Note: Descriptions are shown in the official language in which they were submitted.
21~3~01
Merck Patentgesellschaft
mit beschrankter Haftung
6100 D a r m s t a d t
1,4-Benzodioxane derivative~
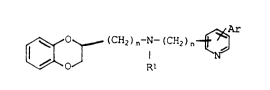
The invention relates to 1,4-benzodioxane deriva-
tives of formula I
~ - ~ Rl I
wherein
Rl is H or A,0 Ar is a phenyl radical which is unsubstituted or mono-
su~stituted or disubstituted by A, F, Cl, Br, I, CN,
OH and/or OA or by a methylenedioxy group,
A is alkyl having 1-6 C atoms, and
m and n are in each case independently of one another 1
or 2,
and to their salts and to their metabolites occurring in
the metabolism.
Similar compounds are disclosed in DE 36 09 142.
The object of the invention was to find novel
compounds capable of being used for the preparation of
drugs.
It has been found that the compounds of formula
I and their biocompatible acid addition salts possess
valuable pharmacolo~ical properties. Thus, in particu-
lar, they are active on the central nervous system,e3pecially as serotonin agonists and antagonists. They
inhibit the binding of tritiated serotonin ligands to
hippocampal receptors (Cossery et al., European J.
Pharmacol. 140 (1987), 143-155). They also modify the
accumulation of DOPA in the corpus striatum and the
accumulation of 5-HTP in the nuclei raphes (Seyfried et
al., European J. Pharmacol. 160 (1989), 31-41). They have
analgesic and hypotensive effect~; thu~, in catheterised,
21 03~01
conscious, spontaneously hypertensive rats (strain:
SHR/Okamoto/NIH-Mo-cHg-Kisslegg; method: q.v. Weeks and
Jones, Proc. Soc. Exptl. ~iol. Med. 104 (1960), 646-648),
the directly measured blood pressure is lowered after
oral administration of the compounds. They are also
useful for prophylaxis and control of the sequelae of
cerebral infarction (Apoplexia cerebri) such as stroke
and cerebral ischaemia.
Compounds of formula I and their biocompatible
acid addition salts can therefore be used as active in-
gredients for anxiolytics, antidepresqants, neuroleptics,
and/or antihypertensives, and also as intermediates for
the preparation of other pharmaceutical active ingre-
dients.
The invention relates to the 1,4-benzodioxane
derivatives of formula I and to their biocompatible acid
addition salts and their metabolites occurring in the
metabolism.
The radical A is alkyl having 1, 2, 3, 4, 5 or
6 C atoms, especially 1 or 2 C atoms, preferably methyl
and also ethyl, n-propyl, isopropyl, n-butyl, isobutyl,
sec-butyl or tert-butyl. OA i9 preferably methoxy and
also ethoxy, n-propoxy, isopropoxy, n-butoxy, isobutoxy,
sec-butoxy or tert-butoxy.
The radical Ar is preferably unsub~tituted phenyl
but can also be monosubstituted or disubstituted phenyl.
If phenyl iR disubstituted, i~ is possible for the
~ubstituents to be identical or different. Preferred
substituents on the phenyl group are F, Cl, methoxy, CN
or methyl. The substituents are in the ortho, meta and/or
para position, disubstituted phenyl radicals preferably
being ortho- and para-substituted. Specifically, Ar is
preferably phenyl, o-, m- or p-trifluoromethylphenyl, o-,
m-, or p-methoxyphenyl, o-, m- or p-fluorophenyl, o-, m-
or p-methylphenyl, o-, m- or p-cyanophenyl or 2,4-dimeth-
oxyphenyl, but also o-, m- or p-ethoxyphenyl, o-, m- or
p-~romophenyl, 2,3-, 2,5-, 2,6-, 3,4- or 3,5-dimethoxy-
phenyl, 2,3- or 3,4-methylenedioxyphenyl.
21Q`~Ol
- 3 -
The parameters m and n are in each case indepen-
dently of one another 1 or 2, preferably in each case 1.
R' is partic~larly preferably methyl, but also
ethyl, propyl, isopropyl, n-butyl, sec-butyl or tert-
butyl.
Accordingly, the invention relates particularly
to those compounds of formula I in which at least one of
said radicals has one of the meanings indicated above,
especially one of the preferred meanings indicated above.
