Note: Descriptions are shown in the official language in which they were submitted.
~2--
Boehringar Mannheim GmbH. ~47(:)/S;~A~
. ..
sment~? as w 11 a~ new ol?~icalI:~-sotive isoindolincne~
~h~ ~ubject o~ the~ pr~ent invention is the U3
5 of tric~oli¢ isQm~o1e der~ tive~ as anti-viral
m~di~am~nti~9 8~ w~ll as new opti~call~-~ctive iso-
dolin~ne~,
!I!h~ inve~?n c~.nc~rns the u~e a:~ tricyclic isoindol~
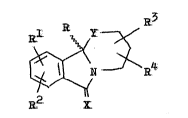
dl~rivatives ~f the~ ~sene~31 î~rmu~a I
.
DB ~ ZN~R4
:~ R~
~i fo~ th~? prep~ti~n Q~ ~eaicam~t~ w~bh ~nti~vira~l o~
,,
:j Anti--ret~oviral scti~n" whersb~ in îormu1s :~ ca~ bo
~, an ox~g~n o,r ~ul~hur~ a~o~,~ thl~ imi~o ~roup ~l~ or i~n
--a~lk~limin~l group, 3E cl3n b~ an px~;en ~
15 ~u1phur~ atom. o~ the~ 197 ~5~al~.~1amino~ ~$-sIk~l--
carbonglamin~ aulRhin~l or aul~ho~ roup~" R ci~n bc
a h~ a~o~, 8 at~ ht-chained. ~r bran~h~i~"
.i
2~tu~atedl oir uns~lrat~:d ~liphis tie radic~l with 1 - 9
atQm~, whieh can b~. ~ubatit~tedi b~ ~en~19, or
ifie~ & G~alk~ r G~6 ~
me3~G~ptQ-C~ alk~l ~ad~il or ~i~;nifie~ ~ phen~I
r~n~: whi~h is ~o~-ibl~ ~ub~ti'cuked cine or more: timas
b~ ~1~6-a1k~1~. !I~;--a~;kox~ 6 a~ lmerG~pt~
::: : ~
2 ~
--3~
Gl-OE~ kylaulphin~l ~ C. ~ lk~lsulphon~ Z~6-
gI~. ~2 (~ lk~ 2-~6-alken~lox;sr,. G ~: -
al~0n~lmeE~ P~0 ~ ~2--~6--a~n~IOX~ t ~2-G6 a~ n~l-
m~rcaptt~ amino,, ~ lk~lami2l~7~ 6~1kgl~ in~9.
5 ~6-a~kglcarbo~lamin~ L~6-alkglaminocarbo~ly,
: ~ ~mi~o~rb0~17 ~:;~i6-alkox~arbonyl, hgdrox~ ben~ylox~p
phe~gIma~capto~, phengI~acy9 nitro, c~no~7 hsl~gen~,
tri~lu~ometh~l~ ~zido~7 form~I~min~ carb~x~l o~ phengl
or. ~ignifl~s a m~n~, bi- Dr ~-~ic~cli¢ carb~c~clic~ring
~Q ~th, in all,5 7 - 15 C.-~toms ~r a heterocgclic m~no-
~bi- o~ tr.i~clic ~ing s~stem~ wh~r.~b~1 in each C~9, 5
or.6 rin~ atom~: are pr~sent per rin~ 3~t~m and, i~ th~
ca~.of he:teroc~clc~9 1 - ~ or l.- 5 h~teroatom~ ar~
: present,, re~pectiv~ a~d the hetero~toms can bQ
nitrog~n" ~u~hur or ox~e~,5~ ignifie~ ~ hgdro~n
~tom, a st~aight~chsined.or branched, ~atur~.ted o.~
~` u~t~sted ~liph3tic r~dical with 1 - 6 G-atoms cr
-alk~xg, ~ ~ -al~lm~rc~ptoO ~ ~ -alk~l~ulph~n~l,.
elk~ .ulph0nyl, 3mi~o7 ~1- G6-alk~l~min~/ di
2~, ~ G6-al-kgla~i~ u~phQnam~o,~ GL-~6-alkoxycQrbangl,;
.~ c~rbox~l~halogen9/h~droxgl,.ni~ro~ cy~,. &zido, ph~ngl
or benz~lo~ h~a the ~me.meaning a~ , whereb~
: the- radicals ~ d R2~f ind~.psndentIy of ~ noth~r,
can be the:Yame ~r ~ ër~nt,, R~ ~ignifie~ h~dr~gen,
C ~ 6-slk~ 6-alko~g-, C~-~6-alk~lm~rcapto, ami~,
, . . .
~ ~6 slk~l~min~, di~ sl~y amin~ halo~en, cgan~,.
hydrox~l,. csrboxyI ~r ~ ~ -alk~xy¢arbongl~. ~4 ha~ the.
~me me~ning a~ R3~.w~ereb~ the radical~ ~3 an~ R4,
,',
~, ', .
i~dependently of one another, can be ~he s~ne or
different, as well as their tautomers, enantiomers,
diasterom~rs and physiologically acceptable salts.
; Co~pou~d~ of ~he.furmula ~ in which Y signi~i~s a~
~-ulphur ~tom.and X sn oxy~en atom~ are known Xrom BE-~-
659~528 (cfo al50 US 3~646,0Z2) and p~ssess an anti-
infl~mmatory, a~nti-convul~ive ~nd ~nslgesic action.
Isoindolinone deriv~tives of the formula. ~, in which
5igni~ie~ the NH ~roup, are de~cribed in the Patent,.
~peci~ic~tion~ GB~1~059,17~ (Chem.Abs~r,.~ 72, 1~1 531 ~2)
- an~ PE-A-l~h45,443; Ne.th.Appl. 6,61~,264 (Chem, ~bs~r~ -
67~, 82 204 Q:) or US 3,590,043 ~i~h anti-infls~ma;tor~,
anal~esic~. blood pr~ssur~ lowerin~ pas~olytic and
anti-tussive activity, as wsll as in the publicAtion
~n~ Asoc., ~uim~ ~rgent. 70~, 651 (1982~(cf~ Chem. Abstr~
97" ~27 596 g), J. Hcterocgcl.Che~.. ~ , 23 (1980) ~nd
: . . .
.~Chem~. Ph~rm~ Bull~. 20, 69 (1972) wi~h co~responding
s~nt~esis procedures~.
Compounds of the formula I, ih which X a~d ~ sig-
ni~ an~ ox~en atom~ are described i~ter alia in J,
~ Hete~ojcgcl.. ..Chem, _6, 1441 (198~), J. ~eteroc~cl. Che~,
.~i 21, 293 (198~), J.. A~,Chem~Soc,. 96,. 4~9 (1974) and J~Am,
. Chem.S~c..... 96, 507 tl974), In U,S. 3,336,30G ~n anti-
convulsive uction is ~ttributed to such deri.~stives.
.,
Compounds wi~h similar structure and simil.dr
action spectrum to compounds of the formula I are
. .,
.
.. ..
':
..
