Note: Descriptions are shown in the official language in which they were submitted.
- 21 21 0:~2
Medical Probe Device
Technical Field
This invention is directed to a unique device for
penetrating body tissues for medical purposes such as tissue
destruction and fluid substance delivery, for example. The
device penetrates tissue to the precise target selected in
order to deliver energy to the tissue and/or deliver
substances. It limits this activity to the precise
preselected site, thereby minimizing trauma to normal
surrounding tissue and achieving a greater medical benefit.
This device is a catheter-like device for positioning a
treatment assembly in the area or organ selected for medical
treatment with one or more stylets in the catheter, mounted
for extension from a stylet port in the side of the catheter
through surrounding tissue to the tissue targeted for medical
activity.
Backqround Art
Treatment of cellular tissues usually requires
direct contact of target tissue with a medical instrument,
usually by surgical procedures exposing both the target and
intervening tissue to substantial trauma. Often, precise
placement of a treatment probe is difficult because of the
location of a target tissue in the body or the proximity of
the target tissue to easily damaged, critical body organs,
nerves, or other components.
Benign prostatic hypertrophy or hyperplasia (BPH),
for example, is one of the most common medical problems
experienced by men over 50 years old. Urinary tract
obstruction due to prostatic hyperplasia has been recognized
since the earliest days of medicine. Hyperplastic enlargement
of the prostate gland often leads to compression of the
urethra, resulting in obstruction of the urinary tract and the
subsequent development of symptoms including frequent
urination, decrease in urinary flow, nocturia, pain,
discomfort, and dribbling. The association of BPH
W094/04220 PCT/US93/0743;
2121032
-2-
with aging has been shown to exceed 50% in men over 50 years
of age and increases in incidence to over 75% in men over 80
years of age. Symptoms of urinary obstruction occur most
frequently between the ages of 65 and 70 when approximately
65% of men in this age group have prostatic enlargement.
Currently there is no proven effec~ive nonsurgical
method of treatment of BPH. In addition, the surgical
procedures available are not totally satisfactory. Cur-
rently patients suffering from the obstructive symptoms of
this disease are provided with few options: continue to cope
with the symptoms (i.e., conservative management), submit to
drug therapy at early stages, or submit to surgical
intervention. More than 430,000 patients per year undergo
surgery for removal of prostatic tissue in the United
States. These represent less than five percent of men
exhibiting clinical significant symptoms.
Those suffering from BPH are often elderly men, many
with additional health problems which increase the risk of
surgical procedures. Surgical procedures for the removal of
prostatic tissue are associated with a number of hazards
including anesthesia associated morbidity, hemorrhage,
coagulopathies, pulmonary emboli and electrolyte imh~ ces.
These procedures performed currently can also lead to
cardiac complications, bladder perforation, incontinence,
infection, urethral or bladder neck stricture, retention of
prostatic chips, retrograde ejaculation, and infertility.
Due to the extensive invasive nature of the current
treatment options for obstructive uropathy, the majority of
patients delay definitive treatment of their condition.
This circumstance can lead to serious damage to structures
secondary to the obstructive lesion in the prostate (bladder
hypertrophy, hydronephrosis, dilation of the kidney pelves,
etc.) which is not without significant consequences. In
addition, a significant number of patients with symptoms
sufficiently severe to warrant surgical intervention are
poor operative risks and are poor candidates for
prostatectomy. In addition, younger men suffering from BPH
W094/04220 2 1 2 1 0 ~ 2 PCT/US93/07437
who do not desire to risk complications such as infertility
are often forced to avoid surgical intervention. Thus the
need, importance and value of improved surgical and
non-surgical methods for treating BPH is unquestionable.
High-frequency currents are used in electrocautery
procedures for cutting human tissue especially when a
bloodless incision is desired or when the operating site is
not accessible with a normal scalpel but presents an access
for a thin instrument through natural body openings such as
the esophagus, intestines or urethra. Examples include the
removal of prostatic adenomas, bladder tumors or intestinal
polyps. In such cases, the high-frequency current is fed by
a surgical probe into the tissue to be cut. The resulting
dissipated heat causes boiling and vaporization of the cell
fluid at this point, whereupon the cell walls rupture and
the tissue is separated.
Destruction of cellular tissues in situ has been used
in the treatment of many diseases and medical conditions
alone or as an adjunct to surgical removal procedures. It
is often less traumatic than surgical procedures and may be
the only alternative where other procedures are unsafe.
Ablative treatment devices have the advantage of using a
destructive energy which is rapidly dissipated and reduced
to a non-destructive level by conduction and convection
forces of circulating fluids and other natural body
processes.
Microwave, radiofrequency, acoustical (ultrasound) and
light energy (laser) devices, and tissue destructive
substances have been used to destroy malignant, benign and
other types of cells and tissues from a wide variety of
anatomic sites and organs. Tissues treated include isolated
carcinoma masses and, more specifically, organs such as the
prostate, glandular and stromal nodules characteristic of
benign prostate hyperplasia. These devices typically
include a catheter or cannula which is used to carry a
radiofrequency electrode or microwave antenna through a duct
to the zone of treatment and apply energy diffusely through
_ 4 _ 2121032
the duct wall into the surrounding tissue in all directions.
Severe trauma is often sustained by the duct wall during this
cellular destruction process, and some devices combine cooling
systems with microwave antennas to reduce trauma to the ductal
wall. For treating the prostate with these devices, for
example, heat energy is delivered through the walls of the
urethra into the surrounding prostate cells in an effort to
kill the tissue constricting the urethra. Light energy,
typically from a laser, is delivered to prostate tissue target
sites by "burning through" the wall of the urethra. Healthy
cells of the duct wall and the healthy tissue between the
nodules and duct wall are also indiscriminately destroyed in
the process and can cause unnecessary loss of some prostate
function. Furthermore, the added cooling function of some
microwave devices complicates the apparatus and requires that
the device be sufficiently large to accommodate this cooling
system.
Application of liquids to specific tissues for
medical purposes is limited by the ability to obtain delivery
without traumatizing intervening tissue and to effect a
delivery limited to the specific target tissue. Localized
chemotherapy, drug infusions, collagen injections, or
injections of agents which are then activated by light, heat
or chemicals would be greatly facilitated by a device which
could conveniently and precisely place a fluid supply catheter
opening at the specific target tissue.
Disclosure of the Invention
It is the principal object of this invention to
provide a device for penetrating tissue, through intervening
tissues to the precise target tissue selected for a medical
action such as tissue destruction and/or substance delivery,
limiting this activity to the precise preselected site,
thereby minimizing the trauma and achieving a greater medical
benefit.
One principal object of this invention is to provide
a device for tissue destruction of body tissues which delivers
~ 5 ~ 2121032
the therapeutic energy directly into a target tissue while
minimizing effects on its surrounding tissue.
Another principal object of this invention is to
provide a device for introducing fluid treatment agents,
particularly flowable liquids, with greater precision and ease
to a specific location in the body.
Another object of this invention is to provide a
thermal destruction device which gives the operator more
information about the temperature and other conditions created
in both the tissue targeted for treatment and the surrounding
tissue. In addition, it will provide more control over the
physical placement of the stylet and over the parameters of
the tissue destruction process.
