl, dialkynylamino-
carbonyl, cycloalkylaminocarbonyl, cycloalkenylamino-
carbonyl, cycloalkynylaminocarbonyl, arylaminocarbonyl,
hetarylaminocarbonyl, heterocyclylaminocarbonyl, alkyl-
S thio, alkenylthio, alkynylthio, cycloalkylthio, arylthio,
hetarylthio, heterocyclylthio, cycloalkenylthio, cyclo-
alkynylthio, alkylamino, alkenylamino, alkynylamino,
cycloalkylamino, arylamino, hetarylamino, heterocyclyl-
amino, cycloalkenylamino, cycloalkynylamino, alkylcarbon-
yl, alkenylcarbonyl, alkynylcarbonyl, cycloalkylcarbonyl,
arylcarbonyl, hetarylcarbonyl, heterocyclylcarbonyl,
cycloalkenylcarbonyl, cycloalkynylcarbonyl, alkylsulfox-
yl, alkenyl~ulfoxyl, alkynylsulfoxyl, cycloalkyl~ulfoxyl,
aryl 8ul foxyl, hetaryl~ulfoxyl, heterocyclyl~ulfoxyl,
cycloalkynylsulfoxyl, cycloalkenyl~ulfoxyl,
alkylsulfonyl, alkenyl~ulfonyl, alkynylsulfonyl,
cycloalkylQulfonyl, arylsulfonyl, hetarylsulfonyl,
heterocyclyl~ulfonyl, cycloalkenylsulfonyl,
cycloalkynylsulfonyl, alkyl~ulfinyl, alkenyl~ulfinyl,
alkynyl~ulfinyl, cycloalkyl~ulfinyl J arylsulfinyl,
hetarylsulfinyl, heterocyclylsulfinyl, cycloalkenyl-
sulfinyl, cycloalkynyl~ulfinyl, alkylcarbonyloxy,
alkenylcarbonyloxy,~lkynylcarbonyloxy,cycloalkylcarbon-
yloxy, cycloalkenylcarbonyloxy, cycloalkynylcarbonyloxy,
arylcaxbonyloxy, hetarylcarbonyloxy, heterocyclylcarbon-
yloxy, alkylcarbonylamino, alkenylcarbonylamino, alkynyl-
carbonylamino, cycloalkylcarbonylamino, cycloalkenyl-
carbonylamino, cycloalkynylcarbonylamino, arylcarbonyl-
amino, hetarylcarbonylamino or heterocyclylcarbonylamino,
R9 may be substituted by 1-4 identical or different
substituent3 R10, and R~ i~ hydrogen, halogen, cyano,
nitro, haloalkyl, alkyl, haloalkoxy, alkenyl, al~ynyl,
cycloalkyl, aryl, hetaryl, heterocyclyl, cycloalkenyl,
cycloalkynyl, alkoxy, alkenyloxy, alkynyloxy, cycloalk-
oxy, aryloxy, hetaryloxy, heterocyclyloxy, cycloalkenyl-
o~y, cycloalkynyloxy, alkoximino, alkenyloximino,
alkynyloximino, cycloalkoximino, cycloalkenyloximino,
2100~6
- 6 - O.Z. 0050/43411
cycloalkynyloximino, aryloximino, hetaryloximino, hetero-
cyclyloximino, alkoxycarbonyl, alkenyloxycarbonyl,
alkynyloxycarbonyl,cycloalkoxycarbonyl,aryloxycarbonyl,
hetaryloxycarbonyl, heterocyclyloxycarbonyl,
cycloalkenyloxycarbonyl, cycloalkynyloxycarbonyl,
alkylaminocarbonyl, dialkylaminocarbonyl,
alkenylaminocarbonyl, dialkenylaminocarbonyl,
alkynylaminocarbonyl, dialkynylaminocarbonyl,
cycloalkylaminocarbonyl, cycloalkenylaminocarbonyl,
cycloalkynylaminocarbonyl, arylaminocarbonyl,
hetarylaminocarbonyl, heterocyclylaminocarbonyl, alkyl-
thio, alkenylthio, alkynylthio, cycloalkylthio, arylthio,
hetarylthio, heterocyclylthio, cycloalkenylthio, cyclo-
alkynylthio, alkylamino, alkenylamino, alkynylamino,
cycloalkylamino, arylamino, hetarylamino, heterocyclyl-
amino, cycloalkenyl~mino, cycloalkynylamino, alkylcarbon-
yl, alkenylcarbonyl, alkynylcarbonyl, cycloalkylcarbonyl,
arylcarbonyl, hetarylcarbonyl, heterocyclylcarbonyl,
cycloalkenylcarbonyl, cycloalkynylcarbonyl, alkylsulfox-
yl, alkenylsulfoxyl, alkynylsulfoxyl, cycloalkylsulfoxyl,
aryl6ulfoxyl, hetarylsulfoxyl, heterocyclylsulfoxyl,
cycloalkynylsulfoxyl, cycloalkenylsulfoxyl,
alkylsulfonyl, alkenylsulfonyl, alkynylsulfonyl,
cycloalkylsulfonyl, aryl~ulfonyl, hetarylsulfonyl,
heterocyclylsulfonyl, cycloalkenylsulfonyl,
cycloaIkynylsulfonyl, alkylsulfinyl, alkenylsulfinyl,
alkynyl~ulfinyl, cycloalkylsulfinyl, arylsulfinyl,
hetarylsulfinyl, heterocyclylsulfinyl, cycloalkenyl-
sulfinyl, cycloalkynylsulfinyl, alkylsulfinyl,
alkenyl~ulfinyl, alkynyl~ulfinyl, cycloalkylsulfinyl,
arylsulfinyl, hetarylsulfinyl, heterocyclylsulfinyl,
cycloalkenylsulfinyl, cycloalkynylsulfinyl, alkyl-
carbonyloxy, alkenylcarbonyloxy, alkynylcarbonyloxy,
cycloalkylcarbonyloxy, cycloalkenylcarbonyloxy, cyclo-
alkynylcarbonyloxy, arylcarbonyloxy, hetarylcarbonyloxy,
heterocyclylcarbonyloxy, alkylcarbonylamino, alkenyl-
carbonylamino, alkynylcarbonylamino, cycloalkylcarbonyl-
2100~46
- 7 - O.Z. 0050/43411
amino, cycloalkenylcarbonylamino, cycloalkynylcarbonyl-
amino, arylcarbonylamino, hetarylcarbonylamino, hetero-
cyclylcarbonylamino, cycloalkylalkoxy,
cycloalkenylalkoxy, cycloalkynylalkoxy, arylalkoxy,
hetarylalkoxy or heterocyclylalkoxy,
Rl may be substituted by 1-4 identical or different
substituent~ R11, and Rl is hydrogen, halogen, cyano,
nitro, haloalkyl, alkyl, haloalkoxy, alkenyl, alkynyl,
cycloalkyl, aryl, hetaryl, heterocyclyl, cycloalkenyl,
cycloalkynyl, alkoxy, alkenyloxy, alkynyloxy, cycloalk-
oxy, aryloxy, hetaryloxy, heterocyclyloxy, cycloalkenyl-
oxy, cycloalkynyloxy, alkoximino, alkenyloximino,
alkynyloximino, cycloalkoximino, cycloalkenyloximino,
cycloalkynyloximino, aryloximino, hetaryloximino, hetero-
cyclyloximino, alkoxycarbonyl, alkenyloxycarbonyl,alkynyloxycarbonyl,cycloalkoxycarbonyl,aryloxycarbonyl,
hetaryloxycarbonyl, heterocyclyloxycarbonyl,
cycloalkenyloxycarbonyl, cycloalkynyloxycarbonyl,
alkylaminocarbonyl, dialkyl2~minocarbonyl,
alkenylaminocarbonyl, dialkenylaminocarbonyl,
alkynylaminocarbonyl, dialkynylaminocarbonyl,
cycloalkylaminoc~rbonyl, cycloalkenylaminocarbonyl,
-- cycloalkynylaminocarbonyl, arylaminocarbonyl,
hetaryl~minocnrbonyl, heterocyclylaminocarbonyl, alkyl-
thio, ~lkenylthio, alkynylthio, cycloalkylthio, arylthio,
hetaryithio, heterocyclylthio, cycloalkenylthio, cyclo-
slkynylthio, alkylamino, alkenylamino, alkynylamino,
cycloslkylamino, arylamino, hetarylamino, heterocyclyl-
smino, cycloalkenylamino, cyclo~lkynylamino, alkylcarbon-
yl, alkenylcarbonyl, alkynylcarbonyl, cycloalkylcarbonyl,
arylc~rbonyl, hetarylcarbonyl, heterocyclylcarbonyl,
cycloalkenylcarbonyl, cycloalkynylcarbonyl, alkyl~ulfox-
yl, hlkenylsulfoxyl, alkynylsulfoxyl, cycloalkylsulfoxyl,
arylsulfoxyl, hetaryl ulfoxyl, heterocyclylsulfoxyl,
cycloalkynylsulfoxyl, cycloalkenylsulfoxyl,
alkylsulfonyl, alkenylsulfonyl, alkynylsulfonyl,
cycloalkylsulfonyl, arylsulfonyl, hetaryl~ulfonyl,
`- 2iO0546
- 8 - O.Z. 0050/43411
heterocyclyl~ulfonyl, cycloalkenylsulfonyl,
cycloalkynylsulfonyl, alkylsulfinyl, alkenyl 8ul finyl,
alkynylsulfinyl, cycloalkylsulfinyl, arylsulfinyl,
hetarylsulfinyl, heterocyclylsulfinyl, cycloalkenyl-
S sulfinyl, cycloalkynylsulfinyl, alkylcarbonyloxy,alkenylcarbonyloxy,alkynylcarbonyloxy,cycloalkylcarbon-
yloxy, cycloalkenylcarbonyloxy, cycloalkynylcarbonyloxy,
arylcarbonyloxy, hetarylcarbonyloxy, heterocyclylcarbon-
yloxy, alkylcarbonylamino, alkenylcarbonylamino, alkynyl-
carbonylamino, cycloalkylcarbonylamino, cycloalkenyl-
carbonylamino, cycloalkynylcarbonylamino, arylcarbonyl-
amino, hetarylcarbonylamino, heterocyclylcarbonylamino,
cycloalkylalkoxy,cycloalkenylalkoxy,cycloalkynylalkoxy,
arylalkoxy, hetarylalkoxy or heterocyclylalkoxy,
R1l may be substituted and is hydrogen, halo~en, cyano,
nitro, haloalkyl, alkyl, haloalkoxy, alkenyl, alkynyl,
cycloalkyl, aryl, hetaryl, heterocyclyl, cycloalkenyl,
cycloalkynyl, alkoxy, alkenyloxy, alkynyloxy, cycloalk-
oxy, aryloxy, hetaryloxy, heterocyclyloxy, cycloalkenyl-
oxy, cycloalkynyloxy, alkoximino, alkenyloximino,alkynyloximino, cycloalkyloximino, cycloalkenyloximino,
cycloalkynyloximino, aryloximino, hetaryloximino, hetero-
cyclyloximino, alkoxycarbonyl, alkenyloxycarbonyl,
alkynyloxycarbonyl,cycloalkoxycarbonyl,aryloxycarbonyl,
hetaryloxycarbonyl, heterocyclyloxycarbonyl,
cycloaïkenyloxycarbonyl, cycloalkynyloxycarbonyl,
alkylaminocarbonyl, dialkylaminocarbonyl,
alkenylaminocarbonyl, dialkenylaminocarbonyl,
alkynylaminocarbonyl, dialkynylaminocarbonyl,
cycloalkylaminocarbonyl, cycloalkenylaminocarbonyl,
cycloalkynylaminocarbonyl, arylaminocarbonyl,
hetarylaminocarbonyl, heterocyclyl~minocarbonyl, alkyl-
thio, alkenylthio, alkynylthio, cycloalkylthio, arylthio,
hetarylthio, heterocyclylthio, cycloalkenylthio, cyclo-
alkynylthio, alkylamino, alkenylamino, alkynylamino,cycloalkylamino, arylamino, hetarylamino, heterocyclyl-
amino, cycloalkenylamino, cycloalkynylamino, alkyl-
210~4~
- 9 - O.Z. 0050/43411
càrbonyl, alkenylcarbonyl, alkynylcarbonyl,
cycloalkylcarbonyl, arylcarbonyl, hetarylcarbonyl,
heterocyclylcarbonyl, cycloalkenylcarbonyl,
cycloalkynylcarbonyl, alkyl~ulfoxyl, alkenylsulfoxyl,
alkynylsulfoxyl, cycloalkylsulfoxyl, arylsulfoxyl,
hetarylsulfoxyl, heterocyclyl~ulfoxyl,
cycloalkynylsulfoxyl, cycloalkenylsulfoxyl,
alkylsulfonyl, alkenylsulfonyl, alkynylsulfonyl,
cycloalkylsulfonyl, arylsulfonyl, hetaryl~ulfonyl,
he~erocyclylsulfonyl, cycloalkenylsulfonyl,
cycloalkynylsulfonyl, alkylsulfinyl, alkenylsulfinyl,
alkynyl~ulfinyl, ~ycloalkylsulfinyl, axylsulfinyl,
hetarylsulfinyl, heterocyclylsulfinyl, cycloalkenyl-
sulfinyl, cycloalkynyl~ulfinyl, alkylcarbonyloxy,
alkenylcarbonyloxy,alkynylcarbonyloxy,cycloalkylcarbon-
yloxy, cycloalkenylcarbonyloxy, cycloalkynylcarbonyloxy,
arylcarbonyloxy, hetarylcarbonyloxy, heterocyclylcarbon-
yloxy, alkylcarbonylamino, alkenylcarbonylamino, alkynyl-
carbonylamino, cycloalkylcarbonylamino, cycloalkenyl-
carbonylamino, cycloalkynylcarbonylamino, arylcarbonyl-
amino, hetarylcarbonylamino, hetsrocyclylcarbonylamino,
cycloalkylalkoxy,cycloalkenylalkoxy,cycloalkynylalkoxy,
. arylalkoxy, hetarylalkoxy or heterocyclylalkoxy,
and their plant-tolerated acid addition products and base
addition products not only have high fungitoxic, insec-
ticidal, nematocidal and acaricidal activity but al~o are
vQry well tolerated by plants.
Acids for acid addition products are, for
example, mineral acids, for example hydrochloric acid,
hydrobromic acid, phosphoric acid, sulfuric acid or
nitric acid, cr carbo~ylic acids, such as formic acid,
acetic acid, oxalic acid, malonic acid, lactic acid,
malic acid, succinic acid, tartaric acid, citric acid,
salicylic acid, p-toluenesulfonic acid or
dodecylbanzenesulfonic acid, and also protic compounds
generally, eg. ~accharin.
Base~ for base addition products are, for
21005~6
- 10 - O.Z. 0050/43411
example, potassium hydroxide, sodium hydroxide, potassium
carbonste, ~odium carbonate and ammonium hydroxide.
The novel compounds of the formula I may be
obtained in the preparation as mixtures of ~tereoisomers
(E/Z isomer6, diastereomer~, enantiomer~), which can be
separated into the individual components in a convention-
al manner, for example by crystallization or chromatog-
raphy. Both the individual isomers and mixtures thereof
can be used a~ fungicide~, acaricides, namatocides or
insecticides and are embraced by the present invention.
~he stated alkyl radicals are preferably of 1-10
carbon atoms, are substituted or uneub~tituted and are,
for ex~mple, methyl, ethyl, propyl, n-propyl, i~opropyl,
butyl, n-butyl, isobutyl, tert-butyl, ~ec-butyl, pentyl,
pent-l-yl, pent-2-yl, pent-3-yl, 2-mathylbut-1-yl, 2-
methylbut-2-yl, 2~methylbut-3-yl, 3-methylbut-1-yl, 2,2-
dLmethylprop-l-yl, hexyl, hex-l-yl, hex-2-yl, hex-3-yl,
l-methylpentyl, 2-methylpentyl, 3-methylpentyl, 4-methyl-
pentyl, 1,l-dimethylbutyl, 1,2-dimethylbutyl, 1,3-
dimethylbutyl, 2,2-dimethylbutyl, 2,3-dimethylbutyl, 3,3-
dimethylbutyl, l-ethylbutyl, 2-ethylbutyl, 1,1,2-tri-
methylpropyl, 1,2,2-trimethylpropyl, l-ethyl-l-methyl-
propyl, l-ethyl-2-methylpropyl, heptyl, hept-l-yl, hept-
2-yl, hept-3-yl, hept-4-yl, l-methylhexyl, 2-methylhexyl,
3-methylhexyl, 4-methylhexyl, 5-methylhexyl, l-ethyl-
pentyl, 2-ethylpentyl, 3-ethylpentyl, l-propylbutyl, 1-
isopropylbutyl, octyl, oct-l-yl, oct-2-yl, oct-3-yl, oct-
4-yl, l-methylheptyl, 2-methylheptyl, 3-methylheptyl, 4-
methylheptyl, 5-methylheptyl, 6-methylheptyl, l-ethyl-
hexyl, 2-ethylhexyl, 3-ethylhexyl, 4-ethylhexyl, 1-
propylpentyl, 2-prcpylpentyl, nonyl, non-1-yl, non-2-yl,
non-3-yl, non-4-yl, non-5-yl, l-methyloctyl, 2-methyl-
octyl, 3-methyloctyl, 4-methyloctyl, 5-methyloctyl, 6-
methyloctyl, 7-methyloctyl, 4-Me~hyl-2-propylpentyl,
decyl, dec-l-yl, dec-2-yl, dec-3-yl, dec-4-yl, dec-5-yl,
1-ethyloctyl, 2-ethyloctyl, 3-ethyloctyl, 4-ethyloctyl,
5-ethyloctyl, 6-ethyloctyl or 2-propylheptyl.
