Note: Descriptions are shown in the official language in which they were submitted.
WO 93/0503 ~ 2 1 1 7 0 7 ~pCr/J"92fO1117
DESCRIPTION
QUINAZOLINE DERIVA~IVES AND THEI~ PREPARA~ION
~ he pre~ent in~ntion rel~te~ to no~el quinazoline
deriv~ti~e~ and ph~rmaceutically ~cceptable salts thereof.
MorQ particularly, it relate~ to novel quinazoline
deri~ati~es and pharmaceutic~lly ncceptable salts thereof,
which display effects on the peripheral or central nervou~
~ystem, to processe~ for the preparation thereof, to a
ph~rm~ceutic~l compo~ition comprising th~ 8ame, to a u~e
of the s2me a~ a medicament ~nd to a method of the
t~erapeuti~ tre~tment of di~e~se~ in a human beinq or
~nimal~
INDUSTRIAI, APPl ICAB~LIT~r
A~cordingly, one o~ect of the pre~ent invention is
to provide no~rel ~inazoline deriYative~ anc'.
pharm~ceutically acceptable ~alts thereof, w~ich di~play ::
effect~ on the peripheral or centr~l nervous system, in
particulhr on the peripheral nervous sy~tem.
~nother ob~ect of the present invention is to pro~ide ~:
processe~ for the preparation of novel quin~zoline
deri~atives and ~lts the~eof.
A further ob~ect of the present invention is to
pro~ide a ph~rmaceutical composition comprisin~, as an
. ~cti~e ingred~ent, said quinazoline deri~ative~ and
:
Wo93/oso3s - 2 - pCT/JP92/01117
~ 211707~
pharmaceutically accept~ble ~alts thereof.
Still further object of the present in~ention i5 to
pro~ide ~ use of said quinazoline deri~ati~e~ and
phnrmaceutlcally accept~ble salt~ thereof as a dopamine
recep~or ~gonist, 5-HT recQptor antagoni~t, e~pecially
5-HT2 receptor antagonist; ~1 receptor antagoni~t; ~nd the
like and a method of the therapeutic treatment of dopAm~ne
receptor; 5-HT receptor, espec~ally 5-HT2 receptor;
~l-receptor; mediated diseases, particularly hyperten~ion,
c~rd~ovascular disorder ( Q . g . angina pectori~) myocardial
infarctlon, etc.), Parkin~onism, ~nd the like, in a human .
being or animal.
lS DISCLOSURE OF INVENTION
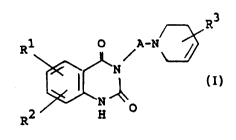
The ob~ect quinazoline derivati~es are no~el and can
bs represented by the following general formula:
Rl O A-N~
~,~ ( I ) ~
2S R2 H -
in which Rl is ~ub~tituted heterocyclic-(lower~alkyl,
and -
~2 i~ hydrogen, h~logen, nitro, A~;no,
protected amino, hydroxy~mino, lower
alkyl, hydroxy, protected hydroxy,
~ulfamoyl, carboxy, protected carboxy,
mercapto, option~ ubstituted
heterocyclic-carbonyl, optionally
2 1 1 7 ~ 7 5 pcT/Jps2/o~
substituted heterocyclic-~lower)alkyl,
lower alkylthio, hydroxy(lower)alkyl
or protected hydroxy(lower~alkyl,
R3 is aryl which may have suitable .
substituentls), an~
A is lower alkylene, ~-:
and pharmaceutically acceptable salts thereof. .
Suitable salts of the object compound (I) are
pharmaceutlcally acceptable~ conventional non-toxic salts
and may include a salt with a base such as an inorganic :~
base salt, for-example, an alkali me~al salt (e.g. sodium
salt, potassium salt, etc.), an alkaline earth metal salt
le.g. calcium salt, magnesium salt, etc.), an ammonium
salt, an organic base salt, for example, an organic amine
sa~t (e.g. triethylamine salt, pyr~dine salt, picoline
salt, ethanolamine salt, triethanol~m~ne salt,
dicyclohexylamine salt, N,N'-dibenzylethylenediamine salt,
etc.); a salt with an acid such as inorganic acid addition
salt (e.~. hydrochloride, hydrobromi~e, sulfa~e, -~
phosphate, etc.), an organic acid addition salt (e.g. :.
formate, acetate, trifluoroacetate, maleate, tartrate,
fumarate, methanesulfonate, benzenesulfonate, etc.);
a salt with a basic or acidlc amino acid (e.g. arginine, :~
aspartic acid, glutamic acid, etc.); and the like.
According to the present invention, the ob;ect
compound (I) or pharmaceutically acceptable salts thereof
can be prepared.by the processes as illustrated by the
foll~w~ny reAction scXemes.
3S
w093/0503~ - 4 - pCT/JP92/01117
~ 211707~
Process l :
CO(X)2
R3 (III) ; 0 A-N
~II) (I) :~
or salts thereof or salts thereof
Process 2 :
Reduction
of nitro
~ R3 group of 3
1 O A-N ~ Ra R A-N
a ~
~I-a) (I-b)
or salts thereof or salts thereof
Process 3 :
Introduction
R3 of the R3
l o / -N ~ amino- RI o A-N~
~ ~ N protective ~ ~ ~ /
~ ~ N ~ o , ~ N~o
c d
c) ~I-d)
35or salts thereof or sa1ts thereo~
W~g3/0503~ 5 2 11 70 7,~ pcT/JP92/01117
Process 4 -
1 ~ N~ A N ~ ~
R2 R2 H ;
(I~) tI) -:
or salts thereof or salts thereof
Process 5 :
~, ~
Removal
of the
1~ , ~ R3 hydroxy- ,R3 ~-
1 o A-N ~ :ot ceive ~; o A-N
(I-e) (I-f)
or salts thereof or salts thereof
Process 6 :
Removal
R3 of t~e
Rl O A-N ~ carboxy- ~l o A-N
~ N protective ~\rJ~ N
g grO r in ~ N~ O
g) ' (I-h)
3S or salts thereof or salts thereof
W093/0s03~ - 6 - PCT/JP92101117
` 21l707~
wherein Rl, R2, R3 and A are eac~ as defined above,
R2 is nitro,
~ is hydroxyamino or amino,
Rc is am~no,
- Rd is protected amino,
Re is protected hydroxy or protec~ed hydroxy- ~:
(lowerJalkyl,
R~ is hydroxy or hydroxy(~ower)a~kyl, ;~
Rg is protected carboxy,
~ is car~o~y~ ;
R i~ esterified carboxy, and
X isa leavmg group.
The compounds (II~ and (I~) used in the Processes l
and 4 are new and can be prepared, for example, by the
following methods or a conventional manner.
Method A :
.
21;) ~R3
H2N-A N~
Rl O Rl O R3
\~0 (VI ) ~ ~
2 N~o or salts 2 2 :-
R thereof R
~V) ~II)
or salts thereof or salts thereof
~ -
`
w093/0503~ 2 1 1 7 0 7 ~ pCT/Jpg2/o~
Method B :
Reduction :~
l o R3 of the R1 o R
S ~ \~ g: o~
(VII) ~II)
or salts thereof or salts thereof
'
Method C :
H2N-A-N/~ ~.
1 O ~ R1 O R3
R ~ ~ ~ N~-A-N
NH-R4or salts NR-R~
R2 thereof R~
IVIII) (IV)
or its reactive derivative or salt~ thereof
at the carboxy group,
or salts thereof
Method D :
R3 ~:
H2N-A-N ~ 1 o R3
NH-A-N
NO2 or salts NO2
R thereof R2
~IX) ~VII)
or its reactive derivative or salts thereof
at the carboxY group,
or salts thereof
w043~0~03~ 8 PCT/JP92/01117
2117075
in whieh Rl, R2, R3, R4 and A are ea~h as de~ined
above.
some of the starting materials of ~he abo~e Method A ~:~
~re new and can be prepared, for example, accordin~ to the
me~hod of Preparation a~ mentioned below, or in a
convent~onal manner.
