Note: Descriptions are shown in the official language in which they were submitted.
W~ 92t21283 2 - . ~ ~ ~ PCT/U592/44654
OPTICAL CEREBRAL OXIMETER
TECHNICAL FIELD
This invention relates generally to in vivo
spectrophotometric methods and apparatus, for examining
and/or monitoring biological tissue, substances and/or
conditions in living subjects, in particular humans.
More particularly, the invention relates to the novel
application of such in vivo methods and apparatus to
provide a new form of biomedical device for
non-invasively monitoring oxidative metabolism in
mammalian (e.g. human) subjects on an in vivo basis, a
specific and preferred embodiment of which comprises
means for so-monitoring regional oxygen saturation in the
brain, and for providing a quantitative readout thereof
in terms familiar tee medical practitioners, i.e., percent
oxygen saturation.
BACKGROUND
Bpectrophotometry has, of course, long been used as
a valuable investigative tool in various scientific
fields, particularly biological and medical research, and
various applications of the underlying principles
utilizing selected wavelengths of light in the near
infrared range (often referred to as N.I.R.
spectrophotometry) have for quite some time been utilized
for certain in vivo procedures and/or investigation on
human beings. For example, a frequently-encountered such
device is the pulse oximeter conventionally used in
hospitals and other medical facilities to provide a
direct indication of arterial oxygen saturation by means
of a clip or the like which fastens to an appendage such
as the ear or finger of the patient. As has been noted
by a small but growing field of investigators, the
potentialTy~useful applications of N.I.R. in vivo
spectrophotometry are considerably broader and more
diverse than this, however, due to the interesting and
' useful characteristic of ?t.I.R. wavelengths in being able
to pass through ("transmiss") biological substance such
as human skin, bone, and tissue for at least a length of
several centimeters, and a useful: brief description and
WAD 92/21283 ~ 11 p '~ ~ ~ PCT/US92104654
-2-
commentary as to this is set forth in patents attribut-
able in at least part to the present inventor (see for
example U.S. Patent No. 4,570,638j, as well as in the
various references of record therein. In the latter
regard, particular reference is made to the patents
issued to Jobsis et al, e.g. U.S. Patents Nos. 4,281,645,
.4,223,680 and 4,321,930.
While previous developments in the general field of
N.I.R. in vivo spectrophotometry, as noted above, have no
doubt provided interesting and at least potentially
useful insights and information heretofore, many
important,further developments and applications no doubt
remain to be made, and certain of these are likely to be
of considerable importance~to medical practitioners. For
example, accurate, meaningful, non-intrusive monitoring
of brain status and viability is a most important need
which prior technology has not sufficiently satisfied.
As is well known and widely appreciated, the brain is a
delicate and easily-damaged portion of human anatomy,
while at the same time being the epicenter of
neurological and physiological function. Brain damage
through injury or cerebral vascular disease is responsi-
ble for numerous deaths and serious illnesses each year,
involving on the order of at least 100,000 surgical
procedures annually in recent years. Brain vitality is
primarily a function of oxidative metabolism, and the
pres~ominant cause of neurological dysfunction and
w
malfunction relates to the lack of sufficient brain
oxidation, typically as a result of obstruction or
othex~rise insufficient arterial blood flow to the brain.
Of course, this can occur even during surgery, and it has
been estimated that at least 2,000 patients die each year
in the United States alone due to anesthetic accidents,
while numerous other such incidents result in brain
damage of some degree; at the same time, certain major
and complex surgical procedures, particularly of a
neurological, cardiac or vascular nature, may require
induced low blood flow or pressure conditions, which
W~ 92/2~2~3 ~ PCTlUS92/fl46S4
-3-
inevitably involves the potential of insufficient oxygen
delivery to the brain. At the same time, the brain is
the human organ which is most intolerant of oxygen
deprivation, and brain cells will die within a few
minutes if not sufficiently oxygenated. Moreover, such
cells are not replaced, and thus involve irreversible
-brain damage which may potentially result in paralysis,
disability, or even death.
