Note: Descriptions are shown in the official language in which they were submitted.
- 1 -
2~.1~.1C~
PHENYLACETIC ACID DERIVATIVES, PROCESS FOR THE PREPARATION
THEREOF AND CORRESPONDING USE.
D E S C R I P T I O N
This invention relates to phenylacetic acid derivatives,
particularly [2-(2,6-dichloroanilino)phenyl]acetic acid, com-
monly known as diclofenac, a product known for its analgesic
and antiinflammatory properties. The invention also relates
to a process for the preparation of said derivatives and to a
process for the use thereof in the preparation of [2-(2,6-
dichloroanilino)phenyl]acetoxyacetic acid, also known for its
analgesic and antiinflammatory properties.
STATE OF THE ART
Diclofenac (formula I) is a product having analgesic and
antiinflammatory properties, known since the 60's and which
has been extensively used in sodium salt form orally, rectal-
ly and parenterally. In view of some of its adverse side
effects (gastrointestinal problems, mainly) numerous attempts
have been made to prepare diclofenac derivatives which, while
maintaining or improving its analgesic and antiinflammatory
properties, have weaker adverse side effects, or allow other
forms of administration (e. g. topical). One of the diclofenac
derivatives used in therapeutics is (2-(2,6-dichloroanilino)-
phenyl]acetoxyacetic acid (formula II), described for the
first time in 1984 (EP-P-0 119 932). Nevertheless, the design
and preparation of agents having good analgesic and antiin
flammatory properties, with little or no side effects and
good ways of administration, is still a problem of interest
in modern therapeutics.
~CH2-COONa CH2-COO-CH2-COON
NH ~ NH
CI. CI C1 C1
~I) CII)
.,
' - 2 -
The preparation of [2-(2,6-dichloroanilino)phenyl]acet-
oxyacetic acid of formula II suffers from the chemical draw-
back of obtaining a free carboxylic group in the presence of
a carboxylic acid ester which has not to be modified. Two
solutions have been proposed to solve this problem: (a) carry
out hydrogenolysis of the formula II benzyl ester (EP-P-0 119
932), and (b) treat certain esters of the. formula II ester
with iodine trimethylsilane, prepared in situ from chloro-
methylsilane and anhydrous sodium iodide, in an inert at-
mosphere, using acetonitrile as solvent (ES-P-2 020 146).
Such esters are prepared, in turn, from diclofenac by reac-
tion in a heterogenous medium and phase transfer conditions
(ES-P-2 020 145).
With regard to process (a), as mentioned in ES-P-2 020
146, the main drawback of the hydrogenolysis process lies in
the need to have special equipment to handle the pressurized
gases and the concomitant risk of the industrial use of
hydrogen and catalysts. Furthermore, the viability of the
method is restricted to the use of the formula II esters
susceptible of hydrogenolysis and, more precisely, the benzyl
ester.
Process (b) apparently represents a solution to the
problems inherent in process (a), although chlorotrimethyl-
silane and sodium iodide, both relatively expensive, are used
as reactants and acetonitrile, highly toxic, as solvent.
Also, by-products or mixtures of solvents (acetonitrile/-
water) are generated, with disposal problems.
It will be gathered from the foregoing that the prepara
tion on an industrial scale of [2-(2,6-dichloroanilino)phen
yl]acetoxyacetic acid is still a serious technical problem.
DESCRIPTION OF THE INVENTION
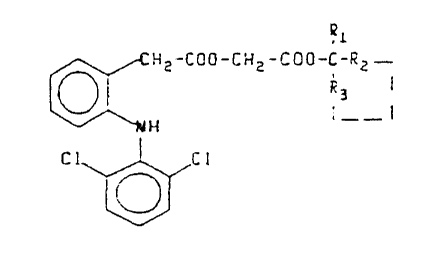
The object of the present invention are the tertiary
alkyl esters of formula III and their pharmaceutically accep-
table solvates, where R~, Rz and R3 are the same or different
and are either methyl, ethyl or propyl radicals; or Rz and
Rs, which are attached as shown by -(R2....Rs)-, jointly form
a -(CHZ)n- radical where n = 4 or 5. Excepted is the case
where R~ - RZ -~R3 , = CHs . Particularly preferred are the
product wherein R~ is methyl and R2 and R3 are both ethyl and
- 3 -
- the product wherein R~ is methyl and Rz and R3 are jointly a
-(CHz)s- radical. Also an object of this patent are the above
products for use in therapy, particularly for use as anal-
gesic or antiinflammatory agents.
