Note: Descriptions are shown in the official language in which they were submitted.
W093/04727 PCT/GB92/0}552 j~
2116534
BALLOON - CATHETER
The present invention relates to surgical devices
generally, and in particular to surgical devices suitable
for use in the thermal or photo-mediated treatment of body
tissues, such as the prostate gland.
The prostate gland is located at the base of the
bladder, where it surrounds a portion of the urethra, the
duct by which urine is conveyed from the bladder to the
exterior. The function of the prostate is to produce a
fluid which becomes a part of the ejaculated semen (also
carried through the urethra). Prostatic disease, both
benign and malignant, is a common urological problem which
accounts for a major part of the health care expenditure in
developed countries. As men grow older, the tissue of the
. . .
prostate often begins to enlarge, a condition called
hyperplasia. As the bulk of the prostate enlarges, the
- gland begins to constrict the portion of the urethra
passing through the prostate thereby preventing the normal
flow of urine, a condition known as benign prostatic
hypertrophy or hyperplasia (BPH). As BPH ~develops,- the
constricted regions within the prostatic urethra can from
time to time obstruct the flow of urine. The signs of BPH
are difficulty in starting urination, dribbling following
urination, reduced force of the stream of urine, a tendency
to urinate frequently in small amounts, as well as pain and
discomfort, and an increase in urinary tract infections.
The symptoms are common with 75 to 80% of men over the age
SUBSTITUTE SHEET
Wog3/04727~ c~ PCT/GB92/01552j~
21ifi~1 ! `!
of fifty affected.
In fact, recent statistics apparently reveal that
a 50 year old man in the USA has a 20 to 25% chance of
undergoing a prostatectomy during his remaining lifetime.
It is currently estimated that about 500,000
prostatectomies are performed each year in the United
States alone. See the Harvard Medical Health Letter,
September 1988, Vol. 13, No. 11, pp.l to 4 and Castaneda et
al., "Prostatic Urethra: Experimental Dilation in Dogs",
RadioloaY, June 1987, pp. 645 to 648, and Castaneda et al.,
"Benign prostatic Hypertrophy: Retrograde Transurethral
Dilation of the Prostatic Urethra in Humans", Radiologv,
June 1987 pp. 649 to 653.
When the obstructive symptoms of BPH become
; 15 bothersome, the constricted portions of the urethra are
usually reopened surgically. Current accepted treatment
;~ for BPH involves either open or transurethral surgery,
which is costly and is associated with an acceptable but
; undesirable degree of mortality (estimated from 1.3 to 3.2%
- - see- Castaneda et al., "Benign Prostatic Hypertrophy:
~etrograde Transurethral dilation of the Prostatic Urethra
in Humansn, Radioloav, June 1987, pp. 649 to 653) and with
~; a significant degree of morbidity, especially in less fit
patients.
One popular surgical procedure for treating BPH, as
an alternative to open surgery, is a transurethral
resection of the prostate, or TURP. The transurethral
resection involves inserting a resectoscope through the
~ SUBSrlTUTE SHEET
W093/04727` ~ '' PCT/GB92/01~2 ;~
~` 2116S3~ ;
urethra. A spring wire, adapted to carry an electric
current, is inserted through the resectoscope for use in
removing tissue. The wire carries one current for cutting
away the tissue and another current for cauterizing, the
remaining tissue to minimize bleeding. Typically, as much
as 30cm3 (about 2 cubic inches) of tissue are removed in
this way.
An alternative but similar procedure is the radial
prostatectomy, usually limited to the tissue at the bladder
neck region and known as a bladder neck incision, or BNI.
In this procedure, ~radial cuts, parallel to the
longitudinal direction of the prostatic urethra, are made
transurethrally into the prostate with the wire of a
resectoscope (such a procedure has been suggested for
removing constrictions of a stenotic region of upper air
passageways by vaporizing tissue with a laser beam to form
radial cuts in the stenotic region of the air passageways -
see Shapshay et al., "Endoscopic Treatment of Subglottic
'and Tracheal Stenosis by Radial Laser Incision and
Dilationn, Annals of Otoloav. Rhinoloav & Larvnqoloav, Vol.
' 96",~No'.--6,~November-December 1987, pp. 661-to 664).,~
The TURP and BNI/prostatectomy surgical,techniques
are neither trivial nor inexpensive. The procedure carries
the same risks as many other general surgical procedures,
including those associated with the use of general
anaesthesia. Other surgical hazards include stricture
formation at the urethra or bladder neck, post-manipulation
~ SUBSTITUTE SHEET
W093~04727 PCT/GB92/01552
2116S34 ` ~
pain or bladder spasm, urinary tract infections and
reactive urethral swelling which can cause urinary
obstruction and epididymitis. Other complications include
infection and retrograde ejaculation. Further, the post-
S operative care following a TURP procedure requires aprolonged hospital stay, creating substantial costs for
medical care. In addition, some men have reported sexual
dysfunction following the resection. Certain men have also
become incontinent as a result of the surgery because of
inadvertent damage done to the distal sphincter muscle
apparatus lying at the apex of the prostate and extending
downstream in the wall of the membranous urethra, and
responsible for controlling urine flow. The surgery
usually results in moderate discomfort with some post-
operative bleeding being usual.
As a result of the trauma that many men experiencefrom TURP, the relatively long in-patient care required for
post-operative recovery, the possibility of other less well
~ understood effects on cardiac function, and the possibility
; 20 of- increased long term mortality compared to open
prostatectomy (see Roos et al., "Mortality and Reoperation
- After Open and Transurethral Resection of the Prostate for
Benign Prostatic Hyperplasia", New Ena. J. Med., Vol.320,
pp. 1120 to 1124, 1989), alternative techniques of treating
`~ BPH and other prostate disorders are being investigated.
Heat treatment has for many years been investigated
as a way of destroying diseased tissue. Microwaves and
radiofrequency ultrasound have been used to treat enlarged
SUBSTITUTE SHER
W093/04727}~ PCT/GB92/01552
2116534
prostate tissue, the applied heat causing the swelling to
subside. These techniques generally involve inserting a
probe comprising an antenna capable of emitting microwaves
into the patient's rectum at a point adjacent t~ the
S prostate gland. The emitted microwaves are capable of
penetrating the wall of the rectum and are focused into the
prostate. The problem with this techniques is largely one
of accuracy, that is, directing the energy into the target
tissue without heating adjacent normal tissue, and
delivering sufficient energy to raise the diseased tissue
to the requisite temperature to cause cell death. Because
of this, several treatments are normally required before
the prostate returns to its normal size, thereby making
this process both time-consuming and expensive.
Laser surgery utilises laser light transmitted
through flexible optic fibres, either illuminating the
target in a hollow viscus or inserted percutaneously,
through needles, directly into the centre of the target
lesion, thereby minimising the effects on surrounding
tissues. Interstitial~hyperthermia induced by lasers was
; firs~ dèscribed by Bown, S.G., "Phototherapy of;r$umours",
World T. Surq, Vol.7, pp.700 to 709, 1983 and subsequently
by Hashimoto, E. et al., "In Depth Radiation Therapy by YAG
Laser for Malignant Tumours in the Liver Under Ultrasonic
~Imaging", Gastroenterolov, Vol.88, p.l663, `1985;
Godlewski, G. et al., "Deep Localized Neodymium (Nd)-YAG
Laser Photocoagulation in Liver Using a New Water Cooled
and Echoguided Handpiece", Lasers in SurqerY and Medicine,
: `:
SUBSTITUTE SHEET
W093/04727 - PCT/GB92/01552,~
211C53~ 6
Vol. 8, pp.S01 to 509, 1988, and Steger, A.C. et al.,
"Intestinal Laser Hyperthermia: A New Approach to the Local
Destruction of Tumours", British Medical Journal, Vol. 299,
p.365, 1989.
The use of laser radiation has been described for
removing tissue of the prostate gland so as to remove
tumours or all or part of the gland as.an alternative to
the electrocautery resection technique described above.
See US Patent No. 4,672,963 and Smith, J. A. et al., "Laser
Photoradiation in Urologic Surgery", The Journal of
Uroloav; Vol. 31, April 1984, pp. 631 to 635, cited
therein.
The device described in US Patent No. 4,672,963
uses a computer to continuously adjust the amount of laser
radia~tion transmitted to the prostate during the procedure.
The `device includes an ultrasonic probe, inserted
,
~ ~ transurethrally or positioned externally, for imaging the
, ~:
-~ ` prostate in real time during the procedure so as to provide
real time data regarding the destruction of the prostate
~ 20 tissue, thereby enab}ing the computer to adjust the laser
-~ ~ ; '~radiàtion accordingly. ~Transrectal and external ultrasonic
ging of the-prostate~are we}l known as further suggested
by Sanders, R.C. et al., "Update on Prostatic Ultrasound",
Uroloaic Radio}oav, - 1967; Fleischer, A.C., "Prostatic
25 ~ Endo onography - A Potential Screening Test", Diaanostic
,- ~
~ ~ aaina, April 1987, pp.78 to 82: and Lee, F., '`Prostatic
r ~, ~ ' ~
Evaluation By Transrectal Sonography: Criteria for
Diagnosîs of Early Carcinoma", RadioloaY, Vol. 158, pp.91
: :~ SUBSTITUTESHEET
W093/04727`~' 2 1 1 6 ~ 3 ~ PCT/GBg2/UI552
to 95, January 1986.
