Note: Descriptions are shown in the official language in which they were submitted.
HOECHST ARTIENGESELLSCBAF'T HOE 92/F 405 R Dr. VF/do
Description
Substituted beazoylguanidines, process for their prepara-
tion, their use as pharmaceutical or diagnostic, and
pharmaceutical containing them
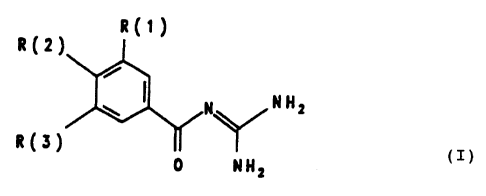
The invention relates to beazoylguanidines of the formula
I
R{t)
R(2)
N N H=
R(3)
0 NHZ
in which
R(1) = hydrogen, F, C1, Br, I, -N02, -CAN, R(16)-CPH2p-Oq wherein p is
zero or 1, q is zero, 1, 2 or 3, R(16) is C~F2~+~ and r is 1,
2 or 3, R(4)-S0~ or R(5)R(6)N-SOZ-, where
m is zero, 1 or 2,
R(4) and R(5) are (Cl-Cs) -alkyl, (C3-C6) -alkenyl,
-CaHsa-R ( 7 ) i CF3.
n is zero, 1, 2, 3 or 4,
R(7) is (C,-C,)-cycloalkyl, phenyl which is
unsubstituted or substituted by 1-3 substituents
selected from the group comprising F, Cl, CF3,
methyl , me thoxy or NR ( 8 ) R ( 9 ) , where R ( 8 ) and R ( 9 )
are H or Cl-C,-alkyl, sad R(5) can also mean H,
R(6) is H or (C1-C,)-alkyl, where R(5) sad R(6)
together can be 4 or 5 methylene groups, of which
one C8s group can be replaced by oxygen, S, NH,
N-CH3 or N-benzyl,
R(2) _ (Cl-C9)-heteroaryl which is linked via C or N and
which is unsubstituted or substituted by 1-3
substitueats selected from the group comprising
F, Cl, CF,, CH3, methoxy, hydroxy, amino, methyl-
amino or dimethylamino: or
-SR(10), -OR(10), -NR(10)R(11), -CR(10)R(11)R(12);
_ 2 - ~1~.~~~6
R(10) _ -C,H,a- (Cl-C9) -heteroaryl which is unsbustituted or
substituted by 1-3 substitueats selected from the
group comprising F, Cl, CF3, C83, methoxy,
hydroxyl, amino, methylamiao or dimethylamino,
a is zero, 1 or 2,
R(11) and R(12) independently of one another have
the definition given for R(10) or are hydrogen or
(Cl-C,) -alkyl,
R (3) has the definition given for P (1) or is (C,-C6) -
alkyl or -X-R(13) is which
X is oxygen, S, NR(14), where R(14) is H or
(Cl-C3) -alkyl,
R (13 ) is H, (Cl-C6) -alkyl, (Cs-C,) -cycloalkyl,
-CbH,b-R (15) where b is zero. 1, 2, 3 or 4, it
also being possible for R(13) and R(14) together
to be 4 or 5 methylene groups and for a CHs group
to be replaced by oxygen, S, NH, N-CH, or N-
beazyl, and
R(15) is phenyl which is uasubstituted or substi
tuted by 1-3 substituents selected from the group
comprising F, Cl, CF,, methyl, methoxy or
NR(8)R(9) , where R(8) and R(9) are H or (C1-C,)
alkyl,
with the exception of compounds I in which are simultaneously R(1), R(4)-SOm
or R(S)NSOZ, and R(2) (C~-C9)-heteroaryl, and the pharmaceutically acceptable
salts thereof.
