Note: Descriptions are shown in the official language in which they were submitted.
The invention relate~ to benzotriazole~ which contain at
least 2 fluorine atoms per molecule and correspond to the
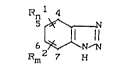
formula
6 ~ (I
Rm2 7 H
in which
n and m = 1 or 2,
R1 represent~ Cl-C~-fluoroalkyl, fluoro-C1-C6-alkoxy,
fluoro-Cl-C6-alkylthio, fluoro-C1-C6-alkyl-
sulphonyl, substituted C6-Cl2-phenyl or sub~ti-
tuted C6-Cl2-phenoxy, and
R2 represent~ hydrogen, fluorine, chlorine, bromine,
nitrile, Cl-Cc-alkyl, Cl-C6-alkoxy, Cl-C6-alkyl-
thio, C1-C6 - alkylsulphonyl, Cl-C~-fluoroalkyl,
fluoro-C1-C6-alkoxy, fluoro-C1-C6-alkylthio or
fluoro-C1-C6-alkyl~ulphonyl, or
15 R1 and R2 together represent -0-X-0-, in which X denotes
a fluorinated C1-C2-alkylene radical,
with the proviso that R1 does not represent 5-CF3 if R2
denote~ ffl drogen.
Le A 29 290 - 1 - ~ -
3 0 2
The substituents Rl and Rl are preferably located - if n -
m = 1 - in the 4,6, the 5,6 or the 5,7 position.
The terms "fluoroalkyl", "fluoroalkoxy" and "fluoroalkyl-
thio" do not exclude an additional sub~titution of the~e
radical~ by chlorine.
The term "fluoro-Cl-C6-alkoxy" preferably encompas~e~
difluoromethoxy,trifluoromethoxy,chlorodifluoromethoxy,
1,1,2,2-tetrafluoroethoxy, 2-chloro-1,1,2-trifluoro-
ethoxy, pentafluoroethoxy and 1,1,2,3,3,3-hexafluoro-
propoxy.
The term "fluoro-C,-C6-alkylthio" preferably encompas~es
difluoromethylthio, trifluoromethylthlo, chlorodifluoro-
methylthio,1,1,2,2-tetrafluoroethylthio,2-chloro-1,1,2-
trifluoroathylthio, pentafluoroethylthio and 1,1,2,3,3,3-
hexafluoropropylthio.
The term "fluoro-Cl-Cc-alkylsulpho~yl" preferably encom-
pas~esdifluoromethylsulphonyl,trifluoromethyl~ulphonyl,
chlorodifluoromethylsulphonyl, 1,1,2,2-totrafluoroethyl-
~ulphonyl, 2-chloro-1,1,2-trifluoroethyl~ulphonyl,
p~ntafluoroethyl~ulphonyl and 1,1,2,3,3,3-hexafluoro-
propylsulphonyl.
: ` : :
Tho tsrm "substituted C6-C,2-phenyl" preferably encom-
passes2-chloro-4-tri41uoromethyl-phenyl,2,6-dichloro-4- -
trifluoromethyl-phenyl, 2-fluoro-4-trifluoromethyl-
phenyl, 2-chloro-6-fluoro-4-trifluoromethyl-phenyl,
Le A 29 290 - 2 - ;
-`~`` 211~30~
2-chloro-4-cyano-phenyl and 2,6-dichloro-4-cyano-phenyl.
The term "substituted C6-Cl2-phenoxy" preferably
encompasses 2-chloro-4-trifluoromethyl-phenoxy, 2,6-
dichloro-4-trifluorcmethyl-phe~oxy, 2-fluoro-4-tri41uoro-
methyl-phenoxy, 2-chloro-6-fluoro-4-trifluoromethyl-
phenoxy, 2-chloro-4-cyano-phenoxy a~d 2,6-d~chloro-4-
cyano-phenoxy.
