Note: Descriptions are shown in the official language in which they were submitted.
- 3 ~ 3 ~ 1 0 E : ~q ~ r,, ~ 5 ~ ,j ;,~ F ~ O ~ o ~
3 ~
C:yclic Compollndæ and Their U~e
~L~
The pre ent illvention relate~ to novel cyclic
5 compounds havin~ e~cel lent pharmacolo~ l a~tions,
their in~rm~diates for ~he ~ynth2sis and salt~
the~eo~ .
Mo~ ~pocifically, the pre~ent in~ention relate~
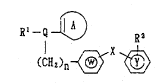
to a compound xepr~sen~ed by the ganer~l ~onnula
, ~
R~ 2
X I (I)
(Cl~2~n~ ~
l5 ~hi3rein R' i~ an option~lly ~u~titute~i h~roc~rbon
re~idue which i~ o~tionally bound ~hrou~h a }letero-at~m
or an o~tiona~ ly jubs~i~ut~d acyl group; R2 iS an
opt~onally Rubstitu~ed 5-7 mem~re!d heterocycli~
~e~idue h~Yinq, a~ a gr~sup cap~lbl~ o con~3tituting the
20 xing, ~xbo~yl group, thiocarbonyl gro~p, an optionall~{
~7cidizerd oulfur ~to~ or ~ group aon~r~rt~iblc lntc~ ~th3m~
Q i8 C~I or N; X is ~ di~ect bond or a spacer h~rlng an
atomia l~ngth of two or les~ between t~e ~in~ Y ~nd the
ring W; W and ~ ar~ each an op~lonally ~ub~tituced
25 ~omatic hydrocarbc~n resi~lue op~ionally con~alnln~ a
hot~r~-atom or an option~l ly sau~ atuted het~ocy~lic
re~i~ue; n i6 an in~ege;c of 1 or ~; the rin~ A i~ an
optionally sub~tituted 5-8 member~d cyclic group, and
two of ~ho 3ub~l~ltuants axe ~p~ onally bound to e~ch
30 otht~r to ~orm a ringl or el 313.l th~ o~ d to an
an~lioton~ln anta~eni~tic a~ent containing same.
~19~
~he renLn-angiotan~in ~y~t~n is~ in~Tol~red in ~h~a
3S hoTaeo~tatic ~unc~ion to contrGl fiy~;temic blood
pre~urs, the volume o~ ~od~ ~luld, balance among ~he
... ..
: : ,, ,: ; ~
3~ 3 1i~ T.iK-~ E.~S .S.`.7i.~ 3~d~5 :~ 1'3:
- 2 ~ 35
electrolytes, etc., a~oclated -~ith the aldo~te~one
~y~t~m. P~ela~cion between th~ r~nin-~ngioten$1n sy~te~
and hyperten~ion has bean clar~ i~ied by the development
of ~he inhi~itor~ of angiot~n~in II (AII ) conYertlng
erlzyms ~A~E) whi~h produce angiot~n~in II havLng a
strong vasocoIl~tXictive action. Since angiotensin II
con~ict~ blood ves~el ~o ol~vate bloc;~d pre:3s~ vi~l
the angio~n~in ~I rec~ptor6 on the c~llular ~e~bran~,
an~1ot~n~in II a~tagonists, llke the P.CE inhibito~,
Chn be u~æd $or the th~rapy o~ hyperten3ion cau~ed by
angioten~in II. It ha been reported tha~ a num~r o~
nngl~ol;cs~oirl II ~n~lo~ue4 ~uch ~ ~arala6in~ ~S~rl,
I1~8] AII ~nd t~e like 3?oS9~ss poten~ angioterl~in I~
antaç~oniSIn. It ha~, however, ba~n reported that,
lS p~ptide an~agoni~ s shows a ~hort t~3rm action by
pa~enteral admini~tra~ion and thay ar~ ine~featlve by
o~el ~dmini~t~tion t~l~ A. Ond~ttL a~d D. w. Cu6hman,
~nnual Report~ in ~edisinal Chemist~y, 13, 82-91
~ 1978 ) ~ .
0~ th~ other hAnd, ~or ~ Jing th~ problerns
ob~erv~ ln t~ peptlde angloton~ in I I ~n~qoni~t3,
~tudio~ on n~n-pep~id~ an~iot~n~in II antagoni~t~ have
been developed. In ~he ea~lie~t ~tudia6 in thi~ ald,
imid~zole derivatiYe~ ha~ring angi~>t~n~in II ~n~agoni6m
2S h~e b~3en disclo~d ln J~paslese p~t~ant unexa~nined
public~tion liaO. 56-71073 (JPA 56-71073), ~P~ 56-71074,
3PA 57-98270 a~d JPA 5~-1S77~8, USP 4,355,040 and
4,340,598, et~::. Later, improved imidazole derivat~ve3
are dlacls~d in EP-0~53310, EP-0291~69, EP-0324377,
~P--~03~S~, WO--~100277, J~A 63--~3~68 and ~?A x--117B7~;
pyrroLa, py~ole ~nd tri~ol- dorivativQE; in EP-
032384l, ~P-040g332 and ;rPA 1-287071, benzi~aidazole
d~rivatives~ in USP 4,880,804, ~P 03g2317, EP-039~732,
EP-Q4~0~3S ~n~ 3PA ~ ~3.~64~ azaLnd6~ne derivative~ in
. 3~ 03g~7315 p~J:;m~do~ d~rivati~rG~ i~ E2-04073J.2;
;7yrldine deri~ative~ in EP-0475206 and ~P-0499415, ~nd
,, .
.. . .
'~
3 3 ~ 3 1 3 ~ : , ; T .. ~ .. .... E .. S . ~ e . ~ . 3 ~ , 3:
- 3 ~
qulnazolinon~ deriv~ive~ in EP-~411766; ~J ~ngioten~in
II antagoni~t~.
Howe~ra~, Ln order to b~come a 2racticall~ u3eful
ther~pelltic agent, angioten~in II ant~oni6t~ are
5 r~ui~ed to ha~Je a ~rong and long actln5~ an~ioten~in
I~ an~cagoni~tia and hypo~ens~ve action by oral
admlnL6;tratlon. ~ ~how~L in EiO Ar kno~n li~r~tu~e
re~xence~, the pro~erable st~uctural ~eature a~ ~
~tron~ angio~en~i~ II antagoni~t is ~on~iclera~d to have
10 an ~cid gro~p, for ~xample, ~et~zol~ group or carboxyl
group on the biphanyl 0id~ chain, e~poei~lly ~&trazole
grc~up ao ~o~t pr~D~uxabl~ ona ~nd clirical t4~ of
compound~ h~ving the ~etra~olo group ~or ~nti-
hy~ert~n~os ~:gent i~ conduc1:ed ~Y. Chri~ten, B.
W~ber~ 3. Nus~berger, R. J. Lee~ P.B,l!~.W,~.
r~an~, and H. R. ~runner, A~. J~ Hyp~r en~.~ 4,
~5~S (199l~]. ~ow~v~rr ~o~po~nd~ ha~in~ tet~ol~ rin~
~nd azide compounds ~ be used for synth~ ing th~
have been known as involving a dan~e~ of explooion,
whlch bec~m~s a ~ u~ prubl~m ~o the larg~ scale
~repa~atlon ~nd indu~ial p~odu~tion.
Th~ pre~en~ inventor~ conqLd~red ~hat co~ound~
actin~ to cont~l ranin-an~ioten~Ln sy~e~ as w~ll as
b~ing clinically u~e~ul for the ~r~atm~nt sf
2~ ~lrcul~tory d~ease~ ~uch ~ hypR~t~n~io~, e~rdiop~thy
~hypercardia, h~art failure, cardia~ infar~io~, etc.),
cerebral a~oplexy, a~ improving ~erebral ~un~ion, ar~
~equ~d to ha~ an angiot~n~i~ II recep~o~.
~nta~onis~ic ~ctivit~ ~nd also hav~ a s~ron~ and l~ng
30 el~ive ~n~iotsn~Ln ~1 antagc~ni~tio as:t~Yity ~nd
hy~ot~n~ive actLon by oral a~ministxation, ~nd they
hav~ ~de ex~en~iv~ and intensivo ~tudi~.
A~ a ~sult, the present in~rento~ h~v~ fo~d a
no~r~l cycL c compo~s haY1ng~ a. ~;?o~eslt an6~iot~nain II
35 :~ece~tor ~nt2l~0ni~tic ~c~ivi~ a~ well as a lon~ ctlng
and ~t~ony AII antag~nis~ nd anti-hyp~rt~n~
~ ~ - .' , 1 . .. , . . -
. .
,
33~ 7~ ..... 3~ s.~ ; C-~ C~
. .
2 ~ 3 ~
~ction~ by oral administ~ation. 'rhe pre~arl~ in~entors
h~ve f ur~her ~eveloped ~tudies to accoDlpli6h the
}~e~ent in~ention.
5 ~L~
The pre~ent invention i~ l:o provi~a the no~l
cy~ c compounds having a h~terocy~ c x~idu~
~ubstitutable for a ~tron61 acid gr~up such ~ totrazole
or car~oxrlic group, which ha~ a strong an~iotensin II
10 ~nt~gonistic action and antihyE~erten~lv~ action arld
whlch can be put to pract~cal use xa~is~. ctorily a~ a
m~di~inal ag~n~.
~oxe ~poc~fically, tht3 pre~ent inventiotl relate~
~o
15 ~l) C~Qpound~ repre~nt~d by the fox~tula (I):
~ t Q ~
~1H2~n~ ~>
[~rh~r~in Rl io An ~ptionally ~ >st~ tutt3d h~droaa~on
res~d~l~ whi¢h i~ optior~ally bound throuc~h ~ h~teI~o-~tom
and or an opt~onally sub~tituted i~cyl s~roup; * i~ An
~ptio~lly ~ub~itu~d $-7 meml:~er~d h~erocyclic
25 l~e~idue havia~g, a~. a g~oup ~ap~bl~ of ~on~tituting l;he
~ing, ~rbonyl s~roup, thic~c~rbonyl group, ~n optionally
o~:idi~ sulfur atom or a grou~ conv~rtiblo into ~em;
Q i~ CB or M~ Y i~ a dire~t ~ond or a pA~e~ avin~ an
atonl~ length o~ two or 7 e~ ~tw~n the ring Y ~nd the
30 ~ing w; rlng~ w and Y ar~ each an optlonally
~ub~tituked ar~matic hydrocArbon residue o~tionally
contaLn~nq a h~t~ro-atom ~r an optionally sul~8~ttuted
h~ero~5rclic res~due$ n i~ an intQgor o~ 1 or 2; the
y A i~ a~ c~p~ior~ ubs*~t~tec~ 5~ ~d~ a~rclic~
35 ~ro~p~ and two of tho ~ub0tit-~n~ ar~ option411~ ~und
l:o ~as:12 other to for~n a ring~ or a ~alt ~h~res~,
', ' : .
.: "' ,: : ' :
'.'. - . ' ,
.
93}1~ 3~1~E !-~ E~.~ ?.~ 3~^~r ~ Al;J; 7''``1
_ 5 ~ fi l ?3 S
pre~erably,
~2) a compound of the abo~e general formula (I),
wherein th~ atom adjac~n~ to the po~ition wher~ th~
rlng A is bound to Q i~ a ca~bon atom sub~tituted ~ith
S a group capabl6~ of liber~ting proton or a group
conver~ible thereinto in ~ living hod~, or a ~alt
thQr~o~ ~ and an ~rlgioten~in ~I anta~c)nisti~ a~snt
containing a co~pound o~ ~1 ) or ~ s~lt thexeo~ .
Ref~rring to th~ abo~,re-mentioned ~Qne3:~1 formula
10 ~ I ), example~ of thç~ hydrocarbon r~sidue represented by
R~ include alkyll alkenyl, alkynyl, cycloalkyl,
oyclo~lkenyl, ~ryl ~nd ~r~lkyl gro~lpa. Among th~m,
~lkyl, alkenyl and cycloalkyl group~ a;ce pr~e~a~le.
The hydroc~rbon r2~idue ln~y be bound to Q t~.rough i~
15 het~sro ~to~, or Jnay b~ ~urther ~ub~titu~3d with ~
Eiub~titu~nl; uch as an optionally bound throuçlh the
hotoro ~tom as~dJor opt~ onall~ ~ub~ utc~d hyd~oc~rbon
r~idu~ ropre~nt hy Rl.
~he alk~Tl group repre6en~d by Rl is a 6traight-
20 c:hain or branched a~kyl group ha~ring 1 to 2~bout ~carbon ~tom~ (C1.8), as exempll~1~3d ~y methyl, ethylr
prc,pyl, ioopropyl, butyl, ~.~obutyl " ~o~-butyl, t-~u~l,
pentyl, i-p~ntyl, h~sxyl, heptyl O~ o~yl.
The alk~nyl ~roup repre~ented ~ 18 a ~traight-
25 chain or branch~cl alk~nyl g~oup havi~g 2 to about 8c~r~on at~, a~ ~x~mpli~iecl by virlyl~ propenyl, 2
I:~u~on~rl, 3-~ut~nyl, i~lo~ut~nyl 9~ :2^ octonyl .
q~hs alkynyl gr~oup ~epre~en~ ~y . Rl 1~ a ~alght- :
::h~in or bsan~hod ellkynyl group h~alJing 2 to about 8
3~ ~ark:on ~toms, a3 ~a;emplif~ed by ethynyl, 2-propinyl, ~-
~nLtynyl ~ 2--p~ntyn~ ~ 2~ ty~yl -
The cyclc:~alkyl grou~ r~pre~nt~d by ~1 iB a
cycloalkyl group having 3 ~ ~bou~ 6 c~rbon ~tom~
~x~mplifi2d hy cyclQprop~L, cyclo~utyl., cyclalpontyl c~r~.
3~ cy~lohe~at~rl .
l!h~ cya lo~lkenyl g;~oup ~pL~s~n~ed by Rl i~ a
.
,
.~
- 6 ~
cy~lo~lkQnyl group having .3 ~o 6 car~on a'com~, 3.5
exemplified ~yclopropenyl, cyclopentenyl or
cyclohexenyl .
~ he ab~e~ ntioneQ alkyl, alkenyl, alkynyl or
S cycloalkyl or cycloalkenyl g.roup may option~lly be
3l~b6titu~ed with hydroxyl ~roup, an optionally
~ub~ti~utG~d amlrl:~ gxoup (~.g. ~mlno, N-Cl ~, alkyl~in~
or N~ di-Cl 6 e,lkyl~mino), halogen, C! 4 .,lkoxy group,
O:C C~ 4 alkylthio group.
The aralkyl S~roup r~pres~n~ed by R~ or
o~ple, ~ phenyl~C~4 alkyl ~uch a~ b~nzyl or
phenethyl, and ~h~ roup repr~ented by Rl is, for
example, phenyl. The aralkyl o:r a~yl group ~ay ~e
optianally s~ub9titllted wit:h, ~n an ~ption~l po~i~lon af
15 it~ ~ryl rir~g, fc~r example, halogen (~.g. F, Cl or Br),
nltro, an opt~on~lly aub~t~uted amîno group ~
amino, ~-CI ~ alkylamino o~ NrN-di-C,.4 alkylamlno)r Cl "
~lXox~ (e.g. m~thox~ or Qthoxy)~ C~ 4 alkylthio (e~g.
~R~t~ylth~o o~ ethylthLo~ o~ C~ 4 alkyl (~.g. ~thyl ~r
2 0 ethyl ) .
~ nong the ~bov~-exe~plifled ~aroups repre~ented b~
~, opt i onaily ~ubstitutad alk:~l or 21kenyl group~
(e.g. C!~ lkyl or ~C2,~ al~enyl g~oup optionally
~b~tit~t~d wlth ~ydrc~xyl ~roup"~mino group, halog~n
~5 or Cl,4 alk~xy g~oup) a~ pree~ble.
y ~;ptionally bound throu~h a hat~ro-atc~m
~e.g. nltro~s~n [~R~3 ~9 ~tand~ ~Gr hydrogen or
optionally ~ub~tituted lower ~C1 ~) alkyl~, oxygen or
~ulur t-~(~ h de~ote~ Qn integor o~ 0 to 2)~
30 81;C. ), e.nd, ~mong thQm, o~;>tionally ~ub~titute~ Alkyl or
alXanyl group optionally bound through a heter~-atom
~e.~. methyl, ~thyl, propyl, i~opropyt ~ acathyl~
snothylam~no, ~ ~thy~;aT~:na; propyl~ J, p:coEff~nyl~m~nc~
iYo~ro~la~ino, al lylarl~ino, butyryla~ino~
35 i60~uty~ylamino, dirn~th~rlamino~ ~3thyl~t~ylaJnino,
3 ~-- Y ~ G i~ _ ~ ,, ; . . . K _ C .i ~ E: . . ~ S .i .: . . F a: r g t _ _ ; JA ~ .;, A ~ ;, _, 3
~ethoxy, ethoxy, propoxy, isopropoxy, propenyloxy,
allylo~y, butoxyf i~obutox~ c-bu~oxy, t-butoxy, l-
butenyloxy, 3-butenyloxy, ~okutenyloxy, pentoxy,
i~spen~o~yO h~x~loxy, methylthio, ethylthlo,
propyl~hio~ is~propylthio, allylthi~, butyl~hio,
isobutylthio; ~ec-but~lthio/ t-bu~ylthio, 2-
but~nylthio~ 3-but~n~lthio, i~obut~nylthio, pcntylthlo,
iso~entylth~o, hexyl~hio, ~hoxym~thyl, ~ethoxye~hyl,
i~opropoxy~ethyl, t-b~tho~g~thyl, ~ethylthiomethyl,
ethylthioe~hyl, t-buthylthioethyl, e~) ar~
pre~r~ble.
~ a~pl~ o~ th~ ~ayl g~oup ~p~nt~d ~y
include ~lkanoyl, alken~yl, al~ynoyl,
cycloal~ylcarbonyl, aralkynoyl and b~nzoyl group~.
Among them/ ~lkanoyl, alkenoyl ~d cyclo~lkylc~rbonyl
: group~ are pre~rable. Th~ acyl y~ou~ may be furth~r
sub~t~uted wlth, fo~ ox~m~l~, a~ o~tionall~
sub~tltut~d hydrocarbon re~idua which i~ optionally
bound through a ho~ro-atom ropre~ented by abo~-
mentioned Rl.
~ he ~lko.noyl g;c~up r~p~;ose-n~d by ~ a
~traight-c:hain or branchel:l alkanoyl ~:roup having 1 to
a~out ~ carbon atom~, a~ exempl~fled ~ ~ormyl, acety~
propiony~, b~ty~yl, isobutyryl, v~leryl, i~ov~ler~l,
hex~noyl, h~ptanoyl or octanoyl.
!rh~ ~lke~ up ~ep;~on~ed by R~
~tra~ht-c~qn or bran~h~d alk~n~l grou~ ha~ing 3 t~
about 8 carl~on ato~nS, a~ exempll led br acryloyl,
m~sth~cryloyl ~ C~otolloyl, 2~ ut~noyl, 3-butenoyl, 2-
3~ ponk~o~l~ 3-penten~yl, 2-he~enoyl, 2-heptenoyl or 2-
octG~noyl .
~ rhQ alky~oyl group repre~;er3~ed by Rl i~ a
8traight-ch~in or branch6~d allcynoyl gr~up ha-ring 3 to
ab~ut 8 c~rb~n t~tom~ ~x~pl~ d b~ ;p:copionc~lf a-
35 butynoyl, 2~pen~ yl c~ 2-~ctync~yl.
I?he ~ycloalkylc!a~bonyl ~rou~ r~prs~erl~ed by ~ i6
: .
,
''
. . . ,, ;: . ~, i:,, ~ , . :
!~
'
3 3~ 1 3 ? 7 :~; T.~E--~ D.4 ~ ` 2 ~ 3 ^ ~ ; C ~ 3 ~
3 5
a ~ycloalkylcarbonyl g~oup ha~ing ~ to about 7 carbon
atoms, as ~xemplifi~d by cyclo~repylcarbonyl,
cyclobutylcarbonyl, c~clopentylcarbon~l or
cycloh~x~laarbonyl.
S ~h~ above mention~d alkanoyl, ~lkenoyl, ~lkynoyl
or cycLoalkylcarbonyl group may option~lly be
subs~L~u~ed wlth, for example~ ~ydroxyl gr~up~ an
optionally ~ub6tituted amLno group [e.g. amino, N-Cl.4
alkyl~min~, or N,N-di-Cl4 ~lkyl~ino~, halog~n, ~1~4
alkoxy group or Cl,4 alkylthio ~roup.
Exa~pl~s of option~ sub~ uted arom~ic
hy~ocar~on ~e~i~u~ o~ h~tarocyclic re~idue optionally
con~ainlng a ho~ero-ato~, which are ~epr~ented by tho
ring Y and the rin~ W, includs aromatic hydrocarbon
ro~idue~ such as ph~nyl, and 4- ~o 7-~embered
monocyclic or condan~ed heteroc~clic r~idu~
~ont~ini~g o~ ~r mo~o o~ ~, S ~nd 0~ ~or oxA~plo,
pyridyl, pyrimidin~l, pyrids2inyl, pyxaziny~, thi~nyl,
~uryl, pyrrolyl, im~dazolyl, ~yra~ol~ othiazolyl,
isoxaz~lyl, ~sobenzofuranyl, b~nzofuran~l,
indolizinyl r lsoindolyl r 3~-lndolyl, indolyl, lH-
incl~oLyl, purln~1~ 4~1-quinolisinyl ~ i6clquinolyl ~
~uinolyl, phthal~inyl, naphthyridinyl, ~uinoxalinyl,
quina~olinyl, cinnolinyl and pteridinyl. ~mong them,
ph~nyl i~ e~erable one.
~he abo~o-mentiQn~d ri~q Y ha~ ~ ~ubsti~u~nt
re~re~nted ~y Ra as exem~lified by an ~pti~nally
~ubatituted 5- to 7-mamb~r~d (pr~f~r~bly 5- to 6-
~bered) ~onoayclic het~ocyc}i~ residue eontaining
one or mo e o~ N, 5 an~ 0 (p~fe~a~ly N-conta~nLng
ho~co~:ocyclic ~:o~i~lu~ h~vlng hydro~n clto~ c~ p~bl~ o
b~ing deprotonate~) or a g~ou~ ~on~ertible ther~into.
X~a~ples o~ ~ho ~roup rQpr~ented by R2 are ~hown
be~
~ .
,,~. , -~ . ,
.