2 5 Preferred compounds of the formula I are those in which:
R(1) = hydrogen, F, C1, -CAN, R(16)-CpH2p-Oq wherein p is zero or 1, q is
zero, 1, 2 or 3, R(16) is C~F2~~~ and r is 1, 2 or 3, R(4)-S0~ or
R(5)R(6)N-SOz-, where m is zero, 1 or 2,
R (4) and R (5) are (Cl-C,) -alkyl, (C3-C,) -alkenyl,
-CaHsn-R(7) Or -CF3,
n is zero or l,
R(7) is (C3-C6) -cycloalkyl, phenyl which is un
substituted or substituted by 1-3 substituents
selected from the group comprising F, Cl. CF3,
methyl , methoxy or NR ( B ) R ( 9 ) , where R ( 8 ) sad R ( 9 )
are 8 or methyl,
- 3 -
R(5) also having the meaning of H, and
R(6) is H or methyl,
R(3) - hydrogen, methyl, cyano, -CF,, F or C1,
and the remaining radicals are as defined above, with the exception of
compounds I in which are simultaneously R(1), R(4)-S0~ or R(5)NSO2, and R(2)
(C~-C9)-heteroaryl, and the pharmaceutically acceptable salts thereof.
Particularly preferred compounds I are those it which:
R(1) - hydrogen, F, Cl, -CAN, -CF3, R(4)-SOm or
R(5)R(6)N-SO,, where m is zero, 1 o r 2, R(4) is
methyl or CF3, or R(5) and R(6) independently of
one another are H or methyl
R (2) - (C1-C9) -heteroaryl which is linked via C or N and
which is unsubstituted or substituted by a radi-
cal selected from the series comprising F, Cl,
CF3, CH3, methoxy or dimethylamino; or
-SR(10) , -OR(10) , -NR(10)R(11) ,
-CR(10)R(11)R(12);
R (10) is -C,Hsa- (Cl-C9) -heteroaryl, which is unsub-
stituted or substituted by a radical selected
from the series comprising F. Cl, CF3, CH3, meth-
oxy or dimethylamino;
a is zero, 1 or 2, and
R(11) and R(12) independently of one another are
hydrogen or methyl
R(3) is methyl, cyano, trifluoromethyl. F, Cl or
hydrogen,
with the exception of compounds I in which are simultaneously R(1), R(4)-SOm
or R(5)NS02, and R(2) (C~-C9)-heteroaryl, and the pharmaceutically acceptable
salts thereof.
(Cl-C9)-Heteroaryl is to be understood ae meaning, in
particular, radicals which are derived from phenyl or
naphthyl and in which one or more CH groups are replaced
by N and/or in which at least two adjacent CH groups
(while forming a five-membered aromatic ring) are
,.,.
- 4 -
replaced by S, NH or O. Moreover, it is also possible for
one or both atoms of the condensation site of bicyclic
radicals (as in indolizinyl) to be N atoms.
Heteroaryl means. in particular, furanyl, thienyl,
pyrrolyl, imidazolyl, pyrazolyl, triazolyl, tetrazolyl,
oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, pyridyl,
pyrazinyl, pyrimidinyl, pyridazinyl, indolyl, indazolyl,
quinolyl, isoquinolyl, phthalazinyl, quinoxalinyl,
quinazolinyl or cinnolinyl.
If one of the substituents R(1) to R(22) contains one or
more asymmetric centers, they can have either the S or
the R configuration. The compounds can exist as optical
isomers, diastereomers, racemates or mixtures of these.
The alkyl radicals which have been mentioned can be
straight-chain or branched.
The invention furthermore relates to a process for the
preparation of the compound I, which comprises reacting
compounds of the formula II
R(1)
R(2)
(II)
R(3)
0
with guanidine, R(1) to R(3) having the abovementioned
meaning and L being a leaving group which can readily be
substituted by a nucleophile.
The activated acid derivatives of the formula II, in
which L is an alkoxy group, preferably a methoxy group,
a phenoxy group, a phenylthio, methylthio or 2-
pyridylthio group, or a nitrogen heterocycle, preferably
1-imidazolyl, are obtained advantageously in a manner
known per se from the carbonyl chlorides (formula II,
- 5 -
L = C1) on which they are based and which, in tura, can
be prepared in a manner known per se from the carboxylic
acids (formula II, L = OH) on Which they are based, for
example thionyl chloride.