2 ~ 3
5~
k~Lc~n ~rom the earlier German Ps~ent Application
P 40 35 8090,7. ~he there ~cribed compound~
repre:~3ent tric~clic i~oi~doIino~e d~rivatives in
which the rin~: condensed on in the 2 9 3-position
5 the i~oindolinone ring is 8 thi~z~le ring (thiaz~lo-
~,3-~-isoindolinon~3 deritr~tive~.. The corr~p0nding
opticaIl~-~ctl~re ~orm~ ~re de~ribed in the earlier
GQ~m~n ~4;;E~plicati~n P 4Q 37 674.5~.
q!he~ cQmpou~d~ o~ the pre~ent i7~ve~tion di~pli~
1~ vsluable pharmacological. pr~pertie3. In pa~ticu~ar~.,
the~ ar~ suit~ble f~r the therapg~ and proph~.ls~ci~ of
ction~ which ~re c~u~e~ b~ I~NA viEuses~ uch ~8
~e..g.~ tha h~r.pe~ plax viru~, the c~ tome 3;aloviru~
~:! pspil~:omavir~ , th~ v~ric.ella ~oster YirU~ ~r
~5 ~ Ba~r ~riru~ or R~N~ viruse~, ~uch 8~P tag~D
:,~ vi;~u~e~: Ql:!' e~peci~l3.g retrovirua~3~ such as the ~n~o-
viru~? E[~rIV-I andi II:, a~P we.~l. a~.the lentiviru$e~
~.
vi~n~ hum~n immurl~ deficien~g viru~; llllV-l snd -2
~h~e comp~unds ~ the ~ormula I appear to be
~i ff~pecia~lg ~uitabl~ $or k~:re t~eatDIent o-f t~e cli~ici~
m~if6!~ti~tio~ the r~.tro~irisl EI~ i~fection in
hum~n~ ch a.~ the pc~ ta~nt ~:~n~ra~d: l~mph-
lenopa~h3~ ,; the ~dvt~n~.e ~ of the~
r~la~d comp1e~ 2 and the clinical~ ~om~let~
25 picture; of l!~I~So
:~ ~ha compo~nd~ of the gener~l f~rmula I acc~:rdin3:
t.o the inventio~ po-~e~s sn ~u~6.tandin~ nki-~riral
action and ~3re e5pe~iaII~ suit~bla for/ the treatment
.. . . ..
2 ~
- ~6-
o~ viral or retroviral inf~ctions~ Viral inf~ctivn~
of msmmals, espeoi~ of human~, are wide~pre~dO
mt~ ~ intensive ~ndeav~urs, hitherto it ha~
; not been po~ible to mske available chemotherapeuti~
whi~h inter~e~e cau~all~ or s~mptomaticall~ with the
vixall~ or ~etr~vir~ cau~e~ ~ppesr~nce~ of d:isea~io
. with reco~isa~l.e sub~tsnti~l gucGe5~ A~.pre~ont,
: it i~ ~t p~ssibl~ t~ cura-.certain ~iral di~ease~,
~u~h 3~ for ~æ~mple the ~cqui~ed immune d~f~Gie~
:. 10 s~.~d~oma ~ $.~, the ~IDS.-r~lated comple~ (A~ nd
-1 th~ir prelimin~r~-~ta~e~" her.pe~ ~g~msgslovi~us~
~CMYr~ ïnf~.uen~a! and other viru~ infe:ction~ ~r
.1 ch~mother~p~utic~ fsvQuri~bl~- ~o in~luenc~ theiP
mpto~ t pre~.ent~ ~ample~ ~or th~ t~eatment
~5 of ~ the~e:i~ av~ila~lQ ~lmo~t e~clu~ive~
1~
i azide-3'-desoæ~th~midine (:hZ~)~ kno~n a~ Zidovu~ine ~
. ~ ~.
.~ on ~trovir ~.. Howev~r~ ~Z~ is characterise~ b~
i
e~y~ rrow ther~pe~tiG ~peclirum or~ by ve~ ever~3
to~i~iti~ ead~ ~ppearing~ the the~apeuti¢ rang~
~chq,M,~ç ~9~ ~ In~e~ ?~ 427-4
~heioomp~dD o~ th~ ge~s~al f~rm~ o n~t p~ e~
th~e ~i~&dv~t8~e~ ~hsg ~t ant~-virall~ without
be~n~ ~to~oai~ i~ pharm~col~cal~g r~ nt d~e~
I:t~e~u~d~ n~w b~3 d~m~.tr~$e~.th~.t e~m~ouDd~ ~Y
-~ 25 ~he ~enerD~ ~rmula I in~ibit.the mul~ip~c~ n ~f
and;~N~ viru~e~, re~pe~t~ ?,~t the ~t~g~
th~.vlru~-~p~ ic.DN~.~nd.~A.tran~Gri~ 3~
p~G~t~v~ Vi~ the inhib~tion ~ ths ~nz~me rev~r~e
~7~ 210~6~
tran~criptase~ the substar-ces influence the mu].l;i-
p~icstion of retroviru~es (cf. Proc. Natl.. Ac~d, Sci.
~ U~A 83,, 1911,. 1986 ~nd.Nsture 325, 7737 198~).
: ~ince ~ very gre~!, ne~ ~XiAt.9 .-or ~.hemothera-
peutic~ which inter~ere as 3peci~ically ~s pos~ibl~
with retroviD~lly csused.diseaees or their ey~ptom~
withou-t in~luencin~ the norm~lly occurring n~tur~l
bodg functI;on~,the ~sid compounds could be us~d
; advant~geousl~ prophglacticsllg o.r ther~peuticall~
in the tre~tment of ~isease~i:in which a-retrovir~l
infection is of pathophysiolo~ical, symptomatic or
clinical rele.vance~
The subject of the inve~tion are especisll~ tho~e.
compou~ds of the for~ula I in which Y si~nifie~ a.
: 15 ~.ulp,hur atom or the ~roup~ -SO or -SO2-~ It has been
shown th~t these co~po~nds~show an ~speciall~ ~ood
antiviral or an~i-retroviral activity,
Compounds of the formuD~ I hsve hither~o onlg
:
been known ~ r~cemates_ In comparison with the
`~ 20 racemates, the opticall~-active ~`orms, especiallg
the R_~enantiomers, po3sess a higher pharmacolo~ical
effec~ivenes~ and also repre~ent a subject of the
~` :
~ p~esent i~vention.
~ , .
The separa~tion ~f the rsce.ma.tes into the .:
en~ntiome~rs can be carried out an~lgtically, semi-
prepar~tivel~ ~nd prepara~ivel~ chro~stographicallg
: on suitable optic~ sctive phases with conventional
elution agents. As optically-~ctiv~ ph~s~s,. there are~
, : .
.
~ .~ , , .
, ,, '' ' ; ~ .; ' ' `'~' '
21~963
sultable~ ~or e~ample,. optic~ ctive polyacr~
amides or pol,yme thacrylamides, in part al50 on ~ilic~
gel (e~g. GhiraSpher(P~) of Merck, Chiralpsk(R) OT~OP
of ~aker), cellulose esterJc~rbamates ~e~g, ~ir~cel(~3
5 OBtOY of Bsker/Daicel)~.p~ b~sed on c~clo~xtrin
or crown ethers (e.g~. Crownpak(R) of Daicel!) or
microcr~s~alline ~ellulose triacetate (Merck),
Tn the definitions of R or Rl and R2, an s-liphatic
radical signifies a straight-chained or b~snched al~
10 a~ke~yl ~r alk~yn~l radical with 1 - 9, pre~erablg
2 - 7 or 1 - 6 carbon 8tom~l respectively, ~uch as
. the prop~l,.i~oprop~l, but~l,. isobutgl9 pentyl,
-hex~l or, in the ca~e o~ ~ ~ sl~o the hept,yl rad.i.c~.l.
As unsaturated rsdicals, there come into Questior
15 C2-C7_ or a2~C6-81kenyl or alk~ngl radical~,
prefe.rsbly- C~ C5, such a3 e,g.. the all~l, di~t~y:l-
i
~ allyl, buten~l, isobutengl, penten~l or propyngl
:,
radic~I~
In the de~inition of R, a~ sliphat~c radical,.
: ; 20 which can be ~ubstituted bg phengI,~ i~ especisll~
., ~ .
a phen;yl~ 6-alkgl grotlp ~, such a~ e . ~5, th~
benz;yl., pheneth~l~, phenylpropyl or phen~y-lbutgl
radica~ . :
~, :
If R signi~:ie~ a phenyl ring,. thi~ ean be
~5 substituted one, ~wo or three timea~ Independentl~ o~
one anotper, the. substitucnts can ~tand in the o-,
m or p-positionr '~wice s~bstituted phenyl rings are
- prefe.rably the ~ and 3,~-substituted derivatives.
A carbocgclic rin~ with 7 - 15 C-Dtoms can be.