In the above and subsequent de criptio~s of the
present spe~ificat~on, suitable examp~es and illustrations
of the various definitions whlch the present invention
includes within the scope thereof are explained in detail
~s follow~.
The ter~ "lower" is intended to mean 1 to 6 carbon
atom(s), preferably l to 4 carbon atom(s), unless
1~ otherwise indicated.
Suitable "lower alkyl" m~y include strai~ht or
branched one such as methyl, ethyl, propyl, isopropyl,
butyl, t-butyl, pentyl, hexyl, and the like.
Su~table "lower alkoxy" may include straight or
branched one such as metho~y, ethoxy, propoxy, isopropoxy, :
butoxy, t-butoxy, pentyloxy, hexyloxy, and the ~ike.
Suitable "aryl which may have suitable :::
substituent~s)" may include phenyl, tolyl, xylyl, cumenyl,
mesity~, naphthyl, and the li~e, each of which may be .
subst~tute~ by one or more, preferably one or two
subst~tuent(s) such as halogen te.g. fluorine, chlorine,
bromine, iodine), ~ower alkyl as mentioned above te.g. ~-
met~yl, etc.), and the like, in which more preferred
example ma~ be phenyl which is substituted or
unsubstituted by a group ~onsisting of halogen and lower :~
alkyl, and the most preferred one may be phenyl.
Suitable "protected carboxy" may include carbamoyl,
esterified carboxy wherein "e~terified carboxy" ~an be
referred to the ones as mentioned below, and the like.
~5 Su~-table examples of the ester moiety of an
211707~
wos3~0so3~ _ 9 _ PCT/JP92~0tl17
esterified carboxy may be the ones such as lower alkyl
ester (e.g. methyl ester, ethyl ester, propyl ester,
isopropyl ester, butyl ester, iso~utyl ester, t-~utyl
ester, pentyl ester, hexyl ester, etc.) which may have at -
least one suitable substituent(s), for example, lower
alkanoyloxyllower)alkyl ester te.g. acetoxymethyl ester,
propionyloxymethyl ester, but~ryloxymethyl ester, ~-~
valeryloxymethyl estèr, piva~oyloxymethyl ester,
hexanoyloxymethyl ester, }-(or 2-)acetoxyethyl ester,
1-~or 2- or 3-)acetoxypropyl ester, l-~or 2- or 3- or
4-)acetoxybutyl ester, l-(or 2-)pro~ionyloxyethyl ester,
1-(or 2- or 3-)propionyloxypropyl ester, 1-(or
2-)butyryloxyethyl ester, 1-(or 2-)isobutyryloxyethyl
ester, l-(or 2-)piv~-~oyloxyethyl ester, 1-(or
1~ 2-)hexanoyloxyethyl ester, iso~utyryloxymethyl ester,
2-ethyIbutyryloxymethyl ester,
3,3~dimethy~butyryloxymethy} ester, l-(or
2-)pentanoy~oxyethy~ ester, etc.1, lower
alkanesulfonylllower)alkyl ester (e.g. 2-mesylethyl ester,
etc.), monolor dl or-tri)halo~lower)alkyl ester (e.g.
2-iodoethyl ester, 2,2,2-trichloroethyl ester, etc.);
lower alkoxycarbonyloxyllower)alkyl ester ~e.g.
methoxycarbonyloxymethyl ester, ethoxycarbonyloxymethyl
ester, propoxycarbonyloxymethyl ester,
t-butoxycar~onyloxymethyl ester, 1-tor
2-)methoxycarbonyloxyethyl ester, 1-(or
2-)ethoxycarbonyloxyethyl ester, 1-(or
2-)isopropoxycarbonyloxyethyl ester, etc.~,
phthalidylidene(lower)alkyl ester, or (5-lower
alkyl-2-oxo~ dioxol-4-yl)~lower)alkyl ester te.g.
(5-methyl-2-oxo-1,3-dioxol-4-yl)methyl ester,
(S-ethyl-2-oxo-~,3-dtoxol-4-yl)methyl ester,
(5-propyl-2-oxo-1,3-dioxol-4-yl)ethyl ester, etc.];
lower alkenyl ester ~e~g. vinyl ester, allyl ester, etc.);
3~ lower alkynyl ester (e.g. ethynyl ester, propynyl ester,
WO 93/0503~ - 10 - PCI`/JP92/01117
211707~
ete.); arllower)alkyl ester [e-g- mono- or di- or :
triphenyl(lower)alkyl ester, ete.] which may have at least .
one sultable substituent(s) (e.g. lower alkoxy, nitro, ..
hydroxy, lower alkyl, ete.), for example, mono- or di- or
S triphenyl(lower)alkyl ester which may have (lower~alkoxy
le.g. benzyl ester, benzhydryl ester, trityl ester, 0
phenethyl ester, 4-methoxybenzyl ester,
3,4-dLmethoxybenzyl ester, bis(methoxyphenyl)methyl ester,
ete.], nitrophenyl(lower)alkyl ester (e.g. 4-nitro~enzyl
ester, ete.), [hydroxyl-(lower)alkylphenylllower)alkyl
ester ~e.~. 4-hydroxy-~,5-di-t-butylbenzyl ester, etc~);
aryl ester whieh may h~ve at least one suita~le
substituent(s) (e.g. phenyl ester, 4-ehlorophenyl ester, ~.
tolyl ester, t-butylphenyl ester, xylyl ester, mesityl
ester, eumenyl ester, ete.); phthalidyl ester; and the
like.
More preferable example of the proteeted earboxy thus
defined may be earb~moyl and lower alkoxyearbonyl.
Suitable "proteeted amino" may inelude amino
proteeted.. by a eonventional amino-protective ~roup as -~
mentioned below.
Suitable "amino-proteetive group" may inelude aeyl
sueh as aliphatie aeyl, aromatie aeyl, heteroeyelic aeyl
2~ and aliphatie aeyl substituted with aromatie or
heteroeye~le ~roup(s) derived from earboxylie, earbonic,
sulfonie ~nd earbamic aeids. .................................... .
The aliphatie ae~l may include saturated or ~.
unsaturated, aeyelie or eyelie ones, for example, alkanoyl -.
~0 sueh as lower alkanoyl le.g. formyl, aeetyl, propionyl,
butyryl, isobutyryl, valeryl, isovaleryl, pivaloyl, :::
hexanoyl, ete.), alkylsulfonyl sueh as lower alkylsulfonyl
(e.~. mesyl, ethylsulfonyl, propylsulfonyl, .
lsopropylsulfonyl, butylsulfonyl, isobutylsulfonyl,
3S pentylsulfonyl, hexylsulfonyl, ete.), earbamoyl,
211707~
w093/0503~ - 11 - PCT/JP92/0lll7
N-alkylcarbamoyl (e.g. methylcarbamoyl, ethylcarbamoyl,
etc.), alkoxycarbonyl such as lower alkoxyca~bonyl (e.g.
methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl,
butoxycarbonyl, t-butoxycarbonyl, etc.),
alkenyloxycarbonyl such as lower alkenyloxycarbonyl (e.g.
vinyloxycarbonyl, allyloxycarbonyl, etc.), alkehoyl such
as lower alkenoyl (e.g. acryloyl, methacryloyl, crotonoyl,
etc.3, cycloalkanecarbonyl such as
cyclo~lower)alkanecarbonyl (e.g. cyclopropanecarbonyl,
cyc~opentanecar~onyl, cyclohexanecarbonyl, etc.), and the
like.
