Note: Descriptions are shown in the official language in which they were submitted.
- 1 2112250
T-TL~
~ew ~im2 ~ ino~ croc~ l'.can cl~.os~honic ac cs zr.c
... . _ _
salts ~ e~~^ , tne~r Drocuction ~rocess and r~lz~Q-c
~:~_r~ace~-.ical com~csi iors.
.
~ ~E5~.~_?TI0~
~ r' Q 1 ~ O - ~_~n - T ,~ ~e n ' isr
The ~QS~nt invention relates to new dimet:~yl-
amino-h;icro~y--lkan diphosphonic ac ds anc the szl.s
thereof.
Furthermore the invention relates to a method for
their production, as well as the pharmaceutical
compositions containing these product.s.
Description o~ the relevant art
:: ` ~ :
It is known ~the~ h1gh comple~ing ac-ivity shown
with res?ect to~divalent and poly~alent metallic ions
:: .
~ by the diphosphonic~ acids and their water soluble
i ~ 20 ~ salts, in particular~o~f the group comprising the ~mino-
hydroxy-alkan-d1phos?honic acids, eS~en if used in
substo1chiometric ~ quantities. With res?ect ~o
~ polyphosphates, which~show like prope~,ies an can also
l~ be used in subs~oichiometric quantities, they ha~ the
25~ ~advantage, as it is well known, of being stable to tne
hydrolysis. Thanks to this property the above group or
compounds has been used with advantage, inter aliz, in
the phar~aceu'ical field for the treatment OL diseases
; associat-d wit~ dis~ rbances ol the bone metabolism,
~ 30 s~ch as 0s,~3~0rosis, Paset's cise~se, osteol~ys~s~ ~ ~ caused by tumourand hyperparathyrodis~, osteoarthrosis,
arthritis and tne like.
~ ~ ~ 211225;0
The l- t~7 .-o-l- h ~C-O:C ~ )utan-~ o s~r.or.i c ac G-`.d
i _ ~ C ~ a t _ . 2s (s2G Ital_c.. ~c ~ o-1-0108/ _. t..2
na~e o~ t:-~ same ap?licar.t) h-s ~ ven par.icula~l~
2--~c_ ~ ~n -.is ~_s?ect. The a-c.-- c_~.-cunc is ~c= J-
S ~ t c_~.c_..__~__~..s ...~ ._ t:;_.. _ ..__s;m~ ci~.os?~onic c~m?ou.cs .iith cor-2s?0-.d-nsl;
lowe~ u~wanted side ef ects.
3 .e_h c-.-nv-h~;c_,:;;-al.~an c~ O__^Gl-'C ac~__ cnC
their cerivz~ives are also knowr~ wnich snow similar
pro?erties and ac~i.vity when al~l g.oup is prop~l or
butyl. In particular, ~S patent ~0.40s459a discloses,
inter alia, the 3-dime~hylamino-hydroxy-propan-1,1-
. diphosphonic acid, its preparation a~d uses, while US
patent No.4624947 dlscloses the 4-dimethylamlno-
lS hydrQxy-butan-l,l-diphosphonic acid, :its preparation
~: and uses t unaer the form both or the acid and of its
..
water soluble salts. .The latter compounds .are .also
described as .possb1le ~ radionuclie caxriers to
particular biological~tissues,: such as for example the
bone~ t1ssue, for the eYecution ol- scintigraphic
ana~Yses (see ~or ins~ance Eurooe~nt patents No.96932
and 96933).
The known ~Frocesses for the preoaration o~
phosphonic acids and in particular o,~ amino-hydroxy
alkan-di~hos~honic . aci~s, :~com?r se re~c~ing the
corr~espondin~ amino-carboxylic acid witn a mixture of
phosphoxous acid and ~ phosphorus halide, such as
phosphorus t~ichlor1de, and hydrol~zi nG the poly~ eric
products thus obtained in orde- to give the wanted
3D ~dipnos?honic acid to be recover2G unce_ purifiec ~orm,
: for e~am51e, b~I cr~stalliz~tion.
