Note: Descriptions are shown in the official language in which they were submitted.
. WO92/1721l PCT/US92/02911
:~ 2~ 977~q `
. .
RETROVIRUS }N~IBITION WITH ANTISENSE NUCLEIC ACIDS
~ COMPLEMENTARY TO PACKAGING 6EQUENCES
-~ BACRGROUND OF T~E INVEN~ION
Field of the Invention
~ This invention, which is in the field of virology
-~ and medicine, relates to DNA sequences encoding an
antisense RNA molecule capable of hybridizing with a
retrovirus packaging sequence and thereby inhibiting a
retrovirus infection, the antisense RNA sequences,
~¢ hosts transfected with the DNA sequences, and methods -
for rendering cells and animals resistant to
retrovirus infection.
. ~ _
Descri~tion of the Backqround Art
A. RETROVIRUSES
Retroviruses are a major threat to the health of
humans and animals. This is-most dramatically
demonstrated by the role of HIV-1 in the worldwide
AIDS epidemic. To date, no effective treatment or
cure for retroviral infections has been found. It is
widely accepted that the damage caused (both directly
and indirectly) by retroviruses as infectious agents
has become the most serious globaL challenge to
medical science in recent times. Hence, the need for
effective methods and compositions for prevention or
treatment of retrovirus injections is abundantly
clear.
Retroviruses comprise a large class of RNA
viruses which have the property of "reverse
transcribing" their genomic RNA into DNA which can
integrate into the host cell genome. Many members of
this virus class are tumorigenic. These viruses
require only a limited number of genes and genetic
regulatory sequences to complete their cycle of
infection in host cells or organisms (1-4). This
SUBSTITUTE SHEET
.~
. . . : , ~
~'' WO92/17211 2 1 ~ 7 7 g 9 PCT/US92/02911
remarkable molecular efficiency partially explains the
effectiveness of retroviruses, such as human
immunodeficiency virus (HIV), as pathogens. The
existence of distinct genes and gene products also
suggests targets for molecular attenuation of
retroviral replication in host organisms.
Retroviruses are small, single-stranded positive-
sense RNA viruses. Their genomes contain, among other
things, the sequence for the RNA-dependent DNA
polymerase, reverse transcriptase. Many molecules of
reverse transcriptase are found in close association
with the genomic RNA in the mature viral particle.
Upon entering a cell, this reverse transcriptase
i l5 produces a double-stranded DNA copy of the viral
genome, which is inserted into the host cell's
, .
chromatin. Once inserted, the viral sequence is
called a provirus. In some ways retroviraI
integration resembles that of various eucaryotic
mobile genetic elements such as Copia and 412 of
Drosophila or Ty-l in yeast. In the case of these
transposable elements and retroviruses, long stretches
of highly conserved "sequence are" flanked by inverted
repeats. Also, integration of any of these entities
results in the production of short, direct repeats of
the host cell's chromatin.
Although the complete details of the integration
of retroviral DNA into the genome of its host cell
(formation of proviral DNA) have not been worked out,
certain facts about it have been established.
Retroviral integration is directly dependent upon
viral proteins. Linear viral DNA termini (the LTRs)
form the structure allowing integration of the
proviral DNAo There is a characteristic duplication
of short stretches of the hosts DNA at the site of
integration.
, ~
..~
.
.:
"` SUBSTITUTE SHEET
.-
, . ~ . . . . . . .,. . : . . ,:
.~ .
:` ~ ` . ~ ,. . ' .
~ , .
;~ ~ . . . .
~ W092~17211 3 2~ ~7 PCT/USg2~0291l
` -
- The retroviral protein directly involved in
inserting the viral DNA into the host DNA is called
the i~tegrase protein (IN). The sequence of the IN
: .
protein is encoded in the 3' part of the viral
polymerase gene. After translation it is
proteolytically processed from the larger precursor
molecule to yield an active protein (of 46kd in Mo-
;n MLV; in avian viruses and the human immunodeficiency
virus it is 32kd). During integration the IN proteinremoves bases from the 3' hydroxyl termini of both
strands of the reverse transcriptase produced viral
~` DNA. These 3' ends are covalently attached to 5'-
phosphoryl ends of the host cells' DNA. The IN
`~ ~5 protein of all retroviruses is thought to have an
endonuclease activity which results in the production
~i of a staggered cut in the host DNA at the site of
'- integration. The filling in of this staggered cut by
~ cellular enzymes after the ligation of the viral DNA
.,.
to the host DNA results in the duplication of the
,i short sequences of the host DNA.
Progeny viral genomes and mRNAs are transcribed
from the inserted proviral sequence by host cell RNA
~, polymerase II in response to transcriptional,
regulatory signals in the terminal regions of the
proviral sequence, the long terminal repeats or LTRs.
".j The host cell's protein production machinery is used
to produce viral proteins, many of which are inactive
until processed by virally encoded proteases.
Typically, progeny viral particles bud from the cell
surface in a non-lytic manner. Retroviral infection
does not necessarily interfere with the normal life
cycle of an infected cell or organism. While most
~; classes of DNA viruses may be implicated in
, 35 tumorogenesis, retroviruses are the only taxonomic
, group of RNA viruses that are oncogenic. Various
"~
,,~ ,
SUBSTITUT~ SHEET
~`, ~ , . , ~ . ,
, . - , ~. ~. ~
i,i .
.` WO92/17211 21 0 ~7 8 ~ _ 4 - PCT/US92~02911
: .:
retroviruses such as the Human Immunodeficiency virus
(HIV), which is the etiological agent responsible for
~'! acquired immune deficiency syndrome in humans, are
, 5 also responsible for several very unusual diseases of
~i the immune systems of higher animals.
-. All retroviruses share common morphological
- characteristics. They are enveloped viruses typically
around lOOnm in diameter. The envelope is derived
; lO from the cytoplasmic membrane of the host cell as the
~;~ maturing virus buds from that cell. It is covered by
qlycoprotein spikes, coded for by the viral genome.
I' The cytoplasmic membranes of infected cells actively
~ transcribing the proviral sequences and processing
; ~5 viral proteins have viral envelope glycoproteins
inserted into them. In some cases, these
glycoproteins have been shown to cluster on certain
regions of the cell membrane. Furthermore, viruses
tend to bud preferentially from these regions.
The envelope encloses an icosahedral capsid, or
~ nucleoid, composed of proteins coded for by the virus.
-j, The capsid contains a ribonucleoprotein complex that
includes the genomic RNA, reverse transcriptase, the
^,9 integrase protein and certain other factors necessary
for the production of the double-stranded DNA copy of
^;~ the viral genome. It is not uncommon for the capsid
;,~. to contain small amounts of non-viral RNA other than
~'~ the cellular tRNAs which are always present. The
;~ cellular tRNAs are base-paired to specific regions on
~- 30 the viral genome and play an important role in reverse
transcription as will be described later. There is
also an inner coat composed of core proteins found
;~ between the nucleocapsid and the envelope.
'~i There are several classification schemes
applicable to retroviruses. What is, perhaps, the
` simplest classification scheme is based on morphology.
i.
,.................................. .
;i `
SUBSTITUTE SHEET
.. .
.. . . .
r : ~ .
~ , ~
.1 ` .
WO 92/1721 1 PCl'/US92/0291 1
., , .. :
. .~, . ,
,~ 21~77~q
:; This morphological classification scheme of
retroviruses is based on structural similarities
apparent in electron micrographs. In this scheme,
retroviruses are divided into four groups or types of
particles designated as A-type, B-type, c-type and D-
type.
The A-type particles are non-infectious and are
i found only within cells. They range in size from 60-
`'! 10 90nm. They do not have an encapsulating membrane.
They may be found intracisternally or
intracytoplasmically. Their classification is further
subdivided on this basis. The role of the
intracisternal A-type particles is unknown, but the
intracytoplasmic particles appear to be immature, or
precursor forms, to B-type mouse mammary tumor
~i- virions. It has also been speculated that they might
be retrotransposons.
B-type particles exhibit very prominent spikes on
their envelope surface, and their nucleoids are
eccentrically located. Mouse mammary tumor virus is
the primary example of this type of retrovirus.
C-type particles represent the largest
morphological class of retroviruses. The envelope
spikes of the C-type viral particles vary greatly in
size and quantity, but all viruses of this type have a
~; centrally located core in the mature virion. Moloney
murine leukemia virus is a typical C-type virus.
-~ The D-type particles exhibit the same
eccentrically located nucleoid as the B-type.
~ However, the spikes of the D-type are noticeably
'~ shorter than those of the B-type. Examples of this
type have only been found in primates.
To understand many of the concepts presented
3S herein, it is necessary to understand the organization
of a typical retrovirus. The Moloney murine leukemia
.~
SUBSTITUTE SHEET
~ .
"` :
~s ~ . ,
,.; , ~
,~..`~
..",
::"
-j WO92/17211 21V77 89 -- 6 -- PCl/US92/02911
.... . .
.
virus (Mo-MLV) will serve as an example. The sequence
of the entire virus is known, see Shimnick, et al.,
Nature, 293:543-48 ~1981) and Miller and Verma, J.
Virol., 49:214-22 (1984). As with all retroviruses,
Mo-MLV carries two copies of its genomic RNA in the
mature viral particle. The diploid RNA genome of
~i retroviruses is unique among viruses and is a
necessary component of the reverse transcription
process. The identical subunits of the viral genomic
~` RNA exhibit several characteristics of a eucaryotic
. mRNA molecule. The 5' end of the molecule carries a
typical in ~WA cap structure (m7 G5 'ppp5 'Gm). A poly-
`~!, A tail of about 200 residues is attached to the 3'
~,~ 15 end, and several internal adenosine residues are
- methylated.
~;3 Besides the cap and the poly-A tail, three
primary coding regions and six functional regions can
be identified on the viral RNA. A copy of the highly
conserved LTR, or long terminal repeat, region is
found at both ends of the molecule. Like the diploid
~ genome, these are needed for successful reverse
,~j transcription. The 5' LTR region includes sequences
:~ having promoter and enhancer activity. The LTR region
also contains a poly-A addition signal. The 5' LTR
region is followed by the U5 region. Next, is the L,
or untranslated leader,~ sequence. The L region
;~ includes the primer binding (PB) site for the
` initiation of negative strand DNA synthesis, the PB-
;'` 30 site. A molecule of tRNA, which is used as a primer
in the initiation of negative strand DNA synthesis, is
base-paired to the PB-site. It also includes the
site, which is required for encapsidation of the viral
. .,~
genome.
~ 35 Next are the three major coding regions: qaa, or
s the group-specific antigen gene, which code for the
.~ .
;~
~;.,
X SUBSTITUTE SHEET
~i
, ~,
;'`
.. . ` ~ : . ~ `
'f~'~` ` :' ~ `
,. : ` ` ~ ' ~ '' : .
~ W092/17211 ~ 7`~ 1 ~ 7 7 ~9 PCT/US92/02911
-
viral core proteins; ~ol, which encodes the viral
ymerase (or reverse transcriptase); and env, which
encodes the envelope proteins and glycoproteins.
` 5 These are followed by the PB+ site, which binds a
primer used in positive strand DNA synthesis. Next,
; is the U3 region, which contains the viral enhancer
and promoter. The U3 region is followed by the second
~:~ copy of the LTR region.
`~s lO The mature viral particle of MLV contains nine
major proteins. These are produced by the post-
translational processing of primary translational
products. These proteins are typically named
according to a system introduced in 1974. In this
system, the symbol "p" (for protein) or "gp"
(glycoprotein) is followed by a number showing the
j~ approximate molecular weight of the protein in kDa as
;~j determined by sodium dodecyl sulfate- polyacrylamide
gel electrophoresis (SDS-PAGE) (23). Four internal -~
structural proteins are products of the viral qaq
-~ gene. They are: p30, the major capsid protein; pl5, a
hydrophobic matrix protein; plO, a basic protein found
in the nucleocapsid; and pl2, an acidic phosphoprotein
often designated as ppl2, whose virion location and
function have yet to be determined.
