Note: Descriptions are shown in the official language in which they were submitted.
2 ~ O ~ ~ 9 7 o . z . ooso/43543
N-HyDRoxy-N-pMENyLcARBoxAMIDEs~ THEIR PREPARATION
AND COMPOSITIONS CONTAINING THEM FOR CONTROLLING ~-
E~ARMFUL FUNGI
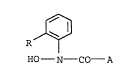
The present invention relates to N-hydroxy-
N-phenylcarboxamides of the formula I
R ~ . I
HO - N - CO - A
where the substituents have the following meanings: ..
R is C2-C12-alkyl, C2-C12-alkoxy, C3-C12-alkenyl,
C3-Cl2-alkenyloxy~ C3-C6-alkynyl, C3-C6-alkynyloxy,
where these groups can be partially or completely
halogenated; C3-C7-cycloalkyl, C4-C7-cycloalkenyl, :'.:.
C3-C7-cycloalkoxy or C4-C~-cycloalkenyloxy, where .
these rings can carry one to 3 Cl-C4-alkyls; phenyl
which can carry one to five halogen atoms and/or one ~ .
to three of the following radicals: Cl-C4-alkyl, :
C1-C4-haloalkyll C1-C4-alkoxy, C1-C4-haloalkoxy,
C1-C4-alkylthio or C1-C4-haloalkylthio; -:
A is a cyclic radical from the group consisting of the
formulae Al to A7:
tR3)n
Al A2 A3 A4
7~C~ ~ 6
~5 A6 A7
where the substituen~s have the following meanings:
20 X is -CH2-, -S-, -SO- or -S2-;
Y is -O- or -S-' ::-
R1, R2, R4, R' and R7 are halogen, Cl-C4-alkyl or
,::: , '~,~"''' '~.,.', i,, "-"" ", , ,,,; .:- .", , " ~" ;~
'. , ' ' '; ' ' . ' , ! . ' : ,
,~ ~ ,', " '.' ."; ' ' ' ', . ,',
' ' ' ~ , ' ,, ",, ' '' ' ' ', ' ,'i'
2106~7
- 2 - O~Z. 0050~43543
C,-C4-haloalkyl;
R3 and R6 are hydrogen, halogen or C,-C4-alkyl;
n is 1 or 2, where the radicals R3 can be different if
the value of n is 2.
The invention additionally relates to the
preparation of these compounds, compositions containing
them and their use for controlling harmful fungi, in
particular Botrytis.
N-(2-Chlorophenyl)-2-chloronicotinamide is known
from the literature as a fungicidal active compound
(DE-A 2 417 216).
It is an object of the present invention to
provide novel fungicidally active compounds having an
improved spectrum of action.
We have found that this object is achieved by the
compounds I defined at the beginning.
We have additionally found processes for pre-
paring these compounds, compositions containing them and
their use for controlling harmful fungi.
The compounds I are in general obtained by ~-
reacting a carboxylic acid halide of the formula II in a
manner known per se (eg. J. March, Advanced Organic
Chemistry, 2nd Ed., 1977, 382 ~f., McGraw-Hill) with an
N-hydroxyaniline of the formula III in the prPsence of a
2~ base.
Hal-CO-A + R ~ - - ~ R~
HO - NH HO - N - CO - A
II III I
~ he radical Hal in the formula II is a halogen
~uch as chlorine, bromine or iodine, in particular
chlorine or bromine.
This reaction i8 customarily carried out at
temperatures from -20C to 100C, preferably -10~C to
: ". '
.. . .. . . . . . .
.. .. . . : .. , . :
, .... ,, ,. . - : ,, . . : . : . :
., , ,, , " ., . , . . . . : :.
, . ., .. :, . , ... , : . . .
., . , . ,:, . .. .. . ., .. , ~ : . :
:;, ., ' .: ' ': ' '. .' , , ' ' . . , ' ~ ' ' ,
,, , . ~. . ., ~, . " . ,
..... . ", , , i , ~, ...... . . .
210~497
_ 3 _ o.z. 00~0/435~3
50C.
Suitable solvents are:
aliphatic hydrocarbons such as pentane, hexane, cyclo-
hexane and petroleum ether, aromatic hydrocarbons such as
toluene, o-, m- and p-xylene, halogenated hydrocarbons
such as dichloromethane, chloroform and chlorobenzene,
ethers such as diethyl ether, diisopropyl ether, tert-
butyl methyl ether, dioxane, anisole and tetrahydrofuran,
nitriles such as acetonitrile and propionitrile, ketones
such as acetone, methyl ethyl ketone, diethyl ketone and
tert-butyl methyl ketone, alcohols such as methanol,
ethanol, n-propanol, isopropanol, n-butanol and tert-
butanol, and also dimethyl sulfoxide and dimethyl-
formamide, particularly preferably toluene, xylene and
tetrahydrofuran.
Mixtures of the solvents mentioned can also be
used.
Suitable bases are generally inorganic compounds
such as alkali metal and alkaline earth metal hydroxides
such as lithium hydroxide, sodium hydroxide, potassium
hydroxide and calcium hydroxide, alkali metal and
alkaline earth metal oxides such as l.ithium oxide, sodium
oxide, calcium oxide and magnesium oxide, alkali metal
: and alkaline earth metal hydrides such as lithium
hydride, sodium hydride, potassium hydride and calcium
hydride, alkali metal amides such as lithium amide,
sodium amide and potassium amide, alkali metal and
alkaline earth metal carbonate~ ~uch as lithium carbonate
and calcium carbonate and also alkali metal hydrogen-
carbonates such as sodium hydrogencarbonate, and organo-
metallic compounds, in particular alkali metal alkyls
such as methyllithium, butylli~hium and phenyllithium,
alkylmagnesium halides such as methylmagnesium chloride
and also alkali metal and alkaline earth metal alkoxides
such as sodium methoxide, sodium ethoxide, potassium
ethoxide, potassium tert-butoxide and dimethoxymagnesium,
additionally organic bases, eg. tertiary amines such as
, -, . . ... . . . . . . .
, :, .. .
,~. , , ,. :
':' : , ' : .. ', . ' ' ' '' : ,
.. . .
21~6~97
- 4 - O.Z. 0050/43543
trimethylamine, triethylamine, tri-isopropylethylamine
and N-methylpiperidine, pyridine, substituted pyridines
such as collidine, lutidine and 4-dimethylaminopyridine
and also bicyclic amines.
Sodium hydro~encarbonate, sodium carbonate,
triethylamine ~nd pyridine are particularly preferred.
The bases are in general employed in equimolar
amounts based on the compound II. However, they can also
be used in an excess of from 5 mol% to 30 mol%,
preferably 5 mol% to 10 mol%, or - in the case of the use
of tertiary amines - if appropriate as a solvent.
The star~ing materials are in general reacted
with one another in equimolar amounts. It may be advan-
tageous for the yield to employ II in an excess of from
1 mol% to 20 mol~, preferably 1 mol% to 10 mol%, based on
III.
The starting substances of the formulae II and
III needed for preparing the compounds I are known in the
literature (Houben-Weyl, Methoden der org. Chemie
(Method~ of Organic Chemistry), Vol. 10/1, pp. 1138-1148)
or can be prepared according to the literature cited.
With respect to their use :in fungicidal composi-
tions, suitable compounds of the formula I are those in
which the substituents have the following meanings:
25 R is C2-Cl2-alkyl such as ethyl and straight-chain or
branched propyl, butyl, pentyl, hexyl, heptyl,
octyl, nonyl, decyl, undecyl and dodecyl, particu-
larly strai~ht-chain or branched C3-C10-alkyl such as
propyl, 1-methylethyl, butyl, 1-methylpropyl,
2-methylpropyl, l,1-dimethylethyl, n-pentyl,
l-methylbutyl, 2-methylbutyl, 3-methylbutyl,
1,2-dimethylpropyl, 1,1-dimethylpropyl,
2,2-dimethylpropyl, 1-ethylpropyl, n-hexyl,
1-methylpentyl, 2-methylpentyl, 3-methylpentyl,
4-methylpentyl, 1,2-dimethylbutyl, 1,3-dimethyl-
butyl, l,3-dimethylbutyl, 2,3-dimethylbutyl,
1,1-dimethylbutyl, 2,2-dimethylbutyl, 3,3-dimethyl-
,, ,- .. , . ............. , ; . .: .
, ~ . ., , . . . , , , , . ,. . : .
., ,. , , , . . . , ', . , ', ', . : ~.. '. .'' :' : : ' . ;
...... . , ... . . '. ' .'. ' :. '. :
2 1 ~ 7
- 5 - O.Z. 0050/43543
butyl,l,1,2-trimethylpropyl,1,2,2-trimethylpropyl,
l-ethylbutyl, 2-ethylbutyl, 1-ethyl-3-methylpropyl,
n-heptyl, 1-methylhexyl, l-ethylpentyl, 2-ethyl-
pentyl, 1-propylbutyl, octyl, l-methylheptyl,
2-methylheptyl, l-ethylhexyl, 2-ethylhexyl,
l-propylpentyl, 2-propylpentyl, nonyl, l-methyl-
octyl, 2-methyloctyl, 1-ethylheptyl, 2-ethylheptyl,
l-propylhexyl, 2-propylhexyl, decyl, l-methylnonyl,
2-methylnonyl, l-ethyloctyl, 2-ethyloctyl, 1-propyl-
heptyl and 2-propylheptyl, in particular propyl,
l-methylethyl, butyl, l-methylbutyl, 2-methylbutyl,
l,l-dimethylethyl, pentyl, 1-methylbutyl, hexyl,
heptyl and l-methylheptyl, where these groups can be
partially or complete1y halogenated, ie. the
hydrogens of these groups can be partially or com-
pletely replaced by halogens such~ as fluorine,
chlorine and bromine, in particular fluorine and
chlorine, for example haloalkyl such as chloro-
methyl, dichloromethyl, trichloromethyl, fluoro-
methyl, difluoromethyl, trifluoromethyl, chloro-
fluoromethyl, dichlorofluoromethyl, chlorodifluoro-
methyl, l-fluoroethyl, 2-fluoroethyl, 2,2-difluoro-
ethyl,2,2,2-trifluoroethyl,2-chloro-2-~luoroethyl,
2-chloro-2,2-difluoroethyl, 2,2-dichloro-2-fluoro-
ethyl, 2,2,2-trichloroethyl and pentafluoroethyl;
C2-Cl2-alkoxy such as ethoxy and ~traight-chain or
branched propoxy, butoxy, pentoxy, hexyloxy, heptyl-
oxy, octyloxy, nonyloxy, decyloxy, undecyloxy and
dodecyloxy, particularly straight-chain or branched
C2-C10-alkoxy such as ethoxy, propoxy, l-methyl-
ethoxy, butoxy, l-methylpropoxy, 2-methylpropoxy,
1,1-dimethylethoxy, n-pentoxy, 1-methylbutoxy,
2-methylbutoxy,3-methylbutoxy~1,2-dimethylpropoxy,
1-ethylpropoxy, n-hexyloxy, 1-methylpentoxy,
2-methylpentoxy, 3-methylpentoxy, 4-methylpentoxy,
1,2-dimethylbutoxy, 1,3-dimethylbutoxy,
'.~ . :, . , ,' ; ' ' '
,. . . . . . . .