Some preferred groups of compounds can be expressed by
the following partial formulae Ia to Ig, which correspond
to formula I and in which the radicals and parameters not
dsscribed in greater detail are as defined for formula I,
but in which:5 in Ia Ar is p-fluorophenyl and m and n are in each case
l; .
in Ib Ar is phenyl and m and n are in each case 1;
in Ic Ar i8 p-methoxyphenyl and m and n are in each
case 1;0 in Id Ar i8 phenyl and is linked to the 4-position of
the pyridyl radical;
in Ie Ar is phenyl, p-methoxyphenyl or p-fluorophenyl
and is linked to the 5-position of the pyridyl
radical;5 in If Ar i~ phenyl, p-met~oxyphenyl or p-fluorophenyl
and R1 is B;
in Ig Ar is phenyl, p-methoxyphenyl or p-fluorophenyl
and R1 i~ methyl.
The invention further relates to a process for
the preparation of 1,4-benzodioxane derivative~ of
formula I and their salts, characterised in that a
compound of formula II
~ CH2)~X II
21 03'"01
-- 4 --
wherein m is as defined and
X is Cl, Br, I, OH or an OH group functionally modi-
fied to form a reactive group,
i~ reacted with a compound of formula III
Ar
~ (CH2) ~ III
N
wherein
R1, Ar and n are as defined,
or in that a compound of formula IV
~ r (CH2)~--NRIH IV
10 wherein
R' and m are as defined,
is reacted with a compound of formula V
(C~2) ~,X
Ar~ V
N
wherein Ar, n and X are as defined,
or in that a compound which has formula I excPpt that one
or more hydrogen atoms have been replaced by one or more
reducible groups and/or one or more additional C-C and/or
C-N bonds is treated with a reducing agent,
or in that a compound which has formula I except that one
or more hydrogen atoms have been replaced by one or more
solvolysable groups is treated with a solvolysing agent,
and/or in that a secondary amine of formula I (Rl = H) is
optionally converted by alkylation into a tertiary amine
of formula I (Rl = A) and/or in that a group Ar is
converted into another group Ar and/or in that a
21~3~
resulting base of formula I i8 converted into one of its
salts by treatment with an acid.
The compounds of formula I are otherwise prepared
by methods known per se, such as those described in the
literature (e.g. in the standard works such as ~ouben-
Weyl, Methoden der Organischen Chemie (Methods of Organic
Chemistry), Georg-Thieme-Verlag, Stuttgart; Organic Re-
actions, John Wiley & Sons, Inc., New York; German Offen-
legungsschrift 36 09 142), namely under reaction
conditions such as those which are known and suitable for
said reactions. It is also possible to make use of
variants known per se, which are not mentioned in greater
detail here.
If desired, the starting materials for the
claimed process can also be formed in situ in such a way
that they are not isolated from the reaction mixture but
are immediately reacted further to give the compounds of
formula I.
It is possible to obtain a compound of formula I
~0 by reaction of a compound of formula II with a compound
of formula III.
In the 1,4-benzodioxane derivatives of formula
II, X is preferably Cl or Br, but it can also be I, OH or
an OH group functionally modified to form a reactive
group, especially alkylsulfonyloxy having 1-6 C atoms,
e.g. methanesulfonyloxy or arylsulfonyloxy having 6-10 C
atom~, e.g. benzenesulfonyloxy, p-toluenesulfonyloxy,
naphthalene-l- or -2-sulfonyloxy. The paramet~r m is
preferably 1.
In compounds of formula III, R~ is preferably H
or methyl, n i8 preferably 1 and Ar is preferably phenyl
which is unsubstituted or substituted by F, Cl, ~r,
methyl or methoxy.
The compounds of formulae II and III are known in
some cases; the unknown compounds of formulae II and III
can easily be prepared in analogy to the known compounds.
Thus, it is possible, for example, to obtain
compounds of formula II by reduction o~ benzodioxanes
2103~1
-- 6 --
which carry a COOH, COOA, -CH2COOH or -CH2COOA group in
the 2-position.
For the preparation of the compounds of formula
III, for example, the methods known per se can be used
for the preparation of primary and secondary amines.
The reaction of the compounds II and III proceeds
according to methods such as those known from the litera-
ture for the alkylation of amines. The components can be
melted together in the absence of a solvent, in a sealed
tube or an autoclave if necessary. It i~ also possi~le,
however, to react the compounds in the presence of an
inert solvent. Examples of suitable solvents are hydro-
carbons such as benzene, toluene or xylene; ketones such
as acetone or butanone; alcohols such as methanol,
ethanol, isopropanol or n-butanol; ethers such as tetra-
hydrofuran (THF) or dioxane; amides such as dimethyl-
formamide (DMF) or N-methylpyrrolidone; or nitriles such
as acetonitrile, or else, if desired, mixtures of these
solvents with one another or mixtures with water. It can
be favourable to add an acid-binding agent, for example
an alkali metal or alkaline earth metal hydroxide, car-
bonate or bicarbonate or another alkali metal or alkaline
earth metal salt of a weak acid, preferably a potas~ium,
sodium or calcium salt, or to add an organic base such as
triethylamine, dimethylaniline, pyridine or quinoline, or
an excess of the amine component of formula III. The
reaction time i8 between a few minutes and 14 days,
depending on the condition~ used, and the reaction
temperature is between about 0 and 150, normally between
2Q and 130.