Preferred compounds I corre~pond to the ~ormulae
(IIt F3C ~ N(III) F3C ~ y
Cl 'N Br
H H
(IV) F3C ~ tVt F3CO
F3C N'N Cl
H H
(VIt F3CO ~ .N(VII) F3CS ~ e
Br 'N C1 N'N
H H :~
(VIIIt F3C ~ N(IX) F3C
F3CO N'N F3C 'N
H H
. ~
Le A 29 290 - 3 -
~4302
tX ) F3CS~ N (XI ) F~CS~ 1l 17
B r ~N~N ~N'N
- H ¦ H
Br
(XII)Cl~ N (XIII) FClCH-CF20~N
FClCH-CF21~N'N FCICH-CFz~N'N
( X I V ) HCFz - CFz~ CF3~N ( XX )
,CF2
~CF2 H ¦ H
Cl
(XV) CF3-CHF-CF2~CF3~N (XXI )
CF3 - CHF- CFz ~N :
H ¦ H :
Br
(XVI ~ ~0~ 1 CF
~N~N ~ N (XXII) -~
}~ ¦ H
CF3 : ~ -
( XV I I ) F2 C~O~ ;~
F2C~ H ~ -:
Le A 29 290 - 4
`~
~,l lA31~2
(XVI I I ) ClFC~'O~
C 1 FC~o N'N
H
( XIX ) F2C'O~
C 1 FC`o N'N
The fluorinated benzotriazoles according to the in~ention
may be obtained by reacting the corre~ponding o-phenyl-
enediamine~ with a nitrosating agent (a.g. sodiuM
nitrite).
The conden~ation of the o-phenylenediamine~ with a
nitrosating agent is preferably ef~ected in solution. All
solvents which are in~rt und~r the rea~tion conditions
are, in pri~ciple, euitable a~ ~olvent or di~peraant or
diluent. Mixtures of solvents may al~o be u~ed. Solvents
which may be mention~d by way of example are: water,
alcohols, ketone~, organic acid~, such a~ fo~mic acid,
acetic acid and propionic acid, DMF, toluenq, chloro-
benzene, d~chloromethane, trichloroethyle~e, and the
like.
Water in combination with an organic acid, for example,
ia advantag~ous ~incQ the reaction i~ carried out in th~
acid p~ range.
However, th~ choice of the ~ol~ent/diluent aleo depend~
Le A 29 290 - 5 -
~114302
on the nature of the n~tro~ating agent. For example, if
an alkali metal nitrite, such as sodium nitrite, 18 used,
then this will be readily soluble in water, indicating
this mode of implementation to be advantageou~. In a
similar manner, the method can also be carried out in
other solvents u~ing other nitroaating agents, such as
methyl nitrite or i~o-amyl nitrite.
Further nitrosating agents are nitrogen oxide~, nitrosyl-
sulphuric acid or nitrosyl chloride.
.
The addition of the nitrosating agent is preferably
effected at a low temperature, that i8 at 0 to 10C, but
can also be carried out at temperature~ below or above
this. It i8 likewi~e possible to complete the reaction at
an elevated temperature. For this purpose, a te~perature
range from 40 to 100C proved to be advantageous.
' ' ~---:
In order to obtain a product which i~ as pure as
poa~ible, the nitrosating agent i~ employed in molar
eguivalent~ or ~n a slight excess. In this way, complete
reaction is achieved and sepaxation of ~tarting material
and product i8 di~pensed wlth. A larger exoese of nitros-
ating agent does not interfere with the reaction and can
be readily separated off, owing to the great difference
in solubility.
It favours the working u~ of the sample if a reaction
medium is collected in which the product is poorly
soluble 20 that it can be ~eparated off, for example by
,'~' ,. '~
' ' ~ .
~e A 29 290 - 6 -
.
3 ~ 2
filtration.
The o-phenylenediamine~ which are ~uitable for use a~
starting material~ can be prepared in a variety of ways,
independently of the nature of the R1 and R~ radicals:
If o-phenylenediamines are to be prepared in which both ~-
R~ and R2 represent donor group~ in the 4 and 5 positions,
e.g. compound~ in which R1 represent~ polyfluoroalkoxy or
polyfluoroalkylthio and Rl and/or R2 represents fluorine,
chlorine, bromine, alkyl, alkoxy or bisfluoroalkylamino,
a benzene derivative of the formula
R ~
~ (XXIII~, -
R2m
can then be dinitrated and the nitro groups sub~equently
reduced. The dlnitration can be carried out, for example,
using HN03/H,SO~ mixtures, which can optionally also
contain oleum, at temperatures from, for example, 0 to
100C. The reduction can be carried out, for exa~ple,
using iron in the presence of aqueous hydrochloric acid
and ethanol at te~peratures from, for example, 50 to
100C, or catalytically using elemental ~ydrogen, e.g. 1
to 100 bar, and in the pres2nce of cataly~ts containing
metal~ or compounds of metal~ from the 8th subgroup of
the per~odic systam, in particular nickel or palladiu~,
at, for example, 25 to 100C.
