Note: Descriptions are shown in the official language in which they were submitted.
i~2100~2p
AZ IDO-CYCI~ODEXTRIN
This invention relates to methods of synthesis of
aminocyclodextrins: Cyclodextrins are widely known as food
and drug additives, as catalysts in chemical and industrial
processes, and in numerous spectroscopic, analytical and
preparative procedures, (see Li and Purdy, Chem..Rev. 1992,
92, 1457-1470. Their inclusion compounds are similarly
widely known) see Saenger, Angew. Chem. Int. Ed. Engl.
1980, 19, 433-362.
The present invention primarily relates to processes
of preparing substituted cyclodextrins. Specific reagents
are utilized to produce specific products. The present
invention secondarily provides novel compounds prepared by
the process. Although the invention will be described and
referred to as it relates to processes of preparation of
aminocyclodextrins and novel aminocyclodextrins prepared
2o thereby, it will be understood that the principles of this
invention are equally applicable to similar processes and
products, . and accordingly it will be understood that the
invention is not limited to such processes and products.
BACKGROUND OF THE INVENTION
Cyclodextrins are cyclic alpha-1,4-oligosaccharide
starch derivates) Alpha, beta, gamma, and delta
cyclodextrins are known, containing six, seven, eight, and
nine.glucose units.respectively. Their importance lies in
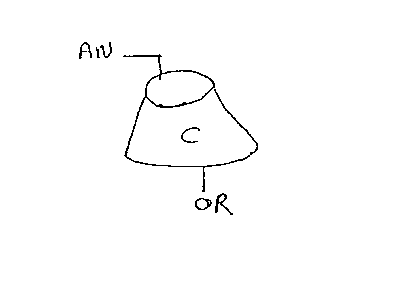
3o their enzymic properties attributed to their hollow
truncated cone structure having primary 6-hydroxyls at the
narrower end, and secondary 2- and 3-hydroxyls at the wider
end, a relatively hydrophobic interior cavity and a
relatively hydrophilic exterior.
The cyclodextrins form inclusion complexes and its is
believed that these inclusion complexes and similar
compounds modify the chemical and physical environment
1
is
i~2100820
affecting chemical reactions to induce chirality in
otherwise achiral reactions. The cyclodextrins are
themselves inherently chiral being composed of chiral D-
glucose units.
Substituted aminodeoxy cyclodextrins are particularly
noted for their chiral catalytic effects (Tagaki et al.,
Tetrahedron Lett., 1990, 31, 3897-3900, Parrot-Lopez et
al., Tetrahedron: Asymmetry, 1990, 1,.367-370, Angew. Chem.
Int. Ed. Engl., 1992, 31, 1381-1383.
Azidodeoxy cyclodextrins are suitable precursors for
aminodeoxy cyclodextrins.
It is a principal object of the invention to prepare
azidodeoxy cyclodextrins.
In accordance with one aspect of one embodiment of the
present invention there is provided a compound of formula
.FOA'~"~A
P~
'C
ofd
wherein C is cyclodextrin, A is azido and n is 0, 1, 2 or
3,R is alkyl of 1 to 6 carbon atoms, alkenyl of
2 to 6 carbon atoms, or azidoalkyl of 1 to 6 carbon atoms,
with the proviso when R is azidoalkyl, n is 0.
In another aspect of the present invention; there is
provided a process of preparation of a compound of formula
Faa nu~A ,I
~1'v ..
C '
r
2
T~:-:
2~0~82~
wherein C is cyclodextrin, A is azido and n is 0, 1, 2 or
3, R is hydrogen, alkyl of 1 to 6 carbon atoms, alkenyl of
2 to 6 carbon atoms, or azidoalkyl of 1 to 6 carbon atoms,
with the proviso when R is azidoalkyl, n is 0, comprising
when n is 1, 2, or 3 reacting a cyclodextrin of formula
FoR nu~.A
G
o~
wherein C is cyclodextrin, R is hydrogen, alkyl of 1 to 6
carbon atoms, or alkenyl of 2 to 6 carbon atoms, with
alkali metal azide triphenyl p~hosphine, and carbon
tetrabromide, when n is 0 reacting cyclodextrin with alkali
metal hydride in a first step, and the product thereof with
haloazidoalkane in a second step.
Having thus generally described the invention,
reference will now be made to the Examples.
EXPERIMENTAL DATA
Example 1
2-O-Allyl-alpha-cyclodextrin
To a solution of dried a-cyclodextrin (2.88 g, 2.96
mmol) in DMSO (30 ml) was added lithium hydride (35 mg; 1.5
eq). The mixture was stirred under Argon until the
solution became clear (24 hours). To this solution was
added allyl bromide (256 ~C1, 1 eq) and lithium iodide (10
mg). The mixture was allowed to stand at 55°C for 4 hours.
