Note: Descriptions are shown in the official language in which they were submitted.
CHLORIDE ASSISTED HYDROMETALLURGICAL COPPER EXTRACTION
FIELD OF THE INVENTION
This invention relates to a hydrometallurgical
treatment of copper sulphide ores or concentrates in the
presence of chloride ions.
BACKGROUND OF THE INVENTION
Effective hydrometallurgical treatment of
copper sulphide ores, such as chalcopyrite (CuFeS2) has
been a long standing goal in the copper mining industry
which has thus far eluded success. The problem lies in
the fact that the severe conditions required for the
effective leaching of copper from these ores results in
oxidation of the sulphide in the ore or concentrate to
sulphate, resulting in the generation of acid which
requires expensive neutralization, rendering the process
impractical and uneconomical. Attempts have been made to
render the sulphide concentrate leachable under relatively
milder conditions under which the sulphide would only be
oxidized to elemental sulphur and not all the way through
to sulphate. These attempts include the pretreatment of
the concentrate prior to the pressure leaching step to
render the sulphide concentrate more readily teachable,
and the leaching of the concentrate in the presence of
chloride ions, such as described in U.S. Patent 4,039,406.
In this process, the copper values in the concentrate are
transformed into a solid basic copper sulphate from which
the copper values must then be subsequently recovered, as
described in U.S. Patent 4,338,168. In the process
described in patent 4,039,406 a significant amount (20-
25%) of sulphide in the ore or concentrate is still
_ z~~~~~~
oxidized to sulphate, resulting in greater oxygen demand
during the pressure leach and the generation of sulphuric
acid.
It is accordingly an object of the present
invention to provide a hydrometallurgical copper
extraction process wherein the oxidation of sulphide in
the ore or concentrate to sulphate is reduced.
SUr'~1ARY OF THE INVENTION
According to the invention, there is provided a
process for the extraction of copper from a sulphide
copper ore or concentrate, comprising the steps of
subjecting the ore or concentrate to a first leaching at
an elevated temperature and pressure in the presence of
oxygen and a lixiviant comprising an acidic solution of
chloride and bisulphate or sulphate ions to produce an
insoluble basic copper salt: leaching the basic copper
salt produced by said first leaching step in a second
leaching with an acidic sulphate solution to dissolve the
basic copper salt to produce a leach liquor containing
copper sulphate in solution: subjecting said leach liquor
to a solvent extraction process to produce a copper
concentrate solution and a raffinate comprising protons
and bisulphate or sulphate ions in solution; extracting
protons and bisulphate or sulphate ions from said
raffinate to produce a sulphuric said solution; and
recycling said sulphuric acid solution to said first
leaching at elevated temperature and pressure to serve as
a source of said bisulphate or sulphate ions in said
lixiviant.
According to a preferred embodiment, the
raffinate is subjected to electrodialysis to effect said
~~.~1~14
extraction of protons and bisulphate or sulphate ions
therefrom.
Also according to the invention, there is
provided a process for the extraction of copper from a
sulphide copper ore or concentrate, comprising the steps
of subjecting the ore or concentrate to a first leaching
at an elevated temperature and pressure in the presence of
oxygen and a lixiviant comprising an acidic solution of
chloride and bisulphate or sulphate ions to produce an
insoluble~basic copper salts leaching the basic copper
salt produced by said first leaching step in a second
leaching with an acidic sulphate solution to dissolve the
basic copper salt to produce a leach liquor containing
copper sulphate in solution; subjecting said leach liquor
to a solvent extraction process to produce a first copper
concentrate solution and a raffinate comprising protons,
copper ions and bisulphate or sulphate ions in solution;
extracting copper ions and bisulphate or sulphate ions
2o from said raffinate to produce a second copper concentrate
solution; recycling said second copper concentrate
solution to said first leaching at elevated temperature
and pressure to serve as a source of said bisulphate or
sulphate ions in said lixiviant; and subjecting said first
copper concentrate solution to electrowinning to recover
copper values therefrom.
Further according to the invention, there is
provided a process for the extraction of copper from a
3o sulphide copper ore or concentrate, comprising the steps
of subjecting the ore or concentrate to a first leaching
at an elevated temperature and pressure in the presence of
oxygen and a solution of chloride ions and at an acidic pH
to produce an insoluble basic copper salt: adding to said
solution, during said first leaching, a source of
bisulphate or sulphate ions selected from the group
CA 02101514 2002-11-27
- 4 -
consisting of sulphuric acid, copper sulphate and a
metal sulphate which hydrolyzes at said acidic pH and
providing sufficient of said source of bisulphate or
sulphate ions to react said source of bisulphate or
sulphate ions with said ore or concentrate so that
there is a nett consumption of bisulphate or sulphate
ions from said source of bisulphate or sulphate ions to
form said basic copper salt; and leaching said basic
copper salt in a second leaching with an acidic
sulphate solution to dissolve the basic copper salt to
produce a leach liquor containing copper sulphate in
solution.
Also according to the invention there is
provided a process for producing a concentrated
solution from a raffinate from a solvent extraction
process, comprising the step of subjecting said
raffinate to electrodialysis using a membrane which is
selected to permit the passage of preselected ions for
producing said concentrated solution.
In the present specification, elevated
temperature means a temperature above room temperature
(25°C), preferably from about 125°C to about 175°C, and
elevated pressure means an oxygen partial pressure
above atmospheric pressure, preferably from about 50
psig (445kPa) to about 250 psig (1825kPa).
Further objects and advantages of the
invention will become apparent from the description of
a preferred embodiment of the invention below.
BRIEF DESCRIPTION OF THE DRAWINGS
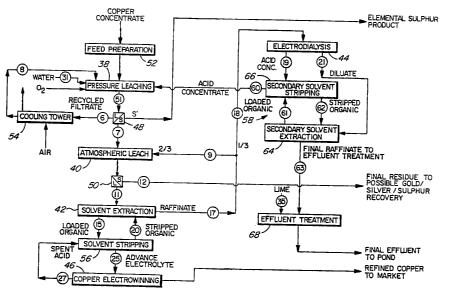
Figure 1 is a flow diagram of a
hydrometallurgical copper extraction process according
to the invention.