Accordingly, the availability of immediate and
accurate information concerning the state of brain oxygen
saturation is of critical importance to anesthesiologists
and surgeons, as well:as other involved medical practi-a
tioners, particularly since the patients involved are
typically in an unconscious state and thus unable to-
provide information by ordinary physical response. Up
until the present time, however, the instrumentalities
available for use, including such things as
electroencephalograph ("EEG"), arterial pulse oximeter
and blood pressure monitors, etc., and even invasive
catheter monitoring of blood oxygen content, acidity,
etc. by penetration of the jugular bulb (jugular vein) do
not provide accurate, ongoing, timely (instantaneous)
information as to cerebral (brain) blood oxygenation
state, particularly since the brain blood supply is
extensive, diffuse, pervasive, and largely venous in
nature rather then arterial. Of course, it is also thus
devoid of conventional pulsative characteristics essen-
' tial to the operation of conventional oximeters.
Accordingly, such devices are not appropriate for
cerebral usage, and of course they are typically made to
be applied only to peripheral tissue or appendages in any
event, i.e...~ a finger or an ear lobe, and are not
utilized in conjunction with venous blood. Of course,
jugular bulb catheters are highly invasive and relatively
traumatic: at the same time, they merely provide blood
samples which are removed and analyzed in another
location, at a subser~uent point in time, and thus only
WO 92/2123 ~ ~~ ~ PCT/US92/04~54
-4-
address the state of venous blood after it has left the
brain.
BRIEF SUM~~ARY OF INVENTION
In a specific and particular sense, the present
invention provides a spectrophotometric cerebral
oximeter, which non-invasively and harmlessly provides
-accurate and continuous real-time information as to the
oxygenation state of the human brain, on an in vivo
basis, without attendant patient stress or discomfort of
any nature. More broadly considered, the present
invention provides in vivo spectrophotometric methods and
apparatus adaptable to other relatively analogous
biomedical procedures and functions, for monitoring
oxidative metabolism and/or other physiologic function,
condition, or state.
In the particular preferred embodiment disclosed,
the invention provides an in vivo, spectrophotometric
cerebral oximeter which will non-invasively provide
continuous monitoring of cerebral oxidation, and will do
so in a form and format of a nature immediately under-
standable and familiar to physicians, i.e., percent
oxygen saturation. Further, the cerebral oximeter so
provided operates by examining (sampling) the cerebral
blood supply throughout the complete vascularization
(arterial, venous, and capillary systems) within the area
of investigation, and the particular region investigated
is or may be selectively accessed in accordance with the
invention, i.e., the tissue volume examined is regional
in nature and of a generally predetermined extent and
location, constituting less than the entire brain or
other area. Still further, the apparatus and methodology
in accordan,~e with the invention includes the provision
of a convenient and readily-usable sensor which may for
example be used in a number of different locations,
and/or moved from one location to another, for compara-
tive consideration of the regions selectively accessed
and examined, whether cranial or otherwise.
VV~ 92/21283 PCi'/U~92/t~46S4
_5_
Accordingly, the cerebral oximeter in accordance
with the invention examines, and measures, blood oxygen
saturation (and thus, oxidative metabolism) in the entire
array of blood vessels present in the cranial region
being monitored, which in the brain may generally be
considered as comprising (by volume) approximately 75
-percent venous, 20 percent arterial, and 5 percent
capillary. Thus, the cerebral oximeter provided in
accordance with the invention addresses not only oxygen
delivery via hemoglobin molecules moved arterially, but
in addition addresses the general, overall state of
cerebral oxygen consumption, which is of course directly
ralated to brain vitality and state, and indicative of
continued viability. As already indieated, the invention
provides such information on an instantaneous real-time
basis, and as a result provides critical immediate
information capable of clearly and quantitatively
indicating the need for urgent measures to provide
increased or decreased cerebral oxygen supply or
consumption (metabolic activity), momentary responses to
which may well prevent serious neurologit~al or other
trauma or injury.