In summary, the medical probe device of this
invention comprises a catheter having a control end and a
probe end. The probe end includes a stylet guide housing
having at least one stylet port in a side wall thereof and
guide means for directing a flexible stylet outward through
the stylet port and through intervening tissue at a
preselected angle to a target tissue. The housing can include
an array of such ports. The preselected angle is preferably
from 20~ to 160~ with the central axis of the stylet guide
housing. The total catheter assembly includes one or more
stylet guide lumena communicating with respective stylet ports
and a stylet positioned in each of said stylet guide lumena
for longitudinal movement from the respective port through
intervening tissue to target tissues.
The stylet can be an electrical conductor enclosed
within a non-conductive layer, the electrical conductor being
a radiofrequency electrode. Preferably, the non-conductive
layer is a sleeve which is axially or longitudinally movable
on the electrical conductor to expose a selected portion of
the electrical conductor surface in the target tissue.
In a still further embodiment, the stylet is a
cannula having a longitudinal, central treatment fluid supply
lumen extending therethrough, and the catheter has a treatment
- 6 - ~1 2 1 032
fluid transport lumen communicating with the treatment fluid
supply lumen.
An ultrasound reflector such as a bubble or an
ultrasound transducer can be embedded or otherwise attached to
the probe end or a portion of the stylet to provide a signal
for use in positioning the catheter and stylet.
When the stylet includes a radiofrequency electrode,
optimally at least one temperature sensor such as a thermistor
or fiber optic cable can be attached to the probe end, stylet
guide housing and/or stylet.
In one preferred embodiment, the stylet guide
defines a stylet path from an axial orientation in the
catheter through a curved portion to a lateral orientation at
the stylet port, the curved path optionally having a radius
which is sufficient to deflect the deployed, extended stylet
to the desired angle, that is, a radius of up to 0.5 cm,
depending upon the diameter of the catheter. The stylet guide
means can define a stylet path having a first curved portion
extending in a direction away from the stylet port and a
second curved portion, continuing from the first curved
portion and extending to the stylet port.
For deploying a plurality of stylets, the stylet
guide means can define at least two non-intersecting stylet
paths from parallel axial orientations in the catheter through
curved portions to lateral orientations at stylet ports, the
stylet ports having axes forming an angle of up to 180~. For
treating prostate lobes in one embodiment, the stylet port
axes form an angle of less than 90~ and preferably from 50~ to
7oo~
The non-conductive sleeve can comprise a leading
tip, a rigid proximal control section, and a flexible portion
extending from the leading tip the rigid proximal control
section, whereby the sleeve can be extended through a curved
path from an axial orientation to an orientation extending
outward through a stylet port. The leading tip can be
tapered inward toward its terminal end. The flexible
W094/04220 2 1 ~ 1 0 ~ ~ PCT/US93/07437
portion can optionally be a spiral coil. If the spiral coil
is made of conductive material, it can be enclosed in an
outer non-conductive material.
The distal portion of the catheter can be more flexible
than the proximal portion thereof, facilitating its passage
through curved ducts.
In one embodiment, a control handle is attached to the
control end of the catheter and stylet movement means
attached to a stylet and engaging the handle for longi-
tudinal movement of the stylet in the stylet guide means.
The stylet movement means comprises manual engagement means
for translating manual motion into longitudinal motion of
the stylet in the stylet guide means.
In embodiments where the electrical conductor has axial
movement in the non-conductive sleeve, a non-conductive
sleeve movement means is attached to a non-conductive sleeve
and an electrical conductor movement means is attached to
the electrical conductor enclosed therein. The
non-conductive sleeve movement means translates manual
motion into longitudinal motion of the non-conductive sleeve
in the stylet guide means. The electrical conductor
movement means translates manual motion into longitudinal
motion of the electrical conductor in the non-conductive
sleeve. The non-conductive sleeve movement means and the
electrical conductor movement means engage the handle for
movement thereon. The non-conductive sleeve movement means
and the electrical conductor movement means can include
separate, adjacent manual movement means, mounted on the
handle for both separate and coordinated movement thereon.
The housing can have at least two parallel longitudinal
slots through a wall thereof, the manual movement means each
including a finger engaging surface connected to a slide
extending through one of the longitudinal slots to a
connector in the interior of the housing, the connector
being attached to a respective non-conductive sleeve or
electrical conductor.
W094/04220 PCT/US93/07437
2121032
The method of this invention for applying destructive
energy to a target tissue comprises first introducing a
catheter to a zone adjacent to the tissue to be treated.
Then an electrical conductor is moved from the catheter
through surrounding tissue into a target tissue to be
destroyed. The electrical conductor can be a wire or tube
comprising a conductive surface surrounded by a
non-conductive sleeve for preventing significant transfer of
energy from the conductor in tissue surrounding the sleeve.
Heat is generated in the target tissue from an electric
current or electromagnetic field produced by the electrical
conductor. The volume of tissue being treated is controlled
by moving the non-conductive sleeve to expose a selected
length of electrode in the body tissue to be treated, the
remaining area of the electrode remaining shielded by the
sleeve to protect the intervening tissues. The amount and
duration of the energy delivery is also varied to control
the volume of tissue being treated.
The electrical conductor can be positioned using a
fiber optic viewing system incorporated within the catheter
shaft, positioned to facilitate positioning of the device.
Such a system can also include separate optics for
lumination and viewing, and flushing fluid supply conduits
for flushing the viewing fields.
The electrical conductor can also be positioned in the
tissue to be treated using ultrasound imaging from an
ultrasound transducer positioned at a distance from the
target tissue or ~u~po~ed by the electrical conductor or
non-conducting sleeve.
The extent of heating can be monitored and controlled
during the ablative treatment using temperature sensors
supported by the electrical conductor or nonconductive
sleeve.
In another embodiment of the method of this invention
for treating a target tissue such as the prostate, two
flexible stylets from the catheter are moved through
catheter ports in the sidewall of the catheter and through
9 21 21 032
the urethra wall and surrounding tissue into the prostate
target tissue to be treated, the catheter ports having axes
forming an angle of less than 180~ and for treatment in some
tissue, less than 90~.
In a still further embodiment, a grounding plate is
placed on the skin to direct the electrical current passing
from one or more electrodes in a path through the target
tissue to be ablated.
According to a broad aspect of the present invention
there is provided a medical device for the treatment by radio
frequency ablation of a target volume in prostatic tissue of a
prostate of a human male having a bladder with a base and a
penis with a urethra therein formed by a urethral wall
extending into the base of the bladder along a longitudinal
axis with the tissue of the prostate surrounding the urethra
near the base of the bladder. The medical device comprises an
elongate member having proximal and distal extremities and
having a longitudinal axis and being sized to be able to enter
the urethra and having a length so that when the distal
extremity is disposed in the vicinity of the prostate the
proximal extremity is outside of the urethra. The elongate
member has a sidewall with a passageway therein extending
along the longitudinal axis and characterized in that a radio
frequency electrode of an electrically conductive material is
disposed in the passageway and has a sharpened tip. A sleeve
of insulating material is coaxially mounted on the radio
frequency electrode and slidably disposed in the passageway.
