Note: Descriptions are shown in the official language in which they were submitted.
-~ ~10893~
.j - 1-
'i
4-19330/~
Antiretrovirnl ncvl c~om~
The invention relates to novel antiretroviral compounds, to processes for the preparadon
of those compounds, to pharmaceudcal compositions comprising those compounds, tothose compounds for use in a therapeutic method of treating the human or animal body
Md to the use of those compounds for the therapeutic treatment of the human or animal
body or for the preparation of pharmaceutical compositions.
According to modern science, a whole range of diseases are caused by retroviruses.
As far as is known at present, AIDS is a disease of the immune system caused by the
retrovirus HIV ("Human Immunodeficiency Virus"). According to esdmates by the
WHO, the disease affects about 10 million people, is continuing to spread and in virtually
all cases results in the death of the patient.
Hitherto the retroviruses HrV- 1 and HIV-2 (HIV representing "Human Immunodeficiency
Virus") have been idendfied as a cause of the disease and they have been characterised by
molecular biology. From the point of view of treatment, in addition to previous limited
ways of mitigating the symptoms of AIDS and certain preventive measures, there is
particular interest in the search for compositions that interfere with the reproduction of the
virus itself but do not damage the intact cells and tissues of the patient.
Retroviral protease is a proteolytic enzyme that, owing to an aspartate residue in the active
centre, is regarded as an aspartate protease and participates in the maturation of new
infectious vilions in infected cells in the reproductive cyclei of a number of retroviruses.
For example, HIV-1 and HIV-2 each have in their genome a region that codes for a"gag-protease". That "gag-protease" is responsible for the correct proteolytic cleavage of
the precursor proteins that are produced from the genome regions coding for the "Group
Specific Antigens" (gag). During the cleavage, the structural proteins of the virus core are
liberated. The "gag-protease" itself is a component of a precursor protein encoded by the
pol-genome region of HIV- 1 and HIV-2, which protein also contains the regions for the
r~
21089
- 2-
`~,!
~, "reverse transcriptase" and the "integrase" and is thought to be clenved by autoproteolysis.
The "gag-protcnse" cleaves the major core protein p24 of l-llV-1 atld ~ 2 preferentially
N-term;nally of proline residues, for examplc in the divalent residues Phe-Pro, Leu-Pro or
`I Tyr-Pro. It is n protenso hllving a catalyticnlly nctive nspartnte residlle in the nctive centre,
`$ ll so-cnlled nsp~lrtntc protense.
!l
i~ Because of the central role of the "gag-protense" in the processing of the mentioned
"core-proteins", it is assumed that effective inhibition of that enzyme in vivo w~ll suppress
~:', the assembly of mature virions, so that corresponding inhibitors can be used therapellt-
. ically.
In general, attempts have been made for some time to provide compounds for controlling
retroviral diseases, such as AIDS, that are effective in vivo as inhibitors of the said
retroviral gag-proteases, especially the gag-protease of HIV-1 (HIV-l-protease), and also
of those of EIIV-2 and other AIDS viruses.
3 `
The principal aim at present is to make available such compounds having the best possible
pharmacokinetic properties.
A requirement for therapeutic effectiveness in vivo is the achievement of good
bioavailability, for example good absorptive capacity andlor a high blood level, also in the
case of enteral, such as oral, administration, in order thus to ob~ain sufficiently high
concentrations in the infected cells andtor good distribution in the organism.
The object of the present invention is to provide structurally novel antiretroviral
compounds having improved pharmacodynamic prnper~es, which compounds preferably
exhibit better absoIption than do the corresponding unmodified antiretroviral compounds
themselves in the case of enteral, especially oral, administration, and/or which result in
blood levels, especially of the unmodifie(l antiretroviral compound, that are higher than
those obtainable with corresponding administration of the unmodified antiretroviral
compounds themselves.
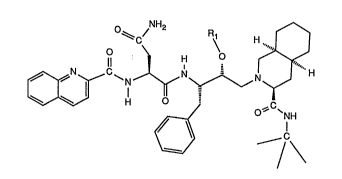
The compounds of the invention are those of formula I
.~.;.
t,' ``` 210~9~
- 3 -
~C/ R~
e~ NH
l \ ,: `.~
wherein Rl is acyl, and salts thereo ;~ ~ :
Euroyean Patent Application EP 0 432 695 (published 19 June 1991) refers to the
compound of formula ~
,, ~, . . ,:
~8~ ~N\~--N~
-I \ ` , ~
which is also included generically in EP O 346 847 (published 19 June 1991) and for
which a method of synthesis is described in EP 0 432 694 (published 11 December 1989).
That compound is also referred to and studies are made of, for example, its action in vitro,
on cell cultures and in vivo as well as its structure/action relationships in the following
publications: Tucker, T. J., et al., J. Med. Chem. 35, 2525-2533 (1992); Roberts, N. A., ~ `
et al., Biochem. Soc. Transact. 20, 513-~16 (1992); Krausslich, H.-G., J. Virology 66,
567-572 (1992); Martin, J. A., et al., Biochem. Biophys. Res. Commun. 176, 180 - 188
(1991); Krohn et al., J. Med. Chem. 34, 3340-3342 (1991); Holmes, H. C., et al., Antiviral
-
2 0 8 9 3 ~
., .:
- 4 -
Chem. & Chemother. 2, 287-293 (1991); Craig, J. C., et al., Antiviral Chem. &
Chemother. 2, 181-186 tl991); Craig, J. C., et al., Antiviral Res. 16, 295-305 (1991);
~oberts, N. A., et al., Science 248, 358-361 tl990); Overton, H. A., et al., Virology 179,
:3 508-511 (1990); Muirhead, G. J., et al~, Brit~ J~ Clin~ Phannacol~ 34(2), 170P (1992);
Williams, P~ E. O., et nh, B~it~ J~ Clin~ PharmacoL 34(2), 15~P (1992); Shnw et al~ rit~ J~
Clin~ Pharmacol~ 34(2), 166P (1992); Johnson, V~ A~, et nl., J~ Infectiolls Dis. 166(5), 1143
(1992); Phylip, L. H.,17EBS Lett. 314(3), 449 (1992); EP 0 432 695 (published
19~06.1991); EP 0 513 917 (published 19~11.1992); Thompson, W. J., J. Am~ Chem~ Soc~
; 115, 801 (1993); and EP 0 346 847 (published 20~12~1989)~
,
There is no mention or suggestion in any of those publicadons of compounds that contain
a different radical instead of the hydrogen at the free hydroxy group in compounds of
formula II.
: .
Surprisingly, it has now been found that when administered to mammals, especially when
administered ornlly, compounds of formula II are present in the blood in significandy
higher concentrations when a compound of formula I having a hydroxy group modified by
Rl is adrninistered than when the corresponding free compound of forrnula II is adminis-
tered~
The general terms and names used in the description of the present invention preferably
have the following meanings:
The compounds of formula I according to the invention CM be present in vaIious isomeric
forms if the radical Rl can be present in various isomeric forms~ For example, any
asymmetric carbon atoms that may be present in the substituents Rl may each
independently of the others be in the (R)-, (S)- or (R,S)-configuration and/or ~ere may be
cis/trans isomerism at multiple bonds, such as double bonds. Accordingly, the compounds
of formula I may be in the form of isomeric mixtures, especially in the form of diastereo-
isomeric mixtures or racemates, or in the forrn of pure isomers, especially pureenantiomers.
The term "lower" used in the definition of groups or radicals, for example lower aL1cyl,
lower alkoxycarbonyl etc., means that, unless expressly otherwise defined, the groups or
radicals so defined contain up to and including 7, and preferably up to and including 4,
carbon atoms. In thc case of lower aLlcenyl and lower alkynyl, "lower" denotes tht
-`` 21089~
`~i
,~
: ¦ presence of from at least 2 to a maximum of 7, preferably from 2 to 4, carbon atoms, and
in the case of lower alkenoyl or lower alkynoyl from 3 to 7, preferably 3 or 4, carbon
atoms.
;l
~eyl Rl has, for exnmple, up to 25, prefernbly up to 19, carbon ntoms mld ;s especinlly the
ncyl ~roup of n carboxylic acid boncled via its carbonyl or the ncyl group of anunsubstituted or substituted amino acid, also aminoearbonyl or the radical of nnN-substituted carbamic acid bonded via its aminocE~rbonyl group or the radical of a semi-
;~ ester of carbonic acid bonded via carbonyl.
,! ` ' ~ ~
-l Preferred acyl groups Rl of a carboxylic acid are, for example, unsubstituted alkanoyl,
aL~cenoyl or alkynoyl, or substituted all~anoyl, aL~cenoyl or alkynoyl, especially octanoyl, ~;
decanoyl, dodecanoyl or palmitoyl, unsubstituted or substituted lower alkanoyl, lower
alkenoyl or lower alkynoyl, :
wherein the substituents are selected, for example, from one or more radicals, preferably
from up to three radicals, especially from one radical or two radicals selected from the
group consisting of hyciroxy, lower alkoxy, lower alkoxy-lower alkoxy, lower alkoxy~
lower alkoxy-lower alkoxy, phenoxy, naphthyloxy, phenyl-lower alkoxy, 2-halo-lower
alkanoyl, such as 2-chloroacetyl, amino-, lower aLkylamino- or cli-lower aLIcylamino-
lower alkoxy-2-lower alkanoyl, such as dimethylamino-lower aL~coxyacetyl, amino-, ;~
lower alkylamino- or di-lower aL~cylamino-lower aL~coxy-lower aL~coxy-2-lower alkanoyl,
such as dimethylamino-(2-lower alkoxyethyl)acetyl, lower alkanoyloxy, phenyl-lower
alkanoyloxy, such as benzoyloxy or phenylacetoxy, halogen, such as fluorine, chlorine,
bromine or iodine, especially fluorine or chlorine, carboxy, lower aL~coxycarbonyl,
phenyl-lower aLIcoxycarbonyl, such as benzyloxycarbonyl, carbamoyl, lower alkyl-carbamoyl, hydroxy-lower alkylcarbamoyl, di-lower alkylcarbamoyl, bis(hydroxy-lower ~ ~ ~
alkyl)carbamoyl, carbamoyl the nitrogen atom of which Is a constituent of a 5- to ~ ~ -
7-membered heterocyclic ring that may contain a further hetero atom selected from
oxygen, sulfur, nitrogen and lower alkyl-substituted, such as methyl- or ethyl-substituted,
nitrogen, for example pylrolidinocarbonyl, morpholinocarbonyl, thiomorpholino-
carbonyl, piperidin-1-ylcarbonyl, piperazin-l-ylcarbonyl or 4-lower aLkylpiperazin-1-yl-
carbonyl, such as 4-methylpiperazin- 1-ylcarbonyl; cyano, oxo, cycloaLkyl, for example
C3-C8cycloalkyl, such as cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl, bicyclo-
alkyl, for example C6-CI2bicycloalkyl, such as decahydronaphth-2-yl, endo- or exo-
2-norbornyl, bicyclo[2.2.2]oct-2-yl or bicyclo[3.3.1]non-9-yl, tricycloaUcyl, for example
Cg-CI4tricycloalkyl, such as 1- or 2-adamantyl, cycloalkenyl, for example C4-C8cyclo-
21~893~
, - 6-
'I
aLkenyl, such as 1-cyclohexenyl or 1,4-cyclohexadienyl, bicycloalkenyl, for example
5-norbornen-2-yl or bicyclo[2.2.2]octen-2-yl, heterocyclyl, which is a saturated, partially
I saturated or unsaturated ring containing from 3 to 7, prefernbly from 5 to 7, ring atoms
nnd up to four heteroatorns independently selected from nitrogen, sulfilr nnd oxygen, ` `
prcfernbly 1 or 2 o:P tho mentioned heteroatoms, the ring being present as such or in once
or twice, prefernbly once, benzo-, cyclopentn-, cyclohexn- or cycloheptn-:fused form,
heterocyclyl being unsubsdtuted or substituted especilllly by lower alkyl, lower alknnoyl,
hydroxy, lower alkoxy, phenyl-lower alkoxy, such as benzyloxy, hydroxy-lower alkyl,
such flS hysroxymethyl, halogen, cyano and/or by trifluoromethyl, for example pyrrolyl, ~ ~ .
2,5-dihydropyrrolyl, furanyl, thienyl, tetrahydrofuranyl, cyclohepta[b]pyrrolyl, ~ ::
pyrrolidinyl, imidazolyl, imidazolidinyl, pyrazolinyl, pyrazolidinyl, tliazolyl, such as
1,2,3-, 1,2,4- or 1,3,4-triazolyl, tetrazolyl, such as 1- or 2-tetrazolyl, tetrahydro-oxazolyl,
tetrahydro-isoxazolyl, tetrahydro-thiazolyl, tetrahydro-isothiazolyl, indolyl, isoindolyl,
quinolyl, isoquinolyl, benzimidazolyl, benzofuranyl, pyridyl, pyrimidinyl, piperidinyl,
piperazin-1-yl, morpholino, thiomorpholino, S,S-dioxothiomorpholino, 1,2-dihydro- or
1,2,3,4-tetrahydroquinolyl, or 1,2-dihydro- or 1,2,3,4-tetrahydro-isoquinolyl, the
mentdoned radicals being unsubstituted or substituted as above, especially by lower
aLkyl, for example as in 4-lower aLkyl-piperazin- l-yl, such as 4-methyl- or 4-ethyl-
piperazin-1-yl, by lower aLkanoyl, for example as in 4-lower aLkanoyl-piperazin-1-yl,
such as 4-acetyl-piperazin-1-yl, or by hydroxy-lower alkyl, for example as in S-hydroxy-
methylfuran-2-ylcarbonyl; and aryl, preferably C6-CI2aryl, for example phenyl,
naphthyl, such as 1- or 2-naphthyl, indanyl, such as 1- or 2-indanyl, indenyl, such as
inden- l-yl, or fluorenyl, such as fluoren-9-yl, aryl being unsubstituted or mono- or poly-
substituted, preferably mono-subsdtuted, for example, by lower aLkyl, for example
methyl, halo-lower alkyl, such as chloro- or bromo-methyl, halogen, for example
fluorine or chlorine, hydroxy, lower aLkoxy, such as methoxy, lower alkanoyloxy,carboxy, lower aLkoxycarbonyl, phenyl-lower aLkoxycarbonyl, carbamoyl, mono- or di-
lower aLkylcarbamoyl, mono- or di-hydroxy-lower aLkylcarbamoyl, halo-lower aLkyl,
such as triiluoromethyl, heterocyclyl-lower aLkyl wheriein heterocyclyl is as de~lned
above, especially heterocyclylmethyl wherein heterocyclyl is bonded via a ring nitrogen
atom, for example piperidinomethyl, piperazin-1-ylmethyl, 4-lower aLkyl-piperazin-1-yl-
methyl, such as 4-methyl- or 4-ethyl-piperazin-1-ylmethyl, 4-lower aLkanoyl-piperazin-
l-ylmethyl, such as 4-acetyl-piperazin-1-ylmethyl, morpholinomethyl or thiomorpho-
linomethyl, cyano and/or by nitro, and especially phenyl substituted in the p-position by
one of the mentioned radicals;
for example lower aLkanoyl, such as ~ormyl, acetyl, propionyl, butyryl, methylpropionyl,
. 21083~4
.,
~.
pivaloyl, n-pentanoyl, hexanoyl or heptanoyl, such as n-heptanoyl, hydroxy-loweralkanoyl, for example ~-hydroxypropionyl, lower alkoxy-lower alkanoyl, for example
lower nlkoxyacetyl or lower nlkoxypropionyl, such as methoxyncetyl, 3-methoxypropionyl
or n-butoxyacetyl, lower nlkoxy-lower nlkoxy-lower nlkanoyl, such ns 2-(2-methoxy-
othoxy)ncotyl, lower nlkoxy-lowor nlkoxy-lower nlkoxy-lower nlknnoyl, such ns
2-(2-(2-metlloxyethoxy)ethoxy)acetyl, phenoxy-lower nlknnoyl, for exnmple phenoxy-
ncetyl, nnpllthyloxy-lower alkanoyl, for exarnple a- or ,B-naphthyloxyacetyl, phenyl-lower
alkoxy-lower alkanoyl, such as benzyloxyacetyl, 2-halo-lower nlkanoyl, such as ~-chloro-
acetyl, amino-, lower alkylamino- or di-lower alkylamino-lower alkoxy-2-lower alkanoyl,
such as dimethylamino-lower alkoxyacetyl, amino-, lower alkylamino- or di-lower alkyl- : ~
amino-lower alkoxy-lower alkoxy-2-lower alkanoyl, such as dimethylamino-(2-lower ~ : :
alkoxyethoxy)acetyl, lower alkanoyloxy-lower alkanoyl, for example lower aL~canoyloxy-
acetyl or lower alkanoyloxypropionyl, such as acetoxyacetyl or ~-acetoxypropionyl, halo- :
lower alkanoyl, for example a-haloacetyl, such as a-chloro-, a-bromo-, a-iodo-, a,a,a-tri- ~ :
fluoro- or a,a,o~-trichloro-acetyl, or halopropionyl, such as ~-chloro- or ~-bromo ~ :
propionyl, carboxy-lower alkanoyl, for example carboxyacetyl or 3-carboxypropionyl,
lower alkoxycarbonyl-lower alkanoyl, for example lower alkoxycarbonylacetyl or lower
alkoxycarbonylpropionyl, such as methoxycarbonylacetyl, ~B-methoxycarbonylpropionyl,
ethoxycarbonylacetyl, ~-ethoxycarbonylpropionyl, tert-butoxycarbonylacetyl or ,B-tert- ` ~:
butoxycarbonylpropionyl, carbamoyl-lower alkanoyl, for example carbamoylacetyl or :
~B-carbamoylpropionyl, lower alkylcarbamoyl-lower alkanoyl, for example methyl- ~ :
carbamoylacetyl or ,B-(N-lower aLkyl)carbamoylpropionyl, such as ,B-(N-methyl)-,,B-(N-ethyl)-"B-(N-(n-propyl))-carbamoyl- or ~-(N-(n-hexyl))-carbamoyl-propionyl, di-
lower aLkylcarbamoyl-lower aLIcanoyl, for example dimethylcarbamoylacetyl,
,B-(N,N-(di-lower alkyl)carbamoyl)propionyl, such as ~-(N,N-dimethyl)-"B-(N,N-di-
ethyl)-"B-(N,N-di-(n-propyl)-carbamoyl)- or ,B-(N,N-di-(n-hexyl))-carbamoyl-propionyl,
,B-pyrrolidinocarbonylpropionyl, ~-morpholinocarbonyipropionyl"B-thlomorpholin~
carbonylpropionyl, ~-piperidin- 1 -ylcarbonylpropionyl"B-piperazin- l -ylcarbonylpropionyl
or ,B-(4-lower alkyl-piperazin-1-ylcarbonyl)-propionyl, such as ,B-(4-methylpiperazin-1-yl-
carbonyl)propionyl, oxo-lower aL~canoyl, for example acetoacetyl or propionylacetyl,
hydroxy-carboxy-lower aLkanoyl, for example a-hydroxy-a-carboxy-acetyl or
a-hydroxy-,B-carboxypropionyl, hydroxy-lower alkoxycarbonyl-lower alkanoyl, for
example a-hydroxy-a-ethoxy- or-methoxy-carbonylacetyl or a-hydroxy-,B-ethoxy- or-methoxy-carbonyl-propionyl, a-acetoxy-a-methoxycarbonyl-acetyl, dihydroxy-carboxy-
lower alkanoyl, for example a"B-dihydroxy-,B-carboxy-propionyl, dihydroxy-lower
alkoxycarbonyl-lower alkanoyl, for example oc,~-dihydroxy-~-ethoxy- or -methoxy-
:'3
21~93~
carbonyl-propionyl, a"B-diacetoxy-,B-methoxycarbonyl-propionyl, a-naphthyloxy-
carboxy-lower alkanoyl, for example 2-a-naphthyloxy-4-carboxy-butyryl, a-naphthyl-
oxy-lower alkoxycarbonyl-lower alkanoyl, for example o~-naphthyloxy-ethoxycarbonyl-
acetyl, 2-a-naphthyloxy-ethoxycarbonyl-propionyl or 2-a-naphthyloxy-4-tert-butoxy-
carbonylbutyryl, a-naphthyloxy-benzyloxycarbonyl-lowcr alkMIoyl, for exalnple
2-a-naphthyloxy-3-benzyloxycMrbonyl-propionyl, a-naphthyloxy-carbamoyl-lower
alkanoyl, for example 2-a-naphthyloxy-4-carbamoyl-butyryl, a-naphthyloxy-cyano-lower
alkanoyl, for example a-naphthyloxy-cyano-acetyl or 2-a-naphthyloxy-4-cyanobutyryl,
a-naphthyloxy-oxo-lower alkanoyl, for example 2-a-naphthyloxy-4-oxo-pentanoyl,
heterocyclyl-lower alkanoyl, for example unsubsdtuted or substituted pyIrolylcarbonyl,
for example 2- or 3-pyrrolylcarbonyl, furylcarbonyl, for example 2-furylcarbonyl,
5-hydroxymethyl-furan-2-ylcarbonyl, thienylcarbonyl, for example 2-thienylcarbonyl,
pyridyl-lower aLlcanoyl, such as pyridylcarbonyl, for example 2-, 3- or 4-pyridylcarbonyl,
pyridylacetyl, for exMmple 2-pyridylacetyl, or pyridylpropionyl, for example 3-(2-pyridyl)-
propionyl, quinolylcarbonyl, such as quinolin-2-ylcarbonyl, isoquinolinylcarbonyl, such as
isoquinolin-3-ylcarbonyl, unsubstituted or substituted indolylcarbonyl, for example 2-, 3-
or 5-indolylcarbonyl, l-methyl-, S-methyl-, 5-methoxy-, 5-benzyloxy-, 5-chloro- or
4,5-dimethyl-indolyl-2-carbonyl, cyclohepta[b]pylrolyl-5-carbonyl, pyrrolidin-(2- or
3-)yl-carbonyl (pyrrolidinyl-2-carbonyl (= prolyl) preferably being in the D- or L-form),
hydroxypyrrolidinylcarbonyl, for example 3- or 4-hydroxypyrrolidinyl-2-cMrbonyl, oxo-
pyrrolidinylcarbonyl, for example 5-oxopyrrolidinyl-2-carbonyl, piperidinylcarbonyl, for
example 2-, 3- or 4-piperidinylcarbonyl, 1,2,3,4-tetrahydroquinolylcarbonyl, for example
1,2,3,4-tetrahydroquinolyl-2-, -3- or-4-carbonyl, or 1,2,3,4-tetrahydroisoquinolylcarbonyl,
for example 1,2,3,4-tetrahydroisoquinolyl-1-, -3- or -4-carbonyl, imidazolyl-lower
alkanoyl, such as imidazolylcarbonyl, for example imidazol-l-ylcarbonyl or imidazol-
4-ylcarbonyl, imidazolylacetyl, for example 4-imidazolylacetyl, or imidazolylpropionyl,
for example 3-(4-imidazolyl)propionyl, morpholinocarbonyl, thiomorpholinocarbonyl,
morpholinoacetyl, thiomorpholinoacetyl, 4-lower alkyl-l-piperazinoacetyl, such as
4-methyl-piperazinoacetyl, indolylacetyl or benzofuranylacetyl, lower aLI~enoyl, for
example acryloyl, vinylacetyl, crotonoyl or 3- or 4-pentenoyl, lower aLIcynoyl, for example
propioloyl or 2- or 3-butynoyl, cycloaLt~ylcarbonyl, for example cyclopropyl-, cyclobutyl-,
cyclopentyl- or cyclohexyl-carbonyl, bicycloalkylcarbonyl, for example decahydro-
naphthyl-2-carbonyl, endo- or exo-norbornyl-2-carbonyl, bicyclo[2.2.2]oct-2-ylcarbonyl
or bicyclo[3.3.1]non-9-ylcarbonyl, tricycloaL~cylcarbonyl, for example 1- or 2-adamantyl-
carbonyl, cycloaLcenylcarbonyl, for example l-cyclohexenylcarbonyl or 1,4-cyclohexa-
dienylcarbonyl, bicycloaLtcenylcarbonyl, for example 5-norbornen-2-ylcarbonyl or
:
2108~3~ -
bicyclo[2.2.2]octen-2-ylcarbonyl, cyclopropylacetyl, cyclopentylacetyl, cyclohexylacetyl
or 3-cyclohexylpropionyl, cycloalkyl-lower alkenoyl, for example cyclohexylacryloyl,
cyclonlkenyl-lower alknnoyl, for example l-cyclohexenylacetyl or 1,4-cyclohexndienyl- ~ "
acetyl, phenyl-lower nlkanoyl, for exnmplo benzoyl, phenylncetyl or 3-pllenylpropionyl, ; ` `
that is unsubstituted or mono- or poly-substituted in the phenyl radicnl by lowor nlkyl, for
exnmple methyl, halo-lower alkyl, such as chloro- or bromo-methyl, hnlogen, :for example
~luorine or chlorine, hydroxy, lower alkoxy, for example methoxy, piperidinomethyl,
piperazin- l-ylmethyl, 4-lower alkyl-piperazin-l-ylmethyl, such as 4-methyl- or 4-ethyl-
piperazin-l-ylrnethyl, 4-lower alkanoyl-piperazin-l-ylmethyl, such as 4-acetyl-piperazin-
l-ylmethyl, morpholinomethyl, thiomorpholinomethyl, cyano and/or by nitro, or :
a-naphthyl- or ,3-naphthyl-lower alkanoyl wherein naphthyl is unsubstituted or mono- or
poly-substituted by lower alkyl, for example methyl, phenyl, halogen, for example :
chlorine, hydroxy, lower alkoxy, for example methoxy, and/or by nitro, and lower .
alkanoyl in phenyl-, a-naphthyl- or ~-naphthyl-lower alkanoyl may be unsubstituted or
substituted, for example, by hydroxy, lower aL~coxy, lower aL"anoyloxy, carboxy, lower : :
alkoxycarbonyl, phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, carbamoyl, ~:
lower aL~cylcarbamoyl, di-lower alkylcarbamoyl, cyano and/or by oxo and may be ~ :
branched, for example 4-chloromethyl-, 4-bromomethyl-, 4-fluoro-, 4-chloro-,
4-methoxy-, 4-morpholinomethyl-, 4-thiomorpholinomethyl-, 4-cyano- or 4-nitro-benzoyl,
a-naphthylacetyl"3-naphthylacetyl, lower alkylphenylacetyl, such as 4-methyl-
phenylacetyl, lower alkoxyphenylacetyl, such as 4-methoxyphenylacetyl, 2-lower
alkoxy-2-phenylacetyl, such as (R)- or (S)-2-methoxy-2-phenylacetyl, 3-(p-hydroxy- ~ :
phenyl)-propionyl, diphenylacetyl, di(4-methoxyphenyl)acetyl, triphenylacetyl, 3-a- or
3-,3-naphthylpropionyl, 3-phenyl- or 3-oc-naphthyl-2-hydroxy-propionyl, 3-phenyl- or
3-a-naphthyl-2-lower alkoxy-propionyl, such as 3-phenyl- or 3-a-naphthyl-2-neo-
pentyloxy-propionyl, 3-phenyl-2-pivaloyloxy- or-2-acetoxy-propionyl, 3-a-naphthyl-2-pi-
valoyloxy- or -2-acetoxy-propionyl, 3-phenyl- or 3-a-naphthyl-2-carboxymethylpropionyl,
3-phenyl- or 3-a-naphthyl-2-lower aLkoxycalbonyl-propionyl, such as 3-a-naphthyl-
2-ethoxycarbonyl-propionyl, 3-phenyl- or 3-a-naphthyl-2-benzyloxycarbonylmethyl-propionyl, 3-phenyl- or 3-a-naphthyl-2-carbamoyl-propionyl, 3-phenyl- or 3-a-naphthyl-
2-tert-butylcarbamoyl-propionyl, 3-phenyl- or 3-a-naphthyl-2-cyano-propionyl, 3-phenyl-
or 3-oc-naphthyl-2-cyanomethyl-propionyl, 3-phenyl- or 3-a-naphthyl-2-acetonyl-pro-
pionyl, 2-benzyl- or 2-a-naphthylmethyl-4-cyano-butyryl, 4-phenyl- or 4-(x-naphthyl-3-
carboxy-butyryl, 4-phenyl- or 4-a-naphthyl-3-benzyloxycarbonyl-butyryl, 2-benzyl- or
2-a-naphthylmethyl-4-oxo-pentanoyl, phenyl-lower aLkenoyl, for example ,B-phenyl-
acryloyl or ,B-phenylvinylacetyl, naphthylcarbonyl, for example a- or ~B-naphthylcarbonyl,
:: ~ 210~93~ ~
!i'j -- 10 --
~ indenylcarbonyl, for example 1-, 2- or 3-indenylcarbonyl, or indanylcarbonyl, for example .
el 1- or 2-indanylcarbonyl.
,`
Preferred acyl groups Rl of n semiester of cnrbonic ncid nre, for exnmple, Imsubstituted or
. substituted nlkoxycMbonyl, especinlly lower nlkoxycarbonyl, for exnmple methoxy-,
ethoxy~ or tert-lower alkoxy-carbonyl, such as tert-butoxycMbonyl, 2-halo-lower alkoxy-
carbonyl, for example 2-chloro-, 2-bromo-, 2-iodo- or 2,2,2-trichloro-ethoxycarbonyl;
aryl-lower alkoxycarbonyl, for example arylmethoxycarbonyl, wherein aryl preferably has
from 6 to 14 carbon atoms, is unsubstituted or mono- or poly-subsdtuted, prefeMbly
mono-substituted, for example, by lower alkyl, for example methyl, halo-lower alkyl, such
as chloro- or bromo-methyl, halogen, for example fluorine or chlorine, hydroxy, lower
a]koxy, such as methoxy, lower alkanoyloxy, carboxy, lower alkoxycarbonyl, phenyl-
lower a]koxycarbonyl, carbamoyl, mono- or di-lower alkylcarbamoyl, mono- or di-
hydroxy-lower alkylcarbamoyl, halo-lower a]kyl, such as trifluoromethyl, heterocyclyl-
lower alkyl wherein heterocyclyl is as defined above as a subsdtuent of lower aL~canoyl,
especially heterocyclylmethyl wherein heterocyclyl is bonded vfa a ring nitrogen atom, for
example piperidinomethyl, piperazin-1-ylmethyl, 4-lower aLkyl-piperazin-1-ylmethyl,
such as 4-methyl- or 4-ethyl-piperazin-1-ylmethyl, 4-lower a]kanoyl-piperazin-1-ylmethyl,
such as 4-acetyl-piperazin- 1-ylmethyl, morpholinomethyl or thiomorpholinomethyl, cyano
andlor by nitro, and is especially phenyl, 1- or 2-naphthyl, fluorenyl or phenyl mono- or
poly-substituted by lower alkyl, for example methyl or tert-butyl, lower alkoxy, for
example methoxy, ethoxy or tert-butoxy, hydroxy, halogen, for example fluorine, chlorine
or bromine, and/or by nitro, for example phenyl-lower alkoxycarbonyl, such as benzyloxy-
carbonyl, 4-methoxybenzyloxycarbonyl, 4-nitrobenzyloxycarbonyl, diphenyl-lower
aLkoxycarbonyl, such as diphenylmethoxycarbonyl, di(4-methoxyphenyl)methoxy-
carbonyl, trityloxycarbonyl or fluorenyl-lower aLtcoxycarbonyl, such as 9-fluorenyl-
methoxycarbonyl; or also heterocyclyl-lower alkoxycarbonyl wherein heterocyclyl is as
defined above as a substituent of aLI~anoyl, for example filran-2-ylmethoxycarbonyl or
pyridin-2-, -3- or -4-ylmethoxycarbonyl. The definitions falling under the definition of
acyl groups Rl of a semiester of carbonic acid may preferably be omitted from all the
definitions of compounds of formula I mentioned hereinbefore and hereinafter.
,
A preferred acyl group Rl of an N-substituted carbamic acid is an aminocarbonyl radical
wherein the amino group carries one or two substituents selected independently of one
another from
~.,. .
210~931
.j - 11-
unsubstituted or substituted lower alkyl, the substituents of which are selected from those
mentioned above for substituted lower alkanoyl nnd are present in the number defined
therein, prefernbly substituents selected from hydroxy, lower alkoxy, phQnoxy,
naphthyloxy, lower nlkanoyloxy, phenyl-lower alkanoyloxy, SUCII as benzoyloxy orphenylacetoxy, halogen, such as fluorine, chlorine, bromine or iodine, especinlly fluorine
or chlorine, cnrboxy, lower alkoxycarbonyl, phenyl-lower aLkoxycnrbonyl, such asbenzyloxycarbonyl, carbamoyl, lower alkylcarbamoyl, hydroxy-lower alkylcarbamoyl,
di-lower aLkylcarbamoyl, bis(hydroxy-lower aLkyl)carbamoyl, cyano, oxo and C6-Cl2aryl,
t`or example phenyl, naphthyl, such as 1- or 2-naphthyl, indanyl, such as 1- or 2-indanyl, : :
indenyl, such as inden-l-yl, or fluorenyl, such as fluoren-9-yl, aryl being unsubstituted or
mono- or poly-subsdtuted, preferably mono-substituted, for example, by lower aLkyl, for ::
example methyl, halo-lower aLkyl, such as chloro- or bromo-methyl, halogen, for example
fluoAne or chloAne, hydroxy, lower alkoxy, such as methoxy, lower alkanoyloxy,
carboxy, lower alkoxycarbonyl, phenyl-lower aLkoxycarbonyl, carbamoyl, mono- or di-
lower aLkylcarbamoyl, mono- or di-hydroxy-lower aLkylcarbamoyl, halo-lower alkyl, such
as trifluoromethyl, cyano and/or by nitro, especially phenyl substituted in the p-position by
one of the mentioned radicals; especially unsubstituted lower aLkyl, such as methyl or
ethyl;
and aryl which preferably has from 6 to 14 carbon atoms and is unsubsdtuted or mono- or
poly-subsdtuted, preferably mono-subsdtuted, for example, by lower alkyl, for example
methyl, halo-lower alkyl, such as chloro- or bromo-methyl, halogen, for example fluorine
or chloAne, hydroxy, lower alkoxy, such as methoxy, lower alkanoyloxy, carboxy, lower
alkoxycarbonyl, phenyl-lower alkoxycarbonyl, carbamoyl, mono- or di-lower alkyl- .
carbamoyl, mono- or di-hydroxy-lower alkylcarbamoyl, halo-lower alkyl, such as tri-
fluoromethyl, heterocyclyl-lower alkyl wherein heterocyclyl is as de~med above as a
subsdtuent of lower alkanoyl, especially heterocyclylmethyl wherein heterocyclyl is
bonded l~ia a Ang nitrogen atom, for example pipeAdinomethyl, piperazin-l-ylmethyl,
4-lower alkyl-piperazin-l-ylme~hyl, such as 4-methyl- or 4-ethyl-piperazin-1-ylmethyl,
4-lower alkanoyl-piperazin-l-ylmethyl, such as 4-acetyl-piperazin-1-ylmethyl, morpho-
linomethyl or thiomorpholinomethyl, cyano and/or by nitro, preferably colTespondingly
subsdtuted phenyl or 1- or 2-naphthyl, the radical of an N-subsdtuted carbamic acid
carrying not more than one of the mentioned aryl radicals at the nitrogen atom; an acyl
group Rl of an N-substituted carbamic acid is especially mono- or di-lower aLkylamino-
carbonyl, such as N-methyl-, N-ethyl-, N,N-dimethyl- or N,N-diethyl-aminocarbonyl, or ~:
phenyl-lower alkylaminacarbonyl wherein phenyl is unsubstituted or substituted by the
.
