Note: Descriptions are shown in the official language in which they were submitted.
4-19412/A
. :
Antiretroviral HYdrazine Derivadves
The invention relates to novel andretroviral compounds, to processes for the preparation
of those compounds, to novel intermediates for the preparation of those compounds,
especially those having antiretroviral acdvity, to pharmaceutical composidons comprising
those compounds, to those compounds for use in a therapeutic method of treating the
human or animal body and to the use of those compounds for the dlerapeutic treatment of
the human or animal body or for the preparation of pharmaceutical composidons. ; -
A whole range of diseases is caused by retroviruses. -~
As far as is known at present, AlDS is a disease caused by the retrovirus HIV ("Human
Immunodeficiency Virus"). Millions of people are already infected. The disease is
constantly spreading and in virtually all cases results in the death of the patient.
Hitherto the retroviruses HIV-l and HIV-2 (HIV ~epresenting "Human Immunodeficiency
Virus") have been identified as a cause of the disease. From the point of vi~w of treatment,
the search for compositions that interfere with the reproduction of the virus itself but do
not damage the intact cells and dssues of the patient is especially interesdng.
Retroviral protease is a proteolytic enzyme that, owing to an aspartate residue in the acdve
centre, is regarded as an aspartate protease. It participates in the maturation of new
infecdous virions in infected cells in the reproducdve cycle of a number of retroviruses.
For example, HIV- 1 and HIV-2 each have in their genome a region that codes for a
gag-protease. That enzyme is responsible for the correct proteolydc cleavage of the
precursor proteins that are produced from the genome regions coding for the "Group
Specific Antigens" (gag). During the cleavage, the structural proteins of the virus core are
liberated. The gag-protease itself is a component of a precursor protein encoded by the
pol-genome region of HIV-l and HIV-2, which protein also contains the regions for the
reverse transcriptase and the integrase and is thought to be cleaved by autoproteolysis.
21~,~ i3~7
- 2 -
The gag-protease cleaves the major core protein p24 of HIV- 1 and HIV-2 preferentially
N-terminally of proline residues, for example in the divalent residues Phe-Pro, Leu-Pro or
Tyr-Pro.
Because of the central role of the gag-protease in the processing of the mentioned core
proteins, it is assumed that effective inhibition of that enzyme in vivo will suppress the
assembly of mature virions, so that corresponding inhibitors can be used therapeutically.
In general, attempts have been made for some time to provide compounds for controlling
retroviral diseases, such as AIDS, that are effective in vivo as inhibitors of the said
retroviral gag-proteases, especially the gag-protease of HIV-l (HIV-l-protease), and also
of those of HIV-2 and o~her AIDS viruses.
The principal aim at present is to make available such compounds having the best possible
pharmacokinetic properties.
A requirement for therapeutic effectiveness in vivo is the achievement of good bio-
availability, for example good absorptive capacity and/or a high blood level, also in the
case of enteral, such as oral, administration, in order thus to obtain sufficiently high
concentrations in the infected cells.
The object of the present invention is to provide compounds having excellent antiretro-
viral activity, especially very good bioavailability.
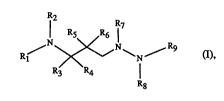
The compounds according to the invention are compounds of formula
R~ ~N~ 3 (I),
R3 R4
R8 ~ ..
wherein Rl and Rg are each indepemlently of the other hydrogen; acyl; unsubstituted or
substituted alkyl; sulfo; or sulfonyl substituted by unsubstituted or substituted alkyl, aryl
or by heterocyclyl, with the proviso that not more than one of the radicals Rl and Rg is
hydrogen; and
2 ~ 7
- 3 -
R2 and R8 are each independently of the other hydrogen or unsubstituted or substituted
alkyl;
R3 and R4 are each independently of the other hydrogen, unsubstituted or substituted
aLlcyl, unsubstituted or substituted cycloaLkyl or aryl;
Rs is acyloxy;
R6 is hydrogen; and
R7 is unsubstituted or substituted aLtcyl, unsubstituted or substituted cycloaL~cyl or aryl;
and salts of the mentioned compounds where salt-forming groups are present, with the
exception of the compound wherein Rl and Rg are each acetyl, R2, R3, R4, R6 and R8 are
each hydrogen, Rs is acetoxy and R7 is 2,2-[N-ethoxycarbonylmethyl)-N-methyl]-
hydrazin- 1 -ylcarbonylmethyl.
In the description of the present invention, the term "lower" used in the definition of
groups or radicals, for example lower alkyl, lower aL~;oxy, lower aL~canoyl etc., means that, -
unless expressly otherwise defined, the groups or radicals so defined contain up to and
including a maximum of 7, and preferably up to and including 4, carbon atoms. In the case
of lower alkenyl or lower alkynyl, from 2 to 7, preferably from 3 to 7, and especially 3
or 4, carbon atoms are present.
Unless indicated to the contrary, where substituted the radicals Rl, R2, R3, R4, R7, R8
and/or Rg are mono- or poly-substituted, especially mono- to tri-substituted, for example
mono-substituted, by identical or diffaent substituents.
If a radical that is defined by referring back to another substituent is defined "independ-
ently" of the radical used for the definition, it means that if both radicals are present in a
compound they need not be identical, although they can, however, be identical.
The carbon atoms in compounds of formula I substituted by R3 and R4 and by Rs and R6
may, if they are asymmetric, be in the (R)-, (S)- or (R,S)-configuration, as may also any
othe~ asymmetric carbon atoms present. Accordingly, the present compounds may be in
the form of isomeric mixtures or in the form of pure isomers, especially in the form of dia-
stereoisomeric mixtures, pairs of enantiomers or pure enandomers. Preferred compounds
of formula I are those wherein the carbon atoms substituted by R3 auld by Rs have the
(S) configuration and any other asymmetric carbon atoms that may be present are,independently of one another, in the (R)-, (S)- or (R,S)-configuration.
' ' '' '' ' ' ` ;' ` ' ` ' ~ ~ ` ` ' ` ~ ' `
2 ~ 7
- 4 -
Unless otherwise indicated, the general terms and names used in the description of the
present invention preferably have the following meanings:
Acyl Rl or Rg has, for example, up to 25, preferably up to 19, carbon atoms and is
especi~lly the acyl group of a carboxylic acid, of a semiester of carbonic acid, of an un-
substitu~ed or N-substituted carbamic acid or of an unsubstituted or substituted amino
acid.
Pre~erred acyl groups Rl or Rg of a carboxylic acid are unsubstituted or substituted
alkanoyl, alkenoyl or alkynoyl having up to 19 carbon atoms, for example n-decanoyl, or
preferably
lower alkanoyl, such as formyl, acetyl, propionyl, butyryl or pivaloyl; or also or especially
3,3-dimethylbutyryl; or substituted lower alkanoyl wherein preferably up to four,
especially (except in the case of halogen which may be present up to three times as a
substituent) up to two, substituents may be present, especially one substituent (except in
the case of halogen which may be present up to three times as a substituent), the
substituents being selected especially from
cycloalkyl which has preferably from 3 to 7 carbon atoms, especially in cycloalkyl-
lower al~anoyl wherein lower alkanoyl is as defined above, for example cycloaL~cyl-
carbonyl, for example having a total of from 4 to 8 carbon atoms, such as cycloI)ropyl-,
cyclobutyl-, cyclopentyl- or cyclohexyl-carbonyl, or 2-cyclohexyl- or 2-cyclopentyl-
acetyl,
cycloaL~cenyl which has preferably from 3 to 7 carbon atoms, especially in cyclo-
alkenyl-lower alkanoyl, such as cycloalkenylcarbonyl, for example having from 4 to 8
carbon atoms, such as l-cyclohexenylcarbonyl, 1,4-cyclohexadienylcarbonyl or l-cyclo-
hexenylacetyl or 1,4-cyclohexadienylacetyl,
bicycloalkyl which contains preferably from S to 10 carbon atoms, especially in
bicycloalkyl-lower alkanoyl, for example bicycloalkylcarbonyl, for example having from
8 to 11 carbon atoms, such as decahydronaphthyl-2-carbonyl, bicyclohexyl-, bicyclo-
heptyl-, bicyclooctyl-, bicyclononyl- or bicyclodecyl-acetyl or -3-propionyl,
bicycloalkenyl, preferably having from 8 to 12 carbon atoms, especially in bicyclo~
alkenylcarbonyl, such as S-norbornen-2-ylcarbonyl or bicyclo[2.2~2]octen-2-ylcarbonyl, :
tricycloalkyl, wherein tricycloalkyl contains, for example, from 8 to 10 carbon atoms, -
especially in tricycloalkyl-lower alkanoyl, for example tricycloalkylcarbonyl, for example
having from 8 to 11 carbon atoms, such as 1- or 2-adamantylcarbonyl,
aryl which has preferably from 6 to 14 ring carbon atoms, such as in phenyl, indenyl,
~-.. ~ : ...... .
211~7 ~7
indanyl, naphthyl, anthryl, phenanthryl, acenaphthyl or fluorenyl, and may be l~nsubsti-
tuted or mono- to tri-substituted especially by lower alkyl, for example methyl, ethyl or
propyl, halo-lower alkyl, for example trifluoromethyl, phenyl, 1- or 2-naphthyl, hydroxy,
lower alkoxy, for example methoxy, carbamoyl-lower aLkoxy, N-lower alkylcarbamoyl-
lower alkoxy or N,N-di-lower aLkylcarbamoyl-lower aLkoxy, amino, mono- or di-lower
alkylamino~ lower alkanoylamino, for example pivaloylamino, halogen, for examplefluorine, chlorine or bromine, carboxy, lower alkoxycarbonyl, such as tert-butoxy-
carbonyl, phenyl-, naphthyl- or fluorenyl-lower alkoxycarbonyl, such as benzyloxy-
carbonyl, lower alkanoyl, sulfo, lower alkylsulfonyl, for example methylsulfonyl,
phosphono, hydroxy-lower alkoxyphosphoryl or di-lower alkoxyphosphoryl, carbamoyl,
mono- or di-lower alkylcarbamoyl, sulfamoyl, mono- or di-lower alkylaminosulfonyl,
nitro and/or by cyano, especially in aryl-lower alkanoyl, wherein phenyl may be present
up to three times, for example in diphenyl-, dibenzyl- or triphenyl-lower aLkanoyl, such as
diphenyl-, dibenzyl- or triphenyl-acetyl, and wherein lower alkanoyl may be unsubstituted
or substituted, for example by carboxy; lower aLkoxycarbonyl, for example methoxy-,
ethoxy-, n-propoxy-, isopropoxy-, n-butoxy-, isobutoxy-, sec-butoxy-, tert-butoxy-,
n-pentyloxy-, isopentyloxy-, neopentyloxy-, tert-pentyloxy-, n-hexyloxy-, isohexyloxy- or
n-heptyloxy-carbonyl; aryl-lower alkoxycarbonyl wherein aryl has from 6 to 12 carbon
atoms, for example benzyloxycarbonyl; carbamoyl, carbamoyl substituted at the nitrogen
atom by one or two radicals selected from lower alkyl, such as methyl, ethyl, propyl, iso-
propyl, n-butyl, isobutyl, sec-butyl and tert-butyl, for example in N-methyl-carbamoyl,
N-n-butylcarbamoyl or N,N-dimethylcarbarnoyl, from carboxy-lower alkyl or lower
alkoxycarbonyl-lower alkyl, for example in the form of carboxymethylcarbamoyl
(glycinylcarbonyl) or in the form of tert-butoxycarbonylmethylcarbamoyl, from di-lower
alkylamino-lower alkyl, for example 2-dimethy1aminoethyl, from hydroxy-lower alkyl, for
example hydroxymethyl or hydroxyethyl, and from di-lower alkoxy-lower alkyl, forexample 2-(2,2-dimethoxyethyl); and/or by cyano and is unbranched or branched, selected
especially from phenyl-lower alkanoyl, such as benzoyl, phenylacetyl or 3-phenyl-
propionyl, which may be unsubstituted or mono- or poly-substituted at the phenyl ring, for
example by lower alkyl, for example methyl, phenyl, halogen, for example fluorine or
chlorine, hydroxy, lower alkoxy, for example methoxy, and/or by nitro, such as 4-chloro-,
4-methoxy- or 4-nitro-benzoyl, naphthylcarbonyl, such as a- or ,B-naphthylcarbonyl or
1,8-naphthalenedicarbonyl bonded to the amino group via both carbonyl groups, indenyl-
carbonyl, such as 1-, 2- or 3-indenylcarbonyl, indanylcarbonyl, such as 1- or 2-indanyl-
carbonyl, phenanthrenylcarbonyl, such as 9-phenanthrenylcarbonyl, a-naphthylacetyl,
~-naphthylacetyl, lower alkylphenylacetyl, such as 4-methylphenylacetyl, lower alkoxy-
? "',~,~, ., . ' ', . . ' ~ : , ~ . . .
~.' : . .
~. . . . .,,, .. ,.. ,~, ~ .
2112~)17
- 6 -
phenylacetyl, such as 4-methoxyphenylacetyl, 3-(p-hydroxyphenyl)-propiGnyl, diphenyl-
acetyl, di(4-methoxyphenyl)acetyl, triphenylacetyl, 2,2-dibenzylacetyl, 3-a- or
3-,B-naphthylpropionyl, phenyl-lower alkanoyl wherein the lower alkanoyl radical is
substituted by carbarnoyl, for example 2-carbamoyl-3-phenylpropionyl, such as 2(R,S)-
carbamoyl-3-phenylpropionyl, 3-a-naphthyl-2-carbamoylpropionyl, 3-phenyl- or 3-a-
naphthyl-2-tert-butylcarbamoyl-propionyl, 3-phenyl- or 3-a-naphthyl-2-(2-dimethyl-
aminoethyl)carbamoylpropionyl, 3-a-naphthyl-2-(carboxy- or tert-butoxy-carbonyl-)-
methylcarbamoyl-propionyl, 3-phenyl- or 3-a-naphthyl-2-(3-hydroxy-2-propyl)-
carbamoyl-propionyl, 3-phenyl- or 3-a-naphthyl-2-(2,2-dimethoxyethyl)carbamoyl-
propionyl and 3-phenyl- or 3-a-naphthyl-2-(5-amino-5-carboxypentyl)-carbamoyl-
propionyl, especially phenyl-lower alkanoyl, such as phenylacetyl, or phenyl-lower
alkanoyl wherein the lower alkanoyl radical is substituted by carbamoyl, such as 2(R,S)-
carbamoyl-3-phenylpropionyl,
heterocyclyl which is preferably a single or double ring system having from 3 to 10
ring atoms, is bonded via a carbon atom or, especially, via a nitrogen atom and contains up
to 3 further hetero atoms selected from oxygen, nitrogen, sulfur, and sulfur linked to 1 or 2
oxygen atoms; the mentioned ring system may also be fused with 1 or 2 phenyl or
naphthyl radicals, it being possible for naphthyl also to be fused-on by two sides, or with 1
or 2 cycloalkyl radicals, cycloalkyl preferably having from 5 to 7 ring atoms; and which
may be unsaturated or partially or fully saturated, for example thienyl, furyl, pyrrolyl,
imidazolyl, pyrazolyl, oxazolyl, thiazolyl, tetrazolyl, pyr.,dyl, pyrazinyl, pyrimidinyl,
pyridazinyl, indolyl, benzimidazolyl, quinolyl, isoquinolyl, 3,1-benzofuranyl, cyclohexa-
[b]pyrrolyl, cyclohexa[b]pyridyl, cyclohexa[b]pyrazinyl, cyclohexa[b]pyrimidinyl,
pyrrolidinyl, pyrrolirlyl, imidazolidyl, piperidyl, piperazinyl, morpholinyl, thiomorpho-
linyl, S,S-dioxo-thiomorpholinyl, indolinyl, isoindolinyl, 4,5,6,7-tetrahydroindolyl,
1,2,3,4-tetrahydroquinolyl or 1,2,3,4-tetrahydroisoquinolyl, with heterocyclyl, for example
one of the last-mentioned radicals, being unsubsdtuted or substituted by lower alkyl, for
example methyl, phenyl, 1- or 2-naphthyl, phenyl-lower alkyl, for example benzyl,
hydroxy-lower aL~cyl, for example hydroxymethyl or 2-hydroxyethyl, lower alkoxy-lower
alkyl, for example methoxymethyl or 2-methoxyethyl, lower alkanoyloxy-lower aLkyl, for
example acetoxymethyl, phenyl- or naphthyl-lower alkanoyloxy-lower alkyl, for example
benzoyloxy-, phenylacetoxy- or 1- or 2-naphthoyloxy-methyl, -2-ethyl or -2-(2,2-di-
methylethyl), lower alkoxycarbonyloxy-lower alkyl, for example tert-butoxycarbonyloxy-
lower alkyl, phenyl-lower alkoxycarbonyloxy-lower alkyl, for example 2-benzyloxy-
carbonyloxyethyl, amino-lower alkyl, for example aminomethyl, hydroxy, lower aLkoxy,
for example methoxy or ethoxy, amino, lower alkylamino, for example methyl-, ethyl- or
~,~: , . .
~' ` ' ` `` ~`' :
21.~2~7
- 7 -
tert-butyl-amino, di-lower alkylamino, for example dimethyl- or diethyl-amino, carboxy,
lower alkoxycarbonyl, for example methoxy-, isopropoxy-, sec-butoxy- or tert-butoxy-
carbonyl, phenyl- or naphthyl-lower alkoxycarbonyl, for example benzyloxycarbonyl,
halogen, for example fluorine, chlorine, bromine or iodine, especially chlorine or bromine,
lower alkanoyl, for example acetyl or pivaloyl, carbamoyl, mono- or di-lower aLkyl-
carbamoyl, for example N-methylcarbamoyl, N-n-butylcarbamoyl or N,N-dimethyl-
carbamoyl, hydroxy- or carboxy-lower alkylcarbamoyl, for example hydroxy- or
carboxy-methylcarbamoyl or hydroxy- or carboxy-ethylcarbamoyl, ni~ro, oxo and/or by
cyano; especially in heterocyclyl-lower alkanoyl wherein lower alkanoyl is unsubstituted
or substituted independently by one of the substituents defined above under aryl-lower
alkanoyl Rl or R9; with heterocyclyl-lower alkanoyl being selected especially frorn
pyrrolylcarbonyl, for example 2- or 3-pyrrolylcarbonyl, thienylcarbonyl, such as 2-thienyl-
carbonyl, furylcarbonyl, such as 2-furylcarbonyl, indolylcarbonyl, such as 2-, 3- or
5-indolylcarbonyl, 4,5,6,7-tetMhydroindolyl-2-carbonyl, quinolyl-lower aLkanoyl, for
example quinolylcarbonyl, such as 2-, 3- or 4-quinolylcarbonyl, isoquinolylcarbonyl, such
as 1-, 3- or 4-isoquinolylcarbonyl, piperidylcarbonyl, such as piperidinocarbonyl or 2-, 3-
or 4-piperidylcarbonyl, piperazinylcarbonyl, such as pipeMzin-l-ylcarbonyl,
morpholinyl-lower alkanoyl, for example morpholino-lower alkanoyl, such as
morpholinocarbonyl, thiomorpholinyl-lower alkanoyl, for example thiomorpholino-lower
alkanoyl, such as thiomorpholinocarbonyl, S,S-dioxothiomorpholinylcarbonyl, such as
S,S-dioxothiomorpholinocarbonyl, 1,2,3,4-tetrahydroquinolylcarbonyl, such as 1,2,3,4-
tetrahydroquinolyl-2-, -3- or -4-carbonyl, 1,2,3,4-tetrahydroisoquinolylcarbonyl, such as
1,2,3,4-tetrahydroisoquinolyl-1-, -3- or -4-carbonyl, tetrazolyl-lower alkanoyl, such as 3- : :
(tetrazol-l-yl)-propionyl, and pyridyl-lower alkanoyl, for exarnple pyridylcarbonyl, such
as 2-, 3- or 4-pyridylcarbonyl, or pyridylacetyl, such as 2-, 3- or 4-pyridylacetyl; and :
heterocyclyl-lower alkanoyl being selected most especially from morpholinocarbonyl,
thiomorpholinocarbonyl, quinolin-2-ylcarbonyl, 3-(tetrazol-1-yl)-propionyl, 2-pyridyl-
carbonyl and 2- or 3-pyridylacetyl,
hydroxy, especially in hydroxy-lower alkanoyl, such as 3-hydroxypropionyl or
2-hydroxy-3-methylpentanoyl, ~:
hydroxy-lower alkoxy, especially in hydroxy-lower alkoxy-lower alkanoyl, such as3-hydroxy-n-propoxycarbonyl,
lower alkoxy, especially in lower alkoxy-lower alkanoyl, for example lower alkoxyacetyl
or lower alkoxypropionyl, such as methoxyacetyl, ethoxyacetyl or 3-methoxypropionyl,
lower alkoxy-lower alkoxy, especially in lower alkoxy-lower alkoxy-lower alkanoyl,
such as 2-methoxymethoxy-3-methylpentanoyl or also or especially 2-(methoxy)-
: :` - ` ' ` `~ ~ -` - ~ . .4.~
. ,: . .
.
. ,, ~`'., ~ , , - .
2 1 ~ 7
- 8 -
ethoxyacetyl,
lower alkanoyloxy, especially in lower alkanoyloxy-lower aLkanoyl wherein lower
alkanoyloxy is, for example, acetoxy, propionyloxy, butyryloxy, isobutyryloxy orpivaloyloxy, such as acetoxyacetyl or 3-acetoxypropionyl,
amino that is not in the a-or ,B-position relative to the bonding carboxy group of the acyl
radical, for example in amino-lower aLlcanoyl wherein the amino group is not in the a- or
~-position, such as 5-aminopentanoyl,
lower alkanoylamino that is not in the a- or ,I~-position relative to the bonding carboxy
group of the acyl radical, especially in lower alkanoylamino-lower alkanoyl wherein the
amino group is not in the a- or ,B-position of the lower alkanoyl radical, such as
5-pivaloylamino-pentanoyl,
lower aL~oxycarbonylamino that is not in the a- or ,B-position relative to the bonding
carboxy group of the acyl radical, especially in
lower alkoxycarbonylamino-lower aL~canoyl wherein the amino group is not in the a- or
,B-position of the lower alkanoyl radical, such as 5-(tert-butoxycarbonylamino)-pentanoyl,
phenyl-lower alkoxycarbonylamino that is not the in a- or ,B-position relative to the
bonding carboxy group of the acyl radical, especially in
phenyl-lower alkoxycarbonylamino-lower alkanoyl wherein the amino grollp is not in the
a- or ,B position of the lower aL~canoyl radical, such as 5-benzyloxycarbonylamino-
pentanoyl or 6-benzyloxycarbonylaminohexanoyl,
amino substituted at the amino nitrogen atom by heterocyclyl-lower alkanoyl wherein
heterocyclyl is independently as defined above as a substituent of lower aL~canoyl Rl or
Rg, especially by N-morpholino- or N-thiomorpholino-carbonyl, especially in hetero
cyclyl-lower alkanoylamino-lower alkanoyl, for example N-morpholino- or N-thio-
morpholino-carbonylamino-lower aL~canoyl, such as N-morpholino- or N-thiomorpholino-
carbonylamino-acetyl,
preferably up to 3 halogen atoms, especially in halo-lower aL~canoyl containing up to 3
halogen atoms, for example a-haloacetyl, such as a-fluoro-, a-chloro-, a-bromo-, a-iodo-,
a,a,a-trifluoro- or a,a,a-trichloro-acetyl, or halopropionyl, such as ~B-chloro- or ,B-
bromo-propionyl,
carboxy, especially in carboxy-lower aL~canoyl, for example carboxyacetyl or ,B-carboxy-
propionyl,
lower aL~coxycarbonyl, especially in lower alkoxycarbonyl-lower alkanoyl, for example
lower aLlcoxycarbonylacetyl or lower alkoxycarbonylpropionyl, such as methoxycarbonyl-
acetyl, 3-methoxycarbonylpropionyl, ethoxycarbonylacetyl, 3-ethoxycarbonylpropionyl or
3-tert-butoxycarbonylpropionyl,
2112~
sulfonyl, especially in sulfonyl-lower alkanoyl, such as 3-sulfonylpropionyl,
carbamoyl, especially in carbamoyl-lower aL~canoyl, such as carbamoylacetyl or
3-carbamoylpropionyl,
lower aLlcylcarbamoyl, especially in lower aLIcylcarbamoyl-lower alkanoyl, for example
lower alkylcarbamoylacetyl or methylcarbamoyl-lower alkanoyl, such as methyl-
carbamoylacetyl,
di-lower alkylcarbamoyl, especially in di-lower alkylcarbamoyl-lower alkanoyl, for
example di-lower alkylcarbamoylacetyl or dimethylcarbamoyl-lower alkanoyl, such as
dimethylcarbamoylacetyl,
carbamoyl substituted at the nitrogen atom by a radical selected from ethylene,
trime~hylene, tetramethylene and pentamethylene wherein a carbon atom may have been
replaced by nitrogen, oxygen, sulfur or by sulfur mono- or di-substituted by oxygen,
especially in correspondingly N-substituted carbamoyl-lower aLkanoyl, it also being
possible for the radical so formed to be fully or partially unsaturated, for example in the
form of piperidino-, pyræin-l-yl-, piperazin-1-yl-, pyrimidin-l-yl-, pyridæin-1-yl-, ~ -~
morpholino-, thiomorpholino- or S,S-dioxothiomorpholino-carbonyl-lower alkanoyl, such
as in morpholinocarbonyl-acetyl, 3-(morpholinocarbonyl)-propionyl or 3-(morpholino-
carbonyl)-2-isobutyl-propionyl,
N-heterocyclyl-lower aL~cylcarbamoyl or N-lower alkyl-N-heterocyclyl-lower aL~cyl-
carbamoyl, especially in N-heterocyclyl-lower aL~cylcarbamo~,rl-lower aL~anoyl or N-lower
aL~cyl-N-heterocyclyl-lower aLkylcarbamoyl-lower aL~anoyl wherein heterocyclyl is
preferably selected from pyrrolyl, furyl, thienyl, imidazolyl, pyrazolyl, oxazolyl, thiazolyl,
pyridyl, pyrazinyl, pyrimidinyl, indolyl, quinolyl and isoquinolyl, which may also be fully
or partially saturated, from morpholinyl and thiomorpholinyl, such as N-methyl-
2-(N-2-pyridylmethyl)-carbamoylacetyl, 2-(N-morpholino-lower alkylcarbamoyl)-lower
alkanoyl, such as 2(R,S)-(N-(2-morpholinoethyl)-carbamoyl)-3-methyl-butyryl, or 2-(N-
(pyridyl-lower aLkyl)-carbamoyl)-lower aLI~anoyl, such as (2(R,S)-(N-(2-pyridylmethyl)-
carbamoyl)-3-methyl)-butyryl,
oxo, especially in oxo-lower alkanoyl, such as acetoacetyl or propionylacetyl, and
cyano, especially in cyano-lower alkanoyl, such as cyanoacetyl, 2- or 3-cyanopropionyl
or 2-, 3- or 4-cyanobutyryl;
lower aLkenoyl having from 3 to 7 carbon atoms, preferably having 3 or 4 carbon atoms,
such as acryloyl, vinylacetyl, crotonoyl or 3- or 4-pentenoyl, or
lower aL~cynoyl having from 3 to 7, preferably 3 or 4, carbon atoms, for examplepropioloyl or 2- or 3-butynoyl.
~ ~ . . . . - -. . , .,., - .. , ,: - ,-. ; ., -
. ~ . . , . ,,. . .~
;
,'. ' '.:
2l~2a~7
- lo -
Preferred acyl groups Rl or Rg of a semiester of carbonic acid are
lower alkoxycarbonyl, for example methoxy-, ethoxy-, n-propoxy-, isopropoxy-,
isobutc~xy- or tert-lower alkoxy-carbonyl, or also or especially n-propoxycarbonyl, such as
tert-butoxycarbonyl or isobutoxycarbonyl,
2-halo-lower alkoxycarbonyl, such as 2-chloro-, 2-bromo-, 2-iodo- or 2,2,2-trichloro-
ethoxycarbonyl,
aryl-lower alkoxycarbonyl, for example arylmethoxy-carbonyl, wherein aryl has from 6
to 14 carbon atoms and is, for example, phenyl, biphenylyl, 1- or 2-naphthyl, fluorenyl, or
phenyl mono- or poly-substituted by lower alkyl, for example methyl or tert-butyl,
hydroxy, lower alkoxy, for example methoxy, ethoxy or tert-butoxy, halogen, for example
chlorine or bromine, and/or by nitro, for example phenyl-lower alkoxycarbonyl, such as
benzyloxycarbonyl or 4-methoxybenzyloxycarbonyl,
heteroc; clyl-lower aLIcoxycarbonyl wherein heterocyclyl is preferably selected from
pyrrolyl~ furyl, thienyl, imidazolyl, pyrazolyl, oxazolyl, thiazolyl, pyridyl, pyrazinyl, pyri- ;:
midinyl, indolyl, quinolyl and isoquinolyl, which may also be fully or partially saturated,
from morpholinyl and from thiomorpholinyl and may be unsubstituted or substituted, :
especially by lower alkyl, such as methyl, such as furylmethoxycarbonyl, tetrahydrofuryl-
lower alkoxycarbonyl, such as 2-tetrahydrofuryl-methoxycarbonyl, 2-morpholino-ethoxy-
carbonyl, or 2-, 3- or 4-pyridylmethoxycarbonyl,
lower alkenyloxycarbonyl wherein preferably the lower alkenyl radical is bonded to the
bonding oxygen atom via a saturated carbon atom, such as allyloxycarbonyl,
lower aL~coxy-lower alkoxycarbonyl, such as 2-methoxyethoxycarbonyl, or
(lower alkoxy-lower alkoxy)-lower alkoxycarbonyl, such as 2-(2-methoxyethoxy)-
ethoxycarbonyl .
Preferred acyl groups Rl or Rg of an unsubstituted or substituted carbamic acid, are
carbamoyl or
unsubstituted or substituted N-alkyl- or N,N-dialkyl-carbamoyl wherein the alkyl radical
has up to 12 carbon atoms, preferably unsubstituted or substituted lower alkyl- or di-lower
alkyl-carbamoyl, such as methyl-, ethyl-, propyl-, tert-butyl-, dimethyl-, diethyl- or di-
n-propyl-carbaTnoyl, the substituents being selected from phenyl, for example in benzyl-
carbamoyl, N-phenyl-lower alkyl-N-lower alkylcarbamoyl, such as N-benzyl-N-methyl-
carbamoyl, or dibenzylcarbamoyl, heterocyclyl that is independently as defined as a
substituent of lower alkanoyl Rl and Rg, preferably pyridyl, such as 2-, 3- or 4-pyridyl,
more especially in N-heterocyclyl-lower alkyl-N-lower alkylcarbamoyl, for example
N-pyridyl-lower alkyl-N-lower alkylcarbamoyl, such as N-(2-, 3- or 4-pyridylmethyl)-N-
f,~
7.~. .
,Y".',-'. . : '
!y~ .
. ,' . .
W~
- 2 ~ 4 7
methyl-carbamoyl, or in N-heterocyclyl-lower alkylcarbamoyl, for example 2- or
3-pyridyl-lower aL~sylaminocarbonyl, such as 2- or 3-pyridylmethylaminocarbonyl,hydroxy, for example in hydroxymethyl, 2-hydroxyethyl, 3-hydroxypropyl, 2-hydroxy~
propyl, and lower aLkoxy, preferably in lower alkoxy-lower aLkyl, for example methoxy-
methyl or 2-methoxyethyl; or also or especially N-lower aLkyl-N-morpholino-loweraLIcyl-aminocarbonyl, such as N-methyl-N-(2-morpholinoethyl)aminocarbonyl, or
N-morpholino-lower aL~cylaminocarbonyl, such as N-(2-morpholinoethyl)arninocarbonyl.
Preferred acyl groups Rl or Rg of an unsubstituted or substituted amino acid are formed
by the amino acid residues, bonded via the carbonyl of their carboxy group, of an a- or
~-amino acid, especially :
a natural a-amino acid having the L-configuration, such as those normally occurring in ~:
proteins, or an epimer of such an amino acid, that is to say having the unnatural D-
configuration, or a D,L-isomeric mixture thereof, a homologue of such an amino acid, for
example wherein the arnino acid side chain has been lengthened or shortened by one or
two methylene groups, wherein the amino group is in the ,B-position and/or wherein a
methyl group has been replaced by hydrogen, a substituted aromatic amino acid wherein
the aromatic radical has from 6 to 14 carbon atoms, for example a substituted phenyl-
alanine or phenylglycine wherein the phenyl may be mono- or poly-substituted by lower
alkyl, for example methyl, hydroxy, lower aL~coxy, for example methoxy, lower aLkanoyl-
oxy, for example acetoxy, amino, lower aLkylamino, for example methylamino, di-lower
aL~cylamino, for example dimethylamino, lower aLtcanoylamino, for example acetylamino
or pivaloylarnino, lower alkoxycarbonylamino, for example tert-butoxycarbonylarnino,
arylmethoxycarbonylarnino wherein aryl preferably has from 6 to 14 carbon atoms, for
example benzyloxycarbonylamino or 9-fluorenylmethoxycarbonylamino, halogen, for
example fluorine, chlorine, bromine or iodine, carboxy and/or by nitro, a benzo-fused
phenylalanine or phenylglycine, such as a-naphthylalanine, or a hydrogenated phenyl-
zlanine or phenylglycine, such as cyclohexylalanine or cyclohexylglycine.
Those amino acids can be substituted at free amino or hydroxy functions, preferably at a
free amino function, by one of the radicals mentioned above under acyl Rl or Rg as the
acyl group of a carboxylic acid, a semiester of carbonic acid or an unsubstituted or
N-substituted carbamic acid or by one of the radicals mentioned below under
unsubstituted or substituted alkyl Rl, R2, R8 or Rg.
Especially preferred is the radical, bonded via its carbonyl group, of an amino acid
2t12~7
- 12-
selected from glycine (H-Gly-OH), alanine (H-Ala-OH), valine (H-Val-OH), norvaline
(a-aminovaleric acid), leucine (H-Leu-OH), isoleucine (H-Ile-OH), norleucine (a-amino-
hexanoic acid, H-Nle-OH), serine (H-Ser-OH), homoserine (a-amino- y-hydroxybutyric
acid), threonine (H-Thr-OH), methionine (H-Met-OH), cysteine (H-Cys-OH), proline(H-Pro-OH), trans-3- and trans-4-hydroxyproline, phenylalanine (H-Phe-OH), tyrosine
(H-Tyr-OH), 4-aminophenylalanine, 4-chlorophenylalanine, 4-carboxyphenylalanine,,B-phenylserine (,B-hydroxyphenylalanine), phenylglycine, a-naphthylalanine (H-Nal-OH),
cyclohexylalanine (H-Cha-OH), cyclohexylglycine, tryptophan (H-Trp-OH), indoline-
2-carboxylic acid, 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, aspartic acid
(H-Asp-OH), asparagine (H-Asn-OH), aminomalonic acid, aminomalonic acid mono- : :
amide, glutamic acid (H-Glu-OH), glutamine (H-Gln-OH), histidine (H-His-OH), arginine
(H-Arg-OH), Iysine (H-Lys-OH), ~-hydroxylysine, ornithine (oc,~diarninovaleric acid),
3-aminopropanoic acid, a,~-diarninobutyric acid and a"B-diaminopropionic acid,
especially preferably the radical of an aliphatic amino acid selected from valine, alanine,
Ieucine and isoleucine, or an amino acid selected from glycine, glutamic acid and
asparagine, it being possible for each of the mentioned amino acids (with the exception of
glycine) to be in the D-, ~ or (D,L)-form, preferably in the L-form (with the exception of
Val which may also be in the (D)- or (D,L)-form), wherein
the a-amino group may be unsubstituted or mono- or di-N-alkylated, for example by
lower aLkyl, such as methyl or n-propyl, or by amino-lower alkyl, such as 3-aminopropyl,
or may be N-acylated by one of the acyl radicals mentioned above under acyl Rl as a
radical of a carboxylic acid, of a semiester of carbonic acid or of an unsubstituted or
N-substituted carbamic acid, preferably by lower alkanoyl, such as acetyl; by aryl-lower
alkanoyl wherein aIyl is selected from phenyl, indenyl, indanyl, naphthyl, anthryl,
phenanthryl, acenaphthyl and fluorenyl and may be unsubstituted or mono- to tri-substituted especially by lower alkyl, for example methyl, ethyl or propyl, halo-lower
aLkyl, for example trifluoromethyl, phenyl, 1- or 2-naphthyl, hydroxy, lower alkoxy, for
example methoxy, carbamoyl-lower alkoxy, N-lower alkylcarbamoyl-lower alkoxy or
N,N-di-lower alkylcarbamoyl-lower alkoxy, amino, mono- or di-lower aLkylamino, lower
alkanoylamino, for example pivaloylamino, halogen, for example fluorine, chlorine or
bromine, carboxy, lower alkoxycarbonyl, such as tert-butoxycarbonyl, phenyl-, naphthyl-
or fluorenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, lower alkanoyl, sulfo,
lower alkylsulfonyl, for example methylsulfonyl, phosphono, hydroxy-lower alkoxy-
phosphoryl or di-lower alkoxyphosphoryl, carbamoyl, mono- or di-lower aLkylcarbamoyl,
sulfamoyl, mono- or di-lower alkylaminosulfonyl, nitro and/or by cyano, and wherein
Iower alkanoyl may be unsubstituted or substituted especially by carboxy, lower alkoxy-
2 ~ 7
- 13-
carbonyl, for example methoxy-, ethoxy-, n-propoxy-, isopropoxy-, n-butoxy-, isobutoxy-,
sec-butoxy-, tert-butoxy-, n-pentyloxy-, isopentyloxy-, neopentyloxy-, tert-pentyloxy-,
n-hexyloxy-, isohexyloxy- or n-heptyloxy-carbonyl, aryl-lower alkoxycarbonyl wherein
aryl has from 6 to 12 carbon atoms, for example benzyloxycarbonyl, carbamoyl,
carbamoyl substituted by one or two radicals selected from lower alkyl, such as methyl,
ethyl, propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl, isopentyl,
neopentyl, tert-pentyl, n-hexyl, isohexyl or n-heptyl, for example in N-methylcarbamoyl,
N-n-butylcarbamoyl or N,N-dimethylcarbamoyl, from carboxy-lower alkyl or lower
alkoxycarbonyl-lower alkyl, for example in the form of carboxymethylcarbamoyl
(glycinylcarbonyl) or tert-butoxycarbonylmethylcarbarnoyl, from di-lower alkylamino-
lower alkyl, for example 2-dimethylaminoethyl, from hydroxy-lower alkyl, for example
hydroxymethyl or hydroxyethyl, and from di-lower alkoxy-lower aL~cyl, for example 2-
(2,2-dimethoxyethyl), and/or by cyano and is unbranched or branched, with phenyl-lower
alkanoyl, such as phenylacetyl, or phenyl-lower alkanoyl wherein the lower alkanoyl
radical is substituted by carbamoyl, such as 2-carbamoyl-3-phenyl-propionyl, being
especially prefeIred; by heterocyclyl-lower alkanoyl wherein heterocyclyl is independ- :
ently as defined as a substituent of lower alkanoyl Rl and is especially morpholino, thio-
morpholino, pyridyl, quinolyl or tetrazolyl, more especially pyridylcarbonyl, such as 2-, 3-
or 4-pyridylcarbonyl, morpholinocarbonyl, thiomorpholinocarbonyl, S,S-dioxothio-morpholinocarbonyl, indol-2-ylcarbonyl, quinolin-2-ylcarbonyl, pyridylacetyl, such as 2- ~ -
or 3-pyridylacetyl, imidazolylacetyl, such as imidazol-l-ylacetylj morpholinylacetyl, such
as morpholinoacetyl, pyridylpropionyl, such as 3-(2- or 3-pyridyl)propionyl, pyrrolidinyl-
propionyl, such as 3-(4-pyrrolidinyl)propionyl, morpholinylpropionyl, such as 3-morpho-
linopropionyl, or tetrazolylpropionyl, such as 3-(tetrazol-1-yl)-propionyl; b~ halo-lower
alkanoyl containing up to 3 halogen atoms, for example a-haloacetyl, such as a-fluoro-,
a-chloro-, a-bromo-, a-iodo-, a,a,a-trifluoro- or a,a,a-trichloro-acetyl, or halopropionyl,
such as ,B-chloro- or ,B-bromo-propionyl; by lower alkoxy-lower aLkoxy-lower alkanoyl; by
lower alkoxycarbonyl, such as tert-butoxycarbonyl; by aryl-lower alkoxycarbonyl wherein
aryl has from 6 to 14 carbon atoms and is selected, for example, from phenyl, naphthyl
and fluorenyl, for example phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, or
9-fluorenylmethoxycarbonyl; by heterocyclyl-lower aL~coxycarbonyl wherein heterocyclyl
is selected especially from pylrolyl, furyl, thienyl, imidazolyl, pyrazolyl, oxazolyl,
thiazolyl, pyridyl, pyrazinyl, pyrimidinyl, indolyl, quinolyl and isoquinolyl, which may
also be fully or partially saturated and unsubsdtuted or subsdtuted especially by lower
alkyl, such as methyl, for example 2-furylmethoxycarbonyl, tetrahydrofuryl-lower alkoxy-
carbonyl, such as 2-tetrahydrofuryl-methoxycarbonyl, or 2-morpholino-ethoxycarbonyl;
.j.,, ~ ,. ,, . . . ... :
211~7
- 14-
by lower aLkenyloxycarbonyl (preferably with a saturated carbon atom at the bonding
oxygen atom), such as allyloxycarbonyl; by lower alkoxy-lower alkoxycarbonyl, such as
2-methoxyethoxycarbonyl; by (lower alkoxy-lower alkoxy)-lower alkoxycarbonyl, such as ~:
2-(2-methoxyethoxy)ethoxycarbonyl; by carboxy-lower alkanoyl, such as 3-carboxy-propionyl; by lower aIkoxycarbonyl-lower aLkanoyl; by amino-lower alkanoyl substituted
at the amino nitrogen atom by heterocyclyl-lower alkanoyl wherein heterocyclyl is
preferably independently as defined above as a substituent of lower alkanoyl Rl or Rg,
especially by N-morpholino- or N-thiomorpholino-carbonyl, for example N-morpholino-
or N-thiomorpholino-carbonylamino-lower alkanoyl, such as N-morpholino- or N-thio-
morpholino-carbonylamino-acetyl; by carbamoyl; by phenyl-lower alkylaminocarbonyl,
such as benzylaminocarbonyl; by N-heterocyclyl-lower alkyl-N-lower alkylcarbamoyl or
N-heterocyclyl-lower alkylcarbamoyl wherein heterocyclyl is independently as defined
above as a substituent of lower alkanoyl Rl or Rg, especially as pyridyl, such as 2-, 3- or
4-pyridyl, especially 2- or 3-pyridyl-lower alkylaminocarbonyl, such as 2- or 3-pyridyl-
methylaminoca}bonyl; or by N-2-, N-3- or N-4-pyridyl-lower aL~cyl-N-lower alkylamino-
carbonyl, such as N-2-, N-3- or N-4-pyridylmethyl-N-methylaminocarbonyl; by hetero-
cyclyl-lower alkylcarbamoyl-lower alkanoyl wherein heterocyclyl is independently as
defined in the definition thereof as a substituent of lowel alkyl Rl, R2, R8 or Rg, for
example 2-(N-morpholino-lower alkylcarbamoyl)-lower alkanoyl, such as 2(R,S)-(N-(2-
morpholinoethyl)-carbamoyl)-3-methyl-butyryl, or 2-(N-(pyridyl-lower alkyl)-
carbamoyl)-lower aLtcanoyl, such as (2(R,S)-(N-(2-pyridylmethyl)-carbamoyl)-3-methyl)-
butyryl; by sulfonyl; by lower alkanesulfonyl, such as methane- or ethane-sulfonyl; by
arylsulfonyl (aryl-SO2) wherein aryl has from 6 to 10 carbon atoms and, for example, is
selected from phenyl and naphthyl and is unsubsdtuted or especially subsdtuted by lower
alkyl, such as methyl, or by lower alkoxy, such as methoxy, such as p-toluenesulfonyl; or
by heterocyclylsulfonyl (heterocyclyl-SO2-) wherein heterocyclyl is preferably selected
from pyrrolyl, furyl, thienyl, imidazolyl, pyrazolyl, oxazolyl, thiazolyl, pyridyl, pyrazinyl,
pyrimidinyl, indolyl, quinolyl and isoquinolyl, which may also be fully or pardally
saturated, from morpholinyl and from thiomorpholinyl and may be unsubsdtuted or subsd-
tuted especially by lower alkyl, such as methyl, such as morpholinosulfonyl, thio-
morpholinosulfonyl, piperidinosulfonyl or piperazinosulfonyl (heterocyclylsulfonyl not
being a subsdtuent in preferred forms);
or is acylated also or especially by lower alkylaminocarbonyl, such as tert-butyl-
aminocarbonyl, N,N-di-lower alkylaminocarbonyl, such as N,N-dimethylaminocarbonyl,
lower alkoxy-lower alkylaminocarbonyl, such as N-(2-methoxyethyl)aminocarbonyl,
N-lower alkyl-N-morpholino-lower alkylaminocarbonyl, such as N-methyl-
- 2:1~2~)/17
N-(2-morpholinoethyl)aminocarbonyl, or N-morpholino-lower alkylaminocarbonyl;
and/or a hydroxy group of the side chain is present in etherified or esterified form, for
example in the fonn of lower alkoxy, such as methoxy or tert-butoxy, aryl-lower aL~coxy,
such as benzyloxy, lower alkanoyloxy, such as acetoxy, or lower alkoxycarbonyloxy, for
example tert-butoxycarbonyloxy. -
Special preference is given to acyl groups Rl or Rg, bonded via the carbonyl group of
the* carboxy function, of an unsubstituted or substituted amino acid selected from phenyl-
alanine, N-(benzyloxycarbonyl)-phenylalanine, tyrosine, tyrosine-O-methyl ether, N-
morpholinocarbonyl-glycine, N-~N-(2-, 3- or 4-pyridyl)methyl-N-methylaminacarbonyl)-
glycine, valine, N-(trifluoroacetyl)-valine, N-phenylacetyl-valine, N-acetyl-valine, N-(3-
phenylpropionyl)-valine, N-(2-carbamoyl-3-phenyl-propionyl)-valine, such as N-(2(R,S)-
carbamoyl-3-phenyl-propionyl)-valine, N-(2- or 3-pyridylacetyl)-valine, N-tetrahydro-
furylmethoxycarbonyl-valine, N-(2-methoxy)ethoxycarbonylvaline, N-3-(tetrazol-1-yl)- :
propionyl-valine, N-(indol-2-ylcarbonyl)-valine, N-(quinolin-2-ylcarbonyl)-valine,
N-methoxycarbonyl-valine, N-ethoxycarbonyl-valine, N-isobutoxycarbonyl-valine,
N-tert-butoxycarbonyl-valine, N-benzyloxycarbonyl-valine, N-(2-furylmethoxy-
carbonyl)-valine, N-allyloxycarbonyl-valine, N-(morpholinocarbonyl)-valine, N-(thio-
morpholinocarbonyl)-valine, N-(S,S-dioxothiomorpholinocarbonyl)-valine, N-(N-2-
pyridylmethyl-N-methylaminocarbonyl)-valine, N-(N-3-pyridylmethyl-aminocarbonyl)- : :
valine, N-(N-2-pyridylmethyl-aminocarbonyl)-valine, N-morpholino-carbonylamino-
acetyl-valine, N-methanesulfonyl-valine, N-morpholinosulfonyl-valine, N-acetyl-leucine,
N-(4-thiomorpholinocarbonyl)-leucine, N-(4-(S,S-dioxothiomorpholino)carbonyl)-leucine,
N-(benzyloxycarbonyl)-leucine, N-asetyl-isoleucine, N-propionyl-isoleucine, N-
(benzyloxycarbonyl)-isoleucine, N-(tert-butoxycarbonyl)-norleucine, N-benzyloxy-carbonyl-glutamic acid, asparagine, glutamine, N-benzyloxycarbonyl-asparagine,
quinolin-2-ylcarbonyl-asparagine and N-(morpholinocarbonyl)-asparagine; or also or
especially the corresponding radicals of N-(3,3-dimethylbutyryl)-valine, N-(n-propoxy-
carbonyl)-valine, N-(2-(2-methoxyethoxy)ethoxycarbonyl)-valine, N-(2-methoxyethoxy-
acetyl)-valine, N-(N,N-dimethylaminocarbonyl)-valine, N-(N-(2-methoxyethyl)amino)-
valine, N-(benzylaminocarbonyl)-valine, N-(2-morpholinoethylaminocarbonyl)-valine or
N-(N-methyl-N-(2-morpholinoethyl)aminosarbonyl)-valine; the amino acid residues
preferably being in the (L)- or (D,L)-form, and in the case of valine also in the (D)-form.
Unsubstituted or substituted alkyl Rl, R2, R8 or Rg is an alkyl radical having from 1
to 20, preferably up to 10, carbon atoms, is branched or unbranched, and is, for example,
21~3~7
- 16-
methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl,
isopentyl, neopentyl, tert-pentyl, n-hexyl, isohexyl, n-heptyl, n-octyl, n-nonyl or n-decyl.
Preference is giYen to lower aLkyl, for exarnple methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl, tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, n-hexyl, isohexyl
or n-heptyl, which is unsubstituted or substituted.
Radicals suitable as substituents in substituted alkyl Rl, R2, R8 or Rg, preferably
substituted lower alkyl, are the radicals mentioned for lower alkanoyl Rl and Rg.
Preference is given especially to unsubstituted lower aLIcyl, such as methyl, ethyl, propyl,
isopropyl, n-butyl, isobutyl or tert-butyl.
AL~cyl-substituted sulfonyl Rl or Rg (aLlcyl-SO2-) preferably contains an unsubstituted or
substituted alkyl radical mentioned under aL~yl Rl, R2, R8 and Rg and is especially
lower aL~anesulfonyl, such as methanesulfonyl, ethanesulfonyl, n-propanesulfonyl or
S-tert-butylsulfonyl, or
aryl-lower aLkyl-substituted sulfonyl (aryl-lower aLkyl-SO2-) that contains, for example,
an unsubstituted or substituted aryl radical as defined for aryl-substituted lower aLIcyl Rl,
R2, R8 and R9 and is selected especially from phenylmethane-, 4-chloro-phenylmethane-,
4-methoxy-phenylrnethane- or 4-nitro phenylmethane-, naphthylmethane-, for example
a- or ,B-naphthylmethane-, 2-phenylethane-, 2-a-naphthylethane-, 2-~-naphthylethane-,
2-(4-methylphenyl)ethane-, 2-(4-methoxyphenyl)ethane-, 3-phenylpropane-,
3-(p-hydroxyphenyl)-propane-, 2,2-diphenylethane- and 2,2-di(4-methoxyphenyl)ethane-
sulfonyl.
Aryl-subsdtuted sul~onyl Rl or R9 (aryl-SO2-) preferably contains an unsubstituted or
subsdtuted aryl radical mendoned in the de~midon of aryl as a subsdtuent of lower
alkanoyl Rl or R9 and is especially benzene- or 1- or 2-naphthalene-sulfonyl that is
unsubstituted or mono- or di-substituted by lower alkyl, such as benzenesulfonyl, 2- or
4-toluenesulfonyl or 1- or 2-naphthalenesulfonyl.
Heterocyclyl-subsdtuted sulfonyl Rl or R9 (heterocyclyl-SO2-) preferably contains
heterocyclyl that is selected from pyrrolyl, furyl, thienyl, imidazolyl, pyrazolyl, oxazolyl,
thiazolyl, pyridyl, pyrazinyl, pyrimidinyl, indolyl, quinolyl and isoquinolyl, which may
also be fully or partially saturated, from morpholinyl and from thiomorpholinyl and may
be unsubsdtuted or subsdtuted, especially by lower aL~cyl, such as methyl, such as
2~12~7
- 17 - ~ -
morpholinosulfonyl, thiomorpholinosulfonyl, piperidinosulfonyl or piperazinosulfonyl. In
especially preferred fonns of the invention, heterocyclylsulfonyl as substituent may be
absent.
Acyloxy R5 has, for example, up to 25, preferably up to 19, carbon atoms and is especially
the acyloxy group, bonded via its carbonyl to the bonding oxygen atom, of a carboxylic
acid or of an unsubstituted or substituted amino acid, also an aminocarbonyloxy group, an
N-substituted aminocarbonyloxy group or an acyl radical of a semiester of carbonic acid
linked via its carbonyl group to the bonding oxygen atom.
A preferred acyloxy group R5 of a carboxylic acid is, for example, unsubstitutedCl-C20aLtcanoyloxy, for example n-decanoyloxy or palmitoyloxy, C3-C20alkenoyloxy or
C3-C20aIkynoyloxy, or substituted Cl-C20alkanoyloxy, C3-C20alkenoyloxy or
C3-C20aL~cynoyloxy, especially lower aL~canoyloxy, octanoyloxy, nonanoyloxy,
decanoyloxy, undecanoyloxy, dodecanoyloxy or also or especially palmitoyloxy;
C3-C7alkenoyloxy; or C3-C7aL~cynoyloxy; or substituted lower alkanoyloxy
wherein the substituents are selected, for example, from one or more radicals, preferably
from up to three radicals, especially from one radical selected from the group consisting
of hydroxy, lower alkoxy, phenoxy, naphthyloxy, lower aLkanoyloxy, phenyl-lower
alkanoyloxy, such as benzoyloxy or phenylacetoxy, halogen, such as fluorine, chlorine,
bromine or iodine, especially fluorine or chlorine, carboxy, lower aLkoxycarbonyl,
phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, carbamoyl, lower aLIcyl-carbamoyl, hydroxy-lower alkylcarbamoyl, di-lower alkylcarbamoyl, bisthydroxy-lower
aLkyl)carbamoyl, cyano, oxo, C3-C8cycloaL~yl, such as cyclopropyl, cyclobutyl,
cyclopentyl or cyclohexyl, C6-Cl2bicycloaL~cyl, such as decahydronaphth-2-yl,
Cg-Cl4tricycloaLkyl, such as 1- or 2-adamantyl, C4-C8cycloalkenyl, such as l-cyclo-
hexenyl or l,~cyclohexadienyl, heterocyclyl which is preferably a saturated, partially
saturated or unsaturated single ring containing from 3 to 7, preferably from 5 to 7, ring
atoms and up to four hetero atoms selected from nitrogen, sulfur and oxygen, preferably
1 or 2 of the mentioned hetero atoms, the ring either being present as such or being once
or twice, preferably once, benzofused, cyclopenta-, cyclohexa- or cyclohepta-fused, and
which may be unsubs.ituted or substituted especially by lower alkyl, lower alkanoyl,
hydroxy, lower alkoxy, phenyl-lower alkoxy, such as benzyloxy, hydroxy-lower aL~cyl,
such as hydroxymethyl, halogen, cyano and/or by trifluoromethyl, for example pyrrolyl,
2,5-dihydropyrrolyl, furyl, thienyl, tetrahydrofuryl, cyclohepta[b]pyrrolyl, pyIrolidinyl,
imidazolyl, imidazolidinyl, pyrazolinyl, pyrazolidinyl, triazolyl, such as 1,2,3-, 1,2,4- or
21120~7
1,3,4-triazolyl, tetrazolyl, such as 1- or 2-tetrazolyl, tetrahydro-oxazolyl, tetrahydro-
isoxazolyl, tetrahydro-thiazolyl, tetrahydro-isothiazolyl, indolyl, isoindolyl, quinolyl,
isoquinolyl, benzimidazolyl, benzofuranyl, pyridyl, pyrimidinyl, piperidinyl, piperazin-
1-yl, morpholino, thiomorpholino, S,S-dioxothiomorpholino, 1,2-dihydro- or 1,2,3,4-
tetrahydroquinolyl, or 1,2-dihydro- or 1,2,3,4-tetrahydro-isoquinolyl, the mentioned
radicals being unsubstituted or substituted as above, especially by lower aL~cyl, for
example in 4-lower aLkyl-piperazin- 1-yl, such as 4-methyl- or 4-ethyl-piperazin- l-yl, by
lower alkanoyl, for example in 4-lower alkanoyl-piperazin-1-yl, such as 4-acetyl-
piperazin-1-yl, or by hydroxy-lower alkyl, for example in 5-hydroxymethylfuran-2-yl-
carbonyl; and aryl, preferably C6-CI4aryl, for example phenyl, naphthyl, such as 1- or
2-naphthyl, or fluorenyl, such as fluoren-9-yl, aryl being unsubstituted or mono- or
poly-substituted, preferably mono-substituted, for example, by lower alkyl, for example
methyl, halo-lower alkyl, such as trifluoromethyl or chloro- or bromo-methyl, halogen,
for example fluorine or chlorine, hydroxy, lower alkoxy, such as methoxy, lower
alkarloyloxy, carboxy, lower alkoxycarbonyl, phenyl-lower aLkoxycarbonyl, carbamoyl,
mono- or di-lower aIkylcarbamoyl, mono- or di-hydroxy-lower aL~ylcarbamoyl, such as
heterocyclyl-lower alkyl wherein heterocyclyl is as defined above as a substituent of
lower alkanoyloxy Rs, especially heterocyclylmethyl wherein heterocyclyl is bonded via
a ring nitrogen atom, for example piperidinomethyl, piperazin- 1-ylmethyl, 4-lower
alkyl-piperazin-1-ylmethyl, such as 4-methyl- or 4-ethyl-piperazin-1-ylmethyl, 4-lower
aLkanoyl-piperazin-1-ylmethyl, such as 4-acetyl-piperazin-1-ylmethyl, morpholino-
methyl or thiomorpholinomethyl, cyano and/or by nitro, and especially phenyl
substituted in the p-position by one of the mentioned radicals;
for example lower alkanoyloxy, such as formyloxy, acetoxy, propionyloxy, pivaloyloxy or
heptanoyloxy, such as n-heptanoyloxy, hydroxy-lower alkanoyloxy, for example
~-hydroxypropionyloxy, lower alkoxy-lower aLkanoyloxy, for example lower
alkoxyacetoxy or lower alkoxypropionyloxy, such as methoxyacetoxy or ,B-methoxy-
propionyloxy, lower alkanoyloxy-lower alkanoyloxy, for example lower allcanoyloxy- ~ -
acetoxy or lower alkanoyloxypropionyloxy, such as acetoxyacetoxy or ,B-acetoxy-
propionyloxy, halo-lower alkanoyloxy, for example a-haloacetoxy, such as a-chloro-,
a-bromo-, a-iodo-, a,a,a-trifluoro- or a,a,a-trichloro-acetoxy, or halopropionyloxy, such
as ~-chloro- or ~-bromo-propionyloxy, carboxy-lower alkanoyloxy, for example carboxy-
acetoxy or 3-carboxypropionyloxy, lower alkoxycarbonyl-lower alkanoyloxy, for example
lower alkoxycarbonylacetoxy or lower alkoxycarbonylpropionyloxy, such as methoxy- :
carbonylacetoxy, ~-methoxycarbonylpropionyloxy, ethoxycarbonylacetoxy"B-ethoxy-
carbonylpropionyloxy, tert-butoxycarbonylacetoxy or ,B-tert-butoxycarbonylpropionyloxy,
- 21~ 2~ ~7
- 19-
carbamoyl-lower alkanoyloxy, for example carbamoylacetoxy or ,B-carbamoylpropionyl-
oxy, lowcr alkylcarbamoyl-lower alkanoyloxy, di-lower aL~ylcarbamoyl-lower alkanoyl-
oxy, hydroxy-carboxy-lower alkanoyloxy, hydroxy-lower alkoxycarbonyl-lower aLkanoyl-
oxy, dihydroxy-carboxy-lower alkanoyloxy, dihydroxy-lower alkoxycarbonyl-lower
alkanoyloxy, heterocyclyl-lower alkanoyloxy, for example pyrrolylcarbonyloxy, such as
2- or 3-pyrrolylcarbonyloxy, furylcarbonyloxy, for example 2-furylcarbonyloxy,
5-hydroxymethyl-furan-2-ylcarbonyloxy, thienylcarbonyloxy, for example 2-thienyl-
carbonyloxy, imidazolylcarbonyloxy, such as 4-imidazolylcarbonyloxy, imidazolyl-acetoxy, such as 4-imidazolylacetoxy, imidazolylpropionyloxy, such as 3-(4-imidazolyl)-
propionyloxy, pyridylcarbonyloxy, for example 2-, 3- or 4-pyridylcarbonyloxy, indolyl-
carbonyloxy, for example 2-, 3- or S-indolylcarbonyloxy, 1-methyl-, 5-methyl-,
S-methoxy-, S-benzyloxy-, S-chloro- or 4,5-dimethyl-indolyl-2-carbonyloxy, quinolyl-
carbonyloxy, such as quinolin-2-ylcarbonyloxy, pyrrolidinylcarboxy, such as pyrrolid-
inyl-3-carbonyloxy, piperidinylcarbonyloxy, for example 2-, 3- or 4-piperidinylcarbonyl-
oxy, morpholinocarbonyloxy, thiomoqpholinocarbonyloxy, morpholinoacetoxy, thio-
morpholinoacetoxy, or 4-lower alkyl-1-piperazinoacetoxy, such as 4-methyl-piperazino-
acetoxy, lower alkenoyloxy, for example acryloyloxy, vinylacetoxy, crotonoyloxy or 3- or
4-pentenoyloxy, lower aL~cynoyloxy, for example propioloyloxy or 2- or 3-butynoyloxy,
C3-C8cycloaLkylcarbonyloxy or C3-C8cycloalkylacetoxy, for example cyclopropyl-, cyclo-
butyl-, cyclopentyl- or cyclohexyl-carbonyloxy, cyclopropylacetoxy, cyclopentylacetoxy
or cyclohexylacetoxy, phenyl-lower alkanoyloxy, for exarnple benzoyloxy, phenylacetoxy
or 3-phenylpropionyloxy, wherein phenyl is unsubstituted or mono- or poly-substituted by
lower aL~cyl, for example methyl, halo-lower alkyl, such as chloro- or bromo-methyl,
halogen, for example fluorine or chlorine, hydroxy, lower alkoxy, for example methoxy,
piperidinomethyl, piperazin-l-ylmethyl, 4-lower alkyl-piperazin-1-ylmethyl, such as
4-methyl- or 4-ethyl-piperazin-1-ylmethyl, 4-lower alkanoyl-piperazin-1-ylmethyl, such as
4-acetyl-piperazin- 1-ylmethyl, morpholino-lower alkyl, such as morpholinomethyl,
thiomorpholinomethyl, cyano andlor by nitro, for example 4-chloromethyl-, 4-bromo-
methyl-, 4-fluoro-, 4-chloro-, 4-methoxy-, 4-morpholinomethyl-, 4-thiomorpholino-
methyl-, 4-cyano- or 4-nitro-benzoyloxy, or lower alkylphenylacetoxy, such as 4-methyl-
phenylacetoxy.
A preferred acyloxy Rs of an acyl radical, linked via its carbonyl group to the bonding
oxygen atom, of a semiester of carbonic acid is, for example, unsubstituted or substituted
alkoxycarbonyloxy, especially- unsubstituted or substituted lower alkoxycarbonyloxy, for
example methoxy-, ethoxy- or tert-lower alkoxy-carbonyloxy, such as tert-butoxy-
'. :' :, : : ~ ~ . . - . . .
