Note: Descriptions are shown in the official language in which they were submitted.
2I~~~~~.
Dr. Thomas P. Abend, CH-9010 St. Gallen
Method and material mixture for manufacture of reactive
hotmelts
The use of hotmelts for solvent free adhering, sealing and
coating of solid or flexible materials is nowadays state of
the art and is, for ecological reasons, preferred. In
accordance with requirements, various thermoplastic polymers
are nowadays used in the formulations, for example, amongst
others, polyethylenes, copolymers of ethylene with vinyl
acetate, copolymers of ethylene with acrylic esters or
. methacrylic esters, polyacrylate or polymethacrylate,
polymers or copolymers of the alpha olefins, copolymers of
styrene with isoprene or butadiene, polyamides and
copolyamides, polyester or copolyester, thermoplastic
polyurethane and epoxy resins.
A problem which still remains unsolved is the
thermoplasticity of the hotmelts or hotmelt adhesives near
the softening point and at temperatures above the softening
point, as well as the resistance of the adhesion to solvents.
Near the softening point, the resistance to thermal
deformation of the bond reduces greatly, and when the melting
point is exceeded, the hotmelt adhesives lose there strength,
which can lead to failure of the adhesive bond.
Hotmelt powders or films for textile applications may be
mentioned as an example: these should, at the lowest possible
application temperatures, result in high bond strength
(shortening of application time, elimination of damage to the
textile fibre, elimination of bleeding or "off-setting"); the
bond shall afterwards, however, withstand the high
temperatures associated with subsequent handling and
treatment processes (pressing, ironing, shaping, drying on
shaped forms or mannekins). The adhesive bonds are here
subjected to temperatures which are of the same intensity or
many times greater than the softening point of the adhesive
' or the previously applied bonding temperature. The
dimensional stability of bonded textile items is threatened
by these treatments.
The same undesirable effects, namely, failure of the adhesive
bond, will occur if the hotmelt adhesive is subjected to
solvents as used in dry cleaning, or excessive washing
temperatures.
There has been no lack of experiments concerning the
reduction or elimination of thermoplasticity and solubility
of the adhesive bonds of hotmelts and hotmelt adhesives.
Thus, moisture reactive isocyanate or silane groups have been
introduced into the polymer. Crosslinked, thermoset polymers
will ensue as a result of the influence of moisture or water
after application of the molten adhesive. A disadvantage is
that these systems must be stored under exclusion of moisture
up to their point of application. In hot curing systems,
crosslinked, thermoset polymers will also arise from
functional polymers with heat reactive crosslinkers, with
peroxides of hydroxy functional or amino functional polymers
with blocked isocyanates, and solid epoxy resins with
dicyandiamides etc.. A disadvantage with the outlined
reactive hotmelt adhesives is that the crosslinking
temperature exceeds 130 to 140°C. Reaction times in practice
require still higher temperatures, which forbids the use of
various, heat sensitive substrates.
The composition of nonreactive, of moisture reactive or heat
reactive hotmelts, their advantages and their disadvantages,
are known to the expert and are described in the technical
CA 02114842 1998-11-03
- 3 -
literature and patent specifications, for example in:
"Schmelzklebstoffe", Vol. 4a (1985), 4b (1986), 4c (1987),
"Schmeltzhaftklebstoffe, Vol. 6a (1990) of the "Klebstoff-
Monographen" by R. Jordan, Publisher: Hinterwaldner Verlag
(Munich) .
The purpose of the invention is to avoid the disadvantages as
they are known, and in particular therefore to produce hotmelts
which:
- are able to be preformed or applied to the surface of
substrates,
- subsequently solidify, through cooling,
- afterwards, after any desired period, can be remelted on the
substrate through the application of heat, if necessary
j oined,
- subsequently, through increasing the temperature further, can
be irreversibly crosslinked.
Surprisingly, a method, which does not possess the
disadvantages known up to now and considerably extends the area
of application of the hotmelts or melting adhesives, of
manufacturing reactive hotmelts applied in bulk or preformed
as well as a solid mixture of substances at room temperature,
has been found.
In accordance with an embodiment of the present invention there
is provided a method of manufacturing a room-temperature shelf-
stable mixture of reactive substances comprising: (A) at least
one thermoplastic polymer having a melting point above 40°C,
so that it is solid at room temperature, and which carries
CA 02114842 1998-11-03
- 4 -
functional groups reactive with isocyanate, (B) at least one
solid polyisocyanate in powder form, suspended in the solid
thermoplastic polymer and having a melting point higher than
that of the thermoplastic polymer, (C) at least one
deactivating agent for reacting with surface isocyanate groups
in the solid polyisocyanate, wherein the polyisocyanate is pre-
mixed with the deactivating agent, and is thereby deactivated,
the pre-mix of polyisocyanate and deactivating agent is then,
at a temperature above the melting point of the polymer but
below the melting point of the deactivated polyisocyanate,
mixed with the polymer, which has been made liquid through
warming, in layers, so that the resulting mixture includes
first layers which predominantly comprise the polymer, and,
between the first layers, second layers which predominantly
comprise the deactivated polyisocyanate and also unbound
deactivating agent, and the mixture is then cooled and
solidified with the layer structure.