It is also possible to obtain a compound of
formula I by reacting a compound of formula IV with a
pyrimidine derivative of formula V.
Some of the compounds of formula IY are known;
the unknown compounds can easily be prepared analogously
to the known compounds. Thus, compounds of formula IV
(R' = H) can easily be prepared by reduction of
corresponding nitriles. It i~ also possible, by reaction
2~03~Ql
-- 7 --
of pyrocatechol or its reactive derivatives with
protected 1 amino-2,3-dihalopropane derivatives and
subsequent removal of the amino protective group, to
obtain compounds of formula IV.
The compounds of formula V can be obtained by
reduction of corresponding pyridinecarboxylic acids,
pyridinecarboxylic acid esters, pyridineacetic acids or
pyridineacetic acid esters.
The reaction of the compounds IV and V as a rule
proceeds by the same methods as are mentioned above for
the reaction of II with III.
Another possibility of preparing compounds of
formula I consists in treating a compound, which basi-
cally correspond~ to formula I, but in which hydrogen
atoms have been replaced by one or more reducible groups
and/or one or more additional C-C and/or C-N bonds, with
a reducing a~ent, preferably at temperatures of between
-80 and +250, in the presence of at least one inert
solvent.
Reducible groups (groups replaceable by hydrogen)
are, in particular, oxygen in a carbonyl group, hydroxyl,
arylsulfonyloxy (e.g. p-toluenesulfonyloxy), N-benzene-
sul~onyl, N-benzyl or 0-benzyl.
In principle, compounds containing only one of
the abovementioned qroups or additional bonds, or com-
pounds containing two or more of the abovementioned
groups or additional bonds adjacent to one another, can
be converted into a compound of formula I by reduction.
This is preferably carried out using nascent hydrogen or
complex metal hydrides or by means of a Wolff-Kishner
reduction or the reductions with hydrogen gas under
transition metal catalysis.
If nascent hydrogen is used as the reducin~
agent, this can be produced e.g. by treating metals with
weak acids or with bases. Thus it is possible e.g. to
use a mixture of zinc with an al~ali metal hydroxide
solution or a mixture of iron with acetic acid. It is
also appropriate to use sodium or another alkali metal in
2~03~1
- 8 -
an alcohol such as ethanol, isopropanol, butanol, amyl or
isoamyl alcohol or phenol. It is also possible to use an
aluminium-nickel alloy in aqueous-alkaline solution,
ethanol being added if necessary. Sodium amalgam or
aluminium amalgam in aqueous-alcoholic or aqueous solu-
tion is also suitable for producing the nascent hydrogen.
The reaction can also be carried out in the heterogeneous
phase, in which case it is convenient to usP an aqueou~
phase and a benzene or toluene phase.
Other reducing agents which can be used to par-
ticul~r advantage are complex metal hydrides such as
LiAlH~, NaBH4, diisobutylaluminium hydride or
NaAl(OCH2CH2OCH3~2H2, and diborane, catalysts such as BF3,
AlCl3 or LiBr being added if desired. Solvents which are
suitable for this purpose are, in particular, ethers such
as diethyl ether, di-n-butyl ether, THF, dioxane, diglyme
or 1,2-dimethoxyethane, and hydrocarbons ~uch a~ benzene.
Solvents which are suitable for a reduction with NaBH~
are primarily alcohols such as methanol or ethanol, as
well as water and aqueous alcohols. Reduction by these
methods is preferably carried out at temperatures of
between -8~ and +150, especially of between about 0 and
about 100.
It is also possible to reduce one or more car-
bonyl groups to CH2 groups according to the Wolff-Kiahner
method, e.g. by treatment with anhydrou~ hydrazine in
absolute ethanol, under pressure, at temperatures of
between about 150 and 250. A sodium alcoholate is ad-
vantageou ly used as the catalyst. The reduction can
also be varied according to the Huang-Minlon method by
carrying out the reaction with hydrazine hydrate in a
high-boiling water-miscible solvent such as diethylene
glycol or triethylene glycol, in the presence of an
alkali such as sodium hydroxide. The reaction mixture is
normally boiled for about 3-4 hours. The water is then
distilled off and the hydrazone formed is decomposed at
temperatures of up to about 200. The Wolff-Kishner
reduction can also be carried out with hydrazine in
2103~Ql
dimethyl sulfoxide at room temperature.
