Note: Descriptions are shown in the official language in which they were submitted.
210~73~
.,
"
PROCESS FOR ~EIGH DCTRACTION OF ZINC FROM
ZINC F13RP IT}3 S
.,
This invention relates to an improved process
for a high extraction of zinc from zinc ferrites and
efficient precipitation of ferric iron as jarosite.
~AC~GROUND OF THE INV~NTIO~
In the conventional roast-leach-electrowin route
for the production of zinc, zinc oxide contained in the
calcine (roasted concentrate) is dissolved in a neutral
leaching stage, leaving undissolved zinc ferrites in the
leach residue. Ferrites dissolution for further zinc
recovery requires high temperature and acid concentration
in a subseguent stage of the process. Iron, which also
dissolves from the zinc ferrites, is precipitated for
disposal through one of the following industrial
processes:
, ,, ~ -
~"-~
:, ,: - ,: . . :
210~73~
- Jarosite
- Conversion (jarosite)
- Goethite
- Hematite
Only the Jarosite and Conversion processes
produce a final jarosite residue; therefore the Goethite
and Hematite processes are not considered in the present
patent application.
In both the Jarosite and Conversion processes,
iron is precipitated at a high temperature (95-105C) as
jarosite. In the Jarosite process, the conventional
practice consists of dissolving the zinc ferrites in a
high-acid-leach and neutralizing with calcine in a
following stage to precipitate iron. This process is well
known and documented and many different fl~wsheets using
jarosite precipitation have been developed in the zinc
industry. The process generally suffers from losses of
zinc undissolved from the calcine added to the iron
precipitation stage and therefore requires additional
steps such as a jarosite acid wash to increase zinc
recovery. In the Conversion process such as disclosed in
Canadian patent No. 1,217,638 granted February 10, 1987,
zinc extraction and iron precipitation take place
simultaneously without the addition of a neutralizing
agent. The major drawback of this process is the limited
initial acid concentration (35-45 g/l) which can be
210~73~ ~
tolerated to achieve efficient iron precipitation. The
leaching rate of zinc ferrites may be increased by
increasing the acid concentration of the solution.
However, increasing the acid concentration also increases
the soluble iron concentration in the final solution.
SnMMaRY OF TH~ INVENTION
In the present invention, it has been found that
high overall zinc extractions (>99~) and low iron
concentration in the final solution can be achieved
through a new conversion-type process. The so-called High
Acid Conversion (HAC) process consists of leaching calcine
containing zinc oxide and zinc ferrite with sulphuric acid
in a neutral leach stage followed by a low-acid-leach
stage with a first solid-liquid separation stage between
the neutral and low acid leach stages to dissolve zinc
oxide while leaving the zinc ferrite substantially
undissolved, separating a ferritic residue in a second
solid-liquid separation stage following the low acid leach
stage, simultaneously dissolving the ferritic residue at
an initial acid concentration of 50-60 g/l and
precipitating iron as jarosite at a temperature of 90C up
to boiling by the addition of ammonia or other alkali
ions, followed by separation of the jarosite residue in a
third solid-liquid separation stage. The overflow of the
third solid-liquid separation stage is neutralized to a pH
of about 1.4-1.8 with neutral leach underflow and/or
", ., ~ i .
~.,-, . ~ , .
~10~7~
calcine to further precipitate iron as jarosite and the
neutralized solution is recycled to the low-acid~leach
stage. It was found that a temperature of 75C in the
neutralization step was sufficient to achieve effective
precipitation of iron as jarosite.
The underflow from the second solid-liquid
separation stage can be washed by counter-current
decantation and/or filtration prior to solids disposal.
' ~ The zinc ferrite leaching is preferably
continued for a period of about five to seven hours with
recycled jarosite seed. The jarosite residue is separated
after such period of about six to seven hours.
The neutralization to pH 1.8 is preferably done
during the first hour of retention in the
neutralization/precipitation stage.
SHORT D~SQIPTION OF THI~ DRAWINS:8
The invention will now be disclosed, by way of
example, with reference to flowsheets illustrated in the
accompanying drawings in which:
¦ 20 Figure 1 is a flowsheet of a conventional leach-
¦ jarosite-process;
Figure 2 is a flowsheet of a conventional leach-
conversion process; and
Figure 3 is a flowsheet of the new high-acid
conversion process.
~ " .