In addition, the cerebral oximeter or other such
apparatus provided in accordance with the invention is
convenient to use, non-invasive and non-traumatic,
produces no attendant side effects, and provides
specific, quantified information of a type not previously
available. At the same time, such apparatus is compact
and relatively portable in nature, may provide direct
visible monitoring vii CRT or other visual display, and
provides digitally storable data which may readily be
maintainec't~,~or future review or comparison or printed out
in hard copy, plotted, etc., and/or periodically accessed
to provide ongoing trend data, for displaying or
analyzing changes which occur over selected periods of
time. As such, the apparatus may be used in such diverse
circumstances as emergency or trauma conditio::.~, whether
in the field (at the scene of accidents, etc. ,nor
WO 9212~2~3 Pf.'T/US92/~654
-6-
example) or in emergency medical centers, intensive care
units, surgical operating rooms, hospital trauma centers,
or at bedside, etc. In particular, however, use during
ongoing surgical procedures is clearly anticipated as
satisfying an existing and important medical need,
particularly during such procedures as brain surgery,
. open heart, organ or other transplant surgery, or that
involving major blood vessels, for example, carotid
endarterectomy; or other bypass surgery, etc., where
blood flow is maintained through heart-lung machines and
there is no arterial pulse present at all in the braiw or
body.. a ..:. : ::. ::
The".foregoing major objectives;'advantages and
considerations of the invention, together with and
including others, will become more apparent following
consideration of the ensuing specification, particularly
taken in conjunction with the appended drawings, briefly
described hereinafter. Once again it is pointed out that
the apparatus and methodology principally described
hereinafter constitutes merely a preferred embodiment of
the underlying invention, and does not specifically
address other and further aspects thereof which will or
may become further appreciated by those skilled in the
art after consideration of the overall disclosure herein.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a pictorial schematic representation
simplistically showing the basic application and utiliza-
tion of apparatus in accordance with the invention:
Fig. 2 is a further pictorial schematic representa-
tion somewhat similar to Fig. 1 showing additional
aspects of the subject matter disclosed;
Fig.. 3;'is an end view of a first optical sensor
assembly for use in conjunction with the invention;
Fig. 4 is a pictorial side view representation of a
different form of optical sensor, of a more preferred
nature;
2~~~'~ 1~J
WO 92/2I2~3 PCT/US92/0465~
Fig. 5 is a schematic representation depicting the
regional examination of the head and brain in accordance
with the invention:
Fig. 6 is a graphical representation illustrating
the spectral absorption characteristics of hemoglobin;
Fig. 7 is a graphical representation showing
_ measured cerebral hemoglobin oxygen saturation in
accordance with the invention in a first test subject;
Fig. 8 is a graphical representation showing
measured cerebral hemoglobin oxygen saturation in
accordance with the invention in a second test subject;
Fig. 9 is a graphical representation showing
cerebral vaseular oxygenation activity contrasted with
extracerebral oxygenation~of the scalp and skull, as
measured by the near and far detectors provided in the
sensor assembly utilized by the invention: and
Fig. 10 is a further graphical representation
showing cerebral oximetry measurements in accordance with
the invention.
DESCRIPTION OF PREFERRED EMBODIMENTS
Oxygen is supplied to the brain by hemoglobin
molecules contained-in the blood supply, to which the
oxygen molecules become bonded during the oxygenation
process which occurs in the lungs as the blood is pumped
by the heart through arteries and capillaries to the
brain. As previously stated, the brain extracts oxygen
from the hemoglobin by oxidative metabolism, and
resulting carbon dioxide molecules are carried away
through the capillaries and veins to the lungs for
reoxygenation. Generally speaking, the optical
spectrophotometry utilized by the invention is based upon
the seler~tive attenuation of particular light spectra in
the near infrared range which is exhibited by oxygenated
hemoglobin as compared to reduced (deoxygenated)
hemoglobin contained in the blood present within the
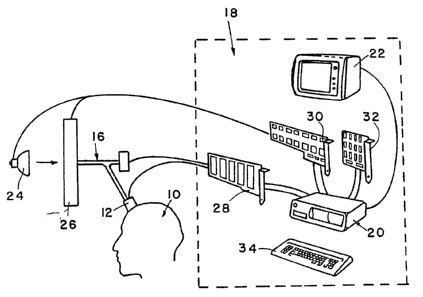
cerebral region under examination. Figs. 1 and 2
pictorially and schematically show the overall or general
application.of the apparatus and methodology of the
CA 02110716 2002-12-12
-8-
invention to the human cerebrum. Thus, Figs. 1 and 2
show a human subject 10 upon whom apparatus in accordance
with the invention is being utilized, such apparatus
comprising a sensor means 12 for applying and receiving
selected light spectra to a particular region 14 of the
brain through or via conductors 16 (which, as
subsequently noted, may be electrical or optical in
nature), from or in conjunction with an infrared
spectrophotometry unit 18 which includes in part a small
digital computer 20 having a monitor 22 on which various
forms of readout information may be presented. As
generally shown in a pictorial and schematic manner by
Fig. 2, the sensor. assembly 12 applies selected light
wavelengths which may emanate from a broadband source 24
(e. g., an incandescent lamp) and be selectively
determined by narrow-bandwidth (monochromatico) filters
26, although as subsequently noted a preferred embodiment
utilizes dedicated light-emitting diodes ("L.E.D.s")
which produce the selected light spectra, and the
computer 20 generally includes an A/D converter section
28, control circuitry 30 (depicted as a circuit board
configured to mount in the expansion slats of computer
20), together with requisite computer memory 32 and an
operator control in the form of a keyboard 34.