In addition to the carbonyl chlorides of the formula II
(L = Cl), other activated acid derivatives of the formula
II can also be prepared, in a manner known per se,
directly from the benzoic acid derivatives (formula II,
L = OH) on which they are based, for example the methyl
esters of the formula II where L = OCH3 by treatment with
gaseous HC1 is methanol, the imidazolides of the formula
II by treatment with carbonyl diimidazole [L - imidaz-
olyl, Staab, Angew. Chem. Int. Ed. Engl. 1, 351-367
(1962)], the mixed anhydrides II with Cl-COOCsHs or tosyl
chloride in the presence of triethylamine in an inert
solvent, and benzoic acids can be activated with dicyclo-
hexylcarbodiimide (DCC) or with O-((cyano(ethoxycar-
bonyl)methylene)amino]-1,1,3,3-tetramethyluronium tetra-
fluoroborate ("TOTU") [Proceedings of the 21 European Peptide Symposium,
2 0 peptides 1990, Editors E. Giralt and D. Andreu, Escome, Leiden, 1991].
A series of suitable methods for the preparation of activated carboxylic
acid derivatives of the formula II can be found in J. March, Advanced
Organic Chemistry, Third Edition (John Wiley & Sons, 1985), p. 350, where
the references are cited.
An activated carboxylic acid derivative of the formula I
is reacted with guanidine in a manner known per se is a
protic or aprotic polar, but inert, organic solveat.
Methanol, ieopropanol or THF have proven themselves in
the reaction of the methyl benzoates (II, L = OMe) with
guanidine, the temperatures being 20°C to the boiling
point of these solveats. In most reactions of compounds
II with salt-free guanidine, the process was advantage-
ously carried out in aprotic inert solvents, such as THF,
dimethoxyethane or dioxane. Even so, water together with
a base such as NaOH, can be used as solvent in the
reaction of II and III.
~,.
- 6 -
If L = Cl, the process is carried out advantageously with
an addition of an acid scavenger, for example in the form
of excess guanidine, to bind the hydrohalic acid.
Some of the basic benzoic acid derivatives of the formula
II are known and described in the literature. The unknown
compounds of the formula II can be prepared by methods
known from the literature, for example by converting
4-(or 5-)halo-3-chlorosulfonylbenzoic acids with aamnonia
or amines into 3-aminosulfonyl-4-(or -5-)halobenzoic
acids, or by converting 4-(or 5-)halo-3-chlorosulfonyl-
benzoic acids with a mild reducing agent, such as sodium
bisulfate and subsequently alkylating the product to give
3-alkylsulfonyl-4-(or -5-)halobenzoic acids, and reacting
the benzoic acids obtained by one of the above-described
process variants to give compounds I according to the
invention.
Some subs tituents can successfully be introduced into the
4- and 5-position by methods which are known from the
literature, namely palladium-mediated cross coupling of
aryl halides with, for example, organostannanes, organo-
boric acids or organoboranes, or organocopper or organo-
zinc compounds.
In general, benzoylguanidines are weak bases and can bind
acid with the formation of salts. Suitable acid addition
salts are salts of all pharmacologically acceptable
acids, for example halides, in particular hydrochlorides,
lactates. sulfates, citrates, tartrates, acetates,
phosphates, methylsulfonates and p-toluenesulfonates.
The compounds I are substituted acylguanidines . The best-
known representative of the acylguanidines is the pyraz-
ine derivative amiloride, which is used in therapy as a
potassium-retaining diuretic. A large number of other
amiloride-type compounds are described in the literature,
for example diemthylamiloride or ethylisopropylamiloride.
CA 02111386 2003-12-11
- 7 -
0 NH
C I II II
~ iN~ ~C~ ~C~
R ~ C C N NHZ
H
~C~N~C~
N N H2
R "
Amiloride: R', R" = 8
Dimethylamiloride: R', R" = CH3
Ethylisopropylamiloride: R' = Cs85, R" = CH(C83)s
Moreover, tests are known which suggest that amiloride
has antiarrhythmic properties (Circulation 79, 1257-63
(1989). However, its broad use as as antiarrhythmic is
restricted by the fact that this effect is only weakly
pronounced and accompanied by a hypoteasive and saluretic
activity, and these side effects are undesired is the
treatment of cardiac arrhythmias.
