Note: Descriptions are shown in the official language in which they were submitted.
WO 93/00163 ~ I 1 0 1 ~ ~ PCl/US92/04121
, -- 1
A~TICLE FOR 8BPARI~TION8 AND P~RIFICATION~;
AI~D MEl~OD OF CONTROI.I.ING POR08ITY TIIFRI~IN
FIELD OF T~IE IN~q!ION
This invention relates to a porous particle loaded
sheet of fibrillated polytetrafluoroethylene (PTFE)
comprising a combination of sorptive particles and
1o energy expandable or expanded polymeric particles, and
a process therefor. The sheet is useful in separations ~;~
and purlfication applications.
BAC~6RO~ND ART
The background art teaches various formulations ,
for blending an aqueous PTFE dispersion with,various '`
additives andtor adjuvants designed for specific
purposes. For example, U.S. Patent No. 4,990,544
teacbes a gasket comprising a fibrillated PTFE resin
20 and a fine inorganic powder dispersed therein. U.S. ,
Patent No. 4,985,296 teaches an expanded, porous P~FE
film containing filler material which is purposely ~'
compressed to provide thin films where space reduction
is desirable.
Assignee's patent application U.S.S.N. 07/639,515
-
(now allowed) discloses a method of controllinq the
porosity in a composite PTFE article by controlling the
amount of lubricant used during processing of the
article. The article also has controlled mean pore
30 size. Desian News, ~Particulates Captured/Carried by
Fibrillated PTFEI', February 9, 1987 (Cahners Publishing
Company), discloses particles carried by fibrillated
PTFE membranes having a porosity in the range of 30 to
70~, and pore sizes from 0.01 to 5.0 microns.
U.S. Patent Nos. 4,971,736, 4,906,378, and
4,810,381 disclose a composite chromatographic sheet-
' like article and method therefor. The artic}e
WO93/00163 ~1 1 01 5 ~ - 2 - PCT/US~/0412
comprises a PTFE f ibril matrix and non-swellable
sorptive hydrophobic particles enmeshed in the matrix.
References cited in these patents relate to other PTFE
matrices containing particulates. U.S. Patent No.
5 4,971,697 teaches a chromatographic article comprising
a PTFE fibril matrix having enmeshed therein a mixture
of non-swellable sorptive particles and hydrated silica
flakes. Hagen, et al., "Membr~ne Approach to Solid
Phase Extractions", Analvtica Chimica Acta, ~ (1990)
10 157-164, relates to particle loaded PTFE matrices
useful in extraction applications.
U.S. Patent No. 4,460,642 teaches a water-
swellable composite sheet of microfibers of PTFE and
hydrophilic absorptive particles enmeshed therein which
15 is useful as a wound dressing.
U.S. Patent No. 4,923,737 discloses a method for a
"metal cloth" prepared from fibrillated PTFE containing
metal or other particles entrapped in the fibrils.
~ n regard to polymers, fibrillated PTFE has also
20 been combined with a polyamide to provide articles by
extrusion blow-molding (U.S. Patent No. 4,966,941) and
with an elastomer to provide articles with increased -~
durability (U.S. Patent No. 4,962,136). U.S. Patent
No. 4,945,125 teaches a process of producing a
25 fibrillated semi-interpenetrating polymer network of
PTFE and silicone elastomer. U.S. Patent No. 4,914,156
describes a blow moldable composition comprising a
polyether, an epoxide polymer, a source of catalytic
cations, and a fibrillatable PTFE. U.S. Patent No.
30 4,902,747 discloses a polyarylate composition
containing fibrillatable PTFE.
U.S. Patent Nos. 4,199,628 and 4,265,952 relate to
a vermicular expanded graphite composite blended with a
corrosion resistant resin such as PTFE with improved
35 impermeability to corrosive fluids at high
temperatures.
W~93/0D163 2 1 1 0 1 S ~ PCT/US92/n4t21
.....
,
.. . .
U.S. Patent No. 4,483,889 discloses the method of
making a composite material comprised of a fibrous
matrix, expandable polymeric microbubbles, and a
formaldehyde-type resin involving distributing the
5 expandable microspheres (either expanded or unexpanded)
into the fiber matrix, expanding the poly~eric bubbles
by ~pplication of heat (in the case where unexpanded
microbubbles were used), and impregnating the resulting
porous matrix with a curable formaldehyde-type resin to
10 give a foam.
U.S. Patent Nos. 3,407,096, 3,407,249, 3,556,161,
and 3,281,511 teach incorporation of extractable or
leachable filler particles to create porosity in an
article.
~MARY OF T~E INVENTION ~-
Briefly, the present invention provides a
composite sheet-like article useful in at least one of
separations and purification applications comprising:
(a) a polytetrafluoroethylene (PTFE) fibril
matrix, and
(b) a combination of sorptive particulate and
energy expandable, or energy expanded, hollow polymeric
particulate enmeshed in the matrix.
Preferably, the weight ratio of PTFE to total
particulate is in the range of 2:98 to 50:50, more
preferably 5:95 to 25:75.
Preferably, the weight ratio of sorptive
particulate to energy expanded or expandable polymeric
30 particulate is in the range of 3:1 to 1000:1, more
preferably 5:1 to 500:1.
In another aspect, this invention provides a
method of controlling interstitial porosity in a
composite sheet-like article useful in at least one of
35 separations and purification applications. The amount
of energy expandable particulate in t~e fibril matrix
..
WO93/00163 - PCT/US92/04121
2~i015~
- 4 -
controls interstitial porosity in the expanded sheet-
like article. -~
What the background has not taught but what this
invention teaches is a composite article comprising, in
S an unexpanded form, a fibrillated PTFE matrix, sorptive
particulate, and energy-expandable hollow polymeric
particles, which composite, on applying energy such as
steam, heat or laser energy, provides an expanded
article whose porosity is greater than that of the
lO unexpanded form of the article. The expanded articles
are porous and efficient articles for separations and
purification applications. - -
In this application:
"sorptive~ means microporous and capable of beinq ;
l5 active in separations and purification applications.
Assignee's copending patent application, U.S.S.N.07/723,064, filed June 28, l99l, discloses composite
articles comprising a fibrillated polyolefin matrix and
energy expandable or expanded particulate enmeshed
20 therein which are useful thermal insulators.
