Note: Descriptions are shown in the official language in which they were submitted.
~_~W093/03790 2 12 2 15 0 PCT/US92/07221
IONTOTHERAPEUTIC DEVICE AND PROCESS
s
CROSS-REFERENCE TO RELATED APPLICATION
~0
This application is a continuation-in-part of U.S.
Application Serial No. 07/587,406 filed September 25, 1990
and of U.S. Application Serial No. 07/046,984, filed May 5,
1987, now U.S. Patent No. 5,042,975, which was a continua-
tion-in-part of U.S. Application Serial No. 890,702 filed
July 25, 1986, now abandoned.
TECHNICAL FIELD
This invention relates to development of an iontothera-
peutic device for regulated transdermal systemic administra-
tion of ionizable pharmaceuticals (including ionizable bio-
pharmaceuticals) and a novel battery device usable as an
element of said device.
It also provides an iontotherapeutic process for trans-
dermal administration of ionizable pharmaceuticals, particu-
' - ` `
larly those which are otherwise transdermally absorbed to a
~` small degree or not at all. The invention also relates to a
t , , ~ , :
polymeric unit dose in which an ionized pharmaceutical is
dispersed. The unit dose is adapted to be assembled as a
part of either the anode or the cathode, depending upon
whether the ionized pharmaceutical is cationic or anionic,
-
so that the ionized pharmaceutical will be delivered trans-
dermally and then be absorbed systemically when the ionto-
therapeutic device is in operation.
~ .
~.
W093/03790 ~ PCT/US92/07221
2122150
~ 5
; BACKGROUND ART
Many pharmaceuticals are required to be administered to
patients by injection. A notable example is insulin, which
cannot be administered orally to be effective in lowering
the elevated blood sugar levels, which are too high in
diabetics (i.e., > 126 mg/dL). Other pharmaceuticals may be
administered orally, but in some cases, there is inefficient
absorption into the bloodstream to permit the pharmac~uti-
cals to achieve their intended therapy. Also, with regard
to oral administration, many orally administered pharmaceu-
ticals undergo a high degree of destruction by the hepato-
gastrointestinal first-pass metabolism. Often the metabo-
lites of the first-pass metabolism cause unwanted biological
activity or toxicity. In oral administration, there are
variables which cause undesirable variations in the extent
of gastrointestinal absorption from subject to subject,
:~
especially in the case of some pharmaceuticals; and there
are also associated problems of uneven blood levels result-
ing from an initial large absorption with attendant un-
desirable side effects or toxicities, and subsequent blood
~ ~
levels which are less than therapeutically optimal.
Recently there has been an increasing interest in
transdermal delivery. However, it is desired that transder-
, . , ~ . ~
mal absorption of a number of pharmaceuticals, particularly
.,
the macromolecular drugs such as insulin and cationic drugs
like propranolol HCl, be improved.
~W093/03~90 21 22l so PCT/US92/07221
~ 5
I The hazard and discomfort of administration of pharma-
¦ ceuticals by injection, especially if therapy is required on
1 lO a frequent basis, such as the subcutaneous injection of
t insulin for diabetes therapy, which is required daily, are
universally known. There has long been a desire to avoid
the necessity of therapy by injection.
Investigations have been carried out to explore the
possibility of delivering certain therapeutic agents topi-
cally by use of a direct current (DC) iontophoresis. For
example, it has been found that fluoride ions can be
assimilated into the structure of a tooth with the aid of DC
iontophoresis. Also, localized "seating" has been caused by
delivering to the skin a sweat-inducing compound, such as
pilocarpine, using a direct current. The induced sweat is
then assayed using an electrode to determine its chloride
ion concentration for diagnosis purposes. A low chloride
content in the sweat indicates that a patient may be suffer-
-' ing from cystic fibrosis. Application of a DC iontophoresis
~ - can be~uncomfortable'particularly when the level of applied
'~ current is at~`a high 'level', in the case of certain pharma-
- ceuticals, in order to achieve'a systemic therapeutic level.
'It is highly desired to provide improved iontotherapeu-
*ic devices and processes and unit dose forms for use there-
in and to provide further thereby therapeutic levels of
' systemically-effective pharmaceuticals efficiently with a
physiologically-acceptable low electric current.
,~
'~
WO 93/037g0 PCI`/USg2/07;~2~!
`- 21221~U
~ S
SU~ARY OF THE INVENTION
A process has been found for administering transder-
mally a systemically effective amount of an ionizable phar-
maceutical in sterile aqueous solution using an iontothera-
peutic device such as provided by this invention. The
ionized pharmaceutical solution can be contained in a unit
dose form such as disposable polymeric matrix unit dose form
in which a dosage amount of an ionized pharmaceutical solu-
tion (pH desirably at least about 1.0, 1.5 or about 2 pH
units above or below the pKa or isoelectric pH of the
ionizable pharmaceutical) is intermixed with a polymer which
is characterized by being compatible with the pharmaceutical
as well as the skin, hydrophilic, and capable of releasing
the pharmaceutical for iontotherapeutic transdermal absorp-
tion. The unit dose form can also comprise a sterile solu-
tion of the ionized pharmaceutical contained within a closed
reservoir unit dose form having a drug-releasing microporous
membrane surface. The unit dose forms are assembled with a
pharmaceutical reservoir electrode and are further adapted
to permit the dissolved, ionized pharmaceutical to be
delivered iontophoretically to the skin of the subject
treated and to provide iontotherapeutic transdermal absorp-
tion of a systemically effective amount of the pharmaceuti-
cal. The unit dose forms are maintained covered to retain
sterility until the desired time of iontotherapeutic admin-
istration. A pharmaceutical reservoir electrode which will
receive such a unit dose form is used as a part of the
~ .