9F~ 9~1C-- G3~ K~C.'. ~.-.TF.:.-S -~S.~ ' Fè:~-r~ -F,'^ 3
~ g _
5 .~' 'f~f~,
~ ~ ff z ,~f ~ f~a ~
Z'~ 2 ~ , z~,
,,~ f O ~
~0
30 ~n the a~ove fo~mula, g~-~H2~ Rg, -O- or -S-; ~-z, ~88
and ~=z~ ~e pectlvely sta~d or a c:æ~b~nyl group, a
oc~r~nyl gxoup o~ ~n optlor~lly ox~d~e~ ~ulrur ~tom
~ æ . ~ . S, S ~ O ~, and S ~ O ) 2 ) ~ ~r~ r~9bly fl ` c~onyl or
thioc~rborlyl ~roup, more pre~rabl~ a car~onyl group.), m
35 ~-nclt~r an int~ger-of-O/ 1 ~r 2, ~n~ R9 ~tarLd~ ~or-hy~r~-or
an op~ionall~ su~atitute~ lower ~lkyl group].
And, b~2side~ ~h~ c~ o~ rbon-c~be1n link~ge AS
,~
g 3 ~ ~ ~ l C : c~ .iK~ E ` - S ~ F ~: ~ r ~ 3 ~
10 ~ r~ ~ ~ 5
in the above Xormula~, a group r~presented by R may
optionally ~e bound to an optionally sub~tituted
a~omatic lhydrocarbon optionally oontaining a hete~o-
atom o~ het~rocyclic re idue, which i~ rep~e~ented by
5 Y, in th~a ca~e o~ g--N~- in the above ~ormulas, through
os~e o~ th~ plu~al number o~ exi~ting nitrogon atom~.
Fo~ e~;ampl~,
when RZ ~ ~, speciflcally
;~ ' 11
~ rN F
4 N~, ~ or. h~
r~ _~
~op~o~ntu that group. Oth~r ~x~npl~ o~ ~ bond@d
~l;hrough the r~itrog~n atom in~lud~
3 ~ f ~ z~
~5 Nr~
tJ~ or Z~Z~ O
Pxe~er~bl~ ~roups repre~ented by R2 are, like 2,5-
dlhy~ro~5-oxo-1) Z,4-o~adiazol-3-~rl or 2r5-dihydro-5-
oxo-l, 2, 4-ti~iadiaz~1-3-yl, ~ho~o ha~ing ~_ or _o~
~roup a~ proton-donor and car3::onyl group, thiocarbolly
~roup or ~ulfinyl group a~ proton a~cel?tor
~l~Qultaneously. And, wh~ le the heterocyclic r~sidue
r~p~o~n~ed by R2 m~y optiontllly ~o~ ~ cond~m~d ring
by ~he l~nkaS~e of ~ubst1tuen~s on t31Q ring, ~r~ 3r~b1e
. . .: : ` ~
33~ 3 13-~7~ .P~E:~.S .S.~ .g~ r~
3 ~
one~ ~e S- to 6-membered het~rocyclic re~idu~s, more
pre~erably 5-memb~red one~. Amonç~ other~, a~ R2,
group~ o~ 'chæ formula
~--I
~whçrein i i8 ~0- o~ -S-,; is G=O, C-S or -StO)T,_, and
10 m is o~ the same meani~g as clefin~d abo~e] l~epecially,
2,5-dihydro-5~o~o-1,2,4-o~adia~ol-3-yl, ~ dihy~ro-5-
thioxo-1,2~4-ox~di~zol-3-yl, 2,5-dihydr~-5~ 2~4-
~chiadi~zol-3-yl ) ar~ p~e~able . In ca~e whera the ring
Y i~ ~or examl?le~ phenyl, ~2~n~y be ~ub~titut~d at any
15 o~ or~ho, mo~a- or para-po~it~on, pre~erably ortho-
pOg i ti~
And, whil~ the abo~e-~ent.ioned heterocyal~
~sldu~ t22) can ~xi~t Ln tautomeric forms as shown
b~low, ~or ex~mple, thre~ t~uto~er9, ~ b and c,
~n ~ ~ Wh~n Z = 0, g ~ O
,
~5 ~ - .
: ~ ~ O O
b e
t~Q hQtR~ocycll~ rssld~ ropr~son~d by th~ formula
r
r'~
HN~ .
.
,
.
~3~! 9,qlO~ ;TA~ED~ PA~E~iTS ~SA:~.q ~e~her~lo~. ; 01;30C~ôOl;~l~/31
-- ~ 2 --
inclu~es all of th~ above-mentioned tautome~ a, b and
P~d, _ ~ff~
5 ~ e~s3nted ~gF~ as ~l~o~n~ h~L~w~ c ~ . Xo~
0
E;xamples o~ ~he ~r-~up repre~;ented by E~10 in~ d~
g~oup~ r~pre~ented b~ the formula -C~ ~4 ) ~oCOR5
~wherein R4 ~tands or hydrogen, Cl5 straight-chain or
lS br~n~hed alkyl g~owp ~e.g. me~h~l, eth~l, n-propyl,
roRyl~ t-butyl, ~-~antyl, l~opentyl, or neopentyl)~
C2-6 st~ai~ht-cha n or bran~ed alkenyl group or C38
~aloalk~l group (e.g. ayalopantyl, ~ycloh~xyl or
cycloheptyl~; ~5 stand~ for C1~ ~t~ai~ht-chaln or
~ranched alkyl group te.g. m~thyl, ~thyl, n-propyl,
ifi~propyl, n-bu~yl, i~o~utyl, ~iec butyl, t-butyl, n-
p~nty~ op~ntyl or n~op~n~yl), C~6 ~tr21ght-chal~ ox
: br~nch~d ~lke~yl group~ C3 ~ cyc~lo~lkyl ~roup te.g.
; cyclopen~yl, cyclohexyl or cy~loh~ptyl~, C~l alk~l
~S group ~u~titut~d with C3~ c~cloalkyl group (e.~.
cyclopentyl, cy~lohexyl or cycloheptyl) or an
optionally ~ubs~lt~t~d ar~l group s~ch a~ phenyl (e.g.
be~zyl, ~chloroben2yl, phen~thyl, ~yclopentylmetnyl o~
a~aloh~x~l~athyl), C~ 3 Alkenyl g~ou~ ~ub~tituted ~ith
C3~ cy~loalkyl ox an o~t.i~nally ~ubstitut~d ~y~ ~rou~
such a~ phenyl ~e.~. a g~ou~ ha~ng alkenyl maiet~ such
. a~ vinyl ~e.~. ~innamyl), p~op~nyl, allyl ~r
i~o~rop~nyl), an op~ionally subs~.ttu~ed ar~l ~roup ~uch
~. as ~he~yl ~e.g. ph~nyl, p ~olyl or r.~ph~hyl), Cl6
! 35 ~tralght-ch~in or branched alko~ group ~ thoxy,
:' .
,~
~-, - -
'
: , . , . . :. ::. , . ,. . ::~ ~
.
~3~ 9~1S~- 33~ K-~C.~ Pr.~ S.~X~ F~:^-r~ ^3~ J j~15. 3:
, ~
- 13 ~ ~ n~
~thoxy, n-propoxy~ isopropoxy, n-butoxy, i~ob~toxy,
~ec-buto~y, t-bu~oxy, n-pentyloxy, l~opentyloxy or
~opRntyloxy), C28 s~raight-chain or br~n~hed
~lkenylQxy group (e.g. allylo~y o~ isobuteny1o~y~, C3,~
S cycloalkylo~y grou~ ~e.g. cyclopentyloxy, cyclohexyloxy
or ~y~lohept~lDxy), Cl,3 alkoxy ~oup substituted wi~h
~3-8 cycloalkyl (~Og. cyclopontyl, cyclohexyl or
cycloheptyl) or with an option~lly ~ubstitu~ed ~r~l
~rou~ such as phenyl ~e.~. a group ha~ing alko~y moie~y
~0 ~u~h a~ methoxy, ethoxy, n-propoxy or isopropoxy) ~.g.
benzyloxy, ph~neth~los~, c~clopent~lmethyloxy or
cyclohexylmethyloxy) ~ C2~3 al~nyloxy ~roup ~ubstltuted
wi~h C~.8 ~y~lo~lkyl (e~g~ cyclopenSyl, ~ycloh~æyl.or
cyclohep~yl~ or wlth an optionally ~ub~ti~uted a~l
group ~uch A~ phenyl ~e.~.. a ~roup h~vin~ al~onyloxy
moi~ty ~u~h ~ ~inyloxy te.g. cinn~yloxy)~
propsnyloxy~ ~llyloxy o~ i~opsopQnylo~ n arylox~
group includ~ny optionally ~ub~titutod phenoxy te.g.
pheno~, p-~lt~ophenoxy or nap~ho~y)~, and an
optionall~ ~u~t~tuted alkyl ~e.~. Cl4 alkyl) or acyl
(e.g, C25 alkanoyl or optionally ~ub8ti~uted ~nzoyl).
Xxample~ o~ Rl lnclud~ methyl, ~t~lyl, p opyl, t-butyl,
methoxym~thyl, tr~phenylme hyl, cy~anosthyl, a~otyl~
p~opionyl, pL~loyloxymethyl, 1-
~5 ~cycloh~xylo~yca~bonyloxy)e~h~l~ (5-~ethyl-a~oxo-1,3
dloxolen-4~yl)methyl, acetoxy~ethyl,
proplonylo~y~othyl ~ n-but~ryloxymoth
isobuty~ylo~ thyl, 1-~ethoxyca~bonyl~xy)~th~
e~ylo~y)ethy~ (isobutyryloxy)ethyl,
cyclohex~lcarbonyloxy~thyl, b~n~oyloxy~ethyl,
cinn~mylc~rboxylme~hy~ an~
: cy~Lop~ntyl~arb~nyloxymothyl. Such group~ m~y inalud~
-~ ~ub~tituent~ which 2re capable o~ ea~ily convertin~
into th~ ~n~t~a~l he~er~y~ r~ldue~r~pro~nted~b~
3S ~ha ~on~ula
:,
.~ .
:. . , . :: ~ .
.
.. . . . .
.
3~J 3,~1~E _i3l17 ~ jT.. :~-L.~ s '`~ .i F~ 3~ - : 0^3^~
. ~
1 4 ~
'~ ~55N
e~th~r chemic~lly or bi.ologicall~ i.e. und~r
~hyoiolo~i~nl aondition~ ~ ~v~ oxam~le, in ViYo r~ iO~L
such a6 osid~tion, ;~eduction or h~drolysis cataly~ed by
in Y2~ enzy~e~) (what i~ c~ d prodrug).
A~3 the ~bo~e-m~ntioned tauto~ner~ of heterocyclic
re~ldue~ ( a, ~ ~nd c ~ and ~he heterocycllc realdue ~ a ~,
b ~ ar~d c ~ titu~d ~lth ~ ~r~ lucl~d in th~
hetæ~ocyclic re~idu~ ~eb~esent~d by the ~ub~titu~nt ~2
in th~ pre~en~ in~ntion, so ~he tautc~ner~ and ~he~r
15 ~ubetituted ~olnpound~ of variou~ h~terocyclic rasldues
de~cril:~d ~n th~ foregoiny are, a~ a m~tte~ of cour~,
in~lladed ~n the ~ub~ tuent R2 in the ~reoent
lnvention. Ar~d, 'che su~st,itu~nt ~2 ll~y h~lY13 urth~3r
~ubstitu~nt~ other than kho~ rep,re~ntod by ~10
20 described ~boYe~, as exemplifietl b;y an optionall~r
~ub~L u~ed allcyl group ~ e . ~ . tde~t~yl and
tr~ phonyl~a~thyl ), halogon ~ ~ F , Cl arld E~g ), ~ltrc~ , -
~ano~ C~,4 alkoxy, and an optiona;Lly ~ub~titu~d ~mino
~oup le.S3. ~nLno, m~thyl~mino and diml~3thyl~ino)~
~5 among oth~r~.
Th~ ring lq lnalude~ optionally sub~itu~ed
z~o~ti~ hydroc~rbon r~idue and hete~ y~lic r~xidu~
~ptlonally con~aini~g on~ or mor~ o~ ~, o and S atc>~n,
~ e~elnpli~ed by ~henyl, pyridyl, pyxa~inyl,
p~lda~inyl, pyx~l21nyl, thienyl, fu~yl, py~rolyl,
~da~olyl, p~ol~l, iooth~a~s~lyl ~nd i~oxa~o~yl
pr~3f erably phenyl ~ .
And, h~ arcm~tic h~rdroc~r~n r~sæidu~ and the
? ho'c~ yclic-rs~dt1~ whiclI m~y opti~ r con~ai~ one
or mor~ o~ N~ O ~nd ~3 atom~ reprQ~nted br tl~e~ ~in~
~nd th~ rinq Y, ma~ optlonally have ~ub~tituent5 a~
~, . ~ . ,
', ,:'
33~ ^-'.133 1i~:7~ D.~ ~ATe: S ~S.~ e~ ; c~3~a ~ 7i3:
, ~
- lS ~
~x~mpli i~d by halogen (~.g. F, ~1 ~nd ~), nitro,
cyano, i-1-4 alkoxy, an op~l on~lly ~ubstl~uted ~mino
group ~.g. amino, methylamino and dimeth~lamino).
6hows that th~ ad~acent ring W (e . g . phenylene
5 grollp) is bound to th~ ring (e.g. phen~l group~
di~ectly cr through a spacer with an atomic chain of 2
ox les~ ~pre~rably direct b~nd). ~s the sp~csr, ~ny
on~3 aan be exemplifi~d, 50 long a~ lt i~ a divalent
chain in which ~he ~umbex of at~n~ con~tit:uting the
lQ straight chain i6 1 or 2, and it ~Zly ha~re a ~ide chain,
~o~e 6peci1call~r C~.,4 alkylena, -CO-, -O-, -S-, -NH-
-Co-ai~- ~ -O-C~- ~ CE~2- ~nd --C~C!H- .
Tha ~ bol n denc~3s ar~ lntege~ of 1 or 2
~ pref er2lbl~r 1 ) .
A~onç;l the compoun~s sho~n by R2, W, X, Y and n
de~cribed a~o~re. repre~ent~d by the form~
~{2~n~ ~ "
2û
tho~e re~re~en~ed by ~he i~ormula
_~ ~3_~X ~ ,
R~' .
~s
}or c~ mpl."
-
' '
,~:
,
.
3,~3~1C~A ~.~.la~ 9t^~ 33_;S' ` ~_
3 5
-- 16 --
~C~ H~ B~
~N
S O ' ~'
-C112 ~ --t~H2~ --CK
o~ N~
~ Oy~
, '' .O ' S
7-~
ar~ ~r0~rabl~
~h~ ~in$ ~ cyclic h~droc~rbon ~ due or
hete~ocyclic ring ha~lng at lea~t os~e ~n~atur~ted bond.
20. ~ al e~ample~ of th~ cycli~: compound~ ~re
~pe~l~ t c~ally ahown ~ bc~ w .
2s d;9 R
3t~ ~ ~3 ~ ~ ~,
35 ~ . i~ v ~ ~
,, ;. . ~, :
, :
'
3 3 ~ 3 P ~ _ h ~,,, ;~; T S ~ S r. ~ .i ? ~ i - r ~ o ~ :
[wherein h and k ind2p~n~ently st~nd fO~ -CH~-, C30,
C-S, S ~ O ~ ," N-E2, O-, -C~=CH~ CH-, -CH-N- ~ -CO-
N ( R9 ~ W ~ R9 ~ -CO-, -N=N-, -N ~ ~9 ) ~ O-N ~ R ' t ~ H=~E~-CO-
U9 ~ stand6 ~or H or an Gption~lly çub~ ut~3d 1~w8
5 ~Cl 4~ ~lkyl group, m and ~ e 0~ th~ same meanln~
def ined above ] .
~hese ar~ m~ example~, and tha pre~ent invention
~hould not be limited thereto.
Tho ~:~o~e~ antion~d ring A ~nay optlonall~y b~
10 ~ ituted with, }~id~ ~he ~tom repre~nted by Q, a
~roul? ~ep;:e~ento~ ~ R~, ~or ~x~mple, a g~oup ~apable
of libl3ra ting proton or a group convextibl~ ther~ to
in~ ~. The sub~itution positlon of ~ pref~ra~ly
the po~itior~ ~d~ac~nt to thcl carbo~ atom to whlGh Q i3
lS bon~ecl.
~ xample~ 0~ the group RJ capable of libera~ing
pro~on o~ a ~oup conYertSble th~.rointo ia vivs~ include
<:ptlonall~ ect~rified or asnidated carboxyl, tetrdæolyl,
txt~llaoro~th~n~ fo~lc 2~41d ~m~ de t -N~3SO2C~
20 pho~phoric a~:id and sulfonic ~cid groups. ~he~e g~o~lp~
~nay option~lly be p~o~ect~cl ~L~h f~n optionally
~ubatltuted lower alkyl group or acyl group, ancl may be
any on~ i~ only th~y a;z~ s~pabl~ of ~o~ing a~ion under
~iolc~gi~al or phy~ lDgl~!al ~o~dition~ I ~OT ~xampl~a,
25 ~Q reaction ~uoh a~ o~ ation, r~cluc ion or
~ydrolysis ~3y in ~iYo en~yme~).
Exa~pl~s c~i optionall~ o~terifi~d or ~nidatsd
carbo~l r~present~d b~ R~ clud~ group~ repr~ ntod
by the formul~ CO-D lwherein D ~t~nd~ ~ox hydrox~rl
30 group, o~tio~ally ~ub~tituto~ a~ino ~e.g. a~ino, N-Cl 4
alkylamino, and N,N-di-CI 4 atkyl~mino) or optionally
~ub~itut~d ~lkoxy ~e~g. Cl 6 alXoxy slroup, whoS~ alkyl
r~oiety i3 optionally ~ub~i'cuted with hydxoxyl group,
o~:ionall~ ~Ub~titUtQ~ amlrlo ~ ~Q~ çr. a~in~
35 dirnethylaminq, die~hylamino, piper~dirLo ~nd
.~ .
: .
- 18 ~
morpholino), halogen, Cl6 alkoxy, Cl6 alkylthio or
optionally substituted dioxolenyl (e.g. 5-methyl-2-oxo-
1,3-dioxolen-4-yl), or group represented by the formula
-o-CH(R4)-oCoR5 [wherein R stands for hydrogen, C16
straight-chain or branched alkyl group (e.g. methyl,
ethyl, n-propyl, isopropyl, n-butyl, isobutyl, t-butyl,
n-pentyl, isopentyl and neopentyl), C26 straight-chain
or branched alkenyl group or C38 cycloalkyl group (e.g.
cyclopentyl, cyclohexyl and cycloheptyl), and R5 stands
for C16 straigh~-chain or branched alkyl group (e.g.
methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl,
sec-butyl, t-butyl, n-pentyl, isopentyl and neopentyl),
C26 straight-chain or branched alkenyl group, C38
cycloalkyl group (e.g. cyclopentyl, cyclohexyl and
lS cycloheptyl)~ C13 alkyl group substituted with C38
cycloalkyl (e.g. cyclopentyl, cyclohexyl and
cycloheptyl) or an optionally substituted aryl group
such as phenyl (e.g. benzyl, p-chlorobenzyl, phenethyl,
cyclopentylmethyl and cyclohexylmethyl), C23 alkenyl
group optionally substituted with C3 8 cycloalkyl or an
optionally substituted aryl group such as phenyl (e.g. I
cinnamyl, etc. having alkenyl moiety such as vinyl,
propenyl, ally, and isopropenyl), an aryl group such as
optionally substituted phenyl (e.g. phenyl, p-tolyl,
~ naphthyl), Cl6 straight-chain or branched alkoxy group
(e.g. methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy,
isobutoxy, sec-butoxy, t-butoxy, n~pentyloxy,
isopentyloxy and neopentyloxy), C2~ straight-chain or
branched alkenyloxy group (e.g. allyloxy and
isobutenyloxy), C38 cycloalkyloxy group (e.g.
cyclopentyloxy, cyclohexyloxy and cycloheptyloxy), Cl3
alkoxy group substituted with C38 cycloalXyl (e.g.
cyclopentyl, cyclohexyl and cycloheptyl) or an aryl
group such as optionally substituted phenyl (e.g.
benzyloxy, phenethyloxy, cyclopentylmethyloxy and
- 19 ~
cyclohexylmethyloxy having alkoxy moiety such as
methoxy, ethoxy, n-propoxy and isopropoxy), C23
alkenyloxy group substituted with C38 cycloalkyl (e.g.
cyclopentyl, cyclohexyl and cycloheptyl) or an aryl
S group such as optionally substituted phenyl (e.g.
cinnamyloxy having alkenyloxy moiety such as vinyloxy,
propenyloxy, allyloxy, isopropenyloxy) and.an aryloxy
group such as optionally substituted phenoxy (e.g.
phenoxy, p-nitrophenoxy and naphthoxy)]}]. And~
examples of the substituent represented by R3 may also
include a group capable of liberating proton or a group
convertible thereinto in vivo (e.g. tetrazolyl,
trifluoromethanesulfonic acid amide, phosphoric acid or ~!
sulfonic acid optionally protected with alkyl (e.g. C
alkyl) or acyl te.g. C2s alkanoyl and optionally
substituted benzoyl).
Examples of the substituent R3 include -COOH- and
a salt thereof, -COOMe, -COOEt, -COOtBu, -COOPr,
pivaloyloxymethoxycarbonyl, 1- -
(cyclohexyloxycarbonyloxy)ethoxycarbonyl, (5-methyl-2-
oxo-1,3-dioxolen-4-yl)methoxycarbonyl,
aceto~ymethyloxycarbonyl, propionyloxymethoxycarbonyl,
n-butyryloxymethoxycarbonyl,
isobutyryloxymethoxycarbonyl, 1-
(ethoxycarbonyloxy)ethoxycarbonyl, 1-
(acetyloxy)ethoxycarbonyl, 1-
(isobutyryloxy)ethoxycarbonyl,
cyclohexylcarbonyloxymethoxycarbonyl,
benzoyloxymethoxycarbonyl, cinnamyloxycarbonyl and -
cyclopentylcarbonyloxymethoxycarbonyl. As such groups
as above, mention is made of any one capable of
liberating proton or a group convertible thereinto
under biological or physiological conditions (e.g. in
vivo reaction such as oxidation, reduction or
hydrolysis catalyied by in vivo enzymes). R3 may be
carboxyl group or a prodrug thereof.
- 20 _ 2~0~3~
R3 may also be groups convertible into anion in
vivo, for example, biologically or chemically.
And, a compound, in which R3 iq a group capable of
liberating proton or a group convertible thereinto
(e.g. optionally protected carboxyl group, tetrazolyl
group, carbaldehyde group and hydroxymethyl group; and
cyano group) chemically (e.g. oxidation, reduction or
hydrolysis), is useful as synthetic intermediaté.