";i .
r
2~ 33 ~I
~ - 12-
., .
radicals mentioned in the definition of aryl, for example by lower alkyl, for example
methyl, halo-lower alkyl, such as chloro- or bromo-methyl or trifluoromethyl, halogen, for
example fluorine or chloline, hydroxy, lower alkoxy, such ns methoxy, cnrboxy l~nd/or by
cyano, proferably by up to throe of those substituents selcctcd indepenclently o~ one
nnother, espccinlly by one of those substituellts, for exnmplo in the p-position, such ns in
N-bonzyl-, N-(4-fluorobenzyl)-, N-(4-chlorobenzyl)-, N-(4-trifluoromethylbellzyl)- or
N-(4-cynnobonzyl)-aMinocarbollyl; especially preferred is aminocarbonyl substituted by
only one radical at the nitrogen atom, for example N-lower alkylaminocarbonyl, such as
N-methyl- or N-ethyl-aminocarbonyl, or phenyl-lower alkylaminocarbonyl wherein
phenyl is unsubstituted or substituted by the radicals mentioned in the definition of aryl,
for example by lower alkyl, such as methyl, halo-lower alkyl, such as chloro- or bromo-
methyl or trifluoromethyl, halogen, such as fluorine or chlorine, hydroxy, lower aLIcoxy,
such as methoxy, carboxy and/or by cyano, preferably by up to three of those substituents
selected independently of one another, especially by one of those subsdtuents, for example
in the p-position, such as in N-benzyl-, N-(4-fluorobenzyl)-, N-(4-chlorobenzyl)-, N-(4-tri-
fluoromethylbenzyl)- or N-(4-cyanobenzyl)-aminocarbonyl. The definitions falling under
the definition of acyl groups R~ of an N-substituted carbamic acid, and the radical
aminocarbonyl Rl may preferably be omitted from all the definitions of compounds of
formula I mentioned hereinbefore and hereinafter.
Preferred acyl groups Rl of an unsubstituted or substituted arnino acid are formed by the
amino acid residues of an c~-"B-, ~- or ~amino acid that is bonded via its carbonyl group,
especially
of a natural o~-arnino acid having the L-configuration, such as those normally occurring
in proteins, or an epimer of such an amino acid, that is to say having the unnatural
D-configuration, or a D,L-isomeric mixture thereof, a homologue of such an amino acid,
for example wherein the amino acid side chain has been lengthened or shortened by one or
two methylene groups, wherein the amino group is in the ,B-, ~- or ~-position andlor
wherein a methyl group has been replaced by hydrogen, a substituted aromatic amino acid
wherein the aromatic radical has from 6 tO 14 carbon atoms, for example a substituted
phenylalanine or phenylglycine wherein the phenyl may be mono- or poly-substituted by
lower ~kyl, for example rnethyl, hydroxy, lower alkoxy, for exarnple methoxy, lower
alkanoyloxy, for example acetoxy, amino, lower alkylamino, for example methylamino,
di-lower alkylamino, for example dimethylamino, lower alkanoylamino, for example :
acetylamino or pivaloylamino, lower alkoxycarbonylarnino, for example tert-butoxy-
carbonylamino, arylmethoxycarbonylamino wherein aryl preferably has from 6 to 14
j~ 210~93`~ ~ ~
~ - 13 -~
carbon atoms, for example benzyloxycarbonylamino or 9-fluorenylmethoxycarbonyl-
amino, halogen, for example fluorine, chlorine, bromine or iodine, carboxy Md/or by
nitro, a benzo-fused phenylalanine or phenylglycine, such as a-naphthylalarline, or a
hydrogenated phenylalnnine or phenylglycine, SllCh as cyclohexylalMine or cyclohexyl-
¦ glycine. Within the context of the mentioned definitions, ncyl groups of nmino acids thnt
havc nlrcndy been mentioned, such ns prolyl, indoline-2-carbonyl, 1,2,3,4-tetrahydroiso-
quinoline-3-carbonyl and trans-3- nnd Irans-4-hydroxyprolyl which have already appeared
in the definition of substituted lower alkanoyl Rl, are excepted, but only with the sole aim
of avoiding overlapping of the definitions.
Those amino acid radicals may be substituted at free amino or hydroxy f~mctions,preferably at a free amino function, by one of the radicals mentioned above under acyl R
as the acyl group of a carboxylic acid or a semiester of carbonic acid, by unsubstituted or
substituted alkyl, especially lower alkyl, such as methyl, ethyl, isopropyl, n-propyl or
n-butyl,
wherein the substituents are selected, for example, from one or more radicals, preferably
from up to three radicals, especially from one radical selected from the group consisting
of hydroxy, lower alkoxy, phenoxy, naphthyloxy, lower alkanoyloxy, halogen, such as
fluorine, chlorine, bromine or iodine, especially fluorine or chlorine, carboxy, lower
alkoxycarbonyl, phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, carbamoyl,
lower alkylcarbamoyl, hydroxy-lower aLkylcarbamoyl, di-lower aLkylcarbamoyl,
bis(hydroxylower alkyl)carbamoyl, cyano, oxo, cycloalkyl, for example C3-C8cyclo-
aLkyl, such as cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl, bicycloalkyl, for
example C6-CI2bicycloalkyl, such as decahydronaphth-2-yl, endo- or exo-2-norbornyl,
bicyclo[2.2.2]oct-2-yl or bicyclo[3.3.1]non-9-yl, tricycloaLlcyl, for example
C9-CI4tricycloalkyl, such as 1- or 2-adamantyl, cycloalkenyl, for exarnple C4-C8cyclo-
alkenyl, such as 1-cyclohexenyl or 1,4-cyclohexadienyl, bicycloaLtcenyl, for example
5-norbornen-2-yl or bicyclo[2.2.2]octen-2-yl, heterocyclyl, which is a saturated, partially
saturated or unsaturated ring containing from 3 to 7, preferably from 5 to 7, ring atoms
and up to four heteroatoms independently selected from nitrogen, sulfur and oxygen,
preferably 1 or 2 of the mentioned heteroatoms, the ring being present as such or in once
or twice, preferably once, benzo-, cyclopenta-, cyclohexa- or cyclohepta-fused form,
heterocyclyl being unsubstituted or substituted especially by lower alkyl, lower aLIcanoyl, `~
hydroxy, lower alkoxy, phenyl-lower alkoxy, such as benzyloxy, hydroxy-lower aL~cyl,
such as hysroxymethyl, halogen, cyano and/or by trifluoromethyl, for example pyrrolyl,
2,5-dihydropyrrolyl, furanyl, thienyl, tetrahydrofuranyl, cyclohepta[b]pyrrolyl, ~;
: ' :,~
~ .
~ 2~8934
~:3 - 14-
pyrrolidinyl, imidazolyl, imidazolidinyl, pyrazolinyl, pyrazolidinyl, triazolyl, such as
,~ 1,2,3-, 1,2,4- or 1,3,4-triazolyl, tetrazolyl, such as 1- or 2-tetrazolyl, tetrahydro-oxazolyl,
tetrahydro-isoxazolyl, tetrahydro-thiazolyl, tetrahydro-isothinzolyl, indolyl, isoindolyl,
. quinolyl, isoquinolyl, benzimidazolyl, benzofurnnyl, pyridyl, pyrimidinyl, piperidinyl,
.~ pipcrnxin-l-yl, morpholino, thiomorpholino, S,S-dioxothiomorpholino, 1,2-dihydro- or
1,2,3,4-tetrnhy(lro-quinolyl, or 1,2-dihydro- or 1,2,3,4-tetrnhytlro-isoqllinolyl, the
mcntioned rndicals being unsubstituted or substituted as mentioned nbove, especially by
lower alkyl, for exnmple as in 4-lower alkyl-piperazin-l-yl, such as 4-methyl- or
4-ethyl-piperazin-1-yl, or by lower alkanoyl, for example as in 4-lower alkanoyl-
. piperazin-l-yl, such as 4-acetyl-piperazin-1-yl, and aryl, preferably C6-Cl2aryl, for
example phenyl, naphtllyl, such as 1- or 2-naphthyl, indanyl, such as 1- or 2-indanyl,
indenyl, such as inden-l-yl, or fluorenyl, such as fluoren-9-yl, the mentioned aryl
, radicals being unsubsdtuted or mono- or poly-substituted, preferably mono-substituted,
for example, by lower alkyl, for example methyl, halo-lower alkyl, such as chloro- or
bromo-methyl, halogen, for example fluorine or chlorine, hydroxy, lower alkoxy, such as
methoxy, lower alkanoyloxy, carboxy, lower alkoxycarbonyl, phenyl-lower
alkoxycarbonyl, carbamoyl, mono- or di-lower alkylcarbamoyl, mono- or di-hydroxy-
lower alkylcarbamoyl, halo-lower alkyl, such as trifluoromethyl, heterocyclyl-lower
alkyl wherein heterocyclyl is as defined above, especially heterocyclylmethyl wherein
heterocyclyl is bonded via a ring nitrogen atom, for example piperidinomethyl,
piperazin- l-ylmethyl, 4-lower alkyl-piperazin- l-ylmethyl, such as 4-methyl- or 4-ethyl- ;
piperazin-l-ylmethyl, 4-lower alkanoyl-piperazin-l-ylmethyl, such as 4-acetyl-piper-
azin-l-ylmethyl, morpholinomethyl, thiomorpholinomethyl, cyano and/or by nitro,
especially phenyl substituted in the p-position by one of the mentioned radicals;
especially by a correspondingly substituted lower alkyl radical, especially correspondingly
- substituted methyl, preferably benzyl, diphenylmethyl, trityl, 2-, 3- or 4-pyridylmethyl, or
may be substituted by one of the radicals mentioned as protecting groups in the section
relating to processes, or may be derivatised at carboxy groups~ :
Especially preferred is the residue, bonded via its a-carbonyl group, of an amino acid
selected from glycine (H-Gly-OH), alanine (H-Ala-OH), 2-aminobutyric acid, 3-amino-
butyric acid, 4-aminobutyric acid, 3-aminopentanoic acid, 4-aminopentanoic acid,5-aminopentanoic acid, 3-aminohexanoic acid, 4-aminohexanoic acîd or 5-aminohexanoic
acid, valine (H-Val-OH), norvaline (o~-aminovaleric acid), leucine (H-Leu-OH), isoleucine
(H-Ile-OH), norleucine (a-aminohexanoic acid, H-Nle-OH), serine (H-Ser-OH),
homoserine (a-amino-~-hydroxybutyric acid), threonine (H-Thr-OH), methionine
~ ~! 2 ~ O ,8 9 3 ~
~1 :
:~ - 15-
, ~ (H-Met-OH), cysteine (H-Cys-OH), phenylalanine ~I-Phe-OH), tyrosine (H-Tyr-OH),
-. 4-aminophenylalanine, 4-chlorophenylalanine, 4-carboxyphenylalanine"B-phenylserine
(~-hydroxyphenylalanine), phenylglycine, oc-naphthylalanine (H-Nal-OH), cyclohexyl-
. alanine (H-Cha-OH), cyclohexylglycine, tryptophan (H-Trp-OM), aspnrtic ncid
:~. (I~ sp-OIl), asparagine (~ sn-OH), arninomnlonic acid, aminomnlonic ncid
mononmide, glutamic ncid (H-GIIl-OH), glutamine (H-Gln-OH), histidine (EI-~Iis-OH),
nrginine (H-~rg-OH), lysine (H-Lys-OH)"~-hydroxylysine, omithine (a"~-dinminovnleric
ncid), 3-aminopropanoic acid, a,~-diMminobutyric ncid Md ~"B-diaminopropionic acid,
. especially the residue of an aliphatic amino acid selected from alanine, valine, norvaline,
leucine, 3-aminopropionic acid, 2-aminobutyric acid, 3-aminobutyric acid, 4-aminobutyric
acid, 3-aminopentanoic acid, 4-aminopentanoic acid, S-aminopentanoic acid, 3-amino-
!'` hexanoic acid, 4-aminohexanoic acid or S-aminohexanoic acid and isoleucine or an amino
acid selected from glycine, asparagine, glutamine, methionine, lysine and phenylalanine, it
~: being possible for each of the mentioned amino acids to be in the D-, L- or (DL)-forrn,
.. ~ preferably in the L-form (except in cases where there is no asymmetric carbon atom, for
example in the case of glycine),
an ~-amino group, if present, is unsubstituted or is mono- or di-N-alkylated, for
example by lower alkyl, such as methyl, n-propyl or n-butyl, by amino-lower alkyl, such
as 3-aminopropyl, by phenyl- or naphthyl-amino-lower alkyl, such as 3-phenylamino-
propyl, by phenyl-lower alkyl, such as benzyl, by diphenylmethyl, by trityl and/or by
heterocyclyl-lower alkyl wherein heterocyclyl is as defined above for an acyl group Rl of
a carboxylic acid, especially by heterocyclylmethyl, for example furanyl-lower alkyl, such
as 2-furylmethyl, thienyl-lower alkyl, such as 2-thienylmethyl, imidazolyl-lower aLkyl,
i such as imidazol-4-ylmethyl, or 2-, 3- or 4-pyridyl-lower alkyl, such as 2-, 3- or 4-pyridyl-
` methyl, and/or is N-acylated, for example, by the acyl groups of a carboxylic acid
mentioned above in the definition of Rl, especially by unsubstituted or substituted lower
alkanoyl, as defined above, especially by acetyl, propionyl, pivaloyl, heterocyclyl-lower : :~
aLI~anoyl, as defined above for acyl Rl, for example furan-2-ylcarbonyl, S-hydroxy-
methyl-furan-2-ylcarbonyl, 2-, 3- or 4-pyridylcarbonyl, morpholinocarbonyl, thiomorpho- :
linocarbonyl, indolylacetyl or benzofuranylacetyl, aryl-lower alkanoyl, such as benzoyl or
phenylacetyl, or the acyl groups of a semiester of carbonic acid mentioned above in the :
definition of Rl, especially lower alkoxycarbonyl, such as tert-butoxycarbonyl, or aryl~
lower alkoxycarbonyl, such as benzyloxycarbonyl,
a carboxy group of the side chain is present in free form or in esterified or amidated
form, for example in the form of a lower alkyl ester group, such as methoxycarbonyl or
tert-butoxycarbonyl, an aryl ester group or an aryl-lower aLkyl ester group, wherein aryl is
l,
.
21~93~
- 16-
phenyl, 4-nitrophenyl, naphthyl, fluorenyl or biphenylyl, for example in the form of a
4-nitrophenoxycarbonyl, benzyloxycarbonyl or 9-fluorenylmethoxycarbonyl group, or ;n
the form of a carbamoyl, a lower alkylcarbamoyl, such as methylcarbamoyl, a di lower
alkylcarbamoyl, such as dimethylcMbamoyl, a mono- or di(hydroxy-lower alkyl)-
c~lrbamoyl, such as hydroxymetllylcarbnmoyl or di(hydroxymethyl)cnrbmlloyl, or n mono-
or di-(carboxy-lower alkyl)carbmnoyl group, such as n carboxymethylcarbnmoyl or
di(carboxym~thyl)carbnmoyl group,
an amino group of the side chain that is not in the o~-position is present in free form or in
alkylated form, for exnmple in the form of mono- or di-lower alkylamino, such as n-butyl-
amino or dimethylamino, or in acylated form, for example in the form of lower alkanoyl-
amino, such as acetylamino or pivaloylamino, amino-lower alkanoylamino, such as
3-amino-3,3-dimethylpropionylamino, aryl-lower alkanoylamino wherein aryl has from 6
to 14 carbon atoms, for example phenyl, naphthyl or fluorenyl, and is unsubstituted or
substituted by lower alkyl, hydroxy, lower alkoxy, carboxy, carbamoyl or by sulfamoyl,
such as 4-hydroxyphenylbutyryl, lower alkoxycarbonylamino, such as tert-butoxy-
carbonylamino, arylmethoxycarbonylamino wherein aryl has from 6 to 14 carbon atoms,
such as benzyloxycarbonylamino or 9-fluorenylmethoxycarbonylamino, piperidyl-l-
carbonyl, morpholinocarbonyl, thiomorpholinocarbonyl or S,S-dioxothiomorpholino-carbonyl, and/or
a hydroxy group of the side chain is present in free form or in etheri~led or esterified
form, for example in the form of a lower alkoxy, such as methoxy or tert-butoxy, aryl-
lower alkoxy, especially phenyl-lower alkoxy, such as benzyloxy, lower alkanoyloxy,
such as acetoxy, or lower alkoxycarbonyloxy group, for example a tert-butoxycarbonyloxy ~ :
group. ~
....
Preference is given especially to acyl groups Rl of an unsubstituted or substituted amino
acid selected from alanyl, N-lower alkylalanyl, such as N-methylalanyl, phenylalanyl,
N-(benzyloxycarbonyl)-phenylalanyl, N-(9-fluorenylmethoxycarbonyl)phenylalanyl,
aminoacetyl (glycyl), N-lower alkylaminoacetyl, N,N-di-lower alkylaminoacetyl, N-lower
alkyl-N-phenyl-lower alkylaminoacetyl, N-lower alkyl-N-imidazolyl-lower alkylamino-
acetyl, N-lower alkyl-N-pyridyl-lower alkylaminoacetyl, N-lower alkyl-N-lower alkoxy-
carbonylaminoacetyl, N-phenyl-lower alkoxycarbonyl-N-lower alkylaminoacetyl,
N-morpholino- or N-thiomorpholino-lower alkylaminoacetyl, for example N-methyl-
aminoacetyl, N,N-dimethylaminoacetyl, N-methyl-N-(n-butyl)aminoacetyl, N-methyl-N-
benzylaminoacetyl, N-methyl-N-[(2-, 3- or 4-)pyridylmethyl]-aminoacetyl, such asN-methyl-N-(2- or 3-)pyridylmethylaminoacetyl, N-(imidazol-4-ylmethyl)-N-methyl-
`.` 2108~3~
l - 17-
j aminoacetyl, N-methyl-N-tert-butoxycarbonylaminoacetyl, N-benzyloxycarbonyl-N-lower
alkylaminoacetyl, N-morpholinocarbonylaminoacetyl, 3-aminopropionyl, 2-aminobutyryl,
3-aminobutyryl, 4-aminobutyryl, 4-(N,N-dimethylamino)butyryl, 3-aminopentanoyl,
i 4-aminopentanoyl, S-aminopentanoyl, 3-aminohexanoyl, 4-nminohexanoyl or S-amino-
hexanoyl, valyl, N-phenylacetyl-valyl, N-ncetyl-vnlyl, N (3-phenylplopionyl)-vnlyl, N-t2-,
, ~ 3~ or 4-pyridylcarbonyl)-valyl, N-methoxycMbonyl-vl lyl, N isobutoxycnrbonyl-valyl,
N~tert-butoxycarbonyl-vnlyl, N-benzyloxycarbonyl-valyl, N-(morpholinoclrbonyl)-valyl,
norvalyl, leueyl, N-acetyl-leucyl, N-(2-, 3- or 4-pyridylcMbonyl)-leucyl, N-(benzyloxycar-
,~ bonyl)-leucyl, isoleucyl, N-acetyl-isoleucyl, N-propionyl-isoleucyl, N-(benzyloxy-
curbonyl)-isoleucyl, N-ttert-butoxycarbonyl)-isoleucyl, methionyl, lysyl, glutamyl,
~-(N-benzyloxycarbonyl)-glutamyl, asparagyl and ~-(N-benzyloxycarbonyl)-asparagyl,
the amino acid residues preferably being in the (L)- or the tD)- or (D,L)-form (except in
cases where there is no asymmetric carbon atom, for example in the case of Gly).
In compounds of formula I, if hydroxy or amino groups having a free hydrogen atom are
located at a carbon atom from which a double bond originates, for example in the case of
substituted lower alkenyl or lower aL~ynyl, tautomeric forms (resulting from keto/enol
tautomerism or imine/enamine tautomerism) are possible. Those and similar tautomers the
occurrence of which is familiar to a person skilled in the art are also included within the
scope of the present Application. Preference is given to compounds of formula I in which
tautomerism in the radical R1 cannot occur (e.g. where there is no bonding of -OH or -NH-
to carbon atoms from which a double bond orignates). ~ ~`
The compounds mentioned are in all cases adequately stable compounds, and not unstable ` ~;
compounds which will be immediately recognisable to a person sJcilled in the art.
Adequately stable compounds are especially those compounds that can be isolated, stored
md/or processed, for exampie to form pharmaceutical compositions.
Salts of compounds of formula I are especially acid addition salts, salts with bases or,where several salt-forming groups are present, mixed salts or internal salts, as appropriate.
Salts are especially the pharmaceutically acceptable, non-toxic salts of compounds of
formulaI.
Such salts are formed, for example, from compounds of formula I having an acid group,
for example a carboxy group, and are, for example, salts thereof with suitable bases, such
210~9~
.,
- 1 8 -
1
as non-toxic metal salts derived from rnetals of groups Ia, Ib, IIa and I[b of the Periodic
Table of the Elements, especially suitable alknli metal salts, for example lithium, sodium
,l or potassium salts, or alkaline earth metal salts, for example magnesium or calcium snlts,
also zinc salts or ammonium salts, as well ns snlts formed Wittl or~Mlic amines, such as
unsubstitllted or hydroxy-substituted mono-, di- or tri-alkylamines, espccinlly mono-, di-
or tri-lowor alkylnmines, or Wittl quaternllry ammonium compoullds, for exnmple with
N-mettlyl-N-ethylamille, diethylnmine, triethylamine, mono-, bis- or tris-(2-hydroxy-lower
alkyl)nmines, such as mono-, bis- or tris-(2-hydroxyethyl)nmine, 2-hydroxy-tert-butyl-
amine or tris(hydroxymethyl)methylamine, N,N-di-lower alkyl-N-(hydroxy-lower alkyl)-
amines, such as N,N-dimethyl-N-(2-hydroxyethyl)-amine or tri(2-hydroxyethyl)amine,
N-methyl-D-glucamine, or quaternary ammonium salts, such as tetrabutylammonium
salts. The compounds of formula I having a basic group, for example a tertiary amino
group, can form acid addition salts, for example with inorganic acids, for example a
hydrohalic acid, such as hydrochloric acid, sulfuric acid or phosphoric acid, or with
organic carboxylic, sulfonic, sulfo or phospho acids or N-substituted sulfamic acids, for
example acetic acid, propionic acid, glycolic acid, succinic acid, maleic acid, hydroxy-
maleic acid, methylmaleic acid, fumaric acid, malic acid, tartaric acid, gluconic acid,
glucaric acid, glucuronic acid, citric acid, benzoic acid, cinnamic acid, mMdelic acid,
salicylic acid, 4-aminosalicylic acid, 2-phenoxybenzoic acid, 2-acetoxybenzoic acid,
embonic acid, nicotinic acid or isonicotinic acid, as well as with amino acids, for example
the a-amino acids mendoned hereinbefore, and with methanesulfonic acid, ethanesulfonic
acid, 2-hydroxyethanesulfonic acid, ethane-1,2-disulfonic acid, benzenesulfonic acid,
4-methylbenzenesulfonic acid, naphthalene-2-sulfonic acid, 2- or 3-phosphoglycerate,
glucose-6-phosphate, N-cyclohexylsulfamic acid (forming cyclamates) or with other
acidic organic compounds, such as ascorbic acid. Compounds of forrnula I having acid
and basic groups can also form internal salts.
For isolation or purification purposes, it is also possible to use pharmaceutically unaccept-
able salts.
The terms "compounds" and "salts" expressly include also individual compounds orindividual salts.
The compounds of forrnula I have valuable pharmacological properties. For example,
after administration to a warm-blooded animal, such as a human, there are released from
those cornpounds by a metabolic route compounds of formula II which are described as
' `` 21~893~
- 19-
~.,
antiretroviral inhibitors of aspartate proteases, such as HlV-protease, and which are
suitable especially for the treatment of AIDS as inhibitors of the aspartate proteases of
HIV-1 and/or HIV-2 (and possibly other retrovimses thnt cause symptoms analogous to
~IDS) (see EP 0 346 847 (published 19 June 1991) and the other publications referred to
nbove). The bindin~ o n compound of formuln .U to HIV 1-protease is described, for
cxnmple, in J. Med. Chem. 34, 3340-3342 (1991), nnd the inhibitory action ngninst HIV-1
nnd HIV-2-protenses and the ~ntiviral inhibitory action in cell cultures are described in
Science 248, 3~8-361 (1990). Action against SIV-infected cells is also described(Biochem. Biophys. Res. Commun. 176,180-188 (1991)).
.~
The compounds of formula II are released from the compounds of formula I in the body of
the animal to be treated, preferably a warm-blooded animal including a human.
With the aid of the compounds of formula I it is possible, especially also in the case of
enteral, preferably oral, administration of the compounds of formula I, to achieve
markedly better absorption of the compound of formula II and/or a higher blood concen-
tration of the compound of formula II than is possible on administration of the compound
of formula II itself under otherwise identical conditions. It will also be possible, by means `
of the introduced radical Rl, for example, to influence the distribution of the active
ingredient in the body în a beneficial manner. Thus the problem mentioned at thebeginning of providing precursors of compounds having antiretroviral action with an
improved pharmacodynamic profile for the treatment of retroviral diseases, such as AIDS,
is solved by means of the novel compounds of formula I.
The advantageous pharmacodynamic properties can be demonstrated, for example, asfollows:
'
The compounds of formula I to be investigated or, as control, the comparison compound
of formula II, are dissolved in dimethyl sulfoxide (DMSO) in a concentration of
240 mg/ml. The resulting solutions are diluted with 20 % (w/v) hydroxypropyl-~-cyclo-
dextrin (HF3,BCD) to obtain a concentration of the test compound of 12 mg/ml. That
solution is administered to mice in a dose of 120 mg/kg by means of artificial special
feeding. 30, 60, 90 and 120 min after administration the animals are sacrificed and blood
is removed. Three or four animals are examined per time point. The blood is heparinised
and prepared for analysis using one of the following two methods: according to the first
method3 whole blood is deproteinised by mixing one part by volume of blood with one
~ 210893~
-20-
part by volume of acetonitrile; after centrifugation the supernatant is analysed byreversed-phase HE'LC. According to the second method, an internal standard is added to
the heparinised blood in a final concentrntion of 4 ,uM. Tho blood is cent~i~uged. 0.2~ ml
of plasmn is clrnwn of ~ ~md deproteinisecl with nn equnl volume of ncetonitrile. ~fter
ccntrifugntion the supernntant is concentrnted by drying i~ vacuo nnd the ~sidue is
suspencled in 20 ,ul of 3M NnCl solution ~md 100 ~ll of 0.05M phthlllnte buffer hnving a pH
of 3Ø The suspension is extrncted first with 1 ml, then with 0.2 ml of diisopropyl ether.
The diisopropyl ether solution is concentrated to dryness by evaporntion and the residue is
dissolved in 50 % (v/v) aqueous acetonitrile. The solution is analysed by reversed-phase
HPLC.
`: ~" .
The analysis by reversed-phase HPLC is carried out using a 125 x 4.6 mm Nucleosil~
Cl8-column (reversed-phase material supplied by Macherey-Nagel, Duren, Federal
Republic of Germany, based on silica gel derivatised with hydrocarbon radicals having
18 carbon atoms) equilibrated with a mobile phase of 40 % acetonitrile in water/0.1 %
trifluoroacetic acid. The flow rate is 1 mVmin. Detection is effected at 215 nm.Standards for the compounds in blood are worked up analogously to the blood samples
and used to establish standard curves on the basis of which the in vivo concentrations are
determined.
The following results are obtainable from a comparison of the compounds of forrnula I
with those of for nula II (active component): the concentradon of the active component of
formula II in the blood of mice after oral administration of a compound of formula I, for
example of a compound of formula I wherein Rl is acetyl, is at most time points,especially at all the above-mentioned time points, significantly higher, for example more
than three times higher, than when the compound of formula II is administered inunesterified form. Alternatively, or in addition, thereto the absorption of the compound of
formula I, for example of the compound of formula I wherein Rl is acetyl, is significantly
higher, for example more than four times higher, than the absorption of the compound of
formula II. It is also possible over a prolonged period to maintain a higher blood level
with a compound of formula I than with the compound of formula II.
The compounds of the present invention can also be used as commercially marketable
comparison compounds in the testing of other aspartate protease inhibitors, by using them
practically as standard compounds in order, for example, to provide for other animal
species that are used a measure of the extent to which blood levels obtained are species-
~ 2~ ~93~
~, - 21 -
''~, ~:
^; dependent. Thus on the one hand a compound not mentioned here can be investigated
: using the above-mentioned mouse rnodel, and oil the other a compound of formuln I t~mt is
mentioned here can be used as the comparison compound in n comparison witll the
co~pound to be investigated in other anirnal models..
In the definilions of compoullds of formuln I mentioned below, it nmy be ndvnntngeous,
for exnmple in orcler to replnce rather general definitions with more specific definitions, to
use definitions of radicals from the general defimitions given above or to omit individual
substituents.
, Preference is given to compounds of formula I wherein Rl is octanoyl, decanoyl,
dodecanoyl, palmitoyl, unsubstituted or substituted lower alkanoyl, lower alkenoyl or
lower alkynoyl,
wherein the substituents are selected from one or more radicals, preferably from up to
three radicals, especially from one radical or also two radicals selected frorn the. group
consisting of hydroxy, lower alkoxy, lower alkoxy-lower alkoxy, lower aLkoxy-lower
alkoxy-lower alkoxy, phenoxy, naphthyloxy, phenyl-lower alkoxy, 2-halo-lower
alkanoyl, such as 2-chloroacetyl, amino-, lower aLkylamino- or cli-lower aLkylamino-
lower alkoxy-2-lower alkanoyl, such as dimethylamino-lower alkoxyacetyl, amino-,lower alkylamino- or di-lower alkylamino-lower alkoxy-lower alkoxy-2-lower aLkanoyl,
such as dimethylamino-(2-lower alkoxyethoxy)acetyl, lower alkanoyloxy, phenyl-lower
alkanoyloxy, such as benzoyloxy or phenylacetoxy, halogen, such as fluorine, chlorine,
bromine or iodine, especially fluorine or chlorine, carboxy, lower aLkoxycarbonyl,
phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, carbamoyl, lower aLkyl-
carbamoyl, hydroxy-lower alkylcarbamoyl, di-lower alkylcarbamoyl, bis(hydroxy-lowe~
alkyl)carbamoyl, carbamoyl the nitrogen atom of which is a constituent of a 5- to
7-membered heterocyclic ring which may also contain a further hetero atom selected
from oxygen, sulfur, nitrogen and lower alkyl-substituted, such as methyl- or ethyl-
substituted, nitrogen, for example pyrrolidinocarbonyl, morpholinocarbonyl, thio~
morpholinocarbonyl, piperidin-l-ylcarbonyl, piperazin-l-ylcarbonyl or 4-lower alkyl-
piperazin-l-ylcarbonyl, such as 4-methylpiperazin-1-ylcarbonyl; cyano, oxo, cycloaLkyl,
for example ~3-C8cycloalkyl, such as cyclopropyl, cyclobutyl, cyclopentyl or cyclo-
hexyl, bicycloalkyl, for example C6-Cl2bicycloaL~cyl, such as decahydronaphth-2-yl,
endo- or exo-2-norbomyl, bicyclo[2.2.2]oct-2-yl or bicyclo[3.3. l]non-9-yl, tricycloaLI~yl,
for example Cg-Cl4tricycloaL~cyl, such as 1- or 2-adamantyl, cycloaL~senyl, for example
C4-C8cycloalkenyl, such as l-cyclohexenyl or 1,4-cyclohexadienyl, bicycloaLlcenyl, for
;``l
:i~ .