There are two major envelope-associated proteins
encoded from thé env gepe. They are the glycoprotein,
gp70, and the nonglycosylated plS protein. The pl5
protein is a transmembrane protein which attaches to
gp70 and anchors it to the membrane. The gp70 protein
attaches the mature virion to cell surface viral
receptors.
The viral polymerase protein is also referred to
as p70. In Mo-MLV, this protein appears to be a dimer
held together by noncovalent and disulfide bonds (26).
Besides acting as an RNA-dependent DNA polymerase,
SUBSTITUTE SHEET
h
,.~ . : ~ . , - '
~'. , . ' ~, '
.~ . .
. . ,
,','`' ' ' : ~ ~ ,' ,~
WO92~17211 2 1 0 7 7~ 9 -- 8 ~ PsCT/US92J02911
reverse transcriptase also has RNase H activity, which
results in digestion of the RNA in a RNA-DNA hybrid
into short oligonucleotides. Two other proteins are
thought to be products of the ~ol gene (in at least
some retroviruses). A viral protease, pl4, is
responsible for maturation of other viral proteins.
An integrase protein, p46 (in MuLV), acts to join or
insert the double-stranded DNA copy of the viral
genome into the host cell DNA (27, 28, 29, 30).
Other retroviruses feature additional retroviral
genes. ThUs, in the HTLV-I retrovirus, we find tax
and rex. The product of tax, essential for viral
replication, is a transacting transcription-activating
factor which enhances viral gene expression (Seiki, M.
et al., Proc. Natl. Acad. Sci. USA 80:3618-3622
(1983); Sodorski, J.G. et al., Science 225:381-385
(1984); Chen, I. et al., Science 229:54-58 (1987)).
The rex gene, also required for viral replication, is
a posttranscriptional regulator of viral gene
expression (Hidaka, M. et al. EMBO J. 7:519-523
(1988); Inoue, J. et al., Proc. Natl. Acad. Sci._USA
84:3652-3657 (1988)).
The HIV-1 retrovirus also possesses genes
modulating viral replication, including v~ F~_tatl
rev vpu, and nef (Haseltine, W.A., J. Acquired Immune
DeficieDçy_Syndrome 1:217240 (1988)).
Another required element for retroviral
replication is the cis-acting viral genomic sequences
necessary for the specific encapsidation of the
genomic viral RNA molecules into virus particles (4-
10). These packaging sequences, termed Psi, have been
identified and exploited in the construction of
retroviral vectors designed for gene transfer (10-13).
Functional packaging sequences are absolutely required
for retroviral replication in host cells. When
,;
.~ .
:1 SUBSTITUTE SHEET
?;
;`s,s
~Sj~
'`', ' ," ~ ~ ~ , :
: :~
~ WO92/172~1 PCT/US92/0291l
789
packaging sequences were deleted ~rom the retroviral
genome (to prevent viral multiplication) and the DNA
was transfected into host cells, the cells could
produce all the viral proteins and viral RNA, but
could not package the viral RNA genome into an
infectious particle. However, such cells could serve
as "helper" cells and complement a DNA vector which
contained only the retroviral LTRs and the packaging
- 10 sequences. The "helper" cell provided: (a) the g3g=
encoded proteins needed to package the vector RNA; (b)
the envelope protein to form the capsid; and (3) the
~ reverse transcriptase to convert the RNA genome into
n~i a DNA copy upon arrival into the infected cell. The
sequences required for RNA packaging into virions have
been defined and Shown t~ reside between the 5' LTR
and the beginning of the early portion of the g~
~-- gene.
~.,,~, .
B- ANTISENSE RNA
Gene expression involves the transcription of
pre-messenger RNA from a DNA template, the processing
of the pre-messenger RNA into mature messenger RNA,
;~ and the translation of the messenger RNA into one or
more polypeptides. The use of antisense RNA to
inhibit RNA function within cells and whole organism
has generated much recent interest (14-16). Antisense
RNA can bind in a highly specific manner to its
complementary sequences ("sense RNA"). This blocks
the processing and translation of the sense RNA and
may even disrupt interactions with sequence-specific
RNA binding proteins (17-20). For example, a plasmid
was constructed leaving a promo~er which directed the
transcription of a RNA complementary to the normal
thymidine kinase (TK) mRNA. When such plasmids,
together with plasmids containing a normally expressed
,~ .
SUE~STITUTE SHEET
i,
, ,
~. :
~ ~ ,
, ~ ~
WO92/17211 PCT/US92/02911
-- 10 ~
., 1''~""
2~ ~7r~q
- TK gene, were injected into mutant mouse L cells
lacking TK, the presence of the antisense gene
substantially reduced expression of TK from the normal
~ 5 plasmid (Izant et al., Cell 36:1007 (1984).
- Antlsense oligonucleotides have been shown to be
-~ inhibitory in various viral systems. ZamecniX and
Stephensen, Biochemistry, 75:280-84 (197B) inhibited
Rous sarcoma virus (a retro~irus) production in
`~ 10 cultured CEF cells by adding an oligodeoxynucleotide,
complementary to 13 nucleotides of the 3' and 5' LTRS,
- to the culture medium. The DNA was terminally blocked
to reduce its susceptibility to exonucleases. They
~ speculated that this antisense DNA might act by
- ~5 blocking circulati~atoin, DNA integration, DNA
transcription, translation initiation or ribosomal
association. Note the conspicuous absence of any
reference to interference with packaging.
Chang and Stollzfus, J. Virol., 61:921-24 (1987)
inhibited the same virus by means of antisense RNA,
' which they hybridized to the coding region or to the
~`~r'' 5' or 3' flanking regions of the env gene.
Gupta, J. Biol. Chem., 262:7492-96 (1987)
inhibited translation of the Sendai virus nucleocapsid
~?,` 25 protein (NP) and phosphoprotein (P.C) mRNAs by means
of antisense DNAs complementary to the 5' fla~king
region. Oligon~cleotides complementary to the coding
i region had no effect on translation. They were unable
to explain this difference in effectiveness.
Based on the evidence presented above, a number
~ of laboratories have tried to block transmission of
x~ HIV-1, which is the causative agent of AIDS, by
blocking viral gene expression with antisense RNA.
~` Thus far, none of these efforts has succeeded.
Antisense experiments have been devised and
undertaken by the NIH. S. Amini, Mol. Cell. Biol.
~i
~:!
SUBSTITUTE SHEET
,~, .
,.~ .
.,. ~
~ WO92/1721I PCT/US92/02911
7 7 ~
. .
(1986) 6(7):2305; M. Matsukura, PNAS (1987) 84:7706;
J. Holt, MCB (1988) 8 (2):963; K. Croen, Science News
~-- (1989) 132:356. such experiments are typically
directed to testing the ability of antisense agents to
block retroviral replication. The current focus in
the art emphasizes the notion that blocking
- replication means the blocking of the expression of
- viral proteins, such as the Rev protein. Because of
10 this emphasis, the assays used to test antisense
sequence for antiviral activity measure the expression
of a HIV-l gene product. See S. Gott, J. Virol (1981)
s 38(1):239 (RT Test); J. McDougal, J. Imniunol. Meth.
-~; (1985) 76:171 (antigen detection). RNA northern blot
15 analysis, the expression assay that is most typically
used to test antisense nucleic acid inhibition of HIV-
1 (See Thomas, PNAS, 77:3201, 1980), annot detect
useful antisense sequences that are homologous to the
viral packaging sequence.
Ruden and Gilboa, J. Virol., 63:677-682 (Feb.
1989) inhibited HTLV-I replication in primary human T
cells engineered to express an antisense RNA. One
antisense RNA was directed against the first kilobase
of the tax gene CDNA. The other was a 1.1 kilobase
HindIII-Pst I fragment from the 5' end of the proviral
DNA. The latter target is said to include the 5'
splice site, the tRNA Rrimer binding site, and
"possibly" signals for packaging of genomic virus RNA.
Antisense-encoding DNAs were operably linked to either
the SV40 early promoter or the cytomegalovirus
immediate early promoter. Only the vectors expressing
the antisense RNA under the control of the CMV
~; promoter exerted an inhibitory effect on cell
proliferation, though the SV40 early promoter/anti-tax
gene also depressed viral production.
, ,.i
,:,
. ~
.~ SUBSTITUTE SHEET
'~; :' ' . . .. .
- wos2/l72ll PCT/US9?/0291l
21~7~9 - 12 - -~
Large antisense molecules of the sort advocated
- by Ruden and Gilboa have several disadvantages. They
`~ are difficult to synthesize (particularly if abnormal
bases are incorporated as discussed below). They
conceivably could recombine with the original virus,
another virus, or an oncogene. They are also more
liable to form secondary structures which interfere
with their binding to the viral target. Additionally,
. 10 they are more prone to hybridize to cellular DNA,
^r , thereby possibly blocking expression of essential
genes.
In addition to the use of antisense
oligonucleotides containing normal bases, various
investigators have utilized sequences containing
nucleoside or nucleotide analogues to block gene
, expression. Such analogues have the advantageous
properties of resistance to nuclease hydrolysis and
~r, improved penetration of mammalian cells in culture
(Miller, P.S. et al., Biochemistry 20:1874-1880
(1981)). For example, an oligo(deoxyribonucleoside
phosphonate) complementary to the Shine-Dalgarno
sequences of 16S rRNA inhibited protein synthesis in
E. coli but not mammalian cells (Jayaraman, K. et al.,
Proc. Natl. Acad. Sci. USA 78:1537-1541 (1983)). Such
oligomers complementary to initiation codon regions of
; rabbit globin mRNA inhibited translation in cell-free
systems. (Blake, K.R. et al.. Biochemistrv 24:6139-
6145 (1985)) while oligomers complementary to the
initiation codons of vesicular stomatitis virus mRNAs
inhibited viral but not cellular protein synthesis in
infected L cells (Miller, P. et al., Feder. Proc. 43,
abstr. 1811 (1984)). More recently, an
,,f;, oligo(nucleoside methylphosphonate) complementary to
the splice junction of herpes simplex virus type 1
immediate early pre-mRNAs 4 and 5 was shown to
.,,
t: SUBSTITUTE SHEET
i-
,'.,
. . .
. .
,' ' ~ ' ~ ~ . ' '
`~
` WO92/17211 PCT/US92/02911
13 ~ 2l ~t~7 8 9
.' ;
selectively inhibit viral infection (Smith, c.c.
et al., Proc. Natl. Acad. Sci. USA 83:2787-279
(1986)).
8UMMA~Y OF T~E IN~ENTION
An object of the present invention is to overcome
the deficiencies noted above.
..... .
~ More particularly, the presen~ inventors have
"~.~ l0 taken a novel approach to the inhibition of retrovirus
replication based on the blockade of virus packaging
through hybridization of antisense RNA essentially
~; only to packaging sequences of the viral genome. In
one embodiment, the inventors have constructed
recombinant plasmids in which murine leukemia virus
. proviral Psi (packaging) sequences, under the
~ transcriptional regulation of lymphotropic virus
.~ promoter/regulatory elements from the Moloney-MuLV LTR
or the cytomegalovirus immediate early region, were
inserted in reverse orientation. This gives rise to
production of antisense RNA complementary to Psi,
which achieves complete inhibition of productive virus
infection.
When these antisense sequences.were also
introduced into cells in vitro and stably transformed
.~ çell lines isolated, the cells were resistant to M-
MuLV infection, and pro~duced only virus devoid of
packaqed viral RNA.
^~;, When linear fragments containing the antisense
Psi and the appropriate transcriptional regulatory
sequences from these plasmids were introduced into the
~-~ mouse germ line by zygote microinjection, the presence
of the antisense Psi RNA was detected in the
lymphocytes of these transgenic mice. Upon challenge
with the appropriate retrovirus (M-MuLV) none of the
.~ .
~; SUBSrITUTE SHEET
~, .
. .
'- : : :` - `
.. . .
~ .
. ~ .
.
~:
: WO 92tl721 I PCr~US92/0291 1
1 4
,~ 210778q
antisense Psi transgenic mice developed any symptoms
of leukemia.