~-- ~ , : . , .
210~7
- 6 - O.Z. 0050/43543
2,3-dimethylbutoxy, 1,2-dimethylbutoxy,
2,2-dimethylbutoxy, 3,3-dimethylbutoxy, 1,1,2-tri-
methylpropoxy, 1,2,2-trimethylpropoxy, l-ethyl-
butoxy, 2-ethylbutoxy, 1-ethyl-2-methylpropoxy,
n-heptyloxy, 1-methylhexyloxy, 2-methylhexyloxy,
3-methylhexyloxy, 4-methylhexyloxy, 5-methylhexyl-
oxy, 1-ethylpentoxy, 2-ethylpentoxy, l-propylbutoxy,
octyloxy, l-methylheptyloxy, 2-methylheptyloxy,
1-ethylhexyloxy, 2-ethylhexyloxy, 1-propylpentoxy,
2-propylpentoxy, nonyloxy, l-methyloctyloxy,
2-methyloctyloxy, l-ethylheptyloxy, 2-ethylheptyl-
oxy, l-propylhexyloxy, 2-propylhexyloxy, decyloxy,
1-methylnonyloxy,2-methylnonylox.y,1-ethyloctyloxy,
2-ethyloctyloxy, l-propylheptyloxy and 2-propyl-
lS heptyloxy, in particular ethoxy, propoxy, 1-methyl-
ethoxy, butoxy, 1-methylpropoxy, 2-me~hylpropoxy,
l/1-dimethylethoxy, pentoxy, hexyloxy and 2-ethyl-
hexyloxy, where these groups can ~e partlally or
completely halogenated, ie. the hydrogens o~ these
groups can be partially or completely replaced by
halogens such as fluorine, chlorine and bromine, in
particular fluorine and chlor.ine, for example halo-
alkoxy such as chloromethoxy, dichloromethoxy,
trichloromethoxy, fluoromethoxy, difluoromethoxy,
trifluoromethoxy, chlorofluoromiethoxy, dichloro-
fluoromethoxy, chlorodifluoromethoxy, 1-fluoro-
ethoxy, 2-~luoroethoxy, 2,2-difluoroethoxy,
2,2,2-tri~luoroethoxy, 2-chloro-2-fluoroethoxy,
2-chloro-2,2-difluoroethoxyl 2,2-dichloro-2-fluoro-
ethoxy, 2,2,2-t~ichloroethoxy and penta~luoroethoxy;
C3-C12-alkenyl such as straight-chain or b~anched
propenyl, butenyl, pentenyl, hexenyl, heptenyl,
octenyl, nonenyl, decenyl, undecenyl and dodecenyl,
particularly straight-chain or branched
C3-C10-alkenyl such a5 2-propenyl, 2-butenyl,
3-butenyl,1-methyl-2-propenyl,2-methyl-2-propenyl,
.:
'
. : ,,., :,, , ,.,,, , ; -
, :. , , ,, ., . . ~. . , . ,, , ., ,, ,. , :, . . ..
21~6~7
- 7 - O.Z. 0050/43543
2-pentenyl, 3-pentenyl, 4-pentenyl, 1-methyl-
2-butenyl, 2-methyl-2-butenyl, 3-methyl-2-butenyl,
1-methyl-3-blltenyl, 2-methyl-3-butenyl, 3-methyl-
3-butenyl, 1,1-dimethyl-2-propenyl, 1,2-dimethyl-
2-propenyl,1-ethyl-2-propenyl,2-hexenyl,
3-hexenyl,4-hexenyl, 5-hexenyl,1-methyl-
2-pentenyl,2-methyl-2-pentenyl,3-methyl-
2-pentenyl,4-methyl-2-pentenyl,1-methyl-
3-pentenyl,2-methyl-3-pentenyl,3-methyl-
3 pentenyl, 4-methyl-3-pentenyl, 1-methyl-
4-pentenyl,2-methyl-4-pentenyl,3-methyl-
4-pentenyl, 4-methyl-4-pentenyl, 1,1-dimethyl-
2-butenyl, 1,1-dimethyl-3-butenyl, 1,2-dimethyl-
2-butenyl, 1,2-dimethyl-3-butenyl, 1,3-dimethyl-
2-butenyl, 1,3-dimethyl-3-butenyl, 2,2-dimethyl-
3-butenyl, 2,3-dimethyl-2~butenyl, ~2,3-dimethyl-
3-butenyl, 1-ethyl-2-butenyl, 1-ethyl~3-butenyl,
2-ethyl-2-butenyl, 2-ethyl-3-butenyl, 1,1,2-tri-
methyl-2-propenyl, 1-ethyl-1-methyl-2-propenyl,
1-ethyl-2-methyl-2-propenyl, 1-methyl-2-pentenyl,
2-methyl-2-pentenyl, 1-methyl-3-pentenyl, 2-methyl-
3-pentenyl, 1-methyl-2-hexenyl, 2-methyl-2-hexenyl,
l-methyl-3-hexenyl,: 2-me~hyl-3-hexenyl, 1-ethyl-
2-pentenyl, 2-ethyl-2-pentenyl, 1-ethyl-3-pentenyl,
2-ethyl~3-pentenyl, 1-methyl-2-heptenyl, 2-methyl-
2-heptenyl, 1-methyl-3-heptenyl, 2-methyl-
3-heptenyl, 1-ethyl-2-hexenyl, 2-ethyl-2-hexenyl,
1-ethyl-3-hexenyl, 2-ethyl-3-hexenyl, l-methyl-
2-octsnyl, 2-methyl-2-octenyl, 1-methyl-3-octenyl,
2-methyl-3-octenyl, 1-ethyl-2-heptenyl, 2-ethyl-
2-heptenyl, 1-ethyl-3-heptenyl, 2-ethyl-3-heptenyl,
l-ethyl-2-octenyl, 2-ethyl-2-octenyl, l-ethyl-
3-octenyl and 2-ethyl-3-octenyl, in particular
1-propenyl, 2-propenyl, 1-methylethenyl, l-methyl-
2-propenyl,2-methyl-2-propenyl,l-ethyl-2-propenyl,
1-methyl-2-butenyl, 1-ethyl-2-butenyl, 1-(1-methyl-
ethyl)-2-butenyl, 1-butyl-2-butenyl, 1-methyl-
,
2 1 0 ~ 7
- 8 - O.Z. 0050/43543
2-pentenyl and 1,4-dimethyl-2-pentenyl, where these
groups can be partially or completely halogenated,
ie. the hydrogens of these groups can be partially
or completely replaced by halo~ens such as fluorine,
chlorine and bromine, in particular fluorine and
chlorine, in particular 3-chloro-2-propenyl and
2,3-dichloro-2-propenyl;
C3-Cl2-alkenyloxy such as straight-chain or branched
propenyloxy, butenyloxy, pentenyloxy, hexenyloxy,
heptenyloxy, octenyloxy, nonenyloxy, decenyloxy,
undecenyloxy and dodecenyloxy, partlcularly
straight-chain or branched C3-C10-alkenyloxy such as
2-propenyloxy, 2-butenyloxy, 3-butenyloxy, 1-methyl-
2-propenyloxy, 2-methyl-2-propenyloxy, 2-pentenyl-
oxy, 3-pentenyloxy, 4-pentenyloxy, l-methyl-
2-butenyloxy, 2-methyl-2-butenyloxy, 3-methyl-
2-butenyloxy, 1-methyl-3-butenyloxy, 2-methyl-
3-butenyloxy, 3~methyl-3-butenyloxy, 1,1-dimethyl-
2-propenyloxy, 1,2-dimethyl-2-propenyloxy, 1-ethyl-
2-propenyloxy, 2-hexenyloxy, 3-hexenyloxy,
4-hexenyloxy, 5-hexenyloxy, 1-methyl-2-pentenyloxy,
2-methyl-2-pentenyloxy, 3~methyl-2-pentenyloxy,
4-methyl-2-pentenyloxy, 1-methyl-3-pentenyloxy,
2-methyl-3-pentenyloxy, 3-methyl-3-pentenyloxy,
4-methyl-3-pentenyloxy, 1-methyl-4-pentenyloxy,
2-methyl-4-pentenyloxy, 3-methyl-4-pentenyloxy,
4-methyl~4-pentenyloxy, 1,1-dimethyl-2-butenyloxy,
1,1-dimethyl-3-butenyloxy, 1,2-dimethyl-
2-butenyloxy, 1,2-dimethyl-3-butenyloxy,
1,3-dimethyl-2-butenyloxy, 1,3-dimethyl-3-butenyl-
oxy, 2,2-dimethyl-3-butenyloxy, 2,3-dimethyl-
2-butenyloxy, 2,3-dimethyl-3-butenyloxy, 1-ethyl-
2-butenyloxy, 1-ethyl-3-butenyloxy, 2-ethyl-
2-butenyloxy,2-ethyl-3-butenyloxy,1,1,2~trimethyl-
2-propenyloxy, 1-ethyl-1-methyl -2-propenyloxy,
1-ethy1-2-methyl-2 propenyloxy, 1-methyl-2-pentenyl-
''. .:
,::'
' '".