21~0~4~
~ O.Z. 0050/43411
The stated alkenyl radicals are preferably of
2-10 carbon atoms, are unsubstituted or sub~tituted and
are, for example, ethenyl, propenyl, l-propenyl, 2-
propenyl, butenyl, 1-butenyl, 2-butenyl, 3-butenyl, 1-
methyl-2-propenyl, 2-methyl-2-propenyl, pentenyl, 1-
pentenyl, 2-pentenyl, 3-pentenyl, 4-pentenyl, l-methyl-
2-butenyl, 2-methyl-2-butenyl, 3-methyl-2-butenyl, 1-
methyl-3-butenyl,2-methyl-3~butenyl,3-methyl-3-butenyl,
1,1-dimethyl-2-propenyl, 1,2-dimethyl-2-propenyl, 1-
ethyl-2-propenyl, hexenyl, 1-hexenyl, 2-hexenyl, 3-
hexenyl, 4-hexenyl, S-hexenyl, l-methyl-2-pentenyl, 2-
methyl-2-pentenyl, 3-methyl-2-pentenyl, 4-methyl-2-
pentenyl, l-methyl-3-pent~nyl, 2-methyl-3-pentenyl, 3-
methyl-3-pentenyl, 4-methyl-3-pentenyl, 1-methyl-4-
pentenyl, 2-methyl-4-pentenyl, 3-methyl-4-pentenyl, 4-
methyl-4-pentenyl, 1,1-dimethyl-2-butenyl, l,l-dimethyl-
3-butenyl, 1,2-dimethyl-2-butenyl, 1,2-dImethyl-3-
butenyl, 1,3-dimethyl-2-but~nyl, 1,3-dLmethyl-3-butenyl,
2,2-dimethyl-3-butenyl, 2,3-dimethyl-2-butenyl, 2,3-
dimethyl-3-butenyl, 3,3-dimethyl-2-butenyl, 1-ethyl-2-
butenyl, l-ethyl-3-butenyl, 2-ethyl-2-butenyl, 2-ethyl-
3-butenyl, 1,1,2-trimethyl-2-propenyl, l-ethyl-l-methyl-
2-propenyl, heptenyl, octenyl, nonenyl or decenyl.
The stated alkynyl radical~ are preferabyl of 2-
10 car~on atoms, are ~ubstituted or unsubstituted and
are, for example, ethynyl, propynyl, l-propynyl, 2-
propynyl, butynyl, l-butynyl, 2-butynyl, 3-butynyl, 1-
methyl-2-propynyl, pentynyl, l-pentynyl, 2-pentynyl, 3-
pentynyl, 4-pentynyl, 1-methyl-2-butynyl, 1-methyl-3-
butynyl, 2-methyl-3-butynyl, 1,1-dimethyl-2-methyl-2-
pentynyl, hexynyl, l-methyl-3-pentynyl, 1-methyl-4-
pentynyl, 1-methyl-3-pentynyl, 2-methyl-4-pentynyl, 3-
methyl-4-pentynyl, 4-methyl-2-pentynyl, 1,1-dimethyl-2-
butynyl, 1,1-dimethyl-3-butynyl, 1,2-dimethyl-3-butynyl,
2,2-dLmethyl-3-butynyl, 1-ethyl-2-butynyl, 1-ethyl-3-
butynyl, 2-ethyl-3-butynyl, 1-ethyl-1-methyl-2-propynyl,
heptynyl, octynyl, nonynyl, or decynyl.
21~03~
- 12 - O.Z. 0050/43411
The stated halogens are fluorine, chlorine,
bromine or iodine.
The stated cycloalkyl radicals are preferably of
3-10 carbon atoms, are substituted or unsubstituted and
are, for example, cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl, cyclooctyl, cyclononyl, cyclo-
decyl, bornanyl, norbonanyl, dicyclohexyl, bicyclo-
[3.3.0]octyl~ bicyclo[3.2.1]octyl, bicyclo[2.2.2]octyl or
bicyclo[3.3.1]nonyl.
The stated cycloalkenyl radical6 are preferably
of 3-10 carbon atoms, are unæubstituted or substituted
and are, for example, cyclopropenyl, ~yclobutenyl,
cyclopentenyl, ~yclohexenyl, cycloheptenyl, cyclooctenyl,
cyclononenyl, cyclodecenyl, bornenyl, norbonenyl,
lS bicyclo~3.3.0]octenyl, bicyclo[3.2.1]octenyl,
bicyclo[2.2.2]octenyl or bicyclo[3.3.1]nonenyl.
The ctated cycloalkynyl radicals are preferably
of 6-10 carbon stom6, are unsubstituted or substi~uted
and ara, for example, cyclohexyne, cycloh~ptyne, cyclo-
octyne, cyclononyne, cyclodecyne, cycloundecyne or cyclo-
dodecyne.
The stated haloalkyl radical~ are preferably of
1-4 carbon atoms, are unsub~tituted or substituted and
are, for example, chloromethyl, dichloromethyl, tri-
chloromethyl, fluoromethyl, difluoromethyl, trifluoro-
methyl, chlorofluoro~ethyl, dichlorofluoromethyl, chloro-
difluoromethyl, l-fluoroethyl, 2-fluoroethyl, 2,2-di-
fluoroethyl, 2,2,2-trifluoroethyl, 2-chloro-2-fluoro-
ethyl,2-chloro-2,2-difluoroethyl,2,2-dichloro-2-fluoro-
ethyl, 2,2,2-trichloroethyl or pentafluoroethyl.
The stated aryl radicals are preferably of 6, 10
or 14 carbon atoms, are un6ubstitut~d or substituted and
are, for example, phenyl, naphthyl, l-naphthyl, 2-
naphthyl, anthracenyl, 1-anthracenyl, 2-anthracenyl or 9-
anthracenyl.
The tated hetaryl radicals preferably have 5-14
ring atoms, including 1-4 hetero atom~ ~elected from the
2100~46
- 13 - O.Z. 0050/43411
group consisting of N, 0 and S, are un~ubstituted or
substituted and are, for example, furyl, 2-furyl, 3-
furyl, thienyl, 2-~hienyl, 3-thienyl, pyrrolyl, 1-
pyrrolyl, 2-pyrrolyl, 3-pyrrolyl, isoxazolyl, 3-
isoxazolyl, 4-isoxazolyl, 5-isoxazolyl, isothiazolyl, 3-
isothiazolyl, 4-isothiazolyl, 5-isothiazolyl, pyrazolyl,
l-pyrazolyl, 3-pyrazolyl, 4-pyrazolyl, 5-pyrazolyl,
oxazolyl, 2-oxazolyl, 4-oxazolyl, 5-oxazolyl, thiazolyl,
2-thiazolyl, 4-thiazolyl, 5-thiazolyl, Lmidazolyl, 1-
imidazolyl, 2-imidazolyl, 4-imidazolyl, 5-imidazolyl,
1,2,3-thiadiazolyl, 1,2,4-thiadiazolyl, 1,2,5-thiazolyl,
1,3,4-thiadiazolyl, tetrazolyl, 1,2,3,4-thiatriazolyl,
1,2,3,4-oxatriszolyl, pyridyl, ~-pyridyl, ~-pyridyl, 4-
pyridyl, pyridazinyl, 3-pyridazinyl, 4-pyridazinyl,
pyrimidinyl, 2-pyrimidinyl, 4-pyrimidinyl, 5-pyrimidinyl,
pyrazinyl, 2-pyrazinyl, 3-pyrazinyl, 1,2,4-triazinyl,
1,3,5-triazinyl or 1,2,4,5-tetrazinyl.
Ad~acent 6ubs~ituents of the heteroaromatics may
be condensed to form an aromatic or heteroaromatic ring,
80 that hetaryl al60 includes fus2d ring systems, eg.
benzofuranyl, isob~nzofuranyl, l-benzothienyl, 2-benzo-
thienyl, indolyl, isoindolyl, benzisoxazolyl, benzoxazol,
. benzi~othiazolyl, benzothiazolyl, 2-benzothiazolyl, 4-
benzothiazolyl, 5-benznthiazolyl, 6-benzothiazolyl, 7-
benzothiazolyl, indazolyl, benzimidazolyl, benzofuranyl,
dibenzofuranyl, dibenzothienyl, acridinyl,
phenanthridinyl, carbazolyl, quinolyl, i6iquinolyl,
phthalazinyl, quinazolinyl, quinoxalinyl, cinnolinyl,
1,5-naphthyridinyl,1,6-naphthyridinyl,1,7-naph~hyridin-
yl, 1,8-naphthyridinyl, pteridinyl, pyrrolopyridinyl,
pyrrolopyridazinyl,pyrrolopyrLmidinyl,pyrrolopyrazinyl,
pyrrolotriazinyl, furopyridinyl, furopyridazinyl, furo-
pyrimidinyl, furopyrazinyl, furotriazinyl, thienopyridin-
yl, thienopyridazinyl, thienopyrimidinyl, thienopyrazin-
yl, thienotriazinyl, Lmidazopyridinyl, ~midazopyridazin-
yl, imidazopyrimidinyl, imidazopyrazinyl, pyrazolo-
pyridinyl, pyrazolopyridazinyl, pyr~zolopyrimidinyl,
210054~
- 14 - O.Z. OOS0/43411
pyrazolopyr~zinyl, isoxazolopyridinyl, isoxazolo-
pyridazinyl, isoxazolopyrimidinyl, i~oxazolopyrazinyl,
oxazolopyridinyl,oxazolopyridazinyl,oxazolopyrimidinyl,
oxazolopyrazinyl,thi~zolopyridinyl,thiazolopyridazinyl,
S thiazolopyrimidinyl, thiazolopyrazinyl, isothiazolo-
pyridinyl, isothiazolopyridazinyl, isothiazolopyrimidin-
yl, isothiazolopyrazinyl, triazolopyridinyl, triazolo-
pyridazinyl, triazolopyrimidinyl or triazolopyrazinyl.
'~he stated heterocyclyl radicals preferably have
3-15 ring atoms, including 1-4 hetero atom~ 3elected from
the group con~isting of N, 0 and S, are saturated or have
parallel un~aturated bonds, are unsub~tituted or
substituted and are, for example, 2-tetrahydrofuranyl,
oxiranyl, 3-tetrahydrofuranyl, 2-totrahydrothienyl, 3-
tetrahydrothienyl, 2-pyrrolidinyl, 3-pyrrolidinyl, 3-
isoxazolidinyl, 4-isoxazolidinyl, 4-isothiazolidinyl, 3-
pyrazolidinyl, 4-pyrazolidinyl, S-pyrazolidinyl, 2-
oxazolidinyl, 4-oxazolidinyl, 5-oxazolidinyl, 2-
thiazolidinyl, 4-thiazolidinyl, 5-thiazolidinyl, 2-
imidazolidinyl, 4-imidazolidinyl, 1,2,4-oxadiazolidin-3-
yl, 1,2,4-oxadiazolidin-5-yl, 1,2,4-thiadiazolidin-3-yl,
1,2,4-thiadiazolidin-5-yl,1,2,4-triazolidin-3-yl,1,3,4-
-- oxadiazolidin-2-yl, 1,3,4-thiadiazolidin-2-yl, 1,3,4-
triazolidin-2-yl, 2, 3-dihydrofur-2-yl, 2,3-dihydrofur-3-
yl, ~,5-dihydrofur-2-yl, 2,5-dihydrofur-3-yl, 2,3-
dihydrofur-3-yl,2,3-dihydrothien-2-yl,2,3-dihydrothien-
3-yl, 2,5-dihydrothien-2-yl, 2,5-dihydrothien-2-yl, 2,4-
pyrrolin-2-yl, 2,3-pyrrolin-3-yl, 2,5-pyrrolin-2-yl, 2,5-
pyrrolin-3-yl, 2,3-isoxazolin-3-yl, 3,4-isoxazolin-3-yl,
4,5-i30x~zolin-2-yl,2,3-i~oxazolin-4-yl,3,4-i~oxazolin-
4-yl, 4,5-isoxazolin-5-yl, 2,3-isothiazolin-3-yl, 3,4-
isothiazolin-3-yl, 4,5-isothiazolin-3-yl, 2,3-
isothiazolin-4-yl, 3,4-isothiazolin-4-yl, 4,5-
isothi~zolin-4 yl, 2,3-i~othiazolin-5-yl, 3,4-
i~othiazolin-5-yl, 4,5-isothiazolin-5-yl, 2,3-
dihydropyrazol-3-yl, 2,3-dihydropyrazol-4-yl, 2,3-
dihydropyrazol-5-yl, 3,4-dihydropyrazol-1-yl, 3,4-
210~
- 15 - O.Z. 0050/43411
dihydropyrazol-2-yl, 3,4-dihydropyrazol-4-yl, 3,4-
dihydropyrazol-5-yl, 4,5-dihydropyrazol-1-yl, 4,5-
dihydropyrazol-3-yl, 4,5-dihydropyrazol-4-yl, 4,5-
dihydropyrazol-5-yl,2,3-dihydrooxazol-2-yl,2,3-dihydro-
oxazol-3-yl, 2,3-dihydrooxazol-4-yl, 2,3-dihydrooxazol-
5-yl, 3,4-dihydrooxazol-2-yl, 3,4-dihydrooxazol-3-yl,
3,4-dihydrooxazol-4-yl, 3,4-dihydrooxazol-5-yl, 3,4-
dihydrooxazol-2-yl, 3,4-dihydrooxazol-3-yl, 3,4-dihydro-
oxazol-4-yl, 2-piperidinyl, 3-piperidinyl, 4-piperidinyl,
3-tetrahydropyridazinyl, 4 tetrahydropyridazinyl, 2-
tetrahydropyr- idinyl, 4-tetrahydropyrimidinyl, 5-tetra-
hydropyrimidinyl, 2-tetrahydropyrazinyl, 1,3,5-tetra-
hydrotriazin-2-yl, 1,2,4-tetrahydrotriazin-3-yl, 1,3-
dihydrooxazin-~-yl, 1,3-dithian-2-yl, oxazol-2-yn-2-yl,
3,4,5,6-tetrahydropyridin-2-yl, 4H-1,3-thiazin-2-yl, 4H-
3,1-benzothiazin-2-yl, 1,1-dioxo-2,3,4,5-tetrahydrothin-
2-yl, 2H-1,4-benzothiazin-3-yl, 2H-1,4-benzoxazin-3-yl,
1,3-dihydrooxazin-2-yl, 1,3-di~hian-2-yl, N-morpholinyl
or dihydroquinazolinyl.
Expressly included are those heterocyclic radi-
cals which carry further functional groups, for ex2mple
oxo or thioxo groups, 80 that the heterocyclyl radicals
-- also include cyclic e~ters, thioester~ or amides or thio
analogs thereof, for example pyrrolidonyl, piperidonyl,
1-azacycloheptan-2-onyl, imidazolidinonyl, diketo-
piperazinyl or thiopyrrolidonyl.