:; ~
211~7
- 20 -
carbonyloxy, 2-halo-lower alkoxycarbonyloxy, for example 2-chloro-, 2-bromo-, 2-iodo-
or 2,2,2-trichloro-ethoxycarbonyloxy; aIyl-lower alkoxycarbonyloxy, for example
arylmethoxycarbonyloxy, wherein aryl preferably has from 6 to 14 carbon atoms, is
unsubstituted or mono- or poly-subsdtuted, preferably mono-substituted, for example, by
lower alkyl, for example methyl, halo-lower aLkyl, such as trifluoromethyl or chloro- or
bromo-methyl, halogen, for example fluorine or chlorine, hydroxy, lower alkoxy, such as
methoxy, lower alkanoyloxy, carboxy, lower alkoxycarbonyl, phenyl-lower aLkoxy-
carbonyl, carbamoyl, mono- or di-lower aLkylcarbamoyl, mono- or di-hydroxy-loweralkylcarbamoyl, heterocyclyl-lower alkyl wherein heterocyclyl is as defined above as a
substituent of lower alkanoyloxy Rs, especially heterocyclylmethyl wherein heterocyclyl
is bonded via a ring nitrogen atom, for example piperidinomethyl, piperazin-l-ylmethyl,
4-lower alkyl-piperazin-l-ylmethyl, such as 4-methyl- or 4-ethyl-piperazin-1-ylmethyl,
4-lower alkanoyl-piperazin-l-ylmethyl, such as 4-acetyl-piperazin-1-ylmethyl,
morpholinomethyl or thiomorpholinomethyl, cyano and/or by nitro, and is especially
phenyl, 1- or 2-naphthyl, fluorenyl, or phenyl mono- or poly-substituted by lower aLkyl,
for example methyl or tert-butyl, lower alkoxy, for example methoxy, ethoxy or tert-
butoxy, hydroxy, halogen, for example fluorine, chlorine or bromine, and/or by nitro, for
example phenyl-lower alkoxycarbonyloxy, such as benzyloxycarbonyloxy, 4-methoxy-benzyloxycarbonyloxy, 4-nitrobenzyloxycarbonyloxy, diphenyl-lower aLkoxycarbonyloxy,
such as diphenylmethoxycarbonyloxy, di(4-methoxyphenyl)methoxycarbonyloxy, trityl-
oxycarbonyloxy or fluorenyl-lower aLIcoxycarbonyloxy, such as 9-fluorenylmethoxy-
carbonyloxy; or heterocyclyl-lower alkoxycarbonyloxy wherein heterocyclyl is as defined
abov~ as a substituent of lower alkanoyloxy R5, for example furan-2-ylmethoxycarbonyl- ~ :
oxy or pyridin-2-, -3- or ~ylmethoxycarbonyl. The definitions falling under the definition
of acyloxy groups R5 of a semiester of carbonic acid may in preferred forms be omitted
from all the definitions of compounds of formula I mentioned hereinbefore and
hereinafter. : -~
A preferred N-substituted aminocarbonyloxy group as acyloxy Rs carries at the nitrogen -
atom one or two substituents selected independently of one another from
unsubstituted or substituted lower alkyl (the substituents being selected from those
mentioned above for substituted lower alkanoyloxy Rs and being present in the number
there-defined, preferably substituents selected from hydroxy, lower alkoxy, lower
alkanoyloxy, phenyl-lower alkanoyloxy, such as benzoyloxy or phenylacetoxy, halogen,
such as fluorine, chlorine, bromine or iodine, especially fluorine or chlorine, carboxy,
lower alkoxycarbonyl, phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl, cyano,
2ll2a47
- 21 -
oxo and phenyl or naphthyl, which are unsubstituted or mono- or poly-substituted,
preferably mono-substituted, for example, by lower alkyl, for example methyl, halo-lower
alkyl, such as trifluoromethyl or chloro- or bromo-methyl, halogen, for example fluorine
or chlorine, hydroxy, lower alkoxy, such as methoxy, lower alkanoyloxy, carboxy, lower
alkoxycarbonyl, phenyl-lower alkoxycarbonyl, cyano and/or by nitro, especially phenyl
substituted in the p-position by one of the mentioned radicals), especially unsubstituted
lower alkyl, such as methyl or ethyl, and
aryl which preferably has from 6 to 14 carbon atoms and is unsubstituted or mono- or
poly-substituted, preferably mono-substituted, for example, by lower alkyl, for example
methyl, halo-lower aL~cyl, such as chloro- or bromo-methyl, halogen, for example fluorine
or chlorine, hydroxy, lower aL~coxy, such as methoxy, lower alkanoyloxy, carboxy, lower
alkGxycarbonyl, phenyl-lower alkoxycarbonyl, halo-lower alkyl, such as trifluoromethyl,
cyano and/or by nitro, the nitrogen atom of the carbamoyl group carrying not more than
one aryl radical;
an acyloxy group Rs of an N-substituted carbamic acid is especially mono- or di-lower
aLlcylaminocarbonyloxy, such as N-medhyl-, N-ethyl-, N,N-dimedlyl- or N,N-diethyl- -
aminocarbonyloxy, or phenyl-lower alkylaminocarbonyloxy wherein phenyl is
unsubstituted or substituted by lower aL~cyl, for example methyl, halo-lower alkyl, such as
chloro- or bromo-methyl or trifluoromethyl, halogen, for exarnple fluorine or chlorine,
hydroxy, lower alkoxy, such as methoxy, carboxy andlor by cyano, preferably by up to
three of those substituents selected independendy of one another, especially by one of
those substituents, for example in dhe p-position, such as in N-benzyl-, N-(4-fluoro-
benzyl)-, N-(4-chlorobenzyl)-, N-(4-trifluoromedhylbenzyl)- or N-(4-cyanobenzyl)-amino-
carbonyloxy; especially preferred is aminocarbonyloxy substituted by only one radical at
the nitrogen atom, for example N-lower aLIcylaminocarbonyloxy, such as N-methyl- or
N-ethyl-aminocarbonyloxy, or phenyl-lower alkylaminocarbonyloxy wherein phenyl is
unsubstituted or substituted by lower aLlcyl, such as methyl, halo-lower alkyl, such as
chloro- or bromo-methyl or trifluoromethyl, halogen, such as fluorine or chlorine,
hydroxy, lower aLtcoxy, such as methoxy, carboxy and/or by cyano, preferably by up to
three of those substituents selected independently of one another, especially by one of
those substituents, for example in the p-position, such as in N-benzyl-, N-(4-fluoro-
benzyl)-, N-(4-chlorobenzyl)-, N-(4-trifluoromethylbenzyl)- or N-(4-cyanobenzyl)-amino-
carbonyloxy. The definitions falling under the definition of acyloxy groups Rs of an
N-substituted carbamic acid, and the radical aminocarbonyloxy Rs may in preferred forms
be omitted from all the definitions of compounds of formula I mentioned hereinbefore and
hersinafter.
" 2112~7
An unsubstituted or substituted amino acid in acyloxy Rs bonded via its carbonyl to the
bonding oxygen atom is preferably formed by the amino acid residues (aminoacyloxy),
bonded via the carbonyl of their carboxy group and an oxygen atom, of an a~ - or~-amino acid, especially
of a natural a-amino acid having the L-configuration, such as those normally occurring
in proteins, or a n epimer of such an amino acid, that is to say having the unnatural
D-configuration, or a D,L,isomeric mixture thereof, a homologue of such an amino acid,
for example wherein the amino acid side chain has been lengthened or shortened by one or
two methylene groups, wherein the amino group is in the ,B-, r- or ~-position and/or
wherein a methyl group has been replaced by hydrogen, a substituted aromatic amino acid
whe~eir. the aromatic rac'.ical has from 6 to 14 carbon atoms, for example a substituted
phenylalanine or phenylglycine wherein the phenyl may be mono- or poly-substituted by
lower aL~cyl, for example methyl, hydroxy, lower alkoxy, for example methoxy, lower
aL~canoyloxy, for example acetoxy, amino, lower aLl~ylamino, for example methylamino, ~ -
di-lower aL1cylamino, for exarnple dimethylamino, lower alkanoylamino, for example
acetylamino or pivaloylamino, lower aL~coxycarbonylamino, for example tert-butoxy-
carbonylamino, arylmethoxycarbonylamino wherein aryl preferably has from 6 to 14carbon atoms, for example benzyloxycarbonylamino or 9-fluorenylmethoxycarbonyl-
amino, halogen, for example fluorine, chlorine, bromine or iodine, carboxy and/or by
nitro, a benz~fused phenylalanine or phenylglycine, such as a-naphthylalanine, or a
hydrogenated phenylalanine or phenylglycine, such as cyclohexylalanine or cyclohexyl- -~ -~
glycine.
Those amino acid residues may be substituted at free amino or hydroxy functions, as
described above for amino acid residues Rl or Rg.
Especially preferred is the residue, bonded via the carbonyl of its carboxy group and an
oxygen atom, of an amino acid selected from glycine (H-Gly-OH), alanine (H-Ala-OH),
2-aminobutyric acid, 3-aminobutyric acid, 4aminobutyric acid, 3-aminopentanoic acid,
4-aminopentanoic acid, 5-aminopentanoic acid, 3-arninohexanoic acid, 4-aminohexanoic
acid or S-aminohexanoic acid, valine (H-Val-OH), norvaline (a-aminovaleric acid),
leucine (H-Leu-OH), isoleucine (H-Ile-OH), norleucine (a-aminohexanoic acid,
H-Nle-OH), serine (H-Ser-OH), homoserine (a-amino-~-hydroxybutyric acid), threonine
(H-Thr-OH), methionine (H-Met-OH), cysteine (H-Cys-OH), proline (H-Pro-OH),
trans-3- and trans-4-hydroxyproline, phenylalanine (H-Phe-OH), tyrosine (H-Ty;-OH),
.~ ~ ......... . . .
r.~
21~2~47
- 23 -
4-aminophenylalanine, 4-chlorophenylalanine, 4-carboxyphenylalanine"B-phenylserine
(,B-hydroxyphenylalanine), phenylglycine, a-naphthylalanine (H-Nal-OH), cyclohexyl-
alanine (H-Cha-OH), cyclohexylglycine, tryptophan (H-Trp-OH), indoline-2-carboxylic
acid, 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, aspartic acid (H-Asp-OH), aspa-
ragine (H-Asn-OH), aminomalonic acid, aminomalonic acid monoamide, glutamic acid(H-Glu-OH), glutamine (H-Gln-OH), histidine (H-His-OH), arginine (H-Arg-OH), lysine
(H-Lys-OH), ~-hydroxylysine, ornithine (a,~-diarninovaleric acid), 3-aminopropanoic
acid, a,~-diaminobutyric acid and a,~-diaminopropionic acid, especially the residue of an
aliphatic amino acid selected from alanine, valine, norvaline, leucine, 3-aminopropionic
acid, 2-aminobutyric acid, 3-aminobutyric acid, 4-aminobutyric acid, 3-aminopentanoic
acid, 4-aminopentanoic acid, 5-aminopentanoic acid, 3-aminohexanoic acid, 4-amino- ~
hexanoic acid or 5-aminohexanoic acid and isoleucine or an amino acid selected from
glycine, asparagine, glutamine, methionine, lysine, histidine, proline and phenylalanine, it
being possible for each of the mentioned amino acids to be in the D-, L- or (D,L)-form,
preferably in the L-form (except in cases where there is no asymmetric carbon atom, for
example in the case of glycine), and
an amino group is unsubstituted or is mono- or di-N-alkylated, for example by lower
alkyl, such as methyl, n-propyl or n-butyl, by pyridyl-lower alkyl, such as 2-, 3- or
4-pyridylmethyl, andlor by phenyl-lower alkyl, such as benzyl, and/or is N-acylated, for
example by unsubstituted or substituted lower aL~anoyl, as defined above for lower
alkanoyloxy Rs~ especially by acetyl, propionyl or pivaloyl, by aryl-lower alkanoyl, for
example phenyl-lower alkanoyl, such as benzoyl or phenylacetyl, by lower aLIcoxy-
carbonyl, such as tert-butoxycarbonyl, or by aryl-lower alkoxycarbonyl, for example
phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl.
Of the last-mentioned residues, preference is given to acyloxy groups Rs of an
unsubstituted or substituted amino acid selected from aminoacetoxy (glycyloxy), N-lower
alkylaminoacetoxy, N,N-di-lower aL~cylaminoacetoxy, N-lower alkyl-N-phenyl-loweralkylaminoacetoxy, N-lower alkyl-N-lower alkoxycarbonylaminoacetoxy and N-phenyl-
lower aLkoxycarbonyl-N-lower alkylaminoacetoxy, for example N-methylaminoacetoxy,
N,N-dimethylaminoacetoxy, N-methyl-N-(n-butyl)aminoacetoxy, N-methyl-N-benzyl-
aminoacetoxy, N-methyl-N-[(2-, 3- or 4-~pyridylmethyl]-aminoacetoxy, such as
N-methyl-N-3-pyridylmethylaminoacetoxy, N-methyl-N-tert-butoxycarbonylamino-
acetoxy, N-benzyloxycarbonyl-N-lower alkylaminoacetoxy, prolyloxy, histidyloxy,
glutamyloxy and asparagyloxy, the amino acid residues preferably being in the (L)- or the
(D)- or (D,L)-form (except in cases where there is no asymmetric carbon atom, for
211~ 7
- 24 -
example in the case of Gly).
Unsubstatuted or substituted alkyl R3, R4 or R7 is preferably one of the radicals
mentioned under alkyl Rl R2, R8 and Rg and is unsubstituted or substituted, especially
by the substituents mentioned for lower alkanoyl Rl or Rg, especially one of those
substituents, and is selected especially from
lower alkyl, for example methyl, ethyl, propyl, isopropyl, n-butyl, isobutyl or tert-butyl,
cycloalkyl-lower alkyl wherein cycloalkyl has, for example, from 3 to 7 carbon atoms,
such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl or cycloheptyl, cycloalkyl being
unsubstituted or mono- to tri-substituted by lower alkyl, such as isopropyl, halo-lower
alkyl, such as trifluoromethyl, hydroxy, lower aL~coxy, amino, mono- ~r di-lower alkyl-
amino, halogen, such as fluorine, chlorine o~ bromine, nitro and/or by cyano and being ~ ~ .
bonded, preferably terminally, to lower alkyl, especially methyl, ethyl, n-propyl,
isopropyl, n-butyl, isobutyl or tert-butyl, for example cyclobutyl-, cyclopentyl-, cyclo-
hexyl- or cycloheptyl-lower alkyl, such as -methyl or -ethyl, preferably cyclohexyl-lower
aLkyl, such as cyclohexylmethyl, and ~:
aryl-lowa alkyl wherein aryl is, for exarnple, independendy as defined for aryl as a
substituent of lower alkanoyl R~ or Rg, which is unsubstituted or substituted as there- ~::
defined, especially phenyl-lower aLIcyl, such as benzyl, 2-phenylethyl, 3-phenylpropyl,
4-fluorobenzyl, 4-cyanobenzyl, 4-trifluorobenzyl, 4-hydroxybenzyl or 4-methoxybenzyl, :::
or: 4-lower alkoxybenzyl (especially having more total alkoxy carbon asoms dlan in
4-methoxybenzyl), such as 4-isobutoxybenzyl, 3,4-di-lower alkoxybenzyl, such as
3,4~imethoxybenzyl, phenyl-lower alkoxybenzyl, such as 4-benzyloxybenzyl,
4-(3,4-di-lower alkoxybenzyloxy)benzyl, lowa alkoxy-lower alkoxybenzyl, such as
4-(2-methoxyethoxy)benzyl, lower alkylenedioxyphenylmethyl, such as 3,4-methylene-
dioxyphenylmethyl, or biphenylylmethyl, such as 4-biphenylylmethyl, especially phenyl-
lower aLkyl, most especially as last defined, or (also or especially) : ~
thienylmethyl or tetrahydropyranylmethyl, such as 2-thienylmethyl or 4-(2,3,5,6-tetra- :
hydro)pyranylmethyl .
Cycloalkyl R3, R4 or R7 is preferably as defined in the definition thereof as a substituent
of lower aL~canoyl Rl or Rg, and is, for example, cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl or cycloheptyl, cycloalkyl being unsubstituted or mono- to tri-substituted by
lower aL~cyl, such as isopropyl, halo-lower alkyl, such as trifluoromethyl, hydroxy, lower
alkoxy, amino, mono- or di-lower alkylamino, halogen, such as fluorine, chlorine or -
bromine, nitro and/or by cyano, such 2S cyclobutyl, cyclopentyl, cyclohexyl or cyclo-
;.;...... .
:, ,, , : .:: : -
:: - . ~ . . :, .. - . , . : ,.
2~2~7
- 25 -
heptyl, especially cyclohexyl.
Aryl ~3, R4 or R7 is preferably independently as defined in the definition thereof as a
substituent of lower aL~anoyl Rl or Rg and, as in tha~ definition, is unsubstituted or
substituted, and is especially phenyl that is unsubstituted or mono- to tri-substituted by
lower alkyl, such as isopropyl, halo-lower alkyl, such as trifluoromethyl, hydroxy, lower
aLl~oxy, halogen, such as fluorine, chlorine or bromine, nitro andlor by cyano, such as
phenyl, 4-methoxyphenyl, 4-fluorophenyl, 4-trifluoromethylphenyl or 4-cyanophenyl.
When, in the compounds of formula I, nitrogen atoms having free hydrogen andlor
hydroxy groups are vicinal with respect to double or t~iple bonds, the corresponding
tautomeric imino and oxo compounds are always also included.
Salts of compounds of formula I are especially acid addition salts, salts with bases or,
where several salt-forming groups are present, can also be mixed salts or inte~nal salts.
Salts are especiaUy the pharmaceuticaUy acceptable, non-toxic salts of compounds of
formula I.
Such salts are formed, for example, by compounds of formula I having an acid group, for
example a carboxy group, a sulfo group, or a phosphoryl group substituted by one or two
hydroxy groups, and are, for example, salts thereof with suitable bases, such as non-toxic
metal salts derived from metals of groups Ia, Ib, IIa and IIb of the Periodic Table of the
~lements, especially suitable aLkali metal salts, for example lithium, sodium or potassium
salts, or aLlcaline earth metal salts, for example magnesium or calcium salts, also zinc salts
or ammonium salts, as well as salts formed with organic amines, such as unsubstituted or
hydroxy-substituted mono-, di- or tri-aL~ylamines, especiaUy mono-, di- or tri-lower aL~cyl-
amines, or with quaternary ammonium compounds, for example with N-methyl-N-ethyl-
amine, diethylamine, triethylamine, mono-, bis- or tris-(2-hydroxy-lower aL~yl)amines,
such as mono-, bis- or tris-(2-hydroxyethyl)amine, 2-hydroxy-tert-butylamine or tris-
(hydroxymethyl)methylamine, N,N-di-lower alkyl-N-(hydroxy-lower alkyl)-amines, such
as N,N-dimethyl-N-(2-hydroxyethyl)-amine or tri~2-hydroxyethyl)amine, N-methyl-D-
glucamine, or quaterna~y ammonium salts, such as tetrabutylarnmonium salts~ The
compounds of formula I having a basic group, for example an amino group, can form acid
addition salts, for example with inorganic acids, for example hydrohalic acids, such as
hydrochloric acid, sulfuric acid or phosphoric acid, or with organic carboxylic, sulfonic,
~ . . . .. . . . . . ........... . . .... . . . .
.
~ . ~ . . ~. ~ , . ..
21~2~3~7
- 26 -
sulfo or phospho acids or N-substituted sulfamic acids, for example acetic acid~ propionic
acid, glycolic acid, succinic acid, maleic acid, hydroxymaleic acid, methylmaleic acid,
fumaric acid, malic acid, tartaric acid, gluconic acid, glucaric acid, glucuronic acid, citric
acid, benzoic acid, cinnarnic acid, mandelic acid, salicylic acid, 4-aminosalicylic acid,
2-phenoxybenzoic acid, 2-acetoxybenzoic acid, embonic acid, nicotinic acid or isonico-
tinic acid, as well as with amino acids, for example the a-amino acids mentioned herein-
before, especially glutamic acid and aspartic acid, and with methanesulfonic acid, ethane-
sulfonic acid, 2-hydroxyethanesulfonic acid, ethane-1,2-disulfonic acid, benzenesulfonic
acid, 4-methylbenzenesulfonic acid, naphthalene-2-sulfonic acid, 2- or 3-phosphoglycer-
ate, glucose-6-phosphate, N-cyclohexylsulfamic acid (forming cyclamates) or with other
acidic organic compounds, such as ascorbic acid. Compounds of formula I having acid ~ ~
and basic groups can also form internal salts. ~ -
For isolation or purification purposes, it is also possible to use pharmaceutically unaccept-
able salts.
The compounds of formula I have valuable pharmacological properties. They have
antiretroviral activity, especially against AIDS, for exarnple against HIV-1 and HIV-2.
They serve as metabolic precursors for compounds of formula II
R2 R7 : ~:
,1~1~ ~R9 (II) ~:
R8
wherein the radicals are as defined for compounds of formula I (analogues of thecompounds of formula I having hydroxy instead of Rs), which are suitable as inhibitors of
retroviral aspartate proteases, especially as inhibitors of the gag-protease of HIV-1 or
HIV-2 (and possibly the aspartoproteases of other retroviruses that cause symptoms
analogous to AIDS), and therefore for the treatment of retroviral diseases, such as AlDS or
its precursors. In that treatment, the compounds of formula II (having hydroxy instead of
Rs in formula I) are released in the body of the animal to be treated, especially a warm-
blooded animal, including a human, from the compounds of formula I.
The compounds of forrnula I preferably have advantageous pharmacodynamic properties
.. . ...
",;. . ~ . ~ . . .
.;;,,. ~, -. . , . -
;i~. , , ~ ... . . . ~ ~ ~ :
2112~3~7
in relation to the compounds of formula II, which can be demonstrated, for example, as
follows:
The compounds of formula I to be investigated and, as control, the comparison compound
of formula II are each dissolved in dimethyl sulfoxide (DMSO) in a concentration of
240 mglml. The resulting solutions are diluted with 20 % (w/v) hydroxypropyl-,B-cyclo-
dextrin (HP~CD) to obtain a concentration of the test compound of 12 mg/ml. Thatsolution is administered to mice in a dose of 120 mg/kg by means of artificial special
feeding. 60, 90 and 120 min after administration the animals are sacrificed and blood is
removed. Three or four animals are examined per time point. The blood is heparinised
and prepared for analysis as follows: an internal standard is added to the heparinised blood
in a final concentration of 4 ~lM. The blood is centrifuged. 0.25 ml of plasma is drawn off
and deproteinised with an equal volume of acetonitrile. After centrifugation the super-
natant is concentrated by drying in vacuo and the residue is suspended in 20 ~11 of 3M
NaCl solution and 100 ~,11 of 0.05M phthalate buffer having a pH of 3Ø The suspension is
extracted first with 1 ml, then with 0.2 ml of diisopropyl ether. The diisopropyl ether
solution is concentrated to dryness by evaporadon and the residue is dissolved in 50 %
(v/v) aqueous acetonitrile. The solution is analysed by reversed-phase HPLC.
The analysis by reversed-phase HPLC is carried out using a 125 x 4.6 mm Nucleosil~
Cl8-column (reversed-phase material supplied by Macherey-Nagel, Duren, Federal
E~epublic of C3ermany, based on silica gel derivatised with hydrocarbon radicals having
18 carbon atoms) equilibrated with a mobile phase of 50 % acetonitrile in water/0.1 %
trifluoroacetic acid. The flow rate is 1 mVmin. Detection is effected at 215 nm.Standards for the compounds in blood are worked up analogously to the blood samples
and used to establish standard curves on the basis of which the in vivo concentrations are
determined.
The following results are obtainable from a comparison of the compounds of formula I
with those of formula II (active component, having hydroxy instead of acyloxy Rs): the
concentration of the active component of formula II in the blood of mice after oral
administration of a compound of formula I, for example of a compound of formula I
wherein Rl is acetyl, may, at most dme points, especially at all the above-mentioned time
points, be significantly higher, for example more than three dmes higher, especially more
than ten times higher and more especially from approximately 20 to approximately 1~0
times higher, than when a compound of formula II is administered in non-esterified form.
21~20~7
Alternatively, or in additionS thereto the absorption of a compound of formula I, for
example of a compound of formula I wherein Rl is acetyl, may be significantly higher, for
example more than four times higher, than the absorption of a compound of formula II. It
is also possible over a prolonged period to maintain a higher blood level with a compound
of formula I than with a compound of formula II.
The obtainable blood concentration of a compound of formula II at the mentioned dme
points is preferably significantly higher than the ED90 determined for the corresponding
compound of formula II in the cell test (see below).
The compounds of formula I, and the compounds of formula II, can also be used asstandards in comparisons of different test systems on different species of animals, which ~ -
represents a further, commercial use. By comparing blood levels in different species of
anima1s, for example, it is possible to compare different animal models.
The compounds of formula II that can be released under physiological conditdons from the ~ ~ -
compounds of the present invention or that serve as stardng materials in the preparation of
compounds of formu1a I, or the salts thereof, have an inhibiting acdon on viral aspartate
proteases, especia11y a gag-protease-inhibitdng acdon. In the tests described below, at
concentrations of 10-6 to 10-9 moVI they inhibit especially the actdon of the gag-protease
of HIV-l and HIV-2 and are therefore suitable as agents against diseases caused by those
retroviruses or by related retroviruses, for example against AIDS or the precursors of
AlDS.
The blood levels of those compounds of formula II can be determined analogously to the
methods mentdoned above for compounds of formula I.
The ability of the compounds of formula II to inhibit the proteolytic activity of, for
example, HIV-l protease can be demonstrated, for example, by the method described by
J. Hansen ee al., The EMBO Journal _, 1785-1791 (1988). In that method, the inhibitdon
of the acdon of the gag-protease is measured on a substrate that is a fusion protein of the
gag-precursor protein and MS-2, expressed in E. coli. The substrate and its cleavage
products are separated by polyacrylamide gel electrophoresis and made visible byimmunoblotting using monoclonal antibodies to MS-2.
In a test that is even simpler to carry out and that gives precise quantitative results, there is
2 1 ~ 7
- 29 -
used as substrate for the gag-protease a synthetic peptide that corresponds to the cleavage
site of the gag-precursor protein. That substrate and its cleavage products can be analysed
by high-pressure liquid chromatography (HPLC).
For example, analogously to the method described by Richards, A.D. ee al., Biol. Chem.
265 (14),7733-7736 (1990), there is used as substrate for a recombinant HIV-1 protease
(preparation in accordance with Billich, S. et al., J. Biol. Chem. 263 (34), 17905 - 17908
(1990)) a synthetic chromophoric peptide (for example HKARVL[N02]FEANleS
(Bachem, Switzerland) or an icosapéptide such as RRSNQVSQNYPIVQNIQGRR
(prepared by peptide synthesis using known methods: J. Schneider et al., Cell 54, 363-368
(1988)) that corresponds to one of the cleavage sites of the gag-precursor protein. That
substrate and its cleavage products can be analysed by high-pressure liquid chromato-
graphy (HPLC).
For that purpose an inhibitor of formula II to be tested is dissolved in dimethyl sulfoxide;
the enzyme assay is carried out by adding suitable dilutions of the inhibitor in 20 mM
~-morpholinoethanesulfonic acid (MES) buffer, pH 6.0, to the assay mix of 67.2 ',lM of
the above-mentioned chromophoric peptide in 0.3M sodium acetate, 0. lM NaCl, pH 7.4,
or 122 IlM of the above-mentioned icosapeptide in 20 mM MES buffer, pH 6Ø The size
of the batches is 100 ~1. The reacdon is started by the addidon of, in the first case, 2 ,ul
and, in the second case,10 ~1 of HIV-l protease and is stopped in the first case after
15 min by the addidon of 100 ~11 of 0.3M HC104 and in the second case after incubation
for one hour at 37C by the addition of 10111 of 0.3M HC104. After centrifugadon of the
sample for S min at 10 000 x g in 100 ,ul (batch with chromophoric pepdde) or 20 ~1
(icosapepdde batch) of the resuldng supernatant and applicadon to a 125 x 4.6 mmNucleosil~9 C18-5,u HPLC column (Macherey ~ Nagel, Duren) and elution, the reacdon
products are quantified by measuring the peak of the cleavage product of formula II at
280 nm (batch with chromophoric peptide) or at 215 nm (batch with icosapeptide),gradient: 100 % eluant 1 -> 50 % eluant ltS0 % eluant 2 (eluant 1: 75 % acetonitrile, 90 ~o
H2O, 0.1 % trifluoroacetic acid (TFA); eluant 2: 75 % acetonitrile, 25 % H2O, 0.08 %
TFA) in the course of 15 min; throughput rate 1 mVmin.
In so doing, it is preferable to determine ICso values (ICso = the concentration that
reduces the activity of HIV- 1 protease by 50 % compared with a control without an
inhibitor) of approximately from 10-6 to 10-9M, especially from approximately 10-7 to
approximately 10-8M.
2:~2~ ~7
- 30 -
In a further test it can be shown that the compounds of formula II that can be released
from the compounds of formula I protect cells that normally become infected by HIV from
such infection or at least retard such infection. In that test the human T-cell leukaemia
cell line MT-2 (Science 229,563 (1985)), which is extremely sensitive eo the cytopatho-
genic effect of HIV, is incubated with HIV alone or with HIV in the presence of the
compounds of the invention and after a few days the viability of the cells thus treated is
assessed.
For that puIpose the MT-2 cells are kept at 37C in humid air with S % C02 in RPMI
1640 medium (Gibco, Switzerland; RPMI 1640 comprises an amino acid mixture without
L-Gln) supplemented with 10 % heat-inactivated foetal calf serum, L-glutamine, hepes
(2-[4-(2-hydroxyethyl)-1-piperazino]-ethanesulfonic acid) and standard antibiotics. 50 ~
of the particular test compound in culture medium and 100 ~1 of HIV-l in culture medium
(800 TCID50/ml) (TCID50 = Tissue Culture Infectious Dose 50 = dose that infects 50 %
of the MT-2 cells) are added to 4x103 exponentially growing MT-2 cells in 50111 of
culture medium per well on 9~well microtitre plates. Parallel batches on a fu;fther micro-
titre plate with cells and test compound receive 100 ~11 of culture medium without virus.
After incubation for 4 days, the reverse transcriptase (RT) activity is determined in 101l1
of cell supernatant. The RT activity is determined in 50 mM of tris (a,a,a-tris(hydroxy-
methyl)methylamine, ultra pure, Merck, Federal Republic of Germany) pH 7.8; 75 mM of
KCI, 2 mM of dithiothreitol, 5 mM of MgC12; 0.05 % Nonidet P-40 (detergent; Sigma,
Switzerland); 50 ~,lg/ml of polyadenylic acid (Pharmacia, Sweden); 1.611g/ml of dT(12-18)
(Sigma, Switzerland). The mixture is filtered through an Acrodisc filter (0.45 ,u: Gellman
Science Inc, Ann Arbor) and stored at -20C. 0.1% (v/v) [alpha-32P]dTTP is added to
aliquots of tnat solution in order to achieve a final radioactive activity of 10 IlCUml. 10 ~1
of the culture supernatant are transferred to a new 9~well microtitre plate and 30 111 of the
mentioned RT cocktail are added thereto. After mixing, the plate is incubated for from 1.5
to 3 hours at 37C. S 111 of that reaction mixture are transferred to Whatman DE81 paper
(Whatman). The dried filters are washed three times for S minutes with 300 mM ofNaCI/25 mM of trisodium citrate and once with 95 % ethanol and again air-d~fied. Eval-
uation is effected in a Matrix Packard 96-well counter (Packard). The ED90 Yalues are
calculated and defined as the lowest concentradon of the par~cular test compound that
reduces the RT activity by 90 % in comparison with cell batches not treated with tne test
compound. The RT activity is a measure of the reproduction of HIV-l .
2~120~7
In that test, the compounds containing hydroxy instead of Rs exhibit an ED90 of approx-
imately from 10-5 to 10-8M, preferably approximately from 5 x 10-7 to 5 x 10-8M.
In the groups of compounds of formula I mentioned below, it may be advantageous, for
example in order to replace rather general definitions with more specific definitions, to use
definitions of radicals from the above-mentioned general de~mitions.
Preference is given to a compound of formula I wherein
Rl and Rg are each independently of the other hydrogen; lower alkanoyl, such as formyl,
acetyl, propionyl, butyryl or pivaloyl, or also or especially 3,3-dimethylbutyryl; especially
acetyl; aryl-lower alkanoyl wherein aryl has from 6 to 14 carbon atoms, preferably as in
phenyl, indenyl, indanyl, naphthyl, anthryl, phenanthryl, acenaphthyl or fluorenyl, and
may be unsubstituted or mono- to tri-substituted especially by lower alkyl, for exarnple
methyl, ethyl or propyl, halo-lower aLIcyl, for example trifluoromethyl, phenyl, 1- or
2-naphthyl, hydroxy, lower alkoxy, for example methoxy, carbamoyl-lower aLkoxy,
N-lower alkylcarbamoyl-lower alkoxy or N,N-di-lower aLkylcarbams)yl-lower aLtcoxy,
amino, mono- or di-lower aL~sylamino, lower alkanoylamino, for example pivaloylamino,
halogen, for example fluorine, chlorine or bromine, carboxy, lower aLkoxycarbonyl, such
as tert-butoxycarbonyl, phenyl-, naphthyl- or fluorenyl-lower alkoxycarbonyl, such as
benzyloxycarbonyl, lower aLkanoyl, sulfo, lower alkylsulfonyl, for example methyl-
sulfonyl, phosphono, hydroxy-lower aL~coxyphosphoryl or di-lower aL~coxyphosphoryl,
carbamoyl, mono- or di-lower alkylcarbamoyl, sulfamoyl, mono- or di-lower aL~ylamino-
sulfonyl, nitro and/or by cyano, and wherein lower alkanoyl is unsubsdtuted or substituted
by carbamoyl or by carbamoyl subsdtuted at the nitrogen atom by one or two radicals
selected from lower alkyl, carboxy-lower aLkyl, lower alkoxycarbonyl-lower alkyl,
di-lower alkylamino-lower aLkyl, hydroxy-lower alkyl and di-lower alkoxy-lower alkyl,
preferably as described under aryl-lower alkanoyl above in the general definitlons, for
example 4-chloro-, 4-methoxy- or 4-nitro-benzoyl, naphthylcarbonyl, such as a- or
,B-naphthylcarbonyl, or 1,8-naphthalenedicarbonyl bonded to the amino group via both
carbonyl groups, phenyl-lower alkanoyl, such as phenylacetyl or 3-phenylpropionyl, lower
aL~cylphenylacetyl, such as 4-methylphenylacetyl, lower alkoxyphenylacetyl, such as
4-methoxyphenylacetyl, 3-(p-hydroxyphenyl)-propionyl, diphenylacetyl, di(4-methoxy-
phenyl)acetyl, triphenylacetyl, 2,2-dibenzylacetyl, 3-a- or 3-~-naphthylpropionyl,
phenyl-lower alkanoyl wherein the lower alkanoyl radical is substituted by carbamoyl,
such as 2-carbamoyl-3-phenylpropionyl, for example 2(R,S)-carbamoyl-3-phenyl- -
propionyl, 3-phenyl- or 3-a-naphthyl-2-carbamoylpropionyl, 3-phenyl- or 3-a-naphthyl- -: -
2ll2a~7
- 32 -
2-tert-butylcarbamoylpropionyl, 3-phenyl- or 3-a-naphthyl-2-(2-dimethylaminoethyl)-
carbamoylpropionyl, especially phenyl-lower alkanoyl, such as phenylacetyl, or phenyl-
lower alkanoyl wherein the lower alkanoyl radical is substituted by carbamoyl, such as
2-carbamoyl-3-phenylpropionyl, for example 2(R,S)-carbamoyl-3-phenylpropionyl;
heterocyclyl-lower alkanoyl wherein heterocyclyl is thienyl, furyl, pyrrolyl, imidazolyl,
pyrazolyl, oxazolyl, thiazolyl, tetrazolyl, pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl,
indolyl, benzimidazolyl, quinolyl, isoquinolyl, 3,1-benzofuranyl, cyclohexa[b]pyrrolyl,
cyclohexa[b]pyridyl, cyclohexalb]pyrazinyl, cyclohexa[b]pyrimidinyl, pyrrolidinyl,
pyrrolinyl, imidazolidyl, piperidyl, piperazinyl, morpholinyl, thiomorpholinyl, S,S dioxo-
thiomorpholinyl, indolinyl, isoindolinyl, 4,5,6,7-tetrahydroindolyl, 1,2,3,4-tetrahydro-
quinolyl or 1,2,3,4-tetrahydroisoquinolyl, which is bonded via a ring carbon atom or a ring
nitrogen atom, in the case of saturated heterocyclic compounds preferably via a ring
nitrogen atom, especially indolylcarbonyl, such as 2-, 3- or S-indolylcarbonyl, quinolyl-
lower alkanoyl, for example quinolylcarbonyl, such as 2-, 3- or 4-quinolylcarbonyl,
piperidylcarbonyl, such as piperidinocarbonyl or 2-, 3- or 4-piperidylcarbonyl, piperaz-
inylcarbonyl, such as piperazin-l-ylcarbonyl, morpholinyl-lower aLt~anoyl, for example
morpholino-lower aLkanoyl, for example morpholinocarbonyl, such as morpholino-
carbonyl, thiomorpholinyl-lower alkanoyl, for example thiomorpholino-lower aLkanoyl,
such as thiomorpholinocarbonyl, such as thiomorpholinocarbonyl, S,S-dioxothiomorpho-
linylcarbonyl, such.as S,S-dioxothiomorpholinocarbonyl, tetrazolyl-lower aLkanoyl, such
as 3-(tetrazol-1-yl)-propionyl, and pyridyl-lower alkanoyl, for example pyridylcarbonyl,
such as 2-, 3- or 4-pyridylcarbonyl, or pyridylacetyl, such as ~-, 3- or 4-pyridylacetyl, with
heterocyclyl-lower alkanoyl being selected especially from morpholino-lower alkanoyl,
such as morpholinocarbonyl, thiomorpholino-lower alkanoyl, such as thiomorpholin~
carbonyl, pyridyl-lower alkanoyl, such as 2-, 3- or 4-pyridylacetyl, and te~razolyl-lower
alkanoyl, such as 3-tetrazol-1-ylpropionyl; (lower alkoxy-lower aL~oxy)-lower alkanoyl;
amino-lower alkanoyl substituted at the amino nitrogen atom by heterocyclyl-lower
alkanoyl wherein heterocyclyl-lower alkanoyl is independently as defined above for
heterocyclyl-lower alkanoyl Rl or Rg, especially amino-lower alkanoyl substituted at the
amino nitrogen atom by N-morpholino-carbonyl or by N-thiomorpholinocarbonyl, more
especially N-morpholino- or N-thiomorpholino-carbonylamino-lower aLkanoyl~ such as
N-morpholino- or N-thiomorpholino-carbonylamino-acetyl; halo-lower alkanoyl
containing up to three halogen atoms, especially a-haloacetyl, such as a-fluoro-,
a-chloro-, a-bromo-, a-iodo-, a,a,a-trifluoro- or a,a,a-trichloro-acetyl, or halopropionyl,
such as ~B-chloro- or ,3-bromo-propionyl, especially trifluoroacetyl; (N-heterocyclyl-lower
alkylcarbamoyl)-lower alkanoyl wherein heterocyclyl is preferably selected from pyrrolyl,
2112~7
- 33 -
furyl, thienyl, imidazolyl, pyrazolyl, oxazolyl, thiazolyl, pyridyl, pyrazinyl, pyrimidinyl,
indolyl, quinolyl and isoquinolyl, which may also be fully or partially saturated, from
morpholinyl and from thiomorpholinyl, especially 2-(N-morpholino-lower alkyl-
carbamoyl)-lower alkanoyl, such as 2(R,S)-(N-(2-morpholinoethyl)-carbamoyl)-3-methyl-
butyryl, or 2-(N-(pyridyl-lower alkyl)-carbamoyl)-lower alkanoyl, such as 2(R,S)-
(N-(2-pyridylmethyl)-carbamoyl)-3-methylbutyryl; lower alkoxycarbonyl, especially
methoxy-, ethoxy-, isopropoxy-, isobutoxy- or tert-lower alkoxy-carbonyl, for example
methoxycarbonyl, tert-butoxycarbonyl or isobutoxycarbonyl; aryl-lower alkoxycarbonyl
wherein aryl is phenyl, biphenylyl, 1- or 2-naphthyl, fluorenyl, or phenyl that is mono- or
poly-substituted, preferably up to tri-substituted, especially mono-substituted, by lower
alkyl, for example methyl or tert-butyl, hydroxy, lower alkoxy, for example methoxy,
ethoxy or tert-butoxy, halogen, for example chlorine or bromine, and/or by nitro, for
example phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl or 4-methoxybenzyl-
oxycarbonyl, especially phenyl-lower alkoxycarbonyl, such as benzyloxycarbonyl; hetero-
cyclyl~lower alkoxycarbonyl wherein heterocyclyl is selected from pyrrolyl, furyl, thienyl,
imidazolyl, pyrazolyl, oxazolyl, thiazolyl, pyridyl, pyrazinyl, pyrimidinyl, indolyl,
quinolyl and isoquinolyl, which may also be fully or partially saturated, and from morpho-
linyl and from thiomorpholinyl and is unsubstituted or substituted by lower alkyl, such as
by methyl, for example 2-furylmethoxycarbonyl, or tetrahydrofuryl-lower aLkoxycarbonyl,
such as 2-tetrahydrofurylmethoxycarbonyl, especially tetrahydrofuryl-lower alkoxy-
carbonyl, such as 2(R,S)-tetrahydrofurylmethoxycarbonyl; lower aL~cenyloxycarbonyl
wherein the lower alkenyl radical is bonded to the oxygen atom via a saturated carbon
atom, for example allyloxycarbonyl; lower alkoxy-lower alkoxycarbonyl, such as
2-methoxyethoxycarbonyl; (lower alkoxy-lower alkoxy)-lower alkoxycarbonyl, such as
2-(2-methoxyethoxy)ethoxycarbonyl; lower aL~anesulfonyl, for example methane- orethane-sulfonyl, especially methanesulfonyl; heterocyclylsulfonyl (heterocyclyl-SO2-)
wherein heterocyclyl is selected from pyrrolyl, furyl, thienyl, imidazolyl, pyrazolyl,
oxazolyl, thiazolyl, pyridyl, pyrazinyl, pyrimidinyl, indolyl, quinolyl and isoquinolyl, ~ .
which may also be fully or partia11y saturated, from morpholinyl and from thiomorpho- ::
linyl and may be unsubstituted or substituted by lower alkyl, such as methyl, such as -
morpholinosulfonyl, thiomorpholinosulfonyl, piperidinosulfonyl or piperazinosulfonyl;
carbamoyl; N-heterocyclyl-lower alkyl-N-lower alkylcarbamoyl wherein heterocyclyl is
independently one of the radicals mentioned above in the definition of heterocyclyl-lower
aL~anoyl Rl or R9, especially pyridyl, such as 2-, 3- or 4-pyridyl, preferably N-pyridyl-
lower alkyl-N-lower aL~cylcarbamoyl, such as N-(2-, 3- or 4-pyridylmethyl)-N-methyl-
carbamoyl; or also or especially N-lower alkyl-N-(morpholino-lower alkyl)-amino-
~, .~ . . . .
~',r':
,: - , - . - .
- 2:Ll~.~47
- 34 -
carbonyl, such as N-methyl-N-(2-morpholinoethyl)-aminocarbonyl; or an acyl radical of
an amino acid the amino function of which is free or acylated by one of the other radicals
mentioned hitherto for Rl and R9 with the exception of one of the mentioned aminoacyl
radicals, the amino acid residues being selected from the residues, bonded via the carbonyl
of their l-carboxy group, of the amino acids glycine, alanine, valine, norvaline, leucine,
isoleucine, norleucine, seIine, homoserine, threonine, methionine, cysteine, proline,
trans-3- and trans-4-hydroxyproline, phenylalanine, tyrosine, 4-aminophenylalanine,
4-chlorophenylalanine, 4-carboxyphenylalanine"B-phenylserine, phenylglycine,
a-naphthylalanine, cyclohexylalanine, cyclohexylglycine, ~yptophan, indoline-2-
carboxylic acid, 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, aspartic acid, aspa-
ragine, aminomalonic acid, aminomalonic acid monoamide, glutamic acid, glutamine,
histidine, arginine, Iysine, ~-hydroxylysine, ornithine, 3-aminopropanoic acid, a,~-di-
aminobutyric acid and a"B-diaminopropionic acid, more especially the residues of an
amino acid selected from valine, alanine, leucine, isoleucine, glycine, glutamic acid and
asparagine, it being possible for each of the men~ioned amino acids to be in the D-, L- or
(D,Lj-form, preferably in the L-form (with the exception of Val, which may also be in the
(D)- or (D,L)-form); and the a-amino group being unsubstituted or N-acylated by one of
the radicals mentioned above for Rl and Rg, especially by lower aLkanoyl, phenyl-lower
alkanoyl, such as phenylacetyl, phenyl-lower alkanoyl wherein the lower aLIcanoyl radical
is substituted by carbamoyl, such as 2(R,S)-carbamoyl-3-phenyl-propionyl, morpholino-
lower alkanoyl, such as morpholinocarbonyl, thiomorpholino-lower alkanoyl, such as thio-
morpholino-carbonyl, pyridyl-lower alkanoyl, such as 2-, 3- or 4-pyridylacetyl,
quinolinyl-lower alkanoyl, such as quinolin-2-ylcarbonyl, tetrazolyl-lower alkanoyl, such
as 3-tetrazol-1-ylpropionyl, lower alkoxy-lower alkoxy-lower alkanoyl, amino-lower
alkanoyl substituted at the amino nitrogen atom by N-morpholino- or N-thiomorpholino-
carbonyl, for example N-morpholino- or N-thiomorpholino-carbonylamino-lower
alkanoyl, such as N-morpholino- or N-thiomorpholino-carbonylarninoacetyl, halo-lower
alkanoyl containing up to three halogen atoms, for example a-haloacetyl, such as ~ ;
a-fluoro-, a-chloro-, a-bromo-, a-iod~, a,a,a-trifluoro- or a,a,a-trichloro-acetyl, or
hal~propionyl, such as ,B-chloro- or ,B-bromo-propionyl, especially trifluoroacetyl,
2-(N-morpholino-lower alkylcarbamoyl)-lower alkanoyl, such as 2(R,S)-(N-(2-morpho-
linoethyl)-carbamoyl-3-methyl-butyryl, 2-(N-(pyridyl-lower alkyl)-carbamoyl)-lower
alkanoyl, such as 2(R,S)-(N-(2-pyridylmethyl)-carbamoyl)-lower alkanoyl, lower alkoxy-
carbonyl, phenyl-lower alkoxycarbonyl, tetrahydrofuryl-lower alkoxycarbonyl, such as
2(R,S)-tetrahydrofurylmethoxycarbonyl, lower alkenyloxycarbonyl wherein the lower
alkenyl radical is bonded via a saturated carbon atom to the bonding oxygen atom, lower
.' ` . ' '':'
, ~ . ,C.,r.~
:~
:
-, 2~ a~7
- 35 -
alkoxy-lower alkoxycarbonyl, (lower alkoxy-lower alkoxy)-lower alkoxycarbonyl, lower
alkanesulfonyl, morpholinosulfonyl, thiomorpholinosulfonyl, piperidinosulfonyl,
4-methylpiperazinylsulfonyl or piperazinosulfonyl or N-pyridyl-lower alkyl-N-lower
alkyl-carbamoyl, such as N-(2-, 3- or 4-pyridylmethyl)-N-methyl-carbamoyl; or also or
especially N-(phenyl-lower aLkyl)-aminocarbonyl, such as N-benzylarninocarbonyl,N-lower aLkylaminocarbonyl, such as tert-butylaminocarbonyl, N,N-di-lower aLkylamino~
carbonyl, such as N,N-dimethylaminocarbonyl, N-(lower alkoxy-lower alkyl)-amino-carbonyl, such as N-(2-methoxyethoxy)-aminocarbonyl or N-(morpholino-lower aLIcyl)-
aminocarbonyl, such as N-(2-morpholinoethyl)-aminocarbonyl, or an acyl radical of an
amino acid, as defined above, wherein the a-amino group is acylated by one of those
radicals,
with the proviso that not more than one of the two radicals Rl and Rg may be hydrogen,
R2, R4, R6 and R8 are hydrogen,
R3 is lower alkyl, such as isobutyl or n-butyl, C3-C7cycloaL~cyl-lower alkyl wherein
C3-C7cycloalkyl is unsubstituted or mono- to tn-substituted by lower alkyl, such as -.
isopropyl, halo-lower alkyl, such as trifluoromethyl, hydroxy, lower alkoxy, amino, mono-
or di-lower alkylamino, halogen, such as fluorine, chlorine or bromine, nitro andtor by
cyano and is bonded, preferably terminally, to lower alkyl, especially methyl, ethyl,
n-propyl, isopropyl, n-butyl, isobutyl or tert-butyl, such as cyclobutyl-, cyclopentyl-,
cyclohexyl- or cycloheptyl-lower aLIcyl, such as -methyl or -ethyl, especially cyclohexyl-
lower alkyl, most especially cyclohexylmethyl; or is aryl-lower alkyl wherein aryl is
independently as defined in aryl-lower aLkanoyl Rl or Rg, or is also or especially phenyl-
lower alkoxybenzyl, 4-(3,4-di-lower alkoxybenzyloxy)benzyl, lower alkoxy-lower
alkoxybenzyl or lower alkylenedioxyphenylmethyl, and is especially phenyl that may be
unsubstituted or mono- to tri-subsdtuted by lower aLkyl, for example methyl, ethyl or
isopropyl, halo-lower alkyl, such as trifluoromethyl, phenyl, 1- or 2-naphthyl, hydroxy,
lower aL~oxy, for example methoxy, or also or especially isobutoxy, carbamoyl-lower
alkoxy, N-lower alkylcarbamoyl-lower alkoxy or N,N-di-lower alkylcarbamoyl-loweralkoxy, amino, mono- or di-lower alkylamino, lower alkanoylamino, for example
pivaloylamino, halogen, for example fluorine or chlorine, carboxy, lower aLkoxycarbonyl,
benzyl-, naphthyl- or fluorenyl-lower alkoxycarbonyl, lower aLkanoyl, sulfo, lower alkyl-
sulfonyl, phosphono, hydroxy-lower alkoxyphosphoryl or di-lower alkoxyphosphoryl,
carbamoyl, mono- or di-lower alkylcarbamoyl, sulfamoyl, mono- or di-lower alkyl-sulfamoyl, nitro andlor by cyano, especially phenyl-lower alkyl that is unsubstituted or
substituted by the mentioned substituents, especially benzyl, 2-phenylethyl, 3-phenyl-
propyl, 4-fluoro-, 4-tlifluoromethyl-, 4-cyano-, 4-methoxy- or 4-hydroxy-benzyl, or also
.. ~ .
~;. :.. : , '' . - ::
"-... . ;' :': ' " :
2:~l2a~7
- 36-
or especially: 4-lower alkoxybenzyl (especially having more total alkoxy carbon atoms
than in 4-methoxybenzyl), such as 4-isobutoxybenzyl, 3,4-di-lower alkoxybenzyl, such as
3,4-dimethoxybenzyl, phenyl-lower alkoxybenzyl, such as 4-benzyloxybenzyl, 4-(3,4-di-
lower alkoxybenzyloxy)benzyl, lower alkoxy-lower alkoxybenzyl, such as 4-(2-methoxy-
ethoxy)benzyl, lower aL~ylenedioxyphenylmethyl, such as 3,4-methylenedioxyphenyl-
methyl, or biphenylylmethyl, such as 4-biphenylylmethyl, more especially phenyl-lower
alkyl, especially as last defined, or also or especially
thienylmethyl or tetrahydropyranylmethyl, such as 2-thienylmethyl or 4-(2,3,5,~-tetra-
hydro)pyranylmethyl,
Rs is lower alkanoyloxy, octanoyloxy, nonanoyloxy, decanoyloxy, undecanoyloxy,
dodecanoyloxy, hydroxy-lower alkanoyloxy, lower alkoxy-lower alkanoyloxy, lower
aLkanoyloxy-lower aL~canoyloxy, halo-lower aLIcanoyloxy, for example a-haloacetoxy,
such as a-chloro-, a-bromo-, a iodo-, a,a,a-trifluoro- or a,a,a-trichloro-acetoxy,
carboxy-lower alkanoyloxy, lower alkoxycarbonyl-lower alkanoyloxy, carbamoyl-lower
alkanoyloxy, lower alkylcarbamoyl-lower alkanoyloxy, di-lower aLkylcarbamoyl-lower
alkanoyloxy, hydroxy-carboxy-lower alkanoyloxy, hydroxy-lower aLkoxycarbonyl-lower
alkanoyloxy, dihydroxy-carboxy-lower alkanoyloxy, dihydroxy-lower alkoxycarbonyl-
lower alkanoyloxy, pyrrolylcarbonyloxy, such as 2- or 3-pyrrolylcarbonyloxy, furyl-lower -
alkanoyloxy, for example furylcarbonyloxy, such as 2-furylcarbonyloxy, thienylcarbonyl-
oxy, for example 2-thienylcarbonyloxy, imidazolyl-lower alkanoyloxy, for exampleimidazolylcarbonyloxy, such as 4-imidazolylcarbonyloxy, imidazolylacetoxy, such as
4-imidazolylacetoxy, imidazolylpropionyloxy, such as 3-(4-imidazolylpropionyloxy,
pyridyl-lower alkanoyloxy, such as pyridylcarbonyloxy, for example 2-, 3- or 4-pyridyl-
carbonyloxy, indolylcarbonyloxy, for example 2-, 3- or 5-indolylcarbonyloxy, quinolyl-
lower alkanoyloxy, such as quinolinylcarbonyloxy, such as quinolin-2-ylcarbonyloxy,
pyrrolidinylcarboxy, such as pyrrolidinyl-3-carbonyloxy, piperidinylcarbonyloxy, for
example 2-, 3- or 4-piperidinylcarbonyloxy, morpholinocarbonyloxy, thiomorpholino-
carbonyloxy, morpholinoacetoxy, thiomorpholinoacetoxy or 4-lower alkyl-l-piperazin~
acetoxy, such as 4-methyl-piperazinoacetoxy, lower alkenoyloxy (wherein the lower
aLtcenoyl radical is preferably bonded to the bonding oxygen atom via a saturated carbon
atom~, for example acryloyloxy, vinylacetoxy, crotonoyloxy or 3- or 4-pentenoyloxy,
lower alkynoyloxy, for example propioloyloxy or 2- or 3-butynoyloxy, C3-C8cycloalkyl-
carbonyloxy, C3-C8cycloalkylacetoxy, phenyl-lower alkanoyloxy, for example benzoyl-
oxy, phenylacetoxy or 3-phenylpropionyloxy, which may be unsubstituted, mono- orpoly-substituted in the phenyl radical by lower alkyl, for example methyl, halo-lower
alkyl, such as chloro- or bromo-methyl, halogen, for example fluorine or chlorine,
: .
, - .,
., ,
,~..... .. ~ : ~ . .
2 ~
hydroxy, lower alkoxy, for example methoxy, piperidinomethyl, piperazin- l-ylmethyl,
4-lower alkyl-piperazin-l-ylmethyl, such as 4-methyl- or 4-ethyl-piperazin-1-ylmethyl,
4-lower alkanoyl-piperazin-l-ylmethyl, such as 4-acetyl-piperazin-1-ylmethyl,
morpholino-lower alkyl, such as morpholinomethyl, thiomolpholinomethyl, cyano and/or
by ni~ro, especially mono-substituted by one of the mentioned substituents, or is the
residue, bonded via a carbonyloxy group containing the carbonyl from the carboxy group
of the amino acid in question, of an amino acid selected from glycine, alanine, 2-amino-
butyric acid, 3-aminobutyric acid, 4-aminobutyric acid, 3-aminopentanoic acid, 4-amino-
pentanoic acid, S-aminopentanoic acid, 3-aminohexanoic acid, 4-aminohexanoic acid,
S-arninohexanoic acid, valine, norvaline, leucine, isoleucine, norleucine, serine, homo-
serine, threonine, methionine, cysteine, proline, phenylalanine, tyrosine, cyclohexyl- - -
alanine, tryptophan, aspartic acid, asparagine, glutamic acid, glutamine, histidine,
arglnine, lysine, ornithine, 3-aminopropanoic acid, a,y-diaminobutyric acid and
a"B-diaminopropionic acid; especially the radical, bonded via carbonyloxy, of an aliphatic
amino acid selected from alanine, valine, norvaline, leucine, 3-aminopropionic acid,
2-aminobutyric acid, 3-aminobutyric acid, 4-aminobutyric acid, 3-aminopentanoic acid,
4-aminopentanoic acid, S-aminopentanoic acid, 3-aminohexanoic acid, 4-aminohexanoic ~: -
acid, 5-aminohexanoic acid and isoleucine or an amino acid selected from glycine, -
asparagine, glutamine, methionine, lysine, histidine, proline and phenylalanine, it being ::
possible for each of the mentioned amino acids to be in the D-, L- or (D,L)-form,
preferably in the ~form (except in cases where there is no asymmetric carbon atom, for
example in the case of glycine); and wherein an amino group is unsubstituted or is mono-
or di-N-alkylated by lower alkyl, such as methyl, n-propyl or n-butyl, by pyridyl-lower
alkyl, such as 2-, 3- or 4-pyridylmethyl, and/or by phenyl-lower alkyl, such as benzyl,
andlor is N-acylated by lower alkanoyl, especially acetyl, propionyl or pivaloyl, by
phenyl-lower alkanoyl, such as benzoyl or phenylacetyl, by lower alkoxycarbonyl, such as
tert-butoxycarbonyl, or by phenyl-lower aLt~oxycarbonyl, such as benzyloxycarbonyl; or is
also or especially palmitoyloxy;
and
R7 is independently of R3 one of the radicals there-defined, especially lower alkyl, more
especially isobutyl or n-butyl, C3-C7cycloalkyl-lower alkyl, especially cyclohexyl-lower
alkyl, such as cyclohexylmethyl, or aryl-lower alkyl, as described for aryl-lower alkyl R3,
especially phenyl-lower alkyl that is unsubstituted or substituted by the mentioned
substituents, such as benzyl, 2-phenylethyl, 3-phenylpropyl, ~fluoro-, 4-trifluoromethyl-,
4-cyano-, 4-methoxy- or 4-hydroxy-benzyl,
or also or especially ~lower alkoxybenzyl (especially having more total alkoxy carbon
'~
21.~2~7
- 38 -
atoms than 4-methoxybenzyl), such as 4-isobutoxybenzyl, 3,4-di-lower alkoxybenzyl,
such as 3,4-dimethoxybenzyl, phenyl-lower allcoxybenzyl, such as 4-benzyloxybenzyl,
4-(3,4-di-lower alkoxybenzyloxy)benzyl, lower alkoxy-lower alkoxybenzyl, such as4-(2-methoxyethoxy)benzyl, lower aL~cylenedioxyphenylmethyl, such as 3,4-methylene-
dioxyphenylmethyl, or biphenylylmethyl, such as 4-biphenylylmethyl, especially phenyl-
lower allcyl, especially as last defined, or also or especially
thienylmethyl or tetrahydropyranylmethyl, such as 2-thienylmethyl or 4-(2,3,5,6-tetra-
hydro)pyranylmethyl,
or a salt thereof where at least one salt-forming group is present.
Great preference is given to a compound of formula I wherein Rl and Rg are each
independently of the other hydrogen, lower alkanoyl, such as acetyl or propionyl, phenyl-
lower alkanoyl, such as phenylacetyl, phenyl-lower aL~canoyl wherein the lower aLkanoyl
radical is substituted by carbamoyl, such as 2-carbamoyl-3-phenyl-propionyl, morpho-
lino-lower alkanoyl, such as morpholinocarbonyl, thiomorpholino-lower aLkanoyl, such as
thiomorpholino-carbonyl, pyridyl-lower alkanoyl, such as 2-, 3- or 4-pyridylacetyl, . :
quinolyl-lower alkanoyl, such as quinolin-2-ylcarbonyl, tetrazolyl-lower alkanoyl, such as
3-tetrazol-1-yl-propionyl, amino-lower alkanoyl substituted at the amino nitrogen atom by ~
N-morpholino- or N-thiomorpholino-carbonyl, for example N-morpholino- or N-thio- ~:
morpholino-carbonylamino-lower alkanoyl, such as N-morpholino- or N-thiomorpholino- ~ -
carbonylamino-acetyl, halo-lower alkanoyl containing up to three halogen atoms, such as
trifluoroacetyl, 2-(N-morpholino-lower alkylcarbamoyl)-lower alkanoyl, such as 2(R,S)-
(N-(2-morpholinoethyl)-carbamoyl)-3-methyl-buwl, 2-(N-pyridyl-lower alkyl-
carbamoyl)-lower alkanoyl, such as 2(R,S)-(N-(2-pyridylmethyl)-carbamoyl)-3-methyl-
butyryl, lower alkoxycarbonyl, such as methoxy-, ethoxy-, isobutoxy- or tert-lower
alkoxy-carbonyl, phenyl-lower aLl~oxycarbonyl, such as benzyloxycarbonyl, tetrahydro-
fuTyl-lower alkoxycarbonyl, such as 2-tetrahydrofuryl-methoxycarbonyl, lower
alkenyloxycarbonyl (preferably having lower alkenyl bonded via a saturated carbon atom
to the bonding oxygen atom), such as allyloxycarbonyl, lower alkoxy-lower alkoxy-
carbonyl, such as 2-methoxyethoxycarbonyl, (lower alkoxy-lower alkoxy)-lower alkoxy-
carbonyl, such as 2-(2-methoxyethoxy)-ethoxycarbonyl, lower alkanesulfonyl, for
example methane- or ethane-sulfonyl, morpholinosulfonyl, thiomorpholinosulfonyl,N-pyridyl-lower alkyl-N-lower alkylcarbamoyl, such as N-(2-pyridylmethyl)-N-methyl-
aminocarbonyl, or an acyl radical, bonded via the carbonyl of its carboxy group, of an
amino acid selected from glycine, alanine, valine, leucine, isoleucine, glutamic acid and
asparagine in the (D)-, (L)- or (D,L)-form (with the exception of glycine), wherein the
2 ~
- 39 -
a-amino group is unsubstituted or acylated by one of the other radicals Rl or R9mentioned hitherto, with the exception of an acyl radical of an amino acid, greatest
preference beiing given to the acyl radicals of N-morpholinocarbonyl-glycine, N-(N-(2-, 3-
or 4-pyridyl)methyl-N-methylaminocarbonyl)-glycine, valine, N-(trifluoroacetyl)-valine,
N-phenylacetyl-valine, N-acetyl-valine, N-(2-carbamoyl-3-phenyl-propionyl)-valine,
N-(2-, 3- or 4-pyridylacetyl)-valine, N-2-tetrahydrofuryl-[2H]-methoxycarbonyl-valine,
N-(2-methoxy)ethoxycarbonyl-valine, N-(2-methoxyethoxy)ethoxycarbonyl-valine,
N-(3-(tetrazol-1-yl)-propionyl)-valine, N-(quinolin-2-ylcarbonyl)-valine, N-methoxy-
carbonyl-valine, N-isobutoxycarbonyl-valine, N-tert-butoxycarbonyl-valine, N-benzyloxy-
carbonyl-valine, N-(morpholinocarbonyl)-valine, N-(N-(morpholinocarbonyl)amino-
acetyl)-valine, N-(thiomorpholinocarbonyl)-valine, N-(N-2-pyridylmethyl-N-methyl- ~ .
aminocarbonyl)-valine, N-morpholinocarbonylaminoacetyl-valine, N-methylsulfonyl-valine, morpholinosulfonyl-valine, N-acetyl-isoleucine, N-propionyl-isoleucine, :
N-(benzyloxycarbonyl)-isoleucine, glutamic acid, N-benzyloxycarbonyl-glutamic acid,
asparagine, N-benzyloxycarbonyl-asparagine and/or quinolin-2-ylcarbonyl-asparagine,
wherein the amino acid residues are each preferably in the (L)- or (D,L)-form, and in the
case of valine also in the (D)-form; with the proviso that not more than one of the radicals
Rl and Rg is hydrogen,
R2, R4, R6 and R8 are hydrogen,
R3 is lower alkyl, such as n-butyl or isobutyl, cyclohexyl-lower alkyl, such as cyclo-
hexylmethyl, or phenyl-lower alkyl that is unsubstituted or substituted by halogen, such as
fluorine, lower alkoxy, such as methoxy, or by cyano, especially benzyl, 4-fluorobenzyl or
4-cyanobenzyl,
Rs is lower aLkanoyloxy, such as acetoxy, propionyloxy, butyryloxy, pentanoyloxy or
pivaloyloxy, octanoyloxy, decanoyloxy, dodecanoyloxy, carboxy-lower alkanoyloxy, such
as 3-carboxypropionyloxy, furyl-lower alkanoyloxy, such as 2-furylcarbonyloxy,
imidazolyl-lower alkanoyloxy, such as 4-imidazolylcarbonyloxy, 4-imidazolylacetoxy or
3-(4-imidazolyl)-propionyloxy, pyridyl-lower alkanoyloxy, such as 2-, 3- or 4-pyridyl-
carboxy, 2-pyridylacetoxy or 3-(2-pyridyl)propionyloxy, quinolyl-lower aLkanoyloxy, such
as quinolin-2-ylcarbonyloxy, aminoacetoxy (glycyloxy), N-lower aLkylaminoacetoxy, such
as N-methylaminoacetoxy, N,N-di-lower alkylaminoacetoxy, such as N,N-dimethyl-
aminoacetoxy, N-lower alkyl-N-phenyl-lower alkoxycarbonylaminoacetoxy, such as
N-benzyloxycarbonyl-N-methyl-aminoacetoxy, phenyl-lower alkanoyloxy, such as
benzoyloxy, 4-morpholino-lower aLkylbenzoyloxy, such as 4-morpholinomethylbenzoyl-
oxy, 4-halomethylbenzoyloxy, histidyloxy or prolyloxy and
R7 is as last defined for R3, especially lower alkyl, such as isobutyl or n-butyl; cyclo-
2 1 ~ 7
- 40 -
hexyl-lower alkyl; or phenyl-lower alkyl that is unsubstituted or substituted by halogen,
such as fluorine, lower aLlcoxy, such as methoxy, or by cyano; as last defined for R3,
or a salt thereof where at least one salt-forming group is present, still greater preference
being given to those compounds in which Rl and/or Rg are not morpholinosulfonyl or
thiomorpholinosulfonyl.