A handle is secured to the proximal extremity of the elongate
member. Operative means is carried by the handle and
connected to the radio frequency electrode and to the sleeve
of insulating material for causing movement of the radio
frequency electrode and the sleeve of insulating material in
the passageway. Means is carried by the elongate member and
cooperatively coupled into the passageway for causing movement
of the sleeve of insulating material and the radio frequency
electrode therein through a curved path extending at an angle
to the longitudinal axis whereby when the sleeve of insulating
- 9a - 21 21 032
material and the radio frequency electrode are advanced under
the control of the operative means carried by the handle, the
radio frequency electrode is advanced through the urethral
wall so that a preselected length of the radio frequency
electrode has been advanced into the target volume and is
exposed in the target volume and the sleeve of insulating
material is advanced through the urethral wall and extends
through the urethral wall while the preselected length of the
radio frequency electrode is exposed in the target volume so
that when radio frequency energy is supplied to the radio
frequency electrode, prostatic tissue in the target volume
surrounding the preselected length of radio frequency
electrode is ablated while the sleeve of insulating material
protects the urethral wall from radio frequency ablation.
Brief DescriPtion of the Drawinqs
Fig. 1 is a schematic cross-sectional drawing of the
lower male anatomy with one embodiment of the device of this
invention in position for treatment.
Fig. 2 is a side view of the terminal housing
portion of the catheter of this invention with a plurality of
extended stylets.
Fig. 3 is an end view of the terminal housing
portion shown in Fig. 2.
Fig. 4 is a side elevational view in section of an
alternative embodiment of a catheter of this invention.
Fig. 5 is a cross-sectional representation of an
embodiment of a RF electrode stylet according to this
invention.
Figs. 6 and 7 are cross-sectional representations of
an embodiment of the catheter of this invention with a stylet
guide system for adjusting the stylet guide angle.
2 1 2 1 0~2
- 9b -
Figs. 8 and 9 are detailed schematic cross-sectional
views of a RF electrode stylet shown in Fig. 4 with a
partially retracted sleeve positioned to treat tissue targeted
for destruction while shielding intervening tissue from
treatment according to the method of this invention.
Fig. 10 is a schematic view of the assembly of
control system, manual catheter control unit and catheter
according to this invention.
Fig. 11 is an isometric representation of an
embodiment of a manual control system of the system of this
invention.
2121032
-- 10 --
-
Fig. 12 is an isometric representation of an
embodiment of a power and control console of the system of
this invention.
Fig. 13 is a plan view of an alternative four-probe
embodiment of the device of this invention.
Fig. 14 is a side elevational view of the distal
probe end of the catheter shown in Fig. 13.
Fig. 15 is a cross-sectional end view of the probe
end of the device shown in Fig. 14, taken along the line 15-
15.
Fig. 16 is a partial cross-sectional view of the
probe end of the device of this invention, taken along the
line 16-16 of Fig. 15.
Fig. 17 is a cross-sectional view of the control end
of the device shown in Fig. 13, taken along its central axis.
Fig. 18 is a cross-sectional view of the control end
of the device shown in Fig. 17, taken along the line 18-18.
Fig. 19 is a cross-sectional view of the control end
of the device shown in Fig. 17, taken along the line 19-19.
Fig. 20 is a side view of the non-conductive sleeve
connector of the embodiment shown in Figs. 17 and 18.
Fig. 21 is a cross-sectional view of the non-
conductive sleeve connector shown in Fig. 20, taken along the
line 21-21.
Fig. 22 is a side view of the electrical conductor
connector of the embodiment shown in Figs. 17 and 19.
Fig. 23 is a cross-sectional view of the electrical
conductor connector shown in Fig. 22, taken along the line 23-
23.
Fig. 24 is a cross-sectional view of the distal end
of the non-conductive sleeve shown in Figs. 14 and 15, taken
along its central axis.
Fig. 25 is a top view of a two stylet alternative
embodiment of an RF ablation catheter of this invention.
Fig. 26 is a top view of one embodiment of a stylet
tip of this invention.
Fig. 27 is a side view of the single grind electrode
-11- 2121032
tip shown in Fig. 26.
Fig. 28 is an end view of the electrode tip shown in
Fig. 27.
Fig. 29 is a side view of an alternative double
grind electrode tip.
Fig. 30 is an end view of the electrode tip shown in
Fig. 29.
Fig. 31 is a top view of the handle portion of the
ablation catheter of Fig. 25.
Fig. 32 is a side view of the handle portion shown
in Fig. 31 taken along the line 32-32 with the bottom cover
plate partially removed.
Fig. 33 is a bottom view of the handle portion shown
in Fig. 31 with the bottom cover plate removed.
Fig. 34 is a cross-sectional view of the handle
portion taken along the line 34-34 in Fig. 33.
Fig. 35 is a cross-section view of the central
portion of the handle portion shown in Fig. 32 in the stylet
and sleeve retracted position.
Fig. 36 is a cross-sectional view of the central
portion of the handle portion shown in Fig. 32 with the stylet
and sleeve in an extended position.
Fig. 37 is a cross-sectional view of the central
portion of the handle portion shown in Fig. 32 with the stylet
in an extended position and the sleeve partially retracted
therefrom.
Fig. 38 is a schematic view of a deployment of two
stylets in a prostate showing stylet orientation for the
overlapping ablation zone method of this invention.
Best Mode For Carrying Out The Invention
The device of this invention provides a precise
controlled positioning of a treatment stylet in a tissue
targeted for treatment, destruction or sampling from a
catheter positioned in the vicinity of the target tissue.
The term "stylet" as used hereinafter is defined to
include both solid and hollow probes which are adapted to be
21210~
- 12 -
_
passed from a catheter port through normal tissue to a target
tissue. The stylet is shaped to facilitate easy passage
through tissue. It can be a solid wire, thin rod, or other
solid shape or it can be a thin hollow tube or other shape
having a longitudinal lumen for introducing fluids to or
removing materials from a site. The stylet can also be a thin
hollow tube or other hollow shape, the hollow lumen thereof
containing a reinforcing or functional rod or tube. The
stylet preferably has a sharpened end to reduce resistance and
trauma when it is pushed through tissue to a target site.
The stylet can be designed to provide a variety of
medically desired treatments of a selected tissue. As a
radiofrequency electrode, it can be used to ablate or destroy
the target tissue. As a hollow tube, it can be used to
deliver a treatment fluid such as a liquid to a target tissue.
The liquid can be a simple solution or a suspension of solids,
for example, colloidal particles, in a liquid. Since the
stylet is very thin, it can be directed from the catheter
through intervening normal tissue with a minimum of trauma to
the normal tissue.
The device and method of this invention provide a
more precise, controlled medical treatment which is suitable
for destroying cells of medically targeted tissues throughout
the body, both within the external to body organs. The
device and method are particularly useful for treating benign
prostate hyperplasia (BPH), and the device and its use are
hereinafter described with respect to BPH, for purposes of
simplifying the description thereof. It will be readily
apparent to a person skilled in the art that the device and
method can be used to destroy body tissues in any body
cavities or tissue locations that are accessible by
percutaneous or endoscopic catheters, and is not limited to
the prostate. Application of the device and method in all of
these organs and tissues are intended to be included within
the scope of this invention.