The novel compound~ can be prepared, for example,
by the following processes:
For e~cample, the phenol 1 ~EP 251 082) can be
reacted with the corre~ponding alkylating agents under
alkaline condition~ (for exampla, alkali metal carbonates
in dipolar sprotic ~olvents, ~uch a4 dimethylformamide,
N-methylpyrrolidone, tetrnmethylurea, dimethylpropylene-
ure~, dimethylsulfoxide, sulfolane, etc.) to give the
novel compound6 2 (Schame 1)
2100~6
- 16 - O.Z. 0050/43411
Scheme 1
X Y
B O ~
X Y R'02C ORZ
1 ~ 1 2
HO ~ ~ X Y X Y
RlO C ~ oR2
OR~
0 02
la lb
The hetarylation of the phenol 1 i~ known (EP 242
081) bu the alkylation of 1, in particular with unreac-
tive alkylating agent~, to give the ethsrs 2 pre~ents
problems since the cyclization of 1 to la or lb is the
only detectable reaction in ~ome cases (Scheme 1). This
formation of byproducts can be suppre~ed if relatively
highly concentrated solution6 are smployed to incr2a~e
the reaction rate of the bimolecular reaction between the
phenol 1 ~nd ~lkylating agent Hal-B (Hal = Cl, Br or I)
~o give the active ingrEdisnts 2, and the reaction is
carried ou$ in the pre~ence of catalytic amounts of
iodine ~alts (eg. ~aI or RI) in, for example, ~he above-
mentisned dipolar aprotic solvents in order to activate
unreactive alkylating agent~ (~al = Cl or Br).
The active ingredients 2 ~re obtained in a
similar manner by reacting the phenol 1 with alcohols
under the condition~ of the Nitgunobu reaction (Synthesis
1981, 1).
Furthermore, the phenylacetates ~ can be reacted
2100~6
- 17 - O.Z. 0050/43411
under alkaline conditions to give the formylated products
4, which can be alkylated to the novel compounds 3
(Scheme 2).
Scheme 2
X Y X Y X Y
B`o~ _ B`o~f -- B~o~
Rl02 R'O2 OH Rlo2 OR2
3 4 2
Moreover, the ketoesters 5 c~n be ~ub~ected to a
Wittig reaction with (C6H5)3Pt-CH2-O-R2-Cl to give the novel
compound~ 2 (Sche~e 3).
Scheme 3
X Y X Y
B~ ~ ~ B~ ~
RIOz O R102 OR2
- 5 2
The chemical litarature describes many methods
for the synthesis of the ~-ke~oesters 5 (cf. ~or example
L. Weinstock et al., Synthe~ic Communications 11 (1981),
943-946; J. March, Advanced Organic Chamistry, 3rd
Edition (1985), J. Wiley & Son~; R. Larock, Comprehen6ive
Qrganic ~ransformations, 1st Edition 1989, VCH Publi6h-
ers; N. ~ie~er, Reagents for Organic Synthesi~, J. Wiley
& Sons); the synthesi~ of the kstoesters 5 6tar~ing from
benzaldehyde 6 via a cyanohydrin route (EP 422 597),
starting from ortho-haloaromatics 7 (~al = Cl, Br or I)
via a Grignard route (EP 253 213~ 8tarting ~rom benzoates
2100~4~
- 18 - O.Z. 0050/43411
via a P~mmerer rearrangement (J. Amer. Chem. Soc. 1966,
5498; Synthe~is 1982, 41) starting from acetophenones 9
via an oxidation/esterification (Synthetic Communications
21 (1991), 2045; J. Prakt. Chem. 45 (1892), 377) or
starting from benzoyl cyanides 10 (Tetrahedron ~ett.
1980, 3539; Scheme 4) i8 mentioned here merely by way of
example.
Scheme 4
X y X Y X
o~ ~ ~0~ ~0
CHO Hal
R'02C
B~ ~
RIO~C o
X Y X Y
B~ ,1~1 B~ ,1~
H3C O 2 NC O lQ
Furthermore, the novel compounds 2 are obtainable
from the phenylacetates 3 via enhmine formation to give
2100~46
- l9 - O.Z. 0050/43411
lI, hydroly6is of the enamine to give the formyl deriva-
tive 4 (cf. Scheme 2) and alkylation of the formyl
derivative (6imilarly to DE 40 25 892) (Scheme 5).
Scheme 5
~ `0 ~ B~ ~
R'02C R~02C N(AU~1)2 Rl02C OH
3 11 4
X Y
B~oJ~
RIO2C OR~
Moreover, the enol ethers 2 can be prep~red by
eliminating an alcohol RZ-O~ from the acetals 12 (J. Chem.
Soc. Chem. Co~mun. 1980, 838; Chem. Lett. (1976), 796)
and can be obtained from the acrylate~ 13 by 6ucce~6ive
reaction with bromine, an alcoholate R2-O-M (M = Li, Na,
10 ~, Mg) and a protic acid, eg. NaHSO~ (~. Chem. Soc. 1958,
153) (~imilarly to EP 431 323; Scheme 6).
210054~
\
- 20 - O.Z. 0050/43411
Scheme 6
X Y X Y X Y
~ OR: ~0 ~ B
R'02C oR2 R~02C OR2 ~ Rl02C
12 2 l3
Moreover, the propargyl ether 14 i8 a u~eful
intermediate for the synthe~i~ of the novel compounds 15
and 16. For example, the reaction of 14 with aldehyde
oximes Rl-CH~NOH in the presence of NaOCl solution gives
the isoxazole 15, and the reaction of 14 with aryl or
hetaryl halides (HA1 = I or Br) in the presence of a Pd
catalyst gives the acetylene 16 (Scheme 7).
Sch~me 7
N' oJ~ oJ~ ~~
R10 2 OR R10 oR2 R~02C OR~
15 14 16
The Ex2mples which follow illustrate the prepara-
tion of the novel compounds.
E~ANPLE 1
~ethyl ~-(2-(b~nzoylmethoxy)-phenyl)-B-methoxya~rylate
(Table 3, No. 18)
A mixture of 2.1 g (10 mmol) of methyl ~-(2-
hydroxyphenyl)-~-methoxyacryl~te (EP 251 082), 2.0 g (10
mmol) of phenacyl bromide and 1.5 g (11 mmol) of R2CO3 in
5 ml of di~ethylformamide is ~tirred overnight at room
t~mperature. An additional O.6 g of phenacyl bromide and
0.5 g of R2C03 are then added.
2100~46
- 21 - O.Z. 0050/43411
Stirring is carried out for 6 hours at room
temperature (20~C), the reaction mixture i~ dilu~ed with
wster and the aqueous phase i8 extracted three times with
methyl tert-butyl ether. The organic phase i6 dried over
MgSO4 and evaporated down. The residue i8 purified by
column chromatography u~ing hexane/ethyl acetate
mixtures. 1.7 g (52~) of the title compound are obtained
as a colorle~s solid (mp. = 76C).
lH-NNR (CDCl3; ~ in ppm~:
7.95 (d, broad, 2 H, aromatic); 7.5 (m, 4 H, 3 x aroma-
tic, 1 x vinyl); 7.25 (m~ 2 H, aromatic); 7.0 (t, 1 H,
J = 8 Hz, aromatic); 6.9 (d, 1 H, J - 8 Hz, ~romatic);
5.2 (B, 2 H, O-CH2); 3.8 (~, 3 H, O-CH3); 3.65 (8, 3 H,
O-CH3)
EXA~PLE 2
Methyl ~-(2-((N-phenylpyrrolidon-3-yl)-oxy)-phenyl)-~-
methoxyacrylate (Table 3, No. 16)
a) 2-((N-Phenylpyrrolidon-3-yl)-oxy)-benzaldehyde
3.2 g (0.13 mol) of sodium hydride are added to
14.8 g (0.12 mol) of salicylaldehyde in 100 ml of di-
methylformamide wi~h gentle cooling. After evolution of
gas has ceased, 29 g (0.12 mol3 of 3-bromo-N-phenyl-
- pyrrolidone (pr~pared similarly to J. ~ed. Chem. 30
(1987), 1995) are added and stirr$ng i8 carried out
overnight at room temperature. Thereafter, the reaction
mixture i8 diluted wi~h water and the aqueous phase is
extracted thrQe times with methyl tert-butyl ether. The
combined organic phases are dri~d over MgSO4 and evapora-
ted down. The residue i~ purified by column chromatog-
raphy. 12.8 g (39%) of the title compound are obtained
as a pale yellow solid.
H-NMR (CDC13; ~ in ppm):
10.5 (8, 1 H, CHO); 7.0-8.0 (m, 9 H, aromatic); 5.15 (t,
1 H, J = 8 Hz, O-CH); 3.9 (m, 2 H, N-CH2); 2.7 (m, 1 H,
C~H~); 2.4 (m, 1 H, CH~)
b) Methyl 2-((N-Phenylpyrrolidon-3-yl)-oxy)-phenyl-
glyoxylate
2100~46
- 22 - 0.~. OOS0/43411
A mixture of 12.8 g (45 mmol) of the aldehyde
from Example 2a in 100 ml of methylene chloride and S g
(100 mmol) of sodium cyanide and 5 g (94 mmol) of am-
monium chloride in 100 ml of water i~ stirred for 3 hours
at room temperature. Thereafter, the organic phase is
separated off and the aqueous phase is extracted twice
with methylene chloride. The combined organic phases are
dried over ~gS04 find evaporated down. The residue is taken
up in 100 ml of methanol, and 20 ml of 3 N hydrochloric
acid in ether are added. Stirring i8 carried out
overnight at room temperature and the reaction mixture is
then evaporated down undar reduced pressure.
Ths residue i8 taken up in 100 ml of water and
heated at lOO-C for 30 minutes, a viscou~ oil ~eparating
out. The water i~ decanted and the residue is taken up
in methylene chloride. The organic phase is dried over
MgSO4 and evaporated down.
The residue i8 taken up in 100 ml of methylene
chloride, and a solution of 0.3 g of RBr, 0.7 g of
NaH2PO4-H20 and 0.9 g of Na2HPO~ 2H20 in 100 ml of water iB
added. 0.2 g of tetramethylpiperidine N-oxide is then
added, followed by 40 ml of chlorine bleach (co~tains
-- 12.5% of active C12) while stirrin~ vigorously. Stirrin~
i8 continu~d for 1 hour at room temperature, the aqueous
phase i~ ~eparated off, a further 40 ml of chlorine
bleach are added and stirring is continued for a further
hour. The organic phase i8 then separated off, extracted
onco with w~ter, dried over MgSO4 and evaporated down. The
residue iB purified by column chromatography. 6.4 g (42~)
of the title compound are obtained as a beige
solid ~mp. - llQC).
H-NNR (CDCl~; ~ in pp~):
7.9 (d, broad, 1 H, hromatic); 7.7 (d, 2 H, J = 8 Hz,
aromatic); 7.6 (t, broad, 1 H, aromatic); 7.4 (t, 2 H,
J = 8 Hz, aromatic); 7.3 (d, 1 H, J = 8 Hz, aromatic); 7-
7.25 (m, 2 H, aromatic); 5.15 (t, 1 ~, J = 8 Hz, 0-CH);
4.0 (m, 1 H, N-C~hHB); 3.85 (m, 1 H, N-CH~; 3.85 (s, 3
2100~6
- 23 - O.Z. 0050/43411
H, -0-CH3); 2.65 (m, 1 H, -C~hH~); 2.3 (m, 1 H, -CH~)
c) Methyl ~-(2-((N-phenylpyrrolidon-3-yl)-oxy)-phenyl)-
B-methoxyacrylate (Table 3, No. 16)
4.0 g (11 mmol) of methoxymethyltriphenyl-
S phosphonium chloride in 1 g (9 ~mol) of pota~ium tert-
butylate in 50 ml of tetrahydrofuran are stirred for 30
minutes at room temperature. The mixture i~ then cooled
to -20C, 2 g (5.9 mmol) of the ketoester from Example
2b are added and the mixture i~ 810wly allowed to warm
up to room temperature. Stirring is continued for 2 hours
at room temperature, ths re~ction mixture i8 diluted with
water and the aqueou~ phase is extracted three time~ with
methyl tert-butyl ether. The combined organic phases are
drisd over ~gS04 and evaporated down. The residue is
purified by column chromatography using hexane/ethyl
acetate mixtures. 1.4 g (65%) of the trans-icomer of the
title compound are obtained as a pale yellow oil.
H-NNR (CDCl3; ~ in ppm):
7.7 (d, broad, 1 H, aromatic~; 7.5 (~, 1 H, vinyl); 6.9 -
7.45 ~m, 8 H, aromatic); 4.95 (t, 1 H, J = 8 Hz, 0-CH);
3.7 - 4.0 (m, 2 H, N-CH2); 3.8 (8, 3 H, 0-CH3); 3.7 (s, 3
H, 0-CH3); 2.45 (m, 1 H, -C~bH~); 2.25 (m, 1 H, -CH~)
EXAMPLE 3
Methyl c-(2-(3-(meta-methylphenyl)-prop-2-ynyloxy)-
phenyl)-A-methoxyacrylate (Table 3, No. 6)
a) Methyl 2~(prop-2-ynyloxy)-phenylacetate
13 g (O.54 mol) of sodium hydride are added a
little at a time to 75 g (O.45 mol) of methyl ortho-
hydroxyphenylacetate in 500 ml of dimethylformamide.
Gentle cooling i8 carried out. After 30 minutes at room
temperature, the evolution of gas has ceased, and 65 g
(0.54 mol) of propargyl bromide are added. S~irring is
carried out overnight at room tamperature, a~ter which
the reaction mixture i8 diluted with 1 1 of water and the
aqUeOU8 pha8e i8 extracted three times with ~ethyl tert-
butyl ether. ~he combined organic pha~es are dried over
MgSO4 and evaporated down. The regidue i8 di~tilled.
2l0n~46
- 24 - O.Z. 0050/43411
7I.6 g (78%) of the title compound are obtained as a pale
yellow liquid (bp. (0.3 mbar) = 98-100C).
H-NNR (CDCl3; 6 in ppm):
7.25 (m, 2 H, aromatic); 7.0 (m, 2 H, aromatic); 4.7 (d,
1 H, J = about l Hz, O-CH2); 3.7 (s, l H, O-CH3), 3.65 (s,
2 H, CH2); 2.5 (t, 1 H, J = about 1 Hz, =CH~
b) hethyl ~-(2-propynyloxyphenyl)-B-methoxyacrylate
A mixture of 20 g (0.1 mol) of the phenylacetate
from Example 3a, 20 ml of methyl formate and 2.7 g (0.11
mol) of sodium hydride in 100 ml of tetrahydrofuran is
stirred at room temperature. Gas evolution which is
initially 810w and then vigorous occurs. Once the
evolution of gas ha~ cea~ed, the r~action mixture is
diluted with water and the ~queous ph~se is sxtracted
twice with methyl ~ert-butyl ~ther. Thase organic phaseæ
are diEcarded. The aqu~ou~ pha~e i~ then acidified and
i~ extracted three times with me~hyl tert-butyl ether.
The combined ph~ses are ~iltered under suction with a
~ilica gel and evaporated down.
The re~idue i8 taken up in 200 ml of acetone, 2nd
14 g ( n . 1 mol) of R2C03 ~nd 11 g (0.09 mol3 of dimethyl
sulfate are added. Stirring i~ carried out for 4 hours
at room temperature, sfter which the reaction mixture is
evzporated down. The residue i8 taken up in methylene
chloride. The organic pha~e is wa~hed with dilute ammonia
and water, dried over MgSO~ and evaporated down. The
residue i8 purified by column chromatography using
hexane/ethyl acetate mixtures. 11 g (45%) of the trans-
isomer of the title compound are obtainzd a8 a pale
yellow solid.
H-NMR ~CDCl3; ~ in ppm):
7.5 (6, 1 H, vinyl), 7.25 (m, 2 H, aromatic); 7.05 (m, 2
H, aromatic); 4.65 (d, 2 H, J = about 1 Hz, O-CH2); 3.8
(8, 3 H, 0CH3); 3.7 ( , 3 H, O-CH3); 2.5 (t, 1 H, J =
about 1 Hz, =CH)
c) Methyl ~-(2-(3-(meta-methylphenyl)-prop-2-ynylOXy)-
phenyl-~-methoxy~crylate (Table 3, No. 6)
210~5~6
- 25 - O.Z. 0050/43411
A mixture of 2 g (8.1 mmol) of the propargyl
ether from Example 3 b, 1.8 g (8.2 mmol) of meta-iodo-
toluene, 2 g (20 mmol) of triethylamine, 0.3 g of tri-
phenylphosphine, 20 mg of CuI and 60 mg of palladiumlII)
acetate in 20 ml of dimethylformamide is stirred for one
hour at 70C. Thereafter, the reaction mixture i8 diluted
with water and the agueous phase i8 extracted three tLmes
with methyl tert-butyl ether. The combined organic phases
are dried over MgS0~ and evaporated down. The residue is
purified by column chromatography using hexane/ethyl
acetate mixture~. 0.9 q (33%) of the title compound is
obtained aR a crystalline solid (mp. = 76C).