Moreover, it is possible to carry out reductions
by using H2 gas under the catalytic action of transition
metals, such as e~g. Raney Ni or Pd. In this way, e.g.
Cl, Br, I, SH or even OH groups can be replaced by
hydrogen.
Compounds which have formula I except that one or
more H atoms have been replaced by one or more solvolys-
able groups can be solvolysed, especially hydrolysed, to
give the compounds of formula I.
The starting materials for the solvolysis are,
for example, compounds which correspond to formula I,
except that the H atom on the secondary N atom has been
replaced by a solvolysable group. Thus, in particular,
acyl derivatives (which have formula I except that they
contain an acyl group in pla~e of R', preferably an
alkanoyl, alkylsulfonyl or arylsulfonyl group having up
to 10 C atoms in each case, such as methanesulfonyl,
benzenesulfonyl or p-toluenesulfonyl) can be hydrolysed
to give the corresponding compounds of formula I, wherein
R1 = H, e.g. in an acidic or, preferably, neutral or
alkaline medium at temperatures of between O and 200.
Sodium, potassium or calcium hydroxide, sodium or potas-
sium carbonate, or ammonia, i8 conveniently used a~ the
base. The chosen solvents are preferably water; lower
alcohols such as methanol or ethanol; ethers such as THF
or dioxane; sulfones such as tetramethylene sulfone; or
mixtures thereof, especially mixtures containing water.
Hydrolysis can also be carried out simply by treatment
with water alone, especially at the boiling point.
A compound of formula I can furthermore be con-
verted to another compound of formula I by methods known
per se.
Thus, for example, compounds of formula I,
wherein R' = H, can be alkylated by reaction with alkyl
halides. The conditions for reactions of this type are,
as a rule, known and described in the standard works of
the chemical literature (e.g. J. March, Adv. Org. Chem.
2~ 03~01
-- 10 --
3rd Ed., J. Wiley & Sons (1985)~.
In addition, it is possible to convert a radical
Ar into another radical Ar.
Conversely, an OH group can be converted into an
OA group by alkylation methods which are known per se.
Compounds of formula I which contain an OA group
can be converted by ether cleavage to the corresponding
hydroxy derivatives. For example, ethers can be cleaved
by treatment with diisobutylaluminium hydride (DIBAH) or
dimethyl sulfide-boron tribromide complex, e.g. in
toluene, ethers such as THF or dimethyl sulfoxide, or by
fusion with pyridine or aniline hydrohalides, preferably
pyridine hydrochloride at about 150-250.
A base of for~ula I can be converted with an acid
into the corresponding acid addition salt. AcidR which
produce biocompatible salts are suitable for this re-
action. Thus it is possible to use inorganic acids, e.g.
sulfuric acid, hydrohalic acids such as hydrochloric acid
or hydrobromic acid, phosphoric acid3 such as ortho-
phosphoric acid, nitric acid and sulfamic acid, as wellas orqanic acids, i.e. specifically aliphatic, alicyclic,
araliphatic, aromatic or heterocyclic monobasic or poly-
basic carboxylic, sulfonic or sulfuric acids, such as
formic acid, acetic acid, propionic acid, pivalic acid,
diethylacetic acid, malonic acid, succinic acid, pimelic
acid, fumaric acid, maleic acid, lactic acid, tartaric
acid, malic acid, benzoic acid, salicylic acid, 2-phenyl-
propionic acid, citric acid, gluconic acid, ascorbic
acid, nicotinic acid, isonicotinic acid, methanesulfonic
or ethanesulfonic acid, ethanedisulfonic acid, 2- hyd-
roxyethanesulfonic acid, benzenesulfonic acid, p-
toluenesulfonic acid, naphthalenemonosulfonic and naph-
thalenedisulfonic acids and laurylsulfuric acid.
If desired, the free base~ of formula I can be
liberated from their salts by treatment with strong bases
such as sodium or potassium hydroxide or sodium or potas-
sium car~onate.
