Note: Descriptions are shown in the official language in which they were submitted.
~092f226~8 P~T/US92/04772
2 L1~4~3
SELF-THICXENED CLEANING COMPOSITIONS
- Technical field
The present invention relates to cleaning compositions.
The compositions according to the present invention are
self-thickened, in that they do not require the presenc
a thickensr compound; ~urthermore, the compositions
according to the present inv~ntion are physically stable, ~;
even at low temperatures. The compositions accordin~ to
the present invention are USP-fUl for insta~ce as hard
surface cleaning omposltions or as laundry cle~ni~g
compositions.
Backqrvund
Thickened cleaning composltions are well known in the
art, and have found various applications for instance in
~:~ the context of hard surface or laundry cleaniny
compositions.
: The simplest ~ay to make such compositions is to add a
thickener on top~of a non-viscous product. However,
W092/226~8 2 1 1 ~ ~ 1 3 PCT/US92/04772
thickeners can be seen as undesirable in many respects,
such as processing issues, long term product stability
issues as well as other considerations related to formula
cost. It is thus desirable to formulate a cleaning
composition which is viscous, but does not necessarily
contain a thickener.
The art teaches that it is possible to do so by using a
sy~tem where viscosity is built by combining an anionic
sur~ac~ant together with an electrolyte in an aqueous
medium, wherein the viscosity can be adjusted by balancing
both ingredients. This system is very often used in the
formulation of ~5~mpO0S, but has also found application in
. ,,
cleaning compositions. In theory, this solution can be use~
with any anionic surfactant, but the compositions of the
present invention only use an alkyl sul~ake anionic
surfactan~ derived ~rom natural coconut oil; indeed, this ~:
surfactant provides good performance and is particularly
desirable for obvious environmental reasons.
, ~
Thus, self-thickened compositions can be made which
comprise such an anionic surfactant derived from natural
coconut oil and an ~lectrolyte. The ~iscosity of such a
composition is of course sensitive to temperature, and a
new technical problem has been identified in that such
compositions are not physically stable at low temperature,
where the product undergoes phase separation, As a result, -:
the product has an aesthetically unacceptable aspect.
.:
lt has now been found that this problem could be solved :~-
.
;by using a specific electrolyte and adding to the system
above a nonionic surfactant ~rom~a selected class.
;
Canadian patent CA 1 1~4 381 discloses scouring ~:
compositions containing anionic surfactants, an electrolyte
and an ethoxylat~ed alcohol surfactant.
'~
~.
W092/22628 PCI/US92/04772
. 3 2~10~13
European patent EP 116 905 ~eaches the use of ~n
ethoxylated and/or propoxylated alcohol surfactant to
reduce the viscosity of industrial-grade synthetic anionic
surfactant concentrates~
European patent application EP 329 209 discloses
scouring compositions comprlsing a surfactant, an
electrolyke and a solvent to build viscosity.
Summary o~ the invention
The compositions according to the present invention are
self-thickened a~ueous cleaning compositions havi~g a
viscosity of from 50 to 700 cps at~ 60rpm shear r~te at 20~C,
which comprise from 1% to 25~ by weigh~ o~ the total
compositlon of an alkyl sulphate anionic surfactant derived
from natural coconut oil, from 0.1~ to 8~6 ~y weight of the :~
total composition of ammonium salts and ~rom 0.5% to 25% by
weight of the total composition of a compound o~ the
structure:
:
Rl-~ _ ~(R2o~n~R30)m]--R4l wherein :
,.
- Rl is a Cl_2~ alkyl or alkenyl group;
- R2 is a C2_4 aliphatic hydrocarbon chain;
- ~3 is a methyl or ethyl~monosubstituked C2-C4 aliphatic
hydrocarbon chain;
- R4 is a Cl_~5 alkyl or~alkenyl or carboxyl chain, or H;
- n is an integer:of from~ 1 to lO; ..
- m is an integer of ~rom 0 to 20;
.
or mixtures ther,eof~ :
Detailed description of the invention
The compositions according to the pre.sent invention
comprlse from 1%:~to ~5~ by weight o~ the total composition, :~
~: :
WO~/22628 ~ ~o ~l3 PCT/US~2/~4772
preferably ~rom 2% to 8% by weight of the ~otal composition,
most preferably 3% to 5% o~ an alkyl sulphate anionic
surfactant derived from natural coconut oil, or mixtures .
thereof. Suitable alkyl sulphate anionic surfactants for use
herein can be made by the processes well known to the man
skilled in the art. Typically, such surfactants are made
from coconut alcohol which is sulfated and possibly
ethoxyla~ed. Indeed, ethoxylated alkyl sul ~a~e sur~actants
are also suitable for use herein, but the average degree of
ethoxylation o~ the anionic sur~actants to be used herein
should not be more than 2.
The neutrali2ing cation ~or the alkyl sul~ates d~rived
from natural coconut oil to ~e used herein ca~ b~ any o~ the
conventional cations used in detergent technology, such as
ammonium, potassium or unsubstituted or N-substituted
ammonium saltsl or mixtures thereof; preferred are the sodium
salts.
The viscosity of the compositions according to the
invention is obtain~d by using ammonium salts as an
electrolyte; suitable ammonium salts for use in the
compositions according to the inYention include
alkanolammonium salts, ammonium chloride, ammonium ~cetate,
ammonium citrate, ammoniu~ carbonate and the like; it is the
electrolyte together with the above described surfactants
which builds the viscosity. The viscasity of the composition
depends on the proportion of both ingredients, the general
rule being tha~ the more electrolyte is added, the more .
viscosity i5 o~tained. The compositions according to the
present invention comprise from 0.1% to 8% by weight of the
total comp~sition of ammonium salts, preferably from 0.2% to
6%, most preferably 0.3% to 5%. The ratio of ammonium salts :~
to anionic surfactant is adjusted so as to obtain a
composition having a visc05ity of from 50 to 700 cps at 60rpm
shear rate at 20~C, more preferably 80 cps to 350 cps. The
viscosity of the compositions according to the invention
allows to use said:compositions on vertical surfaces.
,,
'
W092~2262~ PCT/US92/04772
21 i~
Such a product comprising the surfactant and the
electrolyte describe~ herein above could be used as such,
however, as ~escribed in the background part, physical
stability of the product is not ~atisfactory under all
conditions, in that the product undergoes phase separation at
low temperatures, around 10~C and below~ It has now been
found that this problem could be solved by simply adding to
the composition a nonionic surfactant from a selected class.
Accordingly, the compositions according to the present
invention comprise ~rom 0.5% to 25~ by weight o~ the total
composition, prefer~bly from 0.6~ to 10~ , most preferably
from 0.7% to 2~ ~f a compound of the formul~
.. . . .
. :
Rl-a _ ~(R2o)n(R3o)m]--R4~ wherein :
- R1 is a C1_2S alkyl or alkenyl chain, preferably
C~ 5, preferably alkyl;
- R2 is a C2_4 aliphatic hydrocarbon chain, preferably
C2;
- R3 is a methyl or ethyl monosubstituted C2-C4 aliphatic
hydrocarbon chain, preferably a methyl substituted
ethylene; : :
- R4 is a Cl 25 alkyl or alkenyl or carboxyl chain, or H, '
preferably H;
- n is an integer o~ from 1 to 10, preferably 1 to 5;
- m is an integer o~ from 0: to 20; preferably 0 to 10, most
preferably 0 to 3,
or mixtures thereof.
:
It is to be understood that, in the chemical formula
i above, R20iand R30 groups~may~appear in any sequence in the
: :~: molecule; also, when n and m are~greater than 1, dif~ere~t
R20 and R30 groups may:appear:in a same molecule. These
surfactant are commercially aYailable from ICI under the
trade name UKANI~ ~M ~,:or ~rom:BAS~ under the trade name
PLURAFAC LF ~. Preferred surfactant where m is 0 are
~: '
; ~:
, ~
: ~ '
W092/2262~ 2 ~ t3 PCT/US92/04772
available from SHELL under the trade name DOBANOL ~ or ~rom
BASF under the trade name LUTEN50L ~. ~
The compositions according to the invention are acidic ;
and have a pH of from 1 to 6, preferably 2.5 to 5. The pH of
the compositions according to the invention is partially
determined by the amount of e~ectrolyte, but the pH of the
~inal composition can be adjusted by the addition of
appropriate acidifiers such as organic or inorganic acids, or
acidic salts which buffer pH to an acid value. ~mples of
suitable acidifiers are sulfuric acid, phosphoric acids,
although somewhat undesirable from an environmental
viewpoint, hydrochloric acid, phosphonic acid, citric acid, ~:
acetic acid, tartaric acid, maleic acid~ succinic acid,
malonic acid and the like, or mixtures thereof.
In a particularly pxeferred embodiment of the invention,
the compositions comprise from 0.5% to 20% by weight o~ the
total composition of citric acid, preferably from 1% to 10~
Indeed, it has been found there are many benefits obtainable
from the addition of citric acid to the compositions ~.
according to the prPsent invention,:such as limescale ~emoval .~.
performance and improved disinfesting properties. When
citric acid is used in the compositions according to the
invention, it is possible to form in~situ the ammonium salts
described herein~above, in this case ammonium citrate by
incorporating ammonium hydroxide~ The presence o~ citric
acid also helps to build the product's viscosity; When high
le~els of citric acid are used:~to obtain optimal
performances, it~:may~be necessary;to adjusk the pH of the
composition by adding an alkalinizing agent~
In a preferred embodime'nt,~the compositions according to
the invention further comprise~from 0.01~ to 0.5~ by weight .:;
of~the total composition of:a~ capped 1j2:- prop~lene
terephthalate polyoxyethylene~polyester of ~he formula: ~
-:
: ~ ' ,':,'
~ ~ ::SUB~ TE SHEE~T
..
W0~2/2~628 ' PCT/US~2/0477~
7 2 1 ~ 3
0 o CH3 o O
CH3(OCH~cH2)n(~c ~ c-CH2CH)uOc ~ Co-(cH2cH2o)n CH3
wherein n is an integer of ~rom about 12 to 43 and u is an
integer of from about 2 to 8, and mixtures thereof. These
polymers have been extensively described as soil release
agents in softening compositions and in liquid detergent
compositions and in EP 185 427 and ~P 220 156. However, it
has now been found that, the above polymers have a regulating
ef~ect on the viscosity of the composikions; indeed, the
viscosity of a ~iven product is ~ypically very sensitive to
temperature, and the compositions according ~o the present
invention, although they remain phase-stable at all
temperatures, tend to become more viscous when temperature
drops. It has now been found tha~ the above polymers make
tha compositionls viscosity Iess sensitive to temperature;
thus the compositions have less tendency to become thicker at
low temperatures.
In a preferred embodiment, the compositions according to
the present invention ~urther comprise from 1% to 15% by
weight of the total composition, preferably from 2% to 10%,
most preferably 3% to 8% of hydrogen peroxide. In the
compositions of the prior art, i,e. viscous systems
consisting of an anionic sur~acta~t and an elec~rolyte, the
addition of hydrogen peroxide leads to a severe drop in
viscosity; another advantage of the compositions according to
the present invention, i.e. comprising the stabilizing
nonionic surfactant from the selected class, is that the
addition of hydrogen peroxide has little e~ect on the
produck's viscosity Thus, bleaching compositions can also
be made according to the present invention.
The compositlons according to the present invention may
also comprise conventional ingredients such as solvents,
chelating agents, fragrance and dyes, provided all these
ingredierlts are compatible with the compositions.
SUBS~TU~E S~EET ~'
'"~
2 l 1 ~ ~ 1 3 PCT/US92/0477~
The compositions according to th~ present invention can
be made by any process where all ingredients are mixed
together. However, in the preferred embodiment where the
compositions comprise a substantial amount of citric acid, it :~
is preferred to use a process which comprises the st~ps of
dissolving the citric acid in water separately ~rom the
remainder o~ th~ composition, adjusting the pH o~ the citric
acid solution to the target pH value o~ the final
composition, and adding said pH-adjusted citric acid ~olution
to the remainder o~ khe composition, the pH o~ which having ~:
been separa~ely adjusted ~o sai~ t~rget pH value. In c~se
the compositions also comprise hydroge~ p~ro~ide, it is
preferred to add the hydrogen peroxide as a final step in the
process, on top of the remainder sf the composition including
the citric acid.
The compositions according to the present invention can
be useful in many di~ferent con~exts where it is desirable to
use a viscous product~ For insta~ce, hard surface cleaning :~
compositions are advantageously formulated as Yiscous
products in order to optimize their application to vertical
surfaces, for instance baththubs or toilet bowls. Indeed,
the product's viscosity prevents the product f rom running too
quickly down said surface.
The compositions according to the present invention can
also be used in the context of laundry cleaning. For
instance, a lau~dry pretreatment composition can be
advantageously ~ormulated as a viscous product for a most
convenient application to the laundry ~abri~s. Indeed, the
viscosity o~ the product allsws for a better control of the
dlspensing of the product and prevents the spreading o~ the :
dispensed product beyond ~he fabric surface being pretreated.
The following examples will further illustrate the ;.
present invention.
SUBSTITU-ll E SI~EET
.
W092/2262~ PCr~US92/0~772
9 2110~1'3
Examples
The following compositions are made, which contain the
listed ingredients in the listed propor~ions (weight %).
Compositions~ 2 1 3 1 4 1 5 1 6
NaCnAS ¦ 3-5 ¦ 3-5 ¦ 3-5 ¦ 4~0 ¦ 2.4 ¦ 3.5
Citric Acid ¦ 5.8 ¦ 6.0 ¦ 5.8 ¦ - ¦ 4.0 ¦ 5.7
Ukanil R ¦ 1.5 ¦ 1.5 ¦ 1.0 ~ 1.0 ¦ 2.0 ¦
Dobanol R 23-3 1 _ I _ I _ I - I - I 0.~
A~unonia ~ 0.6 1 0.~ 1 0.7 ~ I 0.32
Ammonium Chloride ~ I 1.0
Sodium Chloride I _ I _ I - 1 4.0 ¦
Tri Ethanol Amine I - ¦ - ¦ 1-5 ¦ - ¦ ~ ¦ ~ ~
Hydrogen Chloride I _ I _ I _ ¦ 9.051
Hydrogen Peroxide I _ 1 6~0 ¦ ~ I _ I _ I _
S~dium Cumene Sulphonate ¦ 1.0 ¦ - ¦ - I _ l - l - ;
Perfume 1 0.5 1 - I 0.5 1 - I - I 0.4
pH ¦ 4.1 ¦ 4~ ¦ 4.5 ¦ 2,5 ¦ 2.2 ¦ 3.6 ~.
Water & minors I ~lp to 100%
Data
:
The following data illustrates the benefits obtained
from the compositions ac~ording to the present invention. ~ :
composition is made which comprises, by weight o~ the total
composition: .
, .
: Sodium coconut alkyl sulfate:3.5% ;
: Ammonia: O.7S~
:
Citric acid: ~ ; 5.8
pH: : 4.3
: Water & minors: up to 100%
:; ~ .
An identical composition is also made, except it comprises in
addition 1.5% of Ukanil R.
..
S~ r~TU~E SHEET
;
.
W092/22628 . Pcr/us92/o4772
2110~13 lo
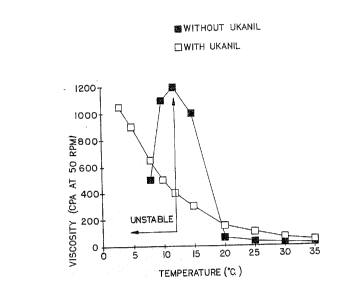
The viscosity of both compositions is then measured at
60rpm shear rake, at different temperatures. The results are
reported in Fig. 1. This graph shows that the composition
without Ukanil R presents the phase stability problem as from .:
13~C and below, whereas in the same time, the composition ..
with Vkanil R raises in viscosity as the temperature
decreases, but remains stable. Also, one can notice that, at
all temperatures, the composition with Vkanil ~ is somewhat .
mor~ viscous than the composition without.
, .
.
, .
: '
~UBSTITUTE SHEI~T