Note: Descriptions are shown in the official language in which they were submitted.
`` ` 210 3 7 9 RollUS 5 2 / O 1 17
- 1 -
POWDER COATING COMPOSITIONS
Field of the Invention
This invention belongs to the field of pouder
coatings. More particularly, this invention relates to
a novel blend of polyester-based powder coating
compositions.
Back~round of the Invention
Plastic materials used in the manufacture of po~der ;~
coatings are classified broadly as either thermosetting
or thermoplas.ic. In the application of thermoplastic
po-~der coatings, heat is applied to the coating on the
substrate to melt the particles of the po~der coating
and thereby permit the particles to flow together and
fo_m a smooth coating.
Thermosetting coatings, when compared to coatings
derived from thermoplastic compositions, generally are
tousher, more sesistant to solvents and detergents, ha~e
better adhesion to metal substrates and do not soften
~hen exposed to ele~ated temperatures. However, the
curing of thermosetting coatings has created problems in
obtaining coat~ngs which have, in addition to the above-
stated desirable characteristics, good smoothness an~
flexibility. ~oatings prepared from thermosetting
powder compositions, upon the application of heat, mz.
cure or set prior to forming a smooth coating, result_..g
in a relati~ely rough finish referred to as an "oranae
- peel" surface. Such a coating surface or finish lacks
the gloss and luster of coatings typically obtained frc..
thermoplastic compositions. The "orange peel" surface
problem has caused thermosetting coatings to be appliec
from organic solvent systems ~hich are inherently
~ r~r~T~ ~U~T
.. : ... , . ., , :. :: ~ '
21 0~709
.
--- 2 --
undesirable because of the envi~onment~l and sa~ety
proble~s occasioned by the e~aporation of ~he solvent
system. Solv~nt-based co~ing oompositions also su~fer
from the disadvantage of rel~tively poor percent
utilization, i.e., in some modes ot applicatiGn, oniy 60
perc~nt a~ less of the solvent-based coating composition
being applied contacts ~he articls or subst~ate being
coated. Thus, a substantial por~ion of ~olvent-based
coatings can be wasted slnce that portion which does not
lo contact the arLicle or substrate being coated o~viously
cannot be reclaimed. . :
In ad~ition to exhibiting good gloss; impact
strength and resistance to solvents and;chemicals,
coating~ derived from thermo8etting coating compo~itio~s
1~ must p~ssess good to axcellent flexibility. For
example, good flexibility is essential ~or powder
~oating compositions use~ to coat sheet (coil) steel
wh~ch is destined to be for~ed or shaped into articles
used in the ~anu~actu~e o~ various household appli~nces
and automobiles wherein the sheet metil is ~lexed or
bent at var~ouq angles.
Powder co~tings based on aromatic poly4s~ers a~e
well-known but generally suffer fro~ poor weather-
ability,
U. S. Paten~ No. 4, 352,924 . d~s~-~ribes icertain
crystalline polyaste~s use~ul in powcle~ coating
co~positions. W0 8~5320 describes powder coati~g
composi'cio~ co~pri61~d of an a~o2~phous polyester, a
se~icrystalli~e pclyester, a~d a polyisocyanate
crosslinking agent..
Brie~ ~s~ri~tion o~ th~ Draw~a
.
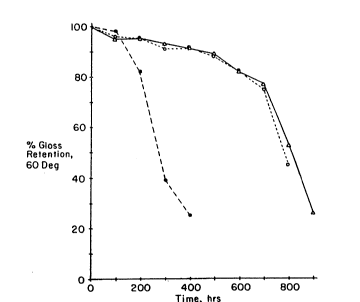
~igure 1 is a gr~p~ o~ QUV weathering o~ t~e powder
coating~ of Comparative Example 1, and Examples 2 and 3,
e S l~ ~Jr
- . . ; ~,
.. . . , . ~ . .. .