International Patent Publication No. Wo 90/13333
discloses a device for use in dilating the urethra of the
prostate gland to relieve the symptoms of pr4state
enlargement. The device generally comprises a catheter
having disposed at the remote end thereof an inflatable
balloon for temporarily dilating at least a portion of the
prostatic urethra and sized so as to compress the tissue of
an average sized prostate gland enlarged by BPH. The
catheter also includes means for transmitting a laser beam
along an axis transverse to the general direction of the
urethra so that the laser beam can be selectively directed
into portions of the tissue compressed by the balloon. The
balloon is preferably made of a material substantially
transparent to the laser beam, the laser being focused by
the surgeon through the wall of the balloon into the
prostatic-tissue. The tissue heated by the laser is
denatured producing a general coagulation necrosis of the
treated region. As the denatured tissue heals, the treated
tissue -region contracts so as to effectively~dilate the
u'rethral'passa~eway, thereby restoring urine ~low.
' The~ pres~ent invention provides various devices
which find general application in the heat treatment of
~ body tissues.` The devices of the invention find particular
-~ 25 utili`ty in'the'treatment of benign prostate hypertrophy and
prostate cancer. However, it should be evident that while
the preferred embodiments of the devices of the invention
are described hereinafter for use in prostatic surgery they
"
~: ~ SUBSTrrUTE SHEET
w093io4727`~ t~ p~ PCT/GB92/01552
211653~ 8
can also be used to treat tissue(s) in other parts of the
body, such as the bladder, lesions within the kidney or
adrenal gland, benign or malignant growths within the
liver, gut, breast, brain, lung, uterus and skin and
S vascular abnormalities throughout the body.
Thermal treatment of diseased prostatic tissue and
in particular laser surgery of the prostate represents an
alte~rnative approach to curative therapy without the
morbidity of radical surgery or radiotherapy. The term
"thermal treatment" as used herein is intended to encompass
both mild heating, commonly referred as 'hyperthermia', as
well as the more vigorous heating used to denature (or
coagulate) the treated tiss~e. Thermal treatment can be
performed by a variety of methods, such as microwave,
radiofrequency and whole body heating, but the advantage of
làser surgery is that the thermal energy can be delivered
deep within the tissues of the body through optic fibres of
~ relatively non-traumatic size~ Thus, while the devices of
-; the invention are suitable for use with a wide variety of
apparatus for administering thermal energy to the body,
including both microwave and other radiofrequency
-generating~ apparatus, they find particular utility with
apparatus for laser surgery. The term "laser surgery" as
used herein generally refers to the delivery of laser
` radiation into the body to effect the thermal warming of
the target tissue, but it is also intended to encompass
photodynamic therapy in which the target tissue is
pretreated with a photoreactive chemical before exposure to
SUBSTITUTE SHEET
w093io4727' ~ 6 S 3 4 PcT/GB92/ols~2 ~
,. ~. .
radiation of the appropriate wavelength to initiate the
photochemical reaction.
According to one aspect of the present invention
there is provided a catheter dimensioned for insertion into
the body and having thereon a balloon, the catheter further
comprising an inlet and outlet to the balloon to allow
pressurisation/inflation of said balloon and the
circulation of a cooling fluid into and out of said balloon
to effect cooling of the outer surface thereof.
Preferably, the cooling fluid is responsible for
inflating/pressurising the balloon. The device ordinarily
comprises at least two conduits disposed along the catheter
and arranged so as to transport cooling fluid to and from
the balloon.
The balloon-catheter devices of the invention find
particular utility in the selective heat treatment of
certàin body tissues where they are used to provide cooling
for those non-target tissues or organs in close proximity
to~the tissue(s) being treated. The cooling fluid is
20- circulated through the device to cool the cuff of the
balloon' which is in~direct contact with ~the tissue(s)
and/or'~orgàns to be protected. Unlike known catheters,
where an opening is provided at the remote end of the
` catheter to`f}ood the site of insertion with a cooling
~; 25` fluid,'such as saline, it is possible to cool those tissues
.,
' in direct contact with the balloon.
~; The balloon-catheters of the invention are
particularly advantageous inasmuch as they can provide
~'
;~ SUBSTITUTE SHEET
:
w093io4727 `~ ' PCT/GB92/01552 A ~
~11653~ lo
simultaneous cooling of non-target tissue(s) together with,
on inflation of the balloon, therapeutic dilation of the
body tissue being treated. The balloon is preferably sized
such that, when inflated, it compresses the target tissue,
thereby generating a zone of relative ischaemia and
increasing the efficiency of the heat treatment. Moreover,
when inserted into an occluded bo~y passageway, e.g., the
constricted region of the prostatic urethra, inflation of
the balloon may, by distending the passageway, contribute
to restoring its original calibre.
Therefore in accordance with another aspect of the
invention there is provided a method of heat treating the
body wherein thermal energy is delivered to a target body
tissue, said method further comprising inserting a balloon-
catheter of the invention into the body, inflating theballoon and circulating cooling fluid therethrough to
effect the cooling of tissues and/or organs in close
proximity to the target tissue.
One preferred application of the balloon-catheter
is in the heat-treatment of the prostate, e.g., to treat
BPH or `prostatic carcinoma, to provide cooling for the
proximal`and/or distal urethral sphincter muscles and most
importantly for the lining of the urethra during removal of
` the diseased tissue. The balloon-catheter is preferably
dimensioned such that it can be inserted into the body via
the urethra, ordinarily through the~operating channel of
conventional endoscope, such that the balloon lies within
; the prostatic fossa. The balloon-catheter may, however be
SU85mUTE SHEET
WO 93io4727``': `` ,`1` i` 'l 21 1 6 5 3 ~ PCT/GB92/01552~
11 `
inserted via any appropriate transrectal, transperineal (or
other transcutaneous route) or transurethral route using
any of a conventional endoscope, cystoscope or
resectoscope. The balloon is preferably sized such ~hat,
when inflated within the prostate fossa, the urethra will
expand so as to compress at least a selected portion of the
prostatic tissue, thereby generating a zone of relative
ischaemia.
The balloon is generally designed to accommodate
temperatures of up to 10 atmospheres (1.01 x 106 Nm~2),
although in situ the balloon pressure would not normally
exceed about 5 atmospheres (5.05 x 105 Nm~2).
The balloon-catheter may a~so comprise one or more
conduits disposed along the catheter and arranged so as to
lS allow the site of insertion to be flushed with a local
anaesthetic gel or solution. The outer surface of the
balloon may be textured so as to retain an anaesthetic gel
or solution applied thereon.
The balloon-catheter may also comprise one or more
conduits disposed along the catheter adapted to receive
vièwing me`ans, e.g., a~viewing fibre optic or rod lens, ~o
allow the surgeon to monitor the insertion manoeuvre and/or
the site of insertion.
The balloon-catheter may also include a smaller,
-~ 25 secondary balloon located either proximal and/or distal to
~ the main balloon through which the cooling fluid is also
;~ circuIated to provide cooling for the urethral sphincter
muscle apparatus.
SU8STITUTE SHEET
WOg3/~727 ~ J, ~ PCT/GB92/01552;~
2~ 53~ 12
The balloon-catheter is preferably configured so as
to facilitate the delivery and temporary implantation of
and/or the secure retention of an optic fibre or other heat
generating probe (referred to hereinafter simply as the
"probe") into a given tissue or organ of the body for the
express purpose of delivering laser/thermal energy thereto.
In one embodiment, the probe may be inserted into
the target tissue independently of the balloon-catheter,
the simple act of inflating the balloon stabilising the
probe by pressing it against and/or compressing the tissue
in which it is inserted. The outer surface of the balloon
may be advantageously textured so as to increase the
purchase of the balloon for the probe.
Alternatively, the probe may be slidably
manipulated through the catheter into the target tissue.
The balloon-catheter may include means to facilitate the
implantation of the probe into the target tissue. In the
simplest embodiment, the implantation means may take the
form of a needle-tipped canula which is slidably
manipulated through the catheter into the target tissue,
- the pro~e--in ~turn being manipulated through the canula.
The balloon-catheter may also include means to deflect the
probe and/or the canula away from the catheter into the
target tissue and/or means to displace the probe
25 predictably forwards into the target tissue or the canula
predictably backwards to expose the probe.
The balloon-catheter or a component part thereof
may be treated so as to render it more echogenic to
SIJBSTITUTE SHEET
W093/04727 ; -~ 2116 5 3 ~ PCT/G W2/01562
ultrasound imaging and/or provided with readable depth
markings so as to allow the surgeon to monitor the depth of
insertion into the body.
According to another aspect of the invention ~here
is provided an applicator device for inserting an optic
fibre or other heat generating probe into the body, the
device comprising:
a housing adapted to be held by the surgeon;
an elongate delivery tube adapted to be inserted
into the body, one end of which is mounted in said housing;
said optic fibre or other heat generating probe
; being disposed within the delivery tube, and
means to protrude the optic fibre or probe from the
delivery tube or means to retract the delivery tube over
lS the $tatic optic fibre or probe so as to expose the
fibre/probe.