The aliphatic acyl substituted with aromatic group~s)
may ~nclude aralkoxycarbonyl such as
phenyl~lower)alkoxycarbonyl (e.g. benzyloxycarbonyl,
phenethyloxycarbonyl, etc.), and the like.
These acyl groups may be further substituted with one
or more suitable subst~tuent~s) such as nitro, halogen as
menttoned below, and the like, and preferable acyl having
such subst~tuent(s) may be nitroaralkoxycar~onyl (e.g.
nitrobenzyloxycarbonyl, etc.), trihalo~lower)alkyl ~e.g. -
trifluoroacetyl, etc.), and the like.
Pref erable example of amino-pro~ecti~e group thus
defined may be :
- lower alkanoyl (e.g. acetyl, etc.); -
- trihalo(lower)aIkanoyl such as trifluoro(lower~-
alkanoyl (e.g. trifluoroacetyl, etc.);
- lower alkoxycarbonyl (e.g. ethoxycarbonyl, etc.);
'0 - carbamoyl;
- N-(lower)alkylcarbamoyl (e.g. N-ethylcarbamoyl,
etc.);
- lower alkylsulf onyl ( e . ~ . mesyl, ethylsulfonyl,
etc.); and the like.
~5
W093/0503~ - 12 PCT/JP92/01117
21170~5
"Protected hydroxy" means a hydroxy group protected
by a convent~onal hydroxy-protective group, and suitable
"hydroxy-protective group" may include lower alkyl as
defined above, acy~ as defined above, ar(lower)alkyl such
as mono-, di- or tr~phenyl(lower)alkyl ~e~g. trityl,
etc.), preferably lower alkyl and triphenyl(lower~alkyl.
Suitable heterocyclic group in "optionally
substituted heterocyclic-carbonyl", "optionally
substituted heterocyclic-(lower)alkyl" and "substituted
heterocyclic-(lower)alky~" may include 3 to 10, preferably
5 or 6-membered heterocycllc group, prefera~ly
heteromonocyclic group containing at least one hetero ato~
such as oxygen atom, nitrogen atom and sulfur atom (e.g.
morphollno, etc.), and the like.
1~ Particularly such "heterocyclic group" means
saturated or unsaturated, monocyclic or polycyclic
heterocyclic group containing at least one hetero-atom
such as an oxygen, sulfur, nitrogen atom an~ the like. --~
More preferable heterocyclic group may be
heterocyclic group such as :
-unsaturated 3 to 8-membered, preferably 5 or
6-membered heteromonocyclic group containing 1 to 4
nitroqen atom~s), for example, pyrrolyl, pyrrolînyl,
imidazolyl, pyrazolyl, pyridyl, and its N-oxide,
pyrimidyl, pyrazinyl, pyr~dazinyl, triazolyl (e.g., -
4H-1,2,4-triazolyl, lH-1,2,3-triazolyl, --~
2~-1,2,3-triazolyl, etc.), tetrazolyl (e.g.,
l~-tetrazolyl, 2~-tetrazolyl, etc.), dihydrotriazinyl
(e.g., 4,5-dlhydro-1,2,4-triazinyl,
2,g-dihydro-1,2,4-tr~azin~l, etc.), etc.;
-saturated 3 to 8-membered, preferably 5 or
6-membered heteromonocyclic ~roup ~ontaining 1 to 4
nitrogen atom~s), for example, azetidinyl, pyrrol~dinyl,
~5 ~midazolidinyl, piperidinyl, ~Lazolidinyl, piperazinyl,
2117075 -
w093/05035 - 13 - PCT/JP92/01117
ete.;
-unsaturated condensed 7 to 12-membered heteroeyelic
group containing 1 to 5 nitrogen atomts), for example,
indolyl, isoindolyl, indolizinyl, benzimidazolyl,
quinolyl, isoquinolyl, indazolyl, benzotriazolyl,
tetrazolopyridyl, tetrazolopyridazinyl ~e.g.,
tetrazolo[1,~-blpyridazinyl, ete.),
dihydrotriazolopyridazinyl, et~.;
-unsaturated 3 to 8-membered, preferably 5 or
6-membered heteromonoeyelie ~roup eontaining 1 to 2 oxygen
atom~s) and 1 to 3 n~trogen atom(s), for example,
oxazolyl, isoxazolyl, oxadiazolyl, ~e.g.,
1,2,4-oxadiazolyl, ~,3,4-oxadiazolyl, 1,2,5-oxadiazolyl,
etc.), etc.;
lS -saturated 3 to 8-membered, preferably ~ or
6-membered heteromonocyclic group eontaining 1 to 2 oxygen
atom(s) and L to 3 nitrogen atom~s), for example,
morpholinyl, ete.'
-unsaturated eondensed 7 to 12-membered heteroey~lie
group eontaininq 1 to 2 oxygen atom(s) and 1 to 3 nitrogen
atomts), for example, benzoxazolyl, benzoxadiazolyl, ete.;
wherein said heteroeyelie group may be substituted by
one or more, preferably one or two suitable substituent(s)
sueh as lower alkyl as mentioned above, l~wer alkoxy as
2~ mentioned above, in whieh more preferable example may be
saturated 5 or 6-membered heteromonoeyelie group
eontaining 1 to 4 nitrogen atom(s), ~r containing 1 to 2
oxygen atomts) and 1 to 3 nitrogen atomls), optional~y
substituted by lower alkyl.
$uitab1e lower alkyl group in "heteroeyelie-
(lower)alkyl`' ean be referred to the ones as mentioned
- above.
More preferable example of substituted
heteroeyelie-tlower)alkyl thus defined may be lower
alkylpiperazinyl(lower)alkyl and morpholinyl(lower)alkyl,
WO 93/0~035 - 14 - PCr/JP92~01117
~11707~
and the most preferable one may be
4-methylpiperazin-1-ylmethyl.
More preferable example of heterocyclic-carbonyl thus
defi~ed may be lower alkylpiperazinylcarbonyl and
morpholinylcarbonyl.
Suita~le "halogen" may be fluorine, chlorine,
bromine, iodine, and more preferred example may be
chlorine.
Suitable "lower alkylene'l may include straight or
branche~ one such as methylene~ ethylene, tri~ethylene,
tetramethylene, pentamethylene, hexamethylene, -
methylmethylene, ethylethylene, propylene, and the like,
in which the most preferred one may be tetramethylene.
Suitab~e "leaving group" may include imidazole, lower
i5 alkylimidazole ~e.g. 2-methylImidazole, etc.), an acid
residue such as halogen as mentioned above (e.g. chlorine,
etc.), and the like. -~
Suitable "lower alkylth~o" may include straight or
branched one such as methylthio, ethylthio, propylth~o,
isopropylth~o, butylthio, t-butylthio, pentylthio, --
hexylthio, and the like.
Suitable "esterified carboxy" means the same ones as
mentioned in the exp~anation of protected carboxy, in
which more preferable ex~mple may be lower alkoxycarbonyl.
Su~table "hydroxy(lower)alkyl" may include
hydroxymethyl, hydroxyethyl, hydroxypro~yl, hydsoxyhexyl, ~-~
and the like.
Suitable "protecte~ hydroxy~lower)alkyl" means
hydroxy(lower)al~yl protected by a conventional
hydroxy-protective group as mentioned in the explanat~on
of "protected hydroxy", in which more preferable example
may be lower aIXoxy~lower)alkyl and -~
triphenyl(lower)alkoxy(lower)alkyl.
~5
~11707~
WO 93/0~03~ - 15 - pCr/JP42~01117
ThQ processes for the preparat$on of the ob~ect
compound (I) of the prQ~nt inventlon ar~ explained in
det~il $n the following. -~.
5 (1) Proc~
The compound (I) or ~alts thereof c~n be prspared by
reacting the compo~nd (II) or salts thereof wi~h the
compound (III).