. ".~ 2~0370~
- 2~
all of w~.ich are describcd in the ~xpe~imental Secticn
below. "QW" weathering is per~ormed by expo~ing the ~-
coating to high i n~ensity ultraviolet radiation, thereby
simulating perfornl~nce o~ the coating in ~e presence Or
5 sunlight, albeit on an accel~rated basis. The plat
indicated by bold dots is the coating of ComparativQ ; ~ -
'
- . : ,'
. , ' ., :.
' ~
,,
,,'
:: :" ' '' ' ' ~
' :'.' . ~ '
.
: ' ' . , ,
PCTIUS ^,2/Ql 1
2 1 0 3 7 ~ ~ ROIUs 1 5 APR 199
Example 1; the plot indicated by open-circled points is
the coating of Example 2; and the plot isdic ted by
~tri~ngle-points n is the coating of Example 3. Percent
gloss retention is plotted versus time.
Summarv of _he Inventlon
~ he present invention provides a novel blend of
polymers having free hydroxyl groups, which, when
combined ~ith a cross-lin~ing agent And cured, provides
coatings which have superior gloss retention when
exposed to ultraviolet radiation.
Detailed De _ri~tion of the Invention
The present invention provides powder cozting
compositions having good to excellent gloss, impact
strength (toughness), flexibility, and weatherability
superior to that of coatings based on aromatic poly-
esters. The time elapsed while subjecting po~dercoatings to Q~V conditions which cause a 50~ loss in
gloss, is referred to herein as ~G50~. As is evident
from Figure 1, the powder coating compositions of the
present invention are far superior, ~ith regard to gloss
retention, than coatings based on aromatic polyesters.
Thus, the present invention provides thermosetting
po~der coating compositions comprising:
(1) a novel blend of polymers having free hydroxy
groups comprised of:
(a) about 10 to 80 weight percent of an aromatic
polyester havins a glass transition tempera-
ture (Tg) of greater than 40C, a hydroxyl
:
r-r ~-~rr~
,
: - : ~-
~, 210 3 7 9 PR~T¦US 9 2 /AORl 11 '76
- 4 -
~ ' '
number of ~bout 20 to 200 and n~ i~herent
viscosity of about 0.1 to 0.5; and
(b) about 20 to 90 weight percent of poly(tetra-
methylene trans-1,4-cyclohexanedicarboxylate
having a hydroxyl number of about 20 to 200,
and an inherent viscosity of ~bout O.l to 0.5;
and
(2) a cross-linking effective ~mount of 2 cross-linking
~gent.
~ he effectiveness of this novel blend becomes
apparent in the comparison of accelerated 0 W weathering
l; of powder coatings ~o_mulated with (i~) a blend of
polymers containing hydroxy end groups ~nd comprised of
50 weight percent of an aromatic polyester and 50 weight
percent o~ poly(tetramethylene trans-1,4-cyclohexane-
dicarboxylate) as described above, and (2) an aromatic
polymer containing hydroxy groups. Each formulation
contained a blocked polyisocyanate as cross-linking
agent and 1 percent of a conventional ultraviolet light
stabilizer in combination with l percent of a hindered
amine light stabilizer (HALS). During Q W exposure,
formulations of (1) and (2) retained 50~ of 60C gloss
after 810 and 280 hours, respectively. This data
illustrates the superior weathering of coatings
formulated with the novel blend of an aromatic polyester
and poly(tetramethylene trans-1,4-cyclohexane-
dicarboxylate) over coatings formulated with only an
aromatic polyester.
Both the ~romatic polyester and the all-aliphatic
polyester may be produced using well kno~n polycondensa-
tion procedures.
.: . . :
P~T/~S 9 2 J ~1'1 f
2103709 ROIUS 15 APR l9~
Poly(tetr~ethylene trans-1,4-cyclohexane-
dicarboxylate) ~ay be prep~red from 1,4-butaDediol and
the ncid or diester of trans-1,4-cyclohex~ne-
dicarboxylic acid. ~hen the diester is used, excess
glycol is prefe~ably used during ester interchange and
is removed unde~ reduced pressure until the desired
viscosity is obtained.