The optic fibre or probe is ordinarily shrouded by
a needle-tipped canula likewise disposed within the
delivery tube and which can be protruded or retracted so as
20 ~ ~ to expose the probe/fibre.-
~
` In a preferred embodiment, at least one of thedelivery tube, canula and optic fibre or probe is separable
from the remainder of the device which can be withdrawn
` from the body leaving the separable component in place
within the body.
The applicator device may also comprise one or more
of the following optional fèatures:
(l) At least a portion of the delivery tube and/or
SUBSIIlUTE SHEET
WOg3/04727 ; i~ ;! PCT/GB92/01552,;,~
2116S3~ 14
canula may be treated so as to render it more echogenic to
ultrasound location.,
(2) At least a portion of the delivery tube and/or
canula may be provided with readable markings to allo~ the
5surgeon to determine the depth of insertion into the body.
(3) Means to flush an irrigant through the
delivery tube to free obstructions etc.
(4) Means to deflect the emerging optic fibre or
probe and/or canula away from the delivery tube.
10(5) Viewing means to allow the surgeon to monitor
the insertion maneouvre and/or the site of insertion.
(6) The delivery tube and/or the canula may be
provided with anchoring means to facilitate their temporary
anchorage within the body.
15, (8) The applicator device may be provided with a
, inflatable balloon on the delivery tube, the tube further
comprising an inlet and outlet to the balloon to allow the
pressurisation/inflation of the balloon and/or the
circu}ation of a cooling fluid into and out of the balloon.
20According to a further aspect of the invention
' there~ i8~ provided a method of heat or photo-treating a
- givèn tissue-or organ of the body in which an optic fibre
or other heat generating probe is inserted, either
~ peroutaneously or through an appropriate body cavity, into
'~" 25the target tissue or organ using an applicator device of
the invention for the purpose of delivering laser/thermal
energy thereto.
~he present invention also relates to the
SUBSTITUTE SHEET
WO 93/04727 2 1 1 6 5 3 4 PCI/GB92/01552 `
combination of a balloon-catheter and an applicator device
of the invention.
According to another aspect of the invention there
- is provided an optic fibre for use in the heat and /or
photo-treatment of a given tissue or organ within the body,
the fibre comprising a core having thereon a cladding and
comprising one or more of the following:
(a) means to facilitate the accurate positioning
of the fibre within the body;
10(b) steering means to allow the direction of fibre
travel within the body to be controlled;
(c) means to facilitate the temporary anchoring of
the fibre in the body, and
(d) one or more temperature sensors disposed along
the fibre to allow the temperature of target and/or non-
target tissue(s) to be monitored.
The positioning means (a) may comprise, for
example:
treating at least a portion of the fibre so as to
render it more echogenic to ultrasound location;
providing at least a-portion of the fibre with an
ultrasound èmitter to aid its ultrasound location;
- providing at least a portion of the fibre with a
~ plurality of readable markings to allow the depth of
insertion to be determined, and
providing a self-tightening or lockable bead which
is sIidably `disposed along the fibre so as to akut a
suitable anatomical landmark.
-: SUBSTITUTE SHEET
w0 93io4i27 ~ ? ~t` ~ `; PCT/GB92/01552 ,~;
~il653~ ` i` `~`
16
The steering means (b) may comprise, for example:
one or more steering wires affixed to the remote
end of the fibre and disposed along the length thereof and
arranged such that by appropriate manipulation of said
S wire(s) the direction of travel of the fibre can be
controlled.
The anchoring means (c) may comprise, for example:
an inflatable balloon arranged such that, when the
fibre is inserted into said tissue or organ, the balloon
can be inflated to secure the fibre in position for the
duration of the treatment, and
one or more protrudable anchoring members disposed
on the fibre ~nd arranged such that, when the fibre is
inserted into said tissue or organ, the anchoring member(s)
can be protruded into the target tissue to secure the fibre
in position for the duration of the treatment. The
anchoring member may take the form of a wing, cage, spike
or hook.
The optlcal fibre may also comprise a collar
,
-20 enshrouding at least a portion of the fibre, the collar
~;~`bearing;the above~described~features (a) to (d).
;`According-to a further aspect of the invention
there is provided a method of heat or photo-treating a
given tissue or organ of the body in which an optic fibre
of the invention is inserted into the body, either
~- percutaneously or through an appropriate body cavity, for
;the purpose of delivering laser radiation to said target
tissue or organ.
:
,
:~ SUBSTmJTE SHEET
WOg3/04727 ~ 2116 5 3 4 PCT/GB92/01552~
Accordinq to another aspect of the present
invention there is provided a cooling jacket for an
ultrasound probe adapted to be inserted into the body of a
patient, the jacket comprising an outer sleeve defining a
central space and having an opening at one end to allow the
remote end of the ultrasound probe to be inserted into the
central space, the opening being dimensioned such that the
sleeve has a sealing fit about the ultrasound probe, the
jacket further comprising an inlet and an outlet to allow
pressurisation/inflation of the jacket and the circulation
of a cooling fluid through the jacket to effect cooling of
the outer sleeve. Preferably, the cooling fluid is
responsible for pressurising/inflating the jacket. The
cooling jacket ordinarily comprises at least two conduits
arra~ged for transporting cooling fluid to and from the
jacket. -
~ ~he cooling jacket may optionally be provided with
an inner sleeve having a complementary fit about the remote
end of the ultrasound probe.
-The cooling jacket is preferably formed of a
compllant matérial, such as latex, to allow for independent
movement of the probe when inserted into the body.
- - The invention also relates to the combination of an
ultràsound probe and the aforesaid cooling jacket.
-- Ultrasound probes provided with such a cooling
jacket find particular utility in the selective heat
treatment of certain body tissues where they can be used to
provide cooling for those non-target tissues or organs in
~ SUBSTITUTE SHEET
WO 93/04727 ` ~ ` PCI`/GB92/01552 ~
21~6~3~ -`
18
close proximity to those being treated, in addition to
their normal imaging function. - The cooling fluid,
typically water, saline or any other physiologically
compatible fluid, is circulated through the jacket to cool
the outer sleeve which is in direct contact with the
tissues and/or organs to be protected.
Therefore in accordance with a further aspect of
the invention there is provided a method of heat treating
the body in which thermal energy is delivered to a target
body tissue or organ wherein an ultrasound probe fitted
with a cooling jacket of the invention is inserted into the
body and cooling fluid is circulated through the jacket to
effect cooling of non-target tissues or organs in close
proximity thereto.
One preferred application of the cooling jacket is
in the heat-treatment of the prostate to provide cooling
for the lining of the rectum. The ultrasouhd probe is
preferably dimensioned such that it can be inserted into
the rectum of the patient via the anus with the cooling
jacket sized such that, when inflated, the outer sleeve of
-- the jacket contacts the~walls of tbe rectum to protect the
lining of the rectal:wall from heat damage.
The cooling jacket is preferably configured so as
to facilitate the delivery and temporary implantation of
-- 25 and/or the secure retention of an optic fibre or other heat
generating probe into the target tissue or organ. The
cooling jacket may comprise one or more conduits extending
through the jacket to a port(s) provided in the outer
SUBSmUTE SHEET
WO 93/04727 !~ r ~ ' 2 1 1 6 5 3 ~ PcT/GB92/ols52
19
sleeve to allow the optic fibre or probe to be manipulated
through the jacket into the target tissue or organ.
Movement of the optic fibre or probe can adventageously be
directed via real-time feedback from visual ultr~sound
images generated by the ultrasound probe of the thermal-
tissue effect.
The surgical devices of the invention will now be
described by way of example with reference to the
accompanying drawings, in which: -
Figure 1 is a simplified anatomical illustration of
the human (male) uro-genital system;
Figure 2 is a longitudinal section through one
embodiment of a balloon-catheter in accordance with the
invention;
Figure 3 is a transverse section along the line A-A
: of the balloon-catheter of Fiqure 2;
Fiqure 4 is a perspective view of the remote end of
an alternative embodiment of balloon-catheter in accordance
with the invention;
Figures 5 and 6 represent simplified-anatomical
illustràtions of the human (male) uro-genital system having
isposëd - within:~the urethra -a~-balloon-catheter in
accordance with the invention;
Figure 7 to 9 are simplified representations of
-~ applicator devices in accordance with the invention:
Figures lOa and lOb are sectional views of the
remote end of the delivery tube of an applicator device in
accordance with the invention;
SUBSTITUTE SHEET
W093/04727 ; .~ '` PCT/GB92!01552 i~ 7
2116534 20
Figures lla to llc illustrate an alternative
embodiment of applicator device;
Figure 12 illustrates another embodiment of
applicator device in accordance with the invention;
SFigure 13 illustrates a further embodiment of
applicator device in combination with a ballcon-catheter of
the invention;
Figure 14 is an anatomical illustration of the uro-
genital system of a patient undergoing laser surgery of the
10prostate and having disposed within the prostatic fossa a
balloon-catheter of the invention;
Figure 15 illustrates another embodiment of
applicator device in combination with a balloon-catheter of
the invention;
15Figure lSa illustrates a perspective viéw of the
remote end of the delivery tube of a modified version of
- the applicator device of Figure 15;
Figure 16 illustrates a further embodiment of
applicator device in combination with a balloon-catheter of
- the invention;~
. Figure-16a: i8 a sectional view along the line B-B
of: the applicator device/balloon-catheter combination of
- Figure 16;
Figures 17a and 17b are perspective views of the
remote end of a canula-having a self-anchoring mechanism
for use with an applicator device in accordance with the
invention;
Figures 18 and 19 are sectional.views of the remote
SUBSTITUTE SHEET
W093/04727 ` ` 2116 5 3 4 PCT/GB92/015~2
21
end of the canula of an applicator device of the invention
having means to limit the extent of maximum travel of an
optic fibre disposed therein:
Figure 20 is a sectional view of a body cavity
having disposed therein an ultrasound probe fitted with a
cooling jacket in accordance with the invention;
- Figures 21 and 22 are simplified anatomical
illustrations of the prostate of a patient undergoing
prostatic surgery having disposed therein the probe/jacket
combination of Figure 18;
Figure 23 is a ~sectional view of the anal passage
of a patient undergoing prostatic surgery having disposed
therein an ultrasound probe fitted with an alternative
embodiment of cooling jacket in accordance with the
invention;
Figure 24 is a perspective view of the proximal end
of the probe/jacket combination of Figure 23;
Figures 25 and 27 are simplified anatomical
illustrations of the prostate of a patient undergoing
prostatic surgery having disposed therein the probe/jacket
combination of Figure 23;
- Figure 26 is a transverse sectional view along the
line C-C of the anatomical illustration of Figure 25;
~ Figure 28 is a transverse sectional view through a
2S probe fitted with a further embodiment of cooling jacket in
accordance with the invention, and
Figures 29 to 41 illustrate various embodiments of
optic fibres modified in accordance with the invention.