H-NMR (CDCl3; ~ in ppm):
7.5 (~, 1 H, vinyl); 6.9-7.4 (m, 8 H, aromatic); 4.9 (s,
2 H~ 0-CH2); 3-8 (8, 3 H, 0-CH3); 3.7 (8, 3 H, 0-CH3); 2.5
(8, 3 H, CH3)
EXANPLE 4
Methyl ~-(2-((3-para-methylphenyliosxazol-5-yl)-methoxy)-
phenyl)-~-methoxyacrylate (Table 3, No. 13)
50 ml of chlorine bleach (contains 12.5% of
active C12) i8 added to a ~olution of 2 g (8.1 ol) of
the propar~yl ether from Example 3 b and 1.7 g (12.5
- mmol) of p-methylbenzaldehyde oxime in 30 ml of methylene
chloride. Stirring is csrried out for 30 minute~ at room
temperhture, the phase~ are separated and the agueous
phase i8 extracted three times with methylene chloride.
The combin~d organic phases ~re extracted once with
water, dried over MgS0~ ~nd ~vaporsted down. The residue
crystallize~ and is stirred thoroughly with methyl tert-
butyl ether/hexane. 1.9 g (62%) of the trans-isomers of
the title compound aro obtained as a pale yellow solid
~mp. = 82C).
H-NMR (CDCl3; ~ in ppm):
7.7 (d, 2 H, J = 8 Hz, aromatic); 7.55 (8, 1 H, vinyl);
7.25 (m, 4 H, aromatic); 7.0S (m, 2 H, aromatic); 6.6 (s,
1 H, i~oxazol); 5.2 (8, 2 H, 0-CH2); 3.85 (8 3 H, 0-CH3);
3.7 (8, 3 H, 0-CH3); 2.4 (8~ 3 H, CH3)
-`` 2100~46
- 26 - O.Z. 0050/43411
The compounds stated in the Tables below can be
prepared in a similar manner.
BASF A~t~en~e~ell~cb~t 920400 O.Z. 0050/43411
27 2100~
Table 1
~0~
H3CO2C ~ CH3
~n Table 1, B may have the following meanings:
1: -C~H2-C(=O ~ m X
II: -CH2-C(=N OCH ~ m
-CH2-C(-N-O-CH2-C ~ m
Xm
IV: -CH2-CH(-OCH ~ X
~ m
V: -CH2-CH(-O-CH2-CI~
VI: -CH2-C(CO)- N:~ m
BASF Aktien5~e~ell~lchaÇt 920400 O.Z. 0050/43411
2100~6
28
Vll: -CH2-C(=O)-N(CH,~ m
Xm
VIII: --C~z-c(=o)-N(--CH3)-CU~X
~X: -CH2-C(=O)-N(-CH2-CI~ m
X: ~N~X~
Xm
XI: ~N~
Xm
XII~ ~N~
XIII: ~N~i
~ASF Aktie~e~ell~chaft 920400 O.Z. 0050/43411
21~0~6
29
O
xv
~N
CH2
XVI: ~ ~m
XV I I: ~ CH~xm
.--
XVII~: ~ ~ m
X
~ m
XIX ~CH~
BASF Akt~en~ ellschaft 920400 O . Z . 0050/43411
2100~
/ m
xx~
X
m
~C~
XXI: l l
~ X
XXII: ~ m
. ~Xm
XXIII: ~CH~"N~J~
o
BASF A~ti-nge~ell~ch~t 920400 O.Z. 0050/43411
31 ~lOQ~
In Table 1 the compounds given below have for example the
following meanings:
H3CO2C~ ~CH
~ ,CH~
II/l.ll:I .
H3C02~ ~ CH3
CH3 CH3
VII/1.66: ~ ~CH~N~
. H3CO2C~ ~CH3 ~
X/l 160:~ ~N~OCH3
H3CO2C `CH3
H3C02C~ ~
BASF A~tie~e~ellschaft 920400 O.Z. 0050/43411
32 2 1 ~ a ~ 4 ~
For each of the groups B (I to XXIII) Xm may have the fol-
lowing meaning
Table 1
~ ~ B
H3CO2C ~ CH3
No. Xm
1 4-C(=O)-C6H5
. . ..
2 2-F
3 3-F
,,
4 4-F
_ _ , .
~ ~ ~
6 2,4,6-F3
2,3,4,5,6-F5
8 2,3-F2 _
9 , ~
3-Cl
. _ . . -
11 4-C1
. ~ __ _ _ _ _
12
_
13 2,4-C12
. . . .. _ . . . .. __
. 14 2,5-C12
. _ - .. __ .. .....
15 __ _
16 3,4-C12
.
17 3,5-C12
18 2,3,4-C13
19 '-----
2,3,6-C13
21 ~ -
22 2,4,6-Ci~
23 3,4,5-Cl3
~ .. _
BASF A~ti~nge~ellrch~ft 920400 O.Z. 0050/43411
33 2 1 0 0 ~ 4 5
No. Xm
24 2,3,4,6-Cl~
2,3,5,6-Cl4
26 2,3,4,5,6-Cl5
27 2-Br
28 3-Br
..
29 4-Br
2~4-Br2
2~5-Br2
32 2~6-Br2
33 2,4,6-Br3
. . .
34 2,3,4,5,6-Br5
__ _
2-I
36
4-I
38 2,4-I2
. ,_ 2-Cl, 3-F
2-Cl, 4-F
_ , , . ,_
412-Cl, 5-F
422-Cl, 6-F
. _
43 2-Cl, 3-Br
. ~
44 2-Cl, 4-Br
_ _ . .. , ..... __
2-Cl, 5-Br
46 . ~
47 .. 2-Br, 3-Cl
48 2-Br, 4-Cl
.... _ _ _
49 2-Br, 5-Cl
_ ,,_ , _
2-Br, 3-F
_ , , _ . _ -
51 2-Br, 4-F
52 2-Br, 5-F
_
53 2-Br, 6-F
54 2-F, 3-Cl
. . , , - ._
. 2-F, 4-Cl
56 2-F, 5-Cl
57 3-Cl, 4-F
58 3-Cl, 5-F
59 3-Cl, 4-Br
_ . ,.
BASF Aktien~sellschaft 920400 O.Z. 0050/43411
34
.
No. Xnl
3-C1, 5-Br
. ~ ...... ... .~ . .
61 3-F, 4-Cl
62 3-F, 4-Br
63 3-Br, 4-Cl
64 3-Br, 4-F
., 2,6-C12, 4-Br
66 2-CH3
. .
67 3-CH3
68 4-CHl
69 2,3-(CH3)2
2,4-(CH3)2
. . , ~ _
71 2,5-(CH3)2
72 2,6-(CH3)2
73 3,4-(CH3)2
74 3,5-(CH3)2
2,3,5-(CH3)3
76 2 3 4-(CH3)3
_
78 2,4,5-(CHl)3
79 2,4,6-(CH3)3
_ 3,4,5-(CHlil
81 2,3,4,6-lCH3)~
82 2,3,5,6-(CH~)4
83 . 2,3,4,5,6-(CH3) r,
84 2-C~Hs
3-C~H'
86 4-C2Hs
87 2,4-(C2Hs)2
88 2,6-(C2Hs)2
89 3,5-(C2Hs)2 _ _
2,4,6-(C2H5)3
91 2-n-ClH7
. ... _ .
92 3-n-C3H7
93 4-n-C3H7
_ .. . , _.~ ,
94 2-i-C3H7
3-i-ClH7
BASF ~Uktien~e~ell~c~u~ft 920400 O.Z. 0050/43411
210~;-,4~
No ~ xm
96 4-i-C3H7
97 2,4-(i-C~H7)~
98 2,6-(i-CIH7)
99 3,5-(i-C3H7j 2
lO0 2,4,6-(i-C3H7)3
. -.. .
101 2-s-C4Hg
. _
102 3-s-C4Hg
103 4-s-C4Hg
104 2-t-C4Hg
105 3-t-C4Hg
;
106 4-t-C4Hg
107 2,3-(t-C4Hs) 2
108 2,4-(t-C4Hg)-
,.
109 2,5-(t-C4Hg),
llO 2,6-(t-C4Hg)~
lll 3,4-(t-C4Hg)~
112 2,4,6-(t-C4Hg)3
. .... ,_ _ _
113 4-n-CgH1g
114 . 4-n-ClzH2s
115 4-n-C1sH31 . .
116 4-(1,1,3,3-tetramethylbutyl)
117 4-(2,4,4-trimethylpropyl)
118 2-t-C4Hg, 4-CH3
.
ll9 2-t-C4Hg, 5-CH~
120 . 2,6-(t-C4Hg)~, 4-CH3
121 2-CH3, 4-t-C4Hg
122 2-CHl, 6-t-C4Hg
123 2-CH-1, 4-i-ClH7
124 2-CH3, 5-i-C3H7
125 3-CH3, 4-i-C3H7
126 2-i-C3H7, 5-CH3
127 2,4-(t-C4Hg)~, 6-i-C~H7
128 2-allyl
129 3-allyl
130 4-allyl
131 2-allyl, 6-CH,
BASF ~ tien~ooellsch~ft 920400 O.Z. 0050/43411
21~ ~ ~ 4 i~
No. X~
132 ~ ly~`
133 3-cyclo-C6H
134 4-cyclo-C6Hll
135 2,4-(cyclo-C6HIl)2, 6-CH3
136 2-CH" 4-cyclo-C6H
137 2-CH.-C6H5
138 3-CH2-C6Hs
139 4-CH -C6H5
140 2-CH2-C6H5, 4-CH3
141 2-CH3, 4-CH7-C6H5
142 2-C6Hs
143 3-C6Hs
144 4-C6Hs
145 4-(2-i-C3H7-C6Hg)
146 4-C6H5, 2,6-(CH3)2
. . ..
147 2-Cl, 4-C6H5
148 2-Br, 4-C6H5
_
149 2-C,H5, 4-Cl
150 2-C6H5, 4-Br
151 2-CH2C6H5, 4-Cl
152 2-CH2C6H', 4-Br
.. 153 2-Cl, 4-CH2C6H5
'5g 2-Br, 4-CH2C6Hr
155 2-cyclo-C6Hll, 4-Cl
156 2-cyclo-C6Hll, 4-Br
157 2-Cl, 4-cyclo-C6H
158 2-Br, 4-cyclo-C6H
159 2-OCH3
160 3-OCH3
161 4-OCH3
162 2-OC~Hs
163 3-O-C2Hs
164 4-O-C2H5
165 2-O-n-C-~H
166 3-O-n-C,H-I
167 4-O-n-C~H7
BASF Aktien~e~ell~chaft 920400 O.Z. 0050/43411
37 210~546
NO. X,l~
168 2-O-i-ClH7
169 3-o-i-C3H7
170 4-o-i-C3H7
171 2-o-n-C6Hl3
172 3-O-n-C6H13
173 4-O-n-C6H13
174 2-O-n-C8HI7
175 3-O-n-Ci3H17
176 4-O-n-C~3HI7
177 2-O-CH2C6H5
178 ~ 3-O-CH2C6H5
179 4-O-CH2C6H5
180 2-O-(CH,)~C~,H5
181 3-O-(CH~)3C6H'
182 4-O-(CH~)3C6H5
183 2,4-(OCH3)2
184 2-CF3
185 3-CF
186 4-CF
187 2-OCFl
188 3-OCF3
189 4-OCF3
190 3-OCH2CHF~
191 -- 2-NO2
192 3-NO2
193 4-NO2
194 2-CN
195 3-CN
196 4-CN
. 2-CH" 3-Cl
198 2-CH3, 4-Cl
. ...
199 2-CH~, 5-Cl
. .
200 2-CH~, 6-Cl
201 2-CH" 3-F
202 2-CH3, 4-F
203 2-CHl, 5-F
- ~ASF Aktien~esell~chaft 920400 ~.Z. 0050~43411
38 210~5~6
No. Xm
., . ~
204 2-CH3, 6-F
205 2-CH3, 3-Br
206 2-CH3, 4-Br
207 2-CH3, 5-Br
208 2-CH3, 6-Br
209 2-Cl, 3-CHl
.
210 2-Cl, 4-CH3
211 2-Cl, 5-CH3
. . .
212 2-F, 3-CH3
213 2-F, 4~CH3
214 2-F, 5-CH3
215 2-Br, 3-CH3
216 2-Br, 4-CH3
. . _.__
217 2-Br, 5-CH3
218 3-CHl, 4-Cl
219 3-CH3, 5-Cl
220 3-CH3, 4-F
22i 3-CHl, 5-F
222 3-CHl, 4-Br
.. .,.__
223 3-CH3, 5-Br
224 3-F, 4-CH3
225 3-Cl, 4-CH3
226 3-Br, 4-CH3
. . . _ _
227 2-Cl, 4,5-(CH3)2
228 2-Br, 4,5-(CH3)2
229 _ 2-Cl, 3~5-(cH3)2
230 2-Br, 3,5-(CH3)~
231 2,6-Cl" 4-CH3
32 2,6-F2, 4-CH3
233 2,6-Br2, 4-CH3
234 2,4-Br~, 6-CH
235 2,4-F2, 6-CH3
236 2,4-Br~, 6-CHl
- _ . , , ,, _ . -.
237 2,6-(CHl) , 4-F
. _
238 2,6-(CH3)-, 4-Cl
.. ~
239 2,6-(CHI) , 4-Br
BASF Aktien~e~ell~chaft 920400 O.Z. 0050/43411
39 ~ i 0
No. X" - _
290 3,5-(CHI)), 4-F
241 3,5-(CH~)2, 4-Cl
242 3,5-(CH3)~, 4-Br
243 2,3,6-(CH3)3, 4-F
244 2,3,6-(CH~)3, 4-Cl
245 2,3,6-(CH3)3, 4-Br
246 2,4-(CH~ , 6-F
247 2,4-(CH~) , 6-Cl
248 2,4-(CH3)`, 6-Br
249 2-i-C3H7, 4-Cl, 5-CH3
250 2-Cl, 4-NO2 _ -
251 2-NO2, 4-Cl
252 2-OCH3, 5-NO2
253 2,4-Cl~, 5-NO2
254 . 2,4-C12, 6-NO2
255 2,6-Cl~, 4-NO~
256 2,6-Br2, 4-NO
257 2,6-J2, 4-NO~
258 . 2-CH3, 5-i-C3H7, 4-Cl
259 2-CO2CH3
_ 260 3-CO;CHl
261 4-CO CH3
262 2-CO~(C2Hs)
263 3-CO~(C2H~)
264 4-CO~(C~H~,)
. . .. _ ____
265 2-CO,(n-C~H7)
266 3-CO2(n-C3H7)
.
267 4-CO (n-C~H7)
268 2-CO,(i-C3H1)
. ~
269 __ 3-CO~(i-C3H7)
270 4-CO (i-C~H7)
271 2-CO2(n-C6H13)
272 3-CO,(n-C6HI~)
273 4-CO~(n-C~H~,)
274 2-CHi-OCH~
275 3-CH~-OCH~
BASF Akt~en~e~ell~chaft 920400 O.Z. 0050/43411
2 1 0 ~ c)
No. X",
276 4-CH2-OCH3
277 2-CH2O(C2H5)
_ ~ . .
278 3-CH2O(C2Hs)
.
279 4-CH2O(C2Hs)
280 2-CH2O(n-C3H7)
281 3-CH2O(n-C3H7)
282 . _ 4-CH2O(n-C3H7)
283 2-CH'O(i-C3H7)
284 ~
285 4-CH2O(i-C3H7)
286 2-CHO
287 3-CHO
288 4-CHO
289 2-CO-CH3
290 3-CO-CH3
. __
291 4-CO-CHl
292 2-CO-CH~-CH3
. _
293 3-CO-CH2-CH3
294 4-CO-CH2-CH~
295 2-CO-CH2-CH~-CH3
296 3-CO-CH2-CH2-CHl
297 4-CO-CH2-CH2-CH3
. .. .._ . ,
298 2-CO-CH(CH3)-CH3
-- _ ~..... _ . . . ... _ . ._
299 - 3-CO-CH(CH3)-CH3
. . _ . -- - . .
300 4-CO-CH(CH3)-CH3
. __
301 2-Me-4-CHO
_, _ ,,
302 2-Me-4-CH3-CO
303 2-Me-4-CH3-CH2-CO
304 2-Me-4-CH3-CH4-CH~-CO
_
305 2-Me-4-CH3-CH(CH3)-CO
306 2,S-Me2-4-CHO
307 2,5-Me2-4-CH3-CO
308 2,5-Me~-4-CH3-CH~-CO
309 2,5-Me2-4-CHl-CH -CH2-CO
310 2,5-Me2-4-CH.-CH~CH3)-CO
311~ 2-Cl-4-CHO
BASF AXt~en~esell~chaft 920400 O.Z. 0050/43411
41 210~
~ .....
