Note: Descriptions are shown in the official language in which they were submitted.
W094/~5759 2 ~ 2 1 ~ 9 2 PcTJus93/O~O~O
--1 -- ! ; `",1
AN IMPRO~ED CLEANING COMPOSITION
WHICH INCLU~ES A SULFONATED ALKYLATED AROMATIC
SURFACTANT A~D A NnNJONIC SURFA~TANT
~',
BACKGROUND OF THE INVENTION
This invention is directed to cleaning
compositions and methods useftul in remo~ing ~oils,
particularly those of oil and grease, from surface~
The ~omposition~ useful in the present invention
contain a sulfonated alkylated aromatic ~"SAA") ::~
surfactant and a nonionic sur~aotank.
The largest group of SAA surfactants in use in
cleaning compositions are based on dodecyl benzene
sulfonate; a predominately monoalkylated mono~lfonated ~:
(MAMS) benzene. Another family of SAA ~ur~actant
analogs are based~on diphenyl oxide and are -~
predominately monoalkylated having a relatively short
: (less than:C16) alkyl chain and are predomina~ely
di~ulfonated (MADS ~PO). The SA~ ~urfactants o~ the
present invention,~on khe other hànd, are comprised of
: at least one longer alkyl side chain (greater than or :~
;~ equal to C18)t are predomlnantly monosul~onat~d, and may
:~ contain one or more phenyl: grQups.
,. ..
WO 94/05759 P~/US93/080$0
2I2~ 7~2 -2-
SUMMARY OF THE I NVENT I ON .::
:,' .
One aspect of the present invention is an
improved cleaning composition which offers superior
cleaning of soil~ especially those of oil and grea~e.
More specifically, the removal of oily particulate
stains or soils from surfaces by nonlonio surfae~ant~
can be ~ubstantially improved throu~h the addition of
cerkain ~ulfonated alkylated aromatic ("SAA")
surfactants to the nonionic surfactant.
- The class o~ SAA surfa¢tant analog~ used in the
, cleaning composition of the present invention aontain an ~
aromatic moiety comprising at least one benzene ring. -
The aromatic moiety may contain up to three benzene ;
rings attached in variou~ ~orms. For example ? the ;
aromatic moiety may contain polyoyclic aromatic(~) such
a~ naphthalene, anthracene, and the like~ Additionally,
the aromatic moiety may contain an ethe~ linkage as in
diphenyl oxide or biphenylphenyl ether, or other linking
atoms. Preferred aromatic moietie~ are benzen~
toluene, xylene, naphthalene, dipheny~ oxide, biphenyl,
~ and biphenylphenyl ether.
The SAA oP the present invention contains at
least one~ alkyl group from a C1g up to about C30. For
the purposes of t~his invention, a m~noalkylated SAA
refers to a SAA having one alkyl group with a chain
length of C18 up~to about C30 per aromatic mo;ety and a
30 dialkylated SAA refers to a SAA having two alkyl groups ~`
with chain lengths~;o~ C18 up to about C30 per aromatic
moitety. Thus, a monoalkylated~xylene~has one alkyl
group~of chain length of Clg up to~about C30 and two
,: ~
. ~, ' !
' ' ~ ' ;'
.
' ; ''''
': ~ ' ~ ,`~'
W094/05759 PCT/US~3/080X0
212~7~2
-3-
methyl groups. The SAA of the present invention may be
monoalkylated or dialkylated.
The SAA of the present invention al~o includes
at least one sulfonate group; wherein ~or aromatics i`
con~isting of more than one benzene ring, disulfonate i~
present in less than 50 percent of the total SAA. Thus,
the aromatic moiety in the present invention is
sulfonated to at least about 100 percent and no more
than about 150 percent.
- ~he more pre~erred sulPonated alkylated ^~`
, aromatics ar~ monoalkylated or dialkylated,
monosulfonated benzene, toluene, xylene, naphthalene~
diphenyl oxide, biphenyl, and biphenylphenyl ether. The
SAA of the present invention can be used in its acid or
salt/neutralized form. Thus, the SAQ ~urfactant is
represented by the following formula: -
(R) " ~ r~ so3 X~)n,
~20
where: ,
Ar i5 an aromatic moiety which may be, but is
not limited to, benzene, toluene, xylene,
naphthalene, diphenyl oxide, biphenyl, or
~ , ,
CJ biphenylphenyl ether;
R is an alkyl group having a chain length of ~;
from 18 up to about 30;
.
X~ is hydrogen or a compatible counterion,
which may be, but is not limited to NH4, Na,
Ca~2~, protonated~diethanol amine, or protonated
triethanol;amine;
1 < n' ~ 1~5; and
1 ~ n'l < 2.
~¢,
......
, :
~: .
.. .
WOg4/05759 PCT/US93/080~0
2 1 2 1 1 9 ~ --4~
The present invention also includes a method of
cleaning a tain from a surface of fabric characterized
by contacting the stain with the cleaning composition of
the present invention comprising a ~ulfonated alkylated
aromatic surfactant and a nonionic ~urfactant.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a graph showing the influence of
the per ent of disulfonation o~ one embodiment of the
pre~ent invention on pretreatment cleaning.
Figure 2 is a graph showing the influe~ce o~
the percent of dialkyla~ion of one embodiment o~ the
present invention on pretreatment cleaning.
Figure 3 is a graph showing the influe~ce of
the ratio of the sulfonated alkylated aromatic ;~
surfactant to nonionic surfactant in one embodiment of
the present invention.
Figure 4 i~ a graph showing the influence o~
the type of countsrion on cleaning performance of one
embodiment of the present invention.
Figure 5 is a graph ~howing the influence of
water on the cleaning performance of one embodiment of
the present invention.
Figure 6 is a graph showing the in~luence of
water on the cleaning performance o~ another embodiment
of the present inventio .
~'
"~.
; ;,~
W094/05759 PCT/US93/08080
~12~792 `
-5-
DETAILED DESCRIPTION OF THE INVENTION ;;
The sulfonated alkylated aromatic surfactant
Commercially available monophenyl sulfonated
alkylated aromatic surfactants useful in the present
invention include Ari~tol F sulfonic acid (available
from Pilot Chemical Company) 7 which is a monosulfonated,
C20-24 alkyltoluene (monoaromatic~ and is greater than
90 percent monoalkylated.
Commeroially available alkylated aromatic
compounds may be predominantly mono~ul~onated to be
included as SAA surfaotants o~ the presenk invention.
An example of such compounds includes Aristol A
synthetic sulfonata~le oil available ~rom Pilot Chemical
Company. Ariitol A is a C20-24 alkylbenzene
(monoaromatic) which is at least 80 p~rcent
monoalkylated.
The SAA surfactant o~ the pre~ent invention
~ will be monoalkylated or dialkylated and monosulfonated
tor no more than 50 mole peroent disulfonated for the
polyaromatics). The SAA surfaotants o~ the present
inven~ion can be prepared by a Friedel-Craft~ reac~ion
of an alp~a olefin with an aromatic moiety such as
diphenyl oxide. The weight percent of the monoalkylated
and dialkylated d~iphenyl~oxide (DPO) is determined by -~
gas chromatography~of the distilled alkylated diphenyl ~
oxide~(ADPO). The alkylates~(ADPO)~can then be ~`;
sul~ona~ted wikh;either chlorosulfonic acid or sulfur
trioxide and can be u~ed in the acid ~r salt/neutralized
~orm. The~preferred polyaromatic SAA surfactant analog
of the~present invention has a greater mole percent of
~monosulfonation relative~to~disul~onation. The
pre~erred sulfonating agen~ choqen to produce such an
,
::
: ~
W094/05759 pcr/uss3/o~oBo
21217~
analog in a laboratory environment is chlorosu~fonic ~;
acid. However, a different sulfonating agent, ~or e
example sulfur trioxide, may be preferred in a ;
produotion environment. The percentage of the various
sulfonated components (for example disulfonated to
unsulfonated, monosulfonated and higher sulfonated) is
determined by liquld chromatography.
The Nonionic Surfactant
.:
Nonionic surfactants suitable for inclusion in
the present cleaning compositions and methods are cho~en
to impart compatibility and enhance interfacial
interrelations between the cleaning ¢omposition, the
stain, and the surface from which the stain is to be
removed. Suitable nonionic aurfaotants include, but are
not limited to: ethylene oxide adducts of alcohols,
alkyl phenols~ fatty acids~ Patty acid amides and fatty
acid esters.