SUBSTITUTE SHEET
~y ~ ~
Wo9j/04727 i'~l,.J~ PCT/GB92/01552f c.
211653~ 22
Figure l, is a simplified anatomical illustration
of the human (male) uro-genital system. The prostate (2)
is a gland found in male mammals surrounding the urethra
(4) in the region where it leaves the bladder (6). It
S releases a fluid containing various substances, including
enzymes and an anti-coagulating factor, that contribute to
the production of semen which is released through the penis
(8) via the urethra (4) at sexual orgasm. The size and
secretory function of the prostate (2) are under hormonal
control. Urine enters the bladder (6) from the ureters
(not shown) and is discharged to the exterior through the
urethra (4) under the control of the proximal and intrinsic
distal sphincter muscles (lO and 12 respectively) which are
situated at the neck of the bladder t6) and at the apex of
the prostate (2), the latter marked endoscopically by the
presence of the verumontanum, and extend into the
membranous urethra (4). The position of the seminal
vesicles (14) and the pubic symphysis (16) is also shown.
Benign prostatic hypertrophy (BPH) is generally caused by
the transitional (or central) zone (18) of the prostate (2)
enlarging, usually with age,- thereby compressing and
- restricting the -outflow-of urine from the bladder (6).
Malignant prostate cancer, now the second commonest cause
of death from malignant disease in the UK and the commonest
r: 25 cause of death from malignant disease in US males, is
normally associated with the peripheral zone (20) of the
prostate (2), sometimes referred to as the "true prostate".
The prostate (or other target tissue or organ) is
SUBSTITUTE SHEET
W093/~727 ' '; 21 16 5 3 ~ PCT/GB~2/01552
23
scanned by the most appropriate method, ordinarily
transrectal ultrasound (TRUS), to identify the target areas
within the prostate, that is, BPH of the transitional zone
and peripheral zone lesions in the case of prostatic
carcinoma.
The target areas may be marked in the scanner's
memory as a series of three-dimensional co-ordinates which
allow for the precise insertion of the optic fibre or other
heat generating probe (and/or any other appropriate
surgical instrument/device) into the area(s) of interest.
Of course, the patient must remain in or be repositioned in
the position used for the original scanning procedure which
may be immediately prior to the actual operation or at some
earlier occasion.
One surgical device of the invention comprises a
catheter dimensioned for insertion into the body and having
thereon a balloon, the catheter further comprising an inlet
and an outlet to allow pressurisation/inflation of the
balloon and circulation of a cooling fluid into and out of
the balloon. The balloon-catheter is primarily intended to
-^ -be used in the selective thermal treatment of diseased body
tissue to cool those non-target tissues in close proximity
to the tissue being treated. They find particular utility
in the treatment of benign prostatic hypertrophy and
carcinoma of the prostate where they are employed to
provide cooling for the sphincter muscles and most
importantly the lining of the urethra or rectum (depending
on whether the balloon lies in the prostatic urethra or
SUBSTITUTE SHEET
W093/04727`~ P~ ~ PCT/GB92/01552 ~,~
2116534 24
rectum).
The balloon-cather is normally hand held and
inserted into the body via the transrectal, transperineal
(or other transcutaneous route) or transurethral route
using a resectoscope, endoscope or cystoscope. The
balloon-catheter is preferably sized so as to allow for its
insertion through the operating channel of a conventional
endoscope if it is to be inserted via a body cavity, such
as the urethra. The balloon-catheter may be sufficiently
rigid so as to allow for its insertion through the skin,
e.g., the skin of the perineum. Insertion through the skin
would ordinarily be guided by the use of biopsy guides and
channels.
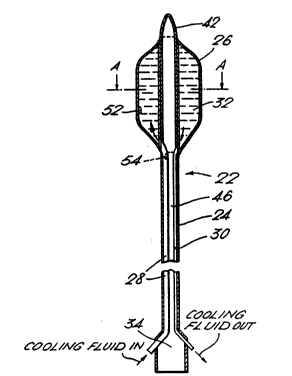
The balloon-catheters of the invention (denoted
lS generally by (22)) will now be described in greater detail
with reference to Figures 2 to 6. Each device (22)
generally comprises an elongate catheter (24) dimensioned
so as to be capable of insertion into the body cavities of
the patient and havinq thereon a balloon (26). Both ends
of the balloon (26) are secured to the catheter (24) to
form a hermetic seal between the balloon (26) and the outer
- - wall of the catheter (24). -
~
The balloon is preferably sized such that when it- is~inflated within the prostatic urethra, the urethra of an
~average sized hypertrophied prostate gland will expand so -
as to compress the prostatic tissue, preferably without
exceeding its elastic limit. An inflated balloon having a
diameter of about 12 to 25 mm is normally adequate for the
SUBSTITUTE SHEET -
W093/04727'`'~ ' 2 1 1 6 5 3 ~ PCT/GB92/01552
' 25
average sized hypertrophied prostate gland to achieve the
desired compression of the prostatic tissue, although this
dimension may vary with smaller and larger prostate glands
with the balloon being sized accordingly.
Th`e balloon may be made of various known materials
already used in other medical procedures, such as those
described in US Patent No. 4490421. The balloon is
generally designed to accommodate up to about lO
atmospheres (l.Ol x lo6 Nm~2) and preferably no more than
about 6 atmospheres (6.06 x 105 Nm~2) of water pressure and
should be capable of holding that pressure for at least 5
minutes. Preferably the balloon material is designed to
rupture should the pressure exceed the aforesaid limits in
order to ensure that ruptures at even greater pressures
would not occur causing potential harm to the patient. It
should be evident that the material used for the balloon
for other procedures in other body passageways, e.g., the
rectum, may be of a type which is more compliant, such as
; a latex material, so as to more readily conform to the
shape of the passageway, and may operate at other pressures
' depénding on''the''balloon size and application.
The balloon-catheter (22) generally comprises at
least two conduits (28 and 30j for transporting a cooling
~' `fluid (32) to and from the balloon (26). The two conduits
; 25 '(28 and 30) may be connected at their distal ends, thereby
~; forming a closed loop for the cooling fluid (32) or, more
preferably, both conduits (28 and 30) may open at their
distal end into the balloon (26) as shown in Figure 2. In
SUBSTITUTE SHEET
W093~0472~ PCT/GB92/01552 -
~ill;53.1
26
this preferred arrangement, the cooling fluid (32),
typically water, saline or any other physiologically
compatible fluid, is responsible for pressurising and
inflating the balloon (26), although if desired se~arate
pressurising/inflation means may be provided. The pressure
differential required to inflate the balloon may be
provided by a suitable valve mechanism positioned at the
proximal end of the conduit draining fluid from the balloon
or, alternatively and as shown, the conduit(s) (30)
draining the cooling fluid (32) may simply have a smaller
bore than the supply conduit (28) or a constriction to
provide the requisite pressure differential.
The proximal end (34) of the catheter (24) is
adapted to be connected to a suitable fluid supply and pump
mechanism (not shown) to allow the balloon (26) to be
inflated/pressurised and the cooling fluid circulated
therethrough. The device may also be connected to a
æuitable control unit (not shown - but see Figures 7 to 19)
co~prising a pistol or scissor grip to facilitate its in
- gi5~manipulation.
The-cooling fluid is circulated through the device
to cool the outer surface (or cuff) of the balloon which is
in direct contact with the tissue(s) to be protected. This
-~ ` i8 in~marked contrast to known catheters incorporating
cooling means where an opening is provided at the remote
end to simply flood the site of insertion. It will be
appreciated that it is not possible to cool those tissues,
e.g., the lining of the urethra, in direct contact with the
.
SUBSTlTlJTE SHEET
W093/04727 " 2116 S 3 4 PCT/GB92/01552 ~
27
catheter using such devices.
