Note: Descriptions are shown in the official language in which they were submitted.
,. . , .wo g2/2224g ~ ' 'PCr/USs2'iO2610 ` 211û~7
METHOD8 FOR PROVIDING LOCALIZ~D THERAPEUTIC HEAT
TO BIOLOGICAL TI88~E8 AND FL~ID8 ~8ING
GA8 FILL~D LIP080ME8
RELAT~D APPLICATION
This application is a continuation-in-part of
copending application U.S. Serial No. ~81,027, filed
September 11, 1990, and a continuation-in-part of
copending application U.S. Serial No. 569,828, filed
August 20, 1990, which in turn is a continuation-in-part
of application U.S. Serial'No. 455,707, filed December 22,
~;. ;
1989, the disclosures of each'of which are hereby
incorporated herein by reference in their entirety.
BAC~GRO~ND OF T~E INVENTION
Field of the Invention
~ - ~'`The''present'invëntion relates to the use of
`ultrasonic`energy"~to'iheat'biological tissues and fluids,
and more specificàlly, to the use of hyperthermia
potentiators, such as gas filled liposomes prepared by a
vacuum drying gas instillation method, and/or gàs filled
liposomes^substàntially devoid of liquid in the interior
thereof, in combination with ultrasound to facilitate the
selective heating of the tissues and fluids.
Description of thé Prior Art
The usefulness of heat to treat various
inflammatory and arthritic conditions has long been known.
The use of ultrasound to generate such heat for these as
,~, ,, ,.WO g2/22249 ~'PCI~/US92io2610
21 1~ - 2 -
well as other therapeutic purposes, such as in, for
example, the treatment of tumors has, however, been a
fairly recent development.
Where the treatment of inflammation and arthritis
is concerned, the use of the ultrasound induced heat
serves to increase blood flow to the affected regions,
resulting in various beneficial effects. Moreover, when
ultrasonic energy is delivered to a tumor, the temperature
of the tumorous tissue rises, generally at a higher rate
than in normal tissue. As this temperature reac~es above
about 43C, the tumorous cells begin to die and, if all
goes well, the tumor eventually disappears. Ultrasound
induced heat treatment of biological tissues and fluids is
known in the art as hyperthermic ultrasound.
The non-invasive nature of the hyperthermia
ultrasound technique is one of its benefits. Nonetheless,
in employing hyperthermic ultrasound, certain precautions
must be taken. Specifically, one must be careful to focus
the ultrasound energy on only the areas to be treated, in
an attempt to avoid heat-induced damage to the
surrounding, non-targeted, tissues. In the treatment of
tumors, for example, when temperatures exceeding about
43-C are reached, damage to the surrounding normal tissue
is of particular concern. This concern with over heating
~5 the non-target tissues thus places limits on the use of
hyp-rthermic ultraso~nd. Such therapeutic treatments
would clearly be more effective and more widely employed
if a way of targeting the desired tissues and fluids, and
of maximizing the heat generated in those targeted
tissues, could be devised.
` The present invention is directed toward
- `improving the effectiveness and utility of hyperthermic
ultrasound by providing agents capable of promoting the
selective heating of targeted tissues and body fluids.
W0 92/22249 2 1 1 o ,~ 8 ~cr/us92/0261o
811~1ARY OF THE INV~NTION
The present invention is directed to methods for
heat treating biological tissues and fluids which comprise
administering to the tissue or fluid to be treated a
s thera-peutically effective amount of a hyperthermia
potentiator comprising gas filled liposomes prepared by a
vacuum drying gas instillation method, and then applying
ultrasound to that tissue or fluid.
The present invention is also directed to methods
for heat treating biological tissues and fluids which
comprise administering to the tissue or fluid to be
treated a therapeutically effective amount of a
hyperthermia potentiator comprising gas filled liposomes
substantially devoid of liquid in the interior thereof,
and then applying ultrasound to that tissue or fluid.
By using the potentiators of the present
invention, hyperthermic ultrasound becomes a better, more
selective and more effective therapeutic method for the
treatment of tumors, inflammation, and arthritis, as well
as other various conditions.
.
BRI~F DE8CRIPTION OF T~B FIG~RE~
FIG~RE 1 shows an apparatus according to the
present invention for preparing the vacuum dried gas
instilled liposomes and the gas filled liposomes;`
25 ~ substantially~deYoid of liquid in the interior théreof
prepared by the vacuum drying gas instillation`method.
FIG~RE 2 is a graphical representation of the dB
reflectivity of the vacuum dried gas instiiled`liposomes
and the gas filled liposomes substantially devoid of
~ liquid in the-interior thereof prepared by the vacuum
drying gas-instillation method. The data was obtainéd by
scanning with a 7.5 megahertz transducer using an Acoustic
Imaging~ Model 5200 Scanner (Acoustic Imaging, Phoenix,
Arizona), and was generated using the system test software
to measure reflectivity. The system was standardized
~W092/2224s ~ ; PCT/US92io2~l0
.
21'10~87 -~-
prior to each experiment with a phantom of known acoustic
impedance.
.
DEq!AIL~D DB8CRIPTION OF T~ NTION
The present invention is directed to a method for
heat treating biological tissues and fluids comprising
administering to the tissue or fluid to be treated a
therapeutically effective amount of a hyperthermia
potentiator, and then applying ultrasound to said tissue
or fluid.
The hyperthermia potentiators described herein
comprise gas filled liposomes prepared by a vacuum drying
gas instillation method, and/or gas filled liposomes
substantially devoid of liquid in the interior thereof.
The vacuum drying gas instillation method, which
lS may be employed to prepare both the gas filled liposomes
prepared by the vacuum drying gas instillation method, and
the gas filled liposomes substantially devoid of liquid in
the interior thereof, contemplates the following process.
