Note: Descriptions are shown in the official language in which they were submitted.
P~OC$~ ~O RECOVB!R LIQ~ID ~E~ANB
The invention relates to a process to recover liguid
methane ~rom a feed gas mixture compri6ing essentially
methane, C2~ hydrocarbons, carbon dioxide and nitrogen.
Highly pure liquid methane is used to an increasing
extent as a non-polluting ~uel Por diesel engines in locomo-
tives, buses and trucks. The liquid methane is generally
recovered ~rom natural gas. For example, in U~S. Patent No.
~,761,167, a process to recover methane from a gas ~ixture
containing methane, C2~ hydrocarbons, carbon dioxide and
nitrogen provides ~eeding the gas mixture first to a cryo-
genic distillation stagef in which the C2~ hydrocarbon frac
tion a6 well as a firist carbon dioxide fraction are re-
: covered. The remaining CH4/N2/CO2 gas mixture is then con
veyed to a pressure swing ad~orption stage where the mixture
is freed from carbon dioxide. In a nitrogen separating unit
i i
downs~ream o~ the pressure swing adsorption stage, the re ~-
sultant gas ~ixture is separated to provide the methane pro-
duct fraction a~ well as a nitrogen fraction, and the latter
~ 20 is used to regenerate the adsorber loaded with carbon diox-
'~
1,';
~,~
ide. This process is particularly useful i~t the nitrogen
concentration in the feed gas mixture is approximately of
the same order of magnitude as the methane concentration.
In an alternative to this process, there is employed,
instead of the cryogenic distillation stage, an amine scrub-
bing stage to separate the carbon dioxide from the feed gas
mixture. In this case, is neceit~sary to employ a downstr~i?iam
adsorption unik for drying the resultant water saiturated
~eed gas mixture from the amine washing stage. This proce-
dure has the drawback, however, that the adsorption unit,because of the complPte water saturation of the gas stxeam
exiting from the amine washing stage, must ~e very large.
Furthermore, the sy~tem must provide for the disposal or
recycling of the amine scrubbing agent used in the carbon
dioxide separation stage.
An object of one aspect of this invention is to provide
a process to recover liquid methane, in which, on the one
hand, an amine washing stage can be avoided, and, on the
other hand, an adsorption unit can be used which is smaller
in comparison with prior art procesiies.
To achieve this object according to the invention, a
process is provided wherein:
a) the feed gas mixtur~? is fed fir~t to an adsorption
unit and freed ~rom water in the latter;
b) the dried feed gas mixture is fed to a membrane
~ separation stage where the carbon dioxide is separated down
I to a contenk lower than 2% by volume; and
- 2 -
c) the gas mixture now essentially consisting of
methane, C2~ hydrocarbon~ an~ nitrogen is fed to a low-
temperature distillation stage wherein the C2+ hydrocarbons
as well as the residual content of carbon dioxide are
separated by distillation.
If desired, the resultant purified methane can be
liquefied by an external refrigeration cycle and an expan-
sion step. The resultant liquid methane is then transPerred
to a storage tank. By virtue of boil-off from thP storage
tank, the liquid is gradua].ly depleted in residual nitrogen,
as one method of removing the nitrogen.
The combination according to the invention of adsorp-
tion to dry the feed gas stream, membrane separation to re-
move carbon dioxide, and low-temperature distillation to
recover the liquid methane product stream results in a pro-
cess that is very easy to operate, quick to start and eco-
nomical. In addition to the water contained in the feed gas
mixture, any glycol in the feed gas can also be removed by
the adsorption stage. Adsorption processe~ to dry all types
of gas streams are well known ~rom the literature. Adsorp-
tion agents for the drying step of this invenkion include
but are not limited to silica gel, activated alumina and
molecular sieve (zeolite).
In the membrane separation stage, a reduction of the
carbon dioxide content to less than 2% by volume, generally
about 0.5 to 1.8% by volume, can be achiev~d with the
- selection O:e suitable membranes in one, two or more separa-
- 3 -
. . : . , ,~ :: . . . ~ : , ,.
tion stages, for example, a spinal wound membrane manufac-
tured by Grace Membrane Systems from cellulosic acetate,
described in Chemical Enqineerinq Proqress, January 1989,
pages 41-62, "Economics of Gas Separation Membranes".
In the liquef~ction occurring in the downstream low-
temperature distillation sta~e, the residual carbon dioxide
together with the C2~ hydrocarbons is removed down to a con-
centration of less than 50 ppm with very little additional
expenditure over that generally required for the liquefac-
10 tion of the methane. Moreover, this minor additional expen-
diture is also offset, in that the gas stream exiting from
the membrane separation unit enters the low temperature
distillation stage at about 300 K~ In contrast, in the case
of an amine scrubbing stage up~tream of the low temperature
I distillation stage, the temperature of the exit gas was
¦ approximately 322 K. This 22 differencf again would, of
course, entail an additional expenditure of energy ~or the
liquefaction of the resultant gas within the low-temperature
distillation stage.