Especially preferred is a compound of formula I wherein Rlis lower aLkoxycarbonyl,
halo-lower alkoxycarbonyl, phenyl-lower aLkoxycarbonyl, the monovalent residue, bonded
via carbonyl, of-an aliphatic amino acid selected from valine, alanine, leucine and iso-
leucine or the residue, bonded via carbonyl, of an aliphatic amino acid as defined above
acylated at the amino nitrogen atom by one of the radicals phenyl-lower aL~canoyl,
morpholinyl-lower aL~anoyl, thiomorpholinyl-lower aL~anoyl, pyridyl-lower alkanoyl, -
lower aLkoxycarbonyl and phenyl-lower aLIcoxycarbonyl, all the mentioned amino acids -~
being in the D-, D,L- or L-form, preferably in the L-form, ~:
R2is hydrogen, - ~-R3is phenyl-lower aLIcyl, 4-fluorophenyl-lower aLkyl or cyclohexyl-lower aLkyl,
R4is hydrogen,
R5is lower alkanoyloxy, octanoyloxy, decanoyloxy, dodecanoyloxy, carboxy-lower
aLI~anoyloxy, furyl-lower alkanoyloxy, imidazolyl-lower aLkanoyloxy, pyridyl-lower ~ :
aL~anoyloxy, quinolyl-lower aLkanoyloxy, aminoacetoxy (glycyloxy), N-lower aLIcyl-
aminoacetoxy, N,N-di-lower alkylaminoacetoxy, N-lower aLIcyl-N-phenyl-lower aL~coxy-
carbonylaminoacetoxy, phenyl-lower aL~canoyloxy, ~morpholinomethylbenzoyloxy,
4-halomethylbenzoyloxy, histidyloxy or prolyloxy (=pyrrolidin-2-ylcarbonyloxy),
R6 is hydrogen,
R7is lower aLkyl, cyclohexyl-lower alkyl, phenyl-lower aL~cyl, 4-cyanophenyl-lower aL~cyl
or 4-fluorophenyl-lower aL~cyl,
Rgis hydrogen and
Rg is one of the radicals mendoned for R,, and the asymmetric carbon atoms carrying the
radicals R3 and Rs are in the S-con~lguration, and pharmaceutically acceptable salts
thereof.
Great preference is given to a compound of formula I wherein Rl and Rg are N-methoxy-
carbonylvalyl, R2, R4, R6 and R8 are hydrogen, R3 is benzyl or cyclohexylmethyl, Rs is
lower aL~canoyloxy, especially acetoxy, or pyridylcarbonyloxy, especially 2-pyridyl-
carbonyloxy, and R7 is cyclohexylmethyl or benzyl, and pharmaceutically acceptable salts
thereof, especially an isomer of that compound wherein the carbon atom carrying R3 and
'.'.'"'' .',: ,': " '', ' ' , , . ' ~`'. '
, ~,, . . ' ~ ' ' ~. ., , ': ',
'':`~
2 1 :1 2 ~ L~l 7
- 41 -
the carbon atom carrying Rs are in the (S)-con~lguration.
Great preference is given also to a compound of formula I wherein Rl and Rg are each
independently of the other N-lower aLkoxycarbonyl-valyl, R2 is hydrogen, R3 is phenyl-
methyl or cyclohexylmethyl, R4 is hydrogen, R5 is palmitoyloxy, lower aLkoxy-lower : ;
alkanoyloxy or pyridylcarbonyloxy, R6 is hydrogen, R7 is phenylmethyl or cyclohexyl- :
methyl and Rg is hydrogen, or a pharmaceutically acceptable salt thereof.
Of those compounds, special preference is given to
1 -[2~S)-palmitoyloxy-3(S)-(N-(methoxy-carbonyl)-(L)-valyl)amino-4-phenyl-butyl]-
l-[cyclohexylmethyl]-2-[N-methoxy-carbonyl-(L)-valyl]hydrazine of forrnula I, or a
pharmaceutically acceptable salt thereof; or
1 -[2(S)-(methoxy-acetoxy)-3(S)-(N-(methoxy-carbonyl)-(L)-valyl)amino-4-phenyl-
butyl]-l-[cyclohexylmethyl]-2-[N-methoxy-carbonyl-(L)-valyl]hydrazine of formula I, or
a pharmaceutically acceptable salt thereof; or
1 -[2(S)-(2-pyridyl-carbonyl)oxy-3(S)-(tert-butoxy-carbonyl)amino-4-phenyl-butyl]-
l-[cyclohexylmemyl]-2-[tert-butoxy-carbonyl]hydrazine of formula I, or a phannaceut-
ically acceptable salt thereof.
Especially preferred are, finally, also compounds of formula I wherein Rl is quinolin-
2-yl-carbonyl-(L)-asparaginyl, R2 is hydrogen, R3 is phenylmethyl, 4-lower aL~coxyphenyl-
methyl or 4-benzyloxyphenylmethyl, R4 is hydrogen, Rs is lower alkanoyloxy, such as
butyryloxy, octanoyloxy, decanoyloxy, dodecanoyloxy, palmitoyloxy, lower aL~coxy-lower
aL~canoyloxy, such as methoxyacetoxy, carboxy-lower aLkanoyloxy, furyl-lower alkanoyl-
oxy, imidazolyl-lower alkanoyloxy, pyridyl-lower allcanoyloxy, such as especially
pyridinylcarbonyloxy, for example 2- or 3-pyndinylcarbonyloxy, quinolyl-lower aLkanoyl-
oxy, aminoacetoxy (glycyloxy), N-lower alkylaminoacetoxy, N,N-di-lower alkylamino-
acetoxy, N-lower alkyl-N-phenyl-lower alkoxycarbonylaminoacetoxy, phenyl-lower
alkanoyloxy, 4-morpholinomethylbenzoyloxy, 4-halomethylbenzoyloxy, histidyloxy or
prolyloxy (=py~rolidin-2-ylcarbonyloxy), R6 is hydrogen, R7 is phenylmethyl, 4-lower
alkoxyphenylmethyl or cyclohexylmethyl, R8 is hydrogen and Rg is lower alkoxy-
carbonyl-(L)-valyl, lower alkoxy-lower alkoxy-lower alkoxycarbonyl-(L)-valyl, phenyl-
lower alkoxycarbonyl-(L)-valyl, lower alkanoyl-(L)-valyl, benzylaminocarbonyl orC3-C7alkenyloxycarbonyl, or also lower alkoxycarbonyl, or pharmaceutically acceptable
211~ 0~7
- 42 -
salts thereof, especially a compound selected from
1 -[2(S)-(2-pyridylcarbonyl)oxy-3(S)-(N-quinoline-2-carbonyl)-(L)-asparaginyl)amino-
4-phenylbutyl- 1 -[phenylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazinel
1 -[2(S)-butyryloxy-3(S)-(N-quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenylbutyl-
l-lphenylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine,
l-t2(S)-(2-pyridylcarbonyl)oxy-3(S)-(N-quinoline-2-carbonyl)-(L)-asparaginyl)amino-
4-phenylbutyl-1-[4-methoxyphenylrnethyl]-2-[N-(benzyloxycarbonyl)-(L)-valyl]-
hydrazine, and ~ .
1-[2(S)-(methoxy-acetyl)oxy-3(S)-(N-quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenylbutyl-1-[4-methoxyphenylmethyl1-2-[N-(benzyloxycarbonyl)-(L)-valyl]-
hydrazine; or a pharmaceutica'lly acceptable salt thereof.
Very special preference is given to compounds of formula I selected from
1-[2(S)-propionyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]- . ~ ~:
1-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-va'lyl]hydrazine;
1-[2(S)-butyryloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-'outyl]-1-[cyclo-
hexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;
-C2(S)-pentanoyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-
l-[cyclohexylmcthyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;
1 -[2(S)-octanoyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-
1 -[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;
1-[2(S)-decanoyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-
1-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;
1 -[2(S)-dodecanoyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-
1 -[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;
1-[2(S)-pivaloyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]- ~ -
1-[cyclohexylmethyl]-2-[N-~methoxycarbonyl)-(L)-valyl]hydrazine;
1-[2(S)-(2-furylcarbonyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-
butyl]-l-[cyclohexylmethyll-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;
1-[2(S)-(4-imidazolylcarbonyl)oxy-3~S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-
phenyl-butyll- 1 -[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;1 -[2(S)-(4-imidazolylacetyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-
butyl]- 1-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;
1 -[2(S)-(3-(4-imidazolyl)-propionyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-
phenyl-butyl]- 1 -[cyclohexylmethyl]-2-~N-(methoxycarbonyl)-(L)-valyl]hydrazine;1-[2(S)-benzoyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-
21~2~7
- 43 -
1 -[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;
1-[2(S)-(2-pyridylacetyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl- :
butyl]-l-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;
1-[2(S~-(3-(pyridin-2-yl)-propionyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-
phenylbutyl)]- 1 -[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;
1 -[2(S)-(quinolin-2-ylcarbonyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]- l-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;
1 -[2(S)-(aminoacetyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-~phenyl-butyl]-
l-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;
1-[2(S)-(N-methylaminoacetyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-
4-phenyl-butyl]- 1-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;
1 -[2(S)-(N,N-dimethylaminoacetyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-
phenyl-butyl]- 1 -[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;1-[2(S)-(N-benzyloxycarbonyl-N-methyl-aminoacetyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-
(L) -valyl]hydrazine;
1-[2(S)-prolyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-1-[cyclo-
hexylmethyll-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine;
1 -[2(S)-(4-morpholinomethylbenwyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-
4-phenyl-butyl]- 1 -[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-~L)-valyl]hydrazine;
1-[2(S)-(4-chloromethylbenzoyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-
4-phenyl-butyl]-1-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine; and
1-[2(S)-(3-carboxypropionyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-
butyl]-l-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]hydrazine, or pharmaceut-
ically acceptable salts thereof.
Most preferred of all are the- compounds mentioned in the Examples and their salts.
The compounds of formula I and salts of such compounds having at least one salt-forming
group are obtained by means of processes known per se, for examp~e as follows:
a) a hydroxy compound of formula II
~,,', ' :: ~: ' : :
21~2~7
- 44 -
R2
Rl 7 ; -. ~ .
R~ X<~ ~N~ 9 (II)
R3 R4
R8
wherein the radicals are as defined for compounds of formuia I, is acylated with a
carboxylic acid of formula III ~ ~:
Rs-H (III),
or with a reactive acid derivative thereof, wherein Rs is as defined for compounds of
formula I, free functional groups in the starting materials of formulae II and III that are not ~ -
. to participate in the reaction being if necessary in protected form, and any protecting
groups present are removed, or
b) for the preparation of compounds of formula I wherein Rg is acyl, sulfo, or sulfonyl
substituted by unsubstituted or substitated aL~yl, aIyl or heterocyclyl, and the remaining
radicals are as defined, an amino compound of formula
R5 R5 17
R~ ~ ~N~ (IV),
R~ ~R
R,
wherein the radicals are as defined immediately above, is condensed with an acid of
formula
R9'-OH (V),
or with a reactive acid derivative thereof, wherein R9' is as defined for R9 with the
exception of hydrogen and unsubstituted or substituted allcyl, free functional groups, with
the exception of those participating in the reaction, being if necessary in protected form,
and any protecting groups present are removed, or
2 1 ~ 7
- 45 -
c) for the preparation of compounds of formula I wherein Rl is acyl, sulfo, or sulfonyl
substituted by unsubstituted or substituted aL~cyl, aryl or heterocyclyl, and the remaining
radicals are as defined, an amino compound of formula
H ~ >~1~N ~R8 (VI),
R3 R4 18
wherein the radicals are as defined immediately above, is condensed with an add of
formula
Rl'-OH (VII),
or with a reactive acid derivative thereof, wherein Rl' is as defined for Rl with the
exception of hydrogen and unsubstituted or substituted aL~cyl, free functional groups, with
the exception of d ose participating in the reaction, being if necessary in protected form,
and any protecting groups present are removed, or -
d~ for the preparation of compounds of formula I wherein Rl and Rg are two identicalradicals selected from acyl, sulfo, and sulfonyl substituted by unsu'ostituted or substituted
aLIcyl, aryl or heterocyclyl, and the remaining radicals are as defined, a diamino compound
of formula
H~ ~ ~N~ (VIII),
R3 R4 18 N
wherein the radicals are as defined immediately above, is condensed with an acid suitable
for in~oducing the identical radicals Rl and Rg, or with a reacdve acid derivative thereof, : `
wherein Rl and Rg are as defined immediately above, free funcdonal groups, with the : -
exception of those participating in the reaction, being if necessary in protected form, and
any protecting groups present are removed, or
~,~
2112~7
- ~6 -
e) for the preparation of a compound of formula I wherein in place of R7 there is a radical
R7'' which is unsubstituted or substituted aL~cyl or cycloaL~yl, in a compound of formula I'
Rz
R~ X~N~ ~Rg (I~) ~
R3 R4 R
wherein R7' is hydrogen and the remaining radica1s are as defined, the radical R7" is
introduced by substitution with a compound of formula XII
R7"-X (X~,
wherein X is a leaving gtoup and R7" is unsubstituted or substituted alkyl or cycloaLkyl,
free functional groups, with the exception of those participating in the reaction, being
- optionally in protected foqm, and any protecting groups present are removed, or
f) in a compound of formula I wherein the substituents are as defined above with the
proviso that in the compound of foqmula I in question at least one functional group is
protected by protecting groups, the protecting groups present are removed,
and, if desired, a compound of formula I obtainable in accordance with any one of
processes a) to f) above having at least one salt-fonning group is converted into its salt or
an obtainable salt is converted into the free compound or into a different salt andJor any
isomeric mixtures that are obtainable are separated andlor a compound of formula I
according to the.invention is converted into a different compound of formula 1 according .
to the invention.
The said processes are described in detail below; unless other vise indicated, ~e radicals
Rl, R2, R3, R4, R5, R6, R7, R8 and Rg are as defined for compounds of fo~nula I:Process a) (Acylation of a hydroxy group)
The acylation of the hydroxy group is effected, for example, in a manne~ known per se
using an acid of formula III wherein Rs is as deflned with the exception of aminocarbonyl-
2~120~7
- 47 -
oxy and the radical of an N-substituted carbamic acid bonded via its aminocarbonyloxy
group, or using a reactive derivative thereof. A suitable reactive derivative is, for
example, a carboxylic acid of formula IX
Rs-Zl (IX),
wherein Rs' is one of the acyl radicals occurring in acyloxy, as defined above, and
wherein Zl is reactively activated hydroxy (the compound of formula IX thus contains,
instead of a hydroxy function bonded to the carbonyl group, reactively activated hydroxy,
preferably as defined below). The free carboxylic acid of fonnula III can be activated, for
example, by strong acids, such as a hydrohalic, sulfuIic, sulfonic or carboxylic acid, or by
acidic ion exchangers, for example hydrochloric, hydrobromic or hydIiodic acid, sulfi3ric
acid, an unsubstituted or substituted, for example halo-substituted, alkanecarboxylic acid,
or by an acid of formula III, preferably using an excess of the acid of formula III, if
necessary with the bonding of resulting water of reaction by water-binding agents, with
removal of the water of reaction by azeotropic distillation or with extractive esterification,
by acid anhydrides, especially inorganic acid anhydrides, or more especially organic acid
anhydrides, for example carboxylic acid anhydrides, such as lower aLkanecarboxylic acid
anhydrides (with the exception of formic acid anhydride), for example acetic anhydride, or
by suitable activating or coupling reagents of the type listed below, especially also in situ.
R5'-Zl may especially also be a carboxylic acid azide (Zl = azido; obtainable, for
example, by reaction of a corresponding acid ester via the conesponding hydrazide and
treatment thereof with nitrous acid); a carboxylic acid halide (Zl = halogen, especially ~ ~ -
chlorine or bromine~, especially an acid cnloride or bromide, obtainable, for example, by
reaction with organic acid halides, especially with oxalyl dihalides, such as oxalyl
dichloride, or with inorganic acid halides, for example with acid halides of phosphorus or
sulfur, such as phosphorus trichloride, phosphorus tribromide, phosphorus pentachloride,
phosphorus pentabromide, phosphorus oxychloride, phosphorus oxybromide, thionyl
chloride or thionyl bromide, or especially under mild conditions wit'n tetra-lower aLtcyl-
a-halo-enamines, for example tetramethyl-a-halo-enamines, especially l-chloro-
N,N,2-trimethyl-1-propenamine (preferably by reaction in inert solvents, especially
chlorinated hydrocarbons, such as methylene chloride or chloroform, or ethers, such as
diethyl ether, dioxane or tetrahydrofuran, at preferred temperatures of from -78 to 50C,
especially from -60 to 30C, for example from -10C to room temperature (cf. Devos, A.,
et al., J. C. S. Chem. Commun. 1979, 1180-1181, and Haveaux, B., et al., Org. Synth. ~
26 (1980)), it being possible for the resulting acid halide, for example the acid chloride of
, ~ j . . ~ . ................... :: :. , . . ~ :. ,
. ,~
::
2~12~7
- 48 -
formula IX wherein Zl is chlorine, also to be used further in situ, for example by reaction
with the compound of formula II in the presence of teriiary nitrogen bases, such as
pyridine and/or dimethylaminopyridine (DMAP, which is preferably added in catalytic
amounts), at preferred temperatures of from -20 to 50C, especially from 0C to room
temperature); an activated ester wherein Z1 is the radical of an alcohol having electroll-
attracting substituents, especially cyanomethoxy or aryloxy wherein aryl is preferably
phenyl or naphthyl that is mono- or poly-substituted by halogen, nitro and/or by cyano, for
example nitrophenoxy, such as 4-nitrophenoxy or 2,4-dinitrophenoxy, or poly-halo-
phenoxy, such as pentachlorophenoxy; or a symmetrical or, preferably, asymmetrical acid
anhydride which can be obtained, for example, by the action of a salt, for example an
alkali metal salt, of an acid of formula III or its reaction partner, preferably a lower
alkanecarboxylic acid, such as acetic acid, such as the sodium or potassium salt, on a
complementary acid halide, especially, in the case of the reaction of a salt of a carboxylic
acid of formula III, a carboxylic acid halide, for example chloride, such as acetyl chloride,
and, in the case of the reaction of a carboxylic acid halide of formula IX wherein Z1 is
halogen, for example chlorine or bromine, with a salt of a lower alkanecarboxylic acid,
especially sodium or potassium acetate. There may be used as activating and coupling
reagents for activating carboxylic acids of formula III in situ especially carbodiimides, for
example N,N'-di-C1-C4aL~cyl- or N,N'-di-Cs-C7cycloaLIcyl-carbodiimide, such as diiso-
propylcarbodiimide o,r N,N'-dicyclohexylcarbodiimide, advantageously with the addition
of an activating cata1yst, such as N-hydroxysuccinimide or unsubstituted or substituted,
for example halo-, C1-C7aL~yl- or Cl-C7aLIcoxy-substituted, N-hydroxy-benzotriazole or
N-hydroxy-S-norbomene-2,3-dicarboxamide, Cl-C4alkylhaloformate, for example
isobutyl chloroformate, suitable carbonyl compounds, for example N,N-carbonyldiimid-
azole, suitable 1 ,2-oxazolium compounds, for example 2-ethyl-5-phenyl-1 ,2-oxazolium
3'-sulfonate or 2-tert-butyl-5-methyl-isoxazolium perchlorate, suitable acylamino
compounds, for example 2-éthoxy-1-ethoxycarbonyl-1,2-dihydroquinoline, or suitable
phosphoryl cyanamides or azides, for example diethylphosphoryl cyanarnide or diphenyl-
phosphoryl azide, also triphenylphosphine disulfide or l-Cl-C4aL~yl-2-ha1opyridinium
ha1ides, for example 1-methyl-2-chloropyridinium iodide.
If in the compound of formula III two free carboxy groups are present, for example in
carboxy-lower aL~anoic acids, such as 3-carboxypropanoic acid, there may also be present
as activated acid derivative an internal anhydride, for example a succinic anhydride.
Z1 is preferably halogen, such as chlorine or bromine, and acyloxy, for example lower
.. ,. ~. .. - . :
2 1 1 ,~ 7
- 49 -
alkanoyloxy, such as acetoxy.
For the specific case of the introduction of an acyl radical of a semiester of carbonic acid
linked via its carbonyl group to the bonding oxygen atom there are suitable especially the
compounds of for nula IX wherein Zl is halogen, such as chlorine, which can be prepared,
for example, by reaction of the complementaIy alcohols, for example unsubstituted or
substituted alkyl alcohols, aryl-lower alkyl alcohols or heterocyclyl-lower aLkyl alcohols,
as defined in the definition of unsubstituted or substituted alkoxycarbonyloxy, aryl-lower
alkoxycarbonyloxy or heterocyclyl-lower alkoxycarbonyloxy Rs, with phosgene or with
analogues thereof that contain other halogen atoms, especially bromine, instead of
chlorine, preferably in the presence of tertiary nitrogen bases, such as pyridine or triethyl-
amine, and in inert solvents, for example chlorinated hydrocarbons, such as methylene
chloride or chloroform, ethers, such as diethyl ether, tetrahydrofuran or dioxane, or
carboxylic acid amides, such as dimethylformamide. -Also suitable are corresponding
N-carbonyl azolides of formula ~ (Z1 = an N-containing heterocycle, such as l-imidazo-
lido) which are obtained, for example, by reaction with the corresponding N,N'-carbonyl
diazolides, such as N,N'-carbonyl diimidazole, under condidons such as those just
described for phosgene and analogues with other halogen atoms. The reaction of
compounds of formula II with corresponding compounds of formula IX then likewisetakes place under those conditions (c Staab, H. A., Angew. Chemie ~, 407 (1962.
For the specific case of the introduction of arninocarbonyloxy Rs or of an N-substituted
aminocarbonyloxy group Rs there is suitable as activated acid derivadve especially the
corresponding isocyanate of formula IX'
Q-N=C=O (lX')
wherein Q is an amino-protecting group, for example trihaloacetyl, such as trifluoro- or
trichloro-acetyl, or one of the unsubstituted or substituted lower alkyl radicals or aryl
radicals mentioned above in the definition of aminocarbonyloxy Rs wherein the amino
group carries l or 2 substituents, it being possible, when Q is an amino-protecting group,
to obtain after the reaction with the compound of formula II the corresponding compound
of formula I wherein R5 is free aminocarbonyloxy by removal of the protecting group Q as
described below for the freeing of amino protected by acyl, especially by acid hydrolysis,
or, when Q is one of the mentioned substituted or unsubstituted lower alkyl radicals or aryl
radicals, a corresponding compound of formula I having aminocarbonyloxy Rs mono-
.:. .. : : , : - :
... .
2112~7
- so -
substituted at the nitrogen atom. Both aminocarbonyloxy and N-mono-substituted amino-
carbonyloxy Rs can be converted into N-disubstituted aminocarbonyloxy by alkylation
witll a further unsubstituted or substituted lower alkyl radical using suitable starting
materials and conditions analogous to those described below in the "Additional Process
Steps".
The reactions can be carried out under reaction conditions known per se, at customary
temperatures, in the presence or, especially when lower alkanoyl anhydrides are used to
activate the carboxylic acid of formula III, in the absence of inert solvents or diluents, for
example in acid amides, for example carboxylic acid amides, such as dimethylform~nide,
dimethylacetamide or 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (DMPU), or
amides of inorganic acids, such as hexarnethylphosphoric acid triamide, ethers, for
example cyclic ethers, such as tetrahydrofuran or dioxane, or acyclic ethers, such as
diethyl ether or ethylene glycol dimethyl ether, halogenated hydrocarbons, such as halo- ~ `
lower alkanes, for example methylene chloride or chloroform, ketones, such as acetone,
nitriles, such as acetonitrile, acid anhydrides, such as acetic anhydride, esters, such as
ethyl acetate, bisalkane sulfines, such as dimethyl sulfoxide, nitrogen heterocycles, such
as pyridine, or mixtures of those solvents, especially in anhydrous solvents or solvent
mixtures, it being possible to select for the above-mentioned reactions the particular
solvents that are suitable in each case, there being used, as appropriate and expedient, salts
of the compounds used, especially metal salts of carboxylic acids that are used, such as the
aLcali metal or aL~caline earth metal salts, for example sodium or potassium salts, in the
absence or the presence of cata1ysts, such as dimethylaminopyridine, condensation agents
or neutra1ising agents, such as tertiary nitrogen bases, for example pyridine, tnethylamine,
N-methylmorpholine, dimethylarninopyridine or ethyl diisopropylamine, and, depending
on the nature of the reaction andlor the reactants, under atmospheric pressure or in a
closed vessel, under nor nal pressure or under elevated pressure, for example at the
pressure produced in the reaction mixture under the reaction conditions in a closed tube,
and/or in an inert atmosphere, for example under an argon or nitrogen atmosphere.
Preference is given to reaction conditions that are mentioned specifically in any particular
case or, especiaUy, that are analogous to those mentioned in the Examples. The course of
the reaction is advantageously monitored using customary methods of analysis, especially
using thin-layer chromatography. It is possible to select from those reaction condidons
those that are suitable for each of the reactions described in this text, reaction conditions
that are specifically mentioned being especially preferred.
2 1 15 2 a ~ 7
- 51 -
The reaction according to the invention is preferably carried out under mild conditions,
especially at temperatures of from -10C to 60C, for example from 0C to room
temperature or at slightly elevated temperatures up tO about 50C, for example approxim-
ately from 0C to room temperature. Both in the case of the reaction with a carboxylic
acid halide of formula IX wherein Zl is halogen, such as chlorine or bromine, and in the
case of the reaction with an anhydride, especially a symmetIical anhydride (Zl = O-R5'),
the corresponding compound of formula IX (halide and Rs'-O-Rs', respectively) is used
especially in an approximately equimolar amount in relation to the compound of
formula II or in excess, for example from 0.95 to 10 times the molar amount.
Preferred as compounds of formula II for Process a) are the starting compounds of formula
R2 :~-
OH R6 17
R~ ~ ~N~ (II'),
R3 R4
R8 ~ .
wherein the radicals are as defined for compounds of formula I, and the salts of the
mendoned compounds where salt-forming groups are present.
Functional groups in starting materials the reaction of which is to be avoided, especially
carboxy, arnino, hydroxy, mercapto and sulfo groups, can be protected by suitable protect-
ing groups (convendonal protecting groups) which are customarily used in the synthesis of
pepdde compounds, and also in the synthesis of cephalosporins and penicillins as well as
nucleic acid derivadves and sugars. Those protecdng groups may already be present in
the precursors and are intended to protect the funcdonal groups in quesdon against
undesired secondary reacdons, such as acyladon, etherificadon, esterification, oxidadon,
solvolysis, etc.. In certain cases the protecdng groups can addidonally cause the reacdons
to proceed selecdvely, for example stereoselecdvely. It is characterisdc of protecting
groups that they can be removed easily, i.e. without undesired secondary reactions taking
place, for example by solvolysis, reducdon, photolysis, and also enzymadcally, for
example also under physiological conditions, and, especially, that they are not present in
the end products.
The protecdon of functional groups by such protecdng groups, the protecting groups them-
2112~47
- 52-
selves and the reactions for their removal are described, for exarnple, in standard works
such as J. F. W. McOmie, "Protective Groups in Organic Chemis~y", Plenum Press,
London and New York 1973, in Th. W. Greene, "Protective Groups in Organic Synthesis",
Wiley, New York 1981, in "The Peptides", Volume 3 (E. Gross and J. Meienhofer, eds.),
Academic Press, London and New York 1981, in "Methoden der organischen Chemie",
Houben-Weyl, 4th edition, Volume 15/I, Georg Thieme Verlag, Stuttgart 1974, in H.-D.
Jakubke and H. Jescheit, "Aminosauren, Peptide, Proteine" ("Amino acids, peptides,
proteins"), Verlag Chemie, Weinheim, Deerfield Beach and Basle 1982, and in Jochen - `
Lehmann, "Chemie der Kohlenhydrate: Monosac~haride und Derivate" ("The Chemistryof Carbohydrates: monosaccharides and derivatives"), Georg Thieme Verlag, Stuttgart
1974.
A carboxy group is protected, for example, in the form of an ester group which can be
cleaved selectively under mild conditions. A carboxy group protected in esterified form is
esterified especially by a lower aL1cyl group that is preferably branched in the l-position of
the lower aL~yl group or substituted in the 1- or 2-position of the lower alkyl group by suit-
able substituents.
A protected carboxy group esterified by a lower aL~cyl group is, for example, methoxy-
carbonyl or ethoxycarbonyl.
A protected carboxy group esteriffed by a lower alkyl group that is branched in the
l-position of the lower allcyl group is, for example, tert-lower alkoxycarbonyl, for example
tert-butoxycarbonyl.
A protected carboxy group esterified by a lower aLkyl group that is substituted in the 1- or
2-position of the lower a11yl group by suitable substituents is, for example, arylmethoxy-
carbonyl having one or two aryl radicals, wherein aryl is phenyl that is unsubstituted or
mono-, di- or tri-substituted, for example, by lower aLkyl, for example tert-lower aLIcyl,
such as tert-butyl, lower aL~oxy, for example methoxy, hydroxy, halogen, for example
chlorine, and/or by nitro, for example benzyloxycarbonyl, benzyloxycarbonyl substituted
by the mentioned substituents, for example 4-nitrobenzyloxycarbonyl or 4-methoxy-
benzyloxycarbonyl, diphenylmethoxycarbonyl or diphenylmethoxycarbonyl substituted by
the mentioned substituents, for example di(4-methoxyphenyl)methoxycarbonyl, and also
carboxy esterified by a lower alkyl group, the lower aLkyl group being substituted in the 1-
or 2-posidon by suitable substituents, such as l-lower alkoxy-lower alkoxycarbonyl, for
.. .. .
. ~
. , . ~ ~, .
, .,
2112~7
- 53 -
example methoxymethoxycarbonyl, l-methoxyethoxycarbonyl or l-ethoxyethoxy-
carbonyl, l-lower alkylthio-lower alkoxycarbonyl, for example l-methylthiomethoxy-
carbonyl or l-ethylthioethoxycarbonyl, aroylmethoxycarbonyl wherein the aroyl group is
benzoyl that is unsubstituted or substituted, for example, by halogen, such as bromine, for
example phenacyloxycarbonyl, 2-halo-lower aLkoxycarbonyl, for example 2,2,2-trichloro-
ethoxycarbonyl, 2-bromoethoxycarbonyl or 2-iodoethoxycarbonyl, as well as 2-(tri-substi-
tuted silyl)-lower alkoxycarbonyl wherein the substituents are each independently of the
others an aliphatic, araliphatic, cycloaliphatic or aromatic hydrocarbon radical that is
unsubstituted or substituted, for example, by lower alkyl, lower alkoxy, aryl, halogen
and/or by nitro, for example lower alkyl, phenyl-lower alkyl, cycloalkyl or phenyl each of
which is unsubstituted or substituted as above, for example 2-tri-lower alkylsilyl-lower
alkoxycarbonyl, such as 2-tri-lower alkylsilylethoxycarbonyl, for example 2-trimethyl-
silylethoxycarbonyl or 2-(di-n-butyl-methyl-silyl)-ethoxycarbonyl, or 2-triarylsilylethoxy-
carbonyl, such as triphenylsilylethoxycarbonyl.
A carboxy group may also be protected in the form of an organic silyloxycarbonyl group. ~ -
An organic silyloxycarbonyl group is, for example, a tri-lower alkylsilyloxycarbonyl
group, for example trimethylsilyloxycarbonyl. The silicon atom of the silyloxycarbonyl
group can also be substituted by two lower alkyl groups, for exarnple methyl groups, and
by the amino group or the carboxy group of a second molecule of formula I. Compounds
having such protecting groups can be prepared, for example, using dimethylchlorosilane
as silylating agent.
A protected carboxy group is preferably tert-lower alkoxycarbonyl, for example tert-but-
oxycarbonyl, benzyloxycarbonyl, 4-nitrobenzyloxycarbonyl, 9-fluorenylmethoxycarbonyl
or diphenylmethoxycarbonyl.
A protected amino group may be protected by an amino-protecting group, for example in
the form of an acylamino, arylmethylamino, etherified mercaptoamino, 2-acyl-lower
alk-l-enylamino or silylamino group or in the foTm of an azido group.
In a corresponding acylamino group, acyl is, for example, the acyl radical of an organic
carboxylic acid having, for example, up to 18 carbon atoms, especially an unsubstituted or
substituted, for example halo- or aryl-substituted, lower alkanecarboxylic acid or an
unsubstituted or substituted, for example halo-, lower alkoxy- or nitro-substituted, benzoic
acid, or, preferably, of a carbonic acid semiester. Such acyl groups are, for example,
:, . . -. i ~ . : ~ . . - . . ~- - - . -
;: ~
: - :: ~ - -
: ........ -: . - ;.
^ . . . :~ ~ . :: . -- ~ .~ . - . - ~.
. ~
", ~ . , . ~ .. .. . ..
g~.. ::: . . . . .. : . . . .. .. ~ :
21~2~47
- 54 -
lower aL~canoyl, such as formyl, acetyl, propionyl or pivaloyl, halo-lower alkanoyl, for
example 2-haloacetyl, such as 2-chloro-, 2-bromo-, 2-iodo-, 2,2,2-trifluoro- or 2,2,2-tri-
chloro-acetyl, unsubstituted or substituted, for example halo-, lower alkoxy- or nitro-
substituted, benzoyl, such as benzoyl, 4-chlorobenzoyl, 4-methoxybenzoyl or 4-nitro-
benzoyl, lower aLkoxycarbonyl, preferably lower alkoxycarbonyl that is branched in the
l-position of the lower alkyl radical or suitably substituted in the 1- or 2-position, for
example tert-lower alkoxycarbonyl, such as tert-butoxycarbonyl, arylmethoxycarbonyl
having one, two or three aryl radicals which are phenyl that is unsubstituted or mono- or
poly-substituted, for example, by lower aL~cyl, especially tert-lower alkyl, such as
tert-butyl, lower alkoxy, such as methoxy, hydroxy, halogen, such as chlorine, and/or by
nitro, for example benzyloxycarbonyl, 4-nitrobenzyloxycarbonyl, diphenylmethoxy-carbonyl, 9-fluorenylmethoxycarbonyl or di(4-methoxyphenyl)methoxycarbonyl, aroyl-
methoxycarbonyl wherein the aroyl group is preferably benzoyl that is unsubstituted or
substituted, for example, by halogen, such as bromine, for example phenacyloxycarbonyl,
2-halo-lower aL~coxycarbonyl, for example 2,2,2-trichloroethoxycarbonyl, 2-bromoethoxy-
carbonyl or 2-iodoethoxycarbonyl, 2-(tri-substituted silyl)-lower alkoxycarbonyl, for
example 2-tri-lower aLtcylsilyl-lower alkoxycarbonyl, such as 2-trimethylsilylethoxy-
carbonyl or 2-(di-n-butyl-methyl-silyl)-ethoxycarbonyl, or triarylsilyl-lower alkoxy-
carbonyl, for example 2-triphenylsilylethoxycarbonyl.
In an arylmethylamino group, for example a mono-, di- or especially tri-arylmethylamino
group, the aryl radicals are especially unsubstituted or substituted phenyl radicals. Such
groups are, for example, benzyl-, diphenylmethyl- or especially trityl-amino.
In an etherified mercaptoamino group the mercapto group is especially in the forrn of
substituted arylthio or aryl-lower aL~cylthio, wherein aryl is, for example, phenyl that is
unsubstituted or substituted, for example, by lower alkyl, such as methyl or tert-butyl,
lower aIkoxy, such as methoxy, halogen, such as chlorine, and/or by nitro, for example
4-nitrophenylthio.
In a 2-acyl-lower alk- l-enyl radical that can be used as an amino-protecting group, acyl is,
for example, the corresponding radical of a lower alkanecarboxylic acid, of a benzoic acid
that is unsubstituted or substituted, for example, by lower alkyl, such as methyl or tert-
butyl, lower aIkoxy, such as methoxy, halogen, such as chlorine, and/or by nitro, or
especially of a carbonic acid semiester, such as a carbonic acid lower alkyl semiester.
Corresponding protecting groups are especially l-lower alkanoyl-lower alk-l-en-2-yl, for
., .
2ll2a~7
- 55 -
example l-lower alkanoylprop-l-en-2-yl, such as 1-acetylprop-1-en-2-yl, or lower aLtcoxy-
carbonyl-lower alk- l-en-2-yl, for example lower aL~coxycarbonylprop-l-en-2-yl, such as
l-ethoxycarbonylprop-l-en-2-yl.
A silylamino group is, for example, a tri-lower aLIcylsilylamino group, for example
trimethylsilylamino or tert-butyl-dimethylsilylamino. The silicon atom of the silylamino
group can also be substituted by only two lower aL~cyl groups, for example methyl groups,
and by the amino group or carboxy group of a second molecule of forrnula I. Compounds
having sueh protecting groups can be prepared, for example, using the eorresponding ~ -
ehlorosilanes, such as dimethylchlorosilane, as silylating agents.
An amino g~oup can also be proteeted by eonversion into the protonated form; sui~able
eorresponding anions are espeeially those of strong inorganic acids, such as sulfuric acid,
phosphoric aeid or hydrohalie aeids, for example the chlorine or bromine anion, or of
organie sulfonie acids, sueh as p-toluenesulfonic acid.
Preferred amino-proteeting groups are lower aL~coxyearbonyl, phenyl-lower aLkoxyear-
bonyl, fluorenyl-lower alkoxyearbonyl, 2-lower alkanoyl-lower alk-1-en-2-yl and lower
aL~oxyearbonyl-loweralk-l-en-2-yl.
A hydroxy group ean be proteeted, for example, by an aeyl group, for example lower
alkanoyl that is substituted by halogen, such as ehlorine, sueh as 2,2-diehloroacetyl, or
especially by an acyl radieal of a earbonie aeid semiester mendoned for proteeted amino
groups. A preferred hydroxy-proteeting group is, for example, 2,2,2-triehloroethoxy-
earbonyl, 4-nitrobenzyloxyearbonyl, diphenylmethoxyearbonyl or triphenylmethoxy-carbonyl. A hydroxy group can also be proteeted by tri-lower alkylsilyl, for example
trimethylsilyl, triisopropylsilyl or tert-butyl-dimethylsilyl, a readily removable etherifying
group, for example an aL~cyl group, such as tert-lower alkyl, for example tert-butyl, an oxa-
or a thia-aliphatic or -cycloaliphatic, especially 2-oxa- or 2-thia-aliphatic or -cyclo-
aliphatic, hydrocarbon radical, for example l-lower alkoxy-lower alkyl or l-lower alkyl-
thio-lower alkyl, such as methoxymethyl, l-methoxyethyl, l-ethoxyethyl, methylthio-
methyl, l-methylthioethyl or l-ethylthioethyl, or 2-oxa- or 2-thia-cycloalkyl having from
S to 7 ring atoms, such as 2-tetrahydrofuryl or 2-tetrahydropyranyl, or a corresponding thia
analogue, and also by l-phenyl-lower alkyl, such as benzyl, diphenylmethyl or trityl,
wherein the phenyl radicals can be substituted, for example, by halogen, for example
chlorine, lower alkoxy, for example methoxy, and/or by nitro.
~ ; t :: -;: : ': '
,h,.~ - ' ? .; .:, ' '
21~ 20~7
- 56 -
Two hydroxy groups, especially adjacent hydroxy groups, occurring in a molecule, or a
hydroxy group and an amino group that are adjacent to one another, can be protected, for
example, by bivalent protecting groups, such as a methylene group that is preferably
substituted, for example, by one or two lower aLkyl radicals or by oxo, for example
unsubstituted or substituted aLkylidene, for example lower aLkylidene, such as isopropyl-
idene, cycloaL~cylidene, such as cyclohexylidene, a carbonyl group or benzylidene.
A mercapto group, for example in cysteine, can be protected especially by S-allcylation
with unsubstituted or substituted alkyl radicals, by silylation, by thioacetal formation, by
S-acylation or by the formation of asymmetric disulfide groupings. Preferred mercapto-
protecting groups are, for example, benzyl that is unsubstituted or substituted in the phenyl
radical, for example by methoxy or by nitro, such as 4-methoxybenzyl, diphenylmethyl
that is unsubstituted or substituted in the phenyl radical, for example by methoxy, such as
di(4-methoxyphenyl)methyl, triphenylmethyl, pyridyldiphenylmethyl, trirnethylsilyl,
benzylthiomethyl, tetrahydropy;anyl, acylaminometnyl, such as acetamidomet'nyl, iso-
butyrylaceta nidomethyl or 2-chloroacetamidomethyl, benzoyl, benzyloxycarbonyl or
alkyl-, especially lower aL~cyl-aminocarbonyl, such as ethylaminocarbonyl, and also lower
allcylthio, such as S-ethylthio or S-tert-butylthio, or S-sulfo.
A sulfo group can be protected, for example, by lower aL~cyl, for example methyl or ethyl,
by phenyl or in the f~rm of a sulfonamide, for example in the form of an imidazolide.
In the context of this Application, a protecting group, for example a carboxy-protecting
group, is to be understood as being expressly also a polymeric carner that is bonded in a
readily removable manner to the functional group, for example the carboxy group, to be
protected, for example a carrier suitable for the Merrifield synthesis. An example of such
a suitable polymeric carrier is a polystyrene resin, weakly cross-linked by copolymerisa-
tion with divinylbenzene, that carries bridge members suitable for reversible bonding.
The freeing of protected groups is effected as appropriate by the methods described under
Process f) (Removal of protecting groups).
Process b) (Condensation to form an amide bond)
In staning materials of fonnulae IV and V, functional groups, with the exception othe
,.
,. ~ . .- .
:t. - :: .... ~ . . :
: - , . : . .
2l~2a~7
groups that are intended tO participate in the reaction or that do not react under the
reaction conditions, are protected each independently of the others by one of the protecting
groups mentioned under Process a).
The acids of formula V contain a free carboxy or sulfo group or reactive derivatives
thereof, with the result that reactive acid derivatives of the compounds of formula V may
also be present, for example the derived activated esters or reactive anhydrides, and also
reactive cyclic amides. The reactive acid derivatives can also be formed in situ.
Activated esters of carboxylic acids of formula V are especially esters unsaturated at the
linking carbon atom of the esterifying radical, for example of the vinyl ester type, such as : :
vinyl esters (obtainable, for example, by transesterification of a corresponding ester with
vinyl acetate; activated vinyl ester method), carbamoyl esters (obtainable, for examplej by
treatment of the corresponding acid with an isoxazolium reagent; l,2-oxazolium or `~
Woodward method), or l-lower alkoxyvinyl esters (obtainable, for example, by treatment
of the corresponding acid with a lower alkoxyacetylene; ethoxyacetylene method), or
esters of the amidino type, such as N,N'-disubstituted amidino esters (obtainable, for
example, by treatment of the corresponding acid with a suitable N,N'-disubstituted carbo-
diimide, for example N,N'-dicyclohexylcarbodiimide; carbodiimide method), or
N,N-disubstituted amidino esters (obtainable, for example, by treatment of the corres-
ponding acid with an N,N-disubstituted cyanamide; çyanamide method), suitable aryl
esters, especially phenyl esters suitably substituted by electron-attracting substituents
(obtainable, for example, by treatment of the corresponding acid with a suitablysubstituted phenol, for example 4-nitrophenol, 4-methylsulfonylphenol, 2,4,5-trichloro-
phenol, 2,3,4,5,6-pentachlorophenol or 4-phenyldiazophenol, in the presence of acondensation agent, such as N,N'-dicyclohexylcarbodiimide; activated aryl estersmethod), cyanomethyl esters (obtainable, for example, by treatment of the corresponding
acid with chloroacetonitrile in the presence of a base; cyanomethyl esters method),
thioesters, especially unsubstituted or substituted, for example nitro-substituted, phenyl-
thio esters (obtainable, for example, by treatment of the corresponding acid with
unsubstituted or substituted, for example nitro-substituted, thiophenols, inter alia by the
anhydride or carbodiimide method; activated thiol esters method), or especially amino or
amido esters.(obtainable, for example, by treatment of the corresponding acid with an
N-hydroxyamino or N-hydroxyamido compound, for example N-hydroxysuccinimide,
N-hydroxypiperidine, N-hydroxyphthalimide, N-hydroxy-S-norbornene-2,3-dicarboxylic
acid imide, l-hydroxybenzotriazole or 3-hydroxy-3,4-dihydro-1,2,3-benzotriazin-4-one,
2l1 2a~7
- ~8 -
for example by the anhydride or carbodiirnide method; activated N-hydroxy estersmethod). Internal esters, for example ~-lactones, can also be used.
Anhydrides of carboxylic acids of formula V may be symmetric or preferably mixedanhydrides of those acids, for example anhydrides with inorganic acids, such as acid
halides, especially acid chlorides (obtainable, for example, by treatment of the corres-
ponding acid with thionyl chloride, phosphorus pentachloride, phosgene, l-chloro-
N,N-2-trimethyl-1-propenamine treacdon conditions mentioned under Process a or
oxalyl chloride; acid chloride method), azides (obtainable, for example, from a corres-
ponding acid ester via the colresponding hydrazide and treatment thereof with nitrous
acid; azide method), anhydrides with carbonic acid semiesters, for example carbonic acid
lower aLIcyl semiesters (obtainable, for example, by treatment of the corresponding acid
with chloroformic acid lower alkyl esters or with a l-lower alkoxycarbonyl-2-lower
alkoxy-1,2-dihydro~uinoline; mixed O-alkylcarbonic acid anhydrides method~, or
anhyd~ides with dihalogenated, especially dichlorinated, phosphoric acid (obtainable, for
example, by treatment of the corresponding acid with phosphorus oxychloride; phosphorus
oxychloride method), anhydrides with other phosphoric acid derivatives (for example
those obtainable with phenyl-N-phenylphosphoramidochloridate or by reacdon of alkyl-
phosphoric acid amides in the presence of sulfonic acid anhydrides and/or racemisation-
reducing additives, such as N-hydroxybenzotriazole, or in the presence of cyanophos-
phonic acid diethyl ester) or with phosphorous acid derivatives, or anhydrides with
organic acids, such as mixed anhydrides with organic carboxylic acids (obtainable, for
example, by treatment of the corresponding acid with an unsubsdtuted or subsdtuted lower
aLkane- or phenyl-lower alkane-carboxylic acid halide, for example phenylacetic acid
chloride, pivalic acid chloride or trifluoroacetic acid chloride; mixed carboxylic acid
anhydrides method) or with organic sulfonic acids (obtainable, for example, by treatment
of a salt, such as an alkali metal salt, of the corresponding acid with a suitable organic
sulfonic acid halide, such as lower alkane- or aryl-, for example methane- or p-toluene-
sulfonic acid chloride; mixed sulfonic acid anhydrides method) and symmetric anhydrides
(obtainable, for example, by condensation of the corresponding acid in the presence of a
carbodiimide or l-diethylaminopropyne; symmetric anhydrides method).
Suitable cyclic amides of carboxylic acids of formula IV are especially amides having
five-membered diazacycles of aromatic character, such as amides with imidazoles, for
example imidazole (obtainable, for example, by treatment of the corresponding acid with
N,N'-carbonyldiimidazole; imidazole method), or pyrazole, for example 3,5-dimethyl-
~ ... ~ ... , . , ~ . .
2~2~7
59
pyrazole (obtainable, for example, via the acid hydrazide by treatment with acetylacetone;pyrazolide method).
As mentioned, derivatives of carboxylic acids of formula V that are used as acylating
agents can also be formed in situ. For exarnple, N,N'-disubsdtuted amidino esters can be
formed in situ by reacting a mixture of the starting material of formula V and the acid used
as acylating agent, in the presence of a suitable N,N'-disubstituted carbodiimide, for
example N,N'-cyclohexylcarbodiimide. In addition, amino or amido esters of the acids
used as acylating agents can be formed in the presence of the starting material of
formula IV to be acylated, by reacting a mixture of the corresponding acid and amino
starting materials in the presence of an N,N'-disubstituted carbodiimide, for example
N,N'-dicyclohexylcarbodiimide, and of an N-hydroxyamine or N-hydroxyarnide, for
example N-hydroxysuccinimide, where appropriate in the presence of a suitable base, for
example 4-dimethylamino-pyridine. Moreover, activation in situ can be achieved by
reaction with N,N,N',N'-tetraaLkyluronium compounds, such as 0-benzotriazol-1-yl- ~ -
N,N,N',N'-tetrarnethyluronium hexafluorophosphate, 0-(1,2-dihydro-2-oxo-1-pyridyl)-
N,N,N',N'-tetramethyluronium tetrafluoroborate or 0-(3,4-dihydro-4-oxo-1,2,3-benzo-
triazolin-3-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate. Finally, phosphoric acid
anhydrides of the carboxylic acids of formula V can be prepared in situ by reacting an
alkylphosphoric acid amide, such as hexamethylphosphoric acid triamide, in the presence
of a sulfonic acid anhydride, such as 4-toluenesulfonic acid anhydride, with a salt, such as
a tetrafluoroborate, for example sodium tetrafluoroborate, or with another derivative of
hexamethylphosphoric acid triamide, such as benzotriazol-l-yloxy-tris(dimethylamino)-
phosphonium hexafluoride, preferably in the presence of a racemisation-reducing additive,
such as N-hydroxybenzotriazole.
In an analogous manner, many of the reaction types listed above for carboxylic acids of
formula V can also be carried out for compounds of formula V having a terminal sulfonyl
group in the condensation with compounds of formula IV to form sulfonamides.
For example, it is possible to use activated sulfonic acid esters, for example the corres-
ponding aryl esters, such as phenyl esters, especially those substituted by nitro groups, it
beir.g possible for the amine component of formula IV also to be used in the form of an
alkali metal amide, for example an alkali metal arylamide, such as sodium anilineamide,
or an aLkali metal salt of nitrogen-containing heterocycles, for example potassium
pyrrolide.
- , : .:
., . . , ~ . . ...
2 ~ 7
- 60-
lt is also possible to use reactive anhydrides, such as the corresponding symmetric acid
anhydrides (which can be prepared, for example, by reaction of the alkylsulfonic acid
silver salts with alkylsulfonyl chlorides) or, preferably, the corresponding asymmetric acid
anhydrides, for example anhydrides with inorganic acids, such as sulfonyl halides,
especially sulfonyl chlorides (obtainable, for example, by reaction of the corresponding
sulfonic acids with inorganic acid chlorides, for example thionyl chloride, phosphorus
pentachloride), with organic carboxylic acids (obtainable, for example, by treatment of a
sulfonic acid halide with the salt of a carboxylic acid, such as an alkali metal salt,
analogously to the above-mentioned mixed sulfonic acid anhydrides method), or azides
(obtainable, for example, from a corresponding sulfonic acid chloride and sodium azide or
via the corresponding hydrazide and treatment thereof with nitrous acid analogously to the
above-mentioned azide method).
The amino group of compounds of formula IV that participates in the reaction preferably
carries at least one reactive hydrogen atom, especially when the carboxy or sulfonyl group
reacting ~herewith is present in reactive form; it may, however, itself have been
derivatised, for example by reacdon with a phosphite, such as diethylchlorophosphite,
1,2-phenylenechlorophosphite, ethyl dichlorophosphite, ethylenechlorophosphite or tetra-
ethylpyrophosphite. A derivative of such a compound having an amino group is, for
example, also a carbamic acid halide or an isocyanate, the amino group that participates in
the reaction being substituted by ha10carbonyl, for example chlorocarbonyl, or being
modified in the form of an isocyanate group; in the latter case only compounds of
formula I that carry a hydrogen atom at the nitrogen atom of the amide group forrned by
the reaction are obtainable (R8=H).
Condensation to form an amide bond can be carried out in a manner known per se, for
example as described in standard works such as "Houben-Weyl, Methoden der organ-ischen Chemie", 4th edition, Volume 15/lI (1974), Volume IX (1955), Volume E 11
(1985), Georg Thieme Verlag, Stuttgart, "The Peptides" (E. Gross and J. Meienhofer,
eds.), Volumes 1 and 2, Academic Press, London and New York, 1979/1980, or
M. Bodansky, "Principles of Peptide Synthesis", Springer-Verlag, Berlin 1984.
The condensation of a free carboxylic acid of formula V with the corresponding amine of
formula IV can be carried out especially in the presence of one of the customarycondensation agents, or using carboxylic acid anhydrides or carboxylic acid halides, such
j , .. .. - - . - .-
~5~
~`"'' ,' ' ' ' ' '
~: . '- ' " ' : ''
::, ,- . ~' '
2 ~ 7
- 61 -
as chlorides, or activated carboxylic acid esters, such as p-nitrophenyl esters. Customary
condensadon agents are~ for example, carbodiimides, for example diethyl-, dipropyl-,
N-ethyl-N'-(3-dimethylaminopropyl)-carbodiimide or especially dicyclohexylcarbo-diimide, also suitable carbonyl compounds, for example N,N'-carbonyldiimidazole,1,2-oxazolium compounds, for example 2-ethyl-5-phenyl-1,2-oxazolium 3'-sulfonate and
2-tert-butyl-5-methylisoxazolium perchlorate, or a suitable acylarnino compound, for
example 2-ethoxy- 1 -ethoxycarbonyl- 1 ,2-dihydroquinoline, N ,N ,N ' ,N ' -tetraaL~cyluronium
compounds, such as O-benzotriazol-1-yl-N,N,N',N'-tetramethyluronium hexafluoro-
phosphate, also activated phosphoric acid derivadves, for example diphenylphosphoryl
azide, diethylphosphoryl cyanide, phenyl-N-phenylphosphoroamidochloridate, bis(2-oxo-
3-oxazolidinyl)phosphinic acid chloride or 1-benzotriazolyloxy-tris(dimethylamino)-
phosphonium hexafluorophosphate.
If desired, an organic base is added, preferably a ter~iary amine, for example a tri-lower
aLkylamine having buLky radicals, such as ethyl diisopropylarnine or triethylamine, and/or
a heterocyclic base, for example 4-dimethylaminopyridine or preferably N-methylmorpho-
line or pyridine.
The condensadon of acdvated esters, reacdve anhydrides or reacdve cyclic arnides with
the corresponding amines is customarily carried out in the presence of an organic base, for
example simple tri-lower aLIcylamines, for example triethylamine or tributylamine, or one
of the above-mentdoned organic bases. If desired, a condensadon agent is addidonally
used, for example as descIibed for free carboxylic acids.
The condensation of acid anhydrides with amines can be effected, for exarnple, in the
presence of inorganic carbonates, for example a nmonium or aLl~ali metal carbonates or
hydrogen carbonates, such as sodium or potassium carbonate or hydrogen carbonate (if
desired together with a sulfate).
Carboxylic acid chlorides, for example the chlorocarbonic acid derivadves derived from
the acid of forrnula V, or sulfonic acid chlorides are condensed with the corresponding
amines preferably in the presence of an organic amine, for example the above-mendoned
tri-lower aL~cylamines or heterocyclic bases, where ~ppropriate in the presence of a
hydrogen sulfate.
.~
2i ~ 2~7
- 62 -
The condensation is preferably carTied out in an inert, aprodc, preferably anhydrous,
solvent or solvent mixture, for example in a carboxylic acid amide, for example
formamide or dimethylformamide, a halogenated hydrocarbon, for example methylenechloride, carbon tetrachloride or chlorobenzene, a ketone, for example acetone, a cyclic
ether, for example tetrahydrofuran, an ester~ for example ethyl acetate, or a nitrile, for
example acetonitrile, or in a mixture thereof, as appropriate at reduced or elevated
temperature, for example in a temperature range of from approximately -40C to approx-
imately +100C, preferably from approximately -10C to approximately +50C, and in the
case where arylsulfonyl esters are used also at approximately from +100C to +200C, and
where appropriate under an inert gas atmosphere, for example a nitrogen or argonatmosphere.
Aqueous, for example alcoholic, solvents, for example ethanol, or aromatic solvents, for
example benzene or toluene, may also be used. When aLIcali metal hydroxides are present
as bases, acetone can also be added where appropriate.
The condensation can also be carried out in accordance with ~he technique known as solid
phase synthesis which originates from R. Merrifield and is described, for example, in
Angew. Chem. 97, 801 - 812 (1985), Naturwissenschaften 71, 252 - 258 (1984) or in R. A. ~ -
Houghten, Proc. Natl. Acad. Sci. USA 82, 5131 - 5135 (1985).
For the specific case of the introduetion of a radical of a semiester of carbonic acid Rg
there are suitable especially the compounds of formula V',
RX-O-(C=O~-Z2 (V')
wherein R,~ is especially lower aLkyl, halo-lower aLlcyl, aryl-lower alkyl, heterocyclyl-
lower alkyl, lower alkenyl, lower alkoxy-lower aLkyl or (lower aL~coxy-lower aLkoxy)-
lower alkyl, as in the definition of acyl groups R9 of a semiester of carbonic acid, and
wherein Z2 is halogen, such as chlorine, which can be prepared, for example, by reaction
of the complementary alcohols, such as lower alkyl alcohols, halo-lower alkyl alcohols,
aryl-lower alkyl alcohols, heterocyclyl-lower aLkyl alcohols, lower alkenyl alcohols, lower
alkoxy-lower alkyl alcohols or (lower alkoxy-lower alkoxy)-lower alkyl alcohols, with
phosgene or with analogues thereof that contain other halogen atoms, especially bromine,
instead of chlorine, preferably in the presence of tertiaTy nitrogen bases, such as pyridine
or triethylamine, and in inert solvents, for example chlorinated hydrocarbons, such as
~J ~
. j . :
., j:, ` . .
~: ' '` , ` . ~' . : `':
2 1~ ~ 2 ~ ~ 7
- 63 -
methylene chloride or chloroform, ethers, such as diethyl ether, tetrahydrofuran or
dioxane7 or carboxylic acid amides, such as dimethylforrnamide. Also suitable are corres-
ponding N-carbonyl azolides of formula V' (Z2 = an N-containing heterocycle, such as
l-imidazolido) which are obtained, for example, by reaction with the corresponding
N,N'-carbonyl diazolides, such as N,N'-carbonyl diimidazole, under conditions such as
those just described for phosgene and analogues with other halogen atoms. The reaction
of compounds of formula IV with corresponding compounds of fonnula V' then likewise
takes place under those conditions (cf. Staab, H. A., Angew. Chemie _~, 407 (1962)).
For the specific case of the introduction of a radical Rg of an unsubstituted orN-substituted carbarnic acid~ such as carbamoyl or unsubstituted or substituted N-aLlcyl-
carbamoyl, there is suitable as activated acid derivative especially the corresponding
isocyanate of formula V"
W-N=C=O (V")
wherein W is an amino-protecting group, for exarnple trihaloacetyl, such as trifluoro- or
trichloro-acetyl, or one of the unsubstituted or substituted alkyl radicals mentioned above
in the definidon of acyl groups R9 of a substituted carbamic acid, it being possible, when
W is an amino-protecting group, to obtain after the reaction with the compound of
formula IV the corresponding compound of foImula I wherein iRg is carbarnoyl by removal
of the protecting group W as described below for the freeing of protected amino,especially by acid hydrolysis, or, when W is one of the mendoned subsdtuted or
unsubsdtuted lower aLtcyl radicals, a corresponding compound of formula I having arnino- -
carbonyl Rg mono-subsdtuted at the nitrogen atom. Both aminocarbonyl and N-mono-substituted aminocarbonyl Rg can be converted into N-disubstituted aminocarbonyl by
alkylation with a further unsubstituted or substituted lower aL~cyl radical using suitable -
starting materials under condidons analogous to those described below in the "Additional -
Process Steps".
The reactions with the compounds of formulae V' and V" are effected under reaction
condidons analogous to those mentioned for the reaction of compounds of formula IX
and IX' with those of formula II under Process a).
Depending on the starting compounds used, the radicals Rl and Rg in the obtainable
compounds of formula I can be identical or different from one another.
i . . ~ . .. .
2l~a~7
- 64 -
The freeing of protected groups is effected where appropriate by the methods described
under Process f) (Removal of protecting groups).
Process c) (Formation of an amide bond)
In starting materials of formulae VI and VII, functional groups, with the exception of the
groups that are intended to par~icipate in the reaction or that do not react under the
reaction conditions, are protected each independently of the others by one of the protecting
groups mentioned under Process a). ~ -
The process is totally analogous to the process mentioned under ~rocess b) except that
instead of compounds of formula IV those of formula VI are used, and instead of
compounds of formula V those of formula VII are used.
Depending on the starting compounds used, the radicals Rl and Rg in the obtainable
compounds of formula I can be identical or different from one another.
In the reactions in Process b), and a1so in c) and d)j in some cases the acyl radical in
acyloxy Rs may migrate to the nitrogen atom to which Rg is to be linked; analogous
secondary reactions are possible in Processes c) and d).
The freeing of protected groups is effected where appropriate by the methods described
under Process f) (Removal of protecdng groups).
Process d) (~ormation of an amide bond)
In star~ng materials of formula VIII and in the acid suitable for introducing the idendcal
radicals Rl and Rg', or the reacdve derivadves thereof, functional groups that are not
intended to participate in the reacdon or that do not react under the reaction condidons are
protected each independently of the others by one of the protecdng groups mendoned
under Process a).
The acid suitable for introducing the identical radicals Rl and Rg is preferably an acid of
formula V or VII, or it is present in the form of a reacdve derivative of such an acid, as
described above.
.. . . .
-- ` 211 2~7
- 65 -
PTeferred as starting compounds of formula VIII that may be protected by protecting
groups are those which are described as being preferred in the section relating to starting
compounds.
The condi~ions for the process are analogous to those mentioned under Process b), except
that instead of compounds of forrnula IV those of formula VIII are used, and instead of
compounds of formula V those of formula V or VII are used.
The freeing of protected groups is effected where appropriate by the methods described
under Process f) (Removal of protecting groups).
Process e) (Alkylation of a secondary nitrogen atom)
In starting materials of formula I' and in the compound of formula XII suitable for intr~
ducing the radical R7", or the reactive derivatives thereof, functional groups dlat are not
intended to participate in dle reaction or that do not react under the reaction condidons are
protected eaGh independendy of the others by one of the protecting groups mentioned
under Process a).
A leaving group X is especially a nucleofugal leaving group selected from hydroxy esteri-
fied by a strong inorganic or organic acid, such as hydroxy esterified by a mineral acid, for
example a hydrohalic acid, such as hydrochloric acid, hydrobromic acid or hydriodic acid,
or by a strong organic sulfonic acid, such as a lower alkanesulfonic acid that is unsubsti-
tuted or substituted, for example by halogen, such as fluorine, or an aromatic sulfonic acid,
for example a benzenesulfonic acid that is unsubstituted or substituted by lower alkyl,
such as methyl, halogen, such as bromine, and/or by nitro, for example a methanesulfonic
acid, p-bromotoluenesulfonic acid or p-toluenesulfonic acid, and hydroxy esterified by
hydrazoic acid.
The substitution can take place under the conditions of a first-order or second-order
nucleophilic substitution.