, ~ :
, ~ ' , . ' .'~
" ' ' : "; ~ ' ," , . ' , '. " ' ", " ' ' ' '., " ., ' '
. " . ~
2 1 0 ~ 7
_ g _ o z. 0050~43543
oxy,2-methyl-2-pentenyloxy,1-methyl-3-pentenyloxy,
2-methyl-3-pentenyloxy,1-methyl-2-hexenyloxy,
2-methyl-2-hexenyloxy,1-methyl-3-hexsnyloxy,
2-methyl-3-hexenyloxy,1-ethyl-2-pentenyloxy,
2-ethyl-2-pentenyloxy,1-ethyl-3-pentenyloxy,
2-ethyl-3-pentenyloxy,1-methyl-2-heptenyloxy,
2-methyl-2-heptenyloxy,1-methyl-3-heptenyloxy,
2-methyl-3-heptenyloxy,1-ethyl-2-hexenyloxy,
2-ethyl-2-hexenyloxy,l-ethyl-3-hexenyloxy,2-ethyl-
3-hexenyloxy, 1-methyl-2-octenyloxy, 2-methyl-
2-octenyloxy, 1-methyl-3-octenyloxy, 2-methyl-
3-octenyloxy, 1-ethyl-2-heptenyloxy, 2-ethyl-
2-heptenyloxy, 1-ethyl-3-heptenyloxy, 2-ethyl-
3-heptenyloxy, 1-ethyl-2-octenyloxy, 2-ethyl-
2-octenyloxy, 1-ethyl-3-octenyloxy and 2-ethyl-
3-octenyloxy, in particular 2-propenyloxy, 1-methyl-
2-propenyloxy, 2-methyl-2-propenyloxy, 2-pentenyl-
oxy, 3-pentenyloxy, 1-methyl-2-butenyloxy and
1-methyl-2 pentenyloxy, where these groups can be
partially or completely halogenatedt ie. the
hydrogens of these groups can be partially or com-
pletely replaced ~y halogens such as fluorine,
; chlorine and bromine, in particular fluorine and
chlorine, in particular 3-chloro-2-propenyloxy,
: 25 2,3-dichloro-2-propenyloxy and 2,3,3-trichloro-
: 2-propenyloxy;
C3-C6-alkynyl such as 2-propynyl, 2-butynyl, 3-
butynyl, 1-methyl-2-propynyl, 2-pentynyl, 3-pent-
ynyl, 4-pentynyl, 1-methyl-3-butynyl, 2-methyl-3-
butynyl, 1-methyl-2-butynyl, 1,1-dimethyl-2-propyn-
yl, l-ethyl-2-propynyl, 2-hexynyl, 3-hexynyl, 4- :
alkynyl, 5-hexynyl, 1-methyl-2-pentynyl, 1-methyl-3-
pentynyl, 1-methyl~4-pentynyl, 2-methyl-3-pentynyl,
2-methyl-4-pentynyl, 3-methyl-4-pentynyl r 4-methyl-
2-pentynyl, 1,2-dimethyl-2-butynyl, 1,1-dimethyl-3-
butynyl, 1,2-dimethyl-3-butynyl, 2,2-dimethyl-3~
" ,~ .
;' ~ ~ .
' ' , ' :
21~6~97
- 10 - O.Z. OO50/43543
butynyl, 1-ethyl-2-butynyl, 1-ethyl-3-butynyl, 2-
ethyl-3-butynyl and 1-ethyl-1-methyl-2-propynyl, in
particular 2-propynyl, 2-butynyl and 3-butynyl,
where these groups can be partially or completely
halogenated, ie. the hydrogens of these groups can
be partially or completely replaced by halogens such
as fluorine, chlorine and bromine, in particular
fluorine and chlorine, for example 3-chloro-2-pro-
pynyl, 3-chloro-2-butynyl and 4-chloro-3-butynyl;
C3-C6-alkynyloxy such as 2-propynyloxy, 2-butynyloxy,
3-butynyloxy,l-methyl-2-propynyloxy,2-pentynyloxy,
3-pentynyloxy, 3-pentynyloxy, 4-pentynyloxy, 1-
methyl-3-butynyloxy, 2-methyl-3-butynyloxy, 1-
methyl-2-butynyloxy,1,1-dimethyl-2-propionyloxy,1-
ethyl-2-propynyloxy, 2 hexynyloxy, 3-hexynyloxy, 4-
alkynyloxy, 5-hexynyloxy, 1-methyl-2-pentynyloxy, 1-
methyl-3-pentynyloxy, 1-methyl-4-pentynyloxy, 2-
methyl-3-pentynyloxy, 2-methyl-4-pentynyloxy,
3-methyl-4-pentynyloxy~ 4-methyl-3-pentynyloxy,
1,1-dimethyl-2-butynyloxy, 1,1-dimethyl-3-butynyl-
oxy, 1,2-dimethyl-3-butynyloxy, 2,2-dimethyl-3-
butynyloxy,1-ethyl-2-butynyloxy,l-ethyl-3-butynyl-
oxy, 2-ethyl-3-butynyloxy and 1-ethyl-1-methyl-2-
propynyloxy, preferably 2-propynyloxy, 2-butynyloxy,
1-methyl-2-propynyloxy and 1-methyl-2-butynyloxy, 2-
propynyloxy, 2-butynyloxy, 3-butynyloxy and 1-
methyl-2-propynyloxy, where these groups can be
partially or completely halogenated, ie. the hydro-
gens of the~e groups can be partially or completely
replaced by halo~en~ such as fluorine, chlorine and
bromine, in particular fluorine and chlorine, for
example 3-chloro-2-propynyloxy, 3-chloro~2-butynyl-
oxy and 4-chloro-3-butynyloxy;
C3-C7-cycloalkyl such as cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl and cycloheptyl, where these
........ ..
~'''`'.
. , . "
2~0~497
O.Z. 0050/43543
rings can carry one to 3 Cl-C4-alkyl~ such as methyl,
ethyl, propyl, l-methylethyl, butyl, l-methylpropyl,
2-methylpropyl and l, l-dimethylethyl;
C4-C7-cycloalkenyl such as cyclobutenyl, cyclo-
pentenyl, cyclohexenyl and cycloheptenyl, where
these rings can carry one to three Cl-C4-alkyls such
as methyl, ethyl, propyl, l-methylethyl, butyl, 1-
methylpropyl, 2-methylpropyl and 1, l-dimethylethyl;
C3-C7-cycloalkoxy such as cyclopropoxy, cyclobutoxy,
cyclopentoxy, cyclohexyloxy and cycloheptyloxy,
where these rings can carry one to 3 C,-C4-alkyls
such as methyl, ethyl, propyl, l-methylethyl, butyl,
l-methylpropyl, 2-methylpropyl and
1, l-dimethylethyl;
or C4-C7-cycloalkenyloxy such as l-cyclobutenyloxy,
2-cyclobutenyloxy, l-cyclopentenyloxy,
2-cyclopentenyloxy, 3-cyclopentenyloxy,
l-cyclohexenyloxy, 2-cyclohexenyloxy, 3-cyclo-
hexenyloxy, l-cycloheptenyloxy, 2-cycloheptenyloxy,
3-cycloheptenyloxy and 4-cyc:loheptenyloxy, where
these rings can carry one to 3 C,-C4-al3~yls such as
methyl r ethyl, propyl, l-methylethyl, butyl, 1-
methylpropyl, 2-methylpropyl and 1, l-dimethylethyl;
phenyl, which can carry one to five halogen ~uch a~
2 5 ~luorine, chlorine, bromine and iodine, in
particular fluorine, chlorine and bromine, and/or
one to three of the following radicals:
- Cl-C4-alkyl as mentioned above;
- Cl-C4-haloalkyl as mentioned above;
Cl-C4-alkoxy as mentioned above;
- Cl-C4-haloalkoxy as mentioned above;
- Cl-C4-alkylthio such a~ methylthio, ethylthio,
: . . . . . .
: .
'' ' ~ : ., : ,
2~6497
- 12 - O.Z. 0050/43543
propylthio, 1-methylethylthio, butylthio, 1-
methylpropylthio, 2-methylpropylthio and
1,1-dimethylethylthio;
- or Cl-C4-haloalkylthio, particularly C,-C2-halo
alkylthio such as chloromethylthio, dichloro-
methylthio, trichloromethylthio, fluoromethyl-
thio, difluoromethylthio, trifluoromethylthio,
chlorofluoromethylthio, dichlorofluoromethylthio,
chlorodifluoromethylthio, 1-fluoroethylthio, 2-
fluoroethylthio, 2,2-difluoroethylthio, 2,2,2-
trifluoroethylthio, 2-chloro-2-fluoroethylthio,
2-chloro-2,2-difluoroethylthio, 2,2-dichloro-2-
fluoroethylthio, 2,2,2-trichloroethylthio and
pentafluoroethylthio.
15 A is a cyclic radical ~rom the group consisting of the
formulae A1 to A7:
~1 ~ R ~ O Ca3 ~3~ C113
Al A2 A3 A4
R3)~ ~Nr;~
2~5 A6 A7
.:
where the sub~tituentæ have the following meanings:
X is -CH2-, -S-, -SO- or -SO
Y . is -O- or -S~
R1, R2, R4, Rs and R' independently of one another are
halogen such as fluorine, chlorine and bromine,
Cl C4-alkyl as mentioned above, or C1-C4-haloalkyl as
mentioned above; .
R3 and R6 independently o one another are hydrogen,
halogen such as fluorine, chlorine and bromine or
- :,,: : . ~ :: . . , ., .,, . , ., ,.. , . ., .. , ,. . .. .. i i,.. ... ;, , .. , .. . . . :
2~0~97
- 13 - O.Z. 0050~43543
C;-C4-alkyl as mentioned above;
n is 1 or 2, where the radicals R3 can be different if
the value of n is 2.