~: According to US patent No.~A07761 the reaction is
:conducted in the presence or an organic inert
:: ~
, ~
W~93/00348 PCT/~2/00~58
_ 3 _ 2il2250
- substance, for example a chlorinated hydrocarbon such
as chlorobenzene, while the hydrolysis is conducted
with a not oxidizing strong acid. According to another
process described in US patent No.4705651, the same
reaction is conducted without either solvents ox inert
substances and with different reactants molar ratios,
using water as hydrolyzing agent and recovering the
wanted amino diphosphonic ~cid by precipitation with an
alcohol which is added to the hydrolized solution.
According to US patents No.4054598 and No.4624947
the dimethylamino-l-hydroxy-alkan~ diphosphonic
acids can be prepared from the corresponding
dimethylamino-l-amino-alkan-l-hydroxy-l,l-diphosphonic
acids by reaction with nitrous acid, salts thereof and
compounds forming nitrous acid under the reaction
conditions,while the reactant acid can be obtained from
the corresponding nitrile by reaction with phosphorous
: halide under anhydrous conditions in the presence of a
~; sol~ent or an inert inorganlc diluent followed by
hydroly5is in water. As an alternative the reactant
acid can be obtalned by phosphonylation of the
: corresponding dimethylated aminoacid with phosphorous
,, :
:~ ~ acid in the presence of PCl3 or by other
phosphonylation reactions well known to the person
skilled in the art.
It is an object of the present invention to provide
.
new dimethylamino-l-hydrcxy-alkan~ diphosphonic
acids, their derivatives and water soluble salts.
;~: : It is a further object of the present invention to
provide a process for the industrial production of the
~: above mentioned compounds.
Another object of the invention is to provide
pharmaceutical compositions comprising the
.
W093/~0~8 PCTt~2/0~0~8
21122~0
-- 4
dimethylamino-l-hydroxy-alkan-l,l-diphosphonic acids,
their derivatives and water soluble salts according to
the present invention for the administration to
patients suffering from diseases associated with
disturbances of bone metabolism.
Summary of the Invention
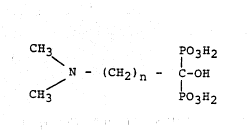
The above ob~ects are reached with the new
dimethylamino-l~hydroxy-alkan-l,l-diphosphonic acids
and salts ~hereof which are characterized by the
formula
CH3 1O3H2
N - ~CH2)n ~ f-OH
CH3 P3H2
wher in n=4 or 5
More particularly, the compounds according to the
~; present invention are the 5-dimethylamino-1-hydroxy-
: pentan-l,l-diphosphonic acid (DAPeDP) and the 6-
dimethylamino-l-hydroxy-hexan-l,l-diphosphonic acid
(DAEDP), as well as their non-toxic, pharmaceutically
acceptable, water-soluble salts.
: The compounds according to the present invention can
be prepared in several ways. Accordiny to known methods
already in: use for compounds of the same class, both
DAPeDP and DABDP can be o~tained respectively from the
5;-dl~ethylamino-1-amino-pentan-1,1-diphosphonic acid
and from the 6-dimethyl-1-amino-esan~ diphosphonic
acid by react~lon with nitrous acid or with reactants
capabIe of forming nitrous acid under the reaction
conditions. The dimethylated amino-alkyl diphosphonic
;~:
acids used as reactants can be obtained by reaction of
5-dimethylamino-valeronitrile and, respectively, of 6-
: ~ :
2~122~0
ci.~-~ ~.O~~?r^~t~ vlt~ Gn, 2mou;~.~ o- ?~r,
si~c:^ ~ c:~e_ t~ e s.olchiome_~ic unce_ ann-,c~ous
conc-_ic-s a..c in the ?res~nce o' an iner~ sollJe-.t or
C' "~ ?rC'-C_ 05_' i -.eC ~ his va~ ls .:---
-or.._-. 30t'^. ~.-Pe~2 _-.d D.'~DP c~n ais~ ~e procuc^~
reac.ins the 5-~lmeth~lamino valeric acid and,
_S___=' ';2 ',', .-e o-d'me_h~-l~mino ca~roic ac~c
H3P03 and P.{3 (wrere ~ = halogen~ either in the
presence o~ or in the absence of solvents or diluting
agents. The molar ratios among the reactants
~aminoacia: H3P03:P~3) are quite variable, generally
comprlsed between 1:1:1 and 1:20:5 acording to ~rhether
'a solvent is use~d or not. Preferred'molar ratio or
'~ 15 1:5:2 in the absence of a solvent is used.
