Note: Descriptions are shown in the official language in which they were submitted.
CA~ 1 J503
330~6-00
AZOXYCYANn~N7nnIOXANE DERIVATIVES
This invention relates to certain ~ y-.~~h~ o~ioxane
derivatives, processes for their preparation, compositions
cont~;ning such compounds and their use as fungicides.
EP-A-0371560 discloses a method of ~ t;ng a fungus, and/or
bacterium, and/or yeast, and/or nematode, at a locus which
comprises treating the locus with a compound of the general formula
R4 ~ 2 (A)
Rs ~ = NX
(o) n
in which R2 and R3 together, or R3 and R4 together, represent an
optionally substituted hydrocarbyloxy chain; the ring is optionally
substituted at any or each of the 1. in;ng sites R5, R6 and R2 or
R ; n le~leSe~ O or l; and X represents a cyano group, a group
-COOH or a salt, ester or amido derivative thereof. The term
"hydrocarbyloxy chain" is used to denote a carbon atom chain
interrupted within the chain by one or more (but preferably one
only) oxygen atom. In each of the examples, the hydrocarbyloxy
chain contains only one oxygen atom and this is located at one end
of the chain.
EP-A-0518436 discloses compounds of the general formula
~1 ~ R1 RZ (B
( ) n~ I ~R3 R4
XN=~ ~/ O
CA27 175~3
in which m is 0 or 1; each of R and R , and R and R if preSent,
independently represents a hydrogen or halogen atom or an
optionally substituted alkyl, cycloalkyl or aryl group, or Rl and
R together or R and R together represent an optionally
substituted alkylene chain; X represents a cyano group, a group
-COOH or a salt, ester or amido derivative thereof; Y represents an
alkyl group or a halogen atom; and n represents 0, 1, 2 or 3; and
their use as fungicides.
It has now been discovered that certain ~u~y~ -1,3-benzo-
dioxane derivatives exhibit good activity against certain --
phytopathogenic fungi.
According to the present invention there is therefore provided
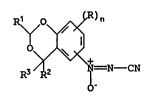
a compound of general formula
R3 2 CN
in which n is 0, 1 or 2; each R, if present, inA~ ..lly
represents a halogen atom or an optionally substituted alkyl or
alkoxy group; R represents a hydrogen atom or a haloalkyl group;
Z5 and R2 and R3 in.~e~_n.~..tly represent a hydrogen atom or an alkyl
or haloalkyl group.
When the compounds of this invention contain an alkyl group,
this may be linear or branched and may contain up to 12, preferably
up to 6 and especially up to 4, carbon atoms.
~hen any of the foregoing substituents are designated as being
optionally substituted, the substituent groups which are optionally
present may be any one or more of those customarily employed in the
development of pesticidal compounds, and/or the 'ificAtion of
such compounds to influence their structure/ activity, persistence,
penetration or other property. Specific examples of such
' CA2~ 1 -i7 5 ~ ~
- 3 -
substituents include, for example, halogen atoms, nitro, cyano,
hydroxyl, alkyl, haloalkyl, alkoxy, haloalkoxy, amino, alkylamino,
dialkylamino, formyl, alkoxycarbonyl, carboxyl, alkanoyl,
alkylthio, alkylsulphinyl, alkylsulphonyl, carbamoyl and alkylamido
groups. When any of the foregoing substituents represents or
contains an alkyl substituent group, this may be linear or branched
and may contain up to 12, preferably up to 6, and especially up to
4, carbon atoms. Typically, 0-3 substituents may be present, most
commonly 0 or 1.
Preferably, each R, if present, indep~-ld~ ly represen,ts a
halogen atom or a Cl 6 alkyl or Cl 6 alkoxy group, each group being
optionally substituted by one or more substituents selected from
halogen atoms, nitro, cyano, hydroxyl and amino groups. It is also
preferred that n is 0 or 1. When n is 1, R is preferably located
lS at the 8-position of the 1,3-benzodioxane ring.
More preferably, R represents a halogen (especially chlorine
or bromine) atom or a Cl 4 alkyl (especially methyl) or Cl 4 alkoxy
(especially methoxy) group, each group being optionally substituted
by one or more substituents selected from halogen atoms and
hydroxyl groups.
It is preferred that R represents a hydrogen atom or a Cl 6
haloalkyl group.
More preferably, R represents a hydrogen atom or a Cl 4 halo-
alkyl group, the or each halogen atom in the haloalkyl group
preferably being a fluorine or chlorine atom.
It is also preferred that R2 and R3 jn~ y represent a
hydrogen atom or a Cl 6 alkyl or Cl 6 haloalkyl group.