For example, a compound of formula XII, especially a compound of formula XII wherein
X is a leaving group having a high polarisability of the electron shell, for example iodine,
can be used in a polar aprotic solvent, fi~r example acetone, acetonitrile, nitromethane,
,~
..,,~
F . . ' ~ ' , ~ . :
2~ 1~2~47
- 66 -
dimethyl sulfoxide or dimethylformamide. The reaction may alternatively be carried out
in water to which, where appropriate, an organic solvent, for example ethanol, tetrahydro-
furan or acetone, has been added as solubiliser. The substitution reaction is calried out at
room temperature or at reduced or elevated temperature, for example in a temperature
range of from approximately -40 to approximately 100C, preferably from approxirnately
-10 to approximately 50C, and where appropriate under an inert gas, for example under
a nitrogen or argon atmosphere.
The freeing of protected groups is effected where appropriate by the methods described
under Process f) (Removal of protecting groups).
Process f~ (Removal of protecting groups)
The removal of protecting groups that are not constituents of the desired end product of
formula I, for example the carboxy-, amino-, hydroxy-, mercapto- andlor sulfo-protecting
groups, is effected in a manner known ~ se, for example by means of solvolysis,
especially hydrolysis, alcoholysis or acidolysis, or by means of reduction, especially
hydrogenolysis or chemical reduction, as well as by photolysis, as appropAate stepwise or
simultaneously, it being possible also to use enzymatic methods. The removal of ~e
protecting groups is described, for example, in the standard works mentioned under
Process a) in the section relating to protecting groups.
For e~sample, protected carboxy, for example tert-lower alkoxycarbonyl, lower alkoxy-
carbonyl substituted in the 2-posidon by a trisubstituted silyl group or in the l-position by
lower alkoxy or lower alkylthio, or unsubstituted or substituted diphenylmethoxycarbonyl
can be converted into free carboxy by treatment with a suitable acid, such as formic acid,
hydrogen chloride or trifluoroacetic acid, where appropriate with the addition of a nucleo-
philic compound, such as phenol or anisole. Carboxy can also be freed from loweraLtcoxycarbonyl by means of bases, such as hydroxides, for example aL~cali metalhydroxides, such as sodium hydroxide or potassium hydroxide. Unsubstituted or
substituted benzyloxycarbonyl can be freed, for example, by means of hydrogenolysis, i.e.
by treatment with hydrogen in the presence of a metal hydrogenation catalyst, such as a
palladium catalyst. In addition, suitably substituted benzyloxycarbonyl, such ~s 4-nitro-
benzyloxycarbonyl, can also be converted into free carboxy by reduction, for example by
treatment with an aL~cali metal dithionite, such as sodium dithionite, or with a reducing
metal, for example zi~c, or a reducing metal salt, such as a chromium(II) salt, for example
k~
~; :
2 1 ~ 7
- 67 -
chromium~II) chloride, customarily in the presence of a hydrogen-yielding agent that,
together with the metal, is capable of producing nascent hydrogen, such as an acid,
especially a suitable carboxylic acid, such as an unsubstituted or substituted, for example
hydroxy-substituted, lower alkanecarboxylic acid, for example acetic acid, formic acid,
glycolic acid, diphenylglycolic acid, lactic acid, mandelic acid, 4-chloromandelic acid or
tartaric acid, or in the presence of an alcohol or thiol, water preferably being added. By
treatment with a reducing metal or metal salt, as described above, 2-halo-lower aLkoxy-
carbonyl (where appropriate after conversion of a 2-bromo-lower alkoxycarbonyl group
into a corresponding 2-iodo-lower alkoxycarbonyl group) or aroylmethoxycarbonyl can
also be converted into free carboxy. Aroylmethoxycarbonyl can also be cleaved bytreatment with a nucleophilic, preferably salt-forming, reagent, such as sodium thio-
phenolate or sodium iodide. 2-(tri-substituted silyl)-lower alkoxycarbonyl, such as 2-tri-
lower alkylsilyl-lower alkoxycarbonyl, can also be converted into free carboxy by
treatment with a salt of hydrofluoric acid that yields the fluoride anion, such as an alkali
metal fluoride, for example sodium or potassium fluoride, where appropriate in the - ~-
presence of a macrocyclic polyether ("Crown ether"), or with a fluoride of an organic
quaternary base, such as tetra-lower aLkylammonium fluoride or tri-lower alkylaryl-lower
alkylammonium fluoride, for example tetraethylammonium fluoride or tetrabutyl-
ammonium fluoride, in the presence of an aprotic, polar solvent, such as dimethyl
sulfoxide or N,N-dimethylacetamide. Carboxy protected in the form of organic silyloxy-
carbonyl, such as tri-lower alkylsilyloxycarbonyl, for example trimethylsilyloxycarbonyl,
can be freed in customary manner by solvolysis, for example by treatment with water, an
alcohol or an acid, or, furthermore, a fluoride, as described above. Esterified carboxy can
also be freed enzymatically, for example by means of esterases or suitable peptidases, for
example esterified arginine or lysine, such as lysine methyl ester, using trypsin.
A protected amino group is freed in a manner known ~ se and, according to the nature of
the protecting groups, in various ways, preferably by solvolysis or reduction. Lower
alkoxycarbonylamino, such as tert-butoxycarbonylamino, can be cleaved in the presence
of acids, for example mineral acids, for example a hydrogen halide, such as hydrogen
chloride or hydrogen bromide, or sulfuric or phosphoric acid, preferably hydrogen
chloride, or strong organic acids, such as trihaloacetic acid, for example trifluoroacetic
acid, or formic acid, in polar solvents, such as water, or ethers, preferably cyclic ethers,
such as dioxane; 2-halo-lower alkoxycarbonylamino (where appropriate after conversion
of a 2-bromo-lower alkoxycarbonylamino group into a 2-iodo-lower alkoxycarbonylamino
group), or dissolved directly in a liquid organic carboxylic acid, such as formic acid,
.~
a~
..., . ` . ' :
2112 V (~ r~
- 68 -
aroylmethoxycarbonylamino or 4-nitrobenzyloxycarbonylamino can be cleaved, for
example, by treatment with a suitable reducing agent, such as zinc in the presence of a
suitable carboxylic acid, such as aqueous acetic acid. Aroylmethoxycarbonylamino can
also be cleaved by treatment with a nucleophilic, preferably salt-forming, reagent, such as
sodium thiophenolate, and 4-nitrobenzyloxycarbonylamino also by treatment with an
aLkali metal dithionite, for exarnple sodium dithionite. Unsubstituted or substituted
diphenylmethoxycarbonylamino, tert-lower alkoxycarbonylamino or 2-(tri-substituted
silyl)-lower alkoxycarbonylamino, such as 2-tri-lowçr alkylsilyl-lower alkoxycarbonyl-
arnino, can be cleaved by treatment with a suitable acid, for example formic acid or -
trifluoroacetic acid; unsubstituted or substituted benzyloxycarbonylamino can be cleaved,
for example, by means of hydrogenolysis, i.e. by treatment with hydrogen in the presence
of a suitable hydrogenation catalyst, such as a platinum or palladium catalyst;
unsubstituted or substituted triarylmethylamino or formylamino can be cleaved, for
example, by treatment with an acid, such as a mineral acid, for example hydrochloric acid,
or an organic acid, for example formic, acetic or trifluoroacetic acid, where appropriate in
the presence of water, and an arnino group pt~tected in the form of silylamino can be
freed, for example, by means of hydrolysis or alcoholysis. An amino group protected by
2-haloacetyl, for example 2-chloroacetyl, can be freed by treatment with thiourea in the
presence of a base, or with a thiolate salt, such as an aLkali metal thiolate of thiourea, and
subsequent solvolysis, such as a1coholysis or hydrolysis, of the resulting substitution
product; and amino is freed from trifluoroacetylamino, for example, by hydrogenolysis
with bases, such as alkali metal hydroxides or carbonates, such as Na2CO3 or K2CO3, in
polar solventsi for example alcohols, such as methanol, at temperatures of from 0 to
100C, especially at from 40 to 80C. An amino group protected by 2-(tri-substituted
silyl)-lower aL~oxycarbonyl, such as 2-tri-lower alkylsilyl-lower alkoxycarbonyl, can also
be converted into the free amino group by treatment with a salt of hydrofluoric acid that
yields fluoride anions as indicated above in connection with the freeing of a correspond-
ingly protected carboxy group. Likewise, silyl, such as trimethylsilyl, bonded directly to a
hetero atom, such as nitrogen, an be removed using fluoride ions.
Amino protected in the form of an azido group is converted into free amino, for example,
by reduction, for example by catalydc hydrogenation with hydrogen in the presence of a
hydrogenation catalyst, such as platinum oxide, palladium or Raney nickel, by reducdon
using mercapto compounds, such as dithiothreitol or mercaptoethanol, or by treatment
with zinc in the presence of an acid, such as acetic acid. The catalytic hydrogenation is
preferably carried out in an inert solvent, such as a halogenated hydrocarbon, for example
2~2~A7
~9
methylene chloride, or in water or in a mixture of water and an organic solvent, such as an
alcohol or dioxane, at approximately from 20C to 25C, or with cooling or heating.
A hydroxy or mercapto group protected by a suitable acyl group, a tri-lower alkylsilyl
group or by unsubstituted or substituted l-phenyl-lower alkyl is freed analogously to a
correspondingly protected amino group. A hydroxy or mercapto gTOUp protected by
2,2-dichloroacetyl is freed, for example, by basic hydrolysis, and a hydroxy or mercapto
group protected by tert-lower alkyl or by a 2-oxa- or 2-thia-aliphatic or -cycloaliphatic
hydrocarbon radical is freed by acidolysis, for example by tTeatment with a mineral acid or
a stTong carboxylic acid, for example trifluoroacetic acid. Mercapto protected by pyridyl-
diphenylmethyl can be freed, for example, using mercury(II) salts at pH 2-6 OI by zinc/- -
acetic acid or by electrolytic reduction; acetamidomethyl and isobutyrylamidomethyl ca
be freed, for example, by reaction with mercury(II) salts at pH 2-6; 2-chloroacetamido-
methyl can be freed, for example, using l-piperidinothiocarboxamide; and S-ethylthio,
S-tert-butylthio and S-sulfo can be freed, for example, by thiolysis with thiophenol, thio-
glycoiic acid, sodium thiophenolate or 1 ,4-dithiothreitol. Two hydroxy gTOUpS or an
adjacent amino and hydroxy group which are protected togetheT by means of a bivalent
protecting gTOUp, prefeTably, for example, by a methylene group mono- or di-substituted
by lower aL~cyl, such as lower aLIcylidene, for example isopropylidene, cycloalkyiidene, for
example cyclohexylidene, or benzylidene, can be freed by acid solvolysis, especially in
the presence of a mineral acid or a strong organic acid. A tri-lower aL~ylsilyl g~oup is
likewise removed by aeidolysis, for example by a mineral acid, preferably hydrofluoric
aeid, or a strong earboxylie aeid. 2-halo-lower alkoxycarbonyl is removed using the
above-mentioned redueing agents, for example a redueing metal, sueh as zine, redueing
metal salts, sueh as ehromium(II) salts, or using sulfur compounds, for example sodium
dithionite or preferably sodium sulfide and earbon disulfide.
A sulfo group protected in the form of a sulfonic acid ester or sulfonamide is freed, for
example, by acid hydrolysis, for example in the presence of a mineral acid, or preferably
by basic hydrolysis, for example with alkali metal hydroxide or alkali metal carbonate, for
example sodium carbonate.
When several protected functional groups are present, if desired the protecting groups ean
be so selected that more than one such group can be removed simultaneously, for example
by acidolysis, such as by treatment with trifluoroacetic acid, or with hydrogen and a
hydrogenation catalyst, such as a palladium-on-carbon catalyst. Conversely, the groups
`~
~~~` 2~l2al7
- 70 -
can also be so selected that they cannot all be removed simultaneously, but rather in a
desired sequence, the corresponding intermediates being obtained.
Additional Process Steps
In the additional process steps, which are optional, functional groups of the starting
compounds that are not to take part in the reaction may be in unprotected or protected
form, for example may be protected by one or more of the protecting groups mentioned
above under Process a). The protecting groups may be retained in the end products or
some or all of them may be removed according to one of the methods mentioned under
Process f).
Salts of compounds of formula I having at least one salt-forming group may be prepared in
a manner known per se. For example, salts of compounds of formula I having acid groups
may be formed, for example, by treating the compounds with metal compounds, such as
alkali metal salts of suitable organic carboxylic acids, e.g. the sodium salt of 2-ethyl-
hexanoic acid, with organic aLkali metal or aLcaline earth metal compounds, such as the
corresponding hydroxides, carbonaees or hydrogen carbonates, such as sodium or
potassium hydroxide, carbonate or hydrogen carbonate, with corresponding calciumcompounds or with ammonia or a suitable organic amine, stoichiometric a~nounts or only
a sma11 excess o the salt-forming agent preferably being used. Acid addition salts of -
compounds of formula I are obtained in customary manner, e.g. by treating the compounds
with an acid or a suitable anion exchange reagent. Internal salts of compounds of formula I
containing acid and basic salt-fonning groups, e.g. a free carboxy group and a free amino
group, may be formed, e.g. by the neutralisation of salts, such as acid addition salts, to the
isoelectric point, e.g. with weak bases, or by treatment with ion exchangers.
Salts can be converted in customary manner into the free compounds; metal and
ammonium salts can be converted, for example, by treatment with suitable acids, and acid
addition salts, for example, by treatment with a suitable basic agent.
Stereoisomeric mixtures, that is to say mixtures of diastereoisomers andlor enantdomers,
such as, for example, racemic mixtures, can be separated in a manner known per se by
suitaUe separadng processes into the corresponding isomers. For example, mixtures of
diastereoisomers can be separated into the individual diastereoisomers by fracdonal
crystallisation, chromatography, solvent paltition etc. Racemates can be separated from
~12~7
one another, after conversion of the optical antipodes into diastereoisomers for example by
reaction with optically active compounds, e.g. optically active acids or bases, by
chromatography on column materials covered with optically active compounds or byenzymatic methods, e.g. by selective reaction of only one of the two enantiomers. This
separation can be carried out either at the stage of one of the starting materials or with the
compounds of formula I themselves.
The configuration at individual chirality centres in a compound of formula I can be select-
ively reversed. For example, the configuration of asymmetric carbon atoms that carry
nucleophilic substituents, such as amino or hydroxy, can be reversed by second-order
nucleophilic substitution, optionally after conversion of the bonded nucleophilic substit-
uent into a suitable nucleofugal leaving group and reaction with a reagent that introduces
the original substituent.
In a compound of formula I, an aryl radical present in R7 and/or R3, especially a phenyl
radical, can be hydrogenated for example by catalytic hydrogenation, especially in the
presence of heavy metal oxides, such as rhodium/platinum mixed oxides, for example with
the Nishimura catalyst, preferably in a polar solvent, such as an alcohol, for example
methanol or ethanol, at temperatures of from 0 to 80C, especially from 10 to 40C, and at
a hydrogen pressure of from 1 to 10 atm, preferably at a~proximately normal pressure.
In a compound of formula I, a C3-C7ali~enyl radical or C3-C7aLlcynyl radical present in R
and/or Rg, cspecially an allyl radical, for example in allyloxycarbonyl, can be hydrogen-
ated, f~ example by catalytic hydrogenation in the presence of metal, such as platinum or
especia11y palladium, catalysts, which are free or, preferably, on a ca~ier, such as carbon
or silica gel, especially in the presence of palladium on activated carbon, preferably in a
polar solvent, such as a lower alkanol, for example methanol or ethanol, at temperatures of
from 0 to 80C, especially from 0 to 40C, and under a hydrogen pressure of a maximum
of 10 atm, preferably under approximately normal pressure.
In an obtainable compound of formula I an amino or carboxamide group may be substi-
tuted or a carboxy group that is free or in reactive form may be esterified or amidated.
The substitution of a carboxamide group or of another primary or secondary amino group,
for example in order to introduce radicals such as unsubstituted or substituted allcyl Rl,
R2, R8 and/or Rg into compounds of formula I in which at least one of these radicals is
21~ 2~7
- 72 -
hydrogen, to introduce unsubstituted or substituted aLkyl, as defined for N-substituted
aminocarbonyloxy Rs, as a substituent at the nitrogen atom of aminocarbonyloxy Rs, or to
introduce unsubstituted or substituted aLkyl as a substituent in unsubstituted or substituted
N-aLkyl- or N,N-diaLkyl-carbamoyl Rg or Rl, is effected, for example, by aLIcylation with
suitable starting materials.
Suitable agents for aLlcylating a carboxamide group in a compound of formula I are, for
example, diazo compounds, for example diazomethane. Diazomethane can be
decomposed in an inert solvent, the free methylene formed reacting with the carboxamide
group in the compound of formula I. The decomposition of diazomethane is carried out
preferably catalytically, for example in the presence of a noble metal in finely divided
form, such as copper, or a noble metal salt, for example copper(I) chloride or copperlII)
sulfate.
Further aL~cylating agents are selected from compounds of formulae
Rl"-X (Xa),
R2'-X (Xb),
Rg''-X (XIa) and
R8'-X (XIb),
wherein the radical X is a leaving group and Rl'' and R9" have the definitions given for Rl
or R9 with the excepdon of hydrogen, acyl, and sulfo that is unsubstituted or substituted as
above and R2' and R8' have the definitions given for R2 and R8 with the exception of
hydrogen. A leaving group X is especially a nucleofugal leaving group selected from
hydroxy esterified by a strong inorganic or organic acid, such as hydroxy esterified by a
mineral acid, for example a hydrohalic acid, such as hydrochloric, hydrobromic or hydr-
iodic acid, or by a strong organic sulfonic acid, such as an unsubstituted or substituted, for
example halo-substituted, such as fluoro-substituted, lower aL~canesulfonic acid, or an
aromatic sulfonic acid, for example a benzenesulfonic acid that is unsubstituted or
substituted by lower alkyl, such as methyl, by halogen, such as bromine, and/or by nitro,
for example a methanesulfonic, trimethanesulfonic or p-toluenesulfonic acid, and hydroxy
esterified by hydrazoic acid.
The reaction can be carried ou~ under the conditions of a first-order or second-order
nucleophilic substitution.
2112 347
- 73 -
For example, one of the compounds of formulae Xa, Xb, XIa and XIb can, especially
when the radical X is a leaving group with a high polarisability of the electron shell, for
example iodine, be used in a polar aprotic solvent, for example acetone, acetonitrile, nitro-
methane, dimethyl sulfoxide or dimethylformamide. The reaction can also be carried out
in water to which, where appropriate, an organic solvent, for example ethanol, tetrahydro-
furan or acetone, has been added as solubiliser. The substitution reaction is carried out if
desired at reduced or elevated temperature, for example in a temperature range of from
approximately -40 to approximately 100C, preferably from approximately -10 toapproximately 50C, and if desired under an inert gas, for example under a nitrogen or
argon atmosphere.
For the esterification or amidation of a carboxy group in a compound of formula I, if
desired the free acid can be used or the free acid can be converted into one of the above-
mentioned reactive derivatives and reacted with an alcohol, with ammonia, or with a
primary or secondary amine, or, in the case of esterification, the free acid or a reactive
salt, for example th~i caesium salt, can be reacted with a reactive derivative of an alcohol.
Por example the caesium salt of a carboxylic acid can be reacted with a halide or sulfonic
acid ester corresponding to the alcohol. The esterification of the carboxy group can also be
carried out with other customary alkylating agents, for example with diazomethane, allyl
halides, su1fonic acid esters, Meerwein salts or l-substituted 3-aryltriazenes.
A free arnino group preisent in a compound of formula I can be acylated, for example to
introduce one of the radicals acyl, sulfo and substituted sulfonyl mentioned for Rl or Rg.
The acylation is ~aTTied out according to one of the methods mentioned above under
Process b), c) or d) for condensation or according to one of the methods mentioned for
protecting groups or, for example, according to one of the processes mentioned in
Organikum, 17th edition, VEB Deutscher Verlag der Wissenschaften, Berlin (East) 1988.
In an obtainable compound of formula I wherein the substituents are as defined and at
least one free hydroxy group is present and the remaining functional groups are in pro-
tected form, the free hydroxy group can be acylated or etherified.
The acylation can be carried out with suitable acylating reagents according to one of the
methods mentioned under Processes a) to d) or according to one of the methods mentioned
for protecting groups.
~ ~, . . .... . . . .
2 ~ 7
- 74 -
The etherification can be carried out with the above-mentioned alkylating agents and
under the same reaction conditions, for example with diazomethane, alkyl halides,
sulfonic acid esters, Meerwein salts, l-substituted 3-aryltriazenes, etc..
In an obtainable compound of formula I a sulfinyl or sulfonyl group can be produced from
a thio group, and the corresponding sulfoxide or sulfone from a sulfide, by oxidation.
The oxidation to the sulfonyl group or to the sulfone can be carried out with most of the
customary oxidising agents. The oxidising agen~s used are preferably those that oxidise
~he thio group or the sulfide sulfur selectively in the presence of other functional groups of
the compound of formula I in question, for example amino or hydroxy groups; examples
of such oxidising agents are aromatic or aliphatic peroxycarboxylic acids, for example
peToxybenzoic acid, monoperphthalic acid, m-chloroperbenzoic acid, peracetic acid,
performic acid or trifluoroperacetic acid. The oxidation with peroxycarboxylic acids is
carried out in the customary solvents suitable therefor, for example chlorinated hydro-
carbons, for example methylene chloride or chloroform, ethers, such as diethyl ether,
esters, such as ethyl acetate or the like, at temperatures of from -78C to roomtemperature, for example from -20C to +10C, preferably about 0C. The peroxycarb-
oxylic acid can also be formed in situ, for example with hydrogen peroxide in acetic acid
or formic acid that may or may not contain acetic anhydride, for exarnple with 30 % or
90 % hydrogen peroxide in acetic acid/acetic anhydride. Also suitable are other peroxo
compounds, for example potassium peroxomonosulfate in lower alkanoVwater mixtures,
for example methanoVwater or ethanoVwater, or in aqueous acedc acid at temperatures of
from -70C to +30C, for example from -20C to room temperature, and also sodiummetaperiodate in methanol or methanoUwater mixtures at temperatures of from 0C to
50C, for example approximately room temperature. If stoichiometric amounts of the
mentioned oxidising agents are used it is also possible-for the corresponding sulfinic acids
or sulfoxides to be obta~ned. There are suitable for that purpose, for example, sodium
metaperiodate in methanol or methanoVwater mixtures at temperatures of from -15C to
room temperature, for example approximately 0C, m-chloroperbenzoic acid in methylene
chloride, chloroform or ethyl acetate at temperatures of from -78C to 10C, preferably
from -30C to 0C, also tert-butylhypochlorite in lower alkanols, for example methanol, or
hydrogen peroxide in acetone or acetic acid at temperatures of approximately 0C, or the
above-mentioned potassium peroxomonosulfate at low temperatures.
2 ~ 7
- 75 -
If desired, the corresponding thio compound or the corresponding sulfide can be obtained
by reducing a sulfonyl group or a sulfone radical in an obtainable compound of formula I,
for example with diisobutylaluminium hydride in ether or tetrahydrofuran.
In an obtainable compound of formula I having a sulfinyl group, that group can be reduced
to a thio group. Selective reducing agents that leave other functional groups in the
compound of formula I, for example the amide function, unchanged are preferred.
Examples of such selective reducing agents are dichloroborane, which is preferably used
in tetrahydrofuran or dimethoxyethane at temperatures of from -30C to ~10C, triphenyl-
phosphine in boiling carbon tetrachloride, trichlorosilane or hexachlorodisilane, iron
pentacarbonyl, also sodium hydrogen sulfite in aqueous/alcoholic solvents, for exarnple
water/methanol, water/ethanol or also water/tetrahydrofuran, at temperatures of from
-10C to +50C, also sodium borohydride in the presence of cobalt(II) chloride or also
hydrogen in the presence of catalytic amounts of palladium, for example palladium/carbon
in boiling ethanol.
In a compound of formula I, any protecting groups present or suitable radicals Rl or Rg,
i.e. especially those representihlg acyl, can be removed according to one of the processes
mentioned under Process f), especially by hydrolysis, for example in the presence of
bases, such as alkali or aLkaline earth metal hydroxides, for example sodium hydroxide, or
acids1 such as organic acids or mineral acids, for example hydrogen halide, such as -
hydrogen chloride. The hydrolysis is carried out under customary conditions, for example
in aqueous solution or in anhydrous solvents, especially in ethers, such as dioxane, at
temperatures of from -50C to the reflux temperature of the reaction mixtures in question,
for exarnple from 0C to 50C, preferably in the presence of a protective gas, such as
argon or nitrogen.
Startin~ materials:
The present invention relates also to novel starting materials and/or intermediates and to
processes for their preparation. The starting materials used and the reaction conditions
selected are preferably those that result in the compounds described as being preferred.
Attention is drawn to the fact that in the case of starting compounds of formula IV, VI
and VIII, the acyl radical of acyloxy R5 may migrate to a nitrogen atom; Processes b), c)
and d) above should therefore be regarded as being subject to that reservation.
Processes a), e) and f) and their starting compounds are preferred, especially Processes a)
. ,.. ~ .. ~ ... , . , - ~ ,. - -
:.: . .
.~,': ~ ' ~ . - ''' ; -
. ~, .
2~12~47
- 76 -
and f).
In the preparation of all starting materials free functional groups that are not to participate
in the reaction in question may be in unprotected or protected form, for example they may
be protected by the protecting groups mentioned above under Process a). Those protecting
groups may be freed at appropriate times by the reaction described under Process f). The
protecting groups are introduced, for example, as in the description of Process a).
The hydroxy compounds of formula II used as starting materials, and other staIting
compounds, are obtained analogously to the processes described in the European Patent
Application having the Publication No. EP O 521 827 (published on 7th January 1993),
preferably using the correspondingly substituted compounds, for example by adding a
hydrazine derivative of formula
R17
7 (XIII),
R8
~''
wherein the radicals are as defined above, to an epoxide of formula
R2
R~ >~ (XIV),
R3 R4
:
preferably of formula ~ ~ ~
R2 H .:
R~ ~"~ (XIV A)
.
R3
wherein each of the radicals is as defined above, free functional groups, with the excep~on
~ .: :. . . . : :
211~ 17
- 77 -
of those participating in the reaction, being if necessary in protected forrn, and removing
any protecting groups present. When the compound of formula XIV A is used, the
preferred starting materials of formula II' are obtained, as mentioned under Process a).
Depending on the definition of R7, the arnino group of the hydrazine derivative of
formula XIII participating in the reaction has preferably at least one free hydrogen atom; it
may, however, itself have been derivatised in order to increase the reactivity of the
hydraz,ine derivative.
The addidon of the compounds of formula XIII to the epoxides (oxiranes) of formula XIV
is effected preferably under the conditions customaTy for the addidon of nucleophiles to
epoxides.
The addidon is effected especially in aqueous soludon and/or in the presence of polar
solvents, such as alcohols, for example methanol, ethanol or ethylene glycol, ethers, such
as dioxane, amides, such as dimethylformamide, or phenols, such as phenol, also under --
anhydrous condidons, in non-polar solvents, such as benz,ene or toluene, or in benz,ene/-
water emulsions, where appropriate in the presence of acid or basic catalysts, for example
aLkaline solutions, such as sodium hydroxide soludon, or in the presence of solid-phase
catalysts treated with hydraz,ine, such as aluminium oxide, in ethers, for example diethyl
ether, generally at temperatures of approximately from 0C to the reflux temperature,
preferably from 20C to 130C, where appropriate under reflux, under elevated pressure,
for example in a bomb tube, it also being possible for the boiling temperature of tlhe
reaction mixture to be exceeded, and/or under an inert gas, such as niTrogen or aTgon, it
being possible for each of the two compounds of formulae XIII and XIV to be present in
excess, for example in a molar ratio of from 1:1 to 1:100, preferably in a molar ratio of 1:1
to 1:10, especially in a ratio of 1:1 to 1:3.
The starting materials of formulae XIII and XIV are known or, if novel, can be prepared
according to processes known per se, for example compounds of formula XIII can be
prepared from hydrazine or suitable derivatives thereof, and compounds of formula XIV
can be prepared from suitable amino acids or analogues thereof, for example those having
one of the mentioned side chains R3.
The compounds of formula XIII can be obtained, for example, from hydrazine or suitable
derivatives thereof of formula
- 2~2~7
- 78 -
H2N-NH-Rll (XV)
wherein Rl I is hydrogen or an amino-protecting group, as described above under
Process a), especially tert-lower alkoxycarbonyl, such as tert-butoxycarbonyl, aryl-lower
alkoxycarbonyl, such as benzyloxycarbonyl or 9-fluorenylmethoxycarbonyl, or one of the
above-mentioned acyl amino-protecting groups by (in o~der to prepare via the subsequent
steps a compound of formula I wherein in place of R7 there is the radical R7~ which is
unsubstituted or substituted alkyl or cycloalkyl) alkylating with a compound of
formula XII to introduce R7~, as defined under Process e), under conditions as described
under Process e), or introducing a radical R7 by reaction of a suitable carbonyl compound
with the free amino group of the compound of formula XV or an acylated derivative
thereof and subsequent reduction of the resulting hydrazone to form a hydrazine derivative
of formula
RTNH NH R~1 (XVI),
wherein the radicals in all of the mentioned compounds are as defined hereinbefore and
functional groups in participating reagents that are not to take part in the reaction are, if : .
desired, protected, and removing the protecting group R~ 1. unless it corresponds to one of : .
the radicals R9 in compounds of formula I, and then introducing a radical R9 other than . -
hydrogen, if desired by condensation under the conditions mentioned above for Process b) ~ ~ ~
with an acid of fonnula V or by alkylation with a compound of formula XIa, as defined .-.
above, and/or introducing a radical R8 other than hydrogen by alkylation with a compound
of formula XIb, as defined above, under conditions analogous to those mentioned above
for aL~cylation in the "Additional Process Steps". ~ :
The carbonyl compounds suitable for the introduction of R7 that are used for ~e prepara- .
tion of compounds of formula XVI are known aldehydes or ketones that can be prepared
by processes knownper se or are commercially available, the reactive carbonyl group of
which is, after reaction with compounds of formula XV and subsequent reduction, a
component of one of the mentioned radicals R7, preferably lower alkane aldehydes, cyclo-
hexyl-lower alkane aldehydes or phenyl-lower alkane aldehydes. :
The reaction of those carbonyl compounds with the compounds of formula XVI to form
the corresponding hydrazones is carried out under the conditions customaIy for the
reaction of carbonyl compounds with arnines, preferably in polar organic solvents, for
-` 2~2~47
-79-
example ethers, such as tetrahydrofuran or diethyl ether, alcohols, such as methanol or
ethanol, carboxylic acid amides, such as dimethylformamide, or esters, such as ethyl
acetate, or in aqueous solution, preferably in methanol, and also in the presence or absence
of acid catalysts, for example carboxylic acids, such as formic acid or acetic acid, or
sulfonic acids, such as p-toluenesulfonic acid, at temperatures of from 0C to the reflux
temperature of the reaction mixture, preferably at temperatures of from 20C to the reflux
temperature of the reaction mixture.
The reduction of the resulting hydrazones is carried out preferably by hydrogenation in the
presence of a suitable catalyst. Suitable catalysts used for the hydrogenation include
metals, such as nickel, iron, cobalt or ruthenium, and noble metals and their oxides, such
as palladium or rhodium and their oxides, where appropriate applied for example to a
suitable support, such as barium sulfate, aluminium oxide or activated carbon, or in the
form of skeleton catalysts, such as Raney nickel. Customary solvents for the catalytic
hydrogenation are, for example, water, alcohols, such as methanol or ethanol, esters, such
as ethyl acetate, ethers, such as dioxane, chlorinated hydrocarbons, such as dichloro-
methane, carboxylic acid amides, such as dimethylformamide, or carboxylic acids, such as
glacial acetic acid, or mixtures of those solvents. The hydrogenation is carried out at
temperatures of from 10 to 250C, preferably from room temperature to 100C, and at
hydrogen pressures of from 1 to 200 bar, preferably from 1 to 10 bar, in the customary
apparatus.
Especially preferred for the preparation of compounds of formula XV are reaction condi-
tions analogous to those described in J. Chem. Soc. Perkin I, 1712 (1975).
The compounds of formula XIV can be obtained, for example, by the reduction of amino
acids ~cnown per se that are commercially available or can be prepared by processes
known per se, or their analogues, of forrnula
Rlo--NH COOH
X (XVII)
R3 R,~, .
wherein Rlo is hydrogen or one of the amino-protecting groups mentioned under
Process a), especially tert-lower alkoxycarbonyl, such as tert-butoxycarbonyl, aryl-lower
`, 2112~
- 80 -
alkoxycarbonyl, such as benzyloxycarbonyl or 9-fluorenylmethoxycarbonyl, or one of the
acyl arnino-protecting groups mentioned under that process, and R3 and R4 are as defined
for compounds of forrnula I, preferably the reduction of amino acids of formula
Rlo--NH~COOH
-- (XVII A), - :
R3 :
wherein the radicals are as defined, to aldehydes of fonnula
Rlo--NH ~CHO : `
R3 R4
~ "
wherein the radica1s are as def~ed, preferably to the aldehydes of formula
:''
Rlo--NH~CHO
(XVIII A), ~ ; ~
R3 - ~ -
wheran the radicals are as defined (obtainable, for example, from compounds of
formula XVII A), by reaction of those aldchydes with an ylide compound, preferably a :
sulfur-ylide compound, to form an epoxide of formula
':
o
Rlo~NH ~ l
X \1 (Xl[X)7
R3 R4
wherein the radicals are as defined, preferably to compounds of formula
--` 2~2a~7
,
Rlo--NH ~ ¦
\l (XIX A),
-
R3
(obtainable, for example, from compounds of formula XVIII A), wherein the radicals are
as de9ned, removal of the protecting gf~Up Rlo, unless it corresponds to a radical Rl in
compounds of formula I, and (to introduce Rl other than hydrogen) acylation of the amino
group of the resulting compound with an acid of formula VII wherein Rl' is as defined,
under the conditions described for Process b), or alkylation of the amino group of the
resulting compound with a reagent having a nucleofugal leaving group X of formula Xa,
wherein Rl" is as de9ned, and/or (to introduce R2 other than hydrogen) by aLkylation with
a compound of formula Xb wherein R2' is as defined, under the conditions described for
additional process steps, the compounds of formula XIV A preferably being obtained from
a starting material of formula XIX A.
The reduction of amino acids of forrnula XVII or XVII A to the corresponding
aldehydes XVIII and xvm A is carried out, for example, by reduction to the corres-
ponding alcohols and subsequent oxidation to the mentioned aldehydes.
The reduction to the alcohols is carried out, for example, by hydrogenation of the amino
acid halides or other activated carboxylic acid derivatives (for example with activated
hydroxy analogously to compounds of formula IX, as defined under Process a)) under the
conditions mentioned for the hydrogenation of hyd~azones obtained from compounds of
formula XVI, or with complex hydrides, such as sodium borohydride. The subsequent
oxidation of the resulting alcohols is preferably carried out using oxidising agents that
allow the aldehydes of formula xvm or xvm A to be obtained selectively (i.e. without
further oxidation of the aldehydes to the carboxylic acids), for example using potassium
ferrate (K2FeO4) in aqueous solvents or manganese dioxide in organic solvents, or organic
chromic acid derivatives, such as pyridinium dichromate or tert-butyl chromase, in inert
organic solvents, for example chlorinated hydrocarbons, such as methylene chloride or
chloroforrn, in the presence or absence of basic amines, for example tri-lower alkylamines,
such as triethylamine, at temperatures of from -50 to 100C, preferably at from -10 to
50C, for example as described in European Patent Application EP-A-0 236 734, or
,i ~ - ~ . . . . . . .. .
-` 2112~7
- 82-
especially by oxidation of the hydroxy group with a sulfoxide, such as dimethyl sulfoxide,
in the presence of a reagent that activates the hydroxy group, for example a carboxylic
acid chloride, such as oxalyl chloAde, in an inert solvent, for example a chlorinated hydro-
carbon, such as dichloromethane, andlor an acyclic or cyclic ether, such as tetrahydro-
furan, at from -80 to 0C, for example from -78 to -50C.
Direct reduction of the arnino acids to the aldehydes is also possible, for example by
hydrogenation in the presence of a partially contaminated palladium catalyst or by reduc-
tion of the corresponding arnino acid ester, for example the lower alkyl ester, such as ethyl
ester~ with complex hydrides, for example borohydrides, such as sodium borohydride, or
preferably aluminium hydrides, for example lithium aluminium hydride, lithium tri(tert-
butoxy)aluminium hydride or especially diisobutylaluminium hydride, in non-polarsolvents, for example in hydrocarbons or aromatic solvents, such as toluene, at from -100
to 0C, preferably from -70 to -30C, and subsequent reaction to form the corresponding ; ~ -
semicarbazones, for example with the corresponding acid salts of semicarbazones, such as
semicarbazide hydrochloride, in aqueous solvent systems, such as alcohoVwater, for
example ethanoVwater, at temperatures of from -20 to 60C, preferably from 10 to 30C,
and reaction of the resulting semicarbazone with a reactive aldehyde, for example
formaldehyde, in an inert solvent, for example a polar organic solvent, for example a
carboxylic acid amide, such as dimethylformamide, at temperatures of from -30 to 60C,
preferably from 0 to 30C, and then with an acid, for example a strong mineral acid, such
as hydrogen halide, in aqueous soludon, if desired in the presence of the previously used
solvent, at temperatures of from -40 to 50C, preferably from -10 to 30C. The corres-
ponding esters are obtained by reacdon of the amino acids with the correspondingalcohols, for example ethanol, analogously to the conditdons used in the acyladon in
Process a), for example by reaction with inorganic acid halides, such as thionyl chloride,
in organic solvent mixtures, such as mixtures of aromatdc and alcoholic solvents, for
example toluene and ethanol, at temperatures of from -50 to 50C, preferably from -10 to
20C (if necessary using protecting groups).
The preparation of compounds of formulae XVIII and XVIII A is carried out preferably
under conditions analogous to the reaction condidons mendoned in J. Org. Chem. 47,
3016 (1982) or J. Org. Chem. 43,3624 (1978).
A sulfur-ylide suitable for the reacdon of compounds of formula XVIII or XVIII A to
form the epoxides of formula XIX or XIX A is, for example, a dialkylsulfonium
7 21 l2 0~ 7
- 83 -
methylide, for example dimethylsulfonium methylide, an aLl~yl- or phenyl-dialkylamino-
sulfoxonium methylide, for example methyl- or phenyl-dimethylaminosulfoxonium
methylide, or a dialkylsulfoxonium methylide, for example dimethyl- or diethyl-sulf-
oxonium methylide.
The sulfur-ylide compound in question is expediendy prepared in situ from the corres-
ponding sulfonium or sulfoxonium salt and a base, for example sodium hydride, in a
dipolar aprotic solvent, for example dimethyl sulfoxide, or an ether, for example tetra-
hydrofuran or 1,2-dimethoxyethane, and then reacted with the compounds of
formula XVIll or XVIII A. The reaction is normally carried out at room tempeMture, with
cooling for example to -20C, or with gende heating for exarnple to 40C. The sulfide,
sulfinamide or sulfoxide formed simultaneously is removed during the subsequent
aqueous working-up.
The reaction with a sulfur-ylide is preferably carried out analogously to dle condi~ons
mentioned in J. C)rg. Chem. 50, 4615 (1985).
The compound of formula XIX (preferably XIX A) can also be obtained from a compound
of formula XVIII (preferably XVIII A), as defined above, by reaction thereof widl a tri-
lower aLkyl-silylmethyl-Grignard compound, for example prepared from the corres-ponding halo-methylsilane, such as chloromethyl-trimethylsilane, in an inert solvent, for
example an ethcr, such as dioxane or diethyl ether, at temperatures of from 0 to 50C, for
example from room temperature to approximately 40C, subse~quent elimination with
removal of the silyl radical and formation of a double bond, for example by means of a
Lewis acid, such as BF3, an amino-protecting group Rlo present preferably also being
removed, in an inert solvent, for example an ether, such as diethyl ether, or a halogenated
hydrocarbon, such as dichloromethane, or a mixture thereof, at temperatures of from
-50C to the reflux temperature, especially from 0 to 30C, if necessary acylation once
more to introduce an amino-protecting group as Rlo, as defined above, and oxidation of
the resulting double bond to the oxirane, preferably with a percarboxylic acid, for example
m-chloroperbenzoic acid, in an inert so1vent, for example a halogenated hydrocarbon, such
as dichloromethane, at temperatures of from -20C to the reflux temperature of the
mixture, for example at from 10 to 30C.
The preferred star~ing material of formula II' in Process a), or a salt thereof, is prepared,
for example, by adding a hydrazine derivative of the above-defined formula XVI to an
. , . . i ' ~ ~ ' ' ' '
- 2~12~7
- 84-
epoxide of the above-defined formula XIV A, and if desired converting a compound of
formula II' obtainable in accordance with the above process having at least one salt-
forming group into its salt or converting an obtainable salt into the free compound or into
a different salt and/or where appropriate separating obtainable isomeric mixtures and/or
removing protecting groups present in a compound of formula II and/or converting a
compound of formula II according to the invention into a different compound of formula II
according to the invention.
The preparation and conversion of salts, the separadon of isomeric mixtures, the removal
of protecting groups and the conversion of compounds of formula II' are effectedanalogously to the processes described above for compounds of formula I.
The starting materials of Processes b), c) and d) can be prepared in accordance with ~ ~ :
processes known per se; for example, analogously to Pr~cess a), if necessary with the use :
and removal of protectdng groups, compounds of formula IV can be prepared from : :~
hydrazine derivatives of forrnula XIII wherein Rg is hydrogen and the remaining radicals
are as defined for compounds of formula IV, and epoxides of formula XIV wherein the
radicals are as defined for compounds of formula IV, with subsequent acylation of the
resulting compound of formula IV'
¦ HO R6 1 7
R~ ~N~N~ av )~
R3 R4 ¦ . -
R8
especially of formula IV"
R2
HO R6 T7
~N~><~N ~H (IV")
~ `~ N
R3 R4
R8
- 21~2~47
- 85 -
wherein the radicals are as defined for compounds of formula I and wherein instead of the
radical R5 present in a compound of formula IV there is a free hydroxy group, with a
carboxylic acid of formula III or an activated carboxylic acid derivative thereof, as
described under Process a) (yields starting material of formula IV for Process b));
compounds of forrnula VI can be prepared from hydrazine derivatives of formula XIII
wherein the radicals are as defined for compounds of formula VI, and epoxides offoImula XIV wherein Rl is hydrogen and the remaining radicals are as defined forcompounds of formula VI, with subsequent acylation of the resulting compound of
formula VI'
R2 R
HO R6
H~ >~N~ ~Rg (VI )~
R3 R4
R8
especially of formula VI"
R2
¦ HO R6
H~ ~N~ ~Rg (VI )~
R3 R4
R8
wherein the radicals are as defined for compounds of formula I and instead of the radical
Rs present in a compound of formula VI there is a free hydroxy group, with a carboxylic
acid of formula m or an actiyated carboxylic acid derivative thereof, as described under
Process a) (yields starting material of formula VI for Process c)); and compounds of
formula vm can be prepared from hydrazine derivatives of formula xm wherein Rg is
hydrogen and the remaining radicals are as defined for compounds of formula VIII, and
epoxides of formula XIV wherein Rl is hydrogen and the remaining radicals are asdefined for compounds of formula IX, with subsequent acylation of the resulting
compound of formula vm~
.. -.. . : ~ ~. ~.. . ............. . .
S, ~
-- 2 ~ 4 7
- 86- :
R~
¦ HO R6 7 ~ ~
H~ >~N~ ~H (VIII ),
R3 R4
R8
especially of formula VIII" ~ :
R2
¦ HO R17 ~ 1
~N~ ~N~ (VIII"),
R3 4
R8
wherein the radicals are as defined for compounds of formula I and wherein instead of the
radical Rs present in a compound of formula IV there is a free hydroxy group, with a
carboxylic acid of formula m or an activated carboxylic acid derivative thereof, as
described under Proceiss a) (yields stardng material of formula vm for Process d. In the
mendoned preparatdon processes for the preparadon of the preferred compounds of
formula IV (via IV"), VI (via VI") and vm (via vm~) wherein the carbon atoms ca~Tying . - .
R3 and R5 are both in the (S)-con~lguradon, preference is given to the use of the epoxides
of formula XIV A.
Compounds of fonnulaI' wherein the subsdtuents are as defined above, can be prepared,
for example, from a compound of formula xm~
H
HN Rg
\N~ (Xm'),
R8 ~:
wherein the radicals are as deflned for compounds of formula I, by reaction with a
compound of formula XIV, especially XIV A (which results in the preferred compounds
of fonnula I' wherein ~e carbon atoms car~ying R3 and Rs are both in the (S)-configur-
adon), as described for the reacdon of compounds of formula XIII with those of
~. .
2112~47
- 87 -
formula XIV or XIV A, and subsequent acylation of the resulting compound of formula I"
R2
¦ HO R6 l
R~ >~ ~N~ 9 lI ),
R3 R4 ¦
R8
especially of formula I" ',
R2
¦ HO R6
R~ ~N~N~ 9 (I )~
R3 4
R8
wherein the radicals are as defined for compounds of formula I', vith a compound of
formula m or an activated carboxylic acid derivalive thereof (preparation of the radical
acyloxy R5) under reaction conditions analogous to tnose described under Process a) (in
that case there is to be used instead of the compound of formula II an analogouscompound containing R7 instead of R7'); as in that case, any functional groups present that
are not intended to participate in the reaction are where necessary in protected form and
can be freed after the reaction.
Of the starting compounds of fonnula II or II', the compound of formula
H3C CH3
A3C J~,N~N~ ~JX~ \CN (11
b H3C CH3 ; ~ :
. ~
~ '. .'. . ' . ~ . I .' ' '' . i
21 12~7
- 88 -
is especially preferred. On the one hand, that compound is a preferred intermediate in
Process a), but on the other it is itself a preferred compound according to the invention,
since it has good pharmacological activity, especially on the basis of its unexpectedly
good activity in the cell test described above, which indicates that it will be highly
effective in vivo.
Further compounds of formula II according to the invention which have advantageous
pharmacological properties (especially those described in the introduction) and to which
the present Application therefore preferably relates are given below (the present invention
also relates to the corresponding processes for the preparation of those compounds, to
pharmaceutical compositions comprising those compounds, to those compounds for use in
a therapeutic method for the treatment of the human or animal body and to the use of those
compounds in the therapeutic treatment of the human or animal body or in the preparation
of pharmaceutical compositions, in each case analogously to the corresponding aspects of --
the invention relating to compounds of formula I; the same applies to compounds of
formula II"):
Preference is given to compounds of formula II wherein
Rl is quinolin-2-yl-carbonyl-(L)-asparaginyl,
R2 is hydrogen,
R3 is pheny1methyl, 4-lower alkoxyphenylmethyl or 4-benzyloxyphenylmethyl,
R4 is hydrogen,
R6 is hydrogen,
R7 is phenylmethyl, 4-lower aL~coxyphenylmethyl or cyclohexylmethyl,
R8 is hydrogen and
Rg is lower aLlcoxycarbonyl-(L)-valyl, lower allcoxy-lower aL~coxy-lower alkoxycarbonyl-
(L)-valyl, phenyl-lower aL~oxycarbonyl-(L)-valyl, lower alkanoyl-(L)-valyl, benzylamino-
carbonyl or C3-C7aL~enyloxycarbonyl, or also lower alkoxycarbonyl, or phannaceutically
acceptable salts thereof, more especially to one of those compounds of formula II selected
from the compounds having the names:
1-[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-
l-[cyclohexylmethyll-2-[N-(benzyloxycarbonyl)-(L)-valyl]hydrazine (especially
preferred), 1-[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-
phenyl-butyl]-1-[cyclohexylmethyl]-2-[tert-butoxy-carbonyl]hydrazine,
3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-1-[cyclohexyl-
methyl]-2-LN-methoxy-carbonyl-(L)-valyl]hydrazine (especially preferred),
, , ~ ~ ~ ` -~'' ' ' ''
``: 2112()~7
- 89 -
1 -[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-
1 -[cyclohexylmethyl]-2-[3,3-dimethylbutyryl]hydrazine,
1-~2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-
1 -[cyclohexylmethyl]-2-[tert-butylamino-carbonyl]hydrazine,
1-[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-
l-[cyclohexylmethyl]-2-[benzylamino-carbonyl]hydrazine,
1 -[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-
1 -[benzyl]-2-[N-methoxy-carbonyl)-(L)-valyl]hydrazine,
1 -[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-
l-[benzyl]-2-[N-ethoxy-carbonyl)-(L)-valyl]hydrazine (especially peferred),
1 -[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyU-
l-[benzyl]-2-[N-benzyloxy-carbonyl)-(L)-valyl]hydrazine,
1 -[2~S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-~L)-asparaginyl)amino-4-phenyl-butyl]-
l-[benzyl]-2-[N-allyloxy-carbonyl)-(L)-valyl]hydrazine,
1 -[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-~L)-asparaginyl)amino-4-phenyl-butyl] -
1-[4-methoxyphenylmethyl]-2-[N-methoxy-carbonyl)-(L)-valyl]hydrazine (especiallypreferred), 1-[2(S)-hydroxy-3(S)-(N-(quinoline-2~arbonyl)-(L)-asparaginyl)amino-4-
phenyl-butyl]-1-[4-methoxyphenylmethyll-2-[N-benzyloxy-carbonyl)-(L)-valyl]hydrazine
(especially preferred), 1-[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)-
amino-4-phenyl-butyl]-1-[4-methoxyphenylmethyl]-2-[N-allyloxy-carbonyl)-(L)-valyl]-
hydrazine (especially preferred),
1-[2(S)-hydroxy-3(S)-(N-(quino1ine-2-carbonyl)-(L)-asparaginyl)amino-4-(4-methoxy-
phenyl)-butyl]-l-[benzylJ-2-[N-methoxy-carbonyl)-(L)-valyl]hydrazine,
1-~2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-~;L)-asparaginyl)amino-4-(4-methoxy-
phenyl)-butyl]- 1 -[benzyl]-2-[N-benzyloxy-carbonyl)-(L)-valyl]hydrazine,
1-[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-(4-benzyloxy-
phenyl)-butyl]- l -[benzyl]-2-[N-methoxy-carbonyl)-(L)-valyl]hydrazine,
1-[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-(4-benzyloxy-
phenyl)-butyl]- 1 -[benzyl]-2-[N-allyloxy-carbonyl)-(L)-valyl]hydrazine,
1 -[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(~)-asparaginyl)amino-4-phenyl-butyl]-
1 -[4-methoxyphenylmethyl]-2-[N-methoxy-carbonyl)-(L)-valyl]hydrazine,
1 -[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-
1 -[4-methoxyphenylmethyl]-2-[N-allyloxy-carbonyl)-(L)-valyl]hydrazine,
1 -[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-
1-[4-benzyloxyphenylmethyl]-2-[N-methoxy-carbonyl)-(L)-valyl]hydrazine,
1-[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-
- . - - - ~ -- ~ . . ~ , . . . .
... : .: , -
,i,., ~ . . : .
~ `' ' ' - .
21 :~2~47
- 90
1 -~4-benzyloxyphenylmethyl]-2-[N-allyloxy-carbonyl)-(L)-valyllhydrazine,
1-[2(S)-hydroxy-3(S)-(N-quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenylbutyl-
l-[phenylmethyl]- -[tert-butoxy-carbonyl]hydrazine,
1 -[2(S)-hydroxy-3(S)-(N-quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenylbutyl-
l-[phenylmethyl]-2-[N-(2-(2-methoxyethoxy)ethoxycarbonyl)-(L)-valyl]hydrazine
(especially preferred),
1-[2(S)-hydroxy-3(S)-(N-quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenylbutyl-
1 -[4-methoxyphenylmethyll-2-[tert-butoxy-carbonyl]hydrazine,
and
1 -[2(S)-hydroxy-3(S)-(N-quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenylbutyl-
1 -[4-methoxyphenylmethyl]-2-[N-(ethoxycarbonyl)-(L)-valyl]hydrazine,
or a pharmaceutically acceptable salt of each of those compounds.
Preference is given also to compounds of formula II wherein Rl is lower aLlcenyloxy-
carbonyl-(L)-valyl, the radicals R2, R4, R6 and R8 are each hydrogen, R3 is phenylmethyl,
R7 is cyclohexylmethyl and Rg is N-lower alkenyloxycarbonyl-(L)-valyl, or pharma-
ceutically acceptable salts thereof, especially a compound having the name
1-[2(S)-hydroxy-3(S)-(N-allyloxycarbonyl-(L)-valyl)amin~4-phenylbutyl]-1-[cyclohexyl-
methyll-2-[N-allyloxycarbonyl-(L)-valyllhydrazine, or a pharmaceutically acceptable salt
thereof.
Preference is given also to compounds of forrnula II wherein Rl is lower alkoxy-lower
aL~coxy-lower alkoxycarbonyl-(L)-valyl, the radicals R2, R4, R6 and R8 are each hydrogen,
R3 is phenylmethyl, R7 is cyclohexylmethyl and Rg is lower alkoxy-lower alkoxy-lower
aL~coxycarbonyl-(L)-valyl, o~ pharmaceutically acceptable salts thereof, especially a
compound having the name 1-[2(S)-hydroxy-3(S)-(N-(2-(2-methoxyethoxy)ethoxy)-
caTbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[cyclohexylmethyl]-2-[N-(2-(2-methoxy-ethoxy)ethoxy)carbonyl-(L)-valyl]hydrazine (especially preferred), or a pharmaceutically
acceptable salt thereof.
Preference is given also to compounds of formula II wherein Rl is lower alkoxy-lower
alkoxy-lower alkanoyl-(L)-valyl, the radicals R2, R4, R6 and R8 are each hydrogen, R3 is
phenylmethyl, R7 is cyclohexylmethyl and Rg is lower alkoxy-lower alkoxy-lower aIkoxy-
carbonyl-(L)-valyl, or phaTmaceutically acceptabh salts thereof, especially a compound
having the name 1-[2(S)-hydroxy-3(S)-(N-(2-methoxyethoxy)acetyl-(L)-valyl)amino-4-phenylbutyl]-1-[cyclohexylmethyl]-2-[N-(2-methoxyethoxy)acetyl-(L)-valyl]hydrazine,
l .!
" ' ' ' ' .' ~. ~- ., . , ,,, , .. ,.. ' '.' .' ',' ` ''' . ~
"~'' ~ ''~ ~''" ~''"'' ' . ; :
. ~
~i , . ': ~ . ..
,'.. ' ' ' : '; . : :.
"i: .' . ' : . .~ '
21~2~7
- 91 -
or a pharmaceutically acceptable salt thereof.
Preference is given also to compounds of formula II wherein Rl and Rg are each independ-
ently of the other acetyl-(L)-valyl, methoxycarbonyl-(L)-valyl, ethoxycarbonyl-(L)-valyl,
dimethylaminocarbonyl-(L)-valyl, (2-methoxyethyl)aminocarbonyl-(L)-valyl,
N-(2-morpholin-4-yl-ethyl)aminocarbonyl-a,)-valyl or N-(2-morpholin-4-yl-ethyl)-N-methyl-aminocarbonyl-(L)-valyl, the radicals R2, R4, R6 and R8 are each hydrogen, R3
is phenylmethyl, and R7 is thien-2-ylmethyl or 2,3,5,6-tetrahydropyran-4-ylmethyl, or
pharmaceutically acceptable salts thereof, especially a single compound or several
compounds selected from~ 2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyVamino-4-phenyl-
butyl]-l-[thien-2-ylmethyl]-2-[N-acetyl-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L~-valyl)amino-4-phenylbutyl]- 1-[thien-2-yl-
methyl]-2-[N-methoxycarbonyl-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[thien-2-yl-
methyi]-2-[N-ethoxycarbonyl-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenylbutyl]-
l-[thien-2-ylmethyll-2-~N-(N,N-dimethylaminocarbonyl)-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(~-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-phenyl-
butyl]-l-Lthien-2-ylmethyl]-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl]-
hydrazine; : ;
1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-ylkthyl)aminocarbonyl)-(L)-valyl)amino-
~phenylbutyl]- l -[thien-2-ylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-
(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methyl-aminocarbonyl)-(L)-
v~lyl)amino~phenylbutyl]-1-[thien-2-ylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)-N-methylaminocarbonyl)-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-[2,3,5,6-tetrahydro-
pyran-4-ylmethyl]-2-[N-acetyl-(L)-valyl~hydrazine;
1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-
1-[2,3,5,6-tetrahydropyran-4-ylmethyl]-2-[N-methoxycarbonyl-(L)-valyl]hydrazine;1-[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]- 1-[2,3,5,6-tetra-
hydropyran-4-ylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenylbutyl]-
1-[2,3,5,6-tetrahydropyran-4-ylmethyl]-2-[N-(N,N-dirnethylaminocarbonyl)-(L)-valyl]-
hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-phenyl-
~ ........ . . . ......................... .
~. . .
2 ~ 7
- ~2 -
butyl]-1-[2,3,5,6-tetrahydropyran-4-ylmethyl]-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-
(L) -valyl]hydrazine;
1 - [2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-(L)-valyl)amino-
4-phenylbutyl]- 1 -[2,3,5,6-tetrahydropyran-4-ylmethyl]-2-[N-(N-(2-(morpholin-4-yl)-
ethyl)aminocarbonyl)-(L)-valyl]hydrazine
and 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methyl-aminocarbonyl)-
(L)-valyl)amino-4-phenylbutyl]- 1-[2,3,5,6-tetrahydropyran-4-ylmethyl]-2-
[N-(N-(2-(morpholin-4-yl)ethyl)-N-methylaminocarbonyl)-(L)-valyl]hydrazine,
or a pharmaceutically acceptable salt thereof.
Preference is given also to compounds of formula II wherein Rl and Rg are each independ-
ently of the other acetyl-(L)-valyl, methoxycarbonyl-(L)-valyl, ethoxycarbonyl-(L)-valyl,
dimethylaminocaTbonyl-(L)-valyl, (2-methoxyethyl)aminocarbonyl-(L)-valyl,
N-(2-morpholin-4-yl-ethyl)aminocarbonyl-(L)-valyl or N-(2-morpholin-4-yl-ethyl)-N-methyl-aminocarbonyl-(L)-valyl, the radicals R2, R4, R6 and R8 are each hydrogen, R3
is phenylmethyl, and R7 is 4-hydroxyphenylmethyl, 4-methoxyphenylmethyl, 4-isobutoxy-
phenylmethyl, 4-benzyloxyphenylmethyl, 3,4-dimethoxyphenylmethyl, methylene-
4,5-dioxyphenylmethyl, 4-(2-methoxyethoxy)phenylmethyl or 4-biphenylylmethyl, orpharmaceutically acceptable salts thereof, especially a single compound or several
compounds selected from the compounds having the names~
1-[2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-hydroxyphenyl-
methyl]-2-[N-acetyl-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(*methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-hydroxy-
phenylmethyl]-2-[N-methoxycarbonyl-(L)-valyl]hydrazine (especially preferred);
1-[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbuty1]-1-[4-hydroxy- -
phenylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(~L)-valyl)amino-4-phenylbutyl]-
1-[4-hydroxyphenylmethyl]-2-[N-(N,N-dimethylaminocarbonyl)-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-phenyl-
butyl~-1-[4-hydroxyphenylmethyl]-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl]-
hydrazine;
1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-ylkthyl)aminocarbonyl)-(L)-valyl)amino-
4-phenylbutyl]-1-[4-hydroxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)amino-carbonyl)-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methyl-arninocarbonyl)-(L)-
valyl)amino-4-phenylbutyl]-1-[4-hydroxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)-
?"~
~,~i. . ` i
` -
: `; ' ~ .:
2~ 12~A7
- 93 -
ethyl)-N-methylaminocarbonyl)-(L)-valyllhydrazine;
1-[2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]~ 4-methoxyphenyl-
methyl]-2-~N-acetyl-(L)-valyl]hydlazine (especially preferred);
1 -[2(S~-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-
1-[4-methoxyphenylmethyl]-2-[N-methoxycarbonyl-(L)-valyl]hydrazine (especially
preferred);
1-[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-methoxy-
phenylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]hydrazine (especially preferred);
1 -[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenylbutyl]-
1-[4-methoxyphenylmethyl]-2-[N-(N,N-dimethylaminocarbonyl)-(L)-valyl]hydrazine
(especially preferred);
1 -[2(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-phenyl-
butyl]- 1 -[4-methoxyphenylmethyl]-2-[N-(N-(2-methoxyethyl)arninocarbonyl)-(L)-valyl]-
hydrazine;
1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-(L)-valyl)amino-
4-phenylbutyl]-1-[4-methoxyphenylmethyl]-2-[N-(N-(2-(molpholin-4-yl)ethyl)amino-
carbonyl)-(L)-valyl]hydrazine; ~:
1 -[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methyl-aminocarbonyl)-(L)-
valyl)amino-4-phenylbutyl]-1-[4-methoxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)-
ethyl)-N-methylaminocarbonyl)-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-benzyloxyphenyl-
methyl]-2-[N-acetyl-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-benzyl-
oxyphenylmethyl]-2-[N-methoxycarbonyl-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-benzyloxy-
phenylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-(NjN-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenylbutyl]-
1 -[4-benzyloxyphenylmethyl]-2-[N-(N,N-dimethylaminocarbonyl)-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-phenyl-
butyl~-1-[4-benzyloxyphenylmethyl]-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-
(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-ylkthyl)aminocarbonyl)-(L)-valyl)amino-
4-phenylbutyl]- 1 -[4-benzyloxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)amino-
carbonyl)-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methyl-aminocarbonyl)-(L)-
valyl)amino-4-phenylbutyl]-1-[4-benzyloxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)-
~ ... . . . . . . .
~. "~' - ' ' ,:
~,~.: , . . .
- 2:L:L~4
- 94 -
ethyl)-N-methylaminocarbonyl)-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-L4-(3,4-dimethoxy-
benzyl)oxyphenylmethyl]-2-[N-acetyl-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]- 1-[4-(3,4-di-
methoxybenzyl)oxyphenylmethyl]-2-[N-methoxycarbonyl-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-(3,4-di-
methoxybenzyl)oxyphenylmethyl]-2-[N-ethoxycarbonyl-(L)-valyUhydrazine;
1-[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenylbutyl]-
1-[4-(3,4-dimethoxybenzyl)oxyphenylmethyl]-2-[N-(N,N-dimethylaminocarbonyl)-
(L)-valyl]hydrazine;
1-[2~S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)atninocarbonyl)-(L)-valyl)amino-4-phenyl-
butyll-1-[4-(3,4-dimethoxybenzyl)oxyphenylmethyl]-2-1N-(N-(2-me~hoxyethyl)amin~
carbonyl)-(L)-valyl]hydrazine; :
1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-(L)-valyl)amino- . ~:
4-phenylbutyl]-1-[4-(3,4-dimethoxybenzyl)oxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-ylht~hyl)aminocarbonyl)-(L)-valyl]hydrazine; :~ .
1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methyl-aminocarbonyl)-~L)- ~ ~:
valyl~amino~phenylbutyl]-1-[4-(3,4-dimethoxybenzyl)oxyphenylmethyl]-2-
[N-(N-~2-(morpholin-4-yl)ethyl)-N-methylaminocarbonyl)-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-isobutoxyphenyl-
methyl]-2-[N-acetyl-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-iso-butoxyphenylmethyl] -2-[N-methoxycarbonyl-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]- 1 -[4-isobutoxy-
phenylmethyl]-2-~N-ethoxycarbonyl-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-(N,N-dimethylamimocarbonyl)-(L)-valyl)amino-4-phenylbutyl]-
1 -[4-isobutoxyphenylmethyl] -2-[N-(N,N-dimethylaminocarbonyl)-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-phenyl-
butyl]-1-[4-isobutoxyphenylmethyl]-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl]-
hydrazine;
- 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-(L)-valyl)amino-
4-phenylbutyl]-1-[4-isobutoxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)amino-
carbonyl)-(L)-valyl]hydrazine;
1-[2(S) hydroxy-3(S)-(N-(N-(2-(molpholin-4-ylkthyl)-N-methyl-aminocarbonyl)-(L)-valyl)amino-4-phenylbutyl]-1-[4-isobutoxyphenylme~hyl]-2-[N-(N-(2-(morpholin-4-yl)-
ethyl)-N-methylaminocarbonyl)-(L)-valyl]hydrazine;
. , ... ,.................. .. . : .
. ~
,. .
.
