Note: Descriptions are shown in the official language in which they were submitted.
21 166S2
WO 93/07150 Pl~/EPgl2/0;!283
TETRAPHENYLPORPHY~ DERIVATIVES
This in~ention relates generally to catalytic,
antibody controlled processes in which tetraphenylporphyrin
catalysts are used. The invention also relates to
tetraphenylporphyrin derivativesr haptens containing them,
antigens containing the haptens, and antibodies raised to
the antigens, which ha~e application in the processes.
Metalloporphyrins are able to act as catalysts for many
types of chemical reactions; especially oxidative
transformations suZch as hydroxylati.on reactions,
dealkylation reactions, epoxidation reactions, deZsaturation
reactions and the like (~or example, Dixon, ~. and Webb,
E.C.; Enzymes, 3rd Ed.; Academic Press, 1979; Collins, J.R.
et al; l991; J Am. Chem. Soc., ~13, 2736-2743 and Rettie,
A.E. et al; 1988; J. BioZl. CheZm., 263, 13733-13738). However
the selecti~ity of chemo- and/or regio-selecti~e attack (or
oxidation~ of mekalloporphyrins is usually poor and cannot
be predeZZtermined for any given substrate. Usually when a
metalloporphyrin catalyst is used, a complex mixture of
isomeZric anZ~l non-isomeric products will be obtained, which
are difficult to separate.
To control product formation, it has beeZn recently
proposed to use meta~lopor~hyrin~ as catalysts or cof~ct~rs
in antibody mediated reactions; the antibody bringing site-
salectivity to the process ~Schwabacher, A.W., Weinhouse, M.
I., Auditor, M.M a~d LZer~er, R.A., 1989; J. Am. Chem. SocZ.,
111, 2344 to 2346). The authors' proposal is to immunise an
animal with a complex of a substrate and a metalloporphyrin
chosen to~bind to the substrate. Antibodies having binding
sites that are complementary to both the pcZZrphyrin and the
substxate are then isolat ed. It is then proposed to bind a
porphyrin catalys~ to such an antibody so that only
substrate that is correctly orientated can be bound and
reacted.
Schwabacher et al managed to prepare antibodies to Fe3
35 and Co3~ complexes of synthetic meso-ketra-kist4-
carboxyphenyl)porphine by coupling the complexes to keyhole
'
WO93/071~0 2 11 6 6 ~ 2 PCT/EP92/02283
limpet hemocyanin (KLH) or bo~ine serum albumin (BSA) ~nd
applying standard monoclonal techniques. However no further
steps of the proposed process were carried out.
EP 0305870 discloses a similar concept, in general
terms, in which an immunoproximity catalyst ~or chemical
reactions is preparQd by selecting a hapten which
coxresponds to, but is different from, a transition state
complex of the reactant and a catalyst. An i~mune response
is then stimulated using an antigen derived from the hapten
to produce antibodies to the hapten. The antibodies are
then isolated. '7Converting" hapten~ are then u~ed to
covalently bind the catalyst to the antibodies to produce
"modified" antibodi~s. The modifiad antibodies are then
isolated for uYe. These modified antibodies catalyze
cleavage of bonds in the target molecules; much in the
manner of an enzyme. The antibodies are said to ~peed up
the reaction and to introduce ~ite-~pecificity~
EP 0305870 ~uggests that the catalysts could be general
acid-base catalysts, nucleophilic catalysts, electrophîlic
catalysts and metal catalysts. No speci~ic mention is made
of metalloporphyrins. Also, for many proce~ses, the
isolation of a transition state complex for many of these
catalysts may well prove to be difficult, if not impossible.
In any e~ent, EP 0305870 does not disclose any specific
proce~ examples which illustrate that the modified
antibodie~ ha~ in fact been prepared.
PCT patent publication WO 92~01781 discloses a similar
proces~ in which metalloporphyrin derivati~eY are used as
cofactors or catalysts. Also propo~ed in general terms are
porphyrin3 derivativi~d with alkyl gxoup^~ so that the
resultant antibody would have alkyl or aryl binding sites~
The derivat.iveY are u~ed to generate haptens that mimic the
actual catalyst and the ~ubstrate in the relative
orientation and spacing needed for the reaction to proceed.
The haptens are then used to generate antibodie3 which have
binding sites complementary to the catalyst and the
substrate in the correct orientation. Unlike the process
~116~2
WO93/07150 PCT/EP92f02283
described in EP 0305870, the antibodies need not have the
catalyst covalently bound to them prior to useO
Unfortunately it is not a simple matter to create
haptens from the metalloporphyrin derivati~es disclosed in
the PCT publication. This i5 because coupling of a
substrate to the porphyrin is not possible because there axe
no convenient points of attachment on the porphyrin.
Accordingly it is an object of thi~ invention to
provide a metalloporphyrin derivative that can be readily
attached to a substrate to provide a hapten. It is also an
ob~ect to pro~ide haptens containing the metalloporphyrins,
antigen~ containing the haptens, antibodie~ raised to the
antiyens, processes using th~ antibodies, and catalysts for
use in the processes.