The nonionic surfaotant may be monomeric or
polymeric. Suitable nonionic surfactants include those ;~
taught in the EncyclopediaofChemicalTechnology, 3rd ;:
Edition, Vol. 22, pages 360-377. Preferred surfactants
~25 are ethylene oxide~adducts of alcohols and alkyl
phenols~ Preferred nonionic surfactants selected for
" .
inclusion in clean;ing compositions of the pre~ent
.
invention will preferably have a hydrophilic lipophilic ~-
balance (HLB) value in the~range of~from 8 to 15, more
pre~erably 8 to 13, most preferably 9 to 13. ~;
j ~ .
ExàmpIes of commercially available nonionic
surPactants suitable for the present invention include:
Neodol alcohol ethoxylates avallable from Shell
Chemical; Igepal nonylpnenol ethoxylates available from
.''
,
WO ~4/0575~ ~'Cr/US93/080X()
2121792
--7--
Rhone Poulenc; and Tergitol nonylphenol ethoxylates '~
available from Union Carbide.
The Cleanin~ Composition
The SAA surfactant and nonionic surfac~ant
("NS") are typically present in a weight percen~ of
about 5~ ~ SAAt(SAA ~ NS) < 60%. The more preferred
weight percent for sulfonated alkylaked aromatîc to
nonionic surfactant is about lO~ ~ SAA/~SAA ~ NS) ~
50%; the mo~t preferred weight p~cent is about 15% s
SAA/(SAA ~ NS) ~ 30~. Thus, the weight ratio of'
sulfonated alkylated aroma~ic surfactant present to
nonionic surfactant pre~ent i5 typi¢ally from about 1:19
to about 3-2. Preferably, thi~ weight ratio i~ from
about 1:9 to abou~ 1:1, and more preferably from abdut
1.5:8.5 to about 3:7.
`:
The optimal cleaning per~ormance of the -.
composition occurs when the water con~ent o~ the
composition remains below ~bout 30 weight p~r¢ent of the
total oompo~ition. Also the addition o~ hydrocarbon,
such as hexadecane, to the formulation in an amount of ;
about 60 weight percent organic solvent (ba~ed on the
total ~ormulation weight compri~ed o~nonionic
25 ~urfactant, SAA surfaotant:and~organic solvent) may `;
enhance cleaning performance but the addition of
hydrocarbon unpredictably affects:the cleaning
per~ormance e~ficacy of the~ur~actant system.
30 Compositions according to the present invention ~:
: may take the form~of~a liquid,:emulsioni dispersion or
solution, semi-solid or scft solid or stick. Solid
: stick compositions may be formed by dispersing the
: various disclo3~ed compositions into a semi-hard carrier
mediurn. An example of a~semi-hard carrier medium is ~ :
WOg4/05759 PCT/~S93/~8080
2~21792 8
'~
sodium ~tearate. Solid stick pre-spotting in stain
rsmoving compositions and methods ~or making and using
such a solid stick are described in the U.S. Patents
4,842,762; 4,396,521; and 37664,962~
Methods for cleaning a ioil or sta.in on a
surface or fabric are characterized by conta¢ting the
soil or stain with any of the cleaning compositions of
the present invention described hereinabove. Contaoting ;
the 90il or ~tain with the cleaning composition ma~ mean
contacting the area of the sur~ace or fabric where the
stain or ioil re~ides, in addition ~o directly upon the
, stain itielf. The term "stain" includes any substance
which i~ embedded or not embedded, solid or li~uid, wet
or dry, and at or beneath the surface or fabric~
After any of the cleaning composit~ons of the
present invention have been applied to~ or contacted
with the ~tain, the stain m~y be removed by wiping with
a substrate, ~uch as a wet cloth or sponge; or by
contacting the stain with water, such as by wash.ing with -~
a substantially aqueous media~ In the caQe of fabric ;~:
the composition treated stain i~ preferably laundered
with water and more preferably with an aqueous ~olution
of mostly water and a conventional laundry detergent~
,
The cleaning formulations of the present .
: invention are evaluated by measuring thei efficacy of the
formulation as a prewash staln remover treatment ~or
30 soiled fabric. The cleaning formulation i~ applied to ~.
: ' prestainedlfabric and the fabric is washed and evaluàted
for cleaning efficacy by~a reflectance measurement of
the washed fabric compared to that of the unstained
fabric:and the stained fabric.
,
.
: ~,
WOgq/05~59 2 1 2 1 7 9 2 PCT/US93/08080
_g _ -
.~ ~ ,. .
The following examples illustrate (1) the
preparation of the SAA surfactants of the pre~ent
invention; (2) the performance o~ these surfactants with
nonionio ~urfactants as a~clèaning composition and (3)
the performance ~f commercially available SAA
surfactant~ with nonioni~ ~urfaetants as compone~ts o~
the cleaning compositîon.
Examples
The SulPonated Alkvlated Aromakic Surfactants
I. PREPARATION OF DIPHEN~L OXIDE OR BIPHENYL
SULFONATED ALKYLATED:AROMATIC SURFACTANTS (SAA). ;
Alkylates prepared (percentages are by weight):
'15 ~ C6 monoalkylated diphenyl oxide (DPO)
(comparative:example - not an:example of the ;
invention)
* C14 dialkylated DPO (oomparative example ~ not ~:~
: an example of the invention)
: 20 * C16 monoalkylated DPO ~comparative example -
not an example of the inven:tlon)
; * C18 dialkylated DPO
* C20-24 monoalkylated DPO
* C20-24 75%/25% mono/di~:alkylated DPO
* C24_28 80%/20% mono/di a~lkylated DPO
: ~ : * C30+ 80%/20% mo~no/di alkylated DPO
* C20 2~ 80%:/20% mono/di alkylated biphenyl (BP) ::
,
(IAll of the alpha olefins used to prepare the above
alkylates were obtained from Chevron Corporation,
: therefore, the chain length:specifications are those
furnlshed by Chevron.) ~
,:
,., ~...
:. ~
.
,:
:
WOg4/057s() PCT/US93/08080
2121 7~ -10-
General procedure to prepare alkylated aromatic
compounds:
Preparation of C20-24 monoalkylated diphenyl
oxide (DPO): Into a 5 liter round-bottomed flask (RBF)
equipped with a magnetic stirrer, an addition funnel, a
conden3er, and a nitrogen inlet is placed warm DPO,
(2.040 kg, 12.00 moles, 3 mole equivalents with re~pect
to the olefin). To this light yellow solution is added
the Lewis acid catalyst~ aluminum chloride~ AlCl3, (24.0
g, 0.180 moles, 0.045 mole equivalents with respect to
1~ the olefin). This i~ then ~ea~ed to 70-7S C with
stirring ~or 45 minutes ~o a~ to completely di~olve the
AlCl~. The color vf the reaction mixture will gradually
darken during this time until it is red brown.
Concurrently, the C20-24 alpha olefin (1.185 kg9 4.0d
moles) is melted and placed into a~ addition funnel that
has been wrapped with heating tape. The addition o~ the
olefin i begun once all o~ the AlCl3 dissolves into the
DPO. The rate of olefin addition is to maintain the
temperat~re at 70-75 C (over about 5 hour~).
Once all of the olefin i9 added, external
heating is reapplied and the reaction mixture is post ~;
reacted at 70 C for an additional hour. At the end of
the hour~ heating is discontinued and a 25% aqueous
sodium hydroxide ~olution (349 g~ is introduced. The ~;
reaction mixture is allowed to cool and stir overnight
during which time the color changes from a rather dark
red brown to a light yellow.
The stirring is discontinued and the phase~ àre
allowed to separate. The organic phase is decanted away
from the basic aqueous phase, lS placed into several 2 ~"
liter ~eparatory funnels and is washed several times
with distilled water until the pH of the resulting water
.. ...
'. ' ~
- ';
"
. ..
W o 94/057s9 ~ 1 21 7 9 ~ Pcr/uss3/ososo
- 1 1- 1 .,;,
is neutral. The organic phases are then combined and -~;
~to~ed in plastic con~ainers unt~l ~eeded.