~' The product obtained in this way is subjected to
hydrolysis wnich can be perrormed both with a non-
oxidizing strong acid in aqueous solution and }or a
: :
longer time with~ water only. The 5-dimethylamino
2a valeric acid and the 6-dimethylamino caproic acic can
be obtained;by dialkylation according to methods ~nown
' in'the`art.
est ma'de or car-vLnq out the inventlon
A~art rrom the above described known processes LOr
pr'oducin~; t:-~e com~ou~ds of the ~res2nt inven~ion,
accord~ng to the same invention there is provided an
origl~nl method ~;p~rtlcularly suitable, even on
industrial scale, for the production OL compounds OL
; the for~ula:
CH3 P03H2
N - (CH2)n - C-OH
CH3 P3~2
wherein n is eaual to 4 or 5'.
W093/00~ ~ ;, PCT/~2/00058
i. ! , 211~ 6 - ! 'T~e method consists in reacting the 5-amino
valeric acid, or respectively, the 6-amino caproic acid
with formic acid and formaldehyde 40~ solution with
molar ratios generally comprised between 1:2:2 and
51:10:4 under reflux for a time variable irom 0.5 and 3
hours. The mixture of reaction is then concentrated
under vacuum and at a temperature of 100-120C until
distillation has gone to end and the residual product
thus obtained is directl~ passed to the reaction with
H3P03 and PX3 in the previously indicated, known molar
ratio without the need of isolating any intermediate
product. The product resulting from the reaction can be
easily recrystallized from water if necessary.
The above described method allows for the final
product to be obtained from the correspon ing
aminoacids which are more easily available and less
expensive than the corresponding dimethylamino
substituted acids that are thus formed in situ and
reacted with~ no need for their purification, this
obviously resulting ln a considerable overall saving of
the process. ; ~
Practica1~ examples of the method according to the
invention are given herebelow.
Example l
25~ ~131;,2 ~g (l~ mole~ of 6-amino-caproic acid are
dissolved in 190 ml (5 moles) of 99% formic acid and
170 ml (2,2 molesjiof a 40% solution formic aldehyde;
` the solution is kept under reflux for 3 hours and then
concentrated under vacuum up to about llO~C until the
end of distillation. The reaction apparatus is placed
nder nitrogen and 246 g (3 moles) of H3PO3 are added;
the temperature is raised to 90C and 363 ml (4,14
moles) of PCl3 are gradually added. The mixture is kept
:~ :
,~ ~
j:
W093/~0~8 PCT/~2/0~058
- 7 - 21~ 22~
under low reflux for 3 hours and then cooled. 500 ml of
distilled water are then slowly added and heat is
provided at low rate until an omogeneous solution is
obtained; after 6 hours reflux, the solution is
filtered with decolorizing coal, con~entrated and
treated with methanol until a white powder is obtained
which is then filtered, washed with distillëd water and
dried at 40~C under vacuum.
164.8 g (O,54 moles) of 6-dimethylamino-hydroxy-
hexan-l,l-diphosphonic acid are obtained whose identity
and high purity are confirmed with the analyses.
Example 2
206 g (1,5 moles~ of PCl3 ar~ dissolved in a
mixture of 5-dimethylamino-valerianic acid ~145,2 g, 1
mole) and H3PO3 (246 gr 3 moles3 by a dropwise addition
under anh~drous condltions~ under ~acuum and stirring;
the solution is kept under slow PCl3 reflux for 3 hours
and then caused to cool.
600 ml of distilled water are cautiously added
while maintaining the reaction vessel in a ice bath;
once the solution is become homogeneous, it is refluxed
for 6 hours and ~hen treated with decolorizing char~oal
a~d filtered. An equal vol~me of methanol is then added
and the solu~lon is left under stirring for 24 hours.
The obtained product:is filtered washed with distilled
water and dried at 60~C.
: 168.9 g (0,58 mo~es~ of 5-dimethylamino-hydroxy-
pentan-l,l-diphosphonic acid are obtained, the identity
is confirmed by the elementary, alkalimetric and
- ~ 30 spectroscopic analyses. The yield is equal to 58%.
: Examele 3 ~
~: ~ 206 g (1,5 moles) of PCl3 are slowly added under
stirring to a suspension obtained by mixing 159.2 g tl
.. . . . ...
