Note: Descriptions are shown in the official language in which they were submitted.
W~92~1~7~1 rCT/US9~/03328
9 ~ ! :
DESCRIPTION
P~iOCES8 FOR ~ED~CING ~ITROGEN OXIDEB ~ 8IONB ~ND
I~PRO~I~G ~E COMBUSTION EFFICIE~CY OF A T~RBI~E ~`-
Related Application
This application is a continuation-in-part of copend
ing ~nd commonly assigned U.S. Patent ~pplication
entitled "Improved Combustion Efficiency Water-ln-Fuel .-
Oil Emulsion11, having Serial No. 07/603,266, filed in the
name of Sprague on October 24, 1990, which in turn is a
continuation of U.S. Patc-nt Application Serial No.
07/348,296, filed May 5, 1989, : now abandoned, the
disclosures o~ which are incorporated herein by
reference.
~ The present ln~enti~n~relates t~o a~process which:~will :
~improve the combustion ~-ef~lciency ~ o`~ a gas;~turbine:in
order to reduce the ~emissiLons~o~f ~troge~ oxides (NOx~
~and visi~le~emissions~(~p:artic~lates,~which lead~to plume : : ~:
opacity~ ~:o~the~:atmosphe~e~
~ as or combustio~tur~ines have bèén~utilized~:by~many
utillties as peaking~ units~;to~rapidly~;bring àdditional
electrical generation~on:line~a:s~:requlred~and~,;henc~e,~are~
prefer~ed~ for many appli~ations~ Unfortunately~ the:~
:
:
'W~ 71)1 rCT/VS92/03328
2 1~ 9 n9 b -2-
temperatures at which gas turbines opexate tend to cause
the production of thermal NOX, the temperatures being
so high that free radicals of oxygen and nitrogen are
formed and chemically combine as nitrogen oxides.
Nitrogen oxides are troublesome pollutants and comprise a
major irritant in smog. It is further believed that
nitrogen oxides can cause or enhance the process known as
photochemical smog formation through a series of
reactions in the presence of sunlight and hydrocarbons.
Moreover, nitrogen oxides are a significant
contributor to acid rain and have been implicated in the
undesirable warminy of the atmosphere through what is
known as the "greenhouse effect" and in the depletion of
the ozone layer. In addition, gas turbines often emit a
visible plume, which is highly undesirable since it
causes concern among the population in areas surrounding
the facility.
Although the use of emulsified oils (primarily
produced from relatively simple mechanical technigue~
for combustion improvemen~s has been suggested in the
past, they have not:been~applied to gas turbines, and the
oils emulsified are usually~ heavy oils (i.e., #5: oilj.
Because gas turbines are peaking units, the fuel is
required to remain emulsified for at least 30 days in a
holding tank, and at least 2 hours during in-line
mixing . This has been dif f icul~ to accomplish,
especially when using mechanical emulsion methodology.
In addition, gas turbines are very sensltive to
corrosion, which often leads the practitioner to avoid
introducing emulsified water into the combustion zone~
~'.
W~ 7~l PCT~US~2/03328
S
It has been known for some time that the injection of
wa~er directly into the combustion zone (also referred to
as the "combustion can") of a gas turbine at water to
fuel ratios of greater than l:l can control the emission
of NOX and reduce opacity to a limited extent. The
direct injection of water into the combustion can,
though, requires extensive mechanical modificatiQn of the
gas turbine (with high capital cost) and in~olves thP ::
injection of large volumes of demineralized w~tter which
results in ex~ensive additional maintenance and out ye
time because of the the~mal shock to the combustion can.
What is desired, therefore, is a process which
permits the reductic:~n of effluent nitrogen oxides and
plume opacity from a gas turbine wi~hout the thermal ~.
shock, large capital costs, and other drawbacks of ;
injecting water directly .into the combustion can.
Brlef De ~
The present invention will be better understo~d and
its ad~antages more apparent in view of the *ollowing .-
de~ailed description, especialIy when read with reference
to the appended drawings, wherein:
'
FIGURE 1 is a schematic~ illu~stration of a gas turbine
fu~l supply system :having an emulsification æystem -.
according to the present::invention installed therein;
~ IGU~E 2 is a schematic illustration of an
emulsification system according to the present invention
as installed in a gas turbine fuel supply system, and
~ -.