2~ ~2~7
1-[2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-~4-(2-methoxyethoxy)-
phenylmethyl~-2-[N-acetyl-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amin~4-phenylbutyl]-
1-[4-(2-methoxyethoxy)phenylmethyl]-2-[N-methoxycarbonyl-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-
1-[4-(2-methoxyethoxy)phenylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenylbutyl~-
1-[4-(2-methoxyethoxy)phenylmethyll-2-[N-(N,N-dimethylaminocarbonyl)-(L)-valyl]-hydrazine;
1-[2(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-phenyl- - ~ -:
butyll-1-[4-(2-methoxyethoxy)phenylmethyl]-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-
(L) -valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-(L)-valyl)amino-
4-phenylbutyl]- 1-[4-(2-methoxyethoxy)phenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)-
aminocarbonyl)-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-(N-(2-(moIpholin-4-yl)ethyl)-N-methyl-aminocarbonyl)-(L)-
valyl)amino-4-phenylbutyl]-1-[4-(2-methoxyethoxy)phenylmethyl]-2-[N-(N-(2-(morpho-
lin-4-yl)ethyl)-N-methylaminocarbonyl)-(L)-valyl~hydrazine;
1-[2(S)-hydroxy-3(S)-(N-acetyl-SL)-valyl)amino-4-phenylbutyl]-1-[methylene-3,4-dioxy-
phenylmethyl]-2-[N-acetyl-SL)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-SL)-valyl)amino-4-phenylbutyl]-1-[methyl-
ene-3,4-dioxyphenylmethyl]-2-[N-methoxycarbonyl-SL)-valyl]hydrazine;
1 -[2~S)-hydroxy-3SS)-(N-ethoxycarbonyl-(L)-valyl)amino~phenylbutyl]- 1 -[methylene-
3,4-dioxyphenylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenylbutyl]-
l-[methylene-3,4-dioxyphenylmethyl]-2-[N-SN,N-dimethylaminocarbonyl)-SL)-valyl]-hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-SN-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-phenyl-
butyl]-l-[methylene-3,4-dioxyphenylmethyl]-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-
(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-SN-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-SL)-valyl)amino-
4-phenylbutyl~- 1-[methylene-3,4-dioxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)-
aminorarbonyl)-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methyl-aminocarbonyl)-(L)-
valyl)amino-4-phenylbutyl]-1-[methylene-3,4-dioxyphenylmethyl]-2-[N-(N-(2-(morpho-
lin-4-yl)ethyl)-N-methylaminocarbonyl)-(L)-valyl]hydrazine;
2112~l17
- 96 -
1-~2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-f3,4-dimethoxyphenyl-
methyl]-2-[N-acetyl-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]- 1 -[3,4-di-
methoxyphenylmethyl]-2-1N-methoxycarbonyl-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-ethoxycaTbonyl-(L)-valyl)amino-4-phenylbutyl]- 1 -[3,4-di-
methoxyphenylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl3hydrazhle;
1-[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenylbutyl]-
1-~3,4-dimethoxyphenylmethyl]-2-[N-(N,N-dimethylaminocarbonyl)-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-phenyl-
butyl]-1-[3,4-dimethoxyphenylmethyl]-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-
(L)-valyl]hydrazine; .
1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-(L)-valyl)amino-
4-phenylbutyl]- 1-[3,4-dimethoxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)amino-
carbonyl)-(L)-valyl]hydrazine;
1-~2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methyl-aminocarbonyi)-(L)-
valyl~amino4-phenylbutyl]-1-[3,4-dimethoxyphenylmethyl]-2-[N-(N-(2-(morpholin-
4-ylkthyl)-N-methylaminocarbonyl)-(L)-valyl]hydrazine;
1-[2(S~-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-biphenylylmethyl]-
2-[N-acetyl-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-bi-
phenylylmethyl]-2-[N-methoxycarbonyl-(L)-valyl]hydrazine;
1-[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-~L)-valyl)amino-4-phenylbutyl]-1-[4-biphenyl-
ylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenylbutyl]-
1-[4-biphenylylmethyl]-2-[N-(N,N-dimethylaminocarbonyl)-(L)-valyl]hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-phenyl-
butyl~-1-[4-biphenylylmethyl]-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl]-
hydrazine;
1 -[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-(L)-valyl)amino-
4-phenylbutyl]- 1 -[4-biphenylylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)amino-
carbonyl)-(L)-valyl]hydrazine and
1 -[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methyl-aminocarbonyl)-(L)-
valyl)amino-4-phenylbutyl] -1 -[4-biphenylylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)-
N-methylaminocarbonyl)-(L)-valyl]hydrazine,
or pharmaceutically acceptable salts thereof.
~;,,.,.. ; .---., ,. ~ ...... .. . . .
~,-"~
2 ~ 7
- 97 -
Preference is given also to compounds of formula II wherein Rl and R9 are each methoxy-
carbonyl-(L)-valyl, the radicals R2, R4, R6 and R8 are each hydrogen, R3 is phenylmethyl,
and R7 is 4-lower alkoxyphenylmethyl or 4-benzyloxyphenylmethyl, :
or pharmaceutically acceptable salts thereof.
Preference is given finally to the following compounds of fonnula II:
1-~2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[cyclohexyl-
methyl]-2-~N-ethoxycarbonyl-(L)-valyllhydrazine, 1-[2(S)-hydroxy-3tS)-(N-trifluoro-
acetyl-(L)-valyl)amino-4-phenylbutyl]- 1 -[cyclohexylmethyl]-2-[N-trifluoroacetyl-
(L)-valyl]hydrazine,
1 -[2(S)-hydroxy-3(S)-(N-(methoxy-carbonyl)-(L)-valyl)amino-4-cyclohexyl-butyl]-l-[cyclohexylmethyl]-2-[N-methoxy-carbonyl-(L)-valyl]hydrazine,
1-[2(S)-hydroxy-3(S)-(N-(n-propoxy-carbonyl)-(L)-valyl)amino-4-phenyl butyl]-l-[cyclo-
hexylmethyl]-2-[N-(n-propyl)oxy-carbonyl-(L)-valyl]hydrazine,
1-[2(E~)-hydroxy-3(S)-(N-(methoxy-carbonyl)-(L)-valyl)amino-4-phenyl-butyl]-1-[cyclo-
hexylmethyl]-2-[N-methoxy-carbonyl-(L)-valyl]hydrazine,
1-[2(R)-hydroxy-3(R)-(N-(methoxy-carbonyl)-(L)-valyl)amino-4-phenyl-butyl]- 1 -[cyclo-
hexylmethyl]-2-[N-methoxy-carbonyl-(L)-valyl]hydrazine,
1-[2(S)-hydroxy-3(S)-(benzyloxy-carbonyl-amino)-4-phenyl-butyl]-1-~phenylmethyl]-
2-[N-methoxy-carbonyl-(L)-valyl]hydrazine,
1 -[2(S)-hydroxy-3(S?-(N-(methoxy-carbonyl)-(L)-valyl)amino-4-cyclohexyl-butyl]-1 -[phenylmethyl]-2-[N-methoxy-carbonyl)-(L)-valyl]hydrazine,
1 -[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-cyclohexyl-butyl]-
1-[4-methoxyphenylmethyl]-2-[N-methoxy-carbonyl)-(L)-valyl]hydrazine,
1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-cyclohexyl-butyl]- 1-[4-iso-
butoxyphenylmethyl~-2-[N-methoxy-carbonyl)-(L)-valyl]hydrazine,
1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-cyclohexyl-butyl]-
1 -[4-ethoxyphenylmethyl]-2-[N-methoxy-carbonyl)-(L)-valyl]hydrazine,
1 -[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-cyclohexyl-butyl]-
1-[4-benzyloxyphenylmethyl]-2-[N-methoxy-carbonyl)-(L)-valyl]hydrazine, and/or
1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenyl-butyl]- 1 -[cyclo-
hexylmethyl]-2-[2-pyridylcarbonyl]hydrazine,
or a pharmaceutically acceptable salt thereof ih each case.
The preparation of compounds of formula II', especially the preparation according to the
invention of the compound of formula II", is effected, for example
21 1~
- 98 -
i) analogously to the reaction of starting materials of formula XIII with those of
formula XIV A, as described in the preparation of compounds of formula II; in the case of
compounds of formula II', the radicals in the starting compounds are as defined for
compounds of formula I, whereas in the case of compounds of formula II", in the starting
materials Rl and R9 are each N-methoxycarbonyl-(L)-valyl, R2, R4, R6 and R8 are
hydrogen, R3is benzyl and R7is cyclohexylmethyl, or
ii) analogously to Process b), wherein there are used instead of the starting materials of
formula IV those of formula IV', especially IV", as defined above; in the case of the
preparation of compounds of formula II', the radicals are as defined for compounds of
formula IV', whereas in the case of the preparation of compounds of formula II", in the ~ ~ :
starting materials R, and R9' are each N-methoxycarbonyl-(L)-valyl, R2, R4, R6 and R8
are hydrogen, R3is benzyl and R7is cyclohexylmethyl, or
iii) analogously to Process c), wherein there are used instead of the starting materials of
formula VI those of formula VI', especially VI", as defined above; in the case of the
preparation of compounds of formula II', the radicals are as defined for compounds of
formula VI', whereas in the case of the preparation of compounds of formula II", in the
starting materials Rl' and R9 are each N-methoxycarbonyl-(L)-valyl, R2, R4, R6 and R8
are hydrogen, R3is benzyl and R7is cyclohexylmethyl, or
iv) analogously to Process d), wherein there are used instead of the starting materials of
formula VIII those of formula VIII', especially VIII", as defined above; in the case of the
preparation of compounds of formula II', the radicals are as deSned for compounds of
formula VI~', whereas in the case of the preparation of ~ompounds of formula II", in the
starting materials Rl' and Rg' are each N-methoxycarbonyl-(L)-valyl, R2, R4, R6 and R8
are hydrogen, R3is benzyl and R7is cyclohexylmethyl, or
v) analogously to Process e), wherein there are used instead of the starting materials of
formula IV those of formula IV', especially IV", as defined above; in the case of the
preparation of compounds of formula II', the radicals are as defined for compounds of
formula I", whereas in the case of the preparation of compounds of forrnula II", in the
starting materials Rl and R9 are each N-methoxycarbonyl-(L)-valyl, R2, R4, R6 and R8 are
hydrogen, R3is benzyl and R7"is cyclohexylmethyl, or
iS~., I , ' ~ '
2l~a~7
- 99 -
vi) where protected starting materials are used, for example in one of the above-mentioned
processes, by removal of the protecting groups analogously to Process f).
If desired, a compound of formula II', especially II", obtainable in accordance with one of
the above Processes i) to vi) can be converted Into its salt, or an obtainable salt can be
converted into the free compound or into a different salt, and/or isomeric mixtures tnat
may be obtainable can be separated and/or a compound of forrnula II', especially II",
according to the invention can be converted into a different compound of formula II',
especially II", according to the invention. The conditions correspond to those described
above for additional process steps carried out using compounds of formula I.
The compounds of formula II" are prepared according to the invention preferably from
compounds of formula VIII" wherein R2, R4, R6 and R8 are hydrogen, R3 is benzyl and R7
is cyclohexylmethyl, and from N-methoxycarbonyl-(L)-valine or a reactive acid derivative
thereof (corresponding to the compounds of formulae V and VII wherein Rg' and Rl' are
N-methoxycarbonyl-(L)-valyl), analogously to Process d), as described above.
The acids of formulae ~I, V, VII and IX, the acid derivatives thereof, for exarnple of
forrnulae V', V" and IX', and the compounds having nucleofugal groups of forrnulae Xa,
Xb, XIa, XIb and XII are commercially available, known or, if they are novel, can be
prepared in accordance with processes known per se, for example analogously to the
processes mentioned in the Examples, using suitable starting materials. The same applies
to all further starting materials.
There may be mentioned by way of example the preparation of an aryl-lower aLkanoic acid
(a compound of formula m) substituted by heterocyclylmethyl, wherein heter~cyclyl is
bonded via a ring nitrogen atom, which is preferably effected by reacting an aryl-lower
alkanoyl radical substituted by halomethyl, such as chloro- or bromo-methyl, such as
chloromethylbenzoyl or bromomethylbenzoyl, with a corresponding heterocyclic nitrogen
base, such as piperidine, piperazine, l-lower alkylpiperazine, l-lower aL~canoylpiperazine
or especially morpholine or thiomorpholine, with nucleophilic substitution of the halogen
atom.
Amino acid derivatives of formula III, V or VII wherein the a-amino group is alkylated by
a radical selected from phenyl-lower alkyl and heterocyclyl-lower alkyl can be prepared,
for example, by reductive amination of the amino acid (protected, if necessary, at further
. , , : : .
` ` 21:~0~7
- lo~-
groups that are not intended to participate in the reaction) having a primary or secondary
a-amino group, with a phenyl-lower aL~cyl ketone or aldehyde, such as benzaldehyde, or
heterocyclyl-lower alkyl ketone or aldehyde, for example heterocyclyl aldehyde, for
example furan aldehyde, such as furan-2-aldehyde, or pyridine aldehyde, such as
pyridine-3-aldehyde, for example with catalytic hydrogenation, for example in the
presence of a heavy metal catalyst, such as Raney nickel, under normal pressure or under
pressures of from 1 to 100 bar, preferably at approximately 100 bar, or with reduction by
means of complex boron hydrides, such as sodium cyanoborohydride.
The isocyanates of formulae lX' and V" can be prepared, for example, from the corres-
ponding amine precursors by conversion of the amino group into the isocyanato group, for
example by reaction with phosgene with heating, for example under reflux conditions, or
by the dropwise addition of the primary, secondary or tertiary amine, in liquid form or
dissolved in a solvent, to an excess of phosgene in a suitable solvent (toluene, xylene,
ligroin, chlorobenzene, a-chloronaphthalene, etc.) with cooling (for example at from -50
to 0C), there being formed as intermediate a mixture of carbamoyl chloride and amine
hydrochloride which is then phosgenated further at elevated temperature (for example at
from 50C to the reflux temperature) until complete dissoludon is obtained, HCl being
eliminated.
The following applies generally to all the processes mentioned hereinbefore and herein-
after:
As a result of the close relationship between the compounds of formula I and their salts
and stardng materials (stardng compounds and intermediates) in free form and in the form
of their salts, hereinbefore and hereinafter any reference to the free compounds and their
salts should be understood as including the corresponding salts and free compounds,
respecdvely, where appropriate and expedient.
All the process steps listed above can be carried out under reaction condidons known
per se, preferably those specifically mentdoned, in the absence or, customarily, the
presence of solvents or diluents, preferably those that are inert towards the reagents used
and are solvents therefor, in the absence or presence of catalysts, condensatdon agents or
neutralising agents, for exarnple ion exchangers, such as cation exchangers, for example in
the H+ form, and, depending on the nature of the reacdon and/or of the reactants, at
reduced, normal or elevated temperature, for example in a temperature range from approx-
2~1~0~7
- 101 -
imately -100C to approximately 190C, preferably from approximately -80C to approx-
imately 150C, for example from -80 to -60C, at room temperature, from -20 to 40C or
at the reflux temperature, under atmospheric pressure or in a closed vessel, if necessary
under pressure, and/or in an inert atmosphere, for example under an argon or nitrogen
atmosphere.
Isomeric mixtures occurring at any stage of the reaction may be separated into the
individual isomers, for example diastereoisomers or enantiomers, or into any desired
mixtures of isomers, for example racemates or diastereoisomeric mixtures, for example
analogously to the methods described under the "Additional Process Steps".
In certain cases, for example in the case of hydrogenation, it is possible to achieve stereo-
selecdve reactions, which, for example, enable individual isomers to be obtained more
easily.
The solvents from which those suitable for a particular reaction can be selected include,
for example, water, esters, such as lower aL~yl-lower aLl~anoates, for example ethyl
acetate, ethers, suGh as aliphadc ethers, for example diethyl ether, or cyclic ethers, for
example tetrahydrofuran, liquid aromatic hydrocarbons, such as benzene or toluene,
alcohols, such as methanol, ethanol or 1- or 2-propanol, nitriles, such as acetonitrile,
halogenated hydrocarbons, such as methylene chloride, acid amides, such as dimethyl-
formamide, bases, such as heterocyclic nitrogen bases, for example pyridine, carboxylic
acid anhydrides, such as lower alkanoic acid anhydrides, for example acetic anhydride,
cyclic, linear or branched hydrocarbons, such as cyclohexane, hexane or isopentane, or
mixtures of those solvents, for example aqueous solutions, unless the description of the
process indicates otherwise. Such solvent mixtures can also be used in the working-up,
for example by chromatography or partitioning.
The compounds, including their salts, can also be obtained in the form of hydrates, or their
crystals may, for example, include the solvent used for crystallisation. ; ~ -
The invention relates also to those forms of the process in which a compound obtainable
as intermediate at any stage of the process is used as starting material and the remaining
process steps are carried out, or in which a starting material is formed under the reaction
conditions or is used in the form of a derivative, for exarnple in protected forrn or in the
form of a salt, or a compound obtainable by the process according to the invention is
, . . . .. . . . .
~ . . . .
2 ~ 4 7
- 102-
produced under the process conditions and further processed in si~u. In the process of the
present invention it is preferable to use those starting materials that lead to the compounds
(of formula I or II) described in the introduction as being especially valuable. Special
preference is given to reaction conditions analogous to those mentioned in the Examples.
Where necessary, protected starting compounds can be used at any stage of the process
and the protecting groups removed at suitable stages of the reaction.
Protecting groups, their introduction and their removal are as described under Processes a)
and f).
Pharmaceutical ComPositions: -
The invention relates also to pharmaceutical compositions comprising compounds of
forrnula I or (especially those compounds that are described as being preferred) of formula
II.
The pharmacologically acceptable compounds of the present invention may be used, fDr
example, for the preparation of pharmaceutical compositions that comprise an effective
amount of the active ingredient together or in admixture with a significant amount of
inorganic or organic, solid or liquid, pharmaceutically acceptable carriers.
The invention relates also to a phar naceutical composition that is suitable for administra-
don to a warm-blooded animal, especially a human, for the treatment or prevendon of a
disease that responds to inhibidon of a retroviral protease, especially a retroviral aspartate
protease, such as HIV-I- or HIV-II-gag protease, for example a retroviral disease such as
AIDS, comprising an amount of a compound of fo~nula I or formula II" or a pharmaceut-
ically acceptable salt thereof, effective for the inhibition of the retroviral protease, together
with at least one pharmaceutically acceptable carrier.
The pharmaceudcal composidons according to the invendon are those for enteral, such as
nasal, rectal or oral, or parenteral, such as intramuscular or intravenous, administradon to
warm-blooded animals (humans and animals), that comprise an effecdve dose of thepharmacological active ingredient, alone or together with a significant amount of a
pharmaceutically acceptable carrier. The dose of the active ingredient depends on the
species of warm-blooded animal, the body weight, the age and the individual condidon,
individual pharmacokinedc data, the disease to be treated and the mode of administration.
- - -- - - - -- - ~, . - ., -
. , .. ,,. .,.. .. ~ , ,.
, ,,; , " ,,
. . ..
. .,
, ~
`` 2:~2~7
- 103-
The invention relates also to a method of treating diseases caused by viruses, especially by
retroviruses, for example AIDS, which comprises administering a therapeutically effective
amount of a compound of formula I or formula II" according to the invention, especially to
a warm-blooded animal, for example a human, who on account of one of the mentioned
diseases, especially AIDS, requires such treatment. The dose to be administered to warm-
blooded animals, for example humans of approximately 70 kg body weight, is from
approximately 3 mg to approximately 3 g, preferably from approximately 10 mg to
approximately 1.5 g, for example approximately from 100 mg to 1000 mg per person per
day, divided preferably into-l to 3 single doses which may, for example, be of the same
size. Usually, children receive half of the adult dose.
The pharmaceutical compositions comprise from approximately 1 % to approximately95 %, preferably from approximately 20 % to approximately 90 %, active ingredient.
Ph~l.laceutical compositions according to the invention may be, for example, in unit dose
for n, such as in the form of ampoules, vials, suppositories, dragées, tablets or capsules.
The pharmaceutical compositions of the present invention are prepared in a manner known
per se, for example by means of conventional dissolving, lyophilising, mixing, gr~nulating
or confectioning procésses.
Solutions of the active ingredient, and also suspensions, and especially isotonic aqueous
solutions or suspensions, are preferably used, it being possible, for example in the case of
lyophilised compositions that comprise the active ingredient alone or together with a
carrier, for example mannitol, for such solutions or suspensions to be produced prior to
use. The phannaceutical compositions may 'oe sterilised and/or may comprise excipients,
for example preservatives, stabilisers, wefflng and/or emulsifying agents, solubilisers, salts
for regulating the osmotic pressure and/or buffers, and are prepared in a manner known
per se, for example by means of conventional dissolving or lyophilising processes. The
said solutions or suspensions may comprise viscosity-increasing substances, such as
sodium carboxymethylcellulose, carboxymethylcellulose, dextran, polyvinylpyrrolidone or
gelatin.
Suspensions in oil comprise as the oil component the vegetable, synthetic or semi-
synthetic oils customa~y for injection purposes. There may be mentioned as such
especially liquid fatty acid esters that contain as the acid component a long-chained fatty
.. . , . . . ~ . . . . .
~, ` '
if.''' ` ' ~ . ': ' : ~ : "
~112~7
- 104-
acid having from 8 to 22, especially from 12 to 22, carbon atoms, for example lauric acid,
tridecylic acid, myristic acid, pentadecylic acid, palmitic acid, margaric acid, stearic acid,
arachidic acid, behenic acid or corresponding unsaturated acids, for example oleic acid,
elaidic acid, erucic acid, brasidic acid or linoleic acid, if desired with the addition of anti-
oxidants, for example vitamin E"B-carotene or 3,5-di-tert-butyl-4-hydroxytoluene. The
alcohol component of those fatty acid esters has a maximum of 6 carbon atoms and is a
mono- or poly-hydric, for example a mono-, di- or tri-hydric, alcohol, for example
methanol, ethanol, propano}, butanol or pentanol or the isomers thereof, but especially
glycol and glycerol. The following examples of fatty acid esters are therefore to be
mentioned: ethyl oleate, isopropyl myristate, isopropyl palmitate, "Labrafil M 2375"
(polyoxyethylene glycerol trioleate, Gattefossé, Paris), "Miglyol 812" (triglyceride of
saturated fatty acids with a chain length of C8 to Cl2, Huls AG, Germany), but especially
vegetable oils, such as cottonseed oil, almond oil, olive oil, castor oil, sesame oil, soybean
oil and more especially groundnut oil.
The injection compositions are prepared in customary manner under sterile conditions; the
same applies also to introducing the composidons into ampoules or vials and sealing the
containers.
Pharmaceutical compositions for oral administration can be obtained by combining tlhe
active ingredient with solid ca~iers, if desired granulating a resuldng mixture, and
processing the mixture, if desired or necessary, after the addition of appropriate excipients,
into tablets, dragée cores or capsules. It is also possible for them to be incorporated into
plastics c~iers that allow the active ingredients to diffuse or be released in measured
amounts.
Suitable carriers are especially fillers, such as sugars, for example lactose, saccharose,
mannitol or sorbitol, cellulose preparations and/or calcium phosphates, for example
tricalcium phosphate or calcium hydrogen phosphate, and binders, such as starch pastes
using for example corn, wheat, rice or potato starch, gelatin, tragacanth, methylcellulose,
hydroxypropylmethylcellulose, sodium carboxymethylcellulose and/or polyvinyl-
pyr~olidone, and/or, if desired, disintegrators, such as the above-mendoned starches, also
carboxymethyl starch, crosslinked polyvinylpyrrolidone, agar, alginic acid or a salt
thereof, such as sodium alginate. Excipients are especially flow condidoners andlubricants, for example silicic acid, talc, stearic acid or salts thereof, such as magnesium or
calcium stearate, and/or polyethylene glycol. Dragée cores are provided with sui~able,
~1 ~2~47
- 105-
optionally enteric, coatings, there being used, inter alia, concentrated sugar solutions
which may comprise gum arabic, talc, polyvinylpylrolidone, polyethylene glycol and/or
dtanium dioxide, or coating solutions in suitable organic solvents, or, for the preparation
of enteric coatings, solutions of suitable cellulose preparations, such as ethylcellulose
phthalate or hydroxypropylmethylcellulose phthalate. Capsules are dry-filled capsules
made of gelatin and soft sealed capsules made of gelatin and a plasticiser, such as glycerol
or sorbitol. The dry-filled capsules may comprise the active ingredient in the form of
granules, for example with fillers, such as lactose, binders, such as starches, and/or
glidants, such as talc or magnesium stearate, and if desired with stabilisers. In soft
capsules the acdve ingredient is preferably dissolved or suspended in suitable oily
excipients, such as fatty oils, paraffln oil or liquid polyethylene glycols, it being possible
also for stabilisers and/or andbacterial agents to be added. Dyes or pigments may be added
to the tablets or dragée coatings or the capsule casings, for example for idendfication
purposes or to indicate different doses of active ingredient. :
The following Examples serve to illustrate the invendon but do not limit the scope thereof
in anyway.
Temperatures are given in degrees Celsius (C). Where no temperature is specified, the
reaction takes place at room temperature. The Rf values, which indicate the ratio of the
seepage propagation of the substance in question to the seepage propagation of the eluant
front, are determined on thin layer silica gel plates by thin layer chromatography (TLC) in
the following solvent systems:
TLC eluant systems
A chloroform/methanoVwater/ 75:27:5:0.5
acetic acid
B chloroform/methanoUwater/ 90:10:1:0.5
acedc acid
C chloroform/methanoVwater/ 85:13:1.5:0.5
acedc acid
D chloroform/methanol 8:1
E chloroform/methanol 95:5
F hexane/ethyl acetate 2:1
G methylene chloride/diethyl ether/ 20:20:1
; ~
, j . ~ . ... , :
'.:
. :
2 0 ~ 7
- 106-
methanol
H methylene chloride/diethyl ether 1:1
toluene/ethyl acetate 2:1
K chloroform/methanol 5:1
J methylenechloride/diethylether S:l
L hexane/ethyl acetate 4:1
M hexane/ethyl acetate 5:1
N hexane/ethyl acetate 1:1
O ethyl acetate
P methylene chloride/ethanoUNH3aq. 90:10:1
Q methylene chloride/diethyl ether 10:1 : -~
R hexane/ethyl acetate 3:1
S methylene chloride/diethyl ether 20:1
T: chloroform/methanol 30:1
U: chlorofonn/methanol 15:1
V: methylenechloride/diethylether/ -;
hexane 1:1:3
W: methylene chlonde/diethyl ether 20:1 ~ ~ :
X: methylene chlonde/methanol 40:1
Y: toluenetethylacetate 4:1 ~ ~
Z: methylenc chloride/methanol 30:1 -
A': methylenc chlondetmedlanol 15:1
B': methylene chlo~ide/methanol 10:1
C': hexanetethyl acetate 1:3
D': ethyl acetate/edlanol 100:3
E': ethyl acetatetethanol 20:1
F': ethyl acetatetethanol 10:1
G': methylene chloride/methanol 9:1
H': ethyl acetatethexane 3:2
I': methylene chloride/methanol 12:1
J': methylene chloride/methanol 19:1
K' methylene chloride/diethyl ether/
methanol 10:10:1
The abbreviation "Rf~A)", for example, indicates that the Rf value was determined in
solvent system A. The ratio of solvents to one another is always given in parts by volume.
2112~7
- 107-
HPLC gradients:
20 % ~ 100 % a) in b) for 35 min
II 0 % ~ 40 % a) in b) for 30 min
III 20 % ~ 60 % a) in b) for 60 min
IV 10 % ~ 50 % a) in b) for 60 min
V 20 % ~ 100 % a) in b) for 20 min
Eluant a): acetonitrile + 0.05 % TFA; eluant b): water + 0.05 % TFA. Column
(250 x 4.6 mm) filled with "Reversed-Phase" material Cl8-Nucleosil~ (5 ,um
average particle size, silica gel covalently derivatised with octadecylsilanes,
Macherey & Nagel, Duren, FRG). Detection by W-absorption at 215 nm. The
retention times (tR~,t) are given in minutes. Flow rate 1 ml/min. -
- The same abbreviations are used to identify the eluant systems in flash chromatography
and medium-pressure chromatography.
The other short forms and abbreviations used have the following meanings:
abs. absolute (indicates that the solvent is anhydrous)
atm physical atmospheres
(unit of pressure) - 1 atm corresponds to 1.013 bar
Boc tert-butoxycarbonyl --
BOP benzo~iazol-l-yloxy-tris(dimethylamino)phos-
phonium hexafluo~ophosphate
brine saturated sodium chloride solution
DCC dicyclohexylcarbodiimide
DIPE diisopropyl ether
DMAP dimethylaminopyridine
DMF dimethylformamide -
DMSO dimethyl sulfoxide
EDC N-ethyl-N'-(3-dimethylamino propyl)-carbodiimide
hydrochloride
ether diethyl ether
h hour(s)
HBTU O-benzotriazol- 1 -yl-N,N,N 'N'-tetramethyluronium
hexafluorophosphate
~,," " ", ,~ ,", , " ~
;, ~ ", :" ~ ", ~: " ,;;
': ~' :'' ' : ' ' ., ' ' ,' ' ` ' "` :;., ~ ' ,, . .: : ,,
`` 2 ~ 7
- 108-
HOBt l-hydroxybenzotriazole
HV high vacuum
min minute(s)
MS mass spectroscopy
NMM N-methylmorpholine
RE rotary evaporator
RT room temperature
TFA trifluoroacetic acid
THF tetrahydrofuran
Z benzyloxycarbonyl
Mass spectroscopic data ale obtained either by conventional MS or according to the
"Fast-Atom-Bombardment" (FAB~MS) method. The mass data refer in the former case to
the unprotonated molecule ion (M)+ or to the protonated molecule ion (M+H)+.
The values for proton nuclear resonance spectroscopy (lH-NMR) are given in ppm (parts
per million) based on tetramethylsilane as the internal standard. s = singlet, d = doublet,
t = triplet, q = quartet, m = multiplet, dd = double doublet, br = broad.
The valuçs for IR spectra are given in cm~l, and the solvent in question is in r~und
brackets. Where given, s indicates a strong, m a medium and w a weak intensity of the
band in question.
The residue referred to as -[PheNNPhe] is the divalent residue of 3(S)-amino-4-phenyl-1-
(N-benzylhydrazino)-butan-2(S)-ol and has the formula
~,
.,, . , . . . ,. . . ,.. .. --, -- ~ .. ...... . .. . . ... . ~.. ~ .. .i . ... . .. ; , . ..
2112~7
- 109-
The residue referred to as -[PheNNCha] is the divalent residue of 3(S)-amino-4-phenyl- 1-
(N-cyclohexylmethylhydrazino)-butan-2(S)-ol and has the formula
-NH
-- ~NH-
The residue referred to as -[PheNNLeu] is the divalent residue of 3(S)-amino-4-phenyl~
(N-isobutylhydrazino)-butan-2(S)-ol and has the formula
OH ~ ;
-NH ~ N
-- ~NH~
~ ::
~ .
The residue reiferred to as -[PheNNNle] is the residue of 3(S)-amino4-phenyl-1-~N-n-
butylhydrazino)-butan-2(S)-ol and has the for nula . -:
2~120~7
- 110-
OH
-NH J~ ~NH-
The residue referred to as -[PheNN(p-F)Phe] is the divalent residue of 3(S)-amino4-
phe~yl-l-(N (p-fluorophenylmethyl)-hydrazino)-butan-2(S)-ol and has the fonnula ~ -
~\~ F
OH
-NH ~ ~NH-
The residue referred to as -[(p-E7)PheNN(p-~;)Phe] is the divalent residue of 3(S)-amino~
(p-fluorophenyl)-l-(N-(p-fluorophenylmethyl)-hydrazino~-butan-2(S)-ol and has the
formula
~\~ F
OH ~
-NH ~1~ ~NH- -
. ~'.
F~
The residue referred to as -[PheNN(p-CN3Phe] is the divalent residue of 3(S)-amino-
:
2~12~7
- 111 ,~
4-phenyl-1-(N-~p-cyanophenylmethyl)-hydrazino)-butan-2(S)-ol and has the formula ~ ~ -
~N
OH
-NH ~ ~NH~
The residue referred to as -[ChaNNLeu] is the divalent residue of 3(S)-amin~4-cyclo-
hexyl-l-(N-isobutyl-hydrazino)-butan-2(S)-ol and has the formula
L :~
OH
-NH ~1~ ~NH~
s
The divalent radical of 1-[2(S)-acetoxy-3(S)-a nino~phenylbutyl]-[l-cyclohexylmethyl]-
hydrazine has the formula
J~o ~
-NH ~ NH-
:
The abbreviations customarily used in peptide chemistry are used to name divalent
2112~)~7
- 112-
The abbreviations customarily used in peptide chemistry are used to name divalent
residues of natural a-amino acids. However, contrary to customary peptide nomenclature
in which the amino terminus is on the left and the carboxy terminus is on the right, amino
acids that are to the right of the residues -[PheNNPhe], -~PheNNCha], -[PheNNLeu],
-[PheNNNle], -[PheNN(p-F)Phe], -[(p-F)PheNN(p-F)Phe], -lPheNN(p-CN)Phe] and
-[ChaNNLeu] in the compound names, have the bonding carboxy group on the left, which
is indicated by an arrow ( ) symbolising the reversal of the direction of bonding
(inversion). The configuration at the a-carbon atom, if it is known, is indicated by the
prefix (L3- or (D)-. Tyrosine residues etherified at the phenolic hydroxy group by the
radical R are designated Tyr(OR). Nle denotes the residue of norleucine.
ReferenceExample 1: Boc-rPheNNPhel-Boc:
A solution of 300 mg (1.14 mmol) of (2R)-[l'(S)-Boc-amino-2'-phenylethyl]oxirane (J.
Org. Chem. 50, 4615 (1985 and 253 mg (1.14 mmol) of tert-butyl-3-benzyl-carbazate (J.
Chem. Soc., Perkin I, 1712 (1975)) in 4 ml of methanol is heated under reflux for 12 h.
After cooling the reaction mixture to 0 a large portion of the title compound precipitates.
The mother liquor is concentrated by evaporation and the residue is dissolved in a small
amount of methylene chloride. After the dropwise addition of hexane a further amount of
the ti~e compound is obtained in the form of a white precipitate. FAB-MS: (M+H)+~86,
tRet(I)=26.8 min, Rf(E)=0.70.
Reference Example 2: ~(L)-Val-rPheNNPhel~((L)-Val-Z):
191 mg (0.76 mmol) of Z(L)-valine, 336 mg (0.76 mmol) of BOP and 103 mg
(0.76 mmol) of HOBt are dissolved in 5 ml of a 0.3M solution of NMM in DMF, and after
10 min 100 mg (0.25 mmol) of H-[PheNNPhe]-H.3HCl are added and the mixture is
stirred for 2 h at RT under a nitrogen atmosphere. The reaction mixture is concentrated by
evaporation, and the residue is dissolved in methylene chloride and washed twice with
satur~ted sodium hydrogen carbonate solution. The organic phases are filtered through
cotton wadding and concentrated by evaporation and the residue is purified by means of
chromatography on silica gel with methylene chloride/ether (1:1). Lyophilisation of the
product-containing fractions from dioxane yields the title compound in the form of a white
solid. FAB-MS: (M+H)+=752, tRet(I)=27.8 min, Rf(E)=0.45.
The starting material is prepared as follows:
a) H-rPheNNPhel-H.3HCl: -
21120~7
- 113-
A solution of 280 mg (0.58 mmol~ of Boc-[PheNNPhe]-Boc from Reference Example 1 in
10 ml of 4N hydrogen chloride in dioxane is stirred for 2 h at RT under a nitrogen atmos-
phere and then lyophilised. Lyophilisation once more from dioxane/tert-butanol yields the
title compound in the form of a flocculent solid. FAB-MS: ~M+H)+=286, tRet(II)=-23.1 min, Rf(C)=0.17.
Reference Example 3: Boc-(L)-Val-rPheNNPhel, ((L)-Val-Boc):
The tide compound is obtained in a manner analogous to that described in Reference
Example 2 from 50 mg (0.13 mmol) of H-[PheNNPhe]-H.3HCl, 83 mg (0.83 mmol) of
Boc-(L)-valine, 168 mg (0.38 mmol) of BOP,51 mg (0.38 mmol) of HOBt and 2.5 ml of
0.3M NMM in DMF after chromatographic purification on silica gel with chloroform/-
methanol (95:5) and lyophilisation from dioxane. FAB-MS: (M+H)+=684,
tRet(I)=27.4 min, Rf~E)=0.38.
Refereince ExamPle 4: Boc-~PheNNChal-Boc:
The tide compound is obtained analogously to Reference Example 1, from 231 mg
(0.88 mmol) of (2R,3S)-l-E3-Boc-amino-2-phenylethyl]oxirane and 200 mg (0.88 mmol)
of tert-butyl-3-cyclohexylmethyl-carbazate, in the form of a white precipitate from
hexane. FAB-MS: (M+H)+--492, tRet(l)=30.4 min, Rf(E)=0.78.
The starting matelial is prepared as follows:
a) tert-Butvl-3-cYclohexYlmethvl-carbazate:
10.2 g (45.1 mmol) of cyclohexylcarbaldehyde-tert-butoxycarbonylhydrazone, dissolved
in 400 ml of methanol, are hydrogenated in the presence of 5.1 g of 5 % platinum on
carbon at RT and 4 atrn hydrogen pressure. When dle reaction is complete, the catalyst is
removed by filtradon and the filtrate is concentrated by evaporation. The residue is
dissolved in methylene chloride and washed with water. Concentradon by evaporation of
the organic phase yields the title compound in the fo~rn of a colourless resin. lH-NMR
(200 MHz, CDC13): 6.1 (s, br, lH),3.9 (s, br, lH), 2.65 (d, 2H), 1.8-0.75 (m, l lH), 1.45
(s,9H), tRet(I)=32.0 min, Rf~E)=0.75.
b) Cvclohexvlcarbaldehvde-tert-butoxycarbonvlhvdrazone:
A soludon of 10.8 g (81.2 mmol) of tert-butylcarbazate and 10.1 g (90 mmol) of cyclo-
hexylcarbaldehyde in 400 ml of ethanol is heated under reflux for 2 h. Half of the solvent
is then removed by distilladon and the title compound is precipitated by the addidon of
~ .. - . . . "~
2112~7
- 114-
water. It is directly further used in a).
Reference Example 5: H-(L)-Val-LPheNNPhel f(L)-Val)-H.3HCl:
A solution of 40 mg (0.06 mmol) of Boc-(L)-Val-[PheNNPhe]~((L~-Val)-Boc from Refer-
ence Example 3 in 4 ml of 4N hydrogen chloride in dioxane is stirred at RT for 1 h. The
mixture is then diluted with dioxane and, after lyophilisation, the title compound is
obtained in the form of the hydrochloride. FAB~MS: (M+H)+=484, tRet(II)=25.8 min,
Rf~A)=0.45.
Reference Example 6: N-ThiomorpholinocarbonYl-(L~-Val-~PheNNPhel (N-thio-
morpholinocarbonyl-(L)-Val):
35 Ill (0.25 mmol) of triethylamine and 16 mg (0.1 mmol) of (4-thiomorpholinylcarbonyl)-
chloride are added in succession at RT to a solution of 20 mg (0.03 mmol) of H-(L)-Val-
[PheNNPhe] ((L)-Val)-H.3HCl from Reference Example 5 in 0.5 ml of DMF, and the
mixture is stirred for 1 h at RT. The reacdon mixture is diluted with chloroform and
washed with saturated sodium hydrogen carbonate solution. The organic phase is filtered
through cotton wadding and concentrated by evaporation, and the residue is chromato-
graphed on silica gel with a gradient of chlo¢oform/methanol (15:1 -> 8:1). The product
fractions are concentrated by evaporation and precipitated with methylene chloride/DIPE.
Lyophilisation from dioxane yields the tide compound in the form of a flocculent solid.
FAB-MS: (M+H)+--742, tRet(I)=21.6 min, Rf(D)=0.54.
The starting material is prepared as follows:
a) (4-ThiomorPholin~lcarbonvl)chloride:
A solution of 10 g (97 mmol) of thiomorpholine in 200 ml of toluene is added dropwise at
0 to a solution of 85 ml (165 mmol) of 20 % phosgene in toluene and the white suspen-
sion is stirred for 1 h at RT. Excess phosgene is driven off by introducing nitrogen, the
suspension is filtered, and the filtrate is concentrated by evaporation. The title compound
is obtained in the form of a yellow oil. IR (CH2Cl2, cm~l): 1735, 1450, 1440, 1405, 1370,
1290, 1180.
Reference Example 7: N-MolpholinocarbonYl-(L)-Val-rPheNNPhel (N-morpholino-
car~onYl-(L)-Val):
210 ~,ll (1.52 mmol) of triethylamine are added to a solution of 100 mg (0.25 mmol) of
H-[PheNNPhe]-H.3HCl from Reference Example 2a), 163 mg (0.76 mmol) of N-morpho-
,',s, ~
21~2~7
- 115-
linocarbonyl-(L)-valine and 288 mg (0.76 mmol) of HBTU in 2 ml of DMF and the
mixture is stirred for 16 h at RT under a nitrogen atmosphere. The reaction mixture is fully
concentrated by evaporation, and the residue is dissolved in methylene chloride and
washed with saturated sodium hydrogen carbonate solution. The organic phase is filtered
through cotton wadding, concentrated by evaporation and chromatographed on silica gel
with methylene chloride/methanol (15:1). The title compound is precipitated frommethylene chloridefhexane and, after lyophilisation from dioxane/tert-bu~anol, is obtained
in the form of a flocculent solid. FAB-MS: (M+H)+=710, tRet(I)=16.3 min, Rf(E)=0.16.
The starting material is prepared as follows:
a) N-MorPholinocarbonvl~L)-valine:
2.7 g (8.4 mmol) of N-morpholinocarbonyl-(I,)-valine-benzyl ester are dissolved in 75 ml
of ethyl acetate and the solution is hydrogenated for 3 h in the presence of 500 mg of 10 %
palladium on carbon at 1 atm hydrogen pressure and RT. The catalyst is filtered off and,
after concentrating the solvent by evaporation, the title compound is obtained in the form
- of a colourless oil. lH-NMR (300 MHz, CD30D): 4.15 (m, lH),3.65 (m, 4H), 3.40 (m,
4H), 2.12 (m, lH), 0.95 (2d, 6H).
b) N-Morpholinocarbonvl-(L)-valine-benzyl ester:
0.8 ml (8.1 mmol) of (morpholinocarbonyl)chloride (preparadon: J. Med. Chem. 31, 2277
(1988)) and 4.1 ml (24.1 mmol) of N-ethyldiisopropylamine are added to a solution of 4 g
(10.5 mmol) of (L~-valine-benzyl ester 4-toluenesulfonate in 56 ml of methylene chloride
and the mixture is stirred at RT for 24 h. The reaction mixture is diluted wi~ ethyl acetate
and washed in succession with lN hydrochloric acid, water, saturated sodium hydrogen
carbonate solution-and brine. The organic phase is dried over sodium sulfate and concen-
trated by evaporation. Chromatography on silica gel with ethyl acetate yields N-morpho-
linocarbonyl-(L)-valine-benzyl ester in the form of a colourless oil. The ester is directly
further used in a).
Reference Exarnple 8: Phenvlacetvl-(L)-Val-~PheNNPhel (N-PhenYlacetvl-(L)-Val):
The title compound is obtained analogously to Reference Example 7 from 100 mg
(0.25 nmmol) of H-[PheNNPhe]-H.3HCI from Reference Example 2a), 143 mg
(0.61 mmol) of phenylacetyl-(L)-valine (preparation: Mem. Tokyo Univ. Agric. 20, 51
(1978)), 230 mg (0.61 mmol) of HBTU and 200 ',11 (1.42 mmol) of triethylamine after
chromatographicpurification with methylene chloride/ether/methanol (20:20:1) and
X~
:` . . . : ~
2~l2a~7
- 116-
lyophilisation from dioxane/tert-butanol. FAB-MS: (M+H)+=720, tRet(I)=23.7 min,
Rf~G)=0.21.
Reference Example 9: N-(3-PvridYlacetYl)-(L)-Val-~PheNNPhel (N-(3-pyridylacetyl)-
(L)-Val):
The tide compound is obtained analogously to Reference Example 7 in the form of a white
solid from 100 mg (0.25 mmol) of H-[PheNNPhe]-H.3HCl from Reference Example 2a,
576 mg (1.52 mmol) of HBTU,358 mg (1.52 mmol) of N-(3-pyridylacetyl)-(L)-valine and
316 lli (2.3 mmol) of triethylamine after chromatographic puIification with chloroform/-
methanol (5:1) and lyophilisation from dioxane/tert-butanol. FAB-MS: (M+H)+--722,
tRet(II)=27.9 min, Rf(A)=0.71.
The starting material is prepared as follows:
a) N-(3-PyridYlacetyl)-(L)-valine:
3.4 g of N-(3-pyridylacetyl)-(L)-valine-tert-butyl ester are dissolved in 20 ml of ~ifluoro-
acetic acid/methylene chloride (1:1) and the solution is stirred at RT for 16 h. The reaction
solution is fully concentrated by evaporation and the residue is digested with DIPE. The
title compound is obtained in the form of a white amorphous solid. lH-NMR (200 MHz,
CD30D): 8.9-8.6 (m, broad, lH), 8.5 (m, lH),7.95 (m, lH3, 4.33 (m, lH), 3.93 (s, 2H),
2.2 (m, lH), 0.98 (2d, 6H).
b) N-(3-PvridYlacetYl)-(L)-valine-tert-butYl ester:
4.2 ml of triethylamine are added dropwise at 0 to a solution of 3.36 g (16 mmol) of (L)-
valine-tert-butyl ester HCl, 2 g (14.5 mmol) of 3-pyridylacetic acid and 2.17 ml(14.3 mmol) of cyanophosphonic acid diethyl ester in 20 ml of DMF. The reaction mixtuIe
is stirred for 48 h at RT, and then diluted with methylene chloride and washed with lO %
citric acid as well as saturated sodium hydrogen carbonate solution. The organic phase is
filtered through cotton wadding and, after removal of the solvent by evaporation, yields
N-(3-pyridylacetyl)-(L)-valine-tert-butyl ester, which is directly further used in a).
Reference Example 10: Boc-(L)-Val-rPheNNChal_((L)-Val)-Boc:
The title compound is obtained analogously to Reference Example 7 in the form of a
flocculent solid starting from 500 mg (1.25 mmol) of H-[PheNNCha]-H.3HCl, 1.08 g(4.98 mmol) of Boc-(L)-valine, 1.89 g (4.98 mmol) of HBTU and 1.39 ml (9.96 mmol) of
triethylamine after chromatographic purification on silica gel with methylene chloride/-
2ll2a~7
- 117-
ether (1:1) andlyophilisation from dioxane. FAB-MS: (M+H)+=690, tRet(I)=29.3 min,
Rf(H)=Q.48.
The starting material is prepared as follows:
a) H-rPheNNChal-H.3HCl:
1.10 g (2.2 mmol) of Boc-OE'heNNCha]-Boc from Reference Example 4 are dissolved in
20 ml of 4N hydrogen chloride in dioxane and the solution is stirred at RT for 3 h.
Lyophilisation of the reacdon solution yields the title compound in the form of the
hydrochloride. FAB-MS: (M+H)+=292, tRet(II)=27.3 min.
ReferenceExample 11: Z-(L)-Val-rPheNNChal~((L)-Val)-Z:
The title compound is obtained analogously to Reference Example 2 from 50 mg
(0.12 mmol) of H~[PheNNCha]-H.3HCl from Reference Example 10a, 94 mg (0.37 mmol~ -
of Z(L)-valine, 165 mg (0.37 mmol) of BOP,51 mg (0.37 mmol) of HOBt and 2.5 ml of
0.3M NMM in DMF after chromatographic purification on silica gel with methylene
chloride/ether (1:1) and lyophilisation from dioxane. FAB-MS: (M+H)+=758,
tRet(I)=29.1 min, Rf~H)=0.55.
Reference Example 12: Boc-rPheNNLeul-Boc:
The dtle compound is obtained analogously to Reference Example 1, starting from 1.0 g
(3.8 mmol) of (2R)-~l'(S)-Boc-amino-2'-phenylethyl]oxirane and 715 mg (3.8 mmol) of
tert-butyl-3-isobutyl-carbazate (preparadon: J. Chem. Soc., Perkin I, 1712 (1975, in the
form of a precipitate from hexane. FAB-MS: (M+H)+=452, tRet(I)--27.2 min, Rf(I)=0.55.
Reference Example 13: Z-(L)-Val-rPheNNLeul~((L)-Val)-Z:
The title compound is obtained analogously to Reference Example 2 star~ng from 60 mg
(0.17 mmol) of H-[PheNNLeu]-H-3HCl, 125 mg (0.50 mmol) of Z-(L)-valine,221 mg
(0.50 mmol) of BOP, 67 mg (0.50 mmol) of HOBt and 3.3 ml of 0.3M NMM in DMF
after chromatographic purification on silica gel with methylene chloride/ether (1:1) and
lyophilisation from dioxane. FAB-MS: (M+H)+= 718, tRet (I)=26.8 min, Rf(O=0.38.
The starting material is prepared as follows:
a) H-~PheNNLeul-H.3HCI:
The title compound is obtained analogously to Reference Example 10a), in the form of a
:- : ,: .
2~120~7
- 118-
lyophilisate, from 1.21 g (2.48 mmol) of Boc-[PheNNLeu]-Boc from Reference Example
12. FAB-MS: (M+H)+=252, tRet(II)=20.9 min, Rf(K)=0.23.
Reference Example 14: H-(L)-Val-~PheNNChal (~L)-Val)-H.3HCl:
The tiile compound is obtained analogously to Reference Example 10a), from 632 mg
(0.91 mmol) of Boc-(L)-Val-[PheNNCha]~((L)-ValJ-Boc from Reference Example 10, in
the form of the hydrochloride after lyophilisation. FAB-MS: (M+H)~=490,
tRet(II)=29.4 min, Rf(K)=0.23.
Reference Example 15: N-(3-PyridYlacetvl)-(L)-Val-rPheNNLeul (N-(3-PYridvlacetYl)-
(L~-Val):
The title compound is obtained analogously to Reference Example 9 from 90 mg
(0.25 mmol) of H-[PheNNLeu]-H.3HCl from Reference Example 13 a), 358 mg
(1.52 mmol) of N-(3-pyridylacetyl)-(L)-valine,576 mg (1.52 mmol) of HBTU and 316 ~
(2.5 mmol) of triethylamine after chromatographic purification with methylene chloride/-
methanol S15:1) and lyophilisation from dioxane/tert-butanol/water. FAB-MS: (M+H)+=
688, tRetaV)= 15.5 min, Rf(D)= 0.37. ~ -
Reference Example 16: N-TrifluoroacetY!-rPheNN(P-F)Phel-Boc:
A solution of 4.0 g (15.4 mmol) of 2(R)~[l'(S)-(trifluoroacetylamino)-2'-phenylethyl]-
oxirane and 3.89 g (16.2 mmol) of teTt-butyl-3-(p-fluorophenyl-methyl)-carbazate in 35 ml
of methanol is heated at 80C for approximately 20 h in a bomb tube. The reaction
mixture is concentrated by evaporation, the residue is dissolved in a small amount of -
dichloromethane, and the title compound is precipitated therefrom using hexane (refrig~
erator). Column chromatography (SiO2, methylene chloride/ether 95:7) yields further - -
product. TLC Rf(J)=0.57; tRet(I)=24.3 min; FAB-MS (M+H)+-500.
The starting materials are prepared as follows:
a) N-3(S)-(Boc-amino)-2(R.S)-hYdroxv-4-PhenYI-l-trimethYlsilyl-butane:
Under a nitrogen atmosphere, 24.7 g (1.02 mol) of magnesium are placed in 100 ml of abs.
ether and over a period of 35 min a small amount of iodine and, at ~he same time, 132.5 ml
(0.95 mol) of chloromethyltrimethylsilane and 300 ml of ether are added, the temperature
being maintained at 38C by means of an ice bath. The reaction mixture obtained is then
stirred for 1.5 h at RT. After the mixtu~e has been cooled to -60C, a suspension of 48.6 g
(0.195 mol) of N-Boc-phenylalaninal (preparation: DJ. Kempf, J. Org. Chem. 51, 3921
~::, . .
~,, . :
2ll~a~7
- 119-
(1986)) in 1.1 l of ether is added over a period of 40 min. Over a period of 90 min the
reaction mixture is warmed to RT and stirred for a further 90 min at that temperature. The
mixture is then poured onto 2 1 of ice-water and 1.5 1 of 10 % aqueous citlic acid. The
separated aqueous phase is extracted twice with 500 ml of ether. All ether extracts are
washed with 500 ml of 10 % citric acid and twice with brine. After drying over sodium
sulfate the residue is concentrated in vacuo and the resulting title compound is further
used without additional purification. TLC Rf(L)=0.6; FAB-MS (M+H)+=338.
b) l-PhenYI-3-buten-2(S)-amine:
35.6 ml (0.28 mol) of an approximately 48 % solution of boron trifluoride in ether are
added at 5C, over a period of 10 min, to a solution of 18.8 g (0.055 mol) of 3(S)-(Boc-
amino)-2(R,S)-hydroxy-4-phenyl-1-trimethylsilyl-butane in 420 ml of methylene chloride.
The reaction mixture is then stirred at RT for 16 h, cooled to 10C and, over a period of
20 min, 276 ml of a 4N sodium hydroxide solution are added. The aqueous phase isremoved and extracted t~,vice with 400 ml of methylene chloride each time. The combined
organic ext~acts are washed with brine and dried over sodium sulfate. The title product is
further used without additional purification. TLC Rf (C)= 0.15; IR (methylene chloride)
(cm~l): 3370, 3020, 2920, 1640, 1605.
c) N-Trifluoroacetvl- l -phenYl-3-buten-2(S)-amine:
17.0 ml (121 mmol) of trifluoroacetic acid anhydride are added dropwise, at 0C, to 11.9 g
(81 mmol) of 1-phenyl-3-buten-2(S)-amine dissolved in 210 ml of methylene chloride and
70 ml of pyridine. The mixture is stirred for 0.5 h at 0C and then extracted twice with
dilute HCl, water and brine. The aqueous phases are washed a further twice with medlyl-
ene chloride, dried with sodium sulfate and concentrated by evaporation: TLC Rf(M)=0.4.
d) 2(R)-rl'(S)-(Trifluoroacetvlamino)-2'-phenYlethvll-oxirane:
54.28 g (314 mmol) of m-chloroperbenzoic acid are added to a soludon of 14.5 g
(60 mmol) of N-trifluoroacetyl-l-phenyl-3-buten-2(S)-amine in 600 ml of chloroform and
the mixture is stirred for 24 h at RT to complete the reaction. The reaction mixture is
washed twice with 10 % sodium sulfite solution, twice with saturated sodium carbonate
solution, water and brine. The aqueous phases are extracted a further twice with methylene
chloride and the combined organic phases are dried with sodium sulfate and concentrated
by evaporation to yield the tide compound, which is used in the next step without being
further purified: TLC Rf(N)=0.6.
~ . , . : . .
~,. . -.. , ~ .
~ .
.~ .
. . :
. ~' . ' ` ~ . ~ : ;
21~47
- 120-
e) p-Fluorophenvlcarbaldehvde-tert-butoxycarbonvlhYdrazone:
32 g (242 mmol) of tert-butylcarbazate and 30 g (242 mmol) of p-fluorobenzaldehyde in
300 ml of ethanol are reacted analogously to Reference Example 4 b) for 3 h at 80C to
form the title compound, which crystallises on cooling and diluting with water: TLC
Rf(N)=0.48; tRet(I)=19.4 min.
f) tert-ButYI-3-(P-fluorophenvl-methYI)-carbazate:
55 g (231 mmol) of p-fluorophenylcarbaldehyde-tert-butoxycarbonylhydrazone in 500 ml
of THF are hydrogenated with 5.5 g of palladium (5 %) on carbon analogously to Refer-
ence Example 4 a~ to yield the tide compound: lH-NMR (200 MHz, CD30D): 7.35 (dd, 8
and 6 Hz, 2 H), 7.05 (t, 8 Hz, 2 H), 3.9 (s, 2 H), 1.45 (s, 9 H).
Reference Example 17: N-Morpholinocarbonvl-(L)-Val-~PheNN(p-F)phel-Boc:
A mixture of 185 mg (0.80 mmol) of N-morpholinocarbonyl-(L)-valine (for preparation
see Reference Example 7 a, 270 mg (0.67 mmol) of H-[PheNN(p-F)Phe]-Boc, 311 mg
(0.70 mmol) of BOP and 95 mg (0.70 mmol) of HOBT is dissolved at RT in 6.8 ml of0.3M NM~/DMF and stilred for S h at RT. The reaction mixture is concentrated by ;;
evaporation under HV and the residue is partitioned between 4 portions of methylene ;
chloride, 2 portions of lM sodium carbonate solution, water and brine. The combined
organic phases are dried over sodium sulfate, concentrated by evaporation and purified by
column chromatography (SiO2, ethyl acetate): TLC Rf(0)=0.38; tRet(I)--21.8 min;
FAB-MS (M+H)+=616.
The starting material is prepared as follows:
a) H-rPheNN(p-F)Phel-Boc:
At 70C, 15 ml of a lM aqueous solution of potassium carbonate are added drop~,vise to a
solution of 0.3 g (0.6 mmol) of N-trifluoroacetyl-[PheNN(p-F)Phe]-Boc (for preparation
see Reference Example 16) in 50 ml of methanol under a nitrogen atmosphere and the
mixture is stirred for 25 h at that temperature. The reaction mixture is concentrated by
evaporation under HV, methylene chloride is added to the residue and the mixture is
washed twice with water and brine. The aqueous phases are extracted twice with
methylene chloride and the organic phases are dried with sodium sulfate and concentrated
by evaporation. The crude product is used in the next step without being further purified:
tRe~(l)=16.2 min.
'~ ' ;
~" . ' " ' , .
21~ '~47
- 121 -
Reference Example 18: N-Morpholinocarbonyl-(L)-Val-rPheNN(p-F~Phel- ((L)-Val)-Z:129 mg (0.34 mmol) of HBTU are added to a solution of 86 mg (0.34 mmol) of Z-(L)-Val
and 160 mg (0.31 mmol) of N-morpholinocarbonyl-(L)-Val-[PheNNtp-F)Phe]-H in 2.7 ml
of 0.25M NMM/CH3CN (0.25M NMM in CH3CN). After 4 h at RT the mixture is con-
centrated by evaporation and the residue is partitioned between 3 portions of methylene
chloride, 2 portions of saturated sodium hydrogen carbonate solution and brine. The
organic phases are dried with sodium sulfate and concentrated by evaporation to yield the
title compound which is obtained in pure form after digestion from methylene chloride/-
ether 1:1: TLC Rf(P)=0.4; tRet(I)=22.4 min; FAB-MS (M+H)+=749.
The starting material is prepared as follows:
a) N-Morpholinocarbonvl-(L)-Val-rPheNN(P-F)Phel-H:
210 mg (0.34 mmol) of N-morpholinocarbonyl-(L)-Val-[PheNN(p-F)Phe]-Boc (Reference
Example 17) are dissolved in 105 ml of fonnic acid and the solution is stirred for 4 h at
-RT and then concentrated by evaporation. The residue is taken up in methylene chloride
and the solution is washed with saturated sodium hydrogen carbonate soludon and brine.
Extraction of the aqueous phases with 2 portions of methylene chloride, drying dhe organic
phases with sodium sulfate and concentrating by evaporation yields the tide compound,
which is used in the next step without being further purified: tRet(I)=12.9.
Reference Example l9: N-Morpholinocarbonvl-(L)-Val-lPheNN(p-F)Phel~((L)-Val)-H:
160 mg (0.21 mmoV of N-morpholinocarbonyl-(L)-Val-[PheNN(p-F)Phe] ((L)-Val)-Z
(Reference Example 18) in 6 ml of ethanol are hydrogenated widh 40 mg of palladium (10
%) on carbon at normal pressure. Filtration through Celite~' (siliceous earth, filter aid
from Fluka, Buchs, Switzerland), concentration by evaporation and Iyophilisation from
dioxane yields the dde compound: tRe,(hydrochloride, I)=13.4 min; FAB-MS
(M+H)~=615.
Reference Example 20: N-MorPholinocarbonvl-(L)-Val-~PheNN(P-F)Phe l~((L)-Val~(N-morpholinocarbonvl-Glv):
54 mg (0.143 mmol) of HBTU are added to a solution of 26.9 mg (0.143 mmol) of N-morpholinocarbonyl-glycine and 80 mg (0.130 mmol) of N-morpholinocarbonyl-~L)-Val-
[PheNN(p-F)Phe]~((L)-Val)-H in 1.1 ml of 0.25M NMM/CH3CN and the mixture is stirred
for 16 h at RT. The mixture is concentrated by evaporation and the residue is partitioned
between 3 portions of ethyl acetate, water, 2 portions of saturated sodium hydrogen
. ~ , ., - , .
!i ` : ` ~ :
" .::
2112~7
- 122-
carbonate solution, water and brine. The organic phases are dried with sodium sulfate and
concentrated by evaporation to yield the title compound which, after dissolving in a small
amount of DMF and precipitating with DIPE, is obtained in pure forrn: tRe,(I)=15.1 min;
FAB-MS (M+H)+=785.
The starting material is prepared as follows:
a) N-Morpholinocarbonyl- lvcine-benzvl ester:
Analogously to Reference Example 7 b),7.69 g (22.8 mmol) of glycine-benzyl ester 4-
toluenesulfonate and 2.8 g (19 mmol) of (morpholinocarbonyl)chloride in 118 ml of
methylene chloride and 9 ml (53 mmol) of N-ethyldiisopropylamine are reacted for 18 h.
The title compound is obtained in pure form after extraction with methylene chloride and -
digestion with hexane: tRe,(I)= 11.6 min.
b) N-Morpholinocarbonvl-~lycine:
Analogously to Reference Example 7 a), 4.8 g (18.3 mmol) of N-morpholinocarbonyl-
glycine-benzyl ester in 100 ml of ethyl acetate are hydrogenated with 1 g of palladium
(10 %) on carbon to yield the dde compound: lH-NMR (300 MHz, CDCl3): 3.88 (s, 2 H),
3.64 (s, 4 H), 3.50 (s, 2 H),3.35 (s, 4 H).
Reference Example 21: Z-(L)-Val-rPheNN(P-F)Phel-Boc:
463 mg (1.22 mmol) of BTU are added to a solution of 335 mg (1.33 mmol) of Z-~L)-
Val and 448 mg (1.11 mmol~ of H-[PheNN(p-F)Phe]-Boc (for preparation see Reference
Example 17 a)) in 9.4 ml of 0.25M NMM/CH3CN (0.25M NMM in CH3CN). After
stirring for 16 h at RT, the mixture is concentrated by evaporation and the residue is
partitioned between 3 portions of methylene chloride, 2 portions of saturated sodium
hydrogen carbonate solution and brine. l~e organic phases are dried with sodium sulfate
and concentrated by evaporation to yield the title compound, which is purified by column
chromatography (siO2, hexane/ethyl acetate 4: 1 ~ 1: 1): tRe~(I)=26.6 min; FAB-MS
(M+H)+=637.
ReferenceExamPle22: Z-(L)-Val-rPheNN(p-F)Phel~((L)-Val)-Boc:
AnAlogously to Reference Example 18, 165 mg (0.76 mmol) of Boc-(L)-Val and 371 mg
(0.69 mmol) of Z(L)-Val-[PheNN(p-F)Phe]-H in 6 ml of 0.25M NMM/CH3CN are reactedwith 289 mg (0.76 mmol) of HBTU to yield the title compound, which can be crystallised
directly from the reaction solution and filtered off: tRe,(I)=27.2 min; FAB-MS
~1 ~ 2047
- 123
(M+H[~+=736.
The starting material is prepared as follows:
a) Z-~ Val-lPheNN(p-F)Phel-H:
Analogously tO Reference Example 18 a),440 mg tO.69 mmol) of Z(L)-Val-
[PheNN(p-F)Phe]-Boc are deprotected with 212 ml of formic acid to yield the title
compound: tRet(I)=17.8 min.
Reference ExamPle 23: Z(Ll-Val-lPhe~N(p-F)Phel ((L)-Val)-H:
Analogously to Reference Example 18 a), 250 mg (0.34 mmol) of Z(L~-Val-
[PheNN(p-F)Phe] ((L)-Val)-Boc (Reference Example 22) are deprotected with 50 ml of ~ ~ -
formic acid to yield the title compound: tRet(I)=18.0 min; FAB-MS (M~H)+=636.