More preferably, R2 and R3 1n~pppn~en~ly represent a hydrogen
atom, Cl 4 alkyl or Cl 4 haloalkyl group, the or each halogen atom
in the haloalkyl group preferably being a fluorine or chlorine
atom.
Preferably, the a~ ..o group -~I-N-CN is located at the
o
~ 5-; 6- or 7-position of the 1~3-hpn7oAiny~np ring, the 6-position
being especially preferred.
CA2i i 750~
- 4 -
A particularly preferred sub-group of compounds of formula I
is that in which n is 0 or 1; R represents a chlorine or bromine
atom or a hydroxymethyl or methoxy group; each of R and R
in~p~n~ently represents a hydrogen atom or a trichloromethyl
S group; and R3 represents a hydrogen atom.
It should also be noted that compounds of general formula I
could be in any of the following isoelectronic forms:-
Ar-~T=N - CN ; Ar 1: ~T CN ; Ar N\ ~ CN ,~ "
where Ar represents R y O ~ ) n
R3 ~
, and the scope of the present invention covers all such forms. In
addition, it should be appreciated that the compounds of formula I
have chiral centres and are therefore capable of existing as
different optical isomers. The present invention thus includes
both the individual isomers and mixtures of such isomers.
The present invention also provides a process for the
preparation of a compound of formula I as defined above which
comprises treating a compound of general formula
~ ~ (II)
R3 R2 - CN
in which n, R, R1, R2 and R3 are as defined above, with a mixture
comprising hydrogen peroxide and methanoic acid and/or with
- C~2i 1 7503
peroxymethanoic acid.
~ here a mixture of hydrogen peroxide and methanoic acid is
used, the concentration of the hydrogen peroxide is suitably less
than about 75wt%, preferably less than about 50wt~ and, more
S preferably, less than 40wt~. In a preferred ~ the
concentration of hydrogen peroxide is about 30wt~,
The process is preferably carried out at or above, more
preferably, above, ambient temperature. The process may be carried
out at a temperature in the range from 25~C to 75~C, preferably in
the range from 30~C to 50~C.
Preferably, the compound of general formula II is mixed with
methanoic acid and hydrogen peroxide at ambient temperature. The
mixture is preferably then heated, suitably at about 60~C, for a
number of hours. The mixture may then be cooled, for instance, in
an ice bath. The desired product may then be isolated by standard
techniques.
A general process for the preparation of compounds of formula
II is provided by R.J.W. LeFevre and H. Vine, J. Chem. Soc.,
(1938), 431.
The present invention further provides an alternative process
for the preparation of a compound of formula I as defined above
which comprises reacting a compound of general formula
R~3 ~ n (III)
in which n, R, Rl, R2 and R3 are as defined above, with cyanamide
or a metal salt thereof and an oxidising agent.
The oxidising agent may be any compound which, in conjunction
with cyanamide or a metal salt thereof, generates a cyanonitrene.
Preferred examples include in~oben7Gn~ diacetate,
CA21 1 75C3
- 6 -
dibromoisocyanuric acid and cyclic or linear N-halogen or
pse~-~Ah~l~gen amides or imides, especially N-bromosuccinimide.
If a metal salt of cyanamide is used in the process of the
invention, it is preferred that the metal salt is an alkali metal
salt or an alkaline earth metal salt. Alternatively, such metal
salts can be generated in situ by reacting cyanamide with an alkali
metal hydroxide or an alkaline earth metal hydroxide. The use of
monosodium cyanamide is especially preferred. Alternatively, this
may be replaced by a concentrated aqueous solution of cyanamide
with sodium hydroxide which effectively generates monosodium
cyanamide in situ.
Suitably, the reaction takes place in the presence of an
organic solvent, preferably dimethylformamide or a halogenated
hydrocarbon, for example, dichloromethane. The reaction is
preferably effected at a temperature in the range from -20~C to
50~C.
A compound of formula III may be prepared as follows, where Ar
is as defined above:-
(A) (B)
Ar - NOz >Ar - NHOH >Ar - N = O
V IV I-I
A
(C)
Reaction A may, for example, be effected by reaction of the
nitro compound with hydrazine hydrate, in the presence of a
hydrogen transfer catalyst, for example rhodium on carbon, suitably
in the presence of an inert polar organic solvent, for example,
tetrahydrofuran, preferably with cooling; or be effected using
water, stannous chloride as reducing agent, an inert, polar organic
solvent, for example tetrahydrofuran, under an inert atmosphere,
for example nitrogen, in the presence of sodium acetate, suitably
at ambient temperature.
i~2 i 1 750 ~
Reaction B may suitably be effected by treatment of the
hydroxylamine derivative with an oxidising agent, for example an
Fe compound, suitably ferric chloride. The reaction may be
effected in a mixed water/polar organic solvent, preferably with
cooling.