First, in accordance with the process, the liposomes are
placed under negative pressure (that is,- reduced pressure
or vacuum pressure). Next, the liposomes are incubated
under that negative pressure for a time sufficient to
remove substantially all liquid from the liposomes,
thereby re~ulting in substantially dried liposomes. By
re~oval of substantially all liquids, and by substantially
.... i. . ~
dried~liposomes, as those phrases are used herein, it~is
~eant that the liposomes are at least about 90% devoid of
liquid,~preferably at least about 95% devoid of liquid,
;~m~ost~preferably about 100% devoid of liquid. Finally, the
liposomes are instilled with selected gas by applying the
gas~to the liposomes until ambient pressures are achieved,
thus resulting in the subject vacuum dried gas instilled
,
liposomes of the present invention, and the gas filled
liposomes of the invention substantially devoid of liquid
in the interior thereof. By substantially devoid of
liquid in the interior thereof, as used herein, it is
W09~22249 2 1 1 0 4 ~ 7 PCT/US92/026to
meant liposomes having an interior that is at least about
90% devoid of liquid, preferably at least about 95% devoid
of liquid, most preferably about 100% devoid of liquid.
Unexpectedly, the liposomes prepared in
accordance with the vacuum dried gas instillation method,
and the gas filled liposomes substantially devoid of
liquid in the interior thereof, possess a number of
surprising yet highly beneficial characteristics. The
liposomes of the invention exhibit intense ecogenicity on
lo ultrasound, result in good heating of surrounding tissues
and/or fluids on ultrasound, are highly stable to
pressure, and/or possess a long storage life either when
stored dry or suspended in a liquid medium.
The ecogenicity of the liposomes allows the
monitoring of the liposomes following administration to a
patient to determine the presence of liposomes in a
desired region. The ability of the liposomes to result in
heating of the surrounding region is of obvious importance
to the therapeutic applications of the invention.
~ The ~tability of the liposomes is also of great
practical importance. The subject liposomes tend to have
greater stability during storage than other gas filled
liposomes produced via known procedures such as
pressurization or other techniques. At 72 hours after
formation, for example, conventionally prepared liposomes
often are essentially devoid of gas, the gas`having
~diffu~ed~out of the~liposomes 'and/or-the"liposomes`~'aving
ruptured and/or fused, resulting in a concomitant~ loss in
heating potential. ~In comparison, gas filled liposomes of
the present invention generally have a shelf life
stability;of`greater than about three weeks,'preferably a
shelf life ~tability of greater than about four weeks,
more preferably a shelf life stability of greater than
about five weeks, even more preferably a shelf life ;-
stability of greater than about three months, and often ashelf life stability that is even much longer, such as
over six months, twelve months or even two years.
; .' ~ !J~' SwOg2/22~9~ PCT/US92/02610
211~ 48 1 6 --
Also unexpected is the ability of the liposomes
during the vacuum drying gas instillation process to fill
with gas and resume their original circular shape, rather
than collapse into a cup-shaped structure, as the prior
art would cause one to expect. See, e. g., Crowe et al.,
Archives of BiochemistrY and BioPhysics, Vol. 242, pp.
240-247 (1985); Crowe et al., Archives of Biochemistry and
Biophvsics, Vol. 220, pp. 477-484 (1983); Fukuda et al.,
J. Am. Chem. Soc., Vol. 108, pp. 2321-2327 (1986); Regen
et al., J. Am. Chem. Soc., Vol. 102, pp. 6638-664~ (1980).
The liposomes subjected to the vacuum drying gas
instillation method of the invention may be prepared using
any one of a variety of conventional liposome preparatory
techniques which will be apparent to those skilled in the
art. These techniques include freeze-thaw, as well as
techniques such as sonication, chelate dialysis,
homogenization, solvent infusion, microemulsification,
spontaneous formation, solvent vaporization, French
pressure cell technique, controlled detergent dialyzing,
and others. The size of the liposomes can be adjusted, if
decired, prior to vacuum drying and gas instillation, by a
variety of procedures including extrusion, filtration,
sonication, homogenization, employing a laminar stream of
a core of liquid introduced into an immiscible sheath of
liquid, and similar methods, in order to modulate - -
re~ultant liposomal biodistribution and clearance, with
1 - extrusion under pressure through pores of defined size
¦ being the preferred means-of adjusting the size of the
liposomes. The foregoing techniques, as well as others,
are discu~sed, for example, in U.S. Patent No. 4,728~578;
U.K. Patent Application GB 2193095 A; U.S. Patent No.
4,728,575; U.S. Patent No. 4,737,323; International
Application PCT/US85/01161; Mayer et al., Biochimica et
Biophvsica Acta Vol. 858, pp. 161-168 (1986); Hope et
al., Biochimica et BioPhysica Acta. Vol. 812, pp. 55-65
(1985); U.S. Patent No. 4,533,254; Mayhew et al., Methods
.
W092/22249 2 1 1 ~ ~ 8 ~CTiUS92/026l0
in EnzYmoloqy. Vol. 149, pp. 64-77 (1987); Mayhew et al., `
Biochimica et 8iophysica Acta. Vol 755, pp. 169-74 (1984);
Cheng et al, Investiaative RadiologY. Vol. 22, pp. 47-5s
(1987); PCT/US89/05040, U.S. Patent No. 4,162,282; U.S.