': : -~
. ~- 2~0~93~
-22-
example S-norbornen-2-yl or bicyclo[2.2.2]octen-2-yl, heterocyclyl, which is a saturated,
partially saturated or ~msaturated ring containing from 5 to 7 ring atoms and up to four
heteroatoms independently selected from nitrogcn, sulfur and oxygen, preferably 1 or 2
of the mentioned heterontoms, the ring bein~ present ns such or in once benw-,
cyclop~nta-, cyclohexn- or cyclohepta-fused form, heterocyclyl being unsubstituted or
substitutcd especinlly by lower alkyl, lower alknnoyl, hydroxy, lower alkoxy,
phenyl-lower alkoxy, such as benzyloxy, hydroxy-lower alkyl, such as hysroxymethyl,
hulogen, cyano and/or by trifluoromethyl, for example pyrrolyl, 2,~-dihydropyrrolyl,
furanyl, thienyl, tetrahydrofuranyl, cyclohepta[blpyrrolyl, pyrrolidinyl, imidazolyl,
imidazolidinyl, pyrazolinyl, pyrazolidinyl, triazolyl, such as 1,2,3-, 1,2,4- or1,3,4-triazolyl, tetrazolyl, such as 1- or 2-tetrazolyl, tetrahydro-oxazolyl, tetrahydro-
isoxazolyl, tetrahydro-thiazolyl, tetrahydro-isothiazolyl, indolyl, isoindolyl, quinolyl,
isoquinolyl, benzimidazolyl, benzofuranyl, pyridyl, pyrimidinyl, piperidinyl, piperazin-
l-yl, morpholino, thiomorpholino, S,S-dioxothiomorpholino, 1,2-dihydro- or
1,2,3,4-tetrahydro-quinolyl, or 1,2-dihydro- or 1,2,3,4-tetrahydro-isoquinolyl, the said
radicals being unsubstituted or substituted as above, especially by lower alkyl, for
example as in 4-lower alkylpiperazin-l-yl, such as 4-methyl- or 4-ethyl-piperazin-1-yl,
by lower alkanoyl, for example as in 4-lower alkanoylpiperazin-1-yl, such as 4-acetyl-
piperazin- l-yl, or by hydroxy-lower alkyl, for example as in 5-hydroxymethyl-
furan-2-yl-carbonyl, and C6-Cl2aryl, for example phenyl, naphthyl, such as 1- or2-naphthyl, indanyl, such as 1- or 2-indanyl, indenyl, such as inden-1-yl, or fluorenyl,
such as fluoren-9-yl, wherein aryl is unsubstituted or mono- or poly-substituted,
preferably mono-substituted, for example, by lower alkyl, for example methyl, halo-
lower aLcyl, such as chloro- or bromo-methyl, halogen, for example fluorine or chlorine,
hydroxy, lower alkoxy, such as methoxy, lower alkanoyloxy, carboxy, lower alkoxy-
carbonyl, phenyl-lower alkoxycarbonyl, carbamoyl, mono- or di-lower aL~cylcarbamoyl,
mono- or di-hydroxy-lower alkylcarbamoyl, halo-lower aLtcyl, such as trifluoromethyl,
heterocyclyl-lower alkyl wherein heterocyclyl is as de~med above, especially hetero-
cyclylmethyl wherein heterocyclyl is bonded via a ring nitrogen atom, for exalnple
piperidinomethyl, piperazin-1-ylmethyl, 4-lower aLkyl-piperazin-1-ylmethyl, such as
4-methyl- or 4-ethyl-piperazin- l-ylmethyl, 4-lower alkanoyl-piperazin- 1-ylmethyl, such
as 4-acetyl-piperazin- l-ylmethyl, morpholinomethyl or thiomorpholinomethyl, cyano
and/or by nitro, especially phenyl substituted in the p-position by one of the mentioned
radicals;
lower alkoxycarbonyl, for example methoxy-, ethoxy- or tert-lower aLtcoxy-carbonyl, such
as tert-butoxycarbonyl, 2-halo-lower alkoxycarbonyl, for example 2-chlor~-, 2-bromo-,
ii 2108~3~
.
. -23 -
..
-iodo- or 2,2,2-trichloro-ethoxycarbonyl; aryl-lower alkoxycarbonyl, for exa~nple
arylmethoxy-carbonyl, wherein aryl has from 6 to 14 carbon atoms and is unsubstituted or
mono- or poly-substituted, preferably mono-substituted, for example, by lower alkyl, for
example methyl, hnlo-lower alkyl, such as chloro- or bromo-methyl, halogen, for exnmple
flllorine or chlorine, hydroxy, lower nLkoxy, such as methoxy, lower alknnoyloxy,
cnrboxy, lower alkoxycarbonyl, phenyl-lower al~co~ycarbonyl, carbamoyl, mono- or di-
lower alkylcarbnrnoyl, ~nono- or di-hydroxy-lower nlkylcarbamoyl, halo-lower nlkyl, such
as trifluoromethyl, heterocyclyl-lower alkyl wherein heterocyclyl is as defined above as a
substituent of lower alkanoyl, especially heterocyclylmethyl wherein heterocyclyl is
bonded via a Ang nitrogen atom, for example piperidinomethyl, piperazin-l-ylmethyl,
4-lower aLlcyl-piperazin- l-ylmethyl, such as 4-methyl- or 4-ethyl-piperazin- l-ylmethyl,
4-lower alkanoyl-piperazin-l-ylmethyl, such as 4-acetyl-piperazin-1-ylmethyl,
morpholinomethyl or thiomorpholinomethyl, cyano and/or by nitro, and is especially .:
phenyl, 1- or 2-naphthyl, fluorenyl, or phenyl mono- or poly-substituted by lower alkyl,
for example methyl or tert-butyl, lower alkoxy, for example methoxy, ethoxy or tert-
butoxy, hydroxy, halogen, for example fluorine, chloAne or bromine, and/or by nitro, for
example phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, 4-methoxybenzyloxy-
carbonyl, 4-nitrobenzyloxycarbonyl, diphenyl-lower alkoxycarbonyl, such as diphenyl-
methoxycarbonyl, di(4-methoxyphenyl)methoxycarbonyl, trityloxycarbonyl, OI' fluorenyl-
lower alkoxycarbonyl, such as 9-fluorenylmethoxycarbonyl; or heterocyclyl-lower alkoxy-
carbonyl wherein heterocyclyl is as defined above as a substituent of lower aLlcanoyl, for
example furan-2-ylmethoxycarbonyl or pyridin-2-, -3- or -4-ylmethoxycarbonyl; or the
residue, bonded via its a- carbonyl group, of an amino acid selected from glycine, alanine,
2-aminobutyric acid, 3-aminobutyric acid, 4-aminobutyric acid, 3-aminopentanoic acid,
4-aminopentanoic acid, 5-aminopentanoic acid, 3-arninohexanoic acid, 4-aminohexanoic
acid, 5-aminohexanoic acid, valine, norvaline, leucine, isoleucine, norleucine, serine,
homoserine, threonine, methionine, cysteine, phenylalanine, tyrosine, 4-aminophenyl- .
alaDine, 4-chlorophenylalanine, 4-carboxyphenylalanine"~-phenylserine, phenylglycine,
a-naphthylalanine, cyclohexylalanine, cyclohexylglycine, tryptophan, aspartic acid, aspa-
ragine, aminomalonic acid, aminomalonic acid monoamide, glutamic acid, glutamine,
histidine, arginine, lysine, ~-hydroxylysine, ornithine, 3-aminopropanoic acid, o~ di-
aminobutyric acid and a"B-diaminopropionic acid, especially the residue of an aliphatic
amino acid selected from alanine, valine, norvaline, leucine, 3-aminopropionic acid,
2-aminobutyric acid, 3-aminobutyric acid, 4-aminobutyric acid, 3-aminopentanoic acid,
4-aminopentanoic acid, 5-aminopentanoic acid, 3-aminohexanoic acid, 4-aminohexanoic
acid, 5-aminohexanoic acid and isoleucine, or of an amino acid selected from glycine,
~`:
10893~
~, - 24 -
..,.j
:.
asparagine, glutamine, methionine, lysine and phenylalanine, it being possible fnr each of
the mentioned amino acids to be in the D-, L- or (D,L)-form, preferably in the L-form
(except in cases where there is no asymmetric carbon atom, for example in the case of
glyc;ne),
an a-nmino grollp, if present, is unsubstitutcd or is mono o~ di-N-nlkylnted by lower
nlkyl, SllCh ns methyl, n-propyl or n-butyl, by ~mino-lowcr alkyl, sucl ns 3-aminopropyl,
by phenyl- or mlphthyl-amino-lower alkyl, such as 3-phenylaminopropyl, by phenyl-lower
alkyl, such as benzyl, by diphenylmethyl, by trityl, and/or by heterocyclyl-lower alkyl
wherein heterocyclyl is as defined above for substituted lower aLlcanoyl R~, especially by
heterocyclylmethyl, for example furanyl-lower alkyl, such as 2-furylmethyl, thienyl-lower
alkyl, such as 2-thienylmethyl, imidazolyl-lower alkyl, such as irnidazol-4-ylmethyl, or 2-,
3- or 4-pyridyl-lower alkyl, such as 2-, 3- or 4-pyridylmethyl~ and/or is N-acylated, for
example, by the unsubstituted or substituted lower alkanoyl radicals mentioned above in
the definition of R1, especially by acetyl, propionyl, pivaloyl, heterocyclyl-lower alkanoyl,
as defined above for substituted lower alkanoyl Rl, for example furan-2-ylcarbonyl,
S-hydroxymethyl-furan-2-ylcarbonyl, 2-, 3- or 4-pyridylcarbonyl, morpholinocarbonyl,
thiomorpholinocarbonyl, indolylacetyl or benzofuranylacetyl, aryl-lower aL~canoyl, such as
benzoyl or phenylacetyl, lower alkoxycarbonyl, such as tert-butoxycarbonyl, or aryl-lower
alkoxycarbonyl, as defined above, for example phenyl-lower allcoxycarbonyl, such as
benzyloxycarbonyl,
a carboxy group of the side chain is present in free form, in the form of a lower aL~yl
ester group, such as methoxycarbonyl or tert-butoxycarbonyl, an aryl ester group or an
aryl-lower alkyl ester group, wherein aryl is phenyl, 4-nitrophenyl, naphthyl, fluorenyl or : .
biphenylyl, for example in the form of a 4-nitrophenoxycarbonyl, benzyloxycarbonyl or ~ -
9-fluorenylmethoxycarbonyl group, or in the form of a carbamoyl, a lower aLkyl-
carbamoyl, such as methylcarbamoyl, a di-lower alkylcarbamoyl, such as dimethyl-carbamoyl, a mon~ or di-(hydroxy-lower alkyl)carbamoyl, such as hydroxymethyl- ~ :
carbamoyl or di(hydroxymethyl)carbamoyl group, or a mono- or di-(carboxy-lower alkyl)-
carbamoyl group, such as a carboxymethylcarbamoyl or di(carboxymethyl)carbamoyl : ~:
group,
an amino group of the side chain that is not in the a-posi~ion is present in free form, in ~ ;
the form of mono- or di-lower aLkylamino, such as n-butylamino or dimethylamino, lower
alkanoylamino, such as acetylamino or pivaloylamino, amino-lower alkanoylamino, such
as 3-amino-3,3-dimethylpropionylamino, aryl-lower alkanoylamino wherein aryl has from
6 to 14 carbon atoms, for example phenyl, naphthyl or fluorenyl, and is unsubstituted or
substituted by lower aLIcyl, hydroxy, lower aLlcoxy, carboxy, carbamoyl or by sulfamoyl,
!~ ~
210893~
. . .
, - 25 -
.~'
such as 4-hydroxyphenylbutyryl, lower alkoxycarbonylamino, such as tert-butoxy-
carbonylamino, arylmethoxycarbonylamino wherein aryl has from 6 to 14 carbon atoms,
sueh as benzyloxyenrbonylamino or 9-fluorenylmethoxyenrbonylnrnino, piperidyl-l-
Mbonyl, morpholinocarbonyl, thiornorpholinocnrbonyl or S,S-dioxothiomorpholino-
enrbonyl, nnd/or
a hydroxy gronp of the side ehain is present in free form, in the form of a lower alkoxy,
sueh lls methoxy or tert-butoxy, phenyl-lower alkoxy, sueh ns benzyloxy, lower all~anoyl-
oxy, sueh as aeetoxy, or lower alkoxyearbonyloxy group, for exarnple a tert-butoxy-
enrbonyloxy group, "
and to salts of those compounds, especially pharmaceutically acceptable salts thereof.
Preference may also be given to the compounds of formula I, and salts thereof, wherein R
is aminoearbonyl or an aminocarbonyl radical wherein the amino group carries one or two
substituents selected independently of one another from
unsubstituted or substituted lower alkyl, the substituents of which are selected fromhydroxy, lower alkoxy, phenoxy, naphthyloxy, lower alkanoyloxy, phenyl-lower
alkanoyloxy, such as benzoyloxy or phenylacetoxy, halogen, such as fluorine, chlorine,
bromine or iodine, especially fluorine or chlorine, carboxy, lower alkoxycarbonyl,
phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, carbamoyl, lower alkyl-
carbarnoyl, hydroxy-lower alkylcarbamoyl, di-lower alkylcarbamoyl, bis(hydroxy-lower
alkyl)carbamoyl, cyano, oxo and C6-C12aryl, for example phenyl, naphthyl, such as 1- or
2-naphthyl, indanyl, such as 1- or 2-indanyl, indenyl, such as inden-1-yl, or fluorenyl, such
as fluoren-9-yl, aryl being unsubstituted or mono- or poly-substituted, preferably mono-
substituted, for example, by lower alkyl, for example methyl, halo-lower alkyl, such as
chloro- or bromo-methyl, halogen, for example fluoline or chlorine, hydroxy, lower
alkoxy, such as methoxy, lower aL~canoyloxy, carboxy, lower aL~coxycarbonyl, phenyl- ~ : I
lower alkoxycarbonyl, carbamoyl, mono- or di-lower alkylcarbamoyl, mono- or
di-hydroxy-lower alkylcarbamoyl, halo-lower aLIcyl, such as trifluoromethyl, cyano and/or
by nitro, especially phenyl substituted in the p-position by one of the mentioned radicals;
especially unsubstituted lower aL~cyl, such as methyl or ethyl;
and aryl which has from 6 to 14 carbon atoms and is unsubstituted or mono- or poly-
substituted, preferably mono-substituted, by lower aLtcyl, for example methyl, halo-lower
alkyl, such as chloro- or bromo-methyl, halogen, for example fluorine or chlorine,
hydroxy, lower alkoxy, such as methoxy, lower aL~canoyloxy, carboxy, lower aL~coxy-
carbonyl, phenyl-lower aL~coxycarbonyl, carbamoyl, mono- or di-lower aL~cylcarbamoyl,
~. 210~93~
- 26 -
mono- or di-hydroxy-lower alkylcarbarnoyl, halo-lower alkyl, such as trifluoromethyl,
heterocyclyl-lower alkyl wherein heterocyclyl is as defined above as a substituent of lower
alkanoyl, especially heterocyclylmethyl wherein heterocyclyl is bonded via n ring nitrogen
ntom, for example piperidinomethyl, pipernzin-l-ylmethyl, 4-lower alkyl-pipernzin-1-yl-
methyl, such as 4-methyl- or 4-ethyl-piperazin-1-ylmetllyl, 4-lower lllknnoyl-piper-
axin-1-ylmethyl, such ns 4-acetyl-piperazin-1-ylmethyl, morpllolillomethyl or thiomorpho-
linomethyl, eyano and/or by nitro, preferably eorrespondingly substituted phenyl or 1- or
2-nnphthyl, not more than one of tlle substituents of the arninoearbonyl radieal being aryl;
Rl being espeeially aminoearbonyl, mono- or di-lower alkylaminoearbonyl, such as N-
methyl-, N-ethyl-, N,N-dimethyl- or N,N-diethyl-aminoearbonyl, or phenyl-lower alkyl-
aminocarbonyl wherein phenyl is unsubstituted or substituted by the radicals mentioned in
the definition of aryl, for example by lower allyl, for example methyl, halo-lower alkyl,
such as chloro- or bromo-methyl or trifluoromethyl, halogen, for example fluorine or
chlorine, hydroxy, lower alkoxy, sueh as methoxy, earboxy and/or by eyano, preferably by
up to three of those substituents selected independently of one another, especially by one
of those substituents, for example in the p-position, such as in N-benzyl-, N-(4-fluoro-
benzyl)-, N-(4-chlorobenzyl~-, N-t4-trifluoromethylbenzyl)- or N-(4-eyanobenzyl)-amino-
earbonyl; espeeially aminoearbonyl substituted by only one radical at the nitrogen atom, :
for example N-lower alkylaminocarbonyl, sueh as N-methyl- or N-ethyl-aminocarbonyl,
or phenyl-lower alkylaminocarbonyl wherein phenyl is unsubstituted or substituted by the :
radicals mentioned in the definition of aryl, for example by lower aUcyl, such as methyl,
halo-lower alkyl, sueh as ehloro- or bromo-methyl or trifluoromethyl, halogen, such as .
fluorine or ehlorine, hydroxy, lower alkoxy, such as methoxy, carboxy and/or by cyano,
preferably by up to three of those substituents selected independently of one another,
especially by one of those substituents, for example in the p-posi~ion, such as in :
N-benzyl-, N-(4-fluorobenzyl)-, N-(4-chlorobenzyl)-, N-(4-trifluoromethylbenzyl)- or
N-~4-cyanobenzyl)-aminocarbonyl.
Preference is given especially to compounds of fonnula I wherein R1 is unsubstituted or
substituted lower aLIsanoyl, lower aLIcenoyl or lower aLIcynoyl, ~ :
wherein the substituents are selected from one or more radicals, preferably from up to
three radicals, especially from one radical selected from the group consisting of hydroxy,
lower alkoxy, phenoxy, naphthyloxy, lower aLlcanoyloxy, phenyl-lower alkanoyloxy,
such as benzoyloxy or phenylacetoxy, halogen, such as fluorine, chlorine, bromine or
iodine, especially fluorine or chlorine, carboxy, lower aL~coxycarbonyl, phenyl-lower
alkoxycarbonyl, such as benzyloxycarbonyl, carbamoyl, lower alkylcarbamoyl,
.,,
~ 210~93~
- 27 -
'' J,
hydroxy-lower alkylcarbamoyl, di-lower alkylcarbamoyl, bis(hydroxy-lower alkyl)-carbamoyl, carbamoyl the nitrogen atom of which is a constituent of a 5- to 7-membered
heterocyclic ring that mny nlso contain a filrther hetero ~Itorn solected fiom oxygen,
lllfur, nitrogen ancl lower allcyl-substituted, such ns met~lyl- or ethyl-substituted,
nitrogcn, for example pyrrolidillocarbollyl, morpholinocarbonyl, thioltlorpholino-
carbonyl, piperidin-l-ylcarbonyl, piperazin-1-ylcarbonyl or 4-lower nlkylpipernzin-l-yl-
carbonyl, such as 4-methylpiperazin-1-ylcarbonyl; cyano, oxo, cycloalkyl, for example
C3-C8cycloalkyl, such as cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl, bicyclo-
alkyl, for example C6-C12bicycloaLkyl, such as decahydronaphth-2-yl, endo- or exo-
2-norbornyl, bicyclo[2.2.2]oct-2-yl or bicyclo[3.3.1]non-9-yl, tricycloalkyl, for exarnple
C9-Cl4tricycloalkyl, such as 1- or 2-adamantyl, cycloalkenyl, for example C4-C8cyclo-
aLkenyl, such as 1-cyclohexenyl or 1,4-cyclohexadienyl, bicycloaL~cenyl, for example
5-norbornen-2-yl or bicyclo[2.2.2]octen-2-yl, heterocyclyl, which is preferably a
saturated, partially saturated or unsaturated ring containing from 5 to 7 ring atoms and up
to four heteroatoms independently selected from nitrogen, sulfur and oxygen, preferably
1 or 2 of the mentioned heteroatoms, the ring being present as such or in once benzo-,
cyclopenta-, cyclohexa- or cyclohepta-fused form, heterocyclyl being unsubstituted or
substituted especially by lower alkyl, lower alkanoyl, hydroxy, lower alkoxy,
phenyl-lower alkoxy, such as benzyloxy, hydroxy-lower aL~cyl, such as hysroxymethyl,
halogen, cyano andlor by tritluoromethyl, for example pyrrolyl, 2,5-dihydropyrrolyl,
furanyl, thienyl, tetrahydrofuranyl, cyclohepta[b]pyrrolyl, pyrrolidinyl, imidazolyl,
imidazolidinyl, pyrazolinyl, pyrazolidinyl, triazolyl, such as 1,2,3-, 1,2,4- or ~ ~
1,3,4-triazolyl, tetrazolyl, such as 1- or 2-tetrazolyl, tetrahydro-oxazolyl, tetrahydro- ~ -
isoxazolyl, tetrahydro-thiazolyl, tetrahydro-isothiazolyl, indolyl, isoindolyl, quinolyl,
isoquinolyl, benzimidazolyl, benzofuranyl, pyridyl, pyrimidinyl, piperidinyl, piperazin-
1-yl, morpholino, thiomorpholino, S,S-dioxothiomorpholino, 1,2-dihydro- or 1,2,3,4-
tetrahydro-quinolyl, or 1,2-dihydro- or 1,2,3,4-tetrahydro-isoquinolyl, the mentioned
radicals being unsubstituted or substituted as above, especially by lower aL1cyl, for
example as in 4-lower aL~yl-piperazin- 1-yl, such as 4-methyl- or 4-ethyl-piperazin- 1 -yl,
by lower alkanoyl, for example as in 4-lower alkanoyl-piperazin- 1-yl, such as 4-acetyl-
piperazin-1-yl, or by hydroxy-lower aL~yl, for example as in 5-hydroxymethyl-
furan-2-yl-carbonyl, and C6-CI2aIyl, for example phenyl, naphthyl, such as 1- or2-naphthy2, indanyl, such as 1- or 2-indanyl, indenyl, such as inden-1-yl, or fluorenyl,
such as fluoren-9-yl, wherein aryl is unsubstituted or mono- or poly-substituted,
preferably mono-substituted, for example, by lower alkyl, for example methyl, halo-
lower alkyl, such as chloro- or bromo-methyl, halogen, for example fluorine or chlorine,
.~.: - , . . .
2lo89~l~
- 28 -
hydroxy, lower alkoxy, such as methoxy, lower aL~canoyloxy, carboxy, lower alkoxy-
carbonyl, phenyl-lower alkoxycarbonyl, carbamoyl, mono- or di-lower alkylcarbamoyl,
mono- or di-hydroxy-lower alkylcarbamoyl, halo-low~r alkyl, such ns trifluoromethyl,
heterocyclyl-lower alkyl wherein heterocyclyl is ns defined above, especially hetero-
cyclylmethyl wherein heterocyclyl is bonded via n rin$ nitrogen ntom, for exnmple
piperidinomethyl, pipernzin-l-ylmethyl, 4-lower nlkyl-piperazin-l-ylmethyl, such ns
4-methyl- or 4-ethyl-piperazin-1-ylmethyl, 4-lower alkanoyl-piperazin-l-ylmethyl, such
as 4-acetyl-piperazin-1-ylmethyl, morpholinomethyl or thiomorpholinomethyl, cyano
~md/or by nitro, especially phenyl substituted in the p-position by one of the mentioned
radicals;
lower alkoxycarbonyl, for example methoxy-, ethoxy- or tert-lower alkoxy-carbonyl, such
as tert-butoxycarbonyl, 2-halo-lower alkoxycarbonyl, for example 2-chloro-, 2-bromo-,
-iodo- or 2,2,2-trichloro-ethoxycarbonyl; aryl-lower alkoxycarbonyl, for examplearylmethoxy-carbonyl, wherein aryl has from 6 to 14 carbon atoms and is unsubstituted or `:
mono- or poly-substituted, preferably mono-substituted, for example, by lower alkyl, for
example methyl, halo-lower alkyl, such as chloro- or bromo-methyl, halogen, for example
fluorine or chlorine, hydroxy, lower alkoxy, such as methoxy, lower alkanoyloxy, ; . : :
carboxy, lower alkoxycarbonyl, phenyl-lower aLkoxycarbonyl, carbamoyl, mono- or di- ~ .
lower alkylcarbamoyl, mono- or di-hydroxy-lower alkylcarbamoyl, halo-lower alkyl, such
as trifluoromethyl, heterocyclyl-lower alkyl wherein heterocyclyl is as defined above as a - ~:
substituent of lower alkanoyl, especially heterocyclylmethyl wherein heterocyclyl is
bonded via a ring nitrogen atom, for example piperidinomethyl, piperazin-l-ylmethyl,
4-lower alkyl-piperazin-l-ylmethyl, such as 4-methyl- or 4-ethyl-piperazin- l-ylmethyl, ~ :
4-lower aLkanoyl-piperazin-l-ylmethyl, such as 4-acetyl-piperazin-1-ylmethyl, morpho-
linomethyl or thiomorpholinomethyl, cyano and/or by nitro, and is especially phenyl, 1- or
2-naphthyl, fluorenyl, or phenyl mono- or poly-substituted by lower alkyl, for example
methyl or tert-butyl, lower alkoxy, for example methoxy, ethoxy or tert-butoxy, hydroxy, -:
halogen, for example fluorine, chlorine or bromine, and/or by nitro, for exarnple phenyl~
lower alkoxycarbonyl, such as benzyloxycarbonyl, 4-methoxybenzyloxycarbonyl, 4-nitro- :~
benzyloxycarbonyl, diphenyl-lower aL1coxycarbonyl, such as diphenylmethoxycarbonyl,
di(4-methoxyphenyl)methoxycarbonyl, trityloxycarbonyl, or fluorenyl-lower aLkoxy-
carbonyl, such as 9-fluorenylmethoxycarbonyl; or heterocyclyl-lower aLkoxycarbonyl
wherein heterocyclyl is as defined above as a substituent of lower alkanoyl, for example
furan-2-ylmethoxycarbonyl or pyridin-2-, -3- or -4-ylmethoxycarbonyl; or the residue,
bonded via its o~-carbonyl group, of an amino acid selected from glycine, alanine,
2-aminobutyric acid, 3-aminobutyric acid, 4-aminobutyric acid, 3-aminopentanoic acid,
: J
2lass~ .
i - 29 -
~.,
4-aminopentanoic acid, S-aminopentanoic acid, 3-aminohexanoic acid, 4-aminohexanoic
j acid, S-aminohexanoic acid, valine, norvaline, leucine, isoleucine, norleucine, serine,
homoserine, threonine, methionine, cysteine, phenylalnnine, tyrosine, 4-aminophenyl-
alanine, 4-chlorophenylalanine, 4-carboxyphenylalaninc"~-phellylserille, phenylglycine,
a-nnpllthylnlanine, cyclohexylalnnine, cyclohexylglycille, tryptophMI, nspnrtic ncid, nspn-
1 r~lginc, nminomnlonic ncid, nminomalonic ncid mononmitle, glutamic ncid, glll~nmine,
histidine, nrgininc, lysine, ~-hydroxylysine, ornitbine, 3-Mnh~opropnnoic acid, a,~-di-
nminobutyric acicl and a"B-diaminopropionic acid, especially the residue of nn aliphatic
, Mmino acid selected from alanine, valine, norvaline, leucine, 3-aminopropionic acid,
2-aminobutyric acid, 3-aminobutyric acid, 4-aminobutyric acid, 3-aminopentanoic acid,
4-aminopentanoic acid, 5-aminopentanoic acid, 3-aminohexanoic acid, 4-aminohexanoic
, acid, S-aminohexanoic acid and isoleucine, or of an amino acid selected from glycine,
.1 asparagine, glutamine, methionine, lysine and phenylalanine, it being possible for each of ~`
the mentioned amino acids to be in the D-, L- or (DL)-form, preferably in the L-form
(except in cases where there is no asymmetric carbon atom, for example in the case of . :
glycine), ~ `
an a-amino group, if present, is unsubstituted or is mono- or di-N-alkylated by lower ~;;
alkyl, such as methyl, n-propyl or n-butyl, by arnino-lower alkyl, such as 3-aminopropyl,
by phenyl- or naphthyl-amino-lower alkyl, such as 3-phenylaminopropyl, by phenyl-lower
alkyl, such as benzyl, by diphenylmethyl, by trityl and/or by heterocyclyl-lower alkyl
wherein heterocyclyl is as defined above for substituted lower alkanoyl Rl, especially by
heterocyclylmethyl, for example furanyl-lower alkyl, such as 2-furylmethyl, thienyl-lower
alkyl, such as 2-thienylmethyl, or 2-, 3- or 4-pyridyl-lower alkyl, such as 2-, 3- or
4-pyridylmethyl, and/or is N-acylated, for example, by the unsubstituted or substituted
lower alkanoyl radicals mentioned above in the definition of Rl, especially by acetyl,
propionyl, pivaloyl, heterocyclyl-lower alkanoyl, as defined above for substituted lower
alkanoyl Rl, for example furan-2-ylcarbonyl, S-hydroxymethyl-furan-2-ylcarbonyl, 2-, 3-
or 4-pyridylcarbonyl, morpholinocarbonyl, thiomorpholinocarbonyl, indolylacetyl or
benzofuranylacetyl, aryl-lower alkanoyl, such as benzoyl or phenylacetyl, lower aLlcoxy-
carbonyl, such as tert-butoxycarbonyl, or aryl-lower alkoxycarbonyl, as defined above, for
example phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl,
a carboxy group of the side chain is present in free form, in the form of a lower aLkyl
ester group, such as methoxycarbonyl or tert-butoxycarbonyl, an aryl ester group or an
aryl-lower alkyl ester group, wherein aryl is phenyl, 4-nitrophenyl, naphthyl, fluorenyl or
biphenylyl, for example in the form of a 4-nitrophenoxycarbonyl, benzyloxycarbonyl or
9-fluorenylmethoxycarbonyl group, or in the forrn of a carbamoyl, a lower aLkyl-
210~93~
,,
- 30 -
'I
, carbamoyl, such as methylcarbamoyl, a di-lower alkylcarbamoyl, such as dimethyl-
carbamoyl, a mono- or di-(hydroxy-lower alkyl)carbamoyl, such as hydroxymethyl-
carbamoyl or di(hydroxymethyl)carbamoyl, or a mono- or di-(carboxy-lower alkyl)-carbamoyl group, such as a carboxymethylcarbamoyl or di(carboxyme,thyl)carbarnoyl
:` gl'Ollpl
an nmino group of the side chain that is not in the o~-position is present in free form, in
the form of mono- or di-lower alkylamino, such as n-butylamino or dimethylarnino, lower
alkalloylamino, SUC}l as acetylamino or pivaloylamino, amino-lower alkanoylatnino, such
as 3-amino-3,3-dimethylpropionylamino, aryl-lower alkanoylamino wherein nryl has from
6 to 14 carbon atoms, for example phenyl, naphthyl or fluorenyl~ and is unsubstituted or
substituted by lower alkyl, hydroxy, lower alkoxy, carboxy, carbamoyl or by sulfamoyl,
such as 4-hydroxyphenylbutyryl, lower alkoxycarbonylamino, such as tert-butoxy-
carbonylamino, arylmethoxycarbonylamino wherein aryl has from 6 to 14 carbon atoms,
l such as benzyloxycarbonylamino or 9-fluorenylmethoxycarbonylamino, piperidyl-l-
carbonyl, morpholinocarbonyl, thiomorpholinocarbonyl or S,S-dioxothiomorpholino-
carbonyl, and/or
a hydroxy group of the side chain is present in free form or in the form of a lower
alkoxy, such as methoxy or tert-butoxy, phenyl-lower alkoxy, such as benzyloxy, lower
alkanoyloxy, such as acetoxy, or lower alkoxycarbonyloxy group, for example a tert-
butoxycarbonyloxy group,
. and to salts of those compounds, especially pharmaceu~ically acceptable salts thereof.
Preference may also be given to the compounds of formula I, and salts thereof, wherein R
is aminocarbonyl or an aminocarbonyl radical wherein the amino group carries one or two
substituents selected independendy of one another from
unsubstituted or substituted lower alkyl, the substituents of which are selected fiom
hydroxy, lower alkoxy, phenoxy, naphthyloxy, lower alkanoyloxy, phenyl-lower
alkanoyloxy, such as benzoyloxy or phenylacetoxy, halogen, such as fluorine, chlorine,
bromine or iodine, especially fluorine or chlorine, carboxy, lower alkoxycarbonyl,
phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, carbamoyl, lower alkyl-
carbamoyl, hydroxy-lower alkylcarbamoyl, di-lower alkylcarbamoyl, bis(hydroxy-lower
aL~cyl)carbamoyl, cyano, oxo and C6-CI2aryl, for example phenyl, naphthyl, such as 1- or
2-naphthyl, indanyl, such as 1- or 2-indanyl, indenyl, such as inden-1-yl, or fluorenyl, such
as fluoren-9-yl, aryl being unsubstituted or mono- or poly-substituted, preferably mono-
substituted, for example, by lower alkyl, ~or example methyl, halo-lower alkyl, such as
chloro- or bromo-methyl, halogen, for example fluorine or chlorine, hydroxy, lower
: :~
~ 2~893~
- 31 -
`'l
alkoxy, such as methoxy, lower alknnoyloxy, cnrboxy, lower allcoxycarbonyl, phenyl-
lower alkoxycarbonyl, carbamoyl, mono- or di-lower nlkylcnrbnmoyl, mono- or
di-llydroxy-lower nlkylcarbamoyl, hnlo-lower nlkyl, such as trifluoromethyl, cyano (md/or
by nit~o, especially phenyl substituted in the p-pOSitiOII by one of the mentioned radicals;
especinlly Imsubstitllte(l lower alkyl, sllcll ns methyl or ethyl;
and from aryl which has from 6 to 14 carbon atoms and is ullsllbstituted or mono- or
poly-substituted, preferably mono-substituted, by lower alkyl, for example methyl, halo-
lower alkyl, such as chloro- or bromo-methyl, halogen, for example floorine or chlorine,
hydroxy, lower alkoxy, such as methoxy, lower alkanoyloxy, carboxy, lower alkoxy- ~ .
carbonyl, phenyl-lower alkoxycarbonyl, carbamoyl, mono- or di-lower alkylcarbamoyl,
mono- or di-hydroxy-lower alkylcarbamoyl, halo-lower alkyl, such as trifluoromethyl, :
heterocyclyl-lower alkyl wherein heterocyclyl is as defined above as a substituent of lower :
alkanoyl, especially heterocyclylmethyl wherein heterocyclyl is bonded via a ring nitrogen
atom, for example piperidinomethyl, piperazin-1-ylmethyl, 4-lower alkylpiperazin-l-yl-
methyl, such as 4-methyl- or 4-ethyl-piperazin-1-ylmethyl, 4-lower alkanoyl-piper-
azin-1-ylmethyl, such as 4-acetyl-piperazin-1-ylmethyl, morpholinomethyl or thiomorpho-
linomethyl, CyMo and/or by nitro, preferably correspondingly substituted phenyl or 1- or
2-naphthyl, not more than one of the substituents of the aminocarbonyl radical being aryl;
especially aminocarbonyl, mono- or di-lower alkylaminocarbonyl, such as N-methyl-,
N-ethyl-, N,N-dimethyl- or N,N-diethyl-aminocarbonyl, or phenyl-lower aLkylamino-
carbonyl wherein phenyl is unsubstituted or substituted by the radicals mentioned in the
definition of aryl, for example by lower alkyl, for example methyl, halo-lower alkyl, such
as chloro- or bromo-methyl or trifluoromethyl, halogen, for example fluorine or chlorine,
hydroxy, lower alkoxy, such as methoxy, carboxy and/or by cyano, preferably by up to
three of those substituents selected independently of one another, especially by one of
those substituents, for example in the p-position, such as in N-benzyl-, N-(4-fluoro-
benzyl)-, N-(4-chlorobenzyl)-, N-(4-trifluoromethylbenzyl)- or N-(4-cyanobenzyl)-amino-
carbonyl; especially arninocarbonyl substituted by only one radical at the nitrogen atom,
for example N-lower alkylaminocarbonyl, such as N-methyl- or N-ethyl-arninocarbonyl,
or phenyl-lower alkylaminocarbonyl wherein phenyl is unsubstituted or substituted by the
radicals mendoned in the de~mition of aryl, for example by lower alkyl, such as methyl,
halo-lower alkyl, such as chloro- or bromo-methyl or trifluoromethyl, halogen, such as
fluorine or chlorine, hydroxy, lower aL~coxy, such as methoxy, carboxy and/or by cyano,
preferably by up to three of those substituents selected independently of one another,
especially by one of those substituents, for example in the p-position, such as in
-r~
`` 2~893~
,i
- 32 -
; l N-benzyl-, N-(4-fluorobenzyl)-, N-t4-chlorobenzyl)-, N-(4-trifluoromethylbenzyl) or
- ~ N-(4-cyanobenzyl)-aminocarbonyl.