In accordance with another embodiment of the present invention
there is provided a room-temperature shelf-stable mixture of
reactive substances, comprising: (A) at least one thermoplastic
polymer having a melting point above 40°C, so that it is solid
at room temperature, and which carries functional groups
reactive with isocyanate, (B) at least one solid polyisocyanate
in powder form, suspended in the solid thermoplastic polymer
and having a melting point higher than that of the
thermoplastic polymers (C) at least one deactivating agent for
reacting with surface isocyanate groups in the solid
polyisocyanate, the mixture including first layers which
predominantly comprise the polymer, and, between the first
layers, second layers which predominantly comprise the
polyisocyanate and the deactivating agent, wherein a first part
of the deactivating agent is bound to the surface of the
isocyanate particles and a second part of the deactivating
CA 02114842 1998-11-03
- 4a -
agent is unbound in the mixture.
These preformed or, if necessary, nonformed, solid reactive
hotmelts are shelf stable at room temperature and retain latent
thermoreactivity. After any period of time, they can be made
liquid again through heating of the multilayer of substrate and
preapplied hotmelt or hotmelt adhesive, joined, and then
irreversibly crosslinked through raising of the
20
~,.-.
- 5 -
temperature above the "activation temperature". These
hotmelts serve the purpose of bonding, sealing, laminating or
coating of wood, plastics, metals, glass, textiles, synthetic
non-wovens, cardboard, paper, films, foils and so forth.
Polymer reactive systems, carrying hydroxyl or amino groups,
and desactivated, solid, isocyanates in powder form are known
from patent specifications EP-062 780, EP-100 507, DE-32 30
757, DE-34 03 499, DE-34 03 500 as well as the technical
literature, for example as contained in Blum, R.; Schupp, H.,
Prog. Org. Coat. 1990, 18(3), S. 275 - 288.
In the method described, a homogeneous mixture of a liquid
polyol or polyamine and a solid isocyanate, with surface
desactivated by amines, is produced which will not react at
room temperatures. On attaining or exceeding the so-called
"activation temperature", or reaction temperature, which lies
above 55°C, preferably above 80°C, the reaction between solid
isocyanates and functional groups of the polymer will
commence, if necessary accelerated by catalysts. This
reaction will lead to crosslinked, high molecular weight
polyurethane or polyurea.
As an "activation temperature", that (minimum) temperature is
denoted at which the viscosity of the hotmelt begins to
increase through the reaction of the desactivated isocyanate
with the hydroxyl, or the polymer which contains hydroxyl or
amino groups, under formation of urethane or urea groups.
This temperature can, through commencement of exothermal
reactions between the named components, for example be
ascertained by using thermoanalytical methods, for example
DSC (Differential Scanning Calorimetry).
It has been suggested in, amongst others, DE-32 30 757, that
solid polyols with a softening point in the region of 45 to
2iI484~
- 6 -
65°C be mixed with the desactivated, solid isocyanates. After
homogenising and degassing of the mixture, the melt is
allowed to solidify, and then brought to a granulated
condition through a suitable grinding process. At a suitable
time, the reactive blend, stable when stored at room
temperature, can be transferred to a mold heated to
approximately 70 to 100°C. After melting, the viscous melt
will solidify at 100 to 120°C, through the impact of heat.
Instructions concerning the continuous method, presented here
in accordance with the invention, for creation of an
inhomogenous, latent reactive hotmelt and its use for
achieving a permanent and heat resistant bond with one or
more substrate surfaces, do not arise out of this patent.
In the following, the components will be indicated as
follows:
- Polymer A, for thermoplastic polymer with functional,
isocyanate reactive groups,
- Isocyanate B, for solid di- or polyisocyanate,
- Desactivating agent C, to include bound desactivating
agent C' and unbound desactivating agent C " , which
together form the desactivating potential,
- Crosslinking or chain extension components D, for solid
or liquid isocyanate reactive compounds with
molecular weight up to 500,
- Additives E, for isocyanate nonreactive organic or
inorganic compounds.