The balloon-catheter (22) may advantageously
include a smaller secondary balloon (shown in dotted
outline (36) in Figure (4)) situated just below the main
balloon (26) through which the cooling fluid (32) is also
circulated. The secondary balloon (36) overlies the
sphincter active area at the apex of the prostate (2)
leading to the urethra (4), to protect the distal sphincter
muscle (12). The second balloon (36) can also be used as
an aid to positioning the device (22) by providing a
palpable marker which can be felt transrectally by the
surgeon, thereby confirming that the main balloon (26) has
been advanced into the prostatic urethra.
The balloon-catheter (22) may include another
secondary balloon (shown in dotted outline (38) in Figure
6) situated above the main balloon (26) which lies in the
- neck of the bladder (5) and when inflated, anchors the
device (22) in position. Cooling fluid (32) may be
- ~
circulated through the balloon (38) to provide cooling for
~; ~ 20 the proximal sphincter muscle (10)~
When-treating- a patient;suffering from BPH, the
-balloon-catheter; (22) is- typically inserted into the
; urethra (4), as shown in Figures 5 and 6, such that the
~ ~ balloon ~26) lies within the prostatic region of the
-f ~ 25 urethra (4). The patient is appropriately anaesthetized,-~ -
for example, with a topical anaesthetic applied liberally
within the urethra (4) or by injection o local anaesthetic
agents about the target tissue or by regional, spinal or
~ ~ SUBSTITUTE SHEET
~i16~31 r ~ PCT/GB92/01552 ~ rj
general anaesthetic. The balloon-catheter may
advantageously include a conduit (not shown) extending
through or along the catheter to allow the prostatic
urethra to be flushed at periodic intervals with an
anaesthetic gel or solution. Subsequent expansion of the
balloon presses the anaesthetic gel into contact with the
tissue of the prostatic fossa.
The outer surface of the balloon (26) may be
advantageously textured so as to retain an anaesthetic gel
or paste applied thereon.
An additional mild sedative and prophylatic anti-
biotics may also be given. The penis (8) is then
appropriately prepared and draped and the tip (42) of the
catheter (24), with the balloon(s) (26, 36 and 38)
deflated, inserted through the urethra (4) as shown in
Figures 5 and 6.
e manoeuvre can be observed, either
endoscopically, ultrasonically or fluoroscopically using
any of the appropriate apparatus known in the art, to
~ 20 ensure precise positioning of the balloon. The balloon-
-~ ~^ catbeter~may advantageously include a-channel or conduit
-(not shown)~;extending through or along the catheter for a
flexible viewing fibre optic or rigid rod lens to allow the
surgeon to endoscopically monitor the passage of the device
.
along the urethra.-
Once, tbe balloon is properly positioned, it is
inflated, normally to a pressure of from 2 to 5 atmospheres
(2.02 x 105 to 5.05 x 105 Nm~2). The balloon is preferably
~ ~ SUBSrlTUTE SHEET
W093/04727 211 6 5 3 4 PCT/GB92/01552
29
sized such that when inflated it compresses at least a
selected portion of the prostate to generate a region of
relative ischaemia, the compressed tissue having less blood
to absorb the laser radiation and/or to remove the heat
S generated by tissue warming, thereby producing a greater
heating effect per Joule of energy administered. Moreover,
the cooling fluid circulating through the balloon cools the
walls of the prostatic urethra allowing the surgeon to
increase the amount of energy applied to the prostate to
effect the destruction of the target tissue, while
preserving the lining of the urethra.
The balloon-catheter is preferably constructed of
ultrasonic-visible material to facilitate its positioning
via ultrasound imaging. The balloon-catheter or a portion
thereof, e.g., the catheter tip, may also be treated using
one or more conventional techniques known in the art, such
as, etching, sand blasting etc., for rendering it device
more echogenic to ultrasound, as explained hereinafter with
reference to the optic fibres shown in Figures 29 to 31.
In a highly preferred embodiment, the balloon-
` ;catheter is arranged so as to~facilitate the delivery and
the implantation of an optic fibre for administering las~er
radiation to the target tissue, although any other fine
calibre, heat generating probe could be employed, e.g., a
2S microwave antenna or a heated wire element. Referring to
Figure 6, the fibre (44) can, in the simplest embodiment,
be slidably manipulated through the lumen (46) of the
catheter (24) and out of a suitable aperture (48) provided
SUBSTITUTE SHEET
wo 93lo476i ~ `~3; ~ J ! ~t-, f . ,,~ PCI`/GBg2/015~i2 ~ I
in the remote end thereof into the target tissue.
Alternatively, where the fibre is inserted into the
prostatic tissue independently of the balloon-catheter, the
simple act of inflating the balloon(s) can be used to
stabilise the fibre by pressing it against the wall of the
prostatic urethra and/or compressing the tissue in which it
is ir.serted. In the former case, the outer surface of the
balloon may advantageously be textured so as to increase
the purchase of the balloon on the fibre.
10The balloon-catheter preferably includes means to
facilitate the implantation of the fibre into the tissue.
In the simplest embodiment, the applicator means may
comprise a needle-tipped canula (not shown) extending
through the lumen of the catheter which can be used as a
guide to facilitate the accurate delivery of the optic
fibre, the fibre being manipulated through the needle-
-~ tipped canula into the target tissue. Once guided into
position, either by ultrasonic and/or endoscopic or other
guidance, then either the canula would be retracted while
maintaining the-fibre optic at the desired position or, the
flbre would'be~protracted from the canula. Once treatment
has' ceased,''then the fibre can be retracted from the
protracted position into the needle-tipped canula and
removéd`from the body. In a preferred embodiment, the
balloon is'~inflated once the fibre has been correctly
positioned and includes a groove or slot into which the
fibre and/or implantation device sits enveloped in the
balloon.
SUBSmUTE SHEET
WO 93io472i `- 2 1 1 6 5 3 4 ~
31
Preferred applicator devices are described
hereinafter with reference to Figures 7 to 19.
Where several points are to be treated at one time,
the balloon-catheter may allow for the insertion of a
S canula at each of the predetermined target points, after
which a fibre is passed down the individual canulae. As
before, each fibre is protruded (or the canula retracted)
by a predetermined distance to allow it to stand clear of
the canula.
When the fibre optic is manipulated through the
catheter, the device is desirably provided with means to
deflect the fibre (and/or the canula) away from the balloon
and into the target tissue. In the embodiment shown in
Figures 2 to 4, the fibre (44) is threaded through the
lumen of the catheter (24) into a channel (50) provided in
~ the bàlloon (26) which is configured so as to deflect the
emerging fibre (44), in this particular embodiment, in a
direction approximately perpendicular to the direction of
travel Q f the prostatic urethra.
`~ For treatment of benign prostatic hypertrophy, the
thermal--ië~nergy must ~be`directed-to~the main mass of the
= lateral lo~e of the transitional zone but sufficiently far
away from the sphincter areas , the pelvic plexus nerves
controlling sexual potency and the lining of the urethra to
avoid damage to those structures. The balloon-catheter is
ordinarily provided with one or more temperature sensors,
to allow the surgeon to monitor the temperature of the
tissues being protected. In the embodiment shown in Figure
~ - ~ SUBSTITUTE SHEET
W093/04727~ ?- t, :,~ PCT/GB92/01552
2 1 1 6 ~ 3 4 32
2, the device (22) is provided with at least one
thermocouple (52) at a position approximately equivalent to
the middle point of the prostatic urethra so as to monitor
the temperature of the urethral lining and preferably a
second thermocouple (54) at a position approximately
equivalent to the level of the distal sphincter muscle.
The leads extending from the therocouples (52 and 54) have
been omitted in the interests of clarity.
The laser delivery system preferably includes a
laser capable of providing a deeply penetrating continuous
or pulsed wave beam. A preferred example is a neodymium-
YAG laser because its radiation is not absorbed so strongly
by body tissue as some other lasers. The neodymium-YAG
laser also provides a more pronounced scattering, with a
larger volume tissue effect occurring. Examples of such
laser delivery systems include the PEGASUS Laser System,
commercially available from Pfizer Laser System Inc. and
the MEDILASE 2 Laser System commercially available from MBB
Incorporated.
~ Tbe~balloon-catheter may include means (not shown)
for- ultrasonically rviewing at least a portion; of the
- prostatic tissue so-that the portions of the prostate to be
irradiated can be selected by the user, and the selected
``tissue exposed to the laser radiation can be observed and
monitored by the user in real time during the course of the
treatment. This observation is particularly facilitated by
the fact that the treated tissue becomes strongly
echogenic, that is, visible by ultrasound imaging during
SUBSmUTE SHER
WO93io4727` ~ 2116 53 9 PCT/GB~2/01552
33
the course of the treatment, allowing modification of the
position of the fibre and/or the duration of heating if
necessary. Thus, as the zone of visible .change expands
from the~fibre (or other heat source), the position of. the
fibre may be changed to encompass a larger volume of
tissue, usually by gradually withdrawing the fibre.
The device may, for example, include a u/s
emitter/receiver which receives the reflected u~s waves
from the target region and converts them into an electric
signal which correlates with the temperature of that
region. The temperature data so produced can be projected
onto a colour monitor as coloured areas or bands
: representing zones of warmer or cooler tissue which can be
used as a visual guide to the operator in the positioning,
movement and repositioning of the optic fibre (or other
heat generating probe) and/or the increase/decrease of the
laser~energy being delivered.