W094/04220 2 ~ 2 1 ~ 3 2 PCT/US93/07437
BPH is a condition which arises from the benign
replication and growth of cells in the prostate, forming
glandular and stromal nodules which expand the prostate and
constrict the opening of the prostatic urethra. Glandular
nodules are primarily concentrated within the transition
zone, and stromal nodules within the periurethral region.
Traditional treatments of this condition have included
surgical removal of the entire prostate gland, digital
removal of the adenoma, as well as transurethral resection
of the urethral canal and prostate to remove tissue and
widen the passageway. one significant and serious
complication associated with the latter method is iatrogenic
sterility. more recently, laser treatment has been employed
to remove tissue, limiting bleeding and loss of body fluids.
Balloons have also been expanded within the urethra to
enlarge its diameter, with and without heat, but have been
found to have significant limitations.
Microwave therapy has been provided with some success
by positioning a microwave antenna within the prostatic
urethra and generating heat in the tissue surrounding the
urethra with a microwave field. Coolants are sometimes
applied within the catheter shaft to reduce the temperature
of the urethral wall. This necessitates complicated
mechanisms to provide both cooling of the immediately
adjacent tissues while generating heat in the more distant
prostatic tissue. This techn;que is similar to microwave
hyperthermia. Similarly, radiofrequency tissue destruction
with electrodes positioned within the urethra has limited
applicability since it necessarily exposes the urethral wall
to destructive temperatures. To avoid this, low temperature
settings required to protect the urethra must be so low that
the treatment time required to produce any useful effect is
unduly extended, e.g. up to three hours of energy
application.
One embodiment of the device of this invention uses the
urethra to access the prostrate and positions RF electrode
stylets directly into the tissues or nodules to be
2121032
-14-
destroyed. The portion of the stylet conductor extending
from the urethra to the target tissue is enclosed within a
longitudinally adjustable sleeve shield which prevents
exposure of the tissue adjacent to the sleeve to the RF
current. Thus the ablative destruction is confined to the
tissues targeted for destruction, namely those causing the
constriction. Other aspects of the invention will become
apparent from the drawings and accompanying descriptions of
the device and method of this invention. It will be readily
apparent to a person skilled in the art that this procedure
can be used in many areas of the body for percutaneous
approaches and approaches through body orifices.
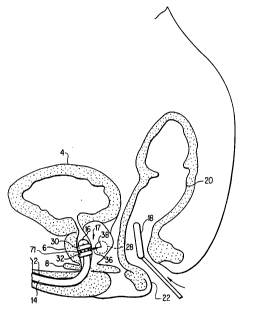
Fig. 1 is a schematic cross-sectional drawing of the
lower male anatomy during use of the device and method of
this invention. The urethra 2 extends from the urinary
bladder 4 through the prostate 6 and urogenital diaphragm 8.
BPH is a condition characterized by constriction of the
portion of the prostatic urethra caused primarily by
proliferation of benign glandular and stroma cells in the
prostate. These nodules press the wall of the urethra
inwardly, restricting the urethral diameter, and can press
normal tissue outwardly, possibly enlarging the prostate.
Traditional treatments short of removal of the prostate have
included either removal of tissue from the urethra to
enlarge its lumen by resection or laser tissue destruction,
or by expansion and heating of the tissue surrounding the
urethra to a temperature which causes cell death. The
latter method is intended to reduce the swelling or
enlargement of the prostate, and restore the urinary passage
to at least a portion of its former diameter.
In the method of this invention, a catheter 14 with a
stylet guide 16 is passed upwardly through the urethra into
the prostate. The position of the guide 16 is precisely
controlled, using an ultrasound image, for example, obtained
from signals received from the conventional ultrasound
transducer 18 inserted into the rectum 20 adjacent to the
prostate through the anal opening 22. The guide 16 facilitates
W094/04220 2 1 2 1 0 3 2 PCT/US93/07437
easy positioning of the stylet 17 into a precise location
under ultrasound imaging. optionally, fiber optics can be
used to position the stylet guide.
The terminal portion of the catheter 14 can optionally
have one or more dilation balloons 30 and 32. Stylet sleeve
36 can be extended through the urethra and other tissue to
be protected, and an RF electrode 38, as shown for example
in this figure, can be extended deep into the target tissue
28.
Fig. 2 is a side view and Fig. 3 is an end view of the
terminal portion of one embodiment of a catheter of this
invention. one or more stylet ports 40 are positioned
between the unexpanded annular balloons 30 and 32. An
ultrasound transponder 42 can be positioned at the terminal
end 44 for producing signals and images which can be used
for precise positioning of the stylet guide housing 16 in
the prostate. Alternatively, an ectogenic bubble can be
incorporated into the distal housing to aid in sonographic
location of the stylet guide. One or more temperature
sensors 46, which can be conventional thermistors,
thermocouples or optical fibers, are positioned along the
catheter to provide a temperature profile of the urethra
adjacent to and preferably on both sides the stylet section.
This temperature profile can be used by the operator to
prevent the temperature of the urethral wall from reaching a
level which would cause cell destruction. These figures
show both balloon segments 30 and 32 and six stylets 36 in
an extended position.
The catheter can be rotated about its central axis
prior to stylet deployment to orient one or more of the
stylets toward tissues to be treated. After the catheter
terminal housing 16 is advanced to a treatment position in
the prostatic urethra, the annular balloons 30 and 32 can be
~p~n~ed in the urethra to stabilize the catheter and dilate
the urethral lumen. The stylets are extended through the
urethral wall and intermediate tissue until they are
positioned in the tissue targeted for treatment. The tissue
- 16 - 21 21 032
targeted for BPH treatment may be nodules, normal tissue or
both. The stylet passageways leading to ports 40 have an
orientation such that their terminal axis forms an angle "all
which can be from about 20~ to 160~ and preferably from about
30~ to 150~ with the central axis of the catheter in a plane
therethrough. As will be explained in greater detail
hereinafter with regard to one embodiment of this invention, a
non-conducting sleeve is then moved to expose the target
tissue to controlled heating by an electric current to a
destructive temperature above 45~C and preferably within the
range of from 55~ to 99~C.
Fig. 4 is a cross-sectional view of a catheter with
an extended stylet of one embodiment of this invention, and
Fig. 5 is a cross-sectional enlarged view of the stylet tip
shown in Fig. 4. In this embodiment, the catheter 48 is
connected to a stylet guide housing 50 with a nose 52. The
stylet 54 comprises a solid core needle 56 coaxially
positioned within a tube 58, both of which are preferably
constructed of a highly flexible, conductive metal such as a
nickel-titanium alloy, tempered steel, stainless steel,
beryllium-copper alloy and the like. Nickel-titanium and
similar highly flexible, shaped memory alloys are preferred.