More generally, the present invention is directed
to an antisense molecule capable of specifically
hybridizing to the packaging sequence of a retrovirus
;~ and thereby inhibiting essentially only the packaging
~- of the genomic RNA of the retrovirus. Preferably, the
-~ antisense molecule is less than about 100 bases, and
` lo more preferably less than about 60 bases, and it may
be DNA, RAN, or an analogue thereof. The antisense
molecule may be administered directly like a drug or,
~,: if an RNA, it may be generated in vivo in the subject
through expression of an introduced gene. Where the
-~: 15 antisense molecule is administered directly, it may be
composed of a nuclease-resistance RNA or DAN analogue
that penetrates the cell membrane.
~ The invention includes recombinant DAN molecules
.~ comprising a DNA sequence which is transcribable into
such an antisense molecule and hosts transformed or
transfected with the above DNA sequence, preferably a
~' mammalian cell host, most preferably a human cell.
The invention further relates to a method for
rendering a cell resistant to productive infection by
~, 25 a retrovirus comprising inserting into the genome of
the cell a DNA sequence, operably linked to a
promoter, wherein the DNA sequence is transcribable
;j, into an antisense RNA molecule which hybridizes to the
packaging sequence and thereby inhibits the packaging
of the genomic RNA of the retrovirus, thus rendering
the cell resistant to productive infection.
`~. The present invention includes a method for
rendering a vertebrate animal, such as a mammal,
resistant to productive infection by a retrovirus
comprising inserting into the genome of essentially
all of the germ cells and somatic cells of the mammal
'~:
,...
- ,? SUBSTITUTE SHEET
., .
... , .
. . ' , . . .
~,.,:' ~ : ' .
, . .
~ WO92~17211 ~ 77~q PCT/US92/02911
.. . !
a DNA sequence containing the packaging sequence of
i the retrovirus or a segment thereof in reverse
`~ orientation operably linked to a promoter and
regulatory elements, wherein the DNA sequence or
segment is transcribable into an antisense RNA
molecule capable of inhibiting the packaging of the
genomic RNA of the retrovirus, thereby rendering the
mammal resistant to infection. The DNA se~uence is
~; 10 preferably introduced into the mammal or its ancestor
at an embryonic stage.
~,~ The invention therefore relates also to a
transgenic non-human mammal essentially all of whose
germ cells and somatic cells contain the above DNA
~' 15 sequence, a transgenic in which said DNA seauence has
been introduced into the mammal or its ancestor of
~, said mammal at an embryonic stage. Also intended is a
chimeric animal, including a human, at least some of
whose cells contain the above DNA sequence.
~,Y~ 20
;ii
;~ .
:~i
~1
. .
~ 30
.,~ .
i'
SUE~STITUTE SHEET
., .
, . .
; . . . ~
.,` .
~:.
' :
; WO92/17211 PCT/US92/02911
- 16 - -
',` 210'77~q
BRIEF DESCRIPTION OF ~E DRAWING8
Fiqure 1 is a schematic diagram showing the
~ construction of plasmids pLPPsias (A) and pCPPslas
i 5 (B)-
Fiqure 2 is a partial sequence (SEQIDNO:l) of the
~ Moloney-Murine leukemia virus (M-MuLV) in the region
.' including the packaging sequence. The CAP, primer
~;, binding site, splice donor site, core packaging
sequence, and the beginning of the qiq gene are
"~; marked.
We have found that antisense molecules
~ complementary to the primer binding site or to the
,~ splice donor site, but not to the packaging sequence
~- 15 of MoMLV, did not show significant inhibition of the
virus infection while tha best inhibition observed was
with anti-sense oligos complementary to the open bold
~;~ sequence, i.e., the core (bases 301-350) of packaging
. r' site.
Fiqure 3 is a partial sequence (SEQIDNO:2j of the
"~,, genomic RNA of bovine leukosis virus, with the region
"~ (341-415) expected to include the packaging signal
indicated by open bold letters.
Figure 4 shows the results of the plaque assay.
Figure 5 is a partial sequence (SEQIDNO:3) of
HIV-1 in the region including the packaging sequence.
~i
.~, .. .
.
~,~,.
SUBSTITUTE SHEET
,~
: - - , : . .
.
'- WO92/17211 PCI'/US92/02911
- 17 ~ ~ 9
.:~
DE~;CRIPTION OF THE PREFERRED ENBODIMENq~13
Interference with the specific interactions
- between the Psi sequences of viral genomic RNA
~' 5 necessary for packaging and virion capsid proteins
will block the retroviral replication cycle. This
interference may be accomplished through the use of
i~ antisense nucleic acids (DNA or RNA) complementary to
,~ a part of the packaging sequences, which hybridizes
lo thereto and thereby inhibits replication.
Antisense sequence directed against a retroviral
` gene would not block replication as effectively as the
'~ antisense molecules of the present invention. An
antisense RNA molecule directed against a retroviral
` 15 gene competes with normal messenger RNA for binding to
-~ ribosomal RNA, while one directed against the
packaging sequence competes with a qaa-encoded core
protein for binding to genomic RNA. RNA-RNA
-i interactions are stronger than RNA-protein
20 interactions.
In order to test the efficacy of such antisense
RNA in blocking retroviral replication in cells and in
whole animals, the present inventors constructed
transgenic mice expressing RNA sequences complementary
25 to the Psi sequences of Moloney murine leukemia virus
'~r~. (M-MuLV). It was discovered that such animals
completely resisted challenge with this leukemia
virus.
The present invention is therefore directed to
~;~ 30 the use of antisense RNA and DNA molecules
s complementary to retroviral packaging sequences as
agents for the prevention and treatment of diseases in
which the causative agent is a retrovirus.
The antisense RNA and genes coding therefor are
` 35 intended to encompass sequences capable of hybridizing
to the packaging sequence of a retrovirus. The
.
,v
SUBSTITUTE SHEET
v
.
...
` . ~ `
~"; .
:'' ~ . .
- WO92/1721l PCT/US92/0291l
- 18 - ^
~1 ~77.~Cl
preferred retroviruses of the present invention
include both human and other animal retroviruses.
Preferred human retroviruses, include Human
Immunodeficiency Virus-1 (HIV-1) (or Human T-Cell
Lymphotropic Virus-3 or Lymphadenopathy Associated
Virus), Human Immunodeficiency Virus-2 (HIV-2), Human
T-Cell Lymphotropic Virus-I (HTLV-1), and Huma~ T-Cell
Lymphotropic Virus-2 (HTLV2). Also intended within
the scope of the present invention are additional
retroviruses of other animal species, most
particularly agriculturally important animals such as
cows and chickens, and pets such as dogs and cats.
A non-limiting list of additional retroviruses
included within the scope of the present invention is
provided in Table I, below. Retroviruses are
described in detail in weiss, R. et al. (eds), RNA
Tumor Viruses. "Molecular Biology of Tumor Viruses,"
Cold Spring Harbor Laboratory, Cold Spring Harbor,
N.Y., 1984, which is hereby incorporated by reference.
The target packaging sequence to which the
antisense RNA of the present invention is to hybridize
in order to inhibit retrovirus infection is be
selected based on examination of the sequence of the
retroviral genome. Since the packaging sequences of
all retroviruses studied are located between the
primer binding site just 3' of the 5' LTR and the
initiation sequences of the first coded viral protein
(usually the qaa protein coding sequences), sequences
within this region (such as, for example bases 200-340
in the proviral genome of HIV-l, isolate ELI) are
preferred candidates for antisense targeting. Because
of the homology in this region among various
retroviruses, an antisense sequence designed for one
particular virus may be used successfully to inhibit
replication of another virus.
.,
SUBSTITUTE SHEE~T
i,i .
., .. : - : ~ : . . `
. : . .
` ;` W092/1721l PCT/US92/02911
2 1 1~ 7 ~ ~ q
i..~,
~ Thus, for example, preferred antisense
; aligonucleotides are specific for the region between
;- bases 280-330 of the Mo-MuLV sequence since it is
homologous to HIV-l. since different isolates of HIV-
1 have the same packaging sequences, but at slightly
~ different positions within the genome, the same
-, sequences would be effective in all HIV-1 strains.
',$~ One preferred anti-sense sequence is one which is
- 10 substantially complementary to all retrovirus
; packaging sequences, thus serving as a "universal"
; inhibitor. A sequence complementary to the core
packaging sequence from HIV-I (see J.Virol., 63:4085,
,~ 1989), may be worth considering in this regard.
Alternatively, oligonucleotide sequences may be
~-~ produced that are "consensus" packaging sequences
-~ having a high degree of complementarity to the
packaging sequences of a set of retroviruses, or which
are specific to a particular retroviruses.
Shown below is an exemplary oligonucleotide
: sequence according to the present invention, useful
for inhibition of HIV-1, which are complementary to
viral genomic RNA, viral mRNA, as well as the viral
DNA sequences. For the packaging sequence of HIV-1,
25 see (Lever J. Virol., 63(9):4085-4097 (1989); Rorman,
et al., (Proc. Natl. Acad. Scl. USA 84:2150-2154
(1987).
SEQ ID NO: 4 is a 44mer sequence shown below,
which is complementary to the HIV-1 packaging
;~i30 sequence.
CTCATGCGGTTTTAAAACTGATCGCCTCCGATCTTCCTCTCTC
The sequence begins (5') immediately after the
S.D site, and ends (3') just prior to the gag/Met
site. The underlined 19 bases are complementary to
the "core" of the HIV packaging sequence. A 19-mer
....
~ .
SUBSTITUTE SHEET
o
: . . ,
~: .
~6 ~ W092tl~211 ~ ~77~Cl 20 - PCT/US92tO2911
.
antisense molecule complementary to this core region,
and a 27-mer complementary to the core and to the four
flanking bases on either side, likewise are useable in
the present invention. The invention is not limited
to any of these packaging site-targeting sequences,
but rather includes shorter and longer sequences.
Table IV identifies a number of packaging
sequences of interest.
The antisense molecule (e.g., RNA) of the present
invention preferably has 100% complementarity to at
least a significant subsequence of the packaging
sequence (on one strand of the viral DNA) for which it
is targeted. Thus, the DNA strand encoding this RNA
should be 100% homologous to the DNA strand which is
complementary to the packaging sequence. In another
embodiment, the sequence may have a lower degree of
- homology, such as at least about 60 or 80%. The
homology must be sufficient such that the antisense
RNA hybridizes to the target packaging sequences with
sufficient affinity to achieve its purpose, i.e.
inhibition of viral packaging.
The efficiency of such hybridization is a
function of the length and structure of the
hybridizing sequences. The longer the sequence and
the closer the complementarity to perfection, the
stronger the interaction. As the number of base pair
mismatches increases, the hybridization efficiency
will fall off. Furthermore, the GC content of the
packaging sequence DNA or the antisense RNA will also
affect the hybridization efficiency due to the
additional hydrogen bond present in a GC base pair
compared to an AT (or AU) base pair. Thus, a target
subsequence richer in GC content is preferable as a
target.
.~ .
;~ .
` SUBSrITUTE SHEET
., ,
, . , ~ , . . .
' ':
W092/17211 PCT/US92/02911
- 21 -
2107789
It is desirable to avoid sequences of antisense
RNA which would form secondary structure due to
intramolecular hybridization, since this would render
the antisense RNA less active or inactive for its
intended purpose. One of ordinary skill in the art
will readily appreciate whether a sequence has a
tendency to form a secondary structure. Secondary
structures may be avoided by selecting a different
target subsequence within the packaging site.
An oligonucleotide, between about 15 and about
100 bases in length and complementary to the target
subsequence of the retroviral packaging region may be
synthesized from natural mononucleosides or,
, ~,
~r15 alternatively, from mononucleosides having
substitutions at the non-bridging phosphorous bound
oxygens. A preferred analogue is a methylphosphonate
ianalogue of the naturally occurring mononucleosides.