Among the groups described as R3, preferable ones
include carboxyl, esterified carboxyl (e.g. methyl
ester, ethyl ester or an ester formed by bonding of a
group represented by the above-mentioned fonmula -O-
CH(R4)-oCoR5 to carbonyl) and optionally protected
tetrazolyl, carbaldehyde and hydroxymethyl.
The ring A may optionally have, besides the groups
represented by Q and R3, further substituents, as
exemplified by halogen (e.g. F, Cl and Br), nitro,
cyano, an optionally substituted amino group [e.g.
amino, N-Cl4 alkylamino (e.g. me~hylamino), N,N-di-Cl4
alkylamino (e.g. dimethylamino), N-arylamino (e.g.
phenylamino)~ alicyclic amino (e.gO morpholino,
piperidino, piperazino and N-phenylpiperazino)], groups
represented by the formula -U-R6 [wherein U stands for
a bond, -O-, ~S- or -CO- and R6 stands for hydrogen, an
25~ optionally substituted lower alkyl group (e.g. Cl_4
alkyl optionally substituted with hydroxyl group, an
optionally subs~ituted amino group (e.g. aminG) ~
halogen, nitro, cyano or Cl-4 alkoxy group]~ groups
represented by the formula -(CH2)1-CO D' [wherein D'
stands for hydrogen, hydroxyl group, optionally
substituted amino (e.g. amino, N-Cl4 alkylamino and N~
N-di-Cl4 alkylamino), or optionally substituted alkoxy
(e.g. Cl6 alkoxy group whose alkyl moiety is optionally
substituted with hydroxyl group, optionally suhstituted
amino ~e.g. amino, dimethylamino, diethylamino,
- 21 - 2~
piperidino and morpholino and morpholino), halogen, Cl6
alkoxy, C16 alkylthio, or optionally substituted
dioxolenyl (e.g. 5-methyl-2-oxo-1,3-dioxolen-4-yl) or
groups represented by the formula -OCH(R7)OCOR8
[wherein R7 stands for hydrogen, C16 straight-chain or
branched alkyl group (e.g. methyl, ethyl n-propyl,
isopropyl, n-butyl, isobutyl, t-butyl, n-pentyl,
isopentyl and neopentyl) or C5 7 cycloalkyl group
~cyclopentyl cyclohexyl and cycloheptyl)~ and R8 stands
for Cl-6 straight-chain or branched alkyl group (e.g.
methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl,
sec-butyl, t-butyl, n-pentyl, isopentyl and neopentyl),
C28 alkenyl group (e.g. vinyl, propenyl, allyl and
isopropenyl), C5 7 cycloalkyl group (e.g. cyclopentyl
cyclohexyl and cycloheptyl), C13 alkyl group
substituted with C5 7 cycloalkyl group (e.g.
cyclopentyl, cyclohexyl and cycloheptyl) or an aryl
group such as phenyl (e.g. benzyl, p-chlorobenzyl, I
phenethyl, cyclopentylmethyl and cyclohexylmethyl), C~ 3 ,.
alkenyl group substituted with C5 7 cycloalkyl (e.g.
cyclopentyl, cyclohexyl and cycloheptyl) or an aryl
group such as phenyl (e.g. cinnamyl having alkenyl
moiety such as vinyl, propenyl, allyl or isopropenyl),
an optionally substituted aryl group such as phenyl
(e.g. phenyl, p-tolyl and naphthyl), Cl6 straight-chain
or branched alkoxy group (e.g. methoxy, ethoxy, n
propoxy, isopropoxy, n-butoxy, isobutoxy, sec-butoxy,
t-butoxy, n-pentyloxy, isopentyloxy and neopentyloxy),
C28 straight-chain or branched alkenyloxy group (e.g.
allyloxy and isobutenyloxy), C57 cycloalkyloxy group
(e.g. cyclopentyloxy, cyclohexyloxy and
cycloheptyloxy), C13 alkoxy group substituted with Cs7
cycloalkyl ~e.g. cyclopentyl, cyclohexyl and
cycloheptyl) or an aryl group such as optionally
substituted phenyl (e.g. benzyloxy, phenethyloxy,
,~ - .
- 22 -
cyclopentylmethyloxy and cyclohexylmethyloxy having
alkoxy moiety such as methoxy, ethoxy, n-propoxy and
isopropoxy), C23 alkenyloxy group, substituted with C5 7
cycloalkyl (e.g.cyclopentyl, cyclohexyl and
S cycloheptyl) or an optionally substituted aryl group
such as phenyl (e.g. cinnamyloxy having alkenyloxy
moiety such as vinyloxy, propenyloxy, allyloxy and
isopropenyloxy)) and an aryloxy group such as
optionally substituted phenoxy (e.g. phenoxy, p-
nitrophenoxy and naphthoxy)], and Q denotes 0 or 1] ortetrazolyl, trifluoromethanesulfonic acid amide,
phosphoric acid or sulfonic acid, each optionally
protected with alkyl (e.g. Cl4 alkyl) or acyl (e.g. C25
alkanoyl and optionally substituted benzoyl).
One or two of these substituents may optionally be
substituted simultaneously on optional positions of the
ring. When two or more of these substituents exist,
(preferably the case where two substituents exist in
two ring-forming groups adjacent to each other), they
may be bonded to each other to form a 5- to 8-membered
aromatic hydrocarbon residue or heterocyclic residue
(preferably aromatic ring such as phenyl), taken
: together with the two ring-forming atoms. These rings
may furthex substituted with any of the above-described
substituents.
Among the heterocyclic rings as ring A, the
following, for example, are preferable:
. .
~ ' ''' :,
~ ~6~ 3~
-- 23 --
~ ~ ' ~R; ~ ~NIt, ~ R3
S~i ~ N~ ~N~ ~N N~
R~ ~ R3R~ ~R~ '
N~N 1~ R~
3 ~ N-N~,
R' ~ ~X~ ~N~
~3
~ 3
(pre~erably, thiophene, pyrazole, pyridine, pyrimidine,
:~ pyrazine, quinoline, isoquinoline (especially pyridine,
pyrimidine)~ skeleton~ :
wherein R3 is of the same meaning as.defined~abo~e].
Among the compounds represented by the formula
25 ~ pre~erable ones:are:represented by the formula
~ ~
Rl ~N,~
~H~,:: : : I
~ :J~ ~
' [wharein Rl stands for an optionally substituted Cl6
: alkyl group which may be bound through a hetero-atom
(e.g~ O, N~Hj, and S) (preferably C24 alkyl group), R2
stands for oxo- or thioxo-substituted oxadiazolyl or .
~ ` !
: .
,: ~
.
. .
- 24 _ 21~
thiadiazolyl optionally protected with an optionally
substituted Cl4 alkyl group (e.g. methyl,
triphenylmethyl, methoxymethyl, acetyloxymethyl,
methoxycarbonyloxymethyl,
cyclohexyloxycarbonyloxyethyl, and pivaloyloxymethyl)
or an acyl group (e.g. C25 alkanoyI and benzoyl), and
examples of the cyclic group represented by the ring A
include those shown by the following formula,
,~ ~ ~ ~ ~S ,
B3 R3 ~ R~ R
S 0~ N~, ~I,N~, N~N N~,
~ R3 ~ R3 R5 Xs
~ '
R3 R~ Rg R3 R ~ ~3
2- ~ R3
in the above formula, R3 stands for groups represented
: by the formula -CO-D" [wherein D~' stands for hydroxyl
group, amino, N-Cl4 alkylamino group, N,N-di-C~4
~ alkylamino group or C14 alkoxy group whose alkyl moiety
may op~ionally substituted with hydroxyl group, amino,
halogen, C26 alkanoyloxy group (e.g. acetyloxy and
pivaloyloxy), l-Cl6 alkoxycarbonyl group (e.g.
methoxycarbonyloxy, ethoxycarbonyloxy and
cyclohexyloxycarbonyloxy) or Cl_4 alkoxy group] or
tetrazolyl optionally protected with Cl4 alkyl group or
i
.,
.:
- 25 - 2~Q~
an acyl group (e.g. C25 alkanoyl group and benzoyl)].
And, compounds represented by the above-mentioned
formula (Ia) wherein R2 stands for N-
hydroxycarbamimidoyl (-C(=N-OH)-NH2) are useful
intermediates for synthesizing compounds of the formula
(Ia) wherein R2 is oxo- or thioxo substituted
oxadiazolyl or thiadiazolyl.
Production Method
The compounds of the general formula (I) can be
produced by, for example, methods as illustrated below.
Reaction (a)
~l\q~ )n~ ~ R' ,1~)
H ~CH2~n
R~
(])
~wherein A, Rl, R2, Q, W, X, Y and n are of the same
; meaning as defined above, and L stands for a halogen
atom or a substituted sulfonic acid ester group.
The above-mentioned reaction (a) is alkylation
using an alkylating agent ~III) in the presence of a
base.
The alkylation is conductedj employing
approximately 1 to 3 moles each of the base and the
alkylating agent relative to one mole of the compound
(II), usually in an organic solvent such as amides
(e.g. dimethylformamide or dimethylacetamide),
sulfoxides (e.g. dimethylsulfoxide), nitriles (e.g.
acetonitrile), ketones (e.g. acetone or ethyl methyl
ketone) and ethers (e.g. tetrahydrofuran or dioxane).
Examples of the base include butyl lithium, sodium
hydride, potassium t-butoxide, potassium carbonate and
,
.
, :. .
, . .
- 26 _ 21 0
sodium carbonate.
As the alkylating agent (III), use is made of, for
example, substituted halides (e.g. chloride, bromide
and iodide) and substituted sulfonic acid esters (e.g.
p-toluenesulfonic acid ester, benzenesulfonic acid
ester and methanesulfonic acid ester).
While the reaction conditions vary with the
combination of the base and the alkylating agent then
employed, it is preferable to conduct the reaction
usually at temperatures ranging from -78C to 100C for
about l to about 50 hours.
Reaction (b)
~5 R'-~-(C~2)n~ ~ ~ (Cllz)~
~ ~ R~
(])
[wherein A, Rl, R2, L, Q, W, X, Y and n are of the same
meaning as defined aboveJ.
The above-mentioned reaction (b) is arylation by
allowing an arylating gent (V) to act on the compound
(IV) in the presence of a base. I
~25 The arylation is conducted, employing I to 3 moles
; ~ ~ each of the base and the arylating agent IV) relative
to l mole of the compound (IV), usually in an organic
sol~ent such as amides (e.g. dimethylformamide or
dimethylacetamide), sulfoxides (e.g.
~30 dimethylsulfoxide), nitriles (e.g. acetonitrile),
kstones (e.g. acetone or ethyl methyl ketone~ and
ethers (e.g. tetrahydrofuran or dioxane).
Examples of the base include bu~yl lithium, sodium
hydride, potassium t butoxide, potassium carbonate and
sodium carbonate.
As the arylating agent (V~, uce is made of, for
1.
:' .' ' :. . . '
., . ,, ' .
- 27 ~ 3~
example, substituted halogenides such as chloride,
bromide and iodide, and substituted sulfonic acid
esters such as p-toluenesulfonic acid ester,
benzenesulfonic acid ester and methanesulfonic acid
ester.
While the reaction conditions vary with the
combination of the base and the arylating agent then
employed, it is preferable to conduct the reaction
usually at temperatures ranging from about -78C to
about 100C for about 1 to about 50 hours.
Reaction (c~
a~ Rl~Q,I~ ' '''
~: 15 ~CHa)n ~ (~H ) ~_
[~}X~N ~X~C~NoH
(la) (Ib)
~o ~ \Q~
~C~2)n
~X~ C~
: 25
wherein A, R1, W, Y, Z and n are of the same meaning
: as defined above].
I The above-mentioned reaction (c) is to obtain the
oxadiazole compound (Ic) by converting the cyano
compound (Ia) into the~amidoxime (Ib) followed by ~he
ring formation.
The reaction for obtaining the compound (Ib) is
conducted by using approximately 2 to 10 moles of
hydroxylamine relative to 1 mole of the compound (Ia)
-in a conventional organic solvent.
Examples of the solvent include amides (e.g.
... .
,
- 28 - ~ n~
dimethylformamide and dimethylacetamide), sulfoxides
(e.g. dimethylsulfoxide3, alcohols (e.g. methanol and
ethanol), ethers (e.g. dioxane and tetrahydrofuran) and
halogenated hydrocarbons (e.g. methylene chloride and
S chloroform).
When an inorganic acid salt (e.g. hydroxylamine
hydrochloride or hydroxylamine sulfate3 or an organic
acid salt (e.g. hydroxylamine oxalate) is employed as
hydroxylamine source, the reaction is conducted in the
presence of a suitable base (e.g. potassium carbonate,
sodium carbonate, sodium hydroxide, triethylamine,
sodium methoxide, sodium ethoxide and sodium hydride)
of about equimolar amountO ~ h~-G~se~-~f-us-i~-n~
While the reaction conditions vary with the reagent or
solvent then employed, the reaction is preferably
conducted at about 50C to about 100C for about 2 to
about 24 hours, after the hydroxylamine hydrochloride
is treated with sodium methoxide ox triethylamine in
dimethyl sulfoxide.
The thus-obtained amidoxime (Ib) is allowed to
react with chloroformate (e.g. methyl ester and ethyl
ester) in a conventional organic solvent (e.g.
chloroform, methylene chloride, dioxane,
tetrahydrofuran, acetonitrile and pyridine) in the
presence of a base (e.g. triethylamine, pyridine,
potassium carbonate and sodium carbonate) to give an o-
acyl compound.
Preferably, the reaction is usually conducted by
using 2 to 5 mole~ of ethyl chloroformate relative to
one mole of the amidoxime (Ib) in the presence of about
2 to about 5 moles of triethylamine in tetrahydrofuran
at temperatures ranging from 0C to room temperatures
for about l to about 5 hours.
By heating thus-obtained o-acyl amidoxime in a
conventional organic solvent, the cyclized compound
(Ic) is easily obtained.
. .
..
, '~ " '~ ' , ' ` '
- 29 - 2~Q~3~
Examples of the solvent include aromatic
hydrocarbons (e.g. benzene, toluene and xylene), ethers
(e.g. dioxane and tetrahydrofuran) and halogenated
hydrocarbons (e.g. dichloroethane and chloroform).
Prefarable reaction conditions comprise heating o-acyl
amidoxime compound for about l to about 3 hours under
reflux in xylene.
Reaction (d)
~ ~ R~\~ ~ R
L C~2~ -~ CH
~Il) (~'1) ~ (Id) ~ H
C~13
[wherein ~, Rl and Q are of the same meaning as defined
above].
The above-mentioned reaction (d) is to obtain the
oxadia~olone (Id) by hydrolyzing the compound (VI)
produced by alkylation of the compound (II) with the
alkylating agent (Xd) obtained in the reaction (k)
described later.
Examples of the organic solvent include ethers
`, 25 (e.g. dioxane and tetrahydrofuran) and alcohols (e.g.
methanol and ethanol).
As the alkali, mention is made of sodium
hydroxide, potassium hydroxide and lithium hydroxide.
Preferably, the compound ~VI) is reacted at 0C to
room temperatures for about O.S to about 2 hours with
about 2 to about lO moles of a 0.5 to lN aqueous
solution of sodium hydroxide.
Reaction (e)
., ,, ,,;
:
_ 30 ~ 3~
R~
C0OR3 ~ I ~OOI~
~2)n ~c~}2)n
~x~R2 ~X_~Ra
(1~
[wherein Rl, R2, R9, Q, W, X, Y and n are of the same
: 10 meaning as defined above].
: : The above-mentioned reaction (e) is to obtain
carboxylic acid (If) by alkali hydrolysis of the ester
compound (Ie).
: This reaction is conducted by using alkali in an
15~ amount of about 1 to about 3 moles relative to one mole
: of the compound tIe) usually in a solvent such as
~ aqueous alcohols (e.g. methanol, ethanol and methyl
: cellosolve).
Examples of the alkali include lithium hydroxide,
~ sodium hydroxide and potassium hydroxide.
: The reaction is conducted at room temperature to
; about 100G for about 1 to about 10 hours, preferably
at~about the boiling point of:the solvent for about 3
I to:about 5 hours.
25 ~ Reaction (f) ~ :
COOT~ ~ > i coOR~
30 ~ C~2)n ~ 2)n
,
~X~' ~ ~X~2' ~ ~'
([g)
[wherein A, R1, R2, Q, W, X, Y and n are of the:same
meaning as defined;above,~and R1l stands for optionally
: substituted alkyl group shown by the afore-mentioned
;
.
.
,' , .. ' , ,. ' '
- 31 ~
Rl ] .
The above reaction (f) is alkylation by an
alkylating agent in the presence of a base.
The alkylation is conducted by using 1 to 3 moles
of the base and about 1 to about 3 moles of the
alkylating agent relative to 1 mole of the compound
(If) usually in a solvent such as dimethylformamide,
dimethylacetamide, dimethyl sulfoxide, acetonitrile,
acetone and ethyl methyl ketone.
Examples of the base include sodium hydroxide,
potassium t-butoxide, potassium carbonate and sodium
carbonate.
Examples of the alkylating agent include
substituted halides (e.g. chloride, bromide and iodide)
and substituted sulfonic acid esters (e.g. p-
toluenesulfonic acid ester).
While the reaction conditions vary with
combination of the base and the alkylating agent then
employed, it is preferable to conduct the reaction at
0C to room temperatures for about 1 to about 10 hours.
And, when chloride or bromide is employed as the
alkylating agent, it is pre~erab:Le to add potassium
iodide or sodi~Dm iodide to the reaction system to
accelerate the reaction.
Reaction (g)
R' ~Q,¢~ :
~C~2)n (CH2)n
~ ~Nh2 ¦~X~
[wherein A, R1, Q, W, X, Y and n are of the same the
32
meaning as defined above]
The above-mentioned reaction (g) is to obtain
oxathiadiazole (Ih) by cyclization of the amidoxime
compound (Ib) obtained by the reaction (c).
The compound (Ih) is obtained by allowing the
amidoxime (Ib) to react with thionyl chloride in a
conventional organic solvent (e.g. dichloromethane,
chloroform, dioxane and tetrahydrofuran) in the
presence of a base (e.g. pyridine and triethylamine).
It is preferable to conduct the reaction, adding
about 2 to about 10 moles of thionyl chloride to the
reaction system, under cooling at 0C to -30C, in the
presence of about 1 to about 3 moles of pyridine
relative to one mole of the amidoxime compound (Ib),
using dichloromethane as the solvent, for about 0.5 to
about 1 hour.
Reaction (h)
I
.
,
',
; .. . .. .... . .
: . ,. . ~; 1:
: ' , ' . . . .
-: : , ;
.
_ 33 _ ,2 ~ O ~
R~ ) L~H2~ (J I la~
H C~2~)
(ll) (YII) ~OnH2
; ~ 10 Rl~ R'~Q~
~2~ C~ ~ f
~N-C-OEt Rl 200C-N=C-~Et
(Y l l l ) ~lX~
: R ~4~)
CH2
: : : : Cwherein A, Rl, L and Q are of the same meaning as
defined:above, and Rl2:stands~for a lower (~Cl8) alkyl
25~:~`group~3.~
: In the above-mentioned reaction scheme (h), the
compound:(VII) alkylated~:with~the alkylating agent:
(IIIa) obtained in~the reaction (l) to be illustrated
later is~allowed t:o react with about 1 to about 2 times
::: 30 :: as much moles of triethyl oxonium tetrafluoroborate in
halogenated hydrocarbon (e.g. methylene chloride and
chloroform) for about 0.5 ~to about 2 hours at
temperatures ranging from O~C to room temperature to
', : give the imidate (VIII) in a good yield.
Subsequently, the:imidate (VIII) is allowed to
react with about 1 to about 2 times as much moles of
_ 34 _ 2~&~.~à
chloroformate (e~g. chloromethyl formate and
chloroethyl formate~ in a conventional organic solvent
(e.g. benzene, toluene, methylene chloride, chloroform,
dioxane and pyridine) in the presence of about 1 to
about 2 times as much moles of a base (e.g. 2,4,6-
trimethylpyridine, triethylamine, dimethylpyridine,
methylpyridine and diethylaniline). The N-
alkoxycarbonyl compound ~IX) is obtained in a good
yield by conducting the reaction in toluene at about 80
to about 100C for about 1 to about 3 hours. The N-
alkoxycarbonyl compound (IX) thus obtained is cyclized
by the reaction of about 2 times as much moles of
hydroxylamine hydrochloride with a base (e.g. sodium
methoxide, sodium ethoxide and potassium carbonate) in
alcohol (e.g. methanol and ethanol). The reaction is
preferably conducted at temperatures ranging from 50C
to about the boiling point of the solvent then employed
for about 3 to about 10 hours.
: Reaction (i)
R' \QJ~
` I
(C~2)n (~2)"
~; ~ 25 ~ ~ ~ C~ ~ 0
~: : (Ib) (li) H
[wherein A, Rl, Q, W, X, Y and n are of the same
meaning as defined above].
The above-mentioned reaction (i) is to obtain the
:: thiadiazole (Ii) by cyclization of the amidoxime (Ib)
. obtained by the afore-mentioned reaction (c).
The reaction to obtain the compound (Ii) is
conducted, by using about 1 to about 2 moles of 1,1'-
thiocarbonyldiimidazole, in a conventional organic
~ 35 ~ 3 ~
solvent in the presence of 1 to 10 eguivalents of a
Lewis acid (boron trifluoride diethyl ether, stannous
chloride, stannic chloride, zinc chloride, cuprous
chloride and silica gel).
Examples of the solvent include ethers ~e.g.
dioxane and tetrahydrofuran) and halogenated
hydrocarbons (e.g. methylene chloride and chloroform).
Alternatively, it is preferable that the compound
(Ib) is dissolved in a mixture of methanol and
hloroform,to which is added 1,1~-
thiocarbonyldiimidazole whole stirring together with
silica gel at temperatures ranging from 0C to room
temperature, followed by allowing the reaction to
proceed for about 0.5 to about 2 hours at about room
temperatures.
Reaction (j)
~'~Q,~ Q~)
(C~2)n (cH2)n
- ~ ~ X ~C~N~
(Ib) ~I j) H
.;
[wherein A, R1, Q, W, X, Y and n are of the same
meaning as defined above].
The above-mentioned reaction (j) is to obtain the
thioketone derivative (Ij) by subjecting the amidoxime
(Ib) obtained in the afore-mentioned reaction (c) to
cyclization.