The needle 56 is axially or longitudinally movable within the
tube 58. The tube 58 is enclosed within a non-conductive
sleeve 60 which is longitudinally movable along the tube 58.
The guide housing 50 has a guide channel 61 which is curved to
permit longitudinal advancement of the flexible stylet.
The sleeve 60 is connected to an annular cylinder 62
connected with a longitudinal thrust tube 64. Longitudinal
movement of the thrust tube 64 causes a corresponding
longitudinal movement of the sleeve 60 along the tube 58. The
sleeve movement is used to vary and control the length of tube
58 and needle 56 exposed to surrounding tissue and control the
amount of energy delivered to the target tissue. The
material, insulating properties, dielectric properties and
- 17 - 2 1 2 ~ 0~2
thickness of the sleeve 60 are selected to prevent heating
energy delivery to tissue in contact therewith by shielding
the tissue from the conductor. If the tissue is to be heated
using radiofrequency current (300 to 750 kHz), the sleeve 60
must have sufficient thickness required to prevent both
current flow and capacitance coupling with the tissue.
Figs. 6 and 7 are cross-sectional, fragmentary
representations of an embodiment of the catheter of this
invention with a stylet guide system for adjusting the stylet
guide angle. The stylet guide housing 124 has a stylet port
126. Within the guide housing 124, a stylet positioning block
128 is positioned for axial movement under the action of a
torque and thrust rod 130. The stylet positioning block 128
has a curved stylet lumen containing a stylet 132.
Optionally, a low friction, flexible guide tubing 134 extends
from the positioning block 128 to the port 126. In the
position shown in Fig. 6, the positioning block 128 is in a
retracted position, orienting the stylet to extend at an acute
angle "b" of approximately from about 20~ and preferably 30~
up to 90~ with respect to the central axis of the guide
housing. Advancement of the stylet 132 through the block 128,
guide tubing 134 and port 126 directs the stylet into tissue
along the dotted line path 136.
Advancement of the positioning block 128 as shown in
Fig. 7 forces the stylet 132 through a curved path having a
smaller diameter through guide tubing 134 to the port 126.
The stylet 132 is then directed an obtuse angle "b" which can
be as high as about 160~ with respect to the guide housing
axis. Advancement of the stylet through the guide block 128,
guide tubing 134 and port 126 in this configuration directs
the stylet into tissue along the dotted line path 138.
As shown in Figs. 6 and 7, the angular projection
of the stylet 132 can be oriented over a wide range of angles
in a plane through the central axis of the stylet guide
housing. It will be readily apparent that rotation of the
2 1 2 1 032
- 18 -
torque and thrust rod 130 about its central axis will cause a
corresponding rotation of the stylet guide housing and
deflection of the stylet in directions outside of the axial
plane. This combined with axial movement of the catheter 124
to an optimum position in a duct and rotation of the catheter
about its central axis yields an infinite variety of stylet
orientation angles. A combination of these movements provides
greater choices of stylet angles so that the stylet can be
advanced to target tissue at any angle from the catheter.
Figs. 8 and 9 are detailed schematic cross-sectional
views of a RF electrode stylet shown in Fig. 4 in use. After
the catheter is positioned in the urethra, the stylet 54 is
advanced from the stylet guide housing 50 through the
prostatic urethra wall 71 to the target tissue 73 to be
treated (outlined with a dotted line). Then, stylet sleeve 60
is retracted to the position shown in Fig. 8, exposing the
portion of the RF electrode positioned in the target tissue
73. RF current is then directed from the electrode 56 and 58
through tissue 73 to conventional grounding plates (not
shown). In selected instances, more directed ablation can be
obtained by using one or more of the stylets as the
indifferent electrode and another of the styles as the active
electrode, thereby using only stylets to complete the dipole
and not using a grounding plate. The RF treatment is
continued until the cells in the target tissue 73 have been
destroyed.
Fig. 9 is a detailed schematic cross-sectional view
corresponding to Fig. 8 in an optional second step following
the procedure described above. Following destruction of the
cells in target tissue 73, the RF electrode sleeve 60 can be
retracted along the stylet electrode 58 to the stylet guide
housing 50, exposing a length of RF electrode 74 leading from
the target tissue through prostatic urethral wall 71.
Sufficient RF current is then applied to cauterize the surface
21210~2
-- 19 --
of the tissue 76 (shown by dotted lines) immediately in
contact with the entire exposed surface of the electrode 58.
For example, this can be achieved with a higher voltage and
shorter duration treatment than is applied to destroy the
cells of the target tissue. The stylet is then fully
withdrawn into the housing 50, leaving a drainage duct leading
from the area of the target tissue 73 to the prostatic
urethra. This can provide drainage of the products of the
treated target tissue 73 during the healing process.
The transurethral needle ablation (TUNA) process of
this invention is a process whereby a physician in a unique
procedure delivers radiofrequency to the hyperplastic tissues
of the prostate which develop in men with the condition known
as BPH, or Benign Prostatic Hyperplasia. This procedure is
unique in that it is the first transurethral procedure which
selectively provides the ability to limit the treatment to
the constrictive tissue and spare the normal prostatic tissue.
This procedure also minimizes the trauma sustained by the
surrounding prostatic urethra, especially when compared to
previously known procedures for relieving obstructive uropathy
due to BPH. The procedure could possibly be carried out
under local anesthesia only, depending upon the rate of
energy delivery and degree of pain sensation experienced
by the patient. When local anesthetic is adequate, the
procedure can be performed in the physician's
W094/04220 PCT/US93/07437
~ 2121032
-20-
office. Local anesthetic could be delivered or applied in
the form of a lubricant containing a topical anesthetic such
as lidocaine mixed with K-Y jelly.
If substantial pain will be experienced by the patient,
the patient must be sedated in addition to application of
topical local anesthetic. This procedure can be provided on
an outpatient basis and would require a short term (2-6
hour) observation. If the procedure and patient require
greater pain control, then spinal anesthesia or a general
anesthesia may be used for patients which qualify for their
use. This would mandate that the procedure be carried out
in the operating room, would require a recovery room, and
could possibly require inpatient care in certain
circumstances. The previously known prostate resection
(TURP) generally requires use of general or spinal
anesthesia and in-patient hospital care following the
treatment.
The BPH method of this invention can be carried out in
the following manner, using a RF electrode stylet embodiment
of this invention. A male patient is given the appropriate
preprocedure preparation which would usually require a
fleets enema or bowel preparation. This would clear the
rectal vault of stool in order to better place a rectal
ultrasound probe, if used, and to assure better
visualization. Appropriate anesthetic, would then be
administered. A conventional grounding plate is then placed
in contact with the patient. The rectal probe would then be
inserted to the level of the prostate in order to obtain an
ultrasound image of the prostate. The procedure could be
done without the use of rectal ultrasound, using only direct
visualization at the discretion of the operator. The
urethral catheter would then be inserted in a fashion
similar to that used for inserting a Foley catheter. First
the glans and the penile shaft would be bathed in betadine
or other disinfectant. The rest of the groin adjacent areas
are draped with sterile towels in the usual fashion. Then
using aseptic or sterile technique, the shaft of the penis
212~0~2
-21-
is grasped in one hand while the catheter is inserted into
the urethral meatus and advanced until it has reached to
desired position in the prostatic urethra. The catheter
movement during its advancement through the urethra can be
monitored directly with the ultrasound image. If direct
visualization with fiber optics is used, the appropriate
landmarks are located and identified, i.e., verumontanum and
bladder neck, etc. If this has not been accomplished
earlier, the various electrical and mechanical connections
between the catheter and the control assembly are connected
at this stage.