Experiments oa isolated animal hearts have also suggested
that amiloride has aatiarrhythmic properties (Eur. Heart
J. 9 (suppl. 1) : 167 (1988) (book of abstracts) . For
example, it has bees found oa rats' hearts that arti-
ficially induced ventricular fibrillation can be
suppressed completely by amiloride. Ia this model, the
abovementioned amiloride derivative ethylisopropyl-
amiloride was even more potent than amiloride itself.
US Patent 5,091,394 (80E 89/F 288) describes benzoyl-
guaaidiaes which have a hydrogen atom in the position
which corresponds to the radical R(1). Canadian patent
application number CA 2,089,439 proposes 3,5-substituted
benzoylguanidines in which, however, the substituents
R(2) and R(3) do not have the meanings claimed in the
present invention.
~~~.~~~~6
g
US Patent 3,780,027 claims acylguanidines whose structure
is similar to those of the compounds I and which are
derived from commercially available loop diuretics, such
as bumetanide. Accordingly, a powerful ealuretic activity
is reported of these compounds.
It was therefore surprising that the compounds according
to the invention have no undesired, disadvantageous
saluretic properties, but have a very good activity
against arrhythmias as they occur, for example, in
connection with oxygen deficiencies. Due to their pharma-
cological properties, the compounds are highly suitable
for use as antiarrhythmics with a cardioprotective
component for the prophylaxis and treatment of cardiac
infarctions and for the treatment of angina pectoris, in
which context they also preventively inhibit, or reduce
greatly, the pathophysiological processes in the
formation of ischemia-induced damage, in particular when
ischeania-induced cardiac arrhythmias are triggered. Due
to inhibition of the cellular Na'/H+ exchange mechanism,
the compounds of the formula I according to the
invention, which have a protective activity against
pathological hypoxic and ischemic situations, can be used
as pharmaceuticals for the treatment of all acute or
chronic damage triggered by ischemia or for the treatment
of directly or collaterally induced diseases. This
applies to their use as pharmaceuticals for surgical
intervention, for example in connection with organ
transplants, where the compounds can be used for the
protection of the organs in the donor before and during
their removal, for the protection of removed organs, for
example in their treatment with, or storage in,
physiological baths, and for the transfer into the
recipient organism. Equally, the compounds are valuable
protective pharmaceuticals when angioplastic curative
interventions are carried out, for example on the heart
or on peripheral blood vessels. Due to their protective
activity against ischemia-induced damage, the compounds
are also suitable as pharmaceuticals for the treatanent of
ischemias of the nervous system, in particular, the
central nervous system, where they are suitable, for
example, for the treatment of apoplexes and brain edemas .
Moreover, the compounds of the formula I according to the
invention are also suitable for the treatment of forms of
shock, for example allergic, cardiogenic, hypovolemic and
bacterial shock.
Moreover, the compounds of the foraaula I according to the
invention are distinguished by powerful inhibitory action
on cell proliferations, for example fibroblast prolifera-
tion and proliferation of the smooth vascular muscle
cells. This is why the compounds of the formula I are
suitable as valuable therapeutic agents for diseases in
which cell proliferation is a primary or secondary cause,
and they can therefore be used as antiatherosclerotics,
agents against diabetic late complications, cancers,
fibrotic disorders, such as pulmonary fibrosis, hepatic
fibrosis or renal fibrosis. organ hypertrophies and
hyperplasias, in particular in prostatic hyperplasia or
prostatic hypertrophia.
The compounds according to the invention are valuable
inhibitors of the cellular sodium proton antiporter
(Na'/H' exchanger) . Which is elevated in a large number of
diseases (essential hypertension, atherosclerosis,
diabetes and the like) even in those cells which are
readily accessible to measurements, such as in
erythrocytes, thrombocytes or leukocytes. The compounds
according to the invention are therefore suitable as
outstanding and simple scientific tools. for example in
their use as diagnostics for determining, and dis-
tinguishing between, certain forms of hypertension, but
also of atherosclerosis, diabetes, proliferative dis-
orders and the like. Moreover, the compounds of the
formula I are suitable for preventive therapy for pre-
venting the genesis of hypertension, such as of essential
hypertension.
to -
In contrast to the known compounds, the solubility in
water of the compounds according to the invention is
significantly improved. They are therefore much better
suited to intravenous administration.