The sensor assembly 12 may as a general matter be in
accordance with one embodiment that is shown for example in
Fig. 3. It may be noted that, as shown in Fig. 3, such a sensor
assembly 12' generally comprises a housing or other support 36
which carries a light-emitting element 38, a first light-
detector or receiver 40 (i.e., the " near " receiver) and a
second such detector or receiver 42 (the "far " receiver)
which is disposed a predetermined and particular distance away
from the source 38 and the " near " receiver 40. In the
,ii
CA 02110716 2002-12-12
-g-
sensor assembly 12" is more elongated
in overall shape and preferably has a somewhat flexible
support 136 which carries the light source 138 and the
near and far receivers 140, 142, respectively, all
arranged in a longitudinal array, disposed along a common
linear axis.
It may be noted that in this preferred form the
source 138 comprises a pair of separate (but
commonly-mounted} light-emitted diodes which provide at
least two particularly-selected wavelengths (described in
more detail subsequently herein), and the receivers 140
and 142 comprise photodivdes. As a result, the entire
sensor assembly 12" is relatively small and compact,
lightweight, and thin, as well as being at least modestly
flexible; of course in this form the conductor array 16'
comprises electrical conductors, since the operative
elements are electro-optical emitters and detectors. Of
course, such components operate with very low levels of ,
electrical excitation, and the actual conductors 16' are
each insulated from one another and carried within an
insulating outer sheath 116.
Regardless of the particular form of sensor assembly
12 which is utilized, the inclusion and relative spacing
of the source 38, 138, near receiver 40, 140, and far
receiver 42, 142 are of importance to the proper
function and performance of apparatus. in accordance with
the invention.
In general,
however, the near receiver (40, 140) is close to but
spaced a particular distance from the source (38, 138) so
that the photons (light energy) which it detects in
,: , ,
11 ~ '~ .1 f;
WO 92!21283 PCTIL'S92/046~4
-10-
response to the emission of selected light spectra by the
source will traverse primarily only the skin (scalp) and
bone ( skul l ) of the subj ect 10 , whereas the °' far"
receiver (42, 142) is disposed a particular further
distance from the source whereby the light energy
(photons) which it receives samples a deeper tissue
volume comprising primarily brain tissue. This selected
brain tissue volume which is sampled, as generally
delineated by the curving line designated 114 which
illustratively depicts the mean optical path of the
photons received at the far receiver 42, 142, constitutes
the selected region 14 noted previously (Fig:el), and it
will be~observed that such region constitutes a particu~
lar internal volume within the overalllbrain content
whose location is determined by the relative disposition
and separation of the source 38, 138, near receiver 40,
140, and far receiver 42, 142, together with the relative
placement and location of the sensor assembly 12 upon the
head of the subject 10.
Of course, there are practical limits to the maximum
distance at which the far receiver (40, 140) may be
disposed relative to the source (38, 138), since the
level of light energy used must be less than that which
would be harmful, while at the same time there must be
more than merely trivial amounts of light energy received
at the far receiver, in order to obtain meaningful data
from the spectral modulation or attenuation of the light
by the substance transmissed. As presently envisioned,
it is probably not effective or useful to dispose the far
receiver directly opposite (across the entire skull
width) from the source, by which the complete width or
diameter o-f,'the brain is transmissed, and it will be
noted that in the configurations discussed above and
depicted in the drawings, both the near and far receivers
operate more in a "reflectance" mode than a
"transmission" mode as those terms are conventionally
used (i.e., they are disposed along mean optical paths
'which are curved, and_are relatively close to the
i
CA 02110716 2002-12-12
-11-
source). Of course, as already indicated, this is
directly consistent with monitoring regional brain
function, which represents the preferred embodiment of
the invention. By way of example, in a particular such
preferred embodiment the distance between the source and
near receiver is approximately 0.3 inches, while the
distance between the source and far receiver is approxi-
mately 1,0 inches.
Generally speaking, some of the basic principles
underlying the invention may be appreciated by reference
to Fig. 6, which shows the known absorbtivity of
hemoglobin to selected N.I.R. light wavelengths. As
there illustrated, the spectral absorption characteris-
tics of oxygenated hemoglobin describe a family of curves
which intersect, and reverse, at a wavelength of approxi-
mately 800 nanometers ("nm"), which constitutes the
isobestic point (typically considered to be at 815 nm).