~ he preferred ~ aliphatic poly(tetramethylene
trar.s-1,4-cyclohexanedicarboxylate) polyester of this
invention has a Tm in the range of about 110 to 150C, a
hvàroxyl number in the range of about 25 to 65, an acid
number of not more than 10 and an inherent ~iscosity of
about C.10 to 0.40. This crystalline polyester
component may ~lso contain a branching agent, such as
trimethylolpropane, to increase the cross-link denslty
of the final coating in a concentration of up to 10 mole
percent based on the total moles of polyol; i.e., up to
10 mole percent of trimethylolpropane residues and from
100 to 90 mole percent of 1,4-butanediol residues. As a
further preferred aspect of this in~ention, up to about
10 mole percent of the 1,4-butanediol residues may be
replaced with diol residues containing from 2 to about
10 carbon atoms. Examples of such glycol residues
include residues of ethylene glycol, propylene glycol,
1,3-propanediol, 2,4-dimethyl-2-ethylhexane-1,3-diol,
2,2-dimethyl-1,3-propanediol, 2-ethyl-2-butyl-1,3-
propanediol, 2-ethyl-2-isobutyl-1,3-propanediol, 1,3-
butanediol, 1,5-pentanediol, 1,6-hexanediol, thio-
diethanol, 1,2-, 1,3- and 1,4-cyclohexanedimethanol,
2,2,4,4-tetramethyl-1,3-cyclobutanediol, 1,4-xylylene-
diol and the like. In this regard 2,2-dimethyl-1,3-
propanediol is especially preferred. When trans-1,4-
cyclohexanedicarboxylic acid is referred to herein, it
is intended to mean at least 70~ trans-isomer.
~r
.
.
.... . ~.. ; .
PCTllJS S2/01176
2103709 RQIUS 15 APR l99~
As noted above, the aromatic polyester ~Component
l(a)) will nec~ssarily have a Tg of gre~ter than 40C.
~he acceptAbly upper limit is generally dictated by the
practicalities of the curing system; in other words, the
upper limit could be as high s 150-180C, however, as
the Tg of the romatic polyester increases, the
performance limitations of the oven used for curing
becomes more critical.
~he preferred aromatic polyester component of the
composition provided this invention has a Tg greater
than 55C, a hydroxyl number in the range of about 25 to
80, an acid number of not more than 15 and ~n inherent
viscosity of about 0.15 to 0.4. The aromatic polyester
component preferably is comprised of (1) diacid residues
of which at least 50 mole percent ~re terephthalic acid
residues, (2~ diol residues of which at lèast 50 mole
percent are derived from 2,2-dimethyl-1,3-propanediol
and (3) up to 10 mole percent, based on the total moles
of (2) and (3), of trimethylolpropane residues. These
preferred aromatic polyesters are commercially avail-
able, e.g., under the names Rucote~ 107 brand resin sold
by Ruco Polymer Corp. and Cargill Resin 3000 sold by
Cargill, Inc.
The relative amounts of the aromatic polyester anc
the all-aliphatic polyester can be varied substantiall-
depending on a number of factors such as the particular
polyesters employed, the cross-linking agent and the
amount thereof being used, the degree of pigment
loading, the properties required of the coatings to be
prepared from the compositions, etc. As provided above,
the compositions of this invention comprise a blend of
about 10 to 80 weight percent of the Aromatic polyester
and 20 to 90 weight percent of the all-~liphatic poly-
ester. The blend of polymers containing free hydroxv
groups provided by this invention preferably is
.. . .. .
21Q37~9 ~û~ls 15 AP~ ~197g~
- 7 -
comprised of about 20 to 75 weight percent of the
~romatic polyester ~nd ~5 to 80 weight percent of the
all-aliphatic polyester.