General procedure ~or topping alkylated aromatic
compounds 7 simple distillation:
The removal of the unreacted DP0 (diphenyl
oxide) (ca. 1.3 kg, 2.0 mole equivalents) ls
accompli~hed through the use o~ a simple vacuum
distillation. The wa~hed organic phase portion from
above (ca 3.5 liters) is placed into a 5 liter RBF
equipped with a magnetic stirrer, a simple distillation
head apparatu~ and a vacuum take o~ adapter. The
, reaction mixture is stirred and the vacuum is slowly ~ ;
applied in order to minimize bumping. rhe distillation ~;;
proceeds as follows (Table 1): ,
.
2~
: ~,
:: : :
: : ~
: .:
l; 30
: l~
W~ 94/057~;9 PCI/IJS93/OXO~O 21217Y~
--1 2--
o
c a
E~ a
~ .,
o .~:
a ~ ~ ~ ~ ~ ~ ~
~ E ,
W ~; '
o ~ Z; a ~1 e. ~ ~" ", '
o
O a _~ ~ a` x ~ z ;c x z
(n u~ 0 0 ,:
o
W ~ 3 ~ 3 :-
00 0 0 0
O ~ Z O O O O
o o o o
C U~ ~ U ~ ~ U
o ~ ~
p~ ,.
0 ~ ~ 0
U U U
o o o ~ , a~ o
E ~D ol~ ~rZ ~r 'I r.l ~ a~ o
." ~ o
U~ O
ol z 4 N ~ ~~ 7~ ~ N N
I~ ~ V O ~ r~ rl ~ N ~ æ
o ~
~ N ~ .n
O ~ ~ :
~ o
~I ,
¢ ''
, ,
'; ~
~,
.;
~0~4/0575~ PCT/~S93/0~080
2~2:1792
-13--
The pot material is then used as the topped
material in subsequent sulfonation reactions or
alternatively could be fractionally distilled to obtain
~ractions that conkain varying degree~ vf alkylated
materials (i.e. mono/di/higher alkylate).
General procedure for the isolation o~
mono/di/higher alkylated aromatic compound3 using a
vacuum frac~ional distillation:
Topped material (0.9 liter~ placed into a 2
liter RBF equipped with a magnetic stirrer, a 14 inch
vigreux ~ractionating column and a vacuum distillation
head, The contents are stirred and the vacuum i~
applied. The distillation proceeds a~ follow~ (Table
2): :.
~
;::~
, :.
WO 94/05759 PCI/US93/0~ 8~ ~
2 1 2 1 7 9 2 --1 4-- !
O ,¢ ~ a ~ ~ ~ c, ;
E~ a ; i;
V~ .
o O '.
~ O O~ Q
u ~" oc~o~cr~ I` ~ 117 :~;
O ~ ~ "
31 ~ X
0 2;~5i; O O ~ ~ X ,,~
O "
.u ~
~ , ,~
c~ ~ Z ~ ~ ; æ v
O a
U ~ -.
V I U ~
10 0 ~1 ~ I G
_~ N ~¢ Q~ 3 ` '
:~ V ~ ~ ~ ~ 0:
_~ ~D ~ v v v v ,~ ~
d X .
L~ a ~ ,
0 -- , .
LO , . ,',
~u ~6~ ~u~
,,, .~: , .;
0 ~
_I,~ rl , o ,,,
,_1 G r~ N N N N N N
N N N D N ~ N
.. . .
N~
Ij" :~ ~,''
" ~
';''`
.~ `'.
.~
'.
~g4/0575g PCT/US93/0~080
2~2~7~
-15-
The pot material is used as dialkylated DP0
based material in subse~uent sulfonation reactions and
in the GC standard solution.
Sulfonates prepared:
* C6 monoalkylated monosul~onated DP0 (comparative
example - not an example of the invention)
14 dialkylated, monosulfonated DP0 ~oomparative
example - not an example of the inventio~)
* C16 monoalkylated, monosulfonated DP0
(comparative example - not an exampl~ of the
invention)
* C1~ monoalkylated, monosulfonated DP0
* C18 dialkylated, monosulfonated DP0
* C20~24 topped material, monosulfonated DP0
* C20-24 monoalkylated, disulfonated DP0 ::
(comparative example - not an example of the
invention)
* C20-24 monoalkylated, monosul~onat0d DP0
* C2~_28 topped material, monosulfonated DP0
* C30+ topped material, mono.~ulfonated DP0
* C20-24 topped material, monosulPonated BP :~
General procedure for the monosulfonation of
aroma~ic materials~
C20-24 monosulfonated monoalkyl~ted ~P0: Into
a 1 liter RBF equipped with a magnetic stirrer,
additional ~unnel, Claisen adapter, water condenser,
nitrogen inlet and a h~drochloric ~cid exit i~ placed
; Ithe C20-24 monoalkylated DP0 (t75.0 g, 0.380 moles).
This is diluted with chloro~orm (350 mL) and cooled to
O~C via an ice bath. To the addition funnel is added
chloroform (50 mL) and chlorosulfonic acid 525.3 mL, :~
0.380 moles). The oontents oP the addition ~unnel are
.....
WO 9'1/0575g PCI /US93/~)8081)
2~2~7~2 -16- . !
mixed and added lnto the reaction vessel over a 2 h ~;
period. ~uring this time the reaction mixture's color
goes ~rom nearly colorless to red-brown. Once all the ;~
the chlorosulfonic acid .solution is added, the reaction
mixture i~ allowed to 510wly warm to ambient temperature
and stir overnight. The following day an aliquot is
removed and analyzed by liquid chromatography to
determine the degree o~ ~ulfonation. When it is
apparent that the reactlon i~ complete, the sitir bar is
removed and the contents are ¢oncentrated in ~acuo (5~10
tor~) utilizing a rotary evaporator. The sample is then
subjected to reduced presisures (0.1-0.5 torr, vacuum
pump) and a final sample is analyzed b~ liquid
chromatography. Both liquid chromatography analyses are
consistent~and show that the percent~of unsul~onated and
monosulfonated p~oduct to the disulfonated material i9
about 96 to 4.
General procedure for the purification of
monoal.~ylated, monosulfonated aromatic materials
(example C20~24 DPO based MAMS)~
C20~24 DPO~based MAMS: ~A portion of the crude
reaction mixture described above is taken (70 g) and
dis~olved in methylene chloride, Me~l2, (300 mL) and ~;
placed on top oP~a Plash silica gel column (10 inch x 2
inch) that has been packed u~ing MeCl2. Some solid
material may ~ail~to dissolve into the MeCl2~ and is
assume~ to be inorganic salts ~uch as sodium sulfate and
sodium chloride.~ The elusion;method and the subsequent
analysis (TLC, elusion with MeC12;ior liquid
chromatogriaphy) o~ the Practions obtained are shown in ;~
Table 3. `
,
:
:
WC) g4/1)575g PCr/US93/080~021217~2 :
--17--
_l :
a
X
a ,,''
~d
W ~
o M e z Z ~ ~ ;C 5 ~ e
, ~
~ .
~n ,~C
. E~ ~ ~ N 0 ~ ~ If) In o O C~ :
8 ~ ~ ~ ~ ~ z o 3 Z o
0~
o : .
W
~; O C~ O O O O o o o c:~ o o ,
O O O O O O ~ o o
~ W , ':.
, ~.
a
N
~ ^ ~ ~ ~ U U U U
a ~ N S aE
e Z~ U~ U ~ o: O O x
W o: - '
, 13 ; , ' ~
o .,-.:
O IS: ,
, ~
.
,
W094/0s7ss PClIUS93/~OXO
2~217~
-18-
The column chromatograph is discontinued when
the MADS component is detected eluding from the column.
Fractions are combined based upon their purity.
Fractions 1 through 7 contain unreacted alkylate and are
di~carded. Fraction 8 through 11 contain only pure
C2~_24 MAMS and are combined to afford 45 g of product.
General procedure for the disulfonation reaction of
aromatic materials: ; ;
C2a_24 disulfonated monoalkylated DPO: To a
125 mL addition funnel is added liquefied ~ulfur
trioxi~e, S03, (56.6 g, 0.707 moles, 2.3 equivalents
, based on ADPO talkylated diphenyl oxide)). This is
~ealed and transferred to a bottom outle~, stopcock
controlled, 500 mL 3-necked RBF that i5 equipped with a
stlr rod, a nitrogen inlet and contains MeC12 (150 mL).