As illustrated, the absorbtivity of reduced
(deoxygenated) hemoglobin rises progressively at lower
wavelengths as a function of the relative absence of
oxygen, the highest such curve thus rep resenting fully
deoxygenated hemoglobin and the lowermost such curve
representing fully oxygen-saturated hemoglobin. As
shown, these curves describe a peak in the general range
of about 760 nm, as well as a valley or dip at approxi-
mately 730-740 nm. Accordingly, as is already known, by
monitoring the optical response at selected wavelengths,
i.e., by comparing intensity of light received at
wavelengths less than the isobestic point with that
received at the latter, and making appropriate computa-
tions, the oxygen content of sampled hemoglobin may be
determined. In accordance with the invention, such
sampling is preferably carried out at wavelengths
representing points of most gradual change, rather than
WO 32!21283 ~ ''~ ~ ~ PCTlUS92l04654
-12-
points representing steepest slopes: accordingly, a first
sampling wavelength may be in the range of about 735 nm,
and another may be at approximately 760 nm. Since the
specific point at which isobestic conditions exist may
vary somewhat as a result of a number of factors, the
reference wavelength is preferably selected to be at
approximately 805 nm.
In view of the foregoing, it will be appreciated
that the primary focus of this description of preferred
embodiments is based upon N.I.R. spectrophotometric
procedures directed toward measurement: of oxyhemoglobin
and deoxyhemoglobin, in order to provide a cerebral .:
oximeter as..noted,:above, i.e., an apparatus for providing
quantified information as to regional oxygen saturation
in the composite vasculature of the brain, and the
following further description sets forth mathematical
descriptions and characterizations of the underlying
rationale and procedure for such a device. It should be
expressly noted, however, that the underlying invention
is not necessarily limited to this specific application,
and indeed is believed to have direct or meaningful
application to other in vivo procedures which are or may
be primarily attributed to or defined in meaningful part
by other well-characterized chromophores, particularly
(but not necessarily) in other somewhat analogous
regional areas or domains, where information relative to
biological processes in such a reasonably defined and
distinctive area is important, and it is necessary or
useful that such information be free of distortions
attributable to hemoglobin or other attributes character-
izing the satin, bone, and dura which is superficial to
the more deeply-located region to be investigated.
With further and continuing reference to the
particular preferred embodiment under discussion, it will
be appreciated that the methodology of the invention
utilizes diffused near-infrared spectroscopic procedures
of a generally transmission-mode character for quantita-
tive evaluation of tissue which is highly scattering and
i~~ 92/21283 ' ~ ~ 6 PCT/US92/0~651t
-13-
partially absorptive in nature, utilizing spatial
resolution for region definition. Since wavelength-
specific attenuation of light propagated through such
tissue is a function of the chromophores, their
extinction coefficients, their concentrations, and the
distance photons travel in the tissue, the basic
relationship may be analogized too, and expressed in
accordance with, the Beer-Lambert relationship as set
forth below, even though this is in fact deemed specifi-
cally descriptive of homogeneous non-scattering media:
Itw> '~ It~~e
In the foregoing expression, the quantity I(w)
represents intensity of transmitted light at wavelength
w, the term I(w)0 represents the intensity of the inci-
dent light at wavelength w, the term a represents the
molar extinction coefficient of the light-absorbing
mol~cul~ (chromophore), the term C represents the content
of such chromophore in the tissue under examination, and
the term s represents the photon pathlength in the tissue
of interest. By use of this relationship, a fundamental
approximation is obtained for interpreting the N.I.R.