More generally, the mononucleoside is any analogue
~J~20 whose use results in oligonucleotides which have the
~,~advantages of (a) an improved ability to diffuse
. ~,.,
through cell membranes and/or (b) resistance to
nuclease digestion within the body of a subject
~(Miller, P.S. et al., Biochemistry 20:1874-1800
-~25 (i981)). Such nucleoside analogues are well-known in
~;the art, and their use in the inhibition of gene
expression are detailed~in a number of references
(Miller, P.S. et al., supra; Jayaraman, K. et al.,
supra; Blake, K.R. et al., supra; Miller, P. et al.,
feder. Proc. 43, abstr. 1811 (1984); Smith, C.C. et
al., su~ra).
`~J' Basic procedures for constructing recombinant DNA
and RNA molecules in accordance with the present
invention are disclosed by Sambrook, J. et al., In:
Molecular Clonina: A LaboratorY Manual, Second
'Edition, Cold Spring Harbor Press, Cold Spring Harbor,
.`~.SU8ST1TUTE SHEET
," ~
'' - .. ', ' :. .
, . . .
,. -
: ~'
i; WO92~17211 21 ~77gq - 22 - PCT/US92/02911
~, . .
NY (1989), which reference is herein incorporated by
~;~ reference.
r,~ Oligonucleotide molecules having a strand which
-~ 5 encodes antisense RNA complementary to the target
~ retrovirus packaging sequences can be prepared using
.¢ procedures which are well known to those of ordinary
skill in the art (Belagaje, R., et al., J. Biol. Chem.
254:5765-5780 (1979); Maniatis, T., et al., In:
lo Molecular Mechanisms in the Control of Gene
Expression, Nierlich, D.P., et al., Eds., Acad. Press,
NY (1976); Wu, R., et al., Prog. Nucl. Acid Res.
Molec. Biol. 21:101-141 (1978); Khorana, R.G., science
~,; 203:614-625 (1979)). Additionally, DNA synthesis may
-`~ 15 be achieved through the use of automated synthesizers.
'-7' Techniques of nucleic acid hybridization are disclosed
' r, by Sambrook et al. (su~ra), and by Haymes, B.D., et
al. (In: Nucleic Acid Hybridization, A Practical
~ Approach, IRL Press, Washington, DC (1985)), which
`,,r~ 20 references are herein incorporated by reference.
An "expression vector" is a vector which (due to
the presence of appropriate transcriptional and/or
translational control sequences) is capable of
expressing a DNA (or cDNA) molecule which has been
cloned into the vector and of thereby producing an RNA
or protein product. Expression of the cloned
~;,3~, sequences occurs when the expression vector is
introduced into an appropriate host cell. If a
'`n ~ prokaryotic expression vector is employed, then the
appropriate host cell would be any prokaryotic cell
~'~ capable of expressing the cloned sequences.
~ Similarly, when a eukaryotic expression vector is
,~¦ employed, then the appropriate host cell would be any --
~' eukaryotic cell capable of expressing the cloned
sequences.
. . ~,
:,.'c~
.~ .
SUBSTITUTE SHEET
.`~
`: WO92/17211 PCT/US92/02911
- 23 -
21~ ~7 ~9
DNA sequence encoding the antisense RNA of the
- present invention may be recombined with vector DNA in
accordance with conventional techniques, including
5 blunt-ended or staggered-ended termini for ligation,
restriction enzyme digestion to provide appropriate
~; termini, filling in of cohesive ends as appropriate,
alkaline phosphatase treatment to avoid undesirable
,3' joining, and ligation with appropriate ligases.
lO Techniques for such manipulations are disclosed by
Sambrook et al., suDra, and are well known in the art.
~" A nucleic acid molecule, such as DNA, is said to
be "capable of expressing" a mRNA if it contains
nucleotide sequences which contain transcriptional
15 regulatory information and such sequences are
"operably linked" to nucleotide sequences which encode
~; ,
the RNA. The precise nature of the regulatory regions
needed for gene expression may vary from organism to
organism, but in general include a promoter which
;~ 20 directs the initiation of RNA transcription. Such
regions may include those 5'-non-coding sequences
involved with initiation of transcription such as the
TATA box.
If desired, the non-coding region 3' to the gene
25 sequence coding for the desired RNA product may be
obtained by the above-described methods. This region
~ may be retained for its transcriptional termination
3 regulatory sequences, such as those which provide for
termination and polyadenylation. Thus, by retaining
30 the 3'-region naturally contiguous to the coding
sequence, the transcriptional termination signals may
be provided. Where the transcriptional termination
signals are not satisfactorily functional in the
expression host cell, then a 3' region functional in
t~e host cell may be substituted.
.
SUBSTITUTE SHEET
~ .
, ~ ~
.: . ;: ~ . ~ .
W092/1721l PCT/US92/02911
24
'.: 21977gq '' '
- .,
,. .
! Two DNA sequences (such as a promoter region
sequence and a coding sequence) are said to be
operably linked if the nature of the linkage between
the two DNA sequences does not (1) result in the
~- introduction of a frame-shift mutation in the region
- sequence to direct the transcription of the desired
-~ gene sequence, or (3) interfere with the ability of
-~ the gene sequence to be transcribed by the promoter
region sequence. A promoter region would be operably
linked to a DNA sequence if the promoter were capable
of effecting transcription of that DNA sequence. In
order to be "operably linked" it is not necessary that
~? two sequences be immediately adjacent to one another.
s 15 For production of the DNA sequences of the
present invention in prokaryotic or eukaryotic hosts,
the promoter sequences of the present invention may be
either prokaryotic, eukaryotic or viral. Suitable
~ii promoters are inducible, repressible, or, more
preferably, constitutive. Examples of suitable
~. prokaryotic promoters include promoters capable of
;;, recognizing the T4 polymerases (Malik, S. et al., J.
Biol. Chem. 263:1174-1181 (1984); Rosenberg, A.H. et
al., Gene 59:191-200 (1987) Shinedling, S. et al., J.
~ 25 Molec Biol. 195:471-480 (1987) Hu, M. et al., Gene
`~? 42-?1-30 (1986), T3, Sp6, and T7 (Chamberlin, M. et
al., Nature 228:227-231 (1970); Bailey, J.N. et al.,
;~ Proc. Natl. Acad. Sci. (U.S.A.) 80:2814-2818 (1983~;
; Davanloo, P. et al., Proc. Natl. Acad._Sci ~U.S.A.)
-~s 30 81:2035-2039 (1984)); the PR and PL promoters of
~ bacteriophage lambda (~ Bac~terl31~g~ Lambda,
s Hershey, A.D., Ed., Cold Spring Harbor Press, Cold
~ Spring Harbor, NY (1973); Lambda II, Hendrix, R.W.,
-~` Ed., Cold Spring Harbor Press, Cold Spring Harbor, NY
~35 (1980)); the trp. recA, heat shock, and lacZ promoters
;~of E. coli.; the int promoter of bacteriophage lambda;
'~
SU8ST1TUTE SHEET
.~
. .
?,~
: ~ :
WO92/17211 PCT/US92/02911
- 25 -
:~: 21077~
the bla promoter of the ~-lactamase gene of pBR322,
and the CAT promoter of the chloramphenicol acetyl
transferase gene of pPR325, etc. Prokaryotic
promoters are reviewed by Glick, B.R., (J. Ind.
Microbiol. 1:277-282 (1987)); Cenatiempo, Y.
(siochimie 68:505-516 (1986)); Watson, J.D. et al.
(In: Molecular Biology of the Gene, Fourth Edition,
Benjamin Cummins, Menlo Park, CA (1987) and Gottesman,
s. (Ann. Rev. Genet. 18:415-442 (1984)).
Eukaryotic promoters include the promoter of the
mouse metallothionein I gene (Hamer, D., et al., J.
Mol. Appl. Gen. 1:273-288 (1982)~; the TK promoter of
Herpes virus (McKnight, S., Cell 31:355-365 (1982));
the SV40 early promoter (senoist~ c., et zl., Nature
(London) 290: 304-310 (1981) and the yeast aal4 gene
promoter (Johnston, S.A., et_al., Proc. Natl. Acad.
Sci. (USA) 79: 6971-6975 (1982); Silver, P.A., et al.,
Proc. Natl. Acad. Sci. (USA) 81:5951-5955 (1984)).
For preparation of vectors for use in inhibiting
retrovirus infection, in susceptible eukaryotic cells
or in whole animals, eukaryotic promoters must be
utilized, as described above. Preferred promoters and
additional regulatory elements, such as
polyadenylation signals, are those which should yield
maximum expression in the cell type which the
retrovirus to be inhibited infects. Thus, for
example, HIV-l, HIV-2, HTLV-l and HTLV-2, as well as
the Moloney murine leukemia virus, all infect lymphoid
cells, and in order to efficiently express an
antisense RNA complementary to the packaging sequence
of one (or more) of these viruses, a transcriptional
control unit (promoter and polyadenylation signal) are
selected which provide efficient expression in
lymphoid cells (or tissues). As exemplified below,
preferred promoters are the cytomegalovirus immediate
.
.,
~ SUBSrITUTE SHEET
.~, .
:
.` , ,
L
~ WO92/17211 PCT/~S92/02911
` 7 ~ 9 - 26 ~
....
early promoter t32), optionally used in conjunction
` with the bovine growth hormone polyadenylation signals
(33), and the promoter of the Moloney-MuLV LTR, ~or
use with a lymphotropic retrovirus. A desirable
feature of the Moloney-MuLV LTR promoter is that it
has the same tissue tropism as does the retrovirus.
The CMV promoter is likewise expressed primarily in
lymphocyte. The metallothionein promoter has the
advantage of inducibility. The SV40 early promoter
;'l exhibits high level expression in vitro in bone marrow
cells.
-~ An antisense RNA molecule may be injected into
the human or other animal subject to be protected or
treated by any compatible route of administration,
e.g., intravenously, intramuscularly, subcutaneously
or intraperitoneally, or administered by ingestion or
inhalation. Special dosage forms, such as slow
release capsules or implants, may be used when
appropriate. Alternatively an antisense DNA molecule
~ may be provided. DNA is more readily synthesized ln
-~ vitro than RNA.
The antisense molecule may be an analogue of DNA
or RHA. The present invention is not limited to use
25 f any particular DNA or RNA analogue, provided it is
capable of adequate hybridization to the complementary
genomic DNA of a packaging sequence, has adequate
resistance to nucleases, and adequate bioavailability
p and cell take-up. DNA or RNA may be made more
resistant to in vivo degradation by enzymes, e.g.,
,~ nucleases, by modifying internucleoside linkages
(e.g., methylphosphonates or phosphorothioates) or by
incorporating modified nucleosides (e.g., 2'-0-
methylribose or 1'-alpha anomers).
The naturally occurring linkage is
SUBSTITUTE SHEET
t
:. ` ' ~ .'
~' '
~ WO92/17211 - 27 -2 1 ~ 7 7 ~ PcT/US92/02911
.~.
. 3'0
, . O' - P = O.
: ~ 05'
- :.
;~ 5
-.:. Alternative linkages include the following:
- 3 o
S -- P = O
.~
~; 05'
~ ~ 10
: ,, ~1,
.... 3'0
CH3 - P = 0
:-~ 051
x'~
,~
` 15
NR2 -- P -- o
05~
:~ (where R is hydrogen and/or alkyl)
3'0
i 20 Ro - P = o
:~ 05'
~ (where R is hydrogen or alkyl)
:. i 3~0
S~ -- P = S.
05'
~ lt is also possible to replace the 3'0-P-05' with
.~ s
other linkages such as 3'0-CH2C(0)-05', 3'0-C(0)-NH5',
and 3'C-CH2 CH2 S-C5'.
:j The entire antisense molecule may be formed of
such modified-linkages, or only certain portions, such
; as the 5' and 3' ends, may be so affected, thereby
~ providing resistance to exonucleases.
. j .
~ Antlsense molecules suitable for use in the
::.~ present invention include but are not limited to
.~ 3s dideoxyribonucleoside methylphosphonates, see Mill, et
:
~ al., Biochemistry, 18:5134-43 (19i9),
.~
, ~ .
`g SUBSTITUTE Sl-IEET
.
:
.. . . .. .
.. . . . ~ . . ~ . ` . . . -
`:. , , ~ ,,:, . : . .