Le A 29 290 - 7 -
3 0 '~
If o-phenylenediamine~ are to be prepared ln which Rl hae
the meaning ~tated in relation to formula (I) and is
located in the 4 position and R2 repre~ent~ Cl or Br in
the 5 position, for example, a nitrobenzene derivati.ve of
the formula,
~1
~ I (XXIV),
R; 7~ ~a 1 ~ .
m :
in which
Hal repre~ents ~luorine, chlorine or bromine,
~ "~
can then be reacted with ammonia, thereby exchanging the
Hal group for an amino group, and tha resulting nitro~
aniline can be reduced. The exchange of halogen for an
amino group can, for example, be carried out u~ing liquid :: ~:
ammonia in the pre~ence of water and a tetraalkyla~monium :~
salt at te~peratures from, for exa~ple, 80 to 200C in a
pressure ~e~sel. Tha nltroanilin~ aan be reduced, for :-.
example, in analogy with the above-de~cribed raduction of
dinitro compou~ds. :~:
If o-phenylenediamlnes are to be prepared in which Rl ha~
the meaning ~ta~ed in relation to formula (I) and i~
locatod in the 4 position and R' represent~ chlorine or:~
bromine in the 6 position, for example, a nitroa~iline of
the formula
Le A 29 290 - 8
21~302
R 1 ~NO 2
L~ 11 (xxv)~
~NH2
can then be reacted with a chlorinating or brominatlng
agent, thereby introducing a chlorine or bromine atom
into the meta position to the nitro group, and the nitro
group can be ~ubsequently reduced. Elemental chlorine and
elemental bromine, and other cu6tomary chlorinating and
brominating agent~, are ~uitable chlorinating or bromin-
ating agents. Suitable solvents are, for example, water,
dilute mineral acids, acetic acid, chloroalkane~ and
trifluoroacetic acid, and suitable temperature~ are, ~or
example, those from -20 to +50C. The reduction can be
effected, for example, in analogy with the above-de~-
cribed red~ction of dinitro compound~.
o-Phenylenediamines which are ~ub~tituted three or four
times can be prepared in analogy with the above-de~cribed
proce~se~.
Mo~t of the abovementioned o-phenylenediamine~, and
proce~ec for their preparation, are described ln ~erman
Patent Application P 42 37 564.9 and in ~P-A 251 013.
The fluorinated benzotriazole~ according to the invention
are valuable intermediates for preparing active compound~
for pharmaceutical~ and plant-protection agent~.
The compounds of the formula (I) according to the
Le A 29 2gO - g - ~ '
~11 4302
invention also themselves exhibit a strong effect against
pests and can be employed in practice for combatting
unwànted perniciou~ organism~. The acti~e compounds are
suitable for use as plant-protection agent~, in parti-
cular as fungicides.
Fungicidal agent~ in plant protection are employed for
combating Plasmodiophoromycetes, Oomycete~, Chytridio-
mycetes, Zygomycetes, Ascomycetes, Basidiomycetes and
Deutero~ycetes.
Some causati~e organisms of fungal disea~es which come
under the generic names listed above may be mentioned a~ ~-
examples, but not by way of limitation: ~
Pythium species, such as, for example, Pythium ultimum; - --
Phytophthora ~pecies, such as, for example, Phytophthora
infe~tans; -~
P~eudoperonospora species, ~uch as, or example, P~eudo-
perono~pora humuli or Pseudop~ronospora cubeni~; -
Pla~mopara species, such as, for example, Plasmopara
viticola;
Peronospora species, such as, for example, Peronospora
pi8i or P. bras~icae;
Erysiphe species, such as, for example, Erysiphe
gramini~;
Spha~rotheca ~pecies, such a~, for example, Sphaerotheca
fuliginea;
Podo~phaera species, such a~, for example, Podosphaera
leucotricha;
Le A 29 290 - 10 -
--`~
3 0 2
Venturia species, ~uch a~, for example, venturia
inaequalis;
Pyrenophora species, such as, for example, Pyrenophora
teres or P. graminea (conidia form: Drechslera, syn:
Helminthosporium):
Cochliobolus speciee, such as, for example, Cochliobolus
sativus (conidia form: Drech31era, 0yn: Helmintho-
sphorium);
Uromyces species, such as, for example, Uromyces appendi-
culatuo;
Puccinia species, ~uch as, for example, Puccinia recon-
dita;
Tilletia species, such as, for example, Tilletia caries;
Ustilago species, such as, for example, Ustilago nuda or
Ustilago avenae;
Pellicularia species, such a~, for example, Pellicularia
sa~akii;
Pyricularia species, such as, for example, Pyricularia
oryzae;
Fusarium speciee, such as, for example, Fusarium
culmorum;
Botrytis species, such ae, for example, Botrytis cinerea;
Septoria species, such as, for example, Septoria nodorum;
Leptosphaeria ~pecies, such as, for example, Lepto-
sphaeria nodorum;
Cerco~pora species, such as, for example, Carcosporacanescens:
Alternaria ~pecies, such a~, for example, Alternaria
bra~sicae and
Pseudocercosporella spec~e~, such a~, for example,
Le A 29 290 - 11 - ~:
~ ~ 4~.~02
P~eudocercosporella herpotrichoides.