With respect to the biological action,
particularly preferred compounds of the formula I are
those in which R has the abovementioned meanings and A is
a cyclic radical from the group consisting of the formu-
lae Al to A7, where X and Y have the abovementioned
meaning and the substituents are the following radicals:
R1 is halogen such as fluorine, chlorine and bromine,
methyl or C1-haloalkyl such as chloromethyl,
dichloromethyl, trichloromethyl, fluoromethyl,
difluoromethyl,trifluoromethyl,chlorofluoromethyl,
dichlorofluoromethyl and chlorodifluoromethyl;
R2 is halogen ~uch as fluorine, chlorine and bromine or
C,-haloalkyl such as chloromethyl, dichloromethyl,
trichloromethyl, fluoromethyl/ difluoromethyl,
trifluoromiethyl,chloro~luoromethyl,dichlorofluoro-
methyl and chlorodifluoromethyl;
R3 is hydrogen or methyl;
n is 1 or 2, where the radicals R3 can be different if
: the value of n is 2;
R4 is halogen such as fluorine, chlorine and ~romine or
~methyl;
- 25 Rs is methyl or C1-haloalkyl such as chloromethyl,
dichloromethyl, trichloromethyl, fluoromethyl,
: difluoromethyl,trifluoromethyl,chlorofluoromethyl,
dichlorofluoromethyl and chlorodifluoromethyl;
: ~ R6 i~ hydrogen, halogen such as ~luorine, chlorine and
bromine or methyl;
R7 is halogen ~uch a5 fll~orine, chlorine and bromine,
: methyl or CL-haloalkyl ~uch aS ~hloromethyl,
dichloromethyl, trichloromethyl, fluoromethyl,
difluoromethyl,trifluoromethyl,chlorofluoromethyl,
dichlorofluoromethyl and chlorodifluoromethyl.
In particular, those compounds of the formula I
are preferred in which R has the abovementioned meaning
.
.. , .,, .. .... ,. .,.,., ,,, . ., . , . . .... . , .. ,... .. , ~ ..... . . . . .
, . . - . , , ::. :,. . , . . , . . . .. . : . ~ .
, ~. : , " . . .. . . . . . . , . ~ .:
., , . . . , , , . " . . . . . .
,- j :. . . : . , :: ,
", "' : .," " , ' ' ' ' , ,; . . .,, i~ . ~ . . .... . .
; 2iO~9~
- 14 - O.Z. 0050/43543
and A is a cyclic radical from the group consisting of
the formulae A1 to A7, where X and Y have the above-
mentioned meaning and the substituents are the following
groups:
Rl is chlorine, bromine, iodine, methyl or trifluoro-
methyl;
R2 is chlorine or trifluoromethyl;
R3 is hydrogen or methyl;
n is 1 or 2, where the radicals R3 can be different if
the value of n is 2;
R4 is chlorine or methyl;
Rs is methyl, difluoromethyl or trifluoromethyl;
R6 is hydrogen, chlorine or methyl;
R7 is chlorine, methyl or trifluoromethyl.
Particularly preferred compounds of the formula I
are summarized in the following Tables A to ~.
. .. - . .......... .. ... . . .
. .. .: ~ . . . .. : .
. , ; . . ,
.
sAsF Aktienge~ellCcha~t 920557 0050/43543
~1~6~97
Table A
!i ~1 Rl
R ~ ~ I.l
HO - N - C0
_ .
CF3 i-C3H7
CF3 n-C3H7
. _ .
CF3 n-C4Hg
, ~_
CF
CF3 . i-C4Hg
CF3 tert.-C4Hg
CF3 n--CsH~
CF3 sec-CsH11 .
CF3 _ n-C6H13 .
CF3 n-C7H1s
CF3 sec.-C7Hls _ _ _ _
CF3 l-methylvinyl -
_ , _
CF3 2-methylvinyl
.. ., , _
CF3 allyl :
.
CF3 2-methylallyl
. . ,
CF3 2-ethylallyl
.. .
CF3 l-methylallyl
. . . . . .. .
: CF3l-ethylallyl
CF3 1-methyl-2-butenyl
_ .
CF3 1-ethyl-2-butenyl
CF3 1-isopropyl-2-butenyl
. , , _
C~; l-n-butyl-2-butenyl
CF3 1-methyl-2-pentenyl
CF3 1, 4-dimethyl-2-pentenyl
CF3 propargyl
CF3 2-butynyl :~
CF3 3-butynyl
CF3 ethox~
CF3 propoxy
. CF3 l-methylethoxy
:
.
,
. .
.: . : . . ; . :, . .
, ~ .";,.. , .. ~ ,,., : .
, . . .
BASF A3ctiengegell~c~ t 920557 O. Z . 0050/43543
2101~97
16
. _._
R R
.
CF3 n-butoxy
. _ _
CF3 l-methylpropoxy
. ~_ :-
CF3 2-methylpropoxy
CF3 l,l-dimethylethoxy
CF3 n-pentyloxy
CF3 n-hexyloxy
CF3 2-ethylhexyloxy
. _ . . _ .
CF3 2-propenyloxy
CF3 2-butenyloxy -
CF3 2-methyl-2-propenyloxy .
CF3 2-pentenyloxy
CF3 3-pentenyloxy
_ . . ._ _
CF3 3-chloro-2-propenyloxy
CF3 2,3-dichloro-2-propenyloxy
CF3 2,3,3-trichloropropenyloxy
CF3 2-propynyloxy
. . . . ~. .
CF3 2-butynyloxy
. .
CF3 3-butynyloxy
~ , .
CF3 1-methyl-2-propynyloxy
CF3 cyclopropyl ~
.- . .
CF3 cyclobutyl
..
CF3 cyclopentyl
CF3 cyclohexyl . .
. __
CF3 2-cyclopentenyl
.~ l-cyclopentenyl
. . . _ , _ _
CF3 2-cyclohexenyl
. _
CF3 l-cyclohexenyl
CF3 ~
CF3 cyclohexyloxy :-
.--. _ _ .
CF3 2-cyclopentenyloxy
. _ . . _
: CF3 2-cyclohexenyloxy
. .......
CF3 phenyl
. ._. _ - ~ .
Cl i-C3H7 _ _ -- -
Cl n-C3H
Cl n-C4Hg
Cl sec.-C4Hg
~5 Cl i-C4Hg _
Cl tert.-C4Hg
,
::. . : , :. .
.. . . ..
: , ' : ~ ' . ' ':. ''.`, . . ` .
: . `; ' . ' . . , ' . . ' ,; . . , , ' , ,
,, . ... . ~ . .. : . : : . .
.
,' ' ; '.`.` : '~, ,
. . . .
sASF Aktieng~sell~icha~t 920557 o.z. 0050/43543
2~06~7
Rl R
._ _
Cl n-C5Hll
Cl sec~-c5Hl 1 _
Cl n-C6H13
.... __ _ _
Cl n-C7Hls :
.,,
Cl sec.-C7Hls
_ _
Cl l-methylvinyl
Cl 2-methylvinyl : -
Cl allyl . .
Cl 2-methylvinyl
Cl 2-ethylallyl
Cl l-methylallyl
Cl l-ethylallyl
Cl l-methyl-2-butenyl
Cl l-ethyl-2-butenyl
Cl l-lsopropyl-2-butenyl
Cl l-n-butyl-2-butenyl
. ~ .
Cl methyl-2-pentenyl
_
Cl 1,4-dimethyl-2-pentenyl
.
Cl propargyl
25 . _ __ _ . .
Cl 2-butynyl . -
. . - . ~
Cl 3-butynyl -
. _ _
Cl ethoxy
Cl propoxy
Cl l-methylethoxy .
Cl n-butoxy :
_ _ _ ,
Cl l-methylpropoxy
Cl 2-methylpropoxy
Cl l,l-dimethylethoxy
Cl n-pentyloxy
, . . . .
Cl ~ n-hexyloxy
. .
Cl 2-ethylhexyloxy
_ _
Cl 2-propenyloxy
. ..
Cl 2-butenyloxy j
. . _ _
Cl 2-methyl-2-propenyloxy
: Cl 2-pentenyloxy
Cl 3-pentenyloxy
45 _ Cl 3-chloro-2-propenyloxy
2,3-dichloro-2-propenyloxy
.:
,: ' .:. :' .: ' ' ' . :, . . .. . . . . . .
~, , ~ ' ,''. ; , ', ' '', .' '' , . ' ,''::, ', . `' .
BASF Aktien~esellschaft 920557 O.~. 0050/43543
21~g~97
18
.. ._ _
. __ _ _ _
Cl 2,3,3-trichloropropenyloxy
Cl 2-propynyloxy -
_
Cl 2-butynyloxy
__ .
Cl 3-butynyloxy
_ .
Cl l-methyl-2-propynyloxy
Cl cyclopropyl
~ . . _ _ .. ._ ._
lU Cl cyclobutyl
Cl cyclopentyl
Cl cyclohexyl
Cl 2-cyclopentenyl
Cl l-cyclopentenyl
Cl 2-cyclohexenyl
Cl l-cyclohexenyl
Cl cyclopentyloxy
Cl cyclohexyloxy
___ _
Cl 2-cyclopentenyloxy .-
. . _ . _ _ .
Cl 2-cyclohexenyloxy
Cl phenyl
~ASF Aktienge~iell~chaft 9205~ ~ a ~ ~ 9~.Z. 0050/43543
19
Table B
R ~ R2 N I.2
HO - N - CO ~
R R
_ . . .
Cl ~
Cl n-C3H7
. _ _
Cl n-C~jHg
Cl sec.-C4Hg
. , _
Cl i-C~Hg
Cl tert.-C~Hg
. _ .. , .. _ _ . _ _
Cl n-C5Hll - -:
~ ,,
Cl sec.-CsHll _ _ -
Cl n-C6Hl3
. . .
Cl n-C7Hl5 : ::
- Cl : :
Cl l-methylvinyl
Cl 2-methylvinyl
: Cl allyl
Cl 2-methylallyl ;-
~__ .
Cl 2-ethylallyl .~ .
.. _ _ _ _~_ _
Cl ~ ~ :
Cl l-ethylallyl .
Cl l-methyl-2-butenyl
: Cl l-ethyl-2-butenyl
Cl l-isopropyl-2-butenyl
Cl l-n-butyl~-2-butenyl :
Cl l-methyl-2-pentenyl
. ,
Cl 1,4-dimethyl-2-pentenyl -: ::
Cl propargyl
.~ _
Cl 2-butynyl . ~:. .
Cl 3-butynyl
.. ._ __ _ _ _ . _ ~ .. .. _ _ ___ . _ .:
Cl ethoxy :.
. . ~ .~ _ _ _ _.
. Cl propox~
~ S ,, .. ,.. _ . . _
Cl l-methylethoxy
. . ~.. _._ _
Cl n-butoxy
. . .
, , , . . , . , . :; , : ,,: ; .
.,
BASF Aktien~e~ell~chaft 920557 O.z. 0050/43543
20 2106~7
R2 ' ,,.___ _
Cl l-methylpropoxy
_
Cl 2-methylpropoxy
Cl l,l-dimethylethoxy
. ~
Cl n-pentyloxy
. _ .