Patent No. 4,310,505; U.S. Patent No. 4,921,706; and
Liposome TechnoloqY, Gregoriadis, G., ed., Vol. I, pp. 29-
37, 51-67 and 79-108 (CRC Press Inc., Boca Raton, FL
1984). The disclosures of each of the foregoing patents,
publications and patent applications are incorporated by
reference herein, in their entirety. Although any of a
number of varying techniques can be employed, preferably
the liposomes are prepared via microemulsification
techniques. The liposomes produced by the various
conventional procedures can then be employed in the vacuum -
drying gas instillation method of the present invention,
to produce the liposomes of the present invention.
The materials which may be utilized in preparing
liposomes to be employed in the vacuum drying gas
instilla-tion method of the present invention include any
of the materials or combinations thereof known to those
skilled in the art as suitable for liposome construction.
The lipids used may be of either natural or synthetic
origin. Such materials include, but are not limited to,
lipids such as fatty acids, lys~lipids,
dipalmitoylphosphatidylcholine, phosphatidylcholine,
- phosphatidic acid, sphingomyelin, cholesterol, cholesterol
- ~ hemi~u¢cinate, tocopherol-^hemisuccinate,;
phosphatidylethanolamine, phosphatidyl-inositol, ~?
lysolipids, sphingomyelin, glycoæphingolipids,
glucolipids, glycolipids, sulphatides, lipids with ether
~ and ester-linked fatty acids, polymerized lipids, diacetyl
; phosphate, ~tearylamine,! di~tearoylphosphatidylcholine,
phosphatidylserine, sphingomyelin, cardiolipin,
phospholipids with short chain fatty acids of 6-8-carbons
in length, synthetic phospholipids with asymmetric acyl
chains (e.g., with one acyl chain of 6 carbons and another
acyl chain of 12 carbons), 6-(5-cholesten-3~-yloxy)-1-
W092/22U9. ! P~T/US92/02610
.. . .. .
2110~87
- 8 -
thio-~-D-galactopyranoside, digalactosyldiglyceride, 6-(5-
cholesten-3~-yloxy)hexyl-6-amino-6-deoxy-1-thio-~-D-
galactopyranoside, 6-(5-cholesten-3~-yloxy)hexyl-6-amino-
6-deoxyl-1-thio-~-D-mannopyranoside, dibehenoyl-
phosphatidylcholine, dimyristoylphosphatidylcholine,dilauroylphosphatidylcholine, and dioleoyl-
phosphatidylcholine, and/or combinations thereof. Other
useful lipids or combinations thereof apparent to those
skilled in the art which are in keeping with the spirit of
the present invention are also encompassed by the present
invention. For example, carbohydrate bearinq lipids may
be employed for in vivo targeting, as described in U.S.
Patent No. 4,310,505. Of particular interest~for use in
the present invention are lipids which are in the gel
state (as compared with the liquid crystalline state) at
the temperature at which the vacuum drying gas
instillation is performed. The phase transition
temperatures of various lipids will be readily apparent to
those skilled in the art and are described, for example,
in Li~osome Technolooy, Gregoriadis, G., ed., Vol. I, pp.
1-18 (CRC Press, Inc..Boca Raton, FL 1984), the
disclosures of which are incorporated herein by reference
in their entirety. In addition, it has been found that
the incorporation of at least a small amount of negatively
charged lipid into any liposome membrane, although not
required,.is beneficial to providing highly stable
liposomes. By!at least a~mall amount, it is meant-about
1 mole.:percent of the total lipid.; Suitable negatively
charged lipids will be readily apparent to those skilled
30-.. in the art, and include, for example phosphatidylserine
. and fattyJacids. Most.preferred for the combined reasons
of ultimate~hyperthermia potentiation, ecogenicity, and
stability..following the vacuum drying gas instillation
process are liposomes prepared from
dipalmitoylphosphatidylcholine.
By way of general guidance, dipalmitoyl-
phosphatidylcholine liposomes may be prepared by
. ! wo 92/22~9 2 1 1 0 4 8 ~CTiUS92/02610
suspending dipalmitoylphosphatidylcholine lipids in
phosphate buffered saline or water, and heating the lipids
to about 50-C, a temperature which is slightly above the
45-C temperature required for transition of the
dipalmitoyl- phosphatidylcholine lipids from a gel state
to a liquid crystalline state, to form liposomes. To
prepare multilamellar vesicles of a rather heterogeneous
size distribution of around 2 microns, the liposomes may
then be mixed gently by hand while keeping the liposome
solution at a temperature of about 50-C. The temperature
is then lowered to room temperature, and the liposomes
remain intact. Extrusion of
dipalmitoylphosphatidylcholine liposomes through
polycarbonate filters of defined size may, if desired, be
employed to make liposomes of a more homogeneous size
distribution. A device useful for this technique is an
extruder device (Extruder Device , Lipex Biomembranes,
Vancouver, Canada) equipped with a thermal barrel so that
extrusion may be conveniently accomplished above the gel
state-liquid crystalline transition temperature for
lipids. - ' `"
Alternatively, and again by way of general
guidance, conventional freeze-thaw procedures may be used
to produce either oligolamellar or unilamellar
25 dipalmitoyl-phosphatidylcholine liposomes. After the `~'
-- freezè-thaw procedures, extrusion procedures as described
-;above may then be performed on'the`liposomes'.' -
- - The liposomes thus prepared may then be subjected
to the vacuum drying gas instillation process'of`the
present invention, to produce the vacuum dried gas
- instilled liposomes, and the gas filled liposomes
~ubstantially devoid of-liguid in the interior thereof, of
-the invention. In accordance with the process of the
invention, the liposomes are placed into a vessel æuitable
for subjecting to the liposomes to negative pressure (that
is, reduced pressure or vacuum conditions). Negative
pressure is then applied for a time sufficient to remove
W092/22249 '~PCT/US92io2610
- 2110~8 1 -^`
-- 10 --
substantially all liquid from the liposomes, thereby
resulting in substantially dried liposomes. As those
skilled in the art would recognize, once armed with the
present disclosure, various negative pressures can be
employed, the important parameter being that substantially
all of the liquid has been removed from the liposomes.