Ri
CH2-C00-CH2-C00-C-R2 -
R3 I
NH I -- I
C1 Cl
(III>
Also an object of the present invention are the phar-
maceutical compositions comprising a therapeutically effec-
tive amount of one of the above products and adequate amounts
of pharmaceutically acceptable excipients, as well as the use
of said products for the manufacture of analgesic or
antiinflammatory drugs.
It is also an object of the present invention to provide
a process for the preparation of a tertiary alkyl ester of
formula III or a pharmaceutically acceptable solvate thereof,
where R~ , Rz and R3 are the same or different and are either
methyl, ethyl or propyl radicals; or RZ and R3, which are
attached as shown by -(Rz . . . .R3 )-, jointly form a -(CHz )n
radical v~here n = 4 or 5; comprising the reaction of a diclo-
fenac sodium or potassium salt with the corresponding alkyl
haloacetate of formula X-CHZ-COO-CR~RzR3, where X is chlorine
or bromine; the process being characterized in that the reac-
tion is carried out in an homogenous medium, in the presence
of a aprotic solvent in which the starting compounds are
soluble.
In a preferred embodiment of the above process, the
sodium salt of diclofenac of formula I is reacted with a
slight excess of the corresponding tertiary alkyl haloacetate
(e. g. chloroacetates of tert.-butyl, 1-ethyl-1-methylpropyl
or 1-methylcyclohexyl), in the presence of a high polarity j
aprotic solvent (e.g. dimethylformamide). The reaction is
carried out preferably at a temperature of 50 to 70'C. At the
end of the reaction, it is allowed to cool to room tempera-
- - 4 -
ture and an excess of water is added, whereby the major por-
tion of the desired product precipitates out and is there-
after separated by filtration. After purification by recrys-
tallization in aliphatic hydrocarbon type solvents (e. g.
petroleum ethers), very pure products with a defined melting
point are obtained.
Further to their intrinsic therapeutic utility, the
formula III tertiary alkyl esters are also useful as syn-
thesis intermediates for the preparation of [2-(2,6-dichloro-
anilino)phenyl]acetoxyacetic acid of formula II, commercially
used as analgesic and antiinflammatory agent. Thus, also an
object of this invention is a process for the use of the
formula III derivatives for the preparation of the formula IT_
acid from a formula III tertiary alkyl ester or a solvate
thereof, where R~, Rz and R3 are methyl, ethyl or propyl
radicals; or R2 and R3, which are attached as shown by-
(R2 . . . .R3 )-, jointly form a -(CHZ )r,- radical where n = 4 or
5; this process of use being characterized in that the for-
mula III tertiary alkyl ester is treated with an acid or
mixture of acids in a solvent. In preferred embodiments of
this process, the reaction is carried out in an anhydrous
medium, the acids used are trifluoroacetic, formic, acetic,
sulphuric, hydrochloric, methane sulphonic, p-toluene sul-
phonic or sulphonic acids bonded to perfluorinated resins and
the solvent is the acid or mixture of acids itself or is of
the hydrocarbon type (e. g. toluene), halogenated hydrocarbon
(e. g. chloroform or methylene chloride), ketone (e. g, methyl-
ethylketone, or methylisobutylketone) or ether (methyltert.-
butylether or dioxane). Particularly preferred is the case
in which the starting product is R~ - RZ - R3 - CHs.
Under the preferred reaction conditions, the formula III
tertiary alkyl ester is reacted at a temperature ranging from
-10°C to the reflux temperature of the solvent; small amounts
up to large excesses of acid (e. g. using the acid or mixture
of acids itself as solvent) are used. A preferred process for
the isolation of the product consists of adding an excess of
water at room temperature to dilute the acid used, after hav-
ing concentrated, the solvent used, when necessary, to separ-
ate the end product by filtration and washing with water or
... 5
an appropriate solvent. If the reaction is carried out with
an acid bonded to a resin, in the first place said resin is
removed by filtration under solubility conditions of the
reaction product. The last stage of the process consists of
purifying the product by recrystallization in an appropriate
solvent (e. g. a hydrocarbon or an ester).