Cl n-hexyloxy
Cl 2-ethylhexyloxy
Cl 2-propenyloxy
Cl 2-butenyloxy
Cl 2-methyl-2-propenyloxy
Cl 2-pentenyloxy
Cl 3 -pentenyloxy
Cl 3-chloro-2-propenyloxy
. - ~
Cl 2,3~dichloro-2-propenyloxy
. _
Cl 2,3,3-trichloropropenyloxy
__ _
Cl 2-propynyloxy
Cl 2-butynyloxy
~ _ . _
Cl 3-butynyloxy
Cl l-methyl-2-propynyloxy : .
. _ . _
Cl cyclopropyl
Cl cyclobutyl
Cl cyclopentyl
Cl cyclohexyl
Cl 2-cyclopentenyl
: 30 Cl l-cyclopentenyl
Cl 2-cyclohexenyl
Cl l-cyclohexenyl
Cl cyclopentyloxy
Cl cyclohexyloxy
: ~ Cl 2-cyclopentenyloxy
Cl 2-cyclohexenyioxy :
Cl . i-C3H7 ~:
Cl n-C3H7 ,, ,~
Cl n-C4Hg
.. ._
Cl sec.-C~Hg
. , . __ .___ . ....... _ .. _ . _ _
Cl i-C4Hg
, . . , . . _ _ . . .
Cl tert.-C~Hg
._ ------ . ..... . __
Cl n-CsHll
. __ . .
Cl sec.-C5Hll _
, . .. .
~ ,: , . . .
.
.: . .
. .
BASF Al~tien~esellE~cha*t 920557 O.Z. 0050/43543
21 21~97
~ ...,
R2 R
, _ - - - . ,
Cl n-C6H13
.
Cl n-C7H1s
5 ~
Cl sec . -C7H1 5
. .
Cl ethoxy
Cl propoxy
Cl l-methylethoxy
Cl n-buto ~ =
Cl l-methylpropoxy
Cl 2-methylpropoxy
. _ .
Cl l,l-dimethylethoxy
. ._ _.
Cl n-pentyloxy
_ , _ _ _
Cl n-hexyloxy
. .._
Cl cyclopentyl
. . ~ ._ .. _ _
Cl phenyl
:
: . .
'' ' '. ~ ' ' ' '
.. . . :
,
B~SF AXtien~esell3cha~t 920557 O.Z. 0050~43543
22 ~ 1 a fi ~ 9 7
Table C
~ ,.
~ I X ~
HO - N - CO ~ \ ~ I.3
rO '
H3C
X R
, _
CH2 i-C3H7
CH2 ~
CH2 --n-C4Hg
CH2 sec. C4Hg
CH2 i-C4Hs
_ _ . .
CH2 tert.-C4Hg
. .
CH2 n-CsHll
... _ . .
CH2 sec.-CsHl1
. .
CH2 n-C6Hl3
. _ _ . . ~
CH2 n-- C7Hls
: 25 CH2 sec.-C7H1s
. _ ,
CH2 1-methylvinyl
: , . .
CH2 2-methylvinyl
. . __ ,
CH2 allyl -:i
. . . . . _ _ . .. __ , . : -
CH2 2-methylallyl
,. ._ _
CH2 2-ethylallyl
- _ _ _
CH2 1-methylallyl
~ . -- - . .
: : ~CH2 1-ethylallyl
___ ,
5CH2 1-methyl-2-butenyl
3 ~. _ _
CH2 1-ethyl-2-butenyl
... _ . .. _
CH2 1-isopropyl-2-butenyl ~ :
. . .
CH2 1-n-butyl-2-butenyl
. .__
: CH2 1-methyl-2-pentenyl
4~ _ ._
v CH2 1,4-dimethyl-2-pentenyl
CH2 propargyl
. . _
CH2 2-butynyl
. .
CH2 3-butynyl
. __ .. ~
CH2 ethoxy
CH2 propoxy
~, : ,
~ . s~sF Aktienge~ell~chaft 920557 O.Z. 0050/43543
23 21~ 7
X R
__
CH2 l-methylethoxy
CH2 n-butoxy :
. . _ __ _ _
CH2 l-methylpropoxy
2 2-methylpropoxy
CH2 l,l-dimethylethoxy
CH2 n-pentyloxy
CH2 n-hexyloxy
...... ... .... _
CH2 2-ethylhexyloxy
CH2 2-propenyloxy
CH2 2-butenyloxy
CH2 ~~~~ 2-methyl-2-propenyloxy
CH2 2-pentenyloxy
CH2 3-pentenyloxy :
~ _
CH2 3-chloro-2-propenyloxy
CH2 2,3-dichloro-2-propenyloxy
CH2 2,3,3-trichloropropenyloxy
CH2 2-propynyloxy
CH2 2-butynyloxy -
_____
CH2 3-butynyloxy
CH2 1-methyl-2-propynyloxy
_ _
CH2 cyclopropyl
..... _ . _ ,
CH2 cyclobutyl
.. _ ~._ ._
CH2 cyclopentyl
_
CH2 cyclohexyl
CH2 2-cyclopentenyl
CH2 l-cyclopentenyl
. ~ ____
CH2 2-cyclohexenyl :
_ . ._
CH2 l-cyclohexenyl
CH2 cyclopentyloxy
. : . .
CH2 cyclohexyloxy -
, . .
CH2 2-cyclopentenyloxy
_. __ _ ~ __
CH2 2-cyclohexenyloxy ... - .
. _ . . .~.
_ _ _ i-C3H7
S n-C3H7 ,_ -- : .
_ _ n-cgH9
s sec . -C4Hg
_ ,
45 . _ i-C4Hg
tert.-C~Hg :.. -
. .
BASF AXtiengesellncha~t 920557 O.Z. 0050/43543
2106~97
24
, . _ .
S n-CsHl 1
__
5 . sec.-CsH11
n-C6Hl3 -
S n-C7H1s
.
S sec.-c7Hl S
_ _
S ethoxy
propoxy
_ _ 1-me~hylethoxy
S n-butoxy
S 1-methylpropoxy
S 2-methylpropoxy
_ _ 1,1-dimethylethoxy
S n-pentyloxy - .
S n-hexyloxy
. cyclopentyl
S phenyl
. _ ''
'
:
.
~0 ,
,, , , , , " ,, ,, ": , , , , ,,, ~ ~ ",
' .; '" '",',;". , ', . ., ;-'';' ' ' ', ,':. ' ' i, , ,:,, " ; ,
., : ,, , '
, . . . . . . . .
~ B~SF ~ktiengesell~chaft 920557 O.Z. 0050~43543
21~6~7
Table D
HO - N ~ CO - ~ R3 I.4
H3C
~ _ _
R R Y
. . _ _ _ __
Hi-C3H7 , ,, O
Hn-C3H7
Hn-C4Hg O
.... __ . _ _
H s ec.-CgHg O
i-C4Hg .
~ tert.-C4Hg
20 . . . _ . _
H n-CsHll O
. __ _ .
H sec.-C5Hll , . .-
H n-C6Hlg O
_ . .~ ., ._ .. _ . .__ :.
H n-C7H15 O
_ _ . :-
... sec.-C7Hls
H ethoxy O .
. ... . _ . ...
: : H : propoxy O :-:
, . . . _ _ .
~_ H l-methylethoxy: . . _
: 30 H : n-butoxy O
.... _ ... - .. .. _ _ ..
H l-methylpropoxy O ..
. A ~ r~ .
: H l,l-dimethylethoxy O
_ .. . _ _ , , .
H n-pentyloxy O .:~
35 . ___ _ .. ._ __ _ ..
~: n-hexyloxy O .
H cyclopentyl O
. _ ",~ ,., , _. ___ _
H cyclohexyl O ~ .
.... ..... _ _
H 2-cyclopentenyl O
: 40 _ l-cyclopentenyl
. : ~ 2-cyclohexenyl O
. _ _ . , ~ .
H l-cyclohexenyl ~ :
H cyclopentyloxy O
H cyclohêxyloxy O
_.
_H _ 2-cyclopentenyloxy O :
'. ': .
,,' ,'' '," ''' .,,', ''.'' ' ' , ' ' ''' . ' ' ' " ' ' '; "' ', ' " ' ' '. " ' ' " ' ' ' ' ' ' ' , ' " ',; ' :; ' ' " " ' " :
,~ . . . . ... .. ... . .
BASF ~ktien~e~ellschaft 920557 O.Z. 0050/43543
26 2~6~97
R3 ~
H 2-cyclohexenyloxy O
_ . .
CH3 i-C3H7 O
__ _ _ _
CH3 n-C3H7 O
_
CH3 n-C4Hg O
, _ .
CH3 sec.-CgHg
CH3 i-C4Hs O
CH3 tert.-C4Hg _
CH3 n-C5Hll
CH3 sec.-C5Hll O
CH3 n-C6H13 _ O
CH3 n-C7Hls O
.
CH3 sec.-C7H1 5 O
_ . . _._ _
CH3 ethoxy O
.___ _ . _ -- _.
CH3 propoxy O
_ . ~
CH3 l-methylethoxy O
20 _ _ _ _
CH3 n-butoxy O
CH3 l-methylpropoxy
.. ~_ ~.-.
CH3 2-methylpropoxy O
CH3 l,l-dimethylethoxy O
CH3 n-pentyloxy
CH3 n-hexyloxy __
CH3 cyclopentyl .
3_ phenyl O
:
:, . : , . . ,, . :.
'" ~ : '' ', ,',',"' '' ',' ~, ', '" '' , '' ' . ,' ' -': :' , ' :
.: ,: ' ' , ' .' . , '. :' , . .
;: . ..... . .
BASF Aktien~e~ell~chaft 920557 O.Z. 0050/43543
27 210~497
Table E
~ R4
R ~ I.5
HO - N - CO y
_
R R Y
.
CH3 i-C3H7
3 n-C3H7 O
CH3 n-C4Hg _
CH3 sec.-CqHg O
CH3 i-C~Hg O : -
CH3 tert.-C4Hg : . .
~Y; ~ _ O ':",,
_ _ _ sec.-C5Hll _ , - .
CH3 n-C6Hl3 O
. ._ . ~ r _ _
CH3 n-C7Hls O .. -
_ _ . .~. .