Reaction C may be effected by irradiating the nitro compound,
which is preferably dissolved in an inert organic solvent, for
example benzene. The irradiation may be effected using a medium
pressure mercury lamp.
Details of the above processes may be found in applicants'
co-pending European patent applications nos. 92120580.3 and
TS 8009 EPC. Other methods suitable for preparing compounds of
formula I, and further descriptions of the methods described
herein, may be found in The Journal of Antibiotics, Jan. 1975,
p.87-90 and June 1986, p.864-868; in Eur,J.Med.Chem.-Chim.Ther.,
1982, 17, No. 5, p.482 -484, and 1980, 15, No.5, p.475-478, and
1977, 12, No.l, p.59-62; in J.Chem.Soc.,Chem.Commun., 1984,
p.323-324; in Chem.Ind. (Milan), 1977, 59(5), p.385; in Gaaetta
Chimica Italiana, 106, 1976, p.ll07-1110; in Tetrahedron Letters,
No. 38, 1974, p. 3431-3432; and in U.S. Patents Nos. 4558040 and
4550121.
The compounds of general formula I have been found to have
fungicidal activity. Accordingly, the invention further provides a
fungicidal composition which comprises a carrier and, as active
ingredient, a compound of formula I as defined above. A method of
making such a composition is also provided which comprises bringing
a compound of formula I as defined above into ~CSOriAtiOn with at
least one carrier. Such a composition may contain a single
compound or a mixture of several compounds of the present
invention. It is also envisaged that different isomers or mixtures
of isomers may have different levels or spectra of activity and
thus compositions may comprise individual isomers or mixtures of
isomers.
- - A composition according to the invention preferably contains
from 0.5 to 9s% by weight of active ingredient.
CA ~ l ~ 7~D~
- 8 --
A carrier in a composition according to the invention is any
material with which the active ingredient is formulated to
facilitate application to the locus to be treated, which may for
example be a plant, seed or soil, or to facilitate storage,
transport or handling. A carrier may be a solid or a liquid,
including a material which is normally gaseous but which has been
compressed to form a liquid, and any of the carriers normally used
in formulating fungicidal compositions may be used.
Suitable solid carriers include natural and synthetic clays
and silicates, for example natural silicas such as diatomaceous
earths; magnesium silicates, for example talcs; magnesium aluminium
silicates, for example attapulgites and vermiculites; ~1 'ni
silicates, for example kaolinites, montmorillonites and micas;
calcium carbonate; calcium sulphate; ammonium sulphate; synthetic
hydrated silicon oxides and synthetic calcium or ~1 ~ni
silicates; elements, for example carbon and sulphur; natural and
synthetic resins, for example coumarone resins, polyvinyl chloride,
and styrene polymers and copolymers; solid polychlorophenols;
bitumen; waxes, for example beeswax, paraffin wax, and chlorinated
mineral waxes; and solid fertilisers, for example superphosphates.
Suitable liquid carriers include water; alcohols, for example
isopropanol and glycols; ketones, for example acetone, methyl ethyl
ketone, methyl isobutyl ketone and cy~loh~Y~n~n~; ethers; aromatic
or araliphatic hydrocarbons, for example benzene, toluene and
xylene; petroleum fractions, for example, kerosine and light
mineral oils; chlorinated hydrocarbons, for example carbon
tetrachloride, perchloroethylene and trichloroethane. Mixtures of
different liquids are often suitable.
Fungicidal compositions are often formulated and transported
in a concentrated form which is all-se~.-..lly diluted by the user
before application. The presence of small amounts of a carrier
which is a surface-active agent facilitates this process of
dilution. Thus preferably at least one carrier in a composition
- according to the invention is a surface-active agent. For example
-
CA 2 i 1 7503
the composition may contain at least two carriers, at least one of
which is a surface-active agent.
A surface-active agent may be an emulsifying agent, a
dispersing agent or a wetting agent; it may be nonionic or ionic.
Examples of suitable surface-active agents include the sodium or
calcium salts of polyacrylic acids and lignin sulphonic acids; the
r~n~ncAtion products of fatty acids or aliphatic amines or amides
containing at least 12 carbon atoms in the molecule with ethylene
oxide and/or propylene oxide; fatty acid esters of glycerol,
sorbitol, sucrose or pentaerythritol; r~n~GneAt~e of these with
ethylene oxide and/or propylene oxide; con~nQAti~n products of
fatty alcohol or alkyl phenols, for example ~-octylphenol or
p-octylcresol, with ethylene oxide and/or propylene oxide;
sulphates or s~lrh~nAt c of these con~n~Ation products; alkali or
alkaline earth metal salts, preferably sodium salts, of sulphuric
or sulphonic acid esters contAining at least 10 carbon atoms in the
molecule, for example sodium lauryl sulphate, sodium secondary
alkyl sulphates, sodium salts of snlrh~nAt~d castor oil, and sodium
alkylaryl c~lrh~nAt~s such as dodecylbenzene sulphonate; and
polymers of ethylene oxide and copolymers of ethylene oxide and
propylene oxide.
