Note: Descriptions are shown in the official language in which they were submitted.
7 ~ ~
HAIR COLORANTS WITH SUBSTANTIVE DYES
This invention relates to hair-dyeing preparations containing at least two
different substantive hair dyes which are distinguished by particularly uniform dye
absorption, even on damaged hair.
In addition to oxidation dyes, which are formed through the oxidative coupling
s of one or more primary intermediate components with one another or with one or
more coupler components, substantive hair dyes play a particularly important part in
the dyeing of hair. Substantive hair dyes have the advantage that they may be used
without the addition of oxidizing agents. The substanLive dyes used are primarily
compounds belonging to the group of nitrobenzene derivatives.
o Good hair-dyeing preparations have to form the desired shades of color in suf-
2 ~ 7 ~ ~
~Icient intensity. In addition, they must readily be absorbed by human hair without
excessive staining of the scalp. The hair colors produced with them must show high
stability to light, heat, perspiration, shampoos and ~he chemicals used in the permanent
waving of hair. Finally, ~hey should be both toxicologically and dcrmatologically safe.
5 Unfortunately, many substantive hair dyes have the disadvantage that they are un
evenly absorbed onto the hair, the more seriously damaged hair ends generally being
colored more intensively than the younger and less damaged hair roots. For this rea-
son, many basically good dyes are unsuitable for practical application.
It has now been found that combinations of two or more substantive dyes pro-
10 vide for the much more uniforrn dyeing even of hair which has been damaged by re-
ductive and oxidative treatments, for example by permanent waving and bleaching, if
the combination contains at least one nitrodiphenylamine dye corresponding to formula
1.
Accordingly, the present invention relates to hair-dyeing preparations contain-
15 ing at least two substantive dyes in a cosmetic carrier, characterized in that they con-
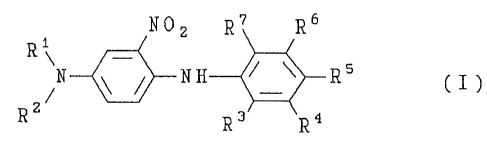
tain a first substantive dye (A) corresponding to forrnula I:
N2 R7 R6
R~ R; ( I )
25 in which R~ and R2 are hydrogen, C,4 alkyl groups or C2, hydroxyalkyl groups and
one of the moieties R3 to R7 is an -SO3H or a -COOH group and the other moietiesare hydrogen, chlorine, Cl.,, alkyl groups, C" alkoxy groups or groups of the formula
-NR8R9, where R8 and R9 are hydrogen, C,~ alkyl groups, C2 ~ hydroxyalkyl groupsor, together with the nitrogen atom, forrn a piperidine, morpholine, piperazine or
30 pyrrolidine ring,
or one of its water-soluble salts and at least one second substantive dye (B) having a
different stNcture.
The substantive dyes (A) corresponding to forrnula I are known from European
Patent Application EP 0 280 187 Al. Through the presence of these dyes, the uni-
~s forrnity of absorption of the many other substantive dyes is distinctly better than could
21~7~3~
,~'` ~
have been expec[ed from the absorption of the individual dyes of lhe combination.
In most cases, lhe combination of dye (A) corresponding to formula I and a sccond
dye (B) shows more uniform absorption than the components (A) and (B) on their
own. At the vcry least, however, the uniformity of dye absorption is higher than cor-
s responds to the mixture rule.
Substantive dyes varying in structure may be used as the second dye (B). Sub-
stantive dyes from the group of aromatic nitro compounds or anthraquinone com-
pounds are particularly preferred. However, the effect according to the invention of
improved uniformity of absorption by combinaticn with dyes corresponding to formula
10 I is also observed with other substantive dyes, for example azo dyes, triphenylmethane
dyes or indophenols.
Particularly suitable substantive dyes (B) are mentioned in the Exarnples, par-
ticularly under B1 to B9.
Particularly suitable substantive nitrodiphenylamine dyes (A) corresponding to
formula I are
A1: 2-nitro-4-aminodiphenylamine-2'-carboxylic acid and
A2: 2-nitro-4-amino-4'-dimethylaminodiphenylamine-2'-
carboxylic acid.
Further examples can be found in European Patent Application EP 0 280 187
20 Al.
The hair-dyeing preparations according to the invention are also distinguished
by improved fastness to washing. Hair colors produced with a combina~ion of a nitro-
diphenylamine dye (A) corresponding to formula I and a second substantive dye (B)
of a different structure are lightened to a lesser extent after washing of the hair than
26 hair colors obtained with dye (B) in the absence of a dye corresponding to formula
I.