_ 5 _ ~1~~~1~
Figure 2 is a flow diagram of an electrodialysis
stage of the process of Figure 1.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENT
The stages of the process comprise, principally,
a pressure leaching stage 38 in an autoclave, an
atmospheric leaching stage 40, solvent extraction and
stripping stages 42 and 56, an electrodialysis stage 44
and an electrowinning stage 46.
After each of the leashing stages 38 and 40,
thickening and/or filtration is carried out as indicated
at 48 and 50, respectively, to separate the liquids and
solids.
The process will now be described in greater
detail by way of a specific example in which a
chalcopyrite concentrate is treated.
The copper concentrate is first ground in a ball
mill, during a feed preparation step 52, to reduce the
size of the particles to about 80%-98% minus 325 Tyler
mesh or smaller. Although satisfactory results are
obtainable without regrinding, it has been found that
there is a small but significant improvement with
regrinding.
The concentrate is leached in the pressure
leaching stage 38 at an elevated pressure and temperature
with a lixiviant containing no copper or up to about 15
grams per litre copper, 6-18 grams per litre chloride and
about 15-35 grams per litre sulphuric acid. About 80% of
the lixiviant is leach liquor which is recycled (Stream
8). The remaining 20% comprises concentrated sulphuric
CA 02101514 2002-11-27
- 6 -
acid which is recycled from the electrodialysis stage
44, as will be described in more detail below.
The temperature of the leach 38 is about
150°C. It has been found that if the temperature is
too high, eg. above about 175°C significant sulphur
oxidation to sulphate takes place. The pressure is
about 200 psig (1480kPa). This is total pressure
comprising oxygen pressure as well as the steam
pressure. The retention time is about 0.5-2.5 hours
and the process is normally carried out in a continuous
fashion in the autoclave. The process can also be
carried out in a batch-wise fashion and, in fact, the
processes in the Examples below have been carried out
in this way.
The solids content is maintained at about 15-
20~, i.e. 170-250 grams per litre solids as determined
by the heat balance and viscosity limitations. A
higher percentage solids Would require some form of
heat removal to prevent the temperature from rising
above the desired limit of about 150°C.
As referred to above, the lixiviant used in
the pressure leach 38 is made up partly of recycled
lixiviant from a previous pressure leach (Stream 8),
which is very low in acid, i.e. pH 3 to pH 5, but
augmented by an acid concentrate (Stream 60) which is
recycled from the electrodialysis stage 44. The
immediate effect of adding the acid concentrate to the
lixiviant is to increase the acidity of the lixiviant
which is fed to the autoclave for the pressure leaching
stage 38, but the most important effect, surprisingly,
has been found to be that the addition of the acid, or
more specifically the sulphate ions, actually
suppresses the oxidation of sulphur emanating from the
concentrate during the pressure leaching stage 38.
- ~~.~~.~J~~
Typically the oxidation of sulphur that is
experienced if no acid recycle is used is about 25%-30% of
the feed sulphur in the concentrate, as is the case with
the process described in U.S. Patent 4,039,406. However,
if acid recycle is used, it has been found that the
sulphur oxidation to sulphate is reduced to about 5-10%.
This improvement has substantial beneficial effects on the
hydrometallurgical extraction process. The oxidation of
sulphur to sulphate creates additional costs in several
ways, such as additional oxygen required for the reaction,
additional reagent required to neutralize the acid so
formed by the oxidation and provision must be made fox
heat removal due to the oxidation of sulphur to sulphate
which is very exothermic. This actually limits the
throughput of the autoclave in which the pressure leaching
stage 38 takes place.
The chemistry of the reaction in the pressure
leaching stage 38 is believed to be altered by the
addition of the acid as follows:
No acid addition:
CuFeSZ + ~/40z + 2/3H20 -~ [1/3CuSO4~z/3Cu(OH)a] + 1/zFezo~
+ s/3S° l1)
With acid addition:
CuFeSz + 5/40z + 1/sH20 + 1/3HZS04 -~ [1/3CuS04~z/3Cu(OH)z]
+ t/zFe203 + 2S° (2)
In both reactions, the copper is precipitated in
the form of a basic copper salt, which has been found to
comprise about 90% of basic copper sulphate, which
contains a sulphate anion, as indicated in the reaction
- ~~~~.514
equations, but about 10% of basic copper chloride is also
formed. In the first reaction it appears that the
sulphate of the basic copper sulphate is supplied by
oxidation of the feed sulphur in the concentrate, whereas
in the second reaction it appears to be supplied by the
sulphate ions in the acid recycle, thus obviating the need
for the oxidation of sulphur to sulphate. Thus in the
second reaction, there is a nett consumption of sulphate
ions to form the basic copper salt.
In actual test work, there is more sulphur
oxidation than is predicted by either reaction. The first
reaction predicts one sixth or 16.7% of the sulphur to be
oxidized whereas experimentally about 25%-30% is found.
With acid addition, experiments indicate that about 5-10%
sulphur is oxidized to sulphate, rather than the zero
oxidation that would be predicted if the second reaction
as written was the only reaction taking place. Therefore,
these reaction equations do not reflect exactly what is
happening in the pressure leaching stage 38 but are only
an approximation.
In order to take advantage of the beneficial
effect of the sulphuric acid recycle to inhibit the
oxidation of sulphur, it is necessary to find efficient
ways of adding acid into the autoclave, which has limited
ability to absorb acid because the bulk of the leach
liquor is recycled. However, there is some loss of leach
liquor in the pressure leaching stage 38, due to venting
(steam losses) and due to leach liquor carried off in the
filter cake after the pressure leach stage 38 (Stream 7).
It has been found that about 20% of the volume of leach
liquor is lost during each cycle in this fashion. The
amount of sulphuric acid needed to suppress sulphur
oxidation has been found experimentally to be about 25
grams per litre. Therefore, since this amount of acid
- 9 - ~~,~15i4
must be contained in 20% of the volume, it must come in a
concentrated form, i.e.