The compound of formula I can possess one or
21~3601
-- 11 --
more centres of asymmetry. When prepared, they can
therefore be obtained as racemates or else in the
optically active form if optically active starting mate-
rials are used. When synthesised, compounds possessing
S two or more centres of asymmetry are generally obtained
as mixtures of racemates, from which the individual
racemates can be isolated in the pure form, for example
by recrystallisation from inert solvents. If desired,
the racemates obtained can be mechanically or chemically
resolved into their optical antipodes by methods known
per se. Preferably, diastereoisomers are formed from the
racemate by reaction with an optically active resolving
agent. Examples of suitable resolving agents are
optically active acids such as the D and L forms of
tartaric acid, dibenzoyltartaric acid, diacetyltartaric
acid, camphorsulfonic acids, mandelic acid, malic acid or
lactic acid. The different forms of the diastereoisomers
can be resolved in a manner known per se, e.g. by frac-
tional crystallisation, and the optically active com-
pounds of formula I can be liberated from the diaster-
eoisomers in a manner known per se.
The invention further relates to the use of the
compound~ of formula I and their biocompatible salts for
the manufacture of pharmaceutical preparations, espe-
cially by a non-chemical route. For this purpose, they
can be converted into a suitable dosage form together
with at least one excipient or adjunct and, if approp-
riate, in combination with one or more additional active
ingredients.
The invention further relates to compositioIIs,
especially pharmaceutical preparations, containing at
least one compound of formula I and/or one of their bio-
compatible salts. These preparations can be used as drugs
in human or veterinary medicine. Possible excipients are
organic or inorganic substances which are suitable for
enteral (e.g. oral~, parenteral or topical administration
and which do not react with the novel compounds, examples
of such excipients being water, vegetable oils, benzyl
21 336(t~
- 12 -
alcohols, polyethylene glycols, gelatin, carbohydrates
such as lactose or starch, magnesium stearate, talc and
petroleum jelly. Tablets, coated tablets, capsules,
syrups, juices, drops or suppositories are used in
S particular for enteral administration, solutions, prefer-
ably oily or aqueous solutions, as well as suspensions,
emulsions or implants are used for parenteral administra-
tion, and ointments, creams or powders are used for
topical administration. The novel compounds can also be
lyophilised and the resulting lyophilisates used e.g. to
manufacture injectable preparations.
The preparations indicated can be sterilised
and/or can contain adjuncts such as lubricants, preserva-
tives, stabilisers and/or wetting agents, emulsifiers,
salts for influencing the osmotic pressure, buffer sub-
stances, colourants, taste correctors and/or flavourings.
If desired, they can also contain one or more additional
active ingredients, e.g. one or more vitamins.
The compounds of formula I and their biocompat-
ible salts can be used for the therapeutic treatment ofthe human or animal body and for controlling diseases.
They can be used for treating disorders of the central
nervous system, such as tension, depressions and/or
psychoses, and side-effects in the treatment of hyper-
tension (e.g. with a-methyldopa). The compounds can also
be used in endocrinology and gynaecology, e.g. for the
therapeutic treatment of acromegaly, hypogonadism,
secondary amenorrhoea, premenstrual syndrome and un-
desired puerperal lactation, and also for the prophylaxis
and therapy of cerebral disorders (e.g. migraine),
especially in geriatrics in a manner similar to certain
ergot al~aloids and for controlling the sequelae of
cerebral infarction (Apoplexia cerebri), such as stroke
and cerebral ischaemia.
In these treatments, the substances of the inven-
tion are normally administered analogously to known,
commercially available preparations (e.g. brcmocriptine,
dihydroergocornin), preferably in dosages of between
~1~3~1
.
- 13 -
about 0.2 and 500 mg, especially of between 0.2 and 50 mg
per dosage unit. The daily dosage is preferably between
about 0.001 and 10 mg/kg of body ~eight. The low dosages
(about 0.2 to 1 mg per dosage unit; about 0.001 to 0.005
mg/kg of body weight) are particularly suitable for use
as anti-migraine preparations; dosages of between 10 and
50 mg per dosage unit are preferred for the other indi-
cations. However, the particular dose for each indivi-
dual patient depends on a very wide variety of factors,
for example the activity of the particular compound used,
age, body weight, general state of health, sex, diet,
time and method of administration, rate of excretion,
drug combination and ~everity of the particular disease
to which the therapy is applied. Oral administration is
preferred.
In the following Examples, "working-up in con-
ventional manner" means: Water is added if necessary,
extraction is carried out with methylene chloride, the
organic phase is separated off, dried over sodium sulfate
and filtered, the filtrate is evaporated and the residue
i~ purified by chromatography on silica gel and/or by
crystallisation. All temperatures are given in C. The
Rf values were determined by thin layer chromatography on
silica gel. The eluent used is given together with the
respective values.
~xamPle 1
A solution of 0.9 mol of 2-chloromethyl-4-phenyl-
pyridine hydrochloride ("A") [m.p. 174-176; obtainable
by reduction of 4-phenylpyridine-2-carboxylic acid with
LiAl~4 to give 2-hydroxymethyl-4-phenylpyridine (m.p.