BR~EF DE~CRrPTION OF TRE DRAWING
In the accompanying Drawing:
FIG. l is a plot of time vs. distance traveled for
25 a solvent front in thin layer chromatography (TLC) in
articles of the invention in which the proportion of
expanded particulate to sorptive particulate is varied;
FIG. 2 is a plot of a TLC solvent front rate vs.
percent unexpanded particulate and vs. percent expanded
30 particulate;
FIG. 3 is a plot of percent numbers of pores vs.
pore size in unexpanded and expanded articles of the
invention;
FIG. 4 is a plot of flow rates vs. percent
35 expanded particulate in articles of the invention;
FIG. 5 is an enlarged perspective view of a
portion of an unexpanded article of the invention;
W093/00163 2 1 1 0 1 5 ~ PCT/US92/~12t
_ 5 _ ;
FIG. 6 is an enlarged perspective view showing an
article of the invention in use in a column;
FIG. 7 is a cross-sectional view, greatly
enlarged, of t~e energy expandable sheet-like article
5 of the invention;
FIG. 8 is a cross-sectional view, greatly
enlarged, of the energy expanded sheet-like article of
FIG. ?. -
- ~ ~
10 D~TAILED DE8CRTP$ION OF T~E DRA~ING
FIGS. 1-4, see~Example l, below.
FIG. 5 is a perspective view of partially r~olled
sheet lO of the article of the invention. Energy-
.. . . . .
expandable particulate 12 and sorptive particulate 14
15 are enmeshed in PTFE fibril matrix 16.
FIG. 6 is an enlarged perspective view showing
packed column 20 containing article l0 of FIG. 5 which
has been rolled up, placed in chromatographic-column
22, and subjected to energy to expand article l0 so
20 that it snugly fills a portion of the interior cavity
24 of column 22.
FIG. 7 shows one embodiment of a cross-sectional
view, greatly enlarged, of the sheet like article 30 of
the invention having PTFE fibrils 32 in which are
25 enmeshed sorptive particulate 34 and energy expandable
particulate 36.
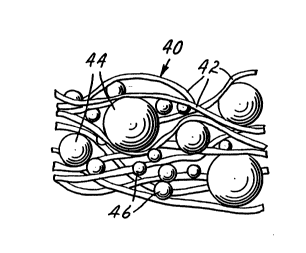
FIG. 8 shows sheet-like article 40 provided after
application of energy to the article of FIG. 7.
Fibrils 42 have expanded particles 44 and s~rptive
30 particles 46 enmeshed therein.
DETA~LED DE8CRIPTION OF PREFDRR~D EMBODIMENT8
Controlled interstitial porosity in shee~-like
-articles comprising a fibrillated PTFE matrix and
35 sorptive particulate enmeshed therein can be achieved -
by further incorporating therein in the range of 0.05
WO93/~163 PCT/US92/~121
211~)15~ 6 -
to 25 weight percent of energy expandable or expanded
hollow polymeric par~icles.
Expandable particulate material useful in the
present invention can be swellable or non-swellable in
S aqueous or organic liquid, and preferably is
substantially insoluble in water or organic liquids. -
In addition, the expandable particulate is not
homogeneous, i.e., it is not a uniform bead of polymer
but rather comprises a polymeric shell having a central
10 core comprised of a fluid, preferably liquid, material.
A further requirement is that the overall dimensions of
the expandable particulate increase upon heating at a
specific temperature. This expansion or intumescence
is different from expansion due to solvent swelling and
15 can occur in the dry state (i.e., in the absence of
solvent).
Expandable particulate includes those materials -~
comprised of a polymeric shell and a core of at least
one other material, either liquid or gaseous, most
20 preferably a liquid at room temperature, in which the
polymeric shell is essentially insoluble. A liquid
core is advantageous because the degree of expansion is
directly related to the volume change of the core
material at the expansion temperature. For a gaseous
25 core material, the volume expansion expected can be
approximated from the general gas laws. However,
expandable particulate comprising liquid core material
offers the opportunity to provide much larger volume
changes, especially in those cases where a phase change
30 takes place, i.e., the liquid volatilizes at or near
the expansion temperature. Gaseous core materials
include air and nonreactive gases and liquid core
materials include organic liquids.
Preferred energy expandable particulate (also
3S called microbubbles, microballoons, and microspheres)
have shells comprising copol~mers of vinyl chloride and
vinylidene chloride, copolymers of vinyl chloride and
W093/00163 2 1 1 0 1 ~ S PCT/US9~/04121
- 7 -
acrylonitrile, copolymers of vinylidene chloride and
acrylonitrile, and copolymers of styrene and
acrylonitrile. Furt~er can be mentioned copolymers of
methyl methacrylate containing up to about 20 percent
5 by weight of styrene, copolymers of methyl methacrylate
and up to about 50 percent by weight of ethyl
methacrylate, and copolymers of methyl methacrylate and
up to about 70 percent ~y weight of orthochlorostyrene.
The unexpanded microspheres contain fluid,
10 preferably volatile liguid, i.e., a blowing agent,
which is conventional for microspheres of the type
described here. Suitably, the blowing agent is 5 to 30
percent by weight of the microsphere. The microspheres
can be added in different manners, as dried particles,
l5 wet cakes, or in a suspension, e.g., in an alcohol such
as isopropanol. The microspheres can also be added in
a pre-expanded form.
The unexpanded particulate desirably is in the
size range of from about 0.5 micrometer to about 200
20 micrometers, preferably from l micrometer to lO0
micrometers, most preferably from 3 micrometers to so
micrometers. After expansion, the volume of tbe
expandable particulate increases by a factor of at
least l.5, preferably a factor of at least 5, and most
25 preferably a factor of at least lO, and may even be as
high as a factor of about lO0.
As an example, Expancel pol~meric microspheres
~Nobel Industries, Sundsvall, Sweden) expand from an
approximate diameter of lO micrometers in the
30 unexpanded fcrm to an approximate diameter of 40
micrometers after expansion. The correspondinq volume
increase is
Vf/Vi = (rf/r~)3 = 43 ,
or 64-fold, where Vf and rf are the final volume and
35 radius of the expandable particulate, respectively, `
after expansion, and Vi and ri are the corresponding
initial values for the unexpanded particulate.
WO93/~163 PCT/US92/~t21
2l~al~fi ''"
- 8 -
Expanded particulate provides increased interstitial
porosity in the sheet material.
Preparation of expandable particulate is normally
accomplished by suspension polymerization. A general
5 description of some of the techniques that can be
employed and a detailed description of various
compositions that are useful as expandable particulate
can be found in U.S. Patent No. 3,615,972. A further
description of compositions useful as expandable
10 particulate in the present invention is given in U.S.
Patent No. 4,483,889.