25 grams per litre 125 grams per litre
20%
There is surplus acid produced in the solvent
extraction stage 42 where the Cuso4 solution is changed
into an HZS04 solution. However, the acid so produced is
very dilute (Stream 17), only about 20-60 grams per litre,
due to the nature of the solvent extraction chemistry.
The difficulty cannot effectively be overcome by simply
evaporating the raffinate (Stream 17) coming from the
solvent extraction stages 42 and 56 to produce a
concentrated acidic solution because it is necessary to
eliminate impurities in the raffinate, such as iron and
zinc, and evaporation followed by recycling would return
the impurities to the pressure leaching stage 38.
This problem has been solved in the present
invention by extracting sulphuric acid from the raffinate
and in the particular embodiment described here, use is
made of electrodialysis to effect this extraction. Thus,
by introducing the electrodialysis stage 44, which will be
described in more detail below, sulphuric acid is
recovered from the raffinate in a concentrated form
suitable for use in the pressure leaching stage 38.
The slurry produced by the pressure leach 38 is
cooled to below 100°C and then filtered 48 to separate the
residue from the leach liquor or lixiviant, which is
recycled through a cooling tower 54 to the leaching stage
38, as noted above,
The residue contains the copper originally
present in the concentrate as insoluble basic copper
grams per litre. Therefore, since
- 1° w ~1~3.~~.~
sulphate and basic copper chloride together with all the
other solid materials, such as '.Fe20~ (hematite) and
elemental sulphur.
There is a gain in tha_ weight of the leach
residue. Typically it has 30-40% more dry weight than the
feed concentrate. It has been found that the leach
residue contains about 0.5-2% chloride, as well as the
copper, iron oxide and sulphur, which is due to the
presence of the basic copper chloride and the basic
copper sulphate. The iron in the chalcopyrite concentrate
is converted almost completely to hematite, while sulphur
is mostly converted to the elemental form with only a
fraction (about 5-10%) being oxidized to sulphate, as
noted above.
The leach liquor produced by the pressure
leaching step 38 has much the same composition as the feed
lixiviant except that there is a drop in the chloride
concentration from about 12 grams per litre to about 7-10
grams per litre, depending on the conditions, due to the
formation of the basic copper chloride.
The filter cake or leach residue is repulped in
raffinate from the subsequent solvent extraction stage 42,
which comprises an acidic sulphate solution containing
about 20-60 grams per litre H2S04 and a small amount of
copper, about 0.5-5.0 grams per litre.
The second leaching stage 40 takes place at
atmospheric pressure and a temperature of about 40°C for a
retention time of about 15-120 minutes. The percentage
solids is typically about 6-11% or about 70-140 grams per
litre, although it is quite possible to operate the
process outside this range. A higher percentage solids in
the second leaching 40 can produce a cu concentration in
- 11 - ~~~i51~
the resultant leach liquor that is too nigh for the normal
solvent extraction process, but this may be overcome by
recycling of aqueous streams within the solvent extraction
circuit. A higher percentage solids during leaching has
the advantage of smaller capacity requirements, but has
the disadvantage of higher entrained losses in the leach
residue. The final acidity of the slurry is about pH
1.5-2.0 or about 1-5 grams per litre HZS04.
During the atmospheric leaching stage 40, the
basic copper salts dissolve almost completely with very
little of the iron going into solution.
Typically, the leach liquor produced after
filtration 50 contains about ~.0-40 grams per litre copper,
depending on the percentage solids feed to the second
leach 40, with less than 1 grams per litre iron and about
0.5-4.0 grams per litre chloride.
The copper extraction has been found to be about
97-98% based on the original feed to the pressure leaching
stage 38. Iron extraction to solution has been found to
be less than about 5%.
The main constituents of the solid residue
(Stream 12) after filtration 50 are hematite and elemental
sulphur, as well as any gold or silver which may have been
present in the original concentrate. The sulphur can be
recovered by screening or flotation to separate it from
the hematite into a high-grade sulphur concentrate, which
can be further treated for recovery of sulphur. The gold
and silver can be recovered by cyanidation after sulphur
is removed from the leach residue.
The copper leached in the atmospheric leaching
stage 40 is extracted by means of solvent extraction 42 to
- 12
produce a loaded copper electrolyte suitable for
electrowinning 46. After the solvent extraction stage 42,
the loaded organic extractant is subjected to washing and
stripping 56. The high copper concentration of about
10-40 grams per litre derived from the atmospheric
leaching stage 40 provides significant advantages over
conventional solvent extraction/electrowinning plants
because much higher loading of the organic is possible,
thus reducing the size of the plant for a given tonnage of
copper. Stripping of the loaded organic is effected by
means of spent acid from the electrowinning stage 46 to
obtain a pure copper sulphate solution which is then
passed to the electrowinning stage 46.
The raffinate (Stream 17) from the solvent
extraction 42 typically contains 0.5-5.0 grams per litre
Cu, less than 1 gram per litre Fe, 20-60 grams per litre
acid as HZS04 and 0.5-4.0 grams per litre chloride.
The raffinate from the solvent extraction stage
42 is divided into two portions. A first portion (Stream
9) comprising about two-thirds of the raffinate is
recycled to the atmospheric leach stage 40. A second
portion comprising the remainder or about one-third of the
raffinate (Stream 18) is sent to the electrodialysis stage
44 to produce a diluate and a concentrate acid solution.
The electrodialysis may be operated with a variety of
membranes to provide the desired selectivity. With non-
selective membranes, virtually all ions in solution, pass
into the concentrate (Stream 19) leaving essentially only
water in the diluate (Stream 21).
However, in the preferred embodiment being
described here, more selective membranes are employed
which allow essentially only monovalent cations and
monovalent arid divalent anions to pass into the
- 13 _ ~~101514
concentrate (Stream 19) . Thus H+, Cl', Sp,~', HS04' ions
will pass into the concentrate leaving behind other ions
such as Cu++, Ca++, Zn++, Mg++, Fe'+ and Fe+++ in the
diluate (Stream 21).
The electrodialysis process is not 100%
efficient, nevertheless, and some of the listed ions will
find themselves going into the opposite stream.