The reaction to obtain the compound (Ij) is
conducted, using about 1 to about 10 moles of 1,1'-
thiocarbonyl diimidazole, in a conventional solvent in
the presence of a base.
As the solvent, use is made of, for example,
I
. ~
. .
- 36 - 2~
ethers (e.g. dioxane and tetrahydrofuran), halogenated
hydrocarbons (e.g. methylene chloride and chloroform),
acetonitrile and acetone.
As the base, mention is made of, for example,
amines (e.g. triethylamine, pyridine, 2,6-
dimethylpyridine, l,S-diazabicyclo[4.3.0]non-5-ene~
1,8-diazabicyclo[5.4.0]-7-undecene).
The reaction is preferably conducted by dissolving
the compound (Ib) in acetonitrile and allowing the
xeaction to proceed at 0C to about room temperatures
for about 10 minu~es to about 24 hours.
The reaction (j) can be conducted also under such
xeaction conditions as the following.
The reaction is conducted, using about 1 to about
10 moles of acetic anhydride relative to 1 mole of the
compound (Ib), in a conventional solvent in the
presence of a base.
As the solvent, use is made of, for example,
halogenated hydrocarbons (e.g. methylene chloride and
chloroform) and ethers (e.g. dioxane and
tetrahydrofuran).
As the base, mention is made of amines (e.g.
triethylamine and pyridine). The reaction is
preferably conducted, dissolving the compound (Ib) in
methylene chloride, at temperatures ranging from 0C to
room temperature for about 1 to about 5 hours.
By allowing 1 mole of O-acetylamidoxime thus
obtained to react with about 3 to about 10 moles of
carbon disuLfide in an organic solvent in ~he presence
of a base, thioketone ~Ij) is obtained.
As the solvent, use is made of amides (e.g. N,N-
dimethylformamide and dimethylacetamide) or dimethyl
sulfoxide.
As the base, mention is made of sodium hydride or
potassium t-butoxide. The reaction of O-
acetylamidoxime with carbon disulfide is preferably
. . .
- 37 _ 2~Q~
conducted in dimethylformamide for about 1 to about 3
hours, adding sodium hydride portionwise to the
reaction system while stirring at room temperatures.
The reaction products obtained as above by the
reactions (a) to ~j) can easily be isolated by
conventional isolation and purification process, for
example, column chromatography and recrystallization.
Incidentally, these compounds (I) can be
converted, by conventional methods, to salts with
physiologically acceptable acids or bases. These salts
include, for example, salts with an inorganic acid such
as hydrochloric acid, sulfuric acid and nitric acid
and, depending on the compounds, salts with an organic
acid such as acetic acid, oxalic acid, succinic acid
: 15 and maleic acid, saIts with an alkali metal such as
sodium and potassium, and salts with an alkaline earth
metal such as calcium.
: The starting compounds can be synthesized by the
methods described as follows.
Reaction (k)
011
C~3 ~ ~~~
~ IX ~l iX b3
: ~C13 ~ N ~3C
30~ ~ ~~~~i' LC~
lxcll [Xd3
~wherein L is of the same meaning as defined above].
The above reaction (k) is to obtain the compound
: '
- 38 - 2~0~
(Xd), by converting the cyano compound (Xa) to the
amidoxime compound (Xb) under substantially the same
reaction conditions as in the reaction (c), then
subjecting the amidoxime compound (Xb) to cyclization
to give the oxadiazole compound (Xc), followed by
subjecting the oxadiazole compound (Xc) to
halogenation.
The amidoxime compound (Xb) obtained from ~he
compound (Xa) by substantially the same procedure as in
reaction (c) is allowed to react with about 1 to about
10 moles of trichloroacetic anhydride or
hexachloroacetone relative to one mole of the amidoxime
(Xb) in accordance with the method described in the
literature reference [F. Eloy, et al., Helv. Chim.
lS Acta, 49, 1430(1966)] to give the oxadiazole compound
(Xc), then the compound (Xc) thus obtained is allowed
to react with a halogenating agent (e.g. N-
bromosuccinimide and N-bromoacetamide) (molar ratio =
about 1 o 1 to 1 : 1.5) in halogenated hydrocarbon
~0 (e.g. carbon tetrachloride) at temperatures ranging
from 50C to the boiling point o~ the solvent then
employed for about 1 to about 3 hours, in the presence
of a catalytic amount of an initiator (e.g. benzoyl
peroxide and azobisisobutyronitrile). This reaction
can also be carried out under irradiation of lightO
Reaction (1)
Mc ~ ~ Mc ~ ~ LC8~
~8 CONB3
30 (X e ~ ~X f ~ ~m a)
[wherein L is of the same meaning as defined above].
The reaction (l) comprises converting carboxylic
acid (Xe) to amide (Xf) by a conven~ional manner, then
leading (Xf) to the halogenide (IIIa).
The carboxylic acid (Xe) is allowed to react with
: ' :
- 39 _ , .
about 2 to about 5 moles of a halogenating agent (e.g.
oxalyl chloride or thionyl chloride) in an organic
solvent (e.gO tetrahydrofuran, chloroform or methylene
chloride) at temperatures ranging from room temperature
to the boiling point of the solvent then used for about
1 to about 20 hours. It is preferable to accelerate
this reaction by the addition of a catalytic amount of
dimethylformamide. The acid halide thus obtained is
preferably allowed to to react with an excess amount of
aqueous ammonia in an organic solvent (e.g.
tetrahydrofuran or dio~ane) at temp~ratures ranging
from 0C to room temperature for about 1 to about 10
hours, so that the amide derivative (Xf) can be
obtained in a good yield.
The reaction to obtain the halide (IIIa) from the
amide derivative (Xf) is conducted preferàbly in
substantially the same manner as described in the
reaction ~k).
Reaction (m)
::
:
.
.
, ' ; .,:
;' : '' ~ '
_ 40 _ ~ 1,35
c~
~Xe) 1~ ~Xg3 it~lN~O~e
C~a~ !> ~C~
(Xh3 ~)N--~lle ~ ~ûR
~ C~
(X i ) ~ (x ~
LC~
R19- ~ *~_
CX k) (X I )
~o
wherein Rl3 stands for an option.ally substituted alkyl
group shown by the above-mentioned R~ le.g. triphenyl
methyl, methoxy methyl and cyanoethyl) or t-
25 butyldimethyl silyl group; and L is of the same meaning
~ as defined above].
-: The reaction (m) is to obtain the oxadiazole
compound (Xi), which comprises leading carboxylic acid
(Xe) to acyl isothiocyanate by a conventional method,
allowing the latter to react with alcohol to give
carbonyl thiocarbamate:(Xg), subjecting the compound
~Xg) to methylation to give carbonata (Xh), then
: allowing the compound (Xh) to react with hydroxylamine,
: followed by cyclization under heating.
In the reaction for obtaining carbonyl
thiocarbamate (Xg), the compound (Xe) is allowed to
:- . . . ~
:: :: ,:, :. ; , .,
., ,
: ~ ;
- 41 ~
react with about 2 to about 5 moles of a halogenating
agent (e.g. thionyl chloride) relative to 1 mole of
(Xe) in halogenated hydrocarbon (e.g. chloroform and
methylene chloride) for about 1 to about 5 hours at
temperatures ranging from 50C to the boiling point of
the solvent then employed to give acid chloride. The
acid chloride thus obtained is allowed to react with
about 2 to about 5 moles of thiocyanate (e.g. sodium
salt and potassium salt) in ether (e.g. dioxane and
tetrahydrofuran3 at temperatures ranging from 50C to
: the boiling point of the solvent then employed for
about 1 to about 3 hours to give isothiocyanate. It is
preferable to subject the isothiocyanate thus obtained
to heating together with about 2 to about 10 moles of
alcohol (e.g. methanol and ethanol) at temperatures
ranging from about 50C to the boiling point of the
solvent then employed for about 15 minutes to one hour.
In the reaction for obtaining
iminomonothiocarbonate (Xh) from the compound (Xg), it
is preferable to allow the compound (Xg) to react with
methyl iodide (molar ratio = 1 : 1 to 1 : 2) in an
organic solvent (e.g. methanol, ethanol,
dim thylformamide (DMF) and acetonitrile), in the
presence of about 1 to about 2 moles, relative to one
25:~ mole of (Xg), of a base (e.g. NaOMe, Na2CO3 and K2CO3)
at temperatures ranging from room temperature to about
50C for about 10 to about 24 hours.
In the reaction for obtaining oxadiazole compound
(Xi) from the compound (Xh), it is preferable to allow
. (Xh) to react with hydroxylamine (molar ratio = about
o 1 : 2) in alcohol (e.g. methanol and ethanol)
at temperatures ranging from room t~mperature to about
50C for about 10 to about 20 hours, followed by
subjecting the reaction mixture to hea~ing in an
organic solvent (e.g. toluene and benzene) in the
presence of about a catalytic amount of an acid (e.g.
.
-
- 42 - 2~ 0~1 3~
p-toluenesulfonic acid) at temperatures ranging from
about 50C to the boiling point of the solv~nt then
employed fox about 1 to about 3 hours~
In the reaction for obtaining the demethylated
compound (Xj) from the compound (Xi), it is preferable
to subject an excess amount of pyridine hydrochloride
and (Xi) to fusing reaction under nitrogen atmosphere
at temperatures ranging from about 150 to about 160C
for about 0.5 to about 1 hour.
In the reaction for obtaining the compound (Xk)
from the compound (Xj), it is preferable to allow the
compound (Xj) to react with an alkylating agent (e.g.
triphenylmethyl chloride, methoxymethyl chloride and
cyanoethyl chloride~ (molar ratio = about 1 : 1 to 1 :
about 2) in an organic solvent (e.g. chloroform,
methylene chloride, dioxane, tetrahydrofuran and
pyridine) in the presence of about 1 to about 2 moles
of`a base (e.g. potassium carbonate, sodium carbonate,
triethylamine and pyridine) at temperatures ranging
from 0C to about room temperature for about 1 to about
3 hours.
The reaction for obtaining the compound (Xl) by
halogenating the compound (Xk) can be conducted in
substantially the same manner as in the reaction for
obtaining the compound (Xd) from the compound (Xc) in
the above reaction (k).
Reaction ~n)
., .
I
,, ' ': .
` - 43 ~ 1 3 ~
~e ~ M
~e; ~00~ C07~t ~Xm~
Me ~ _ ~ ~e ~ -~
~ X n ~ ~ON~(:ONH
' ~~
ED~
~Xp~
Cwherein h is of the same meaning as defined above].
The reaction (n) comprises converting carboxylic
acid (Xe) to semicarbazide (Xn) via hydrazide (Xm) by a
conventional manner, then subjecting (Xn) to
dehydrocyclization to give oxadiazolone (Xo), followed
by leading ~Xo) to the halogenated~compound (Xp).
In the reaction for obtaining hydrazide (Xm) from
carboxylic;acid~(Xe), (Xe) is allowed to react with
25~ about 2 to about 5 moles of a halogenating agent (e.g.
oxalyl chloride and thionyl chloride) in an organic
solvent (e.g. tetrahydrofuran, chloro~orm and methylene
chloride) at temperatures ranging from room temperature
,,. ~
to the boiling point of the solvent then employed for
;about l to about 20 hours. In this case, it is
preferable to add a catalytic amount of
dimethylformamide to accelerate the reaction. The acid
chloride thus obtained is allvwed to react wi~h about 2
to about 5 moles of hydrazine hydrate in an organic
solvent (e.g. tetrahydrofuran and dioxane) at
temperatures ranging from room temperature to about
2 l ~ 6 1 3
- 44 -
50C for about 1 to about 10 hours to obtain (Xm).
In the reaction for producing semicarbazide (Xn)
from the hydrazide (Xm) thus obtained, it is preferable
to allow (Xm) to react with about 2 to 5 moles of
isocyanate (e.g. sodium or potassium salt) in an
aqueous solution in the presence of an acid (e.g.
hydrochloric acid or sulfuric acid) in an amount equal
to that of the isocyanate then employed at temperatures
ranging rom 0C to rosm temperature for about 1 to
about 5 hours.
In the reaction for producing oxadiazolone (Xo)
: from the semicarbazide (Xn) thus obtained, it is
: preferable to heat (~n) in an organic solvent (e.g.
benzene and xylene) at about the boiling point of the
solvent then employed for about 5 to about 20 hours.
:: The reaction for producing the halogenated
compound (Xp) from the oxadiazolone (Xc) thus obtained
is preferably conducted in a manner similar to that
described in the reaction (k) mentioned above.
The compounds (I) and their salts are relatively
less toxic, strongly inhibit the vasoconstrictive and
pressor response induced by angiotensin II, exert a
hypotensive effect in animals, especially mammals (e.g.
human being, dog, rabbit and rat), and therefore the
25~ angiotensin II antagonistic ayent of this in~ention
containing the compound (I) or a salt thereof is useful
: as a therapeutic agent for not only hypertension but
~ also circulatory:diseases such as cardiac diseases
- (hypertxophy of the heart, cardiac insuficiency,
cardiac infarction or the likej, cerebral apoplexy,
nephropathy and arteriosclerosis. And, the compound
(I) is useful also as an agent of improving cerebral
functions observed in Alzheimer~s disease or senile
dementia, through its action on central nervous system,
and further has an action of antianxiety and
antideprementia.
' .
: .
_ 45 _ ~?~ 3 ~
For such therapeutic use as above, the compound
(I) or a salt thereof can he safely administered
orally, parenterally by inhalation, rectally or
topically as pharmaceutical compositions or
formulations (e.g. powders, granules, tablets, pills,
capsules, injections, suppositories, syrups, emulsions,
elixir, suspensions and solutions), comprising at least
one species of the compounds of the present inven~ion
alone or in admixture with pharmaceutically acceptable
carriers, adjuvants, excipients, vehicles and/or
diluents.
Pharmaceutical compositions can be formulated in
accordance with conventional procedures. In the
~ present specification, "parenterally" includes
subcutaneous injection, intravenous injections,
- intramuscular injection, intraperitoneal injection or
instillation. Injectable preparations, for e~ample,
sterile injectable aqueous suspensions or oil
suspensions can be prepared by known procedure in the
fields concerned,~ using a suitable dispersant or
wetting agent and suspending agent. The sterile
injections may be in the state of, for example, a
solution or a suspension, which is prepared with a non-
toxic diluent administrable parenterally, e.g. an
~25 ~ aqueous solution, or with a solvent employable for
ste~ile injection.~ Examples of~usable vehicles or
acceptable solvents include water, Ringer's solution
and an isotonic aqueous saline ~solution. Further, a
sterile non-volatile oiI an usually be employed as
solvent or suspending agent. Any non-volatile oil and
a fatty acid can be used for this purpose, which
includes natural, synthetic or semi-synthetic fatty
acid oiI or fatty acid and natural or synthetic or
semi-synthetic mono- or di- or tri-glycerides.
Rectal suppositories can be prepared by mixing the
drug with a suitable non-irritable vehicle, for
.. . .
, ;. ..... ....
- 46 - 2 ~
example, cocoa butter and polyethylene glycol, which is
in the solid state at room temperatures, in the liquid
state at temperatures in intestinal tubes and melts in
rectum to release the drug.
As a solid formulation for oral administration,
mention is made of powders, granules, tablets, pills
and capsules as referred to above. In such
formulations as exemplified above, the active component
can be mixed with at least one additive, for example,
sucrose, lactose, cellulose, sugar, mannitol, m~ltitol,
dextrin, starch, agar, alginate, chitin, chitosan,
pectin, tragacanth, gum arabic, gelatin, collagen,
casein, albumin, synthetic or semi-synthetic polymer or
glyceride. ConYentionally, these formulations can
contain further additives, for example, an inactive
diluent, a lubricant such as magnesium stearate, a
preservative such as paraben or sorbic acid, an anti-
oxidant such as ascorbic acid, a-tocopherol or
cysteine, a disintegrator, a binder, a thickening
agent, a buffer, a sweetener, a flavoring agent and a
perfuming agent. Tablets and pills can further be
prepared with enteric coating. Examples of liquid
preparations for oral administration include
pharmaceutically acceptable emulsions, syrups, elixirs,
suspensions and solutions, which may contain an
inactive diluent, for example, water, which is
conventionally employed in the field concerned.
The dose of a specific patient is decided
depending on the age, body weight, general health
conditions, sex, diet, dose interval, administration
routes, excretion rate, combinations of drugs and
conditions of the diseases then treated, while taking
them and any other necessary factors into
consideration.
The dose varies with th~ diseases to be treated,
conditions of such diseases, subject patients and
_ 47 - ~Q~.3
administration routes, and it is preferable that a
daily dose of about 1 to about 100 mg (preferably about
1 to about 50 mg) for oral administration or about 0.01
to about 50 mg (preferably about 0.3 to about 30 mg)
for intravenous injection is given once or divided
into two or three administrations wh~n used as an agent
for the therapy of essential hypertension of an adult
human.
Examples
By the following reference examples, working
examples, experimental examples and formulation
examples, the present invention will be illustrated
more concretely, and it is needless to say that they
should not be construed as limiting the invention
thereto.
:,
Reference Example 1
4-Methylbiphenyl-2-carboxamido oxime
To a solution of hydroxylamine hydrochloride (17.9
g) in dimethyI sulfoxide (120 ml) was added a
methanoIic solution of sodium methoxide prepared from
metallic sodium (5.92 g) and anhydrous methanol (50
ml). The mixture was stirred for 10 minutes at room
temperature, to which was added 2~-cyano-4-
25~ m2thylbiphenyl (lO g). The~reaction mixture was
stirred for 5 hours at 100C. The reaction mixture was
partitioned between ethyl acetate and water, and the
aqueous layer was extracted with ethyl acetat~. The
organic layers were combined , washed with water and
; ~30 dried, then the solvent was distilled under reduced
pressure. The residue was purified by column
chromatography on ilica gel to afford the title
compound as white amorphous product (11.2 g, 96%).
H-NMR (200MHz, CDCl3) ~ : 2.39(3H,s), 4.42(2H,br s),
7.22(2H,d), 7.31-7.50(5H,m), 7.56-7.60(lH,m).
IR (KBr) cml: 3490, 3380, 1642, 1575, 1568.
:
.
~:' ,',': :
, ' . ' ,:,
- 48 - ~1 O~l 3
Reference Example 2
5-Trichloromethyl-3-(4'-methylbiphenyl-2-yl)-1,2,4-
oxadiazole
To a benzene (lO0 ml) solution of the compound (10
g) obtained in Reference Example 1 in benzene (lO0 ml)
was added dropwise trichloroacetic anhydride (16.4 g).
The reaction mixture was then heated under reflux for
two hours. The reaction mixture was cooled and
concentrated to dryness. The residue was partitioned
hetween ether and water. The aqueous layer was
extracted with ether. The extract was combined with
the organic layer, which was washed with water and
dried, then the solvent was distilled under reduced
pressure. The residue was purified by column
chromatography on silica gel to af~ord the title
compound as a pale yellow oil (12 g, 77%).
H-NMR (200NHz,CDC13) ~ : 2.38(3H,s), 7.16(4H,s), 7.44-
7.64(3H,m), 7.88 7.93(lH,m).
IR (neat) cml: 3025, 1600, 1580, 1561, 1508.
Reference Example 3
5-Trichloromethyl-3~(4'-bromomethylbiphenyl-2-yl)-
1,2,4-oxadiazole
To a solution of the compound (24.8 g) obtained in
Reference E~ample 2 in carbon tetrachloride (300 ml)
were added N-bromosuccinimide (12.5 g) and ~, ~' -
azobisisobutyronitrile (1.15 g). The mixture was
hea~ed f or two hours under reflux, which was then
cooled. White insoluble material was filtered of f, and
the filtrate was diluted with dichloromethane. The
organic layer was washed with water and dried, then the
solvent was distilled under reduced pressure. The
residue was recrystallized from ether-hexane to afford
the the title compound as colorless crystals (23.0 g,
76%), m.p. 77-79C.
Elemental Analysis for Cl6XlON20BrCl3Ø5H20:
,'~' ,''' ~' , . ~'.
- 49 - ~ 35
C(%) H(%) N(%)
Calcd.: 43.52; 2.51; 6.34
Found : 43.76; 2.33; 6.31
lH-NMR (200MHz,CDCl3) ~ : 4.52(2H,s), 7.23(2H,d),
7.38(2H,d), 7.44-7.65(3H,m), 7.91-7.95(lH,m).
IR (KBr) cm : 1600, 1560, 1475, 1428, 1332.
Reference Example 4
4'-Bromomethylbiphenyl-2-carboxamide
4'-Methylbiphenyl-2-carboxamide (2.1 g), N-
bromosuccinic acid imide (2.5 g) and
azobisisobutyronitrate (AIBN: 82 mg) were added benzene
(20 ml), then the mixture was stirred for 20 hours at
60-70C. Resulting crystalline precipitate was
collected by filtration and washed with isopropyl
ether, which were suspended in water. The suspension
was ~tirred for 30 minutes. Insoluble material was
collected by filtration and dried. Crude crystals thus
obtained were recrystallized from ethyl acetate -
methanol to afford colorless needles (1.6 g, 55%), m.p.
220-221C (d). I
Elemental AnalysiS for C14~l2BrNO: i
C(~) H(%) N(~)
Calcd.: 57.95; 4.17; 4.83
Found : 57.85; 4.16; 4.77
H-NMR (200MHz, DMSO-d6) ~ : 4.75(2H,s~, 7.31-
7.69(10H,m).
IR (KBr) cml: 3150, 3000, 1570, 1540, 1520, 1500,
1300, 665.
Working Example 1
Ethyl 2-[N-propyl-N-[2'-(2,5-dihydro-5-oxo-1,2,4-
oxadiazol-3-yl)biphenyl-4-yl]methyl]aminopyridine-3-
carboxylate
a) Ethyl 2-chloropyridine-3-carboxylate
2-Chloropyridine-3-carboxylic acid (7.~ g) and
. .
., :
, , . :
..
_ 50 - 2~
thionyl chloride (45 ml) were heated for 3 hours in
benzene (65 ml) under reflux. The reaction mixture was
concentrated to leave an oil, which was added dropwise
to ethanol ~40 ml). The mixture was heated for further
one hour under reflux. The solvent was distilled, and
the residue was dissolved in toluene. The solution was
dried over anhydrous magnesium sulfate. The solvent
was distilled to leave the title compound (8.4 g, 90~)
as a pale yellow oil.
lH-NMR (200MHz,CDC13) ~ : 1.42(3H,t), 4.44(2H,q),
7.34(1H,dd), 8.17(1H,dd), 8.52(1H,dd).
b) Ethyl 2-propylaminopyridine-3-carboxylate
The compound (4.6 g) obtained in Working Example 1
a) and propylamine (9 ml) were dissolved in ethanol.