The compositions of the invention may for example be
formulated as wettable powders, dusts, granules, solutions,
leifiAhle concentrates, emulsions, sl~ep~nei~n conr~ntrAtes and
25 aerosols. Wettable powders usually contain 25, 50 or 75% w of
active ingredient and usually contain in addition to solid inert
carrier, 3-10~ w of a dispersing agent and, where necessary, 0-10
w of stabiliser(s) and/or other additives such as penetrants or
stickers. Dusts are usually formulated as a dust concentrate
having a similar composition to that of a wettable powder but
without a dispersant, and may be diluted in the field with further
solid carrier to give a composition usually ~ontl1n1ne ~-10~ w of
active ingredient. Granules are usually prepared to have a size
- between 10 and 100 BS mesh (1.676 - 0.152 mm), and may be
manufactured by A~g1~ ~Lion or impregnation techniques.
CA21 1 7503
- 10 -
Generally, granules will contain ~-7s% w active ingredient and
0-10% w of additives such as stabilisers, surfactants, slow release
modifiers and binding agents. The so-called "dry flowable powders"
consist of relatively small granules having a relatively high
concentration of active ingredient. Emulsifiable concentrates
usually contain, in addition to a solvent and, when necessary,
co-solvent, 1-50% w/v active ingredient, 2-20% w/v emulsifiers and
0-20% w/v of other additives such as stabilisers, penetrants and
corrosion inhibitors. Suspension concentrates are usually
c , ' d so as to obtain a stable, non-s~ ne flowable
product and usually contain 10-75% w active ingredient, 0.5-15% w
of dispersing agents, 0.1-10% w of 5-lcp~n~ine agents such as
protective colloids and thixotropic agents, 0-10% w of other
additives such as defoamers, corrosion inhibitors, stabilisers,
penetrants and stickers, and water or an organic liquid in which
the active ingredient is substantially insoluble; certain organic
solids or inorganic salts may be present dissolved in the
fn lAtin~ to assist in preventing sedimentation or as anti-freeze
agents for water.
Aqueous dispersions and emulsions, for example compositions
obtained by diluting a wettable powder or a c~.,c~..L~a~e according
to the invention with water, also lie within the scope of the
invention. The said emulsions may be of the water-in-oil or of the
oil-in-water type, and may have a thick 'mayonnaise' like
consistency.
The composition of the invention may also contain other
ingredients, for example other compounds possessing herbicidal,
insecticidal or fnngiri~Al properties.
Of particular interest in enhancing the duration of the
protective activity of the compounds of this invention is the use
of a carrier which will provide a slow release of the fungicidal
compounds into the environment of the plant which is to be
protected. Such slow-release fn lAtions could, for example, be
- inserted in the soil adjacent to the roots of a vine plant, or
~ ~ 2 ~
11
could include an adhesive component enabling them to be applied
directly to the stem of a vine plant.
The invention still further provides the use as a fungicide of
a compound of the general formula I as defined above or a
composition as defined above, and a method for combating fungus at
a locus, which comprises treating the locus, which may be for
example plants subject to or subjected to fungal attack, seeds of
such plants or the medium in which such plants are growing or are
to be grown, with such a compound or composition. A locus as
described above may suitably be treated with a compound I at an
application rate in the range O.OS to 4 kg/ha, preferably 0.1 to 1
kg/ha.
The present invention is of wide applicability in the
protection of crop plants against fungal attack. Typical crops
which may be protected include vines, grain crops such as wheat and
barley, apples and tomatoes. The duration of protection is
normally dependent on the individual compound selected, and also a
variety of external factors, such as climate, whose impact is
normally mitigated by the use of a suitable formulation.
The invention is further illustrated by the following
examples.
Example lA
Preparation of 6~ y~lo-1,3-benzodioxane
(n=0;Rl=R2=R3=
(a) Preparation of 6-azocyano-l~3-b~n7oainy~n~
6-Amino-1,3-benzodioxane (4 g, 26.5 mmol) was dissolved in a
mixture of water (6.5 ml) and hydrochloric acid (7.5 ml, 35~ w/w).
After the addition of crushed ice (30 g), the solution was
diazotised by addition of sodium nitrite (1.87 g, 27 mmol). The
chilled solution was then neutralised with sodium hydroxide
(10~ w/w). Trichloromethane (50 ml) was added and the reaction
mixture was cooled to about 0~C. After the addition of potassium
cyanide (1.84 g, 28.3 mmol) dissolved in water (14 ml), the organic
- layer was separated, dried over sodium sulphate, evaporated to
CA 2 i 1 7503
- 12 -
dryness and the resulting orange crystals were filtered to give
2.52 g crude 6-azocyano-1,3-benzodioxane.