Pharmaceuticals which contain a compound I can be admin-
istered orally, parenterally, intravenously, rectally or
by inhaling, the preferred way of administration depend-
ing on the particular symptom of the disease. The com-
pounds I can be used by themselves or together with
galenic auxiliaries, and they can be employed both in
veterinary medicine and human medicine.
A person skilled in the art knows, on the basis of his
expert knowledge, which auxiliaries are suitable for the
desired pharmaceutical formulation. Auxiliaries which can
be used in addition to solvents, gel formers, bases for
suppositories, tableting auxiliaries, and other excipi-
ents for active substances are, for example, antioxi-
dants, dispersants, emulsifiers, antifoams, flavor
improvers, preservatives, solubilizers or colorants.
For an oral dosage form, the active compounds together
with the suitable additives, such as carriers, stabil-
izers or inert diluents, are mixed and foranulated by
customary methods to give suitable dosage forms, such as
tablets, sugar-coated tablets, hard gelatin capsules, or
aqueous, alcoholic or oily solutions. Inert excipients
which can be used are, for example, gum arabic, magnesia,
magnesium carbonate, potassium phosphate, lactose,
glucose or starch, in particular maize starch. Dry
granules or moist granules can be used for the prepara-
tion. Examples of oily excipients or examples of solvents
are vegetable or animal oils, such as sunflower oil or
cod liver oil.
For subcutaneous or intravenous administration, the
active compounds, if desired together with the substances
which are customary for this purpose, such as
- 11 -
solubilizers, emulsifiers or other auxiliaries, are
dissolved, suspended or emulsified. Examples of suitable
solvents are: water, physiological saline or alcohols,
for example ethanol, propanol, glycerol, and also sugar
solutions, such as glucose or mannitol solutions, or else
a mixture of the various solvents which have been
mentioned above.
Pharmaceutical formulations which are suitable for
administration in the form of aerosols or sprays are, for
example, Solutions, suspensions or emulsions of the
active substance of the formula I in a pharmaceutically
acceptable solvent, such as, in particular, ethanol or
water, or else in a mixture of such solvents.
If required, the formulation can also contain other
pharmaceutical auxiliaries, such as surfactants, emul-
sifiers and stabilizers, and a propellant gas. The
concentration of active substance in such a preparation
is generally from about 0.1 to 10, in particular from
about 0.3 to 3 ~ by weight.
The dose of the active substance of the formula I to be
administered and the frequency of administration will
depend on the power and duration of action of the com-
pounds used in addition also on the nature and severity
of the disease to be treated and on the sex, age, weight
and individual responsiveness of the mammal to be
treated.
On average, the daily dosage rate of a compound of the
formula I in the case of a patient of approximately 75 kg
will be at least 0.001 mg/kg, preferably 0.01 mg/kg, up
to not more than 10 mg/kg, preferably 1 mg/kg, of body
weight. If the disease is acute, such as immediately
after suffering a cardiac infarction, even higher and, in
particular, more frequent, doses may be required, for
example up to 4 single doses per day. In particular, for
intravenous administration, such as in the case of a
patient who has suffered an infarction and is under
- 12 -
intensive care, up to 200 mg per day may be required.
List of abbreviations:
MeOH methanol
DMF N,N-dimethylformamide
NBS N-bromosuccinimide
AIBN a,a-azobis-isobutyronitrile
EI electron impact
DCI desorption chemical ionization
RT room temperature
EA ethyl acetate (EtOAc)
DIP diisopropyl ether
MTB methyl tert.-butyl ether
mp melting point
HEP n-heptane
DME dimethoxyethane
FAB fast atom bombardment
CH,Cl, dichloromethane
THF tetrahydrofuran
eq equivalent
Experimental part
General protocol for the preparation of benzoylguanidines
(I)
Variant A: from benzoic acid (II, L = OH)
0.01 M of the benzoic acid derivative of the formula II
is dissolved or suspended in 60 ml of anhydrous THF, and
1.78 g (0.011 M) of carbonyldiimidazole are then added.