.
Reference Example 24: Z-(L)-Val-rPheNN(P-F)Phel ((L)-Val) (N-morpholinocarbonyl-GIY):
Analogously to Reference Example 20, 32 mg (0.17 mmol) of N-morpholinocarbonyl-
glycine (Reference Example 20 b)) and 99 mg (0.16 mmol) of Z-(L)-Val-tPheNN(p-F)-
Phe] ((L)-Val)-H in 1.3 ml of 0.25M NMM/CH3CN are reacted widh 65 mg (0.17 mmol)of HBTU to yield the title compound, which crystallises direcdy from the reaction
solution: tRet(I)=21.1 min; FAB-MS (M+H)+=806.
Reference ExamDle 25: Z(L~-Asn-~PheNN(D-F)Phel-Boc:
3.0 g (7.8 mmol) of Z-(L)-asparagine-p-nitrophenyl ester (Bachem, Bubendorf/Switzer-
land) are added to a solution of 2.09 g (5.2 mmol) of H-[PheNN(p-F)Phe]-Boc (for prepar-
ation see Reference Example 17 a)) in 68 ml of DMF and 2.7 ml (16 mmol) of N-ethyl-
diisopropylamine. The mixture is stirred for 16 h at RT and concentrated by evaporation
under HV, and the residue is taken up in a large amount of methylene chloride (poorly
soluble) and washed with 2 pordons of 5 % potassium carbonate soludon. The aqueous
phases are extracted twice more with a large amount of methylene chloride, and the
combined organic phases are dried with sodium sulfate and concentrated by evaporation.
The title compound is obtained by dissolving the crude product in a small amount of
methanol and precipitating by the addidon of toluene at -20C: tRc~(I)=21.2 min.
Reference Example 26: H-(L)-Asn-~PheNN(D-F)Phel-Boc:
Analogously to Reference Example 19, 0.40 g (0.61 mmol) of Z-(L)-Asn-
- 124-
[PheNN(p-F)Phe]-Boc is hydrogenated in 20 ml of methanol to yield the title compound:
tRe,(Ij=14.9 min.
Reference Example 27: Quinoline-2-carbonyl-(L)-Asn-rPheNN(P-F)Phel-Boc:Analogously tO Reference Example 17, 134 mg (0.78 mmol) of quinoline-2-carboxylic
acid (Fluka, Buchs/Switzerland) in 4 ml of 0.3M NMM~DMF are reacted with 344 mg ~-
(0.78 mmol) of BOP, 105 mg (0.78 mmol) of HOBT and 268 mg (0.52 mmol) of H-(L)-
Asn-[Phe~N(p-F)Phe]-Boc. Since, according to HPLC, there is still some H-(L)-Asn-
[PheNN(p-F)Phe]-Boc present after stirring for 16 h at RT, a further 299 mg of BOP,
70 mg of HOBT, 89 mg of quinaldic acid and 113 ,ul of NMM are added. After a further
16 h the mixture is concentrated by evaporation and the residue is partitioned between 3
portions of methylene chloride, 2 portions of saturated sodium hydrogen carbonate solu-
tion and brine. The combined organic phases are dried with sodium sulfate and concen-
trated by evaporation. The crude product is dissolved in a small amount of DMF, precip-
itated with DIPE and cooled to -20C to yield the title compound: tRet(I)=22.8 min;
FAB-MS (M+H)+=673.
Reference ExamPle 28: Z(L)-Asn-rPheNN(p-OPhel (L)-Val)-Z:
88 mg (0.35 mmol) of Z(L)-Val in 3.8 ml of 0.3 M NMM/DMF are activated with 153
mg (0.35 mmol) of BOP and 47 mg (0.35 mmol) of HOBT and, after 15 min, 144 mg
(0.23 mmol) of Z(L)-Asn-tPheN}I(p-F)Phe]-H-2HCI are added. The reacdon mixture is
stilred for 14 h at RT and then concentrated by evaporation, the residue is dissolved in
ml of methanol and partitioned between 3 portions of methylene chloride and 2 portions
of lM sodium carbonate solution, and the organic phases are dried with sodium sulfate
and concentrated by evaporation. Repeated dissolution of the crude product in a small
amount of DMF and precipitation with DIPE yields the title compound: tRet(I)=æ.2 min;
FAB-MS (M+H)+=785.
The starting material is prepared as follows:
a) Z-(L)-Asn-rPheNN(p-F)Phel-H-2HCl:
Under a nitrogen atmosphere, 2 ml of 4N HCUdioxane (Fluka, Buchs/Switzerland) are
added to a so1ution of 150 mg (0.23 mmol) of Z-(L)-Asn-[PheNN(p-F)Phe]-Boc (Reference
Example 25) in 1 ml of dioxane. The reaction mixture is stirred for 1.5 h at RT and then
lyophilised, and the lyophilisate is directly further reacted.
21.L2~4
- 125-
Reference ExamPle 29: Trifluoroacetvl-rPheNN(p-F)Phel~((L)-Val)-Z:
Analogously to Reference Example 17, 239 mg (0.95 mmol) of Z-(L)-Val in 10.5 ml of
0.3M NMM/DMF are reacted for 15 h with 421 mg (0.95 mmol) of BOP, 129 mg
(0.95 mmol) of HOBT and 0.3 g (0.63 mmol) of N-trifluoroacetyl-[PheNN(p-F)Phe]-H.
Column chromatography (SiO2, methylene chloride/ether 10: 1) and precipitation from
DMF solution with DIPE yields the title compound: TLC Rf(Q)=0.15; tRct(l)=25.9 min;
FAB-MS (M+H)+=633.
The starting material is prepared as follows:
a) N-Trifluoroacetvl-rPheNN(P-F)Phel-H:
At 0C, S ml of trifluoroacetic acid are added to 0.20 g (0.40 mmol) of N-llifluoroacetyl-
[PheNN(p-F)Phe~-Boc ~for preparation see Reference Example 16) in 5 ml of methylene
chloride. The reacdon mixture is stirred for 4 h at 0C and for 2 h at RT and then
concentrated by evaporation. Lyophilisation of the residue from dioxane yields the title
compound, which is further reacted without being pulified: tRet(I)=14.7 min.
Reference Example 30: ~(Ll-Asn-rPheNNPhel-Boc:
Analogously to Reference Example 25, 167 mg (0.34 mmol) of H-[PheNNPhe]-Boc in
3.6 ml of DMF and 0.18 ml (} mmol) of N-ethyl-diisopropylamine are reacted with 0.20 g
(0.52 mmol) of Z(L)-asparagine-p-nitrophenyl ester to yield the title compound, which is
obtained in pure form by column chromatography (SiO2, ethyl acetate): TLC Rf(O)=0.19;
tRet(l)=20.9 min.
The starting material is prepared as follows:
a) N-Trifluoroacetvl-rPheNNPhel-Boc:
Analogously to Reference Example 16, 1.82 g (7.0 mmol) of 2(R)-[l'(S)-(trifluoroacetyl-
amino)-2'-phenylethyl]-oxirane (Reference Example 16 d)) and 1.58 g (7.1 mmol) of tert-
butyl-3-benzyl-carbazate (J. Chem., Perkin I, 1712 (1975)) in 15 ml of methanol are
reacted in a bomb tube to yield the title compound, which is isolated by column chromato-
graphy (SiO2, methylene chloride/ether 50: 1): TLC Rf(J)=0.38; tRet(I)=24.5 min.
b) H-rPheNNPhel-Boc:
Analogously to Reference Example 17 a), 258 mg (0.53 mmol) of N-trifluoroacetyl-[PheNNPhe]-Boc in 60 ml of methanol are reacted with 10.7 ml of lM potassium car-
... .
2~20~7
- 126-
bonate solution to yield the title compound.
Reference Example 31: Z-(L)-Val-~(p-F)PheNN(p-F)Phel-Boc:
Analogously to Reference Example 21, 18 mg (0.070 mmol) of Z-(L)-Val and 27 mg
(0.064 mmol) of H-[(p-F)PheNN(p-F)Phe]-Boc in 0.6 ml of 0.25M NMMICH3CN are
reacted with 26.6 mg (0.070 mmol) of HBTU to yield the title compound, which is
purified by dissolving in a small amount of methylene chloride and precipitating with
DIPE: FAB-MS (M+H)+=655.
The starting material is prep2red as follows:
a) N-Boc-(p-fluoroPhenYlalanine):
In 0.4 1 of dioxane/water 1:1 20 g (109 mmol) of p-fluorophenylalanine (Fluka, Buchs,
Switzerland) are reacted with 35.5 g (163 mmol) of Boc-anhydride and 150 g (1.09 mol)
of potassium carbonate. After 4 h, the reaction mixture is acidified with citric acid solution
and extracted with 3 portions of ethyl acetate. The organic phases are washed with 10 %
citric acid, water and brine, dried with sodium sulfate and concent;ated by evaporation.
Dissoludon of the residue in a small amount of methylene chloride and crystallisation by
the addition of hexane yields the tit1e compound: tRet(I)=16.9 min.
b) N-Boc-(p-fluorophenYlalaninol):
At from -5C to -10C 9.66 ml (69 mmol) of triethylamine are added to a solution of
17.9 g (63 mmol) of N-Boc-(p-fluorophenylalanine) in 73 ml of abs. THF, and a solution
of 9.05 ml (69 mmol) of chloroformic acid isobutyl ester in 44 ml of abs. THF is added
dropwise thereto. After stirring for 0.5 h at RT, the resulting precipitate is filtered off with
suction. The filtrate is added dropwise, with cooling, to 4.77 g (126 mmol) of sodium
borohydride in 28 ml of watér. The mixture is stir~red for 4 h at RT and then acidified with
10 % citric acid, the THF is partially removed by evaporation using a RE and the residue
is partitioned between 3 portions of ethyl acetate,2 portions of 2N sodium hydroxide solu-
tion, water, saturated sodium hydrogen carbonate solution and brine. The organic phases
are dried with sodium sulfate, concentrated by evaporation, dissolved in a small amount of
methylene chloride and crystallised by the addition of hexane to yield the title compound:
TLC Rf(N)=0.36; tRet(I)=16.8 min; lH-NMR (200 MHz, CD3(~D): 7.24 (dd, 8 and 5 Hz,2
H),6.98 (t, 8 Hz, 2 H),3.73 (m, l H),3.47 (d, 5 Hz, 2 H), 2.88 (dd, 13 and 6 Hz, 1 H),
2.62 (dd, 13 and 8 Hz, 1 H), 1.36 (s,9 H).
-127- 21~ 2;~17
c) N-Boc-(P-fluorophenvlalaninal):
Under a nitrogen atmosphere, 4.44 ml (62.4 mmol) of DMSO dissolved in 76 ml of
methylene chloride are added dropwise to a solution, cooled to -60C, of 4.0 ml
(46.8 mmol) of oxalyl chloride in 44 ml of methylene chloride. After siirring for 15 min,
resulting in a clear reaction solution, 8.4 g (31.2 mmol) of N-Boc-(p-fluorophenylalaninol)
in the form of a solution in 185 ml of methylene chloride/l~ 1 are added (~ precipita-
tion) and the mixture is then stirred for 25 min. 17.3 ml (124.8 mmol) of triethylamine
dissolved in 38 ml of methylene chloride are then added. After the mixture has been
stirred for 30 min, 278 ml of a 20 ~o potassium hydrogen sulfate solution are added
dropwise, followed by 220 ml of hexane. The mixture is left to warm to RT, and the
aqueous phase is removed and extracted with 2 portions of ether. The organic phases are
washed with saturated sodium hydrogen carbonate solution, water and brine, dried with
sodium sulfate and concentrated by evaporation to yield the tide compound, which is used
in the next step without being further purified: lH-NMR (200 MHz, CDCI3): 9.63 (s, 1
H), 6.9-7.2 (2m, 4 H),5.04 (m, 1 H), 4.42 (m, 1 H), 3.10 (m, 2 H), 1.43 (s,9 H).
d) N-3(S)-(Boc-amino)-2(R~S)-hYdroxv-4-(P-fluorophenvl)-l-trimethYlsilYl-butane:Analogously to Reference Example 16 a), 1.63 g (67 mmol) of magnesium in 33 ml of
abs. ether are reacted with 8.3 ml (60 mmol) of chloromethyltrimethylsilane to form the
Grignard compound which, after reaction with 13 mmol of N-Boc-(p-fluorophenyl-
alaninal), extraction and column chromatography (SiO2, hexane/ethyl acetate 5:1 ~ 4:1),
yields the title compound in the form of a diastereoisomeric mixture: TLC Rf(L)=0.32;
tRet(I)=24.9 min (22 %)125.5 min (78 %); FAB-MS (M+H)+=356.
e) l-(p-FluoroPhenvl)-3-buten-2(S)-amine:
Analogously to Reference Example 16 b), 1.1 g (3.1 mmol) of N-3(S)-(Boc-amino)-
2(R,S)-hydroxy-4-(p-fluorophenyl)-1-trimethylsilyl-butane in 22 ml of methylene chloride
are reacted with 1.9 ml (15.5 mmol) of an approximately 48 % solution of boron
trifluoride in ether to yield the title compound: IH-NMR (300 MHz, CDCl3): 7.2-7.10 and
7.05-6.9 (2m, each 2 H),5.9-5.8 (m, 1 H),5.2-5.0 (m, 2 H),3.57 (m, 1 H), 2.79 (dd, 12 and
6 Hz, 1 H), 2.62 (dd, 12 and 8 Hz,1 H), 1.7 (sb, 2 H).
f) N Trifluoroacetvl-l-(p-fluorophenvl)-3-buten-2(S)-amine:
Analogously to Reference Example 16 c), 364 mg (2.2 mmol) of 1-(p-fluorophenyl)-3-
buten-2(S)-amine in 1.8 ml of methylene chloride and 5.4 ml of pyridine are reacted with
460 ,Ll (3.3 mmol) of trifluoroacetic acid anhydride to yield the title compound, which
.......
s
2112~7
- 128-
after digestion with hexane is obtained in pure form: TLC Rf~F~=0.58; MS (M)+=261.
g) 2(R)- j l ' (S)-(Trifluoroacetylamino)-2' -(p-fluorophenyl)ethvll-oxirane:
Analogously to Reference Example 16 d3, 359 mg (1.37 mmol) of N-trifluoroacetyl-l-(p-
fluorophenyl)-3-buten-2(S)-amine in 9 ml of chloroform are oxidised with 1.18 g
(6.87 mmol) of m-chloroperbenwic acid to yield the title compound: TLC Rf(R)=0.45.
h) N-TrifluoroacetYl-r(p-F)PheNN(p-F)Phel-Boc:
Analogously to Reference Example 16, 415 mg (1.49 mmol) of 2(R)-[l'(S)-(trifluoro-
acetylamino)-2'-(p-fluorophenyl)ethyU-oxirane and 377 mg (1.57 mmol) of tert-butyl-
3-(p-fluorophenyl-methyl)-carbazate in 9 ml of methanol are reacted to yield the title
compound: TLC Rf(S)=0.53; FAB-MS (M+H)+=518; lH-NMR (300 MHz, CD30D):
7.4-7.3 and 7.3-7.2 (2m, each 2 H),7.05-6.9 (m, 4 H), 4.23 (m, 1 H), 3.90-3.65 (m, 3 H),
3.03-2.78 and 2.74-2.60 (2m, each 2 H), 1.30 (s, 9 H).
i) H-r(P-F)PheNN(P-F)phel-Boc:
Analogously to Reference Example 17 a),285 mg (0.55 mmol) of N-trifluoroacetyl-
[(p-F)PheNN(p-F)Phe]-Boc in 45 ml of methanol are reacted with 14 ml of lM potassium
carbonate solution to yield the title compound: tRet(I)=16.4 min.
Reference ExamPle 32: Z-(L)-Val-r(p-F)Phe~N(p-F)Phel-H:
Analogously to Reference Example 18 a), 215 mg (0.33 mmol) of Z-(L)-Val-
[(p-F)PheNN(p-F)Phe]-Boc are deprotected with 100 ml of formic acid to yield the title
compound: FAB-MS (M+H)+=SSS.
Reference ExamPle 33: Z-(L)-Val-r(p-F)PheNN(p-F)Phel~(N-(N~(2-pvridYlmethyl)-N-
methvlaminocarbonvl)-(L)-Val):
Analogously to Reference Example 18, 23.6 mg (0.089 mmol) of N-(N-(2-pyridyl- -methyl)-N-methylaminocarbonyl)-(L)-valine (for preparadon see EP 402 646 Al, 19th
Dec. 1990) and 45 mg (0.081 mmol) of Z-(L)-Val-[(p-F)PheNN(p-F)Phe]-H are reacted
with 33.8 mg (0.089 mmol) of HBTU in 0.76 ml of 0.25M NMM/CH3CN to yield the dtle
compound which is recrystallised with DMF/DIPE: l~LC Rf(O)=0.39; FAB-MS
(M~H)t=802.
2ll2a~
- 129-
Reference Example 34: Z-(L)-Val-r(p-F)PheNN(p-F)Phel~(N-(2(R,S)-carbamoyl-3-
phenvl-propionyl)(L)-Val):
Analogously to Reference Example 18, 26.0 mg (0.089 mmol) of N-(2(R,S)-carbamoyl-3-
phenyl-propionyl)-(L)-valine (preparation: Synth., Struct., Funct., Proc. Am. Pept. Symp.,
7~h, 85, (1981)) and 45 mg (0.081 mmol) of Z-(L)-Val-[(p-F)PheNN(p-F)Phe]-H (Refer-
ence Example 32) are reacted with 33.8 mg (0.089 mmol) of HBTU in 0.76 ml of 0.25MNMM/CH3CN to yield the tide compound which is recrystallised with DMl;/DIPE:
R~(P)=0.64; FAB-MS (M+H)+=829.
Reference Example 35: AcetYl-Val-rPheNNPhel (N-acetvl-Val):
Analogously to Reference Example 7, the title compound is obtained from 100 mg
(0.25 mmol) of H-[PheNNPhe]-H.3HCl from Reference Example 2a), 121 mg (0.76 mmol)
of N-acetyl-(L)-valine, 288 mg (0.76 mmol) of HBTU and 0.211 ml (1.52 mmol) of
triethylamine in DMF after Iyophilisation from dioxane. FAB-MS: (M+H)+=568,
tRet(I)=15.0 min., Rf(B)=0.46.
Reference ExamPle 36: Z-(D)-Val-lPheNNPhel ((D~-Val)-Z:
Analogously to Reference Example 2, the title compound is obtained from 50 mg
(0.123 mmol) of H-[PheNNPhe]-H.3HCl from Reference Example 2a),95 mg (0.38 mmol)of Z(D)-valine, 168 mg (0.38 mmol) of BOP,51 mg (0.38 mmol) of HOBt and 2.53 ml of
0.3M NMM in DMF after lyophilisation from dioxane. FAB-MS: (M+H)+=752,
tRe,(I)=26.4 min, Rf(H)=0.21.
Reference ExamPle 37: Quinoline-2-carbonvl-Val-rPheNNPhel. (N-quinoline-2-carbonvl-
Val):
145 mg (0.53 mmol) of N-(quinoline-2-carbonyl)-(L)-valine, 235 mg (0.53 mmol) of BOP
and 72 mg (0.53 mmol) of HOBt are dissolved in 3.5 ml of a 0.3M soludon of NMM in
DMF. After 10 min 70 mg (0.18 mmol) of H-[PheNNPhe]-H.HCI (Reference Example
2a)) are added, and the mixture is stirred for S h at RT under a nitrogen atmosphere. The
reacdon mixture is concentrated by evaporadon and the residue is dissolved in methylene
chloride and washed twice with saturated sodium hydrogen carbonate solution, once with
10 % ci'aic acid and once again with saturated sodium hydrogen carbonate soludon. The
organic phases are filtered through cotton wadding and concentrated by evaporation, and
the residue is precipitated twice from methylene chloride/methanol by the addition of
DIPE. Lyophilisation from dioxane yields the title compound in the form of a white solid
(mixture of two diastereoisomers distinguishable by HPLC). FAB-MS: (M+H)+=794,
21120~7
- 130-
tRet(A)=29.1 and 29.3 min, R~(B)=0.81.
a) N-(Quinoline-2-carbonvl)-(L~-valine:
3.28 g (15.9 mmol) of N,N-dicyclohexylcarbodiimide and 2.0 ml (14.5 mmol) of triethyl-
amine are added to a solution of 2.5 g (14.5 mmol) of (L)-valyl-tert-butyl ester and 2.5 g
(14.5 mmol) of quinoline-2-carboxylic acid in 100 ml of methylene chloride~I~F (10:1)
and the mixture is stiIred for 18 h at RT. The reaction mixture is cooled to -18~ and
filtered off from the urea. rhe filtrate is concentrated by evaporation, and the residue is
dissolved in methylene chloride and washed once with saturated sodium hydrogen
carbonate solution and once with water. The organic phases are filtered through cotton
wadding, concentrated by evaporation and, after chromatographic purification on silica gel
with hexane/ethyl acetate (2:1), yield N-(quinoline-2-carbonyl)-(L)-valyl-tert-butyl ester.
2.59 g (12.2 mmol) thereof are left at RT in methylene chloride~IFA (1:1) for 4.5 h. After
concentration by evaporation dhe residue is purified by chromatography on silica gel with
hexane/edhyl acetate (2:1). The product-containing fractions are concentrated by evapora-
tion, dissolved in methylene chloride again, and converted into the hydrochloride of the
tide compound by washing with lN sodium hydroxide solution and lN hydrochloric acid.
IH-NMR (200 MHz, CD30D): 1.05 and 1.07 (2d, J=6Hz, 6H), 2.40 (m, lH),4.65 (m, lH),
7.70 (m, lH),7.85 (m, lH),8.00 (dxd, lH), 8.20 (m, 2H), 8.48 (d, lH).
Reference ExamPle 38: Acetvl-(L)-Val-rPheNNChal~(N-acetYl-(Ll-Val):
Analogously to Reference Example 37, the tide compound is obtained from 160 mg (0.40
mmol) of H-[PheNNCha]-H~3HCI from Refercnce Example 10a), 190 mg (1.19 mmol) of
N-acetyl-(L)-valine,525 mg (1.19 mmol) of BOP, 160 mg (1.19 mmol) of HOBt and 7.9
ml of 0.3M NMM in DMF after precipitation from chloroform/methanol widh DIPE andlyophilisation from dioxane FAB-MS: (M+H)+=574, tRCt(I)=18.1 min, Rf(B)=0.30.
Reference ExamPle 39: N-(3-PYridYlacetvl)-(E)-Val-rPheNNChal (N-(3-pYridylacetYl)-
(L)-Val).3HCl:
Analogously to Reference Example 7, the tide compound is obtained from 100 mg (0.25
mmol) of H-[PheNNCha]-H-3HCl from Reference Example 10a),358 mg (1.52 mmol) of
N-(3-pyridylacetyl)-(L)-valine from Reference Example 9a), 576 mg (1.52 mmol) ofHBTU and 0.316 ml (2.28 mmol) of triethylamine in DMF after chromatographic purifica-
tion on silica gel with methylene chloride/methanol (15:1) and lyophilisation of the
product-containing fractions from dioxane. FAB-MS: (M+H)+=728, tRet(I)=11.3 min,Rf(U)=0.21.
21~ 2~'17
- 131-
Reference Example 40: AcetYl-Ile-rPheNNChal (N-acetvl-Ile):
Analogously to Reference Example 37, the title compound is obtained from 160 mg (0.40
mmol) of H-[PheNNCha]-H-3HCl from Reference Example 10a), 206 mg (1.19 mmol) of
N-acetyl-(L)-isoleucine,525 mg (1.19 mmol) of BOP, 160 mg (1.19 mmol) of HOBt and
7.9 ml of 0.3M NMM in DMF after precipitation from methylene chlonde/methanol bythe addition of DIPE and lyophilisation from dioxane/tert-butanol (mixture of 2 diastereo-
isomers distinguishable by HPLC). FAB-MS: (M+H)+=602, tRe~(I)=20.4 and 20.7 min,Rf(D)=0.33.
Reference Example 41: Thiomorpholinocarbonvl-(L)-Val-rPheNNChal (N-thiomorpho-
linocarbonYl-(L)-Val):
Analogously to Reference Example 6, the title compound is obtained staIting from 70 mg
(0.12 mmol) of H-(L-Val)-[PheNNCha]. (N-(L)-Val)-H-3HCl from Reference Example 14,
58 mg (0.35 mmol) of (4-thiomorpholinylcarbonyl)chloride from Reference Example 6a)
and 0.127 ml of triethylamine in 2 ml of DMF after chromatographic purification on silica
gel with methylene chloride/methanol (95:5) and Iyophilisation of the product-containing
fractions from dioxane. FAB-MS: (M+H)+=748, tRet(I)=24.0 min, Rf(B)=0.70. -~-
Reference Example 42: Z-(L)-Glu-rPheNN(p-F)Phel; ((L)-Glu)-Z:
A solution of 130 mg (0.14 mmol) of Z(L)-Glu(O-tert-butyl)-rPheNN(p-F)Phe] ((L)-Glu(O-tert-butyl))-Z [(Glu(O-tert-butyl) here denotes the radical of glutamic acid ester-
iSed at the ~y-carboxy group by a tert-butyl radical] in 8 ml of methylene chloride/TFA
(1: 1) is stirred for 3 h at RT. The solvent is evaporated off under reduced pressure and the
residue is precipitated from methylene chloride by the addidon of DIPE. The dtlecompound is obtained after lyophilisadon from dioxane/tert-butanol. FAB-MS:
(M+H)+=830, tRet(I)=19.6 min, Rf(B)=0.32.
a) Z-(L)-Glu(O-tert-butYl)-rPheNN(p-F)Phel ((L)-Glu(O-tert-butYI))-Z:
Analogously to Reference Example 37, the dtle compound is obtained from 100 mg
(0.24 mmol) of H-[PheNN(p-F)Phe]-H.3HCI, 245 mg (0.73 mmol) of Z-(L)-glutamic acid
tert-butyl ester, 321 mg (0.73 mmol) of BOP, 98 mg (0.73 mmol) of HOBt and 4.8 ml of
0.3M NMM in DMF after chromatographic purificadon on silica gel with methylene
chlcride/ether (1:1). tRet(I)--30.2 min, Rf(H)=0.17.
: ~:
:- .
: ~ -
?,
i~':'.''. ' , `~' ' ~''` ~
2~120~7
- 132,-
b) H-rPheNN(p-F)Phel-H.3HCI:
Analogously tO Reference Example 2a), the title compound is obtained from 1.77 g(3.51 mmol) of Boc-[PheNN(p-F)Phe]-Boc after Iyophilisation. FAB-MS: (M+H)+=304,Rf(K)=0.19.
c) Boc-rPheNN(p-F)Phel-Boc:
Analogously to Reference Example 1, the tide compound is obtained starting from 2.0 g
(7.60 mmol) of (2R)-[l'(S)-Boc-amino-2'-phenylethyl]oxirane and 2.17 g (9.04 mmol) of
tert-butyl-3-(4-fluorophenyl-methyl)-carbazate from Reference Example 16 f) after
chromatographic purification on si1ica gel with hexane/ethyl acetate (2: l). FAB-MS:
(M~H)+=504, tRet(I)-26.2 min, Rf(F)=0.26.
Reference Example 43: N-(2-PYridvlmethvl)-N-methylaminocarbonvl-(L)-Val-
rPheNN(p-F)-Phel (N-(N-(2-pyridylmethyl)-N-methYlaminocarbonyl)-(L)-Val):
Analogously to Reference Example 37, the tide compound is obtained frc.m 70 mg
(0.17 mmol) of H-[PheNN(p-F)Phe]-H.3HCl from Reference Example 42b), 135 mg ~ ~ -
(0.51 mmol) of N-(N-(2-pyridylmethyl)-N-methylaminocarbonyl)-(L)-valine (preparation
as described in EP 0 402 646 Al of l9th Dec. 1990), 225 mg (0.51 mmol) of BOP, 69 mg
(0.51 mmol) of HOBt and 3.4 ml of 0.3M NMM in DMF after chromatography on silica --
o gel widl methylene chloride/methanol (15:1) and lyophilisadon of the product-containing
fracdons from dioxane. FAB-MS: (M+H)+=798, tRetaV)=35 min, Rf(U)=0.21.
efe~ence Lxample 44: N-(3-(Tetrazol-l-Yl)-proPionYl)-val-rpheNN(p-F)phell ~N-(3-(tetrazol-l-vl)-propionYl)-Val):
Analogously to Reference Example 37, the title compound is obtained from 100 mg ~ -
(0.24 mmol) of H-lPheNN)p-F)Phe}-H.3HCl (fIom Reference Example 42b), 146 mg
~0.61 mmol) of N-(3-(tetrazol-1-yl)-propionyl)-(L)-valine, 268 mg (0.61 mmol) of BOP,
82 mg (0.61 mmol) of HOBt and 4 ml of 0.3M NMM in DMF after precipitadon from
methylene chloride by the addidon of DIPE and lyophilisadon from dioxane (4 diastereo
isomers distinguishable by HPLC). FAB-MS: (M+H)+=750, tRet(III)=30.8; 31.4; 32.4 and
32.8 min, Rf(K)=0.5.
a) N-(3-(Tetrazol-l-vl)-propionvl)-(L)-valine:
Analogously to Reference Example 9b, starting from 4 g (16.4 mmol) of (L)-valine-benzyl
ester HCl, 2.1 g (14.9 mmol) of 3-(tetrazol-1-yl)-propionic acid (preparadon:
US 4 794 109 A of 27th Dec. 1988), 2.4 ml of cyanophosphonic acid diethyl ester and
.
,.~ . .
~, :. - .: . . - . - . -
-` 211 2~
- 133-
4.4 ml of triethylamine in DMF, N-(3-(tetrazol- l-yl)-propionyl)-(L)-valine-benzyl ester is
obtained after chromatographic purification on silica gel with methylene chloride/-
methanol (30: 1). 2.66 g (8.03 mmol) thereof are hydrogenated in methanol/water (9: 1) in
the presence of 530 mg of 10 % palladium on carbon, at 1 atm hydrogen pressure, tO yield
the title compound after precipitation from methanoVDlPE. lH-NMR (200 MHz,
CD30D): 0.9 (d, J=7Hz,6H), 2.1 (m, lH), 2.95 (m, 2H),4.29 (d, J=6Hz, lH), 4.78 (m,
2H), 9.15 (s, lH).
Reference ExarnPle 45: Z-(L)-Val-~PheNN(p-F)Phel ((L)-Val)-Z:
Analogously to Reference Example 37, the title compound is obtained from 100 mg
(0.24 mmol) of H-[PheNN(p-F)Phe~-H.3HCl (from Reference Example 42b), 182 mg
(0.38 mmol) of Z(L)-valine, 321 mg (0.73 mmol) of BOP,98 mg (0.73 mmol) of HOBt -
- and 4.8 ml of 0.3M NMM in DMF after precipitation from methylene chloride by the
addition of D~E and lyophilisation from dioxane. FAB-MS: (M+H)+=770,
tRet(I)=26.3 min, Rf(H)=0.25.
Reference ExamPle 46: Acetvl-Val-lPheNN(P-F)Phel (N-acetYI-Val):
Analogously to Reference Example 37, the title compound is obtained from 80 mg
(0.19 mmol) of H-[PheNN(p-~;~Phe]-H.3HCl from Reference Example 42b), 124 mg
(0.78 mmol) of N-acetyl-(L)-va1ine, 344 mg (0.78 mmol) of BOP, 105 mg (0.76 mmol) of
HOBt and 4.5 ml of 0.3M NMM in DMF after dissolving and reprecipitating twice from
methylene chloride/methanol by the addition of DIPE and Iyophilisation from dioxane/-
tert-butanol. FAB-MS: (M+H)+=586, tRet(I)=15.8 min, Rf (E)=0.32.
Reference Example 47: Acetvl-Val-lPheNN(p-CN)Phel. (N-acetvl-Val):
Analogously to Reference Example 37, the tide compound is obtained in the form of a
mixture of 2 diastereoisomers distinguishable by HPLC from 80 mg (0.19 mmol) of
H-[PheNN(p-CN)Phe~-H.3HCl, 124 mg (0.78 mmol) of N-acetyl-(L)-valine, 344 mg
(0.78 mmol) of BOP, 105 mg (0.78 mmol) of HOBt and 4.5 ml of 0.3M NMM in DMF
af~er precipitation from methylene chloride/methanol by the addition of DIPE and lyophil-
isation from dioxane. FAB-MS: (M+H)+=593, tRet(I)=14.4 and 14.6 min, Rf(D)=0.39.
a) H-~PheNN(p-CN)Phel-H.3HCI:
Analogously to Reference Example 2a), the title compound is obtained from 2.69 g(5.27 mmol) of Boc-[PheNN(p-CN)Phe]-Boc after lyophilisation. FAB-MS: (M+H)+=311,
Rf(K)=0.16.
l .,i.,.~ - - "'~
.''" ~
2~121~41~
- 134-
b) Boc-lPheNN(p-CN)Phel-Boc:
Analogously to Reference Example 1, the title compound is obtained from 2.0 g
(7.60 mmol) of (2R)-[l'(S)-Boc-amino-2'-phenylethyl]oxirane and 1.87 g (7.6 mmol) of
tert-butyl-3-(4-cyanophenyl-methyl)-carbazate after clystallisation from methanoVDIPE.
FAB-MS: (M+H)~=511, tRe,(I)=25 min, Rf(Y)=0.19.
c) tert-ButYI-3-(4-cvanophenYl-methvl)-carbazate:
Analogously to Reference Example 4b), 10 g (76.3 mmol) of 4-cyanobenzaldehyde and
10 g (76.3 mmol) of tert-butylcarbazate in ethanol are reacted to yield 4-cyanophenylcarb-
aldehyde-tert-butoxycarbonylhydrazone. 11.1 gthereofarehydrogenatedin 150mlof
IHF in the presence of 2 g of 10 % palladium on carbon at 2 atm hydrogen pressure to
yield the title compound. lH-NMR (200 MHz, CDCl3): 7.65 (d, J=8Hz, 2H),7.45 (d, J=8
Hz, 2H), 6.08 (s, br, lH), 4.3 (s, br, lH), 4.02 ~s, 2H), 1.45 (s, 9H).
Reference Example 48: Z-(L)-Val-~PheNN(p-CN)Phel ((Ll-Val)-Z: ~-
Analogously to Reference Example 37, the title compound is obtained from 70 mg ~ -
(0.17 mmol) of H-[PheNN(p-CN)Phe]-H.3HCl (from Reference Example 47a)), 125 mg
(0.5 mmol) of Z(L)-valine, 221 mg (0.5 mmol) of BOP, 68 mg (0.5 mmol) of HOBt and
3.33 ml of 0.3M NMM in DMF after precipitation from methylene chloride by the
addition of hexane and lyophilisadon from dioxane. FAB-MS: (M+H)+=777,
tRet(l)=25.3 min, Rf(D)=0.69.
Reference ExamPle 49: Z-(Ll-Ile-rPheNNLeul. ((Ll-Ilel-Z:
Analogously to Reference Example 37, the title compound is obtained from 70 mg
(0.19 mmol) of H-[PheNNLeu]-H.3HCl (from Reference Example 13a)), 154 mg
(0.58 mmol) of Z(L)-isoleucine,257 mg (0.58 mmol) of BOP,79 mg (0.58 mmol) of
HOBt and 3.88 ml of 0.3M NMM in DMF after chromatography on silica gel with
methylene chloride/ether (3:1) and precipitation of the product-containing fractions from
methylene chloride/DIPE and lyophilisation from dioxane. FAB-MS: (M+H)~=746,
tRet(l)=28.2 min, Rf(H)=0.39.
Reference Example 50: IsobutoxYcarbonvl-(Ll-Val-rPheNNLeul~(N-isobutoxycarbonvl-(Ll-Vall:
Analogously to Reference Example 37, the title compound is obtained from 70 mg
(0.19 mmol) of H-[PheNNLeu]-H-3HCI (from Reference Example 13a)), 130 mg
t~'.;`:::. , ', ' ' '~ .. .. . . :.` " ~ :
:. "' . . '.. , :: ' ~: . '' ' ' . . . - : '
21~23~7
- 135-
(0.58 mmol) of N-(isobutoxycarbonyl)-(L)-valine, 256 mg (0.58 mmol) of BOP, 78 mg
(0.58 mmol) of HOBt and 3.9 ml of 0.3M NMM in DMF after chromatography on silicagel with methylene chloride/ether (1:1) and Iyophilisation of the product-containing
fractions from dioxane. FAB-MS: (M+H)+=650, tRet(I)=26.4 min, Rf(H)=0.38.
a) N-(IsobutoxYcarbonvl~-(L)-valine:
11.2 ml (85.3 mmol) of isobutyl chloroformate are added to a solution of 10 g (85.3 mmol)
of (L)-valine in 100 ml of 2N sodium hydroxide solution and the solution is stitred at RT
for 18 h. The reaction solution is washed with methylene chl~lide, acidified with 4N
hydrochloric acid and extracted with methylene chloride. The organic extracts are washed
with brine and filtered through cotton wadding to yield the title compound in the form of a
colourless resin after concentradon by evaporation. lH-NMR (200 MHz, CD30D): 0.95
(m, 12H), 1.9 (m, lH), 2.15 (m, lH), 3.85 (d, J=7Hz, 2H), 4.05 (d broad, lH).
Reference ExamPle 51: N-(3-(Tetrazol-l-Yl)-ProPionvluLl-val-rpheNNLeul (N-3-
(tetrazol-l-vl)-pro~ionvl-(L)-Val):
Analogously to Reference Examp1e 37, the title compound is obtained from 150 mg
(0.42 mmol) of H-[PheNNLeu]-H.3HCl (from Reference Example 13a)), 251 rng
(1.04 mmol) of N-(3-(tetrazol-1-yl)-propionyl)-(L)-valine from Reference Example 44a,
460 mg (1.04 mmol) of BOP, 140 mg (1.04 mmol) of HOBt and 6.9 ml of 0.3M N-methyl-
morpholine in DMF after precipitation from methylene chloride/DlPE and lyophilisation
from dioxane/tert-butanoVwater. FAB-MS: (M+H)+=689, tRet(I)=14.7 min, Rf(K)=0.36.
Reference ExamPle 52: Acetvl-Val-rPheNNLeul~(N-acetYl-Val):
Analogously to Reference Example 37, the title compound is obtained from 70 mg
(0.19 mmol) of H-lPheNNLeu]-H.3HCl (from Reference Example 13a)), 184 mg
(1.16 mmol) of N-ace~l-(L)-valine,512 mg (1.16 mmol) of BOP, 156 mg (1.16 mmol) of
HOBt and 7.8 ml of 0.3M NMM in DMF after precipitation from methylene chloride/-methanol by the addition of DIPE and lyophilisation from dioxane/tert-butanoVwater (2
diastereoisomers distinguishable according to HPLC). FAB-MS: (M+H)~=534,
tR, t(I)=14.7 and 15.1 min, Rf(D)=0.35.
Reference Example 53: Boc-(L)-Val-~PheNNLeul~((L)-Val~-Boc:
Analogously to Reference Example 7, the title compound is obtained from 300 mg
(0.83 mmol) of H-[PheNNLeul-H-3HCI (from Reference Example 13a)),722 mg
(3.33 mmol) of Boc-(L)-valine, 1.262 g (3.33 mmol) of HBTU and 0.927 ml (6.66 mmol)
..
, : .: . . . .. .
;
2112~7
- 136-
of triethylamine in DMF after chromatographic puri~lcation on silica gel with methylene
chloride/ether (1: 1), precipitation of the product-containing fractions and lyophilisation
from dioxane. FAB-MS: (M+H)+=650, tRet(I)=26.3 min. Rf(H)=0.64.
Reference Example 54: H-(L)-Val-rPheNNLeul- ((L3-Val~-H-3HCl: ~ ~ -
Analogously to Reference Example 5, the tide compound is obtained from 396 mg
(0.61 mmol) of Boc-(L)-Val-[PheNNLeu] ((L)-Val)-Boc from Reference Example 53 and
10 ml of 4N hydrogen chloride in dioxane after lyophilisation of the reaction solution.
FAB-MS: (M+H)+=450, tRet(lI)=24.1 min, Rf(K)=0.25.
ReferenceExamPleSS:N-ThiomorpholinocarbonYl-~L)-Val-~PheNNLeu (N-thiomorpho-
linocarbonYl(L~-Val):
Analogously to Reference Example 6, the tide compound is obtained in the form of an
amorphous solid starting from 100 mg (0.16 mmol) of H-(L)-Val-~PheNNLeu3- (L)-Val-
H.3HCl,78.5 mg (0.47 mmol) of (4-thiomorpholinylcarbonyl)chloride from ReferenceExample 6a and 0.172 ml of triethylamine in DMF after chromatographic purification on
si!ica gel with methylene chloride/methanol (95:5), precipitation of the product-containing
fractions from methylene chloride/hexane and lyophilisation from dioxane. FAB-MS:
(M+H)+=708, tRet(I)=21.4 min, Rf(E)=0.45.
Reference ExamPle 56: 2(R~S)-TetrahYdrofurvl-methoxvcarbonvl-(L)-Val-
rChaNNLeul_(N-2(R.S)-tetrahYdrofuryl-methoxvcarbony!-(L)-Val):
Analogously to Reference Example 37, the title compound is obtained from 80 mg
(0.22 mmol) of H-[ChaNNLeu]-H.3HCl, 160 mg (0.65 mmol) of N-(2(R,S)-tetrahydro
furyl-methoxycarbonyl)-(L)-valine, 289 mg (0.65 mmol) of BOP, 88 mg (0.65 mmol) of
HOBt and 4.35 ml of 0.3M NMM in DMF after chromatographic purification on silica gel
with ethyl acetate and lyophilisation of the product-containing fractions from dioxane.
FAB-MS: (M+H)+=712, tRet(l)=22.4 min, Rf(E)=0.21.
a) H-rChaNNLeul-H-3HCl:
Analogously to Reference Example 5, 100 mg (83 %) of the title compound are obtained
from 150 mg (0.33 mmol) of Boc-[ChaNNLeu~-Boc and 10 ml of 4N hydrogen chloride in
dioxane after lyophilisation of the reacdon soludon. Rf(K)=0.26.b)
. . - ~ . .. - .: , , .: . . . . .
! ~, ~ . . , ; ' A. ~: , . ' ~ ' ~ . .
21~2~7
- 137-
Boe-l ChaNNLeul-Boc
A solution of 2~ mg (0.24 mmol) of Boc-[PheNNLeu]-Boc (Reference Example 12) in
15 ml of methanol is hydrogenated for 4 h at 1 atm hy~ogen pressure in the presence of
10 mg of Nishimura-catalyst (Rh(III)- and Pt(IV)-oxide monohydrate, Degussa). The
catalyst is removed by filtration, the solvent is fully concentrated by evapora~ion and the
title compound is obtained after c~ys~allisation from methylene chloride/hexane.tRet(I)=26.7 min, Rf(V)=0.21.
c) N-(2(R~S)-TetrahYdrofurYl-methoxvcarbonYl)-(L)-valine:
Analogously to Reference Example 50a, the title compound is obtained in the form of a
mixture of 2 diastereoisomers from 7 g (60 mmol) of (L)-valine and 9.8 g (60 mmol) of
2(R,S)-tetrahydrofurylmethyl-chloroformate (Heterocycles 27, 1155 (1988)) in 100 ml of
2N sodium hydroxide solution and 30 ml of dioxane. tRet(II)=23.5 and 23.8 min.
Reference Example 57: Z-Val-~PheNNLeul (N-(3-(tetrazol-1-Yl)-propionyl)-Val):
Analogously to Reference Example 37, the title compound is obtained (in the form o~ 2
diastereoisomers distinguishable by HPLC) from 100 mg (0.21 mmol) of Z(L)-Val-
[PheNNLeu]-H,75 mg (0.31 mmol) of N-(3-(tetrazol-1-yl)-propionyl)-(L)-valine from
Reference Example 44a, 137 mg (0.31 mmol) of BOP, 42 mg (0.31 mmol) of HOBt and
2 ml of 0.3M NMM in DMF after precipitation from methylene chloride/hexane and
Iyophilisation from dioxane/teTt-butanol. FAB-MS: (M+H)+=708, tRet(I)-21.1 and
21.1 min, Rf(D)=0.45.
a) Z-(L)-Val-rPheNNLeul-H:
A solution of 250 mg (0.43 mmol) of Z-(L)-Val-lPheNNLeu]-Boc in 5 ml of formic acid is
stirred for 7.5 h at RT. After that time no more starting material can be detected by HPLC
analy~is (tRet(I)=27.5 min), and the reacdon solutdon is concentrated by evaporation. The
residue is dissolved in chloroform and washed with saturated sodium hydrogen carbonate
soludon. The chloroform phase is filtered through cotton wadding and yields the crude
title compound after removal of the solvent by evaporation. tRe~(I)=16.7 min, Rf(K)=0.21.
b) Z-(L~-Val-~PheNNLeul-Boc:
Ana1Ogously to Reference Example 37, the title compound is obtained from 230 mg
(0.653 mmol) of H-[PheNNLeu]-Boc, 247 mg (0.98 mmol) of Z(L)-valine, 434 mg
(0.98 mmol) of BOP, 133 mg (0.98 mmol) of HOBt and 6.5 ml of 0.3M NMM in DMF
after precipitation from methylene chloride/methanol by the addidon of DIPE. FAB-MS:
. ~i , . ~ , ~ , -
2~12~7
- 138-
(M+H)+=585, tR~j,(I)=27.5 min, Rf(C)=0.71.
c)_-IPheNNLeul-Boc:
Analogously to Reference Example 17a), the title compound is obtained starting from
1.27 g (2.84 mmol) of N-trifluoroacetyl-lPheNNLeu]-Boc and 24 ml of lN aqueous
sodium carbonate solution in 90 ml of methanol by precipitation from methylene chloride
by the addition of DIPE. tRet(I)=14.9 min, Rf(K)=0.38.
d) N-Trifluoroacetvl-rPheNNLeul-Boc:
Analogously to Reference Example 16, the title compound is obtained starting from 3 g
(11.57 mmol) of 2(R)-[1 '(S)-(trifluoroacetylamino)-2'-phenylethyl]-oxirane fromReference Example 16d) and 2.3 g (12.15 mmol) of tert-butyl-3-isobutyl-carbazate(preparation: J. Chem. Soc. Perkin I, 1712 (1975)) after chromatographic purification on
silica gel with methylene chloride/ether (20: 1). tRet(I)-24.7 min, Rf(W)=0.36.
Reference Example 58: Acetvl-Val-rPheNNLeul~(N-(2(R.S)-carbamovl-3-phenvl~
~ropionvl)-Val): ~ -
Analogously to Reference Example 37, the title compound is obtained (in the form of 2
diastereoisomers distinguishable by HPLC) from 140 mg (0.3 mmol) of acetyl-(L)-Val-
[PheNNLeu]-H.2HCl, 132 mg (0.45 mmol) of N-(2(R,S)-carbamoyl-3-phenyl-propionyl)-
~L)-valine (preparation: Synth., Struct., Funct., Proc. Am. Pept. Symp.,7th, 85, (1981)3,
199 mg (0.45 mmol) of BOP, 61 mg (0.45 mmol) of HOBt and 3.5 ml of 0.3M NMM in
DMF after precipitation from methylene chloride/DlPE and lyophilisation from dioxane.
FAB-MS: (M+H)~=667, tRet(I)=17.9 and 18.4 min, Rf(D)=0.33.
a) Acetvl-Val-rPheNNLeul-H.2HCl:
Analogously to Reference Example 2a), the tide compound is obtained starting from
230 mg (0.46 mmol) of acetyl-(L)-Val-[PheNNLeu]-Boc after lyophilisation.
tRet(I)=10.5 min, Rf(D)=0.38.
b) Acetyl-Val-rPheNNLeul-Boc:
Analogously to Reference Example 37, the title compound is obtained from 250 mg
(0.71 mmol) of H-[PheNNLeu]-Boc from Reference Example 57c), 170 mg (1.07 mmol) of
N-acetyl-(L)-valine, 471 mg (1.07 mmol) of BOP, 144 mg (1.07 mmol) of HOBt and
7.1 ml of 0.3M NMM in DMF after precipitation from methylene chloride by the addition
of DIPE and Iyophilisation from dioxane. FAB-MS: (M+H)+i-~93, tRet(I)=20.5 min,
211'~7
- 139-
R,(D)=0.59.
Reference Example 59: N-Morpholinocarbonvl-(L)-Val-~PheNNLeul (N-(3-(tetrazol- 1-
vl)-propionvl)-Val~:
Analogously tO Reference Example 37, the title compound is obtained (in the form of 2
diastereoisomers distinguishable by HPLC) from 100 mg (0.19 mmol) of N-morpholino-
carbonyl-(L)-Val-~PheNNLeu]-H-2HCl, 67 mg (0.38 mmol) of N-(3-(tetrazol-1-yl)-
propionyl)-(L)-valine from Reference Example 44a, 124 mg (0.28 mmol) of BOP, 38 mg
(0.28 mmol) of HOBt and 2.1 ml of 0.3M NMM in DMF after precipitation from
methylene chloride by the addition of DIPE and Iyophilisation from dioxane. FAB-MS:
(M+H)+=687, tRet(I)=15.2 and 15.4 min, Rf(D)=0.25.
a) N-MorPholinocarbonvl-(L)-Val-rPheNNLeul-H.2HCl:
Analogously to Reference Example 2a), the tide compound is obtained starting from 279
mg (0.49 mmol) of N-morpholinocarbonyl-~L)-Val-[PheNNLeu]-Boc after lyophilisation.
FAB-MS: (M+H)t=464, tRe,(II)=30.3 min, Rf(D)=0.46.
b) N-MorPholinocarbonvl-(L)-Val-rPheNNLeul-Boc:
Analogously to Reference Example 37, the tide compound is obtained from 250 mg
(0.71 mmol) of H-~PheNNLeu~-Boc (from Reference Example 57c)), 265 mg (1.07 mmol)
of N-morpholinocarbonyl-(L)-valine from Reference Example 7a), 471 mg (1.07 mmol) of
BOP, 144 mg (1.07 mmol) of HOBt and 7.1 ml of 0.3M NMM in DMF after precipitation
from methylene chloridelhexane and lyophilisation from dioxane. FAB-MS: (M+H)+=564,
tRet(l)=21.5 min, Rf(K)=0.69.
Reference Example 60: N-TIifluoroacetY!-rPhe~Leul (N-(2(R.S)-carbamoyl-3-Phenyl-
ProPionyl)-(L)-Val):
Analogously to Referenoe Example 37, the title compound is obtained (in the form of 2
diastereoisomers distinguishable by HPLC) from 136 mg (0.32 mmol) of N-trifluoro-
acetyl-~PheNNLeu]-H.2HCl, 142 mg (0.49 mmol) of N-(2(R,S)-carbamoyl-3-phenyl-prop-
ionyl)-(L)-valine (preparation: Synth., Struct., Funct., Proc. Am. Pept. Symp.,7th, 85,
(1981)), 215 mg (0.49 mmol) of BOP, 66 mg (0.49 mmol) of HOBt and 3.5 ml of 0.3MNMM in DMF after chromatographic purification on silica gel with chloroform/methanol
(15:1), precipitation of the product-containing fractions from methylene chloride/DlPE
and lyophilisation from dioxane/tert-butanol. FAB-MS: (M+H)+=622, tRet(I)=21.6 and
22.0 min, Rf(K)=0.26.
~ ~ V ,
211~ 7
- 140-
a) N-Trifluoroacetyl-lPheNNLeul-H-2HCI:
Analogously to Reference Example 2a), the title compound is obtained starting from
300 mg (0.67 mmol) of N-trifluoroacetyl-[PheNNLeu]-Boc from Reference Example 57d)
after Iyophilisation. Rf(W)<0.1.
Reference ExamPle 61: Z-(L)-Val-rPheNNNlel (N-(2(R,S)-(N-(2-moIpholinoethvl)-
carbamoYI)-3-methvl~-butvrvl):
Analogously to Reference Example 37, the title compound is obtained (in the form of 2
diastereoisomers distinguishable by HPLC) from 100 mg (0.17 mmol) of Z-(L)-Val-
[PheNNNle]-H.2HCI, 69 mg (0.27 mmol) of 2(R,S)-(N-(2-morpholinoethyl)-carbamoyl)-
3-methylbutyric acid (isopropylmalonic acid N-(2-morpholinoethyl)monoamide), 119 mg
(0.27 mmol) of BOP, 36 mg (0.27 mmol) of HOBt and 2.1 ml of 0.3M NMM in DMF
after precipitation from methylene chloride/DIPE and Iyophilisation from dioxane.
FAB-MS: (M+H)+=725, tRet(I)=17.2 and 17.6 min, Rf(D)=0.56.
a) Z(L~-Val-rPheNNNlel-H.2HCI:
Analogously to Reference Example 2a), the dtle compound is obtained starting from
310 mg (0.53 mmol) of Z(L)-Val-[PheNNNle]-Boc after Iyophilisation. tRet(I)=16.4 min,
Rf(U)=0.25.
b) Z-(L~-Val-rPheNNNle~
Analogously to Reference Example 37, the title compound is obtained from 250 mg
(0.71 mmol) of H-[PheNNNle]-Boc, 268 mg (1.07 mmol) of Z-(L)-valine, 472 mg
(1.07 mmol) of BOP, 144 mg (1.07 mmol) of HOBt and 7.1 ml of 0.3M NMM in DMF
after chromatographic purification on silica gel with methylene chloride/methanol (40: 1)
and precipitation of the product-containing fractions from methylene chloride/~IPE.
tRet(I)=25.6 min, Rf(X)=0.17.
c) H-rPheNNNlel-Boc:
Analogously to Reference Example 17a), the title compound is obtained starting from
830 mg (1.85 mmol) of N-trifluoroacetyl-[PheNNNle]-Boc after precipitation from
methylene chloride/DIPE. tRet(I)=15.4 min, Rf(K)=0.54.
2112~7
- 141 -
d)_Trifluoroacetyl-lPheNNNlel-Boc:
Analogously to Reference Example 16, the title compound is obtained starting from 1 g
(3.86 mmol) of 2(R)-[l'-(S)-(trifluoroacetylamino)-2'-phenylethyl]-oxirane from
Reference Example 16d) and 720 mg (3.86 mmol) of tert-butyl-3-butyl-carbazate after
chromatographic purification on silica gel with methylene chloride/ether (20:1).tRet(I)--25.3 min, Rf(Q)=0.43.
e) tert-ButYl-3-butyl-carbazate:
Analogously to Reference Example 4b), the corresponding tert-butoxycarbonyl-hydrazone
(25 g, 99 %) is obtained from 18.0 g (136.2 mmol) of tert-butyl-carbazate and 12.3 ml
(136.2 mmol) of n-butanal in the form of a crude product, which is hydrogenated as
described in Reference Example 4a) in the presence of 10 g of 5% platinum on carbon at
4 atm hydrogen pressure. Chromatographic purification of the crude product on silica gel
with hexane/ethyl acetate (1:1) yields the title compound. Rf(N)=0.44,1H-NMR (200
MHz, CD30D). 0.92 (t, J=7Hz,3H), 1.43 (s, 9H), 1.30 to 1.50 (m, 4H), 2.75 (t, J=7Hz,
2H).
f) 2(R,S)-(N-(2-Mo~holinoethvl~-carbamovl)-3-methvlbutvric acid
Analogously to l~eference Example 9b) there is obtained from 7 g (43.7 mmol) of racemic
isopropylmalonic acid monomethyl ester (Chem. Ber. 119, 1196 (1986)), 6.3 ml
(48.1 mmol) of aminoethyl-morpholine, 6.6 ml (43.7 mmol) of cyanophosphonic aciddiethyl ester and 12.8 ml (91.8 mmol) of triethylamine in DMF, 2(R,S)-(N-(2-morpholino-
ethyl)-carbamoyl)-3-methyl-butyric acid methyl ester (isopropylmalonic acid N-morpho-
linoethylamide methyl ester). This is stirred for 5 h in a mixture of 28 ml of 2N sodium
hydroxide solution and 28 ml of dioxane at RT, acidified with 2N hydrochloric acid and
fully concentrated by evdporation. The residue is digested with ethanol, filtered off, and
concentration by evaporation of the filtrate yields the title compound. lH-NMR (200
MHz, CD30D): 0.95 and 1.00 (2d, J=7H, 6H), 2.25 (m, 4H), 2.70 (m, 6H), 2.75 (d, J=8Hz,
lH), 3.45 (m, 2H), 3.75 (m, 4H).
Reference Example 62: Z-(L)-Val-rPheNNNlel (N-(3-(tetrazol-1-v!)-propionvl)-Val):
Analogously to Reference Example 37, the title compound is obtained (in the form of 2
diastereoisomers distinguishable by HPLC) from 100 mg (0.18 mmol) of Z-~ Val-
[PheNNNle]-H.2HCI (from Reference Example 61a)), 65 mg (0.27 mmol) of N-(3-
(tetrazol-l-yl)-propionyl)-(L)-valine from Reference Example 44a, 119 mg (0.27 mmol) of
BOP, 36 mg (0.27 mmol) of HOBt and 2.1 ml of 0.3M N-methylmorpholine in DMF after
,. . ~ ,-. - ..... . : . - .:
~,:. ~ .. . ~. -- - . . . . .
21.~2~7
- 142-
precipitation from methylene chloride/DIPE and lyophilisation from dioxane/tert-butanol.
FAB-MS: (M+H)+=708, tRet(I)=20.3 and 20.6 min, Rf(D)=0.43.
Reference Example 63: Z-(L)-Val-rPheNNNlel~(N-(2(R.S)-(N-(2-pvridvlmethvl)-
carbamoyl)-3-methvl)-butvryl) (dibenzenesulfonate):
Analogously to Reference Example 37, the tide compound is obtained in the form of the
free amine from 95 mg (0.17 mmol) of Z-(L)-Val-[PheNNNle]-H.2HCl from Reference
Example 61a), 60 mg (0.26 mmol) of (R,S)-isopropylmalonic acid N-(2-picolyl)-mono-
amide, 113 mg (0.26 mmol) of BOP, 35 mg (0.26 mmol) of HOBt and 2.0 ml of 0.3M
NMM in DMF after chromatographic purification on silica gel with methylene chloride/-
methanol (15:13. The free amine is dissolved in methylene chloride, 2 equivalents of
benzenesulfonic acid are added, and precipitation is effected by the addition of DIPE.
Lyophilisation from tert-butanol yields the dibenzenesulf~nate salt (in the forrn of 2 dia-
stereoisomers distinguishable by HPLC). FAB-MS: (M+H)+=703, tRet(I)=17.7 and
18.0 min, Rf(D)=0.54.
a) IsopropYlmalonic acid N-(2-Picolvl)monoamide:
10.6 ml (103 mmol) of N-methylmorpholine are added to a solution of 15 g (93.6 mmol)
of isopropylmalonic acid monomethyl ester (preparation: Chem. Ber. 119, 1196 (1986)) in
150-ml of THF and subsequently 13.5 ml (103 mmol) of isobutyl chloroformate are added
dropwise thereto. After 30 min- 15.3 ml (150 mmol) of 2-picolylamine are added and the
resulting suspension is stirred for 2 h. The reaction mixture is diluted with lN sodium
hydr~xide solution and water and washed with methylene chloride, and the organic phase
is filtered through cotton wadding and concentrated by evaporation. Crystallisation of the
residue yields isopropylmalonic acid N-(2-picolylamide) methyl ester, which is hydro-
lysed in 2N sodium hydroxide solution and dioxane as described in Reference Example
61f) to yield the title compolmd. tRet(II)=16.0 min.
Reference ExamPle 64: Z-(L)-Val-~RheNN(p-F)Phel (N-(3-(tetrazol-1-vl)-propionYl)-(L)-
Val)(benænesulfonate):
Analogously to Reference Example 37, the title compound is obtained in the form of the
free amine from 100 mg (0.16 mmol) of Z-(L)-Val-[PheNN(p-F)Phe]-H from ReferenceExample 22a), 59 mg (0.25 mmo1) of N-(3-(tetrazo1-1-yl)-propionyl)-(L)-valine from
Reference Example 44a, 109 mg (0.25 mmol) of BOP, 33 mg (0.25 mmol) of HOBt and
1.19 ml of 0.3M N-methylmorpholine in DMF after precipitation from methylene
chloride/DIPE. The free amine is dissolved in methylene chloride/methanol, 1 equivalent
2112~7
- 143-
of benzenesulfonic acid is added, and precipitation is effected by adding hexane. Lyophil-
isation from tert-butanol yields the title compound in the form of the benænesulfonate
salt. FAB-MS: (M+H)+=760, tRet(I)=21.6 min, Rf~B)=0.49.
Reference Example 65: MethYlsulfonyl-rPheNNPhel (N phenvlacetyl-(L)-Val):
132 mg (0.28 mmol) of methylsulfonyl-1PheNNPhe]-H~2HCl are reacted analogously to
Reference Example 7 with 197 mg (0.84 mmol) of N-phenylacetyl-(L~-valine (prepara-
tion: Mem. Tokyo Univ. Agric. 20,51 (1978)),317 mg (0.84 mmol) of HBTU and 0.23 ml
(1.67 mmol) of triethylamine in DMF to yield the dtle compound after precipitation from
methanol by the addition of ether. FAB-MS: (M+H)+=581, tRet(I)=20.2 min, Rf(B)=0.64.
a) MethYlsulfonvl-rPheNNPhel-H-2HCI:
Analogously to Reference Example 2a), the dde compound is obtained starting from130 mg (0.28 mmol) of methylsulfonyl-[PheNNPhe]-Boc aftçr Iyophilisation. FAB-MS:
(M+H)+=364, tRet(II)--28.5 min, Rf (K)=0.56.
b) MethvlsulfonYl-rPheNNPhel-Boc:
Analogously to Reference Example 16a), the dde compound is obtained in the forrn of a
diastereoisomeric mixture in a ratio of 4: 1 starting from 1.1 g (4.56 mm~l) of 2(R)-[l '(S)-
(methylsulfonylamino)-2'-phenylethyl~oxirane and 1.11 g (5.02 mmol) of tert-butyl-3-
benzyl-carbazate (preparadon: J. Chem. Soc. Perkin I, 1712 (1975)). By crystallisadon
from methylene chloridelhexane the ratio in favour of the 2S-diastereoisomer is improved
to 10:1. FAB-MS: (M+H)+=464, tRet(I)=21.3 min, Rf(N)=0.26.
c) 2(R)-rl'(S)-(MethYlsulfonylamino)-2'-phenylethvlloxirane: ~'
2.36 g (13.6 mmol) of methanesulfonic acid anhydride and 1.88 ml (13.6 mmol) of
triethylamine are added at 0C to a solution of 1 g (6.8 mmol) of 1-phenyl-3-buten-2(S)-
amine from Reference Example 16b) in 10 ml of methylene chloride and the mixture is
stilred for 1 h. The reaction mixture is washed with water and saturated sodium hydrogen
carbonate soludon and the organic phase is filtered through cotton wadding and concen- - ~ -~
trated by evaporadon to yield 2(S)-methylsulfonylamino-l-phenyl-3-butene. 1 g ~ -
(4.4 mmol) of that crude product is dissolved in 30 ml of methylene chloride,3.05 g
(17.7 mmol) of 4-chloroperbenzoic acid are added at RT and stirring is carried out for
18 h. The reacdon soludon is washed 5 times with 10 % aqueous sodium sulfite soludon,
filtered through cotton wadding and fully concentrated by evaporation. According to
lH-NMR the crude product contains both the (2R)- and the (2S)-epimer in a ratio of 4: 1.
.~.. : . : - ' - ' " '
2112~7
- 144-
lH-NMR (200 MHz, CD30D): 2.30 and 2.52 (2 s, together 3H), 2.6 to 3.2 (m, SH), 3.55
(m, lH),7.32 (m, SH).
Reference Example 66: MethoxYcarbonvl-(L)-Val-rPheNNLeul (N-methoxvcarbonYl-
(L)-Val):
Analogously to Reference Example 37, the tide compound is obtained from 200 mg
(0.55 mmol) of H-[PheNNLeu]-H.3HCl (from Reference Example 13a)), 291 mg
(1.66 mmol) of N-methoxycarbonyl-(L)-valine (preparation: Chem. Lett. 705, (1980)),
735 mg (1.66 mmol) of BOP, æs mg (1.66 mmol) of HOBt and 11 ml of 0.3M NMM in
DMF after precipitation from rnethylene chloride/DD'E and Iyophilisadon from dioxane.
