Note: Descriptions are shown in the official language in which they were submitted.
WO 93tl2262 PCI`/GB92~02279
210~Q12
TITANIUM CONTAINING MAGNESIUM ALLOY PRODUCED BY VAPOUR QUENCHING
This invention relates to alloys which are based upon magnesium
and contain titanium, which are produced by the technique of vapour
quenching. IThese alloys yield improved resistance to corrosion in
comparison with existing alloys of magnesium.
Magnesium is the lightest of the structural metals and hss
significant potential utility for aerospace applications, but
magnesium alloys have somewhat fallen out of favour for use in such
applications, partly because of poor compression properties, but
principally because of their poor corrosion resistance. Conventional
magnesium alloys (that is those produced by ingot metallurgy methods)
have generally poor corrosion resistance due primarily to the
water-solubility of the Mg(0~)2 film which forms on the alloy in damp
envlronments. This problem of surface corrosion is exacerbated by
electrochemical effects as magnesium has a large negative electrode
potential. In commercial purity alloys the presence of particles
containing common impurity elements such as iron, copper and nickel
which have a less negative electrode potential leads to a
susceptibility to pitting and general corrosion. Magnesium alloys are
also subject to electrochemical corrosion when joined to other
structural metals as most have a significantly different electrode
potential.
Major improvements in the corrosion resistance of early magnesium
alloys were obtained by the addition of small quantities of manganese
which reduced the damaging effects of iron, the main impurity, and
certain other impurities by combining these within relatively benign
intermetallic compounds. More recently further improvements in
corrosion resistance have been achieved by reducing the number of
particles present in the alloys through improvements in alloy purity.
In addition, a magnesium alloy has been recently developed which is
produced by a rapid solidification method to a composition by weight
of Mg(base) - 5%Al - 5~Zn - 5%Nd and is claimed to give corrosion
W O 93/12262 ~ O 12 PCT~GB92/02279
properties which are superior to any other currently available wrought
magnesium alloy. The weight loss of this alloy in a saline corrosion
test is comparable to the widely used 2000 series aluminium alloys,
but the alloy does not reveal any redurtion in electrode potential in
comparison to magnesium so it is still susceptible to electrochemical
corrosion.
Some of the alloying ingredients utilised in alloys other than
magnesium alloys have minimal or no solubility within magnesium in the
melt because of the low boiling point of magnesium and the binary
phase diagrams of magnesium with these materials are unknown.
USA patent 4264362 discloses a prior magnesium - titanium alloy
produced in the solid state by a mechanical alloying process. In
contrast to the alloys of this invention, the alloys disclosed in that
specification were alloys in which neither of the two elements was
present in solid solution within the other (the two being in the state
of a mechanical mixture of the elements) and the alloy product was a
supercorroding material intended to react quickly with sea water to
provide exothermic heating.
GB patent 1,382,970 discloses a magnesium-based corrosion
resistant ~lloy additionally comprising aluminium, zinc, manganese and
titanium as well as minute amounts of various doping agents. The
alloys disclosed in this document are produced by conventional ingot
metallurgy and hence those alloys containing titanium do not have the
titanium constituent in solid solution.
~B patent 1,011,585 discloses corrosion resistant magnesium alloys
incorporating, in one example, up to 3% titanium silicide.
US patent 5,024,813 discloses magnesium-titanium alloys produced
by a powder-metallurgical route from a mixtue of magnesium, titanium
and titanium hydride powders. The compositions disclosed are very
broad, ranging from 0.04 to 99.96 wt% of titanium. The sintered
products are not fully dense and therefore doubts remain concerning
the structural properties of these alloys.
W O 93/12262 PCT/GB92/02279
3 2 1 0 ~ ~ 1 2
It is therefore an object of this invention to provide a magnesium
alloy which provides an improvement in corrosion resistance beyond the
level available in current commercial alloys. To achieve this a
reduction in both susceptibility to weight loss in saline environments
and to electrochemical corrosion (by reduction in its electrode
potential) is desirable.
Vapour quenching is a known physical vapour deposition technique
which is used primarily for the production of metal films or coatings
but is also known as a route to the production of alloys having
metastable supersaturated solid solutions on a scale suitable for
quite significant structural applications. This invention is
concerned with the latter application of vapour quenching technology.