",. . . ;,
` wos2/l72ll PCT~US92/029ll
~ 2 ~ 0 7 7 ~9 - 28 - _
oligodeoxynucleotide phosphorothioates, see Matsukura,
et al., Proc. Nat. Acad. Sci., 84:7706-10 (1987),
oligodeoxynucleotides covalently linked to an
intercalating agent, see Zerial, et al., Nucleic Acids
Res., 15:9909-19 (1987), oligodeoxvnucleotide
conjugated with poly(L-lysine), see Leonetti, et al.,
Gene, 72:32-33 (1988), and car~amatelinXed oligomers
ii assembled from ribose-derived subunits, see Summerton,
lo J-, Antisense Nucleic Acids Conference, 37:44 (New
York 1989).
While direct administration of antisense nucleic
. acid drugs provides acute protection, the protective
pe~iod is dependent on the half-life of the molecule.
Moreover, the supply of antisense nucleic acid can be
increased onIy by further administrations. It may
therefore be desirable to provide a self-renewing
source of antisense RNA by introducing a recombinant
~; DNA molecule, capable of transcribing said antisense
i .
~ 20 RNA, into one o~ more cells of the human or animal
.l subject, thus creating a transgenic or chimeric animal
~ having enhanced resistance to retroviral infection.
~ The recombinant DNA may be delivered to the animal by,
c e.g., microinjection of the expression cassette into
25 the animal at the oocyte stage, retroviral vector
transfection of the embryo, or intravenous injection
of the retroviral vectQr into the fetal or postnatal
animal.
Thus, the present invention is also directed to
30 a transgenic eukaryotic animal (preferably a
vertebrate, and more preferably a mammal) the germ
cells and somatic cells of which contain recombinant
genomic DNA according to the present invention which
.. : encodes an antisense RNA capable of hybridizing to a
~j 35 retroviral packaging sequence. "Antisense" DNA is
introduced into the animal to be made transgenlc, or
, .
SUBSTITUTE SHEET
,1 .
.' .
X~
~,
.
-~ W~92/17211 PCT/US92/02911
29 -
` 21~7789 ;
` an ancestor of the animal, at an embryonic stage,
preferably the one-cell, or fertilized oocyte, stage,
and generally not later than about the 8-cell stage.
~` 5 The term "transgene," as used herein, means a gene
-~; which is incorporated into the genome of the animal
and is expressed in the animal, resulting in the
presence of the RNA transcript o~ t~e transgene in the
~ transgenic animal.
`'.j? 10 There are several means by which such a gene can
be introduced into the genome of the animal embryo so
as to ~e chromosomally incorporated and expressed.
The DNA may be microinjected into the male or female
pronucleus of fertilized eggs. It may also be
microinjected into the cytoplasm of the embryonic
cells. The cells may be transfected by a retrovirus
carrying the transgene. The use of retroviral
~: transfection is not limited to the embryonic stage;
;~ the vector may be intravenously or intraperitoneally
:.; io introduced into the fetal or postnatal animal.
`~s Introduction of the desired gene sequence at the
fertilized oocyte stage ensures that the transgene is
present in all of the germ cells and somatic cells of
the transgenic animal and has the potential to be
25 expressed in all such cells. The presence of the
transgene in the germ cells of the transgenic
"founder" animal in tur~n means that all its progeny
~; will carry the transgene in all of their germ cells
and somatic cells. Introduction of the transgene at a
30 later embryonic stage in a founder animal may result
in limited presence of the transgene in some somatic
cell lineages of the founder; however, all t~e progeny
l~ of this founder animal that inherit the transgene
; conventionally, from the founder's germ cells, will
, 35 carry the transgene in all of their germ cells and
~ somatic cells.
:,
"
`; SUBSTITUTE SHEET
" .
, .
f~
:', ~ ' ' :
'~',: ~; . ' ' '
. ~ , .
:, ' ', , ' .
~ W092/17211 PCT/US92/02911
21 077g q '-
,,;
Chimeric mammals in which fewer than all of the
~ somatic and germ cells contain the antisense DNA of
;' the present invention, such as animals produced when
fewer than all of the cells of the morula or blastula
are transfected in the process of producing the
transgenic mammal, are also intended to be within the
scope of the present invention. Chimeric animals may
' be created by "gene therapy". in which the transgene
is typically introduced after birth.
The techniques which may be used include those
disclosed by Wagner, T. et al. (Proc. Natl Acad. Sci.
;~ USA 78:6376-6380 (1981)); Wagner et al., U.S. Patent
4,873,191 (1989); Palmiter, R. et al., Ann. Rev.
~, 15 Genet. 20:465-99 (1986); and Leder, U.S. Patent
4,736,866, the entire contents of which are hereby
incorporated by reference. Analysis of the progeny
-~ mice produced from the microinjected eggs, as well as
offspring of transgenic animals bred conventionally,
-.~, 20 is achieved by DNA extraction and slot blot
hybridization analysis of the DNA for the presence of
~ the transgene (McGrane, M. et al., J. B ol. Chem.
,'J! 263~ 443 11451 (1988)).
-~ Antisense RNA might be delivered to the
lymphocytes of AIDS patients by gene therapy methods.
For example, bone marrow cells may be treated with a
recombinant, replicatio~n deficient, retroviral vector
containing DNA sequences encoding and expressing anti-
:i~ sense RNA complementary to the packaging sequences of
HIV. In this procedure bone marrow cells would beremoved from the patient, treated with the recombinant
retroviral vector and cells in which the DNA genome of
~ the vector had integrated in their chromosomes
.. s selected using FACS cell sorting based upon a light
visualization marker also incorporated into the
~ retorviral vector (i.e., the ~-galactosidase gene).
'.'~
~ .
SUBSTITUTE SHEET
, ', ' . .
... .
i- :
'f
.,.j.:
`,''
WO92/17~11 PCT/US92/0291l
: 2 1
.
These vector integrated bone marrow cells could then
be reintroduced into the patient after oblation of
their other bone marrow cells by irradiation. The
resulting patient would then have only lymphocytes
which encoded the anti-sense RNA sequences to the HIV
packaging sequences and would be resistant to AIDS
just as the transgenic mice described herein are
resistant to M-MuLV.
` 10 For commercial animals the method of choice would
be transgenic introduction into a line of animals, but
several other methods could be used. It is unlikely
that gene therapy approaches like the example given
for human AIDS protection would be used in animals
' 15 because the procedure is too complex and expensive.
Recently it has been demonstrated that DNA can be
introduced into various tissues by attaching it to
proteins which are the ligands for cellular receptors
(Wu, et al., J. Biol. Chem., 263:14621; 1988), or by
unassisted introduction into muscle cells (Wolff, et
al., Science, 247:1465; 1990). Using these methods it
~ would be possible to inject animals with DNA
r~ constructs coding for anti-sense RNA directed against
the packaging sequences of retroviruses. These "DNA
injections" would provide protection against viral
infection within the tissues targeted by the DNA
, delivery system used. This approach would be very
; appealing both for animals and for humans, because it
would involve simply the injection of a DNA molecule
coding for the anti-sense RNA, or this DNA molecule
bound to a receptor targeted ligand. This injection
might have to be given several times a year (for
example, when an outbreak of a retroviral disease
occurs in a given region) but would provide protection
against retroviral infection when needed.
SUBSTITUTE SHEET
,
- .
,
WO 92t 1 72 1 1 pCI / US92/029 1 1
`21~789 - 32 -
. : .
`~. The antisense-RNA encoding DNA may be introduced
when the animal (including human) already is infected,
or prior to infection, as a prophylactic measure. In
the latter case, use of a regulatable promoter may be
desirable.
Having now generally described the invention, the
same will be more readily understood through reference
'''~'!~ to the following examples which are provided by way of
. lo illustration, and are not intended to be limiting of
the present invention, unless so specified.
.,.~,~ .
EXAMPLE I
Plasmids
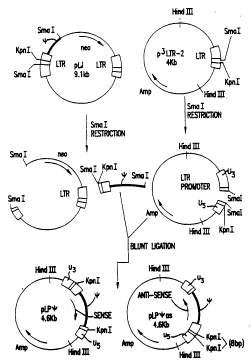
Recombinant plasmids pLPPsias and pCPPsias were
constructed as shown in Fig. 1~. Plasmids pLJ(21) was
cleaved with SmaI and the 540 bp SmaI fragment
containing the M-MuLV sequences isolated. Plasmid
p3'-LTR-2, containing the 3'M-MuLV LTR (22), was
linearized with SmaI and fused to the 540 bp fragment
, from pLJ in both orientations using DNA ligase.
-~ Clones of pLPPsi with the antisense orientation were
selected on the basis of their KpnI digestion
patterns. Plasmid pCMVIE-BGH (30), with an 8 bp BglII
linker introduced at an XmaIII site just 3' of the
CMVIE CAP, was cleaved with both BglII and SmaI and
the 5.4 kb fragment containing the CMV promoter, the
bGH poly-A addition site and a tetracycline resistance
selectable marker isolated. The "sticky ends" of this
fragment were filled in by incubation with
~' deoxynucleoside triphosphates tA,T,G and C) in the
presence of the DNA polymerase I Klenow fragment and
blunt-end ligated to the 540 bp SmaI fragment from pLJ
in both orientations. Clones of pCP~as with the
antisense orientation were selected on the basis of
their KpnI digestion patterns.
`
.,,~ .
;
:
SUBSTITUTE SHEET
,,~ .
~:
:`.,`,, , : . -
, -
i" . ~ . .
. ,;
;: WO 92/1721 1 PCr/US92/0291 1
~ - -- 3 3
21~)7~ 8 q
.~`
. EXAMPLE II
. Transqenic Mouse Production
`, The procedure for the production of transgenic
5 mice by direct microinjection of DNA into the male
.~ pronucleus of fertilized mouse eggs has been described
(24). DNA extraction from mouse tails and slot blot
hybridization analysis of the progeny resulting from
~ these microinjected eggs were performed as previously
;; ~0 described (25).
. EXAMPLE III
Cell Culture
~,~ Mouse NIH 3T3 cells were maintained in Dulbecco's
`, 15 Modified Eagle's medium (DMEM) containing 10% Nu-
serum. Stable cell lines expressing antisense Psi
sequences were established by co-transformation with
:~ pCPPsias (36 ~g) and pSV40neo (0.36 ~g) (ATCC 33964)
~ using a modified DEAE dextran-dimethyl sulfoxide shock
,, 20 procedure (26).
. EXAMPLE IV
~ DNA and RNA Analysis and Enzyme Assays
J The integrity of DNA sequences in transgenic mice
1 25 was analyzed by the method of Southern (27). RNA was
', prepared from lymphocytes by a single step isolation
method (28) using Ficoll-Hypaque gradient isolation
:1 procedure (29). RNA was then subjected to
i electrophoresis at 70V for 4.5h in a 1.2%
30 agarose/formaldehyde gel and transferred onto
nitrocellulose paper for hybridization and
radioautography as described (30). Reverse
- Transcriptase assays were performed as described
previously (31). In order to distinguish the
35 antisense RNA strand, a strand specific RNA probe was
~ used for Northern hybridization assays of lymphocyte
.
SUBSTITUTE SHEET
I
;; . .
... ~ . . . .
: ~
W092/17211 PCT/US92/02911
- 34 - ^
7 g ~ ~ ~
~ . . .
~,
~ RNA. This probe was synthesized from a pSP65 vector
''~ containing the 540 bp SmaI Psi sequence containing
~, fragment inserted in the sense orientation using SP6
~ 5 RNA polymerase and labeled guanosine 5'-triphosphates
s~ (Promega Riboprobe System, Promega Corp., Madison
~ Wisconsin).
``2 EXAMPLE V
LPsias and CPsias Transgenic Mice
Linear DNA fragments containing the M-MuLV
packaging sequences in inverted orientation under the
regulation of the M-MuLV LTR or the Cytomegalovirus
immediate-early promoter were respectively prepared
from pLPPsias as a 2.2kb HindIII fragment or from
pCPPsias as a 2.3 k~ EcoRI-Cla~I fragment.