In one a~pect this invention pro~ide~ a compound of the
formula I:
R,
R2J ~z
O
R, ~ R, I
R~ /~R~
R8 R3f~ 3 R~
R~ ~2
R,
in which:
each R1 i5 independently selected from -H, -F, -Cl,
-Br, -CH3, -COOH, -S03H, -COO-C~6-alkyl, -CH~CH-COOH,
W~93/07150 2 1 1 6 6 5 2 ~CT/~P~/02283
-CH=CH-COO~C16-alkyl, -SO3-C16-alkyl, -NO2, phenyl, -NH2, and
-NH-CO-C16-alkyl;
each R2 and R2' is independently selected from -H, -F,
-Cl, -Br, -CH3, -COOH, -SO3H, -NO2 and phenyl;
each R3 and R3' is independently selected from -H, -F,
-Cl, -Br, CH3, -O-C16-alkyl, -NO2, phenyl, -NH2, and
-NH-CO-C16-alkyl, or
at least one R3 or R3' is independently selected from
(a) -NH-CO-alkylene-3N-imidazole or -NH-CO-alkylene 3-
pyridine, i~ which each alkylene has 2 to 4 carbon atoms;
and (b~ -CO-alkylene-3N-imidazole or -CO-alkylene-
3-pyridine~ in which each alkyl~ne has 3 or 4 carbon atoms;
or
a pair o~ R3 and R3', on oppo~ing phenyl groups, jointly
form (c) -NH-CO-alkylene-3-pyrldyl-5-alk~len~-CO-NH- or
CO-alkylene-3-pyridyl-5-alkylene~CO~, in which each
alkylene ~as 2 to 4 carbon atoms;
R4 is a) a ~ydrogen atom; b3 a linker group containing
a reacti~e centre or group through which the compound of
Formula I may be bonded to another compound; or c) a
removable protecting group;
each Ra and R8' is independently -H, -F, -Cl, -Br or
-CN; and
acid addition salts of the compound and sodium,
potas8iu~ and calcium salts of the compound.
: When R9 i9 a linker group b), it is preferably of the
formula -(CH2j~-R5- (CH2) n~ (~6) ~iA in which Rs is -(CO)--,
-(SO2)~ or -(POOH~-, R6 i9 - 0~ ~S~~ or -~NH~-, each of m, n
and p independently is 0 or l and A is a xeactive leaving
group or centre or, when p is l, A may al~o be a hydrogen
atom.
More pre~erably m, n and p are 0 and A is halogen,
particularly Cl or Br. A particularly preferred linker
group is -COCl, which may ~asily react with a functional
group such a~ -OH or -NH2 on another molecule to form the
bridging gxoup -CO-~- or -CO-NH-.
When R~ is a removable protecting group c), it is
WO93/07150 2 11 6 6 5 ~ PCT/EP92/02283
pref~rably a prctecting group which will protect the >N-NH2
group against oxidation by a reagent such as 2,3-dichloro-
5,6-dicyanobenzoquinone (DDQ), and which is removable by
hydrolysis under acid or alkaline conditions. A preferred
S protecting group is CF3-CO-, which may be removed by mild
alkaline hydrolysis.
Preferably R1 is H, a group which increases the water
solubility of the compound, or a functiQnal group which
p~rrnits the attachment of a carrier protein to the compound.
More preferably, at least one Rl is -NH-CO~CH2-CH2~COOH or
NH2, at least one further R1 i~ -COOH, -COOCH3 or -CH=CH-COOH
and the remainder are H.
Preferably R2 and R2' are all H. Preferably one R3 or
R3', or one pair of R3 and R3' are selected from groups a),
b) and c) as defined above for R3 and R3'. The remaining R3
and R3' are preferably H. R~ and R8' ~re preferably H~
Compound~ of formula I in which R4 is m-C~3C6H4SO2- and
O2NC6~4CO are known, but these two groups are neither linker
group~ since they ha~e no reacti~ atom3 or groups for
20 binding, nor are they protecting groups which can be removed
without destroying the ~N-N< bond.
The Cl6-alkyl may be any branched or unbranched alkyl
group that contains up to 6 carbon atoms. Methyl i5
pre f erred .
25 The use o~ N-amino porphyrin compounds greatly
facilitates the synthesi~ of hapt~ns since a linker group
can be readily attached to the nitrogsn atom that has been
added. Then a desired substrat~ or a functionalized
derivativa of a desired substrate may be attached to th~
linker group.
The i!nvention also provide~ a compound of formula I, as
. . defined above, for use in the preparation of a hapten that
mimics a transition state of a metallopo~phyrin catalyst and
a substrate in a reaction.