Alternatively, the change in the blood flow rate
within the target region can be analysed from the change in
: 20 u/s~signal reflection using the "doppler shift" principle,
-~ to give à velocity value which is~projected.onto the image
; of thé blood vessel on a screen as a blue- colour for
relatively slow moving fluid and red colour for faster
moving fluid. The blood vessels at the periphery of the
heated arèa initially dilate, thereby increasing the rate
~;~ of the blood flow (seen as becoming more red), but as the
:; heated area eventually reaches those vessels, the rate of
blood flow slows (becoming more blue), presumably as they
SU8Sl H UTE SHEET
w093io4727 ~ `' PCT/GB92/01552~.s~
2116534
become thrombosed, eventually ceasing to flow at tissue
death. The aforedescribed methods of using ultrasound to
monitor the thermal tissue effect to control the treatment,
with the position of the fibre/probe and energy delivered
being adjusted to either damage target tissue or to protect
vital structures (such as the vessels running just outside
the prostatic capsule with the nerves that control
potency), represent a further aspect of the invention.
The laser is energized so as to deliver an amount
of radiation sufficient to cause the tissue to coagulate.
The ultrasonic transducers may be used to view the tissue
ultrasonically during and after the treatment to determine
the area and extent of coagulation. The typical result of
such treatment is indicated by the crosshatched region (56)
~ lS shown.in Figure 6. Once all the selected areas have been
: exposed to the laser radiation, the balloon can be deflated
: and the device withdrawn from the urethra. As soon as the
balloon is deflated, body fluids will begin to flow into
the previously-compressed and heated tissue to promote
: 20 healing. ~If desired, the balloon-catheter may be withdrawn
'' and~loaded'with ~a small temporary indwelling stent which
-can' then~be placed~in position and dilated to its full
diameter over the balloon when it is seen, either
~; '- endoscopically -or ultrasonically, to be lying in an
25 `'-`' appropriate position within the prostatic urethra, thereby
over¢oming any consequent oedema that might cause
temporarily increased obstructive symptoms. During post-
operative healing, the damaged tissue will shrink, thereby
~ .
`
SUBS H l UTE SHEET
W093/0472i'`-;'` ' 4 PCT/GB92/01552
` 35
reopening the urethra and restoring the calibre to
something like its normal diameter.
The balloon-catheter may also be provided with
readable depth markings, e.g., as explained hereinafter
with reference to the optic fibre shown in Figure 29, such
that the surgeon can determine the depth of insertion into
the body. In one èmbodiment, radio-opaque markers (not
shown) may be provided at opposite ends of the balloon
which can be seen fluoroscopically to ensure that the
balloon is safely advanced into the prostatic urethra
before inflation so as to minimise the risk of damage to
either sphincter muscle when the balloon is inflated.
The balloon-catheters of the invention are believed
to provide several advantages in the treatment of symptoms
lS of BPH and prostatic carcinoma, in particular, preservation
of' the urethral lining with necrosis of underlying
-obstructive/malignant tissue, as well as reducing the
chances of tissue and muscle damage associated with
impotence and incontinence. Furthermore, by inflating the
bal100n sufficiently to squeeze the tissue so as to
' `co~press' the -tissue without necessarilyfexceeding its
' elastic''limit, the tissue will not be unnecessari~ly damaged
due to tearing. ~
A further aspect of the present invention will now
'~ 25 ^;~ be''described with reference to Figures 7 to l9. ~
~; -- - ' Figures 7 and 8 illustrate the general principles
' of an applicator device (lO0) in accordance with the
present invention. The device generally comprises an
SUBSrlTUTE SHEET
093/04n7 ; ~ 36 PCT/GB92/0l552
elongate, ordinarily rigid but in a preferred embodiment
articulated, delivery tube (102) dimensioned for insertion
into the body of the patient, and a housing (104) which
separates as shown in Figure 7, on operation of the scissor
S grip (106). When treating the prostate, the device may be
inserted through the urethra or the rectum or via the skin
by direct puncture, preferably guided by ultrasound and
usually inserted via the biopsy channel of an ultrasound
probe. A surgical instrument (108), which may be a needle
or optic fibre for delivering laser radiation, is anchored
in one section (110) of the housing (104) such that
operation of the scissor grip (106) causes the instrument
(108) to be protruded from and retracted into the delivery
tube (102).
lS Figure 9 illustrates an alternative arrangement of
; applicator device (112) .in which protraction/retraction of
the instrument (108) is effected by drawing together the
;-~ two sections of the housing (104) as indicated. A
resiliently-fleXible member (114) biases the two sections
of the housing (104) ~apart so that the instrument is
el- ~'ordinarily-retracted within the~..delivery tube .(102). A
`latch~'~mechanism (notishown).may be provided to allow the
two sectiQns of the housing (104) to be locked together,
thereby fixing the instrument (108) in the protracted
position and allowing the surgeon the use of both hands.
In an alternative arrangement (not shown), rather
than protrude the instrument from the delivery tube, the
delivery tube may be retracted over the static instrument
"
SUBSTITUTE SHEET
W093/04727`~- 2116 5 3 4 92/0l552 ~3 ~
37
to leave it standing clear of the device.
When the applicator device is inserted
transurethrally, the delivery tube is preferably provided
with a suitable deflection mechanis~ to steer the emerging
S instrument away from the device and into the tissue of the
body, e.g., the lateral lobes of the prostate. For
example, referring to Figures lOa, and.lOb, the remote end
of the delivery tube (102) may have sufficient flexibility
to allow for the use of a control wire (116) embedded in or
affixed to the end of the delivery tube (102) to control
the angle of deflection of an optic fibre (118) as shown.
Figures 11 to 19 illustrate more sophisticated
applicator devices.
Referring to the applicator device (120) shown in
Figure lla to llc, the delivery tube (102) encloses a
needle-tipped canula (122) having disposed therein an optic
fibre (118) for administer;ng laser radiation to body
tissue(s). The delivery tube (102) is provided with an
adjustable deflector (124) to steer the emerging cannula
~: : io~-: (122)/fibre (118) at a range of angles away-from the device
(120). ~The angle of thè `deflector~(12A) is adjusted by a
lever~(li6)-provided on the housing`(l28). Those skilled in
the art will appreciate that there are many ways of
` `articulating/deflecting the remote"end of the delivery tube
, ~ 25 ``` or cànula to!orientate the fibre (or other instrument) on
. . .. , . ,. . ~ .
insertion.
operation of the scissor grip (106), as shown in
Figure llb, causes the needle-tipped canula (122) to
~: SUBSTITIJTE SHEET
woi3/0472i ~ ?,~ r~ PCT/GB92/01552 ~,~Y~
211B53l `^ `` 38 "
protrude from the delivery tube (102). The fibre (118) is
in turn protruded from the canula (122) by the surgeon
pushing on the finger grip (130) of a collar (132) secured
to the proximal end of the fibre (118), as shown in Figure
5llc. The collar (132) may be adjustable to allow the
surgeon to define the maximum allowable protrusion of the
fibre (118). The scissor grip ~106) will typically allow
for between 3 to 5 cm movement of the canula (122) and full
displacement of the fibre, that is, until the collar (132)
10abuts the housing (128) of the device, a further 0.5 to 2
cm thereover. A latch mechanism (134) may be provided to
alIow the surgeon to lock the collar (132) to the housing
(128) to stabilise the protruded fibre (118) in position.
In an alternative embodiment (not shown), the
15needle-tipped canula or, alternatively, the relevant
- instrument (fibre) or delivery tube, may be disengaged from
: the remainder of the housing. In this manner, the surgeon
can load and implant a succession of canulae into the
' target region, an optic fibre being manipulated through
, each canula, to allow for the simultaneous treatment of two
or,more regions of,the,target-tissue.
A viewing fibre-optic or rod lens (136) may be
mounted atop the housing (128) to allow visual inspection
of the chosen site of insertion and to confirm that the
~-~ 25emerging canula (122) and/or,fibre (118) is entering the
` tissue well away from the sphincter regions. The eyecup
(138) of the rod lens (136) may be connected to a suitable
video monitor.
SUBSTITUTE SHEET
W093/04727 l ~ PCT/GB92/01552~
~ 2116534 -.:
39
Figure 12 illustrates an alternative embodiment of
applicator device (140) in which a rod lens (136), together
with one or more light conducting elements, the needle-
tipped canula (122) and enclosed optic fibre (118) are
S contained within the delivery tube (102). Inlet and outlet
ports (142 and 144) are provided to allow an irrigant, such
as water, saline or any other physiologically compatible
fluid to be flushed through the delivery tube (102) in
order to improve visibility, free obstructions etc.
It will be appreciated that the applicator and
balloon-catheter devices of the invention are closely
related.
Figure 13 illustrates an alternative embodiment of
applicator device (146) in combination with à balloon-
lS catheter (22). The applicator device (146) is inserted
through an orifice (148) provided in the proximal end of
the balloon-catheter (22) which acts, in this embodiment,
as a sheath for the applicator device (146). A window
(149) is provided in the remote end of the balloon-catheter
(22) through which the surgeon can visualize the tarqet
tissuë using ~à rod lens (136) or other similar viewing
`` instrumènt and thouqh which the optic fibre (118) can pass
to the target tissue. Once the fibre is in position, the
applicator device (146) may be withdrawn, as shown in
Figure i4, or left in position as desired. The balloon
(26) -is then inflated by circulating cooling fluid
therethrough and heating commenced. The whole operation,
including the heating stage, would no~mally be monitored by
SUBSmUTE SHEET
'` ' Pcr/GB92/o1ss2~ ,?i,
ultrasound, typically using a TRUS probe (202) inserted
into the patient's rectum. The probe (202) may be provided
with a cooling sheath (not shown) as described hereinafter
with reference to Figures 20 to 28.