The amount of the C20-2l~ monoalkylated DPO (143.6 g7
0.308 moles, 1/2.3 equiv~lents) i~ calculated based on
the amount o~ the S03 weighed out and i5 placed into a 1
20 liter 3-necked RBF equipped with a nitrogen in,et and
overhead stirrer. The alkylate i~ diluted with MeC12
(350 mL) and the 1 liter 3-necked RBF is attached to the
outlet of the S03/MeC12 RBF. The reaction vessel is
cooled to O C via an ice bath. The alkylated DPO
25 solution is stirred and the S03tMeC12 so~Lutlon is slowly
added over 1-5 h being careful not to allow the reaction s
temperature to rise above 4 C. During thi~ time, the
reaction darkens from light yellow to red brown. An
aliquo~ is removed after a post r~action time af 0.5
3~ hours at OiC and is concentrated, neutralized and
dissolved in a water/meShanol mixture. This is analyzed
by liquid chromatography. ~This analysis indicated that
the ratio of unsulfonated/monosulfonated ADPO to
disulfonated ADPO is about 2 to 98.
WO 94/0575') PCI /US93/08080 - ~
2~2i7;~2
-19-
For the cleaning formulations of the present
invention the purity of the monoalkylated, disul~onated
DPO ba~ed species are generally pure enough that no
further puri~ication techniques are required. ;
General procedure for the analys1s of the
alkylates:
The analysi.~ of the alkylates is done uqing a
gas chromatography system comprised of a Hewlett Packard
GC; HP 7673 A autosampler; HP33g6A integrator; HP g114B
external disk drive and a J&W DB~ 15m x 0.32 mm, 0.1 um ;,
film column. The flow rate o~ helium i~ 8~7 ml/min~
The instrument i~ calibrated with an internal standard
and a standard ~olution of components. The percentage3
reported ar,e in weight percent. Each different ~DPO,
(differing by chain length) prepared required
development of a eeparate GC system.
General procedure ~or the analysis of the
sulfonate~ aromati¢ speoies: ~
The analysis o~ the sulfonated aromatic species ~;
is done using a liquid chromato6raphy s~stem compri ed
of a Milton Roy piston pump7 a single port injector, a
250 mm x 4.6 mm glass column packed with Yydac's 301SC
~200 mm) anion exchange resin, ~ LDC UV III monitor (254
~5 nm filter), and a Spectra Phy~ics~Chrom Jet integrator.
The solvent system is a 1:1 methanol ;~
(MeOH):tetrahydro~uran (THF); buffered with 0.2 M acetic
acid (AcOH),~0.2 M~sodium acetate (NaOAc), and 0.1 M
sodium perchlorate (NaCl04).~ The operating pressure is
l kept between 50-80 psi with dampening. The~percentages ; ~-
reported are in area percent.
. . .
.,
',.
W~94/0575g PCT/US93/080~0
~121792 -20~
II. PREPARATION OF CLEANING COMPOSITION/ STAINED
FABRICS/EVALUATION OF CLEANING
Preparation of cleanin~ composition
The cleaning samples prepared all contained
5 alkylated t sulfonated aromatic materials and nonionic ~:
surfactants in various ratios according to ~he figures
and tables which follow in the results section. The
alkylated, ~ulfonated aroma~ic component is the Pormula
as stipulated in the Pigures and table~ which follow.
~ he nonionic surfactant ~omponent o~ the cleaning
formulation is either Neodol alcohol ethoxylate~
available from ~hell Chemical; Igepal nonylphenol
ethoxylateq available from Rhone Poulenc; Ter~itol
nonylphenol ethoxylates available ~rom Union Carbide or
one of three blends prepared from Neodol to impart
various HLB surfactant solutions. Blend one (HLB v~lue
approximately 9) consist~ of approximately 77 weight
percent Neodol 25-3 and approximatelr 23 weight percent
Neodol 25-9; blend two (HLB value approximately 10)
consists of approximately 50 weight percent Neodol 23-3
and approximately 50 weight percent Neodol 23-6.5 ;
blend three ~HLB value approximately 11.4) consi~ts of
approximately 32 weight percent Neodol 25-3 and
approximately 6a weight percent Neodol 25-9.
Prepara~ion o~ fabric
Test ~abrics are:
1!~) Cottsn: #405, Bleached Cotton Sheeting,
48" wide, obtained from Test Fabric
Incorporated, Middlesex, NJ
2) Polyester/Cotton: #7436, Dacron
54W~Cotton, 65/35, Poplin, 60" wide,
'''':
W09~/057s9 P~T/US93/080XO
21217!J~
-21-
obtained ~rom Test Fabric Incorporated,
Middlesex~ NJ
The ~abric is prewashed three times to remove
sizing in a General Electric Washer Model ~WWA8340G. Settings used for a~l cycles are as follow~:
Regular/Normal cycle
Warm wash/cold rinse
Load si~e: large
Generally, one piece of cotton fabric, 45" x 96", and
one piece of polycotton fabric, 6~" x 72~', are laundered
per wash load. Zero pho3phsrous content TIDE0powdered
laundry detergent (Proctor & Gamble) is used for this
prewash and in all phases o~ testing. For each wa~h
15 cycle, 15~-0.5 grams o~ TIDE is used. The fabric is not .
dried between wa~h cycles. After the third wash cycle,
the fabri~ is dried in a General Electric Dryer Model ~
#DDE8200GBLAD on high heat for 70 minutes. After ~`
drying, the fabric is referred to as prewashed ~abric~
The fabric is removed promptly from the dryer and
smoothed with clean hands to remove wrinkles. The `;
prewashed fabric is not ironed. If it is not used in a
test within six weeks it is discarded.
. ~,
Cuttin~ swatches
After drying, the prewashed ~abric is cut into
swatches approximately 3" x 4.5". An Eastman Chick-a- `
dee Model D2 rotary shear ~(MJ Feley Company, Ro~evîlle,
MI) is used for the cutting~procedure. Metal templates
of cleaned aluminum or cleaned galvanized ~heeting are
used as guides for the rotary shear. Fabric salvages
are permissible on the final test ~watch. Once cut into
the 3" x 4.5" test size the prewashed ~abric is referred
i:
'`~
W~g4/0575~ Pcr/us93/o8o~o
2~2~7~2 -22-
to as a swatch or a test swatch. All swatches are
~tored away ~rom light.
Staining o~ swatches
Staining o~ swatches i~ done in the afternoon s
o~ the day before a cleaning test. A 2l~ hours set time
between staining and te~ting i5 recommended to allow the
stain to wick out across the swatch. The ~watches to be
stained are laid out on a bench top on a double
thickness o~ Scott Utllity Wipes ~re~erred to as Soott
wipes)~. All swatches should be ~rom the same prewa~hed
, fabric.
Number of swatches_to st.~}n: Generally ~or' :
each cleaning formulation te~ted two stained cotton
swatches and kwo stained poly~otton swat¢hes are treated
and washed with TIDE and two stained cotton swatches and
two stained polyaotton swatches are treated and washed
without TIDE. Controls are two ~tained cotton swatches
~ and two stained polycotton swatches washed in TIDE only.
Also, three stained ~watches oP each fabric type are
needed as 100% Dirty Standards.
Stain material: The stain material is Wolf' 3
Head~SAE 30W motor oil ~Wol~'s Head Oil Compan~, Qil
City,:PA) used approximately 30 hours in an .. :`
International three cylinder diesel tractor. Sin~e the
stain contai~s parti~ulates, the stock bottle is shaken
at high speed on a reciprocation shaker for five minutes
before it is~applied. If the ~talning process takes
longer than 15 minutes 7 the stock bottle will be
reshaken after 15 minutes.
.:
_~plyinj~ the stain: The stain can be applied
in one of' three ways. All th~ee methods result in
,
: ~ ''
WOg4/0575~ PCT/~S93/08080
2~2:~ 7 92
-23-
approximately 0.18 grams of staîn material being ;
applied. Method 2 or 3 is reco~mended.