.. 1 : . . .
Grenter preference is givcn to cornpounds of formuln I whereill Rl is octnnoyl, decnnoyl,
dodecnnoyl, palmitoyl, unsubstitllted or substitllted lower nlkanoyl,
.. whereill the substituents are selected from OIIC to three radicals, especially from one
radical or also two radicals selected from the group consisdng of hydroxy, lower alkoxy,
lower alkoxy-lower alkoxy, lower alkoxy-lower alkoxy-lower alkoxy, phenoxy,
naphthyloxy, phenyl-lower alkoxy, 2-halo-lower alkMoyl, such as 2-chloroacetyl,
amino-, lower alkylarnino- or di-lower alkylamino-lower alkoxy-2-lower alkanoyl, such
as dimethylamino-lower alkoxyacetyl, amino-, lower aLtcylamino- or di-lower alkyl- :: :
amino-lower alkoxy-lower alkoxy-2-lower alkanoyl, such as dirnethylamino-(2-lower
alkoxyethoxy)acetyl, lower alkanoyloxy, phenyl-lower alkanoyloxy, such as benoyloxy
or phenylacetoxy, halogen, such as fluorine, chlorine, bromine or iodine, especially
fluorine or chlorine, carboxy, lower alkoxycarbonyl, phenyl-lower alkoxycarbonyl, such ~:
as benzyloxycarbonyl, carbamoyl, lower alkylcarbamoyl, hydroxy-lower alkyl-
carbamoyl, di-lower alkylcarbamoyl, bis(hydroxy-lower alkyl)carbamoyl, cyano, oxo, ~ ~
C3-C8cycloalkyl, such as cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl, : . :
C4-C8cycloalkenyl, such as 1-cyclohexenyl or 1,4-cyclohexadienyl, py~rolyl, 2,5-di- . ; . .
hydropyrrolyl, furanyl, thienyl, tetrahydrofuranyl, pyrrolidinyl, imidazolyl, imidazo-
lidinyl, pyrazolyl, pyrazolidinyl, tetrahydro-oxazolyl, tetrahydro-isoxazolyl, tetrahydro-
thiazolyl, tetrahydro-isothiazolyl, indolyl, isoindolyl, quinolyl, isoquinolyl,
benzimidazolyl, benzofuranyl, pyridyl, pyrimidinyl, piperidinyl, piperazin-1-yl, mor-
pholino, thiomorpholino, S,S-dioxothiomorpholino, 1,2-dihydro- or 1,2,3,4-tetrahydro-
quinolyl or 1,2-dihydro- or 1,2,3,4-tetrahydro-isoquinolyl, the said heterocyclic radicals ~ ~ :
being unsubstituted or substituted by lower alkyl, lower aLI~anoyl, hydroxy, lower aL~coxy,
phenyl-lower aL~coxy, such as benzyloxy, hydroxy-lower aLIcyl, such as hydroxymethyl,
halogen, cyano and/or by trifluoromethyl, especially by lower aL1~yl, for example as in
4-lower alkyl-piperazin- l-yl, such as 4-methyl- or 4-ethyl-piperazin-1-yl, by lower
alkanoyl, for example as in 4-lower allcanoyl-piperazin-1-yl, such as 4-acetyl-piper-
azin-1-yl, or by hydroxy-lower alkyl, for example as in 5-hydroxymethyl-furan-2-yl-
carbonyl, and aryl selected from phenyl, naphthyl, such as 1- or 2-naphthyl, indanyl,
such as 1- or 2-indanyl, indenyl, such as inden-1-yl, and fluorenyl, such as fluoren-9-yl,
those radicals being unsubstituted or mono- or poly-substituted, preferably mono-
substituted, for example, by lower alkyl, for example methyl, halo-lower aLkyl, such as
chloro- or bromo-methyl, halogen, for exa nple fluorine or chlorine, hydroxy, lower
,.~,.. . . ~ ",.. . .
i~ 2~0893~
33 -
.~
alkoxy, such as methoxy, lower alkanoyloxy, carboxy, lower alkoxycarbonyl, phenyl-
lower alkoxycarbonyl, carbamoyl, mono- or di-lower alkylcarbMmoyl, mono- or ~ .
di-hydroxy-lower alkylcarbamoyl, halo-lower alkyl, such as trifluoromethyl, piperidino-
methyl, piperazin-1-ylmethyl, 4-lower alkyl-piperazin-1-ylmethyl, such as 4-methyl- or
4-ethyl-pipernzin-1-ylmethyl, 4-lower alkanoyl-pipernzin-l-ylmethyl, such ns 4-ncetyl-
pipernzin-1-ylmethyl, morpholinometllyl, thiomorpllolinomethyl, cyallo mld/or by nitro,
especinlly phenyl substituted in the p-position by one of thc mentioned rndicnls;
or the residue, bonded via its a-carbonyl group, of nn amino ncid selected from glycine,
alnnine, 2-aminobutyric acid, 3-aminobutyric acid, ~-aminobutyric acid, 3-aminopentanoic
acid, 4-Mminopentanoic acid, S-MminOpentMOiC acid, 3-MminohexMoic acid, 4-Mmino-hexanoic acid, S-aminohexanoic acid, valine, norvaline, leucine, isoleucine, norleucine,
serine, homoserine, threonine, methionine, cysteine, phenylalMine, tyrosine, 4-amino-
phenylalanine, 4-chlorophenylalMine, 4-carboxyphenylalanine, ~-phenylserine, phenyl-
glycine, a-naphthylalanine, cyclohexylalanine, cyclohexylglycine, tryptophM, aspartic
acid, asparagine, Mminomalonic acid, Mminomalonic acid monoamide, glutamic acid,glutMmine, histidine, arginine, lysine, ~-hydroxylysine, ornithine, 3-Mminopropanoic acid,
a,~-diMminobutyric acid Md a"l~-diMminopropionic acid, especially the residue of an
aliphatic MminO acid selected from alanine, valine, norvaline, leucine, 3-aminopropionic
acid, 2-Mminobutyric acid Md isoleucine or of an MminO acid selected from glycine,
asparagine, glutMmine, methionine, lysine Md phenylalMine, or of an Mmino acid selected
from 3-Mminobutyric acid, 4-Mminobutyric acid, 3-aminopentMoic acid, 4-aminopentanoic
acid, S-MminopentMoic acid, 3-Mminohexanoic acid, 4-Mminohexanoic acid and S-amino-
hexanoic acid, it being possible in all cases for each of the mentioned amino acids to be in
the D-, L- or (D,L)-form, preferably in the L-form (except in cases where there is no
asymmetric carbon atom, for example in the case of glycine),
M ~-Mmino group, if present, is unsubstituted or is mono- or di-N-allcylated by lower
aLkyl, such as me :hyl, n-propyl or n-butyl, by Mmino-lower aLlcyl, such as 3-aminopropyl,
by phenyl- or naphthyl-aminGlower aLkyl, such as 3-phenylaminopropyl, by phenyl-lower
aLI~yl, such as benzyl, by diphenylmethyl, by trityl, by furMyl-lower aL~cyl, such as 2-fuIyl-
methyl, by thienyl-lower aL~cyl, such as 2-thienylmethyl, by imidazolyl-lower alkyl, such
as imidazol-4-ylmethyl, and/or by 2-, 3- or 4-pyridyl-lower aLIcyl, such as 2-, 3- or
4-pyridylmethyl, Md/or is N-acylated by the unsubstituted or substituted lower alkMoyl
radicals mentioned above in the definition of Rl, especially by acetyl, propionyl, pivaloyl, ~ .
furan-2-ylcarbonyl, 5-hydroxymethyl-furan-2-ylcarbonyl, 2-, 3- or 4-pyridylcarbonyl,
morpholinocarbonyl, thiomorpholinocarbonyl, indolylacetyl or benzofuranylacetyl, .
phenyl-lower aL~anoyl, such as benzoyl or phenylacetyl, lower aL~coxycarbonyl, such as
~ ~ .
21~93~
, .
34
.;3
;~ tert-butoxycarbonyl, or phenyl-lower alkoxycMbonyl, such as benzyloxycarbonyl,
.1 and to pharmaceutically acceptable salts thereof. Greater preference may also be given to
the compounds of formula I and the salts thereof wherein Rl is aminocarbonyl, mono- or
di-lower alkylaminocarbonyl, such ns N-rnethyl-, N-ethyl-, N,N-dimetbyl- or
; ~ N,N-dicthyl-aminocMbonyl, prefcrnbly mono-lower nlkylamillocarbonyl, or phenyl-lower
~`1 nlkylnrninocarbonyl wherein phenyl is unsubstituted or substituted by the ladicals
nncntioned in the definition of Myl, for example by lower alkyl, for example methyl, halo-
lower alkyl, such as chloro- or bromo-methyl or trifluoromethyl, halogen, for example
fluorine or chlorine, hydroxy, lower alkoxy, such as methoxy, carboxy and/or by cyano,
preferably by up to three of those substituents selected independently of one another,
especially one of those substituents, for example in the p-position, such as in N-benzyl-,
N-(4-fluorobenzyl)-, N-(4-chlorobenzyl)-, N-(4-trifluoromethylbenzyl)- or N-(4-cyano-
.~ benzyl)-aminocarbonyl.
33
3 Preference is given more especially to compounds of formula I wherein Rl is
unsubstituted or substituted lower alkanoyl,
wherein the substituents are selected from one to three radicals, especially from one
radical selected from the group consisting of hydroxy, lower alkoxy, phenoxy,
naphthyloxy, lower alkanoyloxy, phenyl-lower alkanoyloxy, such as benzoyloxy or
phenylacetoxy, halogen, such as fluorine, chlorine, bromine or iodine, especially fluorine
or chlorine, carboxy, lower alkoxycarbonyl, phenyl-lower alkoxycarbonyl, such asbenzyloxycarbonyl, carbamoyl, lower alkylcarbamoyl, hydroxy-lower aLtcylcarbamoyl,
di-lower alkylcarbamoyl, bis(hydroxy-lower alkyl)carbamoyl, cyano, oxo, C3-C8cyclo-
alkyl, such as cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl, C4-C8cycloaL~cenyl,
.~ such as l-cyclohexenyl or 1,4-cyclohexadienyl, pyrrolyl, 2,5-dihydropyrrolyl, furanvl,
- thienyl, tetrahydrofuranyl, pyrrolidinyl, imidazolyl, imidazolidinyl, pyrazolinyl, pyrazol-
idinyl, tetrahydro-oxazolyl, tetrahydro-isoxazolyl, tetrahydro-thiazolyl, tetrahydro-
isothiazolyl, indolyl, isoindolyl, quinolyl, isoquinolyl, benzimidazolyl, benzofuranyl,
3 pyridyl, pyrimidinyl, piperidinyl, piperazin- l-yl, morpholino, thiomorpholino,
S,S-dioxothiomorpholino, 1,2-dihydro- or 1,2,3,4-tetrahydro-quinolyl or 1,2-dihydro- or
1,2,3,4-tetrahydro-isoquinolyl, the said heterocyclic radicals being unsubstituted or
substituted by lower alkyl, lower aLIcanoyl, hydroxy, lower aLtcoxy, phenyl-lower aL~coxy,
such as benzyloxy, hydroxy-lower aLIcyl, such as hydroxymethyl, halogen, cyano andlor
by trifluoromethyl, especially by lower alkyl, for example as in 4-lower aL~yl-piperazin-
l-yl, such as 4-methyl- or 4-ethyl-piperazin-1-yl, by lower alkanoyl, for example as in
4-lower alkanoyl-piperazin-l-yl, such as 4-acetyl-piperazin-1-yl, or by hydroxy-lower
. . ~
:` ~
:
'.,J~ ' 21~893-~
- 35 -
alkyl, for example as in 5-hydroxymethyl-furan-2-ylcarbonyl, and aryl selected from . ~ .
phenyl, naphthyl, such as 1- or 2-naphthyl, indallyl, SllCh as` 1- or 2-indanyl, indenyl,
s~lch as inden-l-yl, and fluorenyl, such as fluoren-9-yl, those radicals being unsubstituted
or mono- or poly-substituted, preferably mono-substituted, for example, by lower alkyl,
for exnmple methyl, hnlo-lower alkyl, such ns chloro- or bromo-methyl, halogen, for
examplo fluorine or chlorine, hydroxy, lower alkoxy, such as methoxy, lower alkanoyl-
oxy, earboxy, lower alkoxycarbonyl, phenyl-lower alkoxycarbonyl, carbamoyl, mono- or
di-lower alkylcarbamoyl, mono- or di-hydroxy-lower aL~cylcarbarnoyl, halo-lower aL~cyl,
such as trifluoromethyl, pipelidinomethyl, piperazin-1-ylmethyl, 4-lower alkyl-piper-
azin-l-ylmethyl, such as 4-methyl- or 4-ethyl-piperazin-1-ylmethyl, 4-lower alkanoyl-
piperazin-l-ylmethyl, such as 4-acetyl-piperazin-1-ylmethyl, morpholinomethyl,
thiomorpholinomethyl, cyano Md/or by nitro, especially phenyl substituted in thep-position by one of the mentioned radicals;
or the residue, bonded via its a-carbonyl group, of an amino acid selected from glycine,
alanine, 2-aminobutyric acid, 3-aminobutyric acid, 4-aminobutyric acid, 3-aminopentanoic
acid, 4-aminopentanoic acid, S-aminopentanoie acid, 3-aminohexanoic acid, 4-amino-
hexanoic acid, S-aminohexanoic acid, valine, norvaline, leucine, isoleucine, norleucine,
serine, homoserine, threonine, methionine, cysteine, phenylalanine, tyrosine, 4-amino-
phenylalanine, 4-chlorophenylalanine, 4-carboxyphenylalanine"B-phenylserine, phenyl-
glycine, a-naphthylalanine, cyclohexylalanine, eyclohexylglycine, tryptophan, aspartic
acid, asparagine, aminomalonic acid, aminomalonic acid monoamide, glutamic acid,glutamine, histidine, arginine, lysine, ~-hydroxylysine, ornithine, 3-aminopropanoic acid,
a,~-diaminobutyric acid and a,~-diaminopropionic acid, especially the residue of an
aliphatic amino acid selected from alanine, valine, norvaline, leucine, 3-aminopropionic
acid, 2-aminobutyric acid and isoleucine or of an amino acid selected from glycine,
asparagine, glutamine, methionine, lysine and phenylalanine, or of an amino acid selected
from 3-aminobutyric acid, 4-aminobutyric acid, 3-aminopentanoic acid, 4-arninopentanoic
acid, 5-aminopentanoic acid, 3-aminohexanoic acid, 4-aminohexanoic acid and 5-amino-
hexanoic acid, it being possible in all cases for each of the mentioned amino acids to be in
the D-, L- or (D,L)-form, preferably in the L-form (except in cases where there is no
asymmetric carbon atom, for example in the case of glycine),
an a-a~mino group, if present, is unsubstituted or is mono- or di-N-aLtcylated by lower
alkyl, such as methyl, n-propyl or n-butyl, by amino-lower alkyl, such as 3-aminopropyl,
by phenyl- or naphthyl-amino-lower aLIcyl, such as 3-phenylaminopropyl, by phenyl-lower
alkyl, such as benzyl, by diphenylmethyl, by trityl, by furanyl-lower alkyl, such as 2-furyl-
methyl, by thienyl-lower alkyl, such as 2-thienylmethyl, andJor by 2-, 3- or 4-pyridyl-
. ~ ,.
~ 210~93~
- 36 -
.¦ lower alkyl, such as 2-, 3- or 4-pyridylmethyl, nnd/or is N-acylated by the unsubstituted or
substituted lower alkanoyl radicals mentioned nbove in the definition of Rl, especially by
acetyl, propionyl, pivnloyl, furnn-2-ylcarbonyl, 5-hydroxymethyl-furnn-2-ylcarbonyl, 2-~
3- or 4-pyridylcMbonyl, rnorpholinocarbonyl, thiomorpholinocarbonyl, indolylacetyl or
benzofilrnnylncetyl, pllenyl-lower nlknnoyl, snch ns benzoyl or phenylacetyl, lower
~1 alkoxycarbonyl, sncll as tert-butoxyclubonyl, or phenyl-lower alkoxycarbonyl, such as
I benzyloxycarbonyl, and to ph~umaceuticnlly acceptable salts thereof. Special preference
may also be given to the compounds of formula I and snlts thereof wherein R1 is amino- .
3 carbonyl, mono- or di-lower alkylaminocarbonyl, such as N-methyl-, N-ethyl-, N,N-di-
methyl- or N,N-diethyl-aminocarbonyl, preferably mono-lower alkylaminocarbonyl, or
:~ phenyl-lower alkylarninocarbonyl wherein phenyl is unsubstituted or substituted by the
radicals mentioned in the definition of aryl, for example by lower alkyl, .for example
methyl, halo-lower alkyl, such as chloro- or bromo-methyl or trifluoromethyl, halogen, for
example fluorine or chlorine, hydroxy, lower alkoxy, such as methoxy, carboxy andtor by
cyano, preferably by up to three of those substituents selected independently of one
another, especially one of those substituents, for example in the p-position, such as in
N-benzyl-, N-(4-fluorobenzyl)-, N-(4-chlorobenzyl)-~ N-(4-trifluoromethylbenzyl)- or
N-(4-cyanobenzyl)-aminocarbonyl.
Still greater preference is given to compounds of formula I wherein Rl is octanoyl,
decanoyl, dodecanoyl, palmitoyl, lower alkanoyl, such as forrnyl, acetyl, propionyl,
butyryl, methylpropionyl, n-pentanoyl, pivaloyl, hexanoyl or heptanoyl, hydroxy-lower
alkanoyl, for example ,B-hydroxypropionyl, lower alkoxy-lower alkanoyl, for example
lower alkoxyacetyl or lower alkoxypropionyl, such as methoxyacetyl, 3-methoxypropionyl
or n-butoxyacetyl, lower alkoxy-lower aLkoxy-lower alkanoyl, such as 2-(2-methoxy-
ethoxy)acetyl, lower alkoxy-lower aLkoxy-lower alkoxy-lower alkanoyl, such as
2-(2-(2-methoxyethoxy)ethoxy)acetyl, phenoxy-lower alkanoyl, for example phenoxy-
acetyl, phenyl-lower aLkoxy-lower alkanoyl, such as benzyloxyacetyl, 2-halo-lower
alkanoyl, such as 2-chloroacetyl, amino-, lower aLkylamino- or di-lower alkylamino-lower
alkoxy-2-lower alkanoyl, such as dimethylamino-lower aLkoxyacetyl, amino-, lower aLkyl-
amino- or di-lower alkylamino-lower alkoxy-lower aLkoxy-2-lower alkanoyl, such as
dimethylamino-(2-lower aLkoxyethoxy)acetyl, lower alkanoyloxy-lower alkanoyl, for
example lower alkanoyloxyacetyl or lower aLkanoyloxypropionyl, such as acetoxyacetyl
or ,B-acetoxypropionyl, carboxy-lower aLkanoyl, such as carboxyacetyl or 3-carboxy-
propionyl, oxo-lower alkanoyl, for example acetoacetyl or propionylacetyl, 5-hydroxy-
methyl-furan-2-ylcarbonyl, 2- or 3-pyrrolylcarbonyl, furylcarbonyl, for example 2-furyl-
,~: .- , - . ~: ~ .; . .
; i 2 1 0 ~ 9 3 4
"
- 37 -
;.,~i,
carbonyl, thienylcarbonyl, for example 2-thienylcarbonyl, pyridyl-lower alk~moyl, such as
pyridylcarbonyl, for example 2-, 3- or 4-pyridylcarbonyl, pyridylacetyl, for example
2-pyridylacetyl, or pyridylpropionyl, for example 3-(2-pyridyl)propionyl, quinolyl-
~ carbonyl, such as quinolin-2-ylcarbonyl, isoquinolinylcarbonyl, such ns isoquinolin-
:ll 3-ylcarbonyl, 2-, 3- or S-indolylcnrbonyl, pyrrolidinyl-(2- or 3-)cnrbonyl, 2-, 3- or
`.'j ~-piperi~inylcarbonyl, 1,2,3,4-tetrnhydroquinolyl-2-, -3- or-4-carbonyl, 1,2,3,4-tetrn-
hy(lroisoquillolyl-1-, -3- or -4-cMbonyl, imidnzolyl-lower nlknnoyl, such ns imidazolyl-
carbonyl, for example imidazol-l-ylcarbonyl or imidnzol-4-ylcarbonyl, imidazolylacetyl,
for example 4-imidazolylacetyl, or imidazolylpropionyl, for example 3-(4-imidazolyl)-
. ~ propionyl, pyrazolyl-lower alkanoyl, such as 1-pyrazolylcarbonyl, morpholinocarbonyl,
thiomorpholinocarbonyl, morpholinoacetyl, thiomorpholinoacetyl, 4-lower alkyl-1-piper-
~;~ azinoacetyl, such as 4-methylpiperazinoacetyl, indolylacetyl, benzofuranylacetyl, phenyl-
.. lower alkanoyl, for example benzoyl, phenylacetyl or 3-phenylpropionyl, that is
.1 unsubstituted or mono- or poly-substituted in the phenyl radical by lower aLIcyl, for
~ example methyl, halo-lower alkyl, such as chloro- or bromo-methyl, halogen, for example
fluorine or chlorine, hydroxy, lower alkoxy, for example methoxy, piperidinomethyl,
.~ piperazin-1-ylrnethyl, 4-lower alkyl-piperazin-1-ylmethyl, such as 4-methyl- or 4-ethyl-
piperazin-1-ylmethyl, morpholinomethyl, thiomorpholinomethyl, cyano and/or by nitro,
for example 4-chloromethyl-, 4-bromomethyl-, 4-fluoro-, 4-chloro-, 4-methoxy-,
4-morpholinomethyl-, 4-thiomorpholinomethyl-, 4-cyano- or 4-nitro-benzoyl, 4-methyl-
phenylacetyl, 4-methoxyphenylacetyl, 3-(p-hydroxyphenyl)-propionyl or 2-lower
alkoxy-2-phenylacetyl, such as (R)- or (S)-2-methoxy-2-phenylacetyl; or is the residue,
bonded via thea-carbonyl group, of an aliphatic amino acid selected from alanine, valine,
norvaline, leucine, 3-aminopropionic acid, 2-aminobutyric acid and isoleucine or of an
amino acid selected from glycine, asparagine, glutamine, methionine, Iysine and phenyl-
alanine, or of an amino acid selected from 3-aminobutyric acid, 4-aminobutyric acid,
3-aminopentanoic acid, 4-aminopentanoic acid, 5-aminopentanoic acid, 3-aminohexanoic
acid, 4-aminohexanoic acid and 5-aminohexanoic acid, it being possible for each of the
mentioned amino acids to be in the D-, L- or (DL)-form, preferably in the L-form (except
where there are no asymmetric carbon atoms, for example in the case of glycine); an
a-amino group, if present, is unsubstituted or is mono- or di-N-alkylated by lower aL~cyl,
such as methyl, n-propyl or n-butyl, by phenyl-lower aL~cyl, such as benzyl, by : ~:
' imidazolyl-lower alkyl, such as imidazol-4-ylmethyl, andlor by 2-, 3- or 4-pyridyl-lower
aLtcyl, such as 2-, 3- or 4-pyridylmethyl, and/or is N-acylated by lower aLtcoxycarbonyl, :
such as tert-butoxycarbonyl, or by benzyloxycarbonyl, for example as in alanyl, N-lower
aL~cylalanyl, such as N-methylalanyl, phenylalanyl, aminoacetyl (glycyl), N-lower alkyl-
2 1 0 8 9 3 4
. , .
- 38 -
aminoacetyl or N,N-di-lower aLkylaminoacetyl, such as N-methylaminoacetyl,
N,I~-dimethylaminoacetyl or N-methyl-N-(n-butyl)aminoacetyl, N-lower alkyl-N-phenyl-
lower alkylaminoacetyl, such as N-methyl-N-benzylaminoacetyl, N-lower alkyl-
. 1 N-pyridyl-lower alkylaminoacetyl, for example N-methyl-N-~(2-, 3- or 4-)pyridyl-
methyll-aminoacetyl, such as N-methyl-N-(2- or 3-pyridylmetllyl)mninoac~tyl,
N-(imidnzolyl-lower nlkyl)-N-lower alkylaminoncetyl, such ns N-(imidnzol-4-yl-
methyl)-N-methylalmilloncetyl, N-phenyl-lower nlkoxycnrbonyl-N-lower nlkylamino-acctyl, SllCh as N-benzyloxycarbonyl-N-methylmniQoacetyl, 2-nminobutyryl,
~l 4-(N,N-dimethylamino)butyryl, valyl, norv~lyl, leucyl, isoleucyl, methionyl, lysyl,
glutamyl or asparagyl, wherein the amino acid residues (with the exception of glycine) are
preferably in the (L)-form, and to pharrnaceudcally acceptable salts the eof. Greater
preference may also be given to the compounds of formula I and salts thereof wherein R
is aminocarbonyl, mono- or di-lower alkylaminocarbonyl, such as N-methyl-, N-ethyl-,
N,N-dimethyl- or N,N-diethyl-aminocarbonyl, preferably mono-lower alkylaminocar-bonyl, or phenyl-lower alkylaminocarbonyl wherein phenyl is unsubsdtuted or subsdtuted
by the radicals mentioned in the definidon of aryl, for example by lower alkyl, for
example methyl, halo-lower alkyl, such as chloro- or bromo-methyl or trifluoromethyl,
halogen, for e.xample fluorine or chlorine, hydroxy, lower alkoxy, such as methoxy,
carboxy and/or by cyano, preferably by up to three of those subsdtuents selectedindependently of one another, especially by one of those substituents, for example in the
p-posidon, such as in N-benzyl-, N-(4-fluorobenzyl)-, N-(4-chlorobenzyl)-, N-(4-trifluoro-
methylbenzyl)- or N-(4-cyanobenzyl)-aminocarbonyl.
Sdll greater preference is given especially to compounds of formula I wherein Rl is lower
alkanoyl, such as acetyl, propionyl, butyryl, pivaloyl, hexanoyl or heptauloyl, hydroxy-
lower alkanoyl, for example ~-hydroxypropionyl, lower alkoxy-lower aLIcanoyl, for
example lower alkoxyacetyl or lower alkoxypropionyl, such as methoxyacetyl or .
,B-methoxypropionyl, phenoxy-lower aLkanoyl, for example phenoxyacetyl, lower
alkanoyloxy-lower alkanoyl, for example lower alkanoyloxyacetyl or lower alkanoyloxy-
propionyl, such as acetoxyacetyl or ,B-acetoxypropionyl, oxo-lower aL~canoyl, for example : - .1.
acetoacetyl or propionylacetyl, 5-hydroxymethyl-furan-2-ylcarbonyl, 2- or 3-pyrrolyl-
carbonyl, furylcarbonyl, for example 2-furylcarbonyl, thienylcarbonyl, for example : .
2-thienylcarbonyl, pyridylcarbonyl, for example 2-, 3- or 4-pyridylcarbonyl, 2-, 3- or
5-indolylcarbonyl, pyrrolidinyl-3-carbonyl, 2-, 3- or 4-piperidinylcarbonyl, 1,2,3,4-tetra-
hydroquinolyl-2-, -3- or-4-carbonyl, 1,2,3,4-tetrahydroisoquinolyl-1-, -3- or-4-carbonyl, ;
imidazolylcarbonyl, such as imidazol-1-ylcarbonyl, morpholinocarbonyl, thiomorpholino-
~; i
~:i
~; :
2~ 0893~
7i - 39-
.,
carbonyl, morpholinoacetyl, thiomorpholinoacetyl, 4-lower aL~yl-l-piperazinoacetyl, such
;:,,as 4-methyl-piperazinoacetyl, indolylacetyl, benzofuranylacetyl, phenyl-lower allcanoyl,
.~for example benzoyl, phenylacetyl or 3-phenylpropionyl, that is unsubstitllted or mono or
poly substituted in the phenyl radicnl by lower alkyl, for exnmple methyl, hnlo lower
~1alkyl, sucll as chloro or brorno methyl, ha10gen, Çor exnmple ~luorine or chlorille,
hydroxy, lowcr nlkoxy, for exmnple methoxy, piperidinomethyl, piperazin l ylmethyl,
4 lower nlkyl piperazin l ylmethyl, such as 4 mctbyl or 4 ethyl-pipernzin-1-ylmethyl,
~Imorpllolillomethyl, tlliomorpholinomethyl, cynno nnd/or by nitro, for exnmple 4-chloro-
~methyl, 4 bromomethyl, 4 fluoro-, 4-chloro, 4 methoxy, 4-morpholinomethyl, 4-thio-
morpholinomethyl-, 4-cyano- or 4-nitro-benzoyl, 4-methylphenylacety;l, 4-methoxy-
phenylacetyl or 3-(p-hydroxyphenyl)-propionyl; or is the residue, bonded via itsa-carbonyl group, of an aliphatic amino acid selected from alanine, valine, norvaline,
:~leucine, 3-aminopropionic acid, 2-aminobutyric acid and isoleucine or of an amino acid
31selected from glycine, asparagine, glutamine, methionine, Iysine and phenylalanine, or of
an amino acid selected from 3-aminobutyric acid, 4-aminobutyric acid, 3-aminopentanoic
Jacid, 4-aminopentanoic acid, S-aminopentanoic acid, 3-aminohexanoic acid,
4-aminohexanoic acid and S-aminohexanoic acid, it being possible for each of thementioned amino acids to be in the D-, L- or (DL)-form, preferably in the L-form (except
where there are no asymmetric carbon atoms, for example in the case of glycine); an
a-amino group, if present, is unsubstituted or is mono- or di-N-aLt~ylated by lower aL~cyl,
such as methyl, n propyl or n-butyl, by phenyl-lower alkyl, such as benzyl, and/or by 2-,
3- or 4-pyridyl-lower alkyl, such as 2-, 3- or 4-pyridylmethyl, and/or is N-acylated by
lower alkoxycarbonyl, such as tert-butoxycarbonyl, or by benzyloxycarbonyl, for example : .
as in alanyl, N-lower aLItylalanyl, such as N-methylalanyl, phenylalanyl, aminoacetyl
(glycyl), N-lower alkylaminoacetyl or N,N-di-lower alkylaminoacetyl, such as
N-methylaminoacetyl, N,N-dimethylaminoacetyl or N-methyl-N-(n-butyl)aminoacetyl, `:
N-lower alkyl-N-phenyl-lower alkylaminoacetyl, such as N-methyl-N-benzylaminoacetyl,
N-lower alkyl-N-pyridyl-lower allylaminoacetyl, for example N-methyl-N-[(2-, 3- or
4-)pyridylmethyl]-aminoacetyl, such as N-methyl-N-(3-pyridylmethyl)aminoacetyl, :
N-phenyl-lower alkoxycarbonyl-N-lower aL~cylaminoacetyl, such as
N-benzyloxycarbonyl-N-methylaminoacetyl, 2-aminobutyryl, valyl, norvalyl, leucyl,
isoleucyl, methionyl, lysyl, glutamyl or asparagyl, wherein the amino acid residues (with
the exception of glycine) are preferably in the (L)-form, and pharmaceutically acceptable
salts thereo Greater preference may also be given to compounds of formula I and salts
thereof wherein Rl is aminocarbonyl, mono- or di-lower aL~ylaminocarbonyl, such as
N-methyl-, N-ethyl-, N,N-dimethyl- or N,N-diethyl-aminocarbonyl, preferably mono-
. . . ~ .
;~
210893~ ~
...; ~ .
- 40 -
~.1
~1 lower aL~cylaminocarbonyl, or phenyl-lower aL~cylaminocarbonyl wherein phenyl is
~ unsubstituted or substituted by the radicals mentioned in the definition of aryl, for
example by lower alkyl, for example methyl, halo-lower alkyl, SllCh as chloro- or bromo-
methyl or trifluoromethyl, halo~en, for examplo fluorinc or chlorine, hydroxy, lower
alkoxy, such as methoxy, carboxy and/or by cyano, preferably by Up to thlee of those
substitllellts selected independently of one another, especially by one of those substituents,
for exarnple in the p-position, such ns in N-benzyl-, N-t4 fluorobenzyl)-, N-(4-chloroben-
zyl)-, N-(4-trifluoromethylbenzyl)- or N-(4-cyanobenzyl)-aminocarbonyl.