30 m~no-,. bi~ or tricgclic and have, per ring~ in each
: .
.. . . .. ~ , . . .
' - ~
. ~ .,:
' :, , ':
' ~ . :: . ,
,.~
,
.,, ' ~
.... " " ..
2 ~ 3
_9_
case 5 or 6 C-atQma~ This ~in~ can be saturated9,
unsat~r~to~, partl~: ~atura~e~ or aromaticO ~he
~o~l~win~ ~in~ sy-stem~ are mentiorled. b~ wa~ of
e~amp:le:- the n~,p~Lthylg anthrac~r~l,r, phen3nthrenyl~,
5 fllloren~l, ind3n~1~! indan~ cenaph~h~le~
norbo~n~l 7, ~damant~l ~ing or a ~3-G7-cgcloal~yl or
~5~8; c~closlkeng~l ~roup.
~he heteroc~Glic m~no- bi- or tric~clic rin~
Yys~ms cont~inS; per ri~g ~tem7, 5 or 6 GarbQn atoms"
O whereb7 1.-- 4 er 1 - 5 ~:-atom~, re~pec~ivelg; c~n b~
~epls,:c~dl bg the hetero~om~ ox~;en,. sulp~ur and/or
it~o~enr The ~ln~ tem~ can be ~romatic ~ partly
or completel~ h~dro~enated. !I!he following rin~ sg ~tems
~re mentioned bg wa~ o:f e~,mple. the p~ridine."
:i .
~ 15 pgrimidine" p~rid~zine~,p~razine~ triazine,~ pgrrol~
~i p~ra~ole~, imidazole, t~i~zole, thiazole~ oxazol~-,
oxa~ole" oxadi~zol~:" fura.zan~ uran,~ ~hiophene,7
- .~
;~, indol.s~ ~uino~ine, i~oquin~line,~ cumarone~7 thio~
~,!` nsphthsn~.benzoxazole~-.benzthi~ole" ind~zols~
2Q, ben~imid~zol~7 ~ztris~Qle" chromene9r ~h~hulazin~,
~` quinaz dine, quinQx~ins,, meth~lenediox~benzen~,.
~ c~rbszol~ .cridine,.. phanox~a~iDe, phenothl~zine~
-` phenaz~n~r. purine.~ am9 whareb~- tha u~aturated
or~a~omatiG carb~- and.h~terocycle~ can b~ p~rtly
of com~letelg h~d~o~e~t0~.
.
R.preferablg ~ ie~ uh ubatituted phen~.l or
. phan~ ubati~utad once ~r twi~a bg ~I~G;6-alkyl,
~G;6-alkoxy9; a~ 6-~lXgImercapto,, al-a.6~1kyl-
.
, , ~ . .
... . . . .
~ ~ . . ...
2 1 ~ 3
-lQ
~,Ulp4ingli C l.-&-aI3~laulpho~ 2-c6
G -~;_elk~15 C3~ 6~ ngl~X~s Cl- ~6 al ;r
6-dia~ amin63~ C6-a ~ yI¢arbon;9~ iDs39 Cl-C6
laminocarb~ ; C~ C6-~lkox~c~rb~ngl, amino,.
~, 5 h~drox~l, nitxog~ azido,? triflu~r~methyl~ c~an~ ~r
hal~gen" The: pre~iou~ mention~d "'~ 1" p~rt~ in
! ,
the ~finitiorl~ in ~ueetion prsf~rab:~y ¢ontain up to 4~?
peGi~ J Up ~ 3 a-a~.t~m2~
Garboc~clic ring~. sre. l?r~erabl~ phe~ biphen~lq,
nap~hth~l.7, a~thr~ce~l9, ind~n~l,; inda~ luor~
~cen~.ph~h~len~ phena~thren~l.p n~rb~.rn~ ~nallt~l"
! ~ -C6-c~¢l~aIkyl,; G5~ c~oloalken~ e~peeiall~
pheng~ ria~hth~l snd ind~n~l~
Het~oc~c~ rin~ s~tem~ ~re. pre~er~bly p~rr~
imids~ol~,, fur&~n~ thi~phene, p~ridine,, p~ idi~
~hi~zola~ tri~zine, indole, guin~ina, i30quinolin~,
~ ~uma~e~ thiQnaphth~37~be~z~lid~z~ in~zQliR~
`, m~ibh~l~ned`iox~banzena7 eith~ x~benz~ie~ ~arbszol~
:3 ~G~idi~ nd-p'~e~othiazi~e~ e~pe~iall~ ~hicphene
;, 20 pgrid~
~ Qr~-tha radiGal~ ~l~a.n~ R~?~ ~re pre~er~ h~ rc~n~ ~
ff6 ~ ~2~6~ n~ ~6~I~n~ aDko~
m~ ap~o,~ mi~ lk~x~--
o~rb~ 19~m~n~ ~al~n, h~drox~1? G~an0~snd a~id~,
whe~eb~ the "aI~1" p~rt3 in the previ~u31~ ~e~ion~d
; de~initi~ pr~fer~bl~ c~tain ~p ~.o ~,~es~ia:1Iy ~p
,t~,~ a~tQ~
.... , :
, , - . .. ,; : ~ . .
:~ .. . ..
2 ~
--liLo
Pr~f~r~ed sub~tituellt~ f~,r 1~ aT~d ~4 a~e h~drog~n~"
_C6~D~ aL~C6--~lk~x~ m~r¢~P~ ~
~: c~rb~lD, ~,-alkox~:carbo~l, hal~en, ¢ga~o~ a~d.
h~dr~x~l" whereby tha "slk~l" p~rt of the previou~l~
5 m~rlti~;ne~ d~initi~n~ pr~:Eerabl~ contairl up-t~ 4,
efipe~iall~ up t~ ~: G~;~t0.m~
~ a~d ~ ~re: pr~fer~bl~: ox~ger~ #r ~ulFhurO
B~ halo~;ea3 i~ ~, in ~eneral~ to be u~d~r~t~od ~lu~ri~e "
chlc~ine:; bromir~ ~n~ iodi~e,, pr~fer~bly fluorinct,
1~ ¢hl¢~rine ~n~ bromi~
E~ecial3~r ~refe~ed radic21~ for :1~. a~r~ ~:3~5-
~:~ sI3~1~ 5-alkaD~ 2~4~I" benz~l" phen~thgl~
phen~ phe~iI. m~n~ cr ~i~ubstitsit~ ~6-a~y~,
p 6~-aiL~lme~cap~ all;~ all~lox~"
-; :IL5 ~ ain~a~,.; d~ 6--~lk;y~amin~t, ~minQ" h~drQx~l,
azi~o,~ t~ifluor~me*h~l" G;sra~o or hal~7g~n or ph~n~l
tri~ub~titu'Ged bg methgl. Q.r ha~ogeng~ naphthgl
~nth~ac~ , indenyl,~ ind~ ;l9, ~cen~phth~l~ngl,~
:` ph~n~llthren;g~l." ada~an~l,; c~cl~hex~lp c~cloh~xen~l,,
2~ ~i~r~19~ lthi;~ p;~t~id~l~o pyr~m~d~n;~I, th~a~
in~o~l~ ing~-I." b~nzimidazG7}~1:" methyIç7n0diox
ph~n~ c~rba~Q~ arld. ph~n~,thiazin~
E~ nd~ R2^~. ind:ependentl~ of 0~8 another" are~
e~pe¢ia~ly pr~fe~e~ hg~ en", m~th~ t;hatlf i~C3p~9~1"
2.5 al~1,~. me:tho3~ ?th~:,, me~h~ rcspt~ s~lmerc~pt~,~
meth~lamillo,~ methQxg~c~ 7n~l9~ ~th~x~arb~17, nit~
~zido, ~an~ h~drox~ nd hsla7~se~5. whereb~ chI~ririe:
and bromine-- a~e quit~ e~peei~ srsf~rr0~ halo~e~0
`'
,
i3 ~
Fo,~ R3 and ~4 are es~ecially preieerred meth~l,
~th~l., ieopro~gly.~ methox~, ~th~x~,t meth~lmercapto,,
e-th~lmer~ptoi, meth~ Qino~,~ amino ~ chl~rine ~ bromine.
c~an~,
~' 5 :E~ecislly pre~er.~ed. ar~. Gom~pQuna~ ~f tha genersLl
~rmul~ , in which R,? ~19~ ~ has~e the above-giv~n
me~ g and :E1.2.s, 1~3 and ~4 a~e equa~ t~ ~drogall,, m~th~lL"
~3.t~I" ¢hl~rine"~ bromine" m~.thQx~ or eth~,. wher~b~
~2 1~.4 ~ e~peai~l~y pra~erab1~ r~pr~sellt h~dr~gen alld
lÇ~ h~drogen o~ halog~n
IndependantlL~ of oIle. another, ~h~ ~o11~winE m~aning:~
~f the rad~ ~t EL arld ~ 4 com~ in~? qus~!tii~n
~pe~ . in the mea~ing ~f the invention: X ~n ox~g~
.