As polymers A, solid at room temperature, with melting or
softening point at 4-0°C or higher, the following are suitable
for execution of the method according to the invention:
Hydroxy functional or amino functional, aliphatic or aromatic
polyester, polycaprolactone, polycarbonate, polyacetale,
polyacrylate, polyamide, polyurethane, polyether,
polythioether,
Copolymers of styrene or alpha-methylstyrene with
allylalcohol, low molecular weight copolymers of ethylene or
alpha-olefins with hydroxyethyl- or hydroxypropyl-acrylates
or -methacrylates, and other monomers inert to the hydroxy-
functions such as ethyl-acrylate, butylacrylate,
ethylmethacrylate, butylmethacrylate,
graft polymers as a result of graft reactions of
hydroxyethyl- or hydroxypropyl-acrylate, -methacrylate,
allylalcohol, aminoalkyl- and mercaptoalkyl-alkoxysilane on
polymers of ethylene, alpha-olefins or copolymers of
ethylene, alpha-olefins, or copolymers of ethylene, alpha-
olefins with vinyl acetate, ethyl-, butyl-acrylate or -
methacrylate, hydrolytic products of copolymers of ethylene
with vinylacetate, hydroxy containing derivatives of fatty
acids, liquid above 40°C and solid below this temperature,
hydroxy functional or amino functional prepolymers obtained
through the reaction of low or high molecular weight polyols
or diamines with di- or polyisocyanates.
Blends of the previously mentioned polymers can also be used.
The number of functional groups per molecule of the polymer
shall lie, on average, between 1.5 and 6, preferably between
1.8 and 4, and the molecular weight in the area between 400
and 25'000.
''.-
_ g _
The melting or softening point, respectively the
solidification point of the polymers shall lie above 40°C,
preferably above 55°C. The uppermost limit for the melting or
softening point of the polymer is the "activation or reaction
temperature" of the surface desactivated isocyanate.
The proportion of the polymer components A shall with
advantage amount to at least 40~ weight of the total binder.
Low molecular weight aliphatic or aromatic, hydroxy
functional or amino functional crosslinking or chain
extension agents D, with a molecular weight of 62 to 500 and
a functionality of between 2 and 4, can be used in the blend.
A condition is that they are either solid at temperatures
above 40°C or do not lower the softening point of the polymer
in the hotmelt below 40°C.
Low molecular weight polyols or polyamines which can be used
in the hotmelt as a crosslinking and chain extension agent or
for the production of the hydroxy functional or amino
functional prepolymers are, for example: ethandiol,
propandiol, propylenglycol, dipropylenglycol, butandiol,
hexandiol, decandiol, neopentylglycol,
l,4cyclohexandimethanol, hydroquinondi(2hydroxyethyl)ether,
2,2,4trimethy11,3pentandiol, bisphenol Aethoxylate, bisphenol
Apropoxylate, trishydroxyethylisocyanurate, pentaerythrite,
N,Nbis(2hydroxypropyl)aniline, triethanolamine,
N,N'bis(hydroxyethyl)piperazine, 3,5diethy12,4 and
2,6diaminotoluol, 2,4' and 4,4' diphenylmethandiamine,
3,3'dimethy14,4'diaminodiphenylmethane, 4,4'
diaminodiphenylether or sulphide,
4,4'diaminodicyclohexylmethane.
As organic isocyanates B, mainly the aliphatic,
cycloaliphatic and aromatic, solid at room temperature, poly
21I4~~,2
_ g _
functional isocyanates are considered. In so far as the
polyfunctional isocyanates are liquid at room temperature,
they are converted by chemical reaction to polyisocyanates
which are solid at room temperature. The following may be
named as examples of reaction products: di or polyisocyanates
containing the following groups: ester, urea, biuret,
allophanat, carbodiimid, uretdion, urethan, or isocyanurate
groups.
The solid isocyanates shall be insoluble in the functional
polymer or in the high boiling solvent of the suspension at
temperatures below the activation temperature.
The following have in particular proved themselves for the
intended purpose:
dimerized 2,4 and 2,6 diisocyanatotoluene or 2,4' and
4,4' diisocyanatodiphenylmethane, possessing
uretdione groups.
l,5naphthalindiisocyanate, bitoluylendiisocyanate or
3,3'dimenthy14,4' diphenyldiisocyanate.
modified di or polyisocyanates containing urethane,
urea, uretdion,isocyanurate groups on the basis of
l,6hexandiisocyanate,
lisocyanato3,3,5trimethyl5isocyanatomethylcyclohexa
ne, dimethylxylylen and
tetramethylxylylendiisocyanate, 2,4',
4,4'diisocyanatodiphenylmethane, 2,4 and
2,6diisocyanatotolene.
4,4' diisocyanato3,3'demethyldiphenylurea.
- 10 -
Reaction products of diisocyanates with
trimethylolpropane or other short chain polyols or
polyamines.
Mixtures of solid, powder form isocyanate can also be used.