The good toleration, by plants, of the active compounds,
at the concentration~ required for combating plant
diseases, permit~ trsatment of above-ground parts of
plants, of vegetati~e propagation stock and seeds, and of
the 80il.
-:
In this context, the active compounds according to the
invention may be employed particularly ~uccessfully for
combatting Phytophthora on tomatoea and Venturia species
on apples.
Depending on their particular phy~ical and/or chemical
properties, the active compounds can be converted to the
customary formulations, such as solutions, emulsions,
suspensions, powders, foams, pastes, granules, aerosols,
natural and synthetic materials impregnated with active
compound, very fine capsules in polymeric eub~tance~ and
in coating compositions for seed, and furthermore in
formulations used with burning equipment, such a~ fumi-
gating cartridge~, fu~lgat~ng cans, fumigating coils and
the like, as well as ULV cold mi~t and warm mist formula-
tion~.
These formulation~ are produoed in a known manner, for
example by mixing the active compounds with extender~,
that is, liguid solvents, liquefied gaaes under pressure,
and/or solid carrier~, optionally with the use of sur-
face-active-agents. In the ca~e of the use of water as~an
Le A 29 290 - 12 -
- ~ : f,, ': ' :
. . .
~ 3 0 ~
extender, organic sol~ents can, for example, al~o be uaed
a~ auxiliary ~olvent6. A~ liquid solventa, there are
suitable in the main: aromatic~, ~uch a~ xylene, toluene
or alkylnaphthalenes, chlorinated aromatics or chlorin-
ated aliphatic hydrocarbons, such as chlorobenzenes,chloroethylenes or methylene chloride, aliphatic hydro-
carbon~, such as cyclohexane or paraffins, for example
mineral oil fractions, alcohols, such a~ butanol or
glycol a~ well a~ their ethers and e~ter~, ketone~, such
a~ acetone, methyl ethyl ketone, methyl iaobutyl ketone
or cyclohexanone, strongly polar ~olvents, such a~
dimethylformamide and dimethyl sulphoxide, a~ well as
water; by liquefied gaseous extender~ or carriers are
meant liquids which are ga~eous at ambient temperature
and u~der atmospheric presaure, for example aerosol
propellants, such as halogenated hydrocarbons as well a
butane, propane, nitrogen and carbon dioxide: as ~olid
carriers there are suitable: for example ground natural
min~ral~, suc~ aa kaolins, clay~, talc, chalk, quartz,
attapulgite, montmorillonite and diatomaceous earth, and
ground synthetic minerals, ~uch as highly-disperse
silica, alumina and silicates; as solid carriers for
granule~ ~here are suitable: for example crushed and
fractionated natural rocks euch a~ calcite, marble,
pumice, sep~olitQ and dolomite, as well a~ ~ynthetic
granules of inorganic and organic meals, and gra~ule~ of
orga~ic material such a~ sawdust, coconut shell~, maize
co~ a~d tobacco ~talks; as emulsifying and/or foam-
forming agents there are suitable: for example non-ionic
and anionic emul~ifier~, such a~ polyethylene fatty
Le ~ 29 290 - 13 -
,,, ~ " ~`' ' ' , . '
0 2
alcohol ether~, for example alkyl-aryl polyglycol ethers,
alkylsulphonates, arylsulphonate~ as well as albumen
hydroly~is products; as dispersing agents there are
suitable: for example lignin-sulphite waste liqucrs and
methylcellulo~e.