CH3 seC~-c7Hls O ~:
._ - ._ . . .~
CH3 ethoxy O
._. . ~ . -
CH3 propoxy O .:
. ~ .
CH3 l-me~hylethoxy O
. . . ~ . . -
CH3 n-butoxy O
_ CH3 l-methylpropoxy _ _ ..
30 - - C~I3 _ . O
~CH3 1 l-dimethylethoxy O
.~, . _
CH3 n-pentyloxy O
.~__ . .. ..
: CH3 n-hexyloxy O
. .
: 35 CH3 cyclopentyl O
.. .. _
CH3 cyclopentenyl O
_ . _, ,,, ... .. -- . . . ~ - ,
CH3 i-c3H7 S
~ ... .
CH3 n-C3H7 S
. . _
CH3 --- n-C4Hg S
CH3 sec.-C4Hg S . .:. ~
CH3 i-C4Hg S
.. . _ _ _
CH3 tert.-C4Hg S ~ .
, ~ .
CH3 n-CsHll S
_
CH3 sec.-CsHll S
CH3 n-C6H13 , - _ _
-: ' -: .
~ , . : , . . . . . .
. . , . ~ , . . .
"~ . , - ~ : , .. . . . .
.~ . . . . . ... ..
BASF Aktienge~ell~cha~t 920557 o.z. 0050/~3543
2~064~7
28
R4 _ ~
CH3 n-C7Hls S
CH3 ~- ~ S
CH3 ethoxy S
CH3 propoxy
CH3 l-methylethoxy S
CH3 n-butoxy S
~0 . l-methylpropoxy
. _ . .
CH3 2-methylpropoxy S
.
CH3 l,l-dimethylethoxy S
.
CH3 n-pentyloxy S
. ._
CH3 n-hexyloxy S
_ _ .
CH3 cyclopentyl S
_ _
CH3 cyclopentenyl S
.
-
~:
,~
.:
''"
. . ,:- . . `.' . . :. ' . ' .'. , : ' ': :,: :': : , '
.' . .':, ' , ' ,, . '~ . ', ,'' '. ', . : . ,
. , . : : .
., . . , . ::
' ' ;. ~ , ,'. , , -, . . '.' :': .
. BASF Aktienge~llscha~t 9205~7o.z. 0050J43543
29 210~
Table F
R ~ N CH3 I.6
H0 - N - C0 ~ N
Rs
~ _
Rs R6 R
.~',
CH3 H i-C3H7 _
CH3 n-C3H7
CH3 H n-C4Hg
CH3 H sec.-C4Hg
._.
CH3 H i-C4Hg
CH3 H tert.-C4Hg
. _
CH3 H n-CsH
~ . ___ ~ __ _ __ _
CH3 H sec.-C5Hll
_ _ _ _ . _
CH3 H n-C6H13
. ... _ . _ . .
CH3 H n-C7Hl5 - -
CH3 . _ sec.-C7Hl5 . ~ :
. _ . _ _ , .
C~ l-methylvinyl
CH3 H 2-methylvinyl
CH3 _ aIlyl -:
- _ _ . _~
: 30 CH3 . 2-methylallyl
: CH3 ~ 2-ethylallyl
CH3 H l-methylallyl
,, " .__ . . ,.
CH3 H l-ethylallyl
_ _ _ l-methyl-2-butënyl
35 ~ _ . . . . . -.
CH3 H l-ethyl-2-butenyl
. . . .
CH3 H l-isopropyl-2-butenyl
. . .. . _ __ - . ,. . _ _
CH3 H l-n-butyl-2-bute~yl
, .. _ __ _
CH3 H l-methyl-2-pentenyl .' .
~ O .. _ . __ , . .
CH3 H 1,4-dimethyl-2-pentenyl
. __ . _ __ _ _ .,
CH3 H propargyl ~ ~
. . . _ _ . _ , _
CH3 H 2-butynyl :~
. _ _
CH3 H 3-butynyl :
CH3 H ethoxy .:.
,- _ ---- ~ . ~ . , , .,
CH3 H propoxy : -
;
. .
sASF ~kti~nge~ellscha~t 920557 O.Z. 0050/43543
30 21~97
. __ R6 ,_ _
~.
CH3 H l-methylethoxy
. _ ...
CH3 Hn-butoxy
_ . _ . -
CH3 Hl-methylpropoxy
,
CH3 H2-methylpropoxy
. ...
CH3 Hl,l-dimethylethoxy
_ ... _ ..
CH3 - _ n-pentyloxy
CH3 H n-hexyloxy
CH3 ~ _ 2-ethylhexyloxY
CH3 _ 2-propenyloxy
CH3 ~ 2-butenyloxy
CH3 E~ 2-methyl-2-propenyloxy
CH3 _ 2-pentenyl ~
CH3 H 3-pentenyloxy
CH3 3-chloro-2-propenyloxy
CH3 H 2,3-dichloro-2-propenyloxy
CH3 H 2,3,3-trichloropropenyloxy
. ._ . . ._
CH3 H 2-propynyloxy
._ , ,
CH3 H 2-butynyloxy
_
CH3 H 3-butynyloxy
25 . _ ~ . .
CH3 H l-methyl-2-propynyloxy
. ,
CH3 H cyclopropyl -
.. _
CH3 H cyclobutyl
. . _ _
CH3 H cyclopentyl :
CH3 cyclohexyl
. ._ . _ , ,_ . .
CH3 H 2-cyclopentenyl
CH3 H l-cyclopentenyl
. _ _ ~ _
CH3 H 2-cyclohexenyl
. . ..... __ ~ __ _ _
CH3 H l-cyclohexenyl
,, ~ .__ ._ .
CH3 H cyclopentyloxy
~ ._ _
CH3 H cyclohexyloxy
. .. , _
CH3 , _ _ 2-cyclopentenyloxy
CH3 H 2-cyclohexenyloxY .
CF3 H i-C3H7
_ . _ -- _
CF3 H n-C3H7
~. __A~__ . _.___ _ __ .
CF3 H n-C4Hs
. _
CF3 H sec~-cgH9
CF3 _ _ i-CgHg ~ _
CF3 . ~ tert.-C~Hg
, ,:
. , : .
':1
,
~ASF Aktienge~ellschaft 920557 O.Z. 0050/43543
31 2106~97
R5 -- .
. __ _ _ _ .
CF3 H n-C5Hll
_ .. _
CF3 H sec.-CsHl1 ~-.
_ _ -- .. ___
CF3 H n-C6H13
CF3 H n-C7H15
CF3 -. SeC.-C7Hl5 . _-- -
CF3 H ethoxy
~ CF3 . _ propoxy
CF3 H 1-methylethoxy
.
CF3 H n-butoxy
_ , . . . _ , _
CF3 H 1-methylpropoxy
:
CF3 H 2-methylpropoxy
.-.. _ . ._
CF3 H l,1-dimethylethoxy
.
__3 H n-pentyloxy :
CF3 H n-hexyloxy
CF3 H cyc lopentyl
CF3 H Cyc lopentenyl
3 H phenyl
' -
':
.
.
:
:.
- .
4()
: . .
' '': ,. '" ' ", ` :' '' ';; ,' ," ' ~, ; ~ ,. .
'.'. . '; . ~''. ''' . -, ~.' . ' . . ,: '
,, , , . . , . ::
,:'` . ' ` `' . :. ' :
"' ,
BASF A~tienge~ellschaft 920557 o.z. 0050/43543
- 2~6~7
32
Table G
R J~3 R~ N
HO - N - CO S ~ I. 7
R6
1 0 ` ! _ __ .
R R R
. _ . . .
CF3 Ch3 i-C3H7
CF3 CH3 n-C3H7 , ~ , ,,
CF3 ~ CH3 n-C4Hg
CF3 CH3 sec.-C4Hg : -
CF3 CH3 i-CgHg
CF3 CH3 tert.-C4Hg
2 CF3 CH3 n-CsH11
O _ . ~. . _ . , , ,,
CF3 CH3 sec.-CsHl1 :~
CF3 - CH3 n-C6H13
CF3 CH3 n-C7H1s -- -:
CF3 CH3 sec.-C7H1s _ :-
CF3 CH3 1-methylvinyl
. . - ~ . . . _. ._ .
CF3 CH3 2-met hylviny l --:
CF3 CH3 allyl
. _. _ .... _ . ~ .
CF3 CH3 2-methylallyl - -
. . . _. ~ . ... _
: 30 CF3 CH3 2-ethylallyl : -
_ _
CF3 CH3 1-methylallyl
Lr~ CH3 1-ethylall~l
: ; CF3 ~ CH3 1-methyl-2-butenyl ~-
CF3 CH3 1-ethyl-2-butenyl
_ CH3 1-isopropyl-2-butenyl . .
~ . CH~ l-n-butyl-2-butenyl
___
: CF3 CH3 1-methyl-2-pentenyI -.:
.__ _ _ , _ _
CF3 CH3 1,4-dimethyl-2-pentenyl
40 .
3~-- --- CH3 propargyl
CF3 CH3 2-butynyl :
CF3 CH3 3-butynyl :
CF3 CH3 ethoxy
45CF3 CH3 propoxy ~ -~-~~-- :
CF3 CH~ 1-methylethoxy
, - .: - , " ,, , -, . , : .. ; . ,, . , . . , .. : .
', ' ' ` . '. ' " " '' ' " '.' ', ,' `, ' ., ' . " ' . ' ' ~; ' ', ., ~ ' ; ' " " ' ' ' ' ' ' ' ~ ' '
~AS~ ~Xtienge2ell~ch~ft 920557 ~ ~0~ ~95~/435~3
__ _ ,, _ . . . _ __
R R6 R
_ _ .~ ..
_ _ _ CH3 n-butoxy
CF3 CH3 l-methylpropoxy
CF3 CH3 2-methylpropoxy
CF3 CH3 l,l-dimethylethoxy
CF3 CH3 n--pentyloxy :
_
CF3 CH3 n-hexyloxy
CF3 CH3 2-ethylhexyloxy
CF3 - CH3 2-propenyloxy
_ ._
CF3 CH3 2-butenyloxy
CF3 CH3 2-methyl--2-propenyloxy .:
_ . .__ .
3 CH3 2-pentenyloxy
CF3 CH3 3-pentenyloxy : .
CF3 CH3 3-chioro-2-propenyloxy .