The invention also pro~ides a proce~s for the
preparation of a compound of formula I, as defined above,
comprising the steps of:
WO93/071~0 2 11 6 6 5 2 PCT/EP92/022
a) for a compound of formula I in which R4 is H,
deprotecting a compound of formula I in which R4 is a
protecting group;
b) for a compound of formula I in which R4 is a
linker group, r acting a compound of formula I in which R4
is hydrogen with a precursor of the linker group that
contains two reacti~e centres or groups, one of which is
capable of forming a bond with the >~-NH2 group;
c) for a compound of formula I in which R~ is a
protecting group~ i) protecting the >N~NH2 group of a
compound of ~ormula III
R,
R2~, R2'
R ~,~R"'
R, ; _ ~R~ III
R
R,
in which Rl~ R2, R2', R3~ R3', R8 and R~' are a~ defined above;
and ii) oxidizing the compound of formula III to the
corresponding compound of formula I.
¦ 15 In step a) the depxotection step will depend upon the
I nature of the protecting group, but is preferably carried
out by acid or alkaline hydrolysis. Where the protecting
group is CF3CO-, mild alkaline hydrolysis may be used; for
example using EtOH/KOH or EtOH/Ca(QH) 2 at temperatures in
WO93~071~0 ? 1 1 6 6 ~ 2 PCT/EP92/02283
... 7
the 65C to 75C.
In step b) the precursor of the linker group may be,
for example, X-(C~I2)~- Rs~ (CH2) n (R6)p-A in which X is a
reactive leaving group or atom, preferably Cl or Br, and the
other symbols are as defined abo~e. Where the linker group
is -CO~Cl-, a suitable precursor is phosgene or diphosgene.
In step c), where the protecting group is CF3-CO-, the
compound o formula III in which R4 is hydxogen may be
reacted with txifluoracetic anhydride in a polar non-aqueous
solvent. The oxidation step ii) may be carried out using an
oxidizing age~t such as DDQ in an inert solvent, for example
methylene chloride.
The starting material o~ formula III may be prepared by
reacting the corresponding porphyrin with O-m-toluene-
lS sulphonylhydroxylamine as described in Callot, H. J.; 1979;Tetrahedron, 35, l455 6.
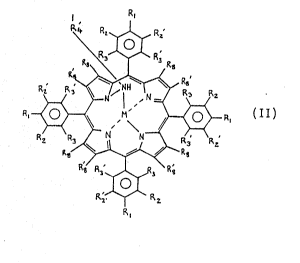
~ n another aspect this invention pro~ides a hapten
comprising a metalloporphyrin cofactor bound to a residue of
a substrate, the hapten mimicking a transition state of a
metalloporphyrin catalyst and the substrate in a reaction,
and in wh.ich the m~talloporphyrin cofactor iY of the ~ormula
II: R,
1, R2.J~, Rz
R ~ R~
~R, II
R~ R ~ 3 R~
O
R2~ R;
R,
~1166~2
WO93/071~0 PCTt~P92/0228~3
in which:
Rl, R2, R2 , R3, R3', R8, and R8' are as defined above for
formula Ii
R4' is a bridging group connecting the metalloporphyrin
catalyst to the residue of the substrate; and
M is a metal ion ha~ing a co-ordination number of at
least 4;
or an acld addition salt thereof or a sodium, potassium
or calcium salt thereo~.
The bridging group may be any suitable bridging group,
with the proviso that it should b~ sel~ct~d such that the
spacial orientation of the metallopvrphyrin cofactor with
respect to the residue is as close as possible to that of
the transition state ~ormed by the corresponding
metalloporphyrin catalyst and the substrate during reaction.
Once the transition state has been identified and the
residue of the subs~rate selected, selection of the bridging
group will be routine.
Pre~erably the bridging group is of the ~ormula
20 ~ (CH2) m - Rs - (C~2) n~ (R6) p~ in which Rs is -~CO)-, -(SO2)- or
(POOH3-, R6 is -O-, S-, or -(NH~-, m is 0~or l, n is 0 or
l and p is 0 or l. Pre~erabLy m, n and p are'0. In a
specific exampl~ the bridging group is -(SO2)-O- o~
--C ( 0)--0--. :
It will be appreciated that the hap~en has the
ad~antag~ that bridging group projects axially from the
cen~rally located amino group. Therefore the hapten can
mo~e closely mimic the relati~e positions of the
correspo~ding substrate and metalloporphyrin catalyst in the
transition state.
'~ ~hen at least one R3 or ~3~ iS independently selected
from (a) -NH--CO-alkylene~3N-imidazole or -NH~CO-alkylene-
3-pyridine, in which each alkylene has 2 to 4 carbon atoms;
and ~b) -CO-alkylene~3N-imidazol~ or CO-alkylene-
3-pyridine, in which each alkylene has 3 or 4 carbon atomsi
or a pair of ~3 and R3' on opposing phenyl groups jointly
form (c) -NH-CO-alkylene-3-pyridyl-5-alkylene-CO-NH- or
21166S2
WO93/07150 PCT/EP92/02283
-CO-alkylene-3-pyridyl~5-alkylene-CO-, in which each
alky.Lene has 2 to 4 carbon atoms; a nitrogen atom in the
heterocyclic ring acts as a fifth ligand for the metal ion
M. The side of the porphyrin that has the fifth ligand is
shielded and cannot come into contact with antibodies raised
to antigens containing the hapten.