Adhesives such as carboxymethylcellulose and natural and
synthetic polymers in the form of powders, granules or
latices, ~uch as gum arabic, polyvinyl alcohol and
polyvinyl acetate, as well as natural pho~pholipids, 0uch
as cephalins and lecithin3, and synthetic phospholipid~,
can be used in the formulations. Other additives can be
mineral and vegetable oils. ~ -
It is possible to use colorants such as inorganic pig-
ments, for example iron oxide, titanium oxide and
Prussian Blue, and organic dyestuffs, such as alizarin
dyestuffs, azo dyestuffs and metal phthalocyanine dye-
~tuffs, and trace nutrients such as saltB of iron,
mangane~e, boron, copper, cobalt, molybdenum and zinc.
:.
The formulations in general contain 0.0000001 to 95% by
weight of active compound, preferably 0.0001 to 90% by
weight.
The active compounds according to the invention can be
pre~ent in the formulations as a mixture with other known ~ -
actlve compounds, such as fungicides, in~ecticides,
acaricides and herbicides, a~ well as in mixture~ with -
fertilizers and growth regulator~. -
~e A 29 290 - 14 - ~ -~
3 0 2
The active compounds can be used as such or in the form
of thei~ formulations or the use f ormB prepared there-
from, such as ready-to-use solutions, suspensions,
wettabie powders, pastee, soluble powder~, dusting agants
and granules. ~hey are u~ed in the customary manner, for
example by watering, ~praying, atomizing, scattering,
du~ting, foaming, bruahing on and the like. It i8
furthermore po~sible to apply the active compounds by the
ultra-low volume method or to inject the active compound
formulation or the active compound itself into the soil.
The seeds of the plants can also be treated.
In the treatment of parts of plants, the active compound
concentrations in the use forms can vary within a sub-
stantial range. They are, in general, between 1 and
0.0001% by weight, preferably between 0.5 and 0.0001% by
weight. -
.
In the treatment of seed, amounts of active compound of
0.001 to 50 g per kilogram of seed, preferably 0.01 to
10 g, are generally reguired.
For the treatment of ~oil, active compound concentration~ `~
of 0.00001 to 0.1~ by weight, preferably 0.0001 to 0.02%
by weight, are required at the place of action.
~ . :
Le A 29 290 - 15 -
~ ~4302
-
Examples
Preparation of starting material~ (o-phenylenediamines)
for the novel benzotriazoles
a) 320 g of 1,2-bis-(2-chloro-1,1,2-trifluoroethoxy)-
benzene were added dropwi~e to 500 g of a mixed acid
containing 33~ by weight of XN03 and 67% by weight
of H2SO~. After one hour at 40C, 250 ml of 20%
strength by weight oleum were added dropwise.
Sub~equently, the mixture was heated to 80C and
then stirred for 15 hour~. A further 120 ml of 20%
strength by weight oleu~ and 250 g of the above-
mentioned mixed acid were then added dropwise. After
6 hours at 80 to 82C, the mixture wa~ cooled down
and poured onto ice. The organic phase wa~ separated
off and wa~hed with water. Following azeotropic
drying with 1,2-dichloroethane, 350 g of 1,2-di-
nitro-4,5-bis-(2-chloro-1,1,2-trifluoroethoxy)-
benzene were obtained, which waa 98% pure by weight
(oil, n20: 1.4832, GC 99.1%).
350 g of this dinitro co~pound were added dropwise
to a mixture consisting of 1.5 1 of ethanol, 50 ml
of water, 30 ml of concentrated aqueous hydrochloric
acid and 470 g o~ iron filings, and thi~ new mixture
then heated to boiling at reflux for a total of
15 hour~. Subsequently, the aooled solution was fil-
tered and concentrated, and the residue was recry~-
tallized from cyclohexane. 216 g of 1,2-diamino-4,5-
Le A 29 290 - 1~ -
-
21 14302
bis-(2-chloro-1,1,2-trifluoroethoxy)-benze~e were
obtained with a melting point of 58 to 60C.
b) 24 g of finely powdered 2-nitro-4-~rifluoromethyl-
mercapto-aniline were di~olved in 50 ml of tri-
S fluoroacetic acid, and 18 g of brom~ne were meterad
in at 20C. ~he mixture was the~ stirred at 20C for
3 hour~ and ~ub~equently at 40C for a further
30 minutes, after which it wa~ poured onto water and
the product wa~ taken up in dichloromethane. 31 g of
6-bromo-2-nitro-4-trifluoromothylmercapto-aniline
resulted after remo~ing the ~olvent.