~tom~,i ~ a sul~hur or Qx~.~en at~m; R ~ 6- ~k~ t
. ~5 n~ h~l i~d~n~.l" pgrid~ thien~,l.or phen~ ou~
:~j wh~r~b~ the~phen~ ~roup? csn be substituted once or
~! b~ ~L G6~~k~ 6~ x~ t;halo~
hgdrogen ~r-hal~gen ~to~; ~2 _ ~4 & h~dr~n ~t~m,
~h~ m0dio~ent~. oont~inin~ ~t,~es~t on~ compouna cf
the f~,rmul~ I f~ th~ trestment of virsl infe~tion~
c~ be aaminis.t~rea enterall~ ~r psrenterall~ in liq!uid
9Qli~. form~ ~her.e hereb~!come.i~t~ ~ue~tio~ the.
usual f~rm~ ~ ad~inist~atiQ~, ~uch a:~ f~r e~mRle
tabl~;ts., Gap~ule~ ~r~g~s~ S~Up~,~ soluti~n~ ~r
2.5 au~pe~i~n~r ~ inJ.~ction mediu~; water i~ pre~er~.bl~
u~d which contai~ the additives u~ual.~n the ¢~ f
in~eetio~ ~olutions~. ~uch as ~tsbili~ing a-~ents~,.
~olubili~ing ~g9nt8 and buf~ere.. ~uch ~daitive~ 8rQ
,:
.. ,` .
:-:,, - , , :....... ,. :
` ;, :
' ' ' ,,: '
2i~ 3
~ 1.3~
e~ tartrate and citrate bu~er~ e.thsnol, complex
formers:, such as eth~lenediamine~b~t~aacetic ~cid and
~ it~ n~n-toxic ~alt~,. hi~h moleculsr poI~mer~,. such ~s
: liquid p~ eth~lene ~xide, fo~ visco~it~ re~ulatio~.
hi~uid`.car~i~r msteri~ls for injection ~clution~ mu~t
be: ~terile and a~e pre~erablg filled into ampoules~
~o~ arr~e~ ma~erials are, for example, starch9
l~cto~e,.~annitol.~; methyl cellulo~e~ tal~9, highl~
di~.r~:~d ~icic ~id~ high m~ecu}~r fattg a¢id
1~ $uch a~ stearic a.cid,/ gelati~, s~ar ~g~r~ lc~um
~; ph~ph~ magnesium ~tearate ~ anima~ a ve~e~tabl~
f~ts9 ~oIid hi~h moIeoularipol~mers,, ~uch a~!p~
~*h~lens ~yCQlS ~ ~tOE.. Compositi~n~ auitable for oral'
~dmini~tation can~jif dcei~ed contsin flavouring or ~ :
~5 ~we~.tening agents. F.or the p~oduction Q~ ~he pac~ag~
unit~ resd~ far u~e" the appropriste medicinal form~"
uah ~ e~g~ ~apaules~ tabl~t~t, d~a~ee~ re con~ectioned
in the ~ppropri~t~ numb~r o~ .ce~.and packe~ into
ppropri~te~ uni~" whereb~ the so produced p~c~age unit~
:~ ~ 2Q are pr~vi~ad with the inform~ti~n for the u~e ~s a;nti
ir21 or anti-r.etrovirsL a~en~, fc.r ex~mple in the fo.rm
the;previou~ly de~ rib~d.paeka~e. in~rt lsaflet.
For the produc~iQn Qf ph~siolo~icall~ compotibl~
s~llt~.,,compound~ of the f~rmu}~ I.which csrr~ ~ basic
grQUp a:re reacted with inorganic ~r organic ~cld~ uch
a:~ e..~.. hgdrochloric a~id,jh~drob~omic acidf ~ulphuric
- .,
acid~. ph~sph~ric acid,~umarlc.acid,~ succinic aGid~q~
tartsric acid, citr.ic acid, Iactic acid or maIslG acid,,
:.,
2 1 ~ i 3
and the acid-addition salt~ isolated. I~ the compoun~
of the formul~ I con~ain an aci~ ~roup~.t~en one obt~ins
tha ph~si`ologicalI~ compatible salt~ b~ ~eaction with
. aIkaIi metsI or alkaline earth metal hgdroxides7 ~uch
5 a~ e 0.~ sodium hgdr~xide, pot~s~ium h~droxlde or
caIcium h~drox.ide~ or with other baffic group-s~ ~uch 8
e-..~r w~h ~mine~:"
-~ The dosaging can depend u~n ~ari~us ~aat~rs, ~uGh
a~ m~de of admini~t~tion" ~pecies, age~or individual
tate. ~ he~Ith~ ~he com~und~ acG~rdi~ to th~ inv~n-
tion ane usuall~ admini~tere~ in am~unt~ a~ Q~ DO mg,.
praferabl~ 0..2 - 80 m~ per da,~. and ~er kg o~ bad~ w0i~ht.
It~ re~erra~ to divide up the dail~ do~ into 2 - 5
admi;ni~t~ati~na, whereb~, in the cas~'~f each ~dminist-
~5 rskiQn9, 1 - 2 tablet~ are ~iVell with an active material
cont~nt Q~ 0~5 - 50n~mg~ The t~blet~ can al~o b~ r~tarded~,
wh~b~ ths number o~ ~dminist~tions per da~ i~ reduced
t~ 3~ ~h~ ve~m~kerial cont~nt o~ the ~etarde~
t~ t ca~ am3~n~ to ~ - lO~ mg~ ~he ~c~.tive m~terial
20 aan ~l~o be ~ive~ b~ eontinuou~ in~u~ion" whereby the
~mOE~ta ~f 5 ~ l~OQ^mg per da~ normally ~u~ic:e.
Th~.oomFo.u~d~ ~ the ~sn~rsl ~ormu~a I ac~Qrdin~
te the i~v~tion are.preRs~.e~ acG~rdin~ to per 3e kn~wn
pro¢e~e~inithat one react~ po~sibl~ ~ubatitute~
~5 benz~c acid:deri~ative~ of the ge~e:raL formula II
,
"
. , .
, ~,j
~ 2
: -~15
:~' ''' ~
(II)~
n2..
.
which Rt, Rl ~nd ~ ha~ the a~ove-given m~ning
~n~ qua~t~ QH or ¢~N, with ~ub~tituted o~
u~ub~titukffd aminoalk~lmar~apt~n~ v~ the ~en~r~l.