5Figure 15 illustrates another embodiment of -
applicator device (150) adapted to deliver a balloon-
catheter (22) to the prostatic urethra. In this
embodiment, the balloon-catheter (22) is inserted through
the delivery tube (102) of the applicator device (150).
10Alternatively and as shown in Figure 15a, the balloon-
catheter (22) may lie in a slot (152) provided in the
remote end of the delivery tube (102). ,
Figures 16 and 16a illustrate a further embodiment
of applicator device (154) in which the delivering tube
: 15(102) is formed with a 'U-shaped' cross-section in which
the balloon-catheter (22) rests. A conduit (156) extending
therethrough is provided for the optic fibre (118) or other
instrument.
~' In an ~lternative embodiment (not shown), the two
20:: devioes may be combined in a,single integral unit. For
'''example~- the:-:-cooling-.balloQn may .be ..provided on the
c~ delivery;itube of.the applicator-device, with the conduits :~
supplying cooling fluid to and from the balloon passing
`;' `'''through,or along the delivery tube. ..
25 ~The applicator.~device or,a portion thereof. may
:~ optionally be provided with readable depth markings and/or
treated to render it more echogenic to ultrasound imaging
as described hereinafter with reference to the optic fibres
~ SUBSTITUTE SHEET
WO 93/oA7i7 ' ~ f ~ I ` `` 2 1 1 6 5 3 ~ Pcr/GB92/0lss2 ;i ~ 1 ~
. ` . .
41
shown in Figures 29 to 32.
The delivery tube and/or the canula may also be
provided with anchoring means as described hereinafter with
reference to the optic fibres shown in Figures 34 to 39.
For example referring to Figures 17a and 17b the needle-
tipped canula (122), which may be disengagable from the
remainder of the applicator device (not shown), may be
provided with an anchoring member to allow for the
temporary anchoring of the canula (122) into the target
tissue during treatment. In the embodiment shown, the
canula (122) is provided with two wire 'wings' (158) which
can be manipulated by the surgeon pushing/pulling on a
control wire (160) from a 'collapsed' state, as shown in
Figure 17a, in which it lies substantially flush with the
surface of the canula (122) in slot (162), to an anchoring
position, as shown in Figure 17b, in which the wings (lS8)
project into the target tissue, thereby securing the canula
:~ (122) in position.
;~ Referrïng to Figures 18a and 18b, the remote end of
the needle-tipped canula (122) may,. in a preferred
~e~bodiment,~ be~ provided with a stop (164) which, as the
bptic fibre (118) is protruded from the canula (122) into
the target tissue, abuts the cladding (166) of the fibre
~:; (118) preventing its further movement. In this manner, the
~ 25 -length of the~fibre core (168) exposed, represented by 'x',
~ will determine the extent of fibre protrusion into the
- ~ target tissue.
Alternatively and referring to Figures l9a and l9b,
'
SUBSTITUTE SHEET
WOg3/04727 ~ PCT/G~92/01552~;y
the optic fibre (118) may be provided with a collar (170)
secured about the fibre (118) a predetermined distance 'y'
from the fibre tip which, as the fibre (118) is protruded
from the canula (122), abuts stop (164). The collar (170)
may be adjustable to allow the surgeon to control the
length of fibre (118) protruded from the canula (122). It
will be appreciated that the stop and collar need not be
positioned at the remote end of the canula/fibre but may be
located at any point along the length thereof.
A further surgical device in accordance with the
present invention will now be described with reference to
Figures 20 to 28.
Figure 20 illustrates a sectional view of the body
cavity (200) of a patient having disposed therein an
: 15 ultrasound probe (202) of conventional type provided with
a cooling jacket (204) in accordance with the invention.
The cooling jacket (204) is ordinarily formed of a
compliant material, such as latex, and comprises an outer
sleeve (206) defining a central space (208). An opening is
~: 20 provided in one end (210) of the cooling jacket (204) to
allow-the remote end of the probe (202) to be inserted into
the ce tral space (208), as shown, the jacket (204) having
a sealing fit thereabout.
The cooling jacket (204) is provided in said one
25` ~end (210) with an inlet (212) which allows for the
introduction of a cooling fluid (214), typically water,
saline or any other physiologically compatible fluid, into
the central space (208), thereby inflating/pressurising the
SUBSmUTE SHEET
WO 93/04727 ' '- 2 1 1 6 5 3 4 /Gs92/0ls52 ~ a ~
43
jacket (204). Preferably, the inlet (212) is connect to a
suitable reservoir of cooling fluid (not shown) and pump
mechanism (also not shown) to allow the cooling fluid (214)
to be circulated through the central space (208) and out of
the cooling jacket (204) via outlet (216) to effect the
cooling of the body tissues in contact with the outer
sleeve (206), in this case, the walls (218) of the body
cavity (200). Alternatively, separate inflation and
cooling means may be provided. The pressure differential
required to inflate the cooling jacket (204) may be
provided by a suitable valve mechanism (not shown) at the
outlet or, alternatively, the outlet (216) may simply have
a smaller diameter than the inlet (212), as shown.
One preferred application of such a device is in
the laser or heat treatment of prostate disorders (see
Figures 20 and 22). The probe (202) is inserted into the
rectum (220) of the patient through the anus (222), with
the cooling jacket (204) deflated and positioned such that
~-' it lies adjacent the prostate (2). The probe (202) allows
the surgeon to visualise the prostate region to facilitate
~;~ - thé location of, for :example, an: optic fibre (224) (or
othèr-- -;heat ~ generating probe) inserted either
transcutaneously through the skin of the perineum (226)
(see Figure 21) or transrectally through the biopsy channel
' ~5~ ~ 25 (228) of the probe (202) and/or to monitor the progress of
the treatment in real-time, while simultaneously providing
: cooling for the walls (230) of the rectum (220). Referring
to :Figure 22, the outer sleeve (206) of the cooling jacket
SUBSrlTUTE SHEET
2 1 1 6 5 3 ~ ` PCT/GBg2/0l5Q ,~
(204) is provided with a port (232), explained hereafter
with reference to figures 23 to 27, through which the optic
fibre (224) passes out of the jacket (204) into the rectal
passageway. The cooling fluid circulating through the
jacket (204) causes it to swell and fill the rectal
passageway, thereby anchoring the probe (2C2) in position.
Figures 23 to 27 illustrate an alternative
embodiment of cooling jacket (204) in which the end wall
(210) of the jacket (204) is provided with an inner sleeve
(234) having a complementary fit about the remote end of
the probe (202), the cooling fluid circulating through the
closed space (208) defined between the outer and inner (206
and 234) sleeves.
The cooling jacket (204) is advantageously provided
with one or more conduits (236) which extend through the
closed space (208) to a port (232) provided in the outer
sleeve (206). Each conduit (236) is dimensioned so as to
allow the surgeon to manipuIate surgical instruments, such
as biopsy knives, viewing fibre optics etc., through the
device into whatever body cavity the probe has been
inserted.- The conduits (236) may be integrally formed of
the same~materia} as the remainder of the cooling jacket
(204), but are more preferably formed from or lined with a
rigid o~ semi-rigid material to allow for the insertion of
edged instruments. Each conduit (236) is, due to the
compliant nature of the outer sleeve (206), afforded a
degree of relative mobility, thereby allowing the probe
(202) to move freely and to scan various areas as desired.
SUI~STlTlJTE SHEET
W093/~727l - 211 6 5 3 4 PCT/GB92/01552
Each port (232) may be provided with a rupturable membrane
(not shown), which may be self-sealing or, it may simply be
left open.
Cooling jacket (204) may also be provided with one
or more integrally formed channels (238) extending through
the outer sleeve (206), into which finer instruments, such
as temperature sensors may be inserted to allow the surgeon
to monitor the temperature of the relevant body cavity.
Referring to Figures 25 to 27, the device may be
used to facilitate the insertion of a optic fibre (224)
into the prostate (2). The optic fibre (224), ordinarily
enclosed in a needle-tipped cannula (not shown), is
manipulated through the device via conduit (236a), through
the wall (230) of the rectum (220) and into the target
prostatic tissue. The cooling fluid (214) is then
circulated through the jacket (204) such that it swells and
fills the rectal passageway, as shown in Figures 27,
thereby anchoring the probe (202) and fibre (224) in
position.
20- Figure 28 represents a section through an
alternative embodiment of~cooling jacket (204), in which
the inner sleeve (234) is formed~as an extension of the
outer sleeve (206). Anchoring filaments (240) may be
provided between the conduits (236) and inner sleeve (234),
as shown.
The advantages of a probe incorporating a sheath in
accordance with the invention can be summarised as follows:
(1) To improve the ultrasound imaging
SUBSTITUTE SHEET
W093/04727 ~ PCT/GB92/01552
21~65~
46
characteristics of the probe by providing an u/s "stand
off" and acoustic window.