Examples of commercially available expandable
hollow polymeric microspheres useful in the present
invention include those made of poly(vinyIidene
15 chloride-co-acrylonitrile) such as Expancel~ 820,
Expancel~ 642, Expancel~ 551, Expancel~ 461, and
Expancel~ 051 polymeric microspheres. Other
commercially available materials having similar
constructions, and comprising, for example, a shell of
20 methacrylonitrile-acrylonitrile copolymer, available as
Micropearl~ F-80K microbubbles (Matsumoto Yushi-Seiyaku
Co., Ltd., Japan), are also useful as expandable
particulate in the present invention.
A wide variety of blowing or raising agents may be
25 incorporated within the polymerization process. They
can be volatile fluid-forming agents such as aliphatic
hydrocarbons including ethane, ethylene, propane,
propene, butene, isobutene, neopentane, acetylene,
hexane, heptane, or mixtures of one or more such
30 aliphatic hydrocarbons preferably having a number
average molecular weight of at least 26 and a boiling
point at atmospheric pressure about the same
temperature range or below the range of the softening
point of the resinous material of the polymeric shell
35 when saturated with the particular blowing agent
utilized.
WO g3/00163 PCI/US92/W121
21101S S
g
Other suitable blowing agents are halocarbons such
as perfluorobutanes, perfluoropentanes,
perfluorohexanes, fluorotrichloromethane,
dichlorodifluoromethane, chlorotrifluoromethane,
S trichlorotrifluoroethane, heptafluorochlorocyclobutane,
and hexafluorodichlorocyclobutane, and tetraalkyl
silanes such as tetramethyl silane, trimethylethyl
silane, trimethylisopropyl silane, and trimethyl-n-
propyl silane, all of which are commercially available.
The shape of the expandable particul~te is
preferably spherical but is not restricted to
spherical, i.e., it may be irregular. Other shapes can
easily be envisioned such as urnlike as described in
U.S. Patent No. 3,615,972. The shape and orientation
15 of the expandable particulate in the composite article
determine the anisotropy of the expansion step. Where
essentially spherical expandable particles are used,
heating leads to isotropic expansion of the composite,
i.e., there is no preferred direction of expansion and
20 all three axes expand uniformly so that the overall
shape of the article does not change, only its size.
Other physical constraints that may have been imposed
on the article, such as during processing or by
anchoring one part of the article prior to expansion,
25 may lead to less than perfect isotropic expansion where
essentially spherical expandable particulate is used.
As a result of the expansion of the expandable
particulate, the volume of the composite article
increases. The percent volume increase is dependent on
30 a number of factors such as the loading of expandable
particulate in the composite and the molecular weight
of the polymeric shell of the expandable particulate.
The decrease in article density is inversely
- proportional to the volume increase of the article.
Thickness of the composite article prior to
expansion can range from about 0.0127 cm to about 0.32
cm, preferably from about 0.018 cm to 0.25 cm, most
W093/~163 2 1 1 0 1 5 ~ PCT/US92/Wl21
-- 10 --
preferably from about 0.025 cm to about 0.127 cm. When
the article is too thin, it has very little structural
integrity while articles having thicknesses outside of
the given range may be difficult to form. Thickness
5 after expansion is dependent on several factors, as
stated above. Thinner articles can be made by
densification as is described in U.S. Patent No.
4,985,286. Alternatively, densification can be
accomplished by solvent extraction of the polymeric
10 microspheres. ~-
Chromatographic sheet-like articles and method of
preparation have been disclosed in U.S. Patent Nos.
4,810,381, 4,906,378, and 4,971,736. Sorptive
particulate useful in the sheet-like articles of the
15 present invention are disclosed therein.
The sorptive particulate material (which can be
one material or a combination of materials) useful in
the present invention is non-swellable in aqueous and
organic media and is substantially insoluble in water
20 or the elution solvent. Not more than 1.0 gram of `
particulate will dissolve in 100 g. of aqueous media or
elution solvent into which particulate is mixed at
20C. The sorptive particulate material can be carbon,
an organic compound, a polymer, or an inorganic oxide
25 such as silica, alumina, titania, zirconia, and other ;~
ceramics, or it can be ion exchange or chelating -
particles. Preferred particulate material are silica
and zirconia, with silica being particularly preferred
because of the ease in bonding a variety of hydrophobic
30 and semi-hydrophobic coatings onto its surface and
because they are commercially available.
Silica is available from Aldrich Chemical Co.
(Milwaukee, WI). Zirconia is available from Z. Tech
Corporation (Bow, NH). Other inorganic oxides are
35 available from Aldrich Chemical Co.
Suitable sorptive particles for the purposes of
this invention include any particle which can be coated
WO93/00163 2 1 1 0 1 5 6 PcT/usg2/o4l2l
.
11 --
with insoluble, non-swellable sorbent material or the
surface (external and/or internal) of which can be
derivatized to provide a coating of insoluble,
non-swellable sorbent material. Preferred supports for
5 such coatings include carbon and inorganic oxide
particles, most preferably silica particles. Such
particles having coated surfaces are well known in the
art, see, for example, Snyder and Kirkland,
"Introduction to Modern Liquid Chromatography", 2d Ed.,
10 John Wiley & Sons, Inc. (1979) and H. Figge et al.,
"Journal of Chromatography" 3Sl (1986) 393-408. The
coatings can be mechanically applied by n situ
crosslinking of polymers or the coatings can be
functiona} groups covalently bonded to the surface of
15 the particles. Many such coated particles are ;~
commercially available (e.g., C18 bonded phase silica,
Alltech, Deerfield, IL).
Sorptive coatings which can be applied to silica
particulate can be either thin mecbanical coatings of
20 insoluble, non-swellable polymers such as crosslinked
silicones, polybutadienes, etc. or covalently bonded
organic groups such as aliphatic groups of varying
chain length (e.g., C2, C8, and Cl8) and aliphatic or
aromatic groups containing amine, nitrile, hydroxyl,
25 chiral, and other functionalities which alter the
polarity of the coating. The silica, or other support
particle, in this case acts primarily as a carrier for
the organic coatings and the particles are
non-swellable. The variation in chemical composition
30 of the coatings provides selectivity in molecular
separations and polarity.
The sorptive particulate material may have a
spherical shape, a regular shape or an irregular shape.
- Sorptive particulate material which has been found
35 useful in the invention has an apparent size within the
range of 0.1 to about 600 micrometers, preferably in
the range of 1 to 100 micrometers. It has been found
W093/00163 PCr/US92/04121
2 ~ 12 -
advantageous in some instanees to employ partieulate
materials in two or more partiele size ranges falling
within the broad range. As an example, partieles
having an average size in the range of 0.1-30
5 mierometers and even up to lO0 mierometers having
ehromatographie aetivity may be employed in eombination
with partieles having an average size in the range l to
250 mierometers aeting as a property modifier.