The mixture is stirred for 2 hours at RT, and 2.95 g
(0.05 M) of guanidine are then introduced into the
reaction solution. The mixture is stirred overnight, and
the THF is then distilled off under reduced pressure
(Rotavapor), water is added, the mixture is brought to pH
6 to 7 using 2N HCl, and the corresponding benzoylguanid-
ine (formula I) ie removed by filtration. The resulting
benzoylguanidines can be converted into the corresponding
~1~.~.~~~
- 13 -
salts by treating them with aqueous, methanolic or
etheric hydrochloric acid or other pharmacologically
acceptable acids.
General protocol for the preparation of benzoylguanidines
(I)
Variant B: from alkyl benzoates (II, L = O-alkyl)
5 amnol of the alkyl benzoate of the formula II and 25
mmol of guanidine (free bass) are dissolved in 15 ml of
isopropanol or suspended in 15 ml of THF, and the solu-
tions, or suspensions, are refluxed until the reaction is
complete (control by thin-layer chromatography typical
reaction time 2 to 5 hours). The solvent is distilled off
under reduced pressure (Rotavapor), the residue is taken
up in 300 ml of EE, and the mixture is washed 3 x using
in each case 50 ml of NaHC03 solution. The ethyl acetate
phase is dried over Na~SOs, the solvent is distilled off
in vacuo, and the residue is chromatographed on silica
gel using a suitable solvent, for example EE/MeOH 5:1.
(Salt formation see Variant A)
Example 1
4-(4-Pyridylthio)-3-methylsulfonylbenzoylguanidine
S
HyCOzS NHi
N~ II N~NH:
0
a) Methyl 4-(4-pyridylthio)-3-methylsulfonylbeazoate
6 mmol of methyl 4-chloro-3-methylsulfonylbenzoate,
18 mmol of R,C03 and 6 amnol of 4-pyridylthiol are stirred
in 30 ml of (anhydrous) DMF for 1 h at 130°C. The mixture
is subsequently poured into 100 ml of saturated aqueous
NaHC03 solution, and this is extracted 3 x using 100 ml
of EA. The ethyl acetate phase is dried over Na~S04, the
~~.~.~.38~
- 14 -
solvent is removed in vacuo, and the residue is
chromatographed on silica gel using MTB. Pale yellow
crystals, mp 112°C,
Rf (MTB) - 0 .17 MS (DCI) : 324 (M + 1)
b) 4-(4-Pyridylthio)-3-methylsulfonylbenzoylguanidine
3.6 amnol of ester 1 a) and 18.1 amnol of guanidine are
reacted in accordance with general protocol B. White
crystals, mp 205°C
Rf (EE/MeOH 5:1) - 0.24 M5 (DCI): 351 (M + 1)
Example 2
4-(2-Pyridylthio)-3-methylsulfonylbenzoylguanidine
N 5
II N~NHi
CH3025 NHi
0
mp 207°C
Rf (EA/MeOH 5:1) - 0.27 MS (DCI): 351 (M + 1)
Example 3
4-(3-Pyridyloxy)-3-methylsulfonylbenzoylguanidine
0
~N~NHi
HyCOzS ~ NHz
0
a) Methyl 4-(3-pyridyloxy)-3-methylsulfonylbenzoate
2 mmol of methyl 4-chloro-3-methylsulfonylbenzoate, 2
mmol of 3-pyridinol and 6 mmol of RzC03 were stirred in
ml of (anhydrous) DMF for 2 h at 130°C. The mixture is
.~. ~.1~.~~8~
- 15 -
subsequently poured into 100 ml of saturated aqueous
NaHC03 solution and this is extracted 3 x using 100 ml of
EA. The ethyl acetate phase is dried over NaaSO,,, the
solvent is removed in vacuo, and the product is further
reacted without further purification.