FIGURE 3 is a graphic representation of the results
of Examples IIa and IIb.
.,
.
W~ tOI PCr/US92/03328
2 1 ~ 6
Disclosure of Invention
The pr~sent in~ention relat~s to a method for
reducing nitrogen oxides emissions and improving the
combustion efficiency of a gas turbine (which term will
be considered to be interchange~ble with ~omb~stion
turbine). In particular, this invention relates to a
process involving the formation of a stable water-and~
fuel oil emulsion, where the oil is a light fuel oil such
as diesel fuel, distillate fuel or ~2 oil. The subject
emulsion can be either a water-in-fuel oil or a fuel
oil-in-water emulsion (although water-in-fuel oil
emulsions ~re preferred for most applications),~and the
introduction of the emulsion into at least one of the
combustion cans of a gas turbine through its fuel system.
Typically, the oil phase in the inventive emulsions
comprise what is conventiona:lly known as diesel fuel,
distillate fuel, or #2 oil, as defined by the Amert an
Society of Testing and Measurement (ASTM~ Standard
Specification for Fuel Oils (~esignation: D 396-86).
Especi~lly preferred are distillate fuels. Included
among these are kerosene and ~et ~uels, both commercial
and military, commonly referred to as ~P4 and JP5,
respectively.
Although demineralized water is n~t re~uired for the
suc~essful control of nitrogen oxides and opacity, the
use of , demineralized wat~r in the emulsion foxmed
accordi~g t~ the process~of this invention is preferred
in order to avoid the deposit of minerals from the wa~er
on the blades and other iL~ernal surfaces of the gas
turbine. In this way, turbine life is extended and
maintenance and outage tlme signifi~antly reduced.
~ /lg7~l PC~`/U~92/03328
2~ 09VYij -
The emulsions used in the fuel system of the gas
turbine advantageously comprise water-in-fuel oil
emulsions having up to about 50% w2ter by w ight. The
emulsions of this type which have the most practical
significance in combustion applications are those having
at least abo~t 5% water and are preferably about 10% to
about 35% water-in-fuel oil by weight. In addition, this
invention also relates to the formation of fuel
oil-in-water emulsions having about 50% to about 8b%
water, which have practical applicability in certain
situations.
Advantageously, the emulsions are prepared such that
the discontinuous phase (i.e., the water in a water-in-
fuel-oil emulsion and the oil in an a fuel oil-in-water
emulsion) has a particle size wherein at lea~t about 70%
of the droplets are below about 5 microns Sauter mean
diameter. More preferably, at least about ~5~, and most
pre~erably at least about 90~, are below about 5 microns
Sauter mean diameter.
Emulsion stability is largely related ~o droplet
si~e. The primary driving force for emulsion separation
is the large energy associated with placing oil molecul s
in close proximity to water molecules in the form of
small droplets. Emulsion breakdown is controlled by how
quickly droplets coalesce. Emulsion stabil~ity can be
e~han~ed by the u~e of surfa~tants and the like, which
~Ict as ~mulsifiers or emulsidn stabilizers. These
generally work by forming repulsiYe layers hetween
droplets prohibiting coalescence~ The gravitational
driving f orce for phase separatlon is much more pro~inent
for large droplets,~ so lemulsions containing large
droplets separate mosk rapidly.
W~ 7~)1 P~T/US92/03328
2 l 09~ 6-
Smaller droplets also settle, but can be less prone
to coalescence, which is the cause of creaming. If
droplets are sufficiently small, the force of gravity
acting on the droplet is small compar~d to thermal
fluctuations or sub~le mechanical agitation forces. In
this case ~he emulsion can beoome stable almost
indefinitely, although given a long enough period of time
or a combination of thermal fluctuations these emulsions
~ill eventually separate~ '
Because of the operating characteristics of gas
turbines, ik is requixed that the water/fuel oil emulsion
exhibit a high degree of st:ability. In most cases, gas
turbines are "peaking" unit:s, as noted,. which do not
operate regularly. Accordingly, an emulsified fuel mcy
sit stagnant for extended pe.riods or with only mild
recirculation in the fuel line. In order to avoid
separation of the emulsion into its components, which can
cause slugs of water to be injected throu~h the burner
nozzle leadin~ to combustion problems and possible engine
damage, an emulsifier or emulsion stabilizer is also
desirable in the water/fuel oil emulsion.