spectra utilized; since there are at Least three signifi-
cant chromophores present in brain tissue, each with
separate extinction coefficients and concentrations, the
above-noted relationship may be modified and expressed as
follows:
N
~~~ yw~iwb ~ ~ aiw, ~l~ys
1
The measurements made at the selected examination
wavelengths may be usefully referenced by subtracting
them from referenced measurements made at second selected
wavelength i.e., the isobestic point of hemoglobin noted
above in connection with Fig. 6. Since the above
relationship refers to absorption at wavelength w,
absorption at a s~cond wavelength w' is subtracted from
WO 92/2123 ~ ~ ~ ~ l ~ ~j PCT/U~~2/04g54
°14°
that at the first wavelength, w, yielding the following
expressions
~ ~g~rzw~~r ~ ~c~~. 3'~ ~ ~~'. jl~'(i)~
~1
The foregoing expression may be simplified by use of
arbitrary definitions; 1.e., everything directly measured
may be defined by the variable M. Since the difference
in extinction coefficient is also a known, it may be
defined by the tens d. Accordingly:
c,~> ~ ° c~;~c~~ + 1~ I~~t~cW~~
c~. ;~ ' ~c.~, ;) ~ ~c~~. ;~
Thus, the expression describing absorption at a
second wavelength w' subtracted from that at a first
wavelength w may be reduced to the following simpler
notation:
~ ~ dc.., ,7Cci~s
s
Consideration of the simplified relationship dust
express~d reveals that the variable of interest,
~chromophore concentration, may be quantified for
oxyh~raoglobin and deoxyhemoglobin if such expression is
solved by making (N+1) measurements of M to solve for
C(p)s (oxyhemogolbin) and C(p)s (deoxyhemogolbin)
independently. These values are proportional to
chromophore content. The value s is a constant, and by
i~~ 92/212$3 2 ~ ~ PCT/1J~92/04fi54
-15-
calculating the ratio of deoxy- to oxy- hemoglobin, this
constant cancels out of the expression. If this is
assumed to be constant, the number of unknowns does not
increase subsequent measurements, and this assumption
appears to be well-supported. Thus:
C~.r~~~ _ ~c~.~Cc~ ~ I-i~
In the foregoing expression, the variable fir
represents the hemoglobin ratio of deoxy- to
oxyhemoglobin, which raay then be used to solve for the
r~gional saturati~n of hemoglobin designated rSHgbo2
below:
1!( 1 + ) _ ~( ~. s) ~ r~I~ ' B
It will therefore be se~n that the term "rSFigboa",
defined as "regional saturation of hemoglobin",
constitutes the ratio of oxygenated hemoglobin to total
hemoglobin in the sampled field (defined region) of the
brain under investigation. As previously stated, this
region will contain both arterial and venous blood, as
well as a saaall capillary content, but the venous blood
will heavily outweigh the arterial blood because the
great majority (on the order of 70-80 percent) of the
cerebral blood is in the venous compartment.
. It will be appreciated that the foregoing
..
relationship may be usefully implemented in computer
software by appropriate algorithm, particularly in view
of the comments and discussion set forth previously
herein in conjunction with Figs. 1-6 inclusive. In this
regard, hov~e~vsr, it is to be emphasized once again that
the invention is preferably implemented by way of the
preferred embodiments noted and the accompanying commen-
tart'; in particular, the transmitted light of wavelengths
w, w', etc. is preferably sequentially applied in short
bursts (pulses) by use of a suitable number of repeti-
tions which alternate application of the selected
d'V~ 92.'2123 ~ ~ ~ ~ ~ ~ PCT/LJS92/04654
-16-
wavelengths. Detection of resulting light for each such
burst thus occurs at both the near and far locations
essentially simultaneously, and is preferably obtained on
a.time-gated basis corresponding to the occurrence of the
pulsed incident light wavelengths, praviding synchron~us
detection/demodulation techniques. Of course, the
- detected light burst intensities at the selected
wavelengths constitute an analog quantity as detected,
and these are preferably converted to digital form for
subsequent processing. The computer 20 noted in
connection with Figs. 1 and 2 is preferably utilized to
control all time-based functions, as well as for the
processing of digitized data in accordance with the
aforementioned algorithm.
It should be expressly noted that differential
processing (in essence, subtraction) of the near-far
detection measurements is considered to be of the essence
ins order to define the selected internal region which is
to be examined, and in particular to exclude the effects
of the sampled near field from the measurements of the
desired far field, thereby eliminating not only boundary
(initial impingement and peripheral penetration) effects
but also those attributable to transmission through the
skin, bone and dura by the selected examination spectra.
This processing may be carried out incrementally, prior
to each iterative spectrophotometric transmission and
detection sequence, since the digitized data may readily
be stored on an increment-by-increment basis and used for
further processing (or storage) as desired. It is
believed useful, however, to accumulate an average for
each particular type of measurement over a given number
of cycles--(,i.e., bursts of investigative light at a
common wavelength, received at a particular sensor), and
then subtractively process the resulting averages in the
manner just noted above.