Under the conditions of the preferred
embodiment, with a selective membrane as described, the
diluate solution (Stream 21) typically contains 1-3 grams
per litre copper and 8-20 grams per litre sulphuric acid,
and the concentrate acid solution (Stream 19) usually
contains less than 4 grams per litre copper, 150-220 grams
per litre H2S04 and 4-10 grams per litre chloride.
With reference to Figure 2, the electrodialysis
stage 44 will now be described in more detail. It
includes an electrodialysis unit 70 which comprises a
number of alternating concentrate and diluate
compartments separated by alternating cationic and anionic
membranes and an anode and a cathode compartment
containing an anode and a cathode, respectively. The
anionic and cationic membranes are selected from suitable
selective membranes for the removal of monovalent and
divalent anions, such as C1', HS04', 5042', and
monovalent cations, such as protons. An electrical
potential difference is applied between the anode and the
cathode to produce a current with a current density in the
range of about 100A/m2 to 1500A/m2.
The electrodialysis stage 44 further comprises a
concentrate recirculation tank 72, a diluate recirculation
tank 74 and a recirculation tank 76 for electrode rinse
solution.
- 14 - ~~.0~~1~
The raffinate (Stream 18) is fed to a raffinate
feed tank 78 from where the raffinate is fed by means of
pumps 80 to the diluate compartments. Circulatory flow of
diluate and concentrate through the diluate and
concentrate compartments, respectively, are produced by
means of recirculation pumps 82 at a linear velocity
sufficient to maintain turbulent flow in the compartments.
A portion of the circulating diluate is withdrawn as the
treated diluate solution with reduced concentrations of
the ions and passed to a diluate tank 84. A part of the
circulating concentrate is also withdrawn and passed to a
concentrate tank 86.
The cathode and anode compartments are rinsed
separately or with a common rinse solution circulated to
both the electrode compartments. The rinse solution is
fed from a rinse solution tank 88 by means of a pump 90 to
the recirculation tank 76, from where it is circulated by
means of pumps 92 to the electrode compartments.
Rinse liquid is bled from the tank 76 to an
effluent treatment pond.
Normally the membranes which are effective for
acid recovery such as monovalent and divalent selective
anionic membranes and monovalent selective cationic
membranes, are not as effective with respect to chloride
ions, resulting in only about 30%-40% recovery of chloride
ions. On the other hand, membranes which are effective
for the recovery of chloride ions are not so effective for
the recovery of acid. Thus, to render bath the acid and
chloride ion recovery effective, it has been found
expedient to carry out the electrodialysis in two stages.
In a first stage, membranes are used which are effective
for acid recovery, and the diluate from the first stage is
- 15 -. ~~.~1514
then treated in a second stage using different membranes
which are effective for the recovery of chloride ions,
such as SELEMIONTa CMR and ASR membranes. The
concentrate from the first stage, which contains the
concentrated acid and some chloride, can then be combined
with the concentrate from the second stage, containing the
chloride concentrate, to form the concentrate (Stream 19).
Typically the concentrate stream (Stream 19)
from the electrodialysis stage 44 will be about 10-20% of
the feed flow, whereas the diluate stream (Stream 21)
comprises the rest or 80-90% and contains the bulk of the
water in the feed stream, as well as any ions that have
been rejected by the membranes, such as Cuz' present in
the feed. It is desirable to recover such CuS04 and this
is effected in the process according to the present
invention by subjecting the diluate stream to an auxiliary
or secondary solvent extraction circuit 58. The circuit
58 comprises an extracting stage 64 and a stripping stage
66, for the extraction and stripping operations,
respectively. The concentrate acid solution from the
electrod.,'_alysis stage 44 can be used as stripping acid in
the stripping stage 66 to strip the copper from the loaded
organic. The acid solution resulting from the stripping
stage 66 is 140-200 grams per litre sulphuric acid which
is recycled to the pressure leach stage 38. Typically the
feed to this circuit (the diluate stream) will contain
about 1.0-3.0 grams per litre copper and the raffinate or
waste stream will contain about 0.05-0.1 grams per litre
copper. The overall extraction of copper from the leach
liquor (Stream 11) in the solvent extraction,
electrodialysis and electrowinning stages, of the process
has been found to be as high as 99.7%.
- is - ~~.~~~J1~
As already noted, in this particular embodiment,
the electredialysis stage 44 is selective, i.e. copper
ions are in the diluate stream (Stream 21).
.5 As noted above, the residue from the pressure
leaching stage 38 (Stream 7) comprises a mixture of
elemental sulphur (S~), hematite (Fez03) and~mainly basic
copper sulphate. This residue is fed to the atmospheric
leaching stage 40 where the basic copper sulphate is
dissolved in acid as far as possible, leaving the hematite
and elemental sulphur components essentially untouched.
This produces a solution of copper sulphate (CuSO,~)
(Stream 11) which is fed to the solvent extraction stage
42, where copper is exchanged with an organic ligand
(R-H), producing acid in the aqueous stream, the raffinate
(Stream 17). The reactions can be summarized as follows:
Atmospheric Leaching Staae:
CuS04 ~ 2Cu (OH) Z + 2H2S04 -~ 3CuS04 + 4HZ0 (3)
Solvent Extraction Staae:
3CuS04 + 6R-H -~ 3RZCu + 3HZS04 (4)
Thus the overall reaction can be represented as follows:
CuS04 ~ 2Cu (OH) 2 + 6R-H -~ 3RZCu + HzS04 + 4H20 (5)
There is thus one extra mole of acid produced
for every three moles of Cu leached in the atmospheric
leach. In order to make use of this extra mole of HZS04,
the raffinate stream from the solvent extraction stage 42
is split, as noted above, so that two-thirds thereof
(Stream 9) are returned to the atmospheric leaching stage
and the remaining one-third (Stream 18) is fed to the
electrodialysis stage 44 to produce the acid concentrate
35 which is fed to the pressure leaching stage 38.