r~ W093/037gO 21 2 21 5 0 PCT/US92/07221
5.
iontotherapeutic device, such as provided by this invention,
which is used to carry out the iontotherapeutic delivery and
transdermal absorption of the ionized pharmaceutical. The
pharmaceutical reservoir electrode is either a cathode or an
anode depending upon whether the pharmaceutical is in
anionic or cationic form, respectively. The iontotherapeu-
tic device provides, in the process, an iontotherapeutically
effective and physiologically acceptable pulse current with
a specific waveform having an amplitude such as up to about
lOmA based on a reservoir electrode skin-contacting area of
about 5 cm2 and an effective frequency of at least about lO
Hz up to about 50 KHz until the subject treated has received
a pharmacologically-effective systemic dosage of the ionized
pharmaceutical.
The pharmaceutical administered by this invention can
be selected from pharmaceuticals which ordinarily are not
transdermally absorbed through intact skin in an effective
- dosage amount, such pharmaceuticals including but not
limited to insulins, vasopressin, heparin, growth hormones,
gIucagon, oxytocin,~ and other macromolecular drugs as well
as a number of others which can be provided in ionized form.
, : , ,
A number of compounds which are naturally-occurring in
humans, or variants thereof, and which often are peptide in
,,,~. , .
nature, are also included within this pharmaceutical group,
many of which can be produced identically or as a related
WOg3/037gO PCT/US92/0~
, 5 212215 6
compound using DNA recombinant or other biological tech-
niques.
Also provided by the invention is a novel iontothera-
peutic device capable of transdermally administering a sys-
temically effective amount of an ionized pharmaceutical.
The device is a lightweight, portable ~ransdermal periodic
iontotherapeutic device for transdermal administration of a
systemically-effective amount of an ionized pharmaceutical,
which is adapted to be worn by a subject being iontothera-
peutically treated, comprising
1) a DC power supply capable of providing an iontothera-
peutically effective and physiologically acceptable DC
current in the range up to about lOmA;
:
2) a periodic waveform generator electrically connected to
the DC power supply and having :integrated circuitry
capable of-providing a) a periodic waveform in the
square, triangular~, sinusoidal, trapezoidal, or other
acceptable geometric form or combination thereof: b) an
, on/off ratio-of;~1/50-to 10/1; and c) a repetition fre-
~-. 45 quency from about 10 Hz to about 50 KHz;
,
, 3) an output circuit electrically connected to said wave-
'~ 50 form generator which a) can provide a periodic DC cur-
~- rent in a pre-selected waveform of said forms, b) moni-
tors current intensity dellvered; c) adjusts and main-
tains the current intensity within predetermined maxi-
mum and minimum levels and d) delivers the current to a
~'
~ WOg3/03790 ~CT/US~ 722l
~` ^` 2122150
reservoir electrode for iontotherapeutic transdermal
administration of said ionized pharmaceutical;
. .
4) a pharmaceutical reservoir electrode which can be pre-
selected to be either the cathode or the anode depend-
ing upon whether the ionized pharmaceutical is anionic
or cationic; said electrode having a receptacle adapted
to receive a unit dose of said ionized pharmaceu~ical
in which said ionized pharmaceutical is in aqueous
solution at a pH at least 1.0 pH unit below or above
the isoelectric point or pKa point of said ionized
pharmaceutical ; said electrode with said received unit
dose adapted to be placed in electrical contact with
- the intact skin to be treated iontotherapeutically;
said electrode having a terminal to receive and to
transmit through said unit dose the said periodic DC
,
- : current and~ said unit dose~adapted to be in electrical
contact with said terminal;~: ~
:5); ~receptor electrode adapted to be in electrical contact
~ 45 with ~the intact skin to~be: treated and forming with
said pharmaceutical ;reservoir electrode a combination
~' of anode and cathode e}ectrodes;
50~
~ : said eleotrodes electrically~connected to said output
-~; circuit and providing when placed upon the skin of a
: subject being treated a current path through the inter-
vening tissue of the subject being treated; and
~ W093/03790 PCT/US92/07221
- 2122150 8
~ 6) a preprogramable control element electrically inte-
¦ grated within said device to preprogram and to control
said iontotherapeutic administration on an automated
basis as in accordance with a physician's prescription
entered into the control element, without interaction
of a subject being treated with the device for said
administration except to permit said subject to stop
operation of the device as in the event of an emer-
gency.
The device will ordinarily have a terminal by which the
transdermal administration carried on by the device can be
monitored using a computer system and a connecting line to
connect the device and the computer system or by which a
3S prescription for administration of a pharmaceutical by the
device can be entered into the programmable control element
by use of a computer system and a connecting line to connect
the control ele~ent with the computer system.