Suit~ble ~alt~ of the compound tII) mny be acid
add~tion salts such as those ~i~en for the compound ~
Suitsble ex~mple of the compound (III) may include
N,N'-c~rbonyldiim$dazole, ~,N'-ca bonylbis(2-methyl-
imid~zol~), phosgene or its reactive equi~alent (e.g.
d~mer or trimer thereof, etr. ), and the llke.
lS Thi~ re~ction can be carried out in a conventional
solvent which doe~ not ~dversely influence the react~on
such a8 dichloromethane, pyridine, N,N-dimethylformam~de,
4-methyl-2-pent~none, tetrahydrofur~n, etc., or a mixture
therQof .
The reaction temper~ture is not critical ~nd the
re~ction iJ usually carr-ed out under from w~rming to
he~ting.
t2) Process 2 :
The compound (I-b) or salts thereof c~n be prepared
by ~ub~ecting the compound tI-a) or salts thereof to
reduction of n~tso group of R~.
Su~table salts of the compounds (I-a) and (I-b) may
be the ~me ~ those for the compound (I).
The pre~ent renction i~ uYually carri~d out by a
con~ent~onal method a~ ment~oned below.
Reduction method :
The reduction method applicable for this reaction may
include con~ent~onal ones which are capable of converting
WO 93/0503~ - 1 6 - PCr/JP92/01 1 17
211707~
a nitro group to ~ hydroxyamino or amino group, for
ex~mple, reduction using tin(II) chloride or zinc powder;
reduction u~ing a combination of a metal (e.g~ zinc, zinc
~m~lgam, QtC. ) or a ~alt of chrome compound (e.g. chromous ~-~
chloride, chromous acetate, QtC. ) and an organ~c or
~ inorganic ~cld (e.g. ~cetic acid, propionic acid,
hydrochloric acid, sulfuric acid, etc.3 oonventional
catAlyt$c reduction in the presence of a conventional
metallic c~taly~t such a~ palladium catalysts (e.g. ~pongy
palladium, pAlladium bl~ck, palladium oxide, palladium on
carbon, collo~dal palladium, p~lladium on barium sulfate,
palladium on barium carbonate, palladium hydroxide on
c~rbon, etc.), nlckel cataly~ts (e.g. reduced nickel,
ntckel oxide, Raney nickel, etc.), platinum cataly~ts
(e.g. pl~tinum plate, sRongy platinum, platinum bl~ck,
colloid~l plat~num, pl~tinum oxide, platinum wire, etc.);
reduction using aluminum am~lgam, electrolytic reduction;
and the like.
Tn case that the c~talytic reduction i-~ applied, the
reaction i~ preferably carried out around neutral
condition. -
Th$~ reaction i8 u~ually carried out in a
conventional sol~ent which does not ad~er~ely influence
ths reaction such a~ water, alcohol (e.g. methanol, -~
eth~nol, prop~nol, etc.), diox~ne, tetrahydrofuran, ~cetic
~cid, buffer ~olutlon (e.g. phosphate buffer, acetate
buffer, etc.-), ~nd the like, or a mixture thereof.
The re~ction temperature i8 not critical ~nd the
re~ction i~ u~u~lly carried out under from warming to
heating.
(3) Proce~s 3 :
The compound (I-d) or salts thereof can be prepared
by introducing ~n ~mino-protecti~e group into the compound
(~-c) or salt~ thereof.
211707~
W093/05035 - ~7 - PCT/JP92/01117
Su~table salts of the eompounds (I-e) and tI-d) may ~-
be the same as those for the eompo~nd (I).
Su~table introdueing Aqent of the amino-protective
~roup used in this reaetion may be a conventional one
whieh is eapable of introdueing the a~ino-proteetive group
sueh as aeyl as mentioned before, for example, lower
alkyl isoeyanate (e.g. ethyl isoeyanate, ete.);
alkali metal eyanate (e.g. potassium eyanate, ete.);
lower alkyl halo(lower)alkanate le.~. ethyl ehloroform~te,
ete.); earboxylie aeid, earbonie aeid, sulfonie aeid and
their reaetive derivative (e.g. an aeid halide, an acid
anhydride, an aetivated amide, an aetivated ester, ete.);
and the like. Preferable e~ample of sueh reaetiYe
derivative may inelude lower alkanoic aeid halide (e.g.
L5 aeetyl ehloride, ete.); lower alkanesulfonyl halide ~e.g.
mesyl ehloride, ethanesulfonyl ehloride, ete.); a mixed
aeid anhydride with an aeid sueh as substi~uted phosphorie
aeid ~e.g. dialkylphosphorie aeid, phenylphosphorie aeid,
diphenylphosp~orie ae~d, dibenzylphosphorie aeid,
~0 halogenated phosphorie aeid, ete.), dialXylphosphorous
aeid, sulfurous aeid, thiosulfur~e aeid, sulfurie aeid
sulfonie aeid (e.g. methanesulfonie aeid, toluenesulfonie
acid, ete.), mono(lower)alkyl ester of carbonie aeid,
aliphatie earboxylie aeid (e.g. pivalie aeid, pentanoic
aeid, isopentanoie a~id, 2-ethylbutyrie aeid,
txiehloroaeetie aeid, ete.), aromatie earboxylie acid
le.g. benzoie aeid, ete.); a symmetrieal aeid anhydride
sueh as lower alkanoic 2nhydride (e.g. acetie anhydride, ~-
ete.), tr~halo~lower)alkanoie anhydride (e.g.
trifluoroaeetie anhydrlde, ete.~; an aetiYated aeid amide
with a heteroeyelie eompound eontaining imino funet~on
sueh as imidazole, 4-substituted imid~zole,
dimethylpyrazole, triazole and tetrazole; an activated
ester ~e.g. p-nitrophenyl ester, 2,4-dinitrophenyl ester,
triehlorophenyl ester, pentaehlorophenyl ester,
WO 93/0503~ - 18 - PCI`/JP92/01117 ~
21170~5 ~
mesylphenyl ester, phenylazophenyl ester~ phenyl
thioester, p-nitrophenyl thioester, p-cresyl thioester,
carboxymethyl thioester, pyridyl ester, p~peridinyl ester,
8-quinolyl thioester, or an ester with a N-hydroxy
compound such as N,N-dimethylhydroxylamine,
l-hydroxy-2-(lH)-p~ridone, N-hydroxysuccinimLde,
N-hydroxyphthalimide, 1-hydroxybenzotriazole,
1-hydroxy-6-chlorobenzotriazole, etc.); and the li~e.
This reaction can be carried out in the presence of a
base or an acid according to the tntroducing agent of the ;-
~mino-protectiYe group to be used.
Suitable base may include an organic or inorganic
base such as alkal~ metal ~e.g. lithium, sodium,
potassium, etc.), alkaline earth metal (e.g. calcium,
etc.), alkali metal hydride le.g. sodiu~ hydride, etc.),
alkaline earth metal hydr~de (e.g. calcium hydride, etc.),
alkali metal hydroxide (e.g. sodium hydroxide,potassium
hydroxide, etc.), alkali metal carbonate (e.g. sodium
carbonate, potassium carbonate, etc.), alkali metal
bicar~onate (e.g. sodium bicarbonate, potassium
bicarbonate, etc.), aIkali metal alkoxide (e.~. sodium --
methoxide, sodium ethoxide, potassium tert-butoxide,
etc.), alkali metal alkanoic acid (e.g. sodium acetate,
etc.), trialkyl~mine (e.g. triethylamine, etc.), pyridine
compound (e.g. pyridine, lut~dine, picoline,
4-dimethylaminopyridine, etc.), guinoline, and the like.
Suitable acid m~y include an organic actd (e.g.
formic acld, acetic-acid, propionic acid, trif~uoroacetic
acid, benzenesulfonic acid, p-toluenesulfonic acid,~ etc.)
and an inorganic acid (e.g. ~ydrochloric acid, hydrobromic
acld, sulfuric acid, phosphoric acid, etc.).