183-184) and reaction with SOC12], 1 mol of 2-amino-
methyl-1,4-benzodioxane and 5 ml of triethylamine is
boiled in 100 ml of acetonitrile for 12 hours, and worked
up in the conventional manner to give N-(1,4-benzodioxan-
2-ylmethyl)-N-(4-phenyl-2-pyridyl)amine (dihydro-
chloride), m.p. 237-238.
The following are obtained analogou~ly by reac-
2103601
tion of "A"with N-(1,4-benzodioxan-2-yl)methyl-N-methylamine:
N-(2,4-benzodioxan-2-ylmethyl)-N-(4-phenyl-2-pyrid-
yl)-N-methylamine;
with N-~2-(1,4-benzodioxan-2-yl)ethyl]amine:
N-(4-phenyl-2-pyridylmethyl)-N-[2-(1,4-benzodioxan-
2-yl)ethyl]amine;
with N-[2-(1,4-benzodioxan-2-yl)ethyl]-N-methylamine:
N-(4-phenyl-2-pyridylmethyl)-N-[2-(1,4-benzodioxan-
2-yl)ethyl]-N-methylamine;
with N-(1,4-benzodioxan-2-ylJmethyl-N-ethylamine:
N-(4-phenyl-2-pyridylmethyl)-N-(1,4-benzodioxan-2-
ylmethyl)-N-ethylamine;
with N-[2-(1,4-benzodioxan-2-yl)ethyl]-N-propylamine:
N-(4-phenyl-2-pyridylmethyl)-N-[2-(1,4-benzodioxan-
2-yl)ethyl]-N-propylamine;
with N-[2-(1,4-benzodioxan-2-yl)ethyl]-N-isopropylamine:
N-(4-phenyl-2-pyridylmethyl)-N-[2-(1,4-benzodioxan-
2-yl)ethyl]-N-isopropylamine.
Example 2
A mixture of 2.1 g of 3-p-fluorophenyl-5-amino-
methylpyridine [obtainable by LiAlH4 reduction of
3-p-fluorophenyl-5-cyanopyridine] and 1.6 g of 2-chloro-
methyl-1,4-benzodioxane ("B"), di~solved in 40 ml of
2S water and 40 ml of acetone, i~ boiled for 16 hours and
worked up in the conventional manner. N-(3-p-Fluoro-
phenyl-S-pyridylmethyl)-N-(1,4-benzodioxan-2-ylmethyl)-
amine is obtained; hydrochloride, m.p. 221-222.
The following are obtained analogou~ly by reac-
tion of "B"with 3-p-methoxyphenyl-5-aminomethylpyridine:
N-(3-p-methoxyphenyl-5-pyridylmethyl)-N-(1,4-benzo-
dioxan-2-ylmethyl)amine, dihydrochloride;
m.p. 228-230;
with 3-phenyl-S-aminomethylpyridine:
N-(3-phenyl-5-pyridylmethyl)-N-(1,4-benzodioxan-2-
ylmethyl~amine, dihydrochloride; m.p. 230-233~;
21~3601
- 15 -
with 3-p-cyanophenyl-5-aminomethylpyridine:
N-(3-p-cyanophenyl-5-pyridylmethyl)-N-(1,4-benzo-
dioxan-2-ylmethyl)amine;
with 3-p-chlorophenyl-5-aminomethylpyridine:
N-(3-p-chlorophenyl)-5-pyridylmethyl)-N-(1,4-benzo-
dioxan-2-ylmethyl)amine;
with 3-p-bromophenyl-5-aminomethylpyridine:
N-(3-p-bromophenyl-5-pyridylmethyl)-N-(1,4-benzo-
dioxan-2-ylmethyl)amine;
with 3-(2,4-dimethoxyphenyl)-5-aminomethylpyridine:
N-[3-(2,4-dimethoxyphenyl)-5-pyridylmethyl]-N-(1,4-
benzodioxan-2-yl-methyl)amine;
with 3-(2,4-dichlorophenyl)-5-aminomethylpyridine:
N-[3-(2,4-dichlorophenyl)-5-pyridylmethyl]-N-(1,4-
benzodioxan-2-ylmethyl)amine;
with 3-p-methylphenyl-5-aminomethylpyridine:
N-(3-p-methylphenyl-5-pyridylmethyl)-N-(1,4-benzo-
dioxan-2-ylmethyl)amine;
with 3-m-methylphenyl-5-aminomethylpyridine:
N-(3-m-methylphenyl-5-pyridylmethyl)-N-(1,4-benzo-
dioxan-2-ylmethyl)amine;
with 3-p-ethoxyphenyl-5-aminomethylpyridine:
N-(3-p-ethoxyphenyl-5-pyridylmethyl)-N-(1,4-benzo-
dioxan-2-ylmethyl)amine;
with 3-(3,4-dimethoxyphenyl)-5-aminomethylpyridine:
N-l3-(3,4-dimethoxyphenyl)-5-pyridylmethyl]-N-(1,4-
benzodioxan-2-ylmethyl)amine.