Some partiele size reduetion may take plaee during
lO the high shear mixing and t~e ealendering operations,
depending upon the friability of the partieulate
material. Thus, while the partieulate material
initially may be rather large, it may ultimately be
redueed to a finer size in the final produet.- ~;
lS Partieles useful in the present invention have
water sorptive eapaeity less than 10% by weight,
preferably less than 1% ~y weight. As noted above,
partieles whieh undergo dimensi~nal ehanges due to
water swellability are less de-~irable. In view of the -
20 teaehings of U.S. Patents 4,565,663 and 4,460,642, it
is surprising that hydrophobie particles and other
non-swellable particles enmeshed in PTFE provide -
superior ehromatographie artieles eompared to
water-swellable hydrophilie partieles enmeshed in PTFE.
As deseribed in the method of U.S. Patent No.
4,153,661, the aetive sorbent partieles useful in the
present invention ean be pre-mixed with a property
modifier whieh ean function, for example, as a
proeessing aid. Representative non-swellable property
30 modifiers (some of which may be soluble in water) can
be eoated partieles (e.g., eation exchange resins),
ealeium earbonate, ammonium earbonate, kaolin, sugar,
polyethylenes, polypropylenes, polyesters, polyamides,
polyurethanes, polyearbonates, zeolites, chitin,
35 vermieulite, elay, eeramies, ion exchange and ehelating
partieles, and the like. These property modifier
materials can be present in an amount in the range of 0
WO93/00163 PCT/US92/04121
`` 211015~
to 28.99 parts per part of PTFE, preferably 0 to 9.00
parts per part of PTFE, provided that the sorbent
non-swellable particles plus property modifiers do not
exceed 29 parts particulate to l part PTFE.
s Other non water-swellable property modifiers may
be advantageously added to the mixture~of the PTFE
aqueous dispersion and the sorptive particulate and
expandable or expanded particulate to provide further
improvement in or modification of the composite article
l0 of the invention. For example, modifier particulate
can include chromatographically inactive materials such
as low surface area glass beads or bubbles to act as
property modifiers and processing aids. It is
desirable from a surface energy standpoint to minimize
15 the PTFE level and at times to alter the level of tbe
active particulate. Coloring or fluorescing
particulate can be added at low levels (up to l0 weight
percent of particulate) to aid in visualizing sample
components to be separated. Chemically active
20 particulate which indicate pH or acidity of the
component bands can be useful for diagnostic purposes.
A limited amount of water-swellable property
modifiers (i.e., up to 30 weight percent, preferably
less than 2S weight percent, more preferably less than
25 l0 weight percent, and most preferably less than l
weight percent, of total---particulate) can be useful as
a processing aid. Representative swellable property
modifiers include starch, chitosan, modified starches
such as SephadexTM and SepharoseTM starches (Pharmacia,
30 Sweden), agarose, polymethacrylates,
styrene-divinylbenzene copolymers, polyacrylamides,
cellulosics, and coated particles (e.g., silica coated
with a polyacrylamide). Water-swellable materials may
be used as a thin coating on non-swellable particulate.
When the particulate is hydrophobic, the preferred
method~of manufacture of the article of the invention
utilizes an emulsion of PTFE with a masking agent added
WO93/00163 PCT/US92/04121
2 1 1 ~ 14 -
to modify the hydrophobic particle surface/water
interaction and allowing rapid wetting of the surface
of the hydrophobic particulate. Preferred masking
aqents are polar organic compounds such as alcohols,
5 amines, acids, etc. with the preferred group being
alcohols due to their efficacious removability as by
solvent extraction or drying after formation of the
article.
Specifically, the PTFE composite sheet material of
10 the invention is prepared by dry blending the
combination of particulates employed until a uniform
dispersion is obtained and adding a volume of masking
agent or lubricant up to approximately one half the
volume of the blended particulate. The blending takes
l5 place along with sufficient lubricant water to exceed -
the sorptive capacity of the particles. Tbe aqueous -
PTFE dispersion is then blended with the
particulate/ma~-;;ing agent mixture to form a mass having
a putty-like or dough-like consistency. The sorptive
20 capacity of the solids of the mixture is noted to have
been exceeded when small amounts of water can no longer
be incorporated into the mass without separation. Care
should be taken to ensure that the ratio of water to
masking agent does not exceed 3:l. This condition
25 should be maintained throughout the entire mixing
operation. The putty-like mass is then subjected to
intensive mixin~ at a temperature maintained below the
expansion temperature of the expandable particulate for
a time sufficient to cause initial fibrillation of the
30 PTFE particles. Minimizing the mixing at the specified
temperature is essential in obtaining optimal
chromatographic transport properties.
Mixing times will typically vary from 0.2 to 2
minutes to obtain the necessary initial fibrillation of
35 the PTFE particles. Initial fibrillation causes
partial disoriented fibrillation of a substantial
portion of the PTFE particles.
W093/00163 2 ~ i O 1 ~ S PCT/US92/04121
¢
-- 15 --
Initial fibrillation will be noted to be at an
optimum within 60 seconds after the point when all
components have been fully incorporated together into a
putty-like (dough like) consistency. Mixing beyond
5 this point will produce a composite sheet of inferior
separations and chromatographic properties.
The devices employed for obtaining the necessary
intensive mixing are commercially available intensive
mixing devices which are sometimes referred to as
l0 internal mixers, kneading mixers, double-blade batch
mixers as well as intensive mi¢xers and twin screw
co~pounding mixers. The most popular mixer of this
type is the sigma-blade or sigma-arm mixer. Some -:
commercially available mixers of this type are those
15 sold under the common designations Banbury mixer, Mogul
mixer, C. W. Brabender Prep mixer and C. W. Brabender
sigma blade mixe_. Other suitable intensive mixing
devices may also be used.
The putty-like mass is then transferred to a
20 calendering device w~ere it is calendered between rolls
maintained below the expansion temperature of the
expandable particulate, preferably at room temperature,
to cause additional fibrillation and consolidation of
the PTFE particles, while maintaining the water level
25 of the mass at least at a level of near the absorptive
capacity of the solids, until sufficient fibrillation
occurs to produce the desired chromatographic sheet
material. Preferably the calendering rolls are made of
a rigid material such as steel. A useful calendering
30 device has a pair of rotatable opposed calendering
rolls each of which may be adjusted toward the other to
reduce the gap or nip between the two. Typically, the
gap is adjusted to a setting of about l0 millimeters
- for the initial pass of the mass and, as calendering
35 operations progress, the gap is reduced until adequate
consolidation occurs. At the end of the initial
calendering operation, the sheet is folded and then
WO93/00163 ~ PCT/US92/~121
21101~5 16 -
rotated 90 to obtain biaxial fibrillation of the PTFE
particles. Smaller rotational angles (e.g., 20 to less -
than 90) may be preferred in some chromatographic or
separations applications to reduce calender biasing,
5 i.e., unidirectional fibrillation and orientation.