Suitable curing or cross-linking a~ents for use
with hydroxyl-functional polyesters re well kno~ in
the ~rt. Preferred cross-linking agents include blocked
isocyanates.
The blocked polyisocyanate compounds of the
compositions of this invention ~re kno~ compounds and
can be obtained from commercial sources or may be
prepared according to published procedures. ~pon being
heated to cure coatings of the compositions, the
compounds are unblocked and the isocyanate groups react
with hydroxy groups present on the amorphous polyester
l; and the all aliphatic polyester to cross-link the
polymer chains and thus cure the compositions to form
toush coatings. Examples of the blocked polyisocyanate
cross-linking component include those which are based on
isophorone diisocyanate blocked with E-caprolactam,
commercially a~ailable under the tradenames HUls 1530
and Cargill 2400.
~ he most readily-~ilable, and thus the pre~erred,
blocked polyisocyanate cross-linking agents or compounds
are those commonly referred to as E-caprolactam-blocked
isophorone diisocyanate, e.g., those described in U.S.
Patent Nos. 3,822,240, 4,150,211 and 4,212,962,
incorporated herein by reference. However, the p-oducts
marketed as -caprolactam-blocked isophorone dii,o-
cyanate may consist primarily of the blocked,
difunctional, monomeric isophorone diisocyan~te, i.e., a
mixture of the cis And trans isomers of 3-isocyanato-
methyl-3,5,5-trimethylcyclohexylisocyanate, the
blocked, difunctional dimer thereof, the blocked,
trifunctional trimer thereof or a mixture of the
monomeric, dimeric and/or trimeric forms. For example,
~ ~ S ~
- , - . . . . . .:
..... ~ ,., ,.,,, . .: .
, .. . . . ~ . .
PCTIUS 92/ Ol 1~6
2103709 ~OIUS 1~ APR 1992
- 8 -
the blocked polyisocyanate compound used as the cross-
linking agent may be a mixture consisting prLmarily of
the - caprolactam-blocked, difunctional, monomeri~
isophorone diisocyanate and the E-caprol ctam-blocked,
trifunctional t~imer of isophorone diisocyanate. The
description herein of the cross-linking agents as
~polyisocyanates~ refers to compounds which contain at
least two isocyanato groups which are blocked with,
i.e., reacted with, another compound, e.g.,
E-caprolactam. ~he reaction of the isocya~ato groups
with the blocking compound is reversible at elevated
temperatures, e.g., about 150C, and above, at which
temperature the isocyaDato groups are available to react
with the hydroxyl groups present on the free hydroxy
groups of the polyester to form urethane linkages.
The amount of the blocked diisocyanate cross-
linking compound present in the compositions of this
invention can be varied depending on several factors
such as those mentioned hereinabove relative to the
amount of components (l)~a) and (l)(b) which are
utilized. Typically, the amount of cross-linking
compound which will effectively cross-link the hydroxy-
containing polymers to produce coatings having a good
combination of properties is in the range of about 5 to
30 weight percent, preferably 15 to 25 weight percent,
- based on the total weight of components (l)(a) and
(l)(b) and the cross-linking compound.
The powder coating compositions of this invention
may be prepared from the compositions described herein
by dry-mixing and then melt-blending components (l)(a)
and (l)(b) ~nd the blocked polyisocyanate compound,
alo~g with other additives commonly used in pouder
coatings, and then grinding the solidified blend to a
particle size, e.g., an average particle size in the
range of about 10 to 300 microns, suitable for producir.g
~ ,~,`~ S~
.
: . : , ,
.. . . . .. . . .