The hair-dyeing preparations according to the invention may also contain two
different dyes (A) of formula I and one or more other dyes (B).
Finally, the hair-dyeing preparations according to the invention may also con-
30 tain oxidalion dye precursors.
To produce the hair-dyeing preparations according to the invention, the substan-tive hair dyes (A) corresponding to formula I and the other dyes (B) are incorporated
2:~77~
,,,
in a suitable cosmetic carrier, for example in creams, emulsions, gels or even surfact-
ant-containing foaming solutions, for example in sham~oos, foarn aerosols or other
preparations suitable for application to the hair.
Standard ingredients of cosmetic preparations such as these are, for example,
wetting agents and emulsifiers, such as anionic, nonionic, cationic, zwitterionic and
ampholytic surfac~n~. Preferred surfactants are, for example, fatty alcohol sulfates,
fatty alcohol polyglycol ether sulfa~.es, n-alkanesulfonates, a-olefln sulfonates, ethylene
oxide adducts with fatty amines, with fatty acids, with fatty alcohols, with aL~yl phe-
nols, with fatty acid partial glycerides, with sorbitan fatty acid esters and with fatty
o acid aL~anolarnides.
Other standard ingredients are water-soluble thickeners such as, for example,
mcthyl or hydroxyethyl cellulose, starch and water-soluble starch derivatives, biopol-
ymers, water-soluble synthetic polymers and copolymers, for example those of acrylic
and methacrylic acid. For formulation as dyeing creams, fatty components such as,
15 for example, fatty alcohols, fatty acids, fatty acid partial glycerides, esters, paraffins
and waxes in emulsified forrn are used as carriers. In addition, the hair-dyeing prepa-
rations may contain known hair-care additives such as, for example, water-soluble cat-
ionic polymers, water-soluble proteins and protein derivatives, pantothenic acid, vita-
mins, plant extracts and fragrances, pH regulators (buffers), electrolytes and water.
The constituents of the carrier are used in the usual quantities for producing
the hair-dyeing preparations according to the invention. For example, wetting agents
and emulsifiers are used in quantities of 0.5 to 30 % by weight while thickeners are
used in concentrations of 0.1 to 10 % by weight, based on the hair-dyeing preparation
as a whole.
In one preferred embodiment, the carrier is an emulsion containing 1 to 5 %
by weight of a Cl~.22 fatty alcohol, 5 to 20 % by weight of an adduct of 5 to 20 moles
of ethylene oxide with 1 mole of a linear, terminal C,2.22 alkyl amine, based on the
weight of the hair-dyeing preparation as a whole.
The substantive dyes (A and B) are used in the hair-dyeing preparations ac-
20 cording to the invention in a total quantity of 0.05 to 5 % by weight and preferably
in a total quantity of 0.2 to 2 % by weight, based on the hair-dyeing preparation as
a whole. The quantity ratio of the dyes tA) corresponding to forrnula I to the dyes
21~779~
(~) of different structure may bc in the range from 1:9 to 9:1 and is pre~rably in ~he
range from 3:7 to 7:3.
Where the hair-dyeing preparation according to the invention conlaillS oxidationdye precursors, it is advisable addi~ionally to incorpor~e a small quantity of a rcduc-
ing agent, for example 0.5 to 2.0 % by weigh~ of sodium sulfite, to stabilizc thc oxida-
tion dye precursors. In this case, an oxidizing agent is added to the hair-dyeing prepa-
ration before it is applied to initiate the oxidative development of the oxidation dye
precursors. Suitable oxidizing agents are, in particular, hydrogen peroxide or adducts
thereof with urea, melamine or sodium borate and also mixtures of such hydrogen per-
oxide adducts with potassium peroxysulfate.
Irrespective of the nature of the cosmetic composition, the hair-dyeing prepara-tions according to the invention may be used, for example, in the form of creams, gels
or shampoos in a mildly acidic, neutral or alkaline medium. The hair-dyeing prepara-
tions are preferably used at a pH value in the range from 6 to 10. They may be ap-
plied at temperatures in the range from 15 to 40 C. After a contact time of about
30 minutes, the hair-dyeing preparation is removed by rinsing out from the hair to be
dyed. The hair is then rewashed in a mild shampoo and dried. There is no need for
rewashing with a shampoo in cases where a carrier of high surfactant content, for ex-
ample a dye shampoo, has been used.
Hair colors of high intensity and good fastness properties, including in particu-
lar good fastness to washing and high stability to fading and changes in color during
shampooing, can be obtained with the hair-dyeing preparations according to the inven-
tion. The following examples are intended to illustrate the invention without limiting
it in any way.