Generally, a negative pressure of at least about 700 mm
Hg, and preferably in the range of between about 700 mm Hg
and about 760 mm Hg (gauge pressure) applied for about 24
to about 72 hours, is sufficient to remove subst~ntially
all of the liquid from the liposomes. Other suitable
pressures and time periods will be apparent to those
skilled in the art, in view of the disclosures herein.
Finally, a selected gas is applied to the .
liposomes to instill the liposomes with gas until ambient
pressures are achieved, thereby resulting in the vacuum
dried gas instilled liposomes of the invention, and in the
gas filled liposomes substantially devoid of liquid in the
interior thereof. Preferably, gas instillation occurs 20 slowly,..that is, over a time period-of at least about 4
hours, most preferably over a time period of between about
4 and about 8 hours. Various biocompatible gases may be
employed. Such gases include air, nitrogen, carbon
dioxide, oxygen,-argon, xenon, neon, helium, or any and
a}l combinations thereof. Other suitable gases will be
apparent to.those skilled in the art, the gas chosen being
only limited by the proposed application of the liposomes.
.; .~ .~T;he above.described method for production of
liposomes is referred to hereinafter as the vacuum drying
gas.instillation process.
.~ If desired, the liposomes may be cooled, prior to
subjecting the liposomes to negative pressure, and such
cooling is preferred. Preferably, the liposomes are
.cooled to below 0-C, more preferably to between about -
10-C and about -20-C, and most preferably to -10-C, prior
to subjecting the liposomes to negative pressure. Upon
reaching the desired negative pressure, the liposomes
,~ W09~22249 PCT/US92/02610
--- 2:110487
temperature is then preferably increased to above ooCl
more preferably to between about lO-C and about 20-C, and
most preferably to lO C, until substantially all of the
liquid has been removed from the liposomes and the
negative pressure is discontinued, at which time the
temperature is then permitted to return to room
temperature.
If the liposomes are cooled to a temperature
- below 0C, it is preferable that the vacuum drying gas
instillation process be carried out with liposomes either
initially prepared in the presence of cryoprotectants, or
liposomes to which cryoprotectants have been added prior
to carrying out the vacuum drying gas instillation process
of the invention. Such cryoprotectants, while not -
mandatorily added, assist in maintaining the integrity of
liposome membranes at low temperatures and also add to the
ultimate stability of the ~embranes. Preferred
cryoprotectants are trehalose, glycerol,
polyethyleneglycol (especially polyethyleneglycol of
molecular weight 400), raffinose, sucrose, and sorbitol,
with trehalose being particularly preferred`.
It has also been surprisingly discovered that the
liposomes of the invention are highly stable to changes in
pressure. Because of this characteristic, extrusion of
the liposomes through filters of defined pore size
following vacuum drying and gas instillation can be '
carried out, if desired, to create liposomes of reIatively
homogeneous-and defined pore size. ' '`
For storage prior to use, the'liposomes of the
pre~ent invention may be ~uspended in an aqueous solution,
~uch as a.saline ~olution (for example, a phosphate `
- buffered ~aline ~olution), or simply water, and 'stored
preferably at a temperature of between about 2-C and about
lO-C, preferably at about 4-C. Preferably, the water is
35 sterile. Most preferably, the liposomes are stored in a
hypertonic saline solution (e.g., about 0.3 to about 0.5
NaCl), although, if desired, the saline solution may be
W092~22249 PCT/USs2/026l0
0 ~ 12 -
isotonic. The solution also may be buffered, if desired,
to provide a pH range of pH 6.8 to pH 7.4. Suitable
buffers include, but are not limited to, acetate, citrate,
phosphate and bicarbonate. Dextrose may also be included
s in the suspending media. Preferably, the aqueous solution
is degassed ~that is, degassed under vacuum pressure)
prior to suspending the liposomes therein. Bacteriostatic
agents may also be included with the liposomes to prevent
bacterial degradation on storage. Suitable bacteriostatic
lo agents include but are not limited to benzalkonium
chloride, benzethonium chloride, benzoic acid, benzyl
alcohol, butylparaben, cetylpyridinium chloride,
chlorobutanol, chlorocresol, methylparaben, phenol,
potassium benzoate, potassium sorbate, sodium benzoate and
sorbic acid. One or more antioxidants may further be
included with the gas filled liposomes to prevent
oxidation of the lipid. Suitable antioxidants include
tocop~erol, ascorbic acid and ascorbyl palmitate.
Liposomes prepared in the various foregoing manners may be
stored for at least several weeks or months. Liposomes of
the present invention may alternatively, if desired, be
stored in their dried, unsuspended form, and such
liposomes also have a shelf life of greater than several
weeks or months; Specifically, the liposomes of the
present invention, stored either way, generally have a
shelf life stability of greater than about three weeks,
preferably~a ~helf life stability of greaterithan about
~ four weeks, more preferably a shelf life stability of
greater than~about five weeks, even more preferably a
shelf life stability of greater than about three months,
and often a shelf life stability that is even much longer,
such~as over six months, twelve months or even two years.
As another aspect of the invention, useful
apparatus for preparing the vacuum dried gas instilled
liposomes, and the gas filled liposomes substantially
devoid of liquid in the interior thereof, of the invention
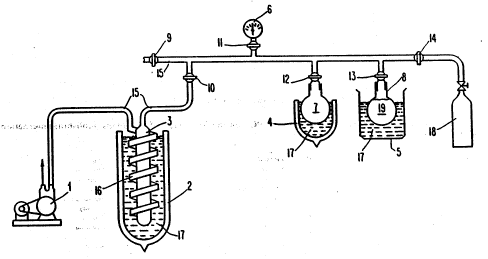
is also presented. Specifically, there is shown in Figure
wos2/2224s ' Pcr/uss2io26l0
~ 2110487
- 13 -
1 a preferred apparatus for vacuum drying liposomes and
instilling a gas into the dried liposomes. The apparatus
is comprised of a vessel 8 for containing liposomes 19.