1) Plastic disposable pipet (Fisher Scientific
C~mpany, Fisherbrand Catalog ~13-711-5).
Fill pipet and hold ik at 45angle about
one inch above the center o~ the ~watah.
Slowly drip on seven drops at the center of
each swatGh.
2) NichirYo Ox~ord model 810C s~ringe pipet ~;
~ (Fi~her S¢ientific Company) with a 6 milli ;~
liter syringe on the end. Di3penser
setting at 2 to give 200 microliters (+
0.8% accuracy as manufactured~ volume
dispensed. Slowly di~pense the stain
material at the center of the swatch with
the tip of the syringe about one inch above
it. ,
3) Weighed amo _ t, Tare out the swatch on a
Scott Wipe on a two decimal place balance
and apply 0.18g ~ 0.02 of stain material
slowly~by pipet to the center of the
swatch.
After~staining: After applying the oil stain,
the swatch~s sit undisturbed overnight on a bench top.
The swatches are~now referred to as stained swatcheq,
30~ l Stained swatches will sit a minimum of 16 hours
after staining before being u~ed in a test. This îs to
allow the stain to 'set'/wick out into the swatch.
Stained swatches not used within 28 hours o~ staining
are disoarded.
' ~"
;:
,~':'''
W094/05759 PCT/US~3/08080
21~1792 -24- ~
Preparation of stained swatches for testin~
:
Stained swatches are inspected before testing.
Any that look unu~ual, i.e. the oil stain did not spread
ou~ or only spread unevenly, are discarded. Extra
stained swatches will not be held over to be used in
another cleaning test.
Two c~tton and two polycotton stained swatches
are laid out on a new clean Scott wipe. If possible,
the technical cleaning formulation is ~tirr~d with a
woode~ applicator stick. A stained ~watch is tared.
The stained swatch is removed to a Scott wipe and :~
approximately 1.5 grams of the technioal cleaning
formulation is applied. A wooden applicator stick i~
15 used to gently spread it over the sur~ace of the stained ;-
area. A rubbing motion i3 .not used. High viscosity
technical cleaning Pormulation may require more sample
and more work to spread them. Low vi~cosity technical
cleaning Pormulatiol1s may be transferred to the stained
swaSch by volum~tric syringe.
. ,~
The technical cleaning formulations is spread ~;
to cover a diameter of 1.5 inches at the center o~ the
stained area and~at least 90% of the full stained area.
The treated stained swa~ch is placed on the balance and
the amount o~ technical cleanlng ~ormulation applied
recorded. The amount applied should be 1.5 ~ 0.2 g.
The balance pan is cleaned and the next stained ~watch
30~ !in the s~et of four lS proces~ed in the same way. Once
the technical cleaning formulation i~ applied~ the
stained swatch is referred to as~ a treated stained
swatch. ~ ~ ~
:~'
:~ ~
:
WOg~/0575g 2 1 2 :l 7 9 2 Pcr/us~3/0~080
-25- i ;;
In order to keep the contact time between stain
and technîcal cleaning ~ormulation in the selected range
of a minimum of 5 minutes and a maximum af 30 minutes,
no more than 25 minutes should lapse between the first
swa~eh and the l~st qwatch in the set is treated.
Ideally, no more than ten minute~ should lapse before
all stained swatches in the set are treated. Five
minutes after the last stained swatch in the set iq
treated~ the treated stained swatches are put into a ;'
prepared a~itator pot of a Terg-O-Tometer ~SEE
PREPARATION OF TERG-O-TOMETER SECTION). The treated
stained swatches are introduced into the wash water in
the order in which they were treated.
15Th~ above process is ~enerally done twice ~or
each technical cleaning formulation with one set wa~hed
in water with TIDE present and the second ~et wa~hed in
water without TIDE present. Stained swatches treated
with different teehnical cleaning formulations are not
washed together.
Preparation of controls
.,~
For each te~t day, control~ are run with TI~E
only. Two cotton stained swatches and two polycotton
stained ~watches are introduced into an agitator pot
prepared with 2.00 ~ 0.01 g TIDE in the water. Controls
are processed the same as technical cleaning formulation
treated ~tained swatches.
Preparation of ter~-o-tométer and
testinF~ with ter~-o-tometer
Deionized water is used in all phases of
testing after the initial prewash o~ the fabric.
..,~
WVg4/05759 PCr/US~3/0~080
~ 2 -26-
A six place Terg-0-Tometer model ~7243S
(Research and Testing Company, Inc. Fairfield, NJ) is A~
used. The Terg-0-Tometer bath is preheated to 120F.
The agitator pots are ~illed with 1700 ~ S0 mL of
deionized water. The wa~h and rinse cycles are run at
120~F and the agitatio~ r~te is 100 rpm. The
temperature is checked in each a~itator pot.
Wash cYcle
Unle~ specified otherwise7 stained ~watche~
are w~shed with TIDE in the water: 0.5 grams of TIDE is
used ~or each swatch. Generally four swatches are
washed in 2 + 0.01 grams o~ TIDE per agitator p~t. The
lot code o~ the TIDE ~ox is recorded. TIDE is added to
the agitator pot, with agitakion on, for approxlmately
two minutes before the swatche~ are added.
For stained swatches with or wi~hout TIDE in
the water: treated stained swatches are added one at a
time to the agitator pot, wikh agitation on, in the
order in whioh they were treated with the treated side
facing away fro~ the agitator shaft. After all the
swatches are a~ded to a pot, a timer is set for 15
minutes. At the end of 15 minutes, agitation is
stopped. The contents of the agitator pot are poured -
through a ~ieve, retaining the washed treated ~tained
swatches (washed swatches). The washed swatches are
retrieved and the excess water ~queezed out o~ them by
hand. They are rinsed for 3-5 second with deionized
water. The washed swatches are then ~eparated from each
other.
'f .
'' .
'~
'
',
: :',."'
W~g4/~5759 PCT/US93/080~0
2~2~792
-27- ,
Rinse cycle
The rinse cycle is the same ~or all ~watches
whether washed with or without TIDE.
The ~eparated washed swatches are immediately
added one at a time to a rinse agitator pot. A rînse
agitator pot contain~ 1700 ~ 50 ~L deionized water at
120F. There is no specific order of addition ~or a
rinse cycle. Agitation and water temperature is the
same as for a wash ¢ycle. A rin~e cycle is five minute~
in length. At the end o~ the five minute~, the contents
of ~he rinse agitator pot is poured through a sieve,
retaining the washed and rin~ed swatches (rinsed
swatches).~ Rinsed swa~ches are separated by hand and
rinsed 3-5 ~econds with deionized water. Excess water
is squeezed out oP the rin~ed swatche~ by hand. The
rinsed swatches of each s0t are smoothed ~lat by hand
onto a single thickness oP Scott wipe. A se¢ond Scott
wipe is u~ed to lightly cover the rin~ed ~watches. The
rinsed swatches are not ironed and are allowed to dry
overnight. -
-- ~,
Determination o~ nercent clean
, .
Percent clean is calculated from reflected
light readings. Reflecta~ce readings are rep~rted as
three coordinates tha~ define a specific point in the ;~
cubic space that is used to described color and hue. ~,
Reflected light readings from a set of 100% Clean
! ~ !S~andardsland ~rom a set of 100% Dirty Standards are
used to determine the range ~or zero to 100% clean. The
reflectance of a rinsed swatch is then placed as a
percentage along this range and~this percentage is
reported as its percent clean.
. .
"'''~
wo94/~s7s~ P~T/US~3/08~80
2 ~r~ 28-
Rin~ed swatches are air dried at least ,~
overnight (16 hours) before refle.ctance readings are ;'
taken. Rinsed swatches not read within 24 hours of test
will be stored in a dark place to prevent deterioration.
A MiniScan~ Spec~rocolorimeter version MS4500L (Hunter
Lab, Reston VA) is used to make re~lectance measurem4nts
and is standardized before each reading session. The
Commission Internationale de l'Eclairage ~CIE) 1931 '''
Tristimulus XYZ Scale (CIE X~Z Sc~le) i~ used as the '
10 reflectance scale with a CIE 1931 standard source '~'~
illuminant C and CIE 1931 2 standard observer. .','
At least two readings for each rinsed swatch is :,."
done with the sen~or of the MlniScan rotated 90 to
minimize the effect of f.bric nap on the reflectance~ ~.
readings. The average of these readings is recorded,
Readings are taken at the center of a rinsed swatch on
the s~de to which the technioal cleaning formulation is
applied. The rin~ed swatch is laid on a piece of white .:,
: :: zo poster board to give a uniform background to all rinsed :~
swatches and to eliminate the tendency of the rinsed ~':
swatch to pillow up. Pillowing up would occur on a soft
background and e~feot the re~leotance angle of the light ''
and therefore the reflectance~reading.