Excessive calendering in thin layer chromatographic or ~-
separations composites reduces the solvent flow rate
resulting in longer run times per separation.
The calendered sheet is then dried under
lO conditions which promote rapid water evaporation yet
will not cause damage to the composite sheet or any
constituent therein. The preferred drying temperature
range is from 20C to about 50C. The most convenient
drying method involves suspending the composite sheet
~5 at room temperature for at least 24 hours. The time -;
for drying may vary depending upon the particular -
composition, some particulate materials having a -~
tendency to retain water more than others.
The resulting composite sheet has uniform porosity
(homogeneous throughout) and a void volume of at least
30~ of the total volume and up to 80%, preferably 40 to
60 percent.
The PTFE aqueous dispersion employed in producing
the PTFE composite sheet of the invention is a
25 milky-white aqueous suspension of PTFE particles.
Typically, the PTFE aqueous dispersion will contain
about 20% to about 70% by weight solids, the major
portion of such solids being PTFE particles having a
particle size in the range of about 0.05 to about 0.5
30 micrometer. Commercially available PTFE aqueous
dispersions may contain other ingredients, for example,
surfactant matsrials and stabilizers which promote
continued suspension of the PTFE particles; these
dispersions are less desirable for separations and
35 purification applications.
Such PTFE aqueous dispersions are commercially
available from E.I. Dupont de Nemours, Inc.,
WO93/nU163 2 1 1 ~ 1 S ~ PCT/US12/WI21
- 17 -
Wilmington, DE, for example, under the tradenames
TeflonTM 30, TeflonTM 30B or TeflonTM 42. TeflonTM 30
and TeflonTM 30B contain about 59% to about 61% solids
by weight which are for the most part 0.05 to 0.5
5 micrometer PTFE particles and from about 5.5% to about
6.5% by weight (based on weight of PTFE resin) of
non-ionic wetting agent, typically octylphenol
polyoxyethylene or nonylphenol polyoxyethylene.
TeflonTM 42 contains about 32 to 35% by weight solids
lO and no wetting agent. Fluon~ PTFE, having reduced
surfactant levels, is available from ICI, Exton, PA.
It is important that the polytetrafluoroethylene
fibrillated networ~ be tight enough to support the
enmeshment of the expandable particulate and sorptive
15 particulate so that the final composite has sufficient
structural integrity to be handled. In the present
invention, the sorptive particulate and energy
expandable particulate do not easily dislodge from the
final composite, i.e., they do not fall out of the
20 article when the article is handled. A further
advantage of a PTFE fibrillated network is that PTFE
fibrils are able to flow or draw out as the expandable
particulate expands, thereby maintaining the structural
integrity of the article. In addition, the poor
25 bonding of PTFE to the expandable particulate also
allows the fibrils to "slide" from a given
microbubble's surface during the expansion step, i.e.,
there is poor adhesion of the fibrils to the polymeric
shell of the microbubble.
The useful range of fibrillated polymer in the
final compositeæ can be from about 2% to about 50% by
weight, preferably from 3% to 40%, and most preferably
from 5% to 25%, based on the total weight of the
composite.
Energy can be provided to the composite article to
cause expansion of the expandable particulate by any of
a number of means, including thermal energy from a heat
W093/OOt63 PCr/US92/04121
.
21101~ - 18 -
source such as an oven, steam, or a heat gun, radiant
energy such as that given off by an infrared light bulb
and a laser such as a carbon dioxide laser, and other
means known to those skilled in the art. Steam is a
5 particularly effective expanding agent.
Useful temperature ranges for the thermal `
expansion step are dependent on the type of poiymer
used in the microbubble and on the particular blowing
agent used. Typical temperature ranges are from about
10 20C to about 200C, preferably from 50C to 175C,
most preferably from 70C to 160C. Nobel Industries
provides a series of expandable bubbles which expand at
different temperatures. A more complete description of
various polymers and blowing agents can be found in
15 U.S. Patent No. 3,615,972. Further discussion of
blowing agents in general can be found in U.S. Patent
Nos. 4,640,933 and 4,694,027.
The length of time required for full expansion of
the composite article to occur is dependent on the
20 particular blowing agent, the nature of the polymeric
shell of the bubble, and the efficiency of heat
transfer to the article. For most microbubbles, about
five minutes in a convection oven set at a temperature
slightly higher than the softening point of the
25 polymeric shell is sufficient to allow full expansion.
In cases where heat transfer to the article is much
more efficient, such as with the use of steam as a heat
source, expansion can occur much more quickly,
generally in less than 30 seconds. It is also
30 possible to only partially expand the microbubbles by
controlling the length of time the composite article is
exposed to the expansion temperature or exposing the
microbubbles to a temperature below the normal
expansion temperature such that individual microbubbles
35 have only expanded to a fraction of their potential
volume. In this way, the final volume and density of
WO93/00163 2 1 1 0 1 ~ ~ PCT/US92/~121
- 19 - ; .'
the article can be controlled, in those cases where
this is desirable.
Care must be taken to avoid over-exposure of the
composite article to elevated temperatures since the
5 blowing agent can be driven from the interior of the
polymeric microbubble faster than air can permeate into
the interior to maintain constant internal pressure,
thus causing a collapse of the structure. Conditions
for expansion of particulate vary depending upon the
lO particulate used and the degree of expansion desired.
Controlling the interstitial porosity of the sheet
material has great utility in composite materials
utilized in the science of separations and
purifications. It is known to provide porosity in
15 sheet materials by using added property modifier
particulate which can be dissolved out of the sheet
material, via washing, leaving voids. Use of
controlled levels of lubricant fluid during the
manufacture also has been shown to control void volume
20 or porosity. The present invention provides sheet-like
articles which have great utility in separations and
purification applications by controlling porosity by
use of expandable particulate.