TLC on silica gel (CHjCN/HO2, 8/2) showed 3 products having
Rf values of 0.28, 0.20, 0.09, and corresponding
respectively to diallyl, monoallyl-a-cyclodextrins, and
starting material. a-cyclodextrin and its derivatives were
precipitated out by the addition of acetone (500 ml). The
3
210~~~0
precipitate was filtered and washed with acetone (100 ml)
to give 3 g of crude product which was purified by flash
chromatography on a silica gel column (4x40 cm) eluting y
with CH3CN/HZO, 9/1 (1 liter) then 8/2 (1.5 liters). The '
pure fractions of monoallyl-a-cyclodextrin were combined,
then concentrated in vacuo to give a solid (900 mg, 30%).
The proton NMR spectra showed that it was a mixture of 2,
and 6-O-allyl-a-cyclodextrins. The latter was present in
about 20% (based on the integration of the alkenyl
protons). Pure 2-O-monoallyl-a-cyclodextrin was obtained
after recrystallization from MeOH/HZO (720 mg, 24%).
ON
- Y O,~-~ ~~~w~-cy.J..tn~.
mp 270°C (dec). Cc
(aJp+55° (c 0.1, H20) . y
IR (KBr) 3400 (OH) .
~H NMR (DMSO-d6, 300 MHz) ~ :3.20 (dd, 1H, Jz_~ = 3.3, JZ_3 =
9.0, H2") 3.22-3.48 (m, 21II, H2, H4, HzO, 3.50-7.70 (m, 18H,
H5, H6) , 3.70-3.82 (m, 5H, H3) , 3.85 (td, 1H, J3_z = Jg_4
9.0, JH3-off - 2.2, I-I3") , 4.16 (dd, 1H, Jd_e = 12.8, Jd_~ = 5.7,
Hd)., 4.28 (dd, 1H, Je.d = 12.8, J~-~ = 5.7, He) , 4.38-4.52 (m,
6H, OH6) , 4.79 (s, 5H, H1) , 4.95 (d, 1H, J~_z = 3.3, H1A) ,
5. 18 (dd, 1~I, Ja_~ = 10.4, Ja_b = 1.8, Ha) , 5.29 (dd, 1H, Jb_~
- 17.3, Jb_a = 1.8, Hb), 5.40-5.70 (m, 11H, OH2, OH3), 5.80-
5.95 (m, 1H, J~_b = 17.3, J~_a = 10.4, J~_d = J~_e = 5.7, Hc) .
'3C NMR (DMSO-d6, 300 MHz) ~: 60.1(C6), 71.9, 72.2 (C2, C5),
72.4 (allyl) , 72.8 (C3") , 73.2, 73.3, 73.4 (C3) , 79.6 (C2") ,
82.2, 82.4 (C4), 82.8 (C4A), 82.8 (C4A), 100.2 (C1A), 101.2,
102.0, 102.1, (C1), 117.7 (allyl), 134.8 (allyl).
3 5 FABMS C39H6403o . 10 3 5 ( M+N a ) .
Example 2
(i) 6 Azido-6-deoxy-alpha-cyclodextrin and
(ii) 6 6' Diazido-6 6'-dideoxy-alpha-cvclodextrin_
4
2144824
~.
~3 ~3 ~3
(ii1
a ! a
To a solution of dried a-cyclodextrin (2.8 g, 2.88
mmol) in DMF (60 ml) were added lithium azide (1.40 g, 10
eq), triphenylphosphine (1.89 g, 2.5 eq) and carbon
tetrabromide (2.39 g, 2.5 eq). The addition of the.latter
caused a mildly exothermic reaction and the solution turned
yellow. The reaction was stirred under Argon at room
temperature for 6 hours. TLC on silica gel (CH3CN/HZO, 8/2)
showed 3 major products having Rf values of 0.35, 0.20,
0.09, and corresponding respectively to diazido, monoazido-
a-cyclodextrins and starting material. After addition of
methanol ~ ( 10 ml ) , the brown solution was concentrated to
about half by rotary evaporation under reduced pressure,
then poured into acetone (500 ml) to precipitate out a-
cyclodextrin and its derivatives. The precipitate was
filtered and washed with acetone (100 ml) to give 3 g of
crude products which were purified by flash chromatography
on a silca gel column (4x40 cm) eluting with CH3CN/HzO, 9/1
(2 liters) then 8/2 (1.5 liters). The pure fractions were
combined, then concentrated in vacuo to give 6-azido-6-
deoxy-a-cyclodextrin (570 mg, 20%), and 6,6'-diazido- .
6,6'dideoxy-a-cyclodextrin (760 mg, 26%).
6-Azido-6-deoxy-a-cyclodextrin y3
mp -190°C (dec) lit. 217°C (dec), Carbohydr. Res., 1971, 18,
29-37)
[a]p + 133 ° (c 0.2, H20) ; (lit. + 128° (c 0.4, H20) ,
Carbohydr. Res., 1971, 18, 29-37)
IR (KBr) 3400 (OH) , 2100 (N3) .
5
.. .
2100820
~H NMR (DMSO-d6, 300 MHz) . ~: 3.20-3.45 (m, 30H, H2,H4,
H20), 3.52-3.70 (m, 18H, H5, H6), 3.71-3.87 (m, 6H, H3),
4.40-4.60 (m, 5H, OH6), 4.72-4.82 (m, 5H, H1), '4.83 (d, 1H,
J~-2 =~3.2, H1A), 5.31-5.49 (m, 6H, OH3), 5.49-5.62 (m, 6H,
OH2).