¦ The substrate may be any molecule upon which an
oxidative transformation, such as a hydroxylation,
dealkylation, epoxidatlon or desaturation reaction, is to be
performed. The residue is a group that corresponds to the
substrate molecule and is bound to the bridging group. The
residue may differ from the substrate in that it may contain
an additional functional group through which it is bound to
the bridging group. Alternatively the residue may bind to
the bridging group through an atom or functional group
existin~ in ~he substrat~. In either case, the residue is
attached to the bridging group in such a way to mimic a
transition state of the ~ubstrate in a reaction pathway with
a metalloporphyrin catalyst.
For example, the substrate may contain a non-activated
primary, secondary or tertiary carbon atom which is to be
hydroxyl~ated. The residue would then comprise the substrate
molecule with a hydrogen rems:~ved from the carbvn atom or
with the hydrogen replaced by a functional group that is
bonded to th~ bridging group. In a specific example, in the
preparation of~Ser8-cyclosporin A from cyclo~porin A (C$A),
the substrate would be cyclosporin A and the residue would
be S~x~-cyc1s~poxin A bonded to the bridging group though
the OH of Ser~. The -O- of the hydroxy may be considered to
3Q be part of the residue or the bridging ~roup.
; ' As an alternatlve example, the residue may contain a
group of the ~orMula >N-alkylene'- in which the alkylene'
:
:~ : may be any branched, unbranched, substituted or
un~ubstituted alkylene radical. In this case, the substrate
will be a group of the formula ~N-alkyl.
: ~ specific example of such a substrate would be
cyclosporin A in which the N-methyl of ~eu4 is to be
~ .
: :
WO93/07150 2 116 ~ 5 2 PCT/EP92/022~
hydroxylated to give N-hydroxymethylleucine4.
In another example, the re~idue may contain a group of
the formula -O-alkylene'- in which alkylene' is as defined
abo~e. The substrate would then have a group of the formula
-O-alkyl and the hapten would mimic a transition state in
the dealkylation and hydroxylation of the -0-.
A specific example of such a substrate would be
ascomycin (which is described in EP 184 162) in which
-O-alkyl corresponds to the methoxy group on the carbon atom
numbered 15. The residue would then be ascomycin but with
one of the hydrogen atoms of the methoxy group replaced by a
bond to the bridging group. The hapten would then mimic a
transition state in the replacement of an alkoxy group with
a hydroxy group on carbon atom 15 of ascomycin.
In another example, the residue may contain an aromatic
group of which a carbon atom is attached to the bridging
group. The substrate would then also contain an aromatic
group and the hapten would mimic a transition state in the
hydroxylation o~ the aromatic ring.
In another example, the residue may contain the group
of the formula
: ~ :
3 ~ or Rl2--Rl3
25 ; ~ H
NH
(in which Rlz and~Rl3 are each independently a substituted
car~on atom) ~which mimics an epoxy ring. In this case the
substrate would~contain~the group Rl2=Rl3 which i~ to be
epoxidated~
In a yet urther example, the residue may contain khe
group ~ ~
Rl4-CH2-C 'H-R,4'
in which Rl4 is H or an`~unsubstituted or substikuted alkyl
:~
: ::: : :
i 2
W093/~7150 PCT/EP92/02283
11
group and Rl4' is an unsubstituted or substituted alkyl
group. The subs~ra~e would then contain a group
Rl9-CH2-CH2-R19' of which the single bond between the CH2~CH2
is to be desaturated. Plainly the substituents on the groups
Rl4 and Rl4' must permit the desaturation of the C-C bond and
hence the removal of a hydrogen atom from one of the carbon
atoms. Specific examples would be the desaturation of
dihydro-MeBmtl cyclosporin A to cyclosporin A and the
desaturation of valproic acid to 4,5-dehydro-valproic acid.
In one preferred example, each of R2, R~', R3 and R3, i~
H and a least one Rl is -NH-C0-C~2-CH2-COOH or ~H2 and ~h~
others are H. The use o~ a hapten in which one Rl is
-NH-CO-CHz-CH2-COOH or NH2 facilitates coupling of a carrier
protein to the hapten. Also the solubility of the hapten
15 can be increa~ed. The solubility of the hapten can also be
increased by substitutin~ the para-position of the phenyl
groups with carboxy or ester groups.
Preferably the metal ion M is such that when it is
,.,~
q coordinated in the hapten, it is inert; particularly to
20 oxygen. For example, the metal iOI' may be Ni2', Zn2~ or Sn4 ~ .
In a further aspect, the invention provides an antigen
comprising a hapten, as defined abo~e, coupled to a carrier
protein that is capable of ~ausing an immunogenic response.
The carrier protein may be connected to the porphyrin
25 portion of the hapte~; especially to one of ~he Rl groups.
Alternatively the carrier protein may be connected to the
rasidue portion of the hapten. The carrier protein m y be
any suita~le protein such as keyhole limpet hemocyanin
(KLH), bovi~e serum albu~in ~BSA) or ovalbumin.