The process is performed by evaporating alloy constituents from
individual or combined sources to produce a flux of vapours, and
causing these vapours to be condensed upon a collector which is
controlled in temperature to a value which causes extremely rapid
quenching of the impinging vapours. All this takes place within a
vacuum chamber. A general description of the vapour quenching process
for the production of alloys is given in two articles by Bickerdike et
a~ in the International Journal of Rapid Solidification, 1985, volume
1 pp 305-325; and 1986, volume 2, pp 001-019. The collector deposit
obtained by vapour quenching can be utilised in various product forms.
It can be taken from the collector and processed intact e.g. by
forging, rolling, or hot pressing to yield a monolithic product or it
can be comminuted to particulate form and subsequently canned and
consolidated by hot isostatic pressing or extrusion.
The invention claimed herein is a magnesium based alloy produced
by vapour quenching which comprises 0.5X to 47% of titanium by weight,
wherein the titanium is substantially held in solid solution in the
alloy as deposited.
We have been able to produce magnesium alloys incorporating a
variety of such ingredients using a vapour deposition process by
evaporating the magnesium base and the alloying ingredients from
separate sources and combining the vapour streams to yield an alloyed
W O 93/12262 P ~ /GB92/02279
2 1 0 ~ 0 1 ~ 4
deposit on the collector. We have found that msgnesium - titanium
alloys produced by this method are capable of yielding a particularly
good resistance to corrosion.
Preferably the alloys have a titanium content in the range 5 to
40% by weight.
We have demonstrated a marked improvement in corrosion resistance
in these alloys compared to prior art materials and compared to
alternative research materials with different alloying ingredients
such as aluminium. chromium and silicon.
The magnesium alloy may contain. in addition to the titanium,
other ingredients such as those used in prior art magnesium alloys
within the following weight limits:
manganese up to 6X; aluminium up to 13%; zinc up to 7X;
zirconium up to 5%; neodymium up to 6%; commercial mixed rare earths
up to 5% yttrium up to 6%; silver up to 3%; thorium up to 5%;
lithium up to 10%; and silicon up to 2%.
Preferably the titanium content of the alloy does not exceed 40Z
in alloys intended for du~ies where low density is important for at
higher titanium contents the alloy density approaches that obtainable
from other alloys such as aluminium-lithium alloys. But it should be
noted that the resistance to corrosion and the electrode potential
improves with increasing titanium content SG a high titanium content
might be preferred for this reason.
A most preferred range of titanium in the alloy is 15 to 28X.
Practical ternary alloys might include titanium in the aforementioned
preferred range with either approximately 5% aluminium (to yield a
physically stronger magnesium based material still having good
corrosion resistance) or else approximately 1% silicon (to increase
corrosion resistance still further).
W O 93/12262 PCT/GB92/02279
2 1 a ~ ~ 1 2
Alloys within the scope of the claims have demonstrated thermal
stability ~as ascertained by differential scanning calorimetry) up to
200-C and can be processed from comminuted particulate form by first
can~ing the particulate and then extruding or hot isostatic pressing
this.
The invention is further described below by reference to examples
of materials made to varying compositions by two alternative vapour
quenching methods and by reference to the drawings, of which:
0 Figure 1 is a schematic cross sectional view of one form of small
scale evaporation equipment;
Figure 2 is a schematic cross sectional view of a second form of
evaporation eguipment;
Figure 3 is a differential scanning calorimetry plot showing the
thermal stability for a test sample of AS32 magnesium-
titanium alloy heated over a temperature range of 50 to 500-C
at a rate of 10-/minute;
Figure 4 is a plot showing the corrosion resistance of heat treated
sample of the magnesium-titanium alloy, and0 Figure 5 is a plot comparing the composition and density for the
magnesium-titanium alloy.