~3C NMR (DMSO-d6), 300 MHz) ~: 51.3 (C6A), 60.0-60.3 (C6),
70.4 (C2A), 72.0, 72.2, 72.3, 72.4, 73.0 (C2,C5), 73.2
(C3A) , 73.3 (C3) , 82. l, 82. 2, 82.4 (C4) , 83'. 2 (C4A) , 101.7
(C1A), 102.0, 102.2, 102.3 (C1).
FARMS C36H5o029N3 ~ 1020 (M+Na) .
6.6'-Diazido-6,6'-dideoxy-a-cyclodextrin
i 5 Analytical reversed HPLC (~.c-Bondapack (T'1Vn C 18 Column, 3 .9x3 00
mm) showed, that it is a mixture of 2 isomers in relative
ratios of 75/25 as calculated from the peak areas on HPLC
chromatogram.
i'13 ~I3 ,
2 0 -!
1
4
a
y~ _ d~az;de
2 5 Analytical reversed phase HPLC of the mixture of the 2 isomers of diazido-
a-
. cyclodextrin. A stepwise gradient elution was applied : 10 % MeOH/H20 for
10 min. , then 50 % Me0HIH20 for 20 min. , column was washed with MeOH
between injections.
Retention times of the peaks 1 and ? are respectively : 19.S0 and 21.9-: min.
36 mg of this~mixture yielded by semi-preparative reversed
phase HPLC (Novapak (TM) C18 Column, 7.8x300 mm) 21 mg, 80% of
6
,~;
..:
r
2~~Q~~~
the major isomer and 6 mg, 70% of the minor one. '
The determination of the structure of the 2 isomers
was done by ~3C NMR and Korner's method. The major isomer -
is the AD isomer and the minor one is the AC isomer.
M~t70
~ ~'~°~''~
1 ~ 2 'J 3
a-cyclodextmn
i
~.r (Si ~ : !a,.I ~ ~' ~- -r
Isomer Determination of Di- and Triazido-a-cyclodextrin by
Korners method (J. Am. Chem. Soc. 1986, 108,4509).
The AD isomer
V~ y~
a
mp 165°C (dec).
[aJp+77 ° (c 0.11, H20) .
IR (KBr) 3400 (OH), 2100 (N3).
~H NMR (DMSO-d6, 300 MI-iz) ~ : 3.11-3.48 (m, 36H, H2, H4,
HzOI), 3.48-3.70 (m, 18H, H5, H6), 3.70-3.90 (m, 6H, H3),
4.42-4 .68 (m, 5H, OI-16) , 4.7G (d, 2H, J~_z = 3. 6, H1) , 4.78
(d, 2I-i, J~_z = 3 . 3 , H1) , 4 . 84 (d, 2H, J~_2 = 2 . , 4 H1A) , 5. 30-
5.72 (m, 12H, OH2, OH3).
~3C NMR (DMSO-d6, 300 MHz) ~ : 51.3 (C6A), 60.0, 60.3 (C6),
70.4 (C2A) , 72.0, (C2) , 72. 1 (C5) , 72.4 ~(C5A) , 73.0, (C3A) ,
73.2 (C3), 82.2, 82.4 (C4), 83.2 (C4A), 101.7 (C1A), 102.0,
102.2 (C1) .
FABMS C36H5aO2aN6 . 10 2 3 ( M+H )
Example 3
(i) 6-Azido-6-deoxy-alpha-cvclodextrin and
(ii) 6 6'-Diazido-6 6'-dideoxy-alpha-cyclodextrin and
7
~~oos~o
..
(iii) 6 6' 6" Triazido-6 6' 6"-trideoxy-alpha-cyclodextrin
To a solution of dried a-cyclodextrin (2.5 g, 2.61
mmol) in DMF (60 ml) were added lithium azide (1.28 g, 10
eq), triphenylphosphine (2.05 g, 3 eq) and carbon
tetrabromide (2.60 g, 3 eq). The addition of the latter
caused a mildly exothermic reaction and the solution turned
yellow. The reaction was stirred under Argon at room
temperature for 6 hours. TLC on silica gel (CH3CN/H20, 8/2)
showed 4 major products having Rf values of 0.50, 0.35;
0.20, 0.09, and corresponding respectively to triazido,
diazido, monoazido-a-cyclodextrin and starting material
After addition of methanol (10 ml), the brown solution was
concentrated to about half by rotary evaporation under
reduced pressure, then poured into acetone (500 ml) to
precipitate out a-cyclodextrin and its derivatives. The
precipitate was filtered and washed with acetone (100 ml)
to give 3 g of crude products which were purified by flash
chromatography on a silica gel column (4x40 cm) eluting
with CH3CN/HzO, 9/1 (2 liter) then 8/2 (1.5 liter) . The
pure fractions were combined, then concentrated in vacuo to
give:
6-Azido-6-deoxy-a-cyclodextrin (470 mg, 18%), 6,6'-diazido
6,6'-dideoxy-a-cyclodextrin (mixture of 2 isomers, 670 mg,
25%), and 6,6',6"-triazido-6,6',6"-tridexoy-a-cyclodextrin
(270 mg, 10%).