Example 3
A ~olution of N-(3-p-methoxyphenyl-5-pyridyl-
methyl)-N-(1,4-benzodioxan-2-ylmethyl)amine in 40 ml of
THF is added dropwise with stirring in an N2 atmo~phere
at 20 to a suspension of 0.6 g of diisobutylaluminium
hydride (DIBAH) in 20 ml of THF. The mixture is stirred
at 20 for 1 hour, decomposed using dilute sodium hyd-
roxide solution and filtered, and the filtrate is worked
up in the conventional manner to give N-(3-p-hydroxy-
phenyl-5-pyridylmethyl)-N-(1,4-benzodioxan-2-ylmethyl)-
amine.
~l Q3~1
- 16 -
Example 4
Analogously to Example 3, starting from N-[3-
(2,4-dimethoxyphenyl)-5-pyridylmethyl]-N-(1,4-benzo-
dioxan-2-ylmethyl)amine, reaction with 1.1 g of DIBAH and
conventional working-up gives N-t3-(2,4-dihydroxyphenyl~-
5-pyridylmethyl]-N-(1,4-benzodioxan-2-ylmethyl)amine.
Example 5
A solution of 2.8 g of N-(3-p-methoxyphenyl-5-
pyridylmethyl)-N-(1,4-benzodioxan-2-ylmethyl)amine (m.p.
228-230) in 40 ml of dimethylformamide is treated with
0.3 g of NaH with ice-cooling and s~irred for 1 hour.
1.5 ml of ethyl iodide are then added, the mixture is
stirred for a further 2 hours and conventional workin~-up
gives N-(3-p-methoxyphenyl-5-pyridylmethyl)-N-(1,4-
benzodioxan-2-ylmethyl)-N-ethylamine.
The following are obtained analogously by
alkylation of the corresponding secondary amines of
formula I:
N-(4-phenyl-2-pyridylmethyl)-N-(1,4-benzodioxan-2-yl-
methyl)-N-ethylamine;
N-(4-p-methylphenyl-2-pyridylmethyl)-N-(1,4-benzodioxan-
2-ylmethyl)-N-ethylamine;
N-~4-phenyl-2-pyridylmethyl)-N-~2-(1,4-benzodioxan-2-
yl)ethyl]-N-ethylamine;
N-(4-p-methylphenyl-2-pyridylmethyl)-N-[2-(1,4-benzo-
dioxan-2-yl)ethyl]-N-methylamine;
N-(4-m-methoxyphenyl-2-pyridylmethyl)-N-(1,4-benzodioxan-
2-ylmethyl)-N-ethylamine;
N~(4-p-methylphenyl-2-pyridylmethyl)-N-[2-(1,4-benzo-
dioxan-2-yl)ethyl]-N-propylamine;
N-(4-p-methoxyphenyl-2-pyridylmethyl~-N-[2-(1,4-benzo-
dioxan-2-yl)ethyl]-N-isopropylamine;
N-(3-p-methoxyphenyl-5-pyridylmethyl)-N-(1,4-benzodioxan-
2-ylmethyl)-N-isopropylamine;
N-(3-phenyl-5-pyridyl)-methyl)-N-(1,4-benzodioxan-2-
ylmethyl)-N-methylamine;
N-(3-p-cyanophenyl-5-pyridylmethyl)-N-(1,4-benzodioxan-2-
ylmethyl)-N~ethylamine;
21~3~01
- 17 -
N-(3-p-chlorophenyl-5-pyridylmethyl)-N-(1,4-benzodioxan-
2-ylmethyl)-N-ethylamine;
N-(3-p-bromophenyl-5-pyridylmethyl)-N-(1,4-benzodioxan-2-
ylmethyl)-N-methylamine;
N-l3-(2,4-dimethoxyphenyl)-5-pyridylmethyl]-N-~1,4-
benzodioxan-2-ylmethyl)-N-ethylamine;
N-(3-p-fluorophenyl-5-pyridylmethyl)-N-(1,4-benzodioxan-
2-ylmethyl)-N-methylamine, Rf = 0.3 (ethyl acetate);
N-[3-(2,4-dichlorophenyl)-5-pyridylmethyl]-N-(1,4-benzo-
dioxan-2-ylmethyl)-N-propylamine;
N-(3-p-methylphenyl-5-pyridylmethyl)-N-(1,4-benzodioxan-
2-ylmethyl)-N-isopropylamine;
N-(3-m-methylphenyl-5-pyridylmethyl)-N-(1,4-benzodioxan-
2-ylmethyl)-N-isopropylamine;
N-(3-p-ethoxyphenyl-5-pyridylmethyl)-N-(1,4-benzodioxan-
2-ylmethyl)-N-isopropylamine;
N-[3-(3,4-dimethoxyphenyl)-5-pyridylmethyl]-N-(1,4-
benzodioxan-2-ylmethyl)-N-butylamine;
N-[3-(2,4-hydroxyphenyl)-5-pyridylmethyl3-N-(1,4-benzo-
dioxan-2-ylmethyl)-N-butylamine.