Those alloy examples which follow and are designated with the
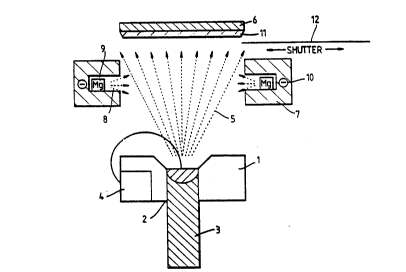
prefix "VM" have been produced using the apparatus depicted in
Figure 1. The apparatus has separate evaporators for the magnesium
and titanium charges. The evaporator for the titanium charge
comprises a water-cooled copper crucible 1 with an underside opening 2
through which is fed a rod charge of titanium 3 and there is an
electron beam gun 4 which is beam steered so that the electrons
impinge upon the topmost end of the rod charge 3 to cause this to be
melted locally. A stream of titanium vapour 5 issues from the melt
pool in an upward direction towards a collector 6 and the flux of
vapours is controlled by adjustment to the power setting of the
electron beam gun 4.
W O 93/12262 PCrtGB92/02279
210~Qi2 6
The evaporator for the magnesium base constituent of the alloy is
a heated block 7 of metal of a generally "U" shaped configuration
which is located above the level of the titanium source crucible l and
is of a size and is positioned such that it encompasses the rising
flux of titanium vapour on three sides thereof. Block 7 has an
inwardly directed slot 8 within which solid charge pieces 9 of
magnesium are placed and the block 7 is heated by means of a radiant
heater lO, extending through the mass of the block, to a temperature
at which magnesium vapour issues forth from the charge pieces 9 by
sublimation. These vapours issue forth laterally through the slot
opening to converge upon the rising flux of titanium vapour 5 and
vapqur mixing takes place by mingling of the vapour streams and by
atomic collisions. Some of the intermixed vapours rise upwards to
condense upon the collector 6 to yield a deposit ll.
The collector is heated to and maintained at a suitable
temperature to ensure that the impinging vapours are quenched to
produce an appropriate microstructure in the alloy deposit ll.
Between the collector plate 6 and the sources of vapours there is a
~0 shutter 12, which is closed in the warm up period of operation of the
equipment and opened when the equipment stabilises. This is a
safeguard measure intended to avoid unwanted collection of deposits
having the wrong compositlon.
In the use of the above-described apparatus in the production of
the magnesium-titanium alloys exemplified below, the apparatus was
controlled to the following parameters:
collector temperature 125 to 200-C
electron beam gun power 2-3kW
magnesium source temperature approximately 550C
vacuum chamber pressure around 5 x lO-; torr
Variation of composition is achieved through alteration of the
respective rates of generation of the titanium and magnesium vapours.
The electron beam gun power is ~aried within the range given above to
increase or decrease the fiux of titanium vapours and the temperature
W O 93/l2262 P ~ /GB92/02279
~ 7 210~12
to which the magnesium source is heated is varied (but remaining below
the melting temperature) to incresse or decrease the flux of magnesium
vapour. Simultaneous manipulation of both these provides control over
the alloy deposition rate.
Using different sources for the two separate ingredients as
described above overcomes the problems of melt insolubility of one
ingredient within the other and overcomes also problems which might
otherwise be caused by gross differences between the vapour pressures
exhibited by different ingredients. Ternary and more complicated
alloys can be produced by , instead of having the rod charge
comprising just titanium, using a pre-alloyed rod sour~-e containing
both the titanium and the other ingredients, except for the magnesium
which is provided for as heated blocks as described before.
The remaining alloys of the examples which follow were produced
using the equipment depicted in Figure 2 and these alloy examples are
identified with a prefix of "AS". More detailed information regarding
th~s form of equipment is given in UK patent application GB 2240852.