~3 ~3 ~1 ~z ~
3
l~~ lii~
tiii~
a a a
_6 6' 6" Triazido-6 6' 6"-trideoxy-a-cvclodextrin
Analytical reversed phase HPLC (~-Bondapak C18 Column,
3.9x300 mm) showed that it is a mixture of 3 isomers in
relative ratios of 70/20/10 as calculated from the peak
areas on HPLC chromatogram.
8
~~o~~
V. 5
~I : X13 4-( )
I
Analytical reversed phase 1-1PLC of the mixture of the 3 isomers of triazido-a
cvclodextrin. A stepwise gradient elution was applied : 10 ~Io ivIeOH/H.''.O
for
10 mina, then ~0 % VIe01-UH?0 for?0 min: , column was washed with MeOH
between injections.
Retention times of the peal, 7. -: (p), and ~ l-1) :u'o respectively :
2'_'.16, '_'=1.03,
and ?6.67 min.
100_mg of this mixture yields by semi preparative reversed
HPLC. (Novapak C18 Column, 7.8x300 mm) 16 mg, 80%, 50.mg,
70~, and 8 mg, 70% of pure isomers.
The determination of the structure of the 3 isomers
was done by ~3C NMR and Korner's method. The isomer 7 on
the HPLC chromatogram correspond to the ACE isomer, the
isomer 4(5) to the ABD or ABE isomer and the isomer 5(4) to
the ABE or ABD isomer.
2 0 ~ R~,d~:~.~.
2 3
~./ 'r 1
' a-cvclodex~=i,
w
' 5 ( l ~6 -=t
~(S) 4-
isomer Determination of Di- and Triazido-a-cyclodextrin by
Korner's method (J. Am. Chem. Soc. 1986, 108,4509).
Example 4
2-O-Allyl-6-azido-6-deoxy-alpha-cyclodextrin and
2 O Ally_1 6 6'- --diazido-6 6'-dideoxy-alpha-cyclodextrin
To a solution of dried 2-O-allyl-a-cyclodextrin (872
mg, 0.86 mmol) in DMF (30 ml) were added lithium azide (420
mg, 10 eq), triphenylphosphine (564 mg, 2.5 eq) and carbon
tetrabromide (715 mg, 2.5 eq). The addition of the latter
caused a mildly exothermic reaction and the solution turned
9
21~Q~~0 ( .
yellow. The reaction was stirred under Argon at room
temperature for 6 hours. TLC on silica gel (CH3CN/HZO, 8/2)
showed 3 major products having Rf values of 0.54, 0.40,
0.20, and corresponding respectively to diazido-monoallyl-
a-cyclodextrin, monoazido-monoallyl-a-cyclodextrin and
starting material. After addition of methanol (5 ml), the
brown solution was concentrated to about 3 ml by rotary
evaporation under reduced pressure, then applied on a
silica gel column (4x40 cm) eluting with CH3CN/HzO, 92/8
(11), 90/10 (1.51), then 85/15 (1.51). The pure fractions
were combined, then concentrated in vacuo to give: 2-O-
allyl-6-azido-6-deoxy-a-cyclodextrin (320 mg, 36%), and 2-
O-allyl-6,6'-diazido-6,6'-dideoxy-a-cyclodextrin (160 mg,
20%) .
2-O-ally~l-6-azido-6-deoxy-a-Cvclodextrin
N3
mp = 175°C (dec). a
[a]p+153° (c 0.1, H20) .
IR (KBr) 3400 (OH), 2100 (N3). O
~H ~NMR (DMSO-d6, 300 MHz) ~ : 3.15-3.49 (m, 20H, H2, H4,
Hz0), 3.49-3.70 (m, 18H, H5, H6), 3.70-3.87 (m, 5H, H3),
3.87-3.90 (m, 1H, H3") , 4. 16 (dd, 1H, Jd_e = 12.8, Jd_~ = 5.7,
Hd) , 4.28 (dd, 1H, J~_d = 12.8, Je_~ = 5.7, He) , 4.38-4.65 (m,
5H, OH6), 4.78, 4.84, 4.96, 5.02 (s, 6H, H1, H1"), 5.17 (d,
1H, Je_~ = 10.3, Ha), 5.29 (d, 1H, Jb_~ = 17.3, Hb), 5.37-5.78
(m, 11H, OH2, OH3), 5.78-5.96 (m; 1H, Hc).
~3C NMR (DMSO-d6, 300 MHz) ~: 51.3 (C6"), 60.1, 60.31, 60.33
(C6) , 70.4, 71.7, 71.9, 7.2.0, 72. 1, 72.3, 72.4 (C2, C5) ,
72.7 (allyl) , 73.0 (C3") , 73. 1, 73.2, 73.3 (C3) , 79.6 (C2A) ,
82.1, 82.2, 82.4, 82.5 (C4), 83.2 (C4a), 101.80 , 101.82
(C1"), 102.00, 102.07, 102.2 (C1), 117.8 (allyl), 134.8
(allyl).