Example 6
0.1 mol of (+)-N-(3-p-fluorophenyl-5-pyridyl-
methyl~-N-(1,4-benzodioxan-2-ylmethyl)amine i8 dissolved
in an ethyl acetate/ethanol mixture (400 ml; 3:1) at 50
and 0.05 mol of L-(+)-mand~lic acid, dissolved in 350 ml
of the same solvent mixture, are added. The reaction
mixture is then cooled to room temperature and allowed to
stand for 64 hour~ for cry~tallisation. The resulting
crystals are separated off and recrystallised from ethyl
acetate/ethanol (3:1) for further purification. (+)-N-(3-
p-Fluorophenylpyridylmethyl)-N-(1,4-benzodioxan-2-yl-
methyl)amine L-(+)-mandelate are obtained. Subsequent
treatment with triethylamine gives R-(+)-N-(3-p-fluoro-
phenyl-5-pyridylmethyl)-N-(1,4-benzodioxan-2-ylmethyl)-
amine, Rf 0.29 (acetone); ~a]20 = +33.3o (methanol).
Example 7
The mother liquors obtained in the
crystallisation according to ~xample 6 are concentrated,
2~ 03601
- 18 -
and the concentrate is treated with 25 ml of methanol and
warmed to 50. 0.27 mol of D-(-)-dibenzoyltartaric acid,
dissolved in 40 ml of methanol, are then added and the
mixture i~ cooled to 0C for crystallisation. Separation
of the crystals and additional purification by
recrystallisation from methanol gives (-)-N-(3-p-fluoro-
phenyl)-5-pyridylmethyl)-N-(1,4-benzodioxan-2-ylmethyl-
amine D-~-)-dibenzoyltartrate. Subsequent treatment with
triethylamine gives S-(-)-N-(3-p-fluorophenyl-5-pyridyl-
methyl)-N-(1,4-benzodioxan-2-ylmethyl)amine, Rf 0.29
(acetone); [a]20 = -28-6o (methanol).
The following Examples relate to pharmaceutical
preparations containing 1,4-benzodioxane derivatives of
formula I or their acid addition salts:
Example A: Tablets
A mixture of 1 kg of N-(3-phenyl-5-pyridyl-
methyl)-N-1,4-benzodioxan-2-ylmethyl)amine, 4 kg of
lactose, 1.2 kg of potato starch, 0.2 kg of talc and 0.1
kg of magnesium stearate is compre~sed to tablet~ in
conventional manner so that each tablet contains 10 mg of
active ingredient.
Example B: Coated tablets
Tablets are formed by compre~sion analogously to
Example A and then covered in conventional manner with a
coating of sucrose, potato ~tarch, talc, tragacanth and
colourant.
Example C: Capsules
2 kg of N-(3-p-methoxyphenyl-5-pyridylmethyl)-N-
(1,4-benzodioxan-2-ylmethyl~amine are filled into hard
gelatin capsules in conventional manner so that each
capsule contains 20 mg of the active ingredient.
Example D: Ampoules
A solution of 1 kg of N-(4-phenyl-2-pyridyl-
methyl)-N-(1,4-benzodioxan-2-ylmethyl)amine dihydro-
21~3~01
-- 19 --
chloride in 60 1 of double-distilled water is filtered
under sterile conditions, filled into ampoules and
lyophilised under sterile conditions and the ampoules are
sealed under sterile conditions. Each ampoule contains 10
mg of active ingredient.
Tablets, coated tablets, capsules and ampoules
containinq one or more of the other active ingredients of
formula I and/or their biocompatible acid addition salts
can be obtained analogously.