Standards; will consist of:three unstained ~':
swatches of each;fabrio (100% Clean:Standards) from the ~;,
~ same prewashed cloth as ~the test set and three stained ','
::: swatches of each~P~abric ~100% Dirty S~andard) from the .'~
3, s,ame skaining day as the ~test set and from the same j , .....: prewashed cloth. Readings are done using the same
~ method:as for the rinsed~swatches~
.,
; . ~
WO9~/05759 2 ~ 217 ~9 2 Pcr/lJs~3/osnxl)
~2g- ,
Determination o~ percent clean is by the
following equation: . :
(1) Percent Clean - .
1*{~(Xr-Xd)~(Yr-Yd)2+(Zr-Zd)2l1/Z / [(Xc~Xd)2~(
Yd)2~c-zd)2]l/2}
Where: ;
Xc = clean standard swatch X value from MiniScan :~
Yc = clean ~tandard ~watch Y value from MiniScan
~c ~ clean standard swatch Z value from MiniScan
Xd ~ dirty standard stained swatch X value from ~.
MiniScan
Yd = dirty standard stained swatch Y value from .
MiniScan
Zd = dirty standard sta;ned swakch Z value ~rom
MiniScan
Xr = Rinsed swatch X value for MiniS~an
Yr - Rinsed swatch Y value for MiniScan
Zr = Rinsed swatch Z value for MiniScan ~ :
:
~ :
' ~ .
" .
''' ,,,
. .
.
W~ 9q/05759 PCI`/US93/080~0
r~ 9 7
3 0
:
III. EVALUATION OF SAMPLESJCLEANING FORMULATION
Cleaning formulations to be tested are prepared
by by combining the desired amount of a sulfonated
alkylated aromatic surfactant, noniorlic ~ur~actant and
any additional additives to be included in the
formulation and mixing them until uniform. The
formulations may require heating ~o facilitate mixing. ..
Formulation Variables .,.. ;
_ . ,
A. Type of Sulfonated Alkylated Aromatic
Surfactant ;~,.
1) Alk~l chain length
Table 4 summarizes the cleaning res~lts for a
~eries oP ~ubstantially monoalkylated monosulfonated
diphenyl oxide (MAMS DPO) ~urfactants and variou~
alkylbenzene ~ul~onic acîds when used in a formulation
consi~ting of 1 part of the ~ulfonated alkylated
2~ aromatic surfactant to 3 parts o~ Igepal C0-520 nsnionic
surfactant. ~ata with TIDE deter~ent without any s~ain ~:
pretreatment are included in the table for compari~on.
A compari~on of the~data ~how that the percent
cleaning obtained with a sulfonated alkylated aromatîc
surfactant containing an alkyl group of C1~ or longer
are considerably ~uperior to the cleanin~ obtained with ;~
the Comparative ExampIe~ or a typical detergent (TIDE~
- 30 The MAMS DPO demonstrates~:the most dramatic improvement
in clea~ning;performance ~rom the C16 to C18 alkyl chain
length. : .:
:
, ,.
'~ ',';',
,`'.' ''
, ~,
" .. : :
; ',
, .
;-
WO ~4/05759 2 ~ ~ 1 7 9 2 PCI`/VS~3/0811)8~
,~, .
- 3 1 - ! ~
,
Table 4
The Influence of Alkyl Chain Length on Cleaning Performance
monot mono/ Percent Clean Percent Clean
5Sulfonated di~ di- ~washed inTide) (Notwashed in Tide)
alkylate sulfonate
Surfactant (weight (areaPolyester/ C Polyester/
percent) percent) Cotton cottc~n Otton cotton
C6MAMS DPO 100/0 84/16 36 2g
10Comparative ~
Example A :
.
C16 MAMS DPO 10010 9317 38 33 44 24
Comparative
Example B -
C18 MAMS DPO 100/0 9713 ~,~1 401 57 26
C20~24 MAMS DPO 74126 86/14 752 4~z ~1 35
C30~ MAMSDPO 80/20 85/15 70 58
~Toluene sulfonic 33 12 5 7 :
acid
Compara~ive : .:
20Example C
C12 Benzene 34 27 37 23 :
sulfonic acid3
Comparative . ~ ~
Example D . .
C18Toluene 97/3 100/0 42 31
25sulfonic acid4 :
C20 24Toluene 93n~ 100/0 772 472 76 30 ~:.
sulfonic acid5
........ ~......... :......... ........... O
Tide alone : 246 116
Control - not an ~:
example of the : .
3 invention I : ! ~.
1. Average of 2 tests. ~ . .
~: 2. Avera~eof6tests.
3. B!O SOFT S-1 ûQ~, Stepan
4. Aristoi (;, Pilot Chernical
: ~ S. ~ristol F, Pilot Chemical
6. Averageof20tests.
WO 94/05759 PCr/USs3/0808~
2 ~ r~ 9 ~ 2 . .
--3 -- :.
:,"
Table 4
The Influence of Alkyl Chain Length on Cleaning Performance
mono/ mono/ Percent Clean Percent Clean
di- di- (washed in Tide) (Not washed in Tide)
Sulfonated alky!ate sulfonate ~.
(welght (ar~a Polyester/ Polyester/
percent) percent) Cotton cotton Cotton cotton
' ",~
Igepal C0-520 25 23 ~ .
alone
Control - not an :
exampl~ of ~he
10 invention
1. Average of ~ tests.
2. Average of 6 tests
3. BlOSOFTS-100~,Stepan
4. AristolG,PilotChemical
5. Aristol F, Pilot Chemical
15 6. Averageof20test5-
In all ca~es the data show that cleaning with
formulations containing the sulfonated alkylated .~;
aromatic ~ur~actant containing an alkyl group of 18
carbons or longer gave better performance than the ;~
nonionic surfactant by it~elf or the nonionic sur~acta~tcontainîng a commonly availa~re linear dodecylbenzene
sulfonic acid ~BIO SOFT S-100~ from Stepan Chemi¢al Co.
Northfield ~L.~ or a commonl~ available h~drokrope (p-
25 toluene sulfonic~acid). .
2) Percent of sulfonation
~.:
The percent of sulfonation is evaluated bypreparing cleaning:formulation~ of Igepal C0-520 with
3i SAA ~ur~jactants oontaining dif~rent ratios af mono- to
d~sul~onated C20-24: alkylate diphenyl oxide that have ~-:been separa~ely pr~epared using the same batch of C20 24
monoa~kylated diphenyl oxide. The predominantly
monosulfonated DPO is analyzed to be 100 percent
monoalkylated~ 96 percent monosulfonated, and 4 percent ~
:.,' '
' ~
,~ ~
,,
W094/0s759 PCT/US93/08080
~2179~ ;
-33-
disulfonated; the predominantly disulfonated DPO is ;~
analyzed to be 100 percent monoalkylated and 90 percent
disulfonated. A puri~ied monosul~onated, monoalkylated
DPO i~ prepared and analyzed to be 100 percent
monoalkylated and 100 percent mono~iulfonated.
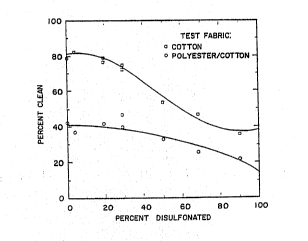
Figure 1 ~ummarizeQ the inPluence o~ the
percent of sul~onation on the cleaning for a formulation
consisting of 1 part of the mono- and di~ulfonated
C20-24 monoalkylate diphenyl oxide sur~actant ~o 3 part~
by weight of Igepal C0-520 nonionic surfactant. Th~
cleanîng per~ormance beginsi to decrease a~ter about 30
percent by weight of the C20-24 monoalkylated diphenyl
oxide is disulfonated.