In order to prevent uncontrolled and spontaneous reaction
between the powder form, solid isocyanate and the functional
polymers, polyols, polyamines on uniting with the liquid
polymer, or afterwards during storage and processing of the
hotmelt adhesive, the powder form, solid isocyanates are
surface desactivated.
The desactivating agent is so selected that it is bonded to
the surface of the isocyanate particles through chemical
reaction, and in this way gives rise to separation of phases
by means of an inert layer between the polyisocyanate
particles and the other reactive components, namely the
hydroxy functional and amino functional polymers and low
molecular weight functional crosslinkers or chain extension
agents.
For the desactivating of the isocyanate groups of the solid
isocyanate, 0,1 to 25, in particular 0,2 to 12 equivalent
percent of the available isocyanate groups of the solid
isocyanate are reacted with the desactivating agent C. In
order to obtain stable hotmelts, an excess of desactivating
agent is always to be used when working. This "unbound"
desactivating agent C " , together with the surface "bound" or
"reacted" desactivating agent C', forms the "desactivation
potential". The unbound desactivating agent C " can differ
from the bound desactivating agent C' in that the surface
desactivation takes place in two stages, commencing with
desactivating agent C'. The ratio of unbound desactivating
2~ t~84;~
- 11 -
agent C " to bound desactivating agent C' can amount to 0.1
to 10.
The optmal concentration of desactivator must be
experimentally ascertained, corresponding to the intended use
of the reactive hotmelt and corresponding to the particle
size of the solid isocyanate. The mean particle size will
dictate the required concentration of desactivator, particles
with smaller particle size and correspondingly more surface
area requiring more desactivating amine.
The quoted literature (Blum, R.; Schupp, H., Prog. Org. Coat.
1990, 18(3), 275 288) and the above quoted patents provide
instructions as to the determination of the optimal
concentration of desactivators.
The solid polyisocyanates B are desactivated, preferably
through reaction with primary and secondary aliphatic amines,
di or polyamines, derivatives of hydrazine, amidine and
guanidine. Further desactivating agents are listed in the
above mentioned patent specifications.
The following have been found sound: ethylendiami.ne,
l,3propylendiamine, diethylentriamine, triethylentetramine,
2,5dimethylpiperazine,
3,3'dimethy14.4'diaminodicyclohexylmethane,
methylonandiamine, isophorondiamine,
4,4'diaminodicyclohexylmethane, diamino and
triaminopolypropylenether (Jeffamine ~ Texaco Chem. Co.),
polyamidoamine (EuretekR, Shering AG, Berlin),
aminoalkylalkoxysilane and mixes of mono,di and polyamine.
The solid, surface desactivated isocyanates are sensitive to
mechanical stress arising from high shear action. Through
excessive shear, mainly in metering and supply pumps, in the
_ 211842
,...
- 12 -
piping, in the static mixers or under the action of doctor
blades, the inert layer separating the reactive phases
(comprised mainly of polyureas) can be destroyed, exposing
the surface of the solid isocyanate. Premature reaction will
be the result. Through the presence of the desactivating
agent C " , the system is "selfhealing": as long as the
desactivating agent C " is present in the system, the exposed
isocyanate surface will react rapidly and preferentially with
the desactivating amines, with spontaneous formation of a new
inert polyurea layer.
As a desactivating agent C " , low molecular weight amine or
polymine, with molecular weights up to 450, has been
particularly proven in that these diffuse more rapidly than
the higher molecular weight polyamine onto the damaged
surface of the solid isocyanate.
The desactivating reaction can also take place in processing
of a master batch, in an inert, light solvent. The solvent
can be removed after completion of the desactivating
reaction, if necessary after introduction of a second
desactivating amine which acts as an unbound desactivating
agent C " .
Within the entire mixture of substances, the relationship of
the isocyanate groups of reactive, solid isocyanate B to the
sum of amino and/or hydroxyl groups of the polymer A, of the
desactivating agent C, and of the lower molecular weight
chain extension agent or crosslinking agent D amounts to 0,5
to 2, preferably 0,8 to 1,5 (data in equivalents)
Liquid phase for the preparation of the suspensions of solid
isocyanate B can be high boiling solvents, liquid or low
melting plasticizers or low melting point, low molecular
weight, if necessary functional polymers or resins. The
~1~48~2
- 13 -
mentioned low molecular weight hydroxy functional chain
extension agents and crosslinking agents D, which are
compatible with the polymers A, are also particularly
suitable. In the case of the desactivating reaction, the
solid isocyanates react preferentially with the
simultaneously present desactivating amines.
The use of low molecular weight and low viscosity solvents or
plasticizers for manufacture of the suspension of solid
desactivated isocyanate can be advantageous: During metering
and pumping, within the piping and during mixing with the
polyol components in the static mixer, the viscosity of the
suspension and, with that, the associated shear stress to the
inert surface of the desactivated isocyanate, is small.