1
- 17 -
An additional benefit of the process according
to the invention is that chloride ions lost from the
pressure leach circuit into the pressure leach residue,
either as insoluble basic copper chloride or as entrained
solution losses in the filter cake, can be recycled along
with the acid concentrate back to the pressure leach. Any
chloride ions present in the pressure leach residue will
report almost quantitatively to the atmospheric leach
liquor and thence to the raffinate after solvent
extraction. If not bled from this circuit they would
quickly build up to higher levels in the atmospheric leach
liquor and eventually transfer to the electrowinning
circuit where chloride is particularly undesirable. By
splitting the raffinate from the solvent extraction stage
42, as noted above, and treating one-third thereof through
the electrodialysis stage 44 and solvent extraction 58,
this effectively recycles the chloride content back to the
pressure leach thereby minimizing any chloride makeup
requirements therein.
The efficiency of the process according to the
invention may be gauged from the overall reaction for the
entire process. If reactions (3) and (4) are combined
with the reactions for pressure leaching (6), solvent
stripping (7) and electrowinning (8), as given below, then
it can be seen that the overall reaction (9) for the
entire process does not produce or consume any acid or
chloride ions. Thus the process neither requires acid nor
neutralization if these reactions are strictly adhered to.
In practice, however, as already mentioned, about 5-10% of
the sulphur in concentrate is oxidized to acid.
Pressure Leachinc,L, Sta~~:
3CuFeSz + ~s/40z + Hz0 + HZS04 1 [CuS04 ~ 2Cu (OH) z) +
3/zFez03 + 6S0 (6)
- 18 - ~~.~~~~.
Solvent Stripping Staae:
3RZCu + 3HZS04 -~ 3CuS04 + 6R-H (7)
Electrowinning Staae:
3CuS04 + 3H20 -~ 3Cu~ + 3/202 + 3HZS04 (8)
Overall Process Reaction:
3CuFeSz + 15/402 -~ 3Cu~ + 3/2Fez03 + 6S~ + 3/202 (9)
With the process according to the present
invention, relatively high copper recoveries, typically 97
to 98% at quite low temperatures in the atmospheric leach,
such as 40°C, have been obtained. Such low temperatures
are known to suppress iron dissolution and test results
have shown only about 200 ppm Fe with 10 grams per litre
Cu in the atmospheric leach solution after filtration 50
(Stream 11). This is a marked improvement over prior art
processes, such as described in U.S. Patent 4,338,168,
which reports only about a 93% recovery at this
temperature and requires higher temperature and/or acid
levels to obtain satisfactory copper recovery values.
Unfortunately, such more severe conditions also dissolves
about 50% of the Fe in the feed to the atmospheric
leaching stage 40 complicating the process by requiring
the addition of a jarosite precipitation process to
separate the Fe from the copper. In the present process
the Fe is rejected in the atmospheric leach residue.
Due to the reduction in sulphur oxidation and
the effective recycling of the sulphuric acid as
described, the process according to the invention does not
require any special neutralization procedure. Since only
about 5-10% of the sulphur is oxidized to sulphate only a
relatively small amount of acid is produced which can
effectively be taken care of by a lime neutralization
process (Stream 35) which is required in any event for the
treatment 68 of the final effluent from the solvent
extraction cycle 58, which is the bleed of impurities such
as Zn and Mg from the circuit.
The results of tests which were carried out for
the various stages of the process will now be given in the
following Examples. In Example 1 the feed to the pressure
leach did not contain acid. In Example 2, an acidic feed
was charged to the leach.
The copper concentrate from a porphyry deposit
in Highland Valley, British Columbia (Stream 1) is
composed of 40.19% copper, 20.50% iron, arid 29.24%
sulphur. In both Examples 1 and 2, the concentrate was
ground to 98%-400 mesh. In Example 1, the charge to the
autoclave had a wet weight of 175.1 grams at 14.4%
moisture. The solution feed to the leach was a
combination of 900 ml of recycled pressure leach filtrate
(Stream 8) containing 1.5 grams per litre copper, less
than 1 ppm iron and 11.47 grams per litre chloride and 100
ml of water. The makeup water (Stream 31) actually
contained 2.8 grams of sodium chloride so that the total
chloride concentration in the leach was 12.0 grams per
litre. The concentrate was leached for one hour at 200
psi and 150°C. Upon completion of the pressure leach, the
slurry (Stream 51) was filtered. The 995 ml of filtrate,
Stream 6, contained 1.0 grams per litre copper, less than
1 ppm iron, 8.3 grams per litre sodium, and 11.6 grams per
litre chloride and had a pH of 3.9. The total wet weight
of the residue from the pressure leach was 323.0 grams. A
91 gram sample was taken for analysis. This sample , on a
2° ~ ~~.~~~i~~
dry basis, contained 32.4% copper, 16.9% iron, 0.49%
sodium and 10.4% elemental sulphur and had a moisture
content of 37.7% moisture.
The residue from the pressure leach was
subjected to an atmospheric leach for an hour at 40°C and
a pH 1.7. The charge to this leach consisted of 231.6
grams of solids at 37.7% moisture and 2120 ml of water
with 30 ml of concentrated HZS04 (Stream 9). The slurry
from this leach, Stream 10 was filtered to obtain 108.4
grams of residue at 36.2% moisture and a 2120 ml filtrate.
The residue was washed once by displacement and resulted
in a 245 ml wash water containing 4.2 grams per litre
copper and 217 ppm iron. The filtrate, Stream 11,
consisted of 23.2 grams per litre copper, 403 ppm iron,
420 ppm sodium, and 1.2 grams per litre chloride. The pH
of the filtrate was 1.7 and had a free acid of 3.1 grams
per litre. The residue from the leach contained 2.52%
copper, 0.16% sodium and 28.9% iron. The results of this
example are given below in Tables 1 and 2.
Example 2
To reduce sulphur oxidation, sulphuric acid was
added to the feed of the pressure leach. The sulphuric
acid provided the sulphur needed to form Basic Copper
Sulphate instead of oxidizing sulphur in the concentrate.
The following equation defines the reaction which is
occurring:
CuFeSz t 5i40z + 9/3H20 f 1/3HZS04 -~ [ 1/3CuSO4 ~ ziscu (oH) z
+ ~ezFezo3 + 2S° (10)
This addition of acid to the feed of the leach
reduces the sulphur oxidation from 28% to 9%. The charge
to the present leach consisted of 183.0 grams of wet
- 21 - ~1~~~~~
concentrate at 16% moisture. Since the actual liquor from
a past pressure leach, Stream 8, and the concentrated acid
from the electrodialysis/solvent extraction, Stream 60,
were unavailable, these feeds were made synthetically.