Unlike the process for the preparation of [2-(2,6-di-
chloroanilino)phenyl]acetoxyacetic acid described in ES-P-2
020 146, which uses alkyl (straight or branched chain) or
arylalkyl esters as starting products, the process of the
invention is specific for tertiary alkyl esters. Thus, unlike
the methyl (-COOCHa), primary alkyl (-COOCH2R), secondary
alkyl (-COOCHRZ), monoarylalkyl (e. g. -COOCHZAr) and other
possible esters, the formula III tertiary alkyl esters in-
volved in the process of the invention undergo, in a highly
specific way, an elimination reaction by treatment with acid,
preferably in an anhydrous medium. In the particular case of
the process for the preparation of [2-(2,6-dichloroanilino)-
phenyl]acetoxyacetic acid (II), this high specificity is
extremely useful, in view of the presence in the molecule of
(II) of a primary alkyl ester group which does not have to
be modified. Thus, the process of the present invention
allows the formula II acid to be prepared with very high
yields (over 909K in some cases), with an extraordinary puri-
ty. Furthermore, the reactants used are common, low-cost
acids and the by-products of the elimination are alkenes, non
toxic and easily removable (volatile). Other notable advan-
tages afforded by this process are its easy conversion to
industrial scale production and the gentleness and safety of
its reaction conditions, which are neither dangerous nor
involve complex technology.
EXAMPLES
Example 1: Preparation of tert. butyl [2-(2.6-dichloroanili-
no)phenYl7acetoxy acetate (Formula III where R~ - Rz - R3 -
CH3 )
10 grams of sodium diclofenac were dissolved in 70 ml of
dimethylformamide (DMF) under a nitrogen atmosphere and the
temperature was~rai,sed to between 50 to 70°C. Then 5.6 g of
tert. butyl chloroacetate were added and the reaction was
.. . , .. ,.
- - 6 -
_" 21~~.~~9
- continued at the same temperature for 2 hours. The mixture
was allowed to cool down to room temperature and an excess of
water was added. The product obtained was filtered and dried,
to give tert. butyl [2-(2,6-dichloroanilino)phenyl]acetoxy
acetate, with an 89% yield. Melting point: 89.5-91.5'C (re-
crystallized from petroleum ether).
Example 2: Preparation of 1-ethyl-1-methvloropvl t2-(2.6-
dichloroanilino)phenvl7acetoxv acetate (Formula III where R~
- CHa and Rz - R3 - CHZCHa)
Operating in a similar way to Example 1, but replacing the
tert. butyl chloroacetate with 1-ethyl-1-methylpropyl chloro-
acetate (previously prepared according to the synthesis
method described in Org. Synth. Coll. Vol. III, p. 142-4),
the title product was obtained with m.p. 78.5-80°C.
Example 3: Preparation of 1-methvlcyclohexyl f2-(2,6-di-
chloroanilino)phenvl7acetoxy acetate (Formula III where R~ -
CHa and -(Rz ,R3 )- - -(CH2 )s-)
Operating in a similar way to Example 1, but replacing the
tert. butyl chloroacetate with 1-methylcyclohexyl chloroacet-
ate (previously prepared according to the synthesis method
described in Ors. Synth. Coll. Vol. ITI, p. 142-4), the title
product was obtained with m.p. 60-62°C.
Example 4: Preparation of L2-(2,6-dichloroanilino)phenvl]-
acetoxyacetic acid (II)
10 g of tert. butyl [2-(2,6-dichloroanilino)phenyl]acetoxy
acetate were stirred under nitrogen in 37 g of trifluoro-
acetic acid at room temperature. After one hour's stirring,
the mixture was concentrated by evaporation at reduced pres-
sure and the residue was dispersed in water. After filtra-
tion, washing and drying at 40°C, the title product was ob-
tained with a 76% yield. Melting point 149.5-151'C (recrys-
tallized from toluene).
In a similar test, treating 10 of tart. butyl [2-(2,6-dichlo-
. . . . ~ , ..
J-
roanilino)phenyl]acetoxy acetate with 34 g of anhydrous for-
mic acid at 50'C for 2 hours, the title product was obtained
with a 91% yield.
. ,
,~.