Advantageously/ the emulsifier utilized comprises a
composition selected from one or more alkan~lamides,: by
which ls gene~ally meant an amide formed by condensation
o~ an alkyl or hydroxyalkyl amine~ or mixtures thereof r
and an organic acid. Pre~erred acids are fatty acids,
such as lauric acid, linoleic acid, oleic acld, stearlc
acid, and coconut oil ~atty acids. Most pre~erred are
alkanolamides having a molar ratio of alkanolamine group
to acid group of from about l:l to about 2~
:''
Surprisingly, these compositio~s can stabilize an
emulsion of up to about 50% water-in-fuel oil, or up to
W~ ~J2/ 1~70 i PCT/US92/03328
2l~sas6
--7-- ~:
abs:~ut 80% fuel oil-in-water in alkanolamid~ amounts as
low as about 0.05% by weight, and even as ~ow as about
0.01% ~y weight. In fact, although there is no true :~
maximum amount of emulsifier which can be used, there is
usually no need for greater than abou~ 1%, or, in fact,
greater than about 0.5% by weight emulsifier in the
subject emulsion. Advantageously, to stabilize an --
emulsion ~f up to about 50% water-in-fuel oil, the noted :~.
alkanolamides should be included in an amount of ~rom ;~
about 0.1% to about 0.3% by weight~
Suitable alkanolamides which can function to .
stabilize the emulsion of the process of the pr~sent
invention include any one or more of the followi~g: :~
cocamide diethanolamine (DEA), lauramide DE~,
polyoxyethylene (POE) cocamide, cocamide monoethanolamide
(MEA), POE lauramlde DEA, olea.mide DEA, linoleamide DEA,
an~ stearamide MEA, as well as mixtures thereof. ~uch
alkanolamides are commercially available under trade
names such as Clindrol ll~O-0, rrom Clintwood Chemical
Company of Chicago, :IIlinois; Schercomid ODA, from Scher
::hemicals, Inc. of: Clifton, New Jersey; Sche~c:omid SO~
also from Scher Chemicals , I:nc .; ~nd Mazamide~, and the
Mazamide series from PPG Nazer Products Corp. of Gurnee,
Illinois. : : :
Other emulsif iers which: may be useful include :
ethoxylated alkylphenols, such as nonyl~ phenol, octyl
phenol, i etc. and salts o~ alkylated sulfates or
sulfonates, such ~ as sodium lauryl sulfate. ~ In addit~ion,
the skilled artisan will recognize that o~her emulsifiers
or blends of emulsifiers, ma~ be also effective at
maintaining the stability: of the inventive ~emulsio~
.;,
The use of ~ the noted emulsifiers prov1des chemicaI
"
. . .
WOg~/l9701 PCT/US92~03328
~ 1 0 ~
-8- :
emulsification, which is dependent on hydrophylic-
lipophylic balance (HLB), as well as on the chemical
nature of the emulsifier. The HLB of an emulsifier is an
expression of the balance of the size and strength of the
hydrophylic and the lipophylic groups of the composition.
The HLB, which was developed as a guide to emulsifiers by :~
ICI ~mericas, Inc. of Wilmington, Delaware can be
determined in a number of ways, most conveniently for the
p~rposes of this in~ention by the solubility or
dispersibility characteristics of the em~lsifier in
water, from no dispersibility (HLB ranye of 1-4) to clear
solution (HLB range of 13 or greater). The emulsifiers
useful in the present invention should most preferably
have an HLB of 8 or less, meaning that after vigorous
agi~ation they form a milky dispersion in water ~HLB
range of 6-8~, poor dispersion in water (HLB range of
4~6), or show no dispersability in water (HLB range of
less than 4).
It is also possible to utilize a physical emulsion .:
stabilizer in combination with the chemical emulsifiers
noted above to maximize the sta~ility of the emulsio~ :
achieved in the process of the present invention. Use of
physical stabilizers also provides economlc benefits due
to their relatively low cost. Altho~gh not wishing to:be
bound by any theo ~, i~ is belie~ed that physical
stabilizers increase ~emulsion stability :either by
increasing ~he solubility of immiscible phases or by
~orming an insoIuble barrier attracted to the oil~water
interface. Exemplary of suitable~ physical stabilizers
are ~axes, cellulose products and~gums such as whalen gum
and xanthan gum. : ~
~,...