Control of porosity greatly enhances the utility
25 of chromato~raphic and separations articles. Two types
of porosity are involved: l) the internal porosity of
the sorptive particles and 2) the flow through or
interstitial porosity of the composite article. Proper
choice of the sorptive particle porosity depends on the
30 intended application, typically 60 to lO0 Angstrom
pores for small molecules such as drugs, pesticides,
pollutants, etc., and 200 to lO00 Angstrom pores for
large biomolecules. Type of particulate includes both
organic and inorganic materials and determines the
35 sorptive or reactive specificity of the composite
article.
W093/00163 PCT/US92/04121
2iiO1~6 20 -
Interstitial porosity controls the distance
between sorptive particles which determines the
diffusion kinetics of the composite, wherein td = d2/2D,
where td is the time required for diffusion of a ~`
5 molecule for distance d and D is the diffusion
coefficient of the medium, e.g., air, water, etc. The
present invention typically utilizes 8-lO micrometer
sorbent particles with 60 Angstrom internal pores and
high surface area, e.g., 100-500 m2/gm, for efficient
lO separations. Interstitial porosity controlled by the
expandable polymeric particulate determines the rate at
which fluids can be passed through the composite
article, i.e., residence time and linear flow velocity.
Typical interstitial porosity for separation articles
15 of the present invention range between O.l and lO
micrometers. The resultant composite article has great ~-
utility in rapid and efficient processing of fluids to
isolate pollutants, drugs, and biomolecules in
environmental and bioprocessing applications.
The articles of the present invention are uniquely
suited in separations devices wbere elimination of
voids and channels is desirable at confinîng surfaces,
such as in cylindrical tubes or columns between flat
restraining plates, or in any confined geometric space.
25 Upon energy expansion, the article, either in the form
of a flat sheet (round, square, or any geometric shape)
or roll, can snugly fill and conform to the shape of a
confined volume. For example, a chromatograph column
or cartridge can be loosely fitted with a rolled sheet,
30 then energy applied to the composite column or
cartridge, causing expansion to provide a snugly
fitting material which eliminates voids and channels in
separations applications.
Objects and advantages of this invention are
35 further illustrated by the following examples, but the
particular materials and amounts thereof recited in
these examples, as well as other conditions and
WO93/00163 21 ~ O 1 S ~ PCT/US92/04121
- 21 -~
details, should not be construed to unduly limit this
invention. Unless otherwise stated, all parts and
percentages are by weight.
S E:~C~IID1~
A group of seven chromatographic sheets comprising
- a PTFE fibrillated matrix and a combination of TLC
silica (Aldrich Chemical Co.) ~nd Expancel 551DU
polymeric particles enmeshed therein was prepared as
10 follows:
Varying ratios of Expancel 551 DU microspheres and
TLC grade silica were dry blended to obtain a range
from 0% to 50% by weight Expancel microspheres with
respect to silica particulate. Sample 1, the 0% sheet,
15 was made by mixing 20 g silica with 7.1 g Fluon PTFE
emulsion containing 27.9 weight percent solids.
Thirty-five gm of 50:50 volume percent
isopropanol:water was then added and blending occurred
to obtain a mass with dough-like consistency. The mass
20 was then calendered with a roll temperature of 38C.
and a gap between the rolls of 0.38 cm. After the
initial calendering, the shee~ is folded and rotated 90
degrees to obtain biaxial fibrillation of the PTFE
particles. The gap between the calendering rolls was
25 redured in increments of 0.13 cm and the sheet
recalendered. Folding, rotating the sheet, reducing
the gap, and recalendering was repeated until the
composite sheet thickness was 0.05 cm. This procedure
was repeated for samples 2, 3, 4, 5, 6, and 7 with the
30 exception that the weights of Expancel
microspheres:silica were 0.04:19.96, 0.20:19.80,
0.60:19.4, 1.20:18.8, 4.00:16.00, and 10.00:10.00 g,
respectively. The seven composite sheets then
contained 0, 0.2, 1.0, 3.0, 6.0, 20.0, and 50.0 weight --
35 percent Expancel microspheres with respect to TLC
silica particulate. The composite sheets were dried at
room temperature for 24 hours.
.. .
WO93/00163 ' PCT/US92/04121
21101S6 22 -
Table l, below, shows the data obtained for
toluene solvent migration rates by incorporating
different levels of Expancel microbubbles in a TLC
(thin layer chromatography) silica formulation. These
5 rates directly correlated to ~flow through"
(interstitial) pore sizes obtained before and after
thermal expansion of the composite article. FIG. l
shows the effect of the weight percent of Expancel
microspheres on TLC elution rates'after expansion. In
10 this example, the amounts of Expancel microspheres were
varied from 0.2 to 50% and a standard TLC test dye
mixture (IV 30-04, available from Analtech, Inc.,
Newark, DE) was used to determine the effect of
Expancel microbubble concentration on the Rf values
(retardation factors), as discussed in C.F. Poole, et
al., "Contemporary Practice of Chromatography",
Elsevier, New York (1984) pp. 625-626, versus that
observed for conventional TLC separations. Plots A
through F in FIG. l represent the following weight
20 percent ratios of Expancel microbubbles to silica: A
(0.2/99.8), B (l/99), C (3/97), D (6/94), E (20/80), F
(50/50). The Rf data indicate that amounts greater
than about 20% gave degraded performance of the silica
TLC application. The preferred range for'TLC was from
25 0.2 to 20%. FIG. 2 shows the TLC solvent front rate
for samples B, C, D, and E, listed a~ove at a solvent
front distance of 40 mm before expansion (plot G) and
after expansion (plot H). The upper curve for the
unexpanded article shows a slight increase in solvent
30 front rate as the percentage of Expancel microbub~les
with respect to silica is increased. This may be due
to the enhanced "wetting" characteristics of the
Expancel particles. The lower curve for the expanded
article shows the unexpected parallel relationship
3S between unexpanded and expanded articles over the l to
20% concentration range. The increased rate is not
directly proportional to the Expancel particulate
WO g3/~163 2 1 1 0 1 ~ 6 PCT/US92/04121
- 23 -
concentration. This indicates that the more preferred
range of Expancel particulate concentration is from 1
to 10% and the most preferred range for TLC
applications is from 1 to 6% with respect to the silica
5 concentration. Table 2, below, lists the data for the
unexpanded and expanded articles.
WO 93/00163 . PCI/US92/W121
211 6
û15 -24-
I r~
I ~
U 1~ ~ N N O O O N 1~
U~ _I ~ O N Itl In ~ 0 ~ ~1 0
~ ~ ~ O O O O ~i ~ 1~1 ~ ~1 ~.`
-C: . . _ _ _
a ~ o o o ~ ~ o o 0 CD o
~ ~ ~1 ~ ~1 1-1 ~ ~0 ~ N 0 0 ~
~ ~ D. ~ . . . . . . . . .