The RF electrode stylet is then deployed under direct
vision or ultrasound imaging into a selected target tissue.
This requires that the physician locate the target area to
be treated, rotate, advance and/or retract the catheter as
necessary to orient the stylet guide port toward the target
area. The stylet, preferably completely surrounded in its
insulating sleeve or sheath, punctures and penetrates the
epithelial lining of the prostatic urethral, traveling
through prostatic tissue to the target tissue, and
penetrating the tissue to the desired depth. Local
anesthetic can be infiltrated into the target tissue through
the central lumen of the stylet as the stylet is advanced.
The insulating sleeve is then retracted the amount required
to expose a precise selected length of the RF electrode in
the target tissue. This amount is selected to effect the
degree and volume of tissue destruction desired, the volume
increasing as the length of the exposed electrode increases.
This volume is selected based on the size of the target
tissue to be ablated and the relative position of the
electrode stylet in the target tissue. The distance the
sleeve is withdrawn can be measured external to the body
using a conventional measuring devices such as a scale.
The electrode stylet is then energized from an RF
energy source by closing a conventional switch. Preferably,
the time and/or power levels are preset by the control unit.
The RF energy is delivered to the target tissue for a
- 22 - 2 1 21 ~2
preselected time, monitoring the advance of the destructive
lesion by the rectal ultrasound image. Impedance is also
monitored, and when or if it exceeds a preset value, the power
supply can be reduced or terminated. The temperature of the
catheter surface adjacent the urethral lining, the sleeve and
even the exposed electrode can also be monitored using
temperature sensors attached to these components to precisely
control the volume of the lesion and prevent excessive heating
of normal tissue.
After the target tissue destruction has proceeded to
the desired stage, the physician has two options. The stylet
electrode can be withdrawn into the catheter to facilitate
quick healing and rapid sealing of the urethral puncture site.
Alternatively, the physician can create a temporary
physiological drainage capillary which would allow any fluid
or debris accumulating in the ablated target tissue to drain
into the urethra. This physiological drainage capillary can
be created after target tissue destruction by withdrawing the
insulating sleeve or sheath back into the urethral catheter as
shown in Fig. 9. The conductive stylet is then energized to a
level sufficient to "sear" or cauterize a small hollow channel
through the tissue. This channel will eventually scar and
fibrose, or it will seal and heal. The conductive stylet is
then entirely withdrawn, and the catheter is slowly and
carefully withdrawn from the urethra. The patient is then
monitored and treated as appropriate for the type of
anesthesia delivered and the condition of the patient.
Fig. 10 is a schematic view of the assembly of the
power and control system 150, a manual catheter control unit
152, catheter 154, and power foot control 156. The power foot
control functions can be accomplished by numerous other
methods to include manual digital switches on control box 150
and by a trigger device on the catheter handle 152. The
manual operation of the catheter assembly is controlled from a
manual control unit shown in greater detail in Fig. 11, with
the power control and temperature displays being provided in
the control system 150 shown in greater detail in Fig. 12.
2121032
- 23 -
Fig. 12 is an isometric representation of an
embodiment of a manual control system of the system of this
invention. The manual control 152 has a pistol grip 158 with
a tube 160 leading to the console shown in Fig. 13. The tube
160 houses RF power supply cables, temperature sensors,
ultrasound transducer power and signal delivery leads, balloon
inflation fluid and vacuum lumens.
Rocker switches 162 and 164 provide control over the
inflation or deflation of balloons 30 and 32 (Figs. 1 and 2).
Tab 166 sliding in groove 168 is connected to a stylet 62,
advancing it into the target tissue as the tab 166 is moved
forward. Rotary dial 170 is attached to the catheter 154 and
can be used to rotate the catheter for orientation of the
stylet or stylets. Window 172 has graduations showing the
percentage of balloon expansion.
Fig. 12 is an isometric representation of an
embodiment of a power and control console 150 of the system of
this invention. The housing of this console has a display
panel 174 with digital readout displays 176 showing power to
the stylet, antenna temperatures, tissue temperatures,
impedance values, and other data, for example. The housing
can support a sealed membrane switch panel 178 having system
control buttons 179. Power cord 180 leads to a standard power
outlet. Cable 182 leads to the manual catheter control unit
152 shown in Fig. 11. Cable 184 leads to an optional power
foot control unit. Cable 185 leads to the grounding patch for
use in unipolar systems.
Fig. 13 is a view of an alternative four-probe
embodiment of the device of this invention. The device
comprises a handle portion 180 and a catheter portion 182.
The catheter portion 182 includes an elongated catheter 184
having a distal catheter probe end 186. A plurality of
stylets 188 extend outwardly from the probe end 186. The end
190 of the handle portion 180 is attached to the proximal end
of the catheter 182, and manual control tabs 192 and 194
mounted thereon for sliding engagement with side walls of the
handle portion. Using the handle 180 for control, the
catheter is introduced into a body duct, vascular structure or
2121032
- 24 -
canal such as the urethra, for example, and pushed up the duct
to the treatment position, for example a position adjacent the
prostate. Stylets 188 are individually and selectively passed
outward from the distal end 190 through surrounding tissue to '
the target tissue to be treated by movement of respective
manual control tab pairs 192 and 194. When the stylets are
electrical conductors surrounded by movable sleeves, the
sleeves can be retracted from the end of the stylets by
movement of manual control tabs 194 as described in greater
detail hereinafter. Preferably, the proximal portion of the
catheter 182 is preferably stiff to facilitate control during
insertion in a body duct, while the distal portion is
preferably flexible to allow the catheter to pass through
curved duct portions.
Fig. 14 is a side partially sectioned view of the
distal probe end of the catheter shown in Fig. 13 with stylets
extended from the side ports, and Fig. 15 is a cross-sectional
end view of the probe end of the device shown in Fig. 14,
taken along the line 15-15. The distal catheter tip 186 is a
stylet guide housing having a lateral surface 196 which merges
with a tapered tip portion 198. The stylets 188 extend
outwardly from the lateral surface 196 and comprise an
electrode 200 and movable surrounding sleeve 202. The
proximal portion 204 of the stylet guide 214 is connected to
the distal end 206 of the catheter stem 208. Further stylet
ports such as the port from which stylet 203 extends are
positioned at a greater distance from the tip 198 than ports
216. The embodiment shown in Figs. 14 and 15 comprises two
sets of stylets, each pair extending from ports in a common
plane perpendicular to the catheter central axis. It will be
readily apparent to a person skilled in the art that other
stylet arrays such as a longitudinal array or a spiral array
can also be used, and these variations are considered to be
fully within the scope of this invention.