To introduce these sequences into mouse germ
lines, the fragments were separately microinjected
into the pronuclei of fertilized mouse eggs (200 to
500 copies per egg). These eggs were subsequently
~-~ transplanted into the oviducts of pseudopregnant
foster recipient mice. When the resulting offspring
were about one month old, a segment of the tail of
each animal was removed and DNA was prepared from it.
~; 25 Transgenic mice were detected by DNA slot blot and
Southern transfer hybridizations. Two lines of
transgenic mice (LPPsias and CPPsias) were analyzed
and used in this study. LPPsias transgenics trace to
a single founder mouse resulting from the
microinjection of the 2.2 kb HindIII fragment from
pLPPsias and CPPsias transgenics trace to a single
founder from the microinjection of the 2.3 kb EcoRI-
ClaI fragment from pCPPsias.
Tail DNA (10 ~g) from the LP~as founder and his
,~ 35 offspring was digested with SacI and EcoRI restriction
endonucleases and CPPsias founder and offspring DNA
~;
1 ~ .
~ SUBSTITUTE SHEET
A
~ WO92/17211 2 ~ ~ ~ 7 8q PCT/US92/02911
:: ;
was digested with Kpn I and then subjected to
electrophoresis at 50V for 9h in a 0.8% agarose gel.
The DNAs were transferred onto nitrocellulose paper by
the technique of Southern (27). Following incubation,
the nitrocellulose paper was hybridized to a nick
translated DNA probe (5 x lO8 to lO X lO8 cpm/~g)
prepared from pBR322 for LPPsias DNA (this probe
hybridizes to the LPPsias sequences because of the
presence of pBR322 sequences in this construction but
-~ does not crosshybridize with endogenous retroviral
....~
sequences present in the animal) or a l.3 kb Sac I~
EcoRI fragment from pCPPsi for CPPsias DNA. After
washing and drying, the nitrocellulose was
autoradiographed by exposure to Kodak film at -70QC
for 16 h. From the Southern blots it was clear that
both LPPsias and CPPsias mice contain complete
transcription units for the production of the 540 b
antisense sequences integrated into their chromosomal
components. For LPPsias DNA, the 1470 bp hybridizing
SacI-EcoRI fragment shown by each LPPsias mouse
,;.,
confirmed the presence of the appropriate sequences
~^ and CPPsias mouse DNA from each animal showed the
characteristic 400 bp KpnI hybridizing fragment.
;, ~
:~ EXAMPLE VI
Expression of Antisense Psi RNA in LPPsias
and CPPs~as Transqenic Mice
The transcriptional units introduced into these
two lines of mice were constructed to provide the
~ 30 appropriate tissue tropism for the transcription of
: the antisense RNA within the lymphoid target tissue
~ for M-MuLV. Within the LPPsias mice the
r'' transcriptional unit, including the U3R
promoter/enhancer elements and the RU5 polyadenylation
signals from the M-MuLV LTR, is essentially identical
to the transcriptional control sequences of the intact
.,,
. ~
, ~
.;~ SUBSTITUTE SHEET
~.j
.~ .
~` .~, . , . . -
,`:` , :
.,. : :~
.
':``` ':' '
W092/17211 PCT/US92/02911
~ 7 7 ~q - 36 - ~
M-MuLV and would be expected to produce the antisense
RNA at the site of viral replication.
The transcriptional control unit within the
CPPsias mice, including the cytomegalovirus
immediately-early promoter (32) and the bovine growth
hormone polyadenylation signals (33), would also be
~, expected to have lymphoid tissue tropism (34). In
order to confirm production of antisense M-MuLV RNA
within the lymphoid tissue of these mice, Northern
hybridization analysis of RNA from lymphocytes
.` isolated from LPPsias and CPPsias mice was performed.
RNA from both CPPsias and LPPsias mice was hybridized
' 15 to a strand specific ~NA probe complementary to the M-
MuLV antisense Psi sequence with a specific activity
of 1-5 x lO8 cpm/~g. The distinct 600 b hybridizing
RNA fragments found in all LPPsias lymphocyte RNA and
the 750b hybridizing RNA in CPPsias mice confirmed the
production of the antisense Psi sequences of M-MùLV in
the white blood cells of LPPsias and CPPsias
transgenic mice.
The different lengths of the antisense RNA in
; LPPsias and CPPsias mice is the result of the
`:
different gene constructions introduced into these two
lines of mice. The CPPsias mice contain a gene
construction including a small portion of exon 5 from
the bovine growth hormone gene and the bovine growth
hormone poly A addition signals resulting in a longer
antisense RNA product.
i~ .
., .
SU8STITUTE SHEET
.. . .
~ , .
~ . . r
WO92/17212 PCT/US92/02911
- - 372~ ~77 8~
. .
- EXAMPLE VI
Inhibition of M-MuLV Re~lication in Cells
Expressina Antisense Psi RNA
A stable cell line expressing antisense Psi
sequences was established by co-transformation of
mouse NIH 3T3 cells with linearized pCPPsias (36 ~g)
, and pSV40neo (0.36 ~g) (ATCC 33694) using a modified
DEAE-dextran dimethyl-sulfoxide-shock procedure (26).
Stable transformants were selected in G4l8DMEM medium,
Southern analysis performed to establish the presence
'~ of presence of integrated CPPsias sequences, and
Northern analysis carried out to confirm the presence
of antisense M-MuLV RNA production by cloned cell
q
lines. Both control mouse NIH 3T3 cells and the
CPPsias transformed cell line were challenged with M-
MuLV (4XlO6 PFU/75 cm plate) for 24 hrs., washed free
of virus, cultured for an additional 48 hrs, filtered
through 0.45 um filters to remove cells and cellular
material from the medium and virus particles
4 20
concentrated from the medium by sucrose gradient
centrifugation (35). RNA was prepared from the viral
pellet by a phenol-chloroform extraction procedure
(35) and the presence of M- MuLV genomic RNA detected
by Northern Analysis using a random primer labeled 540
bp Sma I DNA fragment from pCPPsias tl x lO8 - 5 x lO4
cpm/~g) as a M-MuLV virus specific prob~. No viral
RNA was produced from antisense Psi RNA expressing NIH
3T3 cells after challenge with M-MuLV, while a
substantial amount of viral genomic RNA (8. 3 Rb) was
produced from control NIH 3T3 cells challenged with M-
MuLV.
Reverse transcriptase activity in the supernatant
from the stable cell line expressing antisense Psi RNA
after infection with M-MuLV was measured and compared
to normal mouse NIH 3T3 cells infected with M-MuLV
(3l) . Substantial reverse transcriptase activity was
., .
.,
!.-i
~ SUBSTITUTE SHEET
. .
,,,~ .
.,~, ~ : ~ . ,
... . : : , - -
.,~. ~ : - . :
~ .
,, .
. ;~ W092/17211 PCT/US92/02911
~: 3 8 ~ :
8 9
observed (see Table II) even though this same
supernatant was shown to be devoid of viral gen~mic
RNA.
:. S
EXAMPLE VII
Inhibition of Leukemia In Antisense Psi RNA
Expressing Transgenlc Mice
In order to test the level of inhibition of M-
MuLV induced leukemia in antisense Psi transgenic
~A mice, littermate control and tranegenic mice were
challenged with M-MuLV at birth. Transgenic male
~; LPsias and CPsias mice (Fl hybrids of C57B6 and SJL)
, ,,
were mated to non-transgenic (C57B6/SJL) females.
Within several hours after birth each offspring from
these matings were injected intraperitoneally with 0.l
ml containing l X 105 M-MuLV infectious virions. At 4
weeks of age, a DNA sample from the tail of each mouse
pup was analyzed by slot blot hybridization and each
mouse ear notched to code transgenic and non-
transgenic offspring. At 12 to 14 weeks of age both
transgenic and control mice with sacrificed and
assayed for the presence of leukemia symptoms. Mice
were judged to be leukemic if three criterion were
met; spleen weight in excess of 0.5g, a hematocrit
value lower than 35% and typical leukemia morphology
in Giemsa-stain lymphocytes (36). In a typical
leukemia morphology the number of red blood cells
(RBC) was dramatically decreased, the shape of RBC was
abnormal, the lymphocyte cell number and size
remarkably increased, and the lymphocyte shape changed
into a malignant appearance. In a normal blood cell
morphology, the majority of the cells were red blood
cells and the RBCs were round and smooth looking.
Only one or two lymphocytes could be seen in a typical
slide. In Table III these data for each transgenic
and control mouse are shown. While significant
,,
i SUBSTITUTE SHEET
i, .
;~ ,
. . . ,~
. ~................................. . . :
WO92/17211 PCT/US92/02911
- - 39 -
~ 7 g q
percentage (33%) of the control mouse showed the
symptoms of leukemia, none of the LPPsi as or CPPsi as
-~ mice were judged to be leukemic. Numerous of the
5 control mice were obviously severely impaired, showing
typical leukemia lymphocyte morphology, containing
spleens with weights in excess of 0.5g and several had
spleens larger than l.Og (Fig. 6) while no antisense
^ 2si transgenic mice appeared abnormal prior to
; lO sacrifice or showed enlarged spleens, low abnormal
prior to sacrifice or showed enlarged spleens, low
hematocrit values or leukemic lymphocyte morphology.
The difference in spleen weight between control and
s transgenic mice could be as large as 22 times
(#13/#17).
; Thus, inhibition of packaging resulted in
retention of normal blood cell morphology and norma'
spleen weight and normal hematocrit values after viral
challenge, in dramatic contrast with the control mice.
' 20 The data presented in Table III clearly suggests
a strong inhibition of leukemia initiation which is M-
MuLV replication dependent in mice producing the
antisense RNA. Since the antisense RNA produced in
- these mice only contains sequences complementary to M-
i 25 MuLV packaging sequences and not to coding sequences
i (21), the site of interference in the viral
~? replication cycle is concluded to be the packaging
step. RNA complementary to Psi sequences appears to
be highly effective at competing with the interactions
30 between Psi and capsid protein.
EXAMPLE VIII
Inhibition of Productive Virus I fection in Vitro
in Cells Ex~ressinq Psi Antisense RNA
In order to directly study the effects of
antisense Psi RNA on viral replication in the M-MuLV
system, stable cell lines expressing CPPsias sequènces
.
SUBSTITUTE SHEET
.
:
wo 92/17211 21 a 7 7 8 q 40 - PCI/U592/OZ91 1
~ were produced and challenged with M-MuLV. While
control cells showed substantial quantities of M-MuLV
viral RNA in RNA preparations from the cell
supernatant, supernatants from cell lines expressing
antisense Psi RNA were devoid of M-MuLV genomic viral
RNA suggesting the inability of these cells to produce
functional virus containing packaged viral RNA. In
,~ spite of the lack of viral RNA in the cell
lo supernatant, significant reverse transcriptase
activity was measured by a sensitive reverse
transcriptase assay (31) (Table II) suggesting that
:: the antisense Psi RNA expressing cells are producing
; empty viral particles because of antisense blockage of
~- 15 the packaging process.
EXAMPLE IX
HYpothetical Use of Antisense Sequences Com~lementary
Packaging Sequences of HIV-l in the Treatment AIDS
~ 20 The packaging sequence of HIV-l, isolate ELI is
r,~ located within bases 200-340. There is a significant
`j3 homology between bases 280-330 of this virus and the
~1 packaging sequence of the M-MULV virus.
Oligonucleotides complementary to the packaging
; 25 sequence of the HIV-l region between pase pairs 280--
330 (in the proviral genome of HIV-l, isolate ELI) are
~ synthesized using standard techniques. The largest of
; such oligonucleotides is 50 bases in length and is
complementary to the entire 280-330 sequence.
Alternatively, a 50 base oligonucleotide containing
methylphosphonate analogues of the natural
mononucleosides is synthesized according to known
methods.
~ Doses ranging between about 500 mg and 10 grams
:~, ` 35 of these antisense oligonucleotides, having either the
.. l
; ... .