I~ another aspect this invention provides an antibody,
or a fragment thereof, that binds to a hapten as defined
above. Preferably the antibody i5 produced by monoclonal
: techniques. The antibody, or ragment, has the advar.tage
that it has two binding pockets; one for the porphyrin
portion and the other for the residue portion.
Xn another spect this invention pro~ide~ a process for
- th:e production of antibodies suitable for controlling
.~
.~
~ '
.
wo g~tO71~0 211 6 ~ 5 ~ ` PCT/EP92/022~3
reactions in which a substrate undergoes reaction in the
presence of a metalloporphyrin catalyst to give rise to
specific regioisomers or enantiomeric pure compounds, the
process comprising:
providing a hapten as defined above that corresponds to
a transition state of the substrate and catalyst;
stimulating an immune response in a mammal, preferably
a mouse, for the production of antibodies to the hapten; and
isolating and purifying those antibodies from the
im~une response that are specific for the hapten.
Preferably the antibodies are monoclonal antibodies.
~ The process may further comprise the step of selecting
the antibodies by binding them to haptens as defined above
c that have been immobilised in chromatography columns or
bound to tracer proteins.
In~another aspect this in~ention provides a process for
the oxidation of a sub~trate, in the presence o* a
metalloporphyrin catalyst, to produce a specific regioi~omer
or enantiomer; the process comprislng:
providing an antibody as d~fined above that is specific
for a hapten that mimics a transition state of the substrate
and the catalyst;
providing a metalloporphyrin catalyst that binds to th~
antibody,
providing an oxidizing agant, and
com~ining the antibody, catalyst~ oxidizing agent and
substrate to p~xmit the substrate to react.
Preferably the metalloporphyrin catalyst is coordinated
with a metal ion selected from Fe3~, Cr3~ and Mn~.
The N-amino-porphyrins o~ fvrmula I may be synthesised
` I I by reacting tetraphanylporphyrin with O m-toluenesulfonyl-
hydroxylamina:in a suitable sol~ent such as chloroform to
~: produce N-aminotetraphenylchlo.rin. The N-aminotetra-
phenylchlorin may be isolatPd and purifiQd using
chromatography. A suitable protecting group, for example a
trifluoroacetyl group, may then be introduced to protect the
introduced amino group and the compound oxidized to give N-
WO93/07150 PCT~EP92/02283
(protecting group)amino-tetraphenylporphyrin. The
protecting group may then be removed and a suitable linker
or bridging group added. A similar procedure i5 described
in Callot, H.J.; 1979, Tetrahedron, 35~ 1455 6 in which N-
tosyl-aminotetraporphyrin i9 produced. Callot did not use a
removable pxotecting group and hence did not obtain amino-
tetraphenylporphyrin, but the procedure described can be
readily adapted. Methods of manufacturing poxphyrins with a
fifth ligand are known; for exa~ple Meunier et al; 1988;
Inor~ Cheo , 27, 1~1.
The residu~ of the substrat~ may be produced by first
synthesising or providing the desired product (ie, the
substrate when reacted). This may be done using classical
chemical pathways or by direct hydroxylation using a
porphyrin catalyst. For example, N-hydroxymethylleucine4-
CsA may be produced by reacting CsA over a porphyrin
catalyst in the presence of magnesium monoperoxyphthalate.
The product is then covalently bonded to the bridging group
of the aminoporphyrin, for examp~e by condensation. The
procedure adopted will depend upon the desired product but
will be facilit~t~d by the amination of the porphyrin. The
adduct formed in the condensation step may be isolated and
puri~ied using chromatography.
The adduct is then complexed with a suitable metal ion,
25 for example by dissol~ing a salt of the m~tal ion .in a
suitable solvent and refluxing with the adduct. The metal
ion coordinates between the introduced nitrogen and the
thr~e pyrrol nitrogen atoms o~ the porphyrin. The reaction
scheme for the production of a D~Ser8-CsA hapten is
illustrated below.
:, ....
. , .
6~52
W0 93,~'071~-,0 P~/EP92~02283
14
6~ H2N-0-S02-Ph-Me
~NH N~( CHC,'3 )~N N~
~N~ ~ ~N+
~3 N~F
(CP3C0)20 ~7 N~ P D~Q
DMF/DMAP ~ H CH2C12
b
~ 3 ~ ~3 N~- ~3 NH2
~o
~) EtOH, KO~I 65 ~)
~3 ~
~CI
Diphosgezlc ~
""~ /~ ,CyA-l:)-Se~-8
DMAP, C}i2C'2, f~,r ~ HN--~ --N.^~
[~
,
., .