PERCENT CLEAN DATA FOR FIGURE 1
% Monosulfonated ~ Clean Cot % Clean PEC ~52b~L}
10.0 36 ~2 90.0
31.~ 47 26 68~5
50.0 54 33 ~O.O `;
71.3 75 40 28.7 `
71.3 73 47 28.7
80.8 79 42 19
80.8 77 ~ 42 19.2
96.0 82 37 4.0
100.0 79 42 O.O
3) Percent of alk~lation
The pereent of alkylation i~ evaluated by
preparing ~ormulations with~different ratio~ of mono- to
dialkylated C18 alkylated diphenyl oxide (DPO). The
monoalkylated DPO i~i analyzed to be 99.9% monoalkylated
and 97% monosulfonated; the dialkylated DPO i~ analyzed
~,
:
W~g4/05759 PCT/~S93/08080
~ 1 2 ~ 7 g 2 -34-
to be 100% dialkylated and ~4~ monosulfonated. Figure 2
summarizes the percent of alkylation influence on the
cleaning for a formulation consisting of 1 part of the
mixture of the sulPonated mono~ and dialkylated C18
diphenyl oxide ~urfactant to 3 parts of Igepal C0-520
nonionic surfa¢tant; for polyester~cotton fabric the
cleanin~ performance is essentially independent of the
ratio of the degree of ~lkylation. However, for cotton
~abric, significantly better cleaning performanoe i9
obtained with the dialkylated surfa¢tant than with the
mono~lkylated surfac~ant. Also, a minimum in the
cleaning performance is observed at approximately 30
weight percent of dialkylation. ;~
The influence of increased chain lengkh on
cleaning performance for monosulfonat~d dialkylated
aromatic surfactants is shown in Table 5 for ~oth
diphenyl oxide and benzene based surfactants. This
table illustrates three trends, firstly, the data
demonstrate that cotton cleaning per~ormance is enhanced
by increasing the length of the alkyl group for both
diphenyl oxide~and benzene~based surfactants. Secondly,
~uperiority of the cleaning is observed with the sodium
,
salt o~ C16 dialkylated DPO~compared to the sodium salt
or acid form of the C16 monoalkylated DP0 and the
dodecylbenzene su1fonic acid.~ Finally, a comparison of
Table 4 with Table 5 shows c1eaning performance
enhancement is~preferably obtained with a monosulfonated
long chain monoalkylated~ arom~tic surfactant than by a
similar iequiva1ent weight monosulfonated dialkylated
aromatic surfactant in which its~two shorter alkyl
chains are roughly one~half~the length of the long chain
u~ed to prepare the monosu1fonated monoalkylated
aromatic surfactant.
.,:
; ::
. .
W(~ ~4/OS759 Pl /US93/08080
2~2~7~
-35-
PERCENT CLEAN DATA FOR FIGURE 2
% Monoalkylation % Dialkylatiorl % Clean Cot % Clean PEC
, ~
100.0 0.0 59 39
100.0 0,0 62 llO
100.0 OoO 63 33
00.0 O.,0 60 32
94.4 5.6 57 38
94.4 5.6 61 32 :
89.9 10.1 54 38 ~ .
-8!~.9 10.1 55 36
80.8 19.,2 54 35 ::
80.~ 19.2 ~8 33
70.2~ 29.8 50 33
70 . 2 29 . 8 48 39
50~1 49 . 9 57 39 .:
50 .1 4 9 o 9 60 34
40-0 60.0 59 32
?0
25.0 15.0 66 33
10.0 90.0 75 31
0.0 100.() 77 41
0.0 100.. 0 73 34
;25 .
4) Type of Aromatic Moiety
The in~luence of the type of aromatic moiety on
cleaning performanoe is illustrated in Table 3A for C18 :~:
and C20-24 aljkyl chain lengths u~ed in formulations
consist~ng of 1 part by weight of the monosul~onated
monoalkylated aromatic surfactant to 3 parts by weight
: of Igepal C0-520 nonionic ~urfactant. These data show
that with the C20-24 alkyl:chain length, the type of
aromatic moiety has little effect on the type of :
'-
. . , . ~
W ~ ~/05759 ~'CT/~Sg3/080~0
2 ~ ~ 1 7 ~ ~ ~36-- !
Table 5 ~.
The Influence of ~ialkyl Chain Length on Cleaning Perormance
mono/di- mono/di- Percent Clean
alkylate sulfonate i.
Sulonated Surfactant .
(welght (a~ea Polyester~
perce~t) percent) Cotton cotton
Cl~ DAMS DPOl 0/100 84/16 756 6
~14 DAMS DPOl 0/100 9Z~ 54 2
(c~nparative example)
Clo DAMS DPOl 0/977 9~/6 37 27
(comparative ~xample)
C12 DA BSA2r3 ~90~ 100/0 716 266 :; ~comparative example) dialkylate
Clo DA BSA2 100/0 l00/0 32 28
(comparative example)
C16 MA~S DPo4 100/0 93/7 38 33
~comparative example)
C16 MAMS DPo4 Na ~alt 99.8/0.2 91/9 30 38
(comparative example) .:.
C16 DAMS DPo4 Na salt 3/97 89/11 466 qo6
(comparative example) :~
p-Toluene sulonic 33 12 '~
acid -:(comparative exampl~)
~ - ., .. - .. ~ ............................. ... ,..... , ~ ................... ,"
Dodecylbenzene : 35 286
sul~onic acid5
(comparative example)
Tide alone ~ ~ 248 113 ~:(comparative example)
1. DAMS D20 - Dialkylated~monosulfonated~diphe yl oxide. ~.;
2. DA BSA - Dialkylated benzene sulEonic acid.
3.l Ari~tol E,IPiLot Chemical.
4. MAMS DPO ~ Monoalkylated monosulfonated diphenyl oxide.
5. BIO SOFT 5-10a'~, Stepan.
6. Average of 2 tests. : .:7. Contai~s 3% trialkylated or higher alkylates j~,
c~ea~ing ob~alned. Ilowever, with the C18 alkyl chain
'`'~'.
,`,~
W094/05759 pcr/uss3/oBo8l) :
2121792
-37-
length superior performance is observed with diphenyl
oxide than with the MAMS toluene aromatic moiety.
Tahle 6
The In1uence of ~ype o~ Aromatic Moiety on Cleaning Performance
Percent Cl~an
Sulfonated Surfactantl
CottonPolyester/cotton
C20_24 ~AMS D.iphenyL oxide 756 466
C20_24 ~AMS Biphenyl , 787 507
C20_24 MAMS Toluene2 776 476
,~..................... ".,..... ,.,... ~.. ,.... ~,
C20_2~ MhMS ~enzene3 75 57
.................................... ~..... ,.. ,.,,.. ~,,........... ~
C20_24 MAMS Benzene4 77 52
Clg ~AMS Diphenyl oxide 606 406
Clg MAMS Toluene5 4Z 31
1. MAMS - monoalkylated monosul~onated
2. Aristol F, Pilot Chemical.
3. Aristol A alkylate, Pilot Chemical, sulfonated with one mole o
chlorosulfonic acid per mole of al~ylat~.
4. Aristol A alkylate, Pllot Chemical, ~ul~onated with one mole of
sulfurtrioxide per mole of alkylate~ :
5. Aristol G, Pilot Chemical.
6. Average of 6 tests.
7. Average of 2 tests.
B. Ratio of Sulfonated Alkylated Aromatio
Sur~actant to Nonionic Surfactant
;,:
The influence o~ the ratio of the sul~onated `:-
alkylated aromatic surfactant to nonionic ~urfactant is
.
illustrated in Figure 3 for a MAMS diphenyl oxide which
is 74.2 weight percent monoalkylated of C20-24 and 86
percent monosulfonation. The results in Figure 3 ..
clearly ~how that there is:a synergistie cleaning
performance obtained with the mixed surfaetant system.
~ .
W~94/~s7s9 P~T/US93/08080 :
2 ~ 2 1~ t~ h -38- '
' ,',
The optimum performance is obtained with a weight ratio
of about 1 part of the sulfonated alkylated aromatic
surfactant to 3 parts of the nonionic surfactant.
C. Influence of Ionization of the Sulfonic Acid
The anionic surfaotant~ in salt form are
preferably used in cle~ning formulations for cleaning
~tain~ such as blood~ Cleaning formula~ion~ of the
present inven~ion are prepared u~ing 1 part by weight
monosulfonated monoalkylated aromatic surfactant to 3
parts by weight Igepal C0-520 in which the ~ul~oni¢ acid
is neutralized with various bases. The mono~ulfonated
monoalkylated aromatic ~urfactant consists of either
C20-2~ MAMS toluene ~Aristol F sulfonic acid, Pilot ~,
Chemical) or C20-2l~ MA~S DP0 twith a mono-/disulfonation
ratio of 86/14 and a mono/ dialkylation ratio of
74.2/25.8)~ Each formulation contains 10 weight percent
water based on the total formulation weight. The data
2~ is s~mmarized in Figure 4 and Table 7. The data .~:
demonstrates that neutralization of the sul~onated alkyl ;;~
aromatics in a cleaning composition results in a similar `i~`:
: cleaning performanoe to the unneutralized form~
D. Additlon oÇ Water
The influence of water on~cleaning perÇormance ~:
is illustrated~ in Figures 5 and 6 for formulations
containing Igepal C0-520 nonionic surfactant. Two ;::
dif~erent types~ of alkyl aromatic~surfaetants are used:
In Figùrle 5 the data were obtained Çor a formulation
with 1 part by weight of a MAMS diphenyl oxide with a
mono/disulfonation ra5io o~ 86/14 and a :~
mono/dialkylation ratio of 74.2/25.8 for 3 parts by :~.
weight Or I~epal C0-52Q. In Figure 6, 1 part by weight
' ''~.
" ~
: : ' ,'~
'.''"
~ ')4/057$9 ~ .L 21 7 9 2PCT/US93/08080
39_
Table 7
MAMS MAMS 7
MAMSDP0 MAMS DP0 Toluene Toluene
Counterion %Clean Cotton %Clean PEC %Clean ~Clean
1 H 78 50 76 51
2 Na 66 49 75 53
3 NH4 74 59 66 55
4 Ca/2 77 ~1 55 42
~ DEA 69 45 72 57
6 TEA 67 46 70 55
of C20-24 monoalkylated toluene sulfonic aeid (Aristol
F, Pilot Chemical) was used Por 3 parts by weight of
Igepal cb-520.
Both Figure 5 and 6 show that cleaning better
than that obtained with TIDE alone (Table 4) can be
obtained with water levels o~ up to 80 wt% in the
formulations. These figures also show that a small
amount (about 10%) of water may be beneficial.
.
' '':.
:~
3 0,
,
WVg~/05759 PCTt~S93/0~080
~ 3~ _40~
'' .
. :
E. Type and HLB of Nonionic Surfactant
The in~luence of the type and HLB o~ the nonionic
surfactant is demonstrated in Tables 8 and 9. These
Tables present the data on the cleaning of cotton
~watches in Table 8 and that oP polyester/cotton
swatches in Table 9. The pretreatment formulation~
contain 1 part by weight oP ~ulfonated alkylated
aromatic surfactant per 3 parts by weight nonionic :`~
surfactant. The data demonstrate that cleaning
performance is enhanced by the oleaning oomposition~
comprised of the sulfonated alkylated aromatic
surfac~ants of this invention in combination with eikher ~:
a nonylphenol ethoxylate (Tergitol or Igepal) or a `
linear alcohol ethoxylate (Neodol) over the HLB range of
8.9 to 13Ø ~
,"
, :
`~
~ .
.
: :,
`.' ',~
;
: .:
-~
WO l~4/O5759 ~ ~ 2 1 r7 !3 2 PCl /US93/OXOXO
--41-- !
v cn ,,~,
C
~ C~
.r o 3 1 o ~0
."
~1 o ~ .C ~ ,, ;'
~,~ 3 ~ ~ N ~ ~ N ~1
U
~ ~ O
C U 5S ~o N
v e ~1 ~
U ~) ~
~ U U~ C
W ~ O U~
L~ ~ U ~') ~; ~I) :
UJ V O ~ D N ~D ~ 1,0 ttl 0~ ~` ~ tD C~ ~.
3 ~ o ~ ~ ~ ~ ~
o C ~ ~ ~ ~ ,"
C ~ C E 6
o V V ~ U U
z c ~ a~
~ V ~ U ~ ~
e ~ ¢ v o o ~O O C7 O~
w ~ N N N C~ N e e
U ~
o O o
V ~ ,_, ~,
~, ......
O ~
O ~ c ~ 7 "
~J ~ ~' O N ~I t~ 1 ~ N t~
c c ~ ' g
U U ';
w ~, 0 ~ ~ o~n ~ o O o.
OO r~ G _I O ~tr~ r~
~n o o
s ~
r OID t~ o--~ N ~ ~
C ~ Z Z o u ~ ~ N
O c ~ _~ ~J O , O ~ a m " O ~ ~
O ~ o $ ~ ~.
Z; Z Z
` ~ ' : ,
: .
~ . ~
.
,,.
.
~ .
WOg4/057S9 PCl'~US~3/08080 . ~
2~2~7~ A2- ! ~
'~'
'.~
v cn
O
~ 3 ~ E~
r~ V _
a~_, 11
' ~ C C "'
~ rO 3 2 ,~
W ~g U
4~ ^,. o
.. C ,, ,,
0 ~J D U
$
~ ~ U
0 ~ C
~U V V O U~
~ C V
o o ~ r ~ ~ ~ o ~
." p, Ll ~ O C C
O
C~C '' C
o _I tO t~
c U~ O O Cl~ O ~ Ot7~ U~
I~J V _I _I ~ ~ ~I r~r4 N ~ S Ei E~
W V 1~1 ~ O I ` .C C ' ,'
--I P~ V ~
3 O O
o ~ v ~D
- C ~ ~o ~ ~1 ~,~ r~cn ~ D O ,
U ~O V :C
C E3 ~
Q~ O ~ ~ V . .
o C~ ~ o ~ ~ U
O ` r~ O_1 0 ~
~ C C
V ~ O O .,
~ ~ c
1~ 0 ~ O ~D 1" o--I Nr~
C V O Z otU ~ S r~ U 1~ ;;
n c c ~ ~' " " , ' ,~ ~ O O O 0 1 . ." ~
'~
'
S
- '
'~
WO s4/0575') 212 :~ I 9 2 Pcr/uss3to8o8o
-43- ` :
':
E. Stick Formulation
A stick formulation is prepared with the
following composition:
Norpar 15 4n g
Neodo} 45-7 lZ g
Igepal C0-520 12 g
PEG E-900 (Dow)l 2 g
Na2 C03 2 g
DI Water 6 g . ~:
Aristol F (or ~or DDBSA this 5 g
is BIOSOF~ 5-100~)
Stearic Acid 2 g
Cottonseed Oil 10 g
Enzyme (Proteolytic) 2 g
~utal 93 g
1. Polyglycol E900 (Dow) polyethylene glycol :~
': :
'`
';:
;;.
.', '":
W~94/057ss P~T/US93~08080
2~7~2
-4~
The comparisons in Tables 10 illu~trate that ;:
~imilar cleaning performance is obtained with
compo~itions o~ this invention usinK a ~tick (solid) as
well a~ liquid cleaning formulations.
.
Table l0
CLEANING PE~FORMANCE
OF STIC~ FORMULA~IONS
SAMP~ lZS
1 0 '''''''
,I
S~PLE PERCENT C~EAN
IDENTIFICAT~ON COTTON PoLyEsTER/
Aristol ~F~ 74 51 ..
Stick
: .',
DDBSA Stick 40 29
Tide only no28 ll
Pretreatment
~5 ;
:: ,,
3~
; ..
.,, ':
'''~
-::
W~94/057s~ PCT/US93/080X0
,. 212117~2
-45-
Experimental
Stick hardness i~ mea~ured in accordance with
ASTM standard D-127. The procedure uses an ASTM
penetrometer equipped with a standard cone (150 g)
without additional weight added. Stick hardness is
reflected by the depth the cone penetrates into the
stick in five seconds~ The depth is reported in 0.1 mm.
Thus a higher number de~cribes a softer stick. Each
stick in this ex~mple ha~ a stick hardness of 92
., ,'':
,~
~'
'' ;
:.,