In the process according to the invention, additional
preferred features are optionally employed. In one, the
carbon dioxide fraction recovered in the membrane s~paration
stage is mixed together with the C2, hydrocarbon ~raction
from the low-temperaturP distillation stage as well as with
the boil-off gas from a liquid methane storage tank down-
stream of the process, an~ the resultant mixture is fed to
Sll
- the adsorption unit as regenerati~g gas.
~ 4 ~
.,.,, . . ... .~ ,: , .-. -.- .
In a further development of the invention, it is pro-
posed to compress in one stage or in multiple stages the
carbon dioxide fraction, recovered in the membrane separat-
ing unit, together with the bo~ o~f gas before mixing with
the C2; hydrocarbon fraction.
In another advantageous embodiment of the process, in
the low-temperature distillati.on, a column is provided to
separate a liquid cut of C2~ hydrocarbons and the residual
carbon dioxide. This liquid cut is vaporized by indirect
heat exchange with the gas mixture from the membrane ~epa-
ration stage, said gas mixture essentially consisting of
methane, C2~ hydrocarbons and nitrogen, and having been
previously cooled by a partial stream of a refrigeration
cycle medium used for the low-temperature distillation
stage.
Figure 1 is a box Plowsheet of the process of U.S.
4,761,167;
Figure 2 is a box flowsheet of a prior art process
employing an amine scrubbing stage;
;: 20 Flgure 3 is a box ~lowsheet of the invention;
Figure 4 is a comprehensive schematic flowsheet of the
:':
invention wherein two interchangeable adsorbers are employed
and certain gas streams are recycled to the adsorbers for
regeneration purposes; and
: Figure 5 is a sch~matic comprehensive flowsheet of the
low temperat:ure distillation stage of Figure 4 with ancil-
lary equipment.
- 5 -
,,1
;i
Figure 1 represents a process as it is described in
U.S. Patent No. 4 761 167. In this case, the feed gas
stream is fed to cryogenic distillation stage A by pipe 1
and in the distillation, the C2+ hydrocarbons as well as a
first carbon dioxide stream are recovered by pipe 5. A
CH4tN2tCOz gas mixture is fed to pressure swing adsorptior
stage B by pipe 2. In it, an adsorptive separation of this
gas mixture provides a C02-rich ~raction, discharged by pipe
6, and a CH4/N2-rich fraction, which is fed by pip2 3 to
nitrogell separation unit C. While the methane product
stream is recovered by pipe 4, the nitro~en fraction is re-
cycled by pipe 7 to pressure swing adsorption stage B and
used therein as regenerating gas for the adsorbers loaded
with carbon dioxide.
Figure 2 shows a combination of an amine scrubbing D to
~emove the carbon dioxide, an adsorption unit E to dry the
resultant C02 depleted feed ~as mixture and a cryogenic
distillatio~ A to separate the C2+ hydrocarbons. The carbon
¦ dioxide separated in the amine scrubbing stage D is dis-
charged by pipe 8, the water recovered in adsorption unit E
by pipe 6 and the C2, ~raction separated in cryogenic
distillation A by pipe 5.
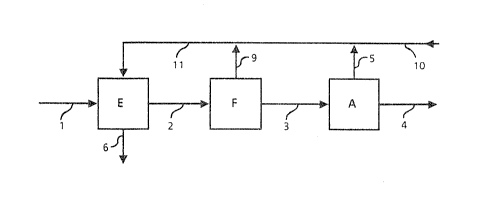
Figure 3 shows the process according to the invention,
comprisin~ an adsorption unit E to dry the feed gas mixture,
a membrane separation unit F to separate carbon dioxide as
~3 well as a low-temperature distillation stage A to separate
- the dried gas stream, partially freed from carbon dioxide,
71
!~ - 6
. .
!~
.:
into a liquid methane product fraction, which i5 discharyed
by pipe 4, as well as in a C2+- and C02-containing ~raction,
which is given o~f by pipe 5. The carbon dioxide fraction
drawn off from membrane separation unit F by pipe 9, to-
gether with the C2~- and C02-rich fraction drawn off from low-
temperature dietillation A by pipe 5 and a boil-off gas
brought in by pipe 10, which comes from a methane tank down-
stream to this process, can be fed by pipe 11 to adsorption
unit E as regenerating gas to regenerate the adsorber loaded
with H20. As the ad~orption material in stage E, it is pre-
~erred to employ molecular sieve (zeolite). As the membrane
material in stage F, it is preferred to employ cellulosic
acetate or a composite material (dimethylsilicone and poly-
ester). Nitrogen in the methane can be removed in the boil-
off gas or by intermediate flashing to a gas/liquid separa-
tor or by a shipping column.