No. xnl
312 2-Cl-4-CH3-CO
313 2-Cl-4-CH3-CH2-CO
314 2-Cl-4-CH3-CH(CH3)-CO
315 2,5-C12-4-CHO
316 2,5-Cl.-4-CH3-CO
317 2,5-Cl2-4-CH3-CH -CO
318 2,5-Cl~-4-CH3-CH2-CH2-CO
319 . 2,5-Cl~-4-CH3--CH(CH3)-CO
320 2-C(=NOCH3)-CH-1
321 3-C(=NOCH3)-CH3
322 4-C(-NOCH3)-CH
323 - 2-C(=NOC-H5)-CH-1
324 3-C(=NOC2H5)-CH3
325 4-C(=NOC2H5)-CH3
326 2-C(-NO-n-C3H7)-CH3
327 3-C(=NO-n-C3H7)-CH3
328 4-C(=NO-n-C3H7)-CH3
329 2-C(=NO-i-C3H7)-CH3
330 3-C(-NO-i-C3H7)-CH3
331 4-C(=NO-i-C3H7)-CH3
332 2-C(=NO-allyl)-CH3
333 3-C(=NO-allyl)-CH3
334 4-C(=NO-allyl)-CH3
.
335 - 2-C(=NO-trans-chloroallyl)-CH3
336 3-C(=NO-trans-chloroallyl)-CH3
. _ _
337 4-C(=NO-trans-chloroallyl)-CH3
338 2-C(=NO-propargyl)-CH3
339 3-C(=NO-propargyl)-CH3
. ... __
340 4-C(=NO-propargyl)-CH3
341 2-C(=NO-n-C4H~)-CH3
. .. , .. _ . __ .
342 3-C(=NO-n-C4H~)-CHl
343 4-C(=NO-n-C4H~ CH3
344 2-C(=NO-CH~-C~H~)-CH3
_
345 3-C(=NO-CH~-C6H~)-CH
.
346 4-C(=NO-CH2-C6H~)-CH3
347 2-CHl-4-CH=NOCH~
_ . _ . -. .
BASF Aktiengeaell~ch~ft 920400 O.Z. 0050/43411
q2 210~a4~
No. Xm
348 2-CH~-4-CH=NOC,Hr
349 2-CH3-4-CH=NO-n-ClH7
.
350 2-CH3-4-CH=NO-i-C3H7
351 2-CH3-4-CH=NO-allyl
352 2-CH3-4-CH=NO-(trans-chloroallyl)
353 2-CHl-4-CH=NO-propargyl
354 2-CH~-4-CH=NO-n-C4Hg
.~ _ 2-CH~-4-CH=NO-CH2-C6Hr,
356 2-CH~-4-(CH~-C=NOCH~)
357 2-CH3-4-(CH3-C=NOC2H5)
358 . 2-CH3-4-(CH3-C=NO-n-C~H7)
359 2-CHl-4-(CH3-C=NO-i-C3H7)
360 2-CHl-4-(CH3-C=NO-allyl)
361 2-CHl-4-(CH3-C=NO-trans-chloroallylj
362 2-CH3-4-(CH3-C=NO-propargyl)
363 2-CH3-4-(CH3-C=NO-n-C4Hg)
364 2-CH3-4-(CH3-C=NO-CH2-C6H5)
365 _ 2-CH3-4-(C2H5-C=NO-CH3)
366 2-CH3-4-(C2Hs-C=NO-C2H5)
.. __ _ .
367 . 2-CH3-4-(C2Hs-C=NO-n-C3H7)
. . _ . .
368 2-CH3-4-(C2Hs-C=NO-i-C3H7
, ..
369 2-CHl-4-(C~H~,-C=NO-allyl)
, .
370 . 2-CH3-4-(C2H5-C=NO-trans-chloroallyl)
. _ _
371 -- 2-CH3-4-(C2H5-C=NO-propargyl)
_ _ _
372 2-CH3-4-(C2H5-C=NO-n-CqHg)
373 2-CHl-4-(C~H~-C=NO-CH~-C~-.H~!)
374 2,5-(CH3)~-4-(CH3-C=NOCHl)
375 2,5-(CH3)2-4-(CH3-C=NOC2HI~)
376 2,5-(CH3).-4-(CH3-C=NO-n-C3H7)
377 2,5-(CH~)~-4-(CH3-C=NO-i-C3H7)
378 2,5-(CHi)~-4-(CH~-C=NO-allyl)
379 2,5-(CH3)2-4-(CH~-C=NO-transchloroallyl)
380 2,5-(CH-~)~-9-(CH3-C=NO-proparyl)
381 2,5-(CH,),~-4-(CH-~-C=NO-n-CqHg)
382 2,5-(CH~),-4-(CH:.-C=NO-CH:-C6Hs)
.
383 2-C~,H~,
_
BASF Akt~enge~ell~ch~ft 920400 O.Z. 0050/43411
43 2100~
No. Xlr~
384 3-C~H5
385 4~C/Hr~
386 2-(2'-F-C6H4)
.
387 2-(3'-F-C6Hq)
388 2-(4'-F-C6H4)
389 3-(2'-F-C6H4)
.
390 3-(3'-F-C6H4)
391 3-(4'-F-C6H4)
, . . ..
392 4-(2'-F-C6H4)
393 4-(3'-F-C6H4)
.. _ . . .
394 4-~4'-F-C6H4)
395 2-(2'-Cl-C6H4)
396 2-(3'-Cl-C6H4)
397 2-(4'-Cl-C6H4)
398 3-(2'-Cl-C6H4)
_ ,.
399 3-(3'-Cl-C6H4)
.
. _. 3-(4'-Cl-C6H4)
401 4-(2~-cl-C6~-)
402 4-(3'-Cl-C6H4)
403 4-(4'-Cl-C6H4)
405 2-(2'-CH3-C6H4)
,~ .. .
406 2-(3'-CH.-C6H4)
_,
407 2-(4'-CH3-C6H4)
408 3-(2'-CH3-C6H4)
409 3-(3'-CH3-C6H4)
410 3-(4'-CH3-C6H4)
411 4-(2'-CH3-C6H4)
----- ..... ._
412 4-(3'-CH3-C6H4)
._ . - .. .. _._
413 4-(4~-cHl-c6H4)
414 2-(2'-CH3-CO-C6H4)
415 2-(3'-CH3-CO-C6H4)
416 2-(4'-CH3-CO-C6H4)
417 3-(2' <~ CC~C ~
418 3-(3'-CH3-CO-C6H4)
419 3-(4'-CH-~-CO-C~H4)
420 4-(2'-CH-.-CO-C~H4)
_
BASF Akt~enge~ellachaft 920400 O.Z. 0050/43411
4~ 21~D~
No. Xm
...__.
421 4-(3'-CH~-CO-C6H4)
422 4-(4'-CHl-CO-C~,Hq)
423 2-(2'-(CH3-C(=NOallyl))-C6Hq)
424 2-(3'-(CH3-C(=NOallyl))-Ct,H4)
425 2-(4'-(CH3-C(=NOallyl))-C6H4)
_ . .
426 3-(2~-(CH3-C(=NOallyl))-C~H4)
427 3-(3'-(CH3-C(=NOallyl))-C6H4)
428 3-(9'-(CH3-C(=NOallyl))-C6H4)
.
429 4-(2'-(CH3-C(=NOallyl))-C6H4)
430 4-(3'-(CH3-C(=NOallyl))-C6H4)
431 . 4-(4'-(CH3-C(=NOallyl))-C6H4)
432 . 2-(2'-CH3O2C-C6H4)
433 2-(3'-CH3O2C-C6H4)
434 2-(4'-CH3O~C-C6H4)
435 3-(2'-CH3O~C-C6H4)
.
436 3-(3'-CH3O2C-C6H4)
-- _ . ~
- _ 3-(4'-CH3O2C-C6H4)
438 4-(2'-CH3O2C-C6H4)
439 4-(3'-CH3O2C-C6U4)
440 4-(4'-CH3O2C-C6H4)
. ~
441 2-(2'-CH3O-C6H4)
-.
442 2-(3'-CH3O-C6H4)
. .
443 , 2-(4'-CH3O-C6H4)
444 3-(2~-CH~O-C~.H4)
. .
445 3-(3'-CHlO-C6H4)
.
446 3-(4~-CH3O-C6H4)
_. _ . , ,,
447 4-(2~-CHlO-C6H4)
.
448 4-(3'-CH3O-C6H4)
449 4-(4'-CH3O-C6H4)
450 2-(2'-O2N-C6H4)
451 2-(3'-O~,N-C6H4)
.
452 2-(4l-O N-C6H4)
._ _ .
453 3-(2'-O2N-C6H4)
. _
454 3-(3'-O2N-C6H4)
_ . _ . ,.~.
455 3-(4~-O,N-C,~H4)
456 4-(2~-O,,N-C~H4)
_
BASF Akt~ensleElell8chaft 920400 0.2i . 0050/43411
2iO05~6
No ~ Xm
_ ; _ _
457 4- (3 ' -0~ N-C6H4)
458 4- (4 '-O2N-C6H4)
459 2- (2 '-NC-C6H4)
460 2- (3 ' -NC-C6H4)
461 2 - (4 ' -NC-C 6Hq )
462 . 3- (2 ' -NC-C6H4)
463 3- (3 ' -NC-C6H4)
464 3- (4 '-NC-C6H4)
465 4 - ~ 2 ' -NC-C6H4)
466 4- (3 ' -NC-C6H4)
_
467 4 - (4 ' -NC-C6H4)
468 2- (2 ' -CF-~ -C6H4)
. . . - . .
469 2- (3 ' -CF~-C6H4)
,,_ , _
470 2 - (4 ' -CF3 -C 6H4)
471 3- (2 '-CF-~-C6H4)
472 3- (3 '-CF3-C6H4)
; ~ 3 (4 ~ -CF3 - ~`6H4)
474 4 - (2 ' -CF3 -C 6H4)
475 4- (3 '-CF3-C6H4)
476 4 - (4 ' -CF3 -C 6H 4)
477 2 --o-c6H5
475 3 -o-c6H5
476 4-O-C6Hs
478 - 2 -O- (2 ' -F-C6H4)
479 2-O- ~ 3 ' -F-C6H4)
480 ~
481 _ 3-O- ~ 2 ' -F-C6H4)
482 3 - O- (3 ' -F-C6H4)
483 3-O- ~4 '-F-C6H4)
.
484 4-O- ~2 ' -F-C6H4)
485 4 -O- ~ 3 ' -F-C6H4)
486 4 -O- ~ 4 ' -F-C6H4)
487 2-O- (2 ' -Cl-Ci,H4)
. ,._
488 2-O- (3 ' -Cl-C~H4)
489 2 - O- (4 ' -C l -C ~H ~ )
.
490 3-O- (2 ' -Cl-C~.H4)
. ._
BASF Aktien~eaellachaft 920400 O.Z. 0050/43411
46 21005~
No. Xl,,
491 3-O-(3'-Cl-C6H4)
492 3-O-(4'-Cl-C6H4)
493 3-0-(4'-Cl-C6H4)
494 4-O-(2'-Cl-C6H4)
495 4-o-(3'-Cl-C6H4)
496 4-o-(4'-Cl-C6H4)
497 2-O-(2'-CH3-C6H4)
498 2-O-(3'-CH3-C6H4)
_
499 2-O-(4'-CH3-C6H4)
.
500 3-o-(2~-cH3--c6H4)
501 3-O-(3'-CH3-C6H4)
502 3-O-(4'-CH3-C6H4)
503 4-O-(2'-CHI-C6H4)
504 4-O-(3'-CH3-C6H4)
505 4-O-(4'-CH3-C6H4)
.. .. . ..
506 2-O-(2'-CH3-CO-C6H4)
507 2-O-(3'-CH3-CO-C6H4)
508 2-O-(4'-CH3-CO-C6H4)
509 3-O-(2'-CH3-CO-C6H4)
. ~
510 3-O-(3'-CH3-CO-C6H4)
.
511 3-O-(4'-CH3-CO-C6H4)
~. . _
512 4-O-(2'-CHl-CO-C6H4)
. _
513 4-O-(3'-CH3-Co-C6H4)
: . _
514 - 4-O-(4'-CH3-Co-C6H4)
515 2-O-(2'-(CH3-C (=NOallyl))-C6Hq)
516 2-o-(3~-(cH3-c(=Noallyl))-c6H4)
517 G (4 C~ r~ llyl ~ t,)
518 3-0-(2'-(C~ NO~IIYII~ L~
519 3-0-(3'-(CH3-C (=NOallyl))-C6H4)
_
520 3-0-(4'- (CHl-C(=NOallyl))-C~H4)
. _
521 4-0-(2'-( CHl-C(=NOallyl))-C6H4)
522 4-O-(3'-(CH3-C(=NOallyl))-C6H4)
523 _ . 4-O-(4'-(CHl-C(=NOallyl))-C6H4)
524 2-O-(2'-CH-,O~C-C6H4)
525 2-O-(3~-CH30,C-C~H4)
.
526 2-O-(4'-CH-~O~C-C6H4)
BASF Akt~en~esell~ch~St 920400 O.Z. 0050/43411
47 21 00~4~
NO. . . .
527 3-0-(2'-CH30 C-C6H4j
528 3-0-(3'-CH30 C-C~H9)
._ _ 3-O-(4'-CH3O2C-C6H4)
530 4-O-~2'-CH3O~C-C6Hq)
531 4~O~(3'~CH3O2C-C6Hq)
532 4-O-(4'-CH~O`C-C6H4)
533 2-O-(2'-CH3O-C6Hg)
534 2-O-(3'-CH3O-C6H4)
535 ~
536 3-O-(2'-CH3O-C6H4)
537 3-O-(3'-CH3O-C6Hq)
538 3-O-(4'-CH30-C6H4)
539 4-O-(2'-CH3O-C6H4)
540 4-O-(3'-CH3O-C6H9)
541 _ 4-O-(4'-CH30-C6Hg)
542 2-O-(2~-o2N-C6H4)
543 2-O-(3'-O2N-C6H9)
544 2-O-(4~-O2N-C6Hg)
545 3-0-(2'-O~N-C6Hq)
546 3-O-(3'-O2N-C6H4)
547 3-O-(4'-O2N-C6H4)
_ .
548 4-o-(2~-o2N-c6H4)
~ 4-O-(3~-O2N-C6Hg)
550 - 4-O-(4'-O2N-C6Hg)
551 2-O-(2'-NC-C6H4)
552 2-0-(3'-NC-C6Hq)
553 2-O-(4'-NC-C6H4)
554 3-O-(2'-NC-C6H4)
555 3-0-(3'-NC-C6Hq)
556 3-0-(4'-NC-C6Hq)
557 4-O-(2'-NC-C6H4)
558 4-0-(3'-NC-C6Hg)
559 4-o-(4'-NC-C6H4)
560 2-O-(2'-CF3-C6Hg)
561 r 2-O-(3'-CF-~-C6H4)
562 2-O-(4'-CF~-C6H4)
BASF Akti~n~e~ellschaft 920400 O.Z. 0050/43411
48 ~ 4 ~
No. X",
563 3-O-(2'-CF3-C6H4)
564 3-O-(3'-CF3-C6H4)
565 3-O-(4'-CF3-C6H4)
.
566 4-0-(2'-CFI-C6H 4)
. - ,
567 4-O-(3'-CF3-C6H9)
568 4-O-(4'-CF~-C~.H4 )
569 2-pyridinyl-2'
570 2-pyridinyl-3'
571 2-pyridinyl-4'
572 3-pyridinyl-2'
573 3-pyridinyl-3'
. . _ . ,
574 3-pyridinyl-4'
. _ . _ , , ,
575 4-pyridinyl-2'
576 4-pyrldinyl-3'
577 4-pyridinyl-4'
578 2-pyrimidinyl-2'
579 2-pyrimidinyl-3'
580 2-pyrimidinyl-4'
_ . .
581 3-pyrimidinyl-2'
582 3-pyrimidinyl-3'
583 3-pyrimidinyl-4'
584 4-pyrimidinyl-2'
585 4-pyrlmidinyl-3'
586 - 4-pyrimidinyl-4'
587 2-pyrazolyl-1'
588 2-pyrazolyl-3'
589 2-pyrazolyl-4'
590 3-pyrazolyl-1~
591 3-pyrazolyl-3'
592 3-pyrazolyl-4~
593 4-pyrazolyl-1'
594 4-pyrazolyl-3'
595 4-pyrazolyl-4'
596 2-isoxazolyl-3'
597 2-isoxazolyl-4'
598 2-isoxazolyl-5'
BAS~ AXtien~q~oll~c~t g20400 O.Z. 00$0~434~1
49 21~05~
I
__
No. X" ¦
... . ... ~ ... __
599 3-i oxazolyl-3'
600 3-li oxazoiyi-4~
601 3-i. oxazoiyl-S'
60~ 4-ilsoxazolyl-3
603 --
.__
604 4 i~oxazolyl-5
605
606 2-i~othi~zolyl-4
._
607 2~ oth~azolyl-5'
.......... . . ... .....