When utilizing both chemical emulsifiers and physical
emulsion stabllizPrs, the physical 5tabilizer is present
.,
WO~ 7nl PCT/US92/033~8
210906
in an amount of about 0.05% to about 5% by weight of the
combination ~f chemical emulsifier and the physical
stabilizer. The resulting combination
emulsifier/stabilizer can then be used at the same levels
noted above for the use of emulsifier alone.
The emulsification provided must be sufficient to
maintain the emulsion to a greater extent than if the
emulsifier was not present and to as great an extent'as
possibli. The actual level of emulsification will vary
depending upon the percentage of oil and water in the
emulsion and the particular fuel oil utilized. ~or
example, when the continuous phase is ~2 oil, it is
highly desired that no more than about 0.1~ free water be
present in the emulsion, and that the emulsion -is
maintained that way at ambient conditions for at least
about two hours. Ambient conditions, that is, the .
conditions to which the emulsion is expected to be
exposed, include the temperature in the gas turbine fuel
feed lines. Such temperatures can be~up to about 65C
more typically up to about 90C and even as high as about~
100 C. ~ '
The emulsion used in the pro~ess of the present
in~ention can be formed usinq a suitable mechanical
emulsifying apparatus which would be familiar to the
skilled artisan. Advantageously, the ~apparatus is an
in-1ine emulsifying device for most efficiency. The
emulsion is. formed by feeding both the water and ~he fuel
oil in the desired proportions to the emulsifying ~.
appara~us, and: emulsi~ier or stabilizer ~he~ used~can~
either be admlxed or dispersed in~o one or b~th of ~he
components before emulsification or can be added to the
emulsion after it is formed.
: : ~
~2~197~1 PCT/U~92/03328
2 1 0 !~ ~ 3 6
--10--
Preferably, the emulsifier and/or sta~ilizer is
present at the time of emulsifying the water and fuel
oil. Most advantageously, any emulsifier or stabilizer
used is pro~ided in the water phase, depending on its
HLB. It has been found that the emulsions noted above
with the chemical emulsifiers can be stabilized at up to
about 50~ water-in-fuel oil for up to 30 days and
longer. In fact, with mild agitation, such as
recirculation, it is believed that th~ emulsic~ can stay
in suspension indefinitely.
Surprisingly, the emulsion can then be introduced
into a c~mbustion ran of the gas turbine through the fuel
feed lines and burner nozzles conventionally used with
such combustion apparatus. There lS no need for
modification of the gas turbine fuel feed lines or
combustion can to accommodate the emulsion used in the
process of this invention.
Figures 1 an~ 2 illustrate a gas turbine fuel supply :
system having installed:therein an emulsification system
for ~he practice of ~he:process:of the present:invention
and a schematic illustration;:of~ the emuls~ification system:
:itself. As illu~.at~d~ in~ Figure l:,~an:~emulsification~
system lO can ~ be installed~ln~;a~ gas~:turbine~fuel supply
system lOO between the~ h~eater l22 and~the~final:filter
124~ hough emulsifi~ation:~system~lO~is:illus~ra~ed~as
being installed: ~in~ this~pos~ition:in~uel supply ~sys~em~
lOO, it will ~ be recogniz~ed b~y the skilled arti6an~t~at
other positions~ may; ;be ~ more~ ~advantaqeous in ~terms of
emulsion stability:~ in: ~other :~fuel ~:supply ~ ~;system;~
embodiments, and :emulslfi:catlon : system~: lO~can:~:be
installed at virtual~ly any::po~lnt alon~ fuel supply~system~
lOO :for operability.~Indeed~, it~will also be~recogniz~d~
that~ heater ~122 ~and~final~filter 124 are preferr~d: :
.,
,
' ~ `''
W~Z~1')7~1 P~T/US9~/03328
2~'.`3~3~
components of fuel supply system 100 and conventionally
utilized~ but not critically needed.
Fuel supply system 100 is typical of many gas turbine
f~el supply syst~ms and generally comprises a fuel supply
line 110 which is fed by a fuel tank or other holding or
storage apparatus (~ot shown). Fuel flowing through fuel
supply line 110 proceeds through a set of initial filters
112a and 112b, and is then fed to individual fuel sup~ly
systems 120, 220, and 320 which feed engines controlled
by fuel supply system 100. For ease of understanding,
fuel supply system 120 which feeds engine manifold 130 is
specifically illustrated. Supply systems 2~0 and 320 are
equivalent in operation.