The catheter stem 208 includes an outer tubular
housing 210 which encloses a plurality of stylets stems 212
disposed in a parallel relationship. As can be seen from Fig.
15, the individual stylets are directed outward in paths which
- 25 - 2 1 2 i 032
have axes forming angles with each other. Oppositely disposed
stylets can form an angle of up to 180~ while in the
configuration shown, the axis of adjacent stylets can form an
angle of up to 90~ for example.
Fig. 16 is a partial cross-sectional view of the
probe end of the device of this invention, taken along the
line 16-16 of Fig. 15. The stylet is directed through a
stylet guide means 214 in the distal catheter end 186 which
leads from a path in the proximal end 204 of the stylet guide
214 parallel with other stylet guides to a lateral orientation
through stylet port 216. To facilitate longitudinal movement
of the stylet through the guide path, the guide path
preferably has a curved portion 218 extending to the port 216.
The curved path optionally has a radius which is sufficient to
deflect the deployed, extended stylet to the desired angle,
that is, a radius of up to 0.5 cm, depending upon the diameter
of the catheter. Optimally, the guide path also has a reverse
curved portion 220 which extending from the axially parallel
path in the proximal end 204 outwardly away from the port 216
to the beginning of the curved path 218.
The distal tip 198 of the catheter can have a hollow
space or bubble 222 which reflects ultrasound, permitting its
easy identification with ultrasound generated by a rectal
probe as shown in Fig. 1. Alternatively, a transponder can be
mounted in the distal tip 198.
Fig. 17 is a cross-sectional view of the handle and
control end of the device shown in Fig. 13, taken along its
central axis. The control handle 180 is attached to the
control end of the catheter stem 208. The handle 180
comprises a housing having a distal end forming an axial
sleeve 224 enclosing the proximal end 226 of the catheter stem
208. The proximal end 226 is held in place by setscrew 228
extending through the sleeve 224. Manual engagement means 192
and 194 engage lateral handle housing walls 230 and 232, and
are mounted for sliding engagement with respective slots 234
and 236 in the respective housing walls. They translate the
manual motion into longitudinal motion of the stylet in the
stylet guide means.
,.
- 26 - ~1 2 i O ~ 2
Fig. 18 is a cross-sectional view of the control end
of the device shown in Fig. 13, taken along the line 18-18 of
Fig. 17. Referring to both Figs. 17 and 18, finger engaging
sleeve movement tabs 192 are connected to connecting slide
portion 238 extending through a respective longitudinal slot
234 and an inner portion 240 which forms a sliding engagement
with the interior surface of the handle wall 230. Slot 242 in
the connecting slide portion receives a pin 244 extending
through a sleeve connector 246. Axial movement of the tab 192
thus effects an axial movement of corresponding sleeve 248 in
the handle. Each side of the handle can have a pair of
longitudinal, parallel slots to accommodate manual tabs for
both sleeve and electric conductor.
Fig. 19 is a cross-sectional view of the control end
of the device shown in Fig. 13, taken along the line 19-19 of
Fig. 18. Referring to Figs. 17 and 19, finger engaging
electrical conductor movement tab 194 is connected through a
connecting slide portion 250 extending through a respective
longitudinal slot 236 to an inner portion 252 which forms a
sliding engagement with the interior surface of the handle
wall 232. Slot 254 receives a pin 256 extending through an
electrical conductor connector 258. Axial movement of the tab
194 thus effects an axial movement of the corresponding
electrical conductor 260 in the handle.
Movement of adjacent tabs 192 and 194 advance the
corresponding sleeve and electrical conductor together through
the corresponding stylet guide, out the corresponding stylet
port, and through intervening tissue to the target tissue to
be ablated. Reverse movement of the sleeve tab 192 then
retracts the sleeve to expose a selected area of the
electrical conductor surface in the tissue, preparatory to
ablation.
Fig. 20 is a side view of the non-conductive sleeve
connector of the embodiment shown in Figs. 17 and 18, and Fig.
21 is a cross-sectional view of the non-conductive sleeve
connector shown in Fig. 20, taken along the line 21-21.
Connecting pin 244 extends through a hole in the sleeve
212~032
- 27 -
_
connector 246. An axial edge of the sleeve connector 246 is
connected to the proximal end portion 248 of the sleeve.
Fig. 22 is a side view of the electrical conductor
connector of the embodiment shown in Figs. 17 and 19, and Fig.
23 is a cross-sectional view of the electrical conductor
connector shown in Fig. 22, taken along the line 23-23.
Connecting pin 256 extends through a hole in the electrical
conductor connector 258. An axial edge of the electrical
conductor connector 258 is connected to the proximal end
portion 260 of the electrical conductor.
Fig. 24 is a cross-sectional view of the distal end
of the non-conductive sleeve shown in Figs. 15-17 taken along
its central axis. The non-conductive sleeve 202 comprises a
tapered leading tip 262 and a rigid proximal portion 264. A
flexible portion 266 extends between the leading tip 262 to
the rigid proximal portion 264. The flexible portion 266 can
be any flexible configuration such as a spiral coil, wire
braid, stainless steel tube, or any other flexible
construction which yields a catheter which has the required
flexibility and torque strength. If the flexible portion 266
and the rigid proximal portion 264 are made of conductive
materials such as metal, they can be covered with an
insulating sleeve 268. The annular ridges 270 in the rigid
proximal portion and the flange 272 in the tip engage the
sleeve 268, securing the sleeve in place. The inner lumen 274
of the non-conductive sleeve 202 receives the electrical
connector 200. A temperature sensor such as thermistor 271
can be mounted on the tip to provide local temperature
information. An ultrasound transponder 273 can also be
mounted on the tip to provide a signal useful for precise
positioning of the stylet tip in a tissue to be ablated.
Fig. 25 is a top view of a two stylet preferred
embodiment of an RF ablation catheter of this invention. The
flexible catheter 300, attached to handle 302, has a terminal
stylet guide 304 with two stylets 306 and 308. The handle has
stylet sleeve tabs 356 and electrode tabs 358 as will be
described in greater detail hereinafter. The handle is also
connected to a visual monitor 301 and RF power connector 303,
- 28 - 2121032
transponder connector 305 and thermocouple connector 307. The
portion of the catheter 300 leading from the handle 302 to the
stylet guide tip 304 can optionally have a graduated
stiffness. For example, the catheter can be designed to be
more stiff near the handle and more flexible near the tip, or
any other stiffness profiles. The catheter can be constructed
of an inner slotted stainless steel tube with outer flexible
sleeve such as is described in U.S. Patent No. 5,322,064. It
can also be made of coiled or braided wire to which an outer
sleeve is bonded.
Fig. 26 is a top view of the stylet tip of the
embodiment shown in Fig. 25, Fig. 27 is a side view of the
single grind electrode tip shown in Fig. 26, and Fig. 28 is an
end view of the electrode tip shown in Fig. 27. In this
embodiment, the sharpened tip 326 and leading cutting edges
328 and 330 are formed by grinding one surface of the tip, the
cutting edges forming an angle, "d", of from 15~ to 45~ and
preferably from 25~ to 35~ with a line parallel with the
central axis of the tip. The proximal surface of the tip
forms a shoulder 332 which the leading or distal edge 334 of
the sleeve 336 abuts, preventing movement of the sleeve 336
over the sharpened tip. The sleeve 336 can also support
temperature sensors such as a thermistor 338 and an ultrasound
transponder 340.