Further, the device desirably has one or more addi-
tional terminals by ~which the control element can be con-
nected by a connecting line with a sensor to sense a skin
, condition or with a separate sensor to sense a level of an
~' 50 entity in the body (which correlates with a need for admin-
,~ istration of the pharmaceutical), the sensor(s) held in
intimate contact with the subject's body and signals said
control element on need for administration or skin condi-
~,'
_~WO 93/03790 2 1 2 2 1 5 0 PCI/US92/07221
g
~ tion. For example, in insulin iontotherapy, the signal can
i transmit the nature of need for insulin administration.
Further, the invention provides a process for adminis-
tering an ionized pharmaceutical by use of the above defined
device and carrying out the following steps:
1) enter~ng a prescription or other instructions into the
control element of said device using a computer system;
2) assembling a dosage unit containing a pharmaceutically
acceptable aqueous solution of said peptide into a
receptacle of a reservoir electrode of a transdermal
periodic iontotherapeutic system, which electrode is a
cathode or anode depending upon whether such ionized
peptide is anionic or cationic, said solution having a
pH at least about 1.0 pH unit below or above the iso-
~- 35 electric point of said peptide;
3) placing the cathode and anode electrodes of said trans-
dermal periodic iontotherapeutic system in electrical
contact with tbe intact skin to be treated; and
. ~
4) applying an iontotherapeutically effective, periodic DC
current of up to about~ lOmA based on a reservoir elec-
trode/skin-contacting area of about S cm2 using a) a
periodic waveform in the square, triangular, sinu-
- soidal, trapezoidal, or other acceptable geometric
form, or combinations thereof, b3 a physiologically
acceptable repetition frequency of at least about 10
::
~ W093/037~ PCTlUS92/07r2~1
21221~0 lO
s
Hz, and c~ an on/off ratio of from 1/50 to 10/1; said
process providing a systemically èffective absorption
of said peptide pharmaceutical from said solution at a
rate of at least 500 percent from that provided by
passive diffusion transdermal absorption from said
: 15
solution during an administration time of at least 2
hours
The above defined process desirably is carried out
wherein a sensor is held in intimate contact with the body
of subject being treated such as in intimate contact with
the skin of the person being treated and said sensor trans-
mits one or more signals to the control element of the
device such as a physiological factor of the subject being
treated which correlates with the pharmaceutical administra-
tion carried out by the~ device or a skin condition which
relates to the transd-rma;l adminiseration
RIBF ~ DESCRIPTION OF TIIE DRAWINGS ~ ~
~, ~J~ FIC l~is a diagr m portr ying a device of the inven-
tion ~in operation ~to effect iontotherapeutic transdermal
absorption of an ~ionized pharmaceutical and its uptake into
50~ ; the~bloodstream of the subject treated
FIG 2 is a~block diagram~ of a transdermal periodic
iontotherapeutic device parent application Serial No
~- 07/046,984
,
`~ 2 1 2 2 1 ~ 0
. 1 1
FIG. 3 is a block diagram of a transdermal periodic
iontotherapeutic device coming within the invention.
FIGi 4 is a detailed circuit diagram for th,e Square-
Wave Generator shown in FIGS. 2 and 3.
FIG. 5 is a detailed circuit diagram for the Trape-
zoidal-Triangular Wave Generator shown in FIGS. 2 and 3.
FIG. 6 is a detailed circuit diagram for the Sinusoidal
Signal Generator shown in FIGS. 2 and 3.
FIG. 7 is a detailed circuit diagram for the Output
Circuit shown in FIGS. 2 and 3.
FIG. 8 is a block diagram of a wristwatch-type minia-
turized periodic iontotherapeutic device coming within the
invention, in which the drug reservoir electrode is posi-
tioned away from the main portion of iontotherapeutic
device.
FIG. 9A and 9B are diagrams illustrating a wristwatch-
type miniaturized transdermal periodic iontotherapeutic
,system with the drug reservoir electrode positioned directly
in the lower portion of the iontotherapeutic device and with
~multifunctional programmability.
FIG. 10 is a block diagram of a portable transdermal
, periodic iontotherapeutic device.
FIG. ll and llA~ are detailed circuit~diagrams of the
device shown in FIG. 10.
FIG. 12 is a detailed circuit diagram showing an elec-
tronic timer element which can bç used to control the ionto-
therapeutic administration,
3/037g0 ~2 2~l~n PCT/US92/0
12
FIG. 13 is a schematic diagram of a wrist-type ionto-
therapeutic device coming within the invention showing a
belt-type battery power supply and a sensor for blood sugar
monitoring.
FIG. 14 is a schematic diagram showing an iontothera-
peutic device of this invention in interface with a computer
system through a connecting line (e.g., interface cable/
telephone line).
FIG. 15 is a schematic diagram of an iontotherapeutic
device of this invention using a belt or ~and to attach to
the subject being treated.
FIG. 16 is a graph comparing the effects of periodic
wave mode and DC mode on the transdermal absorption of insu-
lin and on the reduction of blood glucose level (B.G.L.) in
~; 35 the diabetic hairless rats.