Preference is given especially to compounds of formula I wherein Rl is oct~moyl,:j decanoyl, dodecanoyl, palmitoyl, lower alkanoyl, such as acetyl, propionyl, butyryl,
methylpropionyl, n-pentanoyl, pivaloyl, hexanoyl or heptanoyl, lower alkoxy-lower
alkanoyl, such as methoxyacetyl, 3-methoxypropionyl or 4-butoxyacetyl, lower alkoxy-
lower alkoxy-lower alkanoyl, such as 2-(2-methoxyethoxy)ethoxyacetyl, lower alkoxy-
lower alkoxy-lower alkanoyl, such as 2-(2-(2-methoxyethoxy)ethoxy)acetyl, phenoxy-
lower alkanoyl, such as phenoxyacetyl, phenyl-lower alkoxy-lower alkanoyl, such as
benzyloxyacetyl~ 2-halo-lower alkanoyl, such as 2-chloroacetyl, amino-, lower allcyl-
amino or di-lower alkylamino-lower alkoxy-2-lower alkanoyl, such as dimethylamino-
lower alkoxyacetyl, amino-, lower alkylamino- or di-lower alkylamino-lower alkoxy-
lower alkoxy-2-lower alkanoyl, such as dimethylamino-(2-lower alkoxyethoxy)acetyl,
2-lower alkoxy-2-phenylacetyl, such as (R)- or (S)-2-methoxy-2-phenylacetyl, furyl-
carbonyl, for example furan-2-ylcarbonyl, pyridyl-lower alkanoyl, for example pyridyl-
carbonyl, such as 2-, 3- or 4-pyridylcarbonyl, pyridylacetyl, such as 2-pyridylacetyl, or
pyridylpropionyl, such as 3-(pyridin-2-yl)propionyl, quinolylcarbonyl, such as quinolin
2-ylcarbonyl, isoquinolylcarbonyl, such as isoquinolin-3-ylcarbonyl, pyrrolidinyl-
2-carbonyl, especially L- or D-prolyl, imidazolyl-lower alkanoyl, such as 4-imidazolyl-
carbonyl, imidazolylacetyl, for example 4-imidazolylacetyl, or imidazolylpropionyl, such
as 3-(4-imidazolyl)propionyl, pyrazolyl-lower alkanoyl, such as 1-pyrazolylcarbonyl, - ~:
phenyl-lower aLIcanoyl, for example benzoyl, phenylacetyl or 3-phenylpropionyl, 4-chloro- ~ ~.
methylbenzoyl, 4-morpholinomethylbenzoyl, 4-thiomorpholinomethylbenzoyl, amino- ~ ~ :
acetyl, N-lower aLtcylaminoacetyl or N,N-di-lower aLtcylaminoacetyl, such as N-methyl-
aminoacetyl, N,N-dimethylaminoacetyl or N-methyl-N-(n-butyl)aminoacetyl, N-loweraLlcyl-N-phenyl-lower alkylaminoacetyl, such as N-methyl-N-benzylaminoacetyl, N-lower
aLkyl-N-benzyloxycarbonylaminoacetyl, such as N-methyl-N-benzyloxyaminoacetyl,
N-imidazolyl-lower aL1cyl-N-lower alkylaminoacetyl, such as N-(imidazol-4-ylmethyl)-
N-methylaminoacetyl, N-lower alkyl-N-pyridyl-lower alkylaminoacetyl, for example
2~ ~93~
- 41 -
N-methyl-N-[(2-, 3- or 4-)pyridylmethyl]-aminoacetyl, such as N-methyl-N-(2- or 3-pyri-
dylmethyl)aminoacetyl, or 4-(N,N-dimethylamino)butyryl, and to pharmaceutically
acceptable salts thereof.
Pro~crence is giVCIl more especinlly to compoull~ls of fomluln I wherein Rl is lower
nlkanoyl, such ns ncetyl, propionyl, butyryl, pivnloyl, hexnnoyl or heptnnoyl, furylcnr-
bonyl, for example furml-2-ylcarbonyl, pyridylcarbonyl, for example 2-, 3- or 4-pyridyl-
carbonyl, phellyl-lower alkanoyl, for example benzoyl, phenylncetyl or 3-phenylpropionyl,
4 morpholinomethylbenzoyl, 4-thiomorpholinomethylbenzoyl, aminoacetyl, N-lower
alkylaminoacetyl, N,N-di-lower alkylaminoacetyl, such as N-methylaminoacetyl,
N,N-dimethylaminoacetyl, N-methyl-N-(n-butyl)aminoacetyl, N-lower alkyl-N-phenyl-
lower alkylMminoacetyl, such as N-methyl-N-benzylaminoacetyl, or N-lower alkyl-
N-pyridyl-lower alkylaminoacetyl, for example N-methyl-N-[(2-, 3- or 4-)pyridyl- -
methyl]-aminoacetyl, such as N-methyl-N-(3-pyridylmethyl)aminoacetyl, and to pharma-
ceutically acceptable salts thereof.
Very special preference is given to compounds of ~ormula I wherein Rl is lower alkMoyl,
such as acetyl, lower alkoxy-lower alkanoyl, such as methoxyacetyl, pyridylcarbonyl, such
as pyridin-2-ylcarbonyl, or furylcarbonyl, such as furM-2-ylcarbonyl, and to pharmaceut-
ically acceptable salts thereo
Preference is given more especially to compounds of formula I wherein R1 is lower
alkMoyl, such as acetyl, or furylcarbonyl~ such as furan-2-ylcarbonyl, and to phannaceut-
ically acceptable salts thereof. - -
Preference is given very especially also to compounds of formula I wherein R1 is lower
alkoxy-lower alkanoyl, such as methoxyacetyl, or pyridylcarbonyl, such as pyridin-2-yl-
carbonyl, and to pharmaceutically acceptable salts thereof.
Preference is given most especially to compounds of formula I wherein R1 is lower
alkanoyl, especially acetyl, and to pharmaceutically acceptable salts thereof.
Special preference is given to the compounds of formula I mentioned in the Examples and
to the pharmaceutically acceptable salts thereof.
The compounds of the formula I and salts of such compounds having at least one
~ 210893~
~ - 42 - :
.;;~ .
salt-forming group are obtained in accordance with processes known per se, for example: ~
:
:~ a) by react;ng n compound of formula II, as defined above, with a carboxylic ncid of
forrnula I~l
,
I~l'OH (~).
wherein Rl is as defined, or with a reactive derivative thereof, free functional groups in
the starting materials of formulae II and m that are not intended to par~icipate in the
reaction being if necessary in protected form, and removing any protecting groups present,
cr
b) by amidating an amino compound of formula IV
H~ ~N
C
'I ;
wherein Rl is as defined, or a reactive derivative thereof, with a carboxylic acid of
formula V
O .:
~ ~OH (V)
or with a reactive acid derivative thereof, free functional groups in the staIting materials of
fonnulae IV and V that are not intended to participate in the reaction being if necessary in
I
Ll~
~i,
'! 2 1 0 8 9 3
! - 43 -
, I protected form, and removing any protecting groups present, or
` I c) by amidating an amino compound of formula VI
H ~
o c~ (Vl)
.
, . -~
wherein Rl is as defined, or a reactive derivative thereof, with a carboxylic acid of
formula VII ~ ;
... ..
. NH2 ,
~ ~N)\l~
or with a reactive acid derivative thereof, free functional groups in the starting materials of
formulae VI and VII that are not intended to participate in the reaction being if necessary
in protected form, and removing any protecting groups present,
and, if desired, converting a compound of formula I obtainable in accordance with the
above process having at least one salt-forming group into its salt and/or converting an
~. 2~Q8~3~
`~`1 - 44 -
obtainable salt into the free compound or into a different salt and/or separating any
isomeric mixtures of compounds of formula I that are obtainable and/or converting a
compound of formula I according to the invention into a different compound of forrmlla I
according to the invention.
The proccsses defined nbove are clescribed in cletail below:
.~
~! Process a~ Prep~arntion of ~m ncylnted compound
The preparation of an ester is effected, for example, in a manner known per se using an
`I acid of forrnula III wherein Rl is as defined with the exception of aminocarbonyl and the
radical of an N-subsdtuted carbamic acid, or using a reactive derivative thereof. A
suitable reactive derivative is, for exarnple, a compound of forrnula IIIa
Rl-Zl (~a), ~
., ,~ .
wherein Rl is as last defined and Zl is especially reactively activated hydroxy. The free
carboxylic acid of formula III can be activated, for exarnple, by strong acids, such as a
hydrohalic, sulfuric, sulfonic or carboxylic acid, or by acidic ion exchangers, for exarnple
hydrochloric, hydrobromic or hydriodic acid, sulfuric acid, an unsubstituted or substituted,
for example halo-substituted, alkanecarboxylic acid, or by an acid of formula ~
preferably using an excess of the acid of formula m, if necessary with the binding of
resulting water of reaction by water-binding agents, with removal of the water of reaction
by azeotropic distillation or with extractive esterification, by acid anhydrides, especially
inorganic acid anhydrides, such as carboxylic acid anhydrides, such as lower aLlcane-
carboxylic acid anhydrides (with the exception of formic acid anhydride), for example
acetic anhydride, or by suitable activating or coupling reagents of the type listed below,
especially also in sftu. Rl-Zl may also be a carboxylic acid azide (obtainable, for
example, by reaction of a corresponding acid ester via the corresponding hydrazide and
treatment thereof with nitrous acid); a carboxylic acid halide, especially an acid chloride ~ -
or bromide, obtainable, for example, by reaction with organic acid halides, especially with
oxalyl dihalides, such as oxalyl dichloride, or especially with inorganic acid halides, for
example with acid halides of phosphorus or sulfur, such as phosphorus trichloride,
phosphorus tribromide, phosphorus pentachloride, phosphorus pentabromide, phosphorus
oxychloride, phosphorus oxybromide, thionyl chloride or thionyl bromide, or especially
under mild conditions with tetra-lower aLIcyl-a-halo-enamines, such as tetramethyl-
cc-halo-enamines, especially l-chloro-N,N,2-trimethyl-1-propenamine (preferably by
210~93~
~,
- 45 -
reaction in inert solvents, especially chlorinated hydrocarbons, such as methylene chloride
or chloroform, or ethers, such as diethyl ether, dioxane or tetrahydrofilran, at preferred
temperatures of from -78 to 50C, especially from -60 to 30C, for example from -10C to
room temperature (cf. Devos, ~., et al., J. C. S. Chem~ Commun. 1~79, 1180-1181, and
Hnvenux, P~., et al., Org. Synth. ~, 26 (1980)), it being possible for the resulting acid
halide, ~or example the ncid chloride of formula IIIn wherein Zl is chlorine, also to be
further processed in situ, for example by reaction with the compound of formula II in the
presence of tertiary nitrogen bases, such as pyridine and/or 4-dimethylaminopyridine
(DMAP, which is preferably added in catalydc amounts), at preferred temperatures of
from -20 to 50C, especially from 0C to room temperature); an activated ester wherein Z
is especially cyanomethoxy, nitrophenoxy, such as 4-nitrophenoxy or 2,4-dinitrophenoxy,
or poly-halophenoxy, such as pentachlorophenoxy; or a symmetrical or, preferably,
asymmetrical acid anhydride which can be obtained, for example, by the reaction of a salt,
for example an alkali metal salt, su~h as the sodium or potassium salt, of an acid of
formula III or its co-reactant, preferably a lower alkanecarboxylic acid, such as acetic
acid, with a corresponding complementary acid halide, especially, in the case of the
reaction of a salt of a carboxylic acid of formula III, a carboxylic acid halide, for example
chloride, such as acetyl chloride, and, in the case of the reaction of a carboxylic acid
halide of formula IIIa wherein Zl is halogen, for example chlorine or bromine, with a salt
of a lower alkanecarboxylic acid, especially sodium or potassium acetate. There may be
used as acdvating and coupling reagents for acdvadng carboxylic acids of formula III in
situ especially carbodiimides, for example N,N'-di-Cl-C4alkyl- or N,N'-di-Cs-C7cyclo-
alkyl-carbodiimide, such as diisopropylcarbodiimide or N,N'-dicyclohexylcarbodiimide,
advantageously with the addidon of an acdvadng catalyst, such as N-hydroxysuccinimide
or unsubsdtuted or substituted, for example halo-, Cl-C7alkyl- or Cl-C7alkoxy-substituted,
N-hydroxy-benzotriazole or N-hydroxy-S-norbornene-2,3-dicarboxamide, Cl-C4alkylhalo-
formate, for example isobutyl chloroformate, suitable carbonyl compounds, for example
N,N-carbonyldiimidazole, suitable 1,2-oxazolium compounds, for example 2-ethyl-5-
phenyl- 1 ,2-oxazolium 3 '-solfonate or 2-tert-butyl-5-methyl-isoxazolium perchlorate,
suitable acylamino compounds, for example 2-ethoxy-1-ethoxycarbonyl-1,2-dihydro-quinoline, or suitable phosphoryl cyanamides or azides, for example diethylphosphoryl
cyanamide or diphenylphosphoryl azide, also triphenylphosphine disulfide or
l-CI-C4alkyl-2-halopyridinium halides, for example 1-methyl-2-chloropyridinium iodide.
Zl is preferably halogen, such as chlorine or bromine, and acyloxy, for example lower
alkanoyloxy, such as acetoxy.
-
`~ 2~0~934
-46-
~j
For the specific case of the introduction of an acyl radical of a semiester of carbonic acid
linked via its carbonyl group to the bonding oxygen atom there are suitnble especially the
compounds of formula IIla wherein Zl is halogen, such as chlorine, which can be
~¦ prepared, for cxample, by reaction of the complementary nlcohols, for exnmple
unsubstituted or substituted alkyl alcohols, aryl-lower alkyl alcohols or h~terocyclyl-lower
alkyl alcohols whereirl the radicals are as defined in the definition of unsubstituted or
substituted alkoxycarbonyl, aryl-lower alkoxycarbonyl or heterocyclyl-lower
alkoxycarbonyl Rs, with phosgene or with analogues thereof that contain other halogen
atoms, especially bromine, instead of chlorine, preferably in the presenc:e of tertiary
nitrogen bases, such as pyridine or triethylamine, and in inert solvents, ~or example
chlorinated hydrocarbons, such as methylene chloride or chloroform, ethers, such as
diethyl ether, tetrahydrofuran or dioxane, or carboxylic acid amides, such as dimethyl-
formamide. Also suitable are corresponding N-carbonyl azolides of formula lIIa (Zl = an
N-containing heterocycle, such as 1-imidazolido) which are obtained, for example, by
reaction with the corresponding N,N'-carbonyl diazolides, such as N,N-carbonyl
diimidazole, under conditions such as those just described for phosgene and analogues
with other halogen atoms. The reacdon of compounds of formula II with corresponding
compounds of formula ma then likewise takes place under those conditions tcf. Staab,
H. A., Angew. Chemie ~, 407 (1962)).
For the specific case of the introducdon of aminocarbonyl Rl or of an N-substituted
carbarnic acid radical as acyl group Rl there is suitable as activated acid derivative
especially the corresponding isocyanate of formula IIIb
.
Q-N=C=O (lIIb)
wherein Q is a protecting group, for example trihaloacetyl, such as trifluoro- or trichloro-
acetyl, or one of the unsubstituted or substituted lower alkyl radicals or aryl radicals
mentioned above in the definition of an aminocarbonyl radical Rl wherein the amino
group carries 1 or 2 substituents, it being possible, when Q is an amino-protecting group
to obtain after the reaction with the compound of formula II the corresponding compound
of formula I wherein Rl is free aminocarbonyl by removal of a protecting group Q as
described below for the freeing of amino protected by acyl, especially by acid hydrolysis,
or, when Q is one of the mentioned substituted or unsubstituted lower aLkyl or alyl
radicals, a corresponding compound of formula I having aminocarbonyl mono-substituted
:~ ~
i 2~93~
47 -
,
at the nitrogen atom. Both aminocarbonyl and N-mono-substituted aminocarbonyl can be
`~¦ converted into N-disubstituted aminocarbonyl by alkylation, as described below in the
`;l "Additional Process Steps".
'''I , .
'rho reactions can be carried out under renction conditiolls known per se, at customary
temperatures, in the presence or, especially when lower alkanoyl anhydrides are used to
activnte the carboxylic acid of formula III, in the nbsence of inert solvents or diluents, for
example in acid amides, for example carboxylic acid amides, such as tlimethylformamide,
dimethylacetamide or 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (DMPU), or
amides of inorganic acids, such as hexamethylphosphoric acid triamide, ethersj for
example cyclic ethers, such as tetrahydrofuran or dioxane, or acyclic ethers, such as
diethyl ether or ethylene glycol dirnethyl ether, halogenated hydrocarbons, such as
halo-lower alkanes, for example methylene chloride or chloroforrn, ketones, such as
acetone, nitriles, such as acetonitrile, acid anhydrides, such as acetic anhydride, esters,
such as ethyl acetate, bisalkane sulfines, such as dimethyl sulfoxide, nitrogen hetero-
cycles, such as pyridine, or mixtures of those solvents, especially in anhydrous solvents or
solvent mixtures, it being possible to select for the above-mentioned reactions the
particular solvents that are suitable in each case, there being used, as appropriate and
expedient, salts of the compounds used, especially metal salts of carboxylic acids that are
used, such as the alkali metal or alkaline earth metal salts, for example sodium or
potassium salts, in the absence or the presence of catalysts, condensation agents or
neutralising agents and, depending on the nature of the reaction and/or the reac~ants, under
atmospheric pressure or in a closed vessel, under normal pressure or under elevated
pressure, for example at the pressure produced in the reaction mixture under the reaction
conditions in a closed tube, and/or in an inert atmosphere, for example under an argon or
nitrogen atomsphere. Preference is given to reaction conditions that are analogous to
those mentioned in the Examples~ Furthermore, the acylating agent, for exarnple a
carboxylic acid halide or a carboxylic acid anhydride, can itself serve as solvent. The
course of the reaction is advantageously monitored using customary methods of analysis,
especially using thin-layer chromatography.
.~
Unless otherwise indicated, the reaction according to the invention is preferably carried
out under mild conditions, especially at temperatures of from 0C to 60C, for example at
¦ room temperature or at slightly elevated temperatures up to about 45C, for example
approximately at room temperature. If the reaction is carried out with a compound of
formula Rl-Zl wherein Z1 is halogen, or with an activated derivative of a compound of
. . .
:'3 2~93~
- 48 -
"
formula II, such as the corresponding chlorocarbonic acid ester, then, for the preparation
of a compound of formula I wherein Rl is aminocarbonyl or the radical of an
N-substituted carbamic acid, the reaction is advantageously carried out in the presence of
an acid-binding agent, such as a non-ncylntable bnse, especially n tertiary nitrogen bnse,
sucll ns N-mothylmorpholine, 4-dimethylnminopyridine, triethylmnine or ethyl
diisopropylnmine. Both in the cnse of the reaction with a carboxylic acid hnlide of
fonnula IlIa wherein Z1 is halogen, such as chlorine or bromine, and in the cnse of the
reaction with an anhydride, especially a symmetrical anhydtide (Zl = O-Rl), there is used
especially M excess of the corresponding compound of forrnula I~a (halide and R,-O-Rl,
respecdvely), ~or example a more than 1~05-~old excess. Reaction conditi~ns that are
specifically mentioned in each case are preferred.
The protecting groups for functional groups in starting materials the reaction of which is to
be avoided, especially carboxy, amino, hydroxy and/or mercapto groups, include
especially those protecting groups (conventional protecting groups) which are customarily
used in the synthesis of peptide compounds, and also in the synthesis of cephalosporins
and penicillins as well as nucleic acid derivatives and sugars~ Those protecting groups
may already be present in the precursors and are intended to protect the functional groups
in question against undesired secondary reactions, such as acylation, etherification,
esterification, oxidation, solvolysis, etc~ In certain cases the protecting groups can
additionally cause the reactions to proceed selectively, for example stereoselecdvely. It is
a characteristic of protecting groups that they can be removed easily, i.e. without
undesired secondary reactions taking place, for example by solvolysis, reduction,
photolysis, and also enzymatically, for example also under physiological conditions~ Only
those radicals that are not present in the end products are referred to as protecting groups~
The protection of functional groups by such protecting groups, the protecting groups them- -
selves and the reactions for their removal are described, for exarnple, in standard works
such as J. F. W. McOmie, "Protective Groups in Organic Chemistry", Plenum Press,London and New York lg73,in Th~ W~ Greene, "Protective Groups in Organic Synthesis",
Wiley, New York 1981, in "The Peptides", Volume 3 ~. Gross and J~ Meienhofer, eds~),
Academic Press, London and New York 1981, in "Methoden der organischen Chemie",
Houben-Weyl, 4th edition, Volume 15/I, Georg Thieme Verlag, Stuttgart 1974, in H.-D.
Jakubke and H~ Jescheit, "Aminosauren, Peptide, Proteine" ("Amino acids, peptides,
proteins"), Verlag Chemie, Weinheim, Deerfield Beach and Basle 1982, and in Jochen
Lehmann, "Chemie der Kohlenhydrate: Monosaccharide und Derivate" ("The Chemistry
,?~
~ 2 1 ~ 8 9 3 ~
, 49
of Carbohydrates: monosaccharides and derivatives"), Georg Thieme Verlag, Stuttgart `
1 974.
~ carboxy group is protected, for exnmple, in the form of nn ester group whicll cnn be
clenved selectivoly under mild conditions~ A cnrboxy ~roup protected in osterified folm is
esterified especinlly by n lower nlkyl group thnt is prefernbly branched in the 1-position of
tho low~r nlkyl group or substitllted in the 1- or 2-position of the lower alkyl group by suit-
nble substituents.
,, A protected carboxy group esteri~led by a lower alkyl group is, for example, methoxy-
,i carbonyl or ethoxycarbonyl.
A protected carboxy group esteri~led by a lower aLtcyl group that is branched in the
1-position of the lower alkyl group is, for example, tert-lower alkoxycarbonyl, for example
tert-butoxycarbonyl.
A protected carboxy group esterified by a lower alkyl group that is substituted in the 1- or
2-position of the lower alkyl group by suitable substituents is, for example, arylmethoxy-
carbonyl having one or two aryl radicals, wherein aryl is phenyl that is unsubstituted or
mono-, di- or tri-substituted, for example, by lower alkyl, for example tert-lower aLkyl,
such as tert-butyl, lower alkoxy, for example methoxy, hydroxy, halogen, for example
chlorine, and/or by nitro, for example benzyloxycarbonyl, benzyloxycarbonyl substituted
by the mentioned substituents, for example 4-nitrobenzyloxycarbonyl or 4-methoxy-
benzyloxycarbonyl, diphenylmethoxycarbonyl or diphenylmethoxycarbonyl substituted by
the mentioned substituents, for example di(4-methoxyphenyl)methoxycarbonyl, and also
carboxy esterified by a lower alkyl group, the lower allyl group being substituted in ~e 1-
or 2-position by suitable substituents, such as 1-lower aLIcoxy-lower aLtcoxycarbonyl, for
example methoxyme~hoxycarbonyl, 1-methoxyethoxycarbonyl or 1-ethoxyethoxy-
carbonyl, 1-lower alkylthio-lower alkoxycarbonyl, for example 1-me~hylthiomethoxy-
carbonyl or 1-ethylthioethoxycarbonyl, aroylmethoxycarbonyl wherein the aroyl group is
benzoyl that is unsubstituted or substituted, for example, by halogen, such as b~omine, for
example phenacyloxycarbonyl, 2-halo-lower aL~coxycarbonyl, for example 2,2,2-trichloro-
ethoxycarbonyl, 2-bromoethoxycarbonyl or 2-iodoethoxycarbonyl, as well as 2-(tri-substi-
tuted silyl)-lower aLcoxycarbonyl wherein the substituents are each independently of the
others an aliphatic, araliphatic, cycloaliphatic or aromatic hydrocarbon r~dical that is
unsubstituted or substituted, for example, by lower aLkyl, lower aL~coxy, aIyl, halogen
: 1
. --
1 ~ 2 ~ 0 8 9 3 4, 5 0 --
~?.. 1 :,
nnd/or by nitro, for example lower alkyl, phenyl-lower alkyl, cycloalkyl or phenyl each of ~ `
which is unsubstituted or substituted as above, for example 2-tri-lower alkylsilyl-lower .~`
alkoxycnrbonyl, such as 2-tri-lower alkylsilylethoxycarbonyl, for example 2-trimethyl-
silylethoxycarbonyl or 2-(di-n-butyl-methyl-silyl)-ethoxycarbonyl, or 2-trinrylsilylethoxy-
carbonyl, such ns triphenylsilylethoxycnrbonyl.
carboxy group is nlso protected in the form of nn organic silyloxycarbonyl group. An
org~mic silyloxycarbonyl group is, for example, a tri-lower alkylsilyloxycarbonyl group,
for example trimethylsilyloxycarbonyl. The silicon atom of the silyloxycarbonylgroup
can also be substituted by two lower alkyl groups, for example methyl groups, and an
amino group or carboxy group of a second molecule of formula I. Compounds having such
protecting groups can be prepared, for example, using dimethylchlorosilane as silylating
agent. ~`
A carboxy group is also protected in the form of an internal ester by a hydroxy group that
is present in the molecule at a suitable distance from the carboxy group, for example in the
~y-position relative to the carboxy group, i.e. in the form of a lactone, preferably a
~-lactone.
A protected carboxy group is preferably tert-lower aLkoxycarbonyl, for examplei tert-but-
oxycarbonyl,benzyloxycarbonyl,4-nitrobenzyloxycarbonyl,9-fluorenylmethoxycarbonyl
or diphenylmethoxycarbonyl.
A protected aimino group is protected by an amino-protecting ,group, for example in the
form of an acylamino, arylmethiylamino, etherified mercaptoamino, 2-acyl-lower aLk-1-
enylamino or silylamino group or in the form of an azido group.
7 :
In an acylamino group, acyl is, for example, the acyl radical of an organic carboxylic acid
having, for example, up to 18 carbon atoms, especially an unsubstituted or substituted, for
exaimple halo- or aryl-substituted, lower alkanecarboxylic acid or an unsubstituted or
substituted, for example halo-, lower alkoxy- or nitro-substituted, benzoic acid, or,
preferably, of a carbonic acid semiester. Such acyl groups are preferably lower alkanoyl,
such as formyl, acetyl, propionyl or pivaloyl, halo-lower aL~canoyl, for example 2-halo- ~ ;
acetyl, such as 2-chloro-, 2-bromo-, 2-iodo-, 2,2,2-trifluoro- or 2,2,2-trichloro-acetyl,
unsubstituted or substituted, for example halo-, lower alkoxy- or nitro-substituted,
benzoyl, such as benzoyl, 4-chlorobenzoyl, 4-methoxybenzoyl or 4-nitrobenzoyl, lower
?~2 21~3~
- 5 1 -
alkoxycarbonyl, preferably lower aLkoxycarbonyl that is branched in the 1-position of the
lower alkyl radical or suitably substituted in the 1- or 2-position, for example tert-lower
alkoxycarbonyl, such as tert-butoxyc~lrbonyl, arylmethoxycnrbonyl having one, two or
three aryl rndicals which ~ue phcnyl thnt is unsubstitllted or mono- or poly~substituted, for
cxample, by lower nlkyl, cspecially tert-lower alkyl, such ns tert butyl, lower alkoxy, SllCh
ns methoxy, hydroxy, hlllogell, such ns chlorine, and/or by nitro, for example benzyloxy-
carbonyl, 4-nitrobenzyloxycarbonyl, diphenylmethoKycarbonyl, 9-fluorenylmethoxy-carbonyl or di(4-methoxyphenyl)methoxycarbonyl, aroylmethoxycarbonyl wherein thearoyl group is preferably benzoyl that is unsubstituted or substituted, for example, by
halogen, such as bromine, for example phenacyloxycarbonyl, 2-halo-lol,ver alkoxy-
carbonyl, for example 2,2,2-trichloroethoxycarbonyl, 2-bromoethoxycarbonyl or 2-iodo-
ethoxycarbonyl, 2-(tri-substituted silyl)-lower alkoxycarbonyl, for example 2-tri-lower
alkylsilyl-lower alkoxycarbonyl, such as 2-trimethylsilylethoxycarbonyl or 2-(di-n-butyl-
methyl-silyl)-ethoxycarbonyl, or triarylsilyl-lower alkoxycarbonyl, for example
2-triphenylsilylethoxycarbonyl.
In an arylmethylamino ~roup, for example a mono-, di- or especially tr~-arylmethylamino
group, the aryl radicals are especially unsubstituted or substituted phenyl radicals. Such
groups are, for example, benzyl-, diphenylmethyl- or especially trityl-amino.
In an etheAfied mercaptoamino group the mercapto group is especially in the form of
substituted arylthio or aryl-lower alkylthio, wherein aryl is, for example, phenyl that is
unsubstituted or substituted, for exarnple, by lower alkyl, such as methyl or tert-butyl,
lower alkoxy, such as methoxy, halogen, such as chlorine, and/or by nitro, for example
4-nitrophenylthio.
In a 2-acyl-lower alk-l-enyl radical that can be used as an amino-protecting group, acyl is,
for example, the corresponding radical of a lower alkanecarboxylic acid, of a benzoic acid
that is unsubstituted or substi~uted, for example, by lower alkyl, such as methyl or tert-
butyl, lower alkoxy, such as methoxy, halogen, such as chlorine, and/or by nitro, or
especially of a carbonic acid semiester, such as a carbonic acid lower aL~cyl semiester.
Corresponding protecting groups are especially 1-lower alkanoyl-lower aL~-1-en-2-yl, for
example l-lower alkanoylprop-l-en-2-yl, such as 1-acetylprop-1-en-2-yl, or lower aLkoxy-
carbonyl-lower alk-l-en-2-yl, for example lower alkoxycarbonylprop-1-en-2-yl, such as
1 -ethoxycarbonylprop- 1 -en-2-yl.
:
.
:i
i
210~93~ `
- 52 -
'~ A silylamino group is, for example, a tri-lower alkylsilylamino group, for example
,~ trimethylsilylamiDo or tert-butyl-dimethylsilylamino. The silicon atom of the silylamino
group can also be substituted by only two lower ~LIkyl groups, for example methyl groups,
and by the amino group or carboxy group of a second molecule of formula I~ Compounds
;1 having such protecting groups cMn be prepared, for exMnple, using the corresponding
~¦ chlorosilanes, such ns dimethylclllorosilane, as silylnting ngents.
An amino group can also be protected by conversion into the protonated form; suitable
~j corresponding anions are especially those of strong inorganic acids, sut:h as sulfilric acid,
phosphoric acid or hydrohalic acids, for example the chlorine or bromine anionj or of
~;, organic sulfonic acids, such as p-toluenesulfonic acid.
.:~
Preferred amino-protecting groups are lower alkoxycarbonyl, phenyl-lower alkoxycar-
bonyl, fluorenyl-lower alkoxycarbonyl, 2-lower alkanoyl-lower alk-1-en-2-yl and lower
alkoxycarbonyl-lower alk-l-en-2-yl, especially tert-butoxycarbonyl and benzyloxy-
carbonyl.
A hydroxy group can be protected, for example, by an acyl group, for example lower
alkanoyl that is unsubstituted or substituted by halogen, such as chlorine, such as acetyl or
2,2-dichloroacetyl, or especially by an acyl ratlical of a carbonic acid semiester mentioned
for protected amino ,groups. A preferred hydroxy-protecting group is, for example,
2,2,2-trichloroethoxycarbonyl, 4-nitrobenzyloxycarbonyl, diphenylmethoxycarbonyl or ~1
triphenylmethoxycarbonyl. A hydroxy group can also be protected by tri-lower aL~cylsilyl,
for example trimethylsilyl, triisopropylsilyl or tert-butyl-dimethylsilyl, a readily
removable etherifying group, for example an alkyl group, such as tert-lower aL~cyl, for
example tert-butyl, an oxa- or a thia-aliphatic or -cycloaliphatic, especiaUy 2-oxa- or
2-thia-aliphatic or -cycloaliphatic, hydrocarbon radical, for example l-lower aL~oxy-lower
aLIcyl or l-lower aLIcylthio-lower alkyl, such as methoxymethyl, l-methoxyethyl, l-ethoxy-
ethyl, methylthiomethyl, l-methylthioethyl or l-ethylthioethyl, or 2-oxa- or 2-thia-cyclo-
alkyl having from 5 to 7 Iing atoms, such as 2-tetrahydrofuryl or 2-tetrahydropyranyl, or a
corresponding thia analogue, and also by l-phenyl-lower aL~cyl, such as benzyl, diphenyl-
methyl or trityl, wherein the phenyl radicals can be substituted, for example, by halogen,
for example chlorine, lower aLIcoxy, for example methoxy, andlor by nitro.
Two hydroxy groups, especially adjacent hydroxy groups, occurring in a molecule, or a
hydroxy group and an amino group that are adjacent to one another, can be protected, for
I
':1
210893~
53 -
example~ by bivalent protecting groups, such as a methylene group that is preferably
: ~ substituted, for example, by one or two lower alkyl rndicals or by oxo, for exnmple
unsubstituted or substituted alkylidene, for example lower alkylidene, such as isopropyl-
idene, cyeloalkylidene, sueh as eyelohexylidene, a earbonyl group or benzylidene~
"
~ hydroxy group in n position ndjac~nt to a earboxy ~roup ean be proteeted by the
formntion of an internal ester (lnetone), espeeially a ~-laetone~
~''`i
Preferenee is given to a proteeted hydroxy group protected by tri-lower alkylsilyl or in the
form of a laetone, espeeially by tert-butyl-dimethylsilyl~
A mercapto group, for example in cysteine, can be protected especially by S-aLkylation
with unsubstituted or substituted aLkyl radicals, by silylation, by thioacetal formation, by
S-acylation or by the formation of asymmetrie disulfide groupings. Preferred mercapto-
protecting groups are, for example, benzyl that is unsubstituted or substituted in the phenyl
radical, for example by methoxy or by nitro, such as 4-methoxybenzyl, diphenylmethyl
that is unsubstituted or substituted in the phenyl radical, for example by methoxy, sueh as
dit4-methoxyphenyl)methyl, triphenylmethyl, pyridyldiphenylmethyl, trimethylsilyl,
benzylthiomethyl, tetrahydropyranyl, acylaminomethyl, such as acetamidomethyl, iso-
butyrylacetamidomethyl or 2-ehloroacetamidomethyl, benzoyl, benzyloxycarbonyl oralkyl-, especially lower alkyl-aminocarbonyl, such as ethylaminocarbonyl, and also lower
alkylthio, such as S-ethylthio or S-tert-butylthio, or S-sulfo.