In case that the introducing agent of the
a~no-protective group used in a free fonm or its salt
in this reaction, the reaction is preferably carried out
211707~
w093/0~03~ - 19 - pCT/JP92/oll17
in the presence of a condensing agent such as a
car~odi~mide compound le.g. N,N'-dicyclohexylcarbodiimide,
N-cyclohexyl-N'-(4-diethylaminocyclohexyl)carbodiimide,
N,~'-diet~ylcarbodiimide, N,N'-diisopropylcarbodiimide,
N-ethyl-N'-~3-dimethylaminopropyl)carbodiimide, etc.~, a
ketenimine compound ~e.g. N,N'-carbonyLbis-(2-
methy-imidazole), pentamethyleneketene-N-cyclohexylimine,
diphenylketene-N-cyclohexylimine, etc.); an olefinic or
acetylenic ether compounds (e.g. ethoxyacet~lcne,
~0 ~-chlorovinylethyl ether), a sulfonic acid ester sf
N-hydroxy~enzotriazole derivative le.g.
1-(4-chlorobenzenesulfonyloxy)-6-chloro-lH-benzotriazole,
etc.], a combination of trialkylphosphite or
triphenylphosphine and carbon tetrachloride, disulfide or
diazenedicarboxylate (e.g. diethyl diazenedicarboxylate,
etc.), a phosphorus compound (e.~. ethyl polyphosphate,
isopropyl polyphosphate, phosphoryl chloride, phosphorus
trichloride, etc.), t~ionyl chloride, oxalyl chloride,
N-ethylbenzlsoxazolium salt, N-ethyl-~-phenylisoxazolium-
3-sulfonate, a reagent (referred to a so-called ~'Vilsmeier
reagent") formed by the reaction of an amide compound such
as N,N-di(lower)alkylformamide (e.g. dimethylformamide,
etc.), ~-methylformamide or the like with a halogen
compound such as thionyl chloride, phosphoryl chloride,
phosgene or the like.
The react~on is usually carried out in a conventional
solvent w~ich does not adversely influence the reaction
such as water, acetone, dichloromethane, alcohol (e.g.
methanol, ethanol, etc.), tetrahyd ofuran, pyridine, :
N,N-dimethylformamide, etc., or a mixture thereof, and
further in case that the amino-introducing agent is in
l~quid, it can also be used as a solvent. ~-
The reaction temperature is not critical and the
reaction is usually carried out under from coolin~ to
3~ heating.
w093/0503~ z O PCT/JP92/olll7
- 21170~
~4) Process 4 :
The compound ~I~ or salts ~hereof can be prepared by -~
re~cting the compound (IV) or sa~ts thereof with a base. ::
Suitable salts of the compound (IV) may be the ~ame ~-~
as those fsr the compound (I).
Suitable base used in this reaction may be the same
as those siven in the explanation of Process 3.
Thls reaction is usually carried out in a
conventtonal sol~ent which does not adversely influence
the react~on such as tetra~ydrofuran, dioxane, water,
methano~, et~a~ol, etc., or a mixture thereof.
The reaction temF~rature is not critical, and the
reaction is usually carried out under from warming to
heating.
:
(~) Process ~ :
The compound (I-f) or salts thereof can be prepared
by sub~ecting the co~pound (I-e) or salts thereof to a
removal reaction of the hydroxy protecti~e ~roup in R2. -
Suitable salts of the compounds (I-e) a~d (I-f) may
be the same as those for the compound (1).
The present reaction is usually carried out by a
con~entional method such as hydro~ysis, reduction, and the
like. :-
li)- Hydrolysis :
The hydrolysis is preferably carried out ~n the
presence of a base or an acid. Suitable base may include
an alkali metal hydsoxide (e.g. sodium hydroxide,
potassium ~ydroxide, etc.)~ an alkaline easth met~l
hydroxide (e.g. magnesium hydroxide, calci~m hydroxide,
etc.), alkali metal hydride (e.g. sodium hydride,
potassium hydride, etc.), alkaline earth metal hydride
~e.g. calcium hydride, etc.), alkali metal alkoxide (e.g.
3~ sodium methoxide, sodium ethoxide, potassium t-butox~de,
W093/0503~ 2 1 1707~ pCT/JP92t0l1t7
etc.), an alkali metal carbonate (e.g. sodium carbonate,
potassium carbonate, etc.), and alkaline earth metal
carbonate (e.g. magnesium carbonate, calcium carbonate,
etc.), an alkall metal ~icarbonate (e.g~ sodium
~iearbonate, potassium bicarbonate, etc.), and the liXe.
Sultable aeid may include an organir aeid (e.g.
formic aeid, acetic acid, propionic acid, trifluoroacetic
acid, benzenesulfonic aeid, p-toluenesulfonic aeid, etc.)
and an inorganic acid (e.q. hydrochloric acid, hydrobromie
acid, sulfuric acid, phosphoric acid, etc.). The acidic
hydrolysis using trifluoroacetic acid is usually
accelerated by addition of cation trappir.g agen~ (e.g.
phenol~ anisole, ete.).
This reaction is usually carried out in a
conventional solvent which does not adversely influence
the reaetion sueh as water, dlchloromethane, aleohol (e.g.
methanol, ethanol, ete.), tetrahyarofuran, dioxane,
acetone, etc., or a mix~ure thereof. A liquid base or
aeid ean be also used as the solvent.
The reaetion temperature is not eritieal and the
reaction is usually carried out unaer from coolin~ to -~
heatin~.
lii) Reduet~on :
~5 The reduction method applicable for this removal ~-
reaetion may inelude, for example, reduction by using a
combination of a metal (e.g. zinc, zinc amalgam, etc.) of
a salt of ehrome compound (e.g. chromous chloride,
chromuos acetate, etc.) and an organic or inor~anic acid
(e.g. acetie acid, propionic acid, hydrochloric acid,
sulfurie acid, etc.); and conventional catalytic reduction
in the presence of a conventional metallic catalyst such
as pal~adium eatalysts (e.g. spongy palladium, palladium
blac~, palladium oxide, palladium on earbon, colloidal
palladium, palladium on barlum sulfate, palladium on
w093/0503~ ~2 PCT/JP92/01117
` 2I17075
barium carbonate, palladium hydroxide on carbon, etc.),
nickel catalysts (e.g. reduced nickel, ni~kel oxide, Raney
nickel, etc.), platinum catalysts ~e.g. platinum pla~e,
spongy p~atinum, platinum black, colloidal platinum,
p~atinum oxide, platinum wire, etc.), and the like.
In case that the catalytic reduction is applied, the
reaction is preferably carried out around neutral --
conditlon.
Th~s reaction is usually carried out in a
conventional solvent wh~ch does not adversely influence ~-
the reaction such as water, alcohol (e.g. methanol,
ethanol, propanol, etc.), d~oxane, tetrahydrofuran, acetic
acid, buffer solution (e.g. phosphate buffer, acetate
buffer, etc.), and the li~e, or a mixture thereof.
lS The re~ction temperature is not critical and the
reaction is usually carried out under from cooling to
warming.
The removal reaction can be selected according to the
~0 kind of hydroxy-protective group to be removed.
~ .
(6) Process 6 :
The compound (I-h) or salts thereof can be prepared
by sub~ect~ng the compound ~I-g) or salts thereof to a
2S removal reaction of the carboxy-pro~ec~ive group in R2.
Suitable salts of the comyounds (I-g) and (I-h) may
be the same as those for the compound (I).
This reaction is usually carried out by a
conventional metho~ such as ~ydro~ysis, reduction and the
like.