Rf (MTB) - 0.15 MS (DCI): 308 (M + 1)
b) 4-(3-Pyridyloxy)-3-methylsulfonylbenzoic acid
2 mmol of ester 3 a) are dissolved in 20 ml c~~ MeOH, and
5 equivalents of 2N aqueous NaOH are added. The solution
is stirred for 3 h at RT, 50 ml of 0.3 M aqueous RHsPO,,
solution are added, and the mixture is extracted 3 x
using 50 ml of EA. The ethyl acetate phase is dried over
NaZSO, solution, the solvent is removed in vacuo, and the
product is further reacted without further purification.
Rf (EE/MeOH 5:1) - 0.14 MS (DCI) : 294 (M+1)
c) 4-(3-Pyridyloxy)-3-methoxysulfonylbenzoylguanidine
2 aimol of benzoic acid 3 b), 2.2 amnol of carbonyldiimid-
azole and 10 amnol of guanidine are reacted in accordance
with the general protocol A.
White crystals mp 202°C
Rf (EA/MeOH) 5:1) - 0.38 MS (DCI): 335 (M + 1)
The title compounds of Examples 4 to 10 are synthesized
analogously to Example 3:
Example 4
4-(2-Pyridyloxy)-3-methylsulfonylbenzoylguanidine
_ 16 -
N 0
II N~N H i
CH30x5 NH2
0
amorphous;
Rf (EA/MeOH 5:1) - 0.41 MS (DCI): 335 (M + 1)
Example 5
4-(4-Pyridyloxy)-3-methylsulfonylbenzoylguanidine
0
N N NH2
CH30ZS NHZ
0
amorphous;
Rf (EA/MeOH 3:1) - 0.22 MS (DCI) : 335 (M + 1)
Example 6
4-(7-Isoquinolinoxy)-3-methylsulfonylbenzoylguanidine
0
N~ I ~ NHZ
/ N
H3C02S NHZ
0
mp 103 to 105°C
Rf (EA/MeOH 5:1) - 0.23 MS (DCI): 385 (M + 1)
Example 7
4-(5-Isoquinolinoxy)-3-methylsulfonylbenzoylguanidine
- 1' -
N' ~
,0
j
NHZ
N
H3COZS ~ NH2
0
amorphous;
Rf (EA/toluene/MeOH 3:3:1) - 0.21 MS (DCI): 335 (M + 1)
Example 8
4-(5-Quinolinoxy)-3-methylsulfonylbenzoylguanidine
n o
N
~NH2
N
H3COZS NHZ
0
amorphous;
Rf (EA/MeOH 5:1) - 0.23 MS (DCI): 385 (M + 1)
Example 9
4-(6-Quinolinoxy)-3-methylsulfonylbenzoylguanidine
0
NHZ
I N
N
H3COZS ~ NHZ
0
amorphous;
Rf (EA/toluene/MeOH 3:3:1) - 0.24 MS (DCI): 385 (M + 1)
- is - ~~.1~~~
~a~l. to
4-(8-Quiaoliaoxy)-3-m.thylsulfonylbeazoylguaaidiae
~~N
0
NHZ
N
H3COZS NHz
0
amorphous;
Rf (EA/MeOH 5:1) . 0.23 ~8 (DCI): 385 (M + 1)
General protocol A for the preparation of 4-(1-pyrrolo)-benzoic
acids:
0.01 mole of a 4-aminobenzoiC acid ere dissolved in 20 ml giac~aal acetic acid
and then
0.01 mole of 2,5-dimethoxy-tetrahydrofuran are added. The rt>i~re is stirred
for i s
minutes at room temperature and far 1 hour while boiUng. Then the mixture is
allowed
to cool and is poured into 200 ml of water. The ctystamne precipitation is
filtered off , is
washed a few times with water and the obtained 4-(1-pyrrplo)-benzoic acid is
dried in
the air.
The subsequent reaction of a ~-(1-pyrrolo)benzoic add with guar>idine after a
preceding
activation with carbonyldiimidazol results according to the genera! protocol A
for the
preparation of benzoyl guenidines in the oorrespondinp 4-(1-pyrrob)benzoyl-
guanidines
respectively their sans:
Example 11:
3,5-dichloro-4-(1-pyrrolo)benzoyl-guanidine-hydrochloride
is prepared according to General protocol A from 3,5-dichloro-4-(1-
pyrrolo)benzoic
aad, colorless crystals, f=p. 274° C.