The pressure leach was charged with 1000 ml of feed
solution having a chloride concentration of 12.0 grams per
litre and a free acid concentration of 27.0 grams per
litre. The concentrate was leached for an hour at 200 psi
and 150°C. The slurry from this leach, Stream 51, was
filtered. The 1025 ml filtrate, Stream 6, contained 6.0
grams per litre copper, 20 ppm iron, 6.0 grams per litre
sodium, and 10.4 grams per litre chloride. The pH of the
filtrate was 3.1. The residue weighed 331.5 grams wet
from which a 48 gram sample was taken. The residue had a
moisture content of 32.6% and contained 25.4% copper,
13.6% iron, 0.23% sodium and 18.41% elemental sulphur.
As in Example 1, the residue from the pressure
leach was subjected to an atmospheric leach.
Approximately 284 grams of wet residue from the pressure
leach was combined with 3500 ml of acidic water (Stream
9). The residue was leached for an hour at 40°C at a pH
of 1.5. The slurry from this leach was once again
filtered to obtain 3205 ml of filtrate, Stream 11, and
132.2 grams of wet residue. The residue was washed with
385 ml of water and produced a wash water with 3.38 grams
per litre copper and 143 ppm iron. The filtrate contained
11.9 grams per litre copper and 580 ppm iron. The
residue had a moisture content of 28.1% and consisted of
1.65% copper, 0.03% sodium and 16.23% iron.
- 22 -
Comparison of Examples 1 and 2
To compare the effect of adding acid to the feed
of the pressure leach on the leaching of copper, the
following tables illustrate the copper extraction and the
sulphur oxidation for both tests.
Table 1: Copper extraction for tests Examples 1 and 2
Feed Copper ~ Residue Copper ~ X
Example ~-- ~-------T----.--.~.~ T-.~.~ Extraction
I
~ ~ Dry wt.(g) ~ X Cu ~ g Cu ~ Dry Nt. (g) ~ X Cu ~ A Cu
I
O 1 ~ 150.0 ~ 40.19 ~ 60.2 ~ 96.2 ~ 2.52 ~ 2.42 ~ 96.0
1 - f---~ ~--~ -1
2 ~ 153.7 ~ 40.19 ~ 61.8 ( 110.9 ~ 1.65 ~ 1.83 ~ 97.0
~ ~ i i i i i i
With the addition of sulphuric acid to the feed
of the pressure leach, the extraction of copper increased.
The percentage copper remaining behind in the residue of
the atmospheric leach, Stream 12, decreased from 2.52% to
1.65%. The extraction of copper increased from 96% to 97%
in Example 2.
Table 2: % Sulphur oxidation
X Sulphur Oxidation
Example ~ Sulphur Balance Method ( Acid Generation Method
1 4-
a 1 ~ 28 ~ n.a
2
i
As can be seen, the amount of sulphur oxidation
was reduced dramatically from Example 1 to Example 2.
~~fl~~~~
- 23 -
Examples 3 to 6
In order to determine the optimum acid addition
to the feed of the pressure leach, four tests (Examples 3
to 6) were ran consecutively. The goal of these tests was
to vary the acid concentration, Stream 60, so that the
copper in the feed, Stream 8, and in the filtrate, Stream
6, after the pressure leach were in equilibrium. The
following table summarizes the results of this work.
Table 3: Results of Acid Addition
I ~ Acid in feed (g/L) ~ Cu in Feed~ Gain/LosspH
(g) ~ Cu in Filt.(g) ~
Example ~ (Streams 8 ~ (Streams 8 ~ (Stream~ in FiltrateFilt.
6) (
~ ~ and 60 combined) hand 60 combined)~ (g)
3 ~ 27.3 ( 4.1 ~ 9.7 ~5.6
~ 3.2
4 ~ 22.5 ~ 7.0 ~ 10.38 ~ 3.3 ~ 3.7
;
5 ~ 24.0 ~ 8.1 ~ 7.1 ~ -1.0 3.3
~
6 ~ 23.0 ~ 6.7 ~ 4.0 ~ -2.7 3.6
~
Note: Pressure Leach Conditions - 60
min, 150C,
300 psig total pressure (about psi oxygen
245
partial pressure), 225 grams per
litre
concentrate
At 27.3 grams per litre acid in the feed, the
filtrate gained 5.6 grams copper indicating that too much
acid had been added to the feed. At 23.0 grams per litre
acid in the feed, the filtrate lost copper indicating that
too little acid had been added to the feed. Based on
these results, the equilibrium acid concentration in the
feed to the pressure leach was set at 25.0 grams per
litre.
The difference in the acidity of the feed and
filtrates indicates that the acid was consumed during the
leach. This is an indication that the sulphate content of
the Basic Copper Sulphate is principally derived from the
~~.~~.5~.~~
- 24 -
sulphuric acid in the feed and not from oxidation of
sulphur in the concentrate.
To further illustrate that 97% of the copper is
leached in this process, the following table summarizes
the copper extractions for the tests. The extractions of
copper were above 97% in all the tests except Example 4.
The sulphur oxidations were all below 7.4%
Table 4: Copper extractions for Examples 3-6
I ~ X Cu ~ X Sulphur
Example ~ Extraction Oxidation
~ f
~ J i
3 ~ 97.2 ~ 6.73
i
i
I 4 ( 95.4 ~ 7.39
~-
I 5 ~ 97.7 ~ 5.64
~
- ~-
6 ~ 98.0 I 7.36 I
Examt~le 7: Solvent Extraction and Strip_ping~ Process
A solvent extraction and stripping test was
performed and used as an example to demonstrate the
solvent extraction and solvent stripping unit operations
of the process of Figure 1.