~ormul~
2~ R4
'
; or ~iamine~ ~f the ~e~a~l formul~ IU
.~1
H-~ ____f~
in w~ioh ~a~ ~ h~ve.the 3b~ ivan ~ani~gr in
~ ~0. ~itabl~ inert ~01Yent ~.t ~oo~ temper~tur:~ to r~flu~
;~ ~emper3t~r.e., ~o~ib~ in the pr~en~ie ~ ~atal~tiG~
'I
~m~unk~ of acid~ oluene~ulp~oniG aei~,lor,;
r th~ e98~ bhat ~ " brings ~r~l magne~ium
br~mid~ t~ r.eac~ion wi~h ~C3 bromoprop~ p~h~-
imid~ at ~ o r~ g.tempera~u~e.in an i~rt~
~olv~t?i~ th~ is de~¢ribe~,,f~r e~amp-le~
m~ 26~ nd po3~ibl~
~- sub~q~nt~ co~Yerta ¢ompou~d~ the ~ormula I into
; /~
J! other compp.und~ he fQrmu~a I ~nd ~ub~equentI~-
. . .
2 ~ 3
puri~ chromato~raphicall~ or by recr~t~llis~tion~
Racemate~-can be ~eparated into the sntipades b~
; ohr~mato~raph~ on ~uitable op~tic~ act~e ph~e~a
e.4.~ e~luIo~e tri~c~ate~
- 5 The sub~e~uent conver~i~n of cam~ound~ of ~he
fo:rmuls I into oth~r comp~unds o~ the formula I-
con¢e~n~ the prep~ati~n ~f d~rivatives with X = S ~r
N-alk~ ine,~ C~mp~unds:wi.th ~ 3- S ar~ preparaa by-
~e~ctio~ o~ c~m~ou~ds of the ~Qrmuls I,.in which ~
Q ~i~nffie~ an Qx~en ~tom~, with ~ul~hur~gr~p-tran~ -
fer~ co~p~.und~ ch a~ ~.~ Iawe~ reag~nt~
~G~m~und~ h ~ D ~ al~ limi~ a,r~prz~re~ b~
reactio~ ~ the c~rr~po~d.in~ n~ compsand3 ~f the
g~ne~L ~rmula I ~,~h alk~ ine~ aC~a~ to per s~
kn~ meth~ mpGu~d~ pf ~Pe ~rmul~ I ~ith ~ -
N~lk~limi~o~ a~e.pr~p~re.~ b~ ~31k~1a~ti;on o~f co~u~
Q~ the f~smu~2 I in whi~h ~ ~Ispresent~ ~ nit~2g~n ~tom~
~ urtherm~r~,; the ~ub~e~ent co~ve~.~ion con~ern~
g~ the ~xid~ .n ~f compound~ the ~e~e~sl f~rmul~
,Z:: I w th ~ J ~ to the corre~Ec~din~ ~u~Ehin~l hn~.
.~~u~E~.ngl ~ ati~ea w~th ~ ~ S~, ~ ac~ording t~,
per 5e kn~wn met~ods~
~` ~h~ ben~l4 ac~d d~rivati~ Q~ the general ~ormul~ :
. ~;~ ., .
~r~ .wn f~o~,th~ Iit~atur~:~n~ ~re pre~aP.e~ e~ by
~5 ~ried~ t~ a~ylatiQn Q~f ~ub~t~tut~d or un~ub~tit-
-;~u~d.phthaI.ic scid anh~aride with p~.s~lbl~ ~ub~titu~d
~rene~:in ~ ~e~nce ~f a I~is acid (~ lumi~ium.
chlori~e~ ~r b~ re~ction o~ Grignar~ re~gent~ ~/f the.
~.
::;
; ~ :"
::
2 ~
--17
gene~ mu1s
P~gBr ~V~
in which R:" with the excepti~ of h~d~ogen~ ha~ ~h~s-
~bov~ iven mearlin@:~ wi~h ph:th~lic ~cid ~nh~rid~,
5 which i~ pc~ib1~ ~ub~titu~.e~C~,5 i~ ~uitable i~er~
~olve~t~ a~t 10w tem~rs.ture~,.
~ mp~u~d~ ~ th~ ~rmu1~ uGh a-~ e
m2rGaPtOP~OP~ n9 ~E~Y, ~him~ l~cta 4697 752" 1~96
~nd ~ a,r~. kn~wn compound~ and can b~ pre~ar~d
ac~rding t~ QG6rdures kn~n f~om the :Lit~a~re o~
a re c~mm~rcis11~ ~nrai1abl~
q~he pre~ a~i~bn of tha com~?Qilad~ of the ~ rmul~ I
tal~e~ ee ~;nalQ~Gu~ Q th~ pr~psr~ti~n elf thi~zolo-
~ i3~in~0~li~0nes which ar~ kn~wn f~o~ th~. prio~
15 a~t, C~ U~ 3~,3~4~113;, lUS-Pat.~n~ 3$,~46~02~
U~$~ ,86Q"~.85" ~3~Plg1all ~ste~b ~q;?~caltion 564r592;
.':! Jv O~g~ 15Q6 (19~52" ~ w~ J~ Q~, Chem~,
`J ~ .. - ~5 (1969~
En ~he~ meani~; ~ the; p~ e~t invention~ ap~rt.
om th~ compaun~F men~ioned in t~e Ex~mp~e~ an~ ~ho~a
b~ c~mbin~*icn of al3~ m~anin~ the substi~.ue~t~ :
m~nti6Dned in ~he cl~im3, the f~llow~s c~mp~und~ o~
~, formul~ I com~ into~ q~e~ti~n whi~h ca~n bæ preis~
`i r~;ce~ic mi$.ture or ~ optic~ a-ctiv~Ee form or ~1`3
:.: :
25 pur~ R~ and ~nan~
ib -meth~1-3`~4-dih~xa-~H-J.:~,.~h~ ~zino-~`,.3-~_
in~Io1-6(~ b E[.3~-~n~
, ,'. .
2 ~
-~8
2~ lQb--~h~sn9~-1~3~ 1ih;5~dro.~ 7--t;hi~3ZillO-~.3
i~oindol-6(1~-bH3~n~
3~ lOb~(3-t~ romethylphesn~ dih~a:ro-2~-~rq,~7-
thi~ z ino~ 3~ oind~1-6 ( 10-bEI~ -on~
.
5 4 ,. I~)b~ chlorophen~ 3 ,~4-~ihy~ 2E~ ~thi~æino-
oindol-6(10 bE~_ons! -
5 ~ l~b~ m~.tho~heng-~) ~3 ~-d1h~ro-2~ ~th~3 zirlo-
~53 ~--isso~indol-6~ b~ on~
6, ~!Qb--phe~ 9,3~,4~51~Sb--tstr~h~rop~rimido~~9;1-~7-
oindol 6(2E.~ o~
7_ l~b-b~nzg~ ,3~91~b-~-e~ah~.drop~rimido~
i~o~ol-6~2H). one.
8~ b~ ¢h1~rophen~r,r1~-1r~4,10b-~e:~r~h~d:r~p~r1mi~o-
2,1. ~ i~oind~1-6(~E~.-~nQ
I5 9.~ 1~b (4-m~thy1mercapto~h~n~1~-19~,4~t~b-tetr~h~d:r~-
p~rimido-~ o,indol-6(2~!-ona
IO~ IDb-~*.-eth~ phe~1)-1,,3,.,49,~b-tetrshgdr~p~rimid~-
is~indo1-6(2E.)-one.
11L.O~ b~(4-~luorophenyl)-17~3~410b-tetrah~d:ropyrimiao:~
~! 2Q ~ ,~ indo1-6~2~3~-Qn~
.~ ; 12~ l-m~th~ b phe~ 3~4~ b-t~t~dh~drop~rimi~o-
isoinaol.-6(2H~.-on~
13. 1-m~.th~ b-(4-chloroph~nyl)-1~3,4,10b-t~t~-
h~dr~p~rimido~ oindo1-6(2H~ on~
25 14~ prop~l~lOb-phe~1,1,3,.~9,10b-tetr3h~drop;srrimid~-
oindol-6(2H~-on~
15.~ thy1-lOb-phe~ ,,4,10b tetrah~dropgrimido-
,~ oilld~1-6(2H~-on~
- .
, .