~ ~CTIUS 92/ Ol 17
- 2103709 ROIUS 15 At'~
powder coatings~ For example, the ingredients of the
powder coating composition may be dry blended ~d then
melt blended in a ~rabender extruder at 90 to 130C,
granulated and finally ground. The melt blending should
be cArried out at a temperature sufficiently low to
prevent the unbloc~ing of the polyisocyaDate cross-
linking compound and thus voiding premature cross-
linking. To minimize the exposure of the blocked
polyisocyanate to elevated temperatures, components
(l)(a) and (l)(b) may be blended prior to the
iDcorporation therein of the blocked polyisocyanate
compound.
Typi~al of the additi~es which may be present in
the powder coating compositions include benzoin, used to
reduce entrapped air or volatiles, f~ow aids or flow
control agents which aid the formation of a smooth,
glossy surface, catalysts to promote the cross-linking
reactio~ between the isocyanate groups of the c-oss-
linking agent and the hydroxyl groups on the polyme-s,
stabilizers, pigments and dyes. Although it is possible
to cure or cross-link the composition without the use of
a catalyst, it is usually desirable to employ a c2talyst
to aid the cross-linking reaction, e.g., in an amount of
about 0.05 to 2.0 weight percent cross-linking catalyst
based on the total weight of components (l~(~) and
(l)(b) and the cross-linking agent. Suitable catalysts
for promoting the cross-linking include organo-tin
compounds such as dibutyltin dilaurate, dibutyltin
dimaleate, dibutyltin oxide, stannous octanoate and
similar compounds.
~ he powder coating compositions preferably cont~in
a flow aid, al50 referred to as flow control or le~ieling
agents, to enh~nce the surface appearance of cured
coatings of the powder coating compositions. Such flo~
aids typically comprise acrylic polymers and a-e a~ail-
S~S~ S~
2103709 ROIIJS 15 APR ~
- 10 -
able from se~eral suppliers, e.q., Modaflow from
Monsanto Company ~nd Acronal from BASF. Other flow
control agents ~hich may be used iDclude Modarez ~FP
available from Synthron, EX 486 a~ailable from Troy
Chemical, BYK 360P available from BYK Mallinkrodt and
Perenol F-30-P available from HenXel. A specific flo~
aid is an acrylic polymer having a molecular weight of
about 17,000 and contnining 60 mole pexcent 2-ethylhexyl
meth~crylate residues and about 40 mole percent ethyl
acrylate residues. The amount of flow aid present may
preferably be in the range of about 0.5 to 4.0 weight
percent, based on the total weight of components (l)~a)
and (l)(b) and the cross-linking agent.
The powder coating compositions~may be deposited on
various metallic and non-metallic substrates by kno~-n
techniques for powder deposition such as by means of a
po~der gun, by electrostàtic deposition or by deposition
from a fluidized bed. In fluidized bed sintering, a
preheated article is immersed into a suspension of the
powder coating in air. The particle size of the po~der
coating composition normally is in the range of 60 to
300 microns. The powder is maintained in suspension bi
p ssing air through a porous bottom of the fluidized bed
chamber. The articles to be coated are preheated to
about 250 to 400F (about 121 to 205C) and then
brought into contact ~ith the fluidized bed of the
powder coating composition. The contact time depends on
the thickness of the coating that is to be produced and
typically is from l to 12 seconds. The temperature of
the substrate ~eing coated causes the powder to flo~ and
thus f~se together to form a smooth, uniform,
continuous, uncratered coating. The temperature of the
preheated ~rticle also affects cross-linking of the
coating composition and results in the formation of a
tough coating ha~ing a good combination of properties.
5,gs~l~v~ S~
210 3 7 0 9 ~OIus 12~ AP~
- 11 -
Coatings having a thickness between 200 ~nd 500 microns
may be produced by this method.
. The compositions also may be applied using an
electrostatic process wherein a powder coating composi-
tion having a particle size of less than 100 microns,preferably about 15 to 50 microns, i5 bloun by means of
compressed air i~to an applicator in which it is charged
with a voltage of 30 to 100 kV by high-voltage direct
current. The charged particles then are sprayed onto
the grounded article to be coated to which the p~rticles
adhere due to the electrical charge thereof. The coated
article is heated to melt and cure the powde~ particles.