FABMS C39H630z9N3 . 10 6 0 ( M+Na ) .
~~~Q~~O
2 O-allyl-6 6'-diazido-6 6'-dideoxy-a-Cvclodextrin
N~ N
mp = 172°C (dec).
[a]o+131° (c 0.1, HZO) .
IR (KBr) 3400 (OH), 2100 (N3).
~H NMR (DMSO-d6, 300 MHz) H4, HZO)
~ : 3.20-3.48 (m, H2, ,
3.48-3.69 (m, l8Fi, FiS, H6) , 3.69.3.88 H3) 3.88-
(m, 5H, ,
3.98 (m, 1H, H3A) ( 4. 16 (dd, 1H, = 12.8, = Hd)
Jd_e Jd_~ 5.7.,,
4.28 (dd, 1H, Je_d = 12.8, Je_~ = Fie) , 4.50-4.70
5.7, (m, 4H,
OH6), 4.77, 4.79, 4.83, 4.95, 5.00(s, 6H, H1A),5.18
H1,
(d, 1H, Je_~ = 10.3 Ha), 5.29 (d, Jb_~ _ 17.3,Hb), 5.37-
5.78 (m, llH, OH2, 1H, (m, 1H,
OH3), 5.78-5.96 Hc).
~3C NMR (DMSO-d6, 300 MHz) ~: 51.3 (C6A), 60.0, 60.4 (C6),
70.4; 71.8, 72.0, 72.1, 72.2, 72.3, 72.4 (C2, C5); 72.7
(allyl), 73.0 (C3A), 73.3, 73.5, 73.6 (C3), 79.4 (C2A),
82.3, 82.5, 83.2, 83.3 (C4), 101.8, 101.9 (C1A), 102.0,
102.2 (C1), 117.7 (allyl), 134.8, 134.9 (allyl).
FABMS C39HszOzsN6 : 1085 (M+Na)
. Example 5
2-O-All~l-6-azido-6-deoxy-alpha-cvclodextrin and
2-O-All~l-6 6'-diazido-6 6'-dideoxy-alpha-cyclodextrin and
2-O-A11y1-6 6' 6"-triazido-6 6' 6"-trideoxy-alpha-
cyclodextrin
To a solution of dried 2-O-allyl-a-cyclodextrin (740
mg, 0.73 mmol) in DMF (30 ml) were added lithium azide (358
mg, 10 eq), triphenylphosphine (574 mg, 3 eq) and carbon
tetrabromide (728 mg, 3 eq) . The addition of the latter
caused a mildly exothermic reaction and the solution turned
yellow. The reaction was stirred under Argon at room
temperature for 6 hours. TLC on silica gel (CH3CN/H20, 8/2)
showed 3 major products having Rf values of 0.68, 0.54,
11
~1~~8~4 -.
0.40, and corresponding respectively to triazido-monoallyl-
a-cyclodextrin, diazido-monoallyl-a-cyclodextrin and
monoazido-monoallyl-a-cyclodextrin. After addition of
methanol (5 ml), the brown solution was concentrated to
about 3 mol by rotary evaporation under reduced pressure,
then applied on a silica gel column (4x40 cm) eluting with
CH3CN/HZO, 92/8 (11), 90/10 (1.51), then 85/15 (1.51). The
pure fractions were combined, then concentrated in vacuo to
give: 2-O-allyl-6-azido-6-deoxy-a-cyclodextrin (200 mg,
26%), 2-O-allyl-6,6'-diazido-6,6'-dideoxy-a-cyclodextrin
(260 fig, 330) and 2-O-allyl-6,6',6"-triazido-6,6'-6"-
tridexoy-a-cyclodextrin (214 mg, 27%).
2 O a11L1-6 6' 6"-triazido-6 6' 6"-tridexoy-a-CVClodextrin:
1~3 1V 3
a
mp = 168°C (dec).
[a]p+110° (c 0.11, MeOH) .
IR (KBr) 3400 (OI-i) , 2100 (N3) .
~H NMR (DMSO-d6, 300 MHz) ~ : 3. 18-3.42 (m, H2, H4, Hz0) ,
3.45-3.64 (m, 18H, H5, H6), 3.64-3.82 (m, 5H, H3), 3.82-
3.95 (m, 1H, H3") , 4.12 (dd, 1H, Jd_e = 12.8, Jd_~ = 5.7, Hd) ,
4.25 (dd, 1H, Je_d = 12.8, Je_~ = 5.7, He),, 4.50-4.70 (m, 3H,
OH6), 4.72, 4.80, 4.90, 4.98 (s, 6H, H1, H1"),.5.11 (d, 1H,
Je_~ = 10.3, Ha) , 5. 25 (d, 1H, Jb_~ = 17. 3, Hb) , 5.35-5.70 (m,
11H, OH2, OH3) , 5.75-5.95 (m, 1H, IIc) .