3,5-dichloro-4-(1-Pyrrolo)benzoic aad (Fp. 178 - 181 ° C) is prepared
from 3,5-dimethy!-
4-aminobenzoic acid according to the Genera! protocol for the preparation of 4-
(1-
pyrrolo)-benzoic acids.
Example 12:
3-chloro-5-methyl-~4-(1-pyrrolo)benzoyl-guanidine-hydrochloride
is prepared according to General protocol A from 3-chloro-5-methyl-4-(1-
pyrrolo)ben-
zoic acid, colorless crystals, Fp. 236 - 238°C.
- 19 -
3-chloro-5-methyl-4-(1-pyrrolo)benzoic acid (Fp. 160 - 162°C, dec.) is
prepared from 3-
chloro-5-methyl-4-aminobenzoic acid according to General protocol for the
preparation
of 4-(1-pyrrolo)-benzoic acids.
Example 13:
3,5-dimethyl-4-(1-pyrrolo)benzoyl-guanidine-hydrochloride
is prepared according to General protocol A from 3,5-dimethyl-4-(i-
pyrrolo)benzoic
acid, colorless crystals, Fp. 261 - 263° C.
3,5-dimethyl-4-(1-pyrrolo)benzoic add (Fp. 197 - 200°C) is obtained
from 3,5-dimethyl-
4-aminobenzoic ead according to General protocol for the preparation of 4-(1-
pyrrolo)-
benzoic aads.
General protocol for the preparation of 4-[1-(1,3,4 triazolyl)jbenzoic aads
0.01 mole of a methylester of a 4-aminobenzoic acid are suspended in 40 ml
boiling
phosphoroxichloride (POCK} while Stirring, then 0.044 mole of N,N'-
diformyihydrazine
are added and then it is re~(axed for 45 minutes. The excess POCI3 is
distilled olf, and
then the residue which is mostly dark and viscous is treated with 200 ml of an
aqueous
saturated solution of sodium acetate. The precipitation is filtered off and is
washed
some times with water.
0.01 mole of the obtained 4-[1-(1,3,4-triazolyl)jbenzoic acid meihylester is
hydrolyzed
with a boiling mixture of 18 ml of pure acetic acid and 36 ml of 20 per cent
aqueous
hydrochloric aad for 6 hours; thus, the corresponding 4-[1-(1,3,4-
triazolyl)Jbenzoic acid
is obtained.
The subsequent reaction of a 4-[1-(1,3,4-triazolyl)Jbenzoic acid with
guanidine after
activation with Carbonyldiimidazol furnishes the respective 4-[1-(1,3,4-
triazolyl)jbenzoyl-
guanidine respectively their salts according to the General protocol for the
preparation
of benzoyiguanidines.
Exempla 14:
3,5-dibromo-4-[1-(1,3,4-triazolyl)]benzoyl-guanidine.dihydrochloride
is obtained according to General protocol A from 3,5-dibromo-4-[1-(1,3,4-
triazolyl)Jbenzoic aad, colorless crystals, Fp. >290°C.
The 3,5-dibromo-4-[~-(1,3,4-triazolyl)beruoic acid (Fp. 281 - 282°C) is
obtained from
3,5-dibromo-4-[1-(1,3,4-triazolyl))benzoic acid methylester (Fp: 202 -
204°C) according
to the General protocol for tfie preparation of 4-[1-(1,3,4-triazolyl)Jbenzoic
acids.
_ 2~ _
Example 15:
4-imidazolyl-3-trifluoromethyl-benzoylguanidine-dihydrochloride: colorless
crystals, Fp.
244 - 48° C, deC.
synthesis:
a) 4-~uoro-3-tri~uoromethyl-benzoylguanidine from 4-fluoro-3-trifluoromethyl-
benzoic
acid and guanidine according to variant A, colorless powder, Fop. 136 -
38° C.
b) 4-imidazolyl-3-trifluoromethyl-benzoylguanidine-dihydrochloride from a) by
heating
(120°C) in DMF in the presence of 2 eq imidazole for 7 hours,
distilling off the solvent
and treating the remainder with water. Formation of the salt according to
variant A.