. ~ .
The removal of protecting groups that are not constituents of the desired end product of
formula I, for example the carboxy-, amino-, hydroxy- and/or mercapto-protecting groups,
is effected in a manner known 1~ se, for example by means of solvolysis, especially
hydrolysis, alcoholysis or acidolysis, or by means of reduction, especially hydrogenolysis ~ ~ -
or by means of other reducing agents, as well as by photolysis, as desired stepwise or
simultaneously, it being possible also to use enzymatic methods. The removal of the
protecting groups is described, for example, in the standard works mentioned above in the
section relating to protecting groups.
' "~
For example, protected carboxy, for example tert-lower alkoxycarbonyl, lower aL~coxy-
carbonyl substituted in the 2-position by a trisubstituted silyl group or in the 1-position by
lower aL~oxy or by lower aLIcylthio, or unsubstituted or substituted diphenylmethoxy-
carbonyl can be converted into free carboxy by treatment with a suitable acid, such as
" " ~ " ~ ; " ~ ~,"" ,,~
54 210~93~
formic acid, hydrogen chloride or trifluoroacetic acid, where appropriate with the addition
of a nucleophilic compound, such as phenol or anisole. Unsubstituted or substituted
benzyloxycarbonyl can be frecd, for example, by means of hydrog~nolysis, i.e. bytreatment with hydrogen in the presence of a metal hydrogenadon catalyst, such ns n
pallndium cntnlyst. In ~Iddition, suitnbly substituted benzyloxycnrbonyl, such ns 4-nitlo-
benzyloxycarbonyl, can be converted into free carboxy nlso by reduction, for exnmple by
trentment with an nlknli metal dithionite, such as s~lium dithionite, or with A reducing
metal, for example zinc, or a reducing metal salt, such as a chromium(l:l) salt, for example
chromiumal) chloride, customarily in the presence of a hydrogen-yielcling agent that,
together with the metal, is capable of producing nascent hydrogen, such as an acid,
especially a suitable carboxylic acid, such as an unsubstituted or substituted, for example
hydroxy-substituted, lower aL~anecarboxylic acid, for example acetic acid, formic acid,
glycolic acid, diphenylglycolic acid, lactic acid, mandelic acid, 4-chloromandelic acid or
tartaric acid, or in the presence of an alcohol or thiol, water preferably being added. By
treatment with a reducing metal or metal salt, as described above, 2-halo-lower aLkoxy-
carbonyl (where appropriate after conversion of a 2-bromo-lower alkoxycarbonyl group
into a corresponding 2-iodo-lower alkoxycarbonyl group) or aroylmethoxycarbonyl can
also be converted into free carboxy. Aroylmethoxycarbonyl can be cleaved also bytreatment with a nucleophilic, preferably salt-forming, reagent, such as sodium thio-
phenolate or sodium iodide. 2-(tri-substituted silyl)-lower alkoxycarbonyl, such as 2-tri-
lower alkylsilyl-lower alkoxycarbonyl, can be converted into free carboxy also by
treatment with a salt of hydrofluoric acid that yields the fluoride anion, such as an aLIcali
metal fluoride, for example sodium or potassium fluoride, where appropriate in the
presence of a macrocyclic polyether (`'crown ether"), or with a fluoride of an organic
quaternary base, such as tetra-lower aLkylammonium fluoride or tri-lower alkylaryl-lower
alkylammonium fluoride, for example telraethylammonium fluoride or tetrabutyl-
ammonium fluoride, in the presence of an aprotic, polar solvent, such as dimethyl
sulfoxide or N,N-dimethylacetamide. Carboxy protected in the form of organic silyloxy-
carbonyl, such as tri-lower alkylsilyloxycarbonyl, for example trimethylsilyloxycarbonyl,
can be freed in customary manner by solvolysis, for example by treatment with water, an
alcohol or an acid, or, furthermore, a fluoride, as described above. Esterified carboxy can
also be freed enzymatically, for example by means of esterases or suitable peptidases, for
example esterified arginine or lysine, such as lysine methyl ester, using trypsin. Carboxy
protected in the form of an internal ester, such as ~-lactone, can be freed by hydrolysis in
the pIesence of a hydroxide-containing base, such as an alkaline earth metal hydroxide or
especially an alkali metal hydroxide, for example NaOH, KOH or LiOH, especially LiOH,
210~3~
- 55 -
the correspondingly protected hydroxy group being freed at the same dme.
A protected amino group is freed in a manner known per se and, according to the natllre of
the protecting groups, in various ways, preferably by solvolysis or reductiotl. Lower
nlkoxycarbonylamino, such as tert-butoxycnrbonylnmino, cnn bc clenved in the presence
of ncids, for example mineral ncicls, for exnmple a hytlrogen halide, sucll ns hydrogen
chloride or hydrogen bromide, especially hydrogen bromide, or sulfilric or phosphoric
ncids, preferably hydrogen chloride, in polar solvents, such as water or a carboxylic acid,
such as acetic acid, or ethers, preferably cyclic ethers, such as dioxane, and 2-halo-lower
alkoxycarbonylamino twhere appropriate after conversion of a 2-bromo-lower alkoxy-
carbonylamino group into a 2-iodo-lower alkoxycarbonylamino group), aroylmethoxy-
carbonylamino or 4-nitrobenzyloxycarbonylamino can be cleaved, for example, by
treatment with a suitable reducing agent, such as zinc in the presence of a suitable
carboxylic acid, such as aqueous acedc acid. Aroylmethoxycarbonylamino can be cleaved
also by treatment with a nucleophilic, preferably salt-forming, reagent, such as sodium
thiophenolate, ansl 4-nitrobenzyloxycarbonylamino also by treatment with an alkali metal
dithionite, for example sodium dithionite. Unsubstituted or subsdtuted diphenylmethoxy-
carbonylamino, tert-lower alkoxycarbonylamino or 2-(tri-subsdtuted silyl)-lower alkoxy-
carbonylamino, such as 2-tri-lower alkylsilyl-lower alkoxycarbonylamino, can be freed by ~;
treatment with a suitable acid, for example formic acid or trifluoroacedc acid;
unsubstituted or substituted benzyloxycarbonylamino can be freed, for example, by means
of hydrogenolysis, i.e. by treatment with hydrogen in the presence of a suitablehydrogenation catalyst, such as a palladium catalyst, preferably in polar solvents, such as
di-lower alkyl-lower alkanoylamides, for example dimethylforrnarnide, ethers, such as
cyclic ethers, for example dioxane, or alcohols, such as rnethanol, ethanol or propanol,
methanol being especially preferred; unsubstituted or subsdtuted triarylmethylamino or
formylamino can be freed, for example, by treatment with an acid, such as a mineral acid,
for example hydrochloric acid, or an organic acid, for example forrnic, acetic or tlifluoro~
acedc acid, where appropriate in the presence of water; and an amino group protected in
the form of silylamino can be freed, for example, by means of hydrolysis or alcoholysis.
An amino group protected by 2-haloacetyl, for example 2-chloroacetyl, can be freed by
treatment with thiourea in the presence of a base, or with a thiolate salt, such as an aLkali
metal thiolate of thiourea, and subsequent solvolysis, such as alcoholysis or hydrolysis, of
the resulting substitudon product. An amino group protected by 2-(tri-subsdtuted silyl)-
lower alkoxycarbonyl, such as 2-tri-lower alkylsilyl-lower aLkoxycarbonyl, can be
converted into the free amino group also by treatment with a salt of hydrofluoric acid that
`'~
i~ ~
` 21~93~
.
- 56 -
:
yields fluoride anions, as indicated above in connection with the freeing of a correspond-
, ingly protected carboxy group. Likewise, silyl, such as trimethylsilyl, bonded directly to a
`l hetero atom, such ns nitrogen, can be removed using fluoride ions.
,.j
~mino protected in the forrn of an nzido group is converted into free amino, for example,
by reduction, for example by cntalytic hydrogenntion with hydrogen in the presence of a
;I hydrogenation catalyst, such ns platinum oxide, palladium or Raney nickel, by reduction
using mercapto compounds, such as dithiothreitol or mercaptoethanol, or by treatment
with zinc in the presence of an acid, such as acetic acid. The catalytic hydrogenation is
preferably carried out in an inert solvent, such as a halogenated hydrocarbon, for e~ample
~j methylene chloride, or in water or in a mixture of water and an organic solvent, such as an
alcohol or dioxane, at approximately from 20C to 25C, or with cooling or heating.
A hydroxy or mercapto group protected by a suitable acyl group, by a tri-lower alkylsilyl
group or by unsubstituted or substituted 1-phenyl-lower alkyl is freed analogously to a
correspondingly protected amino group. ,f~ hydroxy or mercapto group protected by ` ~ `
2,2-dichloroacetyl is freed, for example, by basic hydrolysis, and a hydroxy or mercapto
group protected by tert-lower alkyl or by a 2-oxa- or 2-thia-aliphatic or 2-oxa- or 2-thia-
cycloaliphatic hydrocarbon radical is freed by acidolysis, for example by treatment with a
mineral acid or a strong carboxylic acid, for example trifluoroacetic acid. Mercapto
protected by pyridyldiphenylmethyl can be freed, for example, using mercury(II) salts at
pH 2-6 or by zinc/acetic acid or by electrolytic reduction; mercapto protected by
acetamidomethyl and isobutyrylamidomethyl can be freed, for example, by reaction with
mercury(II) salts at pH 2-6; mercapto protected by 2-chloroacetamidomethyl can be freed,
for example, using 1-piperidinothiocarboxamide; and S-ethylthio, S-tert-butylthio and
S-sulfo can be freed, for example, by thiolysis with thiophenol, thioglycolic acid, sodium
thiophenolate or 1,4-dithiothreitol. Two hydroxy groups or an adjacent amino andhydroxy group that are protected together by means of a bivalent protecting group,
preferably, for example, by a methylene group mono- or di-substituted by lower aLlcyl,
such as lower aL~cylidene, for example isopropylidene, cycloalkylidene, for example cyclo-
hexylidene, or benzylidene, can be freed by acid solvolysis, especially in the presence of a
mineral acid or a strong organic acid. A tri-lower alkylsilyl group is likewise removed by
acidolysis, for example by a mineral acid, preferably hydrofluoric acid, or a strong
carboxylic acid. 2-halo-lower alkoxycarbonyl is removed using the above-mentioned
reducing agents, for example a reducing metal, such as zinc, reducing me~al salts, such as
chromiumaI) salts, or by sulfur compounds, for example sodium dithionite or preferably
, . , . . ~ ~ ~,
1 210893~
- 57 -
sodium sulfide and carbon disulfide. Esterified hydroxy groups, for example lower
alkanoyloxy, such as acetoxy, can be freed also by esterases, and ncylnted amino, for
example, by suitable peptidases.
Tho temporatures for f~ccing the protected functionnl groups Me preferably from -80 to
100C, especially from -20 to 50C, for example from 10 to 35C, such ns in the rogion of
room temperaturc.
I
When several protected functional groups are present, if desired the protecting groups can
be so selected that more than one such group can be removed simultaneously, for example
by acidolysis, such as by treatment with trifluoroacetic acid, or with hydrogen and a
hydrogenation catalyst, such as a palladium-on-carbon catalyst. Conversely, the groups
can also be so selected ~hat they cannot all be removed simultaneously, but rather can be
removed in a desired sequence, the corresponding intermediates being obtained.
The starting compound of formula II is obtainable from 2-[3(S)-amino-2(~)-hydroxy- -
4-phenylbutyl~-N-tert-butyl-decahydro-(4aS,8aS)-isoquinoline-3(S)-carboxamide (which
can be prepared analogously to EP 0 432 695, Example 33) which can also be obtained,
for example, by reacting a compound of formula VIII
.',
: , ~
~i ~ Nl 1~\o (VIII), ~;
H
wherein Pl is an amino-protecting group, as defined above under Process a), especially
tert-butoxycarbonyl or benzyloxycarbonyl, that can be prepared in accordance with Evans
et al., J. Org. Chem. 50, 4615-4625 (1985), with N-tert-butyldecahydro-(4aS,8aS)-iso- - ~-
quinoline~3(S)-carboxamide (prepared in accordance with EP 0 432 694), for example in a
polar solvent, such as an alcohol, for example methanol or ethanol, at elevated
temperature, for example from 40 to 90C, such as reflux temperature, there being
obtained a compound of formula IX
~;,`, 210893l~
58-
P, ~
:~ ~ NH
wherein Pl is as last defined, and subsequently removing the amino-protecting group P~
for example in the case where P1 is tert-butoxycarbonyl by removal with an acid, such as a . :~.
mineral acid, for example a hydrohalic acid, such as hydrochloric acid, or in the presence :
of a strong organic acid, such as formic acid, in organic solvents, for example an ether, for
; example a cyclic ether, such as dioxane, or in the case of a liquid organic acid, such as
formic acid, without a solvent; or in the case where Pl is benzyloxycarbonyl by hydrogen-
ation in the presence of a catalyst, for example a noble metal catalyst, for example bonded
to a carrier, such as carbon, silica gel or aluminium oxide, preferably palladium on active
carbon, under normal pressure or elevated pressure, preferably approximately under
normal pressure, in a polar solvent, for example an alcohol, such as methanol or ethanol,
nt from 0 to 50C, preferably approximately at room temperature; the resulting compound
[N-tert-butyl-decahydro-2-[[2(R)-hydroxy-4-phenyl-3(S)-amino]butyU-(4aS,8aS)-iso-
quinoline-3(S)-carboxamide}, or a salt thereof, for example the sait of one of the last-
mentioned acids, is amidated either with L-asparagine N-protected by an amino-protecting
group P2, for example o~-(N-benzyloxycarbonyl)-L-asparagine, or with a reactive acid
derivative thereof, especially the p-nitrophenyl ester, as described for ~he amidation under
Process b), preferably in the presence of a tertiary nitrogen base, such as triethylamine or
N-ethyldiisopropylamine, especially in an acid amide, for example dimethylformamide, at . `~
temperatures of from 0 to 50C, p~eferably approximately at room temperature, there
being obtained the compound of formula X
,~ 2lnss~
-59 -
H 0~ 0 ~NH
'. `.
wherein P2 is an amino-protecting group, as defined under Process a), especially benzyl-
oxycarbonyl; the compound of folmula X is then converted into the corresponding free
amino compound by removal of the protecting groups, in the case where P2 is benzyloxy-
carbonyl, for example, by hydrogenation in the presence of a catalyst, for example a noble "~
metal catalyst, for example bonded to a carrier, such as carbon, silica gel or aluminium
oxide, preferably palladium on active carbon, under normal pressure or elevated pressure, ::
preferably under normal pressure, in a polar solvent, for example an alcohol, such as .~
methanol or ethanol, at from 0 to 50C, preferably approximately at room temperature, . ~ ~:
which amino compolmd is then amidated by reaction with quinoline-2-carboxylic acid . .
under reaction conditions analogous to those mentioned under Process b), to yield the
compound of ~ormula lI.
Alternatively, the compound of formula II can also be obtained in accordance with the
preparation processes mentioned in EP 0 432 695 (published on 19.06.1991).
The starting compounds of formula m are commercially available or known, or ~hey can
be prepared in accordance with processes knownper se.
There may be mentioned by way of example the formation of aryl-lower aL~anoyl
substituted by heterocyclylmethyl, wherein heterocyclyl is bonded via a ring nitrogen
atom, which is preferably effected by reacting an aryl-lower aLkanoyl radical substituted
by halomethyl, such as chloro- or bromo-methyl, such as chloromethylbenzoyl or bromo-
methylbenzoyl, with a corresponding heterocyclic nitrogen base, such as piperidine,
piperazine, 1-lower aLkylpiperazine, 1-lower aLkanoylpiperazine or especially morpholine ~ ~ :
21~8~
j - 60 -
;,`'`.
J or thiomorpholine, with nucleophilic substitution of the halogen atom.
.,
Amino acid derivat;ves of forrnula III wherein the a-nmino group is alkylnted by n radicnl
selcctcd from phenyl-lower nlkyl nnd heterocyclyl-lower alkyl cnn be prepnred, for
oxnmple, by rcductive aminntion of the amino ncid (protected, if necessmy, at filrther
~ro~lps that nre not intended to p~uticipnte in the reaction) ~mving a prim~lry or secondary ~`
a-amino group, with n phenyl-lower alkyl ketone or aldehyde, such as benzaldehyde, or
heterocyclyl-lower alkyl ketone or aldehyde, for example heterocyclyl aldehyde, for
example filran aldehyde, such as furan-2-aldehyde, or pyridine aldehyde, such aspyridine-3-aldehyde, or imidazolyl aldehyde, such as imidazol-4-yl aldehyde (if necessary
N-protected, for example by trityl which can be removed as described above in the
compound of formula III or the protected end product of formula I, as described above),
for example with catalytic hydrogenation, for example in the presence of a heavy metal
catalyst, such as Raney nickel, under normal pressure or under pressures of from 1 to 100
bar, preferably at approximately 100 bar, or with reduction by means of complex boron
hydrides,suchassodiumcyanoboronhydride.
The isocyanates of formula IIIb can be prepared, for example, from the corresponding
amine precursors by conversion of the amino group into the isocyanato group, for example
by reaction with phosgene with headng, for example under reflux conditions, or by the
dropwise addidon of the primaty, secondary or tertiary amine, in liquid form or dissolved
in a solvent, to an excess of phosgene in a suitable solvent (toluene, xylene, ligroin,
chlorobenzene, a-chloronaphthalene, etc.) with cooling (for example at from -50 to 0C),
there being formed as intermediate a mixture of carbarnoyl chloride and amine hydro-
chloride which is then phosgenated further at elevated temperature (for example at from
50C to the reflux temperature) until complete dissolution is obtained, HCl being
eliminated.
Process b) Amidation (Condensation to form an amide bond)
In starting materials of formulae IV and/or V, functional groups that are not intended to
participate in the reaction are protected if necessary by protecting groups. The protecting
groups and the introduction thereof are as described above under Process a).
The carboxylic acid of formula V is either in a form having a free carboxy group or is in
the form of a reactive derivative thereof, for example in the form of an activated ester
i
' 1
:
9 3 ~
; - 61 -
.,
derived from the free carboxy compound, in the form of a reactive anhydride, or in the
form of a reactive cyclic amide. The reactive derivatives can also be folmed in SitU.
Activated esters of the compound of formula V having a carboxy group are esyecially
csters unsnturate(l at the linking carbon atom of the esterifying rnclical, for example of the
vinyl ester type, such a~ vinyl esters (obtainable, for example, by trnnsesterificntion of a
corresponding ester with vinyl acetnte; activated vinyl ester method), carbamoyl esters ~ `
(obtninnble, for example, by treatment of the corresponding acid with an isoxazolium
reagent; 1,2-oxazolium or Woodward method), or l-lower alkoxyvinyl esters (obtainable,
for example, by treatment of the corresponding acid with a lower alkoxyacetylene; ethoxy-
acetylene method), or esters of the amidino type, such as N,N'-disubsti~uted amidino
esters (obtainable, for example, by treatment of the corresponding acid with a suitable
N,N'-disubstituted carbodiimide, for example N,N'-clicyclohexylcarbodiimide; carbo~
diimide methocl), or N,N-disubstituted amidino esters (obtainable, for example, by
treatment of the corresponding acid with an N,N-disubstituted cyanamide; cyanamide
method), suitable aryl esters, especially phenyl esters substituted by electron-aetracting
substituents (obtainable, for example, by treatment of ehe corresponding acid with a `
suitably substituted phenol, for example 4-nitrophenol, 4-methylsulfonylphenol, 2,4,5-tri-
chlorophenol, 2,3,4,5,6-pentachlorophenol or 4-phenyldiazophenol, in the presence of a
condensation agent, such as N,N'-dicyclohexylcarbodiimide; activated aryl estersmethod), cyanomethyl esters (obtainable, for example, by treatment of the corresponding
acid with chloroacetonitrile in the presence of a base; cyanomethyl esters method), thio- `
esters, especially unsubstituted or substituted, for example nitro-substituted, phenylthio ~ ~
esters (obtainable, for example, by treatment of the corresponding acid with unsubstituted ~ ~ `
or subseituted, for example nitro-substituted, thiophenols, in~er alia by the anhydride or
carbodiimide method; activated thiol esters method), or especially amino or amido esters
(obtainable, for example, by treatment of the corresponding acid with an N-hydroxyamino
or N-hydroxyamido compound, for example N-hydroxysuccinimide, N-hydroxypiperidine,
N-hydroxyphthalimide, N-hydroxy-5-norbornene-2,3-dicarboxylic acid imide, l-hydroxy-
benzotriazole or 3-hydroxy-3,4-dihydro-1,2,3-benzotriazin-4-one, for example by the
anhydride or carbodiimide method; activated N-hydroxy esters method). In~ernal esters,
for example 3~-lactones, can also be used.
Anhydrides of the acid may be symmetric or preferably mixed anhydrides of that acid, for
example anhydrides with inorganic acids, such as acid halides, especially acid chlorides
(obtainable, for example, by treatment of the corresponding acid with thionyl chloride,
`i`` 21089~
. - 62 -
.. , ~ .,
phosphorus pentachloride or oxalyl chloride; acid chloride method), azides (obtainable, for
example, from a corresponding acid ester via the corresponding hydrazide by treatment
thereof with nitrous acid; azide method), anhydlides with carbonic acid semiesters, for
example carbonic acid lower nlkyl semiesters (obtninable, for exarnple, by trentment of the
corrcsponding ncid with chloroformic ncid lower nlkyl esters or with n 1-lower nlXoxy-
cnrbonyl-~-lower alkoxy-1,2-dihydroquinoline; mixed O-alkylcarbonic ncid nnhydrides
mcthod), or nnhydrides with dihnlogenated, especinlly dichlorinated, phosphoric ncid
(obtninable, for exnmple, by treatment of the corresponding acid with phosphorus oxy-
chloride; phosphorus oxychloride method), anhydrides with other phosphoric acid
derivatives (for example those obtainable with phenyl-N-phenylphosphoramidochloridate ~ ;
or by reaction of alkylphosphoric acid amides in the presence of sulfonic acid anhydrides
and/or racemisation-reducing additives, such as N-hydroxybenzotriazole, or in the
presence of cyanophosphonic acid diethyl ester) or with phosphorous acid derivatives, or
anhydrides with organic acids, such as mixed anhydrides with organic carboxylic acids
(obtainable, for example, by treatment of the corresponding acid with an unsubstituted or
substituted lower alkane- or phenyl-lower alkane-carboxylic asid halide, for example
phenylacetic acid chloride, pivalic acid chloride or trifluoroacetic acid chloride; mixed
carboxylic acid anhydrides method) or with organic sulfonic acids (obtainable, for
example, by treatment of a salt, such as an alkali metal salt, of the corresponding acid with
a suitable organic sulfonic acid halide, such as lower alkane- or aryl-, for example
methane- or p-toluene-sulfonic acid chloride; mixed sulfonic acid anhydrides method) and
symmetric anhydrides (obtainable, for example, by condensation of the corresponding acid
in the presence of a carbodiimide or 1-diethylaminopropyne; symmetric anhydridesmethod).
Suitable cyclic amides are especially amides having five-membered diazacycles ofaromatic character, such as amides with imidazoles, for example imidazole (obtainable,
for example, by treatmellt of the corresponding acid with N,N'-carbonyldiimidazole;
imidazole method), or pyrazole, for example 3,5-dimethylpyrazole (obtainable, for
example, via the acid hydrazide by treatment with acetylacetone; pyrazolide method).
As mentioned, carboxylic acid derivatives that are used as acylating agents can also be
formed in situ. For example, N,N'-disubstituted amidino esters can be formed in situ by
reacting a mixture of the starting material of formula IY and the acid of formula V used as
acylating agent, in the presence of a suitable N,N'-disubstituted carbodiimide, for example
N,N'-cyclohexylcarbodiimide, the reaction being carried out, for example, in the presence
8~3~
`. .
63-
, .
of a suitable base, such as triethylamine. In addition, amino or amido esters of the acids
used as acylating agents can be formed in the presence of the starting material of
formula IV to be acylated, by reacting n mixture of the corresponding acid and amino
starting materials in the presence of an N,N'-disubsdtuted cMbodiimide, for example
N,N'-dicyclohexylcarbodiimide, and of an N-hydroxynmine or N-hydroxyamide, for
example N-hydroxysuccinimide, where approprinte in the presence of a suitable base, for
example 4-dirnethylamino-pyridine. Moreover, activntion in situ can be achieved by
reaction with N,N,N',N'-tetraalkyluronium compounds, such as 0-benzotriazol-1-yl-
N,N,N',N'-tetramethyluronium hexafluorophosphate. Finally, phosphoric acid anhydrides
iil~ f the carboxylic acids of formula V can be prepared in SitU by reacting an alkyl-
phosphoric acid arnide, such as hexamethylphosphoric acid triamide, in the presence of a
sulfonic acid anhydride, such as 4-toluenesulfonic acid anhydride, with a salt, such as a ~ :
tetrafluoroborate, for example sodium tetrafluoroborate, or with another derivative of
hexamethylphosphoric acid triamide, such as benzotriazol-l-yloxy-tris(dimethylamino)-
phosphonium hexafluoride, preferably in the presence of a racemisation-reducing additive,
such as N-hydroxybenzotriazole.
' ' .
The amino group of compounds of formula IV that participates in the reaction preferably
carries at least one reactive hydrogen atom, especially when the carboxy group reacting
therewith is in reactive form; the compound of formula IV may, however, itself be in the
form of a reactive derivative, i.e. with the amino group in reactive form, for exarnple by
reaction with a phosphite, such as diethyl chlorophosphite, 1,2-phenylene chlorophosphite,
ethyl dichlorophosphite, ethylene chlorophosphite or tetraethyl pyrophosphite. Aderivative of such a compound having an amino group is, for example, also a carbamic
acid halide, the amino group participating in the reacdon being substituted by halo-
carbonyl, for example chlorocarbonyl.
Condensation to form an amide bond can be carried out in a manner known per se, for
example as described in standard works such as "Houben-Weyl, Methoden der
organischen Chemie", 4th edition, Volume 15/II (1974), Volume lX (1955) Volume E 11
~1985), Georg Thieme Verlag, Stuttgart, "The Peptides" (E. Gross and J. Meienhofer,
eds.), Volumes 1 and 2, Academic Press, London and New York, 1979/1980, or
M. Bodansky, "Principles of Peptide Synthesis", Springer-Verlag, Berlin 1984.
The condensation of a free carboxylic acid with the corresponding arnine can be carried
out preferably in the presence of one of the customary condensation agents. Customary
2~8~3~
, - 64
condensation agents are, for example, carbodiimides, for example diethyl-, dipropyl-,
N-ethyl-N'-(3-dimethylaminopropyl)-carbodiimide or especinlly dicyclohexylcarbo-diimide, also suitable cnrbonyl compounds, for exnrnple cMbonylimidazole, 1,2-oxazol-
ium compounds, for exnmple 2-ethyl-5-phenyl-1,2-oxazolium 3'-sul~onnte and 2-tert-
butyl-S-methylisoxnzolium perchlorate, or n suitable ncylnmino compolmd, for ex~rnple
2-ethoxy- 1 -ethoxycnrbonyl- 1 ,2-dihydroqllinoline, N,N,N ' ,N ' -tetrnnlkyluronillm
compounds, such as O-benzotriazol-l-yl-N,N,N',N'-tetramethyluroniult1 hexa~luoro-
phosphate, also activated phosphoric acid derivatives, for example diphenylphosphoryl
azide, diethylphosphoryl cyanide, phenyl-N-phenylphosphoroamidochloridate, bis(2-oxo-
3-oxazolidinyl)phosphinic acid chloride or l-benzotriazolyloxy-tris(dimethylamino)-
phosphonium hexafluorophosphate.
If desired, an organic base is added, for example a tri-lower alkylamine having bulky
radicals, for example ethyl diisopropylamine, and/or a heterocyclic base, for example
pyridine, 4-dimethylaminopyridine or preferably N-methylmorpholine.
The condensation of acdvated esters, reactive anhydrides or reactive cyclic amides with
the corresponding amines is customarily carried out in the presence of an organic base, for
example simple tri-lower alkylamines, for example triethylamine or tributylamine, or one
of the above-mentioned organic bases. If desired, a condensation agent is additionally
used, for example as described for free carboxylic acids.
The condensation of acid anhydrides with amines can be effected, for exarnple, in the
presence of inorganic carbonates, for example ammonium or aLtcali metal carbonates or
hydrogen carbonates, such as sodium or potassium carbonate or hydrogen carbonate(customarily together with a sulfate), and the reaction of sulfonic acid halides, such as
sulfonic acid chlorides, can be effected in the presence of hydroxides, for example aL~cali
metal hydroxides, such as sodium hydroxide or potassium hydroxide.
Carboxylic acid halides, for example the chlorocarbonic acid derivative derived from the
acid of formula V, are condensed with the corresponding amines preferably in thepresence of an organic amine, for example the above-mentioned tri-lower aLkylamines or
heterocyclic bases, where appropriate in the presence of a hydrogen sulfate.
The condensation is preferably carried out in an inert, aprotic, preferably anhydrous,
solvent or solvent mixture, for example in a carboxylic acid amide, for example
~ 21~893~
65 -
.~ -,; .
fonnamide or dimethylformamide, a halogenated hydrocarbon, for example methylenechloride, carbon tetrachloride or chlorobenzene, a ketone, for example ncetone, n cyclic
ether, for example tetrahydrofuran, an ester, for example ethyl ncetate, or a nitrile, for
example acetonitrile, or in a rnixture thereof, as approprinte at reduced or elevated
temperature, for exnmple in n tempernture rRnge of from approxitnately -40C to approx-
irnntcly ~100C, pr~fernbly from npproximately -10C to npproximately ~50C, and
without an inert gas or under nn inert gas atmosphere, for example a nit~ogen or argon
atmosphere.
Aqueous, for example alcoholic, solvents, for example ethanol, or aromatic solvents or
mixtures of solvents, for example benzene or toluene, may also be used. When aLIcali
metal hydroxides are present as bases, acetone may also be added where appropriate. ~ ~ -
~ '.: ''
The condensation can also be carried out in accordance with the technique known as solid
phase synthesis which originates from R. Merrifield and is described, for example, in
Angew. Chem. 97, 801 - 812 (1985), Naturwissenschaften 71, 25~ - 258 (1984) or in R. A.
Houghten, Proc. Natl. Acad. Sci. USA 82, 5131 - 5135 (1985).
Functional groups protected by protecting groups in the resulting compounds of formula I ~ `
having protected functions are freed where appropriate in accordance with one or more of
the methods mentioned under Process a).
A starting compound of formula IV is preferably prepared from a compound of
formula VI, which can be prepared as described under Process c), by amidation either with
L-asparagine N-protected by an amino-protecting group P2, ~or example a-(N-benzyloxy-
carbonyl)-L-asparagine, or with a reactive acid derivative thereof, especially the p-nitro-
phenyl ester, preferably in the presence of a tertiary nitrogen base, such as triethylamine or
N-ethyldiisopropylamine, as described above, especially in an acid amide, for example
dimethylformamide, at temperatures of from 0 to 50C, preferably at room temperature,
there being obtained the compound of formula IV N-protected by P2, which compound is
converted into the compound of formula IV by removal of the protecting group P2 under
the conditions mentioned for the removal of protecting groups from a compound offormula IX.
;
~i~
,. i - ~
8 9 3 4
- 66 -
Process c) Amidation (Condensation to form an amide bond)
~j In stardng materials of formulae VI and/or VII, fimcdonnl groups that are not intended to
,~ participate in the reacdon are protected if necessary by protecting groups. The protecdng
~roups nnd the introduction thereof are as dcscribcd above undor Process a).
.~
The free carboxylic acids nnd the reactive derivatives thereof and the processes used for
amidation (condensation) are totally analogous to those described under Process b), except
that amino compounds of formula VI are used instead of those of formuln IV and
carboxylic acids of formula VII are used instead of those of formula V.
Funcdonal groups protected by protecdng groups in the resuldng compounds of formula I
having protected funcdons are freed where appropriate in accordance with one or more of
the methods mendoned under Process a).
It should be noted that this reacdon may possibly be obstructed by acyl migration of the
radical Rl to the free amino group in the compound of formula VI. The process variant is
therefore preferably restricted to those stardng materials and reacdon conditions that allow
reaction to take place without troublesome acyl migradon.
A stardng compound of formula VI is preferably prepared by reacting a compound of the
above-defined formula I~ with a carboxylic acid of formula III, which is likewise defined
above, or with a reactive derivadve thereof, under reacdon condidons analogous to those
described for Process a), there being obtained a P1-protected derivadve of a compound of
formula VI from which the protecdng group, for example tert-butoxycarbonyl or benzyl-
oxycarbonyl, is removed, preferably as described for the removal of protecting groups
from a compound of formula I~, to yield the compound of formula VI.
A stardng compound of formula VII is obtained, for example, by amidating with
quinoline-2-carboxylic acid a carboxy-protected derivatdve of L-asparagine (protected, for
example, by one of the protecting groups for protected carboxy mentdoned under
Process a)) under the above-mendoned amidadon conditions, preferably the conditions
mentioned above for the preparation of compounds of formula II ~rom a compound of
formula X after removal of the protecting group P2, and removing the carboxy-protecting
group to yield the compound of formula VII.
. . ~ .
`~ 2~0893~ ~ -
- 67 -
Process variant c) is preferably not used to obtain compounds of formula I; one of
i Processes a) and b) is preferably used instead. ~ `
All the other starting compounds mentioned for one of the nbove-mentioned proccsses are
commercinlly availablc or known, or can be prepn~(l in nccordance with processes known
per se.