If desired, the apparatus may include an ice bath 5
containing dry ice 17 surrounding the vessel 8. The ice
bath 5 and dry ice 17 allow the liposomes to be cooled to
below 0C. A vacuum pump 1 is connected to the vessel 8
via a conduit 15 for applying a sustained negative
pressure to the vessel. In the preferred embodiment, the
pump 1 is capable of applying a negative pressure of at
least 700 mm Hg and preferably a negative pressure in the
range of about 700 mm Hg to about 760 mm Hg (gauge
pressure). A ~anometer 6 is connected to the conduit 15
to allow monitoring of the negative pressure applied to
~5 the vessel 8.
In order to prevent liquid removed from the
liposomes from entering the pump 1, a series of traps are
connected to the conduit 15 to assist in collecting the
liquid (and liquid vapor, all collectively referred to
herein as liquid) drawn from the liposomes. In a
preferred embodiment, two traps are utilized. The first
trap is preferably comprised of a flask 7 disposed in an
ice bath 4 with dry ice 17. The second trap is preferably
comprised of a column 3 around which tubing 16 is
helically arranged. The column 3 is connected to the
- conduit 15 at its top end and to one end of the tubing 16
- at its bottom end. The~other end of the tubing l6''is
connected to the conduit l5. As shown 'in Figure 1, an ice
bath 2 with dry ice 17 surrounds the column 3 and tubing
16. If desired, dry ice 17 can be replaced with liquid
nitrogen, liquid air or other cryogenic material. The ice
baths 2 and 4 assist in collecting any liquid and'
- condensing any liquid vapor drawn from the liposomes for
collection in the traps. In preferred embodiments of the
present invention the ice traps 2 and 4 are each
maintained at a temperature of least about -70C.
WO g2/22249 ~, ~ PCr/USg2/02610
'"'21'10'4~87
A stopcock 14 is disposed in the conduit 15
upstream of the vessel 8 to allow a selected gas to be
introduced into the vessel 8 and into liposomes 19 from
gas bottle 18.
Apparatus of the present invention are utilized
by placing the liposomes 19 into vessel 8. In a
preferable embodiment, ice bath 5 with dry ice 17 is used
to lower the temperature of the liposomes to below ooc,
more preferably to between about -10C and about -200C,
and most preferably to -lo-C. With stopcocks 14 and 9
closed, vacuum pump 1 is turned on. Stopcocks lo, 11, 12
and 13 are then carefully opened to create a vacuum in
vessel 8 by means of vacuum pump 1. The pressure is
gauged by means of manometer 6 until negative pressure of
at least 700 mm Hg and preferably in the,range of between
about 700 mm Hg and about 760 mm Hg, is achieved. In
preferred embodiments of the present invention, vessel 7,
cooled by ice bath 4 with dry ice 17, and column 3 and
coil 16, cooled by ice bath 2 with dry ice 17, together or
20 individually condense liquid vapor and trap liguid drawn
from-the liposomes so as to prevent such liquids and
liquid vapor from entering the vacuum pump 1. In
preferred embodiments of the present invention, the
temperature of ice traps 2 and 4 are each maintained at a :
temperature of at least about -70-C. The desired negative
pres~ure is.generally maintained for at least 24 hours as
liquid and liquid vapor is r.emoved from;the-liposomes 19
. in vessel~.8-and frozen in vessels 3 and .7. Pressure -
within-the system is monitored using manometer 6 and is
generally maintained for about 24 to about 72 hours, at
which time substantially all of the liquid has been-
removed~:from~.the liposomes. At this point,:stopcock 10 is
slowly clo~ed and vacuum pump 1 is turned off. Stopcock
14 i8 then opened gradually and gas is slowly introduced
into the system from gas bottle 18 through stopcock 14 via
conduit 15 to instill gas into the liposomes 19 in vessel
8. Preferably, the gas instillation occurs slowly over a
; W092/22249 2 1 1 o ~ ~ 7PCT/US92/02610
time period of at least about 4 hours, most preferably
over a time period of between about 4 and about 8 hours,
until the system reaches ambient pressure.
The vacuum dried gas instilled liposomes and the
gas filled liposomes substantially devoid of liquid in the
interior thereof of the present invention have superior
characteristics for use as hyperthermia potentiators. The
subject liposomes provide good heating of surrounding
tissues and/or fluids on ultrasound, are highly stable to
pressure, and/or generally possess a long storage life
either when stored dry or suspended in a liquid medium.
In use, the hyperthermic potentiators of the present
invention are administered to a biological tissue or to
biological fluids, whereupon ultrasound is then applied to
the biological matter. The methods of the invention are
particularly useful when employed in relation to such
biological matter as tumor tissue, muscle tissue or blood
fluid.
The liposomes employed may be of varying sizes,
but preferably are of a size range wherein they have a
A~ mean outside diameter between-about 30 nanometers and
about lO microns, with the preferable mean outside
diameter being about 2 microns. As is known to those
skilled in the art, liposome size influences
biodistribution and, therefore, different size liposomes
are selected for various purposes. For intravascular use,
or,example,~liposome~size is generally no larger than
about 5 microns, and generally no smaller than about 30
nanometers, in mean outside diameter. For non-vascular
uses, larger liposomes, e.g., between about 2 and about lO
- micron mean outside diameter may be employed, if desired.