155 g of the nitroaniline prepared in thi~ way were
heated to boiling at reflux for 15 hours in 700 ml
of etha~ol together with 15 ml of water, 10 ml of
concentrated aqueous hydrochloric acid and 70 g of
iron fil~ngs, and the mixture wa~ then ~iltered; the
filtrate wa~ freed o~ ~olvent under reduced pre~ure
and the solid crude product wa~ recry~tallized from
cyclohexane. 112 g of 6-bromo-4-trifluoromethyl-
mercapto-1,2-diamino-benzene were obtained with a
melting point of 60 ~o 61C.
The remaining o-phenylenediamines which are reguired
for preparing the benzotriazole~ according to the
invention may be prepared in an a~alogoua man~er.
Le A 29 290 - 17 -
.: : . . .. .. .
3 0 2
senzotriazole XVI
~o~
F2C ~ (XVI)
o~A~N'N
10 g of diamine of the formula
~o~H2
were added to a mixture consisting of 75 ml of water and
6 ml of glacial acetic acid and the whole mixture waa
then stirred for 30 minute~. After cooling the mixture
down to 0C, 8 g of sodium nitrite, dissolved in 25 ml of
water, were rapidly added dropwise. Subsequently, the
mixture was ~tirred at 0 to 5C for 1 hour and then at
80C for 3 hours. After cool~ng down to room temperature,
the solid material was filtered off with suction and then
washed with a little water. After drying, 9.6 g of the
desired product were obtained with a melting point of 203
to 204C.
Le A 29 290 - 18 -
. .
~` ~
^ ~llA302
The following were obtained in an analogou~ manner:
F -o ~
~ ~ N~ m.p.:l47-lq8c
Cl~
~ m.p.: 151-152 C
C l ~N~
CF~
Cl~N~ m.p.: 159-160 C
H ~:
CF3~ m.p.: 167 - 168 C
H :
CF3S ~
~N~ m.p.: 228-229 C
Br
FCl~
lll I m.p.:l59-160 C
CH - CF20~N~
Cl
Le A 29 290 - 19 -
-- 211 ~302
F
C 1 - CH - CF`20~
Il I ~ m.p.: 153-154 C
C 1 - CH - CF20f ~N~
F
CF8 ~
,J~ m.p.: 10 7 - 1 0 9 C
~ N
HCF2 CF20~ ~ -
J~ N m.p.: 77-78 C
~,~ N
CF ~0~
C l~N m.p.: 124 - l Z5 C
CF ~S~
ll ,l m.p.: 125-127C
Le A 29290 - 20 -
4302
A~lication
Venturia test (apple)/protective
Solvent: 12.5 parts by weight of acetone
Emul~ifier: 0.3 part by weight of alkylaryl poly-
glycol ether
To produce a suitable preparation of active compound,
1 part by weight of active compound i~ mixed with the
stated amounts of solvent and emulsifier, and the concen- -
trate i~ diluted with water to the desired concentration.
To te~t for protective activity, young plant~ are ~prayed
with the preparation of active compound until dripping
wet. A~ter the spray coating ha~ dried on, the planta are
inoculated with an aqueou~ conidia ~uspension of the
apple scab cau~ative organism (Venturia inaequalis) and
~hen remain in an incubation cabin at 20C and 100%
relative atmospheric humidity for 1 day.
Th~ plants are then placed in a greehhouse at 20C and a
relati~e atmospheric humid~ty of about 70%.
Evaluation i8 carried out 12 days after the inoculation.
In this test, a clearly superior activity compared with
th~ prior art i~ ~hown, ~or example, by the compounds
according to the following preparation example~
Le A 29 290 - 21 -
- ~"~'-.-,
~. 1..~.4302
T a b 1 e
Venturia te~t (apple)/protective
Active compound Efficacy in % of the
- untreated control at an
active compound con-
centration of 0.025% by
weight
F2C ~ ~ 100
Br
F3C~ ~ ~ 78
F3C ~ N ~ 89
Cl
F21 ~ 89
Le A 29 290 - 22 -
-