The solution was heated in a sealed tube for 6 hours at
100C. The reaction mixture was concentrated and the
residue was dissolved in ethyl acetate. The solution
was washed with a sodium hydroxicle solution and dried
20 - over anhydrous magnesium sulfate. The solvent was
distilled to give an oil, which was purified by column
chromatography on silica gel to afford the title
compound (4.1 g, 79%) as a colorless oil.
lH-NMR (20QMHz,CDC13) ~ ~ 1.01(3H,t), 1.38(3H,t), 1.60-
; 25 1.74(2H,m), 3.48(2H,dt), 4.32(2H,q), 6.50(1H,dd),
; 8.01(1H,br), 8.12(1H,dd), 8.28(1H,dd).
: ;
c) Ethyl 2-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
yl)biphenyl-4-yl]methyl-N-propyl]aminopyridine-3-
carboxylate -
To a solution of the compound (0.62 g) obtained in
Working Example 1 c) and 1,3-dimethyl-3,4,5,6-
tetrahydro-2(lH)-pyrimidone (0.73 g) in tetrahydrofuran
(2 ml) was added dropwise, under ice-cooling, lithium
hexamethyl disilaxide prepared from hexamethyl
disilazane ~0.72 g) and a 1.6M butyryl lithium
, ~ "' , ' ~ ' '
.
- 51 ~ 3~
tetrahydrofuran solution (2.8 ml). The mixture was
stirred for 10 minutes, to which was then added
dropwise a solution of the compound (2.0 g) obtained in
Reference Example 3 in tetrahydrofuran (6 ml). The
reaction mixture was stirred for 3 days at room
temperature and the resulting mixture was poured into
lN HCl (10 ml), followed by adjusting at pH 6-7 with lN
NaOH. The resulting solution was extracted with ethyl
acetate, and the extract was washed with water and
dried over anhydrous magnesium sulfate. The solvent
was distilled, and the residue was purified by column
chromatography on silica gel. A crude product thus
obtained was recrystallized from ethyl acetate - hexane
to afford the tile compound (0.20 g, 14~) as colorless
needles, m.p.168-169C.
Elemental Analysis for C26H26N~O4:
C(%) H(%) N(%)
Calcd.: 68.11; 5.72; 12.22
Found : 67.85; 5.72; 12.37
lH-NMR (200MHz,CDC13) ~ : 0.72(3H,t), 1.29(3H,t), 1.42-
1.61(2H,m), 3.17(2H,t), 4.25(2H,q), 4.64(2H,s),
6.61(1H,dd), 7.17(2H,d), 7.30(2H,d), 7.33-7.57(3H,m),
7.77-7.85(2H,m), 8.07(lH,dd).
IR(KBr) cml: 1770, 1725, 1585, 1565, 1495, 1480, 1460,
1440, 1280, 1250, 1225, 1120, 1090, 1060, 760.
Working Example 2
2-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
yl~biphenyl-4-yl]methyl]-N-propyl ] aminopyridine-3-
carboxylic acid
The compound (0.15 g) obtained in Working Example1 c), lN NaO~ (1.5 ml) and methanol (1 ml) were mixed,
and the mixture was heated for 1.5 hour under reflux.
The reaction mixture was diluted with water, which was
adjusted at p~ 3-4 with lN HCl, followed by extraction
with chloroform. The extract solution was washed with
- 52 - ~1 0~1 3~
water and dried over anhydrous magnesium sulfate. The
solvent was distilled and th~ residue was purified by
column chromatography on silica gel. Crude crystals
thus obtained were recrystallized from ethyl acetate
5 methanol to afford the title compound tO.ll g, 79%) as
colorless prisms, m.p.203-204C.
Elemental Analysis for C24H2~N4O4o
C(%) H(%) N(~)
Calcd.: 66.97; 5.15; 13.02
Found : 66.96; 5.17; 13.18
H-NNR (200MHz,DMSO-d6) ~ : 0.74(3H,t), 1.43-
1.61(2H,m), 3.26(2H,t), 4.74(2H,s), 6.80(1H,dd),
7.25(2H,d), 7.35(2H,d), 7.51-7.70(4H,m), 7.86-
7.91(lH,m), 8.23-8.26(lH,m).
IR (KBr) cm : 1780, 1590, 1495, 1470, 1455, 1430,
1380, 1220, 1200, 930, 850, 840, 790, 760, 755, 740.
Working Example 3
Ethyl 2-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-thiadiazol-3-
yl)biphenyl-4-yl]methyl-N-propyl]aminopyridine-3-
carboxylate
a) Ethyl 2-[N-(2'-cyanobiphenyl-4-yl)methyl-N-propyl]
pyridine-3-carboxylate
To a solution of the compound (2.0 g) obtained in
Working Example 1 b) in N,N-dimethylformamide (10 ml)
was added 60% sodium hydride in oil (0.42 ~). The
mixture was stirred for 3 hours at room tempera~ure, to
which was added 4'-bromomethyl-2-cyanobiphenyl (3.1 g),
followed by stirring for further 19 hours at room
temperature. The reaction mixkure was diluted with `
ethyl acetate and dried over anhydrous magnesium
sulfate. The solvent was distilled, and the residue
was purified by column chromatogr~phy on silica geL to
afford the title compound (1.3 g, 34~) as a colorless
oil.
H NMR (200MHz,CDC133 ~ : 0.81(3H,t), 1.37(3H,t),
: . '. ~:, ' . ,
:.
,~ . . .
- 53 ~ 3~
1.59(2H,m), 3.29(2H/t), 4.33(2H,q), 4.79(2H,s),
6.70(lH,dd), 7.38-7.67(7H,m), 7.75(lH,dd), 7.91(lH,dd),
8.26(1H,dd).
IR (neat) cml: 2225, 1715, 1585, 1560, 1480, 1470,
1445, 1410, 1365, 1280, 1225, 1125, 1085, 765.
b) Ethyl 2-CN-[2'-(hydroxycarbamimidoyl)biphenyl-4-yl]
methyl-N-propyl]pyridine-3-carboxylate
To a solution of hydroxylamine hydrochloride (2.6
g) in dimethyl sulfo~ide (DMSO) (25 ml) was added
triethylamine (3.8 g), and the mixture was stirred for
30 minutes at room temperature. To the reaction
mixture was added tetrahydrofuran (50 ml) and resulting
precipitate was filtered off. From the filtrate was
lS distilled off tetrahydrofuran. To a solution of
hydroxylamine in DMSO was added the compound (1.5 g)
obtained in Working Example 3 a), and the mixture was
stirred for two days at 60-65C. To the reaction
mixture was added water, which was extracted with ethyl
acetate. The ethyl acetate solution was extracted with
dilute hydrochloric acid. The aqueous layer was
adjusted to pH 9-10, which was extracted with ethyl
acetate. The extract was washed with water and dried
over anhydrous magnesium sulfate. The solvent was
2S distilled, and the residue was purified by column
chromatography on silica gel to afford the title
compound (1.2 g, 75%) as a colorless oil.
H~NMR (200MH~,CDCl3) ~ : 0.81(3H,t), 1.36(3H,t),
1.59(2H,m), 3.30(2X,t),~4.32(2H,q), 4.38(2H,s),
4.74(2H,s), 6.68(1H,dd), 7.30-7.60(8H,m), 7.89(1H,dd),
8.25(1H,dd).
IR (neat) cm}: 1710, 1650, 1580, 1555, 1480, 1440,
1360, 1280, 1220, 1120, 1080, 760.
c) Ethyl 2-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-thiadiazol-
3-yl)biphenyl-4-yl]methyl-N-propylJaminopyridine~3-
,
: :,
' ~
,
- 54 - ~ a
carboxylate
The compound (1.2 g) obtained in Woxking Example 3
b) and 1,1'-thiocarbonyldiimidazole (0.55 g) were added
tetrahydrofuran (6 ml). The mixture was stirred for 20
minutes at room temperature and the solvent was
distilled off. The residue was dissolved in ethyl
acetate. The solution was washed with water and dried
over anhydrous magnesium sulfate. The solvent was
distilled, and the residue was dissolved in
tetrahydrofuran (6 ml). To the solution was added
boron trifluoride diethyl etherate (0.64 g), and the
mixture was stirred for 30 minutes at room temperature.
The reaction mixture was diluted with water, wh;ch was
extracted with ethyl acetate. The Pxtract was washed
with water, dried over anhydrous magnesium sulfate and,
then the solvent was distilled off. The residue was
purified by column chromatography on silica gel. Crude
crystals thus obtained were recrystallized from ethyl
acetate - hexane to afford the title compound (0.34 g,
26%) as colorless needles, m.p.134-135C.
Elemental Analysis for C26H26N4O3S:
C(~) H(%) N~%)
Calcd.: 65.80; 5.52; 11.81
Found s 66.00; 5.46; 11.87
H-NMR (200MHz,CDCl3) ~ : 0.80(3H,t), 1.37(3H,t), 1.53-
1.64(2H,m), 3.25(2H,t), 4.34(2H,q), 4.75(2H,s),
6.70(1H,dd), 7.25(2H,d~, 7.38-7.60(5H,m), 7.86-
7.94~2H~m), 8.21(1H,dd), 8.43(1H,br s).
Working Example 4
2-[N-2'-(2,5-dihydro-5-oxo-1,2,4-thiadiazol-3-
yl)biphenyl-4-yl]methyl-N-propylamino]pyridine-3-
carboxylic acid
Starting from the compound (0.24 g) obtained in
Working Example 3 c), substantially the same procedure
as in Working Example 2 was followed to afford the
:, .
"
.
- 55 _ 2 ~ 3~
title compound as a colorless powder (0.18 g, 78%),
m.p.91-93C.
Elemental Analysis for C24H22N4O3SØ4H2O:
C(%) H(~) N(%)
S Calcd.: 63.53; 5.06; 12.35
Found : 63.66; 4.95; 12.07
H-NMR(200MHz,CDCl3) ~ : O.86(3H,t), 1.43(2H,m),
3.21(2H,t), 4.29(2H,s), 7.18-7.59(8H,m), 7.74(1H,dd),
8O46(lH,dd), 8.66(lH,dd), 9.65(lH,br).
IR(KBr)cml: 1695, 1580, 1460, 1430, 765.
Working Example 5
Ethyl 2-~N-butyl-N-[2'-(2,5-dihydro-5-oxo-1,2,4-
- oxadia~ol-3-yl)biphenyl-4-yl]methylamino]pyridine-3-
~lS carboxylate
a) E~hyl 2-butylaminopyridine-3-carboxylate
Starting from the compound (2.3 g) obtained in
Working Example 1 a), substantia:lly the same procedure
as in Working Example 1 b) was followed to afford the
title compound as a colorless oil (2.5 g, 93%).
H-NMR(200MHz,CDC13) ~ : 0.96(3H,t), 1.37(3H,t)! 1.36-
1.72(4H,m), 3.46-3.56(2H,m), 4.32(2H,q), 6.49(1H,dd),
7.97(1H,br), 8.11(1H,dd), 8.27(lH,dd).
IR(neat)cm l: 3375, 1690, 1600, 1585, 1520, 1295, 1250,
1125.
,: :
~ ~ :
b) Ethyl 2-[N-butyl~N-(2'-cyanobiphenyl-4-
yl~methylamino]pyridine-3-carboxylate
Starting from the compound (2.4 g) obtained in
Working Example 5 a), substantially the same procedure
as in Working Example 3 a) was followed to afford the
title compound as a colorless oil (2.4 g, 53%).
H-NMR(200MHz,CDC13) ~ : 0.85(3H,t), 1.17-1.31(2H,m),
1.37(3H,t), 1.48-1.63(2H,m), 3.33(2H,t), 4.33(2H,q),
4.78(2H,s), 6.70(1H,dd), 7.38-7.77(8H,m), 7.91(1H,dd),
8.26(1H,dd)~
.
.
". . -, ,.. , :
. .
- 56 ~ .3~
IR(neat)cm : 2230, 1715, 1590, 1560, 1480, 1445, 1410,
1370, 1285, 1~50, 1225, 1125, 1085, 1060, 760.
c) Ethyl 2-[N-butyl-N-(2'-
hydroxycarbamimidoylbiphenyl-4-yl)methylamino]pyridine-
3-carboxylate
Starting from the compound (2.4 gj obtained in
Working Example 5 b), substantially the same procedure
as in Working Example 3 b) was followed to afford the
title compound as colorless prisms (1.0 g, 38~),
m.p.181-182~C (chloroform-methanol).
~lemental Analysis for C26H30N4O3:
C(%) H(%) N(%)
Calcd.: 69.93; 6.77; 12.55
Found : 70.07; 6.73; 12.58
H-NMR(200MHæ,CDCl3) ~ : 0.84(3H,t), 1.14-1.31(2H,m),
1.37(3H,t), 1.55(2~,m), 3.34(2H,t), 4.33(2H,q),
4.38(2H,s), 4.74(2H,s), 6.70(1H,dd), 7.31-7.60(8H,m),
7.90(1H,q), 8.26(1H,dd).
IR(KBr)cml: 3480, 3360, 3220, 1680, 1660, 1580, 1560,
1460, 1430, 1380, 1365, 1305, 12~5, 1260, 1220, 1130,
0l 920, 780, 770.
~: :
; ~ d~ Ethyl 2-[~-butyl-N-[2'-(2,5-dihydro-5-o~o-1~2,4
oxadiazol-3-yl)biphenyl-4-yl]methylamino]pyridine-3-
carboxylate ~
To a solution of the compound (0.5 g) obtained in
Working Example 5 c) and triethylamine (0.44 g) in
chloroform (15 ml) was added dropwise ethyl
chloroformate (O.24 g). The reaction mixture was
stirred for one hour at room temperature, which was
then wa~hed with water and dried over anhydrous
magnesium sulfate. The solvent was distilled under
reduced pressure, and the residue was dissolved in
ethyl acetate (10 ml). The solution was heated with
1,5-diazabicyclo[5,4,0]undec-7-ene (0.67 g) for two
'
.
, . :
.
: , . ., : , ~.
:, ' ' . ''-' .,, .'; ' ., ~ :, .
_ 57 _ ~ 3~
hours under reflux. The reac~ion mixture was adjusted
to pH 4-5, which was washed with water and dried over
anhydrous magnesium sulfate. The solvent was distilled
under reduced pressure. The residue was purified by
column chromatography on silica ~el. The resulting
crude product was recrys~allized from ethyl ace~ate -
hexane to afford the title compound as colorless prisms
(0.38 g, 73%), m.p.146-147C.
Elemental Analysis for C27H28N4O4:
~(%) H(%~ N(%)
Calcd.s 68.63; 5.97; 11086
Found : 68.78; 6.04; 11.88
H~NMR(200MHz,CDCl3) ~ : 0.84(3H,t), 1.13-1.27(2H,m),
1.37(3H,t), 1.47-1.62(2H,m), 3.29(2~,t), 4.32(2H,q),
4.70(2H,s), 6.69(1H,dd), 7.22-7.64(7H,m), 7.84-
7.94(2H,m), 8.13(lH,dd).
IR(KBr)cm : 1775, 1720, 1585, 1565, 1495, 1480, 1455,
1440, 1365, 1280, 1250, 1220, 1170, 1120.
Working Example 6
2-[N-butyl-N-[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
yl) bLphenyl-4-yl]methylamino]pyridine-3-carboxylic
acid
Starting from the compound (0.25 g) obtained in
Working Example 5 d), substantiaily the same procedure
as in Working Example 2 was followed to afford the
title compound as a colorless powder (0.18 g, 75%),
m.p.95-98C. ~
Elemental Analysis for C25H24N4O4Ø5H2O:
C(%) ~(%) N(%)
Calcd.: 66.21; 5.56; 12.36
Found : 66.25; 5.36; 12.26
H-NNR(200MHz,CDCl3) ~ : 0.87(3H,t), 1.24-1.44(4H,m),
3.41(2H,t), 3.41(2H,t), 4.31(2H,s), 7.09(2H,q),
7.22(2H,d), 7.38-7.62(4H,m), 7.72(lH,dd), 8.46(lH,dd),
8.67(1H,dd3.
- 58 ~ fi l 3 ~
IR(KBr)cm s 1780, 1600, 1585, 1495, 1470, 1~30, 1375,
g40, 760.
Working Example 7
Methyl 2-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
yl)biphenyl-4-ylJmethyl-N-valerylamino]thiophene-3-
carboxylate
a) Methyl 2-valerylaminothiophene-3-carboxylate
Valeryl chloride (1.3 g) was added to a solution
of methyl 2-aminothiophene-3-carbo~ylate (1.6 g) [Y.
Kuwada et al. Chem. Abst. 82, 156252(1975)] and
triethylamine (1.2 g) in dichloroethane (20 ml) under
- ice-cooling. The mixture was stirred for two hours.
The reaction mixture was washed with water and dried
o~er anhydrous magnesium sulfate. The solvent was
distilled under reduced pressure. The residue was
purified by column chromatography on silica gel ~o
afford the title compound as a colorless oil (2.3 g,
96%~.
IH-NMR(200MHz,CDCl3) ~ : 0.96(3H,t), 1.33-1.52(2H,m),
1.68-1.83(2H,m), 2.51(2H,t), 3.89(3H,s), 6.72(1H,dd),
;~ 7.19(1H,d), 10.97(1H,~brs).
IR(neat)cm1: 3300, 1700, 1680, 1550, 1500, 1440, 1295,
- 1240,1200, 1165, 1025, 7~0.
~5
b)~ ~Methyl 2-CN-(2'-cyanobiphenyl-4-yl)methyl-N-
valerylamino]thiophene-3-carboxylate
Starting from the compound (2.3 g) obtained in
Working Example 7 a), substantially the same procedure
as in Working Example 3~a)~was followed to afford the
title compound as a colorless oil (4.0 g, 95%).
H-NMR(200MHz,CDCl3) ~ : 0~85(3H,t), 1.17-1.36(2H,m),
1.54-1.69(2H,m), 2.19(2H,t), 3.74(3H,s), 4.53(2H,brs),
5.33(lH,d), 7.12(lH,d), 7.30-7.49(7H,m), 7.59-
7.67(1H,m), 7.75(lH,dd).
IR(neat)cml: 2220, 1720, 1680, 1540, 1480, 1440, 1390,
- sg 2:~Q~
1345, 1275, 1230, 1190, 1155, 1005, 765, 720.
c) Methyl 2-~M-(2'-hydroxycarbamimidoylbiphenyl-4-yl)
methyl-N-valerylamino]thiophene-3-carboxylate
Starting from the compound (4.0 g) obtained in
Working Example 7 b), substantially the same procedure
as in Working E~ample 3 b) was followed to afford the
title compound as a colorless oil (1.5 g, 35%).
lH-NMR(200MHz,CDCl3) S : 0.85(3H,t), 1.17-1.36(2H,m),
1.54-1.69(2H,m), 2.19(2H,t), 3.69(3H,s), 4.45(2H,brs),
4.83(1H,d)j 4.99(1H,d), 7.14(1H,d), 7.20(2H,d), 7.32-
7.59(7H,~)-
IR(neat)cml: 1720, 1650, 1580, 1540, 1440, 1390, 1340,
; 1270, 1230, 1190, 1155, 1005, 750, 715.
d) Methyl 2-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-
oxadiazol-3-yl)biphenyl-4-yl]methyl-N-
valerylamino]thiophene-3-carboxylate
Starting from the compound (0.7 g) obtained in
Norking Example 7 c), substantially the same procedure
as in Working Example 5 d) was followed to afford the
title compound as colorless prisms (0.50 g, 68%),
m.p.154-155C (ethyl acetate - hexane).
Elemental Analysis for C~5H25N3O5S:
25~ C(%~ H(%) N(%)
Calcd.: 63.53; 5.13; 8.55
Found~: 63.61; 5.23; 8.74
H-NMR(200~Hz,CDCl3) ~ :~0.86(3Htt), 1.19-1.38(2H,m),
1.56-1.71t4H,m), 2.25(2H,t), 3.73(3H,s), 4.77(1H,d),
5.08(lH,d), 7.13-7.29(5H,m3, 7.43-7.67(4H,m),
7.89~1H,dd), 8.23(1H,brs).
IR(KBr)cml: 1765, 1715, 1~50, 1460, 1435, 1400, 1270,
1250, 1220, 1180, 1160, 940, 760, 730.
Working Example 8
2-[N-[2'~(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
~ '
' ~ ' '; . ' ' ''
,: ,
- 60 - ~ 1 Q ~ ~ ? 5
yl)biphenyl-4-yl]methyl-N-valerylamino]thiophene-3-
carboxylic acid
Starting from the compound (0.30 g) obtained in
Working Example 7 d), substantially the same procedure
5 as in Working Example 2 was followed to afford the
title compound as colorless prisms (0.20 g, 67%),
m.p.183-185C (decomp.) (chloroform-ether).
Elemental Analysis for C25EI23N3O5S.H2O:
C(%) EI(%) N(%)
Calcd.: 60.59; 5.08; 8.48
Found: 60.56; 4.80; 8.39
H-NMR(200NHz,CDC13) ~ : O.86(3H,t), 1.16-1.38(2H,m),
1.56-1.71(2H,m), 2.17-2.34(2H,t), 3.79(1H,d),
6.00(1H,d), 7.15-7.68(9H,m), 7.92(1H,dd), 9.10(1H,brs).
IR(XBr)cm 1: 1770, 1680, 1640, 1450, 1435, 1395, 1280,
1240, 760, 720.
Working Example 9
Methyl 2-~N-~2'-(2,5-dihydro-5-o~so-1,2,4-thiadiazol-3-
20 yl)biphenyl-4-yl]methyl-N-valery]amino]thiophene-3-
carboxylate
Starting from the compound (0.80 g) obtained in
Working Example 7 c), substantially the same procedure
as in Working Example 3 c) was followed to afford the
title compound as colorless prisms (0.40 g, 4796),
m.p.116-lI7C (ethyl acetate - hexane).
Elemental Analysis for C26H25N3O4S2:
C(96) H(%) N(~)
Calcd.: 61.52; 4.g6; 8.28
Found: 61.43; ~ 4.94; 8.31
H-NMR~200MHz,DCl3) ~: 0.85(3EI,t), 1.17-1.36(2H,m),
1.46-1.69(2EI,m), 2.18(2H,t), 3.80(3H,s), 4.31(1H,d),
5.56(1H,d), 7.15(1X,d), 7.27(4H,s-like), 7.36-
7.62(4H,m), 7.92(1H,dd)~, 8.22(1H,brs).
IR(KBr)cm 1: 1720, 1680, 1660, 1540, 1435, 1395, 1270,
1250, 1155, 760, 720.