Coatings of 40 to 120 microns thickness may be obtained.
Another method of applying the powder c~ating
lS compositions is the electrostatic fluidized bed process
~hich is a combination of the two methods described
above. For example, annular or partially annular
electrodes are mounted over a fluidized bed so as to
produce an electrostatic charge such as 50 to 100 kV.
The article to be coated, either heated, e.g., 250 to
400F, or cold, is exposed briefly to the fluidized
pouder. The coated article then can be heated to effect
cross-linking if the article was not preheated to a
temperature sufficiently high to cure the coating upon
contact of the coating particles with the article.
The powder coating compositions of this invention
may be used to coat articles of various shapes and sizes
constructed of heat-resistance materials such as glass,
ceramic and various metal materials. The compositions
are especially useful for producing coatings on articles
constructed of metals and metal alloys, p~rticularl-
steel articles.
. . ' " : : ' ' : . , . .:. ~ ' ~
~` 2103709
~:XP~RIMENTAL S ECq ION
.
The com~onents of the compositions according to
this i~ven~ion ~ay be mixed by dry blending in a
Henschel ~ixer, followrd by compounding in a 2SK-30
Extruder (Werne~ & Pfleiderer) at 110-130C, grinding,
and screening to obtain powder ~ith average particle
~izc of ab~ut 3g microns.
The powdered compositions were el~ctostatically
deposited on the substrate by use of a powder gun.
After deposition, the powder was heated to a temperature
sur~icient L~ cause its partic}es to flow and ~use
tosether to form a smooth, uniform s~rface. Coatings
-~ere prepared on 7.62 cm by 22.86 cm of 20-gauge,
1~ polis~ed, cold roll ste~l, the surface of which has been
zinc phospha~ed (Bo~.deri~e 37, The Par~er Company).
The artificia} weatherability of the coatings was
detarmined by exposure of the coated panels in a Cycl~c
Ultraviolet Weathering ~ester ~Q W) with ~13 nm
fluorescent tube~. The te5t condition was 8 hours Or
light at 70~C ~nd 4 hours of condensation at 45OC.
The flexibility of the coating~ was determined in
.acco~dance wi~h ASTM 4145-83 a~ ambient emperature by
bendinq or f~lding a coated p2nel back against itsel~,
using a hydraulic jack pre~surized at 700 kg pe~ square
cen~imeter, until the apex o~ ~he bend is as ~lat as can
be ~easonaDly achieved. Thi~ initial bend is ~e~erred
t~ as OT meaning that there i~ nothing ~z~ro thick-
ne~es) between the bent por~ions o~ the panel. The
bend is examined using a lOX magni~ying gla~s and, i~
~ractures of the coating are observed, the panel is bent
a ~econd tim~ ~lT) to ~or~ a thr~e-lay~r ~andwich. Th-
second bend is inspecte~ for coating ~acture and this
p~ocedur~ i~ repeated, forming 4- J 5-, 6-, etc. layer
sandwiches, until a bend exhibit~ no fractur~ of ~he
,
.
s~st~r~ 5 f~/-
:` . . ` . . . . . .
2103709
-- 13 -- .
. .
coa~ing. The re~ult o~ each ~end tes~ i3 th~ minimum
thickness (min~mu~ T-bend) of the b~nd which does not
give any frac~ur~s o~ the coating. Although the bend
test used is excessively :~;evere ~or most purposes ~or
5 which coated articl2s are used, lt provides a means to
compare the ~lexibilities of different powder coating
compositions.
Impact strength was deter~ined by using a G~dner
Lab~ratory, Inc., ~mpact Tester. A weight is dropped
~ithin a slide ~ube from a specified height to hit a
punch having a 1.59 centi~eter dla~ete~ he~ispherical
nose wh~ch is driven into the front ~coated face) or :~
back of the panel. The highest impact which does not
cracX the coating is recorded in cm-kg, front and
15 rev~rsa .