~1166~2
WO 93/07150 15 P~/EP92/02283
~N- Ni(~AC)2 --~N~
=oo o o o =o ~ -C~ =
_ C~IC13, M~OH
0~ _N5~TN~ N~ 65 o= J H~TH$~
o~ o~
~ o 6
~N--N~l N=~N' ~3~N--~ N=~3N~
~3~
,.'~
H~, /
CX~ O o o ~o
H2, 10% Ptl/C O~N~TN~ ;N~
EtOH, HCI - o
_ ~ oS~
~NH2
~3
WO93/07150 2 1 1 6 6 ~ 2 PCT/EPg2/02283
16
Other haptens may be produced by similar methods. For
example, to produce a hapten in which the residue is further
functionalized so that the carrier protein can he attached
to it, the following reaction scheme can be adopted:
M
H~N-O-S02Ph-Me ~ NH2
N~F ~R
(CF3C0)20 ~N=~ DDQ
A R~ HN--~R
DMF/DMAP 6J~H CH2C12
~ HH
M
R~ R R ~R
~ EtOHUKOH
M
M
kOH Diphos8~ne ~N~b-~ l
cDN oN N N~ DMAP, C~I2C1~ X) O O O ~o /
OH C~
O
WO 93/07150 2 ~ 2 P~/EPg2/02283
i ~f~ i\~Tf~'H~ /
~N N N N N --N N N N N-
~ O o O =O ~C~ o o o =o
O~N~2TNJ~ N~ Ni(OAc)2G;~_N~TN~ TN~ I_
~ 'y CHC13~ MeOH ~
R~ S ~R R~ j ~R
'
M M
\irH~k 5~OH
-~ 0~
R ~ _ ~R
R _ H, COOMc, COOH, COONa
M ~ ~ COOMe, CO,OH~, COONa
M
21166~2
WO93/07150 PCT~EP92~022B3
18
The antigen is produced by coupling to the hapten a
carrier protein that renders the hapten/car.rier pro-tein
complex immunogenic. The carrier protein may be covalently
bound to the hapten by providing one of the R~ groups in the
form of an amino group; which then forms a bridge between
the carrier protein and the hapten. Suitable procedures are
disclosed in Richards et ali 1990; C~urr.ent R seaxch in
Photosynthesi~, 3, 6g5-8. The advantage o~ coupling the
carrier protein to the porphyrin portion of the hapten, a~
opposed to the rssidue portion, is its general applicability
since the residue portion need not bear a ~urther ~unctional
group for the attachment of the carrier protein. However i~
a functional group is present in the re~idue portio~ or can
be introduced by synthesis, the carrier protein can be
attached to it. Other procedures ~or binding carrier
proteins to haptens are disclo~ed in Harad~, A et al; 1~90;
Chemistry Letters, 917-918 and 1991; Chemistry L~tters, 953-
~56.
The antigens may then be used to immunise mice. The
spleen cells of the mice that give a good re~po~e are used
with myeloma cells to produce hybridomas~ Those hybridomas
that secrete monoclonal antibodies.~pecific to the haptens
are then selected. The~e hybridoma technique~ are
conventional and suitable techni~ues ar~ disclosed in, for
example, Jacob, ~., Schultz, P.G., Sugasawara, R and Powell,
M; 1987, J. Am. Chem. Soc~, 109, 2174 2176, K~inan, E. et
al; 1990; Pure and Appl. ChemO~ 62, 2013-20~9 and Harada, A
et al; 1990; 5hemistrY Letters, 917-918.
The ~aptens may also be used to i~olate a~d purify the
desired antibodie~ from the antibodies produced by the
~ ~ariousi hybridomas. This i5 a ~ignificant ad~antàge since
radiolabelled antibodies that bind the desired antibody n2ed
not be pr~pared. This can be done by selecting those
antibodies which bind to the haptens; for example by
immobilising the haptens in an affinity chromatograph column
or radiolabelling them and allowing the a~tibodies to bind
to them. Alternatively, con~entional techniques can be used
WO93/07150 ~ 1 1 6 ~ 5 2 PCT/EP92/022~3
19
by raising antibodies against derivatives of the haptens and
using these antibodies in radioimmunoassay procedures.
Once the desired antibodies have been isolated, it is
pos~ible to determine the DN~ sequence coding for the
antibody or to determine khe amino acid sequence of the
antibody. Once this has been done, fra~ments or protein
domains which include the antibody binding regions, can be
built. Procedures for doing this are de~cribed in WO
90~07861.
The selected and purified antibodies may then h~ used
in reactions to produce the desired product in a manner
similar to tha~ described in WO 92/01781. A
metalloporphyr.in catalyst, which can fit inko the pocket of
the a~tibody, i5 provided. The m talloporphyrin catalyst,
the substrat~ and the antibodies are then combined. An
oxygen ~ource is then ~dded under controlled conditions. If
desired, the catalyst may be covalently bound to the
antibody prior to the reaction as known in the art.
Alternatively the catalyst may be added separately ~rom the
antibody and allowed to bind to the antibody during the
process. The substrate will be able to enter the cavity
formed by the antibody and porphyrin only if it is in th~
correct orientation to the catalyst to produre the desired
product.