00 ~ ~ O O O O~1 ~ N ~ ~ . ~.
i~ ~ U _ _ _ ~-
E~ -I ~ r~ O O ~ ~ ~ O O O
~U _~ ~ ~1 ~ OD ~ ~ ~D U) It~
O ~ ~ ~; ¦ N ¦ r~
~ ~ ~ 1~ 1~ t~ r~ ~0 ~ ~ ~ r~
:1 ~ ~ N ~1 ~ 0~ t` D OD (~ ~ OD
1~1 ~ E~ o O O O ~1 N ~ ul ~D CD :
3 _ `;
u~ ~ O O O r~ U) O O
~1 ~ ~ ~D r~ ~ ~O O o~ I~ O~
a.o O O ,, ~ ~ u) ~D tD ,0~
~n . l _ _
~_
~ ~ ~ In O U) O U~ O ~ O U)
_ . ~ O ~ _ ~I _ N N ~ I r
.
U~ O ~
S~BST~T~'rE S~E.~
WO93/00163 2 1 1 0 1 ~ 6 PcT/us92/o4l2l
- 25 -
_ , _ __
TABLE 2
T~C Elut~on Rates Before an~ After Expansion
(~t ~ ~
Wt. % Expancel Before After
Samplemicrospheres Expansion Expansion
5 l (in silica) Time (min) Time (min) 1.
1 0.0 8.70 8.70
2 0.2 `8.00 6.93
3 1.0 8.53 4.50
4 3.0 7.97 3.68
I
10 I 5 6.0 _ ~ 7.47 3.12
6 ~ 20.0 ~ 6.55 ~ 1.13
~ 50.0 6.07 0.68 ``
Increasing the percentage of Expancel microspheres
with respect to the silica particulate decreased the
time for the solvent front to reach the 40 mm distance
from the origin in the unexpanded article and this ~
could be due to the "wetability" or polarity of ~-
20 Expancel particles. The solvent front rate however
increases dramatically after these same formulations
are thermally expanded at 120C for 3 minutes as shown `
in Table 2. This could be due to the increased surface
area of the Expancel microspheres or increased
25 interstitial porosity resulting from the particle
expansion disrupting the spatial cbaracteristics of the -
particle packing. A plot of the data (FIG. 2) in Table
2 shows some unexpected results of expansion. As
noted, the data lines are nearly parallel for the
30 expanded articles (plot H) and unexpanded articles
(plot G). The data further demonstrate that the amount
- of expansion is not a linear function of Expancel
microsphere concentration. FIG. 3 is a plot of the
interstitial porosity before (plot J) and after (plot
WO93/00163 -~ PCT/US92/~121
21101~i~
- 26 -
K) thermal expansion of one of the formulations (6%
Expancel microspheres in TLC silica). The unexpanded
article ~ad a mean pore size of 1.7 micrometers while
the article after expansion had a mean pore size of 5.8
5 micrometers as measured with a Coulter Porometer~
(Coulter Electronics Ltd, Luton, England).
Distribution of flow through pore sizes broadened
considerably after expansion. Table 3 shows the data
obtained for the thickness of the various formulations
10 before and after thermal expansion at 120C for 3
minutes.
-
.. I . ~
TABLE 3 ¦
Compos~t- 8h-et Thic~ness
B~fore ~n~ After Expansion
::
Before After
15 Sample # Wt. % Expansion Expansion ¦
Expancel Thickness (cm) Thickness
microspheres (cm)
(in silica)
~ 0.0 0.051 0.051
I _ I i:
2 0.2 0.038 0.053
I I
3 1-0 0.041 0.061I
I I
4 3.0 0.043 0.066_
_ 6.0 0.048 0.074
20.0 0.048 0.107
7 50.0 0.051 0.130
.
Ex~mple 2:
A series of four composite sheets were prepared by
dry blending varying ratios of Expancel 551DU
microspheres and C18 bonded silica to obtain a range
30 from 1% to 50% by weight Expancel with respect to
W093/~163 2 1 1 0 1 ~ 6 PCT/US92/04121
- 27 -
silica particulate. Sample 8, the 1% sheet, was made
by mixing 0.25 g Expancel microspheres and 24.75 g Cl8
silica with 8.9 gm Fluon PTFE emulsion from ICI
containing 27.9 weight percent solids. Twenty-six g of
5 50:50 volume percent isopropanol:water was then added
and the mix blended to obtain a mass with dough-like
consistency. The mass was then calendered at a roll
temperature of 38OC. After the initial calendering,
the sheet was folded and rotated 90 degrees to obtain
10 biaxial fibrillation of the PTFE particles. The gap
between the calendering rolls was reduced as in Example
1 and the sheet recalendered. Folding, rotating the `~
sheet, reducing gap, and recalendering was repeated
according to Example 1 until the composite sheet -~
15 thickness was 0.05 cm. This procedure was repeated for `~-
samples 9, 10, and 11 witb the exception that tbe
weights of Expancel: C18 silica were 1.25:23.75,
5.0:20.0, and 12.5:12.5 g, respectively. The four
composite sheets then contained 1.0, 5.0, 20.0, and ~-
20 50.0 weight percent Expancel microspheres with respect
to C18 silica particulate. The composite sheets were
dried at ~oom temperature for 24 hours. `
Tbe composite sheets were tben evaluated in solid `~`
phase extraction (SPE) applications. A test compound,
25 Disperse Red 1 (Aldrich Cbemical Co., Milwaukee, WI),
which behaves like many pesticide and drug molecules
with respect to SPE was dissolved in water containing
0.5% methanol to promote wetting of the hydrophobic
particulate. A liter of this solution containing lOo
30 parts per billion (ppb) of the red dye was then
filtered through each composite sheet to determine the
effect-of Expancel microspheres on extraction
efficiency and filtration time. A vacuum of about 700
mm of Hg was used to pull the water solution through a
35 47 x 0.5 mm disc. A cross sectional view of the
composite article was then obtained and the degree of
- WO93/00163 PCT/USg2/~121
21 1 015 ~ 28 -
penetration of the red dye was measured. Table 4 lists
the data obtained for a series of concentration levels
before and after expansion of the articles.
_ _ _ .
TABLE
~e-br~n- ~low ~ Efficiency
Before ~nd After Exp~n~ion
Expancel Before After Dye ~`
microspheres Expansion Expansion Penetra-
Sample(in C1B Time/Liter TimelLiter tion
~ silica) (sec) (sec) (expanded) ~
.,
8 l.0 301 262 < 10%
9 5.0 302 187 ~ 10%
_ ....