The isocyanate functional groups of the components, which if
necessary serve to form the preparation of the suspension or
dispersion of the solid, desactivated isocynate, must
likewise be considered when calculating the NC=/NH+OH ratios.
If necessary, the following, further additives E can be
introduced:
the urethane catalysts known to the expert in the art,
mainly based on organometals. Tertiary amines can
become volatile during open storage of the reactive
hotmelts or react with the carbon dioxide in the
air and so loose some of their effectiveness.
Organometals for the intended purpose are organic
tin (II), tin {IV), iron, lead, cobalt,
bismuthantimon, zinc, and magnesiumcompounds.
Diazabicyclooctane (Dabco) can be used as amine
catalysts, as well as diazabicycloundecene (DBU),
mainly after partial or complete neutralisation
with organic acids or phenols. The catalysts are
~~.~~8~~
- 14 -
employed as a rule in an amount between 0,001 and 3
percent weight, related to the total composition.
Polymers without isocyanate functional groups, small
amounts of solvent, plasticizer, diluents and
resins, pigments, dyestuffs, fillers, pyrogenic
silicic acid, carbon black, short chopped fibers,
metal in powder form, metallic oxides, ferrites,
light and oxidation stabilisers, fungistatic or
bacteriostatic agents, Theological agents, nonsag
additives, surfactants, and adhesive additives. The
use of such additives in hotmelts is state of the
art.
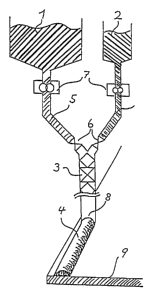
In an initial embodiment of the method according to the
invention, as represented in figure 1, the controlled,
nonhomogenous mixture of both the components according to the
invention,
1 the polymers A
and
2 the suspension of the desactivated, solid
isocyanate B in a carrier liquid 2, which
still contains excessive desactivating
agent C'',
can take place in a completely or partially heated two
component mixing machine with static mixer 3 as commonly used
for processing 2component coatings, sealants and adhesives.
These devices are state of the art, known to the expert in
the art and commercially available.
Static mixers 3 for mixing of two or more components are
nowadays, as an alternative to dynamic mixing heads, state of
the art. For the intended purpose, mainly the static (tube)
.a
,...
- 15 -
mixer from Sulzer AG (Winterthur, Switzerland), in which the
mixing ensues radially and longitudinally within a system of
interspaced and overlapping webs, and the KenicsR static
mixer system (according to US Patent 3,286,992) or the
comparable StatomixR static mixer system from MIXPAC Systems
AG (Rotkreuz, Switzerland), with opposed, helical elements
arranged in a tube which mix longitudinally, have proved
their worth. An element comprises, for example, a lefthand
spiralled helical baffle of a half turn. This is followed by
an element with a righthand baffle of a half turn.
The static mixers also permit mixing of more than two partial
flows, for example a third separate flow of a catalyst
solution or a pigment dispersion.
According to this invention, the static mixers permit
nonhomogenous mixing of the components and the formation of
partial. areas or zones in which use is not made of the number
of mixing elements which would otherwise be necessary for
complete homogenous mixing: homogenous shall mean here that
the concentration of a soluble, compatible, mixable component
in a partial area of lmm2 does nor deviate by more than 2~
from the average value within the entire mixture.
An extrudate 4, which is not completely homogenous, results
at the end of the static mixer. This extrudate comprises
layers or partial areas which are alternately
either rich in polymers A,
or rich in solid isocyanate B and desactivating amines
C.
Through the excess of unbound desactivating agent C " in the
isocyanate rich layers, premanufactured hotmelts are attained
with excellent shelf stability.
- 16 -
It is known to the expert in the art that, for mixing with a
static helical element mixer, 20 to 32 elements, preferably
at least 24 elements, must be used, and with the tube mixer
from the firm Sulzer at least 12 elements, in order to
achieve a homogenous mixture, a condition being that the
viscosity of the components must not vary by more than a
factor of 100 to 1.
Processing, according to the invention, is such that 10 to
22, and preferably 12 to 18 elements, of a baffle mixer are
used in the tube of a static mixer. At a constant flow speed,
this number of elements corresponds approximately to 50 to
90~ of the number of elements which would otherwise be
necessary to create a homogenous, molecularly dispersed
mixture, for example of the desactivating amines C " in the
Polymer A. If other types of static mixers are used, likewise
only 50 to 80~ of the number of elements are used which would
otherwise be necessary to create a homogenous mixture.
The nonhomogenous mixture can be made visible at the end of
the static mixer by adding a dye or pigment to one of the
components. The resulting partial areas will be visible
through the different optical density of the components, and
can be measured.