Fig. 29 is a side view of an alternative double
grind electrode tip, and Fig. 30 is an end view of the
electrode tip shown in Fig. 29. In this embodiment, the
sharpened tip 342 and leading cutting edges 344 and 346 are
formed by grinding both surfaces of the tip. The proximal
surface of the tip forms a shoulder 348 which the leading or
distal edge of a sleeve (not shown) abuts, preventing movement
of the sleeve over the sharpened tip. The forward cutting
edges of this embodiment make little if any contact with the
inner surface of the stylet guide in the catheter tip,
preventing dulling of the cutting edge.
Fig. 31 is a top view of the handle portion of the
ablation catheter of Fig. 25. The handle 302 has an upper
housing plate 350 upon which stylet sleeve positioning slides
- 29 - 2~7~332
352 and electrode positioning slides 354 with manual tabs 356
and 358 are mounted for sliding movement in the direction of
the central axis of the housing. The position of the leading
edges 360 of the slides relative to the graduated markings 362
on the housing plate surface are used to determine the
distance the sleeve and stylet have been advanced from the
stylet guide toward tissue to be treated.
Fig. 32 is a side view of the handle portion shown
in Fig. 31 taken along the line 32-32 with the bottom housing
cover plate partially removed. The proximal end of the
catheter 300 passes through a cylindrical hole 364 in the
cylindrical knurled knob 366 and cylindrical receptor 368
formed by the opposed hemicylindrical surfaces in the distal
ends of the upper housing plate 350 and lower housing plate
370. The proximal end of the knurled knob 366 has a
cylindrical receptor 372 which forms a sliding fit with a
cylindrical projection 374 formed by the distal ends of the
housing plates 350 and 370. Setscrew 376 secures the knob 366
to the catheter 300 so they rotate together as a unit. Pin
378 extends through the knob 366 into an annular groove 380,
allowing rotation but preventing axial movement of the knob
366 relative to the cylinder 374. The angular position of the
knob 366 relative to the housing plate 350 is shown by the
position of the arrow 382 relative to the graduations 384 on
the knob (Fig. 31). Knurled knob 386 threadingly engages hole
388 in the housing plate 350. When the catheter knob 366 has
been turned to rotate the catheter 300 (and the stylet guide
on its end) to a desired stylet orientation, advancement of
the knob 386 against the catheter surface 390 secures its
angular position. The stylets are then advanced through
surrounding tissue to the depth desired, as indicated by
graduations 362.
Fig. 33 is a bottom view of the handle portion shown
in Fig. 31 with the catheter, distal knob and bottom cover
plate removed, and Fig. 34 is a cross-sectional view of the
handle portion taken along the line 34-34 in Fig. 33. Stylet
movement guide plates 392 and 394 are securely mounted in
terminal end receptors 396 in the inner surfaces of upper
2 1 2 1 032
-
housing plate 350. Each of the guide plates 392 and 394 has a
sleeve guide slot 398 and an electrode guide slot 400 therein.
Screws 402 extend through sleeve guide slots 400 and
threadingly engage the sleeve guide blocks 404. Axial
movement of the screws 402 and guide blocks 404 attached
thereto is limited by the length of the slots 398. Sleeve
connector 406 attached to stylet sleeve 408 is secured to the
guide block 404 by screw 410. Slide plate 352 mounted for
sliding movement in a slot 414 in the housing plate 350 is
secured to guide block 404. Screws 416 extend through sleeve
guide slots 400 and threadingly engage the electrode guide
blocks 418. Axial movement of the screws 416 and guide blocks
418 attached thereto is limited by the length of the slots
400. Electrode connector 420 attached to stylet electrode 422
is secured to the guide block 418 by screw 4Z4. Slide plate
354 mounted for sliding movement in a slot 426 in the housing
plate 350 is secured to guide block 418.
Fig. 35 is a cross-sectional view of the central
portion of the handle portion shown in Fig. 32 in the stylet
Z0 and sleeve retracted position (corresponding to the positions
in Fig. 31). Fig. 36 is a cross-sectional view with the
stylet and sleeve in an extended position, and Fig. 37 is a
cross-sectional with the stylet in an extended position and
the sleeve partially retracted therefrom. The stylets are
extended after the catheter is inserted to place the stylet
guides in a position laterally adjacent the target tissue to
be treated and the catheter has been rotated to orient the
stylet guide outlets in the direction of the target tissue.
The stylets are extended through intervening tissue to the
target tissue by moving the manual tabs 356 and 358 toward the
distal end of the handle as shown in Fig. 36. This affects
simultaneous movement of the stylet sleeve 408 and electrode
422. After the extension has proceeded to the extent required
to place the tip of the electrode 422 in the target tissue,
the sleeve 408 is retracted to the position shown in Fig. 37
by moving the manual tab 356 in the proximal direction to the
extent required to expose the desired portion of the electrode
as indicated by graduations 362 (Fig. 31). The RF current is
- 31 - 2 1 2 1 032
then applied to the electrodes until the desired ablation has
been achieved. With this embodiment, two stylets can be
extended, sleeves retracted, and the ablation achieved either
concurrently or sequentially.
Fig. 38 is a schematic view of a deployment of two
stylets in a prostate showing stylet orientation for
overlapping ablation zone method of this invention. For
purposes of illustration but not by way of limitation, the
prostate has been selected for this explanation, and
application of this method and assembly to other areas of the
body are intended to be included.
The tissues to be treated for the treatment of BPH
are located in the transition zone 428 of the prostate. A
catheter of this invention 430 has been inserted up the
urethra 432 to a position adjacent the prostate. Two stylets
434 and 436 have been passed through the urethra wall 432 and
surrounding tissue into the target tissue, and the non-
conducting sleeves 438 and 440 have been retracted to expose a
portion of the respective electrical conductors 442 and 444 at
the end of each stylet. The angle between the axes of the
stylets in this embodiment, "e", is less than 180~, preferably
less than 110~. For most overlapping ablations, angles of 15~
to 90~, and more usually from 20~ to 70~ are most practical.
A grounding plate (not shown) is placed on the body exterior.
Z5 When electrodes 442 and 444 are supplied with RF
current, the circuit from the electrodes to a grounding plate
is closed. The current density flowing through the tissue
passes through the target tissue to be treated, creating
lesions having the approximate cross-sectional shape of
overlapping zones 446 and 448. The current density rapidly
decreases as a function of distance, limiting the size of the
lesions. In this manner, lesions can be caused to overlap to
form a larger lesion, increasing the efficiency of the
treatment. It will be readily apparent that these processes
can be carried out concurrently, as described, or
sequentially, and these variations are intended to be included
in this invention.
- 32 - 2 1 2 1 03 2
. .
Although preferred embodiments of the subject
invention have been described in some detail, it is understood
that obvious variations can be made without departing from the
spirit and the scope of the invention as defined by the
appended claims.