The method of hydrolysis and reduction, and the
reaction condit~ons (e.g. reaction temperature, solvent
etc.) are substantially the same as those illustrated for
removal re~ction of the hydroxy-protective group of the
compound (I-a) in ~rocess 5, and therefore are to be
W0~3/Q~035 2 1 1 7 ~ p~cT/Jp92/olll7
referred to ~aid e~plnnAtion.
The ob~ect compound (I) obt~ined Accord~ng to the
Proce~es 1 to 6 c~n be i~olat~d and purified in a
con~ention~l manner, for example, extraction,
precipitation, fr~ction~l crystall~z~tion,
recryst~lliz~tion, chromatography, and the like.
Method A to C for prep~ring the new ~tarting
compound~ (II) ~nd (IV) or ~lts thereof Are explained ~n
detail in the following.
~,'.
(A) Method A :
The compound (II) or ~a~t~ thereof can be prepared ~y
re~cting the compound (V) or salt~ thereof with the
compound (VI) or 8alt3 thereof.
Su~t~b}e salts of the compound (~) may be s~lts with
bases ~uch as tho~e gi~en for the compound (I).
Suitable salts of the compound (VI) may be the ~2me
acid addition sAlts as tho~e for the co~pound (I).
This re~ction is usuAlly carried out in
con~ention~l sol~ent which does not adversely influence
the reactlon ~uch a~ dichloromethane, pyridine,
N,N-d~methylform~mide, 4-methyl-2-pentanone,
tetrahydrofur~n, etc., or a mixture thereof.
The reaction temperature is not critical and the
reaction i8 u~ually carried out under from warming to
heating.
(B) Met~od B :
The compound ~II) or ~alts thereof can be prepared ~y
reducing the nitro group of the compound (YII) or salts
thereof.
Suitable salts of the compound (YII~ may be the ~ame
a~ those for the compound (I).
The method of reduction ~nd the reaction conditions
35 (Q.g. reaction temperature, sol~ent, etc.) are
WO 93/0503~ - 24 - PCI`/JP92/01117
21171: ~
sub~tantially the s~me a~ thosQ illu~trAted in Proce~s 2, ::
and therefore ~re to be referred to ~aid explanation.
(c) Method C s
T~e compound (IV) or 8~1t~ thereof c~n be prep~red ~y
react~nq the co~pound ~VIII) or it~ re~cti~e deri~Ati~e at
the cnrboxy group, or 8alt~ thereof with tho compound tVI)
or salt~ thereof.
Suitable salt~ of the compound (VIII) may be the ~ame
a~ tho~e for the compound (I).
Su~table r~active der~ati~e of the compo~nd (VIII)
may ~nclude an acid halide, an acid anhydride, an
acSl~ated amide, an acti~sted eYter, And the like.
The suitable example may be an acid chloride; an ac~d ~`
azide; a mixed acid anhydride with an acid such as
sub~tituted pho~phoric acid (Q.g. dialkylphosphoric ac~d,
phenylpho~phoric acld, diphenylpho~phoric ac~d, :~
dibenzylphosphoric acid, halogen~ted phosphoric acid
etc.), di~lkylphosphorou~ acid, lower alkanesulfonic acid
(e.g. meth~ne~ulfonic acid, ethanesulfonic acid, etc.),
~ulforou~ ac~d, thiosulfuric acid, sulfuric acid,
alkylcarbonic acid, aliphatic carboxylic acid (e.g.
pi~alic acid, pentano~c acid, i~opentanoic acid,
2-ethylbutyric acid or trichloroacetic acid, etc.) or
arom~tic c~rboxylic ~cid (e.g. benzoic ACid, etc.);
8 ~ymmetrical ~cid ~nhydride; ~n ~cti~ated amide with
imid~zol~, 4-~ub~tituted imidazole, dimethylpyr~zole,
tri~zole or tetr~zole; or ~n ~cti~ated ester (e.g.
cyanomethyl e~ter, methoxymethyl e~ter,
dimQthyliminomethyl [(cH3)2h~cH-] ester, ~inyl ester,
propargyl ester, p-nitrophenyl e~ter, 2,4-dinitrophenyl
e~ter, trichlorophenyl ester, pent~chlorophenyl e~ter,
me~ylphenyl ester, phenyl~zophenyl e~ter, phenyl
thioe~ter, p-nitrophenyl thioeQter, p-cresyl thioester,
c~rboxymethyl th~oester, pyr~nyl e~ter, pyridyl ester,
w~93/0503~ 2 1 1 7 0 7 SpCT/JP92/01.]7
piperidyl ester, 8-quinolyl thioester, etc.), or an ester
with a N-hyaroxy compound (e.g. N,N-dimethylhydroxylamine,
1-hydroxy-2-(lH)-pyridone, N-hydroxysuccinimide,
N-hydro~yphthalimide, 1-hydroxy-6-chloro-lH-benzotriazole, ;:
etc.), and the l~ke. These reactive deri~ati~es can
optlonal~y be selected from them according to the kind of
the compound ~V~II) to be used.
When the compaund (VIII) is used in free acid form or
its salt for~ in the reaction, the reaction is preferably
1~ carried out in the presence of a conventional condensing
agent such as N,N'-dicyclohexylc~rbodiimide;
N-cyclohexyl-N'-morpholinoethylcarbodiimide; ~;~
N-cyclohexyl-N'-(4-diethylaminocyclohexyl)carbodiimide; ~:
N,N'-d~ethylcarbodlimide, N,N'-diisopropylc~rbodiimide;
~S N-ethyl-N'-(3-dimethylaminopropyl)carbodiimide;
N,N-carbonylb~s-12-methylimid~zole); pentamethyleneketene-
N-cy~lohexylimtne; d~phenylketene-N-cyclohexylimine;
ethoxyacetylene; 1~ oxy-1-chloroethylene; triaIkyl
phosphlte; ethyl polyphosphate; isopropyl polyphosphate;
phosphorus oxychlor~de (phosphoryl chloride); phosp~orus
trichlor~de; thionyl chloriae; oxalyl chloride;
triphenylphosphine; 2-ethyl-7-hydroxybenzisoxazolium salt;
2-ethyl-5-lm-sulfop~enyl)isoxazolium hydroxide
intramo~ecular salt; l-(p-chlorobenzenesulfonyloxy)-6-
2~ chloro-lH-benzotriazole; so-called Vilsmeier reagent
prepared by the reaction of N,N-~imethylformamide with
th~onyl chloride, phosgene, phosphorus oxychlroide, etc.;
or the like.
The reaction may also be carried out in the presence
of an inorgan~c or organic base such as those g~ven in the
explanat~on of Process 3.
The reaction is usually carried out in a conventional
solvent whlch does not adversely influence the reaction
such as water, methanol, ethanol, acetone, dioxane,
acetonitsile, chloroform, methylene chloride, ethylene
W093/0503s - ~6 - PCT/JP92/01117
` 2~1707~
chloride, tetrahydrofuran, ethyl acetate,
N,N-d~methylformamide, pyridine, etc. or a mixture
thereof.
The react~on temperature is not critical, and ~he
S reaction is usually c~rried out under warming to ~eating.
~D) Method D :
The compound (VII) or salts thereof can be prepared
by reacting the compound ~IX) or its react~ve derivativs
at the carbo~y group, or salts thereof with the compound
(VI) or salts thereof.
Suitable salts and reactive derivative of the
compound (IX) may be the same as those for the compound
(V~$~
T~e method of reaction and the reaction cond~tions
te.g. reaction temperature, so~vent, etc.) are
substa~tially the same as those illustrated in Method C,
and therefore are to be referred to said explanat$on.
The object guinazoline derivatives ~I) stLmulate
presynaptic(neuronal) and~or postsynapti~(vascular)
dop~mine receptors that mediate inh~b~tion of neurogenic
release of catecholamine and/or dilatation of renal
vasculature and remission of Parkinsonism, respectively~
Quinazol~ne deri~atives (I) effect ~n the cardiovascular
system as a conseguence of its ~nteraction with
dop2minerglc ~nd adrenergic receptors.