WOg3/0~8 PCT/~2/00058
mole) of 6 ~ Q ino caproic acid with 164 g (2
moles) of H3PO3 in 500 ml of chlorobenzene brought to
90C under a nitrogen stream; the mixture is moderately
refluxed for 3 hours and then is caused to cool.
5500 ml of distilled water are then added slowly
and under stirring, once any solid matter possibly
present is separated and the acqueous phase is refluxed
for 6 hours. After cooling and filtering with
decolorizing charcoal an equal volume of methanol is
added and the mixture is left under stirring for 24
hours. The product obtained is filtered, washed with
distilled water and dried at 60C. 192.3 g (0.63 moles)
of 6-dimethylamino-hydroxy-hexan-1,1-diphosphonic acid
are obtained the identity of which is confirmed by the
elementary alkalimetric and spectroscopic analyses.
~; ~ The yield i~s equal to 63%. The water-soluble
salts of the acids according to the present invention
~ , ~
can be obtained ~by complete or partial neutralization
; with inorganic, organic bases or quaternary ammonium,
such as NaOH,~ ; KOH, NH40H, aIkaline carbonates,
alkanolamines, and the like, and isolating the salt by
a following concentration up to crystallization or,
alternately~ and~;more simply, by precipitation with a
Cl-C3 alcohol~or acetone.
25The diphosphonic acids according to the present
invention and their saline derivatives are suitable for
;~ ~ the preparation of pharmaceutical compositions useful
~ , :
in the therapeutic or pr ventive treatment of diseases
associated~with ~dlsturbances of bone metabolism, such
as osteoporosis, Paget's disease, osteolysis caused by
tumours ~ and hyperparathyroidsm, osteoarthrosis,
arthrytis and the like.
:"
- -
~112250
- 8~ -
Toxicoloqical studies
Acute toxicity of DAEDP and DAPeDP has been tested on CD
rats "Charles River" of a weight equal to 2~0 g. 8 groups
of rats each consistir.g of 8 female rats have been used.
The test compounds are in~culated in the caudal vein by
23G mirage microperfusor with 20 s administering time and
an administered volume of 0.2 ml/100 g b.w. in a sterile,
phisiological solution.
The tested doses have been the following:
- DAEDP : 60 and 30 mg/kg b.w.
- AEDP : 60 and 30 mg/kg b.w.
- DAPeDP : 90 and 45 mg/kg b.w.
APeDP : 90 and 45 ~g/kg b.w.
~15 The animals undergone the test were observed and
controlled as to the death rate, the behaviour and the
general conditions all over the first day o~ treatment and
twice a day the next l4 days to evaluate possible clinical
.
:behaviour modi~ications.
~ 2~0
'`',~:; ~: /
2112250
The toxicological studies which have been carried out
have shown a toxicity of the compounds of the invention
much lower than that of the corresponding non-~ethylated
products. The results of these studies are summarized
herebelow.
a) 6-dimethYlamino-l-hYdroxy-hexan-l,l diphosohonic
acid (DAEDP)
No case of death has been recorded for animals
treated with doses of 60 mg/kg of DAEDP, whereas with an
equal dose of sodic amino-hydroxy-hexan-diphosphonate
(AEDP) a death-rate of 85% has been recorded, analogous to
that recorded in~previous tests on the same product.
Likewise, no c~se of death ~has- been recorded when the
animals`are~ tre~ated~ with a dose of 30 mg/kg of DAEDP,
is whereas a death-rate; of ~5% is recorded when using an
~ , ~
; equal dose of sodic ~amino-hydroxy-hexan-diphosphonate
.(AEDP).~
b) 5-dimethYlamino-l-hydroxypentan-l,l-diphosphonic
;acid ~(DAPeDP) ~ ~
20~ No case~of~ death has been recorded~ for~ animals
treated~with doses~af~90 mg/kg of DAPeDP, whereas with an
equal~ ~dose ~of ~sodic~ amlno-hydroxy-pentan-diphosphonat~
`; (APeDP) a death-rate of 75%-has been recorded, slightly
lower than that~ recorded in previous tests on the same
25~ produc~t. Llk.ewise,~no case of death has been recorded when
the~animals~are treated wlth;45 mg/kg of DAPeDP, whereas a
death-rate of 25%~has~ been recorded when an equal dose of
sodic~ami~no-hydroxy-pentan-diphosphonate (APeDP~ is used.