: ' ', , ~ ~',
-1~-
16~ 3-h~drQx~-lob-phen~ 3~;4~ bDtet~ah~d~op~rîmid
is~indol-6(i2H)-one~
. 3-k~d~ lOb-benz~I-1,,3,~.4,.IOb-tet~ah~dr~pgrimido-
~,~ a7-6(2H~-on~
18.. 9-nit~o-lOb-phen~ 1,3~4,10b-t0~reh~dropyrimido -
~ oindol-6(2H)-One
:19~ lOb-(2~thi~n~ 3~4,10b~ket~eh~dropyrimido~
isoindol-6~2~-vne
2~.~ 3-meth~L-lQb~ chlorophen~ ,3~4slOb-tet~h~dr~--
pyrimido~ oiadol-6(2~3 one
21~ lOb-~4-chloro-3.-aminopheng--~)-1,,3~.,4,~0b-tetrahgd~o-
p~rimido~ is~indol-6(2H)~one
22~. 2-~eth~l~lQb-(4-Ghlorophe~l.S-1,3,~,.10b-tetr~hgdro-
.
~` p~r.i~ido-~ ,1-~ -i~oin~ol~6(2H)~-on~
23D l-meth~l-lOb-(3-nitrophen~l.) 1,3h4,;10b_tet2~h~ro-
p~rimido-~91-~7-~indol-6(2H2-~n3
~24~ 1 e.th~ b ~4-methox~phen~ 4,.10b-~etr~h~d~o-
-.~. pJrimid~ iso:i~dol-6(2~ o~e~
,;~ 2.5~ DDb phen~l-3~4-~ih~dP.o~ zino~
~0i~indol-6(1~b~ ^o.n~
~6~ lQb-~4-t-but~l~h~n~ 3,~-dih~drG~2H.
~ ~,
~ oxazin~ QL~dsl 6-(~I~ E)-~ne
.
`~ 27~ ~Qb-~ meth~l~h~n~ 3~4-dih~d~o- 2H~r~ oxa~zi~o-
- .~ /2, 3~ Qindol~-6( 1Qb~ ~n~
~ , .
.. 2-5~B:~ lob~4-meth~lph0n~l)r3t~-aih~aro-2~I9~-ox~zi~o--
.3-~ -isoind~l-6(10bH~- OD~
29~ lOb~ fluorph~n~ 3,~-dih~dro-2E-~I ~ ox~zin~-
~,.3-~indol!6(10bH~-oh~
: . , . , . , .:
.. . .
, ~
2 ~. O ~ ~ ~ 3
-20~
3~ b~ph~lethengl-3 ~4-dih~ro~ ox~zino-
.3~ oiFldol-6( lObE~ -one~
;lb-phen~l~-th~ 3 ,,4-dih~d;r~2~I-~`9~7-o:g~zino~
,~,3-~d7--i~oindQl-6(15)bEI.3,-One
5 ~2~ lOb-(4 m~thoxg~phen~ 3 ~4-dih~dro-2~
o~c~izino~ 3~ oind~1-6~l~)bl~-o~6:
33.^ IQb~ pip~r~zi~ ,,4~ dih~d~-2HD~`~ x~,zinc~
~ 2,.3~ oi~ 6(:1~bHi)~ne
34-. l~b~ imids z~ 3 ,~4-dih~
Q~ o~æin~ " 3~ ~7~ dol~ 10~ o~
35,~ lQb-~4-morph01in~ dih~ ox~zin
~,3-~7-isoi~dol-6( 1l3bHi~-ona
36~ lOb-prop~ x~ dih~d~-2H~ oxazino-
is~ind~1-6(.1~b~) cline
! ~5 3~ ob~(~-chlGroE?heD~ 3~ d~ih~d~-2~I-~r~xEl!~in~--
,3~ 3oindol!6(1.0b~
~8r lOb~1-pip~idi~o~.~,~dih~dr~ "~7-ox~zino-
9 ~ is~indol~-~(lQ~H~ on~
3.9.~ lQb~ meth~,lphe~,l~ dih~dr~.-2E~
t~in~."3~ oindol-6(J~b~ on~
4.0,.~ 10~ =n~h.tl~$1). ~9r4 dih~dr~. /~ thia~
3~_7~ ~indQl-6 ( IObEl.
41. g~ ob-E?he~ i~d~Q~2H-OE~1~hi~æ~n~
~, ~;,3~-isoi~ Qb~n~
~5 ~æ 9~hlr~r~1Qb--phe~ ~3 a~4-dih~ 7;~h~ n~-
.inao~-6(~ bH~ 3
43" ~-~hI.o~o~IQb~:~-m6t~Iph~n~I)v--3`~,4-~;ih~ro~
,~E,.~thi~in~ ,3-~iso~indoI-6~10b~I~ o~
- . : .; : . ,
.. :,,: : ' :. .
': . ~ ''' ' ''' ~ :
2 ~ s;~
--21~
4~v. l:~b-(3~*-dimeth~l~h~ ,,4-dih~dro~ ,3,7_
thi~zin~,3~3-iso~ind`~16(ll~bH~ crn~
~50. I~)b--ph~n~l--3 ,~-dih~d~--2~ `7-thi3-zin~--~9 3
isloind`~,lQ~6(1.~bH~:~thione
: ~ 5 46V ~l~b~ chlorophen~ 39~4~ihydro-2H~ thiazin
¢~ isoi~dol-6(1QbH)-one
47,~ 9~oet~ b--(3~5~dimeth~1phenyl)--39,4 dihytIro-2H--
/~ thiazi~o~ 3~ in~ol-6(l~bH~-on~
48~ 9-chl~r6~ b~ phth~ 3. ,,4~-dih~ro~
thiazin~,~:i; s ~ ind~ 6(lab~
4.~,~ lOb~~2-naph~ Z,~-dih~ar~2H~~ thiazino
.~,
~:9~ oindol-6-(~b~
50, lObD(.~n~hr~e~-l~y~)-39~ ih~drQ-2~ i7~7-thiszino-
;,~isoi~dcl~ ab~I~ 0~
:IL5 51i, l.Ob-~nthrace~9-gl)~ 4-dih~d~o~9,37-thiazino--
~, 3~ oindol-6( lObH~-one
~i 52~ 9~ th~I~lOb-(3 t~ifluQrome~h~lp~e~L~--3t4-dih~d~o--
;thi~zin~9~,~-iso,ind:cl~ 6(10b~-o~
., 53~ 9-m~t~-lOb-phe~L~39tq;-dih~ro~ 9,~7-thi~zinQ--
in~ol-6(1C~b~ n~
, .... .
5~ lOb-(3w~th~lphen~ 3,,~-aih~dr~ .~l;h~ino-
'' ¢~'7 3-~, -i~ol~1~6~ labE2 -On~
55~ l~b-b~ dih~dro-~EI7~ 3~thi~zino-i!s 3
i~oin~:a.3~-6(10b~
25 56,~ 3, 3 dime thJ 1--~b-phe~ 3 ~$-d:ih~ d~-2H~.
thi~zino~ , 3~ oindo}-6(1~bl~)-on~-
57. l~b-all~1-3,~:ih~d:ro--2~ / ,~thiazino~ 7--
i~oindol-6( l~)bE~-~,n~
,: ..
2 ~ 6 3
-22--
58~ lClb~ den~ 3 ~ dih~:dro~ r~ thiazinaL~
~',3~ oindoi-6(1~bH)-one
5~ 3b-(ïnde~w~-gl~-3~4:-d`ihgdrQ-2H~ thi~zin
,~ oindol-6 ( 1.QbE3--One
60:, lOb~ ae~ y 1 ) ~ dih~dr~ thi3 z ino--
, 3~ Qindol~( l~b~-oaeL
6I~ (phananthren-1-gl). D'3 ~ dih~dr~-2
h~azinQ~ 3 ~ oindote-6(D0~H.)--thio~6
62~ lQb-~phen~nthr~n~ 3 ~-dlh~o ~r,~
~iazi~ ind~ 6(1Gb~ thi~n~
.,
1~ 63.. 9-chl~ b (~-me.th~lphen~ 3~?4 dihy~ro-2H-
, ) . .
thiazin~ oindcle-6(1Qb~-thio~
~; 64~ l~b-(2/n~pht~ -3"4-dih~dr~-2~-~I9,~ -thiazino-
¢~93~ o~ndo~e-6(10bH~ hi3n~
65~. Dgb-(¢7cloh~xe~ ?4-dihg~
thiszino- ~3- ~ -isoindo~1-6(1~bH~-on~
66~ lQb-(2D~ur~ dih~d~-2~- ~ "~ ~ hi~inQ-
o~i~do~l-6( 1~)bH~ n~
67, 1.1 )b~ f ur9~ 3 ~,4-d ih~ 2Q~2H ~ *hia zlnQ-
2t~ ~, 3~ oi~l-6( 1(YbH~ o:n~
. ~ . . . .