~08 3-isothiazolyl-3
_
609 3-i~othiazolyl-4
~10, 3-i~othlazolyl-5
611' - -
i -''-'
613 4-i~othiazolyl-5
..,
614 2-imidazolyl-1'
615 2-lmi~azolyl-2
616
617 3~imidazolyl-l'
618 3-imi~azolyl
61g 3-imidazolyl-4 ~
620
. _ 4~imidazoiyl-2'
622 - =
623 2-oxazolyl-2'
624 2-oxazolyl 4'
. . . .
~25 2-oxazolyl-5'
,~., ~ ~_~
62~ 3-oxazolyl-a'
..
627 3-oxazolyl-4'
628 3~oxazolyl-S~
629 4-oxazolyl-2'
630 _
631 4-oxazolyl-5'
~_ . _ . .
632 2-thiazolyl-2
.__
~33 2-thiazolyl-~'
634 2-thiazolyl-5'
_ ..... ... . ..
BASF A~tien~esell~cha~t 920400 O.Z. 0050/43411
21 0 0 5 4 6
~No. X~,
635 3-thiazolyl-2'
636 3-thiazolyl-9'
637 3-thiazolyl-5'
638 4-thiazolyl-2'
639 4-thiazolyl-4'
640 4-thiazolyl-5'
BASF Aktien~e~ell~ch~ft 920400 O.Z. 0050/43411
51 21~0~6
Table 2
~ ~CH
H3CO2C ~ CH3
No. A
1 pyrrolyl-3
N-CHl-pyrroiyl-3
N-C~,H5-pyrrolyl-3
N-(9'-CH3-C6H4)-pyrrolyl-3
N-(3'-CH3-C6H4)-pyrrolyl-3
6 N-(2'-CH3-C6H4)-pyrrolyl-3
_
7 N-(4'-CH3O-C6H4)-pyrrolyl-3
8 N-(3'-CH3O-C6H4)-pyrrolyl-3
. N-(2'-CH3O-C6H4)-pyrrolyl-3
. N-(g'-NO,-C6H4)-pyrrolyl-3
11 N-(3~-NO~-C6H4)-pyrrolyl-3
12 N-(2'-NO2-C6H4)-pyrrolyl-3
13 N-(4'-CN-C6H4)-pyrrolyl-3
14 N-(3'-CN-C6H4)-pyrrolyl-3
N-(2'-CN-C6H4)-pyrrolyl-3
16 N-(9'-Cl-C~H4)-pyrrolyl-3
17 . _ N-(3'-Cl-C5H4)-pyrrolyl-3
18 N-(2'-Cl-C6H4~-pyr-olyl-3
19 pyrrolyl-2
N-CH,-pyrrolyl-2
21 N-C~H5-pyrrolyl-2
22 N-(4'-CH~-Ct~H4)-pyrrolyl-2
23 ~
29 N-(2'-CH,-C6H4)-pyrrolyl-2
N-(9'-CH.O-C~H4)-pyrrolyl-2
~ASF ~ktien~esellsch~ft 920400 O. Z . 0050/A3411
52 21~5~
. . ,
No. A
26 N-(3'-CH~0-C6H9)-pyrrolyl-2
27 N-(2'-CH30-C6H4)-pyrrolyl-2
28 N-(4'-N02-C6H4)-pyrrolyl-2
29 N-(3'-N0~-C~H4)-pyrroly1-2
N-(2'-N0~-C6H4)-pyrrolyl-2
31 N-(4'-CN-C6H4)-pyrrolyl-2
.
32
33 N-(2'-CN-C6H4)-pyrrolyl-2
34 N-(4'-Cl-C6H4)-pyrrolyl-2
N-(3'-Cl-C6H4)-pyrrolyl-2
36 N-(2'-Cl-C6H4)-pyrrolyi-2
37 furyl-2
38 5-CHl-furyl-2
39 ~
5-(4'-CHl-C6H4)-furyl-2
91 5-(3'-CH-I-C6H4)-furyl-2
42 5-(2'-CHl-C6H4)-furyl-2
93 5-(4'-CH30-C6H4)-furyl-2
94 5-(3'-CH30-C6Hq)-furyl-2
.,.__ . , ,
5-(2'-CH30-C6H4)-furyl-2
._
46 5-(4'-N0~-C6H4)-furyl-2
~. .
47 5-(3'-N02-C6Hg)-furyl-2
, , _ __ _ . _
48 .
49 .. 5-(4'-CN-C6H4)-furyl-2
_
5-(3'-CN-C~,H4)-fury1-2
51 5-(2'-CN-C6H4)-furyl-2
__ ..... . _~ .
52 5-(4'-Cl-C6H4)-furyl-2
. . ~
53 5-(3'-Cl-C6H4)-furyl-2
. __ . ,
54 5-(2'-Cl-C6H4)-furyl-2
. . . _. --
4-CHl-furyl-2
56 4-C,,H~,-fùryl-2
57 4-(4'-CHl-C~,H4)-furyl-2
58 4-(3'-CH,-C~H4)-furyl-2
59 4-(2'-CH-,.-ChH4)-furyl-2
4-(4'-CH30-C~,H4)-furyl-2
61 4-(3'-CH~0-C~.H~)-fury1-2
BASF Aktien~e~ell~chaft 920400 O.Z. 0050/43411
2100~46
53
No. A
62 4-(2'-CH-~O-C6H4)-furyl-2
63 4-(4'-NO,-Ct.H4)-furyl-2
64 . 4-(3'-NO.~-C6H4)-furyl-2
4-(2'-N0;-C~H4)-furyl-2
66 4-(4'-CN-C~H4)-furyl-2
67 4-(3'-CN-C6H4)-furyl-2
68 4-(2'-CN-C6H4)-furyl-2
69 4-(4'-Cl-C6H4)-furyl-2
4-(3'-Cl-C6H4)-furyl-2
71 4-(2'-Cl-C6H4)-furyl-2
_ - __ . . . . .
72 thieny1-2
73 5-CH3-thienyl-2
74 5-C,.Hr~-thienyl-2
5-(4'-CH3-C6H4)-thienyl-2
76 5-(3'-CH3-C6H4)-thienyl-2
77 5-(2'-CH3-C6H4)-thienyl-2
78 5-(4'-CH~O-C6H4)-thienyl-2
79 5-(3'-CHlO-C6H4)-thienyl-2
5-(2~-cH3o-c6H4)-thienyl-2
81 . 5-(4'-NO~-C6H4)-thienyl-2
82 .. __ 5-(3'-NO.-C6H4)-thienyl-2
~83 5-(2'-NO -C6H4)-thienyl-2
84 5-(4'-CN-Ct,H4)-thienyl-2
- 5-(3'-CN-C~,H4)-thienyl-2
86 5-(2'-CN-C~H4)-thienyl-2
~ _ , __
87 5-(4'-Cl-C6H4)-thienyl-2
~ _ ,
88 5-(3'-Cl-C6H4)-thienyl-2
. . _
89 5-(2'-Cl-C6H4)-thienyl-2
.
4-CHl-thienyl-2
. - . _ ~ , ,
91 4-C~.H.. -thienyl-2
92 . 4-(4'-CH?=C6H4)-thienyl-2
93 4-(3'-CHl-C6H4)-thienyl-2
94 4-(2'-CH,-C6H4)-thienyl-2
4-(4'-CH~O-C6H4)-thienyl-2
96 4-(3'-CH,O-C~H4)-thienyl-2
97 4-(2'-CH~O-C~H4)-thienyl-2
BASF A~tion~e~ellBchaft 920400 O.Z. 0050/43411
2100~
: ~ _
No. A
98 4-(4'-NO,-Cf.H4)-thienyl-2
99 4-(3'-NO -C6H4)-thienyl-2
100 4-(2~-NO~-C6Hq)-thienyl-2
101 - - 4-(4'-CN-C6Hg)-thienyl-2
102 4-(3'-CN-C6Hq)-thienyl-2
103 4-~2'-CN-C6Hg)-thienyl-2
104 4-(4'-Cl-C6H9)-thieny1-2
105 4-(3'-Cl-C6Hq)-thienyl-2
106 4-(2'-Cl-C6Hq)-thienyl-2
107 thienyl-3
108 5-CH,-thienyl-3
.
109 5-C,-,Hr;-thienyl-3
, , . ......... . ___
110 . 5-(4'-CH3-C6Hq)-thienyl-3
111 5-(3'-CH-,-C6Hq)-thienyl-3
112 5-(2'-CH~-C6Hq)-thienyl-3
113 5-(4'-CH3O-C6Hg)-thienyl-3
114 5-(3'-CH30-C6Hç)-thienyl-3
115 5-(2'-CH30-C6Hg)-thienyl-3
116 5-(4'-NO2-C6Hg)-thienyl-3
117 5-(3'-NO~-C6H4j-thienyl-3
118 5-(2'-NO~-C6Hg)-thienyl-3
119 5-(4'-CN-CfH4)-thienyl-3
120 .. 5-(3'-CN-C6H4)-thienyl-3
. . .
121 - 5-(2'-CN-C6Hq)-thienyl-3
122 5-(4'-Cl-C6Hg)-thienyl-3
123 5-(3'-Cl-C6Hq)-thienyl-3
124 5-(2'-Cl-C6H4)-thienyl-3
125 pyrazolyl-4
126 N-CH1-pyrazoly1-4
.
127 N-C,.H~,-pyrazolyl-4
128 N-t4'-CH,-ChH4)-pyrazolyl-4
.
129 N-(3'-CH3-C6H4)-pyrazolyl-4
. -
130 N-(2'-CH,-C6H4)-pyrazolyl-4
. ._
131 N-(4'-CH~O-C~,H~)-pyrazolyl-4
. , , __
132 N-(3'-CH~O-Cf,H.;)-pyrazolyl-4
. , .
133 N-(2'-CHIO-C~.H.~,)-pyrazolyl-4
BASF Akti~ngeBellachaft 920400 O.Z. 0050/43411
2100~4~
No. A
134 N-(4~-N0,-C~,H4)-pyrazolyl-4
135 N-(3'-N0~-C6H4)-pyrazolyl-4
136 N-(2'-N0,-C6H4)-pyrazolyl-4
137 N-(4'-CN-C6H4)-pyrazolyl-4
138 . N-(3'-CN-C6H4)-pyrazolyl-4
139 N-(2'-CN-C6H4)-pyrazolyl-4
140 N-(4'-Cl-C6H4)-pyrazolyl-4
141 , N-(3'-Cl-C6H4)-pyrazolyl-4
142 N-(2'-Cl-C6H4)-pyrazolyl-4
143 3-CH,-N-methylpyrazolyl-4
144 _ _ 3-C6H5-N-methylpyrazolyl-4
145 3-(4'-CH,~-C6Hq)-N-methylpyrazolyl-4
146 3-(3'-CH~/-C6H4)-N-methylpyrazolyl-4
.
147 3-(2'-CH,-C6H4)-N-methylpyrazolyl-4
148 3-(4'-CH30-C6H4)-N-methylpyrazolyl-4
149 3-(3'-CH30-C6H4)-N-methylpyrazolyl-4
150 3-(2'-CH,0-C6H4)-N-methylpyrazolyl-4
151 3-(4'-~0 -C6~4)-N-methylpyrazolyl-4
-- . .. . ... . _
152 3-(3'-N0~-C6H4)-N-methylpyrazolyl-4
153 3-(2'-N0 -Cf,H4)-N-methylpyrazolyl-4
154 3-(4'-CN-CbH4)-N-methylpyrazolyl-4
155 3-(3'-CN-C~,H4)-N-methylpyrazolyl-4
156 3-(2'-CN-C6H4)-N-methylpyrazolyl-4
157 3-(4'-Cl-C6H4)-N-methylpyrazolyl-4
158 3-(3'-Cl-C6H4)-N-methylpyrazolyl-4
.. 3-(2'-Cl-C6H4)-N-methylpyrazolyl-4
160 isoxazolyl-5
161 3-CH,,-isoxazolyl-5
162 3-C.. H,~-isoxazolyl-5
163 3-(4'-CHl-C6H4)-isoxazolyl-5
164 3-(3'-CH3-C6H4)-isoxazolyl-5
165 3-(2'-CHl-C6H4)-isoxazolyl-5
- , . ~
166 3-(4'-CH70-Cf,H4)-isoxazolyl-5
167 3-(3'-CH~0-C~H4)-isoxazolyl-5
~ . ~ .
168 3-(2~-CH,0-C6H4)-isoxazolyl-5
.
169 3-(4'-N0.,-C(,H4)-isoxazolyl-5
. . _ , . .
BASF Aktiengeaell~chaft 920400O.Z. 0050/43411
562100~
No . A
170 3-(3'-NO~-C6H4)-isoxazolyl-5
171 . 3-(2'-NO~-C6H4)-isoxazolyl-5
172 3-(4'-CN-C6H4)-isoxazolyl-5
173 3-(3'-CN-C6H4)-isoxazolyl-5
174 3-(2'-CN-C6H4)-isoxazolyl-5
175 3-(4'-C1-C6H4)-isoxazolyl-5
176 3-(3'-Cl-C6H4)-isoxazolyl-5
177 3-(2'-Cl-C~H4)-isoxazolyl-5
178 4-chloroisoxazolyl-5
179 3-CHl-4-chloroisoxazolyl-5
180 3-CsHs-4-chloroisoxazolyl-5
181 3-(4'-CH3-C6H4)-4-chloroisoxazolyl-5
182 3-(3'-CHl-C6H4)-4-chloroisoxazolyl-5
183 3-(2'-CH~-C6H4)-4-chloroisoxazolyl-5
18q 3-(4'-CH3O-C6H4)-4-chloroisoxazolyl-5
185 3-(3'-CH3O-C6H4)-4-chloroisoxazolyl-5
186 3-(2~-CH 10-C6H4)-4-chloroisoxazolyl-5
. . . .
187 3-(4'-NO2-C6H4)-4-chloroisoxazolyl-5
188 3-(3'-NO2-C6H4)-4-chloroisoxazolyl-5
189 3-(2'-NO2-C6H4)-4~ hloroisoxazolyl-5
190 3_ ~
~ . .
191 3-(3'-CN-C6H4)-4-chloroisoxazolyl-5
192 3-(2'-CN-C6H4)-4-chloroisoxazolyl-5
193 - 3-(4'-Cl-C6H4)-4-chloroisoxazolyl-5
194 3-(3'-Cl-C6H4)-4-chloroisoxazolyl-5
195 3-(2'-Cl-C6H4)-4-chloroisoxazolyl-5
196 isoxazolyl-3
197 5-CH~-isoxazolyl-3
198 5-C,.Hs-isoxazolyl-3
199 5-(q'-CH.-ChH4)-isoxazolyl-3
200 5-(3'-CH,-C6H4)-isoxazolyl-3
201 5-(2'-CH~-C6H4)-isoxazolyl-3
202 5-(4'-CH30-C~H4)-isoxazolyl-3
203 5-(3'-CHlO-C6H4)-isoxazolyl-3
204 .~_ 5-(2'-CH~O-C6H4)-isoxazolyl-3
205 . 5-(qi-NO -C6H4)-isoxazolyl-3
BASF ~ktien5leBe11~::haft 920400 O.Z. 0050/43411
57 2100~)46
NO . ¦ A
206 5-(3'-NO~-C6H4)-isoxazolyl-3
207 5-(2'-NO~-C6H4)-isoxazolyl-3
208 5-(4'-CN-C6H4)-isoxazolyl-3
.