- ~ The lipids employed may be selected to optimize
the particular therapeutic use, minimize toxicity and
maximize shelf-life of the product. Neutral liposomes
composed of either saturated or unsaturated phosphatidyl-
choline, with or without sterol, such as cholesterol,
function quite well as intravascular hyperthermia
. W092/22249 ~';PCT/US92io2610
` 2~ 87
- 16 -
potentiators entrapping gas. To improve uptake by cells
.such as the reticuloendothelial system (RES), a negatively
charged lipid such as phosphatidylglycerol, phosphatidyl-
serine or similar materials is added. For even greater
s liposome stability, the liposome can be polymerized using
polymerizable lipids, or the surface of the liposome can
be coated with polymers such as polyethylene glycol so as
to protect the surface of the vesicle from serum proteins,
or gangliosides such as GMl can be incorporated within the
lipid matrix. Liposomes may also be prepared with
attached receptors or antibodies to facilitate their
targeting to specific cell types such as tumors. Most
preferred for reasons of their hyperthermia potentiation
and stability are liposomes prepared from
dipalmitoylphosphatidyl choline.
Where the usage is in vivo, administration may be
carried out in various fashions, such as intravascularly,
intralymphatically, parenterally, subcutaneously,
intramuscularly, intraperitoneally, interstitialiy,
20 hyperbarically, orally, or intratumorly.using a variety of
dosage forms, the particular route of administration and
the dosage used being dependent upon the type of
therapeutic use sought, and the particular potentiating
agent employed.- For example, in tumors with a principal
dominant.arterial supply such as the kidney, these
- hyperthermic.:potentiating agents may be administered
intraarterially. LTypically, dosage is initiated at~-lower
levels and-~increased until~the desired temperature --
increase effect is achieved. Generally, the contrast
agents of the invention are administered in the form of an
aqueous suspension such as in water or a saline~solution
(e.g.,^.phosphate buffered saline). Preferably, the water
is sterile.: Also preferably the saline solution is a
~ hypertonic saline solution (e.g., about 0.3 to about 0.5%
:: 35 NaCl), although, if desired, the saline solution may be
isotonic. The solution also may be bu$fered, if desired,
to provide a pH range of pH 6.8 to pH 7.4. In addition,
~ WOs2/22~9 PCT/US92/02610
211~87
- 17 -
dextrose may be preferably included in the media.
Preferably, the aqueous solution is degassed (that is,
degassed under vacuum pressure) prior to suspending the
liposomes therein.
For in vivo usage, the patient can be any type of
mammal, but most preferably is a human. The method of the
invention is particularly useful in the treatment ~f
tumors, various inflammatory conditions, and arthritis,
especially in the treatment of tumors. The gas filled
lo liposomes prepared by a vacuum drying gas instillation
method and the gas filled liposomes substantially devoid
of liquid in the interior thereof accumulate in tumors,
particularly in the brain, because of the leaky
capillaries and delayed wash-out from the diseased
tissues. Similarly, in other regions of the body where
tumor vessels are leaky, the hyperthermic potentiating
agents will accumulate.
The hyperthe~mic potentiators of the present
invention may be used alone, in combination with one
another, or in combination with other therapeutic and/or
diagnostic agents. In tumor therapy applications, for
example, the hyperthermic potentiators may be administered
in combination with various chemotherapeutic agents.
Any of the various types of ultrasound imaging
devices can be employed in the practice-of the invention,
-;the^particular type or model of the device not being
critical to the method of the invention. Preferably,-
however, devices specially designed for administering
ultrasonic hyperthermia are preferred.- Such devices are
described U.S. Patent Nos. 4,620,546, 4,658,828, and
4,586,512, the disclosures of each of which are hereby
incorporated herein by reference in their entirety. `The
use of a-device designed for administering ultrasonic
- hyperthermia and incorporating resonant frequency (RF)
spectral analyzer is particularly preferred.
Although applicant does not inte~d to be limited
to any particular theory of operation, the hyperthermic
WOg2/22249 PCT/US92/02610
2110~8~
- 18 -
potentiators employed in the methods of the present
invention are believed to possess their excellent results
because of the following scientific postulates.
Ultrasonic energy may either be transmitted
through a tissue, reflected or absorbed. It is believed
that the potentiators of the invention serve to increase
the absorption of sound energy within the biological
tissues or fluids in which they are present, which results
in increased heating, thereby increasing the therapeutic
effectiveness of ultrasonic hyperthermia.
Absorption of sound is believed to be increased
in acoustic regions which have a high degree of ultrasonic
heterogeneity. Soft tissues and fluids with a higher
degree of heterogeneity will absorb sound at a higher rate
than tissues or fluids which are more homogeneous
acoustically. When sound encounters an interface which
has a different acoustic impedance than the surrounding
medium, there is believed to be both increased reflection
of sound and increased absorption of sound. The degree of
absorption of sound is believed to rise as the difference
between the acoustic impedances between the two substances
comprising the interface increases. The potentiators of
the present invention provide high acoustic impedance
differences between the potentiators and any surrounding
liquids and tissues.
Intense sonic energy is also believed to cause
cavitation and,~when cavitation occurs, this in turn is
thought to cau~e intense local heating.~ Gas bubbles are
believed to lower the cavitation threshold, that is,
accelerate the process of cavitation durinq sonication.
- Since the potentiators of the present invention
~ ~provide high acoustic impedance differences-between the
-~ potentiators and the surrounding liquids and tissues, as
well as decrease the cavitation threshold, the subject
potentiators may act to increase the rate of absorption of
ultrasonic energy in the surrounding tissues and fluids
and effect a conversion of that energy into local heat.
W092/22249 ;PCT/VSg2/026l0
- 19- 211~
Additionally, the low thermal conductivity of gas may
serve to decrease local heat dissipation, with the result
that there is both an increase in the rate of heating and
an increase in the final equilibrium temperature.
The potentiators of the present invention may
serve to increase the acoustic heterogeneity and generate
cavitation nuclei in tumors and tissues thereby acting as
a potentiator of heating in ultrasonic hyperthermia.
Because the gas creates an acoustic impedance mismatch
lo with adjacent tissues and adjacent fluids, the g~s acts to
increase the absorption of sound and conversion of the
energy into heat in the surrounding tissues and fluids.
The liposomes of the present invention are
believed to differ from the liposomes of the prior art in
a nùmber of respects, both in physical and in functional
characteristics. For example, the liposomes of the
invention are substantially devoid of liquid in the
interior thereof. By definition, liposomes in the prior
art have been characterized by the presence of an aqueous
medium. see, e.g., Dorland's Illustrated Medical
Dictionarv, p. 946, 27th ed. (W.B. Saunders Company,
Philadelphia 1988). Moreover, the present liposomes
surprisingly result in good heating of surrounding tissues
and/or fluids on ultrasound, and posses a long storage
life, characteristics of obvious importance to the
hyperthermic potentiator applications of the invention.
There are various!other applications for ~
- liposomes of the invention beyond those described in
detail herein. Such-additional uses, for example, include
such application as drug delivery vehicles and as contrast
agents for ultrasonic imaging. Such additional uses and
- other related subject matter are described and claimed in
Applicant's patent applications filed concurrently
herewith entitled "Novel Liposomal Drug Delivery Systems"
and "Gas Filled Liposomes And Their Use As Ultrasonic
Contrast Agents", the disclosures of each of which are
incorporated herein by reference in their entirety.
,j~,..WOg2~22~9 PCT/US92/02610
211048~
- 20 -
The following examples are merely illustrative of
the present invention and should not be considered as
limiting the scope of the invention in any way. These
examples and equivalents thereof will become more apparent
to those versed in the art in light of the present
disclosure, and the accompanying claims.
Examples 1-8 are actual examples that describe
the preparation and testing of the vacuum dried gas
instilled liposomes, the gas filled liposomes being i.
substantially devoid of any liquid in the interior
thereof, of the invention. Examples 9-13 are prophetic
examples meant to be illustrative of how the invention
would operate under the specified conditions.
~XAMPL~8
lS Exam~le 1
Dipalmitoylphosphatidylcholine (1 gram) was
suspended in 10 ml phosphate buffered saline, the
suspension was heated to about 50-C, and then swirled by
hand in a round bottom flask for about 30 minutes. The
heat~source was removed, and the suspension was swirled
for two additional hours, while allowing the suspension to
cool to room temperature, to form liposomes.
The liposomes thus prepared were placed in a
vessel in an apparatus similar to that shown in Figure 1
cooled to ab.out -lO-C, and.then subjected to high negative
vacuum~pressure.~ The-~temperature o.the 1iposomes was
the~n~raised~to about lO-C.. High negative vacuum pressure
was maintained for about 48 hours. After about 48 hours,
nitrogen gas was gradually instilled into the chamber over
a period.of about 4:hours, after which time the pressure
. .returned to ambient pressure. The resulting vacuum dried
:~ gas instilled liposomes, the gas filled liposomes being
substantially devoid of any liquid in the interior ~ :
thereof, were then suspended in 10 cc of phosphate
buffered saline and stored at about 4C.for about three
months.
WO92/2224g PCTiUS92/02610
` 2110(1~7
- 21 -
Example 2
To test the liposomes of Example l
ultrasonographically, a 250 mg sample of these liposomes
was suspended in 300 cc of degassed phosphate buffered
saline (that is, degassed under vacuum pressure). The
liposomes were then scanned in vitro at varying time
intervals with a 7.5 mHz transducer using an Acoustic
Imaging Model 5200 scanner (Acoustic Imaging, Phoenix, AZ)
and employing the system test software to measure dB
reflectivity. The system was standardized prior~to
testing the liposomes with a phantom of known acoustic
impedance. A graph showing dB reflectivity is provided in
- Figure 2.
Example 3
Dipalmitoylphosphatidylcholine (l gram) and the
cryoprotectant trehalose (l gram) were suspended in lO ml -~
phosphate buffered saline, the suspension was heated to
about 50-C, and then swirled by hand in a round bottom
flask for about 30 minutes. The heat source was removed,
and the suspension was swirled for about two additional
hours, while allowing the suspension to cool to room
temperature, to form liposomes.
The liposomes thus prepared were then vacuum
dried and gas instilled, substantially following the
procedures shown in Example l, resulting in vacuum dried
gas instilled liposomes, the gas filled liposomes being
substantially devoid of any liquid in the interior i~
thereof. The liposomes were then suspended in lO cc of
phosphate buffered saline, and then stored at about 4C
for several weeks.
~ Example 4
; To test the liposomes of Example 3
ultrasonographically, the procedures of Example 2 were
substantially followed. The dB reflectivity of the
liposomes were similar to the dB reflectivity reported in
Example 2.
Example 5
W092/22249 PCT/US92/02610
10 ~8~ ~ 22 -
Dipalmitoylphosphatidylcholine (l gram) was
suspended in lO ml phosphate buffered saline, the
suspension was heated to about 50-C, and then swirled by
hand in a round bottom flask for about 30 minutes. The
suspension was then subjected to 5 cycles of extrusion
through an extruder device jacketed with a thermal barrel
(Extruder Device~, Lipex Biomembranes, Vancouver, Canada),
both with and without conventional freeze-thaw treatment
prior to extrusion, while maintaining the temperature at
about. 50 C. The heat source was removed, and the
suspension was swirled for about two additional hours,
while allowing the suspension to cool to room temperature,
to form liposomes.