Fuel supplied through fuel supply line 110 is fed
along engine manifold 130 supply line 120 into heater
122. From there, the fuel flow continues past valve 114
into final filter 124. From ~inal filter 124, the fuel
flow continues along line 12~ through engine pump 136 and
from there into fuel distribution manifold 121 which then
supplles the fuel through primary nozzle 132~ and
secondary nozzle 134 to engine manifold 130~ which is the
combustion æone of the subject gas turbine. In::addition,
fuel supply system 110 further comprises recirculation
lines 123a and 123b and recirculation pump 128 for
recirculation of the fuel through line 123.
! When valve 114 in fuel supply line 120 i~ closed and
val~2s 20 and 22 in ~emulsification system 10 are open,
fuel flowing along fuel supply: line 120 is shunted
through emulsification system lO after heater 1~2, and lS
resupplied to fuel supply line 120 be~ore final filter
124 ior ieeding to englne manifold 130 or recirculation.
.
~Y~ 7~1 PCT/US9~03328
~ 0 9~i -12-
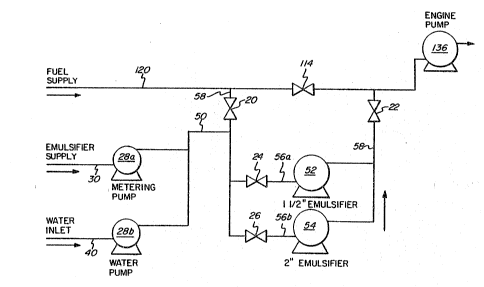
As illustrated in Figure 2, emulsification system lO
comprises an emulsifier supply line 30 which supplies
emulsifier from a tank or other storage means (not shown~
to a metering pump, and is then fed through line 50. In
addition, emulsification system lO comprise~ water inlet
line 40 which feeds water from a tank or other supply
means (not shown) through a water pump 28a to supply line
where it is admixed with emulsifier supplied from
emulsifier supply line 30.
The water/emulsifier fed through line S0 then meets
fuel being fed through line 58 when valve 20 is open and
valve 114 is closed, These are then fed through either
one or both of l l/2 inch emulsifier S2 or 2 inch
emulsifier 54, depending on whether one or both of valves
24 or 26 is open through feed lines 56a and 56b,
respectively. The emulsified water-in-fuel oil is then
fed via line 58 back through fuel supply line 120 when
valYe 22 is open and from there into engine pump 136 and
ir:to engine manifold 130~
Although not wishing to be bound by any theory, it is
believed that the use o~ an emulsion provides striking
a~vantages o~er separate water injection syst~ms because
the water is being provided internal ~o the flame. By
doing so, less water is required to achieve superior
results, which reduces the deleterious effects of
directly introducing large amounts of water to the
~ombustion zone of the gas tur~ine~
Because of the advantages of introducing water
internal to the flame, utilization of the inventive
process r~sults in a redu~ed use of demineralized water
(since the emulsion contains less than the l:l ratio of
water to fuel oil used when water is injected directly
W~J2/1~701 PC~/US92~03328
2 1 0 9 ~ 9 6 ~
-13
into the combustion can), and leads to less thermal
stress which reduces maintenance cost and outage time.
When the emulsified fuel is introduced into the
combustion zone, the heat of vaporization from the
burning fuel causes the emulsified wàter droplets to
become stea~, which creates a secondary atomization.
This secondary atomization improves combustion and
increases the gas volume. In addition, the heat re~uired
to change the water to steam is believed to reduce the
flame temperature of the combustlon which helps to rPduce
formation of nitrogen oxides.
Additionally, use of the water/fuel oil emulsion
results in substantial elimina~ion of the need for an
expensive, independent smoXe suppressant additive.
Typically, such additives are heavy metal ~ased products
which can form ~eposits on the turbine blades, reducing
efficiency and increasing maintenance costs. By the use
:
of emulsions in the process of~this invention, a 90% or
greater reduction in smoke suppressant additive use is
often achieved, which increases ~the blade:life due to
reduced depo its, and~ creates~ less wear on:the turbine
blade coatings. These:~d~antages~ lead;to signifioant~
:~savin~s in operating and~maint~nan~e cos~s.