The thickness of the layers, or expansion of the partial
areas, which are either rich in polymer A or rich in solid
isocyanate B and desactivating amine C, preferably
approximately corresponds to the mean particle size of the
solid, desactivated isocyanate particles and shall not
fundamentally exceed the maximum particle size. The optimal
number of mixing elements in the static mixer must be
ascertained by means of experiments, and is determined by the
viscosity, the rheological characteristics of the components
or the blend and the intended application of the hotmelts.
- 17 -
If blending is carried out with a "paucity" of mixing
elements, an added advantage will be a low pressure drop in
the static mixer.
In the mixing device (figure 1), the static mixer 3, the
piping 5, and metering devices 6 and pump 7 are heated, the
temperature of the heated piping, metering devices, pumps and
mixer being of necessity below the activaction temperature of
the isocyanate.
The resulting stream of the product 4, namely the liquid
hotmelt, exits through dies 8 at the end of the static mixer
3, the cross section of the die determining the cross section
of the solid, uncrosslinked hotmelt. The liquid hotmelt can
be deposited onto the substrate, for example as a coating, if
necessary one or double sided on a carrier or reinforcing
material, as a bead, as a profile; in stripform and as a
powder point coating, through selection of the die and the
method of application. After exiting the die, the adhesive is
cooled, either through ambient air, through the cooling
effect of the item to which the adhesive is applied, or
through a supply of cooled air, gas or inert liquids.
Unsupported reactive hotmelts, for example in the form of a
film, a bead, a net, a granulate or in various forms are
obtained through application of the liquid, uncrosslinked
adhesive onto a belt which supports a nonsticking surface or
a surface provided with a releasing agent. After cooling of
the adhesive, this can be separated from the carrier surface
and immediately, or during application, be applied to the
surface to be adhered, sealed or coated.
Reactive hotmelts in powder form are obtained through
extruding the liquid mixture onto a conveyor belt with a
nonstick surface and cooling it there to a temperature below
its melting point. After solidification, the hotmelt adhesive
- 18 -
can be released from the belt and in a known way, for example
by means of cryogenic grinding, reduced to a granulate or a
powder and if necessary passed through a sieve to produce the
required fractions. The powders according to the invention
serve as reactive hotmelts in powder form for textile
fabrics, in accordance with known methods of application, for
example scatter coating, powder point coating or paste point
coating. They can, however, be used as reactive hotmelt
powder for the adhesion of plastic film, veneer, paper, metal
foil, sheet metal and similar, either with themselves or with
carrier materials, and for coating purposes, such as powder
coating, for example.
In another embodiment of the method according to the
invention, as represented in figures 2 and 3, a moveable belt
is used, primarily a metal belt with a nonsticking
surface, which can be heated, and which moves at a constant
speed beneath the "application heads" for the individual
layers. The mixture according to the invention is created in
such a way that
a) the molten polymer A 1 is applied with a doctor
blade 11 in a layer thickness of 3~ to 200 onto a
moveable conveyor belt coated with adhesive,
b) the solid, surface desactivated isocyanate B, in powder
form 12, is scattered by means of a scattering
device 13, or as a suspension 2 of the desactivated
powder in a carrier liquid with excess
desactivating amine, indeed with said sprinkling
device 13 or doctor blades 11, which can control
and meter the weight or proportion of the applied
isocyanate in relation to the weight of the
polymer,
- 19 -
c) the steps a) and b) are repeated for sufficiently long
until the desired thicknesses of the multilayer
coating 14, comprising polymer rich and isocyanate
rich layers, is attained.
Alternatively, the liquid flows of
(a) Polymer A, and the
(b) suspension of solid, desactivated isocyanate B in a
carrier liquid
are simultaneously extruded in a defined ratio
through the separate openings of a multiple die.
The still liquid or pasty multilayer can be shaped,
pressed, compressed or stretched prior to
solidification.
d) The multilayer 14, whose thickness amounts to between
10~ and several millimeters, can be released from
the carrier either in liquid form or after cooling,
and subsequently reduced by a known method to a
granulate or to a powder with a grain size of up to
500 and if necessary passed through a sieve to
produce the desired fractions. The multilayer can
also be further processed in film, bead or strip
form for adhesive purposes or as a coating
material, if necessary being reinforced by a
carrier material applied to the one side, or in the
form of a reinforcement material held within the
multilayer.