~ wenty degree and 60 degree gloss was measured
using a gloss ~eter (Gardner Laboratory, Inc.) according
to ASTM D-523.
A11 inherent Viscositih~ ~e~e determin~ ~t ~5C in
a ~60~4~ ky weight) mixture of phenol~tetrachloroethane
at a conc~ntration of o . 5 g.~lOO mL. Acid and hydroxyl
numbers a~e deter~ined by titration and are reported
herein ~s ~g of KOH consumed for each gram of polymQr.
The mslting te~pcratures (T~) are determined by
dif~erential scanning calorimetry (DSC) on the s~ond
heating cycle at ~ scanning rate o~ 2~ C per minute
arter t~e sample h~s been heated to ~elt and guenche~ to
below th~ glass transition temperature of the polymer.
Tg value arè reported as the midpo mt of the tran~ition
0 aIId Tm at peaks ~ transitions .
The p~ncil hardness of a coating is that o~ the
hard~s~ that will not cut into the coating accor~ing to
AST~ 3363-74 (reapproved 1980). The results are
expressed according to th~ ~ollowing ~ca1e: ~so~test)
5 ~gST~
- - . . : : .- . : . . , - ;,:
.. . :. ~ - . . . . ..
~`^` 21037~9
-- 13a
6~3, 5B, 4~, 3~, 2B, B, HB, F, H, 2H, 3H, 4~, 5~, 6H
(~ard~st) .
~;~gS~ S ~
- ~ : i - .. : . . ~ : . -: .............. .
,:
- , . ,, . .
~ 210 3 7 0 9 RO¦US 1 ~ R~R 1792
The conical mandrel is performed by beDding the
panel over 15 seconds using a Gardner L~boratory, Inc.,
conical mandrel of specified size accordiDg to
ASTM-522-85. A pass or fail is recorded.
EXA~PLE 1
This example illustrates the typical procedure for
preparing the all-aliphatic polyesters of this in~en-
tion. A 3000 mL, 3-necked, round bottom fl~sk equipped
with a stirrer, a short distillation column, and an
inlet for nitrogen, was charged with dimethyl cyclo-
hexanedicarboxylate (1259.7 g, 6.29 mol), 1,4-butanediol
(997.5 g, 11.08 mol)~ trimethylolpropane (73.9 g,
0.55 moles) and 10 mL of titanium te~raiso-
propoxide/2-propanol solution (100 ppm Ti). The flask
and contents were heated under nitrogen atmosphere to a
temperature of 170C at which point methanol began to
distill rapidly from the flasX. After the reaction
mixture was heated with stirring ~t this temperature for
about 1 hour, the temperature was increased to 200C for
2 hours, raised to 215C for 4 hours, and then to 235C.
After 3 hours at this temperature, ~ ~acuum of 10 mm o'
mercury was applied over a period of 12 minutes.
Stirring was continued under 10 mm of merrury at 235C
for about 3 hours to produce a low melt ~isrosity,
colorless polymer. The resulting polymer has an
inherent ~iscosity of 0.30, a melting point of 130C,
and a hydroxyl number of 30.
EXA~P~E 2
A powder coating composition was prepared from the
following materials:
~l~rD~ T~lTr r~r~_
`~: 2103709
81~3 ~ Polyester of Example 1;
243.7 g Rucote 107, ~ polyester ~ased prima~ily on
te_ephthaliC acid and 2,2-dimethyl-1,3-
5propanediol;
75.0 g Ca~rolactam-blocked isophorone poly-
lsocyan.ate (~uls 153~);
4.0 g Dibutyltin dilaur~te; .
4.0 g ~enzoin;
6.0 g Modaflow ITI;
.0 g Tinuvin 144; and
4.0 g TinuYin 234.
The above ~aterials ~ere ~lt-blended in an AP~
t~in screw extruder at llO~C, ground in a Bantam ~111 t~
which a s~'ea~ o~ liquid nitrogen is fed and classi~ied
2S through a 170 mesh screen on a KEK ~ent~ifugal sifter.
The finely-divid~d, powd6r coating composition obtai~d
had an avera~e ~article size o~ about 50 microns.
The powder coating compo~itlon prepared in
Example 2 was applied elect~ostatically to one side of
the 7.62 c~ by 22~ a6 c~ par.els described above. ~he
cGating ~as the~ cured (cross-linked) by heating the
coated panels at 177C in an oven for 25 minutes. The
curcd coatinqs are about 50 microns thick.
ThQ coatings o~ the panels had both front and back
impact strengths of >1~4 c~-kg, 20 ~nd 60 gloss value~
8~ a~d ~, respcctively, and a pencil hardness ~ ~B.
The coatad pan~ls passed a 0.318 cm c~nical m~ndrel test
and had a T-bend fl~xibility value o~ 1. After 780
- hours of QUV exposure, the coati~g r~tained 50~ o~ ~he
60~ gloss.~ .
~ ~ $5 T7~ ~ ~ ~It~=1
.. ~ .
I - 2103703
- 16 -
XAMPL~ 3
Using t~e procedure described in Example 2, a
powder coating compo6ition was prepared from ~he
following marerials:
163.7 g Polyester of ~xample l;
163 ~ 7 g Rucote 107, a p<:lyegter describçd in
1 û Examp l e 2;
72. ? g cap~olacta~blocked i~ophorone d~ isocyanate
lS 6. 0 g Dibutylt~n dilaurate;
4. ~ g Benzoin;
6. 0 g Moaaflow III;
Using the procedure o~ Example 2, panels we~e
coated wi~h ~his powder coating comDosition and the
coatings were c~red and evaluated. The coatings had
both front ~nd bac~ L~paCt ~trengths of ~184 cm-kg inch-
pounds and 20~ and 60 glosY values of 72 and 91,
re~pectively, and a pencil hardness of B. The coated
panels passed a 0.3~7 c~ conical ~andrel and had a
T-bend ~lex}bility ~alue or 1. After 810 hours of Q W
expGsure, t~e coati~g retained 50t of the 600 gloss.
CoMpp~Tv~M~
A po~der coating composition was prepared ~rom the
~ollowing materials:
s~g~ s *~ r
. . .
21 03709
al6.60 ~ ~u~ote lQ7, a poly~ste~ described in
Example 2;
la3.40 g Caprolacta~-blocked isophorone
~iis~cyanate (Huls 1530);
o.ao g Dibutyl~in dilaurate;
o.oo g 3enzoin;
. 10
15.00 g Mod~flow III;
400.00 g ~itanium dioxide;
10.00 g Tinuvin lC4
' 10.00 g T~nuvi~ 2
Using the procedure of ~xample 2, panels w~re
coat~d with this powder coating composition and the
coatings wa~e cured and eva1uated. The ooatings had
both front and back impa~t strengths o~ ~184 cm-kg and
200 and 60 gloss values of 85 and 9S, respecti~ely, and
j~. a pencil hardness of H. The c~ated panels passe~ ~
O.317 c~ conical ~,andrel and had a T-bend flexibility
~; val~e of 6. Af~er 280 ~ours of Q W exposure, the
;~j. coatin~ retained 50% of the 50 gloss.
Thus, the coatin~s of Examples 2 and ~ p~ssess a G~o
of 780 hours and 810 hours, respective1y. The coating
of Compa~ative Exampl~ 1 pos~csses a G~o Of 280 hours.
:.'
,
-
. ,
,. .. . .
~: ,
.. '~'' ' ' ,
:
:~
.
: .
: "- , . . . : ~
. .
. . ~ . ~ . .
'; ~ ~ : :