~3C NMR (DMSO-d6, 300 MHz) ~: 51.2, 51.3 (C6°') , 60.3 (C6) ,
70.3, 71.7, 71.8, 72.0, 72.1, 72.3 (C2, C5), 72.7 (allyl),
73.0, 73.1, 73.2 (C3), 79.2, 79.3 (C2~), 82.6, 82.8, 83.1,
83.3 (C4), 101.6, 101.7, 101.8 (C1A), 102.00, 102.03, 102.1
(C1), 117.6, 117.7 (allyl), 134.7, 134.8 (allyl).
12
z~ o~g~o
FABMS C39H6~ 027N9 ~ 1110 ( M+Na )
Example 6
Alphahalo-omega-azidohaloalkane
1-Iodo-n-azidoalkanes
Preparation of chloroazidoalkanes:
To a solution of 1-bromo-n-chloroalkane (50 mmol) in
DMSO (50 ml) was added sodium azide (3.25 g, 1 eq) . The
solution was stirred at room temperature for 20 hours, then
diluted with water (100 ml), and extracted with ether
(2x100 ml). The organic layers were combined, then dried
over anhydrous sodium sulfate. The residue obtained after
removal of the solvent was used without further
purification.
1-Chloro-3-azidopropane
Yield 80%
~H NMR (CDC13, 300 MHz) . 2.00 (quint, 2H, J = 6.2), 3.48
(t, 2H, J = 6.4), 3.62 (t, 2H, J = 6.2).
1-Chloro-4-azidobutane
Yield 86%
~H NMR (CDC13, 300 MHz) . 1.60-2.00 (m, 4H), 3.31 (t, 2H,
J = 6.2), 3.54 (t, 2H, J = 6.2).
1-Chloro-5-azidopentane
Yield 90%
~H NMR (CDC13, 300 MHz) . 1.47-1.68 (m, 4H), 1.80 (quint,
2H, J = 7.3), 3.29 (t, 2H, J = 6.4), 3.54 (t, 2H, J = 6.5)
Preparation of iodoazidoalkanes:
A solution of chloroazidoalkane (50 mmol) and sodium
iodide (7.5 g, 2 eq) in acetone was heated at reflux for 20
hours. After removal of the solvent in vacuo, the residue
was diluted with water (30 ml), then extracted with ether
(2x50 ml). The organic layers were combined, dried over
anhydrous sodium sulfate. The residue obtained after
removal of the solvent was purified by distillation.
13
~2oosza
1-Iodo-3-azidopropane
Yield 710
Ebo_~ 20-25°C
~H NMR (CDC13, 300 MHz) ~: 2.04 (quint, 2H, J = 7.3), 3.25
(t, 2H, J = 6.7), 3.43 (t, 2ii, J = 6.6).
1-Iodo-4-azidobutane
Yield 75%
Ebo.~ 60°C
~H NrIR (CDC13, 300 MHz) ~5: 1.65-1.76 (m, 2H), 1.85-1.97 (m,
2H), 3.20 (t, 2H, J = 6.7), 3.31 (t, 2H, J = 6.6).
1-Iodo-5-azidopentane
Yield 80%
EB0.1 70°C
1H NMR (CD13, 300 MHz) ~: 1.42-1.5,6 (m, 2H), 1.56-1.68 (m,
2H), 1.78-1.90 (quint, 2H, J = 7.3), 3.19 (t, 2H, J = 6.8),
3.29 (t, 2H, J = 6.6).
Example 7
2-O-Azidoalkyl-beta-cyclodextrin
To a solution of dried f3-cyclodextrin (2.4 g, 2.11
mmol) in DMSO (15 ml) was added lithium hydride (26 mg, 1.5
eq). The mixture was stirred under Argon until the
solution became clear (24 hours). To this solution was
added 1-iodo-n-azidoalkane (1.5 eq). The mixture was
allowed to stand at 60°C for 10 hours. TLC on silica gel
(CH3CN/HZO, 8/2) showed 3 products corresponding to dialkyl,
monoalkyl-f3-cyclodextrin, and starting material. After
evaporation of DMSO in vacuo, the residue was dissolved in
water (5 ml), then applied on a silica gel column (4x40
cm). Elution with CH3CN/HzO, 9/1 removed the dialkyl,
monoalkyl derivatives, and starting material were eluted
with CFi3CN/HZO, 8/2. The pure fractions of monoalkyl-f3
cyclodextrin were combined, the concentrated in vacuo to
give a solid.
14
21p4~~0
....
a
i~~ ~,
O N3 0 0 N3
2-O-Az idopropyl-fi-cy-clodextrin
Yield 250
mp 210°C (dec).
[a]p+141.3° (c 0.22, MeOH). Q
IR (KBr) 3400 (OII) , 2100 (N3) .
~H NMR (DMSO-d6, 300 MHz) ~ : 1.68-1.85 (m, 2H (propyl)(
3.00-3.48 (m, 30H, fI2, H4, (propyl), Hz0), 3.49-3.90 (m,
30II, H3, H5, H6, (propyl) , 4.40-4..60 (m, 7Hi, OH6) , 4.78-
4 .90 (m, 6I-I, H1.) , 4 . 95-5. 05 (m, 1H, H1A) , 5. 60-6. 10 (m, 13H,
OH2, OH3).