209 5-(3'-CN-C6H4)-isoxazolyl-3
210 5-(2'-CN-C6H4)-isoxazolyl-3
211 5-(4'-Cl-C6H4)-isoxazolyl-3
212 5-(3'-Cl-C6H4)-isoxazolyl-3
213 5-(2'-Cl-C6H4)-isoxazolyl-3
214 isothiazolyl-S
215 3-CH3-isothiazolyl-S
. _ , _
216 3-ChHs-isothiazolyl-S
217 3-(4'-CH3-C6H4)-isothiazolyl-5
218 3-(3'-CH3-C6H4 ) -i sothiazolyl-5
219 3-(2'-CH~-C6H4)-isothiazolyl-5
220 3-(4'-CH~O-C6H4)-isothiazolyl-5
221 3-(3~-CH-~O-C6H4 ) -iSOthiaZO1Y1-5
222 3-(2'-CH3O-C6H4)-isothiazolyl-5
223 3-(4'-NO2-C6H4)-isothiazolyl-5
224 3-(3'-NO~-C6H4)-isothiazolyl-S
225 3-(2'-NO2-C6H4)-isothiazolyl-S
226 . 3-(4'-CN-C6H4)-isothiazolyl-S
227 . 3-(3'-CN-C6H4)-isothiazolyl-S
228 - 3-(2'-CN-C6H4)-isothiazolyl-5
229 3-(4'-Cl-C6H4)-isothiazolyl-5
230 3-(3'-Cl-C6H4)-isothlazolyl-5
231 3-(2'-Cl-C6H4)-isothiazolyl-5
. _
232 oxazolyl-4
. . __
233 2-CH3-oxazolyl-4
. ___ 2~Cr~H~~OXaZOiyl~4
235 2-(4'-CHl-C6H4)-oxazolyl-4
236 2-(3'-CH3-C6H4 ) -OXaZO1Y1-4
237 2-(2'-CHl-C6H4)-oxazolyl-4
238 ~- 2-(4~-CH-1O-Cf H4 ) -OXaZO1Y1-4
239 2-(3'-CH ~O-C~ H4 ) -OXaZO1Y1-4
240 2-(2'-CH3O-Ct,H4)-oxazolyl-4
241 2-(4'-NO -CfH~)-oxazolyl-4
BASF Aktien~eaQllachaft 920400 O.Z. 0050/43411
58 2100a~6
~ _ .
No. A __
242 2-(3'-NO;~-C6Hg)-oxazolyl-4
243 2-(2'-NO~-C6H4)-oxazolyl-4
244 . 2-(4'-CN-C6Hg)-oxazolyl-4
245 2-(3'-CN-C6Hg)-oxazolyl-4
246 2-(2~-CN-C6H4)-oxazolyl-4
247 2-(4'-Cl-C6H4)-oxazolyl-4
248 2-(3'-Cl-C6Hq)-oxazolyl-4
249 2-(2'-Cl-C6Hg)-oxazolyl-4
250 thiazolyl-4
251 2-CH3-thiazolyl-4
252 2-C6H5-thiazolyl-4
253 2-(4'-CHl_C6H4)-thiazolyl-4
254 2-(3'-CH3-C6Hg)-thiazolyl-4
255 2-(2'-CH3-C6Hg)-thiazolyl-4
256 . 2-(4'-CH3O-C6Hg)-thiazolyl-4
267 2-(3'-CHlO-C6Hg)-thiazolyl-4
258 2-(2'-CH30-C~Hg)-thiazolyl-4
_
259 2-(4'-NO;.-C6Hg)-thiazolyl-4
260 2-(3'-NO~-C6Hg)-thiazolyl-4
261 2-(2'-NO,-C6Hg)-thiazolyl-4
262 2-(4~-cN-c6H9)-thiazolyl-4
263 2-(3'-CN-C6Hg)-thiazolyl-4
. . . . .
264 2-(2'-CN-C6Hg)-thiazolyl-4
265 - 2-(4'-Cl-C~Hg)-thiazolyl-4
.
266 2-(3'-Cl-C6Hg)-thiazolyl-4
.
267 2-(2'-Cl-C6H4)-thiazolyl-4
-- . ., _ .
268 N-CH3-1,2,4-triazolyl-5
269 3-CH,!-N-CH3-1,2,4-triazolyl-5
.. _ .. ~
270 3-ChH5-N-CH3-1,2,4-triazolyl-5
.
271 3-(4'-CH3-C6H4)-N-CH3-1,2,4-triazolyl-5
272 3-(3'-CHl-C6Hg)-N-CH3-1,2,4-triazolyl-5
273 . 3-(2'-CH~-C~H4)-N-CH,-1,2,4-triazolyl-5
274 3-(4'-CH10-C~H4)-N-CH-~-1,2,4-triazolyl-5
275 _ 3-(3'-CHlO-C~H4)-N-CH3-1,2,4-triazolyl-5
276 3-(2'-CH~O-C6H4)-N-CHI-1,2,4-triazolyl-5
277 3-(4'-NO~-C~H4)-N-CH-I-1,2,4-triazolyl-5
BASF Aktien~esell~chaft 920400 O.Z. 0050/43411
59 2100a4~
_ .
No. A
278 3-(3'-NO -C6Hq~-N-CH~-1,2,4-triazolyl-5
279 3-(2'-NO~-C6H4)-N-CH3-1,2,4-triazolyl-5
280 3-(4'-CN-C6H4)-N-CH3-1,2,4-triazolyl-5
281 -(3'-CN-C6H9)-N-CH3-1,2,4-triazolyl-5
. -
282 -(2'-CN-C6Hq)-N-CH3-1,2,4-triazolyl-5
283 3-(4'-C1-C6Hq)-N-CH3-1,2,4-triazolyl-5
284 3-(3'-Cl-C6Hq)-N-CH~-1,2,4-triazolyl-5
285 -(2'-Cl-C6H4)-N-CH3-1,2,4-triazolyl-5
286 1,3,4-oxadiazolyl-2
287 5-CH3-1,3,4-oxadiazolyl-2
288 -C6H5-1,3,4-oxadiazolyl-2
289 5-(4'-CH3-C6H4)-1,3,4-oxadiazolyl-2
290 5-(3'-CH3-C6H4)~1,3,4-oxadiazolyl-2
. _ .
291 5-(2'-CH3-C~H4)-1,3,q-oxadiazolyl-2
292 5-(4'-CH30-C6H4)-1,3,4-oxadiazolyl-2
293 5-(3'-CH30-C6H4)-1,3,4-oxadiazolyl-2
294 5-(2'-CH30-C6Hq)-1,3,4-oxadiazolyl-2
295
296 . 5-(3'-NO2-C6Hq)-i,3,4-oxadiazolyl-2
297 5-(2'-NO~-C6Hq)-1,3,4-oxadiazolyl-2
298 5-(4'-CN-C6Hq)-1,3,4-oxadiazolyl-2
299 5-(3'-CN=C6Hq)-1,3,4-oxadiazolyl-2
300 5-(2'-CN-C6H4)-1,3,4-oxadiazolyl-2
301 ~ 5-(4'-Cl-C6Hq)-1,3,4-oxadiazolyl-2
302 5-(3'-Cl-C6Hq)-1,3,4-oxadiazolyl-2
303 5-(2'-Cl-C6H4)-1,3,4-oxadiazolyl-2
304 1,2,4-oxadiazolyl-3
305 5-CH3-1,2,4-oxadiazolyl-3
306 5-C~.HI,-1,2,4-oxadiazolyl-3
_ 5-(4'-CHl-C~H4)-1,2,4-oxadiazolyl-3
308 5-(3'-CH3-C6Hq)-1,2,4-oxadiazolyl-3
309 5-(2'-CH3-C6H4)-1,2,4-oxadiazolyl-3
310 5-(4'-CH30-C6Hq)-1,2,4-oxadiazolyl-3
311 5-(3'-CH-10-C6Hq)-1,2,4-oxadiazolyl-3
312 5-(2'-CH-IO-C6Hq)-1,2,4-oxadiazolyl-3
313 5-(4'-NO~-C6Hq)-1,2,4-oxadiazolyl-3
BASF Aktiengesell~ch~ft 920400 O.Z. 0050/43411
2100~46
r
No. A
314 5-(3'-NO~-C6H4)-1,2,4-oxadiazolyl-3
31S 5-(2'-NO~-C6H4)-1,2,4-oxadiazolyl-3
316 5-(4'-CN-C6H4j-1,2,4-oxadiazolyl-3
317 5-(3'-CN-C~.H4)-1,2,4-oxadiazolyl-3
318 . 5-(2'-CN-C6H4)-1,2,4-oxadiazolyl-3
.
319 5-(4'-Cl-C6H4)-1,2,4-oxadiazolyl-3
320 5-(3'-Cl-C6H4)-1,2,4-~xadiazolyl-3
321 5-(2'-Cl-C6H4)-1,2,4-oxadiazolyl-3
322 1,2,4-oxadiazolyl-5
323 3-CHl-1,2,4-oxadiazolyl-5
324 3-C~H5-1,2,4-oxadiazolyl-5
325 3-(4~-CH3-C6H4)-1,2,4-oxadiazolyl-5
326 3-(3'-CH3-C6H4)-1,2,4-oxadiazolyl-5
327 3-(2'-CHI-C6H4)-1,2,4-oxadiazolyl-5
328 3-(4'-CHlO-C6H4)-1,2,4-oxadiazolyl-5
329 3-(3'-CH,O-C6H4)-1,2,4-oxadiazolyl-5
330 3-(2'-CH~O-C6H4)-1,2,4-oxadiazolyl-5
331 3-(4'-NO2-C6H4)-1,2,4-oxadlazolyl-5
332 . 3-(3'-NO2-C6H4)-1,2,4-oxadiazolyl-5
333 3-(2'-NO2-C6H4)-1,2,4-oxadiazolyl-5
334 3-(4'-CN-C6H4)-1,2,4-oxadiazolyl-5
335 3-~3'-CN-C6H4)-1,2,4-oxadiazolyl-5
336 ,-, 3-(2'-CN-C6H4)-1,2,4-oxadiazolyl-5
337 - 3-(4'-Cl-C6H4)-1,2,4-oxadiazolyl-5
338 3-(3'-Cl-C6H4)-1,2,4-oxadiazolyl-5
339 3-(2'-Cl-C6H~)-1,2,4-oxadiazolyl-5
.~ 1,2,4-thiadiazolyl-3
341 5-CH3-1,2,4-thiadiazolyl-3
342 5-C,.Hr,-1,2,4-thiadiazolyl-3
343 5-(4'-CHl-C6H4)-1,2,4-thiadiazolyl-3
344 5-(3'-CH~-C6H4)-1,2,4-thiadiazolyl-3
345 5-(2'-CH,-C6H4)-1,2,4-thiadiazolyl-3
346 5-(4'-CH~O-C~H4)-1,2,4-thiadiazolyl-3
347 5-(3'-CH,O-C~,H4)-1,2,4-thiadiazolyl-3
348 5-(2'-CH~O-C6Hq)-1,2,4-thiadiazolyl-3
349 5-(4'-NO,-ChH4)-1,2,4-thiadiazolyl-3
~ASF Akt~on~e~ollschaft 920400 O.Z. 0050/43411
61 2100~4~
Nc. A
350 5-(3'-NO~-C~H4)-1,2,4-thiadiazolyl-3
.
351 _ 5-(2'-NO~-C6H4)-1,2,4-thiadiazolyl-3
352 5-(4'-CN-C6H4)-1,2,4-thiadiazolyl-3
353 5-(3'-CN-C6Hq)-1,2,4-thiadiazolyl-3
354 5-(2'-CN-C6H4)-1,2,4-thiadiazolyl-3
355 5-(4'-Cl-C6H4)-1,2,4-thiadiazolyl-3
356 5-(3'-C1-C6H4)-1,2,4-thiadiazolyl-3
357 5-(2'-C1-C6H4)-1,2,4-thiadiazolyl-3
358 1,3,4-thiadiazolyl-2
359 5-CHl-1,3,4-thiadiazolyl-2
360 ~ 5-C6Hs-1,3,g-thiadiazolyl-2
361 5-(4'-CH3-C6H4)-1,3,4-thiadiazolyl-2
362 5-(3'-CH3-C6H4j-1,3,4-thiadiazolyl-2
363 . 5-(2'-CHI-C6H4)-1,3,4-thiadiazolyl-2
364 5-(4'-CH30-C6H4)-1,3,4-thiadiazolyl-2
365 ~
366 _ 5-(2'-CH30-C6H4)-1,3,4-thiadiazolyl-2
367 5-(4'-NO~-C6H4)-1,3,4-thiadiazolyl-2
368 5-(3'-NO2-C6H4)-1,3,4-thiadiazolyl-2
369 5-(2'-NO2-C6H4)-1,3,4-thiadiazolyl-2
370 5-(4'-CN-C6H4)-1,3,4-thiadiazolyl-2
371 5-(3'-CN-C6H4)-1,3,4-thiadiazolyl-2
372 5-(2'-CN-C6H4)-1,3,4-thiadiazolyl-2
373 . 5-(4~-Cl-C6H4j-1~3~4-thiadiazolyl-2
374 5-(3'-Cl-C6H4)-1,3,q-thiadiazolyl-2
375 5
376 pyridlnyl-2
377 pyridinyl-4
378 ~ _
3-79
380 pyridazinyl-2
381 pyrimidinyl-4
. . ._ , ,
382 pyrimidinyl-5
, , , _................... _ ,
383 pyrimidinyl-2
334 pyridinyl-3
-- . . . , _
BASF Aktien~esellschaft 920400 O.Z. 0050/43411
62 21~0~46
Table 3
IR( cm~l )or
No. CompDu~d . m p
H-NMR ~ ppI ) _
7.5 (s, I ~1); 3.8 (s, 3 H);
~0~ ~ 3.65 (s, H)
_
~ ~ ~ 6.6 (s, l H); 3.9 (s, 3 H);
i ~ C113
~ ~ CI 6.6 (s, I H); 3.9 (s, 3 H);
4 H3C02C~ ~~ 3.7 (s, 3 H)
~ CH3
,...~
~C~ K~
BASF Akt~ençl08elll~chaft 920400 O. æ . 0050/43411
63 210054~
No. ~ompound IR( cm~I )or _
IH--NM~ ( PP,n )
~1 ~O~CH3 ~ 76
7 ...._ _ . 63
~13CO2C ~C~3
_ . . . 7 5 (5, 1 H); 3.8 (5, 3 H);
8 ~0~ CH3 3 7 (5~ 3 H)
H3CO2CJ~ ~CH3 ~
~ 6.55 (5,1 H); 3.9 (5, 3 H);
9 ~CH3 3,7 (S~ 3 ~I)
_ __ .
~ 7,5 (5, I H); 3.8 (5, 3 H);
~O~~O~N 3.7 (5, 3 ~)
__ H3CO2CJ~ ~C~l3~3 ,
BASF Alctiengegell8chaft 920400 O. Z . 0050/43411
64 210~
: _ _
Nn. Compound IR ( cm~l 3 or m p
~ NMR ( PP~ 1
. .
11 ~O--~N CH3 67
H3CO2C ~CH3
~01
_ _
13 H3CO ~C(~ ~ 82
. , . _ _ _
14 ~O~ 125
C~C ~CH3N =~ D
BASF Aktien~e~ellschaft 920400 O.Z. 0050/43411
2100~
. . _
la( cm~~ )or
No. Conipound III--NMR ( PP,n ~ m p
.. _
16 ~0~ ~0 3 57 (s 3 H); 3.8 (s, 3 H);
H3C02C CH3
.. .. .
17 ~`~3 3 7 (s 3 HH); 3-8 (s, 3 H);
H3C02C CH3
_ ~0 ~3 _ _ 76
H3C02C C~3
. . . . ~ .
19 ~O~N~0 179
H3C02C O~CH3
~E ~ ~ ~0 116
BASF Akt~enge~ellschaft 920400 O.Z. 0050/43411
66 21 003~ ~
IR ( cm~ I ) or _
No. Compound m p
IH-NMR ( PPn"
1 ~3CO~C~ ~CH3
. .
~ 7.5 (s, I H); 3.75 (s, 3 H);
22 ~O~N 3.65 (s, 3 H)
H3C02C~ '~ CH3~
_ . _
3~ N
. ~ 7.55 (s, I H); 3.8 (s, 3 H);
24 b~o~~ 3.65 (s, 3 H)
H3CO~C~ ~CH3 ~
_ . . ,, . ,_
~5 ~3CO2C~O~ _ 123
BASF Aktien5J~sell~chaft 920400 O.Z. 005n/43411
67 2100a~
No.Compound IR ( cm~ I ) or m p
__ IH--~MR ( PP~ )
~ 2 isomers (approx. 3 1):
26 ~ ~NO--CH~ 7.5 (2 s, I H); 3.8 (2 s, 3 H);
J~ O C113 l l 3.65(2s,3H
H3C02C -- ~CH3 ~
.
H3CO--N~
27 ~oJ 74
~ 13C02C~ ~ CH3
28 }i3C02C~
, . . . - .
. ~ 7.55 (s, l H); 3.8 (s, 3 H);
29 H3C02C~ 3,7 (s, 3 H)
_ ~
~ ~~ 3.65 (s, 3 H)
H3C02C~~C113
. . .
BASF Aktien~el3ell1aCh~ft 920400 0 . Z . 0050/43411
68 2 ~ 0 ~
IR( cm~I )or
No. Compound lH-NMR ( ppm ) m p
_ ~7 45 (~, I II); 3.8 (s, 3 H);
H3CO2C O~CH3
. _
~ ~l6.5 (s, I H); 3.85 (s, 3H);
32 ~O~ 3.65 (s, 3 H)
~3C02C~ .