'~'
All the renctions mentioned cnn be carried out under reaction conditions known per se,
preferably under the conditions mentioned above, at customary tempera~ures, in the '` `
presence or absence of inert solvents or diluents, for example in acid arnides, for example
carboxylic acid amides, such as dimethylformamide, dimethylacetamide or 1,3-dimethyl-
3,4,5,6-tetrahydro-2(1H)-pyrimidinone (DMPU), or amides of inorganic acids, such as
hexamethylphosphoric acid triamide, ethers, for example cyclic ethers, such as tetrahydro-
furan or dioxane, or acyclic ethers, such as diethyl ether or ethylene glycol dimethyl ether,
halogenated hydrocarbons, such as halo-lower alkanes, for example methylene chloride or d
chloroform, ketones, such as acetone, nitriles, such as acetonitrile, acid anhydrides, such
as acetic anhydride, esters, such as ethyl acetate, bisalkane sulfines, such as dimethyl
sulfoxide, nitrogen heterocycles, such as pyIidine, or mixtures of those solvents, especially
in anhydrous solvents or solvent mixtures, it being possible to select for the above-
mentioned reactions the particular solvents that are suitable in each case, there being used,
where appropriate and expedient, salts of the compounds used, especially metal salts of
carboxylic acids that are used, such as the alkali metal or alkaline earth metal salts, for
example sodium or potassium salts, in the absence or presence of catalysts, condensation
agents or neutralising agents and, depending on the nature of the reaction and/or the
reactants, under atmospheric pressure or in a closed vessel, under normal pressure or
under elevated pressure, for example at the pressure produoed in the reaction mixture
under the reaction conditions in a closed tube, and/or in an inert atmosphere, for example
under an argon or nitrogen atmosphere. Preference is given to reaction conditions that are
analogous to those mentioned in the Examples. Furthermore, the acylating agent, for
example a carboxylic acid halide or a carboxylic acid anhydride, can itself serve as
solvent. The course of the reaction is advantageously monitored using customary methods
of analysis, especially using thin-layer chromatography.
: :.
Additional Process Steps
In the additional process steps, which are optional, in the starting compounds functional
~`:
`:s
~, 210~93~
- 68 -
,j~ .groups that are not intended to participate in the reaction may if necessary be in protected
form, for example may be protected by one or more of the protecting groups mentioned
above under Process a). 'rhe protecting groups may be removed nccording to the methods
mentioned under Proc~ss n).
Snlts of compounds of formula I rnay be prepnred in n manner known per se. For example
salts of compounds of formula I having acid groups mny be formed, for example, by
treatment with metal compounds, such as alkali metal salts of suitable organic carboxylic
acids, e.g. the sodium salt of 2-ethylhexanoic acid, with organic alkali metal or alkaline
earth metal compounds, such as the corresponding hydroxides, carbonates or hydrogen
carbonates, such as sodium or potassium hydroxide, carbonate or hydrogen carbonate,
with corresponding calcium compounds or with ammonia or a suitable organic amine,
there preferably being used stoichiometric amounts or only a small excess of the salt-
forming agent. The preferred acid addition salts of compounds of formula I are obtained
in customary manner, e.g. by treatment with an acid, for example an organic acid or an
inorganic acid, or with a suitable ion exchanger. Internal salts of compounds of formula I
having acid and basic salt-forming groups, e.g. a free carboxy group and a free amino
group, may be formed, for example, by the neutralisation of salts, such as acid addition
salts, to the isoelectric point, e.g. with weak bases, or by treatment with ion exchangers.
Salts can be converted into the free compounds in customary manner, metal and
ammonium salts can be converted, for exarnple, by treatment with suitable acids or acid
ion exchangers, and acid addition salts, for example, by treatment with a suitable basic
agent or basic ion exchangers.
The conversion of a salt of a compound of formula I into a different salt is effected, for
example, by converting a salt into the free compound, as last described, and subsequendy
converting the free compound into a different salt, as last described, or by converting a salt
directly into a different salt, for example by converting one salt into another by means of
chromatography, for example by means of gel permeation chromatography, or frs7m a
solution having an excess of the oppositely charged ion required for the formation of the ;
new salt.
Stereoisomeric mixtures, that is mixtures of diastereoisomers and/or enantiomers, such as,
for example, racemic mixtures, can be separated in a manner known per se by suitable
separating methods into the corresponding isGmers. For example mixtures of diastereo-
21~9~l~
- 69 -
~31
isomers can be separated into the individual diastereoisomers by fractional crystallisation,
chromatography, solvent partition etc.. Racemates can be separated, after conversion of
the optical antipodes into diastereoisomers for example by renction with optically active
compounds, e.g. optically active acids or bases, by chromatography on column materials
covered with optically active compounds or by en~ymntic methods, e.g. by selective
rcnction of only one of the two ennntiomers. This separntion can be cnrriecl out either nt
thc stn~o of one of thc starting materials or with the compounds of formuln I themselves.
;.l
In an obtninable compound of formula I an amino or carboxamide group may be substi-
tuted, a carboxy group that is present in free form or in reactive form may be esterified or
amidated, or an esterified or amidated carboxy group may be converted into a free carboxy
group.
The substitution of a carboxamide group or of a primary or secondary amino group, for
example to form di-lower alkylcarbamoyl or mono- or di-hydroxy-lower alkylcarbamoyl,
in compounds of formula I in which the nitrogen atom of the amino groups to be reacted is
bonded to hydrogen, is effected e.g. by aLtcyladon.
Suitable agents for alkylating a carboxamide group in a compound of formula I are e.g.
diazo compounds, e.g. diazomethane. Diazomethane can be decomposed in an inert
solvent, the free methylene formed reacting with the carboxamide group in the compound
of formula I. The decomposition of diazomethane is carried out preferably by catalysis,
e.g. in the presence of a noble metal in finely divided form, e.g. copper, or a noble metal
salt, e.g. copper(I) chloride or copperaI) sulfate.
Alkylating agents are also mentioned in Gennan Offenlegungsschrift 2 331 133, e.g. alkyl ;
halides, sulfonic acid esters, Meerwein salts or l-substituted 3-aryltriazenes, which can be
reacted under the reaction conditions mentioned therein with a compound of formula I
having a carboxamide group.
Further aLkylating agents are selected from corresponding unsubstituted or substituted ~ -
lower aLl~yl compounds that serve to introduce substituted or unsubstituted lower aL~cyl
radicals, as mentioned above for the radical Rl of an N-substituted carbamic acid, carlying
an additional substituent X wherein X is a leaving group. A leaving group is especially a
nucleofugal leaving group selected from hydroxy esterified by a strong inorganic or ~ ;
or=anic acid, such as hydt oxy estenfied by a mbleral acid, e.g. a hydt ohalic acid, such as
~ . ~
` 21~8~3~
- 70 -
.,
hydrochloric, hydrobromic or hydriodic acid, or by a strong organic sulfonic acid, such as
an unsubstituted or substituted, for example halo-substituted, such as ~luoro-substituted,
lower allcanesulfonic acid, or an aromatic sulfonic acid, e~g. a benzenesulfonic acid tlmt is
unsubstituted or substituted by lower alkyl, such ns methyl, by hnlogen, such ns bromine,
nncl/or by nitro, e.g. n methmlesulfonic, trimethnnosulfonic or p-toluenesulfollic ncid, and
hydroxy csterifAled by hyclrnzoic ncid.
For example, one of the compounds having a substiluent X wherein X is a leaving group
with high polarisability of the electron shell, e.g. bromine or iodine, call be reacted in a
polar aprotic solvent, e.g. acetone, acetonitrile, nitromethane, dimethyl sulfoxide or
dimethylformamide. The substitution reaction is carried out if desired at reduced or
elevated temperature, e.g. in a temperature rMge of from approximately -40 to approx-
imately 100C, preferably from approximately -10 to approximately 50C, and if desired
under an inert gas, e.g. under a nitrogen or argon atmosphere.
For the esterification or amidation of a carboxy group in a compound of formula I, for
example for the amidation of a free carboxy group of an amino acid, such as Glu or Asp,
with ammonia, lower alkylamine or di-lower aLIcylamine, if desired the free acid can be
used or the free acid can be converted into one of the above-mentioned reactive
derivatives and reacted with an alcohol, with ammonia, or with a primary or secondary
amine, or, for the purpose of esterification, the free acid or a reactive salt, e.g. the caesium
salt, can be reacted with a reactive derivative of an alcohol. For example the caesium salt
of a carboxylic acid can be reacted with a halide or sulfonic acid ester corresponding to
the alcohol. The esterification of the carboxy group can also be carried out wi~ other
customary alkylating agents, e.g. with diazomethane, aL~cyl halides, sulfonic acid esters,
Meerwein salts or 1-substituted 3-aryltriazenes, etc..
In order to convert an esterified or amidated carboxy group into the free carboxy group, it
is possible to use one of the methods described above for the removal of carboxy-
protecting groups or, if desired, aUcaline hydrolysis under customary reaction conditions,
such as those mentioned in Organikum, 17th edition, VEB Deutscher Verlag der Wissen-
schaften, Berlin 1988.
In a compound of formula I a free amino group can be acylated, for example in order to
introduce one of the radicals mentioned for Rl with the exception of hydrogen. The
acylation is effected in accordance with the methods mentioned above under Process a) or
. ~ . I . . . i ~ .
:;~
: q, , .
;:
~ 210~93`~
:3 - 71 -
one o-f the methods mentioned for protecting groups or, for example, in accordance with
~ one of the processes mentioned in Organikum, 17th edition, VEB Deutscher Verlng der
.~ Wissenschaften, Berlin (Enst) 1988.
i
In n compound of formula I wheroin Rl is nn nrninoncyl rndical having n prilnnry or
. secolldary a-nmino grollp, the a-nmillo group can be alkylnted by n radical selected from
lower alkyl, phenyl-lower alkyl and heterocyclyl-lower alkyl, for example by reductive
.~ arnination of the nmino group of the amino acid residue (protected, if necessary, at further
i' groups that are not intended to participate in the reaction) with a lower aLIcyl aldehyde or
i ketone, a phenyl-lower alkyl aldehyde or ketone or a heterocyclyl-lower alkyl aldehyde or
ketone, for example formaldehyde, a heterocyclyl aldehyde, for exarnple furan aldehyde,
such as furan-2-aldehyde, pyridine aldehyde, such as pyridine-3-aldehyde, or imidazolyl
aldehyde, such as imidazol-4-yl aldehyde (if necessary N-protected, for example by trityl
which can be removed as described above from the protected compound of formula I), for
example with catalytic hydrogenation, for example in the presence of a suitable heavy
metal catalyst, such as Raney nickel, under normal pressure or under pressures of from 1
~! to 100 bar, preferably at approximately 100 bar, or with reduction by means of complex
boron hydrides, such as sodium cyanoboron hydride~ :
In a compound of formula I wherein Rl is 2-halo-lower aLkanoyl, such as chloroacetyl, the
halogen atom may if necessary, especially in the case of ~hlorine or bromine, be converted
by the Finkelstein reaction in a polar aprotic solvent, especially a ketone, such as acetone,
with sodium iodide into the corresponding compound of formula I containing 2-iodo-
lower alkanoyl, especially 2-iodoacetyl. A compound of formula I containing 2-halo~
lower alkanoyl, especially 2-iodoacetyl, can then be converted by reaction with an amino-, ::
lower alkylamino- or di-lower alkylamino-lower alkanol or an amino-, lower aLkylarnino-
or di-lower alkylamino-lower alkoxy-lower aL~anol, preferably in the presence of bases,
such as carbonates or hydrogen carbonates, for example aLIcali metal carbonates or
hydrogen carbonates, such as sodium or potassium carbonate or sodium or potassium
hydrogen carbonate, in the solvents last mentioned, or ~especially in.the case of 2-chloro-
or 2-bromo-lower alkanoyl) the corresponding (if necessary N-protected) alkali metal
alcoholates of the amino-, lower alkylamino- or di-lower alkylamino-lower alkanols or : :
amino-, lower alkylamino- or di-lower alkylamino-lower alkoxy-lower alkanols (which .
can be prepared, for example, from the corresponding alcohol and an aLtcali metal, such as
sodium or potassium, in a suitable, halogen-free aprotic solvent) into the compound of ~ ~:
formula I wherein Rl is arnino-, lower alkylarnino- or di-lower alkylamino-lower alkoxy-
; !
2 1 ~ ~ 9 3 ~
- 72 -
2-lower alkanoyl, such as dimethylamino-lower aLkoxyacetyl, or amino-, lower alkyl-
amino- or di-lower alkylamino-lower alkoxy-lower alkoxy-2-lower alkanoyl, such as
dimethylamino-(2-lower nlkoxyethoxy)acetyl.
In an obtainnble compollnd of formllln I whoreill the substituents are lls defined mld nt
i! Ienst one free hydroxy grollp is present and the remnining functionnl groups nre in
protected form, the free hydroxy grollp cnn be ncylnted or etherified.
The acylation can be carried out using acylating reagents in accordanc~ with one of the
methods mentioned under Processes a) to e) or in accordance with one of the methods
~ mentioned for protecting groups, or in accordance with one of the processes mentioned in
,`3 Organikum, 17th edition, VEB Deutscher Verlag der Wissenschaften, Berlin (East) 1988.
i The etherification can be carried out using the above-mentioned alkylating agents and
under the same reaction conditions, for example with diazomethane, alkyl halides,
~ sulfonic acid esters, Meerwein salts, l-substituted 3-aryltriazines, etc
¦ The above-mentioned reactions can be carried out under reaction conditions that are
known per se, in the absence or customarily in the presence of solvents or diluents,
preferably those solvents and diluents that are inert towards the reagents used and are
solvents therefor, in the absence or presence of catalysts, condensation agents or neutral-
ising agents, depending on the nature of the reaction andlor the reactants at reduced,
normal or elevated temperature, e.g. in a temperature range of from approximately -80C
to approximately 200C, preferably from approximately 20C to approximately 150C,
for example from room temperature to the reflux temperature, in the case of melts at up to
220C, under atmospheric pressure or in a closed vessel, if desired under pressur~, for
example at the preisure produced irl the reaction mixture lmder the reaction conditions in a
closed tube, and/or in an inert atmosphere, e.g. under an argon or nitrogen atmosphere.
The reaction conditions specifically mentioned in each case are prefelred.
Solvents and diluents a~e, for example, water, alcohols, for example lower aLlcyl
hydroxides, such as methanol, ethanol or propanol, diols, such as ethylene glycol, triols,
such as glycerol, or aryl alcohols, such as phenol, acid amides, for example carboxylic
acid amides, such as dimethylformamide, dimethylacetamide or 1,3-dimethyl-
3,4,5,6-tetrahydro-2(1H)-py~imidinone (DMPU), or amides of inorganic acids, such as
hexamethylphosphoric acid triamide, ethers, for example cyclic ethers, such as tetrahydro-
:
. ~
. ~
`~l 210~
- 73 -
.
furan or dioxane, or acyclic ethers, such as diethyl ether or ethylene glycol dimethyl ether,
halogenated hydrocarbons, such as halo-lower alkanes, for example methylene chloride or
chloroform, ketones, such as acetone, nitriles, such as acetonitrile, acid anhydrides, such
as acetic anhydride, esters, such as ethyl acetnte, bisnlknne sulfines, such ns dimethyl
sulfoxide, nitrogen heterocycles, sucll ns pyridine, hydrocnrbons, for exnmple lower
alknnes, sllch as heptane, or nromatic compounds, such as benzenc or tolllelle, or m;xtures
Of those solvents, it being possible to select the pnrticular solvents that nre suitable for
each of the above-mentioned reactions~
In view of the close relationship between the compounds of formula I and the precursors
thereof in free form and in the form of salts, hereinbefore and hereinafter any reference to
the compounds and intermediates should be understood as including the corresponding
salts and free compounds, respectively, as appropriate and expedient, provided that the
compounds contain one or more salt-forming groups~ Starting compounds and intermed-
iates may also be present in protected form, where necessary, it being possible for the
protecting groups to be removed at suitable times. Protecting groups and their removal are
especially as defined above~
The compounds, including their salts, may also be obtained in the form of hydrates, or
their crystals may include, for example, the solvent used for crystallisation~
The invention relates also to those forms of the process in which a compound obtainable
as intermediate at any stage is used as starting material and the remaining steps are carried
out, or the process is interrupted at any stage, or a staIting material is formed under the
reaction conditions or is used in the form of a reactive derivative or salt, or in which a
compound obtainable according to the process of the invention is produced under the
process conditions and further processed in situ. The starting materials used are preferably
those that result in the compounds described above as preferred.
Pharmaceutical Compositions.
The invention relates also to pharmaceutical compositions comprising compounds of
formula I (referred to as active ingredient).
The pharmacologically acceptable compounds of the present invention may be used, e.g.,
for the preparation of pharmaceutical compositions that comprise an effective amount of
2las~3~
-~ - 74 -
j the active ingredient together or in admixture with a significant amount of inorganic or
organic, solid or liquid, pharmaceutically acceptable carriers.
Th~ pharmaceutical compositions according to the invcntion are those for enteral, such as
nasnl, buccnl, rectal or oral, or pnrenternl, such ns intramuscular or intrnvenous, admin-
~1 istrntion to wnrm-blooded nnimals (hllmnns Imd animals), comprising an effective dose of
the ph~umncologicnl active ingredient alone or together with a significant amount of a
pharmaceutically acceptable carrier. The dose of the active ingredient depends on the
species of warm-blooded animal, the body weight, the age and the individual condition,
individual pharmacokinetic data, the disease to be treated and the method of administra-
tion.
The invendon relates also to pharmaceutical compositions and to a method for treating
9 diseases caused by retroviruses, for example AII)S, especially when the disease is caused
3 by HIV-1 or HIV-2, which comprises administering an antiretrovirally effective amount of
a compound of formula I according to the invention, especially to a warm-blooded animal,
for example a human, who requil~s such treatment on account of one of the mentioned
diseases, especially AIDS. The dose to be administered to warm-blooded animals, for
example humans of approximately 70 kg body weight, is f~om approximately 3 mg toapproximately 10 g, preferably from approximately 10 mg to approximately 4 g, for
example approximately from 25 mg to 2.0 g per person per day, divided preferably into 1
to 5, especially 1 to 3, single doses which may, for example, be of the same size. Usually~
children receive half of the adult dose.
The pharmaceutical compositions comprise from approximately 1 % to approximately95 %, pre~erably from approximately 20 % to approximately 90 %, active ingredient.
Pharmaceutical compositions according to the invention may be, for example, in unit dose
form, such as in the form of ampoules, vials, suppositories, dragées, tablets or capsules.
The pharmaceutical compositions of the present invention are prepared in a manner known
per se, for example by means of conventional dissolving, lyophilising, mixing, granulating
or confectioning processes.
It is preferable to use solutions of the active ingredient, and also suspensions or
dispersions, and especially isotonic aqueous solutions, dispersions or suspensions, it being
possible, for exatnple in the case of Iyophilised compositions that cotnpdse the active
i
1 -~
2 1 ~
ingredient alone or together with a carrier, e.g. mannitol, for such solutions, dispersions or
suspensions to be prepared prior to use. The pharmaceutical compositions mny be
sterilised and/or mny comprise excipients, e.g. preservntives, stnbilisers, wetting ngents
and/or emulsifying agents, solubilisers, salts for regulating thc OSIlIOtiC pressure and/or
buffers, nnd are prepnred in a manner lcnown per se, e.~. by menns of conventionnl
dissolving or Iyophilising processes. The said solutions, clispersiolls or suspensions may
comprise viscosity~incrcasing substaulces, such as sodium carboxymethylcellulose,
carboxymethylcellulose, dextran, polyvinylpyrrolidone or gelatin.
Suspensions in oil comprise as the oil component the vegetable, synthe~ic or semi-
synthetic oils customary for injection purposes. There may be mentioned as such
especially liquid fatty acid esters that contain as the acid component a long-chained fatty
acid having from 8 to 22, especially from 12 to 22, carbon atoms, e.g. Iauric acld,
tridecylic acid, myristic acid, pentadecylic acid, palmitic acid, margaric acid, stearic acid,
arachidic acid, behenic acid or corresponding unsaturated acids, e.g. oleic acid, elaidic
acid, erucic acid, brassidic acid or linoleic acid, if desired with the addition of anti-
oxidants, e.g. vitamin E"B-carotene or 3,5-di-tert-butyl-4-hydroxytoluene. The alcohol . ~ .
component of those fatty acid esters has a maximum of 6 carbon atoms and is a mono- or
poly-hydric, e.g. a mono-, di- or tri-hydric, alcohol, e.g. methanol, ethanol, propanol,
butanol or pentanol or the isomers thereof, but especially glycol and glycerol. The
following examples of fatty acid esters are therefore to be mentioned: ethyl oleate, :
isopropyl myristate, isopropyl palmitate, "Labrafil M 2375" (polyoxyethylene glycerol
trioleate, Gattefossé, Paris), "Miglyol 812" (triglyceride of saturated fatty acids with a
chain length of C8 to Cl2, Hiils AG, Germany), but especially vegetable oils, such as
cottonseed oil, almond oil, olive oil, castor oil, sesame oil, soybean oil and more
especially groundnut oil.
The injection compositions are prepared in customary manner under sterile conditions; the
same applies also to introducing the compositions into ampoules or vials and sealing the
containers.
Pharmaceutical compositions for oral administration can be obtained by combining the
active ingredient with solid carriers, if desired granulating a resulting mixture, and
processing the mixture, if desired or necessary, after the addition of appropriate excipients,
into tablets, dragée cores or capsules, or by preparing dispersions, preferably with
phospholipids, which are introduced into vials. It is also possible for the active ingredients
~ 210~93~
. -76 -
to be incorporated into plastics carriers that permit the active ingredients to be diffused or
released in measured amounts.
~ "
'13 Suitnble carriers are especially fillers, such ns sugnrs, e~g. Inctose, saccharose, rnannitol or
sorbitol, cellulose preparntions and/or calcium phosphates, e.g. tricalcium phosphnte or
~! calcium llydrogen phosphnte, nnd binders, such as starch pastes using e.g. corn, wheat,
ricc or potato starcll, gelatin, trngacanth, methylcellulose, hydroxypropylmethylcellulose,
soclium carboxymethylcellulose nnd/or polyvinylpyrrolidone, and/or, if desired, disinte-
~3 grators, such as the above-mentioned starches, also carboxymethyl starch, crosslinked
¦ polyvinylpyrrolidone, agar, alginic acid or a salt thereof, such as sodium alginate. : -
,I Excîpients are especially flow conditioners and lubricants, e.g. silicic acid, talc, stearic
s acid or salts thereof, such as magnesium or calcium stearate, and/or polyethylene glycol.
Dragée cores are provided with suitable, optionally enteric, coatings, there being used,
inter alia, concentrated sugar solutions which may comprise gum arabic, talc, polyvinyl-
pyrrolidone, polyethylene glycol and/or titanium dioxide, or coating solutions in suitable
organic solvents, or, for the preparation of enteric coatings, solutions of suitable cellulose
preparations, such as ethylcellulose phthalate or hydroxypropylmethylcellulose phthalate.
Capsules are dry-filled capsules made of gelatin and soft sealed capsules made of gelatin
and a plasticiser, such as glycerol or sorbitol. The dry-f1lled capsules may comprise the
active ingredient in the form of granules, e.g. with fillers, such as lactose, binders, such as
starches, and/or glidants, such as talc or magnesium stearate, and if desired with stabil-
isers. In soft capsules the active ingredient is preferably dissolved or suspended in suitable
oily excipients, such as fatty oils, paraffin oil or liquid polyethylene glycols, it likewise
being possible for stabilisers and/or antibacterial agents to be added. Dyes or pigments
may be added to the tablets or dragée coatings or the capsule casings, e.g. for
identification purposes or to indicate different doses of active ingredient. - -
Especially preferred as pharmaceutical compositions are phospholipid-stabilised
dispersions of the active ingredient, preferably for oral administration, comprising
a) a phospholipid or several phospholipids of the formula
2108~3~
77 -
.
1C~I2----R
R _O_2CH O
A C~l2~0--P--O--(CnH2n)--N--Rb
0/ 3 RC ..
1~ .
'I wherein RA is Cl0.20acyl, RB is hydrogen or C10 20acyl, Ra~ Rb and RC are hydrogen or
,¦ C14alkyl and n is an integer from two to four, if desired
b) a further phospholipid or several further phospholipids
c) the active ingredient and . ~
d) a pharmaceutically acceptable carrier liquid and if desired further excipients and/or . .
preservatives. '.~
: ::
. ~
The process for the preparation of those dispersions is as follows: a solution or suspension
of components a) and c) or a), b) and c), but preferably of a) and b) in a ratio by weight of :~ :
from 20: 1 to 1: 5, especially from 5: 1 to 1: 1, is converted into a dispersion by dilution . ~ ~ `
with water and the organic solvent is then removed, for example by centrifugation, gel
filtration, ultrafiltration or especially by dialysis, for example tangential dialysis, : ;~
preferably against water, and then, preferably after the addition of excipients or .
preservatives and if necessary with the establishment of an acceptable pH-value by the
addition of pharmaceutically acceptable buffers, such as phosphate salts or organic acids .
(pure or dissolved in water), such as acetic acid or citric acid, preferably from pH 3 to 6,
for example pH 4 - ~, the dispersion obtained is concentrated (unless it a]ready has the
correct active ingredient concentration) preferably to an active ingredient concentration of
from 2 to 30 mg/ml, especially from 10 to 20 mg/ml, concentration preferably being .
effected in accordance wi~ the methods last mentioned for the removal of an organic :
solvent, especially by ultra~lltration, for example using an apparatus for ca~ying out
tangential dialysis and ultrafiltration.
The phospholipid-stabilised dispersion that can be prepared in accordance with that
process is stable for at least several hours at room temperature, is reproducible as regards : ~ : ``
the proportions of the components and is toxicologically acceptable and is therefore
especially suitable for oral administration to humans. :
The size of the particles obtained in the dispersion is variable and is pre~erably from
21Q8934
- 78 -
3 approx. 1.0 x 10-8 to approx. 1.0 x 10-5 m, especially from approximately 10 7 to
~¦ approximately 2 x 10-6 m.
~ .
The nomenclnture for the phospholipids of formllln I nnd the numbering of the carbon
atoms are in accordance with the recommendations of the IUPAaIUlB Commission on
Biochemical Nomenclnture (CBN) (sn-nomenclature, stereospecific mlmbering) given in
thc J~ur. J. of Biochem. 79, 11-21 (1977) "Nomenclntl~re of Lipids".
In a phospholipid of formula A, RA and RB having the definitions C1O 20acyl are
preferably straight-chained Cl0.20alkanoyl having an even number of c~lrbon atoms and
straight-chained C10 20alkenoyl having a double bond and an even number of carbon
atoms.
Straight-chained C10-20aL`Can0Y1 RA and RB having an even number of carbon atoms are,
for example, n-dodecanoyl, n-tetradecanoyl, n-hexadecanoyl or n-octadecanoyl.
Straight-chained C~O-20a1kenOYI RA and RB having a double bond and an even number of
carbon atoms are, for example, 6-cis-, 6-trans-, 9-cis- or 9-trans-dodecenoyl, -tetra-
decenoyl, -hexadecenoyl, -octadecenoyl or -icosenoyl, especially 9-cis-octadecenoyl
(oleoyl).
In a phospholipid of formula A, n is an integer from two to four, preferably t~vo. The
group of the formula -(C~ )- is unbranched or branched aLtcylene, for example l,l-ethyl-
ene, 1,1-, 1,2- or 1,3-propylene or 1,2-, 1,3- or 1,4-butylene. 1,2-Ethylene (n=2) is
preferred.
Phospholipids of formula A al;e, for example, naturally occurring cephalins wherein Ra~ Rb
and RC are hydrogen, or naturally occuning lecithins wherein Ra~ Rb and ~ are methyl,
for example cephalin or lecithin from soybeans, bovine brain, bovine liver or hen's eggs
having different or identical acyl groups RA and RB or mixtures thereof.
Synthetic, substantially pure phospholipids of formula A having different or identical acyl
groups RA and RB are preferred.
The term "synthetic" phospholipid of foImula A defines phospholipids that have a unifo~rn
composition as regards RA and RB Such synthetic phospholipids are preferably the
'~
i"J
~'' ~ -.
,. 1
~,, 2~ 0~93~
r~ ~ 79 ~
l
lecithins and cephalins defined below, the acyl groups RA and Rn f which hnve a defined
structure and are derived from a defined fntty ncid hnvillg n deg~e of purity higher thm~
:~ approx. 95 %- RA and R~3 may be identical or d;fferent nnd may be unsnturnted or
; l SatUrlltOd. RA is preferably sahlrntedl for exnrnple n-hexaclecnnoyl, nnd Rn is preferably
unsaturated, for exarnple 9-cis-octndecenoyl t= oleoyl).
The term "naturally occurring" phospholipids of fonrnula ~ defines phospholipids that do
. not have a uniforrn cormposition as regards RA and RB Such natural phospholipids are
likewise lecithins and cephalins the acyl groups RA and RB Of which ar~ structurally
;1 undefinable and are derived from naturally occurring fatty acid mixtures. ~ ~;
The term "substantially pure" phospholipid defines a degree of purity of more than 70 %
;1 (by weight) of the phospholipid of formula A, which can be detected by suitable
determination methods, for example by paper chromatography.
Special preference is given to synthetic, substantially pure phospholipids of formula A
wherein RA is straight-chained C1O 20aLcanoyl having an even number of carbon atoms and
~1 RB is straight-chained C10 20alkenoyl having a double bond and an even number of carbon
atoms. Ra~ Rb and RC are methyl and n is two.
.
In an especially preferred phospholipid of formula A, RA is n-dodecanoyl, n-tetra-
decanoyl, n-hexadecanoyl or n-octadecanoyl and RB is 9-cis-dodecenoyl, 9-cis-tetra-
decenoyl, 9-cis-hexadecenoyl, 9-cis-octadecenoyl or 9-cis-icosenoyl. Ra~ Rb and Rc are
methyl and n is two.
A very especially preferred phospholipid of formula A is synthetic l-n-hexadecanoyl-
2-(9-cis-octadecenoyl)-3-sn-phosphatidyl choline having a purity of more than 95 %.
Preferred natural, substantially pure phospholipids of formula A are especially lecithin
(L-o~-phosphatidyl choline) from soybeans or hen's eggs.
The terms given in brackets are also customarily used for the acyl radicals in the
phospholipids of formula A: 9-cis-dodecenoyl (lauroleoyl), 9-cis-tetradecenoyl (myrist-
oleoyl), 9-cis-hexadecenoyl (palmitoleoyl), 6-cis-octadecenoyl (petroseloyl), 6-trans-octa-
decenoyl (petroselaidoyl), 9-cis-octadecenoyl (oleoyl), 9-trans-octadecenoyl (elaidoyl)
1 l-cis-octadecenoyl (vaccenoyl), 9-cis-icosenoyl (gadoleoyl), n-dodecanoyl (lauroyl),
2~ 0893~
~ - 80 -
,~ . .
n-tetradecanoyl (myristoyl), n-hexadecanoyl (palmitoyl), n-octadecanoyl (stearoyl),
n-icosanoyl (arachidoyl).
, Other phospholipids are preferably esters of phosphatidic acid (3-sn-phosphatidic ncid)
,~ with the mentioned acyl rndicnls, such as phosphatidyl serine nnd phosphatidyl
ethanolamine.
,i
Sparingly soluble active ingredients may also be present in the form of water-soluble,
pharmaceudcally acceptable salts, as defined above.
The carrier liquid d) comprises the components a), b) and c) or a) and c) as liposomes in
such a manner that for a period of from several days up to several weeks no solids or solid
aggregates, such as micelles, re~form and the liquid comprising the said components can
be administered, preferably orally, if necessary after filtration.
The carrier liquid d) may comprise pharmaceutically acceptable, non-toxic excipients, for
example water-soluble excipients that are suitable for producing isotonic conditions, for
example ionic additives, such as sodium chloride, or non-ionic additives (structure
formers), such as sorbitol, mannitol or glucose, or water-soluble stabilisers for the
liposome dispersion, such as lactose, fructose or sucrose.
In addidon to the water-soluble excipients, the carrier liquid may also compriseemulsifiers, wetting agents or surfactants that can be used for liquid pharmaceutical
formulations, especially emulsifiers, such as oleic acid, non-ionic surfactants of the fatty
acid polyhydroxy alcohol ester type, such as sorbitan monolaurate, monooleate, mono-
stearate or monopalmitate, sorbitan tristearate or trioleate, polyoxyethylene adducts of
fatty acid polyhydroxy alcohol esters, such as polyoxyethylene sorbitan monolaurate,
monooleate, monostearate, monopalmitate, tristearate or trioleate, polyethylene glycol
fatty acid esters, such as polyoxyethyl stearate, polyethylene glycol-400-stearate, poly-
ethylene glycol-2000-stearate, especially ethylene oxide/propylene oxide block polymers
of the Pluronic~ type (Wyandotte Chem. Corp.) or the Synperonic~) type (ICI).
Preferred preservatives are, for example, antioxidants, such as ascorbic acid, or micro-
bicides, such as sorbic acid or benzoic acid. ;
The following Examples serve to illustrate the invention but do not limit the scope thereof
`'~ 2 ~ 3
- 8 1 -
'~, in any way. ~ `
Temperatures are given in degrees Celsius (C). Where no temperntllre is specifled, the
reaction takes place at room temperature. The R~ values, which indicate the ratio of the
seepage propagation of the substance in question to the seopn~e propa~ation of the elunnt
front, nre determincd on thin Inyer silica gel plntes by th;n Inyer chrornato~rnplly (TLC) in
~¦ the followin~ solvent systems:
.
A ethylacetate
B methylene chloride/ethanoVtriethylamine 90:10:2
C methylene chloride~I~lF 1:1
` The abbreviation "Rf(A)", for example, indicates that the Rf value was determined in
solvent system A.
The ratio of solvents and eluants to one another is always given in parts by volume.
,
HPLC gradients:
I 20%~100%a)inb)for35min.
II 20 % ~ 100 % a) in b) for 20 min.
~ ' ,:'.;.
Eluant a): acetonitrile + 0.05 % TFA; eluant b): water + 0.05 % TFA. Column
(250 x 4.6 mm) filled with "Reversed-Phase" material Cl8-Nucleosil~ (5 ~,lm
average particle size, silica gel covalently derivatised with octadecylsilanes,
Macherey & Nagel, Duren, FRG). Detection by UV-absorption at 215 nm. The
retention times (tRe~) are given in minutes. Flow rate 1 mVmin.