The ob~ect compound (I) and ~har~aceutically
accep~able salts thereof of the present invention are
novel and display dopamine receptor stimulating effects;
5-HT receptor antagonism, especially 5-HT2 receptor
antagonisms al receptor antagonism; and the like, and are
useful as a dopam~ne receptor agonist; 5-~ receptor
an~agonist, especlally S-HT2 receptor antagonist; ~1
receptor antagonist; and the like, for treating or
preventing hypertension such as essential hypertension,
2117075
W093/0503~ ?7 PCT/JP92/01117
renal hypertension, pulmonary hypertension, and other :~-
earaiovaseular disorders (e.g. angina peetoris, eongestive
heart failure, myocardial infaretion, etc.); Par~insoniSm; -~'
hyperprolae~inemia; disorders of peripheral perfusion s~eh
as Raynaud's phenomenon, 8ruger's diseases, and
intermittent claudieation; thrombotie an~/or smooth muscle
eell proliferative disease sueh as restenosis after
perculaneous trans~.uminal eoronary an~ioplasty;
hypereholesterolemia, hyperlipemia; urinary disturvanee; .
eardiac hypertrophy; nephropAey sueh as nephritis, renal
failure, ete.; arterioselerosis obliterans; obstruetive "
~hrombus; arterial embolus; Burger-Gruts syndsome;
aeroeyanosis; eh~l~lain; frost~ite; pro1aetin-producing
ovulation d~sorder; prolaetin-produeing pituitary tumor;
puerperal galaetosehesia; galaetorrhea; distal
hypertrophys pituitary gigantism; mental illness such as
melaneholia, anxiety, sehizophrenia, ete.; eerebrovaseular
diseases sueh as eerebral ~nfaretion, cerebral cireulation
insuff~eieney; taehyeardia aeeompanied by sympathetic
hypertoni~; hyperaldosteronism; diabetie eomplieation sueh
as diabetie hypertriglyeeromia, and the like. ''
And further, the objeet eompound II) has vasodilating
aetivity, b~ood fl~w inereasing aetivity sueh as renal .
blood flow inereasing aetiv1ty, and the li~e. ~-
2~ The eompound (I) and pharmaeeutieally aeeeptable ~'
salts thereof may be also useful as an adrenolyt~e,
tran~uilizer', se~at~ve, anti-emetie, hypothermic, skeletal ~.
musele relaxant, anti-inflammatory, hypoglyeemie, ':
anti-viral agent.
~0 Now in order to show the usefulness of the obje~t --
eompound l~) and pharmaeeutically aeeeptable salts, the :~.
pharmaeologieal test data of the representative eompound
of the eompound (I) of this invention are shown in the :
following.
.
WO 93/0503~ - 28 - PCr/JP92/01117 ~
2117075 ~
Test ComPound : r
Co~pound A r The product of Example 1]
Test 1 Dopamine receptor ~DA2 receptor) blnding assay
Test Metho~ 1 :
The affinlty for DA2 receptor of a Test Compound was
determined following in vitro receptor binding assays.
Male rats weighing 150-300g were decapita~ed and the
stratum were dissected from their brains. The tissue was
homogenized in 40 volumes of buffer which consisted of 50
mM Tri~-HCl (p~ 7.~ ~t 25C), ~ mM tetraethylenediamine
tetraacetic acid,5 m~ potassium chloride, 2 mM calcium
chloride, and 1 mM maqnesium chloride. The homogenate was
centrifuged at 50,000 g for 1~ minutes. The pellet was
resuspended in 80 ~olumes of the buffer. The tissue
suspension was centrifuged and suspended again in the same
w~y. 3
Incubation tubes received 100 ~l of t2-N-~2,3(n)- H~-
propyl-N-(2-thiofuranyl)-2'-ethylamino)-5-hydroxy-1,?.,3,4-
tetrahydronaphtha~ene, 10 ~1 of the Test Compound and 0.89
ml of tissue suspension during binding assays. The
concentration of (2-N-[2,3(n)-3H~propyl-N-~2-thiofuranyl)-
2'-ethylamino)-5-hydroxy-1,2,3,4-tetrahydrona~hthalene,
was 1.5 nM. The final tissue concentration of rat
stratum was 160 ~g/ml. The ~ubes were incubated at 25C
for 4S minutes, and then filtered under vacuum through
Whatman GF/B filters and washed three times wi~h 3 ml of
ice-cold buffer. The filters were counted by liguid
scintillat~on counter.
Speciflc binding of ~he (2-N-I2~3(n)-3H~propyl-(N-t2
thiofuranyl)-2'-ethylam~no)-5-hydroxy-1,2,3,4-tetrahydro-
naphtha~ene was determined in the presence of 1 yN
apomorphine. The IC50 value of the Test Compound was
3~ calculated from the data of (2-N-12,3(n)-3H~propyl-N-(2-
w093/05035 2 1 1 7 0 7 ~ pCT/Jpg2/olll7
thiofuranyl) 2'-ethylamino)-5-hydroxy-1,2,3,4-tetrahydro- :
naphthalene~ binding in the presence of 10 9M, 10 8M,
10 7M and 10 6M Test Compound.
Test Result 1 :
Test compound IC50 ~M)
.. . , . ~ . .
Compound A 2~1 x 10
lû
Te~t 2 ~Inh~bition of reserpine-induced DOPA
accumulation] . :~
.:
Test Method 2 :
lS Male SD rats weighin~ 300-400 g were used in this
test. ~ats were pretreated with reserpine (1 mg/kg, S.C.)
17-19 hours before sacrif~ce and then fasted. Test
Compound w~s given orally to the rats 2 hours before -
sacrifi~e. I~-~YdroxYbenzYlhYdrazine (100 mg/k~ p.) was
given 30 mlnutes before sacri~ice.~ E~ch rat was exposed
to miarowaves using a head-focus microwa~e applicator for
1.5 seconds. The whole brain was removed and further
separated tnto the striatum. ::
DOPA was determined as follows; the striatum was :
homogenized in 9 volumes o~ O.lN perchloric acid solution :;
tO.4% EDTA-2Na). The homo~enate was centr~fuged at 10,000
rpm for 1 minute. The supernatant was appl~ed to high
performance liquid chromatography~
:,
~ Test Result 2
. _ _ :
. Test Compound (mg~kg) Inhibit~on (%)
.-
Compound A ~.2
WO 93~0503~ o _ PCI`/JP92/01117
21170~
Test 3 l5-HT2 Bindinq Assay~
Test Method 3 :
The affinity for Serotonin 2 receptor of a test
compound was detenmined following in vitro receptor
b~ nding ~ssays.
Male Wistar rats weighin~ 150-300 g were decapitated
and the frontal cortex were dissected fr~m their brains.
The tissue was homogenized in 1~ volumes of ice-cold
buffer which consisted of 0.32 M Sucrose, 50 mM Tris-HCl
(p~ 7.5 at 25C). The homogenate was centrifuged at 3,300
rpm at 4C for 10 minutes. The supernatant was -
centrifuged at 2~,000 rpm for 20 minutes. The pellet was
resuspended again in the same way, and incubated at ~7~C
for 15 minutes. The tissue suspension was centriAfuged at
20,000 rpm for 20 minutes. The final pellet was
resuspended in 15 ~olumes of ice-cold assay buffer (50 mM
Tris~HCl, 4 mM CaC12, 0.1% Ascorb~c acid, 10 yM pargyline,
pH7.7 at 25-C).