It will be appreciated from the foregoing that the
end result thus obtained will provide a quantified value
for regional oxygen saturation of hemoglobin in the brain
iV0 92/2123 FLT/US92/04654
7_
on an essentially instantaneous, real-time basis, which
may be presented in various forms (e. g., as a numeric
display on the computer monitor, updated at selected
intervals or in accordance with other such garameters),
or in a variety of other forms such as graphs, charts,
etc. As an example of such formats, and to further
illustrate the nature and value of information obtainable
in accordance with the invention, reference is made to
Figs. 7-1~, together with the following commentary
pertaixiing thereto.
Fig. 7 presents a graphical-form chart showing
measured regional cerebral hemoglobin saturation with
respect to ti~ae,.obtained by actual clinical measurement
of a human subject undergoing progressive cerebral
hypoxia. As will be readily observed, a rapid shift from
baseline to abnormal values (less than 55 percent) is
clearly indicated, commencing at about the four minute
point, as a result of the progressive hypoxia, as is the
very rapid return to baseline (and in fact slightly
elevated initial level exceeding baseline) following
corrective patient respiration on one-hundred percent
oxygen. Particular reference should be given to the
arrow indicated on the abscissa scale, which indicates
the point in time at which an analog EEG, retrospectively
evaluated by a clinician on a "blind" basis, first
indicated abnormal theta-delta activity. As may readily
be seen from this, the Blear indications of serious
abnormality provided in accordance with the invention
occurred well over a full minute before the earliest such
EEG indication, and of course this occurs through
ongoing, real-tizae quantified measurement in terms of
percent oscygenation, whereas the EEG chart is retrospec-
tively studied.
Fig. 8 comprises a chart somewhat analogous to that
presented in Fig. 7 and described above, but showing a
longer-duration procedure during which the monitored
patient underwent elective hypothermic cardiac
standstill during surgical repair of a giant intracranial
Pt_°T/~,JS92/04654
i~VO 92/2123
-18-
aneurysm. As will readily be noted, a clearly-
perceptible decline from a baseline value in the range of
60-70 percent saturation commences at approximately 30
minutes, and extends to approximately 45 minutes, during
which time the patient was completely off bypass and had
no cerebral blood flow, and thus no oxygen delivery
(under the aforementioned hypothermic conditions).
Following reperfusion at approximately the 45 minute
point, brain oxygen saturation is shown to rapidly return
toward baseline, and may clearly be monitored during the
highly important ensuing period.
Fig. 9 comprises a different form of chart, -'-
presenting '°optical density" (i.e., attenuative effect)
at the reference wavelength over a period of time, in
seconds, as evidenced by the detected light intensity
information received separately at the near and far
detector locations following introduction of a bolus of
infrared tracer material. This chart thus shows transit
of the tracer through the cerebral vasculature: that is,
selective introduction of the tracer in the internal
carotid artery results in initial presence thereof only
in the deep tissue; thus, ipsilateral spectroscopic
measurements made in accordance with the invention show
(bottom trace) relatively immediate detection of the
tracer at the "far" receiver monitoring the deeper brain
tissue, without any attendant indication at the "near"
receiver (upper trace) which monitors superficial tissue,
etc., until substantially later, after the tracer has
recirculated through the heart and entered the external
carotid system, at approximately fifty seconds after the
initial introduction of the tracer. In this regard, it
will be noted that the far receiver also shows recircula-
tion of the bolus at this second point in time, as well
as graphically displaying the declining persistence of
the tracer within the deep tissue over this interval.
Fig. 10 constitutes a further graphical showing
illustrative of the versatility, usefulness and value of
information provided in accordance with the invention, by
WO 92/21283 PCT/US92/04554
-19-
way of a pair of comparative traces showing (lower trace)
continuous regional cerebral oxygen saturation
(characterizing "deep", i.e., brain, tissue) as compared
to that characterising only the superficial tissue, i.e.,
scalp and skull (upper trace), an actual trauma patient
who suffered a serious closed-head injury and was
continuously monitored. As may readily be observed by
noting the change occurring at the vertical line disposed
at a point representing approximately 27.25 hours after
the onset of monitoring, progressive cerebral
desaturation commences notwithstanding the fact that the
superficial blood supply remains fully oxygenated. It is
to be noted that the first clinical manifestation of--
brain desaturation in this patient occurred more than two
hours later, at approximately 29.5 hours.