FAB-MS: (M~H)+=566, tRet(I)=18.6 min, Rf(U)=0.33.
Reference Example 67: Methoxycarbonvl-(L)-Val-rPheNN(~F)Phel (N-methoxY-
carbonvl-(I,)-Val):
Analogously to Reference Example 37, dhe tide compound is obtained from 200 mg
(0.48 mmol) of H-~PheNN(p-F)Phe~-H-3HCl (from Reference Example 42b)), 255 mg
(1.45 mmol) of N-methoxycarbonyl-(L)-valine (preparation: Chem. Lett. 705, (1980)),
643 mg (1.45 mmol) of BOP, 196 mg (1.45 mmol) of HOBt and 9.7 ml of 0.3M NMM in
DMF after precipitation from methylene chloride/DIPE and lyophilisation f~om dioxane.
FAB-MS: (M+H)+=618, tRet(l)=19.5 min, Rf(U)=0.22.
R rence Example 68: MedhoxYcarbonyl-(O-Val-rPheNN(p-CN)Phel~(N-methoxv-
carbonYl-(L)-Van:
Analogously to Reference Example 37, ~e title compound is obt~ined from 200 mg
(0.48 mmol) of H-[PheNN(p-CN)Phe}-H-3HCl (from Reference Example 47a)), 250 mg
(1.43 mmol) of N-methoxycarbonyl-(L)-valine (preparadon: Chem. Lett. 705, (1980)),
631 mg (1.43 mmol) of BOP, 193 mg (1.43 mmol) of HOBt and 9.5 ml of 0.3M NMM in
DMF after chromatographic purification on silica gel with methylene chloride/methanol
(15:1) and lyophilisation of the product-containing fractions from dioxane. FAB-MS:
(M+H)+=625, tRet(I)=18 min, Rf(U)=0.31.
Reference Example 69: Z-(L)-Val-r(p-F)PheNN(p-F)phel~ (N-(2(R.S)-(N-(2-morPholino-
ethyl)-carbamovl)-3-methyl)-butYrYl):
Analogously to Reference Example 18, 23.0 mg (0.089 mmol) of 2(R,S)-(N-(2-morpho-
linoethyl)-carbamoyl)-3-methylbutyric acid (Reference Example 61 f)) and 45 mg
(0.081 mmol) of Z(L)-Val-[(p-F)PheNN(p-F)Phe]-H (Reference Example 32) are reacted
. .
. - . ..
2112~7
- 145-
with 33.8 mg (0.089 mmol) of HBTU in 0.76 ml of 0.25M NMM/CH3CN to yield the title
compound which is reprecipitated with DMF/DIPE: TLC Rf(P)=0.42; FAB-MS
(M+H)+=795.
Reference Example 70: Z-(L)-Val-r(p-F)PheNN(p-F)Phel~(N-(2(R~S)-(N-(2-pvridvl-
methyl)-carbamoyl)-3-methvl)-butyrvl):
Analogously to Reference Example 18, 21.0 mg (0.089 mmol) of rac. isopropylmalonic
acid N-(2-picolyl)amide (Reference Example 63 a)) and 45 mg (0.081 mmol) of Z-(L)-
Val-[(p-F)PheNN(p-F)Phe]-H (Reference Example 32) are reacted with 33.8 mg
(0.089 mmol) of HBTU in 0.76 ml of 0.25M NMM/CH3CN to yield the title compound
which is reprecipitated with DMF/DIPE: TLC Rf(P)=0.52; FAB-MS (M+H)+=773.
Reference Example 71:
The following compounds can be prepared analogously to one of the afore-mentioned
processes:
a) Z(L)-Val-~(p-E7)PheNN(p-F)Phe] ((L)-Val) (N-morpholinocarbonyl-Gly);
b) N-morpholinocarbonyl-(L)-Val-~(p-F)PheNN(p-F)Phe] ((L)-Val)~(N-morpholino-
carbonyl-~;ly);
c) N-(quinoline-2-carbonyl)-(L)-Asn-~PheNN(p-E;)Phe] ((L)-Val)-Z;
d) N-(morpholinosu}fonyl)-(L)-Val-[PheNNLeu] (N-(morpholinosulfonyl)-(L)-Val);
e) N-(Quinoline-2-carbonvl)-(Ll-Asn-rPheNNChal ((L)-Val)-Z (= 1-~2(S)-hydroxy-3(S)-
(N-(quinoline-2-carbonyl)-(L)-asparagyl)amino-4-phenyl-butyl]-1-[cyclohexylrnethyl]-2- ~;
~N-(benzyloxycarbonyl)-(L)-valyl]-hydrazine):
Under a nitrogen atmosphere, 27 mg (0.107 mmol) of Zvaline in 0.59 ml of a 0.3M
solution of NMM in DMF are activated with 47 mg (0.107 mmol) of BOP and 14 mg
(0.107 mmol) of HOBT and, after 15 min, 50 mg (0.089 mmol) of 1-[2(S)-hydroxy-3(S)-
(N-(quinoline-2-carbonyl)-(L)-asparagyl)amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-
hydrazine are added. The mixture is stirred for 18 h at RT and concentrated by evaporation
under HV. The residue is dissolved in methylene chloride and washed with saturated
NaHCO3 solution, water and brine, the aqueous phases are extracted twice with methylene
chloride, and the organic phases are dried with Na2SO4 and concentrated by evaporation.
Column chromatography (SiO2, ethyl acetate/ethanol 100:3) yields the pure title
compound: TLC Rf(D')=0.21; tR~"(V)=16.7 min; FAB-MS (M+H)+=794.
The starting material is prepared as follows:
~: ,
.~
.... .
,~" ~
2112~7
- 146-
i) l-r2(S)-Hvdroxv-3(S)-(N-(quinoline-2-carbonvl)-(L)-asPara ~vl)amino-4-Phenyl-but
1 -~c~lclohexvlmethyll-h~drazine
Analogously to Example 27 (see below), 921 mg (1.39 mmol) of 1-[2(S)-hydroxy-3(S)-
(N-(quinoline-2-carbonyl)-(L)-asparagyl) amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-
2-[tert-butoxy-carbonyl]-hydrazine (Example 29, see below) in 37 ml of formic acid are
reacted to form dle title compound and used direcdy in the next step.
f) N-(Quinoline-2-carbonyl)-(L)-Asn-[PheNN(p-F)Phe]. (N-(methoxycarbonyl)-(L)-Val)
Example 1: 1-r2(S)-Acetoxv-3(S~-(N-(2-methoxyethoxYcarbonvl)-(L)-valvl)amino-4-
l-butYll- l-rcvclohexvlmethvll-2-~N-(2-medhoxYethoxYcarbonvl)-(L)-valYn -
hvd~azine:
Under a nitrogen atmosphere, 200 mg (0.29 mmol) of N-(2-medloxy-ethoxycarbonyl)-(L)-Val-[PheNNCha] (N-(2-medhoxy-edhoxycarbonyl)-(L)-Val) in 4 ml of THF and 60 ~
(0.43 mmol) of triethylamine are acetylated with 40 ~1 (0.43 mmol) of acetic anhydride for
3 h at RT in the presence of 0.5 mg (0.003 mmol) of DMAP. The reaction mixture is
partitioned between 3 portions of methylene chloride, water, saturated NaHCO3 solution
and brine. The tide compound is obtained from the organic phases after drying with
Na2SO4, concentrating by evaporation and column chromatography (SiO2, methylene
chloride/methanol 30:1): TLC Rf(Z)~0.17; tRe~ -22.5 min; FAB-MS (M+H)+=736.
The ~tarting materials are prepared as follows:
a)N-(2-Methoxv-ethoxvcarbonYl)-(L)-Val-rPheNNChal (N-(2 methoxy-ethoxvcar-
bonYl)-(L)-Val): ~ .
Analogously to Reference Example 2, 820 mg (3.74 mmol) of N-(2-methoxy-ethoxycar-
bonyl)-(L)-valine are activated with 1.65 g (3.74 mmol) of BOP and 505 mg (3.74 mmol)
of HOBT in 25 ml of a 0.3M solution of NMM in DMF and, after 10 min, reacted wi~500 mg (1.25 mmol) of H-[PheNNCha]-H (hydrochloride salt) (see Reference Example10a) for 18 h. The reaction mixture is concentra.ed by evaporation under HV, and the
residue is dissolved in CHCI3 and washed with 10 % citric acid solution, saturated
NaHCO3 solution and brine. The aqueous phases are extracted with 2 portions of C~ICl3,
and the organic phases are dried with Na2SO4 and concentrated by evaporation. Column
chromatography (SiO2, CHCl3/MeOH 30: 1) and precipitation with hexane from a CH2Cl2
solution yields the tide compound: TLC Rf(T)=0.37; tRet(I)=21.5 min; FAB-MS
':~ ~ ' : ~ ,; ' ' , ` '`"'.: ~ ~ :
, , : . ~ i
- 2112~47
- 147-
(M~H)+=694.
b) Chloroformic acid (2-methoxv-ethyl~ ester:
Under a nitrogen atmosphere, 13.3 ml (168 mmol) of 2-methoxy-ethanol are added
dropwise at from 0 to 5C to 100 ml (202 mmol) of a 20 % solution of phosgene intoluene, and the mixture is stirred for 90 min at 0C and for 18 h at RT to complete the
reaction. The reaction mixture is extracted with water, and the organic phase is filtered
through cotton wadding and concentrated by evaporation: IR (CH2Cl2): inter alia 3055w,
2995w, 2935w, 2895w, 2825w, 1775s, 1167s, 1127s; IH-NMR (200 MHz, CDCl3): 3.38
(s, 3 H), 3.64 and 4.44 (2t, J=S Hz, each 2 H).
c) N-(2-MethoxY-ethoxvcarbonvl)-(L~-valine: ''
A solution of 3.06 g (22.1 mmol) of chloroformic acid (2-methoxy-ethyl) ester in 18 ml of
dioxane is added to 2.59 g (22.1 mmol) of L-valine in 26.4 ml of 2N NaOH and themixture is then stiIred for 18 h at RT. The reaction mixture is extracted with chloroform,
and the inorganic phase is acidified with 4N HCl and extracted again with chloroform.
The chloroform phase last obtained is dried and concentrated by evaporation to yield the
tide compound: lH-NMR (200 MHz, CDCI3): 0.92 and 0.99 (2d, J--7 Hz, 6 H), 2.2 (m,
lH), 3.38 (s, 3 H), 3.59 and 4.24 (2m, each 2 H), 4.3 (m, 1 H),5.4 (d, J=9 Hz, HN), 8.5 ~-
(sb, 1 H).
Example 2: 1-r2(S)-Acetoxv-3(Sl-(N-(methoxYcarbonYl)-(L)-valYl)amino-4-phen
butvll-l-~cvclohexv!methY11- 2-~N-(methoxvcarbonvl)-(L)-va1vll-hYdrazine:
Analogously to Example 1, 200 mg (0.33 mmol) of N-(methoxycarbonyl)-(L)-Val-
[PheNNCha]~(N-(methoxycarbonyl)-(L)-Val) in 4 ml of THF and 68 ,ul (0.50 mmol) of
triedlylamine are reacted widl 46 ~,11 (0.50 mmol) of acetic anhydride in the presence of
1.2 mg (0.01 mmol) of DMAP. Precipitation with DIPE from a concentrated solution of
the crude product in medhanol yields the pure tide compound: TLC Rf(A')=0.42;
tRet(I)=22.6 min; FAB-MS (M+H)+=648.
The starting material is prepared as follows:
a) N-(Methoxvcarbonvl)-(L)-Val-lPheNNChal (N-(methoxvcarbonYl)-(L)-Val) (=
1-~2(S)-hvdroxy-3(S)-(N-(methoxYcarbonYl)-(L)-valYI)-amino-4-phenYl-butvl~ rcY
hexvlmethvll-2-rN-(methoxvcarbonYl)-(L)-valvll-hydrazine):
Analogously to Reference Example 2, 1.47 g (8.4 mmol) of N-(methoxycarbonyl)-(L)-
valine are activated with 3.71 g (8.4 mmol) of BOP and 1.13 g (8.4 mmol) of HOBT in
bi"','
2112~47
- 148-
54 ml of a 0.3M solution of NMM in DMF and, after 15 min, reacted with 1.12 g
(2.8 mmol) of H-[PheNNCha]-H (hydrochloride salt) (see Reference Example 10a) for
18 h. The reaction mixture is concentrated by evaporation using a RE at 50C (~ brown
residue), and the residue is dissolved in methylene chloride and washed twice with satur-
ated NaHCO3 solution and brine. The aqueous phases are extracted with 2 poItions of
methylene chloride and the organic phases are dried with Na2SO4 and concentrated by
evaporation. Filtration through silica gel (methylene chloride/methanol 15:1) and precip-
itation twice with DIPE from a concentrated methylene chloride solution yields the tide
compound: TLC Rf(U)=0.33; tRet(I)=21.5 min; FAB-MS (M+H)+=606.
The starting material is prepared as follows:
b) ~ ~MethoxYcarbonvl~-(L)-valine:
5.67 g (60 mmol) of chloroformic acid methyl ester are added to 7.0 g (60 mmol) of
L-valine in 100 ml of 2N NaOH and 30 ml of dioxane ( ~ exothermic reaction) and the
mixture is then stirred for 18 h at RT. The reaction mixture is extracted with methylene
chloride and the aqueous phase is acidified with 27 ml of 4N HCl and extracted again with
methylene chloride. Drying and concentration by evaporation of the latter methylene
chloride phase yields the title compound: tRet(I)--7.2 min; lH-NMR (200 MHz, CD30D):
0.96 (t, J=7 Hz, 6 H), 2.16 (m, l H),3.67 (s, 3 H), 4.06 (m, 1 H),7.07 (d, J=8 Hz,
HNp~ddly e~ch~ged)
Exam~le 3~ 2(S)-(2-PYridvlcarbonvl)oxY-3(S)-(N-(methoxYcarbonvl)-(L)-valvl)amino-
~Phenvl-butY!l- l -~cvclohexvlmethYn-2-1 N-(methoxvcarbonYl)-(L)-valYll -hvdrazine
Under a nitrogen atmosphere,56111 (0.4 mmol) of 1-chloro-N,N,2-trimethyl-1-propen-
amine (B. Haveaw~, A.~ Dekoker, M. Rens, AR. Sidani, J. Toye, L. Ghosez, MMurakami,
M. Yoshioka, and W. Nagata, Organic Syntheses 59, 26 (1980)) are added at 0C to 81 mg
(0.66 mmol) of 2-picolinic acid in 4 ml of methylene chloride. After 45 min at RT, 1.3 ml
of pyridine, 100 mg (0.165 mmol) of N-(methoxycarbonyl)-(L)-Val-[PheNNCha]~ (N-
(methoxycarbonyl)-(L)-Val) (Example 2 a) and a spatula dp of DMAP are added and the
mixture is stirred for 18 h at RT. The dark reacdon mixture is partidoned between 3
portions of methylene chloride, 2 portions of saturated NaHCO3 soludon, water and brine.
Column chromatography (SiO2, ethyl acetate) of the concentradon residue of the
methylene chloride phase dried with Na2SO4 yields the pure tide compound: TLC:
Rf(O)=0.23; tRet(I)=22.5 min; FAB-MS (M+H)+=711.
, `' , ~ ' , .
.; . .
- 2112047
- 149-
Example 4: The following are prepared in accordance with one of the afore-mentioned
processes:
a) 1-[2(S)-propionyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-1-
[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl] -hydrazine
b) 1-[2(S)-butyryloxy-3(S)-(N-(methoxycarbonyl)-~L)-valyl)amino-4-phenyl-butyl]-1-
[cyclohexylmethyl]-2-1N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
c) 1-[2(S)-pentanoyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-1-
[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl~-hydrazine;
d) 1-[2(S)-octanoyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-1-
[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
e) l-t2(S)-decanoyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-1- ~ ~ ;
[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-hydrazine; . :~ .
f) 1-[2(S)-dodecanoyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]- : - -
l-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
g) 1-~2(S)-pivaloyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-1-
[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
h) 1-[2(S)-(2-furylcarbonyl)oxy-3(S)-(N-(methoxycarbonyl)-(L3-valyl)amino-4-phenyl-
butyl]-l-[cyclohexylmethyl]-2-~N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
i) 1-[2(S)-(4-imidazolylcarbonyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-
phenyl-butyl]-l-lcyclohexylmethyll-2-~N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
j) 1-~2(S)-(4-imidazolylacetyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)arnino-4-phenyl-butyl]-l-~cyclohexylmethyl]-2-~N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
k) 1-~2(S)-(3-(~imidazolyl)-propionyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-
4-phenyl-butyl]- 1-~cyclohexylmethyl]-2-~N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
1) 1-~2(S)-benzoyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-1-
~cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
m) 1-~2(S)-(2-pyridylacetyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-
butyl]-l-~cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
n) 1-~2(S)-(3-(pyridin-2-yl)-propionyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-
4-phenylbutyl)]- 1-~cyclohexylmethyl]-2-~N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
o) 1-~2(S)-(quinolin-2-ylcarbonyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-
phenyl-butyl]-l-~cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
p) 1-[2(S)-(aminoacetyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-
butyl]-l-~cyclohexylmethyl]-2-~N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
q) 1-~2(S)-(N-methylaminoacetyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-l-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
2~12~7
- 150-
r) 1-[2(S)-(N,N-dimethylaminoacetyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-
4-phenyl-butyl]-1 -[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
s) 1-[2(S)-(N-benzyloxycarbonyl-N-methylaminoacetyl)oxy-3(S)-(N-(methoxycarbonyl)-
(L)-valyl)amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-
(L)-valyl]-hydrazine;
t) 1-~2~S)-prolyloxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-1-
[cyclohexylmethyl]-2-~N-(methoxycarbonyl)-(L)-valyl]-hydrazine;
u) 1-[2(S)-(4-morpholinomethylbenzoyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)-
amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-
hydrazine;
v) 1-[2(S)-(4-chloromethylbenzoyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-~phenyl-butyl]-l-lcyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-hydrazine;w) 1-[2(S)-(3-carboxypropionyl)oxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-
phenyl-butyl]-l-[cyclohexylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-hydrazine.
Example 5: From the title compounds of Reference Examples 1 to 70, there are prepared
in accordance with one of the above-mentioned processes the monoacetylated derivatives ~;
that contain a 2(S)-acetoxy group instead of the free 2(S)-hydroxy group in the relevant
central divalent radical derivatised from butan-2(S)-ol and designated -[PheNNPhe],
-[PheNNCha], -[PheNNLeu], -[PheNNNle], -[PheNN(p-F)Phe], -[(p-F)PheNN(p-F)Phe],
-[PheNN(p-CN)PheJ or -[ChaNNLeu].
Example 6: The following compounds are prepared in accordance with one of the above-
mentioned processes (the staTting materia1s are indicated in square brackets ([]) (e.g. the
respective Reference Example of which the tide compound is used as starling material:
a) 1-[2(S)-(2-furylcarbonyl)oxy-3(S)-(N-(benzyloxycarbonyl)-(L)-valyl)-amino-
4-phenylbutyl)]-1-[benzyl]-2-[N-(benzyloxycarbonyl)-(L)-valyl]-hydrazine [from the tide
compound of Reference Example 2 and furan-2-carboxylic acid chloride];
b) 1-[2(S)-pivaloyloxy-3(S)-(N-(benzyloxycarbonyl)-(L)-valyl)-amino-4-phenyl-butyl]-
l-[cyclohexylmethyl]-2-[N-(benzyloxycarbonyl)-(L)-valyl]-hydrazine [from the tit1e
compound of Reference Example 11 and pivalic acid anhydride];
c) 1-[2(S)-(N-methylaminoacetyl)oxy-3(S)-(N-(morpholinocarbonyl)-(L)-valyl)-amino-
4-phenyl-butyl]- 1 -[p-fluorophenylmethyl]-2- [N-~benzyloxycarbonyl)-(L)-valyl] -hydrazine
[from the tide compound of Reference Example 18 and N-benzyloxycarbonyl-N-medhyl-
aminoacetic acid with subsequent hydrogenolysis of the resulting 2(S)-(N-benzyloxy-
21120~7
- 151 -
carbonyl-N-methylaminoacetyl compound catalysed by Pd/C];
d) 1-[2(S)-(N-benzyloxycarbonyl-N-methylaminoacetyl)oxy-3(S)-(N-(benzyloxy-
carbonyl)-(L)-valyl)-amino-4-phenyl-butyl]-1-[p-fluorophenylmethyl]-2-[N-(N-(morpho-
linocarbonyl)-glycyl)-(L)-valyl]-hydrazine [from the tide compound of Reference
Example 24 and N-benzyloxycarbonyl-N-methylaminoacetic acid chloride];
e) 1-[2(S)-(N,N-dimethylaminoacetyl)oxy-3(S)-(N-(quinolin-2-ylcarbonyl)-(L)-aspart-
oyl)-amino-4-phenyl-butyl]- 1-[p-fluorophenylmethyl]-2-[tert-butoxycarbonyl]-hydrazine
[from the title compound of Reference Example 27 and dimethylaminoacetic acid
chloride];
f) 1-[2(S)-(2-pyridylcarbonyl)oxy-3(S)-(N-(benzyloxycarbonyl)-(L)-aspaTtoyl)-amino-4-
phenyl-butyl]- l-[p-fluorophenylmethyl]-2-[N-(benzyloxycarbonyl)-(L)-valyl]-hydrazine
[from the tide compound of Reference Example 28 and 2-pyridinecarboxylic acid
chloride);
g) 1-[2(S)-(4-(morpholinomethyl)benzoyl)oxy-3(S)-(N-(benzyloxycarbonyl)-(L)-valyl)-
amino-4-(p-fluorophenyl)-butyl]-1-[p-fluorophenylmethyl]-2-[tert-butoxycarbonyll-
hydrazine [from the title compound of Reference Example 31 and 4-morpholinomethyl-
benzoic acid by way of the acid chloride in the presence of N,N,2-trimethyl-1-chloro-
propen-(l)-arnine],
h) 1-[2(S)-benzoyloxy-3~S)-(N-(benzyloxycarbonyl)-a_)-valyl)-amino-4-(p-fluoro-
phenyl)-butyl]- l-[p-fluorophenylmethyl]-2-tN-(N-(2-pyridylmethyl)-N-methylaminocar-
bonyl)-(L)-valyl]-hydrazine [from the title compound of l~eference l~xample 33 and
benzoyl chloride];
i) 1-[2(S)-(~chloromethylbenzoyl)oxy-3(S)-(N-(benzyloxycarbonyl)-(L)-valyl3-amino-
~(p-fluorophenyl)-butyl]- l-[p-fluorophenylmethyl]-2-~N-(2(R,S)-carbamoyl-3-phenyl-
propionyl)-(L)-valyl]-hydrazine [from the tide compound of Reference Example 34 and
4-chloromethylbenzoic acid in the presence of N,N,2-trimethyl-1-chloropropen-(1)-
amine];
j) 1-12(S)-(imidazol-4-ylacetyl)oxy-3(S)-(N-acetyl-valyl)-amino-4-phenyl-butyl]-l-[benzyl]-2-[N-acetyl-valyll-hydrazine [from the dde compound of Reference Example
35 and 1-tritylimidazolyl-4-acetic acid (prepared from trityl chloride and 4-imidazolyl-
acetic acid in the presence of pyridine) in the presence of N,N,2-trimethyl-1-chloro-
propen-(l)-amine by way of the trityl-protected intermediate with subsequent acidolytic
removal of the trityl protecting group, e.g. using trifluoroacetic acid];
k) 1-[2(S)-(2 pyridylacetyl)oxy-3(S)-(N-(quinolin-2-ylcarbonyl)-valyl)-amino-4-phenyl-
butyl]-l-[benzyl]-2-[N-quinolin-2-yl-carbonyl-valyl]-hydrazine [from the title compound
of Reference Example 37 and 2-pyridineacetic acid in the presence of N,N,2-trimethyl-
2 1 1 ,~ 7
- 152-
l-chloropropen-(l)-amine];
1) 1-[2(S)-(3-pyridylacetyl)oxy-3(S)-(N-acetyl-(L)-valyl)-amino-4-phenyl-butyl]-1-
[cyclohexylmethyl]-2-[N-acetyl-(L)-valyl]-hydrazine [from the fftle compound of
Reference Example 38 and 3-pyridineacetic acid in the presence of N,N,2-trimethyl-1-
chloropropen-( 1 )-amine];
m) 1-[2(S)-(4-pyridylacetyl)oxy-3(S)-(N-(3-pylidylacetyl)-(L)-valyl)-amino-4-phenyl-
butyl]-l-[cyclohexylmethyl]-2-[N-(3-pyridylacetyl)-(L)-valyl]-hydrazine [from the tide
compound of Reference Example 39 and 4-pyridineacetic acid in the presence of N,N,2-
trimethyl-l-chloropropen-(l)-amine];
n) 1-[2(S)-(quinolin-2-ylcarbonyl)oxy-3(S)-(N-(N-(2-pyridylmethyl)-N-methylamino-
carbonyl)-(L)-valyl)-amino-4-phenyl-butyl]-1-[p-fluorophenylmethyl]-2-[N-(N-
(2-pyridylmethyl)-N-methylaminocarbonyl)-(L)-valyl]-hydrazine [from dhe tide
compound of Reference Example 43 and quinoline-2-carboxylic acid in the presence of
N,N,2-trimethyl-1-chloropropen-(1)-amine];
o) 1-l2(S)-(2-pyrrolidinylcarbonyl)oxy-3(S)-(N-(benzyloxycarbonyl)-(L)-valyl)-amino-
4-phenyl-butyl]- 1-[p-fluorophenylmethyl]-2-[N-(benzyloxycarbonyl)-(L)-valyl]-hydrazine
[from the tide compound of Reference Example 45 and proline in dhe presence of N,N,2-
trimethyl-l-chloropropen-(l~amine];
p) 1-[2(S)-propionyloxy-3(S)-(N-~benzyloxycarbonyl)-(L)-valyl)-amino4-phenyl-
butyl]-l-[p-cyanophenylmethyl]-2-[N-(benzyloxycarbonyl)-(L)-va}yl]-hydrazine [from dhe
tide compound of Reference Example 48 and propanoic acid anhydride];
q) 1-[2(S)-butyryloxy-3(S)-(N-(benzyloxycarbonyl)-(L)-isoleucyl)-amino-4-phenyl- butyl]-l-[isobutyl]-2-[N-(benzyloxycarbonyl)-(L)-isoleucyl]-hydrazine [from the tide
compound of Reference Example 49 and butyric acid anhydride];
- r) 1-[2(S)-pentanoyloxy-3(S)-(N-(isobutoxycarbonyl)-(L)-valyl)-amino-4-phenyl-butyl]-
l-Eisobutyl]-2-[N-(isobutoxycarbonyl)-(L)-valyl]-hydrazine [from dhe title compound of
Reference Example 50 and pentanoic acid chlo~ide];
s) 1-[2(S)-decanoyloxy-3(S)-(N-acetyl-valyl)-amino-4-phenyl-butyl]-1-[isobutyll-2-[N-
acetyl-valyl]-hydrazine [from the tide compound of Reference Example 52 and decanoic
acid in the presence of N,N,2-trimethyl-1-chloropropen-(1)-amine];
t) 1-[2(S)-dodecanoyloxy-3(S)-N-valyl-amino-4-phenyl-butyl]-1-[isobutyl]-2-[N-valyl]-
hydrazine [from the title compound of Reference Example 54, protected at the two free
valylamino groups by benzyloxycarbonyl, in the presence of N,N,2-trimethyl-1-chloro-
propen-(l)-amine with subsequent hydrogenolytic removal of the benzyloxycarbonylprotecting groups from the obtainable intermediate];
u) 1-[2(S)-(3-carboxypropionyl)oxy-3(S)-(N-(thiomorpholinocarbonyl)-(L)-valyl)-
: - . ~ .. ; . ~ .,
21:12~ ~7
- 153-
: ,
amino-4-phenyl-butyl]-1-[isobutyl]-2-[N-(thiomorpholinocarbonyl)-(L)-valyl]-hydrazine
lprepared from the htle compound of Reference Example 55 and succinic acid anhydride
in the presence of pyridine];
v) 1-[2(S)-(4-imidazolylacetyl)oxy-3(S)-(N-(benzyloxycarbonyl)-(L)-valyl)amino-4-
phenyl-butyl]-l-lisobutyl]-2-[N-(3-(tetrazol-1-yl)propionyl)-(L)-valyl]-hydrazine
[prepared from the tide compound of Reference ~3xample 57 and 1-trityl-4-imidazolyl-
acetic acid analogously to Example 6 j];
w) 1-[2(S)-(furan-2-ylcarbonyl)oxy-3(S~-(N-acetyl-(L)-valyl)amino-4-phenyl-butyl]-
l-[isobutyl]-2-[N-(2(R,S)-carbamoyl-3-phenyl-propionyl)-(L)-valyl]-hydrazine [prepared
from the title compound of R~eference Example 58 and furan-2-carboxylic acid chloride];
x) 1-[2(S)-pivaloyloxy-3(S)-(N-(benzyloxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-
l-[n-butyl]-2-[2(R,S)-(N-(2-morpholinoethyl)carbamoyl)-3-methylbutyryl]-hydrazine
[prepared from the title compound of Reference Example 61 and pivalic acid anhydride};
y) 1-[2(S)-(N-benzyloxycarbonyl-N-methylaminoacetyl)oxy-3(S)-(N-(benzyloxycar-
bonyl)-(L)-valyl)amino-4-phenylbutyl]-1-[n-butyl]-2-[N-(3-(tetrazol-1-yl)-propionyl)-
valyl]-hydrazine [from the tide compound of Reference Example 62 analogously to
Example 6 d];
z) 1-[2(S)-(N-methylaminoacetyl)oxy-3(S)-(N-(benzyloxycarbonyl)-(L)-valyl)amino-4-
phenyl-butyl]- 1 -[n-butyl]-2-[N-(2(R,S)-(N-(2-pyridylmethyl)-carbamoyl)-3-methyl)-
butyryl]-hydrazine [from dle title compound of Reference Example 63 by way of the
2(S)-N-benzyloxycarbonyl-N-methylaminoacetoxy analogue by hydrogenolysis in accord-
ance with Example 6 c].
Example 7: The following compounds are prepared in accordance with one of the above-
mentioned processes (the starting materials are given in square brackets (O) (e.g. the
respective Reference Example of which the titie compound is used as starting material)):
a) 1-[2(S)-(N,N-dimethylaminoacetyl)oxy-3(S)-((N-benzyloxycarbonyl)-(L)-valyl)-
amino-4-phenyl-butyl]-1-[p-fluorophenylmethyl]-2-[N-(3-(tetrazol-1-yl)-propionyl)-
valyl~-hydrazine [from the tide compound of Reference Example 64 and dimedhylamino-
acetic acid chloride];
b) 1-[2(S~-(pyridin-2-ylcarbonyl)oxy-3(S)-(N-methoxycarbonyl)-(L)-valyl)-amino-4-
phenyl-butyl]-l-[isobutyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-hydrazine [from the tide
compound of Reference Example 66 and 2-pyridinecarboxylic acid chloride].
c) 1-[2(S)-(4-morpholinomethylbenzoyl)oxy-3(S)-(N-methoxycarbonyl)-(L)-valyl)-
amino-4-phenyl-butyl]-1-[p-fluorophenylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-
- . -:
-- 2~12~ll7
- 154-
hydrazine [from the title compound of Reference Example 67 and 4-morpholinomethyl-
benzoic acid (by way of the acid chloride in the presence of N,N,2-trimethyl-1-chloro-
propen-~l)-amine)];
d) 1-[2(S)-benzoyloxy-3(S)-(N-methoxycarbonyl)-~L)-valyl)-amino-4-phenyl-butyl]-l-[p-cyanophenylmethyl]-2-[N-(methoxycarbonyl)-(L)-valyl]-hydrazine [from the title
compound of Reference Exarnple 68 and benzoic acid anhydride].
Example 8: 1-r2(S)-Hydroxv-3(S)-(N-allvloxYcarbonYl-(L)-valvl)amino-4-phenYlbut
1 -rcvclohexYlmethYn-2-rN-allYloxvcarbonvl-(L)-valvll-hvdrazine
Analogously to Reference EY.ample 2, the title compound is obtained from 500 mg
(1.25 mmol) of H-[PheNNCha]-H-3HCI from Reference Example 10a,753 mg
(3.74 mmol) of N-allyloxycarbonyl-(L)-valine, 1.65 g (3.74 mmol) of BOP, 505 mg
(3.74 mmol) of HOBt and 24.7 ml of 0.3M N-methylmorpholine in DMF after precipita-
tion twice from methylene chloride by the addition of DlPE and lyophilisation from
dioxane. FAB-MS: (M~H)+=658, tRet(I)=25.5 min, Rf(H)=0.44.
The starting compound is prepared in the following manner:
a) Allyloxycarbonyl-(L)-valine: The title compound is obtained in the form of a colourless
oil analogously to Reference Example lc) starting from 10 g (85.3 mmol) of (L)-valine
and 10.3 g (85.3 mmol) of allyl chloroformate. lH-NMR (200 MHz, CDCl3): 6.05 - 5.8
(m, lH), 5.35 (s, broad, lH),5.20 (m, 2H), 4.60 (d, broad, J=6 Hz, 2H), 4.33 (m, lH), 2.25
(m, lH), 1.00 (d, J=7 Hz, 3H), 0.93 (d, J=7 Hz, 3H).
Example 9: 1-l2(S)-Hvdroxv-3(S~-(N-ethoxvcarbonv!-(L)-valvl)amino-4-PhenvlbutYll-
1 -rcvclohexvlmethvll-2-rN-edloxvcarbonvl-(L)-valYn-hYdrazine
Anals)gously to Reference Example 2, the dde compound is obtained from 500 mg
(1.25 mmol) of H-lPheNNCha]-H-3 HCl from Reference Example 10a,708 mg
(3.74 mmol) of N-ethoxycarbonyl-(L)-valine, 1.65 g (3.74 mmol) of BOP, 505 mg
(3.74 mmol) of HOBt and 24.7 ml of 0.3M-N-medhylmorpholine in DMF after chromato-
graphic purificadon (SiO2, methylene chloride/diethyl ether (1:1)), precipitation from
methylene chloride by the addition of DIPE and lyophilisation from dioxane. FAB-MS:
(M+H)+=634, tRet(I)=24.2 min., Rf~H)=0.3.
The starting compound is prepared in the following manner:
-- 2~12~47
- 155-
a) N-Ethoxycarbonyl-(L)-valine: Analogously to Reference Example lc, the title
compound is obtained in the form of a colourless oil starting from 10 g (85.3 mmol) of
(L)-valine and 9.3 g (85.3 mmol) of ethyl chloroformate. lH-NMR (200 MHz, CDCl3~:
5.15 (d, broad, lH),4.32 (m, lH), 4.15 (q, J=7Hz, 2H), 2.25 (m, lH), 1.25 (t, J=7Hz,3H)
0.98 (d, J=7Hz, 3H), 0.92 (d, J=7Hz, 3H).
Example 10: 1-r2(S)-HydroxY-3(S)-(N-trifluoroacetYl-(L)-valYl)amino-4-phenvlbut
rcyclohexylmethyn-2-rN-trifluoroacetyl-(L)-valyll-hvdrazine
Analogously to Reference Example 2, the title compound is obtained from 500 mg
(1.25 mmol) of H-[PheNNCha]-H-3HCl from Reference Example 10a7798 mg
(3.74 mmol) of N-trifluoroacetyl-(L)-valine, 1.65 g (3.74 mmol) of BOP, 505 mg
(3.74 mmol) of HOBt and 24.7 ml of 0.3M N-methylmorpholine in DMF after chromato-
graphic purification (SiO2, methylene chloride/diethyl ether (1:1)), precipitation from
methylene chloride by the addition of hexane and lyophilisation from dioxane. FAB-MS:
(M+H)+=682, tRet(I)=26.1 min. Rf(H)=0.65.
The starting compound is prepared in the following manner:
a) N-Trifluoroacetyl-(L)-valine: At 0, 6 ml (42.8 mmol) of trifluoroacetic anhydride are
added drop~,vise to a solution of 8.16 g (38.9 mmol) of (L)-valine-tert-butyl ester and
17.3 ml (124.5 mmol) of triethylamine in 100 ml of methylene chloride and the reaction
mixture is stirred overnight at RT. After washing with 10 % citric acid and saturated
sodium chloride solution, the organic phase is concentrated by evaporation and yields
N-trifluoroacetyl-(L)-valine tert-butyl ester in the form of a yellow oil, which is dissolved
in 40 ml of a (1:1) mixture of methylene chloride and TFA and stirred for S h at RT.
Concentration by evaporation of the reaction solution and digestion of the residue with
hexane yields the tide compound in the form of a white solid. lH-NMR (200 MHz,
CD30D): 4.32 (d, J=6Hz, lH), 2.23 (m, lH), 0.98 (d, J=7Hz, 3H), 0.96 (d, J=7Hz, 3H).
Example 11: 1-r2(S)-HvdroxY-3(S)-(N-(2-(2-methoxyethoxy)ethoxv)carbonvl-L)-valvl)-
amino-4-Phenvlbutvll- l -rcyclohexvlmethvn-2-rN-(2-(2-methoxvethoxy)ethoxv)carbonvl- .
(L)-valvll-hvdrazine
Analogously to Reference Example 2, the title compound is obtained from 500 mg
(1.25 mmol) of H-[PheNNCha]-H-3 HCl from Reference Example 10a,985 mg
(3.74 mmol) of N-methoxyethoxy-ethoxycarbonyl-(L)-valine, 1.65 g (3.74 mmol) of BOP,
505 mg (3.74 mmol) of HOBt and 24.7 ml of 0.3M N-methylmorpholine in DMF after
--` 2112047
- 156-
chromatographic purification ~SiO2, chlorofor n/methanol (30: 1)), precipitation from
methylene chloride by the addition of hexane and lyophilisadon from dioxane. FAB-MS:
(M+~)+=782, tRet(l)=21.2 min, Rf(T)=0.26.
The starting compound is prepared in the following manner:
a) N-(2-(2-Methoxyetnoxy)-ethoxy)carbonyl-(L)-valine: 19.8 ml (168 mmol) of diethylene
glycol monomethyl ether are added dropwise at 0 to 100 ml of a 20% solution of
phosgene in toluene and the mixture is stirred overnight at RT. Excess phosgene is
expelled with nitrogen and the reaction solution is washed with water and concentrated by
evaporation. Analogously to Reference Example lc, 10 g (85.4 mmol) of (L)-valine are
added to tne crude 2-(2-methoxyethoxy)ethylchloroformate (15.6 g, 85.4 mmol) yielding
the tide compound in the form of a colourless resin. lH-NMR (200 MHz, CD30D): 4.18
(m, 2H), 4.05 d, J=6Hz, lH), 3.72 - 3.40 (m, 8H), 3.35 (s, 3H), 2.18 (m, lH), 0.95 (t,
J--7H, 6H).
Exa nple 12: 1-~2(S)-Hvdroxv-3(Sl-(N-(2-methoxYet'noxv)acetvl-L)-valyl)amino-4-
PhenYlbutYll-l-rcYclohexvlmet'nvll-2-rN-~2-methoxyethoxv)acetYl-(~l-valYn-hYdrazine
Analogously to Reference Example 2, the tide compound is obtainçd from 500 mg
(1.25 mmol) of H-~PheNNCha]-H-3 HCl from Reference Example 10a, 873 mg
(3.74 mmol) of N-(2)-mothoxyethoxyacçtyl-(L)-valine, 1.65 g (3.74 mmol) of BOP,
505 mg (3.74 mmol) of HOBt and 24.7 ml of 0.3M N-methylmorpholine in DMF after
chromatographic purification (SiO2, chloroform/methanol (30:1)), prçcipitation from
methylene chloride by the addition of hexane and lyophilisation from dioxane. FAB-MS:
(M+H)+=722, tRet(I)--21.3 min, Rf(l~=0.23.
The stardng compound is prepared in the following manner:
a) N-(2-Methoxyethoxy)-acetyl-(L)-valine: At 0, 6.4 ml (45.7 mmol) of triethylamine are
added to a solutdon of 5 g (23.9 mmol) of (L)-valine tert-butyl ester, 2.91 g (21.7 mmol) of
2-(methoxyethoxy)-acedc acid and 3.55 g (21.7 mmol) of cyanophosphonic acid diethyl
ester in 30 ml of DMF and the mixture is then sdrred overnight at RT. The reactdon
mixture is diluted with methylene chloride, washed in succession with 10 % citric acid,
saturated sodium hydrogen carbonate solution and saturated sodium chloride solution and,
after concentration by evaporatdon of the organic phase, yields 5.1 g of N-methoxyethoxy-
acetyl-(L)-valine tert-butyl ester, which is stirred for 1 h at RT in 22 ml of a (1:1) mixture
2~1~0~7
- 157-
of methylene chloride and TFA. Concentration by evaporation of the reaction solution
yields the title compound in the form of a colourless oil. IH-NMR (200 MHz, CDCl3):
10.0 (s, broad, lH), 7.62 d, broad, lH), 4.55 (m, lH), 4.10 (s, 2H), 3.70 (m, 2H), 3.58 (m,
2H), 3.40 (s, 3H), 2.28 (m, lH), 0.98 (d, J=7Hz, 3H), 0.95 (d, J=7Hz, 3H).
Example 13: The following compounds are obtained analogously to the Examples andprocesses described hereinbefore and hereinafter:
A) 1-[2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-~thien-2-ylmethyl]-
2-[N-acetyl-(L)-valyl]-hydrazine;
B) 1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[thien-
2-y1methyl]-2-[N-methoxycarbonyl-(L)-valyl]-hydrazine;
C) 1-[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[thien-
2-ylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]-hydrazine;
D) 1-[2(S)-hydroxy-3(S)-(N-(M,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-1-[thien-2-ylmethyl]-2-[N-(N,N-dimethylaminocarbonyl)-(L)-valyl]-hydrazine;
E) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-
phenylbutyl]-1-[thien-2-ylmethyl]-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl]-
hydrazine;
~') 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-~yl)ethyl)aminocarbonyl)-(L)-valyl)-
amino-4-phenylbutyl]-1-[thien-2-ylmethyl]-2-[N (N-(2-(morpholin-4-yl)ethyl)amino-
carbonyl)-(L)-valyl] -hydrazine;
G) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methyl-aminocarbonyl)-(L)- ;
valyl)amino-4-phenylbutyl]-1-[thien-2-ylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)-N- -
methylaminocarbonyl)-(L)-valyl]-hydrazine.
ExamPle 14: The following compounds are obtained analogously to the Examples and ~;
processes described hereinbefore and hereinafter:
A) 1-[2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-[2,3,5,6-tetrahydrc~
pyran-4-ylmethyl]-2-[N-acetyl-(L)-valyl]-hydrazine;
B) 1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-
[2,3,5 ,6-tetrahydropyran-4-ylmethyl] -2-[N-methoxycarbonyl-(L)-valyl] -hydrazine;
C) l -[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]- 1-[2,3,5,6-
tetrahydropyran-4-ylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]-hydrazine;
D) 1-[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenyl-
211~0~7
- 158-
butyl]-l -[2,3,5,6-tetrahydropyran-4-ylmethyl]-2-[N-(N,N-dimethylaminocarbonyl)-(L)-
valyl] -hydrazine;
E) 1-[2tS)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-
phenylbutyl]-1-[2,3,5,6-tetrahydropyran-4-ylmethyl~-2-[N-(N-(2-methoxyethyl)amino-
carbonyl)-(L)-valyl]-hydrazine;
F) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-(L)-valyl)-
amino-4-phenylbutyl]- 1 -[2,3,5,6-tetrahydropyran-4-ylmethyl]-2-[N-(N-(2-(morpholin-
4-yl)ethyl)aminocarbonyl)-(L)-valyl~-hydrazine;
G) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methylaminocarbonyl)-(L)-
valyl)amino-4-phenylbutyl]-1-[2,3,5,6-tetrahydropyran-4-ylmethyl]-2-[N-(N-(2-
(mo~pholin-~yl)ethyl)-N-methylaminocarbonyl)-(L)-valyl]-hydrazine.
Example 15: The following compounds are obtained analogously to the Examples andprocesses described hereinbefore and hereinafter:
A) 1-[2(S)-Hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-hydroxyphenyl-
methyl]-2-[N-acetyl-(L)-valyl]-hydrazine:
Analogously to Reference l~xample 37, the title compound is obtained from 200 mg(0.48 mmol) of 1-[2(S)-hydroxy-3(S)-amino-4-phenylbutyl]-1-[4-hydroxyphenylmethylJ- -
hydrazine-3HCl, 175 mg (1.07 mmol) of N-methoxycarbonyl-(L)-valine f~om Example 2b,
473 mg (1.07 mmol) of BOP, 145 mg (1.07 mmol) of HOBt and 8.4 ml of 0.3M N-methyl-
morpholine in DMF after chromatographic purification (SiO2, methylene chloride/diethyl
ether~methanol (10:10:1)), precipitation from methylene chloride by the addition of DIPE
and lyophilisadon from dioxane. FAB-MS: (M+H)+=616, tRet(V)=11.6 min.,
Rf(K')=0.56.
The stardng compounds are prepared in the following manner:
a) 1-[2(S)-Hydroxy-3(S)-amino-4-phenylbutyl~-1-[4-hydroxyphenylmethyl~-
hydrazine-3HCl:
Analogously to Reference Example 2a, the dtle compound is obtained from 470 mg
(0.94 mmol) of 1-[2(S)-hydroxy-3(S)-tert-butoxycarbonylamino-4-phenylbutyU-1-[4-hydroxyphenylmethyll-2-[tert-butoxycarbonyl]-hydrazine after complete concentradon by
evaporadon and digestion of the residue with methylene chloride. lH-NMR (200 MHz,
CD30D): 7.42 - 7.15 (m,7H), 6.92 (d, J=8Hz, 2H), 4.1 - 3.85 (m, 3H), 3.55 (m, lH), 3.1
(m,2H), 2.8 (m, 2H).
~':"` ~
;~
:~
21120~7
- 159-
b) 1-[2(S)-Hydroxy-3(S)-tert-butoxycarbonylamino-4-phenylbutyl]-1-[4-hydroxyphenyl-
methyl]-2-[tert-butoxycarbonyl]-hydrazine:
Analogously to Reference Example 1, the title compound is obtained from 26.2 g
(23.6 mmol) of (2R,3S)-1-~3-Boc-amino-2-phenylethyl]oxirane and 5.63 g (23.6 mmol) of
tert-butyl-3-(4-hydroxyphenyl-methyl)-carbazate after crystallisation from ethyl acetate/-
DIPE. IH-NMR (200 MHz, CD30D): 7.28 - 7.10 (m,7H), 6.70 (d, J=8Hz, 2H), 4.7 (m,
4H), 2.95 - 2.45 (m, 4H), 1.31 (s, 9H), 1.28 (s, 9H). tRe,(V)=15.0 min.
c) tert Butyl-3-(4-hydroxyphenyl-methyl)-carbazate:
Analogously to Reference Example 4a, 14 g (106 mmol) of 4-hydroxybenzaldehyde and
14 g (117 mmol) of tert-butylcarbazate in 125 ml of ethanol are reacted to form
4-hydroxybenzaldehyde-tert-b1ltoxycarbonylhydrazone. (19.8 g,80 %). 9.73 g thereof are
hydrogenated in 200 ml of l~IF in the presence of 0.6 g of 5 % palladium on carbon at
1 atm hydrogen pressure to yield the dtle compound, which is crystallised from hot
methanol. lH-NMR (200 MHz, CD30D): 7.18 (d, J=8Hz, 2H), 6.73 (d, J=8 Hz, 2H), 3.80
(s, 2H), 1.45 (s, 9H).
B) 1-[2(S)-Hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-
hydroxyphenylmethyll-2-[N-methoxycarbonyl-(L)-valyl]-hydrazine
C) 1-[2(S)-Hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-
hydroxyphenylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]-hydrazine
A solution of 2.0 g (4.86 mmol) of 1-[2(S)-hydroxy-3(S)-amino-4-phenylbutyl]-1-[4- ~
methoxyphenylmethyl]-hydrazine-3HCl (Example 15 A~ a)), 2.54 g (13.8 mmol) of ~ ~:
N-ethoxycarbonyl-(L)-valine from Reference Example 9a and 2.13 ml (13.8 mmol) ofcyanophosphonic acid diethyl ester in 45 ml of DMF is cooled to 0 and 4.0 ml
(29.2 mmol) of tliethylamine are added. The reaction mixture is stirred for 6 h at RT under
a nitrogen atmosphere and fully concentrated by evaporation in vacuo. The residue is
dissolved in methylene chloride, washed with saturated sodium carbonate solution, 10 %
aqueous CitliC acid and saturated sodium chloride solution, filtered through cotton
wadding and concentrated by evaporation. Chromatographic purification (SiO2, methylene
chloride/diethyl ether/methanol (10:10:1)) and precipitation twice from methanoV-
methylene chloride by the addition of DIPE yields the title compound. FAB-MS:
(M+H)+=644, tRet(V)=12.8 min, Rf(B)=0.41.
D) 1-[2(S)-Hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenyl-
~5j'~
' ' ~, ,' , ' ' '~ , .~ ' ' '
, :. , , ~ ' '
"~'~ ' . :,
- 2~120~7
- 160-
butyl] - 1 - [4-hydroxyphenylmethyl] -2- [N-(N ,N-dimethylaminocarbonyl)-(L)-valyl]-
hydrazine.
E) 1-[2(S)-Hydroxy-3(S)-(N-(N-(2-methoxyedhyl)aminocarbonyl)-(L)-valyl)amino-4-
phenylbutyl]-1-[4-hydroxyphenylmethyll-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-
valyl] -hydrazine .
F) 1-[2(S)-Hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)arninocarbonyl)-(L)-valyl)-
amino-4-phenylbutyl]- 1 -[4-hydroxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)-
aminocarbonyl)-(L)-valyll-hydrazine.
G) 1-[2(S)-Hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methyl-aminocarbonyl)-(L)-valyl)amino-4-phenylbutyl]-1-[4-hydIoxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)-
ethyl)-N-methylaminocarbonyl)-(L)-valyl] -hydrazine.
E~xample 16: The following compounds are obtained analogously to the Examples and
processes described hereinbefore and hereinafter:
A) 1-~2(S)-Hydroxy-3(S)-(~-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-methoxyphenyl-
medhyl3-2-~N-acetyl-(L)-valyll-hydrazine:
Analogously to Reference Example 37, dhe tide compound is obtained from 200 mg
(0.47 mmol) of 1-[2(S~hydroxy-3(S)-amino-4phenylbutyl]-1-[4-methoxyphenylmethyll-
hydrazine 3HCl, 225 mg (1.41 mmol) of N-acetyl-(L)-valine, 624 mg (1.41 mmol) ofBOP, 191 mg (1.41 mmol) of HOBt and 9A ml of 0.3M N-methylmorpholine in DMF
after chromatographic purification (SiO2, methylene chloride/methanol (12:1 and lyoph
ilisation from dioxane/water/tert-butanol. FAB-MS: (M+H)+=598, tRet(~l)=11.2 min, ,~
Rf(I')=0.25.
The starting materials are prepared in the following manner:
a) 1-12(S)-Hydroxy-3(S)-amino-4-phenylbutyll-1-[4-methoxyphenylmethyl]-
hydrazine-3HCl:
Analogously to Reference Example 2a, the tide compound is obtained from 2.65 g
(5.14 mmol) of 1-[2(S)-hydroxy-3(S)-tert-butoxycarbonylamino-4-phenylbutyl]-1-
[4-methoxyphenylmethyl]-2-[tert-butoxycarbonyl]-hydrazine after lyophilisation.
lH-NMR (200 MHz, CD30D): 7.42 - 7.15 (m,7H), 6.92 (d, J=8Hz, 2H), 4.1 - 3.8 (m, 3H),
3.75 (s, 3H), 3.55 (m, lH), 3.1 (m, br, 2H)~ 2.75 (m, br, 2H).
,. ~
: ~
: , .: . . : . ..
2112047
- 161 -
b) 1-[2(S)-Hydroxy-3(S)-tert-butoxycarbonylamino-4-phenylbutyl]-1-[4-methoxyphenyl-
methyl] -2-[tert-butoxycarbonyl]-hydrazine:
Analogously to Reference Example 1, the tide compound is obtained from 3.13 g
(11.9 mmol) of (2R,3S)- 1-[3-Boc-amino-2-phenylethyl]oxirane and 3.0 g (11.9 mmol) of
tert-butyl-3-(4-methoxyphenyl-methyl~carbazate from Reference Example 29c) (see
below) after crystallisation from methanoVDIPE. lH-NMR (200 MHz, CD3OD): 7.3 - 7.1
(m, 7H), 6.85 (d, J=8Hz, 2H), 3.78 (s, 3H), 3.65 (m, 4H), 2.9 - 2.5 (m, 4H), 1.25 (s, 9H),
1.20 (s, 9H), tRct(V)=16.6 min.
B) 1-[2(S)-Hydroxy-3(S~(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-
methoxyphenylmethyll-2-[N-methoxycarbonyl-(L)-valyl]-hydrazine: ;
Analogously to Reference Example 37, the title compound is obtained from 200 mg
(0.47 mmol) of the title compound of Example 16 A) a), 247 mg (1.41 mmol) of
N-methoxycarbonyl-~)-valine from Example 2b, 624 mg (1.41 mmol) of BOP, 191 mg
(1.41 mmol) of HOBt and 9.4 ml of 0.3M N-methylmorpholine in DMF after chromato-graphic purification (SiO2, methylene chloride/methanol (19:1)) and lyophilisation from
dioxane. FAB-MS: (M+H)+=630, tRet(V)=13.5 min. Rf(A')=0.27.
C) 1-[2~S)-Hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]- 1-
[~methoxyphenylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]-hydrazine:
Analo~ously to Reference Example 37, the title compound is obtained from 200 mg
(0.47 mmol) of the title compound of Example 16 A) a), 267 mg (1.41 mmol) of .
N-ethoxycarbonyl-(L)-va1ine from Example 9a, 624 mg (1.41 mmol) of BOP, 191 mg
(1.41 mmol) of HOBt and 9.4 ml of 0.3M N-methylmo~pholine in DMF after chromato-graphic purification (SiO2, methylene chloride/methanol (12:1)), precipitation from
methylene chloride by the addition of DIPE and lyophilisation from dioxane. FAB-MS:
(M+H)+=658, tRet(V)=15.0 min, Rf~I')=0.55.
.
D) 1-[2(S)-Hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenyl-butyl]- 1 -[4-methoxyphenylmethyl]-2-[N-(N,N-dimethylaminocarbonyl)-(L)-valyl]-
hydrazine.
E) 1-[2(S)-Hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-
phenylbutyl]- l-[4-methoxyphenylmethyl]-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-
valyl] -hydrazine .
,:............... .
`~
2112~47
- 162-
F) 1-[2(S)-Hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)arninocarbonyl)-(L)-valyl)-
amino-4-phenylbutyl]- 1 -[4-methoxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)-
aminocarbonyl)-(L)-valyl] -hydrazine.
G) 1-[2(S)-Hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methyl-aminocarbonyl)-(L)-valyl)amino-4-phenylbutyl]- 1-[4-methoxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-
yl)ethyl)-N-methylaminocarbonyl)-(L)-valyl]-hydrazine.
Example 17: The following compounds are obtained analogously to the Examples andprocesses described hereinbefore and hereinafter:
A) 1-[2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-benzyloxy-phenylmethyl]-2-[N-acetyl-(L)-valyl]-hydrazine;
B) 1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-
[4-benzyloxyphenylmethyl]-2-[N-methoxycarbonyl-(L)-valyl]-hydrazine;
C) l-L2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-benzyi-
oxyphenylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]-hydrazine;
D) l-L2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-1-[4-benzyloxyphenylmethyl]-2-[N-(N,N-dimethylaminocarbonyl)-(L)-valyl]-
hydrazine;
E) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-~L)-valyl)amino-4-
phenylbutyl]-l -14-benzyloxyphenylmethyl]-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-
(L) -valyl] -hydrazine;
E7) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-ylkthyl)aminocarbonyl)-(L)-valyl)-
amino-4-phenylbutyl]-1-[4-benzyloxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)-
aminocarbonyl)-(L)-valyl]-hydrazine;
G) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methyl-aminocarbonyl)-(L)-
valyl)amino4-phenylbutyl]-1-[4-benzyloxyphenylmethyl]-2-[N-(N-(2-(morpholin4-yl)-
ethyl)-N-methylaminocarbonyl)-(L)-valyl] -hydrazine.
Exannple 18: The following compounds are obtained analogously to the Examples and
processes described hereinbefore and hereinafter:
A) i-l2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-(3,4-dimethoxy-
benzyl)oxyphenylmethyl]-2-[N-acetyl-(L)-valyl]-hydrazine;
B) 1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-(3,4-
dimethoxybenzyl)oxyphenylmethyl]-2-[N-methoxycarbonyl-(L)-valyl]-hy~razine;
~112~47
- 163-
C) 1-[2~S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-(3,4-
dimethoxybenzyl)oxyphenylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]-hydrazine;
D) 1-[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenyl-butyl] 1-[4-(3,4-dimethoxybenzyl)oxyphenylmethyl]-2-[N-(N,N-dimethylamino-
carbonyl)-(L)-valyl3-hydrazine;
E) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)arnino-4- ~ :
phenylbutyl]-1-[4-(3,4-dimethoxybenzyl)oxyphenylmethyl]-2-[N-(N-(2-methoxyethyl)-
aminocarbonyl)-(L)-valyl]-hydrazine;
F) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-(L)-valyl)- :
amino-4-phenylbutyl]-1-14-(3,4-dimethoxybenzyl)oxyphenylmethyl]-2-~N-(N-(2-(mor-pholin-4-yl)ethyl)aminocarbonyl)-(L)-valyl]-hydrazine;
G) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methylaminocarbonyl)-(L)-
valyl)amino-4-phenylbutyl]-1-[4-(3,4-dimethoxybenzyl)oxyphenylmethyl]-2-[N-(N-(2-
(morpholin-4-yl)ethyl)-N-methylaminocarbonyl)-(L)-valyl]-hydrazine.
, -:,
Example 19: The following compounds are obtained analogously to the Examples andprocesses described hereinbefore and hereinafter: :~
A) 1-~2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-isobutoxyphenyl- . :
methyl]-2-[N-acetyl-(L)-valyl]-hydrazine; .
B) 1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-~4-iso-
butoxyphenylmethyl]-2-[N-methoxycarbonyl-(L)-valyl]-hydrazine;
C) l-L2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-~4-iso-
butoxyphenyLmethyl]-2-~N-ethoxycarbonyl-(L)-valyl]-hydrazine;
D) 1-[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenyl- ~ :
butyl]-1-~4-isobutoxyphenylmethyl]-2-~N-(N,N-dimethylaminocarbonyl)-(L)-valyl]- ~ :
hydrazine;
E) 1-12(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-
phenylbutyl]-1-~4-isobutoxyphenylmethyl]-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl]-hydrazine;
F) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-(L)-valyl)-
amino-4-phenylbutyl]-1-[4-isobutoxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)-
aminocarbonyl)-(L)-valyl] -hydrazine;
G) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methylaminocarbonyl)-(L)-
valyl)amino-4-phenylbutyl]-1-[4-isobutoxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)-
ethyl)-N-methylaminocarbonyl)-(L)-valyl]-hydrazine.
~ ~ ", ,~
... : ' " :~'' '., ' ` ' ' , ' ' : .
2ll2a47
- 164-
Example 20: The following compounds are obtained analogously to the Examples andprocesses described h~reinbefore and hereinafter:
A) 1-[2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl~-1-[4-(2-methoxy-
ethoxy)phenylmethyl]-2-[N-acetyl-(L)-valyl~-hydrazine;
B) 1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L~-valyl)arnino-4-phenylbutyl]-1-[4-(2-methoxyethoxy)phenylmethyl]-2-[N-methoxycarbonyl-(L)-valyl]-hydrazine;
C) 1-[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-
(2-methoxyethoxy)phenylmethyl~-2-[N-ethoxycarbonyl-(L)-valyl]-hydrazine;
D) 1-[2(S)-hydroxy-3(S)-(N-~N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenyl-
butyl]-1-[4-(2-methoxyethoxy)phenylmethyl]-2-[N-(N,N-dimethylaminocarbonyl)-(L)- -
valyl]-hydrazine;
E) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)arninocarbonyl)-(L~-valyl)amino-4-phenylbutyl]-1-[4-(2-methoxyethoxy)phenylmethyl]-2-[N-(N-(2-methoxyethyl)amino-
carbonyl)-(L)-valyl] -hydrazine;
F) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-(L)-valyl)-
amino-4-phenylbutyl]- 1 -[4-(2-methoxyethoxy)phenylmethyl]-2-[N-(N-(2-(morpholin~-
yl)ethyl)aminocarbonyl)-(L)-valyl] -hydrazine;
G) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(mo~pholin~yl)ethyl)-N-methylaminocarbonyl)-(~)-
valyl)arnino~phenylbutyl3-1-[4-(2-methoxyethoxy)phenylmethyl]-2-[N-(N-(2-
(morpholin-4-yl)ethyl)-N-methylaminocarbonyl)-(L) -valyl]-hydrazine.
Exam~le 21: The following compounds are obtained analogously to the Examples andprocesses described hereinbefore and hereinafter:
A) 1-[2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-~phenylbutyl]-l-[methylene-3,4-
dioxyphenylmethyl]-2-[N-acetyl-(L)-valyll-hydrazine;
B) 1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-
[methylene-3,4-dioxyphenylmethyl]-2-[N-methoxyc~rbonyl-(L)-valyl]-hydrazine;
C) 1-[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-~phenylbutyl]-l-
[methylene-3,4-dioxyphenylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]-hydrazine;
D) 1-[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-] -[methylene-3,4-dioxyphenylmethyl]-2-[N-(N,N-dimethylaminocarbonyl)-(L)-
valyl] -hydrazine;
E) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-
. . . : . : , ., ~ .: `.
. , - - . - . . . . , - . . . . ..
21120~7
- 165-
phenylbutyl]-l-[methylene-3,4-dioxyphenylmethyl]-2-[N-(N-(2-methoxyethyl)amino-
carbonyl)-(L)-valyl] -hydrazine;
F) 1-~2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-(L)-valyl)-
amino-~phenylbutyl]- 1 -[methylene-3,4-dioxyphenylmethyl] -2-[N-(N-(2-(morpholin-4-
yl)ethyl)aminocarbonyl)-(L)-valyl]-hydrazine;
G) 1-~2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methylaminocarbonyl)-(L)-
valyl)amino-4-phenylbutyl3-1-[methylene-3,4-dioxyphenylmethyl]-2-[N-(N-(2-
(morpholin-4-yl)ethyl)-N-methylaminocarbonyl)-(L)-valyl]-hydrazine; ~ .
H) 1 -[2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]- 1 -[3,4-dimethoxy-
phenylmethyl]-2-[N-acetyl-(L)-valyl] -hydrazine;
I) 1-~2(S)-hydroxy-3(S)-(N-rnethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[3,4-di-
methoxyphenylmethyl]-2-[N-methoxycarbonyl-(L)-valyl]-hydrazine; :
J) 1-[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[3,4-di-
methoxyphenylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]-hydrazine;
K) 1-[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl)amino-4-phenyl-
butyl~-1-[3,4-dimethoxyphenylmethyl]-2-[N-(N,N-dimethylaminocarbonyl)-(L)-valyl]-
hydrazine;
L) 1-[2(S)-hydroxy-3(S~(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4- ~ -
phenylbutyl]-1-[3,4-dimethoxyphenylmethyl~-2-[N-(N-(2-methoxyethyl)aminocarbonyl)~
(L)-valyl]-hydrazine;
M) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-y})ethyl)aminocarbonyl)-(L)-valyl)-
amino-4phenylbutyl]- 1-[3,4-dimethoxyphenylmethyl]-2-[N-(N-(2-(morpholin-4-yl)-
ethyl)aminocarbonyl)-(L)-valyl]-hydrazine;
N) 1-[2(S)-hydroxy-3~S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methylaminocarbonyl)-(L)-
valyl)amino-4phenylbutyl]-1-[3,4dimethoxyphenylmethyl]-2-[N-(N-(2-(morpholin-4
yl)ethyl)-N-methylaminocarbonyl)-(L)-valyl]-hydrazine.