Bone resorption inhibition
Tests of inhlbition of bone reabsorption induced by
SUB~TITUT~ S1 1-EET
2ll22sn
- 9a
parathormone in the calve of newborn rats, show a dose-
dependant inhibitory activity which has been found with
all the concentrations used with a maximum of 55.9~ for
DAEDP and 64~ Lor DAPeDP with the highest dose used in the
relevant tests.
The experimental method was the following.
Two day old pups were injected subcutaneously (dorsal
midline) with 50~ Qof sterile saline containing 5/~ Ci of
Ca45. After four days the pups were sacrificed by
decapitation and the calvaria recovered. Thereafter the
hemicalvariae were positioned on top of stainless steel
screens inside wells of a 24 microwell cluster pla~e each
containing a total end-volume of 1.25 mls of culture
.medium. The incubatio~n period was divided into two phases.
The fixst phase lasted 24 hr and involved preincubating
::
the calvariae at 37C in a 5% CO2 atmosphere in the
presence of indvmethacin so as to inhibit osteoclast
; activation by ~prostaglandins. The second one involved
changing the preincubation medium and replacing it with
20~ fresh indomethacin-free medium containing the various test
substances plus~ PTH ~80~mg/ml) and incubating all for 72
hr at 37C in a 5%~ CO2 ~atmosphere. After the incubation
tha calvarlae with their respective medium (1 ml) were
recovered and place~d lnslde~ scintillation vials. Those
25~ vials containing the hemicalvaria were treated with 1 ml
of 85% acetic acid and left standing overnight in order to
dissolve the mineral con~ent of the neonatal bone. 9 ml of
sc1ntillation cocktail was added to each vial and the
radioactivity determined with a Bechman LS 6000~ ~ounter.
30~
C:l 11%~TITI IT~ ~s
-- 21122~0
-- 10
Retinoid Test
The test was carried out using Sprague Dawley albino ~
rats (6 male animals each test group) from Ch"arles River ,
breedin~ having a body weight comprised between 250 and
300 g and an age of about 2 months at the beginning of the
test. Feed consisted of rodent standard diet, i.e.
Altromin R (Rieper). The rat was selected ,~or the study t
being the animal already used for like studies referred to
in previous works (see, for instance, A.Stutzer, H.Fleish,
U.Trechsel: Calc. Tissue int.- (1988) 43:294 299).
The compound of the invention , was administered
intravenously in the penis dorsal vein in a single dose of ,,",~
,0.2 ml/10~ g'b.w. in~a st~erile phis~iological solution. '',~
~ The animals were tiroparatiroidectomized ~according
15 to test n25 of the "Manual for l~boratory work in '``
mammalian phisiologyj F.E.D'amour, F.R.Blood, D.A.Belden '~
Jr., Third edition, .revised) under total anaesthesia ';~',
~; induced by intravenously administering of Nembutal at the ,',"~'
~ concentrat~on of 50 mg/~g~'b.w~
2~0 ~ ~ ~Starting from the~operation day 2; ~ of Tiroxine was , `~
admlnlstered subcutaneous~ly to the animals four times each ,''
week. ';~-
The fourth day from the operation the animals were ''-',,
subjected to blood abstraction by cutting the end portion ,,"'~,'
25 of the ~tail. The plasma~was~separated from-the ~lood that ~"''
ha~ been added with eparine and total Ca dosage was ,,'',
carried out on the plasma by EGTA complexo metric ,,-',
titrat~on with a suitable apparatus ~Calcium analyzer '`~"'
~ Coxning 940). The animaIs with a total Ca lower than 2 mM '~
~(about~8; mg/dl) were redistributed in groups with total Ca
average value as omogeneous as possible.
SUI~TlTUTE S7~EET
-
21122SO
, - lOa
The sæ~e day the animals were treated both with
retinoid ~lOO,~e/rat equal to 25 ~ ~ of retinoid
administe~ec subcutaneously) and with the compounds u~der
test. The treatment with retinoid is carried on for other
t,wo days (5th and 6th day from the ope~ation). The 7th day
from the operation the animals were subjected to blood
abstraction from the abdominal aorta 'under CO2 anaesthesia
and next killed by bleeding. The plasma was separated from
the blood that had been added with eparine and total Ca
dosage was carried out on it as above.