'3C NMR (DMSO-d6, 300 MHz) ~ : 28.9, 47. 6 (propyl) , 60.0
(C6), 68.9 (propyl), 71.9, 72.1, 72.3, 72.5 (C2, C5), 72.7
(C3A), 73.1 (C3), 80.8 (C2A), 81.6, 81.7 (C4), 82.2 (C4A)r
100.2 (Cl"), 101.9, 102.0 (Cl).
FABMS C45H~SO35N3 . 1240 (M+Na) .
2-O-Az idobut~l-f3-cyclodextrin
Yield 25%
mp 210°C (dec),
[a]p+127.1° (c 0.22, Hz0) . Q
IR (KBr) 3400 (OH), 2100 (N3).
'H NMR (DMSO-d6, 300 MHz) ~ : 1.50-1.65 (m, 4H (butyl) ) ,
3.22 (dd, 1H, Jz_~ - 3.3; Jz_3 = 10.0, H2A) , 3.22-3.48 (m,
30H, H1, H4, (butyl) , Hz0) , 3.48-3.82 (m, 30H, H2, H5, H6,
X200820
(butyl)), 4.45 (t, 7H, JH6-OH = 5~2, OH6), 4.78-4.86 (m, 6H,
H1) , 4.96 (d, 1H, J~_Z = 3.5, H1A) , 5. 55-6. 00 (m, 13H, OH2,
OH3).
~3C NMR (DMSO-d6, 300 MHz) . 24.8, 26.5, 50.5 (butyl),
60.0 (C6), 71.3 (butyl), 71.8, 71.9, 72.1 72.3, 72.,5 (C2,
C5) , 72.8 (C3A) , 73. 1, 73.2 (C3) , 80.7 (C2A) , 81.6, 81.8,
81.9 (C4), 82.3 (C4A), 100.4 (C1A), 101.9, 102.0 (C1).
FABMS C46H7~035N3 ~ 12 54 ( M+Na ) .
2-O-Az idopentyl-f3-c~clodextrin
Yield 300
mp 230°C (dec).
[a]p+115.5° (c 0.20, MeOH).
IR (KBr) 3400 (OH), 2100 (N3).
~H NMR (DMSO-d6, 300 MHz) . 1.28-1.40 (m, 2H (pentyl),
1.47-1.60 (m, 4H (pentyl) ) , 3.21 (dd, 1H, JZ_~ = 3.3, J2_3 =
9.7, H2A), 3.21-3.48 (m, 30H, H2, H4, (pentyl), H20), 3.48-
3.82 (m, 30H, H3, H5, H6, (pentyl) ) ) , 4.47 (t, 7H, JH6-OH
5.3, OH6) , 4.78-4.87 (m, 6H, H1) , 4.96 (d, 1H, J~_Z = 3.3,
H1A), 5.55-6.00 (m, 13H, OH2, OH3).
~3C NMR (DMSO-d6, 300 MHz) . 22.5, 28.0, 50.6 (pentyl),
60.0 (C6), 71.7 (pentyl), 71.8, 72.1 , 72.3, 72.5 (C2, C5),
72.8 (C3A), 73.1 (C3), 80.7 (C2A), 81.6, 81.8 (C4), 82.3
(C4A), 100.4 (ClA), 101.9, 102.0 (C1).
FABMS C4~H79035N3 ~ 12 6 8 ( M+Na ) .
Example 8
2-O-Azidoalkyl-alpha-cyclodextrin and
3-O-Azidoalkyl-alpha-cyclodextrin
16
~~a~82~
To a solution of dried a-cyclodextrin (2.4 g, 2.46
mmol) in DMSO (15 ml) was added lithium hydride (30 mg, 1.5
eq). The mixture was stirred under Argon until the .
solution became clear (24 hours). To this solution was
added 1-iodo-n-azidoal)cane (1.5 eq). The mixture was
allowed to stand at 60°C for 10 hours. TLC on silica gel
(CH3CN/HZO, 8/2) showed 3 products corresponding to dialkyl,
monoalkyl-a-cyclodextrin, and starting material. After
evaporation of DMSO in vacuo, the residence was dissolved
in water (5 ml), then applied on a silica gel column (4x40
cm) . Elution with CIi3CN/HZO, 9/1 removed the dialkyl,
monoalkyl derivatives, and starting material were eluted
with CH3CN/H20, 8/2. The pure fractions of monoalkyl-a-
cyclodextrin were combined, then concentrated in vacuo to
give a solid. The '3C NMR spectra showed that the
alkylation had occurred at C-2 and'C-3 positions at almost
the same ratio.
N~
N~~ N3
N. I,
~3
a
a
/~ O
O
2- -O-Azidopro~~yl-a-cyclodextrin and 3-O-Azidopropyl-a-
cyclodextrin
a
Yield 300
a.(3) O~N3
mp 165°C (dec).
[a]p+136.4° (c 0.22, MeOII) .
IR (I<Br) 3400 (OI3) , 2100 (N3) .