For example, the D-~laa of CsA may be converted to D-
Ser~ by using the following p.rocedure. A catalyst, CsA and
antibodi~s rai~ed to the aminoporphyrin-bridging group-D-
Ser8-CsA antigen are th2n mixed in a suitable solvent. An
oxygen source is then added under controlled conditions.
CsA with the correct orientation is able to ~nter the pocket
of thelantibody and offer the methyl group to ~e
hydroxylated to the metal-oxygen group. The hydroxylated D-
Ser8 - CsA is then remo~ed. If necessary, the catalyst is
removed and regenerated.
In another example, the N methylgroup o* leucine4 o~
C~A may be con~erted to 4-N-hydromethylleucine by using the
following procedure. A catalyst, CsA and antibodies raised
~116~52
WO93/07150 ~CT/~P92/022~3
to the aminoporphyrin-bridging group-N-hydromethylleucine9~
CsA antigen are then mixed in a suita~le solvent. An oxygen
source is then added under controlled conditions. CsA with
the correct orientation is able to enter the pocket of the
antibody and bond offer the N-methyl group of leucine4 to
the metal-oxygen group of the porphyrin. The hydroxylated
N~hydromethylleucine4-C~A i~ then removed.
Similar procedures may be used for all other reactions.
The source of oxygen atoms may be ~elected from H2O2,
iodosob~nzene, mayne~ium monoperoxyphthalate, NaOCl, KHSO5
and the like.
It will be appreciated that substrates that have more
than one site that can be hydroxylated, dealkylated,
epoxidated, desaturated and the like can be s~lectively
attacked so that only the desired site is alter~d.
Similar~y substrates that have prochiral centres that, when
reacted, can foxm diast~reomers, can be selectively xeacted
so that only one diastereomer forms~ Similarly single
enantiomer products can be produced from substrates that,
when ordinarily reacted, form racemic mixtures.
Example l: Hapten~formed from N-A~ino-5,l0,l5, ?-
Tetraphenyl-21 ,23H-Porvhvrin-Derivat-ve and
N-HydroxymethYlleucine4-Cyclo~porin A
:: :
1.1. M-Amino-tetraphenyl-chlorin from Tetraphenyl-porphyrin:
l0 g o~ Tetraphenylporphyrin is di~solved in 5Q0 ml warm
chloroform. The solution is then cooled to 20C and 9.8 g
O-mesitylsulfonylhydroxylamine is added to it. The solution
is thenistirred for 20 hours at room temperature. The green
reaction mixture is~then heated to 60C for l hour and 2N
sodium carbonate with chloroform added. The crystailine
residue ~l0 g) is then separat~d using column chro~atography
~500 g Alox N, Activity V). After elution with chloroform,
7.5 g of an adduct is obtained. 68Q mg N amino-tetraphenyl~
chlorin is then eluted using a chloroform : Ethanol (ratio
~116~2
WO93/07150 PCT/EP92/02283
21
100 : 0.6 to 1.0) mixture.
1 . 2 . N~tri fluoroacetylamino-tetraphenyl-chlorin:
631 mg N-amino-tetraphenyl-chlorin is dissolved in 30 ml of
absolute dimethyl-formamide and 2 ml pyridinc. 122 mg of
4-dimethyl-aminopyridin~ ~lmM) is then added and a solution
of 231 mg trifluoro acetic acid anhydride (1.1 mM) in 3 ml
~ethylchloride at 2Q'C is added dropwise over 5 minutes. The
solution is then stirred for 10 minutes. The reaction
mixture is then evaporated and the residue is ~haken with 2N
sodium carbonate and chloroform and then washed o~ce with
water. 850 mg of N~trifluoroacety~amino-tetraphenyl~chlorin
is obtained.
1~3. N-tri~luoroacetylamino-tetraphenyl-porphyrin:
A solution of 850 mg N-tri~luoroac~tylamino-
tetraphenyl-chlorin i~ 50 ml dichloromethane is mixed with
~81 mg ~3 mM) DDQ and refluxed or 5 hours. The reaction
mixture is then sLaken once in 2N sodium carbonate and onc~
in water. The residue (780 mg) is then crystallised out of
ethanol t650 mg) and th~n rec~ystalli~d out o~ an
ch}oroform-etha~ol mixture to give 430 mg of
N-trifluoroacetyl amino-tetr~phenyl-porphyrin.
1.4. N-amino-tetraphenyl-porphyrin:
A suspension of 400 mg o N-trifluoroacetylamino-
tetraphenyl-porphyrin in 40 ml of ethanol is mixed with a
solution of 0~5 g calcium hydroxide in 10 ml of ethanol. The
mixture is then stirred for 30 mi~ute~ at 70 to 75 ~. The
precipitate is then cooled to room t~mperature, filtered and
washed with ethanol. The precipitate is then crystallised
out of a chloroform-metha~ol mixture to give 280 mg of
N-amino-tetraphenyl-porphyrin. Mass spectra peaks:-
MH+ 630, [MH-NH2]H+ 615, and other peaks at 215, 237, 255,
2116652
W093/07150 PCTfEP92/022
22
273, 289, 307, 343, 391, 419, 539, 646 and 730.