This second form of equipment comprises an inner evaporation
crucible 31 surrounded by a another evaporation crucible 32. A charge
33 of the titanium is con~ained within the crucible 31. This crucible
is open topped and the charge of titanium within it is heated by means
25 of an electron beam 37. This electron beam 37 is focussed and steered
by means of a magnet 38 to impinge upon the uppermost surface of the
charge 33 to heat it to a temperature at which an appreciable flux of
titanium vapours escapes from the charge. The other evaporation
crucible 32 holds a charge 34 of magnesium within it and this charge
is heated by a radiant heater 39. Crucible 32 has a lid 4O and there
are nozzles 41 present in the lid through which a flux of magnesium
vapours issues forth. The two crucibles are placed as depicted (one
within the other) beneath a collector 35. The nozzles 41 are so
directed as to cause the individual streams of magnesium vapours to
35 converge towards each other on paths which intersect the direct path
between the other evaporator 31 and the collector 35 so that vapour
mixing between the two species of vapours occurs before either species
W O 93/l2262 PCT/GB92/02279
210~12 8 `-
lands upon the collector. A deposit 36 of alloy is built up on the
lower surface of the collector 35. The lid 40 is heated by a separate
radiant heater 42 to a higher temperature than that of the magnesium
charge. Moreover the nozzles are of re-entrant form as depicted in
the figure and by this combination o~ re-entrant form and higher
temperature, condensation within or upon the nozzle of magnesium
vapours is minimised. The nozzles 41 serve the dual function of
directing the streams of magnesium vapours and choking the volume
flow-rate somewhat allowing the magnesium charge to be superheated to
yield sufficient vapour pressure to disperse surface oxide
contaminants from the surface of the melt without saturating the
deposit with an excess of magnesium. In the deposition of the claimed
magnesium-titanium alloys by this second form of equipment the
evaporator 32 is charged with pieces of magnesium and heated to
produce a melt of metal at a temperature of approximately 750-C. The
temperature of the titanium charge 33 within evaporator 31 is not
controlled directly, instead control of titanium evaporation rate and
hence of alloy deposit composition is achieved by variation of
electron beam power up to a maximum power of 20 kW. Ternary and more
complex alloys can be produced by this second equipment by using a
different form of inner crucible 31 accepting an under-fed rod source
in the manner of the first type of equipment, and using a pre-alloyed
titanium alloy rod charge of appropriate composition.
Vapour-quenched magnesium-titanium binary alloys to the following
compositions have been produced by means of the two equipments
described above. All compositions are given in proportions by weight.
Alloy designation Composition
VM49 Mg base - 9.5X Ti
VM46 Mg base - 21Z Ti
VM47 Mg base - 26.5% Ti
VM48 Mg base - 37% Ti
VM44 Mg base - 47% Ti
ASl9 Mg base - 1.5%Ti
ASZ0 Mg base - Z.0%Ti
AS18 Mg base - 7.4XTi
AS21 Mg base - 8.4ZTi
AS23 Mg base - 15.3ZTi
AS22 Mg base - 22.0XTi
AS14 Mg base - 27.5ZTi
W O 93/12262 PCT/GB92/02279
9 210~1 2
Tensile tests have been performed on specimens prepared from
certain of the alloys. The alloy deposit was first removed from the
collector then comminuted to particles, canned then hot isostatically
pressed and subsequently extruded at 180-C. Tensile properties are as
5 rOllOws:
.... .. ...
Alloy 0.2% Tensileelongation Reduction
proof stress strength(~) in area
(MPa) (MPa) (%~
AS 21 154 226 25 31
AS 23 212 283 3.4 10
AS 22 243 317 1.6 2.5
Corrosion resistance tests have been performed on various examples
of the claimed alloy and also a comparison specimen of puré magnesium
prepared by the sflme vapour quenching route. These materials were all
tested in the as-deposited condition. Some other comparison materials
were tested also in their normal commercially avsilable form. These
additional comparison materials are listed below: WE 43 (nominal
composition: Mg base - 4%Y - 3%Nd ~ other rare earths - 0.5ZZr);
AZ9lE (nominal composition: Mg base - 9%Al - 0.5%Zr - 0.3ZMn); and
EA55RS a rapid solidification route alloy (nominal composition: Mg
base - 5XAl - 5XZn - 5%Nd). The corrosion resistance tests comprised
a total immersion of standard coupons of the material in 600mM/l
sodium chloride solution for a period of 7 days followed by cleaning
(in accordance with ASTM Gl-81) to remove corrosion products and
weighing to determine weight change. A rate of corrosion in terms of
weight loss per day was calculated (mg/dm2 per day) and from this a
corrosion rate expressed in terms of millimetres per year -
representative of the rate of corrosion penetration - was derived
using the following expression:
~orrosion rate = corrosion rate x 0.0365/density of alloy
(mm/year) (mg/dm2 per day)
W O 93/12262 PCT/G W 2/02279
2~ ~40~2 lo
Results of the corrosion resistance tests are given below:
Alloy Corrosion rate (mm/year)
_
As 19 0.30
AS 20 0.33
AS 18 weight gain~
AS 22 0.03
AS 14 weight gain
VM 44 0.005
pure magnesium 0.49
WE43 (commercial corrosion 0.42
resistant Mg alloy)
AZ9lE ( ditto ) 0.24
EA55RS ( ditto ) 0.14
NOTES
~ weight gain indicates that corrosion products are stable.