Example 22: The following compounds are obtained analogously to the Examples andprocesses described hereinbefore and hereinafter:
A) 1-[2(S)-hydroxy-3(S)-(N-acetyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-biphenylyl-
methyll-2-[N-acetyl-(L)-valyl]-hydrazine;
B) 1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenylbutyl]-1-[4-
biphenylylmethyl]-2-[N-methoxycarbonyl-(L)-valyl] -hydrazine;
C) 1-[2(S)-hydroxy-3(S)-(N-ethoxycarbonyl-(L)-valyl)amino-~phenylbutyl]-1-[4-
biphenylylmethyl]-2-[N-ethoxycarbonyl-(L)-valyl]-hydrazine;
: : ; : ~ ~ - : . i - .. . :. i. . ~ . . . ~ .. . .
2112~47
- 166-
D) 1-[2(S)-hydroxy-3(S)-(N-(N,N-dimethylaminocarbonyl)-(L)-valyl~amino-4-phenyl-butyl]-1-[4-biphenylylmethyl]-2-[N-(N,N-dimethylaminocarbonyl)-(L)-valyl]-hydrazine;
E) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-valyl)amino-4-
phenylbutyl]-1-[4-biphenylylmethyl]-2-[N-(N-(2-methoxyethyl)aminocarbonyl)-(L)-
valyl] -hydrazine;
F) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)aminocarbonyl)-(L)-valyl)-
amino-4-phenylbutyl]-1-~4-biphenylylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)amino-
carbonyl)-(L3-valyl] -hydrazine;
G) 1-[2(S)-hydroxy-3(S)-(N-(N-(2-(morpholin-4-yl)ethyl)-N-methylaminocarbonyl)-(L)-
valyl)amino-4-phenylbutyl~-1-[4-biphenylylmethyl]-2-[N-(N-(2-(morpholin-4-yl)ethyl)-
N-methylaminocarbonyl)-(L)-valyl]-hydrazine.
Example 23: l.r2(S)-Hvdroxv-3(S)-(N-(methoxYcarbonYl)-(L)-valvl)amino-4-cvclo-
hexvl-butYn-l-rcvclohexylrnethyll-2-rN-methoxvcarbonvl~ -valYll-hvdrazine:
In the presence of 20 mg of Nishimura catalyst (Rh(m)- and Pt(VI)-oxide monohydrate,
Degussa) 100 mg (0.165 mmol) of 1-[2(S)-hydroxy-3(S)-(N-(methoxycarbonyl)-(L)-
valyl)amino~phenyl-butyl]-l-lcyclohexylmethyl]-2-tN-methoxycarbonyl-(L)-valyl]-
hydrazine (Example 2a~ in the form of a solution in 8-ml of methanol are hydrogenated
under low pressure at RT. Removal of the catalyst by filtration through ~?Celite and
concentration of the filtrate by evaporation yields the title compound: FAB-MS
(M+H~+=612.
Examvle 24: 1-r2(S)-Hvdroxv-3(S~-(N-(n-ProPoxYcarbonyl)-(L)-valvl)amino-4-phen
butv!l-l-rcvclohexYlmethY11-2-rN-(n-proPyl)oxycarbonyl-(L)-val-yll-hydrazine:
~ydrogenation under low pressure at RT of a solution of 100 mg (0.152 mmol~ of
1-[2(S)-hydroxy-3(S)-(N-(allyloxycarbonyl)-(L~-valyl~amino~phenyl-butyl]-l-[cyclo-
hexylmethyl]-2-[N-allyloxycarbonyl-(L~-valyl]-hydrazine (13xample 8~ in 4 ml of
methanol using 50 mg of 5 % Pd/C as catalyst, followed by filtration through (~)Celite
(siliceous earth; Fluka, Switzerland~, concentration of the filtrate by evaporation and
digestion from DIPE, yields dhe pure tide compound: FAB-MS (M+H)+=662.
Example 25: 1-r2(R)-Hvdroxv-3(S)-(N-(methoxvcarbonvl)-(L)-valvl)amino-4-Phenvl-
butYll-l-rcvclohexYlmethvll-2-rN-methoxYcarbonyl-(L)-valvll-hvdrazine
Under a protective gas atmosphere,33 mg (0.186 mmol) of N-methoxycarbonyl-(L~-
valine (Example 2b~ are activated with 82 mg (0.186 mmol~ of BOP and 25 mg
(0.186 mmol~ of HOBT in 1.24 ml of a 0.3M solution of NMM in DMF and, after 15 min,
2 ~ S,~ 7
- 167-
reacted with 25 mg (0.062 mmol) of 1-[2(R)-hydroxy-3(S)-amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-hydrazine (in the form of the hydrochloride salt) for 18 h. The
reaction mixture is concentrated by evaporation under HV and the residue is partitioned
between 3 portions of methylene chloride, 2 portions of saturated NaHCO3 solution and
brine. Column chromatography (SiO2, methylene chloride/methanol 99:1 ~ 15:1) andprecipitation with DIPE from a concentrated solution in DMF yields the pure title
compound: tRe,(V)=14.7 min; FAB-MS (M+H)+=6Q6.
The starting material is prepared as follows:
a) 2~)-[l'(S)-(Trifluoroacetylamino)-2'-phenylethyl]-oxirane
(Alternative to Reference Example 16d):
54.28 g (314 mmol) of m-chloroperbenzoic acid are added to a solution of 14.5 g
(60 mmol) of N-trifluoroacetyl-l-phenyl-3-buten-2(S)-amine (Reference Example 16c) in
600 ml of chloroform and the mixture is stirred for 16 h at RT to complete the reaction.
The reaction mixture is washed twice with 10 % sodium sulfite solution, twice with
saturated sodium carbonate solution, with water and finally with brine. The aqueous
phases are extracted a further twice with methylene chloride, and the combined organic
phases are dried with sodium sulfate and concentrated by evaporation. Column chroma-
tography (SiO2, hexane/ethyl acetate 2:1) yields the title compound in the form of a 6:1
mixture of the (2R)- and (2S)-epimers: TLC Rf(F)=0.41, TLC Rf(N)=0.6;
tRe~(V)=12.6 min; lH-NMR (200 MHz, CDCl3): inter alia 4.08 (m, lnH, H-C(2(S))), 4.47
(m, 6/7H, H-C(2(R))).
b) 1-[2(S)-Hydroxy-3(S)-(triflu~roacetylamino)-~phenyl-butyl]-l-[cyclohexylmethyl]-2-
[tert-butoxycarbonyl]-hydrazine and 1-[2(R)-hydroxy-3(S)-(trifluoroacetylamino)-4-
phenyl-butyl]-l-[cyclohexy~methyl]-2-[tert-butoxycarbonyl]-hydrazine:
Under a nitrogen atmosphere, 2.5 g (9.65 mmol) of 2(R)-[l'(S)-(trifluoroacetylamino)-2'-
phenylethyl]-oxirane (contains 14% of the (2S)-epimer) and 2.2 g (9.65 mmol) of tert-
butyl-3-cyclohexylmethyl-carbazate (Reference Example 4a) dissolved in 31 ml of
methanol are reacted for 18 h at 75C. The reaction mixture is concentrated by evapora-
tion and the residue is chromatographed (SiO2, toluene/ethyl acetate 10: 1). The(2S)-epimer is eluted first as the main product, followed by the (2R)-epimer: (2S)-epimer
TL(~ Rf(I)=0.7; tRe,(V)=18.5 min; Anal: calc. C 59.12 %, H 7.44 %, N 8.62 %; found C
59.10 %, H 7.09 %, N 8.81 %. (2R)-epimer TLC Rf(I)=0.6; tRe,(V)=18.5 min; FAB-MS(M+H)+=488.
: .~ "` . ~ `' . ' .. ' ~ ,: .,.,, ' .:. ' : ` ", . ' '.' ': ` ' :'. '
- 2 1 ~ 7
- 168-
c) 1-12(R)-Hydroxy-3(S)-amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-2-[tert-butoxy-
carbonyl]-hydrazine:
Under a nitrogen atmosphere,326 mg (0.669 mmol) of 1-[2(R)-hydroxy-3(S)-(trifluoro-
acetylamino)-4-phenyl-butyl]-1-[cyclohexylmethyl]-2-[tert-butoxycarbonyl]-hydrazine
dissolved in 52 ml of MeOH are heated to 70C, 17 ml of lM aqueous K2CO3 solution are
added dropwise and the mixture is stirred for 16 h at 70C. The reaction mixture is
concentrated by evaporation and the residue is partitioned between 3 portions ofmethylene chloride, 2 portions of water and brine. Concentration by evaporation of the
organic phases which have been dried with Na2SO4 yields the ~itle compound, which is
used direcdy in the next step: lH-NMR (200 MHz, CD30D): 1.42 (s, 9 H, Boc), 0.8-2.1
(m, 11 H, cyclohexyl), 2.4-3.0 (m, 6 H), 3,1 (m, 1 H), 3.54 (m, 1 H),7.28 (m,5 H).
d) 1-[2(R)-Hydroxy-3(S)-amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-hydrazine (hydro-
chloride salt):
Under a nitrogen atmosphere, 2 ml of 4N HCVdioxane ale added to a solution of 80 mg
(0.204 mmol) of 1-[2(R)-hydroxy-3(S)-amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-2-
[tert-butoxycarbonyl]-hydrazine in 2 ml of dioxane and dhe mixture is stirred for 7 h at RT
and then lyophilised. The crude product is used direcdy in the above reaction.
Example 26: 1-r2(R)-HvdroxY-3(R~(N-(medhoxvcarbonvl)-(L)-valv!~arnino-4-Phenvl-
butYn-l-rcvclohexvlmethY11-2-rN-methoxvcarbonYl-(L)-valyll-hydrazine: .
Unde~ a nitrogen atmosphere, 315 mg (1.80 mmol) of N-methoxycarbonyl-(L)-valine
(Exarnple 2b) are activated with 795 mg (1.80 mmol) of BOP and 267 mg (1.80 mmol) of
HOBT in 12 ml of a 0.3M solution of NMM in DMF and, after 15 min, reacted for 48 h
~,vith 240 mg (0.60 mmol) of 1-l2(R)-hydroxy-3(R)-amino-4-phenyl-butyl]-1-[cyclohexyl-
methyl]-hydra~ne (in dle form of the hydrochloride salt) (can be prepared analogously to
1-[2(S)-hydroxy-3(S)-arnino-4-phenyl-butyl]-1-[cyclohexylmethyl]-hydrazine hydro-
chloride salt (Reference Example 10a, = H-[PheNNCha]-H-3HCl) from (D)-N-Boc-phenyl-
alanine). The reaction mixture is concentrated by evaporation under HV and the residue is
partitioned between 3 portions of methylene chloride, 2 portions of saturated NaHCO3
solution and brine. Column chromatography (SiO2, methylene chloride/methanol 19:1)
and digestion with methylene chloride/DlPE yields the pure title compound: TLC
Rf(B')=0.60; tRet(V)=14.7 min; FAB-MS (M+H)+=606.
. . -, ~ ~ . ~ . ; :- : . . . . . .
, . , j ~ .
` 21120~7
- 169-
Example 27~ 2(S)-Hydroxy-3(S)-(benzyloxycarbonylamino)-4-~henvl-butvl
L~nvlmethvll-2-~N-methoxYcarbonvl-(L)-valyll-hvdrazine:
Under a nitrogen atmosphere, 0.28 mmol of 1-[2(S)-hydroxy-3~S)-(benzyloxycarbonyl-
amino)-4-phenyl-butyl]-1-[phenylmethyl]-hydrazine and 54.3 mg (0.31 mmol) of
N-methoxycarbonyl-(L)-valine lExample 2b) are dissolved in 3.36 ml of 0.25M
NMM/CH3CN, 117.6 mg (0.31 mmol) of HBTU are added and the mixture is stirred for3 h at RT. During that time the pure title compound separates from the initially clear
solution in the form of a precipitate, which is filtered off and washed with 3 ml of
CH3CN/DIPE 1:2: TLC Rf(I)=0.22; tRe,(V)=15.4 min; FAB-MS (M+H)+=577. Further
product can be recovered by concentrating the filtrate by evaporation, paTtitioning the
residue between 3 portions of ethyl acetate, 2 portions of saturated NaHCO3 solution,
water and bIine, drying the organic phases with Na2SO4, concentrating by evaporation and
digesting from DIPE.
The s~arting material is prepared as follows:
a) 1 -[2(S)-Hydroxy-3(S)-(benzyloxycarbonylamino)-4-phenyl-butyl]- 1-[phenylmethyl]-2-
[tert-butoxycarbonyl]-hydrazine:
Under a nitrogen atmosphere, 220 mg (0.57 mmol) of 1-[2(S)-hydroxy-3(S)-amino4-
phenyl-butyl]- l-[phenylmethyl]-2-[tert-butoxycarbonyl]-hydrazine (= H-[PheNNPhe]-Boc,
Reference Example 30b) in 15 ml of dioxane/water 1:1 and 408 mg (2.9 mmol) of K2CO3
are reacted with 120 mg (0.69 mmol) of chlo~oformic acid benzyl ester for 16 h at RT.
KHSO4 solution is added, the mixture is cxtracted with 3 portions of ethyl acetate, and the
organic phases are washcd with water and brine, dried with Na2SO4 and concentrated by
evaporation. Digestion from hexane yields the pure title compound: TLC Rf(I)=0.52;
tRet(V)=17.1 min; FAB-MS (M+H)+=520.
b) 1-[2(S)-Hydroxy-3(S)-(benzyloxycarbonylamino)-4-phenyl-butyl]-1-~phenylmethyl]-
hydrazine:
148 mg (0.28 mmol) of 1-[2(S)-hydroxy-3(S)-(benzyloxycarbonylamino)-4-phenyl-
butyl]-l-[(phenylmethyl]-2-[tert-butoxycarbonyl]-hydrazine dissolved in 7 ml of formic
acid are stirred overnight at RT. The reaction mixture is concentrated by evaporation
under HV, the residue is partitioned between 3 portions of ethyl acetate, 2 portions of
NaE~CO3 solution and brine, and the organic phases are dried with Na2SO4 and
concentrated by evaporation: TLC Rf(I)=0.50; tRet(V)=11.8 min.
:::: :: :- ' : ; , ,
., ~
2112~7
- 170-
Example 28~ 2(S)-Hvdroxy-3(S)-(N-(methoxycarbonYl)-(L)-valyl)amino-4-cYclo-
hexyl-butyll- 1 -rphenvlmethvll-2-~N-methoxYcarbonyl)-L-valYll-hYdrazine:
Analogously to Example 16B, 91.7 mg (0.52 mmol) of N-methoxycarbonyl-(L)-valine
(Example 2b) are activated with 232 mg (0.52 mmol) of BOP and 70.7 mg (0.53 mmol) of
HOBT in 3.5 ml of a 0.3M solution of NMM in DMF and reacted with 70 mg
(0.174 mmol) of 1-[2(S)-hydroxy-3(S)-amino-4-cyclohexyl-butyl]-1-lphenylmethyl]-hydrazine (in the form of the hydrochloride salt) for 18 h. The reaction mixture is concen-
trated by evaporation under HV, and the residue is partitioned between 3 portions of
methylene chloride, 2 portions of saturated NaHCO3 solution and brine. Precipitation with
DIPE3 from a concentrated solution in DMF yields the pure title compound: TLC
Rf(G')=0.61; tRet(V)=14.7 min; FAB-MS (M+H)+=606.
The starting material is prepared as follows:
a) N-3(S)-(Boc-amino)-2(R,S)-hydroxy-4-cyclohexyl-1-trimethylsilyl-butane:
Using 2.94 g of Nishimura catalyst (Rh(II~- and Pt(VI3-oxide monohydrate, Degussa,
Germany) 25 g (81.3 mmol) of N-3(S)-(Boc-amino)-2(R,S)-hydroxy-~phenyl-l-tri-
methylsilyl-butane (for preparation see ~P-532 466-A2, page 42) in 882 ml of methanol
are hydrogenatod under low pressure at RT. Removal of the catalyst by f~tration dlrough
~Celite and concentration by evaporation of the f~trate yields the dde compound: TLC
Rf(I)=0.7; PAB-MS (M+H)+=344.
b) l-Cyclohexyl-3-buten-2(S)-amine:
42.2 ml (336 mmol) of an approximately 48 % solution of boron trifluoride in ether are
added at 5C to a solution of 23.1 g (67.2 mmol) of 3(S)-(Boc-amino)-2(R,S)-hydroxy-4-
cyclohexyl-l-trimethylsilyl-butane in 460 ml of methylene chloride. The reaction mixture
is then stirred for 6 h at RT and 3M sodium carbonate solution is added. The aqueous
phase is removed and extracted twice with methylene chloride. The organic extracts are
washed with brine, dried over sodium sulfate and concentrated by evaporation. The title
product is further used without additional purification.
c) N-Boc-l-cyclohexyl-3-buten-2(S)-amine:
15.2 g (69.5 mmol) of Boc-anhydride are added at RT to 8.2 g (53.5 mmol) of l-cyclo-
hexyl-3-buten-2(S)-amine in the form of a solution in 110 ml of methylene chloride. The
mixture is stirred for 17 h at RT and then extracted t~vice with 10 % citric acid, water and
brine. The aqueous phases are washed a further twice with methylene chloride, dried with
.. . .
",,
:.~, . - .. ~ . ~ ~ . - ..
.,
~, ,. .: .:
,: :
2112~47
- 171 -
sodium sulfate and concentrated by evaporation. Column chromatography (SiO2, hexane/-
ethyl acetate 4:1) yields the tide compound: TLC Rf(I)=0.8.
d) 2(R~-[l'(S)-(Boc-amino)-2'-cyclohexylethyl]-oxirane:
8.5 g (172 mmol) of m-chloroperbenzoic acid are added to a solution of 2.5 g (9.86 mmol)
of N-Boc-l-cyclohexyl-3-buten-2(S)-amine in 65 ml of chlorof~m and the mixt~re is
stirred for 18 h at RT to complete the reaction. The reaction mixture is washed with 10 %
sodium sulfite solution, saturated sodium carbonate solution, water and brine. The aqueous
phases are extracted a further t vice with methylene chloride, and the combined organic
phases are dried with sodium sulfate and concentrated by evaporation. Column chroma-
tography (SiO2, hexane/ethyl acetate 5:1) finally yields the pure tide compound: TLC
Rf(L)=0.22.
e) 1-[2(S)-Hydroxy-3(S)-(tert-butoxycarbonylamino)-4-cyclohexyl-butyll-1-[phenyl-
methyl]-2-[tert-butoxycarbonyl]-hydrazine:
Under a nitrogen atmosphere, 200 mg (0.745 mmol) of 2(R)-[l'(S)-(Boc-arnino)-2'-cyclo-
hexylethyl]-oxirane and 165 mg (0.745 mmol) of tert-butyl-3-benzyl-carbazate (J. Chem.
Soc., Perkin I, 1712 (1975)) in 6 ml of methanol are stirred for 2 days at 75C. Concen-
tration of dhe reaction mixture by evaporation and precipitation with hexane from a
concentrated solution in methylene chloride yields the title compound: TLC Rf(I)=0.54.
f) 1-[2(S)-Hydroxy-3(S)-amino-4-cyclohexyl-butyl]-1-[phenylmethyl]-hydrazine (hydro-
chloride salt):
0.5 ml of 4N HCVdioxane are added, with the exclusion of moisture, to a solution of
90 mg (0.183 mmol) of 1-[2(S)-hydroxy-3(S)-(tert-butoxycarbonylamino)-4-cyclohexyl-
butyl]-l-[phenylmethyl]-2-[tert-butoxycarbonyl]-hydrazine in 0.5 ml of dioxane. After S h
at RT the reaction mixture ig lyophilised and the lyophilisate is directly further used.
Example 29: A) 1-l2(S)-HYdroxv-3(S)-(N-(quinoline-2-carbonYl)-(L)-asparaginYl)amino-
4-ph~nvl-butYll- l-rcvclohexvlmethvll-2-rtert-butoxYcarbonYll-hYdrazine:
At 5C, 940 mg (3.27 mmol) of quinoline-2-carbonyl-(L)-asparagine (in the form of the
hydrochloride salt) are dissolved in 35 ml of TH~, and 736 mg (3.57 mmol) of DCC are
added. After 10 min, 482 mg (3.57 mmol) of HOBT, 0.82 ml (7.44 mmol) of NMM and
1.165 g (2.98 mmol) of 1-[2(S)-hydroxy-3(S)-amino-4-phenyl-butyl]-1-[cyclohexyl-methyl]-2-[tert-butoxycarbonyl]-hydrazine are added and the mixture is stirred at RT for
18 h. The reaction mixture is filtered and the filtrate is concentrated by evaporation. The
,.~ -- `, ~ '
:-. , ,,,: .j. , ;.. ~................ . .
21~2~L7
- 172-
residue is partitioned between 3 por~ions of ethyl acetate, saturated NaHCO3 solution,
water and brine, and the organic phases, which have been dried with Na2S04, are concen-
trated by evaporation and subjected to column chromatography tsio2, ethyl acetate) to
yield the title compound: TLC Rf(O)=0.16; tRet(V)=16.5 min; FAB-MS (M+H)+=661.
The sta~ng compound is prepared in the following manner:
a) Quinoline-2-carbonyl-(L)-asparagine tert-butyl ester:
Analogously to Example 29),5.45 g (31.4 mmol) of quinaldic acid in 161 ml of THF are
reacted with 7.08 g (34.3 mmol) of DCC, 4.63 g (34.3 mmol) of HOBT and 5.38 g
(28.6 mmol) of (L)-asparagine tert-butyl ester (Bachem, Bubendorf/Switzerland).
Filtration, extraction and column chromatography (SiO2, ethyl acetate/hexane 3: 1) yields
the pure title compound: TLC Rf(C')=0.15; tRe,(V)=12.2 min; FAB-MS (M+H)+=344.
b) Quinoline-2-carbonyl-(L)-asparagine (hydrochloride salt):
Under a nitrogen atmosphere, 4.0 g (11.6 mmol) of quinoline-2-carbonyl-(L)-asparagine
tert-butyl ester are dissolved in 40 ml of dioxane, and 40 ml of 4N HCI/dioxane are added.
On stirnng for 17 h at RT, the product is precipitated in the form of a solid. Filtration and
washing with DD?E yields the pure title compound: lH-NMR (200 MHz, CD30D): 3.02
(d, J=6 Hz, 2 H), 5.09 ~t, J=6 Hz, 1 H), 7.92 (m, 1 H), 8.12 (m, 1 H), 8.26 (m, l H), 8.4 (m,
2 H), 9.03 (m, 1 H).
c) 1-[~(S)-Hydroxy-3(S)-amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-2-[tert-butoxy-
carbonyl]-hydrazine:
Under a nitrogen atmosphere, 2.0 g (4.10 mmol) of 1-[2(S)-hydroxy-3(S)-(trifluoroacetyl-
amino)-4-phenyl-butyl]-1-[cyclohexylmethyl]-2-[tert-butoxycarbonyl]-hydrazine
(l~xample 25b) dissolved in 316 ml of MeOH are heated to 70C, 103 ml of lM aqueous
K2CO3 solution are added dropwise, and the mixture is stirred for 18 h at 70C. The
reaction mixture is concentrated by evaporation under HV and the residue is partitioned
between 3 portions of methylene chloride, 2 portions of water and brine. Concentration by
evaporation of the organic phases, which have been dried with Na2SO4, yields the title
compound: lH-NMR (200 MHz, CD30D): 1.42 (s, 9 H, Boc), 0.8-2.1 (m, 11 H, cyclo-
hexyl), 2.35-3.0 (m,7 H),3.51 (m,1 H), 7.25 (m,5 H).
5~';: ' ' ' : . . ' .
,. :: : :
.~.. : . : : ~
~,~, . ~ .. ..
.. . ~ ,: . .. : - ~
~.... .
2112~)~7
- 173-
B) _-r2(S)-HYdroxv-3(S)-(N-(quinoline-2-carbonYl)-~L)-asPara~inyl)amino-4-phen
butvll-l -rphenYlmethYll-2-rtert-butoxvcarbonYll-hydrazine:
At 0C, 3.84 g (13.4 mmol) of quinoline-2-carbonyl-(L)-asparagine (hydrochloride salt)
(Example 29 A) b)) are added to 4.69 g (12.2 mmol) of 1-[2(S)-hydroxy-3(S)-amino-4-
phenyl-butyl]-l-[phenylmethyl]-2-[tert-butoxycarbonyl]-hydrazine in 250 ml of THF.
2.18 g (13.4 mmol) of HOBT, 2.76 g (13.4 mmol) of DCC and 2.14 ml (19.5 mmol) ofNMM are added to the suspension which is then stirred for 30 min at 0C and for 17 h at
RT. The reaction mixture is filtered and the filtrate is concentrated by evaporation to a
residual volume of approximately 50 ml. The fine suspension is taken up in methylene
chloride and washed with NaHCO3 solution and brine, and the aqueous phases are
extracted with 2 portions of methylene chloride. Filtration of the combined organic phases
thr~ugh cotton wadding, concentration by evaporation and precipitadon from a concen-
trated solution in methanoVmethylene chloride with DIPE and finally hexane yields the
pure title compound: TLC Rf(P)=0.41; tRet(V)=14.8 min; FAB-MS (M+H)+=655.
The starting material is prepared as follows:
a) l-r2(S)-Hvdroxv-3(S~-(trifluoroacetvlamino)-~phenvl-butvll-l-rphenYlmet~Yll-2-
rtert-butoxvcarbonYll-hYdrazine: (improved version for Reference Example 30a))
Under a nitrogen atmosphere, 20.49 g (79 mmol) of 2(R)-[l'(S)-(trifluoroacetylamino)-
2'-phenylethyl]-oxirane (Example 25 a) and 17.56 g (79 mmol) of tert-butyl-3-benzyl-
carbazate (J. 'Chem., Perkin I, 1712 (1975)) in 300 ml of ethanol are heated at 80C for
20 h. Cooling and partial concentration by evaporadon undl crystallisation commences,
filtration and washing with a small amount of ethanol yields the pure tide compound:
tRet(V)=16.1' min; FAB-MS (M+H)+=482; lH-NMR (200 MHz, CD30D): 1.30 (s, 9 H),
2.70 (m, 2 H), 2.&3-3.08 (m, 2 H), 3.76 (m, 1 H), 3.85 (s, 2 H), 4.21 (m, 1 H),7.2-7.4 (m,
10H).
b) l-r2(S)-Hvdroxv-3(S)-amino-4-DhenYl-but~,rll-l-rphenYlmethYll-2-rtert-but
carbonvll-hYdrazine: (improved version for Reference Example 30b))
Under a nitrogen atmosphere, 6.0 g (12.5 mmol) of 1-[2(S)-hydroxy-3(S)-(trifluoroacetyl-
amino)-4-phenyl-butyl]-1-[phenylmethyl]-2-[tert-butoxycarbonyl]-hydrazine dissolved in
420 ml of MeOH are heated to 80C, 125 ml of lM aqueous K2CO3 solution are addeddropwise (15 min) and the mixture is stirred for 18 h at 80C. The reaction mixture is
concentrated by evaporation and the residue is partitioned bet veen 3 portions of
methylene chloride, water and brine. Concentration by evaporation of the organic phases,
.. ~ ., ~,. :. ... .. .. . ..
'~
;,.'
- ;:
.... : ~
2 1 1 2 ~
- 174-
which have been filtered through cotton wadding, yields the title compound:
tRel(V)=l 1.5 min; IH-NMR (200 MHz, CD30D): 1.29 (s, 9 H), 2.5-3.05 (m, S H), 3.56 (m,
1 H), 3.8-3.95 (AB, 2 H), 7.1-7.4 (m, 10 H).
C): l-r2(S)-HYdroxv-3(S)-(N-(quinoline-2-carbonYl)-(L)-asPala~einvl)amino-4-phen
butvll-l-rp-(methoxvphenvl)methyn-2-rtert-butoxvcarbonvll-hydrazine:
Analogously to Exa nple 29 B), 7.00 g (16.8 mmol) of 1-~2(S)-hydroxy-3(S)-amino-4-
phenyl-butyl]-l-[p-(methoxy-phenyl)methyll-2-~tert-butoxycarbonyl]-hydrazine in 420 ml
of THF are reacted with 5.3 g (18.5 mmol) of quinoline-2-carbonyl-(L)-asparagine (hydro-
chloride salt) (l~xample 29 A) b)), 3.0 g (18.5 mmol) of HOBT, 3.8 g (18.5 mmol) of DCC
and 5 ml of NMM. The reaction mixture is filtered, the filtrate is concentrated by evapora-
tion, and the evaporation residue is takell up in ethyl acetate and washed twice in each
case with NaHCO3 solution and brine. The aqueous phases are extracted with 2 portions
of ethyl acetate and the organic phases are dried with Na2SO4 and concentrated by
evaporation. Column chromatography (SiO2, ethyl acetate) yields the pure title compound:
TLC Rf~O)=0.59; tRet(V)=14.5 min; FAB-MS (M+H)+=685.
The starting material is prepared as follows:
o ,'~
a) D-(McthoxyDhenvl)-ca~baldehvde tert-butoxvcarbonylhvdrazone: --
Under protective gas, 65 ml (534 mmol) of freshly distilled anisaldehyde are dissolved in
850 ml of ethanol,70.6 g (534 mmol) of tert-butylcarbazate are added and the mixture is
heated for 3 h at 80C. Concentration of the reaction mixture by evaporation yields the
title compound: lH-NMR (200 MHz, CD30D): 1.53 (s, 9 H), 3.82 (s, 3 H), 6.94 and 7.64
(2d, J=9 Hz, each 2 H),7.86 (s, 1 H).
b) tert-ButYl-3-(p-methoxyphenv!-methYll-carbazate:
130 g (520 mmol) of p-(methoxyphenyl)-carbaldehyde tert-butoxycarbonylhydrazone are
hydrogenated in 1.3 1 of THF in the presence of 11.5 g of 5 % Pd/C. Removal of the
catalyst by filtration through ~9Celite and concentration by evaporation of the filtrate
yields the tide compound: TLC Rf(F)=0.3; tRet(V)=8.9 min; IH-NMR (200 MHz,
CD30D): 1.44 (s, 9 H),3.77 (s, 3 H),3.83 (s, 2 H), 6.87 and 7.26 (2d, J=8 Hz, each 2 H).
c) l-r2~S)-Hvdroxv-3(S)-(trifluoroacetvlamino)-4-PhenYl-butvll-l-rP-(methoxvphenyl)
methvll-2-rtert-butoxvcarbonvll-hvdrazine:
Under protective gas, 15 g (57.9 mmol) of 2(R)-[l'(S)-(trifluoroacetylamino)-2'-phenyl-
~- 211~47
- 175-
ethyl]-oxirane (Example 25a) and 14.6 g (57.9 mmol) of tert-butyl-3-(p-methoxyphenyl-
methyl)-carbazate in 220 ml of ethanol are heated for 18 h at 80C. Cooling, concentration
by evaporation and digestion in DIPE yields the title compound: TLC Rf(E~)=0.42;tRe,(V)=15.8 min.
d) l-r2(S)-Hvdroxy-3(S~-amino-4-phenYl-butYll-l-rp-(methoxYphenyl)me~hyll-2-rtert
butoxvcarbonvll -hydrazine:
Analogously to Example 29 B) b), 19.7 g (38.4 mmol) of 1-[2(S)-hydroxy-3(S)-(trifluoro-
acetylamino)-4-phenyl-butyl]-1-[p-(methoxy-phenyl)methyl]-2-[tert-butoxycarbonyl]-
hydrazine in 1 l of methanol are hydrolysed with 384 ml of lM K2CO3 solution to yield
the tide compound: TLC Rf(G')=0.4.
Example 30: 1-r2(S)-HvdroxY-3(S~-(N-(auinoline-2-carbonvl)-(L)-asPara~invl)arnino-4-
PhenYl-butYll- l-rcvclohexYlmethvn-2-rN-methoxvcarbonvl-(L)-valvn-hvdrazine~
Unde~ a nitrogen atmosphere, 100 mg (0.-178 mmol) of 1-[2(S)-hydroxy-3(S)-(N-
(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-
hydrazine (Reference Example 71e) i)) and 34 mg (0.196 mmol) of N-methoxycarbonyl- ~ -
(L3-valine (Example 2b) are dissolved in 1.75 ml of a 0.3M solution of NMM in DMP,
74 mg (0.196 mmol) of HBTU are added and the mixture is stirred at RT. Since, after 3
days, there is still unreacted hydrazine present, a further ~.3 equivalents in each case of
N-methoxycarbonyl-(L}valine and BTU in 0.48 ml of 0.3 M NMM/D~ is added.
After 18 h the reaction mixture is concentrated by evaporation under HV, the residue is
dissolved in methylene chloride and washed with saturated NaHCO3 solution, water and
brine, the aqueous phases are extracted twice with methylene chloride, and the organic
phases are dried with Na2SO4 and concentrated by -evaporation. Column chromatography
(SiO2, methylene chlo~ide/methanol 15:1) and digestion from DIPE yields the title
compound: TLC Rf(A')=0.17; tR",(V)=14.6 min; PAB-MS (M+H)+=718. ~ -
Example 31: 1-r2(Sl-HvdroxY-3(S)-(N-(auinoline-2-carbonvl)-(O-asPara~inYl)amino-4
Phenvl-butvn-l-rcvclohexvlmethvll-2-r3.3-dimethylbutvrvn-hvdrazine:
Under a protective gas atmosphere, 100 mg (0.178 mmol) of 1-[2(S)-hydroxy-3(S)-(N-
(quinoline-2-carbony~)-(L)-asparaginyl)amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-
hydrazine (Reference Example 71 e) i)) and 25 ~1 (0.196 mmol) of 3,3-dimethylbutyTic
acid are dissolved in 1.75 ml of a 0.3M solution of NMM in DMF,74 mg (0.196 mmol) of
HBTU are added and the mixture is stirred at RT. Since, after 18 h, there is still unreacted
hydrazine present, a further 0.3-equivalents in each case of 3,3-dimethylbutyric acid and
2112~7
- 176-
HBTU in 0.5 ml of 0.3N NM~VDMF is added. Working up analogously to Example 30,
column chromatography (siO2, ethyl acetate/ethanol 20:1) and digestion from DIPE yields
the tide compound: TLC Rf(E')=0.23; tRet(V)=15.6 min; FAB-MS (M+H)+=659.
o Example 32: 1-f2(S)-Hydroxv-3(S~-(N-(quinoline-2-carbonyl)-(L)-aspara~invl~amino-4-
Dhenyl-butvll-l-rcyclohexylmethvll-2-rtert-butvlaminocarbonyll-hvdrazine:
Under a protective gas atmosphere, 100 mg (0.178 mmol) of 1-[2(S)-hydroxy-3(S)-(N-
(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]- l-[cyclohexylmethyl]-
hydrazine (Reference Example 71 e) i)) are dissolved in 0.7 ml of THF and reacted with
19 111(0.169 mmol) of tert-butyl-isocyanate for 17 h at RT. The reaction mixture is
concentrated by evaporation, the residue is dissolved in ethyl acetate and washed with 5 %
citric acid solution, water and brine, and the inorganic phases are extracted twice with
ethyl acetate, dried with Na2SO4 and concentrated by evaporation. Column chroma- ~
tography (SiO2, ethyl acetate/ethanol 10:1) yields the tide compound: TLC Rf~F')=0.16; ~ -
tRet~V)=15.3 min; FAB-MS (M+H)+=660. ~ -
Example 33: 1-r2(S)-Hydroxy-3~S)-(N-(quinoline-2-carbonYl)-(L)-aspara~inYl)amino-~
phenvl-butyn- l-rcYclohexylmethY11-2-rbenzYlatninocarbonvn-hvdrazine:
Analogously to Example 32, 100 mg (0.178 mmol) of 1-[2(S)-hydroxy-3(S)-(N-
(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-
hydrazine (Reference Example 71 e) i) in 0.7 ml of TH~ are reacted with 21 ~l
(0.16~) mmol) of benzyl isocyanate. Column chromatography (SiO2, methylene chloride/-
methanol 15:1) and digestion from DIPE yields the title compound: TLC Rf(A')=0.12;
tRe,(V)=15.5 min; FAB-MS (M+H)+=694.
Example 34: 1-r2(S)-ButYryloxy-3(S)-(N-(methoxycarbonYl)-(Ll-valyl)amino4-phen
butvll-l-~cYclohexYlmethvll-2-rN-methoxYcarbonyl-(L)-valvll-hvdrazine:
50 ,ul (0.495 mmol) of butyric acid chloride and 2 mg (0.017 mmol) of DMAP are added
to an ice-cooled mixture of 200 mg (0.33 mmol) of 1-[2(S)-hydroxy-3(S)-(N-(methoxy-
carbonyl)-(L)-valyl)amino-4-phenyl-butyl]- 1 -[cyclohexylmethyl~-2-[N-methoxy-
carbonyl-(L)-valyl]-hydrazine (Example 2a) in 2.6 ml of dioxane and 0.4 ml of pyridine.
After 18 h at RT, according to HPLC there is still 1-[2(S)-hydroxy-3(S)-(N-(methoxy-
carbonyl)-(L)-valyl)amino-4-phenyl-butyl]- 1-[cyclohexylmethyl]-2-[N-methoxy-
carbonyl-(L)-valyl]-hydrazine present in the reaction mixture, and therefore a further 0.75
equivalents of butyric acid chloride and a few granules of DMAP are added. After a
further 18 h, a 3rd portion of 1.5 equivalents of butyric acid chloride is added and the
~ ~ .
21120~7
- 177-
mixture is further stirred for one night at RT (~ HPLC: fully reacted). The reaction
mixture is diluted with ethyl acetate and washed with 2 portions of saturated NaHCO3
solution, water and brine, the aqueous phases are extracted twice with ethyl acetate, and
the organic phases are dried with Na2SO4 and concentrated by evaporation. Digestion of
the oily residue from hexane in an ultrasound bath yields the pure title compound: TLC
Rf(O)=0.67; tRe,(V)=17.2 min; FAB-MS (M+H)+=676.
Exam~le 35: 1-r2(S)-Palmitoyloxv-3(S)-(N-(methoxvcarbonvl)-(L)-valvl)amino-4-
, Phenvl-butYll-l-rcYclohexYlmethY11-2-rN-methoxYcarbonYl-(L)-valYll-hYdrazine:
Analogously to Example 34, 2Q0 mg (0.33 mmol) of 1-[2(S)-hydroxy-3(S)-(N-(methoxy-
carbonyl)-(L)-valyl)amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-2-[N-methoxycar-
bonyl-(L)-valyl]-hydrazine (Example 2a) in 2.6 ml of dioxane and 0.4 ml of pylidine are
reacted with 0.15 ml (Q.495 mmol) of palmidc acid chloride and 2 mg (0.017 mmol) of
DMAP. To complete the reaction a further 0.3 ml of palmitic acid chloride and a small
amount of DMAP are added and the mixture is stirred. Extraction and column chroma- ~ ~ ~
tography (SiO2, ethyl acetate/hexane 3:2) yields the pure title compound: TLC ~-
Rf(H')=0.47; tRe,(V)--25.2 min; FAB-MS (M+H)+=844.
Example 36: 1-r2(S)-(Methoxv-acetoxY)-3(S~-(N-(methoxycarbonYl)-(L)-valyl)amino~
Dhenvl-butvn-l-rcvclohexvlmethvn-2-rN-methoxvcarbonvl-(L)-valvn-hvdrazine:
Analogously to Example 34, 200 mg (0.33 mmol) of 1-[2(S)-hydroxy-3(S)-(N-(methoxy-
carbonyl)-(L)-valyl)amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-2-[N-methoxy-
carbonyl-~)-valyl~-hydrazine (l~xample 2a) in 2.6 ml of dioxane and 0.4 ml of p~vridine
are reacted with 50 111 (0.495 mmol) of methoxyacedc acid chloride and 2 mg
(0.017 mmol) of DMAP. To complete the reaction a further 0.5 equivalents of methoxy-
acetic acid chl~ide and a few granules of DMAP are added and the mixture is stiTred.
Extraction and digestion from DIPE/hexane in an ultrasound bath yields the pure tide
compound: TLC Rf(0)=0.48; tRet(V)=15.6 min; FAB-MS (M+H)+=678.
Example 37: 1-r2(S)-(2-Pvridvl-carbonYl)oxv-3(S)-(tert-butoxvcarbonYl)amin~4-Phen
butyll-l-rcYclohexvlmethY11-2-rtert-butoxYcarbonvn-hydrazine:
At 0C under a nitrogen atmosphere, 500 mg (4.07 mmol) of picolinic acid ~luka;
Buchs/Switzerland) in 25 ml of methylene chloride are converted into the acid chloride
with ().57 ml (4.07 mmol) of 1-chloro-N,N,2-trimethyl-1-propenamine [B. Haveaux, A.
Dekoker, M. Rens, A.R. Sidani, J. Toye, L. Ghosez, M. Murakami, M. Yoshioka, and W.
Nagata, Organic Syntheses 59, 26 (1980)]. After 45 min, 10 ml of THF, 8.3 ml of
..'. - ~
2112~7
- 178-
pyridine, 10 mg of DMAP and 1.00 g (2.03 mmol) of 1-[2(S)-hydroxy-3(S)-(tert-butoxy-
carbonyl)amino-4-phenyl-butyl]- 1 -[cyclohexylmethyl]-2-[tert-butoxycarbonyl] -hydrazine
(= Boc-~PheNNCha]-Boc, Reference Example 4) are added and the mixture is stirred for
16 h at RT. Since according to HPLC there is still 1-[2(S)-hydroxy-3(S)-(tert-butoxy-
carb~nyl)amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-2-[tert-butoxycarbonyl]-hydIazine
present in the reaction mixture, a further 2 equivalents of picolinic acid chloride (prepared
as described above) dissolved in 20 ml of methylene chloride are added. After 18 h at RT ~ -
the mixture is diluted with methylene chloride and washed twice with saturated N~CO3
solution, water and brine, and the organic phases are extracted with 2 portions of
methylene chloride, dried with Na2SO4 and concentrated by evaporation. The brownresidue is dissolved in methylene chloride/ethyl acetate, 10 g of silica gel are added and
the mixture is concentrated by evaporation. The resulting powder is applied to a silica gel
column (hexane/ethyl acetate 1:1). Elution widh hexane/ethyl acetate 1:1 yields the tide
compound: TLC Rf(N)=0.17; tRet(V)=19.9 min; lH-NMR (200 MHz, CD30D): 0.6-1.85
(m, 11 H, cyclohexyl), 1.36 and 1.40 (2s, 18 H, 2 Boc), 2.4-3.1 (m, 6 H), 4.28 (m, 1 H),
5.28 (m, 2 H),7.1 7.3 (m, 5 H),7.72 (m, 1 H), 8.08 (m, 1 H), 8.28 (d, J=7 Hz,1 H), 8.75
(d, J=5 Hz, 1 H).
a) 1-[2(S)-Hydroxy-3(S)-(N-(methoxycarbonyl)-(L)-valyl)amino-4-phenyl-butyl]-
l-[cyclohexylmethyl]-2-[pyridin-2-yl-carbonyl]-hydrazine:
i. Under a nitrogen atmosphere, 200 mg (0.335 mmol) of 1-[2(S)-(pyridin-2-yl-carbonyl)-
oxy-3(S)-(tert-butoxycarbonyl)amino-4-phenyl-butyl]-1-[cyclohexylmethyl]-2-[tert-
butoxycarbonyl]-hydrazine are dissolved in 200 ml of forrnic acid and the solution is
stirred for 16 h at RT and then concentrated by evaporation under HV. The residue is
partitioned between 3 porlions of methylene chloride, saturated NaHCO3 solution and
brine and the organic phases are dried with Na2SO4 and concentrated by evaporation
(kd(V)=11.9 min).
ii. The above residue is dissolved in 5.3 ml of 0.25M NMM in acetonitrile, and 134 mg
(0.763 mmol) of N-(methoxycarbonyl)-(L)-valine (Example 2b) and 313 mg (0.826 mmol)
of HBTU are added. On stirring for 18 h at RT the title compound separates out in the
form of a precipitate and can then be filtered off and washed with a small amolmt of
acetonitrile. tRe,(V)=15.9 min; FAB-MS (M+H)+=554, lH-NMR (500 MHz, DMSO-d6):
0.64 and 0.70 (2s, J=7 Hz, (H3C)2C), 0.81 (m, 2 H",~-C6Hl 1), 1.08 (m, 3 H "~-C6H"), 1.30
(m, H~ -C6H~1), 1.57 (m,3 H~ q-C6Hll)~ 1.74 (m, 1 Heq-C6H~ 1.79 (octet, J=7 Hz,
HC(CH3)2), 1.88 (m, 1 Heq~C6Hll)~ 2.47-2.60 (m, H-Cl, HCH-C6Hll), 2.66-2.84 (m,
H-Cl, HCH-C6Hll, H2C-phenyl),3.44 (m, HC2),3.50 (s, H3C-O), 3.73 (m, HC0~-Val),
21120l17
- 179-
3.94 (m, HC3), 4.94 (s, HO), 6.97 (d, J=9 Hz, HN-Val), 7.05 (m, HCphenyl)~ 7.12 (m, 4
HCpheDy1), 7-51 (d, J=9 Hz, HN-C3), 7.61 (m, Hs-Py), 8.00 (m, H3-Py, H4-Py), 8.61 (d, J=5
Hz, H6-Py, 9.70 (s, HN-N).
Example 38: 1-r2(S)-Hvdroxv-3(S)-(N-(quinoline-2-carbonvl)-(L)-asparaginyl~amino-4-
phenvl-butyll- 1- rbenzYll-2-rN-methoxYcarbonvl)-(L)-valvll-hYdrazine:
Analogously to Example 40, 157.4 mg (0.898 mmol) of N-(methoxycarbonyl)-(L)-valine
(Example 2b) and 453 mg (0.817 mmol) of 1-[2(S)-hydroxy-3(S)-(N-(quinoline-2-car-
bonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-1-[phenylmethyl]-hydrazine in 8 ml of
0.3M NMM/DMF are reacted with 340 mg (0.898 mmol) of BTU. Column
chromatography (SiO2, methylene chloride ~ methylene chloride/medhanol 50:1 ~ 25:1)
yields the tide compound: TLC Rj~(J')=0.16; tRet(V)=13.6 min; FAB-MS (M+H)+=712.
Example 39: 1-r2(S)-Hvdroxv-3(S)-(N-(auinoline-2-carbonyl)-(L)-aspara~inyl)amino-4-
phenvl-butYll-l-rbenzY11-2-rN-ethoxYcarbonyl~-(L)-yalyll-hydrazine:
Under protective gas, 36 mg (0.189 mmol) of N-(edhoxycarbonyl)-(L)-valine (13xample
9a), 35 mg (0.259 mmol) of HOBT and 38 mg (0.198 mmol) of EDC are dissolved in
0.7 ml of 0.3M NMM/DMF and the solution is stirred for 10 min at RT. 100 mg
(0.180 mmol) of 1-~2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-
4-phenyl-butyl]-1-[phenylmethyl]-hydrazine (Example 40a) are added and the mixture is
then stir ed for 18 h at RT. The reaction mixture is concentrated by evaporation under HV,
and the residue is taken up in ethyl acetate and washed with 2 portions of 10 % citric acid
solution, water, saturated NaHCO3 solution and brine. The aqueous phases are extracted
with ethyl acetate and the combined organic phases are dried with Na2SO4 and
concentrated by evaporation. Column chromatography (SiO2, methylene chloride/-
methanol 12:1) yields the dtle compound: TLC Rf(I')=0.25; tRet(V)=14.3 min.
Example40: 1-r2(S)-Hvdroxv-3(S)-(N-(quinoline-2-carbonvl)-(L)-asparaginvl)amino-4-
phenv!-butvll- l-~benzvn-2-~N-benzvloxvcarbonyl)-(L)-valvn-hvdrazine:
Under a nitrogen atmosphere, 50 mg (0.198 mmol) of Z(L)-valine and 100 mg
(0.18 mmol) of 1-[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-
4-phenyl-butyl]-1-[phenylmethyl]-hydrazine are dissolved in 1.8 ml of a 0.3M solution of
NMM in DMF, 75.1 mg (0.198 mmol) of HBTU are added and the mixture is sdrred for18 h at RT. The reaction mixture is concentrated by evaporadon under HV, and theresidue is taken up in ethyl acetate and washed with 2 pordons of 10 % citric acid
solution, water, saturated NaHCO3 solution and brine. The aqueous phases are extracted a
2112~7
- 180-
further twice with ethyl acetate and the combined organic phases are dried with Na2SO4
and concentrated by evaporation. Column chromatography (SiO2, methylene chloride/-
methanol 19:1) yields the title compound: TLC Rf(J')=0.27; tRet(V)=15.7 min; FAB-MS
(M+H)+=788.
The starting material is prepared as follows:
a) 1-~2(S)-HYdroxY-3(S)-(N-(quinoline-2-carbonYl)-(L)-asl)ara~inYl)amino-4-Phenvl-
butvll- 1 -rphenYlmethvll-hYdrazine
1.0 g (1.53 mmol) of 1-[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)-
amino-4-phenyl-butyl]-1-[phenylmethyl]-2-[tert-butoxycarbonyl]-hydrazine (Example 29
B) is dissolved in 10 ml of formic acid under protective gas and stirred for 16 h at RT. The
formic acid is removed by evaporadon under HV, the residue is parddoned between 3
pordons of ethyl acetate, saturated NaHCO3 solution and brine, and the organic phases
are dlied with Na2SO4 and concentrated by evaporation to yield the tdtle compound:
tRe,(V)=10.7 min.
Example41: 1-[2(S)-Hydroxy-3(S)-(N-(quinoline-2-carbonyl)-~L)-asparaginyl)amino-4-
phenyl-butyl]-l-[benzyl]-2-[N-allyloxycarbonyl)-(L)-valyl]-hydrazine is prepared in
accordance with one of the processes mentioned hereinbefore or hereinafter.
Example 42: 1-[2(S)-Hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-
phenyl-butyl]-l-[~methoxyphenylmethyl]-2-[N-methoxycarbonyl)-(L)-valyl]-hydrazine
is prepared in accordance with one of the proc~,sses mendoned hereinbefore or hereinafter.
Example 43: 1-r2(S)-Hvdroxv-3(S)-(N-(guinoline-2-carbonYl)-(L)-asparaginyl)amino-4-
~henvl-butvn-l-rp-(methoxYPhenvl)methvn-2-rN-(benzvloxvcarbonvl)-(L)-valvll-
hvdrazine:
Under a nitrogen atmosphere, 330 mg (1.32 mmol) of Z(L)-valine and 700 mg
(1.197 mmol) of 1-[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-
~phenyl-butyl]-l-[p-(methoxyphenyl)methyl]-hydrazine (Example 49 B) a)) are dissolved
in 11.6 ml of a 0.3M soludon of NMM in DMF, 0.50 g (1.32 mmol) of HBTU is added
and the mixture is sdrred for 18 h at RT. The reacdon mixture is concentrated by evapora-
tion under HV and the residue is taken up in ethyl acetate and washed with saturated
NaHCO3 soludon, water and brine. The aqueous phases are extracted a further twice with
ethyl acetate and the combined organic phases are dried with Na2SO4 and concentrated by
r. ~
r` ~
21.120ll7
- 181-
evaporation. Column chromatography (SiO2, ethyl acetate) yields the title compound after
crystallisation from DIPE. TLC Rf(G')=0.50; tRet(V)=15.4 min.
Example 44: 1-[2(S)-Hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-
phenyl-butyl]-1-[4-methoxyphenylmethyl]-2-[N-allyloxycarbonyl)-(L)-valyl]-hydrazine is
prepared in accordance with one of the processes mentioned hereinbefore or hereinafter.
Example 45: 1-[2(S)-Hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-
(4-methoxyphenyl)-butyl]-1-[benzyl]-2-[N-methoxycarbonyl)-(L)-valyl]-hydrazine is
prepared in accordance with one of the processes mentioned hereinbefore or hereinafter.
Example46: 1-[2(S)-Hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-~(4-methoxyphenyl)-butyl]-1-[benzyl]-2-[N-benzyloxycarbonyl)-(L)-valyl]-hydrazine is
prepared in accordance with one of the processes mentioned hereinbefore or hereinafter.
Example n: 1-[2(S)-Hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-
(4-benzyloxyphenyl)-butyl]-1-[benzyl]-2-[N-methoxycarbonyl)-(L)-valyl]-hydrazine is
prepared in accordance with one of the processes mentioned hereinbefore or hereinafter.
Example 48: 1 -[2(S)-Hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4
(4-benzyloxyphenyl)-butyl]-1-[benzyl]-2-[N-allyloxycarbonyl)-(L)-valyl]-hydrazine is
prepared in accordance with one of the processes mentioned hereinbefore or hereinafter.
Example 49: 1-r2(S)-HvdroxY-3(S)-(N-(quinoline-2-carbonvl)-(L)-asparaninyl)amin~4-
phenvl-butYn- l-r~-(methoxYPhenvl)methYn-2-rN-(ethoxvcarbonyl)-a~)-valvll-hydrazine:
Analogously to Example 39),71.2 mg (0.376 mmol) of N-(ethoxycarbonyl)-(L)-valine(Example 9a), 66 mg (0.487 mmol) of HOBT, 72.1 mg (0.376 mmol) of EDC and 200 mg(0.34 mmol) of 1-[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-
4phenyl-butyl]-1-[p-(methoxyphenyl)methyl]-hydrazine are reacted in 1.33 ml of 0.3M
NMMJDMF. Column chromatography (SiO2, methylene chloride ~ methylene chloride/-
methanol 50:1 ~ 20:1) yields the title compound: tRe,(V)=13.9 min.
The starting material is prepared as follows:
., .. , .~: ~
` 2112~7
- 182-
a) 1-~2(S)-Hvdroxv-3~S)-(N-(quinoline-2-carbonYl)-(L)-aspara~invl)amino-4-Phenvl-
butYll-l-lp-(methoxvphenyl)methvll-hvdrazine
Analogously tO Example 40a~7 4.6 g (6.71 mmol) of 1-[2(S)-hydroxy-3(S)-(N-(quinoline-
2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-1-[p-(methoxyphenyl)methyl]-2-[tert-butoxycarbonyl]-hydrazine (Example 29 C) are reacted in 168 ml of formic acid to
form the title compound: tRet(V)=10.8 min.
Example 50: 1-[2(S)-Hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-
phenyl-butyl]-1-[4-methoxyphenylmethyl]-2-[N-allyloxycarbonyl)-(L)-valyl]-hydrazine is
prepared in accordance with one of the processes mentioned hereinbefore or hereinafter.
Example 51: 1-[2(S)-hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-
phenyl-butyl]- 1 -[4-benzyloxyphenylmethyl]-2-[N-methoxycarbonyl)-(L)-valyl]-hydrazine
is prepared in accordance with one of the processes mentioned hereinbefore or hereinafter.
Examp!e 52: 1-[2(S)-Hydroxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-
phenyl-butyl]- 1- [4-benzyloxyphenylmethyl]-2-[N-allyloxycarbonyl)-(L)-valyl]-hydrazine
is prepared in accordance with one of the processes mentioned hereinbefore or hereinafter.
Example 53: The following are prepared analogously to one of the processes mentioned
hereinbefore:
A) 1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L~valyl)amino-4-cyclohexyl-butyl]-1-[4-methoxyphenylmethyl]-2-[N-methoxycarbonyl)-(L)-valyl]-hydrazine;
B) 1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-cyclohexyl-butyl]-1-[4-
isobutoxyphenylmethyl]-2-[N-methoxycarbonyl)-(L)-valyl]-hydrazine;
C) 1-[2(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-cyclohexyl-butyl]-1-[4-
ethoxyphenyimethyl]-2-[N-methoxycarbonyl)-(L)-valyl]-hydrazine;
D) 1-12(S)-hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-cyclohexyl-butyl]-1-[4-
benzyloxyphenylmethyll-2-[N-methoxycarbonyl)-(L)-valyl]-hydrazine.
Example 54: 1-[2(S)-Hydroxy-3(S)-(N-methoxycarbonyl-(L)-valyl)amino-4-phenyl-
butyl]-l-[cyclohexylmethyl]-2-[2-pyridylcarbonyl]-hydrazine is prepared analogously to
one of the processes mentioned hereinbefore.
.
~ . ' . .: '...... , . :
- 2112~7
- 183-
ExampleSS: 1-l2(S)-Hvdroxy-3(S)-(N-(quinoline-2-carbonYI)-(L)-aspara invl)amino-4-
phenyl-butyll- 1 -rphenylmethvll-2-rN-(methoxY-ethoxY-ethoxYcarbonyl)-(L~-va
hydrazine:
Analogously to Example 40,52 mg (0.198 mmol) of N-(2-(2-methoxy-ethoxy)-ethoxy)-carbonyl-(L)-valine (Example lla)) and lOOmg (0.180mmol) of 1-[2(S)-hydroxy-3(S)-
(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-1-[phenylmethyl]-
hydrazine in 1.8 ml of 0.3M NMM/DMF are reacted with 75.1 mg (0.198 mmol) of
HBTU. The evaporation residue is taken up in methylene chloride and washed with 2
portions of 10 % citric acid solution, water, saturated NaHCO3 solution and brine. The
aqueous phases are extracted a further twice with methylene chloride and the combined
organic phases are dried with Na2SO4 and concentrated by evaporation. Column chroma-
tography (SiO2, methylene chloride/methanol 19:1) yields the dtle compound: TLC
Rf(l')=0.08; tRe,(V)=13.5 min; FAB-MS (M+H)+= 800.
Example 56: 1-r2(S)-(2-Pvridvl-carbonvl)oxy-3(Sl-(N-(quinoline-2-carbonvl~-(L)-aspara-
invl)amino-4-phenvl-butvn- 1 -rphenYlmethvll-2-~N-(methoxYcarbonYl)-(L)-valvll-
hvdrazine
Analogously to Example 37, 51 mg (0.416 mmol) of 2-picolinic acid in 0.8 ml of
methylene chloride are converted with 59111 (0.416 mmol) of 1-chloro-N,N,2-trimethyl-
l-propenamine into the acid chloride. After the addition of 0.5 ml of dioxane and 0.4 ml of
pyridine to the latter, the mixture is reacted widh 148 mg (0.208 mmol) of 1-[2(S)-hydr-
oxy-3(S)-(N-(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-1-[phenyl-
methylJ-2-[N-(methoxycarbonyl)-(L)-valylJ-hydrazine (l~xample 38) in 5 ml of dioxane in
dhe presence of 0.5 mg of DMAP. Since, after 18 h, not all of dhe educt has been acylated
according to HPLC, further acid chloride is added. Column chromatography (SiO2,
methylene chloride ~ methylene chloride/methanol 15:1) yields the tide compound:FAB-MS (M+H)+= 817.
Exam~le 57: 1-r2(S~-Butvroxv-3(S)-(N-(quinoline-2-carbonvl)-(L)-asparaginvl)amino-4-
l~henvl-butyn-l-r~henvlmethvll-2-rN-(methoxYcarbonvl~-(L)-valvll-hvdrazine
Under a nitrogen atmosphere, a small amount of DMAP and 0.2 ml of butyric acid
chloride are added to a solution of 121 mg (0.17 mmol) of 1-[2(S)-hydroxy-3(S)-(N-
(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-1-[phenylmethyl]-2-[N-
(methoxycarbonyl)-(L)-valyl]-hydrazine (E~xample 38) in 3.5 ml of dioxane, 2 ml of
methylene chloride and 0.4 ml of pyridine. Dilution of the reaction mixture withmethylene chloride, washing with 2 portions of saturated NaHCO3 solution, water and
2112~7
- 184-
brine, extraction of the aqueous phases with 2 portions of methylene chloride, drying of
the organic phases with Na2SO4, concentration by evapora~ion and column chroma-
tography (SiO2, methylene chloride/methanol 50:1 -> 19:1) yields the title compound:
TLC Rf(I')=0.5; tRet(V)=15.6 min.
Example 58: 1-r2(S)-(2-Pyridyl-carbonyl)oxy-3(S)-(N-(quinoline-2-carbonYl)-L)-aspara
~inYl)amino-4-~henyl-bu~Yn- l-rp-(me*oxyphenvl)methvll-2-rN-(benzYloxvcarbonvl)-~L) -valyll -hydrazine
Analogously to Example 37), 72.7 mg (0.591 mmol) of 2-picolinic acid in 2 ml of
methylene chloride are converted vith 87 111(0.614 mmol) of 1-chloro-N,N,2-trimethyl-
l-propenamine into the acid chloride. After the addition of 1.36 ml of pyridine to the
latter, the mixture is reacted with 100 mg (0.118 mmol) of 1-[2(S)-hydroxy-3(S)-(N-
(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]- l-[p-(methoxyphenyl)-
methyl]-2-[N-(benzyloxycarbonyl)-(L)-valyl]-hydrazine (Example 43)) in the presence of
1 mg of DMAP. Further acid chloride is added in portions until according to HPLC all of
the educt has been acylated to the title compound.
Example 59: 1-r2(S)-(Methoxv-acetoxv!-3(S~-(N-(quinoline-2-carbonyl)-(L)-asparaginYl)-
amino-~phenYl-butyll-l -rp-(methoxyphenYl)methY11-2-rN-(benzyloxYcarbonyl)-L)
valvn -hydrazine
Analogously to Example 34, 100 mg (0.118 mmol) of 1-[2(S)-hydroxy-3(S)-(N-
(quinoline-2-carbonyl)-(L)-asparaginyl)amino-4-phenyl-butyl]-1-[p-(methoxyphenyl)-
methyl]-2-[N-(benzyloxycarbonyl)-~L)-valyl]-hydrazine (Example 43)) in 2.4 ml ofdioxane and 0.14 ml of pyridine are acylated in the presence of 0.7 mg of DMAP with
38.5 ,ul (0.35 mmol) of methoxyacetic acid chloride to foTm the title compound: tRet(V) =
17.8 min.
Example 60: Gelatine solution: - -
A sterile-filtered aqueous solution, with 20 % cyclodextrins as solubilisers, of one of the
compounds of formula I mentioned in the preceding Examples as active ingredient, is so
mixed under aseptic conditions, with heating, with a sterile gelatine solution containing
phenol as preservative, that 1.0 ml of solution has the following composition:
active ingredient 3 mg
gelatine 150.û mg
phenol 4.7 mg
2 11 ~
- 185-
dist. water with 20 % cyclodextrins
as solubilisers 1.0 ml
Example 61: Sterile dry substance for injection:
S mg of one of the compounds of formula I mentioned in the preceding Examples as acdve
ingredient are dissolved in 1 ml of an aqueous solution with 20 mg of mannitol and 20 %
cyclodextrins as solubilisers. The solution is sterile-filtered and introduced under aseptic
conditions into a 2 ml ampoule, deep-frozen and lyophilised. Before use, the lyophilisate
is dissolved in 1 ml of distilled water or 1 ml of a physiological saline solution. The
solution is administered intramuscularly or intravenously. This formulation can also be
introduced into a twin-chambered injection ampoule.
Example 62: Nasal sPraY:
500 mg of finely ground (<5.0 llm) powder of one of the compounds of formula I men-
tioned in the preceding Examples is suspended as active ingredient in a mixture of 3.5 ml
of Myglyol 812~ and 0.08 g of benzyl alcohol. The suspension is inlroduced into a
container having a metering valve. 5.0 g of Freon 12~ are introduced under pressure into
the container through the valve. The "Freon" is dissolved in the Myglyol/benzyl alcohol
mixture by shaking. The spray container contains approximately 100 single doses which
can be administered individually.
Example 63: Pilm-coated tablets
The following ingredients are used for the preparation of 10 000 tablets each containing
100 mg of active ingredient:
active ingredient 1000 g
corn starch 680g
colloidal silica 200 g
magnesium stearate 20 g
stearic acid 50 g
sodium carboxymethyl starch 250 g
water quantum satis
A mixture of one of the compounds of formula I mentioned in the preceding Examples as
active ingredient, 50 g of corn starch and the colloidal silica is processed with a starch
paste, made from 250 g of corn starch and 2.2 kg of demineralised water, to form a moist
,. ~ . " . . ~ . . .
"
.', '''' ' ~
--` 2112V4'^~
- 186-
mass. This is forced through a sieve having a mesh size of 3 mm and dried at 45 for
30 min in a fluidised bed drier. The dry granules are pressed through a sieve having a
mesh size of 1 mm, mixed with a pre-sieved mixture (1 mm sieve) of 330 g of corn starch,
the magnesium stearate, the stearic acid and the sodium carboxymethyl starch, and
compressed to form slightly biconvex tablets.