Slotted dies 15 or doctor blades 11, which can be heated, can
serve as the "application head" or application device for
layering of the polymer A and the suspension of the
isocyanate B. The doctor blades 11 are able to be adjusted in
such a way that a defined gap can be maintained in relation
21148 ~
- 20 -
to the surface of the preceding layer. The liquid polymer A
or the suspension B is metered by the pumps 7, fed to the
slotted die 15 and extruded onto the strip. When using the
doctor blades, the polymer A and the suspension of
desactivated isocyanate B is fed via heated pumps and piping
in such a way that a rotating bead 16 arises im front of
the doctor blade. The surface of the polymer of the
previously applied layer must still be liquid or tacky when
applying the subsequent layer (suspension of the solid
isocyanate) or scattering of the solid isocyanate.
The adhesion of the various layers in this multilayer shall
be the same or greater that the cohesion within the layers.
As mentioned, hotmelts according to the invention can be
applied to a carrier or reinforcing material, if necessary
applied,single or double sided, as a sealant bead, a profile,
in.strips, as powder points, as a carrier free film, as a
net, as sealant beads or as a powder onto the adhesing,
sealing or coating substrates. On reaching the melting or
softening temperature of the polymer, they become liquid. In
this condition, they can wet the surface of the substrates or
the adhering surfaces. On reaching the activation temperature
of the solid desactivated isocyanate, which should be higher
than the melting or softening temperature of the polymers,
they will irreversibly crosslink to high molecular weight,
thermoset polyurethanes or polyureas.
The heat transfer into the system of hotmelts and/or
substrates) can ensue by conventional means such as heated
air, heated gases, through heat or infrared radiation,
contact heat or the exploitation of the residual heat of the
substrates, or inductively with microwaves, electrical
heating, friction or ultrasonics.
- 21 -
For crosslinking by means of heat produced by induction,
microwaves or electrical heating, it has been proven
advantageous if a metallic filler, carbon black, graphite,
metal oxides or ferrites are added to the hotmelt.
Examples
Materials used:
Polymers Softening Hydroxyl Equival Spec.
Point°C value ent g weight
Polycaprolactone 55 37 1500 1,07
Capa 231 (a)
Polycaprolactone 58 28 2000 1,07
Capa 240 (a)
Dynacoll 7350 (b) 65 30 1866 1,19
Isocyanate ~ NCO Equival Melting Function
ent g point°C ality
Isonate M 143 (c) 29.4 143 < 15 2.1 2.2
Desmodur TT (d) 24.1 174 > 150 2.0 2.1
Amine
Laromin C 260 (e) 119.2 < 0 2.0
Catalyst UL29 (f) Tin (IV)organic compound
Plasticizer BBP Butylbenzylphthalate (g), liquid
(a) Linear Polycaprolactone, Interox chemicals Ltd.
(b) Linear Polyester, Hiils AG
(c) Diphenylmethandiisocyanate, The Dow Chemical Co.
~~.~84
- 22 -
(d) Toluoldiisocyanateuretdion, "Dimeres TDI", Bayer AG,
mean particle size 12~
(e) 3,3' Dimethyl4.4'diamodocyclohexymethane, BASF AG,
(f) Witco Chem., Corp.
(g) Bayer AG
Example 1:
Component 1:
The hydroxy functional Polymer A was manufactured as follows:
(1) Dynapol 7350 3732 g (2 Equiv.)
(2) Isonate 143 143 g (1 Equiv.)
(3) UL29solution, 10~ in BBP 4 g
was, during 12 hours at 80°C, converted to
(4) 3879 g Polymer A (1 Equiv.)
Component 2
Suspension of the solid, desactivated isocyanate
(5) Laromin C 260 6.6 g (0.055 Equiv.)
(6) Benzylbutylphthalate 374.4 g
(7) Desmodur TT 200.0 g (1.15 Equiv.)
(8) Total suspension B 581.0 g (1.095 Equiv.)
Both the components were metered in a two component mixing
unit at a mixing ratio of 1.00 parts/component A to 15
parts/component B by means of heated gear pumps at a
temperature of 70°C and fed in separate, heated piping to a
thermally insulated static spiral element mixer (diameter 10
mm) from Mixpac AG, Rotkreuz, (Switzerland), which was
equipped with 16 elements, namely with opposed spiral
21I4842
- 23 -
elements arranged within one tube. (Inside tube diamater
lOmm, length of one element lOmm) .
The continuously mixed hotmelt was applied as a bead to the
surface of a right angled profile of glass reinforced plastic
(UP). The hotmelt solidified as a result of cooling through
the profile and the ambient air. The latent reactive hotmelt
was capable of being stored for a period in excess of 4 weeks
at room temperature, which means that after 4 weeks storage
at 70°C, the hotmelt was still meltable and exhibited a tacky
surface .
The latent reactive hotmelt, after 7 days storage, was made
liquid through heating at a temperature of 80°C in an oven,
and pressed against another UP profile. The temperature of
the "sandwich" was increased to 115°C. Within 5 minutes the
adhesive joint became firm, and was allowed to remain at this
temperature for a further 30 minutes.