~1047~
D~TAIL D~SCRIP~ION OF TH~ INV~N~ION
In the conventional leach-jarosite process
illustrated in Figure 1, calcine containing zinc oxide and
zinc ferrite is leached with spent electrol~te in a so-
called neutral leach stage 10 to dissolve zinc oxide whileleaving the zinc ferrite substantially undissolved. The
zinc ferrite residue is separated in a solid-liquid
separation stage 12. The overflow from the solid-liquid
separation stage 12 is an impure zinc solution which is
purified in subsequent stages and sent to electrolysis for
zinc recovery. The underflow from the solid-liquid
separation stage 12 is a zinc ferrite residue which is fed
to a so-called high acid leach stage 14 where the zinc
ferrite is dissolved with spent electrolyte and additional
sulphuric acid. The solution from ~he high acid leach
stage 14 is separated in a second solid-liquid separation
stage 16 and neutralized with calcine in a pre-
neutralization stage 18. The neutralized zinc sulphate
solution is separated in a third solid-liquid separation
stage 20. The underflow from the solid-liquid separation
stage 20 is recycled to the high acid leach stage while
the overflow is contacted with calcine and alkali ions
(ammonium, sodium or potassium) in a jarosite
precipitation stage 22. The zinc contained in the zinc
oxide is transformed to sulphates while zinc ferrites
remain undissolved and the soluble iron is precipitated as
~ 21~47~
jarosite. The content of the jarosite precipitation stage
is passed through a fourth solid-liguid separation stage
24. The overflow from the solid-liguid separation stage
is recycled to neutral leach stage 10 while the underflow
; 5is subjected to a jarosite acid wash stage 26 to increase
zinc recovery. The output of the jarosite wash stage is
subjected to a fifth solid-liquid separation stage 28.
The overflow from the solid-liquid separation stage Z8 is
recycled to jarosite precipitation while the jarosite
underflow is discarded.
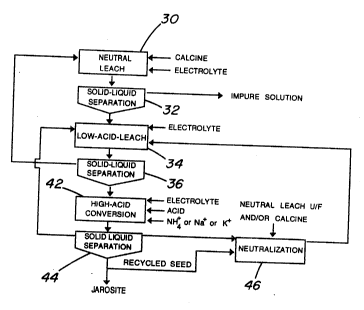
Figure 2 is a flowsheet of a conventional leach-
conversion process wherein zinc extraction and iron
precipitation take place simultaneously without the
addition of a neutralizing agent (pre-neutralization stage
15 18 of the flowsheet of Figure 1). In the flowsheet of
IFigure 2, calcine containing zinc oxide and zinc ferrite
Iis leached with spent electrolyte in a first neutral leach
stage 30 to dissolve most of the zinc oxide while leaving
the zinc ferrite substantially undissolved. The zinc
20ferrite is separated in a solid-liquid separation stage
32. The overflow from the solid-liguid separation stage
32 is an impure zinc solution which is purified in
subsequent stages and sent to electrolysis for zinc
recovery. The underflow from the solid-liguid separation
25stage 32 is fed to a low acid leach stage 34 where it is
leached with spent electrolyte to increase zinc recovery.
"
,f - '
,.-i . . ", , , , . :
,;, ~ ~ ........ . . . .. .
-~ 2~0473~
- The content of the low acid leach stage 34 is separated
out in a second solid liquid separation stage 36. The
overflow from the second solid-liquid separation stage is
recycled to neutral leach while the underflow is fed to a
so-called conversion stage 38 wherein zinc ferrite is
contacted with spent electrolyte and sulphuric acid with
the addition of ammonia or other alkali ions to
simultaneously leach zinc and precipitate iron without the
addition of a neutralizing agent, as disclosed in the
above mentioned Canadian patent No. 1,217,638. The
content of the conversion stage 38 i5 fed to a third
solid-liquid separation stage 40. The overflow from the
solid-liquid separation stage 40 is recycled to low acid
leach stage 34 while the underflow is jarosite which may
be discarded.
The major drawback of this conversion process is
the limited initial acid concentration (35-45 g/l) which
can be tolerated to achieve efficient iron precipitation.
The leaching rate of zinc ferrites could be increased by
¦ 20 increasing the acid concentration of the solution but
¦ increasing the acid concentration also increases the
soluble iron concentration in the final solution. This
second phenomenon results in higher circulating loads of
iron to the low-acid-leach stage 34 and to the neutral
leach stage 30 and is therefore undesirable.
The drawbacks of the normal conversion process
~', ' . ~' ,' ' . , ' ~ ',, '
~,- ," :' ",' " ' ' ' ' ' . ' ., ' ' '
f~ ~10~73~
are overcome in the flowsheet illustrated in Figure 3,
whereby (1) the zinc ferrites are leached with spent
electrolyte and sulphuric acid to give >99~ overall zinc
extraction and (2) ferric iron is precipitated to a final
concentration of <4 g/l soluble iron. The neutral and low
acid leach stages of the new conversion process are
identical to the normal conversion process. However, the
conversion stage is a high acid conversion stage 42
wherein zinc ferrite is leached with spent electrolyte and
sulphuric acid at an initial acid concentration of 50-60
g/l and a high temperature of 9~-105C with the addition
of ammonia or alkali metal ions to simultaneously dissolve
the zinc ferrite and precipitate iron as jarosite. The
content of the high acid conversion stage is separated in
a solid-liquid separation stage 44. The underflow can be
washed by counter-current decantation and/or filtration
prior to solids disposal. The overflow from solid-liquid
separation stage 44 is neutralized with neutral leach
underflow from the first solid~ uid separation stage 32
or calcine to further precipitate iron as jarosite, and
recycled to low acid leach stage 34. Surprisingly a
temperature of about 75C in the neutralization stage 46
was sufficient to achieve effective precipitation of iron
as jarosite to a final concentration of <4 g/l in only
three hours.
The process can be operated using existing
;: ~ . : . .
;~
~ ` ~10~7
.. g
equipment of a conventional Conversion process; no
additional solid-liquid separation equipment is required.
Since jarosite can be precipitated effectively at only
75C, heating of the solution to neutralization is not
necessary. When the capacity of neutral leach solid-
.~
liquid separation stages is considered a bottleneck, the
! use of neutral leach underflow in the neutralization stage
can allow the possibility of increasing plant throughputs
with only minor changes to the piping system; no
additional calcine feeding system is needed if the
i existing one has sufficient capacity.
The invention will now be disclosed with
reference to the following examples:
~xamDle 1
(a) High-Acid Conversion Stage
A slurry was prepared by mixing 750 ml of LAL
(low acid leach) solution (112 g/l Zn, 5.7 g/l Fe, 10.8
g/l H2SO4), 375 mL of conversion circuit solution (95 g/l
Zn, 4.5 g/l Fe, 13.2 g/l H2SOg), 825 ml of spent
electrolyte (40 g/l Zn, 185 g/l H2SO4), 141 ml of water,
j with 280.5 g of LAL solids (ferritic residue; 18.5% Zn,
42.2~ Fe) and 225 g of jarosite seed (1.37% Zn, 35.7% Fe).
The acid concentration was maintained at 50-60 g/l during
the first hour of additions of concentrated sulphuric
acid; the mixture was allowed to react for a total of
seven hours at 98-100C. A total of 28 g of ammonium
: : : , ~ :: ~ :: :
., ~ , .
~ 21 0~ 7~
hydroxide solution containing 29% NH3 was added after two
and three hours. The final solids assayed 1.5% Zn,
corresponding to an overall zinc extraction (neutral
leach, LAL, conversion) of 99.1%. The soluble iron and
S acid contents of the final solution were 15 g/l and 37 g/l
respectively.
(b) Neutralization/Iron Precipitation Stage
One litre of final solution from a High Acid
Conversion test (10 g/l Fe, 31 g/l H2SO4) was mixed with
g of jarosite seed; the slurry temperature was
maintained at 80C. The pH was increased to 1.8 and
maintained constant for one hour by adding Neutral Leach
underflow which contained 344 g/l solids; the slurry was
allowed to react for two additional hours without any pH
adjustment. The final acid and soluble iron
concentrations were 12 g/l and 3.9 g/l respectively. The
final solids consisted of jarosite and zinc ferrites with
traces of Fe2O3 and SiO2.
(c) Low-Acid Leach on Neutralization Residue
The final solids from the neutralization/
precipitation test were subjected to a Low-Acid-Leach
(75C, 14.5 g/l H2SO4, 100 g/l solids) with a sample of
LAL solution (14.5 g/l H2SO4, 2.4 g/l Fe). No iron
dissolution was observed after 30 minutes, showing the
stability of the solids (jarosite and zinc ferrites) under
LAL conditions; the soluble iron and solids concentrations
... :. . : , - . . . .
:~ ~ , . , , , . -
,,. ,. , , . . : ::
;i ~ , - . . . . .
~ 210 4 ~ 3 ~
11 -
remained unchanged at 2.4 g/l and 100 g/l respectively.
Exzm~le 2
The final iron concentration in solution can be
further decreased by increasing the amount of jarosite
seed and/or temperature:
(a) a final concentration of 3.6 g/l iron was
obtained by carrying out a test as in Example 1 (b), with
100 g of jarosite seed;
(b) a test also carried out as in Example 1
(b), but at 90C, resulted in a final iron concentration
of 2.4 g/l.
~xamDle 3
A test was carried out in a way corresponding to
Example 1 (b), but at 90C with an initial solution
containing 15 g/l soluble ferric iron; the final iron
concentration was 1.7 g/l.
The examples mentioned above demonstrate that
higher zinc extractions ~>99%) can be achieved in this new
two-stage Conversion process while controlling the final
soluble iron concentration to <4 g/l. For comparable
overall zinc extractions, the final solution produced by
the conventional Conversion process contains 10.8-11 g/l
soluble ferric iron.
,, ~ ;
, .
,:.~- : ' , . :
,