A copper sulphate filtrate solution from the
atmospheric leach corresponding to Stream 11, and
containing 9.5 grams per litre copper and 3.75 grams per
litre sulphuric acid at pH 1.6 was subjected to solvent
extraction and solvent stripping in the solvent
extraction unit. A 70% to 30% ratio mixture of LIX 84 to
LIX 860 at 30% volume ratio with kerosene was used for the
solvent extraction unit operation. A two-stage extraction
and two-stage stripping were used. A 2:1 organic to
aqueous ratio was used in the two solvent extraction
- 25 -
stages and a 3:1 organic to aqueous ratio was used in the
two stripping stages. Spent acid (Stream 27) containing
32.9 grams per litre copper and 180 grams per litre acid
from the electrowinning was used for stripping the loaded
organic (Stream 15) in the stripping circuit.
The copper sulphate pregnant electrode solution
produced from the stripping circuit (Stream 25) was found
to contain 43.5 grams per litre copper and 146 grams per
litre free acid, and was sent for electrowinning. The
raffinate produced (Stream 17) was found to contain 0.73
grams per litre copper and 19.4 grams per litre sulphuric
acid.
The loaded organic (Stream 15) was found to
contain 7.9 grams per litre of copper, and the stripped
organic (Stream 20) was found to contain 3.39 grams per
litre copper.
E~cample 8A' Electrodialysis (Selective l~iembranes~,
An electrodialysis test was performed and used
as an example to illustrate the electrodialysis unit
operation of the process of Figure 2. In this example,
selective membranes were used, such that only monovalent
rations and mono- and divalent anions were allowed into
the concentrate solution. All divalent rations, such as
Cuz'' or Zn2*, remained in the diluate stream, as well as
higher valent ions.
The raffinate from the solvent extraction
circuit (Stream 18) was subjected to electrodialysis in
the electrodialysis unit 44. The raffinate solution was
found to contain 890 mg/1 copper, 20 grams per litre
sulphuric acid and 1.1 grams per litre chloride.
SELEMIONTM CMV-A cationic and AAV anionic membranes were
- 2s -
used. THE CMV-A cationic membranes are designed to pass
only monovalent cations into the concentrate leaving
divalent and other multi-valent cations behind (in the
dilute). The AAV anionic membranes are designed to pass
both monovalent and divalent anions into the concentrate.
The raffinate solution was passed through an activated
carbon column and a polish filter to remove any organic
and suspended solids. A current was applied between the
electrodes to give a current density of 1000 A/m2. The
temperature was controlled at below 45°C. The electrode
compartments were rinsed with a rinse solution containing
1 molar sulphuric acid.
The final diluate solution (Stream 21) was
analyzed, and was found to contain 0.76 grams per litre
copper, 8.9 grams per litre sulphuric acid, and 0.8 grams
per litre chloride. The final concentrate acid solution
(Stream 19) was found to contain 2.57 grams per litre
copper, 168 grams per litre sulphuric acid and 4.1 grams
per litre chloride. Both the diluate solution and the
concentrate acid solution were treated in the secondary
solvent extraction and stripping process to produce a
final raffinate solution (Stream 63) to the effluent
treatment stage 68 and a concentrate acid solution (Stream
60) back to the pressure leaching stage 38.
Table 5: Results of Example 8A
I I I I I
I I Flow rate I Assay I Distribution(%)
I I I (g/L) I
I I
I I I
I I (ml/min) I Acid I Cu Cl Acid I Cu I
I I (
Cl
I
I FEED I 186 I 20 I 0.89 1.1 100 I 100 I
( I 100
I
~~.T_-~~ i'--~i
I I I I I I I I
I DILUATE I 171 ( 8.9 I 0.76 0.8 41 I 79 I
1 T ~ I I I
67
I
~ --
I CONCENTRATE ( 15 168 I 2.57 4.9 68 ) 23 I
I I I 30
I
z10~.51~~
- 27 -
Example 8B: Electrodial_ysis PNon-selective membranes
A further electrodialysis test was performed and
used as an example to illustrate the electrodialysis unit
operation of the process of Figure 1. However, in this
particular example, a non-selective membrane was used,
i.e. all monovalent and divalent ions were allowed into
the concentrate solution.
The raffinate from the solvent extraction
circuit (Stream 18) was subjected to electrodialysis in
the electrodialysis unit. The raffinate solutions from
the solvent extraction were mixed and used as feed to the
electrodialysis. The raffinate solution was found to
contain 700 mg/1 copper and 20 grams per litre sulphuric
acid. SELEMIONT~ CMV cationic and AAV anionic membranes
were used. These membranes are designed to pass both
monovalent and divalent cations and anions into the
concentrate. The raffinate solution was passed through an
activated carbon column and a polish filter to remove any
organic and suspended solids. The total organic carbon in
the raffinate was reduced from 7.84 ppm to 1.53 ppm after
passing through the activated carbon column and the polish
filter. The solution was then fed to the electrodialysis
unit at a rate of 163 ml/minute. A current was applied
between the electrodes to give a current density of 1000
A/mz The temperature was controlled at below 40°C. The
electrode compartments were rinsed with a rinse solution
containing 1 molar sulphuric acid.
The final diluate solution (Stream 21) was
analyzed, and was found to contain 261 mg/1 copper and 9.5
grams per litre sulphuric acid. The final concentrate
acid solution (Stream 19) was found to contain 7.32 grams
per litre copper and 169 grams per litre sulphuric acid.
_ 28 _ 2141~1~
Both the diluate solution and the concentrate acid
solution was treated in the auxiliary solvent extraction
and stripping process to produce a final raffinate
solution (Stream 63) containing less than 100 mg/1 copper
which is sent to the effluent treatment stage 68.
Table 6: Results of Example 8B
Flow rate ~ Assay ~ Distribution(X)
(g/L)
(ml/min) ~ Acid ~ Cu Cl ~ Acid ~ ~
~ Cu Cl
r
~ FEED ( 163 ~ 20 ~ 0.77 1.42 ~ 100 ~ 100 ~
i ~ ~ ~ 100
-
T 'T T ~ f T-
-1
DILUATE ~ 152 ~ 9.5 ~ 0.26 0.89 ~ 44 ~ 32 ~
~ 58
-~ ~ i ~ -j - +'
CONCENTRATE ~ 11 ~ 1b9 ~ 7.32 8.17 ~ 57 ~ b4 ~
i i ~ ' L L 39
i ~ ~
i
~,~eample 9~ Auxilia~,y Solvent Extraction
A solvent extraction was performed and used as
an example to illustrate the auxiliary solvent extraction
64 and stripping 66 unit operations of the process of
Figure 1.