(b) Preparation of 6-~Gu~y~y~l.u-1,3-benzoxdioxane
The crude 6-azocyano-1,3-benzodioxane (1.5 g, 7.9 mmol)
obtained in (a) above was suspended in a mixture of methanoic acid
(3û ml) and hydrogen peroxide (10 ml, 30% /w). The mixture was
heated to 6û~C for 24 hours and then cooled in an ice bath. The
resulting crystals were then collected and washed with water to
give 0.62 g (38% yield) 6-azoxycyano-1,3-benzodioxane as orange
lû crystals,
m.pt : 157~C, m/e (M ) : 205.
H-NMR(CDC13): S(ppm) e 4.92 (s;ArCH20); 5.35 (s;OCH20);
6.88, 7.95, 8.08 (m;Ar-H)
Example lB
lS Preparation of 6-a~u~y~y~llo-1,3-benzodioxane
(n=O;Rl=R2-R3=H)
6-Nitroso-1,3-benzodioxane (2.5 g, 15.0 mmol) and cyanamide (0.94
g, 21.7 mmol) were dissolved in dichloromethane (190 ml). Under
nitrogen, a solution of inAobPn7pnp diacetate (5.34 g, 16.6 mmol)
2û in dichloromethane (190 ml) was added drop by drop at 0~C. The
mixture was stirred overnight at ambient ~ , ~LUL~, filtered and
evaporated to dryness. Column chromatography on silica using 1:1
petroleum ether: ethyl acetate as eluant yielded 2.3 g (75% yield)
6-azoxycyano-1,3-benzodioxane as a yellow powder, m.pt : 156-158~C,
m/e(M ) : 205.
H-NMR(CDC13): ~ (ppm) - 4.92 (s;ArCH20); 5.35 (s;OCH20);
6.88,7.95,8.08 (m;Ar-H)
Example lC
Preparation of 6~ Ay.y~.~o-1~3-bPn7od1nYanP
3û n=0; Rl-R2-R3-H)
6-Nitroso-1,3-bPn7odi~Y~nP (1.0 g, 6.1 mmol) and
N-bromosuccinimide (1.1 g, 6.2 mmol) were dissolved in dimethyl-
formamide (20 ml). MnnncoAi cyanamide (0.61 g, 9.5 mmol) was
- added. The mixture was stirred for 1 hour at ambient I , ~Lu.
and poured on ice-water (80 ml), filtered, washed with water and
CA21 1 7503
dried to give 0.9 g (71.9% yield) 6-azoxycyano-1,3-benzodioxane as
a yellow powder, m.pt. 156-158~C.
Example 2
Preparation of 6-azoxycyano-8-chloro-l~3-bpn7oaioypnp
(n-l; R-8-Cl; Rl=R2=R3=H)
6-Nitroso-8-chloro-1,3-benzodioxane (3.4g, 17.0 mmol) was dissolved
in dichloromethane (75 ml)., Cyanamide (0.67 g, 16.0 mml) and
io~ohPn7PnP diacetate (6.2 g, 19.5 mmol) were then added. The
mixture was stirred for 1 hour at 0~C and 1 hour at ambient
temperature and the resulting yellow suspension was then filtered
and evaporated to dryness. Column chromatography on silica using
toluene as eluant gave 2.7 g (66% yield)
6-azoxycyano-8-chloro-1,3-benzodioxane as a yellow solid,
m.pt : 158-160~C, m/e (M ) : 239/241
H-NMR (CDC13) : ~ (ppm) = 4.91 (s;ArCH20); 5.40 (s;OCH2O);
7.85, 8.20 (m;Ar-H)
Example 3
Preparation of 6-azoxycyano-8-methoxy-1~3-hPn7nAinYAnP
(n=l;R=8 0CH~, RleR2_R3=H)
6-Nitroso-8-methoxy-1,3-benzodioxane (2.3 g, 11.8 mmol) was
dissolved in dichloromethane (70 ml). Cyanamide (0.5 g, 11.9 mmol)
and dibromoisocyanuric acid (1,3-dibromo-1,3,5-triazin-
2,4,6(1H,3H,5H)-trione; 3.4 g, 11.8 mmol) were then added at 0~C
under nitrogen. The mixture was stirred for 2 hours at ambient
- -rAt..re and the resulting suspension then filtered and
evaporated to dryness. Column chromatography on silica using 2:1
petroleum ether : ethyl acetate as eluant gave 0.8 g (29% yield)
6~ y~ lo-8-methoxy-1,3-bPn7o~ioYAnp as a yellow solid, m.pt :
188-190~C, m/e (M ) :235.