1 20.0 395 81 10% ~
ll 50.0 366 60 90% -
15 l _ ;`~'`
The data show that the presence of unexpanded
Expancel microbubbles which are subsequently expanded
dramatically increased the flow rate as would be
20 expected due to the porosity increase resulting from
expansion, The increase in porosity is especially
important when particulate laden (river, lake) water is
to be filtered or extracted to recover pesticides and
other pollutants. The more open porous structure is
25 less subject to plugging and is desirable for dirty
waters and biological fluids. It appears that SPE
efficiency decreases above 50% Expancel microbubble
levels (penetration is near breakthrough point).
At the breakthrough point, a loss of analyte
30 begins that is undesirable in SPE. FIG. 4 is a plot
(L) of the flow data in Table 4. It is to be noted
that the flow rates were not a linear function of the
expanded Expancel microbubble concentration.
WO93/00163 2 1 1 0 1 S g~ PCT/US92/~121
- 29 -
Table 5 illustrates the relationship observed
between levels of Expancel microbubbles and ability of
the composite articles to extract hydrophobic ..
pollutants such as pbthalates from water. One liter
5 water samples containing trace levels (100 ppb) of
dimethyl-, diethyl-, and dibutyl-phthalates were
filtered through sample articles 8 through 11. The
phthalates were then recovered from the sample ~
composite articles in a concentrated form by elution ~-
10 with a ~mall volume (10 ml) of acetonitrile solvent
which effectively displaced the phthalates from the
hydrophobic C18 bonded silica particulate. Phthalate
concentrates were then analyzed by liquid
chromatography to determine the efficiency of the
15 articles in extracting the phthalates from the aqueous
media.
. . '-'
~:
TABL8 5
20Effect of Exp~ncel Micro~p~eres
Level on C18 8$1ica - 8PE Pht~alates ~ec~very ~%) :-
25Expancel ¦ :
microspheres
Sample (in C18 Dimethyl- Diethyl- Dibutyl-
silica) phthalate phthalate phthalate
8 1 85 99 102
9 5 71 90 100
. _
31 95 99
11 50 0 70 103
. .
The data of Table 5 show the percent recovery of
dimethylphthalate decreased rapidly with the increasing
percent of Expancel microspheres indicating that the
WO93/00163 PCT/US92/04121
2 il al S ~ ~ 30 -
sorptive capacity of the composite was directly
proportional to the Cl8 silica particulate percentage
and that Expancel microspheres were ineffective as a
sorbent for the hydrophobic phthalates. Diethyl and
5 dibutyl phthalate recoveries were less affected because
of their stronger affinities for even low percentages
of the Cl8 silica particulate. These data also
demonstrate that increasing Expancel microsphere
percentages may inversely affect sorptive capacity of
10 the composite article. Other types of expandable
particulate can be utilized to increase interstitial
- porosity and dependent on chemical composition can add
or detract from the net sorptive capacity of the
composite article.
Example 3:
A composite article, sample 12, containing 6%
unexpanded Expancel 551DU polymeric microspheres by
weight of hydrophobic Cl~ bonded silica particulate,
20 made as in Example 2, was cut into a l x 4 cm strip.
The strip was then rolled into a l cm high cylindrical
form (as shown in FIG. 5) and inserted into a
cylindrical column. It was loosely fitting and
individual layers of the wrapping were visually
25 observable. The assembly was then placed in a
convection oven at 120~C for 3 minutes to effect
expansion. After heating, the individual layers had
expanded, fusing together, and the resulting
cylindrical roll as shown in FIG. 6 was firmly held in
30 place in the column without the need for conventional
confining top and bottom particle retaining frits or
porous retaining supports. After cooling, the expanded
column article was wetted with methanol as recommended
in solid phase extraction art described by Hagen et
35 al., _nalvtica Chimica Acta, 236, (l990) 157-164. An
aqueous solution containing a green test dye (McCormick
WO93/~163 2 1 1 0 1 5 ~ PCT/USg2/04121
- 31 -
& Co., Inc., Baltimore, MD) and 0.5% methanol was then
pulled through the column using a vacuum of 700 mm of
Hg, as described in Example 2. Visual inspection
showed that the expanded column article efficiently -.
5 trapped the dye at the top of the column with little
evidence of voids or channeling between the original
individual layers or the particle-wall interface which
is commonly observed with conventional particle packed
columns. It is known in the art to collapse flexible
10 wall columns inward by external radial compression to
help eliminate voids and channels at the column wall
interface. In contrast, the composite sheet of this
invention which comprises a combination of sorptive
particles and expandable microspheres in a PTFE matrix
15 expands radially outward to eliminate voids and
channels at the rigid column wall interface. The
unexpanded composite article of this invention can be ~:
placed in a variety of confining geometrical structures
and subsequently expanded to conform to the geometry of
20 the confining structures to provide devices useful in
the separation and purification sciences.
ExamDle ~
Forced flow planar chromatography is known in the
25 art and consists of a sorbent sheet material clamped
between a rigid plate and a flexible plate which is
akin to a bladder, see, for example, L. Botz et al.,
Journal of I~ id Chromatoara~hv, 13(14), 2809-2828
(l990). The side edges are sealed and fluid pressure
30 is applied to the flexible plate compressing the
sorbent sheet material between the plates to prevent
channeling and eluant/solvent flow over the surfaces of
the sorbent sheet. Elutant is forced into the ed~e of
the sheet through an inlet port using a constant flow
35 pump. The analytes separated by eluant flow through
the-sorbent media exit to a detector through a port at
WO93/00163 PCT/US92/04121
211~1S~ - 32 -
the end opposite of the inlet port. In contrast to the
prior art method, this invention provides rigid plates
(such as glass) between which are sandwiched a PTFE
fibril composite comprising expandable microspheres and
5 sorptive particulate enmeshed therein. Formulations of
silica or Cl8 bonded silica, as in Examples l and 2, can
be used to effect specific separations. A wide range
of other sorptive particulate described in the art can
also be used. Use of a composite article of the
lO invention in such a device eliminates the need to apply
external pressure and the need for a flexible plate.
~he internal pressure generated between the two rigid
plates as a result of expansion of the expandable
particulate in the composite article of the invention
l5 is dependent on both the amount of expandable
particulate in the composite and the degree of -~
expansion. The present invention provides an efficient
means of performing forced flow planar chromatography.
Various modifications and alterations of this
20 invention will become apparent to those skilled in the
art without departing from the scope and spirit of this
invention, and it should be understood that this
invention is not to be unduly limited to the
illustrative embodiments set forth herein.