The diluate solution from the electrodialysis
unit (Stream 21) Was subjected to auxiliary solvent
extraction 64. Diluate solutions from electrodialysis
were mixed and used as feed to this test. The diluate was
found to contain 264 mg/1 copper and 9.0 grams per litre
sulphuric acid at pH 1.02. A 70% to 30% volume ratio
mixture of LIX 84 to LIX 860 at 30% volume ratio with
kerosene was used for the auxiliary solvent extraction and
stripping operations. A one stage extraction and one
stage stripping was used at a 1:1 organic to aqueous ratio
in the extraction stage and a 10:1 organic to aqueous
21~151~
_ 29 _
ratio in the stripping stage. Concentrate acid solution
(Stream 19), containing 5.36 grams per litre copper and
180 grams per litre acid, was used for stripping the
loaded organic (Stream 61) in the stripping circuit.
The copper sulphate solution produced (Stream
60) from the stripping circuit was found to contain 8.8
grams per litre copper and 161.3 grams per litre free
acid, and was recycled back to pressure leaching. The
final raffinate produced (Stream 63) was found to contain
66 mg/1 copper and 12.3 grams per litre sulphuric acid,
and the raffinate is sent to effluent treatment
operation.
The loaded organic (Stream 61) was found to
contain 1.7 grams per litre of copper, and the stripped
organic (Stream 62) was found to contain 1.1 grams per
litre copper.
2 0 Example 10 ~ Copper Electrowinninc_r
A copper electrowinning test was performed and
used as an example to demonstrate the copper
electrowinning unit operations of the process of Figure 1.
A copper sulphate electrolyte produced from the
solvent extraction unit operation (Stream 25) was
subjected to electrowinning in the electrowinning unit.
Copper electrolyte from the solvent extraction stage was
used in this test and the copper electrolyte was 43.5
grams per litre copper and 146 grams per litre sulphuric
acid. About 10 mg/1 of animal glue was added to the
electrolyte solution to provide cathode deposit control
and to counteract the negative effect of trace impurities
on cathode deposit. A current was applied between the
electrodes to give a current density of 300 A/m2. A
~~.0~.~~_~
- 30 -
voltage drop of 2 volts per cell from anode to cathode was
used. The temperature was maintained at 35°C and the unit
was operated for 8.4 hours.
A high quality copper was produced at the
cathodes (Stream 28) at a current efficiency of 97.8%.
The spent acid from electrowinning (Stream 27) was found
to contain 28.5 grams per litre copper and 177 grams per
litre sulphuric acid, and was recycled and used as
stripping acid for the solvent extraction unit.
While the process has been described with
specific reference to a chalcopyrite concentrate, it will
be appreciated that the process can also be applied to
concentrates of other copper sulphide minerals, such as
bornite (Cu5FeS4), covellite (CuS), chalcocite (CuzS),
enargite (Cu3AsS4), tetrahedrite (Cu3SbS3), and the like,
or mixtures thereof.
As an alternative to subjecting the raffinate
from the solvent extraction 42 to the electrodialysis step
44, to produce a concentrated sulphuric acid solution for
recycling to the first leaching step 38, it is also
possible to produce a concentrated copper sulphate
solution by subjecting the raffinate to the
electrodialysis step 44, by the choice of a suitable
membrane and recycling the concentrated copper sulphate as
produced to the first leach 38 to serve as a source of
sulphate ions required for the leach 38. In this case the
electrodialysis is non-selective, i.e. the copper ions are
allowed into the concentrate stream (Stream 19) with the
acid. The reaction taking place in the pressure leach
stage 38 can be represented as follows:
CuFeSz + s/40z + H20 + 1/zCuS04 -~ [ ozCuS04 ~ Cu (OH) z ]
+ t/zFez03 + 2S~ (11)
CA 02101514 2002-11-27
- 31 -
Electrodialysis processes are described in
U.S. Patents 5,064,538, 5,084,180 and 5,110,432. A
description and example of the selective process is
given in U.S. Patent 5,084,180, column 5, line 22 to
line 19, column 6 and Example 5 of U.S. Patent
5,064,538, respectively. This relates to a zinc
sulphate solution, but is also applicable to a copper
sulphate solution. An example of a non-selective
process is given in Example 3 (column 11) of U.S.
Patent 5,110,432.
In yet a further alternative method, the
sulphate ions may be provided from another source,
which may be external to the rest of the process, such
l5 as by the addition of a metal sulphate, which will
hydrolyze under the leaching conditions and thus
produce acid in the situ, such as Fe2(S03)3, to the
pressure leach stage 38, in which case the reaction
taking place can be represented as follows:
CllFeS2 + 5/402 + 2/3H20 + 1/9Fe2 (SOq) 3 ~ [1/3CuS04~2/3Cu (OH) 2]
+ il/laFe203 + 2S~ (12)
While the application of an electrodialysis stage
after a solvent extraction stage for obtaining a
concentrated acid solution for recirculation to the
pressure leaching stage, has been described with
particular reference to the chloride-assisted
hydrometallurgical copper extraction process given as a
particular example above, it will be appreciated that
the combination of solvent extraction and
electrodialysis steps can be utilized in other
applications where a concentrated acid or other
concentrated solution is required, such as for
recirculation to an earlier stage in a process, such as
to a leaching stage as in the
- 32 -
present example. The combination of an electrodialysis
stage with a solvent extraction stage is expected to be
useful in many applications not necessarily including a
pressure leach stage, eg. leaching of an oxide ore, when
confronted with the problem of having to produce a
concentrated acid solution out of the raffinate where the
raffinate has been diluted due to water addition.
while only preferred embodiments of the
invention have been described herein in detail, the
invention is not limited thereby and modifications van be
made within the scope of the attached claims.