Furthermore,~ when ~:compared~ to a ~separate water
injectlon system, the~:use of the process of thls
inventionl leads to improved engine fuel sys~em integrity;
the en~ine burns cooler,iwhich,~ as~noted~ ads~t`o less~
thermal stress;: it is believ~ed that ~he~gas turbine:can~
assume a ~higher ~:load~capacity;~ ~and compliance~; with
environmental regulations is~more~easily ob~ain~b~le.
When the proces5 Of the pres~nt invention is,: for
WO'~2/1~7~1 PCr/US9~/0332~
21~90~6
-14-
lnstance, conducted on a 48 megawatt gas turbine by
installing a manually operated in-line emulsification
system at the fuel oil inlet, it is found that reductions
of Il~trogen oxides of about 75%, which are immediate and
reproducible, are obtained. These results are about 50%
greater than those found when a separate water in~ection
system is used at equivalent water injection rates. In
addition, plume opacity is found to disappear and no .~
operational problems are detected. In fact, inspection ~-
of the engine after use of the emulsion process of this
inventiQn shows no deposits. ;
The following examples further illustrate and explain
the invention. ~:
~ E~E I
An emulsification system is prepared comprising two
rotary emulsifiers and related storage, pumping and
piping apparatus for prPparation and supply of a water-
in-fuel oil emulsion to a Pratt and Whitney Jet engine
burning 30 gaIlons of fuel per minute at full load ..
"
" ~
Baseline emissions tests are run on the engine with~ :
non-emulsified distillate fuel oil, and~ then with ~.
emulsi~ied fuel at water levels:of 10%, 15%, 20%, 25~, :
35~, and 50%. The emulsifier used is oleamide DEA added
at 2.5 gallons per l,000 gallons of fuel ~corresponding
to .25% of emulsi~ier by weight). The emulsion remained ~:
stable (i.e., no visible water separation~ for over two
hours without agitationa
,..
The results of the two tests are then compared and
the foIlowing found: :`
W09~197nl PCT/US9~/03328
~1~9~.i3S
1. When compared to baseline, each incremental
increase in water content reduced nitrogen oxides levels
up to ~o%.
2. At water rontents above 20%, visible opacity
disappeared. ~
3. At water levels above 35~, power output frvm the ::
engine increased ~y approximately 3~ due to greater mass
flow.
4. Blades and guide vanes are found to be cleaner
with the emulsion prepared a~cording to the present
invention.
EX~MPLE IIa
An emulsification system in a~cordance with Figures 1
and 2 ls prepared fox supply to a single TP~M A4 engine `~
operating as par~ of a twinpac~ rated at approximately 35
MW. Flue gas samples are obtained through a three point ~:
pro~e installed on the outlet ~uct with the sample points~
located between the gulde vanes. The~ samples are
combined and the NO and NO2 levels therein measured,
and compared with basellne levels.
: Two tests are run usin~ incre~entally increased
emulsion strengths (water content) and~ the resul~s
plotted in Fi~ure 3.
-EXAMP~ Ib :
~ s reported by ~Becker et al. in "~as Turbine ~ :
Operating Per~ormance a~d Consider~ations for Combi~ne:d
Cycle Conversion~ at ~Hay Road Power Station'i, American
'.
~'
~,
~'
WO~2/l')7()l PCT/US92/0332B
Z ~ U -16-
Power Conference, April, lg90, two 100 MW Siemens model
V84.2 engines with hybrid burners are operated with :~
separate water injection. Flue gas NO and NO2 leYel~
are measured and compared with baseline levels.
Two tests are run using incrementally increased
levels of water injection and the results plotted in ~;~
Figure 3
1
Figure 3 illustrates the fact that use of the process
of the present invention permits equivalent reduc~ion of
ni.trogen oxides with approximately 50% of the amount of
water injected. ;~
rhe above description is for the purpose of teaching
the perso~ of ordinary skill in the axt how to practice
the present invention, and it, is not intended to detail
all of those obvious modifications and variations of it
which will become apparent to the skilled worker upon
reading the description. It is intended, however, that
all such ob~ious modifications and variatlons be included -~
within the scope of :the present in~ention which is
defined ~y the following clalms. ~ .