~`~, SUBSTITUTE SHEE~
. J
'',':`
,-,: ~ ',
` `,'~
. ,
WO92/17211 PCT~US92/02911
- 41 -
2 1 ~ ~ 7 8 q
~` natural or analogue nucleotides, are injected IV into
AIDS patients each day for several weeks.
~.
- 5 EXAMPLE X
HvDothetical Use of Antisense Sequences Complementary
to the
Packaainq Sequences of~ovine LeuXosis Virus
i in the Treatment of Bovine Leukemia
Q Oligonucleotides complementary to the packaging
sequence of the bovine leukosis virus (BLV) between
; base pairs 341 and 417 are synthesized using standard
techniques. The largest of such oligonucleotides is
50 bases in length and is complementary to the 355-405
sequence. Alternatively, a 2-base oligonucleotide
,
containing methylphosphonate analogues of the natural
mononucleosides is synthesi~ed according to known
methods.
Doses ranging between 500 mg and 100 grams of
these antisense oligonucleotides, having either the
natural or analogue nucleotides, are injected IV into
cows infected with the bovine leukosis virus.
:j
EXAMPLE XI
Plaaue Assay Comparing Acute Inhibitory
Effect of Various Antisense Molecules Against Mo-MLV
`I A plaque assay was conducted to determine the
relative inhibitory effect of various antisense
molecules (30 mer, 38 mer, 40 mer, 50 mer and 60 mer)
directed against the packaging sequences ~+ (69-106)
and ~ (216-570) of MO-MLV. The target sequences of
j these molecules are described below:
38mer:
from base 69 to base 106, located at 5'
3I half of U5 region.
60mer:
from base 216 to base 275 50mer:
from base 301 to base 350 40mer:
SUBSTITUTE SHEET
, ., .. ., ~ .
.. ` ~
,. . ' : . ' ~ ~ ' '
~ .
WO92/17211 PCT/US92/02911
; - 42 ~
2107~9
from base 429 to base 468 30mer:
~ from base 541 to base 570 (The base
:~ locations described above are based on the
virus genomic RNA sequence of Shinnick et
al., Nature 293:543-548 (1981).)
, The plaque assay generally followed the method of
Klement, et al., P.N.A.S. 13:753-58 (1969) and Rowe,
; et al., Virology, 42:1136-39 (1970). NIH 3T3 cells
~, 10 were inoculated into a six-well plate. The next day,
they were infected with Mo-MLV, lml of lX106 PFU/well.
After 5-6 days, the cells were irradiated with W for
30-45 secs. to limit their growth, and then Xc cells
were laid atop the NIH 3T3 cells, 0.5 x 105 cells/well.
After 3-4 days, the cells were fixed with 2ml/well of
, methanol (lmin.) and stained with lml/well hematoxylin
(304 mins). Plaques were counted under low
magnification with a dissecting microscope. Results
are shown in Figure 4.
The result of the viral plaque assay with
~ different oligonucleotides has shown that the 50mer
.' oligonucleotide has the best inhibition effect. This
means that in comparison with the control, the 50mer
oligo reduced the plaque number by 6-7 times. The
;~ 25 50mer sequence (RNA 300b-350b, 3'GACAT AGACC GCCTG
GGCAC CACCT TGACT GCTCA AGCCT TGTGG GCCGG 5')
`~ (SEQIDNO:5) comprises a sequence complementary to the
core of the Mo-MuLV packaging sequence.
Significantly, it also comprises a sequence
complementary to the core portion (19bp) of the HIV-1
packaging sequence.
Use of sense strand in a parallel procedure had
no effect, thus clearly showing that the antisense
sequence is responsible.
It is noted that at day 3 in Figure 4, there were
about 7 times the number of plaques in control cells
; ~, ..
.,
;t~i,
` SUBSTITUTE SHEET
., .
~"x ~ ~
"~
,~
WO92/17211 PCT/US92/02911
~ - 43 -
21.~ 7 ~ g 9
~; ,
j as for cells treated with the 50 mer, clearly
'~ demonstrating viral inhibition. The later convergence
~ of plaque contents was expected, as the cells were
:- 5 treated only once with the antisense molecules, which
were gradually degraded by cell nucleases. Use of
resistant analogues, or replenishing the supply of
antisense molecules by subsequent administrations or
by providing for intracellular expression of the
antisense molecule would overcome this problem.
:.:.
:~ .~.,
. . ..
. ..
i Y
: !~
. ",
:~ `
: .,.~
'~
'' 20
. ~ ~
.,.,~
~, 25
,~ .
~ 30
, . . .
: ~
, ~ .
,,
~, 35
.;
- SUBSTITUTE SHEET
. ~, ,
~,
" ~
. ` ` , . ~ . `
~ WO92/~7211 PCT/~S92/02911
` ~ 2 1 0 '7 '7 ~ q ~ 44 ~ ,.
Table I
Animal Retroviruses
Avian Erthyroblastosis Virus
~vian Leukosis Virus (or Lymphoid Leukosis virus)
, 5 Avian Myeloblastosis Virus
~; Baboon Endogenous Virus
,", Bovine Leukemia Virus
''~.' Bovine Syncytial Virus
~, Caprine Encephalitis-Arthritis Virus (or Goat
~ Leukoencephalitis Virus)
.. s Avian Myelocytomatosis virus
~ lO Corn Snake Retrovirus Chicken Syncytial virus
.~ Duck Infectious Anemia Virus
.~ Deer Kidney Virus
:~. Equine Dermal Fibrosarcoma Virus
:j~ Equine Infectious Anemia Virus
Esh Sarcoma Virus
~: Feline Leukemia Virus
.~ Feline Sarcoma Virus
. 15 Feline Syncytium-forming virus
Fujinami Sarcoma Virus
Gibbon Ape Leukemia Virus (or Simian Lymphoma Virus or
~:. Simian Myelogenous Leukemia Virus)
, Golden Pheasant Virus
. Lymphoproliferative Disease Virus
J Myeloblastosis-associated Virus
20 Myelocytomatosis Virus
-. Mink Cell Focus-Inducing Virus
' Myelocytomatosis Virus 13
Mink Leukemia Virus
i Murine Leukemia Virus
;.~. Mouse Mammary Tumor Virus
; Mason-Pfizer Monkey Virus
~, Murine Sarcoma Virus
j~ 25 Myeloid LeUkemia Virus
:1 Myelocytomatosis Virus
1, . Progressive Pneumonia virus
Rat Leukemia Virus Rat~Sarcoma Virus
:~ Rous-Associated Virus 0
:i Rous-Associated Virus 60
3 Rous-Associated Virus 61
,' 30 Reticuloendotheliosis-Associated Virus
Reticuloendotheliosis Virus
. Reticuloendotheliosis Virus-Transforming
.~ Ring-Necked Pheasant Virus
~ Rous Sarcoma Virus
:~ Simian Foamy Virus
;? Spleen Focus-Forming Virus
Squirrel Monkey Retrovirus
;~ 35 Spleen Necrosis Virus
~ Sheep Pulmonary Adenomatosis/Carcinoma Virus
f
,,
.f
SUBSTITUTE SHEET
.
~: .
.~ :
~ WO92/17211 PCT/US92/02911
452 I ~ r~ ~7 ~
..~
~;
. Simian Sarcoma-Associated Virus (or Wooly Monkey
-~ Leukemia Virus)
Simian sarcoma virus (or Wooly Monkey Virus).
. 5TABLE II
~;~ REvERSE TRANSCRIPTASE (RT) ASSAY
. Infecting RT Activity in
, Cell Line Cell/ml Viru~ Titer ~PFU) Supernatant
0 NIH 3T3 lo6 5 X 105 100.0
~'~ Clone 9-5 lo6 5 X 105 39,7
,~ Clone 11-2 105 5 X 105 32.5
,~ Clone 12-6 106 5 X 105 40.1
,.,f~ *In arbitrary units (assay value of normal mouse NIH
,-~ 15 3T3 cells set to lOO)
.:
,l,
`
~~ 20
, x
.
.,~
: 25
,;.,,~ .
'~
.1
, .,
A 30
, ~
.i,
. .~ .
~ 35
';'
.~
... .
SUBSTITUTE SHEET
.il
-
~
, .
. ~ . . ~ - . ,
-- . . . .
, ` . , ~ . .
~- - .
':': ~ ,: . , ~ .~- . ,
W092/172~1 PCT/US92/02911
- 46 -
`~ 21~ l789
TABLE III
~Incidence of leukemia in control and anti-sense w
transgenic mice challenged with Maloney murine
,leukemia virus
Leukemia
~ou~e Tran3genic Hematocrit Weiyht(g) LYmhcyte Leukemla
. 1 NON 49% 0.19
~ 2 NON 33% 0.25
3 NON 49% 0.15
4 NON 46% 0.16
-. 5 NON 43% 0.13
6 NON 35% 0.63
7 NON 35% 0.18
8 NON 42% 0.13
15 9~ NON --- 1.02
~.~ 10 NON 10% 1. 32
:~ 11 NON 49~ 0.12
:~ 12 NON 46% 0.08
,~J 13 NON 30% 1.76
$ 20 18 NON 48% 0 11
19 NON 49% 0 18
22 NON 50% 0.08
`~ 23* NON --- 0. 51
~,~ 24~ NON --- 0. 86
29 NON 39% 0.14
:~' 31 NON 42% 0.13
32 NON 47% 0.11
~y 33 NON 50% 0.11
NON 35% 1.05
~j 36 NON 42% 0.13
'~ 3 7 NON 47% 0.11
38 NON 47% 0.11
39 NON 42% 0.11
NON 31% 0. 87
~ 35 NON 21% 0. 77
.~ 43 NON 48% 0.13
~ 44 NON 35% 0.78
SUBSTITUTE SHEET
. .
(
. : : -
. ,
;, : ~ , .
- ~ .. . .
: `
~ . WO 92/17211 PCltUS92/02911
7 7 ~ q
.,'. .
: Leukemia
Mou~e Transgenic Spleen Liymhocyte Leukemia
~ No. Status Hematocrit Wei~ht(g) ~orphology Pathology
;~ 45 NON 52~ 0.12
48 NON 41~ 0.11
~ 49 NON 49$ 0.18
"~ 52 NON 20~ 0.97 +++1 #
NON 48% 0.11
~ 14 NON 45% 0.lS
:.',. 10 15 CWa~ 46% 0.13
'~ 16 CWas 54% 0.09
17 CWas 45% 0.08
.~
CWas 48% 0.11
21 CWas 47% 0.11
CWas 55% 0.15
26 CWas 46% 0.12
27 CWas 49% 0.10
28 CWas 44% 0.10
CWas 43~ 0.14
34 CWas 48% 0.12
42 CWas 49% 0.14
46 LWas 42% 0.17
'~1
47 LWa~ 46% 0.25
LWas 49% 0.19
51 LWas 48% 0.18
53 LWas 48% 0.17
54 LWas 50% 0.12
LWas ~49% 0.19
56 LWas 49% 0.16
57 LWas 40% 0.09
59 LWa~ 49% 0.13
~; 61 LWas 46% 0.09
62 LWas 44% 0.12
63 . LWas 51% 0.14
64 LWas 46~ 0.13
* died a few hours prior to evaluation
~ definitive leukemia
'
y
.~. ` SUBSTITUTE SHEET
., .
'':~ ' . ~
; wos2/272ll PCT/US92/02911
21~77~9 - 48 ~
' . .
~able IV: -
Table of Packaging Sequences:
~ Each entry includes both a reference for the
,`~!,~ 5 published genomic sequence generally and a reference
for the location of the packaging sequence within the
-~ genome.
1. Reticuloendotlieliosis ~irus (Rev)
Genome: Wilhelmsen, et al. J. Virol. 52:172-182
-~i (1984). bases 1-3149; Shimotohno, et al. Nature
285:550-554 (1980). bases 3150-3607. Packaginq
; Sequence (~):144-base between the Kpn I site at 0.676
kbp and 0.820 kbp relative to the 51 end of the
i' provirus.
~ J. Embretson and H. Temin J. Virol. 61(9):2675-
`: 2683 (1987).
. ,
2. Human immunodeficiency virus type 1 (IIIV-l)
Genome Gallo et al. Science 224:500-503 (1984) .