Incubation tubes received 100 ~l of [ethylen-3Hl-
ketanserin, 10 ~l of the test compound and 0.2 ml of
tissue suspension, and 0.69 ml assay buffer during binding
assays. The f~nal concentration of ~ethylen-3H~-
ketanserin was 0.05 nM. The tubes were incubated at 37~C
for 30 minutes, and then filtered under vacuum through
Whatman GF/B f~lters and washed three times wi~h ~ ml of
ice-cold buffer (50 m~ tris/HCl, pH 7.5 at 25C). The
fllters were counted by liquid scintillation counter.
S~ecific b~nding of [ethylen-3H~-ketanserin was
determined ln the presence of 1 ~M mianserin. The~ICSO
value of the test compound was calculated from the data of
[ethylen-3H~-ketanserin ~inding in the presence of 10 9M,
10 8M, 10 7M, and lO 6M test compound.
3~
2:~171)75
W0~3/0503~ - 31 - PCT/JP92/01117
3~:
..... .......
Test Compound ICsO (M)
Compound A 3.5 x l0-8
For ther~peutic admini3tration, the ob~ect compound
(I) ~nd the phar~aceutically acceptable ~alt~ thereof of
the pres~nt in~ention are used in the form of con~ention~l
ph~rmac~utical prep~ration whirh contains said compound,
as ~n acti~ ingredient, in admixture with :-
pharmaceutically ~ccept~ble carriers such as an organic or
inorganic sol~d or liquid excipient which is suit~ble for-
oral, p~renteral and external admini~tration. :~
The pharmaceutical prepar tion~ m~y be in ~olid form such
a~ t~blet, gr~nule, powder, capsule, or liguid form such
~ ~olution, ~u~pension, ~yrup, emul5 ~on, le nade, ~nd
the like.
If neaded, there may be included in the abo~e
preparation~ auxiliary sub~t~nces, ~tabilizing agents,
wettlng ~gents and other commonly u~ed add~tives ~uch a~
lactose, stearic acid, magne~ium stearate, terra alba,
~ucrose, eorn ~t rch, talc, gelatin, ~gar, pect~n, peanut
oll, ol~e oil, cacao butter, ethylene glycol, tartaric
~cid, citric cld, f~mar~c ~c~d, and the like.
While the do~ge of the compound (I) m~y v~ry from
and ~l~o depend upon the age, conditionQ of the patien~, a
kind of di~ea~es, a kind of tho compound (I) to be
~pplied, etc. In general, amount between about O.OOl mg
and ~bout 300 mg, prefer~bly about O.l mg to about 50 mg
per d~y may be admini~tered to a patient. An averaqe :~
single do~ of about O.OOl mg, O.Ol mg, 0.03 mg, O.l mg,
0.3 mg, 0.6 mq, l.0 mg, 3.0 mg, lO.0 mg, 50.0 mg, lO0.0
W093/05035 - 32 - pCT/JP92~01117
` 2117075
m~, of th~ o~ect compound (I) of tho pre~ent ~n~ntion
may be used as adrsnolytic, hypotens~e, cardio~a~cul~r,
tran~uilizer, sedative, anti-2met~c, hypothermic, skelet~l
mu~clQ rel~xant, antl-inflamm tory, and anti-~iral agent~.
The followtng Prep~rat~on~ ~nd Example~ are gi~en for
the purpo8~ of illustrating this in~ention in more detail.
Prepar~tion, 1~
To a stirred m~xture of 4~ methylpiperazln-l-
ylmethyl)-2-nitrobenzoic ~id (0.28 g),
hydroxybenzotriazole (0.14 g) and dry dime~hylform~mide (5
ml) Wa8 ~dded dicyclohexylcarbodiim~de (O.2l g), and the
mixture W~8 ~tirred for 1 hour At room temper~ture. Then
4-(4-phenyl-l,2,3,6-tetrahydropyridin-l-yl)butylam~ne
hydrochloride IO.27 g) ~nd triethylamine (O.20 g) were
~dded and the stirring wa~ cont~nued for add~tion~l 1
hour. ~N H~drochloric acid (2 ml) and water (20 ml) were
added and the mixture was wa~hed with ethyl acetate,
ad~usted to pH 8 with Aqueou~ ~odium bicarbonate solution
and extracted with ethyl acetate tx2). The combined
organic extracts were dried ~er mngnesium sulfate and
sol~ent wa~ e~aporated to give 4-(4-methylpiperazin-l-
ylmethyl)-2-nitro-N-14-(4-phenyl-l,2,3,6-tetrahydro-
25 pyri~n-l-yl )butyl]benzamide (O.2l g).
NMR (CDC13, ~) s l.78 (4~, m3, 2.23 (2H, m), 2.30
(3H, ~, 2.43 (8H, m), 2.52 ~2H, m), 2.62 (2H,
m), 3.00 (2H, m), 3.28 ~2H, s~, 3.49 (2H, m),
5.75 (lH, m), 7.1-7.4 (7H, m), 7.75 (lH, s),
8.78 (lH, m)
Preparation 1-2)
To ~ st~rred ~ixture of 4-(4-methylp~perazin-l-
ylmethyl)-2-nitro-N-~4-(4-phenyl-l,2,3,6-
tetr~hydropyrid~n-l-yl)butyl]benzamide (0.21 g), ethanol
211707~ :
W093jos03~ - 33 - PCT/~JP92/Otll7
(5 ml) and d~y te~rahydrofur~n (10 ml) was added tin (II) -~
chlorid~ (0.33 g) and the mixtur~ wa3 refluxed for 1 hour. ~-
~fter cooling, chloroform (15 ml) and lN ~odium hydroxide
(25 ml) w~re added ~nd the mixtur~ wa~ stirred. The --~
org~nic layer was ~ep~rated and the remained aqueous l~yer
wa~ ~xtracted with chloroform. The org~nic layer ~nd
extract were combined and dried o~er mAgne~ium ~ulf~te and
evaporated to gi~e 2-amino-4-(4-methylpiperazin-1-
ylmethyl)-N-t4-(4-phenyl-1,2,3,6-tetrahydropyridin-1-yl)-
butyl]benzam~de (O.25 g).
NMR (C~C13, 6) : 1.72 (4~, m), 2.31 (3H, fi), 2.50
(lOH, m), 2.71 (2H, m), 3.15 ~2H, m), 3.33 ~2H,
~), 3.45 (2H, m), 3.75 (2H, m), 5.50 (2H, br ~
6.0~ , m), 6.49 (lH, dd, J-8, lHz), 6.65 (lH, ::
d, J=l~z), 6~41 (lH, m3, 7.2-7.45 (6H, m) -
Ex~mple 1
A mixture of 2-~mino-4-(4-methylpiperazin-1- :~
y~methyl)-N-t4-(4-phenyl-1,2,3,6-tetr~hydropyridin-1-
yl)butyl~benz~mide (0.25 ~ arbonyldilmidazole (0.45 g)
and dry tetrahydrofuran (10 ml) wa~ stirred under reflux
for 14 hour~. After evapor~tion of the sol~ent, the crude
re~idue wa~ taken up with ethyl acetate, washed with
w~ter, dried o~er ma~ne~ium sulfate ~nd e~apora~ed to gi~e
crystal~. Recrystallization from ethanol afforded
7-(4-methylpiperaz~n-1-ylmethyl)-3-[4-(4-phenyl-1,2,3,6-
tetrahydropyrid~n-l-yl)buty~ 2~3~4- ~;
tetrahydroquinazoline-2,4-dione (0.16 g). :-
mp : 167-168C
IR (Nu~ol) : 1700, 1660, 1~20, 1590 cm-l
NMR (CDC~3, ~) : 1.75 (4H, m), 2.30 (3H, s), 2.50
(12H, m), 2.~2 (2H, m), 3.18 (2H, m), 3.56 (2H, :~
~), 4.12 (2H, m), 6.05 (lH, m), 7.00 (lH, s),
7.1S-7.40 (6H, m), 8.03 (lH, d, J~8Hz), 9.28
(lH, br 8 )