68~ lOb-(~2-thienxl~ dih~d~o-2~-~ , ~ thiazino-
~3-~ -is~i~dol-6(1~b~-
69 lOb-(3~thl~n,~ 3,~-dih~ar~-2H ~ I~ ~ thia~i~o~
,3 ~ -i~o~ 1-6~1Qb~ on~-
7Q~ lOb (3-p~rid~1) 3~4-d:ih~dr3-2H~ thiazin~-
~ oi~d~1-6(10bH.~-On~
: 71.~ l~b-~p~rimidin-~ -xl)~3 ~-aih~drQ-2H~
thi~zino~ 3- ~ -i~oindol-6(10b~-one
:~,
,, ~. .,., , -.
. : ,.,. ~ .
. , . :!: ' '
-: ,
2 ~ 3
72. lOb-(~hiazol-4~ ,4-dihydro-2H-/I,37-~hiazino-
~2,~-a7-i~oindol-6(10bH)-One
. 7~ lOb~(in~ol-3-gl)_3,l~-dih~dro-2H /I~37-thi~
;; /~,3 a7-isoindol~6( lOb~ ) -o~e
-~ 5 74. lob-(indol-7-~l)-3~4-dih~dro-2H-~Il37~~hiazin
~ ,3-a7-isoindol-~(lOb~)-one
.. 75. lOb-(quino~in-5-yl)-~,,4-dihydro-~H-~,37-thiazirlo
..
`~................ /~',3-a7-i~oindol~(lObH)-One
.`~ 76~ lob-(bengimidazol-4-Jl)-3~4-dihydrc~-2H-/I~37
~ ~ lO thiazino-~2,3~ oindol-6(10bH)-one ~
,.~,
.i.~ 77~ lOb-~carbazol-4-gl)-3,~4-dihgdro-2H-/I,.37-thiazino-
~ /~,3~a7-isoindol-6(10b~ one:
. -,
::~;; 78~ lob-(phenothiazin~4-gl)-3~4-dihydro-2H~ 37
`'",~ ~ thiazino-/~,3-a7-isoindol-6(10bH)-one
~; 15 79, lob-(4-pyridgl)-3~4-dih~ydro-2H-/I~37-thiazin
~ /2,3-a7-isoindol-~(lObH)-one
'`!; 80, lob-(2-pyrid~l)-3~l4-dihydro-2H-/I~37-th~zirlc)
,3-a7-i~oindol 6(10b~)-one
lob-(phenyl)~3~-dih;2dro-2H~ 37-thlazin
/~,3-a7 isoin~!ol-~(lOb~)-one l-o~ide
~- ~ 82, lOp-(pheny~ 54~dihydro-2H~ 37-t~hia~in
,3-a7-isoindol-6(10bH)~O~e 1,1-dioxide
,
25 i~o~dol-6(10bH~-Qne
. . .
.a ~ mol). 2~benzo~1benzoic acid were di~solv~d
in 80 ml abs.. toluene ~nd, after additio~ of 1,92 g
(21 mmol~ 3-mercaptopropgIbmine, a.c well a~ a ~a~al~ic
':
.
,~ . ,~,~ ........... . ..
) - :. ~.,
~ ~ :
--24--
~mount. ~ p-toluene~uIl~?honic acid,, heate~. undsr reflu~
~or one hour on a~ weter 5~?~1~a.lia~. ~he soIve~t wa~ then
removea in a v~cuum. snd the residue puri~ied b;~ column
chr~m~t~:raph~ on ~ilic~ ;I. 6Q with ethe~/isohex~n~
5 2/~;~;a~ elue~t. ~ie~ld ~..OQ~ ~ (51~ o~ theor~,. m".p~
I64:--167C af ter r~cr~ ~t~ s:ti~n ~rom ethanol~,
he ~-me~c~ptopro~lamine (formuI~ us~: wa~
whth rsî~re~ae t~ Hi~ .. Gh~ a ~6,~ 752 ~lg6~
`-: prep~ bg ra~ti~ commerGisll~ v~i~abl0 3l~br~me~-
:
p~opy~amin~: hgdrabr~mide wi~h c~rbQn di~ulphid~ to giv~
. .,
2~ mer~aptohgdrothiazine, cle~va~e ~ith hg~rcbromi~
~- a~d; lib~r~ti~n of the b~s~ with cau~ic ~o~a so~ution
,
~ : i~ 219~ ldo~
... ~ .
L5 IOb~4}-N~ph.th~1.)~"4-dihycl~o~H ~7--*hiazin~-
~p~ oindo~ 6(~1Qb~ o~ pr~pare~C 811~1~0gO1~5ly
4 E~mEl~ 565~ ~ M~ 2`~8--22~3~C ~d~comF~.)
a~ter $tir~ g up wL~h Qth~r~
Q lQ~ M~.thglphen~ 3 ~ ih~drQ~ 3;i~--thiazi~-
., .
~,3-~soi~L-6(10b~ n~ wa~ p.r2psred ~al~g~s~
to 13~1~a 1 in 4.1~ ~ial~ M;~p~r 149.--15~ a-~t.
~tir~i~6 up with Qth~r
~b~ ~uorophe~ di~ 2H.~thi~zin~--
isoi~ Qb~ prepa~r~-d enalo~u31æ
to E~mp~e 1 itl 63~ ~e~ 6~-~63~ ~f'c~r
~; a~Girring up with eth~r.
~ .
, . . . .
- :, ~, ,
,
~ . -
.,
s ~
~25--
:;; ~
9-~hl~ro~-~b~ph~n~.-1 3 ~ ihydro-2~ thia:zino-
~,~9 3 ~i~oindol-6(1QbH~-One wa~s prepared 3na10gou~1J
t4 :E~ample I i~ ~7~ ~iffld.~ p~ I7~--175C aft~
5 ~tirritlg up wi~h ether~,~
13Eamn-~s! 6
. . ~
lQb~:3~ th~x~pheng1~--3 ,,4-dih~dro 2H~ thia~
~,3-2,7~i~oi~d~-6~:~61bH~. ~na wa~ pr~p~r~d ~x~a1~o.u~
. t~ ~m~ .iel~ I"R`r 165~ ~fter ~tirring
1 up w~ ~th~
, ,
E~am~e~ ~
)b-(~3~ ime.th~1p-h~n~ -3~-~h~ro-~I
`~ thiaz~n~ oindo1-6~10b~ One wa~ prapare~
``~, anfflogous1~ to. :E;x~m~ in 41~ di, M~p~ ZQO0a
~, ~5 a~a~ stir~in~ up wiL~h ethe~,
l~b--~her~ 3 ~4-~:ihg~Qi~X~ox~in~ 3-~-
-, isoindol-6~1Qb~ one~
~ g ~2~ lamQl)v 2-be~zQ~be~zoi¢ aci~ w~r~ dissoIv~d
;~ ln 8Q~ mî ~b~ t~lu~a snd.~ a~t~r ~d~iti~n G;~ 6 g
5),: ~mol),; ~_emin~ proEanolQ~ a$ w~ll a~ c~ a cRta1~ti~
~mou~t ~ p~to~u~ne~Lulp~h~niG ~ei~,. heal~ed und~r- ~ef}u~
~o.r three~ ho,ur~ ~n ~; w~te~ ~arat~r.. !~he ~o1v~nt
then rom~v~ a v~:~u~m3 an~ the re~i~ue; pu~ified bg~
Z~, column c:hrcmat~grsph~ c~ ge~ 6~ w.ith ether,i~'
i~hff~:an0 3,~2 ~ ~1u~t~ Yi~ 4 ~ ~61a~ ~I th~r~ 9
m..p~ lZ9~ Q~-~ a~e~ recr~atallis~ti~n ~rom e~th~n~1.