The other short fonns and abbreviations used have the fiollowing meanings:
abs. absolute (indicates that the solvent is anhydrous)
atm physical atmospheres
(unit of pressure) - 1 atm corresponds to 1.013 bar
Boc tert-butoxycarbonyl
BOP benzotriazol-1-yloxy-tris(dimethylamino)- ;
phosphonium hexafluorophosphate
brine saturated sodium chloride solution
` ` .i~ :
--`' 21~93~
~ `
.2 DIPE - 82 -
y DMAP 4-dimethylaminopyridine
DMF dimethylformamide
;j h hours
HOBt l-hydroxybellzotrinzole
EIPLC hi~h-performnncc liquid chromntoglnplly
; HV high vacuum
min minute(s)
.~' MS mass spectroscopy
NMM N-methylmorpholine
RT room temperature
TFA trifluoroacetic acid
TEIF tetrahydrofuran
TLC thin-layer chromatography
Z benzyloxycarbonyl
Mass spectroscopic data are obtained either by conventional MS or according to the
"~ast-Atom-Bombardment" (FAB-MS) method. The mass data refer in the former case to
the unprotonated molecule ion (M)+ or to the protonated molecule ion (M-~H)+.
The values for proton nuclear resonance spectroscopy (1H-NMR) are given in ppm (parts
per million) based on tetrarnethylsilane as the internal standard. s = singlet, d ~ doublet,
t = triplet, q = quartet, m = multiplet, dd = double doublet.
'::~
Examp!c 1: N-tert-ButYl-decahvdro-2-~2(R)-acetYloxY-4-~henyl-3(S)-rrN-(2 qoinolvl-
car n~l)-L-aspara~inyl~ninolbut~l-(4aS 8aS)-isoquinol;ne-3(S)-carboxamide
Under a nitrogen atmosphere, at RT, 2 crystals of DMAP and 17 111 (0.18 rnmiol) of acetic
anhydride are added to a solution of 81 mg (0.12 mmol) of N-tert-butyl-decahydro2-[2(R)-hydroxy-4-phenyl-3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyUamino]bu~rl]-
(4aS,8aS)-isoquinoline-3(S)-carboxarnide in 2 ml of abs. THF and S0 111 (0.36 mmol) of
triethylamine. After 18 h the reaction mixture is poured onto ice and extracted with 3
portions of ethyl acetate; the organic phases are washed with saturated NaHCO3 solution,
water and brine, dried with Na2SO4 and concentrated by evaporation. Digestion of the
crude product in DIPE yields the title compound: TLC Rf(A)=0.44; tRet=18.9 min; :
FAB-MS (M+H)~-713; 1H-NMR (200 MHz, CD30D): 1.34 (s, (H3C)3C), 1.2-2.0 (m,
ca. 13 H), 2.08 (s, H3CCO), 2.2-2.44 (m, 2 H), 2.6-2.8 (m, 5 H), 2.95-3.13 (m, 2 H), 4.43
:' ::~
.....
.' ~2
5 j
. .
-83- 2~93
(m, HC), 5.30 (m, HC), 6.89 (m, lH), 7.04 (t, J=7 Hz, 2 H), 7.24 (d, J=7 Hz, 2 H), 7.70
(m, 1 H), 7.85 (m, 1 H), 8.02 (d, J=7 Hz, 1 H), 8.17 (m, 2 H), 8.47 (d, J=7 Hz, 1 H).
The starting materials nre prepnred ns follows:
. . I .
ln) N-tcrt-l~utYI-(lccnkY(Iro 2 r2(R~-hvdroxy-4~Qhcnyl 3(~2~rtert~but(~x~Ycnrbollyl-
nmi~D3~a~3(S)~carboxnmlde
Under a nitrogen atmosphere, in nn nmpoule, 1.105 g (4.195 mmol) of 2(S)-[l(S)-(Boc-
amino)-2-phenylethyl]-oxirane (prepared analogously to B.K. Handn, E'.J. Machin, S.
Redshaw, G.J. Thomas - European Patent Appln. 0 346 847 (1989), but using the tert-
butoxycarbonyl group instead of the benzyloxycarbonyl protecting group, or in accordance
with Evans et al., J. Org. Chem. 50, 4615-4625 (1985)) and 1.18 g (4.95 mmol) of N-tert-
butyl-decahydro-(4aS,8aS)-isoquinoline-3(S)-carboxarnide (forpreparation see
K.E.B. Parkes, S. Redshaw, G.J. Thomas, European Patent Appln. 0 432 694 A2 (1990))
in 21 ml of ethanol are heated for 16 h at 90C. Column chromatography (SiO2, hexane/-
ethyl acetate 5:1 ~ 2 % ~iethylarnine) of the evaporation residue yields the title
compound: tRot=18.1 min; FAB-MS tM~H)+=502; lH-NMR (200 MHz, CD30D):
1.1-2.23 (m, 14 H), 1.26 and 1.33 (2 s, 2 tH3C)3C), 2.57-2.70 (m, 2 H), 2.73-2.84 tm, 1 H),
2-93-3- 14 tm, 2 H)~ 3.73-3.90 tm, 2 H), 7.1-7.3 tm, HCArom )~
lb) N-tert-But,Yl~decahYdro-2-l2tE~)-hvdroxY4-phenyl-3(o-aminobutyll-(4as~8as)
~soquinoline-3(S)-carboxamide HCI
Under a nitrogen atmosphere, 0.6 g tl.2 mmol) of N-tert-butyl-decahydro-2-
[2tR)-hydroxy-4-phenyl-3tS)-[tert-butoxycarbonylamino]butyl]-(4aS,8aS)-isoquinoline-
3(S)-carboxamide is dissolved in 12 ml of dioxane; 12 ml of 4N HCl solution in dioxane
are added thereto and the reaction mixture is stirred for 2 h at RT. Lyophilisation yields ` ^
the title compound: IH-NMR (300 MHz, CD30D): 1.37 (s, (H3C)3C), 1.2-1.9 (m, 10 H),
1.9-2.2 (m, 4 H), 2.81-3.03 (m, 2 H), 3.14-3.27 (m, 2 H), 3.44 (m, 1 H), 4.07 (m, 1 H),
4.53 (m, 1 H), 7.27-7.43 (m, HCarom.)
Alternative method of synthesis: The title compound in free form (not the HCl salt) is
obtained by stirring 109 mg (0.217 mmol) of the title compound frorn Example I a),
dissolved in 0.75 ml of concentrated formic acid. After 16 h at RT the formic acid is .
evaporated off under a high vacuum and the residue is partitioned between 3 portions of
ethyl acetate, saturated NaHCO3 solution and brine. Drying of the organic phases with
Na2SO4 and concentration by evaporation yield the title compound: IH-NMR (200 MHz,
~ i~
'~ -84- 2~08~3~
..,
-, CD30D): inter alia 1.32 (s, 9H); 2,27 (d, 12 Hz, lH); 2.37 (dd, 12 Hz, 4 Hz, IH);
2.57-2.78 (m, 3H), 2.99 (d, 12 Hz, lH), 3.1-3.4 (m, 2H), 3.68 (m, lH).
.~.j
lc) N-tert~ut.Yl decnll,vdro-2-r2(R)~lly(lrox,y-4-pll~nyl~3[S)-[[N-b~nzyloxycnrbonyl~
L~nseara~inYllnmn~LIltyll-(~S~XaS)-îsoalllinolillc~3(S)-cnrboxnmide
Dissolvcd in 13 ml of NMM 0.3N in DM~ nnd 2.24 ml (13 mmol) of N-ethyl diisopropyl-
mninc, 0.624 g (1.35 mmol) of N-tert-butyl-decnhydro-2-[2(1~)-hydroxy-4-phellyl-3(S)-
~unino~butyl]-(4nS,8aS)-isoquinoline-3(S)-carboxamide . HCI is renctecl for 4 h at RT with
0.762 g (1.97 mmol) of Z-asparagine-p-nitrophenyl ester (Bnchem, Bubendorf/-
Switzerland). The reaction mixture is concentrated by evaporation under HV and the
residue is partitioned between 3 portions of methylene chloride, 2 portions of 5 % K2CO3
solution and b~ine. Renewed dissolution in a small amount of DMF and precipitation with
ice-cold DIPE yield the title compound: tRet=15.7 min, FAB-MS (M+H)~=650.
ld)N~tert-ButYl~decahvdro-2~[2(R)-hydroxY-4-Phenyl-3ts)-rrL-aspara inYllaminol-
butY11-(4aS,8aS)-isoquinoline-3(S)-carboxamide
Dissolved in 20 ml of methanol, 0.69 g (1.06 mmol) of N-tert-butyl-decahydro-2-[2tR)-
hydroxy-4-phenyl-3(S)-[[N-benzyloxycarbonyl-L-asparaginyl]amino]butyl]-t4aS,8aS)-
isoquinoline-3(S)-carboxamide is hydrogenated in the presence of 140 mg of 10 %
palladium on carbon under 1 atm hydrogen pressure for 5 h at RT. Filtering off the
catalyst and concentradon by evaporation yield the title compound: lH-NMR (300 MHz,
CD3(:)D): 1.33 (s, (H3C)3C), 1.2-2.24 (m, 15 H), 2.45 (dd, Jl=15 Hz, J2=5 Hz, 1 H),
2.55-2.80 (m, 3 H), 3.02-3.14 (m, 2 H), 3.54 (dd, Jl=8 Hz, J2=5 Hz, 1 H), 3.87 (m, 1 H),
4.22-4.32 (m, 1 H), 7.1-7-34 (m, HCa~om.)
' ~
le) N-tert-Butyl-decahydro-2-r2(R)-h~/drox~-4-PhenYl~3(~-r~N-(2-quinolvl-
carbonyl)~L-asPara~inYllaminolbg~yll-(4aS,8aO-isoquinoline-3(S)-carbo~amide
A solution of 246 mg (1.42 mmol) of quinaldic acid, 487 mg (0.945 mmol) of N-tert-
butyl-decahydro-2-[2(R)-hydroxy-4-phenyl-3(S)~[[L-asparaginyl]amino]butyl]-
(4aS,8aS)-isoquinoline-3(S)-carboxamide, 627 mg (1.42 mmol) of BOP and 192 mg
(1.42, mmol) of HOBT is dissolved at RT in 7.5 ml of 0.3M NMM in DMF and stilred for
18 h at RT. The reaction mixture is concentrated by evaporation under HV and theresidue is partitioned between 3 portions of methylene chloride, 2 portions of saturated
sodium bicarbonate solution and brine. Drying of the combined organic phases over
sodium sulfate, concentration by evaporation and column chromatography (SiO2,
methylene chloride/ethanoVacetic acid 90: 10:1 ~ methylene chloride/ethanol/NH3aq
~ 2~1893~
1 -85-
.~ 90:10:1) of the residue yield the title compound: TLC Rf(B)=0.50; tRot=16.0 min;
FAB-MS (M+H)~=671, lH-NMR t300 MHz, CD30D): 1.31 (s, (H3C)3C), 1.1-2.4 (m,
14 H), 2.6-2.9 (m, 5 H), 3.04 (m, 2 H), 3.92 (m, 1 H), 4.23 (m, 1 H), 4.92 (t, J=7 Hz, 1 H),
6.74 (t, J=7 Hz, 1 H), 6.91 (t, J=8 Hz, 2 II), 7.19 (d, J=8 Hz, 2 H), 7.69 (m, 1 H), 7.84 (m,
1),7.99 (t, J=8 Hz, 1 H), 8.14 (d, J=9 Hz, 1 H), 8.17 (d, J=9 Hz, 1 II), 8.48 (d, J=9 Hz,
1 H).
,,'~
.
AlteMatively, the title compound CM nlso be prepared as follows (analogously to EP
.J j 0 432 695, published 19.06.1991, Example 3): a soludon of N-(2-quinolylcarbonyl-M8M
L-asparagine and N-tert-butyl-decahydro-2-[2(R)-hydroxy-4-phenyl-3(S)-aminobu~yl]-
(4aS,8aS)-isoquinoline-3(S)-carboxamide (which can be prepared as described in the :
alternative process under 1 b)) in tetrahydrofuran is cooled to -10C and 3-hydroxy-
1,2,3-benzotriazin-4(3H)-one (Fluka, Switzerland) and dicyclohexylcarbodiimide are
2,~ added thereto; the mixture is stirred at the mentioned temperature for 2 h and at 20C for a
~`3 further 16 h Md then diluted with ethyl acetate and filtered. The filtrate is then washed
with saturated sodium bicarbonate solution and brine and concentrated by evaporation.
~ The residue is purified over a silica gel column with 4 percent by volume methanol in
.~¦ dichloromethane and the title compound is obtained.
Example 2: N-tert-ButYI-decahYdro-2-~2(R)-(furan~2-carbonYIoxv)-4-Phenyl-3(s)~
[~N-(2-quinolYlcarbonYl)-L-asparapinyllaminolbutvll-(4as~8as)-isoquinoline
3(S)-carboxamide
A solution of 8.8,ul (0.06 mmol) of furan-2-carboxylic acid chloride, 0.5 mg of DMAP
and 34,ul (0.2 mmol) of N-ethyl diisopropylamine in 5 ml of dioxane is added to 40 mg
(0.06 mmol) of N-tert-butyl-decahydro-2-[2(R)-hydroxy-4-phenyl-3~S)-[[N-(2-quinolyl-
carbonyl)-L-asparaginyUamino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide
(Example le). After 2 days at RT, the reaction mixture is partitioned between 3 portions
of ethyl acetate, saturated NaHCO3 solution and brine. Drying of the organic phases with
sodium sulfate, concen~ation by evaporation and column chromatography (SiO2, ~ ~ -
methylene chloride/I~ 5:1) yield the title compound: tRe~=l9.0 min; FAB-MS
(M+H)~=765.
Example 3: The following acyl derivatives of N-tert-butyl-decahydro-2~[2(~)-hydroxy-
4-phenyl-3(S)-l[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-iso-quinoline-3(S)-carboxamide can be prepared analogously to one of the Examples given
above or in accordance with the mentioned processes:
`:
: ~ ~
~
210893~
86 -
:~ 3a) N-tert-butyl-decahydro-2-[2(R)-pivaloyloxy-4-phenyl-3(S)-L[N-(2-quinolylcarbonyl)-
~ L-aspnraginyllamino]butyl~-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
;~ 3b) N-tert-butyl-decahydro-2-L2(R)-(N-metllylaminoncetyloxy)-4-phenyl-3(S)-[[N-
(2-quinolylcnrbonyl)-L-nsparnginyl~amino]butyl~-(4nS,8nS)-isoquinolille-3(S)-cnrbox-
. ~ amide,
:,.
3c) N-tert-butyl-decnhydro-2-[2(R)-(N-benzyloxycarbonyl-N-methyla:minoacetyloxy)-
4-phenyl-3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquino-
line-3(S)-carboxamide,
3d) N-tert-butyl-decahydro-2-[2(R)-(N-dimethylaminoacetyloxy)-4-phenyl-3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carbox-
amide,
3e) N-tert-butyl-decahydro-2-[2(R)-(N-n-butyl-N-methylaminoacetyloxy)-4-phenyl-
3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinol~ine-
3(S)-carboxamide,
..
3f) N-tert-butyl-decahydro-2-[2(R)-(N-methyl-N-benzylaminoacetyloxy)-4-phenyl-
3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
3g) N-tert-butyl-decahydro-2-[2(R)-(N-methyl-N-3-pyIidylmethyl-aminoace~loxy)-
4-phenyl-3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquino- . .. :;
line-3(S)-carboxamide,
3h) N-tert-butyl-decahydro-2-[2(R)-(3-py~idylcarbonyloxy)-4-phenyl-3(S)-[[N-
(2-quinolylcarbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(Sj-car-boxamide, and/or
3i) N-tert-butyl-decahydro-2-[2(R)-(4-(N-morpholinyl-methyl)-benzoyloxy)-4-phenyl-
3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-::
3(S)-carboxamide. - ~
. ' ~:.
'~
' ~ "
~ ... ..
~" ~
~ 2~089~
87-
.
..:.,
.. Example 4: The following acyl derivatives of N-tert-butyl-decahydro-2-[2tR)-hydroxy-
4-phenyl-3(S)-[[N-(2-quinolylcarbonyl)-L-asparnginyl]aminolbutyl]-(4aS,8aS)-isoquino-
line-3(S)-carboxamide can be prepared annlogously to one of the :lixamples given above or
in accordance with the mentioned processes:
.~
4a) N-tert-blltyl-dccnhydro-2-[2(R)-propionyloxy-4-pllenyl-3(S)-[[N-(2-quinolyl-
carbonyl)-L-nsparaginylJaminoJbutyl~-(4nS,8nS)-isoquinoline-3(S)-cnrboxllmi(le;
4b) N-tert-butyl-decahydro-2-[2(R)-butyryloxy-4-phenyl-3(S)-[[N-(2-quinolylcarbonyl)-
L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
4c) N-tert-butyl-decahydro-2-[2(R)-methylpropionyloxy-4-phenyl-3(S)-[[N-(2-quinolyl-
carbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
4d) N-tert-butyl-decahydro-2-[2(R)-pentanoyloxy-4-phenyl-3(S)-[[N-(2 quinolyl-
carbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
4e) N-tert-butyl-decahydro-2-[2(R)-octanoyloxy-4-phenyl-3(S)-[[N-(2-quinolylcarbonyl)-
L-asparaginyUamino]butyl]-(4aS,8aS)-isoquinoline-3(S)~arboxamide,
4f) N-tert-butyl-decahydro-2-[2(R)-decanoyloxy-4-phenyl-3(S)-[[N-(2-quinolylcarbonyl)-
L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
:':,..
4g) N-tert-butyl-decahydro-2-[2(R)-dodecanoyloxy-4-phenyl-3(S)-[[N-(2-quinolyl- ~:
carbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
4h) N-tert-butyl-decahydro-2-C2(R)-palmitoyloxy-4-phenyl-3(S)-[[N-(2-quinolyl-
carbonyl)-L-asparaginyUamino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
4i) N-tert-butyl-decahydro-2-[2(R)-(3-carboxypropionyloxy)-4-phenyl-3(S)-[[N-(2-quino-
lylcarbonyl)-L-asparaginyl~amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-c~rboxamide,
4j) N-tert-butyl-decahydro-2-[2(R)-(3-methoxypropionyloxy)-4-phenyl-3(S)-[[N-(2-quino-
lylcarbonyl)-L-asparaginyl]amino]butyU-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
4k) N-tert-butyl-decahydro-2-[2(R)-benzyloxyacetyloxy-4-phenyl-3(S)-[[N-(2-quinolyl-
.~
~; 2~0~93~
~ - 88 -
., carbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
~; 41) N-tert-butyl-decahydro-2-[2(R)- ~ tS)-a-methoxy-a-phellylncetyloxy ) -4-phenyl-3(S)-
. [[N-(2-quinolylcnrbonyl)-L-aspnraginyl~mnino~blltyl~-(4nS,8~1S)-isoquinoline-3($)-cnt-
boxnmide,
:
;1 4m) N-tert-butyl-decnhydro-2-[2(R)- ( (R)-o~-methoxy-a-phenylncetyloxy } -4-phenyl-3(S)-
. 1 ~[N-(2-quinolylcnrbonyl)-L-asparaginyUamino]butyl]-(4aS,8aS)-isoqllinoline-3(S)-car-
boxamide,
. ! 4n)N-tert-butyl-decahydro-2-[2(R)-12-(2-methoxyethoxy)acetyloxy}-4-phenyl-3(S)-[[N-
~2-quinolylcarbonyl)-L-asparaginyl]amino]butyU-(4aS,8aS)-isoquinoline-3(S)-carbox- '
amide,
~ :
40) N-tert-butyl-decahydro-2-[2(R)-ln-butoxyacetyloxy)-4-phenyl-3(S)-[[N-(2-quinolyl-
carbonyl)-L-asparaginyl]amino~butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
4p) N-tert-butyl-decahydro-2-[2(R)-12-(2-(2-methoxyethoxy)ethoxy)acetyloxy)-4-phe- ~ :
nyl-3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]butyU-(4aS,8aS)-isoquinoline-
3(S)-carboxamide, : ~:
4q) N-tert-butyl-decahydro-2-[2(R)-{2-pyridylacetyloxy)-4-phenyl-3(S)-[[N-(2-quinolyl-
carbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
4r) N-tert-butyl-decahydro-2-[2(R)-13-(2-pyridyl)propionyloxy)-~phenyl-3(S)-[[N-
(2-quinolylcarbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carbox-
amide,
4s) N-tert-butyl-decahydro-2-[2(R)-{4-imidazolylcarbonyloxy}-4-phenyl-3(S)-[[N- ;
(2-quinolylcarbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carbox- `
amide,
4t) N-tert-butyl-decahydro-2-[2(R)-14-imidazolylacetyloxy~-4-phenyl-
3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]bu- "';
tyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
.~
:'C'j~
2~893~
.,,.jl .
! - 89-
:;~
4u) N-tert-butyl-decahydro-2-[2(R)-{3-(4-imidazolyl)propionyloxy}-4-phenyl-3(S)-[[N-
, "~ (2-quinolylcarbonyl)-L-asparaginyl]amillo]butyl]-(4aS,8aS)-isoquinoline-3(S)-carbox-
,.j amide,
~'~
~i 4v) N-tert-butyl-decahydro-2-[2(R)-qllinolin-2-ylcarbonyloxy-4-phellyl-
.. ~ 3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]~unino]butyl]-(4aS,8nS)~ isoquinoline-
3(S)-c. rboxamide,
.1
.i.j 4w) N-tert-butyl-decahydro-2-[2(R)-phenoxyacetyloxy-4-phenyl-3(S)-[[N-(2-quinolyl-
~ ~ carbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
.
4x) N-tert-butyl-decahydro-2-[2(R)-pyridine-4-carbonyloxy-4-phenyl-3(S)-[[N-(2-quino-
~. lylcarbonyl)-L-asparaginyl]amino]butyU-(4aS,8aS)-isoquinoline-3(S)-carboxamide, and
i.~. :'
4y) N-tert-butyl-decahydro-2-[2(R)-pyridine-2-carbonyloxy-4-phenyl-3(S)-[[N-(2-quino- ~ :
lylcarbonyl)-L-asparaginyl]amino]butyU-(4aS,8aS)-isoquinoline-3(S)-carboxamide.
::
Example 5: The following acyl derivatives of N-tert-butyl-decahydro-2-[2(R)-hydroxy-
4-phenyl-3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-iso- :
quinoline-3(S)-carboxamide can be prepared analogously to one of the Examples given
above or in accordance with the mentioned processes: :
.
Sa) N-tert-butyl-decahydro-2-[2(R)-{l-pyrazolylacetyloxy}-4-phenyl-3(S)-[rN-(2-quino-
lylcarbonyl)-L-asparaginyUamino]butyU-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
Sb) N-tert-butyl-decahydro-2-[2(R)-pyrazin-2-ylcarbonyloxy-4-phenyl-3(S)-[[N-(2-quino-
lylcarbonyl)-L-asparaginyUarnino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
5c) N-tert-butyl-decahydro-2-[2(R)-isoquinoline-3-carbonyloxy-4-phenyl-
3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]butyl] -(4aS ,8aS)-isoquinoline-
3(S)-carboxamide,
Sd) N-tert-butyl-decahydro-2-[2(R)-aminoacetyloxy-4-phenyl-3(S)-[[N-(2-quinolyl-carbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
Se) N-tert-butyl-decahydro-2-[2(R)- { (L)-pyr~olidine-2-carbonyloxy} -4-phenyl-3(S)-[[N-
~893~
.. "", - 90
(2-quinolylcarbonyl)-L-asparaginyl]amino]butylJ-(4aS,8aS)-isoquinoline-3(S)-carbox-
amide,
Sf~ N-tert-butyl-decahydro-2-[2(R)-{N-(imidazolyl-4-methyl)-N-methylaminoacetoxy)-
4-phenyl-3(S)-[[N-(2-quinolylcarbonyl)-L-asparnginyl~nmillolbutyU-(4aS,8nS)-isoquino-
linc-3(S)-carboxami~
5g) N-tert-butyl-decahydro-2-[2(~)-[N-(pyridin-2-ylmethyl)-N-methyl~minoacetoxy-4-
phenyl-3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]butyll-(4aS,8aS)-isoquino- `
line-3(S)-carboxamide,
5h) N-tert-butyl-decahydro-2-[2(R)-{N,N-(4-dimethylaminobutyryl)oxy}-4-phenyl-3(S)-
[[N-(2-quinolylcarbonyl)-L-asparaginyUamino]butyl]-(4aS,8aS)-isoquinoline-3(S)-car-
boxamide,
~ ,
Si) N-tert-butyl-decahydro-2-[2(R)-benzoyloxy-4-phenyl-3(S)-l[N-(2-quinolylcarbonyl)-
L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
. . .~,
Sj) N-tert-butyl-decahydro-2-[2(E~)-{4-chloromethylbenzoyloxy~-4-phenyl-3(S)-[[N-(2-
quinolylcarbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide,
Sk) N-tert-butyl-decahydro-2-[2(R)-{3-(N,N-dimethylaminopropyl)oxyacetyloxy}- ~ -
4-phenyl-3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]bu~r1]-(4aS,8aS)-iso-quinoline-3(S)-carboxamide, and
51)N-tert-butyl-decahydro-2-[2(E~)-{2-[3-(N,N-dimethylaminopropoxy)ethoxy]- ~ ~;
acetyloxy~-4-phenyl-3(S)-[[N-(2-quinolylcarbonyl)-L-asparaginyl]amino]bu- ~ -
tyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide.
Example 6: N-tert-Butyl-decahydro 2-r2(R)-methoxyacet,~loxy-4-phenyl-
3(0-r~N-(2-guirlolYlcarbon~y!~-L-aspara~invllaminolbutyll-(4as~8as)
quinoline-3(S)-carboxamide
Under a nitrogen atmosphere, at 0C, 4 ',ll (0.046 mmol) of methoxyacetic acid chloride
and 0.2 mg (0.002 mmol) of DMAP are added to a solution of 10 mg (0.015 mmol) ofN-tert-butyl-decahydro-2-[2(R)-hydroxy-4-phenyl-3(S)-[[N-(2-quinolylcarbonyl)-
L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide (Example le) in
~..-. .
`- 21~93~
. ~i
.~ - 91 -
. ;
'r.
0.24 ml of dioxane/pyridine 5:1. Since after 18 h at RT some starting material is still
found with HPLC, a total of 35 tll of pyridine and 35 ~ll of methoxyacetic acid chloride is
added again in 2 portions. When according to HPLC all the starting material hns rencted,
thc renction mixture is pMtit;oned between 3 portions of ethyl ncetnte, snturnted NnHCO3
solution, water nnd brine, nnd the orgnnic phllse is dried in Nn2SO4 nnd concelltrated by
evnporntion. The residue is digested from hexnn~/diisopropyl ether in nn ultrasound bath
nnd left to stnnd at 0C to yield the pure title compoulld: tRCt(II)=13.1 min; FAB MS
(M~H) ~743.
Exampl~ 7: N-tert-But~l decahydro-2-r2(R)-(pYridine-2-carbonvlox~r~ 4-phenyi-
3($)-r~N-(2-quinolYIcarbonYI)-L-aspara~inyllaminolbutYil (4aS~8a3)~iso-
quinoline~3!S)-carboxamide
Under a ni~ogen atmosphere, at 0C, a solution of 30 mg (0.24 mmol) of 2-picolinic acid
(pyridine-2-carboxylic acid) in 1.6 ml of methylene chloride is treated with 20 ,ul (0.144
mmol) of l-chloro-N,N,2-trimethyl-1-propenamine ~B. Haveaux, A. Dekoker, M. ~ens,
AR. Sidani, J. Toye, L. Ghosez, M. Murakami, M. Yoshioka, and W. Naga~a, OrganicSyntheses 59, 26 (1980)] and, in the course of 45 min, at RT, converted into the acid
chloride~ Then 0.4 ml of pyridine, a catalydc amount of 4-dimethylaminopyridine and 40
mg (0.06 mmol) of N-tert-butyl-decahydro-2-[2(R)-hydroxy-4-phenyl-3(S)-[[N-(2-quino-
lylcarbonyl)-L-asparaginyl]amino]butyl]-(4aS,8aS)-isoquinoline-3(S)-carboxamide
(Example le) are added thereto and the reacdon mixture is sdrred for 18 h at RT. Since
according to HPLC some stardng material is sdll present, a further 0.12 mmol of
2-picolinic acid chloride in 0.8 ml of methylene chloride as prepared above, together with
0.2 ml of pyridine, is added thereto. After a further 18 h at RT, the reacdon mixture is
partidoned between 3 portions of methylene chloride, 2 portions of saturated NaHCO3
solution, water and brine, and the organic phases are dried with Na2SO4 and concentrated
by evaporadon. Column chromatography (SiO2, methylene chloride~ 1) and
digesdon from diisopropyl ether yield the pure dtle compound: TLC Rf(C)=0.28;
tRet(II)=13.2 min; FAB-MS (M+H)+=776.
Example 8: Gelatin solution
A sterile-filtered aqueous soludon comprising one of the compounds of formula I men-
tioned in preceding Examples 1 to 7, and an additional 20 % cyclodextrin, and a sterile
geladn soludon preserved with phenol are mixed together with heating under aseptic
condidons in such a manner that 1.0 ml of soludon having the following composidon is
obtained:
'~
`;~
210893~ -
- ~3
- 92 -
: 1
~ !
si active ingredient 3 mg
gelatin 150.0 mg
phenol 4.7mg
dist. water with 20 % cyclodextrins 1.0 ml
;~'''1 " ,
xnmple 9: Stcrilc drY substnllce for ilueetioll
S mg of one of the compounds of formula I mentioned in preceding Examples 1 to 7 are
;1 dissolved in 1 ml of an aqueous solution containing 20 mg of mannitol and 20 % cyclo-
dextrins as solubiliser. The solution is sterile-filtered and under aseptic: conditions
`' introduced into a 2 ml ampoule, deep-frozen and lyophilised. Before use, the lyophilisate
is dissolved in 1 ml of distilled water or 1 ml of physiological saline solution. The solution
is administered intramuscularly or intravenously. This formulation can also be introduced
into double-chamber disposable syringes. - ~;
ExamplelO: NasalsPra~
500 mg of finely ground powder (~5.0 ~Im) of one of the compounds of formula I men-
tioned in preceding Examples 1 to 7 are suspended in a mixture of 3.5 ml of Myglyol
8 12(g) and 0.08 g of benzyl alcohol. The suspension is introduced into a container having a :
metering valve. 5.0 g of Freon 12(~ are introduced under pressure through the valve into ~ :
the container. By shaking, the "Freon" is dissolved in the MyglyoVbenzyl alcohol mixture.
The spray container contains about 100 single doses which can be administered separately.
.
Example 11~ m~coated tablets
For the preparation of 10 000 tablets each comprising 100 mg of a compound of formula I,
the following constituents are processed:
active ingredient 1000 g
cornstarch 680g
colloidal silicic acid 200 g
magnesium stearate 20 g
stearic acid 50 g
sodium carboxymethyl starch 250 g
water quantum satis
A mixture of one of the compounds of formula I mentioned in preceding Examples 1 to 7,
~: 1
$l
: . .
`' -93- 2~08934
. 1
~ 50 g of corn starch and the colloidal silicic acid is processed into a moist mass with a
( i starch paste consisting of 250 g of corn starch Md 2.2 kg of demineralised water. This
; , mass is passed through a sieve of 3 mm mesh size and dried in a fluidised bed drier for
j~ 30 min at 45. The dried granules are pressed through n sieve of 1 mm mesh size, mixed
with n previously sieved mixture (1 mm sieve) of 330 g of corn stnrch, the magnesium
l stearat~, the stearic acid and the sodium carboxymethyl starch nnd compressed to form
j ~ slightly domed tablets.
"-1
Example 12: Orallv ndministrable dispersion 1
625 mg of one of the compounds of formula I mentioned in preceding Examples 1 to 7 and
625 mg of POPC (l-palmitoyl-2-oleoyl-phosphatidyl choline = 1-hexadecanoyl-2-(9-cis-
octadecenoyl)-3-sn-phosphatidyl choline) are dissolved in 25 ml of ethanol. The solution
is diluted with ten times the amount of water. For that pwrpose, the ethanolic solution is
added dropwise at room temperature to the required amount of water at a rate of
10 mVmin. The ethanol is removed from the mixtwre by tangential dialysis ("Cross Flow
~, Filtration") against 1750 ml of water (system: Minitan~, 700 cm2 polyether sulphone
` membrane having an exclusion limit of 100 ~D, Millipore (USA))~ Using the same system,
~, the mixture is concentrated to 15 mg of active ingredient by ultrafiltration. After the
addition of 1.24 mg/ml of citric acid and 1.24 mg/ml of disodium hydrogen phosphate . 2
H20 to adjust the mixture to pH 4.2 and 1 mg/ml of sorbic acid as antimicrobial
preservative, the dispersion is again concentrated to 15 mg/ml and introduced into vials,
for example 20 ml vials. The dispersion particles have a diameter of 0.1 - 2 llm. ~he
;i dispersions are stable at from +2 to 8C for at least six months and are suitable for oral
:~' administration.
~1
?'i~ Example 13: OraDv adrninistrable dispersion 2
Preparation is effected analogously to Example 12, but using 25 mg of the compound of
formula I and 50 mg of POPC to prepare the ethanolic solution.
Example 14: OrallY administrable dispersion 3
Preparation is effected analogously to Example 12, but using 25 mg of the compound of
:~ formula I and 125 mg of POPC to prepare the ethanolic solution.
.~ Example 15: Orally administrable dispersion 4
Preparation is effected analogously to Example 12, but using 50 mg of the compound of
formula I and 50 mg of POPC to prepare the ethanolic solution.
~ 210893~ ::
J:! :
'`: ` '`'
.' Example 16: Orall.~ administrable dispersion
Preparation is effected analogously to one of Examples 12 to 15, but using the compound
. of formula I nnd phosphatidyl choline from soya or phosphatidyl choline from egg yolk
. (70-100 % pure) instend of POPC to prepnre the cthnnolic solution. If desired, nn anti-
: 1 oxidnnt, such ns ascorbic acid, is ndded in a concentrntion of 5 mg/ml.
. ~ . . .
~1
'' '~
. ': .
,~ :
: ` :
:~