Packa~inq sequence (~):19 base pairs between the 5'
~ LTR and the gag gene inîtiation codon. A. Lever, J.
:~ Virol. 63(9):4085-4087 (1989).
s 3. Moloney murine leukemia virus (Mo-MuLV)
; Genome: Shinnick, et al. Nature 293:543-548 (1981).
~ 20 Packaainq sequence (~):350 nucleotides between the
; splice site and the AUG site for coding sequence of
~; gag protein. R. Mann, R. Mulligan and D. Baltimore,
-~ Cell 33:153-159 (1983). Second ~ackaqina sequence
):Only in the 5' half of the U5 region.
J. Murphy and S. Goff, J. Virol. 63(1):319-327 (1989).
.4
~, 4. Avian sarcoma virus (ASV)
;~ 25 Genome: Neckameyer and - Wang J. Virol. 53:879-884
(1985). Packaging sequence (~):150 base pairs between
300 and 600 bases from the left (gag-pol) end of the
provirus. P. Shank and M. Linial, J. Virol.
36(2):450-456 (1980).
5. Rous sarcoma virus (RSV)
Genome: Schwartz, et al. Cell 32:853-869 (1983) .
Packaaing sequence (~):230 base pairs from 120-base
tPB site beginning) to 22-base before gag start codon.
S. Kawai and T. Koyama (1984), J. Virol. 51:147-153.
6. Bovine leukosis virus (BLV)
Genome: Couez, et al. J. Vlrol. 49:615-620, 1984,
`~ bases 1-341; Rice, et al. Virology 142:357-377, 1985,
bases 1-4680; Sagata, et al. Proc. Natl. Acad.
Sci. 82:677-681, 1985, complete BLV provirus.
Packaging sequence: the present inventors predict hat
, i
;-.
SUBSI-ITUTE SHEET
:
d,~
:~ ' ~ ' ' ' . ' '
,' . :
:'' :
:
~'WO92~17211 PCT/US92/02911
-
2 i 0 ~ 7 8C~
~j~it lies between the end of the primer bindin~ site at
.;~."about base 340 and the initiation codon for qa~ at
:~1about base 41-8.
'' 3~j 5
,,~,
i~ r
~ 10 ,
l ~j
:, 15
~r
;~ 20
,~, .
~ Z~5
~ .
:,~ ~.
; 1 .
~,35
'
~,y
~1 ~SUBSTITUTE SHEET
. . .
~' : ' . ~ `' '
~ WO92/172]l 5 PCT/US92/0291l
~ ~ o~
REFERENCES
l. Weiss, R. et al. (eds), RNA_Tumor Viruses .
"Molecular Biology of Tumor Viruses, Cold Spring
Harbor Laboratory, Cold Spring Harbor, N.Y., l9B4.
~ 2. Cobrinik, D. et al., 1987. Avian sarcoma and
-~ leukosis virus pol- Endonuclease recognition of the
. tandem long terminal repeat junction: minimum site
required for cleavage is also required for viral
. 10 growth. J. Virol. 61:1999.2008.
3. Barklis, E., R.C. Mulligan, and R. Jaenisch.
~x 1986. Chromosomal position or virus mutation permits
retrovirus expression in embryonal carcinoma cells.
Cell 47:391-399.
4. ~ann, R. and D. Baltimore. 1985. Varying the
.'5 position of a retrovirus packaging sequence results in
the encapsidation of both unspliced and spliced RNAS.
J. Virol. 54:401-407.
5. Varmus, H. 1987. Reverse Transcription.
Sci. Amer. 56-64.
6. Cone, R.D., A. Weber-Benarous, D. Baorto and
R.C. Mulligan. 1987. Regulated expression of the
3 complete human ~-globin gene encoded by a
transmissible retrovirus vector. Mol. Cell. Biol.
25 7:887-897.
7. Freifelder, D. 1987. Molecular BioloqY:
EukaEY~_ Viruses, Jones and Bartlett Publishers,
Inc., Boston Portola Valley, 2nd ed.
8. Temin, H.M. 1972. RNA-directed DNA
30 synthesis. Sci. Amer.
9. Gilboa, E., S.W. Mitra, S. Goff and D.
Baltimore. 1979. A detailed model of reverse
transcription and tests of crucial aspects. Cell
8:93-100.
10. Conie, R., and R. Mulligan. 1984. High- -
efficiency gene transfer into mammalian cells:
.~
`!
SUBSTITUTE SHEET
... .
.
, .
~ WO92/1~211 PCT/US92/0~911
~7~ ;
. .
Generation of helper-free recombinant retrovirus with
` broad mammalian host range. Proc. Natl. Acad. Sci.
USA 8I:6349-6353.
~ 5 11. Mann, R., R.C. Mulligan, and D. Baltimore.
-~ 1983. construction of a retrovirus packaging mutant
and its use to produce helper-free, defective
retrovirus. Cell 33:153-159.
12. Shank, P.R., and M. Linial. 1980. Avian
~- 10 oncovirus mutant (SE21Qlb) deficient in genomic RNA:
characterization of a deletion in the provirus. J.
Virol. 36: 450-456 .
13. Bender, M.A., T.D. Palmer, R.E. Gelinas, and
A.D. MilIer. 1987. Evidence that the packaging signal
f m Moloney murine leukemia virus extends into the
~; gag region. J. Virol. 61: 639-1646.
14. Weintraub, H., J.G. Izant, and R.M. Harland.
1985. Antisense RNA as a molecular tool for genetic
~ analysis. Trends in Genetics 1:22-25.
i~ 20 15. Travers, A. 1984. Regulation by antisense
~ RNA. Nature 311:416.
,~ 16. Izant, J.G., and H. Weintraub. 1985.
Constitutive and conditional suppression of exogenous
and endogenous genes by antisense RNA. Science
25 229:34S-352.
17. Inouye, M. 1988. Antisense RNA: its
functions and applications in gene regulation - a
~-~ review Gene 72:25-34.
~l 18. Yokoyama, K. and F. Imamoto. 1987.
Transcriptional control of the endogenous MYC
protooncogene by antisense RNA- Proc. Natl. Acad.
Sci. USA 84:7363-7367.
19. Mizumo, T., M.Y. Chou, and M. Inouye. 1984.
;cl A unique mechanism regulating gene expression:
~ 35 Translational inhibition by a complementary RNA
., .
.
j
j SUBSTITUTE SHEEr
-, :
Wo92J17211 ~1 a 7 7~q 52 - PCT/US92/02911
transcript (micRNA). Proc. Natl. Acad. Sci. USA
- 81:1966-1970.
- 20. Chang, L.J., and C.M. Stoltzfus. 1987.
Inhibition of rous sarcoma virus replication of
antisense RNA. J. Virol. 61:921-924.
21. Korman, A.J., J.D. Frantz, J.L. Strominger,
and R.C. Mulligan. 1987. Expressions of human class
II major histocompatibility complex antigens using
.i 10 retrovirus vectors. Proc. Natl. Acad. Sci. USA
84:2150-2154.27.
22. Hayes, 1989. Ph. D. Dissertation, Ohio
~' university.
2! 23. Pasleau, F., M.J. Tocci, F. Leung, and J.J.
Kopchick. 1985. Growth hormone gene expression in
eukaryotic cells directed by the Rous sarcoma virus
long terminal repeat or cytomegalovirus immediate-
-x, early promoter. Gene 38:227-232.
'~t~ 24. Wagner, T., P. Hoppe, J. Jollick, D. Scholl,
R. Hodinka, and J. Gault. 1981. Microinjection of a
~ rabbit Bglobin gene into zygotes and its subsequent
;i expression in adult mice and their offspring. Proc.
Natl. Acad. Sci USA 78:6376-6380.
25. McGrane, M., J. de Vente, J. Yun, J. Bloom,
E. Parks, A. Wynshaw-Boris, T. Wagner, F. Rottman, R.
Hanson. 1988. Tissue-specific expression and dietary
regulation of a chimeric phosphoenolpyruvate
carboxykinase~bovine growth hormone gene in transgenic
mice. J. Biol Chem. 263:11443-11451.
26. Lopata, M.A., Cleveland, D.W. and Sollner-
Webb, B. 1984. High level transient expression of a
cliloramphenicol acetyl transferase gene by DEAE-
dextran mediated DNA transfection coupled with a
dimethyl sulfoxide or glycerol shock treatment. Nucl.
Acids Res. 12:5707- 5717.
J
.
SVBSTITUTE SHEET
: - , . . ~ -`
.
`.~ WO92~17211 ~ 78q PCl/U~i92/02911
. ` i . -- 5 3 --
.` ` .
~,;
27. Southern, E~M. 1975. Detection of specific
sequences among DNA fragments separated by gel
- electrophoresis. J. Mol. Biol. 98:503-507.
28. Chomczynski, P. and N. Sacchi. 1987.
- Singlestep method of RNA isolation by acid guanidinium
thiocyanatephenol-chloroform extraction. Anal.
Biochem. 162:156-159.
29. Bain, B. and K. Pshyk. 1972. Enhanced
reactivity in mixed leukocyte cultures after
separation of mononuclear cells on Ficoll-Hypaque.
Trans~lant. Proc. 4:163-164.
30. Thomas, P. 1980. Hybridization of denatured
: RNA and small DNA fragments transferred to
nitrocellulose. Proc. Natl. Acad. Sci. USA 77:5201-
5205.
. 31. Goff, S., P. Traktman and D. Baltimore.
1981. Isolation and properties of Moloney murine
leukemia virus mutants: Use of a rapid assay for
release of viral reverse transcriptase. J. Virol.
-$ 38:239-248.
32. Steinberg, R.M., D.R. Thomsen, and M.F.
Stinski. 1984. Structural analysis of the major
,; immediate early gene of human cytomegalovirus. J.
- ~,? 25 Virol. 49:190-199
33. Goodwin, E.C. and F.M. Rottman. 1986.
~i Characterization of the minimal bovine and growth
hormone polyadenylation signal. J. Cell ~iochem.
Su~l. l0:170.
'~ 30 34. Roizman, B. (ed.), "The Biology of
Cytomegaloviruses," In: The Herpes Virus, New York,
Plenum Press, 1983.
35. Shields, A., Witte, O.N., Rothenberg, R.,
~ and Baltimore, D. 1978. fligh frequency of aberrant
.,; 35 expression of Moloney murine leukemia virus in clonal
~ infections. Cell 14:601-609.
~5
SUBSTITUTE SHEET
:.
: .
"~
. . ' ~ - . ` , ' -,
.,-: : . . ~ . ; -
,,1
.. - .
. WO 92/1721 I PCr/USg2/0291 1
5 4 ~
,'''' ~ 2~7gq ~' ' ;
~,
` 36. Ruscetti, s., L. Davis, J. Feild, and A.
Oliff. 1981. Friend murine leukemia virus-induced
leukemia is associated witli the formation of mink
~, 5 cell focus-inducing viruses and is blocked in mice
expressing endogenous mink cell focus-inducing
,~ xenotropic viral envelope genes. J. Exp. Med.
54:907-920.
37. Smith, C.C., O.P. Tslo Paul and P.S. Miller
Proc. Natl. Acad. Sci. USA 83:2787-2791 (1986)
(complementary oligonucleotide methods used in
;~ antiviral research)
~' 38. Ruden, T. and E. Gilboa J. Virol 63:677-682
~ (1989)
; 15 The references cited in this specification are
all incorporated by reference~herein, whether
specifically incorporated or not.
While this invention has been described in
- connection with specific embodiments thereof, it will
be understood that it is capable of further
`j modifications. This application is intended to cover
any variations, uses, or adaptations of the inventions
following, in general, the principles of the invention
x and including such departures from the present
disclosure as come within known or customary practice
within the art to which the invention pertains and as
may be applied to the essential features hereinbefore
set forth as follows in the scope of the appended
claims.
,:
:~,
.,.i
~ 35
~ .!
;~
:'i
SUBSTITIJTE SHEET
:.j
"~
; .
: ' - ' :'' ` ' ' - . '''. ` - ':
.