1.5. Condensation of N-hydroxymethylleucine4-Cyclosporin A
with N-amino-tetraphenyl-porphyrin:
A solution of 44.9 m~ of 97% diphosgene is mixed with 2 ml
o~ dichloromethane. rrhe resulting solution is cooled to 0 to
5'C and a solution o~ 244 mg of N-hydrox~methyl-
leucine4-Cyclosporin A in 5 ml dichloromethane is then added
dropwise over 15 minutes. The solution is the~ stirred at
O'C or lS minutes and a solution o~ 126 ~-amino-
tetraphenyl-porphyrin and 25 mg of 4-dimethylaminopyridine
in 1 ml pyridine and 8 ml dichloromethane i5 ~dded rapidly.
The reaction mixtur~ is allowed to react for 2 hours at room
temperature and then 2N sodium carbonate and dichloromethane
is added,. The residue (430 mg) is then purified using column
chromatography (65 g Alox basic, activity II, chloroform)~
340 mg of the condensation product is obtained.
.
1.6. Produotion of a ~ickel-complex of the condensation
: product of step 1.5.:
300 mg of the condensation product is dissolved in a
solution o~ 50 ml chloroform. A solution of 0~8 g of nickel
diacetatetetrahydrat~ l~n 30 ml methano~ is added and the
: mixture re1uxed for an hour. The solution is khen reduced
and shaken once~with chloroform and once with water. The
residu~ is purified using column chromatography (56 ~ silica
gel, acetone~: hexane 1:2). 190 mg of the nicke} complex is
obtained and this i5: recrystallised ~ing tertiary-
butylmethylether and a little petroleum ether.
W~93/07~50 ~ 11 6 6 5 2 pCT/~92/~22~3
Example 2: Hapten of N-amino-5,10,15,~0-tetraphenyl-
21H,23H-porph~rin-derivatlve and Serine8-CYclosPorin A
2.1. N-amino-tetraphenyl-porphyrin is produced in the same
manner as described in Example 1, steps 1.1. to 1.4.
.1
2.2. Condensation of Serine8-Cyclosporin A with
N-amino-tetraphen~l-porphyrin:
244 mg of Serine3-cyclosporin A in S ml methylchloride is
condensed with 126 mg of N-amino-tetr~phenyl-porphyrin in a
manner totally analogous to thak set out in Example 1, step
1.5. 350 mg of the cond~n~ation product is obtained.
2.3. Production of the Nickel-complex of the condensation
product~of step 2.2.:
300 mg of the condensation product is re1uxed with a
solution of 0.8 g of nickel diacetatetetrahydrate in a
manner totally analogous to that set out in example 1, step
1.6. 200 mg of the nickel complex is obtained and this is
recrysta}lised u~ing tertiary-butylmethylether and a little
petroleum ether. Mass spectra p~aks:- MH* 1873 ~nd other
major peaks at 538, 600, 614, 630, 656.
~
Th~ hapten obtained from step 2.3 is activated as its
benzotriazole ester in dimethyl formamide (DMF~ using bist2-
oxo-3-oxazolidinyl]phosphinic chloride (BOP)/hydrvxy-
!~ I benzotriazol (HOBt). This is then add~d to a solution of
protein ~LH, BS~ or ovalbumin) in 2.5:1 DMSO:borate bufferat pH 8.5. A hapten:protein stoichiometry of 5:1 is used to
prevent over-derivativisation and precipitation of the
protein. After 4 hours, the reaction mixture is dialyzed
against phospha~te-bu~fered saline to remove organic
21~52
WO93/07150 PCT/EP92/02283
24
solvents. A conjugate for each protein is obtained
separately.
Example _4_ Generation of antibodies
I~CFl mice are anaesthetized and the peritoneal
cavities surgically opened to acc~ss the spleen. The
surface of the spleen is swabbed with an ethanolic solution
containing KLH conjugate obtained from example 3. The mice
are immunised in a similar m~nner on day 22. Serum titres
are measured on day 27 by ELISA analysis against fre~ hapten
or BSA derivatives absorbed in well& of polystyrene
microtitre plates. The antibodi~5 also h~ve partial
reactivity with conjugate~ of the Ser8-CsA product and a
nickel mono-p-amino-tetraphenylporphyrin control. This
indicates that antibodi~s that recogni~e both the~substrate
and the catalyst components of the hapten are prese~t in the
sera.
ssA a~d KLH conjugates obtained from example 3 are
aflded to Ribi adjuvant and 6 IRCl mice are immunised using
i~p. injections. O~ day 14, the mice are boosted with
conjugate absorbed on bentonite a~d the serum sampled on day
17. Three mice showing high serum titres are boocted with
further conjugate and are sacrificed on day 31. The spleens
from two mice are used i~ standard fusion protocol~ using
Sp2/0 myeloma cells and PEG. A stable clone which secretes
antibody specific for the BSA conjugate is isolated.
The antibodies may be selected and purified using free
hapten immobilised in an ~~inity chromatograph.