33 H3CO2C~ CH3 ~ 123
, , ,,,~ .. ,.. _
~; 13 (sjI H) 3.73 (~, 3 tl);
~ ~ 3 7 (s~ 3 H)
~ ~N ~J 96
_ H3CO2C CH3
BA8F Akti~nge~ellsch~ft 920400 O.Z. 0050/43411
69 210~
IR ( cm~l ) or
No. Compound m p
IH-NMR ( ppm )
.
i~
37 H3C()zC~ ~CH~ cl
_
f ~ ~ CH3 7 A5 (s, 1 H); 3.75 (s, 3 H);
38 ~O~ 3.65 (5~ 3 H)
H3CO2C CH3
.. ,__ ___ . . .. - _ _
. ~ ~ 7.45 (s~ I H); 3.8 (s, 3 H);
39 ~ CH3 3.65 (s~ 3 H)
H3COzC CH3
. . . __
~ CH3 ~l 7.5 (s~ I H); 3.8 (s, 3 H);
~OJ~ 38 (s~ 3 H)
H3CO2C ~ CH3 _
BASF A~ctien~esellEIchaft 920400 O . Z . 0050/43411
210054~
IR( em~l )or
NQ Compound IH-NMR ( PP~ ~ m p
. - .
41 H3Co2c~o~ N--N 106
~ c~oo~
~ OCI13 7.45 (s, IH); 3.75 (s, 3H);
43 ~ ~ 3.7 (s, 3H); 3.6 (s, 3H)
H3C02C~O~CH3
-- e~ 7.46 (s, l l l); 3.75 (s, 3H); .
44 ~~ 3.6(s,3~)
H3CO2C CH3
~~`3~
BASF Alctien~esellschaft 920400 O. Z . 0050/43411
71 2103~
No, ¦ CODIPOU--d ~ NMR ( pp ) F3p
CH3
I 1 ¦ H 7.6 (s, I H); 3.9 (s, 3H);
46 ~~ ~0 3.8 (s, 3H)
H3C02C ~CH3 . .
47 ~o~J~ 7.45 (s, IH); 3.75 (s, 3H);
H3C02C~ ~ ~ CH 3.65 (s, 3H); 3.35 (s, 3H)
48 ~0~ 98
H3C02C 0~CH30
49 . 3.6 (~ 3H)
H3C02C~ ~CII~
~ CH3 _ . _
sn ~O ~N 16~
~3C02C~O~C~13 ~ ~
_ ~ . ~
BASF A)ctiengesell~cha~t 920400 O.Z. 0050/43411
72 2~
No. C-mpou~d IR ( cm~ I ) or m p
~[~=11
~ ~ ~CH3
-1 ~ ,~ 1
~ ~ O~-~Cl 1l9
BASF Aktien~e~ellschaft 920400 O.Z. 0050/43411
73 ~10~4~
No. Compound IR ( cm~ I ) or m
_ __ IH-NMR( PP~ ) P
__ .
56 ~0~N 1 17
H3CO2C ~CH3 ~3
_ .
57 ~0~ N 97
113CO2C ~CH3 ~ _
1~ 7 6 (s, IH); 3.8 (s, 3H);
58 ~O~¢N
H3CO2C CH3 CH
. . _
59 ~O~N 128
3 2 Ci~3 CH3 ~)
36
_ H3C02~ CH3 _
BASF Aktien~eAell~Chaft 920400 O. Z . 0050/43411
74 21~0~6
No. Cou~pound III-NMA ( PP ) m p
_ .
L~ CIIIC~ ~C ~ H3 82
6:~ ~ I 115
H3CO~C ~
6S ~0 O _
H3C02C ~ ~ - ... __.
BASF ~ktienge8ellgchaft 920400 O.Z. û050/43411
21G0~46
IR ( cm~ I ) or
NO. CODIPDUnd m p
IH-~MR ~ ppm )
.. ._ - _ _
66 ~O--~ ` O 135
H3CO2C ~CH3 ~3~ CH3
. . __ - . . ,. __ . _ _
67 ~l3co2c~ ~ ~ 3 6 I c;, 3H )
_
68 [~ C ~ I 7 . 4 ( s, 3 H ),
~ ~o~N~l 1~8
1~ .
BASF Aktien~e~ell~ch~lft 920400 O.Z. 0050/43411
76 2100~4~
IR ( cm~7 ) or _
NQ Compound IU-t~MR ( PP~ ) m p
71 ~O~ç 3 8 ( s, 3 H ),
H3CO2C O~CH3
7 .45 Is, lH);
72 ~0~ ) ) CH3
73 ~ ~ÇN ~ 3 6 5 I s, 3 H ),
H3CO2C ~ CH3
. . ; .5 (s, lH); _
74 ~ ~ÇN ~ 3 6 5 ( 6, 3 H );
H3CO2C ' ~CH3
~0 116
H3CO2C ~CH3 . .
BASF A~cti~ngesellccha~t 920400 O . Z . 0050/43411
77 21~0~46
No. Compound IX ( cm~ I ) or
IU-~MR ( PP~ ) rn
. ~ _ - . _
76 ~ ~N~3 118
H3C02C ~ CH3
~ ~ S ~ R, I U ) ~
C~3 _
2~ Cl ~
~ BASF Aktien~esellachaft 920400 O.Z. 0050/43411
\
78
2100~
The novel compounds are suitable as fungicides.
The fungicidal compounds according to the invention, or
agents containing them, may be applied for instance in the
form of directly sprayable solutions, powders, suspensions
(including high-percentage aqueous, oily or other suspen-
sions), dispersions, emulsions, oil dispersions, pastes,
dusts, broadcasting agents, or granules by spraying, atomiz-
ing~ dusting, broadcasting or watering. The forms of ap-
plication depend entirely on the purpose for which the
agents are being used, but they must ensure as fine a
distribution of the active ingredients according to the
invention as possible.
Normally, the plants are sprayed or dusted with the active
ingredients or the seeds of the plants are treated with the
active ingredients.
The formulations are produced in known manner, for example
by extending the active ingredient with solvents and/or
carriers, with or without the use of emulsifiers and disper-
sants; if water is used as solvent, it is also possible to
employ other organic solvents as auxiliary solvents. Suit-
able auxiliaries for this purpose are solvents such asaromatics (e.g., xylene), chlorinated aromatics (e.g.,
chlorobenzenes), paraffins (e.g., crude oil fractions),
alcohols (e.g., methanol, butanol), ketones (e.g., cyclohex-
anone), amines (e.g., ethanolamine, dimethylformamide), and
water; carriers such as ground natural minerals (e.g.,
kaolins, aluminas, talc and chalk) and ground synthetic
minerals (e.g., highly disperse silica and silicates);
emulsifiers such as nonionic and anionic emulsifiers ~e.g.,
polyoxyethylene fatty alcohol ethers, alkyl sulfonates and
aryl sulfonates); and dispersants such as lignin-sulfite
waste liquors and methylcellulose.
Examples of surfactants are: alkali metal, alkaline earth
metal and ammonium salts of aromatic sulfonic acids, e.g.,
ligninsulfonic acid, phenolsulfonic acid, naphthalenesul-
fonic acid and dibutylnaphthalenesulfonic acid, and of fatty
acids, alkyl and alkylaryl sulfonates, and alkyl, lauryl
ether and fatty alcohol sulfates, and salts of sulfated
BASF Aktien~esellschaft 920400 O.Z. 0050/43411
79 21~0~S
hexadecanols, heptadecanols, and octadecanols, salts of
fatty alcohol glycol ethers, condensation products of
sulfonated naphthalene and naphthalene derivatives with
formaldehyde, condensation products of naphthalene or
naphthalenesulfonic acids with phenol and formaldehyde,
polyoxyethylene octylphenol ethers, ethoxylated isooctyl-
phenol, ethoxylated octylphenol and ethoxylated nonylphenol,
alkylphenol polyglycol ethers, tributylphenyl polyglycol
ethers, alkylaryl polyether alcohols, isotridecyl alcohol,
fatty alcohol ethylene oxide condensates, ethoxylated castor
oil, polyoxyethylene alkyl ethers, ethoxylated polyoxypropy-
lene, lauryl alcohol polyglycol ether acetal, sorbitol
esters, lignin-sulfite waste liquors and methyl cellulose.
Powders, dusts and broadcasting agents may be prepared by
mixing or grinding the active ingredients with a solid
carrier.
Granules, e.g., coated, impregnated or homogeneous granules,
may be prepared by bonding the active ingredients to solid
carriers. Examples of solid carriers are mineral earths such
as silicic acids, silica gels, silicates, talc, kaolin,
attapulgus clay, limestone, lime, chalk, bole, loess, clay,
dolomite, diatomaceous earth, calcium sulfate, magnesium
sulfate, magnesium oxide, ground plastics, fertilizers such
as ammonium sulfate, ammonium phosphate, ammonium nitrate,
and ureas, and vegetable products such as grain meals, bark
meal, wood meal, and nutshell meal, cellulosic powders, etc.
Examples of formulations are given below.
I. A solution of 90 parts by weight of compound no. 1 from
Table 3 and 10 parts by weight of N-methyl-a-pyrrolidone,
which is suitable for application in the form of very fine
drops.
II. A mixture of 20 parts by weight of compound no. 2 from
Table 3, 80 parts by weight of xylene, 10 parts by weight of
the adduct of 8 to 10 moles of ethylene oxide and 1 mole of
oleic acid-N-monoethanolamide, 5 parts by weight of the
calcium salt of dodecylbenzenesulfonic acid, and 5 parts by
weight of the adduct of 40 moles of ethylene oxide and
BASF Alctien~esellschaft 920400 O.Z. 0050/43411
210~4S
1 mole of castor oil. By finely dispersing the mixture in
water, an aqueous dispersion is obtained.
III. An aqueous dispersion of 20 parts by weight of com-
pound no. 3 from Table 3, 40 parts by weight of cyclohexa-
none, 30 parts by weight of isobutanol, 20 parts by weight
of the adduct of 40 moles of ethylene oxide and 1 mole of
castor oil.
IV. An aqueous dispersion of 20 par~s by weight of compound
no. 4 from Table 3, 25 parts by weight of cyclohexanol, 65
parts by weight of a mineral oil fraction having a boiling
point between 210 and 280'~C, and 10 parts by weight of the
adduct of 40 moles of ethylene oxide and 1 mole of castor
oil.
V. A hammer-milled mixture of 80 parts by weight of com-
pound no. 5 from Table 3, 3 parts by weight of the sodium
salt of diisobutylnaphthalene-~-sulfonic acid, 10 parts by
weight of the sodium salt of a lignin-sulfonic acid obtained
from a sulfite waste liquor, and 7 parts by weight of
powdered silica gel. By finely dispersing the mixture in
water, a spray liquor is obtained.
VI. An intimate mixture of 3 parts by weight of compound
no. 6 from Table 3 and 97 parts by weight of particulate
kaolin. The dust contains 3wt% of the active ingredient.
VII. An intimate mixture of 30 parts by weight of compound
no. 7 from Table 3, 92 parts by weight of powdered silica
gel and 8 parts by weight of paraffin oil sprayed onto the
surface of this silica gel. This formulation of the active
ingredient exhibits good adherence.
VIII. A stable aqueous dispersion of 40 parts by weight of
compound no. 8 from Table 3, 10 parts of the sodium salt of
a phenolsulfonic acid-urea-formaldehyde condensate, 2 parts
of silica gel and 43 parts of water, which dispersion can be
further diluted.
IX. A stable oily dispersion of 20 parts by weight of
compound no. 9 from Table 3, 2 parts by weight of the
calcium salt of dodecylben~enesulfonic acid, 8 parts by
BASF Aktiengeeellachaft 920400 O.Z. 0050/43411
81 2100~4~
weight of a fatty alcohol polyglycol ether, 2 parts by
weight of the sodium salt of a phenolsulfonic acid-urea-
formaldehyde condensate and 68 par~s by weight of a paraf-
finic mineral oil.
The novel compounds are extremely effective on a broad
spectrum of phytopathogenic fungi, in particular those from
the class consisting of the Ascomycetes and Basidiomycetes.
Some of them have a systemic action and can be used as
foliar and soil fungicides.
The fungicidal compounds are of particular interest for
controlling a large number of fungi in various crops or
their seeds, especially wheat, rye, barley, oats, rice,
Indian corn, lawns, cotton, soybeans, coffee, sugar cane,
fruit and ornamentals in horticulture and viticulture, and
in vegetables such as cucumbers, beans and cucurbits.
The compounds are applied by treating the fungi or the
seeds, plants, materials or the soil to be protected against
fungus attack with an effective amount of the active ingre-
dients.
Application may be effected before or after infection of the
2S materials, plants or seed by the fungi.
The compounds I are particularly useful for controlling the
following plant diseases:
Erysiphe graminis in cereals,
Erysiphe cichoracearum and Sphaerotheca fuliginea in cucur-
bits,
Podosphaera leucotricha in apples,
Uncinula necator in vines,
Puccinia species in cereals,
Rhizoctonia solani in cotton,
Ustilago species in cereals and sugar cane,
Venturia inaequalis (scab) in apples,
Helminthosporium species in cereals,
Septoria nodorum in wheat,
Botrytis cinerea (gray mold) in strawberries and grapes,
Cercospora arachidicola in groundnuts,
Pseudocercosporella herpotrichoides in wheat and barle
B~SF Aktiengeaell~chaft 920400 O.Z. 0050/43411
.
82 210~4S
Pyricularia oryzae in rice,
Phytophthora infestans in potatoes and tomatoes,
Fusarium and Verticillium species in various plants,
Plasmopara viticola in grapes,
Alternaria species in fruit and vegetables.
The novel compounds may also be used for protecting materi-
als (timber) against, for example, Paecilomyces variotii.
Generally, the fungicidal agents contain from 0.1 to 95, and
preferably from 0.5 to 90, wt~ of active ingredient.
The application rates depend on the effect desired, and vary
from 0.02 to 3 kg of active ingredient per hectare.
When the active ingredients are used for treating seed, ra-
tes of from 0.001 to 50, and preferably from 0.01 to 10, g
per kg of seed are generally needed.
When applied as fungicides, the agents according to the in-
vention may also be present together with other active in-
gredients, for example herbicides, insecticides, growth re-
gulators, other fungicides, or fertilizers.
When admixed with other fungicides, the fungicidal spectrum
of action is in many cases increased.
Use examples
The active ingredient used for comparison purposes was me-
thyl 2-(2-hydroxyphenyl)-3-methoxyacrylate (A) disclosed in
EP 251 082.
Use Example 1
Action on Plasmopara viticola
Leaves of potted vines of the Muller-Thurgau variety were
sprayed with aqueous suspensions containing (dry basis) 80%
of active ingredient and 20% of emulsifier. To assess the
duration of action, the plants were set up, after the
sprayed-on layer had dried, for 8 days in the greenhouse.
Then the leaves were infected with a zoospore suspension of
BASF Aktienge~ chaft 920400 O.Z. 0050/43411
83 210~4~
Plasmopara viticola. The plants were first placed for 48
hours in a water vapor-saturated chamber at 24C and then in
a greenhouse for 5 days at from 20 to 30"C. To accelerate
and intensify the sporangiophore discharge, the plants were
then again placed in the moist chamber for 16 hours. The
extent of fungus attack was then assessed on the undersides
of the leaves.
The results show that active ingredients nos. 3, 4, 5, 6, 7,
8, lO, 11, 12, 13, 1~, 18, 26, 28 und 29 from Table 3, when
applied as spray liquors containing 250 ppm (by weight) of
active ingredient, have a better fungicidal action (90%)
than prior art comparative agent A (40%).
Use example 2
Action on wheat brown rust
Leaves of pot-grown wheat seedlings of the ~Kanzler~ variety
were dusted with spores of brown rust (Puccinia recondita).
The pots were then placed for 24 hours at 20 to 22C in a
high-humidity (90 - 95%) chamber. During this period the
spores germinated and the germ tubes penetrated the leaf
tissue. The infected plants were then sprayed to runoff with
aqueous liquors containing (dry basis) 80% of active ingre-
dient and 20~ of emulsifier. After the sprayed-on layer had
dried, the plants were set up in the greenhouse at 20 to
22~C and a relative humidity of 65 to 70%. The extent of
rust fungus spread on the leaves was assessed after 8 days.
The results of the experiment show that active ingredients
nos. 7, 8, 10 and 31 from Table 3, when applied as a spray
liquor containing 250 ppm of active ingredient, have a bet-
ter fungicidal action (95~) than prior art comparative com-
pound A (0%).