H-NMR (CDC13): S (ppm) - 3.95 (s;OCH3); 4.93 (s;Ar-CH2O);
5.25 (s;OCH20); 7.62 (m;Ar-H)
Examples 4 to 9
By process similar to those described in Examples 1 to 3 above,
- further compounds according to the invention were prepared as
detailed in Table I below. In this table the compounds are
C~ 2 i 1 7503
- 14 -
identified by reference to formula I. Melting point, mass
spectroscopy and H-NMR data for the compounds of Examples 4 to 9
are given in Table lA below.
TABLE I
s
Ex n R Rl R2 R3Position of
No. -N(O)=N-CN group
4 l 8-OCH3 H H H S
0 - -CC13 -CC13 H 6
6 0 - H H H S
7 0 - H H H 7
8 l 8-CH2OH H H H 6
9 1 8-Br H H H 6
TABLE IA
Example M.pt. m/e lH-NMR(CDC13) :~ (ppm)
No. (~C) (M )
4 191 3.99 (s;OCH3); 5.12 (s;ArCH2O); 5.33
(s;OCH2O); 6.85, 7.99 (m;Ar-H)
155 437-449 5.38 (s;ArCHO); 5.72 (s;OCHO); 7.30,
8.32, 8.77 (m;Ar-H)
6 90-93 205 5.10 (s;ArCH2O), 5.25 (s;OCH2O);
7.34, 7.73 (m;Ar-H)
7 145-147 205 4.92 (s;ArCH2O); 5.30 (s;OCH2O);
7.12, 7.73 (m; Ar-H)
-
CA21 1 7503
- 15 -
TABLE IA (continued)
Example M.pt, m/e lH-NMR(cDcl3) : ~ (ppm)
No. (~C) (M )
s
8 235 2.50 (t;OH); 4.72 (d;ArCH2OH); 4.96
(s;ArCH20); 5.35 (OCH20); 7.90, 8.22
(m;Ar-H)
9 150-152 283/285 4.92 (s;ArCH20); 5.43 (s;OCH2O);
7.90, 8.37 (m;Ar-H)
Example 10
The fungicidal activity of compounds of the invention was
investigated by menas of the following eests.
(a) Direct protectant activity against tomato late blight
(Phytophthora infestans; PIP)
The test is a direct protectant one using a foliar spray. The
upper leaf surfaces of tomato plants with two expanded leaves (cv.
First in the field) are sprayed with a solution of active material
in 1:1 water/acetone c~tAinine 0.04~ "TWEEN 20" (Trade Mark; a
polyoxyethylene sorbitan ester surfactant). Plants are treated
using an automated sprayline with an atomising nozzle. The
concentration of the compound is 1000 ppm, and the spray volume is
700 l/ha. After a y~hcc~ ..t period of 24 hours under normal
glAcchn..ce conditions, the upper surfaces of the leaves are
;nACI~1At~d by spraying with an aqueous suspension ~ontAin1ne 2 x
105 zoospores/ml. The inoculated plants are kept for 24 hours in a
high humidity cabinet and 5 days under growth chamber conditions.
The ACC~C is based on the percentage of diseased leaf area
compared with that on control leaves.
(b) Direct protectant activity against broad bean ~rey mould
- - (Botrytis cinerea; BCB)
The test is a direct protectant one using a foliar spray. The
CA21 1 7503
- 16 -
upper surfaces of leaves of broad bean plants (cv The Sutton) are
sprayed with the test compound at a dosage of 1000 ppm using a
Antl t~ sprayline as described under (a). 24 hours after
spraying the leaves are inoculated with an aqueous suspension
conrAinine lO conidia/ml. For 4 days after inoculation plants are
kept moist in a humidity cabinet at 21~C. Disease is assessed 4
days after in~cnlAtion, based on the percentage of leaf surface
area convered by lesions.
(c) Activity against wheat leafspot (Leptosphaeria nodorum;
LN.)
The test is a direct therapeutic one, using a foliar spray.
Leaves of wheat plants (cv Norman), at the single leaf stage, are
inoculated by spraying with an aqueous suspension ~ntAinine l x
106 spores/ml. The inoculated plants are kept for 24 hours in a
high humidity compartment prior to treatment. The plants are
sprayed with a solution of the test compound at a dosage of lO00
ppm using an automated sprayline as described under (a). After
drying, the plants are kept for 6-8 days at 22~C and moderate
humidity, followed by Ae5~ . Aec~c ~ is based on the
density of lesions per leaf compared with that on leaves of control
plants.
(d) Activity a~ainst barley powdery mildew (Erysiphe graminis
f.sp. hordei; EG)
The test is a direct therapeutic one, using a foliar spray.
Leaves of barley seedlings, (cv. Golden Promise) are inoculated by
dusting with mildew conidia one day prior to treatment with the
test compound. The in~cnlAt~d plants are kept overnight at
elAeeh~lee ambient ; .-rAt~.re and humidity prior to treatment.
The plants are sprayed with the test compound at a dosage of 1000
ppm using an ~ ~ t~d sprayline as described under (a). After
drying, plants are returned to a compartment at 20-25~C and
moderate humidity for up to 7 days, followed by A-- -
Aee~c ~ is based on the percentage of leaf area covered by
- sporulation compared with that on leaves of control plants.
CA21 1 7503
- 17 -
(e) Activity a~ainst tomato early blight (Alternaria solani; AS)
This test measures the contact prophylactic activity of test
compounds applied as a foliar spray. Tomato seedlings (cv Outdoor
Girl) are grown to the stage at which the second true leaf is
expanded, The plants are treated using an automated sprayline as
described under (a), Test compounds are applied as solutions or
suspensions in a mixture of acetone and water (50:50 v/v)
cont~inine 0.04% surfactant ("T~EEN 20" - Trade Mark), One day
after treatment the seedlings are innrul~t~d by spraying the leaf
upper surfaces with a suspension of A, solani conidia containing
104 spores/ml, For 4 days after inoculation plants are kept moist
in a humidity compartment at 21~C. Disease is assessed 4 days
after inoculation, based on the percentage of leaf surface area
covered by lesions.
(f) Activity a~ainst wheat eyespot in-vitro
(Pseudocercosporella herpotrichoides; PHI)
This test measures the in vitro activity of compounds against the
fungus causing wheat eyespot. The test compound is dissovled or
5~lqp~n~d in acetone and is added into 4 ml aliquots of half
strength Potato Dextrose Broth dispensed in 25-compartment petri
dishes to give a final concentration of 50 ppm compound and 2.5~
acetone. Each compartment is inncnl~te~ with a 6 mm diameter plug
of agar/mycelium taken from a 14 day old culture of P.
herpotrichoides, Plates are incubated at 20~C for 12 days until
the ~cc~- t of mycelial growth,
(g) Activity a~ainst Fusarium in-vitro (Fusarium culmorum; FSI)
This test measures the in vitro activity of compounds against a
species of Fusarium that causes stem and root rots. The test
compound is dissolved or suspended in acetone and added to molten
half strength Potato Dextrose Agar to give a final r.."~ rn of
50 ppm compound and 3.5~ acetone, After the agar has set, plates
are innc..l~trd with 6 mm diameter plugs of agar and mycelium taken
from a 7 day old culture of Fusarium sp,, Plates are inrl~h~ted at
- 20~C for 5 days and radial growth from the plug is measured.
CA21 1 7503
- 18 -
The extent of disease control in all the above tests is expressed
as a rating compared with either an untreated control or a
diluent-sprayed-control, according to the criteria:-
0 - less than 50% disease control
1 - about 50-80~ disease control
2 - greater than 80~ disease control
The results of these tests are set out in Table II below:-
TABLE II
Example Fungicidal Activity
No. PIP ~CB LN EG AS PhI FSI
1 2 2 1 1 2 2
4 2 2
Example 11
The fungicidal activity of compounds of the invention was further
investigated by means of the following tests.
(a) Antisporulant activity aRainst vine downy mildew
(Plasmopara viticola; PVA)
The test is a direct antisporulant one using a foliar spray. The
lower surface of leaves of vine plants (cv. Cabernet Sauvignon),
approximately 8cm high, are inrcl~lAtpd with an aqueous suspension
rrntAining 5 x 104 zoosporangia/ml. The inoculated plants are kept
for 24 hours at 21~C in a high humidity cabinet, then for 24 hours
in a glAcchr~lce at 20~C and 40~ relative humidity. Infected leaves
are- sprayed on their lower surfaces with a solution of the test
compound in 1:1 water/acetone contAinine 0.04~ "TWEEN 20~ (Trade
Mark; a polyoxyethylene sorbitan ester surfactant). Plants are
sprayed using a track sprayer equipped with 2 air- ' ~.cine
- no~zles. The co~r~ntrAtirn of the compound is 600 ppm and the
spray volume is 750 l/ha. After drying, the plants are returned to
CA21 1 7503
~ - 19 -
the glasshouse at 20~C and 40% relative humidity cabinet for 24
hours to induce sporulation. Assessment is based on the percentage
of the leaf area covered by sporulation compared with that on
control leaves.
(b) Direct protectant activity apainst tomato late blipht
(Phytophthora infestans; PIP)
The test is a direct protectant one using a foliar spray. Tomato
plants with two expanded leaves (cv. First in the Field) are
sprayed with the test compound at a dosage of 600 ppm as described
under (a). After drying, the plants are kept for 24 hours in a
elAc~h~qe at 20'C and 40% relative humidity. The upper surfaces
of the leaves are then inoculated with an aqueous suspension
containing 2 x 10 zoosporangia/ml. The in~c~lat~d plants are kept
for 24 hours at 18~C in a high humidity cabinet and then for 5 days
in a growth chamber at 15~C and 80% relative humidity with 14 hours
light/day, The acc~ is based on the percentage of diseased
leaf area compared with that on control leaves.
(c) Activity apainst tomato early blipht (Alternaria solani;
AS)
The test is a direct prophylactic one using a foliar spray.
Tomato seedlings (cv Outdoor Girl), at the stage at which the
second leaf is expanded, are sprayed with the test compound at a
dosage of 600 ppm as described under (a). After drying, the plants
are kept for 24 hours in a glasshouse at 20~C and 40% relative
humidity followed by inoculation of the leaf upper surfaces with an
aqueous suspension of A. solani conidia c~nt~ininp 1 x 10
conidia/ml. After 4 days in a high humidity cabinet at 21~C,
disease is assessed based on the percentage of leaf surface area
covered by lesions when compared with control plants.
(d)- Direct protectant activity against broad bean prey mould
(Botrytis cinerea; BCB)
The test is a direct protectant one using a foliar spray. Broad
bean plants (cv The Sutton) with two leaf pairs are sprayed with
- the test compound at a dosage of 600 ppm as described under (a).
After drying, the plants are kept for 24 hours in a gl~cch~--c~ at
CA21 1 7503
- 20 -
20~C and 40% relative humidity. The upper surfaces of the leaves
are then inoculated with an aqueous suspension containing 1 x 10
conidia/ml. Plants are kept for 4 days at 22~C in a high humidity
cabinet. The assessment is based on the percentage of diseased
S leaf area compared with chat on control leaves.
(e) Activity against wheat leafspot (Leptosphaeria nodorum; LN)
The test is a direct LII~Ld~ iC one using a foliar spray. ~heat
seedlings (cv Norman), at the single leaf stage, are inoculated
with an aqueous suspension cnntaining 1.5 x 10 conidia/ml. The
inoculated plants are kept for 24 hours at 20~C in a high humidity
cabinet followed by spraying with the test compound at a dosage of
600 ppm as described under (a). After drying, the plants are kept
for 6-8 days in a gl~chn~c~ at 22~C and 70% relative humidity.
Acs~s t is based on the density of lesions per leaf compared
with that on leaves of control plants.
(f) Activity a~ainst wheat eyespot in-vitro
(Pseudocercosporella herpotrichoides; PHI)
This test measures the in-vitro activity of compounds against the
fungus causing wheat eyespot. The test compound is dissolved or
Z0 suspended in acetone and is added into 4 ml aliquots of half
strength Potato Dextrose Broth dispensed in 25-compartment petri
dishes to give a final c~nce,~LLaLion of 30 ppm test compound and
0.825% acetone. The fungal inoculum consists of mycelial fragments
of P. herpotrichoides grown in half strength Potato Dextrose Broth
in shaken flasks and added to the broth to provide S x 10 mycelial
fragments/ml broth. Petri dishes are incubated at 20~C for 10 days
until the ~cs~c t of mycelial growth.
(h) Activity against apple scab in-vitro ~Venturia inaequalis;
VII)
This test measures the in-vitro activity of compounds against
Venturia inaequalis that causes apple scab. The test compound is
dissolved or s~cp~nAed in acetone and added into 4 ml aliquots of
half strength Potato Dextrose Broth dispensed in 25-compartment
petri dishes to give a final concentration of 30 ppm compound and
0.825% acetone. The fungal inoculum consists of mycelial fragments
CA2i 17503
- 21 -
and spores of V. in~q~-~l i.s grown on malt agar and added to the
broth to provide 5 x 10 propagules/ml broth. Petri dishes are
incubated at 20~C for 10 days until the ~cq~c~ ~. of mycelial
growth.
The extent of disease control in all the above tests is expressed
as a rating compared with either an untreated control or a
diluent-sprayed-control, according to the criteria:-
O e less than 50~ disease control
1 e 50~ 80~ disease control
2 - greater than 80~ disease control
The results of these tests are set out in Table III below:-
TABLE III
Example Fungicidal Activity
No. PVA PIP AS BCB LN PHI RSI VII
2 2 2 1 2 2 2
3 2 2 1 2 1 2
6 1 l 2 2
7 2 2 2 1 2 2
8 2 2 2 2
9 l l l 2 2 2
* signifies concentration of test compound e 10 ppm