~E Examples
1. Improving levelling power
1.1 Production of the hair-dyeing preparations
Hair-dyeing preparations of the following composition were prepared:
Tallow al~yl amine(C8-CI8)polyglycol (8 EO) ether 9.0 g
so Oleic acid polyglycerol ester 2.5 g
Ce~yVoleyl alcohol polyglycol ether (5 EO) 3.0 g
2:~77g6
-~ 1,3-Propylene glycol 5.0 g
Substantive dye (or combination of substantivc dyes) 1.0 g
Fragrance 0-3 g
(NH.,)2SO4 1.0 g
Ammonia (conc. in H2O) to pH = 9
Water ad 100 g
The followin~ dyes were used as the substan~ive dyes corresponding to formula
I:
A1 : 2-nitro-4-amino-2'-carboxydiphenylamine
A2 : 2-nitro-4-amino-2'-carboxy-4'-dimethylaminodiphenylamine
The following other substantive dyes were used:
B I : 1,2,3,4-tetrahydro-6-nitroquinoxaline
B 2 : picrarnic acid (2-amino-4,6-dinitrophenol)
B 3 : 1-amino-2-nitro-4-(2,3-dihydroxypropylamino)-5-chlorobenzene
B 4 : 1-amino-2-nitro-4-(2-hydroxyethylamino)-5-chlorobenzene
B 5 : 1,4-bis-(B-hydroxyethylamino)-2-nitrobenzene
B 6 : 4-(3-hydroxypropyl)-amino-3-nitrophenol
B 7 : 1-(2-hydroxyethyl)-amino-4-methyl-2-nitrobenzene
B 8 : 6-chloro-4-nitro-2-aminophenol
B 9 : 1-(2-hydroxyethyl)-amino-2-nitro-4-bis-(2-hydroxyethyl)-
aminobenzene
B 10 : 1-(2-hydroxyethyl)-amino-2-nitro-4-aminobenzene
B 11 : 2-nitro-p-phenylenediamine
B12 : 1-amino-2-nitro-4-bis-(2-hydroxyethyl)-aminobenzene
B13 : 2-amino-4-nitrophenol
B 14 : 1 -amino-2-(2-hydroxyethyl)-amino-5-nitrobenzene
B 15 : 1-amino-2-nitro-4-(2-hydroxyethyl)-aminobenzene
B16 : 1,4,5,8-tetraminoanthraquinone
B 17 : 1-amino-4-methylaminoanthraquinone
B18 : 1,4-diamino-5-nitroanthraquinone
B 19 : 1-methylamino-4-(2-hydroxyethyl)-arninoanthraquinone
B20 : 1,4-diaminoanthraquinone
.~ . . .
~ L ~ 7 r~ ~ ~
B21 : 1-(4'-bis-(2-hydroxye~hyl)-amino)-phenylazo-4'-aminoben-~ene
1.2 Hair dyeing
Grey hair tresses weighing 2 g for a length of 16 to 18 cm were treale~ at the
s hair ends (upper half) with a cold wave preparation (aqueous solution of ammonium
thioglycolate) for 30 minutcs at 27 C, rinsed with warm water, treaLed with 10 ml of
a fixing solution (potassium bromate solution) and rerinsed. The same half was then
bleached for 30 minutes at 27 C with an aqueous preparation of hydrogen peroxide
and ammonium peroxydisulfate. It was then trealed once more with the cold-wave
preparation and the fixing solution. Finally, the tresses as a whole were bleached
once. In this way, two regions damaged to different extents were obtained, namely
seriously damaged hair ends and less damaged hair roots.
The tresses thus pretreated were dyed with the hair-dyeing preparations accord-
ing to 1.1, the dyes or dye combinations listed in Table I being used as the substantive
dyes. The dyeing solutions were left on the hair tresses for about 20 minutes at 27
C, subsequently washed out with a typical shampoo, rinsed with water and dried.
1.3 Determination of uniformity of dyeing (color difference values (DE) between
hair ends and hair roots)
Each hair tress was measured at eight places (4 in the region of the hair roots
and 4 in the region of the hair ends) using a Datacolor color measuring system~ To
this cnd, the sample to be measured was fixed to the spectral photometer in a clamp
and the reflectance spectrometry values were measured over the visible light range
from 390 to 700 nm at intervals of 10 nm and processed in a computer (HP 2113 E
Minicomputer). The computer program determined the standard color values in ac-
cordance with DIN 5033 corresponding to the CIE (Commission Internationale de
l'Eclairage) systern and converted them into color differences according to DIN 6174.