.. .. . :
- 61 ~ fi1
Working Example 10
2-[N-[2~-(2,5-dihydro-5-oxo-1,2,4-thiadiazol-3-
yl~biphenyl~4-yl]methyl-N-valerylamino]thiophene~3-
carboxylic acid
Starting from the compound (0.25 g) obtained in
Working Example 9, substantially the same procedure as
in Working Example 2 was followed to afford the title
compound as colorless crystals (87 mg, 33%)l m.p.l48-
149C (chloroform-ether).
Elemental Analysis for C2sH23N3O4S2Ø25CHCl3:
C(%) H(%) N(%)
Calcd.: 57.94; 4.48; 8.03
Found : 57.92; 4.45; 7.98
lH~NMR(200MHz,CDCl3) ~ : O.87(3H,t), 1.21-1.40(2H,m),
1.57-1.72(2H,m), 2.17-2.40(2H,t), 3.75(1H,d),
6.03~1H,d), 7.03-7.20(5H,m), 7.34(1H,d), 7.40-
7.61(3H,m), 7.93(lH,dd), 10.06(lH,brs).
IR(KBr)cml: 1700, 1680, 1535, 1455, 1430, 1395, 1280,
1260, 1180, 760, 720.
Working Example 11
Methyl 5-methyl-2-[N-butyryl-N-[2'-(2,5-dihydro-5-oxo-
1,2,4-oxadiazol-3-yl)biphenyl-4-
yl]methylamino]thiophene-3-carboxylate
25 ~ a) Methyl 2-butyrylamino-S-methylthiophene-3-
carboxylate
To a solution of methyl 2-amino-5-methylthiophene-
3-carboxylate (2.6 g) [(Y. Kuwada et al. Chem. Abst.
82, 156252 (1975)] in pyridine (29 ml) was added
dropwise under ice-cooling butyric anhydride ~2.8 g).
The reac~ion mixture was stirred for 18 hours at 60C.
The reaction mixture was concentrated and dissolved in
ethyl acetate. The solution was washed with water,
which was dried over anhydrous magnesium sulfate. The
solvent was distilled off, and the residue was purified
by column chromatography on silica gel to~afford the
,: ` ;
- 62 -
title compound as a brownish oil (2 2 g, 62%).
H-NMR(200MHz,CDCl3) ~ : 1.01(3H,t), 1.69-1.8812H,m),
2.37(3H,d), 2.46(2H,t), 3.86(3H,s), 6.82(1H,t),
10.86(1H,brs).
IR(neat)cml: 3300, 1670, 1560, 1540, 1450, 1385, 1275,
1230, 1200, 1175.
b) Methyl 5-methyl-2-[N-butyryl-N-(2'-cyanobiphenyl-
4-yl)methylamino]thiophene-3-carboxylate
Starting from the compound (2.2 g) obtained in
Working Example 11 a), substantially the same procedure
as in Working Example 3 a) was followed to afford the
title compound as a yellowish oil (3,9 g, 100%).
lH-NMR(200MH2),CDCl3) ~ : 0.89(3H,t), 1.61-1.72(2H,m),
2.20(2H,t), 2.40(3H,d), 3.70(3H,s), 4.55(1H,d),
5.27(lH,d), 7.03(lH,d), 7.35(2H,d), 7.4d-7.52(4H,m),
7.64(1H,dt), 7.76(1H,dd).
IR(neat)cm~1: 2220, 1720, 1675, 1440, 1390, 1250, 1190,
1165, 760.
c) Methyl 5-methyl-2~N-butyryl-N-(2'-
hydroxycarbamimidoyl~iphenyl-4-
yl)methylamino~thiophene-3-carboxylate
~ Starting from the compound (3.0 g) obtained in
~ Worki`ng Example ll b), substantially the same procedure
as in Working ~xample 3 b) was followed to afford the
title compound as a colorless oil (1.7 g, 53%).
H-NNR(200MHz,C~Cl3) ~ : 0.89(3H,t), 1.60-1.72(2H,m),
2.20(2H,t~, 2041(3H,d), 3.65(3H,s), 4.45(2H,brs),
4.88(2H,s), 6.99(lH,t), 7.22(2H,d)j 7.35-7.61(7H,m).
IR(neat~cml: 3480, 3370, 1720, 1660, 1580, 1560, 1490,
1440, 1380, 1340, 1250, 1190, 1165~ 1005, 760
d) Methyl 5-methyl-2-[N-butyryl-N-[2'-(2,5-dihydro-5-
oxo-1,2~4-oxadiazol-3-yl)biphenyl-4-
yl]methylamino]thiophene-3-carboxylate
' . . :: ': ' ':
:, ' '' '''' ' .'`'' ~
:,:
.
- 63 ~
Starting from the compound (0.70 g) o~tained in
Working Example 11 c), substantially the same procedure
~; as in Working Example 5 d) was followed to afford the
title compound as coloxless needles (0.45 g, 61%),
m.p.156-157C (ethyl acetate - hexane).
Elemental Analysis for C26H25N3O5S:
C(%) H(%) N(%)
Calcd.: 63.53; 5.13; 8.55
Found : 63.33; 5.08; 8.34
H-NMR(200MHz,CDCl3) ~ : 0.91(3H,t), 1.68(2H,m),
~ 2.26(2H,t), 2.44(3H,d), 3.66(3H,s), 4.52(1H,d),
- 5.24(lX,d), 7.09-7.29(5H,m), 7.43-7.54(2H,m),
`; ~ 7.63(1H,dd), 7.87(1H,dd), 8.45(1H,brs).
IR(KBr)cml: 1760, 1715, 1645, 1490, 1460, 1440, 1400,
1390, 1380, 1250, 1235, 1193, 1160, g40, 760.
Working Example 12
5-Methyl-2 ~N-butyryl~N-[2'-(2,5-dihydro-5-oxo-1,2,4~
;-~ oxadiazol-3-yl)biphenyl-4-yl]met:hylamino]thiophene-3-
`^- 20 carboxylic acid
Starting from the compound (0.25 g) obtained in
Working Example 11 d), substantially ~he same procedure
as in Working Example 2 was followed to afford the
title compound as colorless prisms (0.17 g, 71%),
-~ ~ 25 m.p.210-2I1C (decomp.) (ethyl acetate - hexane).
Elemental Analysis for C25H23N3O5S:
C(%) H(%) ~(%j
Calcd.: ~ 62.88; 4.85; ~ 8.80
Found : 62.65; 4.92; 8.62
IH-~MR(200NHz,CDC13) ~ : 0.89(3H,t)~, 1.57-1.76(2H~,m),
2.22-2.35(2H,m), 2.50(3H,d), 3.76(lH,d), 5.95(lH,d),
6.80(1H,m), 7.20(4H,s-like), 7.41-7.55(2H,m),
7.62(1H,dd), 7.90(1H,dd), 9.20(1H,brs).
IR(KBr)cml: 1760, 1680, 1600, 1560, 1495, 1470, 1460,
1375, 1340, 127~, 1255, 1240, 1180, 1005, 995, 950,
840, 780, 760, 740, 725.-
:
'"'
,
.
~ 6~ - ~i 0
Working Example 13
Methyl 5-methyi-2-[N-butyryl-N-[2'-(2,5-dihydro-5-oxo-
1,2,4-thiadiazol-3-yl)biphenyl-4-
yl~methylamino]thiophene-3-carboxylate
Starting from the compound (0.90 g) obtained in
Working Example 11 c), substantially the same procedure
as in Working Example 3 c) was followed to afford the
title compound as colorless prisms (0.34 g, 35~)
m.p.l31-132C (ethyl acetate - hexane).
Elemental Analysis for C26H25N3O4S2:
C(%) H(%) N(%)
Calcd.: 61.52; 4.96; 8.28
Found : 61.38; 4.98; 8.17
H-NMR(200MHz,CDCl3) ~ : 0.88(3H,t), 1.66(2H,m),
2.19(2H,t), 2.41(3H,d), 3.75(3H,s), 4.41(1H,d),
5.39(lH,d), 7.04(1H,m), 7.17-7.62(7H,m), 7.90(lH,dd),
8.35(1H,brs).
IR(KBr)cm : 1720, 1690, 1660, 1555, 1485, 1460, 1435,
1400, 1345, 1250, 1225, 1190, 1165, 1005, 760.
Working Example 14
5-Methyl-2-[N-butyryl-N-[2'-(2,5-dihydro-5-oxo-1,2,4-
thiadiazol-3-yl)biphenyl-4-yl]methylamino]thiophene-3-
I carbo~ylic acid
25 ~ Starting from the compound (0.20 g) obtained in
Working Example 13, substantially the same procedure as
in Working Example 2 was followed to afford the title
compound as colorless prisms (0.15 g, 83%j, m.p.l93-
195C (decomp.) (ethyl acetate - hexane).
Elemental Analysis for C2sHuN3o4s2
C(%) H(%) N(~)
Calcd.: 60.83; 4.70; 8.51
Found : 60.74; 4.80; 8.35
lH-NMR(200MHz,CDC13) ~ : 0.91(3H,t), 1.55-1.78(2H,m),
2.27-2.46(2H,m), 2.50(3H,d), 3.70(lH,d), 6.01(1~,d),
6.75(1H,m), 7.06(2H,d), 7.17(2H,d), 7.40-7.62(3H,m),
~, ' ., .
.
- 65 - .21 ~ ~ ~ 3 ~
7.92(lH,dd), lO.O9(lH,brs).
IR(KBr)cml: 1680, 1555, 1490, 1460, 1435, 1380, 1340,
1260, 1240, 1180, 760, 720.
Working Example 15
Ethyl 4-[N-butyl-N-[2'-(2,5-dihydro-5-oxo-1,2,4-
oxadiazol- 3-yl)biphenyl-4-yl]methyl]aminopyrimidine-5-
carboxylate
a) 4'-Butylaminomethyl-2-cyanobiphenyl
To a solution of 4~-bromomethyl-2-cyanobiphenyl
(10.0 g3 in tetrahydrofuran (100 ml) was added
; butylamine (26.8 g). The mixture was stirred for 3
hours at room temperature. The reaction mixture was
concentrated and diluted with wa~er, followed by
extraction with chloroform~ The extract was washed
with water and dried. The solvent was distilled under
reduced pressure. The residue was purified by column
chromatography on silica gel to afford the title
compound as a pale yellow oil (10.7 g, quantitatively).
20~ 1H-NMR(200~Hz,CDCl3) ~ : 0.93(3H,t), 1.28-1.60(4H,m),
2.68(2H,t), 3.86(2H,s), 7.39-7.5~(~H,m), 7.64(1H,dt),
; 7.76(lH,d~)-
- IR(neat)cm : 2220, 1480, 760
b) Ethyl 4-chloropyrimidine-5-carboxylate
To a mixture of ethyl 3,4-dihydro-4-oxopyrimidine-
5-carboxylate (3.54 g) (synthesized according to the
s method reported by A.; R. Todd and F. ~ergel on J. Chem.
Soc., 364 (1937)) and triethylamine~(2.13 g) was added
dropwise under ice-cooling phosphorus oxychloride (21
ml), and the mixture was then heated for 1.5 hour under
reflux. The reaction mixture was concentrated to
dryness, which was poured into ice-water, followed by
partitioning between chloroform and a saturated aqueous
solution of sodium hydrogencarbonate. The organic
layer was washed with a saturated aqueous solution of
.
.
': :
- 66 - 2~
sodium hydrogencarbonate and ~hen dried. The solvent
was distilled under reduced pressure. The residue was
purified by column chromatography on silica gel to
afford the title compound as a pale yellow oil (3.42 g,
86%).
H-NMR(200MHz, CDCl3) ~ : 1.44(3H,t), 4.47(2H,q),
9.08(1H,s), 9.13(1H,s).
c) Ethyl 4-[N-butyl-N-(2'-cyanobiphenyl-4-yl)methyl~
aminopyrmidine-5-carboxylate
To a solution of the compound (5.53 g) obtained in
Working Example 15 b) and triethylamine (6.34 g) in
~; tetrahydrofuran (50 ml) was added a solution of the f~
compound (3.90 g) ob~ained in Working Example }5 a) in
tetrahydrofuran (20 ml). The mixture was stirred for
two hours at room tèmperature. ~he reaction mixture
was diluted with water, which was extracted with ethyl
acetate. The extract solution was washed with water
and dried, followed by distilling off the solvent under
reduced pressure. The residue was purified by column
chromatography on silica gel to afford the title
ompound as a pale yellow oil (8.77 g, quantitatively).
H-NMR(200MHz,CDC13) ~ : 0.89(3~,t), ~.81-1.36(5H,m),
1.54-1.69(2H,m), 3.47(2H,t), 4.29(2H,q), 4.88(2H,s),
~25 ~ 7.34(2H,d),~7.40-7.78(6H,m), 8.59(1H,s), 8.63(1X,s).
`; IR(neat~cm1: 2230, 1751, 1S75, 1535, 1145, 765.
d) Ethyl 4-[N-butyl-N-(2'-hydroxycarbamimidoylbiphenyl-
~4-y])methylamino]pyrimidine-5-carboxylate
~30 Starting~rom the~compound (4.00 g) obtained in
Working Example 15 c), substantially the same procedure
as in Working Example 3 b) was followed to afford the
title compound as a pale yellow oil (0.82 g, 11~
H-NNR(200MHz,CDCl3) ~ : 0.89!3H,t), 1.21^1.37(2H,m),
1.33(3H,t),~1.53-1.68(2H,m), 3.48(2H,t), 4.29(2H,s),
4.41(2H,brs), 4.84(2H,s), 7.22-7.60(8H,m), 8.59(1E,s),
: , . . .... , : . .. .
. ':' .. , ~ -: . -
. .
:' ' ~ :' : ,: .. . .
~ :: : . .
,. . . . .
- 67 ~ fil
8.62(1H,s).
IR(neat)cml: 1730, 1715, 1575, 1540, 1370, 1240, 1145.
e) ~thyl 4-[N-butyl-N-[2'-(2,5-dihydro-S-oxo-1,2,4-
oxadiazol-3-yl)biphenyl-4-yl]methylamino]pyrimidine-5-
carboxylate
Starting from the compound (0.78 g) obtained in
Working Example 15 c), substantially the same procedure
as in Working Example 5 d) was followed to afford the
title compound as a colorless powder (0.30 g, 35%),
m.p.65-69C.
Elemental Analysis for C26H27N5O403H2O:
C(~) H(%) N(%)
Calcd.: 65.20 5.81 14O62
Found : 65.16 5.96 14.32
lH~NMR(200~z,CDCl3) ~ : 0.89(3H,t), 1.21-1.36(2H,m),
1.34(3H,t), 1.52-1.67(2M,m), 3.44(2H,t), 4.2g(2H,q),
4.83(2H,s), 7.26(4H,s-like), 7.42(1H,dd), 7.51(1H,dt),
7.62(1H,dt), 7.83(1H,dd), 8.40~1H,s), 8.43(1H,s).
IR(KBr)cm1: 1780, 1730, 1715, 1530, 1540, 1505, 1495,
1475, 1460, 1285, 1240, 1145, 1070, 935, 765.
Working Example 16
4-[N-butyl-N-~2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
yl)biphenyl-4-yl~methylamino]pyrimidine-5-carboxylic
acid
To a suspension of khe compound (0.28 g) obtained
in Working Example 15 e) in tetrahydrofuran (8 ml) -
water (4 ml) was added lithium hydroxide hydrate (0.17
g). The mixture was stirred ~or 24 hours at 70C. The
reaction mixture was adjusted at pH 4 wi~h lN ~Cl,
followed by extrackion with e~hyl acetate. The extract
solution was washed with wa~er and dried. The solvent
was distilled under reduced pressure. Resulting
crystals were recrystallized from chloro~orm - methanol
- hexane to afford the title compound as a colorless
crystal (0.12 g, 46~), m.p.l43-147C (decomp.).
- 68 ~ 3~j
Elemental Analysis for C24H23N5O4Ø5H2O:
C(g6) H(~) N(%)
Calcd.: 63.43 5.32 15.41
Found : 63.67 5.~3 15.53
lH-NMR(200MH2,DMSO-d6) ~: 0.83(3H,t), 1.10-1.62(2H,m),
1.47-1.62(2H,m), 3.42(2H,t), 4.89(2H,s), 7.29(4H,s-
like), 7.51-7.73(4H,m), 8.49(1H,s), 8.56(1H,s).
IR(KBr)cm: 1775, 1635, 1585, 1545, 1535, 1345, 765.
Working Example I7
Ethyl 4-~N-[[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
yl)biphenyl-4-yl]methyl]-N-propylamino]-2-
methylpyrimidine-5-carboxylate
a) 4'-Propylaminomethyl-2-cyanobiphenyl
~` 15 Starting from 4'-bromomethyl-2-cyanobiphenyl (5.4
g), substantially the same procedure as in ~orking
Example lS a) was followed to afford the title compound
as a pale yellow oil (4.8 g, 9696).
lH-NMR(200MHz,CDCl3) ~: 0.95(3Et,t), 1.47-1.66(2H,m),
2.65(2H,~), 3.86(2H,s), 7.38-7.78(8H,m).
IR(neat)cm: 2220, 1480, 1460, 1440, 760
~; bj Ethyl 4-[N-[(2'-cyanobiphenyl-4-yl)methyl3-N-
propylamino]-2~methylpyrimidine-5-carboxylate
To a solution of ethyl 3,4~dihydro-4-oxo-2-
methylpyrimidine-5-carboxylate (3.45 g) (synthesized
according to the method reported by A. R. Todd and F.
Bergel on J. Chem. Soc., 364(1937)) and triethylamine
(4.78 g) in N,N-dimethylformamide t30 ml) was added p-
- 30 ~ toluenesulfonyl chloride (3.78 ~g). The mixture was
stirred for 30 minutes at room temperature. To the
reaction mixture was added a solution of the compound
(4.30 g) obtained in Working Example 17 a) in toluene
(5 ml), and the mixture was stirred for 16 hours at
` 35 room temperature. The reaction mixture was
concentrated under reduced pressure. The residue was
' ,
,. :
:~ . : ,. - :
.":
. . . , : - ... . ~ :,
~ : ' ' ' ~'' ~
- 69 ~ 3~
diluted with water, followed by extraction with ethyl
acetate. The extract was washed with water and dried.
The solvent was distilled under reduced pressure. The
residue was purified by column chromatography on silica
gel to afford the title compound as a yellow oil (4.99
g, 70%).
H~NMR(200MHz,CDCl3~ ~ : 0.85(3H,t), 1.32(3H,t),1.54-
1.74(2H,m)j 2.54(3H,s), 3.40(2H,t), 4.28(2H,q),
4.89(2H,s)/ 7.34-7.78(8H,m), 8.54(1H,s).
IR(neat)cml. 1715, 1575, 1540, 1535, 1510, 1435, 1090,
760.
c) Ethyl 4-[N-[(2'-hydroxycarbamimidoylbiphenyl-4-yl)
methyl]-N-propylamino]-2-methylpyrimidine-5-carboxylate
Starting from the sompound (4.49 g~ obtained in
Working Example 17 b), substantially the same procedure
as in Working Example 3 b) was followed to afford the
title compound as a colorless powder (2.49 g, 51%).
lH-NMR(200MHz,CDCl3) ~ : 0.84(3H,t), 1.32(3H,t), 1.54-
1.72(2H,m), 2.53(3H,s), 3.41(2H,t), 4.28(2H,q),
4.41(2H,s), 4.85(2H,s), 7.24-7.59(8H,m), 8.53(1H,s).
d) Ethyl 4-[N-[[2'-(2,5-dihydro-5-oxo-1,2,4-
oxadiazol-3-yl)biphenyl-4-yl]methyl]-N-propylamin~]-2-
methylpyrimidine-5-carboxylate
Starting ~rom the compound ~0.80 g) obtained in
Working Example 17 c), substantially the same procedure
as in Working Example 5 d) was followed to afford the
title compound as pale yellow needles (0.60 g, 70%),
m.p.176-I77C (ethyl acetate - hexane).
Elemental Analysis for C26H27N5O4:
C(%) H(%) N(%)
Calcd.: 65.95 5.75 14.79
Found: 65.88 5.78 14.56
lH~NMR(200NHz,CDCl3) ~ : 0.82(3H,t), 1.32(3H/t), 1.50-
1.68(2E,m), 2.28(3H,s), 3.33(2H,t), 4.28(2H,q),
` - 70 - ?~ 3~
4.83(2H,s), 7.19(4H,s-like), 7.41(1H,dd), 7.52(1H,dt),
7.62(1H,dt), 7.83(1H,dd)t 8.06(1H,s).
I~(KBr)cmlo 1780, 1725, 1715, 1585, 1535, 1440, 1430,
1260j 1090, 765.
Working Example 18
4-[N-[[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
yl)biphenyl-4-yl]methyl]-N-propylamino]-2-
methylpyrimidine-5-carboxylic acid
Starting from the compound (0.45 g) obtained in
Working Example 17 d), substantially the same procedure
as in Working Example 2 was followed to afford the
title compound as colorless crystals (0.40 g, 8~%),
m.p.182-186C (chloroform-methanol).
Elemental Analysis for C24HZ3N5O405H2O:
C(%) H(%) N(%)
Calcd.: 63.43 5.32 15.41
Found : 63.55 5.40 15.29
1H-NMR(200MHz,DMSO-d6) ~ : 0.76(3H,t), 1.45-1.64(2H,m),
2.41(3H,s), 3.38(2H,t), 4.88(2H,s), 7.27(2H,d),
7.33(2H,d), 7.50-7.74t4H,m), 8.42(1H,s).
IR(KBr)cm1: 1775, 1635, 1585, 1545, 1535, 1345, 765
'
Working Example 19
Ethyl 4-[N-~[2'-(2,5-dihydro-5-oxo-1,2,4-thiadiazol-3
yl)biphenyl-4~yl]methyl]-N-propylamino]-2-
methylpyrimidine 5-carboxylate
Starting from the compound (0.80 g) obtained in
Working Example 17 c), substantially the same pxocedure
as in Working Example 3 c) was followed to afford the
title compound as colorless needles (91 mg), m.p.173-
175C (ethyl acetate - hexane).
Elemental Analysis ~or Cz6H27N5O3s:
C(%) H(%) N(%)
Calcd.: 63.78 5.56 14.30
Found : 63.51 5.72 14.02
..
:, ,
- 71 ~ 3~
H-NMR(200MHz,CDCl3) ~ : 0.83(3H,t), 1.33(3H,t), 1.50-
1.69(2H,m), 2.41(3H,s), 3.33(2H,t), 4.28(2H,q),
4.87(2H,s), 7.19(2H,d), 7.28(2H,d), 7.38(1H,dd~, 7.46-
7.61(2H,m), 7.85(1H,dd), 8.27(1H,s).