~H NMR (DMSO-d6, 3200 MHz) ~ : 1.68-1.82 (m, 2H (propyl),
3.18 (dd, 1H, Jz_~ = 3.3, Jz_3 = 9.4, H2A) , 3.20-3.48 (m, 26H,
17
~~008~0
H2, H4, (propyl), Hz0), 3.48-3.88 (m, 25H, H3, H5, H6,
(propyl) , 3.90 (td, 1H, J3_Z = J3_4 = 8 ~ 9, JH3-OH = 2 ~9, H3A)
5.35-5.75 (m, 11H, OH2, OH3).
On the ~3C NMR spectrum the peaks corresponding to 2-O-
Azidopropyl-a-cyclodextrin were assigned as following:
'3C NMR (DMSO-d6, 300 MHz) ~ : 28.9, 47.6 (propyl) , 60.0
(C6), 68.6 (propyl), 71.9, 72.2 (C2, C5), 72.8 (C3A), 73.2,
73.4 (C3) , 80.6 (C2A) , 81.8, 82.2, 82.4 (C4) , '82.7 (C4A) ,
100.0 (C1f'), 102.1, 102.2 (C1).
FABMS C39Fi6s03oN3 ~ 1078 (M+Na)
2-O-Azidobutyl-a-cyclodextrin, and 3-O-Azidobutyl-a-cyclo-
dextrin
a
2 o z ( 3) Oi~~ N3
Yield 36%
mp 220°C (dec).
[a]o+123.5° (C 0.37, MeOH).
IR (KBr) 3400 (OH), 2100 (N3).
~H NMR (DMSO-d6, 300 MHz) ~ : 1.45-1.65 (m, 4H, (butyl)),
3. 18 (dd, 1H, JZ_~ = 3.0, Jz_3 = 9.8, H2A) , 3. 22-3.48 (m, 26H,
H2, H4, (butyl), Hz0), 3.48-3.82 (m, 23fi, H3, H5, H6), 3.84-
3.96 (m, 3H, H3A, (butyl) ) , 4 . 39-4. 60 (m, 6H, OH6) , 4.74-
4 .84 (m, 5H, H1) , 4 .95 (d, 1H, Ji_z = 3.1, H1A) , 5.32-5.80
(m, lli-i, OH2, OH3) .
On the '3C NMR spectrum the peaks corresponding to 2-O-
Azidobutyl-a-cyclodextrin were assigned as following:
~3C NMR (DMSO-d6, 300 MHz) ~: 24.8-26.6, 50.6 (butyl), 60.0
(C6), 71.0 (butyl), 71.9, 72.2 (C2, C5), 72.8 (C3"), 73.1,
73.2, 73.3, 73.4 (C3), 80.4 (C2A), 81.8, 82.1, 82.4 (C4),
18
2~~~~~0
82.7 (C4~) , 102. 0, 1.02.1 (C1) .
FABI~IS C4~II6~03oN3 . 1092 (M-i-Na) .
2-O-AzidopPntYl a cyclodextrin and 3-0-Azidopentvl-a-
cyclodextrin
a.
2(~~ O N3
Yield 300
mp 235°C (dec).
(a]p+108.5° (c 0.37, MeOH) .
IR (KBr) 3400 (OH) , 2100 (N3) .
III NMR (DMSO-d6) , 300 MHz) ~ : 1. 28,-1.40 (m, 2H (pentyl) ) ,
1. 42-1. 60 (m, 4H (pentyl) , 3 . 12-3. 19 (m, 1H, H2A) , 3 . 19-3.45
(m, 26f-I, H2, H4, (pentyl) , H20) ) , 3.45-3.82 (m, 23FI, H3, H5,
H6), 3.82-3.95 (m, 31i, H3", (pentyl)), 4.4,0-4.58 (m, 6H,
OI16) , 4 .74-4.85 (m, 5I-i, I-I1) , 4 .94 (d, lI-i, J~_z = 3 . 1 H1A) ,
5.33-5.72 (m, 11H, 0132, Oft3) .
On.the '3C NMR spectrum the peaks corresponding to 2-O-
Azidopentyl-a-cyclodextrin were assigned as following:
~3C NMR (DMSO-d6, 300 I~IEIz) ~ . 22.5, 28.0, 28.9, 50.6
(pentyl), 60.0 (C6), 71.4 (pentyl), 71.7, 71.9, 72.2, 72.3
(C2 C5), 72.8 (C3''), 73.2, 73.3, 73.4, 73.6 (C3), 80.4
(C2~') , 81.8, 82. l, 82.4 (C4) , 82.7 (C4~) , 100. 1 (ClA) , 101.9,
102.0, 102.1, 102.2 (C1).
FABMS C4~H69030N3 ~ 1106 (M+Na) .
The novel compounds of this invention have
properties similar to those of the known cyclodextrins.
Although embodiments of the invention have been
described above, it is not limited thereto and it will be
apparent to those s)cilled in the art that numerous
modifications form part of the present invention insofar as
19
~~oo~~o ..
they do not depart from the spirit, nature and scope of the
claimed and described invention.