= CH3 2,3-dichloro-2-propenyloxy ;~
20 CF3 CH3 2,3,3-trichloropropenyloxy
CF3 CH3 2-propynyloxy
CF3 CH3 2-butynyloxy : :
CF3 CH3 3-butynyloxy
CF3 CH3 1-methyl-2-propynyloxy
___ . . .
_ 3 CH3 cyclopropyl .
CF3 CH3 cyclobutyl
. _ . _
CF3 CH3 cyclopentyl .
CF3 CH3 cyclohexyl
CF3 CH3 2-cyclopentenyl .
_ . CH3 l-cyclopentenyl
CF3 CH3 2-cyclohexenyl
CF3 CH3 l-cyclohexenyl
. _ . _ . . -
CF3 CH3 cyclopentyloxy
CF3 C~ cyclohexyloxy :.:
CF3 CH3 2-cyclopentenyloxy .
. _ _ ._v__ . _ , .
o,/ CH3 2-cyclohexenyloxy
CH3 CH3 i-C3H7
4~ _ __ _ _
CH3 CH3n-C3H7 - ~-
CH3 CH3n-C4Hg
-- . ,. . _
CH3 CH3sec . -CgHg
_ - _ _ _ . ___ _. _
CH3 CH3i-C4Hs
A ~. . . .. _.. _ __
~ CH3 CH3 tert.-C~Hg
. . . . ._ ---- ----
CH3 CH3 n-C5H~
,: . : . ., , . . :
- .
, ,,, : : , ;
~, .. . . , . . :: . ~ :
; : : ' : ', , ' ;
. ~ . , , , . , , ,, ~ ,
. .
BASF ~ktiengesell~cha~t 920557 O.Z. OQ50/43543
34 21~6497
~ ~ _
R7 R6 R
_ . ._ __
CH3 CH3 sec.-C5Hll
. _ . .
CH3 CH3 n-C6Hl3
__ _ _ _
CH3 CH3 n-C7Hls _ _
CH3 CH3 sec.-C7Hls
.. _ . _ .. __
CH3 CH3 ethoxy
. . ..
CH3 CH3 propoxy
~ ,
lu CH3 CH3 l-methylethoxy
CH3 CH3 n-butoxy
CH3 CH3 l-methylpropoxy
CH3 CH3 2-methylpropoxy
CH3 CH3 l,l-dlmethyletho
CH3 CH3 n-pentyloxy
CH3 CH3 n-hexyloxy
CH3 CH3 cyclopentyl
_ _ _ . . __ .
CH3 CH3 cyclopentenyl . .
CH3 CH3 phenyl
.
3û
4û
., " , , . : : , .. . . . .
.: . .: . ' ' '
, . . . . . . . .
.
,' , ' ~:
sASF Aktiengesell~chaft 920S57 o.z. 0050/43543
21~497
The novel active ingredients are particularlY suitable for pro-
tecting various materials against degradation or destruction by
bacteria or fungi or from being attacked by and covered with mi-
croorganisms. Examples of materials which can be preserved or mi-
5 crobicidally finished with the novel active ingredients are gluesand adhesives, starch solutions, wax emulsions, clay emulsions,
sizes, finishes, spinning baths, gelatine formulations, putty,
joint sealants, cooling lubricants, drilling oils, fuels, plastic
dispersions, emulsion paints, textiles, leather, raw hides and
10 cosmetics. The compounds are also suitable as anti-slime agents
in the paper industry, in cooling towers and in air moistening
units.
.
The compounds I are also suitable for protecting the following
15 plant species against attack by microorganisms:
cereals (e.g., wheat, barley, rye, oats, rice, sorghum and re-
lated species); beets (e.g., sugar and fodder beets); pomes,
drupes and aggregate fruit (e.g., apples, pears, plums, peaches,
20 almonds, cherries, strawberries, raspberries and blackberries);
legumes (e.g., beans, lentils, peas, soybeans); oil-yielding
crops (e.g., rape, mustard, poppies, olives, sunflowers, coco-
nuts, castor-oil beans, cocoa beans, groundnuts); cucurbits
(e.g., pumpkins, cucumbers, melons); fiber-yielding plants (e.g.,
25 cotton, flax, hemp, jute); citrus fruit (e.g., oranges, lemons,
grapefruit, tangerines); vegetables (e.g., spinach, lettuce, as-
paragus, cabbage varieties, carrots, onions, tomatoes, potatoes,
paprika); laurel species (e.g., avocado, cinnamomum, camphor) or
plants such as Indian corn, tobacco, nuts, coffee, sugar cane,
30 tea, grapes, hops, and banana and rubber trees. For the purposes
of the present invention, the term ~plants~ is also taken to mean
all types of other green growth, whether ornamentals, grassy
areas, embankments, or generally low-growing cover crops.
35 For example the following microorganisms may be combatted with
the novel compounds I:
Straphylococcus aureus, Escherichia coli, Klebsielle pneumoniae,
Citrobacter freundii, Proteus vulgaris, Pseudomonas aeruginosa,
40 Desulfovibrio desulfuricans, Streptoverticillium rubrireticuli,
Aspergillus niger, Aspergillus versicolor, Penicillium funiculo-
sum, Penicillium expansum, Penicillium glaucum, PaecilomyCeS va-
riotii, Trichoderma viride, Chaetomium globosum, Aspergillus ams-
telodami, Phoma pigmentovora, Phoma violacea, Aureobasidium pul-
45 lulans, Saccharomyces cerevisiae, Alternaria tenuis, Stemphyliummacrosporoideum, Cladosporium herbarum, Cladosporium resinae,
Candida albicans, Trichophyton mentagrophytes, Geotrichum candi-
,, - . .. . ~ , . .. .
,, ~ - - . .. . . . . .. . .
.
;, .
,
sASF Aktiengesell~cha~t 920557 O.Z. 0050/~35~3
2105~97
36
dans, Monilia sitophila, Scenedesmus quadricauda, Chlorella vul-
garis, Nostoc muscorium, Oscillatoria limosa and Anabaena
constricta.
5 The novel substances can be converted into conventional formula-
tions such as solutions, emulsions, suspensions, dusts, powders,
pastes and granules. The application forms depend entirely on the
purposes for which they are intended; they should at all events
ensure a fine and uniform distribution of the active ingredient.
10 The formulations are produced in known manner, for example by
extending the active ingredient with solvents and/or carriers,
with or without the use of emulsifiers and dispersants; if water
is used as solvent, it is also possible to employ other organic
solvents as auxiliary solvents. Suitable auxiliaries for this
15 purpose are solvents such as aromatic~ (e.g., xylene), chlori-
nated aromatics (e.g., chlorobenzenes), paraffins (e.g., crude
oil fractions), alcohols (e.g., methanol, butanol), ketones
(e.g., cyclohexanone), amines (e.g., ethanolamine, dimethylforma-
mide), and water; carriers such as ground natural-minerals (e.g.,
20 kaolins, aluminas, talc and chalk) and ground synthetic minerals
~e.g., highly disperse silica and silicates); emulsifiers such as
nonionic and anionic emulsifiers (e.g., polyoxyethylene fatty
alcohol ethers, alkyl sulfonates and aryl sulfonates)i and dis-
persants such as lignin-sulfite waste liquors and methylcellu-
25 lose.
''` ' '~:The fungicides generally contain from 0.1 to 95, and preferably
from 0.5 to 90, wt% of active ingredient. The active ingredients
are used in a purity of from 90 to 100, and preferably from 95 to
30 100, % (according to the NMR/HPLC/GC spectrum).
Usual application concentrations are - based on the weight of the
material to be protected - from 0.001 to 5, and preferably from
0.01 to 2, wt~ of active ingredient; when the active ingredients
35 are used for treating water, in oil production, in drilling and
cutting oils, fuels, in swimming baths, cooling towers, air
moistening units or in the paper industr~, amounts of from 5 to
500 ppm are sufficient. Ready-to-use disinfectant solutions con-
tain for instance from 0.5 to 10wt% of active ingredient.
Examples of such formulations are given below:
~ : '
I. A solution of 90 parts by weight of compound no. 3 and 10
part~ by weight of N-methyl-a-pyrrolidone, which is suitable for
45 application in the form of very fine drops.
.. . . . . .... . ... .. . . . . .
; - , . . ~ . .... . .
',.. ' : ' ' ' ' , ,', :,'", ' ' ,' : ' , ' ~
,,: , . ,-, ,, ., , , ~ .
BASF Aktienges~ll~chaft 920557 O.z. 0050/43543
2 1 ~ 7
37
II. A mixture of 20 parts by weight of compound no. 4, 80 parts
by weight of xylene, 10 parts by weight of the adduct of 8 to 10
moles of ethylene oxide and 1 mole of oleic acid-N-monoethanola-
mide, 5 parts by weight of the calcium salt of dodecylbenzenesul-
5 fonic acid, and 5 parts by weight of the adduct of 40 moles ofethylene oxide and 1 mole of castor oil. By finely dispersing the
mixture in 100,000 parts by weight of water, an aqueous disper-
sion is obtained.
10 III. An aqueous dispersion of 20 parts by weight of compound no.
1, 40 parts by weight of cyclohexanone, 30 parts by weight of
isobutanol, 20 parts by weight of the adduct of 40 moles of
ethylene oxide and 1 mole of castor oil. A mixture of this dis-
persion with 100,000 parts by weight of water contains 0.02wt~ of
15 the active ingredient.
IV. An aqueous dispersion of 20 parts by weight of compound no.
3, 25 parts by weight of cyclohexanol, 65 parts by weight of a
mineral oil fraction having a boiling point between 210 and 280C,
20 and 10 parts by weight of the adduct of 40 moles of ethylene
oxide and 1 mole of castor oil. The mixture of this dispersion
with 100,000 parts by weight of water contains 0.02wt~ of the
active ingredient.
25 V. A hammer-milled mixture of 80 parts by weight of compound no.
2, 3 parts by weight of the sodium salt of diisobutylnaPhthalene-
~-sulfonic acid, 10 parts by weight of the sodium salt of a
lignin-sulfonic acid obtained from a sulfite waste liquor, and 7
parts by weight of powdered silica gel. By finely dispersing the
30 mixture in 20,000 parts by weight of water, a spray liquor con-
taining O.lwt% of the active ingredient is obtained.