IR(KBr)cm1: 1720, 1700, 1585, 1540, 1450, 1435, 1260,
10~0 .
Working Example 20
4-[N-[[2'-(2,5-dihydro-5-oxo-l,2,4-thiadiazol-3-yl)-
biphenyl-4-yl]methyl]-N-propylamino]-2-
methylpyrimidine-5-carboxylic acid
Starting from the compound (0.07 g) obtained in
Working Example 19, substantially the same proc~dure as
in Working Example 16 was followed to afford the title
compound as colorless crystals (55 mg, 81%), m.p. 219-
220C (decomp.).
Elemental Analysis for C24H23N5O3S-H2O:
C(%) H(%) N(%)
Calcd.: 60.11 5.25 14.60
Found : 59.95 5.08 14.56
H-NMR(200MHz,DMSO-d6) ~ : 0.76(3H,t), 1.44-1.62(2H,m),
2.41(3H,s), 3.35(2H,t), 4.85(2H,s), 7.20(2H,d),
7.29(2H,d)l 7.46-7.66(4H,m), 8.42(1H,s).
IR(KBr)cm1: 1685, 1640, 1535, 1340.
2~
J
;WorXing Example 21 ~ -
Ethyl~4-[N-[[2'-(2,S-dihydro 5-thio~o-1,2,4-oxadiazol-
3-yl)biphenyl-4-yl~methyl]-N-propylamino]-2-
methylpyrimidine-5-carboxylate
To a solution of the compound (0.40 g) obtained in
Working Example 17 c) in acetonitrile (10 ml) were
added l,l'-thiocarbonyldiimidazole (0.24 g) and l,8-
diazabicyclo[5.4.0]-7-undecene (0.54 g). The mixture
was stirred for one hour at room temperature. The
reaction mixture was diluted with water and adjusted to
pH4 with lN HCl, followed by extraction with ethyl
- 72 - 21 Ofi t ~a
acetat~. The extract was washed with a saturated
aqueous saline solution and dried. The solvent was
distilled under reduced pressure. ~he residue was
purified by column chromatography on silica gel.
Recrystallization of thus obtained crude crystals from
ethyl acetate - hexane afforded the title compound as
pale yellow needles (0.26 g), m.p.l70-172C.
Elemental Analysis for C26H27N5O3S05H2O:
C(%) H(%) N(~)
Calcd.: 62.63 5.66 14.05
Found : 62.96 5.59 13.81
H-NMR(200MHz,CDC13) ~ : 0.83(3H,t), 1.34(3H,t), 1.47- t
1.65(2H,m), 2.08(3H,s), 3.30(2H,t), 4.35(2H,q),
4.70(2H,s), 7.03(2H,d), 7.14(2H,d), 7.45-7.68(3H,m),
7.81-7.84(lH,m), 8.00(lH,s).
IR(KBr)cml: 1725, 1590, 1545, 1535, 1450, 1340, 1305,
1255, 1090.
Working Example 22
4-[N-[[2'-(2,5-dihydro-5-thioxo-1,2,4-oxadiazol-3
yl)biphenyl-4-yl]methyl]-N-propylamino]-2-
methylpyrimidine-5-carboxylic acid
Starting from the compound (0.20 g) o~tained in
Working Example 21, substantially the same procedure as
in Working Example 2 was followed to afford the title
compound as colorless crystals (0.14 g), m.p.212-214C
; ~ ~ (decomp.) (chloroform-methanol).
Elemental Analysis for C24Hz3N5O3SØ8H2O:
C(%) H(%) N(%
~30 Calcd.: 60.57 5.21 14.71
Found : 60.73 5.11 14.80
H-NNR(200MHz,DMSO-d5) ~ : O.78(3H,t), 1.48-1.66(2H,m),
2.45(3H,s), 3.42(2H,t), 4.91(2H,s), 7.23(2H,d),
7.31(2H,d), 7.47-7.57(2H,m), 7.62-7.70l2H,m)~
8.50(1H,s).
IR(KBr)cml: 1640, 1540, 1490, 1340, 1295, 775.
. .
.
.
.
_ 73 - ~ ~ fi ~
Woxking Example 23
Ethyl 3-[N-[[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
yl)biphenyl-4-yl]methyl]-N-propylamino]-l-
methylpyrazole-4-carboxylate
a) Ethyl 3-[N-(2'-cyanobiphenyl-4-yl)methylamino]-1-
methylpyrazole-4-carboxylate
To a mixture of ethyl 3-amino-1-methylpyrazole-4-
carboxylate (P. Schmidt et al. Hel. Chim. Acta., 1959,
41, 349.) (2.30 g) and potassium carbonat~ in N,N-
dimethylformamide (70 ml~), was added portionwise 4-
bromomethyl-2'-cyanobiphenyl (4.81 g). The solvent was
distilled under reduced pressure. To the residue was
added ice-water and an aqueous ~olution of potassium
carbonate. The mixture was extracted with ethyl
acetate. The extract was washed with water and a
saturated aqueous saline solution, wh.ich was dried over
magnesium sulfate. The solvent was distilled off under
r duced pressure to give crude crystals, which were
recrystallization from ethyl acetate - isopropylether
20 to afford the title compound as white crystals (3.7 g,
76~), m.p.151-152C.
Elemental Analysis for C2lH20N402:
C(%) H(%) N(~)
; Calcd.: 69.98; 5.59; 15.55
Found : 69.72; 5.62; 15.29
H-NMR(200MH~,CDCl3) ~ 1.32(3H,t), 3.74(3H,s),
4.25(2H,g), 4.58(2H,d), 5.73(1H,bd), 7.33-7.70(8H,m),
7-75(1H,d).
IRtnujol)cm : 3390, 2220, 1685, 1565, I555, 1365,
1245, 1160, 1120.
:
b) Ethyl 3-[N-(2'-cyanobiphenyl-4-yl)methyl-N-
propylamino]-1-methylpyrazole~4-carboxylate
To a solution of diisopropylamine (1.05 ml) in
tetrahydrofuran (10 ml) was added at -10C n-butyl
lithium (~.6M hexane solution, 4.1 ml). The mixture
~: : . .. .
;: . - . . ~ ., : ,
. ~: ,,: . . : , ,
.. :.... , . :, . : . ,
' :.
~, ', ,: ,~ :
.: '; ., ' ~- :
: . , . . :, :
74 2 1` ~ ~ ~r 3 ~1
was stirred for 30 minutes at -10C ~ -5C. To the
reaction mixture was added dropwise at -78C a solution
of the compouncl (1.80 g) obtained in Working Example 23
a) in tetrahydrofuran (15 ml). The reaction mixture
was stirred for 30 minutes, to which was added dropwise
at the same temperature a solution of propyl iodide
(0.73 ml) in tetrahydrofuran (3 ml). The reaction
mixture was allowed to warm to room temperature. The
reaction mixture was stirred for further two hours,
which was poured into a saturated aqueous solution of
ammonium clhloride, followed by extraction with ethyl
acetate. The extract was washed with water and a
saturated aqueous saline solution, followed by drying
over magnesium sulfate. The solvent was distilled
under reduced pressure. The residue was purified by
column chromatography on silica gel to afford the title
compound as an oil (1.75 g, 87%).
H-NMR(200MHz,CDCl3) ~: 0.84(3H,t), 1.30(3H,t), 1.50-
1.75(2H,m~, 3.18-3.33(2H,s), 3.75(3H,s), 4.14(2H,q),
4.62(2H,s), 7.36-7.80(9H,m).
IR(neat)cm l 2960, 2220, 1700, 1550~ 1500, 1475, 1440,
1365,~1300, 1245, 1160, 1090.
c) Ethyl 3- L N~(2'-hydroxycarbamimidoylbiphenyl-4-yl)
1 25 ~ methyl-N-propylamino]-l-methyIpyrazole-4 carboxylate
Starting from the compound (1.80 g) obtained in
Norking Example 23 b), substan~ially the same procedure
as in Working Example 3 b) was followed to afford the
title compound as colorless crystals (1.08 g, 55%),
m.p.171-173C (recrystaIlized from ethyl acetate -
isopropylether).
Elemental Analysis for C24H29N5O3Ø2H2O:
C(%) H(%) N(%)
Calcd.: 65.64; 6.75; 15.96
35~ ~ Found: 65.97; 6.90; ~ 15.62
lH-NMR(200MHz,CDCl3) ~: 0.82(3H,t), 1.30(3H,t), 1.46-
.
. . .
, . . ... . . .
.
.
-
- 75 - ~ 3 ~
1.69(2H,m), 3.14-3.28(2H,m), 3.73(3H,s), 4.23(2H,q),
4.40(2H,bds), 4.59(2H,s), 7.29-7.62(8~,m), 7.74(1H,s).
IR(neat)cml: 3470, 3350, 1680, 1640, 1595, 1545, 1495,
1~55, 1190, 1100/ 920, 770.
d) Ethyl 3-[N-(2'-
methoxycarbonyloxycarbamimidoylbiphenyl-4-yl)methyl-N-
propylamino]-l-methylpyrazole-4-carboxylate
To a solution of the compound (0.68 g) of the
working Example 23 c) and triethylamine was added in
- tetrahydrofuran at 0C methyl chlorocarbonate. The
mixture was stirred for two hours at room temperature.
The solvent was distilled under reduced pressure. To
the residue was added ethyl acetate (20 ml), which was
washed with water and a saturated aqueous saline
solution, followed by drying over magnesium sulfate.
The solvent was distilled under reduced pressure, and
the residue was purified by column chromatography on
silica gel to afford the title compound as a powder
(0.72 g, 93%).
H-NMR(200MHz,CDCl3) ~ : 0.82(3H,1:), 1.30(3H,t), 1.48-
1.75(2H,m), 3.22(2H,t-like), 3.75(3H,s), 3.90(3H,s),
4.~4~2H,q), 4.59(4H,bds), 7.28-7.57(7~I,m)~ 7.65(lH,d),
7.76(1H,s).
IR(KBr)cml: 3470, 3340, 2950, 1760, 1700, 1630, 1550,
1500, 1390, 1235, 1160.
e) Ethyl 3-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-
3-yl)biphenyl-4-yl3methyl-N-Propylamino]-1-
methylpyrazole-4-carboxylate
A solution of the compound (0.70 g) of working
Example 23 d) and 1,8-diazabicyclo-[5.4.0]-7-undecene
(0.63 ml) in ethyl acetate (30 ml) was heated for 1.5
hour under reflux. The reaction mixture was washed
with a 10~ aqueous solution of potassium
hydrogensulfate, water and a sa~urated aqueous saline
: ', , ,
,
2~ 0~ 3
- 76 -
solution, successively, followed by drying over
magnesium sulfate. The solvent was distilled under
reduced pressure. Crude crystals thus obtained were
recrystallized from e~hyl acetate - isopropyl ether to
afford the title compound as colorless crystals (0.56
gj 86%), m.p.182-183C.
Elemental Analysis for C~5H~7N5O4.02H2O:
C(%) H(%) N(%)
Calcd.: 64.67; 6.02; 14.62
Found : 64.59; 6.04; 14.66
H-NMR(200MH ,CDCl3) ~ 0 0.84(3H,t), 1.31(3H,t), 1.44
1.65(2H,m), 3.14-3.28(2H,m) ! 3.60(3H,s), 4.23(2H,q),
4.47(2H,s), 7.17-7.68(7H,m), 7.73(1H,s), 7.86(1H,dd),
8.64(1H,bd).
IR(nujol)cm : 1775, 1710, 1565, 1520, 1490, 1325,
1245, 1175, 1130, 1100, 935, 875, 765.
Working Example 24
3-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
yl)biphenyl-4-yl]methyl-N-propylamino] 1-
methylpyrazole-4-carboxylic acid
Starting from the compound (0.23 g) obtained in
Working Example 23 e), substantially the same procedure
as in Working Example 2 was followed to afford the
title compound as colorless crystals (0.16 g, 76%),
m.p.124-126C.
Elemental ~nalysLs for C23H23N5O4Ø2AcEtØ2hexane~
` C(%) H(%) N(%)
Calcd.: 64.12; 5-85; 14.95 - i
Found : 64.06; 6.09; 14.86
H-NMR(200MMz,CDCl3) ~ : 0.93(3H,t), 1.43-1.70(2H,m),
3.13-3.27(2H,m), 3.86(3H,s), 4.19(2H,s), 7.12-
7.31(4H,m), 7.40-7.53(2H,m), 7.56-7.68(lH,m),
7.77(1H,d), 7.93(1H,s), 8-96(1H,bd)-
IR(nujol)cm1: 1770, 1670, 1565, 1510, 1410, 1310,
1240, 1175, 1135, 1105, 940.
`" 2~0~1 3~3
- 77 -
Working Example 25
Ethyl 3-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-thiadiazol-3-
yl)biphenyl-4-yl]methyl-N-propylamino]-1-
methylpyrazole-4- carboxylate
Starting from the compound (0.44 g) obtained in
Working Example 23 c), substantially the same procedure
- as in Working Example 3 c) was followed to afford the
title compound as colorless crystals (0.18 g, 38%),
m.p.147-149C (recryst llized from ethyl acatate -
isopropyl ether).
Elemental Analysis for C25H27N5O3S:
C(%) H(~) N(%)
Calcd.: 62.87; 5.70; 14.66
Found : 62.49; 5.77; 14.30
H-NMR(200MEz,CDCl3) S : 0.83(3H,t), 1.30(3H,t), 1.46-
1.64(2H,m), 3.23(2H,t-like), 3.71t3H~s), 4.24(2H,q),
4.56(2H,s), 7.23(2K,d), 7.34-7.62(5H,m), 7.74(1X,s),
7.90(lH,dd), 8.42(1H,bd).
IR(nujol)cml: 1715, 1690, 1565, 1520, 1445, 1165,
1090.
Working Example 26
3-[N-[2'-(2,5-dihydro 5-oxo-1,2,4-thiadiazol-3-
)biphenyl-4-yl]methyl-N-propylamino]
methylpyrazole-4-carboxylic acid
Starting from the compound (0.12 g) obtained in
Working Example 25, substantialIy the same procedure as
in Working Example 2 was followed to afford the title
compound as colorless crystals ~0.10 g, 90%~, m.p.llO-
112C (recrystallized from ethyl acetate - isopropyl
ether).
Elemental Analysis for C23H23N5O3So0.1 iPr2O:
C(%) H(%) N(%)
Calcd.: 61.65; 5.35; 15.23
Found : 61.47; 5.42; 15`.14
lHONMR( 200MHz,CDC13) ~ : O.79(3H,t), 1.38-1.53(2H,m),
~.
,,, ~.
.. . . .
~ L :: .
. . .
:. ~ ".. ~ .: ' , ,
., ~ .
.. ~ , ' ' .
2 ~ 3 ~
- 7B -
2.96-3.14(2H,m), 3.81(3H,s), 4.27(2H,s), 7.14-
7.63(7H,m), 7.74(1H,s), 7.80(1H,d), 9.40(1H,bd).
IR(nujol)cml: 1695, 1560, 1550, 1503, 1160, 760.
- 5 Working Example 27
Ethyl 3-[N-butyryl-N-[2'-(2,5-dihydro 5-oxo-1,2,4-
thiadiazol-3-yl)biphenyl-4-yl]methylamino]-1-
methylpyrazole-4-carbo~ylate
a) Ethyl 3-[N-butyryl-N-(2'-cyanobiphenyl-4-yl)methyl
amino]-1-methylpyrazole-4-carboxylate
To a solution of the compound (1.80 g) obtained in
Working Example 23 a), triethylamine (1.4 ml) and 4-
dimethylaminopyridine (0.31 g) in 1,2-dichloxoethane
(50 ml) was added dropwise at 0C butyryl chloride
(1.04 ml). The reaction mixture was stirred overnight
and the solvent was distilled off. To the residue was
~added ethyl acetate, which was washed with water, a 10%
aqueous solution of potassium hyclrogensulfate, wat~r
and a saturated aqueous saline solution successively,
followed by drying over magnesium sulfate. The solvent
was distilled off to gi~e crude c:rystals, followed by
recrystallization from ethyl acetate isopropyl ether
to afford the title compound as colo~less crystals
(1.85 g, 86%), m.p.ll8-119C.
Elemental Analysis for C25H26N4O3:
C(%) H(%) N(%)
-Calcd.: 69.75; 6.09; 13.01
Found : 69.77; 6.09; 12.86
~; 1
H-NMR(200MHz,CDC13) ~ : ~.86(3H,t), 1.28(3H,t), 1.45-
1.78(2H,m), 2.14(2H,tj, 3.87(3N,s), 4.20(2H,q),
4~93(2H,s), 7.31-7.68(7H,m), 7.73(1H,d), 7.87(1H,s).
IR(nujol)cm~l: 2225, 1705, 1660, 1545, 1500, 1285,
1245, 1165, 1135, 1110, 1045, 770.
b) Ethyl 3-~N-butyryl-N-(2'- ~
hydroxycarbamimidoylbiphenyl-4-yl)methylamino]-1-
:~ . . .:
:'
.
3 ~
- 79 -
methylpyrazol-4-carboxylate
Starting from the compound (1.07 g) obtained in
Working Example 27 a), substantially the same procedure
as in Working Example 3) was followed to afford the
title compound as a powder (0.50 g, 43~).
lH-NMR(200MHz,CDCl3) ~ : 0.86(3H,t), 1.26(3H,t), 1.54-
1.75(2H,m), 2.18(2H,t), 3.88(3H,s), 4.15(2H,q),
4.29(2H,bds), 4.90(2H,s), 7.20-7.50(7H,m), 7.55(1H,d),
7.86(lH,s~.
IR(neat)cml: 3460, 3370, 2970, 1715, 1705, 1650, 1635,
1545, 1495, 1370, 1240, 1170, 1130, 1040.
c) Ethyl 3-[N-butyryl-N [2'-(2,5-dihydro-5-oxo-1,2,4-
oxadiazol-3-yl)biphenyl-4~yl]methylamino]-1-
methylpyrazol-4-carboxylate
Starting from the compound (0.49 g) obtained in
Working Example 27 b), substantially the same procedure
as in Working Example 5 d) was followed to afford the
titIe compound as a powder (0.45 g, 88%).
Elemental Analysis for C26H27N5O5.0~H2O: j
C(%) H(%) N(%)
Calcd.: 63.33; 5.60; 14.20
Found . 63.27; 5.33; 14.29
H-NMR(200MHz,CDCl3) ~ : 0.89(3H,t), 1.31(3H,t), 1.56-
1.87(2H,m), 2.16(2H,t-like), 3.90(3H,s), 4.12(2H,q),
4.92(2H,bd), 7.13-7.33(4H,m), 7.43-7.70(3H,m),
7.83(1H,d), 8.12(1H,s), 9-18(1H,bd)
IR(nujol)cm : 1780, 1715, 1665, 1640, 1545, 1495,
1400, 1305, 122~, 1170, 1130, 1105, 104~, 940, 760.
~30
Working Example 28
3-[N-butyryl-N-[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-
3-yl)biphenyl-4-yl]methylamino~ methylpyxazole-4-
carboxylic acid
Starting from the compound (0.35 g) obtained in
Working Example 27 c), substantially the same procedure
i
~ .
- 80 -
as in Working Example 2 was followed to afford the
title compound as colorless crystals (0.27 g, 83~),
m.p.220-222C (recrystallized from ethyl acetate -
isopropyl ether).
Elemental Analysis for C24H23N5O5:
C(%) H(%) N(%)
Calcd.: 62.47; 5.02; 15.18
Found : 62.21; 5.14; 15.20
1H-~MR(200MHz,CDCl3) ~ : 0.85(3H,t), 1.50-1.73(2H,m),
2.08(2H,t), 3.97(3H,s), 4.90(2H,bd), 7.20(2H,d), 7.31-
7~67~5H,m~, 7.8~(1H,s), 8.02(1H,d).
IR(nujol)cm :3190, 1790, 1700, 1660, 1545, 1500, 1400, ~j
1255, 1215, 1165, 1140, 935, 760.
~'
Working Example 29
Ethyl 3-[N-butyryl-N-[2'-(2,5-dihydro-5-oxo-1,2,4-
thiadiazol-3-yl)biphenyl-4-yl]methylamino]-1-
methylpyrazole-4-carboxylate
Starting from the compound (0.25 g) obtained in
Working Example 27 b), substantially the same procedure
as in Working Example 3 c) was followed to afford the
title compound as a powder (0.14 g, 52~
H-NMR(200MHz,CDCl3) ~ : 0.87(3X,t), 1.30(3H,t), 1.52- -
74~2H,m), 2.15(2H,t), 3.88(3H,s), 4.21(2H,q),
25 ~ 4.92(2H,s), 7.16-7.63(7H,m), 7.83-7.92(lH,m),
7.94(1H,s), 8.70(1H,bd).
; IR(neat)cm : 3130, 3060, 2960, 1700, 1545, 1495, 1440,
13gS, 1305,~1260, 1215, 1170,~ 1125, 1100.
~ Working Example 30
3-[N-butyryl-N-[2'-(2,5-dihydro-5-oxo-1,2,4-thiadiazol-
3-yl]biphenyl~4-yl]methylamino]-1-methylpyrazole-4-
carboxylic acid -
Starting from the compound (0.14 g) ob~ained in
Working Example 29, substantially the same procedure as_n Working Example 2 was followed to afford the title
!
.. . .
.. ..... ..
. . . . ' . . . .
.. ,. . ~, .... . ..... .. ... ..
,: . .: ... ~.... . .. . .................... . .
. . .
2~ 0$~ 3:)
- 81 -
compound as colorless crystals (79 mg, 61%), m.p.217-
219C (recrystallized from ethyl acetate - isopropyl
ether).
Elemental Analysis for C24H23N504S:
C(%) H(%) N(%)
Calcd.: 60.36; 4.85; 14.67
Found : 60~12; 4.84; 14.36
H-NMR(200MHz,CDCl3) ~ : 0.87(3H,t), 1.54-1.76(2EI,t),
3.98(3H,s), 4.89(2H,bd), 7.09-7.24(4H,m), 7.32-
7.40(1H,m), 7.44-7.62(2H,m), 7.79tlH,s), 7.95(1H,dd),
9.48(lH,bd).
IR(nujol)cml: 3200, 1720, 1695, 1660, 1540, 1505,
1395, 1250, 1210, 1160, 1135, 760.
Working Example 31
Ethyl 5-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
yl)biphenyl-4-yl]methyl-N-propylamino]-1-
methylpyrazole-4-carboxylate
a) Ethyl 5-(2'-cyanobiphenyl-4-yl)methylamino-1-
methylpyrazole-4-carboxylate
` To a solution of ethyl 5-amino-1-methylpyrazole-4-
carboxylate (1.69 g) (P. Schmidt et al. Helv. Chim.