The adhesive joint was able to be separated at room
temperature only through destruction of the joined
"sandwich". The joint withstood temperatures of 140°C,
undamaged.
Example 2:
Component 1
The hydroxy functional polymer was manufactured as follows:
(1) Capa 240 4500 g (2.25 Equiv.)
(2) Isonate 143 179 g (1.25 Equiv.)
(3) UL29solution, 10~ in BBP 5 g
was, during 2 hours at 80°C, converted to
(4) 4684 g Polymer A (1 Equiv.)
melting temperature 58°C.
- 24 -
Component 2
Suspension of a solid, desactivated isocyanate:
(5) Benzylbutylphthalate 341.4 g
(6) Laromin C 260 8.6 g (0.072 Equiv.)
were mixed and
(7) Desmodur TT 200.0 g (1.15 Equiv.)
was introduced and homogenously distributed.
(8) suspension of the solid
isocyanate B 550.0 g (1.078 Equiv.)
Both the components were metered in a two component mixing
unit at a mixing ratio of 100 parts/component A to 12
parts/component B by means of heated gear pumps at a
temperature of 65°C and fed in separate, heated piping to a
thermally insulated static spiral element mixer from Mixpac
AG, Rotkreuz, (Switzerland), which was equipped with 16
elements opposed arranged within one tube. (Inside tube
diamater 13 mm, length of one element 13mm).
The continuously mixed hotmelt was extruded through a slotted
die onto a steel conveyor belt at a layer thickness of 3 mm,
separated from the band after passing through a cooling
section and reduced to centimenter sized pieces. After
complete cooling, the pieces were ground in a powder grinder,
cooled by dry ice. Grain sizes above 500 were recycled
through the grinder. The powder possessed a melting point of
56°C, the activation temperature was determined as being
98°C.
Example 3:
A hydroxy functional prepolymer was manufactured as follows:
(1) Capa 240 4500 g (2.25 Equiv.)
w..
- 25 -
(2) Isonate 143 179 g (1.25 Equiv.)
(3) UL29solution, 10~ in BBP 5 g
was, during 2 hours at 80°C, converted to
(4) 4684 g Polymer A (1 Equiv.)
Suspension of a solid, desactivated isocyanate:
(5) Capa 231 500 g (0.33 Equiv.) and
(6) Laromin C 260 8 g (0.067 Equiv.)
were mixed at 65°C and
(7) Desmodur TT 260 g (1.49 Equiv.)
was introduced and homogenously distributed.
(8) suspension of the solid
isocyanate B 768 g (1.097 Equiv.),
melting temperature above 50°C.
The hydroxy functional prepolymer was applied to a nonstick
steel belt at 70°C, in 8 layers of 100 or 106 g/m2, total
800 or 848 g/m2, using heated doctor blades at a temperature
of 70°C. The liquid surfaces of the individual polymer layers
were in each case immediately layered over with 7 layers of
18,7 or 19,86 g/m2 of suspension 8, total quantity 139 g/m2.
The layers of the suspension were applied from 7 application
heads, likewise comprising doctor blades heated.to 70°C. The
uppermost layer was not coated with the suspension.
At the end of the belt the "multilayer" was separated from
the belt after passing through a cooling section and reduced
to centimeter sized pieces. After complete cooling the pieces
were ground in a powder grinder, cooled by dry ice. Grain
sizes above 500 were recycled through the grinder.
For experiments with powder coating on fabrics, a grain size
range of 80 to 300 was used with the powder examples 2 and
3. The powders were scattered with a coating weight of
2I~~8~~
- 26 -
approximately 30 g/m2 onto a cotton fabric. The powders were
sintered onto the surface of the fabric in a hot air oven at
140°C for a duration of 90 seconds. These coated fabrics can
be stored at room temperature for at least 4 weeks.
The fabrics coated with powder were laminated with untreated
fabric at a temperature of 170°C for 20 seconds in a press
with a pressure of 2 bar, and then further treated at 160°C
for 3 minutes in an air circulation oven.
After 7 days, the samples were washed in boiling water for
one hour, dried, and the resistance to peeling was
determined:
Sample size Peel strength in g per 2.5 cm
according to DIN 54310 before/after after
boiling dry cleaning
Powder according to Example 2 1170 910 1157
Powder according to example 3 950 810 835
The experiments demonstrated that a thermostable crosslink
- took place: the reactive powder, with a melting point of
56°C, was transformed into a thermoset, chemically stable
polyurethane after the activation temperature was exceeded.
Inasmuch as the invention is subject to modifications and
variations, the foregoing description and accompanying
drawings should not be regarded as limiting the invention,
which is defined by the following claims and various
combinations thereof: