Note: Descriptions are shown in the official language in which they were submitted.
W093/216~ 2117 ~ 0 5 PCT/FIg3/001~
M~HD~ ~Yn~Ro~3~DD~H~G~EE~C~.
The object of the invention is a method for storing and
producing electrical energy in the type of electrochemical
cel1, where the cathode is a porous air electrode which takes
oxygen from the ambient air, or to which oxygen is supplied
by other mean~, and the anode is a hydrogen-containing ~etal
hydride, and according to which method the hydrogen stored in
; the metal hydride anode and acting as fuel is oxidized
: lO through an electrolytic solution with oxygen supplied to the
air electrode.
The prior art comprises fuel cells in which hydrogen yas is
s~pplied to a negative electrode, that is, anode, and oxygen
: which is either pure or contained in air, is supplied to a
positive electrode, that is, cathode. In the cell's over~ll
reaction, the only reaction products formed in addition to
e1ectrical energy are water and some heat. The advantage of
fuel cells is good efficiency and unpolluted reaction
products. Their use is9 however, limited for example in
2~ traffic appli~ations, by the handling and storability of
gaseous hydrogen. ~ :
The~prior~art also comprises so-caIled metal air cells,
having a~metal such as zinc, i~on or al~minium as the anode,
;and an air electrode~as the cathode. In these cells, the
metal becomes~oxidized and dissolves in the electrolyte. The
advantage of the cells is a high energy density, and the
di~adYanta~es are e.g. self-dircchargi~g, that is~ corrosion
~f the metal when at rest, and the gradual pas~ivation of the
surface of:the metal. Prior art is represented by e.g. the
; ~ 30 following patents and patent applications: Sweden no. 358772
(HOlM 4/86), WO 88J02931 (HOlM Bjl8), Norway no. 883432
lM 8/l~), Norway no. 156469 ~HOlM 8/12~ and European
patent no. 0124275 (HOlM 8/18).
The prior art is further represented by porous, gas-permeable
2~7~03
W093/21~ PCT/Fl93tO0154
air electrodes, or oxygen electrodes. The disadvantage of
th~se solutions is, however, that the pressure of the
electrolytic solution with respect to the ambient air cannot
be significantly increased due to leaks. It is known to
situat~ the catalyst layer on the surface against the
electrolyte~ The known materials used in air electrod~s
include highly electroconductive carbon, for example r SO~
called carbon black, Teflon as binder, and platinum, silver
or different compounds such as cobolt tetraporphyrine as the
catalyst, The prior art appears, for example, from the
patents and patent applications DE~3332625 A1 (HOlM 4/86~,
DE-34000Z2 A1 (HOlM 4/86), DE-3632701 Al (HOlM 4/86),
~E-372201g A1 (HOlM 4~86), DT-2547491 (HOlM 4/86), DT-2556731
A1 (~OlM 4/86), DE-3331699 A1 (HOlM 4f86), US 4,877,694
(HOlM 4/86), Norway no. 802635 (HOlM 4/86), US-4,927,71
(H01M 4/86), and Sweden no. 324819 ~HOlM 4/86~. These
: electrodes are sometimes called hybride electrodes
especially in connection with metal air cells - which is
not the same as hydride electrodes, which are explained in
greater detail below.- The prior art of the air electrodes
called hybride electrodes appears e.g. from the publications
DE-2658520 Al (HOlM 4/86), DT-2611291 Al (HOlM 4/86~, and
~; DT-2455431 Al (HOlN 4/86).
It is also known to store hydrogen in different metals, metal
alloysr and other compounds, which are collectively called
hydrides. Hundreds of compounds which are able to absorb
large amounts of hydrogen into themselves are known. With the
different compounds, hydrogen absorption amounts of di~ferent
magnitudes are achieved, and their equi~ibrium pressures
- that is, the partial pressure of hydrogen in gas - depend
on the composition of the hydride and on the temperature.
Depending on the compound, the hydrogen a~sorption amounts of
: . hydrides are ~ypically of the order of 1-10 % by weight, and
the equilibrium pressures at room temperature are of the
order of 0.1-10 bar. ~or example the LaNi5H6 hydride and its
different modifications such as LaNi25Co24AloI and MmNi3sCoO7Alo~
have been much studied. The capacity of the hydrides and the
W~93/21~ 2 1 1 7 g O S PCT/~93~01~
prior art appear, for example, from the patents and patent
application~ DT-2003749 ~C01B 6/00), US-4,721,697
(COlB 6/00~, US-4,629,720 (C01B 6/00~, US-4,656,023
(~OlB 6/00), US-4,661,415 (COlB 6/00), US-4,567,032
~C~lB 6/00), US-4,556,551 (C01 B6/00), and Sweden no. 456248
(C01B 6/00)~ The u e of hydrogen-storing materials, that is,
hydride~ as electrodes in batteries is prior art. For
example, Philips have studied and developed a so-called
nickel hydride battery in which hydrogen is stored in a
negative hydride electrode, that is, anode (MHX), with a
nickel electrode as the cathode. The overall reaction of the
discharge is
: ~
MHX + x NiOOH =~ M + x Ni(OH)2
:in other words, the hydrogen transfers from the anode to the
aa~hode. In charying,~the reaction equatiQn is inverse. These
studi~s have been published e.g. in the doctoral thesis:
J.J~G. Wille~s: "~etal hydride electrodes' stability of LaNis-
related compounds", Philips J. of Research, Vol. 39, Suppl.
o. 1 ~1984), and~the composition of the metal hydride has a
patent: US Patent No. 4,487,817 (Dec.~11, 1984). ~at~r prior
ar~ ~of hydride electrodes is represented by patent
: application no. WO 91108167 (COlB 6/00). Using hydride
le~ des~to produce hydrogen directly by means of sunlight
:has also been studied and is known e.g. from the pu~ ation
DE--3~04171 A1 ~Co1B:6/00).
: : : : :
Also of the prior ar~ are fuel cells, the fuel of which,
~' ~ hydrogen~gas, i5 stored in a sep2rate metal hydride container
: ~ fr~ which hydrogen gas can be r~_eased when required and
:~:
suppli~d further to the fuel cell. The hydrogen gas is
~: 30 releas~d from the metal hydride ~y heating the container. In
the United States a fuel cell of this type and a metal
hydride container have been constructed to provide the energy
source for an electric car (~otor Re~iew 1992). As advantages
are seen par~icularly the unpolluting nat~re o~ the fuel cell
because only water vapour is produced as a reac~ion product,
2 1 1 7 9 0 r;~
W093/21~ PCT/Fl93/00154
and the safety of storing hydrogen in metal hydride, which
m2ans that there is no danger of explo~ion. However, a small
dangsr of ~xplosion is created by the hydrogen gas released
from the container and supplied to the fuel cell.
It is also known to store hydrogen in a metal hydride
electrode ac~ing as the anode of an electrochemical cell, and
that the hydrogen used as fuel is oxidized electrochemically,
directly in the metal hydride. An electrochemical cell is
thus used to store and produce electrical eneryy, the cell
~vi~g as the cathode an oxygen or air electrode, to which
pure ~xygen or oxygen contained in air is supplied ~o oxidize
:~ ~he hydrogen used as the fuel. This prior art is described,
for e~ple, in the patents US-3,520,728, US-4,609,599,
US-4,~61,425 and GB~ 76,260. None of these discuss the
problem of self-discharging of hydrogen.
The advantages ~o~rAred with the above-mentioned fuel cell,
: : into which hydrogen is introduced in gaseous form from a
m~al hydride container, are the following: the energy
den~ity is better, because a separate hydrogen electrode is
not required; the apparatus is safer, because hydrogen does
no~ appear in gas~ous form at any stage; the ~ystem for
: controlling:hydroge~ consumption is simpl~r be ause ~he
electri~ current applied determines the consumption nf
hydrogen direc~ly, through the oxidization reaction of
hydrogen; the apparatus is more efficient because one stage
of the process - releasing thP hydrogen from the metal
hydride by heating is eliminated.
The advantag~ousness is based on the fact that the storage
matPrial acts at the same time as an electrode and the oxygen
: : 30 needed for oxidizing the hydrogen is taken di~ectly from the
- ambient air~ Taking oxygen from the air makes possible a high
energy density as it does not have to be stored in the cell
and carried with the cell. In laboratory tests the energy
density has been measured at about 240 Wh/kg, when the weight
of the metal hydride LaNi5 is taken into account. In practice
WO93/21~ 2 1 1 7 g O ~ PCT/~3/~02~
.
this corresponds to an energy density of about 120 Wh/kg,
when the total weight of the cell is taken into account.
The problem with the above-described electroch~mical cell is,
however, its self-discharging, that is, the gradual release
of hydrogen from the hydride, especially during charging.
From the point of view of functioning it is important to
elir;n~te the formation of hydrogen bubbles on the surface of
the metal hydride, as hydrogen tends to escape as bubbles.
One such known cell solution which discusses the self-
disch~xging problem is described in the US patent 3,511,710.
In it, the cathode is an air or oxygen electrode r and
hydrog2n - which is the fuel - is bonded to the hydride, and
the solution also aims a~ reducing the release of hydrogen in
gaseous form, that i5, self-discharg.ing, by using a so-
~ ; 15 called auxiliary voltage in the hydro~en electrode, which is
: ~ preferably at least 40 mV, with respect to the NHE electrode.
US patent no. 4,107;,405 presents a solution in which the aims to reduce the release of hydrogen as a gas, that is self-
: di~harging, by coating the metal hydride with a thin metal
film, or wit~ ions ad~or~ing to the surface of the metalhydride and thus preventing the desorption of hydrogen.
:::
The aim of th~ presènt invention is to achievs a ~impler and
more efficient:~method for eliminating the sel~-discharging of
hydrogen in a metal hydride/air electrode cell. It is
characteristic of the method according to the invention that
to store and produce electrical energy, the pressure of the
electrolytic solution situated inside the porous air
electrode and in contact with the metal hydride electrode, is
ihcreased so that it is higher than the pressure of the
ambient air outside the porous air electrode.
When the pressure of the cell's electrolyte is gradually
increased, it can be seen that hydr-ogen bubbles are first
formed on the hydride electrode. However, this stops when the
WO93/21~ 2117 ~ O j PCT/F193~00154
.
pressure of the electrolyte rises higher than the discharging
pres~ure of ~he hydrogen bonded in the metal hydride. After
this, the release of hydrogen takes place only as molecular
diffu~ion, which is extremely slow compared to discharging in
the form of bubbles. According to the invention, the self-
discharging of the eell is thus prevented.
.
In the solution according to the invention, a gas space is
u~ed which allows for surface changes of the electrolytic
solution as the amount of water changes. During charging,
lO ~xygen gas formed accumulates to the gas space, the pressure
of which settles at a level of at least the ambient air
~ ~ ~ressure~ in order that it could flow out of the cell during
: charging. When the cell is in the state of equilibrium, there
is hydrogen and oxygen in the gas space, the partial pressure
l5 of the hydrogen being equivalent to the equilibrium pressure
of the metal hydride.
.
For example, with a LaNi5 metal hydride electrode the
: equilibrium pressure of hydrogen is 4 bar at a temperature of
40~C~ and ~orre po~;ngly 2 bar at a temperature of 20~C.
20~ ince there is oxygen~gas in the cell, the absolute pressure
of~which is at least 1 bar, the safety val~e operating during
charging:mu~t be designed to open only at pressures exceeding
5 b~rf t~at is, at an overpr ssure of 4 bar, if the apparatus
is designed to func~ion up to the temperature of 40~C.
25 Correspondi~g1y, if the apparatus is designed to function up
n ~ to the temperature of 20~C, the saf ty valve must be designed
to open at pressures exc~ding 3 bar, that is, at an
~' overpr~ssure of 2 bar, to prevent the release of hydrogen in
the form of a gas. Even if the equilibrium pressure of
hydrogen with the metal hydride electrode was below l bar,
: for e~A~le with an LaNi3Co2 electrode, overpressure will
- still be formed in the cell due to the oxygen gas which is
already there.
The pressure of formation of a hydrogen bubble is not only
dependent on the equilibrium pressure, but also on the
WO 93/2166~ 2 1 17 ~! O ;3 P~/F1~3/00154
.
magnitude of the charging current. The higher the charging
current used, the higher the pressure of f ormation of the
hydrogen, which is always at least as high as the e~uilibrium
prgssure. Therefore, in practice, to prevent the formation of
S hydrogen bubbles, significantly higher pressures must be used
than required by the equilibrium pressure. In practice the
opening pressure of the cell ' s safety valve must be sought
experimentally to be such that hydrogen bubbles are not
formed during charging either.
Incre sing the pressure of the electrolyte in the cell with
respect to outside air is possible by means of surface
: :~ension and a su~ficiently small pore size without the
occurrence of leaks through the air electrode. If the radius
of the pore is r = lo-7 m, the surface pressure ~ = 60-10-3 N/m,
~nd the contact angle ~ = oo, the pressure dif~erence between
: the sclution and the ambient air, without that the liquid
.
leakes out of the pores into the ambient air, can be as big
as (Laplace equation~:
~p = 2 - ~ o cos~S/r = 2 - 60 -10 3 ~ l/ 10-7 Pa = 12 bar.
:~ 20 Even at high overpressures, the impermeability to liquid of
~ the air:electrode provided with very small pores~ acc,ording
:~ to the invention, is based on this surface tension
: phenomenon.
To impro~e the capacity of the cell, it is known, for ~mple
from the US-patent 4,609,599 to increase the pressure of the
; ~mbient air or oxygen gas. According to the invention the
cell is, however, pressuri~.ed inside to an overpr~ssure with
respect to the ambient air, the purpose o~ his being to
prevent self-discharging. The oxygen required in the positive
electrode is then obtained directly from the ambient air
which is under normal pressure according to the invention. In
the invention, the oxygen formed in chargin~ also flows out
~s a gas from the cell system.
W093/2l6~ 2 ~17 ~ O ~ PCT/Fl93/00154
The functioning of the method of storing electrical energy is
based on experiments carried out and on theoretical findings
according to which there is very little discharge of hydrogen
as molecular dif~usion without bubbles from the metal hydride
to the elertrolyte and through the air electrode. Similarly,
there is little direct oxidization of hydrogen due to the
hi~h diffusion resistance of the oxygen in the electrvlyte.
Theoretically this can be shown by calculating the rate of
discharge of hydrogen in a case where diffusion resistance is
the only resisting factor. The temperature is 25~C, the
~ thickness of the electrolyte layer is 2 mm, the partial
: pressure of~the hydrogen is 2.5 bar and the surface area of
.he air electrode is 30 cm2. Let us assume that the
electrolyte is saturated with respect to the dissolved
hydrogen on the surface of the metal hydride electrode, and
~ : a~ a distance of 2 mm the hydrogen concentration is zero,
:~ : ; because hydrogen is released through the air electrode. In
these con~itions the hydrogen concentration can be at most
2.1 mol/m3 (solubility = 7.72 710~ mol/(m3Pa)~ and the
diffusion coefficient 1,3 10-9 m2/s (source: Journal.of
Phy~ical Chemistry,~ Vol. 74, No. 8, l9iO, p. 1749~. In this
case the flow of hydrogen from the cell is
:
. (O 2.1)mol/m3
J = -1.3-10-9 m2/s ~ ~ 30-10-~ m2 = 4.1-10-9 mol/s.
0.002 m
hi~ further gives the corresponding amount of ampere-hours
per day, that is, the loss:
, , .
4.1~10-9 mol/s ~ 2~96500 As/mol ~ 24 h/day = 19 mAhlday.
In the cell discussed above there is about 20 g of metal
hydride, and in it 1.4~ by weight of hydrogen, and if no
hydrogen escapes from the cell, the cell gives. 7.44 Ah.
According to the above calculation, the discharge is thus
0.255% / day, and thus in 1.1 years, the cell would discharge
completely by the diffusion mechanism.
WO93/2166~ 2 1 1 7 ~ ~ S PCT/~193/~0}~
In the following is present~d a similar calculation for the
direct oxidization of hydrogen, that is, the diffusion.of
oxygen from the air electrode to the metal hydride electrode
is assessed i~ the same conditions as above. The partial
pressure of the oxygen is 0.21 bar~ The solution is saturated
at the air electrode and the oxygen concentration on the
surface of the metal hydride electrode is zero, because
oxygen is ~ssumed to r~act immediately with hydrogen. In the
said conditions the maximum concentration of oxygen is 0.29
; 10 mol/m3 (solubility -- 12.47-104 mol/(m3 Pa)) and the diffusion
coefficient of the oxygen is 0.4-10-9 m2/s tsource: Journal of
: Physical Chemistry, Vol. 74, NoO 8, 1970, p~ 1749). In this
casa the flow:of oxygen through the electrolyte to the metal
: hydride electrode;is~
(0-0.29)mol/m3
: J: = -0.4-10-9 m2/s - ~ 30-10~4 m2 e 0.174~10-9 mol/s.
0.002 m
The amount of electric~ity lost through dire:ct oxidization per
: day i~s 'chen
20~:0.174-10~9:mo1Js~ 4~-~96500 A5/mol ~ 24~ h/day = l.~l mAh/24h.
With;t~is:m~c-h~;sm, ~he discharging of a cell conta~ning 20
g:of~ metal hydr~ide would~take 12.7 years.
:m ese two mechanisms,~ the diffusion of hydrogen and direct
:oxidization, take~place simultaneously, and thus~as a sum of
the above, the discha:rge is 20.6 mAh/day, which means that
the total discharging of the example cell would take
- approximately one year. The applicant has studied self-
dis~harging in;la~oratory tests, the results of which support
:: ~
~ the above calculations.
~: ::: :
:~:: 30 The pressure inside the cell is created in two different
ways. When there is an auxiliary electrode in the cell, the
oxygen gas forming in it during charging creates pressure
inside the cell, the magnitude of which is regula~ed by means
WO93/2l~ PCTJFl93/00154
of the opening pressure o~ the overpressure valve. During the
discharging of the cell, additional water is formed in the
process thus increa~ing the amount of eiec~rolytic solution
and red~cing the cell's gas space, and therefore increasing
the pressure of the gas. If the air ele~trode operates in two
dire~tions, that is, it is used for both charging and
discharging, then the cell must be pressurized by increasing
pressure from the out~ide.
: Becaus~ the syste~ according to the invention is a closed
~10 one, particular attention must be paid to its cooling.
Cooling can be realized advantageously by circulating the
electrolytic solution. Circulation can take place, for
~: ~x~rle, so that the electrolytic liquid is led to the
: vicinity of the auxiliary electrode, or through the metal
hydride. The resistance loss of ions in the solution can also
be reduced through circu~ation.
Th~ drl~ing foxce of the circulation can be achieved in
different ways. When:using an auxiliary electrode the oxygen
bubbles for~ed during charging create a circulatory motion.
By~cooling~the solution outside the cell, natural circulation
hA$e~ on;the differences of density in the liquid is
achieved:. The~reaction
2 + 2H2O ~ 4e~ =~ 4~
. ~;: ~ : :
taking place in the air electrode~during discharging acts as
a kind of "oxygen pump" which creates circulation.
Circulation may take place through the hydride electrode,
redu~ing the voltage losses occurring in it and at the same
: time cooling it. Cooling may also take place in connection
.
~;~ with an air electrode by providing air flow around the
~:; 30 electrode.
Since the air electrode is porous, carbon dioxide from the
ambient air can diffuse through it. If this disadvantageous
: phenomenon is not prevented, it will cause carbonate
~1~7~0S
. W093/216~ PCT/~93tO0154
formation in ~he electrolytic ~olution and the pores of the
air electrode, thus blocking it. Ts prevent this, the system
is proYided with a carbon dioxide filter through which the
air required by the cell is tak~n. To lengthen the interval
S for changing the filter, the filter is kept exposed to
outside air only when the electric charge of the battery is
discharged.
;~
~: According to the invention, the pressure of the electrolytic
: solution is increased~so that it is higher than the pressure
: lO of the ambient air outside the porous air electrode by
allowing the pressura of the electrolytic solution to rise as
high as desired during~charging. The pressure in the
electrolytic solution is made such as to prevent the
formatlon of hydrogen bubbles on the surface of the metal
:hydride~electrode.
Maintaining ~he overpr~ssure and keeping the electrolytic
~olution inside the air alectrode are effected by allowing
the el~ctrolytic iolution to penetrate into the pores of ~he
ir e1ectrode,~:thus:preventing the escaping of the
electroly~ic~solution out through the air electrode, through
the surface t~nsion~o~ the electrolytic solution forming on
the~pores of the air~eIectrode. ~ .
he metal hydride used in the electrochemical cell is re-
: : hydrogenated in ~he same cell electro~hemically either by
2~5 means of an air electrode or by using a separate auxiliary
:
electrode. The oxygen ~as forming on the separate auxiliary
electrode during charging is allowed tc discharge from the
cell system into~the ambient air~once the overpressure of the
electrolytic solution has become as high as desired.
In the cell, ~he electrolytic liquid can be led through the
metal hydride andlor to the vicinity of the auxiliary
electrode used in charging. In this case the circulatory
motion of the electrolytic liquid in the cell is achieved
during charying by leading the oxygen bubbles forming on the
~ 1 1 7 ~ O ~
W093/21~ PCT/~
auxiliary electrode to the upper part of the cell, thus
creating a circulatory motion when rising up, and/or by
cooling the liquid outside the cell, thus achieving natural
circulation based on the differences of density in the
solution.
~he circul tory motion of the electrolytic liquid in the cell
i8 created during discharging by allowing the OH- ions forming
on tha~ surface of the air electrode against the electrolyte
to ~ject ~he electrolyte supplied to the lower part of the
cell through the hydride electrode and/or by cooling the
solutio~ outside the cell, thus achieving natural circulation
hased on the differences of density in the solution.
The cell or battery is cooled by blowing air around the air
~: electrode. The air coming to the air electrode during
~: 15 discharging is led through the carbon dioxide filter which is
: kep- exposed to outside air only when:the electric charge of
. the battery is discharged.
The object of ~he invention also comprises a new type of
apparatus; for storing and producing elec:trical energy in an
20 ele~;L~o~llemical e::ell with ~one of the disad~antages of known
oells. The cathode:in the apparatus is a porous àir electrode
:which:takes oxygen from the ambient air, or to which oxygen
is supplied by other means, and the anode is a hydrogen-
;~; cont~ining~metal hydride, and in whlch apparatus the hydrogen
25 stored in the~ metal hydride anode and acting as fuel isoxidized ~hrough ~an electrolytic solution with oxygen
~, supplied ~to the air electrode.
::: ' :
~ is characteristic of the invention that the porous air
:: electrode is made to form a container which ran be closed and
withstands overpressure. The formation of hydrvgen bubbles,
which are disadvantageous for the functioning of the cell,
can be eliminated by pressurizing the cell to a pressure
higher than the pressure of formation of the hydrogen
bubbles. The functioning of the apparatus according to the
W093/21664 ~1 1 7~ ~ 5 PCT/~93/00154
.
invention, when using overpressure, is based on the surface
ten~ion phen~menon.
The porous air electrode is made to withstand overpressure by
making îts pores so small that the surface tension of the
electroly~ic solu~ion penetrat~ng into the pores keeps the
overpressure and electrolytic solution inside the air
electrode. The radius of the air electrode pores is for the
: most part smaller than 0. 0001 mm. The air electrode acting as
the cathode i cylindrical.
In connection with the closed air electrode an overpressure
: ~al~e is formed, which - when the metal hydride electrode is
hydrogenated electroche~ically after use - allows the oxygen
gas forming during charging to flow out of the fuel cell
system, while the overpressure still remains on the desired
~evel. A separate auxiliary elec~rode may be located inside
,:
the~air electrode so that after use, the metal hydride
electroda may be ele~trochemically hydrogenated in the same
cell by means of tha auxiliary electrode.
To create circula ion in the electrolytic liquid, a duct is
, ~,
formed in the cell through which the electroly~ic liquid can
: be led through the metal hydride and/or to the vicinity of
he ~uxiliary elec~rode used in charging. Alternatively, a
: : duct m~y be incorporated outside the cell, through which ~uct
he electrolytic liquid can be led through the metal hydride
2:5 and/or to the~ vicinity of the auxiliary electrode usad in
:~ charging.
. ~ ~
To ensure cooling, a cooler for the electrolytic li~uid, such
,
as a heat exchanger is incorporated outside the cell, in
connection with the duct. A fan may also be located in
connection with the cell or batteryn To prevent harmful
carbon dioxide from entering the cell, a carbon dioxide
filter is located in:connection with the battery, through
which filter the air coming to the air electrode is supplied.
To lengthen the service life of the carbon dioxide filter, a
WO 93/21~ 21~ 7 3 o 5 PCT/~93/0015~
: ' .
14
protective flap is formed in connection with it to open the
connection to the outside air only when the electric charge
oP ~he battery is discharged.
The method and ~pparatus according to the invention are not
tied to any particular system or application. The invention
is d~scribed in detail, wi~h examples, in the following, with
reference to the appended drawings.
Figure 1 shows a cross section of one embodiment of the cell
according to the invention with an auxiliary
electrode for charging.
Figure 2 corresponds to figure 1 and shows a second
embodiment of the invention without an auxiliary
electrode.
Figure 3 corresponds to figure 1 and shows a third
~ embodiment of the invention, provided with an
int~rnal electrolyte circulation.
Figur~ 4 ~orresponds to figure 3 and shows a fourth
: ~mhodiment of the invention~ provided with
different type of internal electrolyte circulation.
Figure S corresponds~to figure 3 and shows a fifth
embodiment of the invention, provided with an
: external electrolyte circulati'on.
Figure:6 ~corresponds to figure 5 and shows a sixth
: embodiment of the învention, provided with
: different type of external electrolyte circulation.
~ igure 7 ~shows a system comprising seYeral
:~ electricity generating cells and a separate liquid
~, ~ co~tainer.
Figur~ 8 corresponds to figure 7 and shows a second
embodiment of the system.
Figure 9 shows a third embodiment of the system provided
: : with external electrolyte circulation.
Figure 10 shows a fourth embodiment of the system prvvided
with external electrolyte circulation and cooling~
~35 Fiyure 11 shows a fifth embodiment of the system without
:~external electrolyte circulation.
"
WO93/21~ 2 i 17 ~ ~ 5 PCT/~93/00154
Figure 12 shows a sixth embodiment of the system provided
with external electrolyte circulation, cooling, and
a carbon dioxide filter for incoming air.
Figure 13 shows a seventh embodiment of the ~ystem without
external electrolyte circulation, provided with air
cooling ~nd a carbon dioxide filter for in~oming
air.
~igure 14 shçws diagrammatically a discharge test of an
apparatus according to the invention, where the
voltage and overpr~ssure have been measured as a
function of time.
~igure 15 shows the diagram representing another discharge
test.
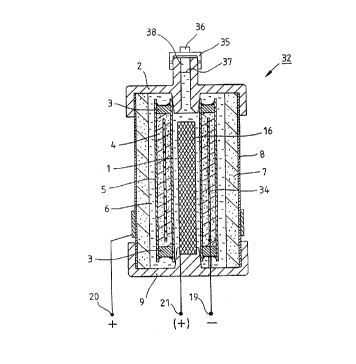
~igure l show~ the cell 32 intended for producing
electricity, the main parts of which are an air electrode 7,
a hy~c~yen containing me al hydride ele-trode 4 which is a
:metal hydride powder cartridge kept intact with binder, an
auxiliary electrode 16, and an electrolyte 6. In the example
solution r~lating to fi~ure 1, the cell 32 is cylindrical~
~Outer~ost on it~i~ a:::porous, electroconductive, cylindrical
sh ~o~y 8 which~:acts as th~ current collector of the air
ele~rode 7. The air~electrode 7, which consists of a
l.y~ hobic layer ~and~-a catalyst layer, is made on the inner
: sur~ce of the~cylindrical mesh body 8. The cataly~t layer of
the air~lectrode 7~is;against the electrolyte 6. The
:: ele~trolyte may be, for example, a concen~rated KOH aqueous
solu~ion.
~:, The hydrogen-containing metal hydride elec~rode 4 consists of
metal hydride powder which is located between ~pacer
; 30 membranes l and 5. Above and below the me~al hydride powder
there are pl~gs 3. A tubular current-collecting net 34 made
of mstal acts as the~current collector of the anode, or metal
~ : hydride electrode 4. The cell 32 is closed with a cover 2
:~ incorporating a safety valve 35 and a supply valve 36. The
lower part of the cell 32 is closed with a base 9.
WO 93/21~ 211 7 3 03 PCT/~93/00154
<;
16
The cell 32 of figure 1, like the other cells presented below
relating to other fig~res, can be discharged by setting a
load of particular magnitude between the ~-)pole 19 and the
(~)pole 20, the load being resistance in the exemplified
case. A load of 1 ohm is used in the discharge test described
below~ When the cell 32 discharges, the current passes from
the air electrode 7, which is in contact with the (+)pole 20
of the cell, thrsugh the external circuit to the metal
hydride lectrode 4 which is in contact with the ~ )pole 19
~;~ 10 of the cell. The overall reaction of the cell is
~2 + 4H(met~hydr.) -=> ~ H20, in other words the hydrogen
bonded to the metal hydride and the oxygen gas react to form
::
water in the electrolytic solution, thus raising the surface
: 37~ of~the e~ectrolytic liquid during the discharging of the
: 15 cell.
The o~erall reaction is the sum of the reactions taking ~lace
~::
in the differen~ electrode5. oxygen reacts in the air
ele~L~o~e~7 according to the reaction ~2 ~ 2 H20 ~ 4e ==> 40H-.
T~e oxygen gas c~ree~to the reaction area, tha~ is, to the
20:~:interPace~of the air~electrode material and the electrolyte
6~ from the air surrounding the;cell by di~fusing through the
porous~,~cylindrical mesh body 8 and the air electrode 7. In
the:o ~ gen reac~ion,~ electrons are used up which arrive at
the reaction area:from an external circuit, and OH- ions are
25 ~fo~med which pass~throug~ the electrolyte to the metal
hydride electrode 4 and react with the hydrogen released from
the ~etal in ac ordance with the reaction OH ~ H =-> H20 ~ e~.
~' The electrons released in the reaction on the surf ace of the
metal hydride electrode 4, that is, the me~al hydride powder,
are conveyed away from the el ctroconductive metal powder by
~m~ans of the current-collecting net 34, and through there to
; the external circuit, and fur*her towards the air electrode
7, to take part again in the reaction of oxygen. In the air
: electr~de 7, the passage of eIectrons to the reaction area is
; 35 made possible by the properties of the air electrode
material; in addition ts being hydrophobic, it is also
~,
::
WO93t216~ ~11 7 ~ ~ 5 PCT/~93/00154
., .
17
relatively electroconductive.
The oxygen gas formed in the process of charging flows
through the overpressure valve 35 into the ambient air. The
opening prPssure of the overpressure val~e 35 has been set to
b~ such that the desired overpressure is formed inside the
cell to prevent the formation of hydrogen bubblesO The
surface 37 of the electr~lytic liquid falls during charging
Qf the cell because in charging, water is dispersed
electrolytically and the oxygen gas formed flows out from the
cell. Correspondingly, during discharging of the cell, the
surface 37 of the electrolytic liquid rises as oxygen enters
the cell, reacting with hydrogen to form water. However, due
to voltage 105s, ~ome water is consumed during the
discharging and charging cycles, and the cell 32, therefore,
incorporates a supply va1ve 36 through which water can be
added to the cell 32 if required.
h Figure 2 shows a s~cond embodim nt of the cell 32 without an
auxiliary electrode. Provided with a suitable catalyst, the
~: air ele~rG~e 7 can also be used in charging. During charging
~ 20 by means of an external voltage source, the reaction in the
: air ~le~L~e 7 takes place, with respect to dischaxging, in
the inverse direction 40H- - > ~2 ~ 2H2O ~ 4e and
correspondingly in the metal hydride electrode 4; 4H2O + 4e~
40~~ + 4H(met.hydr~). The overall reaction in charging is
-': 25 ~H2O ==> 4H(met.hydr.) + ~2- The oxygen gas produced
:~ : discharges into the ambient air through the porous ~ir
electrode 7. The ove~pressure required to prevent the
formation o~ hydrogen bubbles is obtained by increasing the
pressure of the ~as space 38 of the cell 32 through the valve
36 before taking the cell 32 into use, and where required.
Figure 3 shows a cell 32 in which charging is reali2ed by
means of an auxiliary electrode. T~P apparatus differs from
- the apparatus of figure 1 in that circulation of the
electrolyte has been incorporated in it by means o~ a return
duct 39 formed in the cover 2. The oxy~en bubbles formed
WO93f21 ~ 2117 ~ 0 5 ~ ''; PCT/F193/0015~
during charging raise the surface 37 of the electrolytic
liquid, after which the oxygen discharges outside the cell
through the overpres~ure valve 35. The movement of the
bubbles creates circulation of the electrolytic liquid. The
liquid returns to the cell 32 through the return duct 39 and
further to the auxiliary electrode 16 through the metal
hydride 4. The circulation of the electrolyte makes the
operation of the auxiliary electrode more efficient by
preventing the formation o~ an insulating oxygen gas layer on
its surface. The circulation of the electrolyte is
advantageous also during dischargin~ bec~use it reduces
voltage losses in the pores of the hydride electrode.
.
~igure 4 shows the cell of figure 3 but realized with a
different type of internal circulation of the electrolyte.
~he ~olution differs from figure 3 in that some of the
electrolytic liquid coming through the return duct 39 is
:~ suppli~d to the auxiliary electrode 16 through a duct formed
in the base 9.
Fi~ure 5 shows the rell of figure 3 but realized with
external circulation of the electrolyte. The solution differs
from figure 3 in that the return duct 39 is situated outside
the c~ll 32. External circulation gives more even liquid
- distribution.
~igure 6 shows the cell of figure 4 but realized with
25 external circulation of the electrolyte. The solution differs
from figure 4 in that the return duct 39 is situated outside
the cell 32, and the electrolytic liquid is led directly to
the auxiliary electrode.
Figure 7 shows the ~attery 27 consisting of several cells 32
as shown in figure 2, and a separate liquid container 28
connected to the battery by means of piping 29. During
discharging water is formed in the cell, which is then used
up in charging. Therefore, a separate liquid container 28 is
connected to the cells, into which container the water formed
~ W093J21 ~ 21~ 7 ~ ~ S PCT/Fl93/00l54
19
in the discharging process can be transferred and from where
~he water required in charging enters the cells. The battery
can be pressurized through the valve 30 in the liquid
container.
S Figure ~ corresponds otherwise to figure 7 except that the
battery 27 consists of several cells 32 which may be as shown
in figures 1, 2, 3 or ~, and in which the li~uid passes
between the cell 32 and the separate liquid container ~8
: through tha pipe 29 locat d in the upper part of the cell.
~ 10 Figure 9 shows a sys~em in which the cells are provided with
:~ ~ external circulation of the electrolyte, The cells connected
electrically in parallel may be provided with common
electroly~e circulation and a common li~uid container 40, and
with a common overpressure valve 35 and liquid supply valve
360
Fi~ure 10 corresponds otherwise to the system of figure 9
rq~pt that to the return tube 39 o~ the elect~olyte is
con~cted a:hea~ exchanger 41 for ~ooling the electrolyte.
Cooling is impor~ant, for example, bec~use the discharging
20 ~pre su~e of hydrogen~from the metal hydride increases as the
temperature rises. Without cooling,:a higher pressure would
havé to be~:~sed in:~he cell.
Figure 11:corre ponds otherwise to the ~ystem of figure 9
except that:it does;not have external circulation of the
2S electrolyte. Circulation may, however, be a~ranged internally
: in the cell 32, as shown in figure 3 or 4.
igure 12 shows a complete battery system to which a carbon
di~xide filter 42 has been connected. In the system, the
battery is ~ocated in a closed chamber to which air is
~: 30 supplied through the carbon dioxide ~ilter 42. When the
;~ battery is not in use, the carbon dioxide filter is protected
from the effect of carbon dioxide in the air by means of a
protective flap 43 which opens only when the electric charge
W~93/21~ 2 ~ 1 7 ~ o j PCT/~93/001~4
of the battery i5 discharged. To ensure an even supply of
oxygen, a small amount o~ additional air is supplied to the
bat~ery, the said air discharging from the chamber through a
discharge flap 4~. The ~an 4~ causes air to discharge from
the ~-h~her and at the same time it blows cooling air into
the heat exchanger 41 of the electrolytic solution.
The apparatus of f igure 13 is otherwis~ similar to the
apparatus shown in figure 1~, but without electrolyt~
circulations Cooling has been effe~ted by means of the fan
45, by circulating air between the cells.
~ :~
Figure 14 shows diagrammatically an experimental discharge
curve 33 with the voltage as a function of time. In the
discharge test, the ~+) and (-3 poles are connected by means
of a resistance which functions as a load. In this case a~: 15 resistance of 1 ohm was used, and the cell was as in figure
1. The voltage of the cell, as shown in the discharye curve
of figure 14, is measured from the poles of the cell. The
: current pas~ing in the external circuit is obtained by
: calculation on the basis of the cell voltage and t~e known
resi~tance, or load. With a resistance of one ohm, the
current in amperes is the same as the the voltage in volts.
The surface area of the curve is the same as the amount of
electricity obtaîned from the cell in ampere-hours, that is,
7.5 Ah in the case of figure 14. When a curve is formed from
:: 2S this, showing the product of current and voltage as a
function of time, the area of this new curve gives the energy
; obtained from the cell. By dividing the energy obtained by
~, the LaNi mass of the metal hydride, which was 23.0 g in this
casel the energy density obtained for the cell is 242 ~h/kg.
The o~erpressure curve 31 shows that during the test, the
; overpressure in the cell was 1.4 barO
Figure 15 shows diagrammatically the discharge curve 33 with
the voltage of the cell as a function of time. The fact that
little self-discharging takes place has been confirmed
experimentally by leaving the cell at rest for 65 h, that is,
WQ93/21 ~ ~ 1 ~ 7 ~ O~ PCT/~93/00154
.. . .. .
21
2.7 days after charging and ~nly ~hen starting the discharge
test. The cell was disch~rged with a load of 1 ohm. ~he
energy obtained in di~charging was 251 Wh/kg of aCtiYe
m~terial (LaNis), that is, approximately the same amoun~ as in
~ 5 th~ test shown in figure 14, where discharging was started
;~ immediately after charging.
The overpressure used in the test shown in figure 15 was
approximately 1.7 bar, which represents the equilibrium
pr~ssure of hydrogen at a temperature of somewhat below 20~C,
in the ~ase of an LaNi5;metal hydride electrode. The effect of
~: ~ overp~ssure on the formation of gas bubbles was examined in
~ a t~st where the overpressure was increased gradually from
:~ zero:to:three bar within about 1.5 hours. At the beginniny,
the for~ation of bubbles could be seen through the
: 15: tr~n~rArent upper part of the cell, but this ceased at an
overpres ure of 1.7 bar~
' ~:j ' :
~;
::
'~
:: :