Acta., 1959, 42, 349) in tetrahydrofuran (30 ml) was
added at 0C sodium hydride (about 60% in oil,-0.48 g).
~5 The-mixture was stirred for one hour at room
temperature. To the reaction mixture was added
portionwise at OQC 4-bromomethyl-2'-cyanobiphenyl (3.27
g). The mixture was stirred overnight at room
temperature. The reaction mixture was poured into a
saturated aqueous solution of ammonium chloride, which
was extracted with ethyl acetate. The extract was
washed with water and a saturated aqueous saline
solution, ollowed by drying over magnesium sulfate.
The solvent was distilled under reduced pressure to
give crude crystals. Recrystallization from ethyl
acetate - isopropyl ether afforded ~he title compound
. . ; .
- 82 ~
as colorless crystals (2.47 g, 69%), m.p.107-108C.
Elemental Analysis for C2lH20N4O2:
C(%) H(%) N(%)
Calcd.: 69.98; 5.59; 15.55
Found : 70.02; 5.62; 15.65
H-NMR(200MHz,CDCl3) ~ : 1.33(3H,t), 3~78(3H,s),
4.27(2H,q), 4.53(2H,bds), 6.18(1H,bd), 7.40-7.72(8H,m),
7.74-7.83(lH,m).
IR(nujol)cm : 3330, 3050, 2225, 1675, 1585, 1540,
1480, 122~, 1170, 1080, 1040, 770, 760.
b) Ethyl 5-[N-(2'-cyanobiphenyl-4-yl)methyl-N-propyl
amino]-1-methylpyrazole-4-carboxylate
To a solu~ion of the compound (0.51 g) obtained in
Working Example 31 a) in N,N-dimethylformamide (3 ml)
was added, at 0C, sodium hydride (about 60% in oil, 68
mg). The mixture was stirred for 30 minutes at room
temperature. To the reaction mixture was added at 0C
propyl iodide (0.17 ml), and the mixture was stirred
for one hour at room temperature. To the reactlon
mixture was added a saturated aqueous solution of
ammonium chloride, followed by extraction with ethyl
acetate. The extract was washed with water and a
saturated aqueous saline solution, followed by drying
25; over magnesium sulfate. The solvent was distilled off
under reduced pressure and the residue was purified by
column chromatography on silica gel to afford the title
compound as an oil (0.39 g, 69%~. ~
lH-NMR(200M~z,CDC13) ~ : 0.86(3H,t), 1.30-1.52(2H,m),
1-40(3Hrt)r 3-04-3.18(2H,m), 3.60(3H,s), 4.32(2H,s),
4.33(2H,q), 7.29-7.53(6H,m), 7.57-7.68(1~,m), 7.71-
7.78(1H,m), 7.85(1H,s).
IR(neat~cm~: 2960, 2870, 2220, 1705, 1480, 1375r 1225,
1195, 1045.
c) Ethyl S-~N-(2'-hydroxycarbamimidoylbiphenyl-4-yl)
, ":
: . .
:,,
- 83 _ 2~ 3~j
methyl-N-propylamino]-1-methylpyrazole-4-carboxylate
Starting ~rom the compound (1.30 g3 obtained in
Working Example 31 b), substantially the same procedure
as in Working Example 3 b) was followed to afford the
title compound as an oil (0.28 g, 20%).
H-NMR(200MHz,CDCl3) ~ : 0.90(3H,t), 1.32-1.54(2H,m),
1.39(3H,t), 3.17(2H,t), 3.47(3H,s), 4.28(2H,s),
4.33(2H,q), 4.51(2H,bds), 7.17(2H,d), 7.23-7.60(6H,m),
7.81(lH,s).
IR(neat)cml: 3480, 3360, 2970, 1735, 1705, 1645r 1545,
1490, 1375, 12~0, 1195, 1150.
d) Ethyl 5-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
yl)biphenyl-4-yl]methyl-N-propylamino]-l-
methylpyrazole-4-carboxylate
Starting from the compound (0.28 g) obtained in
Working Example 31 c), substantially the same procedure
as in Working Example 5 d) was followed to afford the
title compound as colorless crystals (O.22 g, 76~
m.p.174-175C (recrystallized from ethyl acetate -
isopropyl ether).
Elemental Analysis for C23H27N5O4:
C(%) H(%) N(%)
~ Calcd.: 65.06; 5.90; 15.17
Found : 65.03; 5.97; 14.96
H-NMR(200MHz,CDCl3) ~ : 0.87(3H,t), 1.27-1.50(2H,m),
1.35(3H,t), 3.09(2H,t), 3.55(3H,s), 4.25(2H,q),
4.26(2H,s), 7.23(4H,s-like), 7.36-7.66(3H,m),
7.74(1H,s), 7.80(1H,dd), 8.85(1H,bd).
IR(nujol)cm : 1755, 1700, 1600, 1545, 1485, 1430,
1250, 1195, 1050, 950, 765.
Working Example 32
5-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
yl)biphenyl-4-yl]methyl-N-propylamino]-1-
methylpyrazole-4-carboxylic acid
.. ., , : - .
-. . ,
- 84 - ~1 Ofi1 3~
Starting from the compound (0.14 g) obtained in
Working Example 31 d), substantially the same procedure
as in Working Example 2 was followed to afford the
title compound as colorless crystals (0.11 t, 83%),
m.p.197-198C (recrystallized from ethyl acetate -
isopropyl ether).
Elemental ~nalysis for C23H23N5O4Ø2H2O:
C(~) E(%) N(%)
Calcd.: 63.21; 5.40; 16.02
Found : 63.36; 5.37; 15.75
H-NNR(200MHz,CDCl3) ~ : 0.89(3H,t), 1.32-1.53(2H,m),
3~12(2H,t), 3.76(3H,s), 4.14(2H,s), 7 13(2H,d), 7.20-
7.37(3H,m), 7.44-7.65(2H,m), 7.81(1H,s), 8.06(1H,dd),
9.06(lH,bd).
IR(nujol)cm1: 3170, 1750, 1715, 1545, 1490, 1230,
1190 .
Working Example 33
Ethyl 2-[N-[2'-(~,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)
biphenyl~4-yl]methyl-N-propylamino]quinoline-3-
carboxylate
a) Ethyl 2-propylaminoquinoline-3-carboxylate
An solution of ethyl 2-chloroquinoline-3-
carboxylate (1.1 g) [(K. Shimizu et al., J. Pharm. Soc.
2S Jpn., 87, 672(1967)] and propylamine (1.8 g) in ethanol
20 ml) was heated at 95C fox 8 hours in a sealed
tube. The reaction mixture was concentrated under
reduced pressure, to which was added a saturated ~ i
aqueous solution of sodium hydrogencarbonate, ~ollowed
by extraction with chloroform. The extract was dried
over anhydrous sodium sulfate, then the solvent was
distilled off. The residue was purified by column
chromatography on silica g~l to afford the title
compound as a yellow oil (0.98 g, 80%).
lH-NMR(200MH~,CDCl3) ~ : 1.05(3H,t), 1.44(3H,t), 1.64-
1.83(2H,m), 3.53-3.65(2H,m), 4.39(2H,q), 7.12-
i
.1
':
.
-85_ ~-~Ofil3;`~
7.20(1H,m), 7.53-7.67(3H,m), 7.97(1H,brs), 8.63(1H,s).
IR(neat)cm: 3375, 2955, 1690, 1620, 1285, 1200.
b) Ethyl 2-[N-(2'-cyanobiphenyl-4-yl)methyl-N-
propylamino~quinoline-3-carboxylate
Starting from the compound (1.2 g) obtained in
Working Example 33 a), substantially the same procedure
as in Working Example 23 b) was followed to afford the
title compound as a yellow oil (1.3 g, 64%).
1H-NMR(200(MHz,CDCl3) S: 0.83(3H,t)! 1.41(3H,tj, 1.55-
1.74(2H~m), 3.35(2H,t), 4.38(2H,q), 4.92(2H,s), 7.23-
7.78(12H,m), 8.33(1H,s).
IR(neat)cm ~: 2960, 2220, 1720, 1620, 15~0, 1490, I475,
1430, 1225, 1200, 1070, 755.
~; c) E:thyl 2 [~-(2'-hydroxycarbamimidoylbiphenyl-4-yl)
methyl-N-propylamino]quinoline-3-carboxylate
Starti~g from the compound ~1.3 g) obtained in
Working Example 33 b), substantially the same procedure
as in Working Example 3 b) was followed to afford the
title compound as a y2110w powder (0.77g, 53%), m.p.61-
67C-
H-NMR(200~5Hz,CDCl3) ~ : 0.82(3H,t), 1.40(3H,t), 1.53-
1.73(2H,m),~ 3.34(2H,t), 4.33-4.43(4Hjm), 4.88(2H,s)l -
25~ ~ 7.23 7.14(12H,m), 8.23(1H,s).
IR(neat)cm 1 3400, 3370, 1710, 1620, 1595, 1490, 1430,
1205.
d) Ethyl 2-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiAzoI-
3-yljbiphenyl]methyl-N-propylamino]quinoline-3-
carboxylate
- Starting from the compound (0.35 g) obtained in
Working Example 33 c), substantially~the same procedure
~ as in Working Example 5 d) was followed to a~ford the
title compound as pale yellow prisms (0.27 g, 72%),
m.p.l55-156C (isopropanol).
.
,
- 86 _ ~ OK~
Elemental Analysis for C30H26N4O4:
C(%) H~%) N(%)
Calcd.- 70.85; 5.55; 11.02
Found : 70.55; 5.51; 10.97
H-~MR(200MHz,CDCl3) ~ : 0.83(3H,t), 1.41(3H,t), 1.55-
1.74(2H,m), 3.34(2H,t), 4.38(2H,q), 4.90(2H,s), 7.23-
7.87(12H,m), 8.34(1H,s).
IR(nujol)cml:3130, 1780, 1670, 1490, 750.
Working Example 34
2-tN-~2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
yl)biphenyl-4-]methyl-~-propylamino]quinoline-3-
carboxylic acid
Starting from the compound (0.20 g) obtained in
Working Example 33 d), substantially the same procedure
as in Working E~ample 2 was followed to afford the
title compound as a yellow powder (0.14 g, 75%).
Elemental Analysis for C28H24N4O4Ø5H2O:
C(%) H(%) N(~)
Calcd.: 68.70; 5.15; 11.45
Found : 68.90; 5.01; 11.35
H-NMR(200MHz,CDC13) ~ : O.93(3H,t), 1.45-1.6~3(2H,m),
3.53(2H,t), 4.40(2H,s), 7.05(2H,d), 7.20(2H,d), 7.38-
7.92(6H,m), 7.88(1H,d), 8.08(2H,t), 9.12(1H,s).
IR(nujol)cm : 1780, 1620, 1590, 760.
Working Example 35
Ethyl 2-~N-[2'-(2,5-dihydro-5-oxo-1,2,4-thiadiazol-3-
yl)biphenyl-4-yl]methyl-N-propy].amino]quinoline-3-
carboxylate
To a solution of the compound (0.42 g) obtained in
Working Example 33 c) in dichloromethane (5 ml) was
added dropwise under ice-cooling a solution of I,1'- 1
thiocarbonyldiimidazole (0.18 g) in dichloromethane (3
ml). The mixture was stirred for 15 minutes, to which
were added chloroform (32 ml), silica gel (Merck,
- ~7 ~ a
Art7734, 4 g) and methanol (8 ml). The mixture was
stirred for further 15 hours at room temperature.
Silica gel was filtered off, and the filtrate was
concentrated under reduced pressure. The residue was
5 purified by column chromatography on silica gel. The
product thus obtained was recrystallized from isopropyl
ether to afford the title compound as yellow prisms
(0.19 g, 42~), m.p.124-125C.
Elemental Analysis for C3~H28N4O3S:
C(%) H(~) N(%) S(%)
Calcd.: 68.68; 5.38; 10.68; 6.11
Found: 68.50; 5.34; 10.71; 6.06
H-NMR(200MHz,CDCl3) ~: 0.82(3H,t), 1.42(3H,t), 1.56- J
1.72(2H,m), 3.32(2H,t), 4.40(2H,q), 7.24-7.73(11H,m),
8.00(1H,d), 8.15(1H,brs), 8.34(1H,s).
IR(nujol)cm 1 1715, 1665, 1500, 1200, 1065, 750.
Working Example 36
2-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-thiadiazol-3-
20 yl)biphenyl-4-yl]methyl-N-propylaLmino]~[uinoline-3-
carboxylic acid
Starting from the compound (0.12 g) obtained in
Working Example 35, substantially the same procedure as
in Working Example 2 was followed to afford the title
compound as a yellow powder (0.11 g, 93%), m.p.ll8-
124C
Elemental Analysls for C28H24N4o3S2/3H2O:
C(%) H(%) N(%)
Calcd.: 66.13; 5.02; 11.02
Found o 66.29; 4.80; 10.91
H-NMR(200MHz,CDCl3) ~: 0.87(3H,t), 1.40-1.58(2H,m),
3.32(2H,t), 4.42(2H,s), 7.19(2H,d), 7.29-7.90(9H,m),
7.99(1H,d), 8.11(1E~,d), 8.96(1H,brs), 9.06(1H,s).
IR(nujol)cm: 1695. 1620, 1590, 760.
Experimental Example 1
- 88 _ ?~ ~13~
Inhibitory Effect of Binding of Angiotensin-II to
Angiotensin II Receptor
[Method]
An experiment of inhibition on the binding of
angiotensin II (A-II) receptor was performed by
modified the method of Douglas et al. tEndocrinology,
102, 685-696 (1978)]. An A-II receptor membrane
fraction was prepared from bovine adrenal cortex.
The compound of the present invention (10-6M or 10-
7M) and l25I-angiotensin II (lZ5I-AII~ (2.44KBq/50
microliter) were add~d to the receptor membrane
fraction, and the mixture was incubated at room
temperature for one hour. The receptor-bound and free i
5I-AII were separated through a filter (Whatman GF/B
filter), and the radioactivity of l25I-AII bound to the
receptor was determined.
[Results]
The results relating to the compounds of the
present invention are shown in the table 1.
~xperimental Example 2
Inhibitory Effect of the Compound of the Present
- Invention on AII-induced Pressor Response
~Method]
Jcl:SD rats (9 week old, male~ were used. On the -
previous day of the experiment, rats were applied with
cannulation into the femoral artery and vein under
anesthesia with pentobarbital Na. These animals were
fasted but allowed to access freely to drinking water
until the experiment was started. Just on the day of
conducting the experiment, the artery cannula was
connected with a blood-pressure transducer, and the
average blood pressure was recorded by polygraph.
Before administration of the drug, the pressor action
due to intravenous administration of A-II (100 ng/kg)
as the control was determined. The drugs were orally
- 89 ~ 3 ~
administered, then, at each point of the determination,
A-II was administered intravenously, and the pressor
action was similarly determined. By comparing the
pressor action before and after administration of the
drug, the percent inhibition by the drug was evaluated.
~Results]
The results relating to the compounds of the
present invention are shown in Table 1.
,
:: .
., , . ;..... . :,. .
go ~ o~13~
Table 1
Ex~mplg ¦ St~ucqur~l Formula ~ d~orecept~r Pre1sorRes poo~e [P.~.
[~ inhibitionl O. 3~tkg l~g~3cg
~ 5~ O __ _
~ o-7~
__ ~ _ .___ I
h~ .
, : . I COOI~ ~1(10-~'~)
2 ~ ~ O~I ~ ~10 all~
. ~ S 6 ~` : N T
.~ :
a) +++ > 70% >++ > 5096 >
NT: Not tested
'
;
:, :
- 9~ 3;~
Formulation Examples
When the compound (I) of the present invention is
used as a therapeutic agent for circulatory
disturbances such as hypertension, heart diseases,
cerebral apoplexy and nephritis, i.t can be used in
accordance with, for example, the following
formulations.
1. Capsules
(1) 4-[N-butyl-N-[2'-(2,5-dihydro-5-oxo-1,2,4-
10 oxadiazol-3~yl)biphenyl-4-yl]methyl]aminopyrimidine-5-
carboxylic acid 10 mg
(23 lactose 90 mg
(3) microcrystalline cellulose 70 mg
(4) magnesium stearate 10 mg
one capsule 180 mg
(1), ~2), (3) and a half of (4) are mixed and then
granulated. To the granules is added the remainder of
(4), and the whole is filled into a gelatin capsule.
2. Tablet
(1) 4-[N-butyl-N-~2'-(2,5-dihydro-5-oxo-1,2,4-
oxadiazol-3-yl)biphenyl-4-yl]methyl]aminopyrimidine-5-
carboxyl.ic acid 10 mg
(2) lactose . 35 mg
~` (3) corn starch 150 mg
~4) microcrystalline cellulose 30 mg
(5) magnesium stearate 5 mg
one table230 mg
(1~/ (2), (3), two thirds of (4) and a half of (S)
are mi~ed and then granulated, followed by subjecting
the mixture to compression molding.
3. Injections
(1) 4-[N-butyl-N-[2'-(2,5-dihydro-5-oxo~,2,4-
oxadiazol-3-yl)biphenyl-4-yl]methyl]aminopyrimidine-5-
carboxylic acid disodium salt 10 mg
(2) inositol 100 mg
(3) benzyl alcohol 20 mg
. ~ ~
. : . . :
'~ . 3 ;~
- 92 -
one ampoule 130 mg
(1), (2) and (3) are dissolved in water for
injection to make the whole volume 2 ml, which is
filled into an ampoule. The whole process is conducted
under sterile conditions.
4. Capsules
(1~ 4-[N-butyl-N-[2'-(2,5-dihydro-5-oxo-1,2,4-
thiadiazol-3-yl)biphenyl-4-yl]methyl]aminopyrimidine-5-
carboxylic acid 10 mg
(2) lactose 90 mg
(3) microcrystalline cellulose 70 mg
(4) magnesium stearate 10 mg
one capsule 180 mg
(1), (2), (3) and a half of (4) are mixed and then
granulatedO To the granules is added the remainder of
(4), and the whole is filled into a gelatin capsule.
5. Tablets
(1) 4-[N-butyl-N- r 2'-(2,5-dihydro-5-oxo-1,2,4-
thiadiazol-3yl)biphenyl-4-yl]methyl]aminopyrimidine-5-
20 carboxylic acid 10 mg
(2) lactose 35 mg
(3) corn starch 150 mg
(4) microcrystalline cellulose 30 mg
(S) magnesium stearate 5 mq
one tablet 230 mg
(1), (2), (3), two thirds of (4) and a hal~ of (S)
are mixed and then granulated. To the granules are
added the remainders of (4) and (5), followed by
subjecting the mix~ure to compression molding.
6. Injections
; (1) 4-[N-butyl-N-[2'-(2,5-dihydro-5-oxo-1,2,4-
thiadiazol-3-yl)biphenyl-4-ylJmethyl]aminopyridine-5-
carboxylic acid disodium salt 10 mg
(2) inositol 100 mg
(3) benzyl alcohol 20 mg
one ampoule 130 mg
,- ., .
''', : ,. :
.
.. : . ' : ,
a
- 93 -
(1), (2) and (3) are dissolved in water for
injection to make the whole volume 2 ml, which is
filled into an ampoule. The whole process is conducted
under sterile conditions. 7. Capsules
(1~ 2-[N-[2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-
yl)biphenyl-4~yl]methyl-N-propyl]aminopyridine-3-
carboxylic acid 10 mg
(2) lactose 90 mg
(3) microcrystalline cellulose 70 mg
(4) magnesium stearate 10 mg
one capsule 180 mg
(1), (2), (3) and a half of (4) are mixed and then
granulated. To the granules is added the remainder of -¦
(4), and the whole is filled into a gelatin capsule.
8. Tablets
(1) 2-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)
biphenyl-4-yl]methyl-N-propyl]aminopyridine-3-
carboxylic acid 10 mg
(2) lactose 35 mg
(3) corn starch 150 mg
(4) microcrystalline cellulose 30 mg
(5) magnesium stearate 5 mg
one tahlet 230 mg
(1), (2), (3), two thirds of (4) and a half of (5) are 25 mixed and then granulated. To the granules are added
he remainders of (4) and (5), followed by subjecting
the mixture to compression molding.
9. Injections
(1) 2-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-oxadlazol-3-yl)
biphenyl-4-yl]methyl-N-propyI]aminopyridine-3-
carboxylic acid disodium salt 10 mg
(2) inositol 100 mg
(3) benzyl alcohol 20 mg
one ampoule 130 mg
(1), (2) and (3) are dissolved in water for
injection to make the whole volume 2 ml, which is
:
,. ~ -, - : ,. ,
,: . . . .
, :
., : , ,
:.. . .
,
_ 94 ~ 3à
filled into an ampoule. The whole process is conducted
under sterile conditions.
10. capsules
(1) 2-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-thiadiazol-3-
5 yl)biphenyl-4-yl]methyl-N-propyl]aminopyridine-3-
carboxylic acid 10 mg
(2) lactose 90 mg
(3) microcrystalline cellulose 70 mg
(4) magnesium stearate 10 mg
one capsule 180 mg
(1), (2), (3~ and a half of (4) are mixed and then
granulated. To the granules is added the remainder of
(4), and the whole is filled in a gelatin capsule.
11. Tablets
(1) 2-[N-~2'-(2,5-dihydro-5-oxo-1,2,4 thiadiazol-3-
yl)biphenyl-4-yl]methyl-N-propyl]aminopyridine-3-
carboxylic acid 10 mg
(2) lactose 35 mg
(3) corn starch 150 mg
(4) microcrystalline cellulose 30 mg
`(5) magnesium stearate 5 mg
one tablet230 mg
~ 2), (3), two thirds of (4) and a half of (5)
are mixed and then granulated. To the granules are
added the remainders of (4) and (5), followed by
subjecting the mixture to compression molding.
12. Injections
(1) 2-[N-[2'-(2,5-dihydro-5-oxo-1,2,4-thiadiazol-3-
yl)biphenyl-4-yl]methyl-N-propyl]aminopyridine-3-
~30 carboxylic acid disodium salt ~ 10 mg
(2) inositol 100 mg ~ ¦
(3) benzyl alcohol 20 mg
one ampoule130 mg
(1), (2) and (3) are dissolved in water for
injection to make the whoIe volume 2 ml, which is
filled into an ampouleO The whole process is conducted
.
.
" ., ,.. ,
: : '
:::
-- 95 --
under sterile conditions.
:
:
:
:: :: : : " ; :
,
" ~ ; . ; ~ : - .~
'...... . ,; :: ,.. .. ,... ` .: , ,' ' ~