~ .
.
,,
.,: .. . .: , ,
-~ , . - -, , , ~ , ~ .,
,; ' ' ~
:~ 2 ~ 3 '.~ 3
--26
}~b-M~*hyl.-3~4-dih~dro~2H.-~T~7. oxazinQ~3-~
i~indol-6(1~:tbH~. -One wa~ ~r~p~red ~nalogou~ to
ampl~ 9 in 26~ la. M~p~ 86-89~a, ~t~r stirring
.~......... 5 up wi~h eth~:r,~
. lOb~ Meth~lphe.ngl2-~.5,4-~ih~dr~.-2~ oxa.zino-
3-aj77-is~n~ol-6(2~ lOn~. w~ pr~pa~ed anel~gou-~l~ to
mp~ 3 9 in ~2~ gieId.. M~p~. 111)--114~E af~r recrgst-
atio~ ~rom athanol~
~ample 11
b~ N~phth~ ihydro.-2N~ 7;~xazino-
~` ~,7~ a~.'iso~ol-6(2H~;-o~e w~prepared ~n~gously t~
Examp-~a 9 in 53~ ~ia-~d~ M~p~ 153 158~C a~r recry$t-
~5 ælli~ n from.e~hsnQl5
Ex~mp~ 12.
lOb~(2-~hi~n~ "4-~ dro~2H~ oxazino-
~ ~,3 ,'i~Qin~ 6(2E~-One~wæ~ prep~red ~nalogou~l-~ to
;: Exampl~9 in ~ iel~ M,~p, l~Q~b sfter r~cry~.~al~
~ 2Q ati~ o~th~n~10
,
4~ b~ ~ 9~dih~ 2~ xazino-
~t~ - isoinaoI~(2~2-one W8S prep~red analo~ou~l~
to ~ample 9 ~n 3~Y ~ Id~ ~p~. 67-70~C after ~b~rrin~
2~ up ~ith ~h~r.v
c ~- , ,
~,- E~am~ 4
9-~hl~ lQb~phe~I 3,.~-di~dro-2H.~ oxa!æino-
oindol-6~2H)-~ne wa~ prepar~l analo~ousl~
.. ..
. ~ '. :, . - :,, . . .: ; '
,. .
; .
: ` 2~-d~b~
.~ --2~--
to Example 9 in Ll,5~ ~rield~ M~p.. 13I 134C aftex
stirrin~ up wdth eth~"
'' ~ . ,
lOb-(2~P~ridy,1)-39!4-dihy~ro-2H~ 7-oxszino~
5 ¢.~ oindo~6(~ c3ne wa~ preEsred: an~ sousl~
tc 13xampla 9 in 2726 ~ield.. M~p~ 169C after ~tirrin~
up with ~ th~
b Phen$1. 1,j~ ?4 ,10b--te~r~h~dro-p~rimido~
, ~
I0 isoindol-6(2H)-on~
~: .
~ 8 ~ (21 mmol), 2-benzo~lbenzoic acid w~re di~solv~dL
in 80 ml ab~.. toluene and, af ber additicn o~ 3~7 g
(5Q mm~l) lpl~-diaminopropane, ~ well as of a c~3ta-
l~ric. amourlt ~I p-tol.uena~ulph~ic a'cid~ h~te~ und~r
15 ~Iux î~r thre~ hour~ on a wa,t~r ~ e~ar3tor. !~h~
~olve~t w.a~ th~n ~mo~red in a vacuum and the re~idu~2
~; pu~i~ie:d bg column chrom~to 3;r.aph~ on :3ilic8 ~5~1 60
with ff.ther~isoh~x~ne 2~1 a~ eluent. ~ield 3~ 56q6
of th~or~, m..p.. 18~-18~C aft~ recr~stallisati~n
2Q ~rom ~.l~h~ol~
:
,,
lQ~-(3~th~1phen~ 4 ~ 3b-b~trah~drop~rimido_
Ri~doL 6(2H~-one wa~ pr~pa~ed analQ~;o~
to. :E:xampl~: 17 in 439~ ~ield.. M~p~.~ 12a~G ~f~r ~tirri2~g
25 up with sthe~,
~ E~ampl~ 18
: ! lOb-Phe~xl 3"4-aiha?aro~ ox~zino~
i~oin~ol~6 (.lCtbHl~-thione~,.
. ,
. . .
.. ~ . . ~ . ~
, -. . ~
. .,.~ . ~ . , : ~.
, ~ :
.
,
2 ~ 3 3
- 28-
:~ 2 g 10b-phen21~374-dih,ydro-2H.D)~ ox~zino
3-~ -i30indo1-6(10bH~-one (c~.. Example 9) in
W ml diox~e were mixed with 3.35 g of ~awes~on's
~; r~a.gent a~ ~tirred ~or 7 h ~ 600C (~C control~
A~t.er co~ling, it i~ ~iltered off from precipit.ate,
the filtrata evapor~ke~ in a ~acuum a~nd the ~e~idu~
purifie~b~ oo1umn chr~matogr~ph~ ~n ~îlica ~1. 60
with heptane~meth~1 ethD1 ~etone 6/1 ~ a1uent~
e1d ~D72 g (81~ 0~ th~o~$~9~ p~ 148-~5~~ ~f~r
IQ r~c~ allis~ti~ from o~b~no1
E
-ibitiQn of re.v~e.tra~ript~
h~.3~ ening te~t s~ em contain~ ~he puri~i~d
R~ ~r~m ~ , which had be~n expreis~a b~ genc-
~
te~hnol~ical.method~ in E. coli, ais well as thaaompone~ts ~f the initia~ti~n compIex, au¢h 8~ i~ite-
:;: com~lementar~ 18m~r ~ Q.~u~l~oti~ iB~ pri~r~
~; ~ha ~ ~ ~h~midi~e-5'--~riphosph~t~ ~n¢orp~r~iti~n
w~ mea~.ur~d b~ ~ountin~ in i3 ~.~¢OU~ In kh~
; 2~ ~o~o~win~ ~abl~ thsre.i~ Q~ the I¢50 ~aLue fo~
th~. in~ff~Gmg;i~te~ Gomp~u~a~ ~!hia v~lu~ pondis
~02 th~t GOnGent~ati~n Q~.. the toi~t ~ub~t~ ;whi~h
b~in~3.ebQu~t ~n inhibition o~ the. ~v~r~e tri~n~-
cripta~ bg 5Q~ ompiBri~on substa~Ge~l th~:
va~lue for ~Z~. wa~det~rmined,c.~rre~pondin~
':' '' '' ,'` ' '' ~ ' '
29 -
~ ~ `
~ 21ID~63
~ ~ Re sult s
., .
.-~ substance. of inhibi~on of tha
. ~ the. :Egample ~;5o ~ M_7
1~. 2~2 ~ 6
3 ~_8 x 1~-6
. 5 3 2 x 1~}-5
6 ~ 5 :r 10-6
: 7 1_0 ~1: 10-6
.
,`: 8 5~1 x 11f)-5
; 1~ 1 ~ 3 :x ~.o~5
P'` 11 ~.~ x 10-~
~ '1~ 1,0x~~~
13 1,0 x 1(:~-5
: ' 1~ 7~2 x 1~-6
` ~ 16. 1~0 x 10-5
18 3,5 ~F la~6
`
:~:
.
.
. ,
,,
,
" .
, .:
.. . .
, ¢
.
:,
",
.: