Note: Descriptions are shown in the official language in which they were submitted.
~11~414
WO 93/24214 PCT/US93/04448
1
Catalyst Supports, Supported Catalysts
Methods of Making the Same and Methods of Using the Same
Back round of the invention
The invention relates to catalyst supports,
supported catalysts and methods of making and using them
in heterogeneous catalytic reactions.
Heterogeneous catalytic reactions are widely
used in chemical processes in the petroleum,
petrochemical and chemical industries. Such reactions
are commonly performed with the reactants) and
products) in the fluid phase and the catalyst in the
solid phase. In heterogeneous catalytic reactions, the
reaction occurs at the interface between phases, i.e.,
the interface between the fluid phase of the reactants)
and produ~t(s) and the solid phase of the supported
catalyst. Hence, the properties of the surface of a
heterogeneous supported catalyst are significant factors
in the effective use of that catalyst. Specifically, the
surface area of the active catalyst, as supported, and
the accessibility of that surface area to reactant
chemisorption and product desorption are important.
These factors affect the activity of the catalyst, i.e.,
the rate of conversion of reactants to products. The
chemical purity of the catalyst and the catalyst support
have an important effect on the selectivity of the
catalyst, i.e., the degree to which the catalyst produces
one product from among several products, and the life of
the catalyst.
211~~~~~ . .
WO 93124214 PGT/~1593104448
2
Generally catalytic activity is proportional to
catalyst surface area arid high specific area is therefore
desirable. However, that surfa-ce~area must be accessible
to reactants and products a~s.'well as to heat flow. The
chemisorption of a reactant by a catalyst surface is
preceded by the diffusion of that reactant through the
internal structure of the catalyst and t~.e catalyst
support, if any. The catalytic reaction of the reactant
to a product is followed by the diffusion of the product
away from the catalyst and catalyst support. Heat must
be able to flow into and out of the catalyst support as
well. '
Since the active catalyst compounds are often
supported on the internal structure of a support, the
35 accessibility of the internal structure of a support
material to reactant(s), products) and heat flow is
important. Porosity and pore size distribution are
measures of that accessibility. Activated carbons and__ .,_
charcoals used as catalyst supports have surface areas of
about 1000 square meters per gram and porosities of less__-
than one milliliter per gram. However, much of this
surface area and porosity, as much as 50~, and often
more, is associated with mieropores, i.e., gores with
pore diameters of 2 nanometers or less. These pores can ~
2~ be inaccessible because of diffusion limitations. They -_
are easily plugged and thereby deactivated. Thus, high
porosity material where the poses are mainly in the
2~i~~1~.
WO 93/24214 PC.RI"/US93/04448
3
mesopore (>2 nanometers) or macropore (>50 nanometers)
ranges are most desirable.
It is also important that supported catalysts
not fracture or aT .rit during use because such fragments
may become entrained in the reaction stream and must then
be separated from the reaction mixture. The cost of
replacing attritted catalyst, the cost of separating it
from the reaction mixture and the risk of contaminating
the product are all burdens upon the process. In other
processes, e.g. where the solid supported catalyst is
filtered from the process stream and recycled to the
reaction zone, the fines m°y plug the filters and disrupt
the process.
It is also important that a catalyst,.at the
very least, minimize its contribution to the chemical
contamination of reactants) and product(s). In the case
of a catalyst support, this is even more important since
the support is a potential source. of contamination both
to the catalyst it supports and to the chemical process.
Further; some catalysts are particularly sensitive to
contamination that can either promote unwanted competing
suctions;, i.e., affect its selectivity, or render the
catalyst ineffective, i.e., "poison" it. Charcoal and
s= commercial graphites or carbons made from petroleum
residues usually contain trace amounts of sulfur or
nitrogen as well as metals common to biological systems
and may be undesirable for~that reason.
WO 93/24214 ~ ~ ~ ~ ~~ ~ ;a PC:T/US93/04448
4
While activated charcoals and other carbon°
containing materials have been used as catalyst supports,
none have heretofore had all,of the requisite qualities
of porosity and pore size~:.,d'istribution, resistance to
attrition and purity for the conduct of a variety of
organic chemical reactions.
OBJECTS OF THE INDENTION
It is therefore a primary object of the
invention to provide improved catalyst supports and
supported catalysts for heterogeneous catalytic reactions
for use in chemical processes in the petroleum,
petrochemical and chemical industries.
i
It is a further object of the invention to
provide improved, substantially pure, carbon catalyst
support of high porosity, purity and resistance to
attrition.
It is another object of the invention to ,
improve the activity and selective~y of supported -_ .__
catalysts . _ . _~
It is still a further object of the invention ___
to provide improved methods for preparing supported
catalysts.
It is still a further and related object, of the
invention to improve the economics and reliability of -_
making and using supported catalysts. -_
sUMMARY OF 'f~iE INDENTION
The invention is in a supported catalyst for
conducting a fluid phase catalytic chemical reaction,
~~.~.~~1~1.
WO 93/24214 - PCT/US93104448
processes for performing a catalytic chemical reaction in
fluid phase using the supported catalyst and a process
for making the supported catalyst.
The supported catalyst of the invention
5 comprises a support comprising.a.,carbon fibril aggregate
and a catalytically effective amount of a catalyst
supported thereon. The fibril aggregates comprise a
plurality of carbon fibrils, each comprising multiple,
essentially continuous, generally parallel layers of
ordered graphitic carbon. In a preferred embodiment
these graphitic layers are disposed in substantially
parallel relation to the central axis of the fibril and a
preponderance of the fibrils have an external diameter of
about 3.5 to-75 nanometers and a length-to-diameter ratio
of at least about 5.
Fibril-aggregate-supported catalysts of the
present invention have unique properties. They are
exceptionally macroporous and they are pure and they are
resistant to attrition and consequently can be separated
-20 from a fluid phase reaction medium over a long service
Life. The uniquely high macroporosity of carbon fibril
aggregates, the result o~ their macroscopic. morphology,
greatly facilitates the diffusion of reactants and
products and the flow of heat into and out of the
supported catalyst. This unique porosity results from a
random entanglement or intertwining of ffibrils that
generates an unusually high internal void volume
comprising mainly macropores in a dynamic, rather than
CA 02118414 2002-11-04
78037-40
6
static state. Sustained separability from fluid phase
and lower losses of catalyst as fines also improves
process performance and economics. Other advantages of
the fibril aggregates as catalyst supports include high
purity, improved catalyst loading capacity and chemical
resistance to acids and bases.
BRIEF DESCRIPTION OF THE DRAWINGS
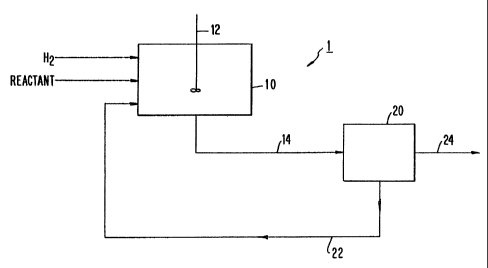
Fig. 1 is a schematic diagram of a catalytic
reaction process performed with fibril-aggregate
supported catalysts of the invention.
DETAILED DESCRIPTION OF THE INVENTION
The carbon fibril aggregates used as catalyst
supports in the invention are formed of a plurality of
carbon fibrils characterized by an outer region of
multiple, essentially continuous, generally parallel
layers of ordered graphitic carbon atoms. The
orientation of these layers with respect to the central
axis of the fibril may vary from substantially parallel
i.e. 0° angle of inclination to substantially
perpendicular i.e., 90° angle of inclination.
In a preferred embodiment of the invention, the
layers of graphitic carbon are in a substantially
parallel relationship and a preponderance of fibrils have
an external diameter of about 3.5 to about 75 manometers,
a length-to-diameter ratio of at least about 5 and
preferably at least 100 or even 1000.
CA 02118414 2002-11-04
78037-40
7
Such carbon fibrils are described in Tennent et
al., U.S. Patents Nos. 5,165,909, 5,578,543, 5,589,152 and
6,235,674, issued on November 24, 1992, November 26, 1996,
December 31, 1996 and May 22, 2001, respectively, ("Novel
Carbon Fibrils, Method for Producing Same and Compositions
Containing Same"), Tennent et al., U.S. Patent No.
5,171,560, issued December 15, 1992 ("Novel Carbon Fibrils,
Method for Producing Same and Encapsulted Catalyst"), Snyder
et al., U.S. Patents Nos. 5,707,916 and 5,877,110, issued
Jan. 13, 1998 and March 2, 1999, respectively, ("Carbon
Fibrils"), Mandeville et al., U.S. Patents Nos. 5,500,200
and 6,375,917, issued March 19, 1996 and April 23, 2002,
respectively ("Fibrils"), and McCarthy et al., U.S. Patent
No. 5,965,470, issued October l, 1999 ("Surface Treatment of
Carbon Microfibers"), all of which are assigned to the same
assignee as the invention here. Other carbon fibrils
include those having a fishbone morphology ("FB") as
described in U.S. Patent No. 4,855,091 to Geus et al..
Carbon fibril aggregates of the invention have a
variety of macroscopic morphologies as determined by
scanning electron microscopy, including bird nest ("BN"),
combed yarn ("CY") and open net ("ON"). Bird nest
aggregates resemble bird nests and are characterized by
individual carbon fibrils randomly entangled with each other
to form entangled balls of carbon fibrils. Combed yarn
aggregates resemble combed yarn and are comprised of bundles
of straight to slighly bent or kinked carbon fibrils having
substantially the same relative orientation with respect to
each other. In other words, the longitudinal axis of each
carbon fibril, despite individual bends or kinks, extends in
the same general direction as that of the surrounding
fibrils in the bundles. Open net aggregates resemble an
open net and
wo 93iZ,a2' ~ 21 ~ ~ ~ 1 ~~
8
PCTlUS93/04448
comprise straight to slightly bent or kinked carbon
fibrils that are loosely entangled with each other.\.Open
net aggregates have a degree of fibril entanglement that
is greater than that observed in combed yarn aggregates
in which the individual fibrils have substantially the
same relative orientation, but that is less than that of
bird nest aggregates. Combed yarn and open net
aggregates are more readily dispersed than bird nest _-
aggregates.
1~ The random entanglement of fibrils in the bird
nest (BN) morphology or the partially (loosely)
intertwined fibrils in the combined yarn (CY) morphology ..
results in a unique porosity and pore structure. Since
the individual fibrils are in the range of 3.5-70
manometers in diameter, the pores formed by the entangled
or loosely intertwined strands are of comparable
dimensions. Additionally, since the strands are not
chemically or physically attached other than by ___
relatively weak van der Waals attractions intermittently
spaced, some movement of the strands or portions of the _-
strands relative to each ~ther can occur either randomly
or on demand. Thus, the pores are in a "dynamic" rather
than static state. This results in a support where
- traditional micropores (<20 ~) are non-existent, and __
whets the entire porosity is available to the fluid -_ - _
medium as meso- or macropores. This is highly
advantageous for performing catalytic reactions.
~~:L~41 ~:
WO 93/24214 PCT/US93/04448
9
Carbon fibril aggregates are prepared by
contacting a carbon-containing gas with a metal catalyst
in a reactor at temperature and other conditions
sufficient to produce them with the above-described
fibril structure and macroscopic aggregate morphology.
Reaction temperatures are 400-850° C, more preferably
600-750° C. Fibril aggregates are preferably prepared
continuously by bringing the reactor to the reaction
temperature, adding supported metal catalyst particles,
and then continuously contacting the supported catalyst
with the carbon-containing gas. Examples of suitable
feed gasesinclude :aliphatic hydrocarbons, e.g.,
ethylene, propylene; propane; and methane; carbon
monoxide; aromatic hydrocarbons, e.g., benzene,
naphthalene; and toluene; and oxygenated hydrocarbons.
Additionally; hydrogen may be included in the feed.
-Preferred catalysts contain iron and, preferably, at
least one element chosen from Group VIa of the Periodic
Table (CAS Version) (e:g.; molybdenum, tungsten, or
__:~0 -chromium), Group VIIA (e.g:; manganese), or the
Lanthanide Series (e~g., cerium).
The macroscopic morphology of the fibril
mggregate, i:e:, whether it is a combed yarn, open net or
bird nest, is controlled by the choice of the catalyst
-25 support that is used in producing the fibril aggregate.
Spherical supports grow. carbon fibrils in random
directions leading to the formation of bird nest
aggregates. Combed yarn and open net aggregates are
211'~~~1~
WO 93/24214 F'~'/US93/04448
prepared using supports having one or more readily -
cleavable planar surfaces, e.g., an iron or iron-
containing metal catalyst particle deposited an a support
material having one or more readily cleavable surfaces
5 and a surface area~of at least Z m2/g.
Preferred support materials for making fibril aggregates
include spherical fumed alumina and activated alumina or
magnesia in the form of aggregates of tabular, prismatic,
or platelet crystals. Such material is commercially
10 available, e.g., spherical fumed alumina from DeGussa,
activated alumina from Strem Chemicals and magnesia from
Alfa Inorganics. The spherical fumed alumina particles
yield primarily bird nest aggregates, while the activated
alumina supports yield primarily combed yarn aggregates
and the magnesia supports yield primarily open net
aggregates.
It is believed that in the formation of combed
yarn and open net fibril aggregates that deposition of a ___
catalyst on a support comprising readily cleavable planar
surfaces allows the carbon fibrils to assist each other __:
as they grow, creating a "neighbor" effect. As the
catalyst particles deposited on the flat surfaces
initiate carbon fibril growth, the individual fibrils are
influenced by their "neighbors". In the case of the '
activated alumina support, this leads primarily to combed
yarn fibril aggregates in which the individual fibrils
have the same relative orientation. The magnesia
supports, although having readily cleavable planar
CA 02118414 2004-07-14
51696-22
11
surfaces, yield primarily open net fibril aggregates in
which the carbon fibrils are lightly entangled because the
magnesia support breaks apart more readily than the
activated alumina support during fibril growth, resulting in
fibril aggregates that are less ordered than the combed yarn
aggregates but more ordered that the entangled fibril balls
of bird nest aggregates. The oxide precursors used to
generate the metal catalyst particles also affect the
tendency of the aggregate-forming catalyst support to break
apart. The more readily the oxide and support can form a
mixed oxide at the interface between them, the more likely
the support is to break apart.
Further details regarding the formation of carbon
fibril aggregates may be found in the disclosure of Moy
et al., U.S. Patent No. 5,456,897.
Improved fibril aggregates for use in the
invention can be made in accordance with methods disclosed
in U.S. Patents Nos. 5,456,897 and 5,726,166, issued
October 10, 1995 and March 10, 1998, respectively, by
David Moy and Asif Chishti entitled "Improved Methods and
Catalysts for the Manufacture of Carbon Fibrils"
21~.~~~.~
WO 93/24214 - ~°CT/iJ~931044618
12
As a catalyst support, carbon fibril aggregates
provide superior chemical and physical properties in
porosity; surface area, separabili.ty, purity, catalyst
loading capacity and chemical resistance to acids and
bases. Carbon fibril aggregates.have porosities as high
as 8 ml/gm of aggregate support, high catalyst loading
capacity, excellent separability from fluid phase owing
to their resistance to attrition below a size of about
0.5 micron, surface areas of about 250 to about 300 m2/gm
and capable of being as high as 1004 m2/gm, high
compositional purity, i.e., freedom from contaminants.
Porosities of from about 2 to about 8 ml/gm and
typically from about 5 to about 8 ml/gm, ensure increased
accessibility to the active catalyst compound supported
on the internal surfaces of the aggregate and confer a
commensurate increase in the effective activity of the
supported catalyst. Porosity is typically measured by
evacuating all gases from a weighed sample of fibrils at
a pressure <O.i mm Hg; absorbing, in vacuo, a liquid,
e.g., distilled water, suitable for such measurements
just to the point of saturation ; removing all liquid
adhered to the fibril particles by filtering it through
i
No. 54 Whatman filter paper with a water aspirator just
to the fonaation of mud cracks in the filter cake; -
weighing the liquid-saturated fibril filter cake; and
calculating the volume occupied by the liquid from the
known weights of the fibrils, the absorbed liquid and the
liquid density.
2118414
WO 93/24214 PCf/U593/04448
13
Carbon fibril aggregate catalyst supports have
a high internal void valume that ameliorates the plugging
problem encountered in various processes. Ntoreover, the
preponderance of large pores obviates the problems often
encountered in diffusion or mass transfer limited
reaetions. The high porosities ensure significantly
increased catalyst life since more catalyst can be loaded
onto the support.
The carbon fibril aggregate catalyst supports
of the invention have improved physical strength and
resist attrition. In normal service they attrit only to
a size of about two microns. When severe attrition does
occur the attritted particles are typically above about
0.5 micron Further, attritted fibril aggregates tend to
self-flocculate; such that even when reduced to particles
of about 0:5 micron they self-flocculate forming larger
size agglomerates, remaining separable as compared to
other 0.5 micron sized particles:
Carbon fibril aggregates, which are highly
.2Q--graphitic, have a higher surface area than other
synthetic graphites, typically 250-300 m2/gm for fzbril
aggregates vs. l0 m2/g for synthetic graphite. If
i - surface areas higher than 250-300 m2/gm are desired,
fibril aggregates can be surface-oxidized to provide
_~ 2.5 surfaces With surface areas above 1000 m2/gm. An
advantage of surface-oxidized carbon fibril aggregates
over activated carbon is that only the surfaces of
individual strands of carbon fibrils are modified and
211~1~.~~
WO 93/24214 PCT/US93/04448
14
structural integrity of the aggregate remains intact,
thereby providing increased surface area without any
diminution in structural integrity.
The chemical purity of-fibril aggregates has a
positive effect on the selectivity of a supported
catalyst since contamination-induced side reactions are
minimized. Carbon fibril aggregates are essentially pure
carbon with only small amounts of encapsulated catalytic
metal compounds remaining from the process in which the
fibril aggregate was formed. The encapsulated fibril-
forming metal compound does not act as a catalyst poison
or as a selectivity-affecting contaminant.
The combination of properties offered by fibril
aggregates is unique. No known catalyst supports combine
such high porosity, high surface area and high attrition
resistance. The combination of properties offered by
fibril aggregates is advantageous in any catalyst system
amenable to the use of-a carbon support. The multiple __
carbon fibrils that make up a'carbon fibril.~ggregate
provide a large number of junction points at which __
catalyst particles can adhere to multiple fibrils in the
fibril aggregate. This provides a catalyst support that
more tenaciously holds the supported catalyst. Further,
carbon fibril aggregates permit high catalyst loadings
per unit weight of fibril and this provides a greater -_ -
resezve capacity of catalyst. Catalyst loadings are
generally greater than 0.01 weight percent and preferably
greater than 0.1 weight percent based on the total weight
WO 93/24214 PCT/US93/04448
of the supported fibril-forming~catalyst. Catalyst
loadings greater than 50 weight percent of active
catalyst based on the total weight of the supported
catalyst are easily within the contemplation of the
5 invention, i.e., loadings in excess of 1n0 weight percent
based on the weight of the f ibxil aggregate-support of
the invention, owing to the porosity of fibril aggregates
and other factors discussed herein.
Because of their high purity, carbon fibril
10 aggregates have the properties of high purity graphite
and, therefore, exhibit high resistance to attack by
acids and bases. This characteristic is ad~rantageous
since one path to regenerating catalysts is regeneration
with an acid or a base. Regeneration processes can be
15 used which employ strong acids or strong bases. Their
high purity also allows them to be used in very corrosive
environments.
METIiOD OF MARINO SUPPORTED CATALYSTS
Supported catalysts are made b_y--supporting a
-2Q.== catalytically effective amount of a catalyst on the
fibril aggregate. The term "on the fibril aggregate"
embraces, without limitation, on, in and within the
aggregate and on the constituent fibrils thereof. The
aforesaid terms may be used interchangeably.
=2-5 - Three basic methods of preparing heterogeneous
supported catalysts are adsorption, incipient wetness
impregnation and precipitation. Supported catalysts may
be prepared by either incorporating the catalyst onto the
~~~~~i~
WO 93/24214 PCT/US93/04448
16
aggregate support or by forming it in situ and the
catalyst may be eithervactive before it is placed in the
aggregate or activated in situ. Desirable active
catalysts are the platinum group (ruthenium, osmium,
rhodium, iridium, palladium and platinum or a mixture
thereof) and, preferably, palladium and platinum or a
mixture thereof.
The catalyst, such as a coordination complex of
a catalytic transition metal, such as palladium, rhodium _._
or platinum, and a ligand, such as a phosphine, can be
adsorbed by slurrying the fibril aggregate in a solution
of the catalyst or catalyst precursor for an appropriate y
time for the desired loading.,
In impregnation by incipient wetness, a
solution of the catalyst or catalyst precursor is
absorbed by the dry fibril aggregate just to the point of
saturation. Since the internal void volumes are very
high (up to 8 cc/g with water) loadings in excess of 2-3
g of active catalyst/g of fibril aggregate can be
obtained (e. g., 2.9 g Zn(OAc)~/g BN fibril aggregate).
Multiple impregnations can result in even higher
loadings.
Alternatively, the catalyst or a catalyst
precursor can be precipitated directly onto the surfaces , v
of the aggregate. This method works well with oxide and
mixed oxide catalysts. For example, a metal oxide can be
first deposited on the fibril aggregate and the active
catalyst then deposited in a second step. This
CA 02118414 2004-07-14
51095-2
17
deposition can be accomplished by loading a precursor of the
metal oxide or catalyst by incipient wetness followed by
addition of a precipitating agent, e.g., a base. Or, the
mixed oxide catalyst can be deposited analogously in a
single step.
Aggregate-supported catalytic metal oxides and
hydrated oxides can be prepared by precipitating them from
aqueous solutions of water-soluble salts of the metals, such
as by adjusting the pH of the solution. The corresponding
sulfides can be made from these oxides and hydrated oxides.
PCT International Publication No. WO 93/24687 (David Moy and
Asif Chishti) entitled "Improved Methods and Catalysts for
the Manufacture of Carbon Fibrils" describes such
precipitation in the context of fibril aggregate-supported
catalysts for making fibrils and fibril aggregates.
Fibril aggregates can be wetted with a catalyst
precursor, such as a metal cation or metal complex in a
suitable solvent system, dried, and then wetted with an
activator, such as a suitable anion in a suitable solvent
system or an acid or base in a suitable solvent, so as to
produce the catalyst in situ within the fibril aggregate
structure. As an alternative, the sequence may be reversed.
Still another method of making a supported
catalyst is by adsorbing a catalyst precursor, such as a
~1~.~~~~.~ .
WO 93!24214 PCT/US93l04448
is
cation or metalate of a catalytic transition metal in
solution, onto the fibril aggregate and then reducing or
oxidizing the precursor to the catalyst, such as to the
metal or metal oxide. .
The catalyst can also be coprecipitated onto
the fibril aggregate along with another material, such as
a material that is compatible with both.the catalyst and
the fibril aggregate where the catalyst is not compatible
with the fibril aggregate. This technique opens the
range of candidate catalysts for support by the fibril
aggregates. For instance, a ferric oxide (Fe203) catalyst
can be coprecipitated with alumina (A1203) ar molybdena
;.
(Mo03) to form a coprecipitated mixed oxide catalyst,
which may be reduced prior to the intended reaction,
i
supported on the fibril aggregate.
Further, the fibril aggregate support may be
pretreated with an acid and/or base before being imparted
with catalytic activity. , -
re ates can be used to su ort
Carbon fibril agg g PP
catalysts other than those whick~ are normally supported
on carbon: A cosupport such as alumina, magnesia,
silica, silica-alumina, silica-magnesia or a zeolite can
be deposited or formed within a fibril aggregate and
provide a support for a catalyst that is amenable to use
only with the cosupport and still be effective because of
the porosity of the fibril aggregate. By the same token
a catalyst and a compatibility material can be
coprecipitated on the fibril aggregate with the catalyst
wl~~~~l~:
WO 93/24214 fCf/US93/04448
19
not otherwise being capable of attachment to a fibril
aggregate. In other words, the porosity of fibril-'
aggregates permit them to hold cosupport or
coprecipitated catalyst material and still have
cuff icient porosity to provide access to reactant(s),
products) and heat f low. Because of the very low bulk
densities of carbon fibril aggregates (0.0~-0.15 g/cc)
and the resistance to attrition to less than 0.5 micron,
the resulting cosupported or mixed oxide catalysts are
suitable for fluidized bed or other catalytic processes
requiring attrition resistance.
In addition, the surfaces of the_individual
fibrils in the aggregate can be modified in order to
~ either increase surface area or to modify their chemical
properties to make them amenable to support of an even
broader range of catalyst materials. It is also
possible, because of the extreme porosity to apply
multiple layers of materials to these surfaces. - '
METHODS OF USING SQPPORTED CATALYSTS
2D - - -- _ -- - Carbon fibril aggregates are candidates f or use
as catalyst supports for catalysts that heretofore
utilized carbon, as a support material. These catalysts
may catalyze substitution - nucleophilic, electrophilic
or free radical; addition - nucleophilic, electrophilic,
=2~--free radical or simultaneous; p-elimination;
rearrangement - nucleophilic, electrophilic or free
radical; oxidation; or reduction reactions. The
foregoing reactions are defined in March, J. Advanced
2~1~t~1%
WO 93/24214 PtT/US93/04448
Organic Chemistry (3rd ed., 1985) at pp. 180-182. See
also Grant and Hackh's Chemical Dictionary (5th ed.
1987). More particularly, carbon fibril aggregates of
. :.
the invention may be used as~,catalyst supports for
5 catalysts for slurried liquid phase precious metal
hydrogenation or dehydrogenation catalysis, Fischer-
Tropsch catalysis, ammonia synthesis catalysis,
hydrodesulfuri2ation or hydrodenitrogenation catalysis,
the catalytic oxidation of methanol to formaldehyde, and fibril-
10 and/or fibril aggregate-forming catalysts. Typical
heterogeneous catalytic reactions and the catalysts that '
are candidates for support on fibril aggregates are set
forth in Table' I below:
211~~~~
WO 93/24214 PCT/US9a/04448
21
Reaction Catalyst
Hydrogenation
Olefin -~ alkane Pt, Pd, Rh, Ru
3H + N -~ 2NH _ _.___. Fe
2H + CO -~ CH OH Cu+/ Zn0
Heptane -~ toluene + 4H Pt
Acetone + H -~ 2-propanol Pt, Copper chromite
H + aldehyde -~ alcohol Pt, Pd, Rh, Ru
nitrobenzene -~ aniline Pd
ammonium nitrate -» hydroxylamine Pd
alkene -~ alkane
substituted alkene -» substituted alkane Pd, Pt, Rh, Ru
Dehydrogenation
_ o _-
2HOCHZCHZOH -~ I . H~ ~ H2 .0
2 0 2,3-dihydrodioxin
cyclohexanone -~ phenol + H Pt
Az~oma ti za ti on -
Pd, Pt, Rh
~ ~ ~
_ _ _ _~ . n. ~ ~.-
~tetcvrydcortrwloee . Itreceee
Pol~meri2ation
- - C H -~ linear polyethylene Cr2+/Sio
Olefin metathesis
.. 2C H -- C H + CH CH=CHCH Mo4+/A1 O
Oxidation
CH OH + ~ O -~ CH O + H O Fe O ~ Mo0
H O + CO ~ H + CO Fe 0 , Ni, Cu0/Zn0
WO 93/24214 PCT/US93/04448
22
g02 -+ CHZCH2 -~ CH~CHO PdCl and similar
salts of noble metals
RCH OH - RCHO + H Pt
Glucose - d-glucuronic acid Pt
OZigomerization
dimethylacetylene dicarboxylate -~ Pd
hexamethyL mellitate
~somerization
o-. ca . c1 --O Pd
1
CI=C1~ ~)
n ~
(~ Ca,tl, ---l i ~),~el,tt, -~;
Carbonylation
- - CO + CH OH - CFi COOH ~ Rh
Decarbonylation
~ca=ccca,~,ca=cao Pd
~ca,ccca,~,ca, co
Xydrosilation --
SiH(CH3)3+cyclooctadiene-1,3 - Pt
3-trimethylsilyl-cyclooctene.
The process of performing a heterogeneous
catalytic chemical reaction in fluid phase with supported
catalysts of the invention comprises contacting a
reactant With a supported catalyst in fluid phase under _ --
suitable reaction conditions. The process may be a batch _-
process or a continuous process, such as a plug flow
process or a gradientless process, e.g., a fluidized bed
process. The supported catalysts of the invention are
particularly useful in catalytic processes where the
~IIB~I~
WO 93/24214 ~ PCT/US93104448
23
reaction environment subjects the supported catalyst to
mechanical stresses suchYas those using liquid phase
slurry reactors, trickle bed reactors or fluidized bed
reactors. The attrition resistance and high loading
capability of the supported catalyst are particularly
beneficial in these environments.
In a batch process, the reactants) are reacted
in the presence of the supported catalyst in a reaction
vessel, preferably under agitation, and then the
supported catalyst is separated from the
reactant(s)/product(s) mixture by suitable means for
reuse, such as by a ffilter or a centrifuge.
Fig. 1 schematically_illustrates a batch
hydrogenation process i-. Supported catalyst is placed in
a batch reaction vessel 10 to which hydrogen and reactant
are added. The vessel is closed and the hydrogenati~.on
reaction performed under agitation. On completion of the
reaction, the vessel contents are passed through line 14
to a filter 20 where the supported catalyst is separated
- - -~0- and returned to the reaction vessel 10 via return line 22
- and the remainder of the vessel contents passed to the
next stage of the process via line 24.
zn a plug flow process, the reactants) pass
through a stationary bed of supported catalyst, such that
-- -_-_ 25 the concentration of products) increases as the
reactants) pass through the catalyst bed. Any supported.
catalyst that becomes entrained in this flow can be
zi~~4~~~
WO 93/24214 PCT/US93/44448
24
separated by suitable means from the
reactant(s)/product(s) stream and recycled into the bed.
In a moving bed or-.fluidized bed process, the
supported catalyst is fluidized or entrained with the
f low of reactants) in the process. The supported
catalyst flows concurrently with the
reactant(s)/product(s). At the end of the reaction step,
any entrained supported catalyst is separated from the
unreacted reactant(s)/product(s) stream, such as by
i
filter, centrifuge or cyclone separator, and recycled to
the beginning of the reaction step. y'
--- In a f luidized bed process, a bed of the
supported catalyst is fluidized but remains within the
bounds of a fixed zone as the reactants) move through
the bed and--~react to farm product(s). In this situation
any supported catalyst that becomes entrained in the
reactant(s)/product(s) stream may be separated by
suitable means and returned to the f luidized bed. __
In a further form of continuous process, the
supported catalyst moves counter-current to the flow of __-_
reactant(s). For example; the reactant may be introduced
as a gas into the base of a vertical reaction vessel and
removed from the top as product(s). The supported -
catalyst is introduced at the top of the vessel and _:__''-
cascades turbulently downwardly through the upward gas
flow to be withdrawn from the bottom for recycle to the
top of the vessel. Any supported catalyst entrained in
the gas flow exiting the vessel could be separated and
~ 11 ~ ~~ .1 !~ Pcrius93io~44~
WO 93124214
recycled to the top of the vessel for recycle into the
reaction vessel.
The fibril aggregate supports of the invention
can also be used as supports for what would otherwise be
5 homogeneous catalysis, a technique sometimes called
supported liquid phase catalysis. Their use as supports
permits homogeneous catalytic processes to be run using
heterogeneous catalysis techniques. In supported liquid
phase catalysis;,the reactants) and catalyst are
icy molecularly dispersed in the liquid phase that is
supported within the,structure of the fibril aggregate.
The high internal volume of fibril aggregates,
as evidenced by their porosity, permits them to be loaded
with a liquid phase catalyst, much like a sponge, and
15 used as a catalyst, but in a solid particle form. Each
catalyst-loaded fibril aggregate can be viewed as a
microreactor in that the interior of the aggregate., is
. _ loaded with a continuous liquid phase containing catalyst
or a plurality of droplets of catalyst ..i,n -solution.
_--20- Consequently, the aggregate behaves both as a solid
.- particle for material handling purposes and as a
homogeneous liquid catalyst for reaction purposes.. The
usefulness of carbon fibril aggregates is aided in this
regard by their chemical stability. The advantages in
-_ - 25 using homogeneous catalyst-loaded fibril aggregates are
the ease of separating the catalyst from the product
stream, ease in carrying out the process, equipment
WO 93/24214 ~ ~ ~ ~ ~ ~ ~ PCT/US93/tD4448
26
sizing and in avoiding corrosion in the condensed liquid
phase.
Fibril aggregates are amenable to use as
supports in the catalysis of substitutions, additions, a-
eliminations, rearrangements, oxidations and reductions.
More specifically, they are useful in hydroformylation
and carbonylatior~ reactions and the blacker process.
In carbonylation reactions, a catalyst-loaded
fibril aggregate is prepared by absorbing a solution of __
la the carbonylation catalyst, such as rhodium chloride and
triphenyl phosphine, in a higher boiling point solvent,
such as mesitylene or pseudocumene, into dry fibril
aggregates, such as bird nest aggregates.
The carbonylation reaction is carried out by
contacting a vapor phase feedstock with the catalyst at
appropriate temperatures and pressures. The feedstock
mixture may be, e.g., carbon monoxide, methyl acetate,
methyl iodide and solvent: The feedstock is absorbed and ___
molecularly dispersed in the catalyst solution and reacts
-- 20 in the liquid phase. The reaction can be carried out in __
a slurry phase reaction as previously described or in a
fixed bed reaction.
The products of reaction, such as acetic -
anhydride and/or acetic acid and byproducts, are removed
from the fibril aggregate particles by vaporization or
filtration.
2n the blacker Process, a catalyst-loaded fibril
aggregate is prepared by absorbing a catalyst, such as
~llg~I
WO 93/24214 POf/US93/04448
z?
palladium chloride, copper chloride, potassium chloride
or litnium chloride, in a solvent such as water, into dry
fibril aggregates. The loaded catalyst is then placed
into a slurry phase or fixed bed reactor and vapor phase .
reactants, such as ethylene, oxygen and hydrogen
chloride, are passed through the~bed at appropriate
partial pressures and temperatures. The products, such
as acetaldehyde and water can be separated from the
catalyst by vaporization or filtration.
EXAMPLES
Examples I through VII describe the preparation
of supported catalysts in accordance with the invention
for various chemical processes and their use in those
processes.
EILAMPLE I.
This example describes the preparation by
incipient wetness of a fibril aggregate-supported
cataxyst for a liquid phase slurry, precious~metal
hydrogenation and the use of the catalyst in the
__- .--2o- hydrogenation of phenol to cyclohexanone/ cyclohexanol.
A sample of BN fibril aggregates (void volume
~of ?.0 cc H20/g), is washed extensively with 6 N HC1,
distilled water, and is then dried. 10.0 g of the dry
- - aggregates are added to a 1 liter 2-neck flask equipped
._ _ _ ~ _ 25 w~.th a 100 cc long-stem addition funnel and a stopcock
attached to a. vacuum source. The fibril sample is
degassed at O.i mm Hg. Eighty cubic centimeters of
0.0135M solution of PdCl2 (from AESI~rR) in 6 N HC1 is
2li~~i~
WO 93/24214 PC'f/US93/04448
28
added~to the addition funnel. While still under vacuum, _ -
the solution is slowly and carefully added to the dry,
degassed fibrils. The flask is shaken frequently to mix
a
the partially wetted fibrils as,the addition is
performed. Addition is contir~~ed until the point of -
saturation is just reached. A total of 72.5 cc is added.
The flask is then placed onto a rotary evaporator and is
dried at 0.1 mm Hg and 80°C for several hours. The dried
catalyst is reduced by reaction with 5% H2 in a 2 inch
quartz tube at 200°C until the gaseous effluent no longer
tests positive for C1°. Nominal loading on the catalyst
is 1.1% palladium by weight.
The test for catalytic activity. is performed in
a 50 cubic centimeter stainless steel~autoclave at 160°C
and a total pressure of 5 atmosphere. Products are
analyzed on a Varian 3700 gas chromatograph using a
capillary SPB-5 column. Ten grams of phenol (Aldrich
Chemical, ACS Reagent) is loaded in the autoclave with __-:
0.2 g of the catalyst. The autoclave is sealed and all
air is removed by purging twice with argon. The _=
autoclave is then heated under an atmosphere of H2 with
stirring (800 rpm) to 100°C, after which the pressure
regulator for the system is set to 5 atm and the
;_
temperature controller set for 160qC. On reaching these
conditions, reaction is allowed to proceed for 15 min. A
1.0 cc sample is withdrawn every 5 min. for analysis.
The rates of formation for cyclohexanone and cyclohexanol
WO 93/24214 P(.'T/US93/04~d48
29
are 16.0 and 0.12 g/g cat-s or liters per .gram of
catalyst-second, respectively.
EXAMPLE II.
This Example describes the preparation of a
fibril aggregate-supported catalyst for the Fischer-
Tropsch process and the use of the catalyst in that
process.
Ten grams of BN fibril aggregates (void volume,
7.0 cc/g) is dispersed with 600 cubic centimeters of
deionized water for 2 minutes in a blaring blender to form
a thick paste. This is then slurried with an additional
1 liter of water in a 3 liter 3-neck indented flask
fitted with a top stirrer. One hundred twenty grams of
41% Fe(N03)3 from Blue Grass Chemical is added to the
slurry and, with vigorous stirring, is neutralized with a
solution of 20 weight percent potassium carbonate to a pH
>~. The slurry is filtered and washed lightly and then
-- is~_dried at 160°C in a convection oven. Nominal loading
of the catalyst is l.i g of iron per gram of carbon
s
- _ f i.brrl- aggregate .
- The catalyst is ground and reduced in H2 at
350°C for 6 hrs. It is added to a fluidized bed reactor
at 340°C and charged with a 6/1 ratio of HZ/CO at 2.5 MPa
- .and at sufficient velocity to maintain a fluidized bed.
_-2~.=-after equilibrium is reached, analysis by gas
chromatography of the liquid product collected downstream
iss
~~.1~~1~
WO X3/24214 PCT/US93/04448
Fraction C5-C10 C11-~18
Olefins 70 60
-Paraffins 13~ 15
5 Oxygenates 12 10
Aromatics .5 15
EXAMPLE IIT.
This example describes the preparation of a
10 fibril aggregate-supported Fe203/Mg0 catalyst for an
ammonia synthesis process and the use of the catalyst in
an ammonia synthesis process.
Magnesia (Martin Marietta MagChem 50) (29.6
grams) is slurried with deionized water at 80°C fo:r 3
15 hrs. and is then allowed to cool. HN fibril aggregates
(3.3 grams) (void volume 7.0, surface area 290 M2/g) is
added to the Mgo/Mg(OH)~ slurry and the mixture stirred
in a blaring blender for 2 min. 67.6 g of a 41% solution
of Fe(N03)3 in deionized water is mixed with a solution of
20 2.17 g ammonium para-molybdate in deio.nized water,
yielding a clear, orange-brown solution. This solution
is ~dded.to the Mg0/Mg(OH)2 fibril aggregate slurry with
vigorous mixing at a steady dropwise rate. The resulting
slurry is black. The slurry i.s filtered and washed twice
25 with 1 N ammonium acetate by reslurrying and refiltering.
The filter cake is dried at 160°C for 18 hrs., then
calcined at 400°C for 4 hrs. The nominal composition of
the calcined catalyst is: 33% Fe203, 6.5% Mo03, 48.5% MgO,
1~% (fibril aggregates.
30 The catalyst is reduced in a H2 stream by
gradually increasing the temperature from 75°C to 425°C
over a 12 hr. period, followed by 24 hrs. at 425°C. At
2~.~~4~~
WO 93/24214 Pt,'T/US93/04448
31
the end of this time, heat is removed. When the
temperature reaches ambient temperature, H2 is purged
with argon. The catalyst is then exposed to a 10%
COZ/argon gas stream to partially reoxidize the reduced
Fe, which is otherwise pyrophoric.
The catalyst is tested for ammonia synthesis in
a fixed bed reactor 8.0 g (23.8cc) of catalyst is loaded
in a 3/4" tube. Reaction is carried out at 480°C and 25
MPa total pressure with a 3/1 ratio of H2/N2. The exit
gas contains 8% NH3, 23% N2 and 690 Hz. This corresponds
to a 15~ conversion to NH3.
ERAI~:PLE IV'.
This example describes the preparation of a i
fibril aggregate-supported catalyst for a
IS hydrodesulfurization process by pore filling and the use
of the catalyst in a hyd.rodesulfurization pracess.
Ten grams of BN carbon fibril aggregates (void
volume_7_ cc/g) is dispersed in a mixture of 600 cc of a
solution that is 0.44 M in Co(N03)~ ~ 6H20 (25.?5% Co0)
and-0~:2_ff~M-in ammonium paramolybdate (81.530 Mo03) in a
Waning blender fir 2 minutes. The,slurry is filtered and
without further washing, dried at 125°C. The ariea
catalyst is calcined at 230°C for 6 hours. The catalyst
exhibits a nominal loading of 2.3 g CoO, 14.1 g Mo03 (6/1
~5 patio-of Mo03/CoO) and 10.0 g fibril aggregates.
The catalyst is activated by reduction at 325°C
with a gas mixture containing 2.5$ H2S in H2 for 2 hrs.
WO 93/24214 ~ ~ ~ ~ ~ ~ PCT/US93/04448
32
0.1 g of catalyst is charged into a 50o cc
stirred autoclave with 300 cc of 1 volo solution of
thiophene in hexadecane. The reactor is charged to 80
atm with H2 and the hydrodesu:lfurization reaction is
carried out at 300°C. one cc samples are withdrawn and
analyzed at 5 min. intervals and a pseudo first order
rate constant for disappearance of thiophene is
determined to be 4.5 x 10-3 L/g cat-s.
- EXAMPhE V.
This example describes the preparation of a
fibril aggregate-supported catalyst for a carbonylation
grocess and the use of the catalyst in a carbonylation
process.
A homogeneous catalyst solution having 0.0015M
of RhCl~, 0.0005 M of Cr(CO)6 and 0.005 M of triphenyl
phosphine dissolved in pseudocumene is absorbed into the
pores of 10 g of BN fibril aggregates (void volume,.7.0
cc/g; surface area 290 m2/g.
The catalyst solution as a suppozted liquid
phase within the fibril aggregates is loaded into a fixed
bed -reactor. The pressure in the reactor is increased
and controlledat 500 p$ig with CO and the temperature is
increased to 200°C. A gas mixture comprising MeOAc, MeI
and. CO saturated with pseudocumene at 200°C and 500 psig
is prepared such that the final composition of the gas
stream is: 64% CO, 25% MeOAc, 10% MeI, 1% pseudocumene.
The gas mixture is fed into the fixed bed reactor
whereupon MeOAc, MeS and CO dissolve in the supported
~11~~1~:-
WO 93/24214 - PCf/~.JS93/04448
33
liquid phase within the fibril aggregates and react to
form acetic anhydride and acetic acid. T~roducts are
removed from the reactor by vaporization.
EXAMPLE VI.
This example descri?aes the preparation of a
fibril aggregate-supported catalyst for the heterogeneous .
blacker process and the use of the catalyst in a
heterogeneous Wacker process.
A homogeneous solution, 0.01 M in PdCl2, 1.0 M
in CuCl2 and 0.001 M in LiCI, is absorbed into the pores
of 10.0 g BN fibril aggregates (void volume, 7.0 cc/g,
surface area 290 m2/g).
The catalyst (83 cc) is loaded into a 1" fixed
bed reactor. A gas stream comprising 60% stream, 31% C2H4
and 9% 02 is fed at 110°C and 60 psig at a total GHSV of
360. Residence time is approximately 10 sec. The
effluent contains 18% CH3CH~, 16% C2H4, 1% 02 and the
balance, steam.
EXAMPLE ~II,.
~ ~ - --This example describes the preparation of a
fibril-supported catalyst for the oxidation of methanol
to formaldehyde and the use of the catalyst in that
i
prowess.
A catalyst is prepared by absorbing a solutiom
attat_is- 0.004 M in ammonium paramolybdate ((NH~)6Mo'024
4H20~, 0.015 M in Fe(N03)3 and 0.5 M in citric acid into
10.0 g BN fibril aggregates (void volume '7.0 cc/g,
surface area 290 m2/g). The impregnated catalyst is
2~.1~41~
WO 93/24214 PCT/US93/04448
34
dried at 110°C and calcined at 240°C for 4 hrs. Nominal
composition of the catalyst is 0.65a Fe203, 2.3% Mo03 and
97% Fibril Aggregates.
The reactor is a quartz tube, 32 mm OD x 1000
mm long fitted with a porous fritted glass disc (l0-20
micron). The bed length is 425 mm. The process is
carried out at 240°C and atmospheric pressure with a gas
stream comprising 6% MeOH/94% air (vol) at a total gas
rate of 125 1/hr. Analysis of products by gas i
chromatography shows a 98o conversion of MeOH with a
selectivity to CH20 of 96%. CO (2.5%), C02 (0.2a) and
methyl formate (0.5%) are also detected.