The liposomes thus prepared were then vacuum
dried and gas instilled, substantially following the
procedures shown in Example l, resulting in vacuum dried
gas instilled liposomes, the gas filled liposomes being
substantially devoid of any liquid in the interior
thereof. The liposomes were then suspended in lO cc of
phosphate buffered saline, and then stored at about 4-C
for several weeks. --` -
Example 6
To test the liposomes of Example 5
ultrasonographically, the procedures of Example 2 were
: 25 substantially followed. The dB reflectivity of the
liposomes were similar to the dB reflectivity reported in
Example.~2~ t 5~ . t '` ' ` ~ f ,,
Example 7 ~
- -In order to test the stability of the Iiposomes ..
of the invention, the liposomes suspension of Example l
:. was passed through 2 micron polycarbonate filters in an
extruder device (Extruder Device~, Lipex Biomembranes,
Vancouver,~Canada) five times at a pressure of about 600
psi. After extrusion treatment, the liposomes were
studied uItrasonographically, as described in Example 2.
Surprisingly, even after extrusion under high pressure,
~ ~W092/22249 PCT/US92/02610
~llO~S7
- 23 -
the liposomes of the invention substantially retained
their echogenicity.
Example 8
The liposomes of Example 1 were scanned by
s ultrasound using transducer frequencies varying from 3 to
7.5 mHz. The results indicated that at a higher frequency
of ultrasound, the echogenicity decays more rapidly,
reflecting a relatively high resonant frequency and higher
energy associated with the higher frequencies.
The following examples, Examples 9-13, are
prophetic examples.
Example 9
A patient with cancer is administered a dose of
gas filled liposomes prepared by a vacuum drying gas
instillation method, the liposomes being substantia~ly
devoid of liquid in the interior thereof. Accumulation of
the liposomes in the tumor is verified ultrasonigraphic-
ally. Focused ultrasonic hyperthermia is then
administered to the tumor and the liposomes therein. The
20 tumor has an increased rate of heating compared to -;
treatment using ultrasonic hyperthermia without the
liposomes.
~xample 10
An ultrasonic device designed for administering
ultrasonic hyperthermia and incorporating an RF spectral
analyzer was employed. After--gas filled liposomes
prepared by a-vacuum drying-~gas~instillation method,-the
liposomes being substantially devoid of liquid in the
interior thereof, are delivered intravenously, the R~
30 - signal in a lesion in the~patient is monitored by the
ultrasonic hyperthermia RF~spectral analyzer. Even though
the concentration-of gas filled liposomes may be too
dilute to visualize on an image ultrasonographically, the
- RF spectral analyzer detects a change in the peak of the
RF spectrum. The peak in the RF spectrum reflects the
harmonic frequency of the gas filled liposomes. This data
enables the operator to the ultrasonic hyperthermia
W092/22249 , PCT/US92/02610
~ 2~ -
equipment to adjust the application of the ultrasonic
energy to coincide with the maximal intra-lesional
concentration of the gas filled liposomes and to evaluate
the disappearance of the gas filled liposomes as
hyperthermia progresses. Assessment of the resonant
frequency also allows the operator to adjust the
frequency, amplitude, duration and pulse repetition rate
to be maximally effective at heating the tumor through the
augmenting effects of the bubbles. Further, the presence
lo of the gas filled liposomes in the lesion allows-the
operator to assess the blood flow through the diseased
tissues, information of great value in determining the
rate of washout of heat from the tumor. Information
regarding blood flow can be obtained either from simple
lS subjective assessment of the images under real time
ultrasound or by quantitative assessment through
integration of the peaks in the RF spectrums reflecting
the resonant frequencies of the gas filled liposomes.
~ ple 11
Gas filled liposomes prepared by a vacuum drying
gas instillation method, the liposomes being substantially
devoid of liquid in the interior thereof and entrapping
oxygen gas, are administered intravenously to a patient
with cancer. Ultrasonic hyperthermia is performed with
pulses of high energy ultrasound and cavitation occurs at
the sites of the bubbles. The oxygen increases the rate
~ of formation of free radicals which help to destroy the
- tumor~tissue.
Example 12 -
- 30 - In a patient with cancer, gas filled liposomes
prepared by a vacuum drying gas instillation method, the
liposomes being substantially devoid of liquid in the
interior thereof and entrapping oxygen, are administered
intravenousIy and the patient is then scanned via
ultrasound. When the peak concentration of liposomes is
in the tumor, the tumor is simultaneously treated with
hyperthermia and radiation therapy. The effect of the
WOg2/22249 r~ c ~ ~c ~ PCT/USg2/02610
2.ll~487
- 2s -
liposomes and hyperthermia magnifies the effectiveness of
the radiation therapy by releasing oxygen to form free
radicals generated by the ionizing radiation and also
formed by cavitation to improve the response of the tumor
to combined radiation and hyperthermia.
Various modifications in addition to those shown
and described herein will be apparent to those skilled in
the art from the foregoing description. Such
modifications are also intended to fall within the scope
of the appended claims.
Exa~ple 13
Gas filled liposomes prepared by a vacuum drying -
gas instillation method, the liposomes beingrsubstantially
devoid of liquid in the interior thereof and entrapping
argon gas, are administered intravenously to a patient
with cancer. Ultrasonic hyperthermia is performed with
pulses of high energy ultrasound and cavitation occurs at
the sites of the bubbles. The argon increases the rate of
formation of free radicals which help to destroy the tumor
tissue.
..~
:
.; :