The results show that the dimethylated derivatives
l'DAEDP and ~APeD,P3 according to the invention are able to
inhibit the bone reabsorption in vivo induced by retinoid.
The compounds have been proven more effective than the
lS corresponding non-dimethylated compounds even if used at
the same doses4
The pharmaceutical compositions according to the
present invention can be prepared in the form of:
- tabletsj capsules, granules, pills, for oral
administration; ~ ~
- drops for oral administration;
- solutions for intra-articular or intravenous
administration; ~ ' '
-'creams for local use.
The above compositlons are advantageously prepared in
comb~nation with ~ inert e~cipients, such as sugars
(saccharose;,~ glucose, lactose), starch and derivatives,
cellulose and derlvatives, fatty acids and salts ~hereof,
polyalcohols, talc, aromatic esters. Typical
:
~ ~ I S~:2T1 T~ I TC Q~l 1:: ~T
~1122~0
- lo~ -
pharmaceutical formulations contaning the 5-dimethylamino-
hydroxypentan-l,l,-diphosphonic acid tDAPeDP) are given
below.
OPERCUL~TE CAPSULES -One capsule contains
DAPeDP 25 mg
lactose 84 mg
hydrolyzed starch 5 mg
talc 5 mg
magnesium stearate 1 mg
DROPS - 10 ml contain
DAPeDP 1 mg
buffer . 1 mg
.
. ~. .~
"
,..
r'
.
:~
: :
~ ~ ' , ',
,~
:
.
~:: ,.' : '- ' -
2TlTI IT~ ~::FT
, W0~3/0~34~ PCT/~2/ ~ 58
11 21122~0
stabilizing, ar~matizing trace
purified water balance
INJECTABLE SOLUTION - One phial contain
DAPeDP 20 mg
sodium chloride 40 mg.
sodium bicarbonate lN soln. 0.15 mg
methyl parahydroxybenzoate 5 mg
purified water 5 ml
INJECTABLE SOLUTION FOR INTR~-ARTICULAR
ADMINISTRATION -
One ampoule contains
DAPeDP 1 mg
: 15 anhydrous sodlum hydroxyde 0,325 m~
lidocaine:hydrochloride 1 mg
glycine ; 20 mg
water pH 6.45 1 mg
~; 20 GRANULAR PROD~CT - One dose contains
~: DAPeDP ~ ~ 200 mg
talc ~ - 10 mg
magne~sium stearate 2 mg
sillca gel 4 mg
25 ~ ~ ~ornstaxch 9 my
: ~ :
"
EFFERVESCENT GRANULAR PRODUCT - One dose contains
DAPeDP : 15 mg
:: : : : :
: : anydhrous sodium car~onate 12 mg
: 30 : sodium bicarbonate 63 mg
anyhydrous citrIc acid 110 mg
sodium saccharinate 5 mg
::
~ saccharose 493 mg
: :
~: ~
W093/00~8 P~T/1T92/000~8
211~2~0 - 12
dehydrated lemmon juice 55 mg
le~mon natural juice 2 mg
3~ CREAM
DAPeDP 3 g
cetyl alcohol 18 g
propylene glycol -10 g
PEG monostearate 4 g
cholesterin-stearate 1 g
linol-linoleic acid 1.~ g
preservative, stabilizers 0.5 g
distilled water 100 g
Similar formulations can be adopted when 6-
1~ dimethylamino-hydroxyhexan-l,l-diphosphonic acid
(DAEDP) is used.
The dosage range of the diphosphonic acids
: ~ according: to the present invention and of the relevant
water-soluble :saline derivative, according to th~
therapeut:ic use and referred to 1 Kg of body weigh~ can
be the~followlng:
Capsules~,~tablets 5 - 400 mg
drops~ ~ 5 - 200 mg
. phia~l~ 1 - 100 mg
:~ subcutaneous~phial 1 - 50 mg
: intramuscular phial 1 - 50 mg
.intra-artlcular phial 0.1 - 5 mg
:
~ 30 ~ ~
: :
:. ::: ~ : ::
~ ~ :
' ~