2 AZ9lE relies for its corrosion resistance upon its high purity
and this low value of corrosion rate may not be representative of
resistance to corrosion in practical situations where contact with
contaminants cannot be avoided.
- The other aspect to corrosion resistance is the electrode
potential exhibited by the material. This value has been measured for
the alloy examPles listed below. The values of electrode potential
given represent the open circuit potentisl (relative to a standard
saturated calomel electrode) exhibited by the alloys in a 600mM/l
solution of sodium chloride after 30 minutes immersion therein.
Material ¦ Open-circuit potential
_
pure Mg -1675mV
VM49 -1378mV
VM46 -1360mV
VM47 -1354mV
VM44 -1301mV
_
W O 93/12262 21~ ~ O 12 PCTJGB92/02279
The experimental results documented above indicate that alloys
containing titanium even at low levels such as 1.5% produce 8 useful
tegree of corrosion resistance compared to both pure magnesium and
prior art alloy WE43. At higher levels of titanium, such as 7.4%
plus, the alloys demonstrate a marked improvement in corossion
resistance compared with the best comparative materials AZ9lE and
EA55RS. Significant reductions in the high negative electrode
potential of pure magnesium are achieved with titanium levels as low
as 8 to 11%. The strength and ductility are reasonable for binary
magnesium based alloys. Additions of Zn (up to 2X) or Al (up to loX)
or Si (up to 2X) can provide significant strengthening with little
adverse effect on predicted corrosion resistance. Also Zr will give a
useful grain refinement effect with consequential strengthening or
toughening and no significant predicted adverse effect on corrosion
resistance. Mn will be expected to yield some increase in corrosion
resistance but no significant increase in strength.
The thermal stability of the alloys is important because most
applications require use at temperatures significantly above room
temperature and it is important that there is no degradation in
mechanical properties and corrosion resistance. An early indication
of the thermal stability of these alloys can be obtained from
differential scanning calorimetry (DSC). DSC of these magnesium
titanium alloys indicates that their short term stability extends to
at least 230-C as is shown in Figure 3.
Corrosion tests on a magnesium - 42.6X titanium alloy which had
been heat treated for one hour at temperatures in the range 140 -
240-C show that there is no detrimental effect on corrosion resistance
as shown in the table below and in Figure 4.
W O 93/12262 P ~ /GB92/02279
2 1~
Corrosion test results for heat treated Mg - 42.6wtX Ti alloy.
Heat Treatment Corrosion Rate
1 hour at mm/year
as deposited 0.315
140' 0.59
160- 0.32
200- 0.33
22~- 0.35
240- 0.35
pure vapour 0.59
deposited magnesium
Maintaining the low density of magnesium alloys is important for -
aerospace applications. It is essential that the levels of titanium
required to reduce the corrosion rate oi the alloys do not increase
the density to unacceptable levels. Analysis shows a maximum value of
around 2.1 g/cm3 should be an objective for these alloys.
Measurements of the density of the deposited alloys, when compared
with the accepted value for pure magnesium and values calculated from
lattice parameter measurement, suggest that there is some porosity but
that alloys containing less than around 28 wtX. of titanium should
have good specific properties. This is shown in the table below and
also in Figure 5. The corrosion tests results indicate that an upper
limit of 28% titanium should not limit the development of a good
corrosion resistant alloy.
W O 93/12262 P ~ /G892/02279
13 ;~ 2
Measured and calculated relative densities for Mg-Ti alloys.
__............ . .
~omposition Measured Relative CPlculated Relative
wtZ Ti Density Density
. . _
0 1.68 1.74
8.4 1.77 .
8.4 1.82
11 1.89
15-3 1.81
18 3 1.91 1.95
21 1 92
243-7 12 93 2.13
27.8 1.99
31.2 . 1.94 .
42.6 2.44