The color difference values (DE) for hair colors obtained with individual dyes
and with dye combinations according to the invention are shown in Table I. The color
difference values of the hair colors obtained with the dye combinations according to
the invention (substantive dye A + substantive dye B) are also shown in the Table.
h 1 ~3 i [~1)
;
Table I
~ Color dif-
Substantive dye, Color tone ference
quantity Hair end Hair xoot DE
Al : 1 g Grey-red Violet-brown 12.57
A2 : 1 g Grey-indigo Indigo 21.1
B1 : 1 g Brown-red Orange 18.35
B1 (0.5g) + Al (0.5g) Red-brcwn R~d-brcwn 6.25
B2 : 1 g Tight orange- Brown-orange 10.2
brown
B2 (0.5g) + Al ~0.5g) Red-brownRed-brown 6.14
B3 : 1 g Red-brcwn Grey-red 9.4
B3 (0.5g) + Al (0.5g) Garnet-brown Grey-red 2.8
E4 : 1 g Raspberry red Brcwn-red 5.8
B4 (0.5g) + Al (0.5g) Red-brcwnGrey-red 5.0
B5 : 1 g Dark purple Grey-magenta 16.7
B5 (0.5g) + Al (0.5g) Ruby Grey-red 10.5
_
B5 : 1 g Dark purple Grey-magenta 16.7
B5 (0.5g) + A2 (0.5g) Grey-magenta Magenta 4.8
B6 : 1 g Coral red Lobster red 4.3
B6 (0.5g) + Al (0.5g) Pcmpeian red Grey red 3.5
B7 : 1 g Yellow-orange Buttercup11.8
yellow
B7 (0.5g) + Al (0.5g) Cinnamon brown Fox red 12.7
_
B8 : 1 g Red-orange Melon orange 18.1
B8 (0.5g) + Al (0.5g) terra di Siena Copper red 8.4
B9 : 1 g Grey-magenta Grey-ruby 5.75
B9 (0.5g) + Al (0.5g) Grey-ru~yDull violet 5.80
B10 : 1 g Dull red Grey-red 12.56
B10 (0.5g) + Al (0.5g) Violet-brown Violet-brown 3.5
l .
B11 : 1 g Red-brown Orange-brcwn 19.9
B11 (0.5g) ~ Al (0.5g) Brown-red Brown-red 3.88
. ......
B12 : 1 g Grey-magenta Light krcwn 11.75
B12 (0.5g) ~ Al (0.5g) Grey-red-brcwn Red-brown 8.87
50 ___ .......... . .
B13 : 1 g Brick red Yellow-orange 10.0
B13 (0.5g) + Al (0.5g) Brown-orange Henna red _
2 ~
.i
Tablel(conti)l~ed)
Color dif-
Sub6tantive dye, Color tone ference
s quantity Hair end Hair root DE
B14 : 1 g Brown-orange Yellcw 13.64
Bl4 (0.5g) ~ Al (0-5g) Orange-brown Orange-krown 10.2
to B15 : 1 g ~ed-brown Dull red- 8.9
brown
B15 (0.5g) ~ Al (0.5g) Red-brown Red-brown 8.4
B16 : 1 g Grey-green Dull green9.15
B16 t0.5g) + Al (0.5g) Grey-ruby Dull ruby 10.6
B17 : 1 g Purple-grey Dull violet 17.65
B17 (0.5g) + Al (0.5g) Grey-red Grey-red 6.6
B18 : 1 g Silver grey Violet-grey11.2
B18 (0.5g) + Al (0.5g) Grey-red Grey-red 2.30
B19 : 1 g Grey-green GFey-tur- 19.9
quoise
B19 (0.5g) + Al (0.5g) Grey-ruby Grey-ruby 7.74
B20 : 1 g Grey-ruby Grey-magenta 9.01
B20 (0.5g) + Al (0.5g) Grey-red-brown Red-brcwn 2.0
~o B21 : 1 g Yellcw Orange-yellow 10.0
B21 (0.5g) + Al (0.5g) Light brown Light brown 7.3
Al (0.33 g)
B10 (0.33 g) Brown-violet Brcwn-violet 5.0
B17 (0.33 g)
Al (0,33 g)
B1 (0.33 g) lerracotta Red-brown 2.0
B4 (0.33 g)
Al (0.33 g)
B1 (0.33 g) Red-brown Red-brown 3.9
B5 (0.33 g)
.
Al ~0.33 g)
B5 (0.33 g) Violet-brown Violet-brown 2.4
B8 (0.33 g)
Al (0.25 g)
~2 (0.25 g)
B5 (0.25 g) Grey-magenta Grey-ruby3.8
B9 (0.25 g)