From the foregoing, the significance and value of
information provided in accordance with the invention is
believed readily apparent, corroborating expectations
based upon appreciation of the fact that cerebral venous
oxygen saturation should constitute an excellent
indicator of the adequacy of cerebral oxygen delivery
and/or cerebral oxygen extraction, and thus of brain
vitality as a general matter. That is, cerebral oxygen
extraction causes rapid changes in cerebral venous oxygen
saturation when cerebral oxygen delivery decreases for
any reason, as for example the presence of systemic
hypoxia, cerebral oligemia, systemic anemia, etc., even
though cerebral oxygen consumption may remain normal. In
this regard, the very advantageous results obtained
through the spatial resolution techniques noted,
providing for specific and independent monitoring of
ingormatic~~~from deep vascular beds or tissue, provides
gor desirable organ-specific or area-specific determina-
tions made well below the skin. Further, although the
specific accuracy and sensitivity of oximetry
measurements in accordance with the invention in
heterog~:neous tissue such as the scalp and adjacent or
near underlying area remain to be seen, and potentially
U
~Y~ 92121283 PCT/US92104fi54
0-
further defined, the usefulness of the resulting informs--
tion is clearly demonstrated by examples such as those
presented in Figs. 9 and 10, as discussed above.
As for specific accuracy of regional oxygen
saturation determinations pursuant to the mathematical
paradigm set forth above, comparative evaluation may
.readily be accomplished for any specific implementation,
and has in fact been done by use of in vitro human blood
which was suitably warmed and artificially oxygenated to
various saturations, and then subjected to comparative
testing with a standard lab cooximeter (using a custom-
ized cuvette with immersible light guides for access by -
apparatus in accordance with the invention). ~y utiliz-
ing linear regression analysis, highly~significant
correlation is shown which supports the underlying
soundness of the mathematical approach discussed above.
Of course, appropriate scale factors may be determined in
this general manner for any desired specific application
of the methodology disclosed herein, and used to
calibrate or correlate the actual output of the
implemented apparatus, for example by conventional
computer data-processing techniques such as embodying the
seals factors in appropriate look-up tables, for example.
It may be noted that such procedures may also provide a
desirable or useful calibration technique in any event.
It should be further pointed out that since the
quantified values of regional hemoglobin oxygen
saturation provided in accordance with the invention
constitute field values, i.e., represent hemoglobin
contained in three separate vascular compartments
(arterial, venous and microcirculatory), these quantified
values represent the weighted average of the three
different vascular compartments. While hydraulic
analysis of the cerebral vascular system, as evidenced by
published information, supports a cerebral blood volume
distribution that is in accordance with that set forth
above, it~may be noted that the specific relative size of
each such blood volume compartment is in fact dynamic in
W~ 92/21283 PCT/US92/04654
-21-
a given patient depending upon the ratio of oxygen supply
to and demand during conditions of physiologic stress,
anatomic location, and in numerous other factors;
consequently, an ideal reference methodology would
simultaneously measure the actual relative blood volume
of these three different compartments, preferably on a
regional basis. Nonetheless, employment of assigned
weighting values in the mathematical paradigm used,
pursuant to the published hydraulic or other analytic
information available, is quite sufficient for purposes
of providing useful clinical instrumentation. Of course,
the presence of extravascular cerebral blood collection,
for example in the subarachnoid, subdural, or
intraparenchymal tissue compartments, could or may
potentially interfere with the strict accuracy of the
quantifications provided, even though relative or trend
data based thereon would seemingly still be of
considerable importance; further, the spatial resolution
capabilities of the inve::~ion may in fact provide a way
to comparatively assess such anomalies, particularly if
they are reasonably well defined. At the same time, the
paradigms set forth above, being primarily designed to
measure and account for extraparenchymal conditions, have
the potential to overcome such problems.
Accordingly, it is believed that a highly useful and
novel methodology is provided by the invention,
particularly, but certainly not exclusively, as applied
in the preferred embodiment discussed herein above, as
well as in other related or analogous applications. It
is to be understood that the foregoing description of a
preferred embodiment of the invention is provided for
purposes.af~description and illustration, and not as a
measure of the invention, whose scope is to be defined
solely by reference to the ensuing claims. Thus, while
those skilled in the art may devise embodiments of the
particular concepts presented in the foregoing illustra-
tive disclosure which differ somewhat from the particular
embodiment shown and described in detail_herein, or may
W~ 92/21283 PCT/US92/04654
-22-
make various changes in structural details to the illus-
trated embodiment, all such alternative or modified
embodiments which utilize the concepts of the invention
and clearly incorporate the spirit thereof are to be
considered as within the scope of the claims appended
herebelow, unless such claims by their language specifi-
cally state otherwise.
h