VI. An intimate mixture of 3 parts by weight of compound no. 1
and 97 parts by weight of particulate kaolin. The dust contains
35 3wt% of the active ingredient.
~ ,
VII. An intimate mixture of 30 parts by weight of compound no.
4, 92 parts by weight of powdered silica gel and 8 parts by
weight of paraffin oil sprayed onto the surface of this silica
40 gel. This formulation of the active ingredient exhibits good
adherence.
VIII. A stable aqueous dispersion of 40 parts by weight of com-
pound no. 2, 10 parts of the sodium salt of a phenolsulfonic
45 acid-urea-formaldehyde condensate, 2 parts of silica gel and 48 ~ -
parts of water, which dispersion can be further diluted.
: , . , , , , . , ." ,
, . , ; . ,. ~ ,
`- ' ' ' ', , , ,' ,: . ' ,
,:, . .. . . . .
:, :
~'.,- '"' ,, , , ': ' ~, , '
" ' : ' : ,. . .
s~SF Aktien~e~ell~cha~t 920557 O.Z. 0050/43543
38 2106~97
IX. A stable oily dispersion of 20 parts by weight of compound
no. 3, 2 parts by weight of the calcium salt of dodecylbenzene-
sulfonic acid, 8 parts by weight of a fat~y alcohol polyglycol
ether, 2 parts by weight of the sodium salt of a phenolsulfonic
5 acid-urea-formaldehyde condensate and 68 parts by weight of a
paraffinic mineral oil.
X. A hammer-milled mixture of 10 parts by weight of compound no.
1, 4 parts by weight of the sodium salt of diisobutylnaphthalene-
19 ~-sulfonic acid, 20 parts by weight of the sodium salt of a
lignin-sulfonic acid obtained from a sulfite waste liquor, 38
parts by weight of silica gel, and 38 parts by weight of kaolin.
By finely dispersing the mixture in 10,000 parts by weight of
water, a spray liquor containing O.lwt% of the active ingredient
15 is obtained.
Used alone, the active ingredients act as low-foaming biocides. A
significant increase in the action of biocidal formulations con-
taining these compounds is achieved if tri-C6- to~Cl2-alkylmethy-
20 lammonium salts, preferably in amounts of from 20 to 40wt~, basedon the weight of compounds of the general formula I, are added.
The active ingredients may also be mixed with other, prior art,
microbicides. In many instances, a synergistic effect is
25 achieved, i.e., the microbicidal action of the mixture is greater
than the added actions of its individual components.
Prior art microbicides may be added to the novel substances in a
weight ratio of from 1:100 to 100:1.
Examples of such active ingredients are as follows:
.
2-(thiocyanomethylthio)-benzothiazole
1-~2-(2,4-dichlorophenyl)-2-(2-propenyloxy)-ethyl]-lH-imidazole
35 2,4,5,6-tetrachloroisophthalodinitrile
methylene bisthiocyanate
tributyltin oxide, naphthenate, benzoate, salicylate
mercaptobenzothiazole
1,2-benzisothiazolone and its alkali metal salts
~0 alkali metal compounds of N~-hydroxy-N-cyclohexyldiazenium oxide
2-(methoxycarbonylamino)-benzimidazole
2-methyl-3-oxo-5-chlorothiazolin-3-one
trihydroxymethylnitromethane
glutardialdehyde
45 chloroacetamide ;~ -
polyhexamethylene bisguanide
5-chloro-2-methyl-4-isothiazolin-3-one ~ magnesium salts
. , , . . , : i : . . , . ., . . , . ". .: .: ,~ . ,.. : . . . . . . : : . :
i:: ., i, ,- . . . . . . . . . . . .
,, , , , , : ' , ' ' . .: : . ' : ' ' ' : ' : . ~ : ' '
,. . ,. , ' ' ,,: . '' ' " . ' ' ' , ; . ' ' '
BASF Aktienge~ell~chaft 920557 O.Z. 0050/43543
39 2 ~ 0 ~ll 9 7
3/5-dimethyltetrahydro-l~3~5-2H-thiadiazine-2-thione
hexahydrotriazine
N,N-methylolchloroacetamide
2-n-octyl-4-isothiazolin-3-one
5 oxazolidines
bisoxazolidines
2,5-dihydro-2,5-dialkoxy-2,5-dialkylfurans
diethyldodecylbenzylammonium chloride
dimethyloctadecyldimethylbenzylammonium chloride
10 dimethyldidecylammonium chloride
dimethyldidodecylammonium chloride
trimethyltetradecylammonium chloride
benzyldimethylalkyl-~Cl2-Cl8)-ammonium chloride
dichlorobenzyldimethyldodecylammonium chloride
15 cetylpyridinium chloride
cetylpyridinium brcmide
cetyltrimethylammonium chloride
laurylpyridinium chloride
laurylpyridinium bisulfate - ~
20 benzyldodecyldi(beta-oxyethyl)-ammonium chloride
dodecylbenzyltrimethylammonium chloride
n-alkyldimethylbenzylammonium ~hloride
(alkyl radical: 40% Cl2, 50% Cl4, 10% Cl6)
lauryldimethylethylammonium ethyl sul~ate
25 n-alkyldimethyl-(l-naphthylmethyl)-ammonium chloride
(alkyl radical: 98% Cl2, 2% C14)
cetyldimethylbenzylammonium chloride
lauryldimethylbenzylammonium chloride
30 Examples of further compounds which may be admixed are:
1,3-dimethylol-5,5-dimethylhYdantoin
dimethylolurea
: tetramethylolacetylenediurea
35 dimethylolglyoxalmonoureine
hexamethylenetetramine
glyoxal
glutardialdehyde
N-methylolchloroacetamide
40 1-(hydroxymethyl)-5,5-dimethylhydantoin
1,3-bis-~hydroxymethyl)-5,5-dimethylhydantoin
imidazolidinylurea
1-(3-chloroallyl)-3,5~7-triaza-1-azonia-adamantan chloride
1,3-bis-(~-ethylhexyl)-5-methy,:1-5-amino-hexahydropyrimidine
45 1,3,5-tris-(hydroxyethyl)-1,3~5-hexahydrotriazine
1,2-dibromo-2,4-dicyanobutane
5-bromo-5-nitro-1,3-dioxane
... . , - . , .
. ~ . . ~ . . . . . ,
:. , ~ : .
,. , : . : .,
':, ,' .: : '. .' . ,. ' ,; . , ` :
, . : .: . ' ' . , '' ' . ' '' ' .
BASF Aktien~esellschaft 9~0557 O.Z. 0050/~3543
43 21 0~97
2-bromo-2-nitropropanediol
1,1'-hexamethylene-bis-[5-(4-chlorophenyl)-biguanide]
4,4-diaminodiphenoxypropane
2-bromo-2-nitropropane-1,3-diol
5 sorbic acid and its salts
p-hydroxybenzoic acid and its esters and salts
zinc-2-pyridinethiol-N-oxide
2-~(hydroxylmethyl)amino]-ethanol
dithio-2,2'-bls(benzmethylamide)
10 5-chloro-2-(2,4-dichlorophenoxy)-phenol
thio-bis-(4-chlorophenol)
o-phenylphenol
chloromethyl-diiodomethylsulfone
p-chlorophenyl-3-iodopropargylformal.
Synthesis examples
The directions given in the synthesis examples below were used,
after appropriate modification of the starting materials, to ob-
20 tain further compounds I. The compounds thus obtained are listedin the tables below with their physical data.
1. N-hydroxy-N-(2-propylphenyl)-2-chloronicotinamide -
~
H3CH2CH2C ~ ~ N
HO - N - CO ~ / \~
~ .
At 0C, 14 ml of water and 19.6 g of sodium bicarbonate are added
to a solution of 15.1 g of 2-n-propylphenylhydroxylamine in 75 ml -
of a 2:1 mixture of ether and ligroin, and 13.6 g of 2-chloroni-
cotinamide is then dripped in while stirring vigorouslY. The mix-
35 ture is stirred overnight at room temperature and then suction
filtered. The residue is stirred for 15 minutes in 10~ strength ~;-
sodium bicarbonate solution, suction filtered, dissolved in ethyl
acetate and dried, and the solvent is evaporated off under re-
duced pressure. ~rom the crude product (14.6 g) there is iso-
40 lated, after recrystallization from ethanol, 12.5 g of 2-chloro-
nicotic acid-N-hydroxy-2-n-propylanilide of m.p. 134-135C.
~ ~ .
,:
qs .
,,, ., ,. ,: .. , , ",., . . ,, . .. ; , ;~ . .. . .
. . . . . .
. . .
~ BASF Aktiengesellschaft ~f20557 . o.z. 0050/43543
41 2106~97 `
Table 1
R~J
HO--N--CO--A
10 ~xample _ Phys. data
CH(CH~)z ' 01~,~ d:~ 3 ~ 107-111C
CH2CH2CH3 ~ pa- ~ ~ 3 ~ 134-135C
3 CH2CH(CH3)2 2-Cl-pyridin-3-yl oil
phenyl 2-Cl-pyridin-3-yl 112-115C
_ CH2CH(CH3)2 2-CH3, 4-CF3-thiazol-4-yl oil
6 phenyl 2-CH3, 4-CF3-thiazol-4-yl173-175C
_ CH2CH(CH3)2 2,4-(CH3)2-thlazol-4-yl oll
~ _ phenyl 2,4-(CH3)2-thiazol-4-yl 58-62C
Examples demonstrating biological action:
Action on Botrytis cinerea
Slices of green paprika pods were sprayed to runoff with aqueous
suspensions containing (dry basis) 80% of the active ingredient
and 20% of emulsifier. After the sprayed-on layer had dried, the
slices were sprayed with a spore suspension [1.7-105 spores per
30 ml; 2% biomalt; water] of the fungus Botrytis cinerea and then
kept for 4 days at 18~C and in high humidity.
~ After this period, the untreated controls exhibited 90% fungus
; attack, whereas the paprika slices treated with 500 ppm of com-
35 pounds nos. 1 and 2 e~hibited 5% attack at most.
At an application rate oE 1000 ppm of compounds nos. 1 and 2 the
paprika slices exhibited no attack at all, whereas the slices
treated with 1000 ppm of 2-chloronicotinic acid-2-chloroanilide
40 exhibited 90% attack, just as the untreated controls.
:;
.~ ..... .. ..... .. . .. .. .. ...
~: :
.