Figure 4 shows an embodiment of the process according
to the invention analogous to Fiqure ~. The drying of the
feed gas mixture from pipe 1 takes place in this case in an
adsorption unit comprising at least two adsorbers E1 and E2.
The dried feed gas mixture is fed by pipe 2 to a membrane
separation unit F, wherein carbon dioxide is separated and
withdrawn by pipe 9~ The resultant gas mixture now essen-
tially consisting of methane, Cz~ hydrocarbons and nitrogen
is fed by pipe 3 to a low-temperature unit A wherein the C2~
hydrocarbons as well as the residual carbon dioxide are sep-
arated by distillation and removed by pipe 5 and the nitro-
~ 7
,, . , , . , ,. . ,, ... . ... ~ . .. - . . ~ -
gen is removed by expansion. The highly pure liquid methane
product stream recovered in khe low-temperature distillation
stage is conveyed by pipe 4 to liquid methane storage tank
S. The boil-off gas escaping from liquid methane storage
tank S is recycled by pipe lOa to the low-temperature unit
A, warmed th~reon to transfer the refrigeration values and
then fed by pipe lOb to comprlessor V, after being admixed
with the CO2-rich process stream in pipe 9 from the membrane
separation unit, After compression, this process stream is
mixed with the Cz-rich ~raction in pipe 5 and fed to adsorb-
ers E1 and E2 by pipe 11 as regenerating gas. The regene-
rating gas loaded with water is discharged from the unit by
pipe 12.
Figure 5 shows a detailed embodiment of the low-tempe-
rature distillation stage of Figure 4. A conventional re-
~rigerant mixture cycle G is used to provide the needed pro-
ces~ refrigeration. The process stream conveyed from mem-
brane separating unit F (fig. 3) by pipe 3 to the low-
temperature distillation is first cooled in heat exchanger
W1 countercurrently to the process streams to be heated.
Before being fed to column K, the resultant cooled proce~s
stream from the membrane separation unit is further cooled
in heat exchanger W4 by a C2-rich liquid stream drawn off
from column K by pipe 20, which in turn is vaporized and is
recycled into rolumn K below the plate holding said liquid
stream. The head of column K is cooled by a partial stream
drawn off in pipe 30 from refrigerant mixture cycle G. At
:. . : . ~ ~
. , , ~ .. : , : : .-: : . : . - : . ., . . . ; , :
the bottom of column K, a c2t hydrocarbon fraction, in which
the residual carbon dioxide is contained~ is drawn off by
pipe 5, heated in heat ex~hanger Wl and then is discharged
from the low-temperature stage. At the head of column K, a
purified methane fraction is recovered, liquefied in heat
exchanger W3 and conveyed by pipe 4 to liquid methane stor-
age tank S. The boil-off gas exiting from this liquid
methane storage tank S is conveyed by pipe lOa back to the
low-temperature unit, heated in the latter in heat exchang
ers W2 and Wl and is then removed by pipe lOh from the low-
temperature unit.
Table 1 contains an exempli~ied material balance re-
latîng to the process represented in Figures 4 and 5. In
this case, the material balance data are calculated at the
points in the process piping to which the respective re~er
énce numbers pertain.
The invention is particularly applicable to the re-
cov2ry of liquid methane from feed gases of the following
composition: ~ by vol~me B.~.P.
CH4 50-97
C~+ 1-15
C02 2-15
N2 0-15
H20saturated
This invention is applic~ble to the production of high-
ly pure liquid methane which i5 defined herein as greater
1 than 99% by volume, preferably greater than 99.5~ by volume.
:f - 9
V 7~ r~
p ~ O o o O O ~ I_
~_
~1,
p
g
O ~ O~ ~ O _
OU~ Ul
o~
o ~ o ..
o o o
~ .
(D
o o r~l ~ ` o
(D .
.~ _ ,o o _ Co 5.
~ _ W oo o ~ Cl~
tD
,_
~ _ oo o ~ o
o~o o ~ W
:: ~n
~;
~o ~
o o ~ _ _
:,,
~n 0 - ~ ~
: - o o o o ~., W
~:
c ~ o ~n ~ ~ o
O O O ~ ~ ~D - ~n
~o o ~ ~ ~ o
o o W ,~, - -
' W _ ~ ~
o o
o
-- 10 --
i
The entire disclosures of all applications, patents,
and publications, cited above, and of corresponding German
Application P 42 37 620.3~ ~iled November 6, 1992, are
hereby incorporated by reference.