(2) It allows for cooling of the wall of the rectum
(or other body cavity) during heat-mediated treatment of,
for example, the prostate.
(3) It can be used to monitor the temperature of
the wall of the rectum (or other body cavity).
(4) It can be used to anchor the probe at the
desired location in the rectum (or other body cavity).
10(5) It allows for the removal of the probe without
disturbing other instruments, e.g., the optic fibre.
(6) It allows for movement of the probe,
; particularly rotational movement, to permit the
'simultaneous' imaging of two or more treatment sites.
15(7) The pressure produced by the inflated sheath on
the internal conduit may serve to secure any instruments
extending therethrough in position.
(8) It allows for the insertion of two or more
instruments, su~h as optic fibres and other heating
elements, at various points of a target organ, e.g., the
- ~- prostate,~without disturbing those previously placed~
--A further aspect of the present invention will now
be described with reference to Figures 29 to 41 which
illustràte a series~of optic fibres modified for use in the
25 laser surgery of body tissue. Each fibre generally
comprises a core and outer cladding. The outer cladding
(300) is ordinarily stripped from the remote end of the
fibre to expose the core tip (302), typically the distal
: ~ SUBSTITUTE SHEET
WO93/0472i` ~;;s~ 1 211653~ PCr/GB92/01552 `t';
.~ .. . .
47
0.5cm thereof, which is then blunted and polished.
Figure 29 illustrates one embodiment of optic fibre
(304) in which the outer cladding (300) is provided with a
plurality of readable depth markings (305) at regular
S intervals, typically every 0.5cm, to facilitate the
accurate positioning of the fibre (304) within the target
tissue when using endoscopic or other visual means of
guidance. The core tip (302) has also been treated using
techniques, such as etching, sand blasting etc., to render
it more echogenic (visible) to ultrasound imaging.
Moreover, by roughening the core tip (302) in this manner,
it is possible to increase the light-emitting surface area
of the fibre (304). Of course, should other modalities of
imaging be used then the fibre may be modified to increase
visil~ility by that imaging modality.
Figure 30 illustrates another embodiment of optic
fibre (306) in which the outer cladding (300) is provided
with an ultrasound emitter (308), e.g., a piezo-ceramic
compound, to increase the visibility of the remote end of
2Q the fibre (306) to ultrasound imaging. The piezo-ceramic
compound is excited by an electric current passed along the
fibre to improve or allow for u/s location. Other devices
known in the art will emit when stimulated by the presence
of~an u/s field. In an alternative embodiment (not shown),
the ultrasound emitter may be provided at regular intervals
along the outer cladding of the fibre in a similar manner
to the optic fibre (304) shown in Figure 29.
Figure 31 illustrates an alterF~ative embodiment of
SUBSTITUTE SHEET
WO93/04727 , , ;~, ? j, ,, PCI`/GB92/01552 ~ t~ ~ 2116531 48 '`
optic fibre (310) in which the core tip (302) is provided
with the ultrasound emitter (308). The core tip (302) is
normally provided with a silicone subbing layer (312) onto
which the ultrasound emitter (308) is coated to avoid
affecting the light propagating properties of the fibre
(310).
Figure 32 illustrates another embodiment of optic
fibre (313) in which the remote end (314) of the outer
cladding (300), typically the last 0.5 cm, has been treated
to render it more echogenic to ultrasound imaging as
described earlier with reference to Figure 29. The fibre
(312) is also provided with a temperature sensor, e.g., a
thermocouple (316), to allow the surgeon to monitor the
temperature of the target tissue. The lead (318) from the
thermocouple (316) may be embedded in or simply affixed to
the outer cladding (300) of the fibre (313). In an
: alternative embodiment (not shown), the fibre may be
provided with a plurality of temperature sensors spaced at
periodic intervals along its length to allow the surgeon to
simultaneously monitor the temperature of the target and
adjacent tissues.,~
Figure..3~3 .illustrates a sectional view .through
another embodiment of optic fibre (320) in which a collar
` (322)` shrouding one or more temperature sensors (316)
surrounds theifibre (320). The collar (322) may be slidably
-'; manipulated by the surgeon from a retracted position (as
shown) to a protracted position indicated in dotted outline
(324) to allow the surgeon to measure the temperature of
SUBSrlTUTE SHEET
wo g3/0472i " ` i 21 16 5 3 4 Pcr/GBg2/0l5s2
49
the body tissue at any point along the length of the fibre
(320). The collar may optionally be provided with
readable-depth markings as described earlier with reference
to Figure 29 and/or treated to render it more echogenic to
ultrasound imaging, for example, the collar may be formed
of a plastics material having embedded therein metal
particles.
Figures 34 to 39 illustrate a series of fibres
incorporating anchoring means to facilitate the secure
retention of the fibres at the target site ~whether it be
in the prostate, bladder, kidney, breast, brain, lung,
uterus, adrenal gland, skin, liver, pancreas, gut etc.
Such means allow for the temporary anchoring of the fibre
(or the cannula or delivering tube of the aforesaid
~applicator devices - see Figures to 7 to 19 if the
anchoring means are applied to them) during treatment,
;~ àfter'which, the anchoring means may be released or stowed
or otherwise rendered inactive to allow the fibre to be
removed or repositioned with further activation of the
anchoring means when the next suitable position of the
; fibre~has been àchieved.'- '~ ~
' '`` Referring to Figure 34, the optic fibre (326) is
provided with an inflatable balloon (328), typically of
about 1 to 5 cm3 volume, which is inflated once the fibre
(326~)`''is'10cated at the target site to anchor the fibre
(326) in position. In the embodiment shown, the balloon
(328) is provided on the outer cladding (300) of the fibre
(326), although a separate collar of the type described
~ SUBSTITUTE SHEET
w0 93io4727 ~ r ~ PCT/GB92/01552~
21165~l S
earlier with reference to Figure 33 could also be used.
The balloon (328) is inflated by passing a fluid,
ordinarily water, saline or similar physiologically
compatible fluid, through a delivery tube (330) into the
balloon (328). The proximal end of the delivery tube (330)
is normally connected to a syringe (not shown) to allow the
surgeon to inflate/deflate the balloon (328) as desired.
Figures 35 to 38 illustrate a series of closely
related optic fibres (332 to 338) in which an anchoring
member, either affixed to or embedded in the outer cladding
of the fibre (or alternatively provided on a collar
described earlier with reference to Figure (33)), can be
manipulated by the surgeon from a 'coilapsed' state, in
which it lies substantially flush with the fibre, to an
anchoring position, in which the anchoring member projects
into the body tissue, thereby securing the fibre in
position. The anchoring member may take a wide range of
forms including the 'cage' (340) of Figures 35 and 35a, the
'wing' (342) of Figure 36, the 'spike' (344) of Figure 37
and~tbe 'hook'~(346) of Figure 38. The anchoring members
(340 to 344) may be made of metal, e.g., a memory alloy, or
plastic, and are typically erected/collapsed by the surgeon
pushing or pulling on a control wire (348) extending along
- the fibre (332 to 338).
Figure 39 illustrates a further embodiment of optic
~ fibre (350) having a 'lockable/tightening' bead (352) that
`~ can be freely pushed along the fibre (350) until it abuts
~ a suitable anatomical landmark, such as the rectal mucosa,
.
~ SUBSmUTE SHEET
wo 93/W727 2116 ~ 3 4 Pcr/GDg2~olss2 ~ - ?~ ~/
51 ' '
whereupon it can be locked/tightened onto the fibre (3so),
thereby aiding the maintenance of the fibre (350) at the
target position. The bead (352) may be palpable to further
indicate to the surgeon that the fibre (350) is correctly
positioned. The bead (352) may be treated to render it
more echogenic as described earlier with reference to
Figures 30 and 31.
Figure 40 illustrates the implantation of a optic
fibre (354) incorporating an anchoring member (342) in the
form of a 'wing'. The fibre (354) is loaded in an
applicator device (356) of the type described previously
with reference to Figure 9. Once the applicator device
(356) has been correctly positioned, the fibre (354) is
protruded from the delivering tube (3s8) by drawing the
body portions (360 and 362) of the housing together as
indicated. The anchoring member (342) may be spring-biased
to the anchoring position (as shown) or, alternatively, the
surgeon may operate a control wire as described previously
with reference to Figures 28 to 32. The surgeon then
relaxes his or her grip on the device (356) allowing the
fibre (354) to partially retract, until the anchoring
member (342) abuts the delivery tube (358) as shown,
preventing further retraction. Once the treatment has been
completed, the fibre (354) is stowed by, in the case of a
spring-biased member, forcibly retracting the fibre (354)
into the delivery tube (358) or, in the case of a wire-
controlled anchoring member by appropriate manipulation of
the control wire.
SUBSTITUTE SHEET
W093/04727 - ~ PCT/GB92/01552 ~,
2116534 52
Figure 41 illustrates a further embodiment of optic
fibre (364) in which a steering wire (366) is attached to
or embedded in the outer cladding (300) at the remote end
of the fibre (364). The proximal end of the steering wire
S (366) is attached to a suitable control unit (not shown)
operable by the surgeon to control the extent of deflection
into the target tissue.
The use of each of the aforesaid devices may be
cumulative with any of the other devices of the present
invention.
.
,
~ .
:
SUBSTITUTE SHEET