FIG. 17 is a graph showing the time course for the
~reduction ~iD the blood glucose level (B.G.L.) in the dia-
betic~hairless rates as the re~sult of transdermal delivery
of insulin from a pharmaceutical reservoir electrode con-
' ~ taining 250 IU of insulin at pH 3.6 by transdermal periodic
iontotherapeutic system with square waveform mode (lmA;
,
on/off = 1/1; frequency = 2 KHz) for 40 min.
: 50 ,, ~
FIG. 18 is a graph showing the effect of the frequency
~-- generated by the transdermal periodic iontotherapeutic sys-
,~, .. -
tem on the reduction in the blood glucose level (B.G.L.) in
the diabetic hairless rates using insulin.
i : ,
_
~ WOg3/037gO PCT/US92/07221
2122150
13
S
FIG. 19 is a graph showing the effect of the on/off
ratio in the transdermal periodic iontotherapeutic system on
the reduction in the blood sugar level (B.G.L.) in the d~a-
betic hairless rats using insulin.
FIG. 20 is a graph showing the effect of the treatment
duration by the transdermal periodic iontotherapeutic system
with drug reservoir electrode at pH 3.6, on the reduction in
the blood glucose level (B.G.L.) in the diabetic hairless
rats using insulin.
FIG. 21 is a graph showing the effect of the treatment
duration by the transdermal periodic iontotherapeutic sys-
tem, with drug reservoir electrode at pH 7.1, on the reduc-
tion in the blood glucose level (B.G.L.) in the diabetic
hairless rats using insulin.
FIG. 22 is a graph showing permeation of vasopressin
facilitated by the transdermal periodic iontotherapeutic
system compared to passive diffusion of a vasopressin solu-
~o tion at pH 5.0 through hairless rat skin.
FIG. 23A is a~graph showing permeation rate of insulin
solution at pH 7.1 through hairless rat skin using no ionto-
therapy as compared to permeation rate shown in FIG. 21B
when using iontother-py (TIDD).
~50 FIG. 24 is a series of graphs showing the comparative
-~ effects of the change in waveform in lowering blood glucose
-- level (B.G.L.) in diabetic hairless rats using transdermal
periodic iontotherapeutic system using insulin solution at
pH 3.68.
~ ,
:`
W093/037gO PCT/US92/07221
2122150 ~`~
14
¦ FIG. 25A is a graph showing lowering of blood sugar
level (B.G.L.) of hairless rats using transdermal periodic
. iontotherapeutic system on Day 1 using insulin soiution at
pH 3.68.
15~ FIG. 25B is a graph showing further lowering of the
blood sugar levels of the same rats on Day 3 using transder-
mal periodic iontotherapeutic-system without further admin-
istration of insulin, indicating that the insulin delivered
X transdermally on Day 1 is stored in the skin tissues and can
25be activated to become available for absorption into
systemic circulation on Day 3 by TPIS.
FIG. 26A is a pair of comparative graphs showing plasma
immunoreactive insulin levels in diabetic rabbits after
administration of insulin solution (pH 7.1) using transder-
35mal periodic iontotherapeutic: system (TPIS) compared with
:~ corre~sponding levels in diabetic rabbits using subcutaneous
;~ administration: (SC). "SZ in~ection" indicates injections to
render rabbits diabetic. ~ ~
`~FIG.~26B is a` pair~of comparative graphs oorresponding
4~5:-to~those of FIG.~ 24A~ showing the réspective reduction of
.:blood glucose Ievels ~(B.G.L.)~. The data show that blood
glucose levels can be controlled at a highly constant level
so~as not to fall substantially, if at all, below normal
levels by TPIS.
, ': .,
~'
::
~ W093/037gO 2 1 2 2 1 5 0 PCT~US92/07221
i:
FIG. 27A is a pair of comparative graphs showing the
increase in plasma insulin concentration after administra-
tion of insulin solution (pH 7.10) using transdermal
periodic iontotherapeutic system (TPIS) compared to using
transdermal iontotherapeutic system (TIDD) in which 4X cur-
rent intensity and 2X administration times are used. TPIS
administration shows more rapid attainment of increased
plasma insulin concentrations.
FIG. 27B is a pair of comparative graphs corresponding
to those of FIG. 25A showing the attained lowering of blood
glucose levels (B.G.L.). The data show a near instantaneous
reduction of blood glucose level from the hyperglycemic
level in the diabetic contro}s using transdermal periodic
iontotherapeutic system (TPIS) ~hereas the reduction using
transdermal iontotherapeutic system (TIDD) is lower than the
normoglycemic level.
FIG. 28 is a pair of comparative graphs showing a
~-~ 40 desired reduction in urine output as indicated by urine
~ os olarity5measurement in anesthetized rabbits using trans-
,~ dermal periodic iontotherapeutic system to administer vaso-
pressin solution (pH 5.0). The corresponding graph shows
: ~ ~
that TPIS is more effective in reducing urine output than
TIDD.
FIG. 29 is a graph showing vasopressin permeation rate
enhancement when the ionic strength of the vasopressin solu-
tion used in TPIS is decreased.
::
wO93/037go ,, PCT/US92/07221
;~ j ' . 3 `
16
` 5 21221~)
~, FIG. 30 is a graph showing enhancement of skin permea-
tion of vasopressin using TPIS with a short skin permeation
lag time. The graph also shows reversibility of skin per-
meation within 2 hours after ceasing TPIS treatment and
again enhancement of skin permeation after reinstituting
TPIS.
~!
~, .
~, 20 DETAIL~D D~SCRIPTION OF THE INVENTION AND THE PREFERRED
ENBODIMENTS
;,
.
FIG. l is a diagram portraying a device of the inven-
~, tion in operation to deliver iontotherapeutically an ionized
;:~
pharmaceutical and its uptake into the bloodstream of the
subject being treated. The figure shows the iontotherapeu-
tic device in electrical contact'with the skin.
3S It also shows the pharmaceutical reservoir electrode in
contact with the skin as well as the other electrode, which
is referred to as the receptor electrode. The electrodes
are in,~,contact with the uppermost skin barrier, called
stratum corneum. The pharmaceutical is transmitted through
, the~stratum corneum and flows into~the dermo-epidermal
' layer.,~ The stratum corneum is the principal absorption rate
limiting barrier. The first portion of the dermis layer is
referred to as the papillary layer, which contains a capil-
lary network of the vascular system. The capillary network
takes up,the transdermally absorbed ~pharmaceutical and the
uptaken pharmaceutical is shown to flow from the capillary
network into the main portion of the vascular system.
WO 93/037gO PCI /US92/07221
2122150
17
FIG. 2 is a block diagram of a transdermal periodic
iontotherapeutic device coming within the invention in which
! the power supply is derived either from the conversion of
the alternate current (AC) from a 120 V-mains (or other
¦ 15 available AC mains) into direct current or from a suitable
battery. The power is turned on manually by a switch or
¦ automatically by a programmable timer. The device also
consists of one or a combination of several electronic
multifunction generators, a drug reservoir electrode and a
receptor electrode. The multifunction generator is
.
assembled with a power supply, to delivery direct current
with periodic waveform of either square, triangular, trape-
zoidal or sinusoidal shape, to an output circuit. The
desired iontotherapeutically-effective waveform can be
selected manually or preprogrammed through a switch (Kl),
and the frequency of the output waveform can be adjusted in
, .-
the range of 10 Hz - 50 KHz. The output circuit then pro-
~ vides a physiologically ~acceptable current, }or example,
;- ranging up to 10 mA, to the pharmaceutical reservoir elec-
~5 trode which contains the~ionized~pharmaceutical to be
délivered transdermally, and a receptor electrode in series.
When desired, the device can be operated to deliver either
;~ ~ DC current alone Iperiodically or continuously), or in com-
~ bination with a periodic waveform.
55~ ~ :
:~
~ '
~' ,
D
~ '
093/037gO PCT/US92/07221
. ,~.
~ 21221~0 18
FIG. 3 is a block diagram of an iontotherapeutic device
x of this invention. It consists of the following elements:
a microprocessor, a multiple waveform generator, a waveform
~- selector, an output circuit, a sensor signal processor, a
.~;
display unit, a power supply with indicator, a reservoir
electrode, and a receptor electrode.
The microprocessor is the center of the device. It has
~, 20 the following functions:
a. receiving and processing the physiological signal(s)
. . ~
from the sensor element;
b. communicating with a computer system via an interface
cable;
c. receiving and exercising commands from the computer
system;
d. storing data and transmitting data to the computer
system;
e. controlling operation parameters of the multiple wave-
form generator, such as frequency and duty cycle of
generated waveforms;
-~
-~ ~ f. selecting the input waveform of the output circuit;
g. controlling the operat~ion parameters of the output
circuit, such as output current amplitude and treatment
cycle;
-
h. monitoring the load impedance of the device and alert-
ing the user of improper operation oonditions.
:
W O 93~03790 P(~r/US92/07221
19 2122I50
The microprocessor is made using a commercial single
chip microcontroller with necessary expanded memory capa-
city, additional input/output ports and signal converters.
A preferred microcontroller is 80C552 single chip microcon-
troller made by Signetics, a subsidiary of Philips Compo-
nents. This microcontroller is very powerful and meets the
requirements of the current application. It has the follow-
ing important features: 16 MHz speed, 8K ROm and 256K RAM
memory, 4 watchdog timer-counters, 6 I/O ports and 8 channel
12 bit A/D, UART and I2C interfaces, and 6 external inter-
rupts.
The multiple waveform generator provides pulse-mode
signals of desired waveforms. It can be realized by using
the circuitry shown in FIG. 6. It can also be made by using
a col ercial integrated circuit ICL8038 made by Motorola
Corporation.
~: :
The waveform selector can be made using a commercial
-~0 electronic analog switch, such as AD7510 made by Analog
Devices.
The~output~circuit can be made by using the circuit
design shown in FIG. 7~or using a three-pin constant current
regulator LM334 made by Natiohal Semiconductor Corporation.
~50i The function of the sensor signal processor is to fur-
ther condition the physiological signals, such as blood
glucose level signals. It provides necessary function, such
as amplification and filtering of the signals. The condi-
tioned signals will be sent to the analog/digital converter
W093/037g0 PCT/US92/07221
'~ 5 2122150-
'ij
of the microprocessor. They will be used for close-loop
j control of iontotherapeutic treatment.
1 10
The power supply unit consists of batte~y elements
', connected in series. The batteries can be either regular
?Ji 15 o~nes or rechargeable ones. A low-batter indicator will be
used to signal the low battery condition.
FIG. 4 is a detailed circuit diagram for the square
wave generator shown in FIG. 2. It employs a microchip 555
timer. The frequency (F) of the square wave is:
F =
tl ~ t2 tl = 0.693 (Pl + P2) C
t2 - 0.693 P1 C
where P's are potentiometers, C is a capacitor, and D's are
diodes. During the operation, the capacitor C is charged
~through the potentiometer Pl and P2 and the diode D for t
seconds and~discharged through potentiometer P1 and diode D2
~o for t2 seconds. Other circuits can be used in place there-
of.
~, FIG. 5 is a detailed circuit diagram for the triagular-
- ~5
~ trapezoidal waveform generator shown in FIG. 2. It consists
:
of an integrator (A) and a regenerative comparator (B) con-
~nec~ted in a positive feedback loop. Precise triangular
waves aré formed by integration of the square wave which is
fed back from the output of the~comparator to the input of
th integrator. The frequency (F) ~of the triangular wave
;~ W093/03790 2 1 2PCT/US92/07221
21
~: 5
1~ F = l tl = Vo+ - vo ) Rl Vo+
. .
tl + t2 R2 C (p2a + P3b)
- tl = Vo+ - Vo ) Rl Vo
R2 C (P2a + P3b)
where Vo+ and Vo~ are the higher and lower trip points of
,, the comparator, respectively. Resistors Rl and R2 control
the comparator trip points. Capacitor C is the integration
capacitor. Potentiometer Pl provides adjustment of the
triangular wave offset. Potentiometers P2 and P3 adjust
frequency and symmetry, respectively.
The third op-amp circuit (C) acts as a damper. It
produces a trapezoidal wave with the same frequency as the
triangular wave. Potentiometer P4 sets the clamping level.
Other circuits can be used in place thereof.
FIG. 6 is a detailed circuit diagram for the sinusoidal
signal generator shown in FIG. 2~ The circuit of the
generator uses two amplifiers: one (A) acts as a non-
inverting integrator, and other (B) acts as an inverting
,~, integrator. They are connected in cascade to form a feed-
"." ~
~, back loop. The frequency (F) of the sinusoidal signal is
. ,A
determined by:
F =
- 2 CP
~ 55
;,,
,
WOg3/037g0 PCT/US92/07221
2122150 22
S
C's and P's are integration capacitors and the variable
resistors, respectively. Resistor Rl is a feedback resis-
tor. Capacitor Cl is used to prevent high-frequency oscil-
lations. Other circuits can be used in place thereof.
FIG. 7 is a detailed circuit diagram for the Output
Circuit shown in FIG. 2. The desired waveform is selected
manually or automatically from the 3 generators through a
switch (Kl) and sent to the inverting amplifier, from which
the signal then goes to the output stage of two transistors.
The output current (dose currentO is adjusted by a potentio-
meter (P?, as monitored by a current meter (A), and is
delivered to the drug reservoir electrode (B). Other
circuits can be used in place thereof.
FIG. 8 is a diagram illustrating the wristwatch-type
miniaturized transdermal periodic iontotherapeutic system
with multifunction programmability. It is designed to have
one or more nuclear batteries and two pieces of microchips:
~-
one for the purpose of genersting different waveforms, as
outlined in FIGS. 4-6, and the other is for the purpose of
45 ~ controlling and to display the output current. The nuclear
batteries provide the energy needed for long-term operation.
For instance, the programmability may include selection of
DC alone or in combination with a periodic waveform, a dose
current for a particularly designated time period. In cer-
5S tain applications, it may be advantageous in operating the
devices of this invention to have the periodic current wave-
~ '
W093/037gO PCT/US92/07221
'~ 2122150
23
form remaining at some constant DC level during the off
cycle. In this design of iontotherapeutic device, the drug
reservoir electrode is positioned outside the device.
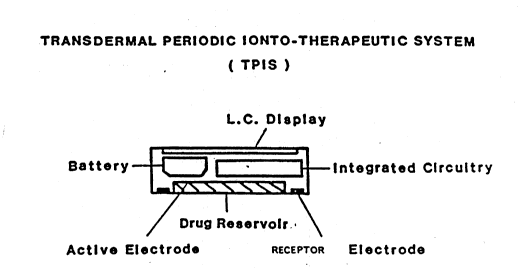
FIG. 9 shows an embodiment of another design of ionto-
therapeutic device. It shows two views of the device. The
first view is a cross-sectional view showing the integrated
circuitry, L.C. display, battery, drug reservoir electrode
positioned directly in the lower central portion of the base
and the receptor electrode encircling the drug reservoir
electrode. The next view shows the bottom view of the
device. In the center portion of the bottom view is shown
the circular drug reservoir portion of the drug reservoir
;~ 30 electrode. The drug or pharmaceutical dissolved in an
aqueous solution is homogéneously dispersed in a polymer
: ~ :
matrix unit dose as described~herein. The pharmaceutical
solution can also be contained in a reservoir-type unit dose
~ ~ having a microporous surface adapted to permit the drug to
r~., '~ be tra D itted.~ Next, there is shown the receptor elec-
~trode,~ as a circular ring positioned in spaced~relationship
from the drug reservoir~electrode. At the top of the cross-
sectional view is shown~a ~liquid crystal display. It can
display a number of functions, including whether or not the
device is in operation, the type ;of periodic current and
waveform being used and other pertinent information of the
transdermal periodic iontotherapeutic~ drug delivery. The
battery employéd as the power source for this invention can
,
:
! wo 93~03790 PCT/US92/07221
i 21221~0
24
be a lithium or other nuclear battery having a voltage, for
example, of from 6 to 12 volts.
FIG. 10 is a block diagram of a portable transdermal
periodic iontotherapeutic device in which the power supply
is derived from a battery source such as one or more 9V
batteries. The power is turned on manually by a switch.
The device can be equipped so that it can be turned on auto-
matically by a programmable timer. The device also cons~sts
of one or a combination of several electronic multifunction
2S generators, a drug reservoir electrode and a receptor elec-
trode. The multifunction generator can provide periodic
waveform of either square, triangular, trapezoidal or sinu-
soidal shape, to an output circuit. The desired iontothera-
peutically effective waveform can be selected manually and
the frequency of the output waveform c~n be adjusted to a
physiologically acceptable frequency of at least 10 Hz and
: : ~
up to about 50 KHz. The output circuit then provides a
physiologically acceptable~ current, ranging up to 10 mA, to
~ the pharmaceutical reservoir electrode, which contains the
4S solution of the ionized pharmaceutical to be delivered
transdermally, and a receptor electrode in series. When
desired, the device can be operated to deliver either DC
current~alone (periodically or continuously), or in combina-
.
tion with a periodic waveform. ~ ~
FIGS. 11 and llA show a detailed circuit diagram for
the portable transdermal periodic iontotherapeutic device
shown in the block diagram of FIG. 1~. Referring to FIG.
::
.
WOg3/03790 PCT/US92/07221
21 221 ~ O
s
11, the following is a description of the circuits and their
functioning:
T,he ~C-to-DC converter and battery voltaqe monitor
lCl, Rl-R4, Cl-C3, Ll and diode IN914 consist of a DC-
~5
to-DC converter which is incorporated in step-up applica-
tion. The output voltage is elevated from 9V battery to 27V
with the proper adjustment of R4. The output voltage of the
battery is monitored by a battery voltage monitor which
includes a zener diode Dl, R5-R7, C4 and C106Yl. When out-
put of 9-V battery drops below minimum acceptable volume of
8.3V, LED lights to indicate the need for recharging.
Pul~ç_generator and constant current output staae
2~ D2 D5, Tl, C5, C6 and R8 are components of a
triangle-wave generator. In this circuit, the charge and
' discharge currents for C6 come through the diode bridge
,
formed by~D2-D5. Bridge D2-D5 consists of four general
'purpose~switching diodes 'with ~low-leakage characteristics,
'- that-serve~to steer currènt in the proper direction through
45 '~ he'current~source'made up of Tl and~R8.
' The pin 3 of IC2~serves as~a source of current for the
timing network, and its state of high or low determines the
direction of current flow~into ~or out of C6 for charge or
discharge. Since bath charge~ and discharge currents flow
~ through the same current regulator circuit,~the currents are
WOg3/037~ PCT/~'S92/07221
21221~0 26 -
equal, and thus times of charge and discharge are equal. As
a result, triangular waves are formed across C6.
The circuit covers the frequency range of a.bout 20 Hz
to 30 KHz. The adjustment of the frequency is done with R8.
The frequency of the triangle waves can be expressed as
f =
5R8C6
The output of the triangle-wave generator is sent to
the pin 3 of IC3 which serves as a comparator. The voltage
comparison is made between pin l and pin 3 of IC3. The
square waves are formed at pin 7 of IC3 with a duty cycle
which is determined by the voltage of the voltage divider
composed of Rlo~Rl2. The higher the voltage applied to pin
2 is, the shorter the "on" time of the square waves, and
vice versa. The duty cycle of the sguare waves covers the
~- ~ range of l/lO to 10/l. The square waves are amplified by
T2-T4;and s-nt to pin ll of IC4.
In aonstant current output stage, IC923 is employed to
serYe as a current regulator. IC923 is originally designed
to be a voltage regulator with an output current limit
resistor R across pin lO and pin 3. The maximum output
current is set as 0.6/R. This feature is adapted to form a
- current regulator. As soon as the condition (VoUt~RL)>Is is
satisfied (where VOUt is the output voltage, RL, load resis-
tance, and Is~ output current preset), the output current
S5
will be kept at the preset level.
:
W093/03790 21 2 21 50 PCT/US92/07221
27
R21 is the minimum current limit resistor. R22 is used
to preset the desired output current. C7 and R20 are used
10to eliminate high frequency noise.
Ou~put current monitor
15Intersil 7106 interfaced with a liquid crystal display
is the heart of the current monitor. R23 is a shunt resis-
20tor. C8 and R24 consist of an RC oscillator which runs at
about 48 KHz and is divided by four prior to being used as
the system clock. C10 and R27 serve as an input filter.
25C~1, C12 and R28 determine the display sensitivity. Cg is
for auto-zero function.
The power is turned on manually by a switch or auto-
matically by a programmable timer. The device also consists
of one or a combination of several electronic multifunction
35generators, a drug reservoir electrode and a receptor elec-
~trode. m e multifunction generator is assembled with a
power supply, to deliver direct current with-periodic wave-
form of either square, triangular, trapezoidal or sinusoidal
shape, to an output circuit. ~The desired iontotherapeuti-
~45~cally effective waveform can be selected manually or pro-
- grammed through a switch ~X1), and the frequency of the
output waveform can be ad~usted in the range of 10 Hz - 50
KHz. The output circuit then provides a physiologically
~;acceptable current, ranging up to 10 mA, to the pharmaceuti-
55cal ~reservoir electrode, which contains the pharmaceutical
formulation to be delivered transdermally, and a receptor
WOg3/037~ PCT/US92/07221
2122150 28
electrode in series. When desired, the device can be
operated to deliver either DC current alone (periodically or
lo continuously)~ or in combination with a periodic waveform.
FIG. 12 is a detailed circuit diagram for the timer of
the multi-channel transdermal periodic iontotherapeutic
dèvice shown in the block diagram of FIG. 12. Referring to
FIG. 12, the following is a description of the circuit, and
their functioning:
Timer
The timer consists of ten IC chips, two relays and
other components, IC8 provides a system clock. ICl, IC3 an
IC5 are quad 2-input multiplexers which consist of four 2-
input multiplexers with common select and enable inputs.
When the select input is at logical "o", the four output
pins assume the values of inputs of pin 1, 5~, 14, 11, other-
wise, ~nputs of pin 3, 6, 13, 10. The inputs of the first
group represent the~ "off" time of the timer which has a
maximum value of 999 minutes. The inputs of the second group
represent the "on" time of the timer which has a maximum
~5 value of~ 99 minutes. m e~values of both "on" and "off" time
needed are set through~BCD thumbwheels.-
IC2, IC4 and IC6 are "decade-down" counters which
receive preset values from multiplexers. The pin 15's of
these counters will become logical "o" when the minimum
~55 count is reached. When all three counters reach the mini-
mum, ICg, a "AND" gate, will turn to be logical "1". This
:
W093/03790 PCT/US92/07221
2122151)
29
pulse is inverted by IClo and goes to reset the system
clock, reloads counters and converts IC7, which consists of
two Flip-Flop's. At the instant when "on" time is finished,
the pin 3 and pin 5 turn to be logical "o", which opens two
relays and turns on the red LED> AT the same time, the pin
2 and pin 6 turns to be logical "1", which will load the
values representing the "on" time to pin 4, 7, 9, 12 of
three multiplexers and turns off the green LED. At the
instant when "off" time is finished, the pin 3 and pin 5
turn to be logical "1", which will load the values repre-
senting the "off" time to pin 4, 7, 9, 12 of three multi-
plexers and turn-- on the green LED. The whole cycle of both
"on" and "off" is repeated for any desired length of time.
The switch K2 is used to interrupt the operation and trigger
the timer.
Pulse aenerator and constant current output staaes
IC13, diode bridge consisting of four IN914, Tl, R28
and C5-C7 are components of a triangle wave generator. In
this cIrcuit,~ the cha~rge and discharge currents for one of
t '' :
` 45 C6-C17 come through the diode bridge for~med by four IN914,
; which serve to steer curren~t in the proper direction through
the current source made up of Tl and R28.
The pin 3 of IC2 serves as a source of current for the
timing network, and its state of high~or low determines the
direction of current flow into or out of the capacitor for
charge or discharge. Since both charge and discharge cur-
:
WOg3/037gO PCT/US92/07221
2122150 30
rents flow through the same current regulator circuit, the
currents are equal and thus times of charge and discharge
lo are equal. As a result, triangular waves are formed across
the working capacitor C.
The circuit covers the frequency range of about 10 Hz
~5
to 30 KHz. The adjustment of the frequency is done by the
selection of the proper capacitor through a multi-stop
switch. The frequency of the triangle waves can be
expressed as
f - 1
SR28C
The output of the triangle wave generator is sent to
the pin 3 of IC14 which serves as a comparator. The voltage
comparison is made between pin 2 and pin 3 of IC14. The
square waves are formed at pin 7 of IC14 with a duty cycle
which is determined by a voltage-divider composed of R322-
-~ R34. The higher the voltage applied to pin 2 is, the short-
.40 er the "on" time of the square waves, and vice versa. The
duty cycle of thé square waves covers the range of 1/10 to
10/1. The square waves are amplified by T2 and T3 and then
45 i - ~
sen~ to three voltage followers T4-T6.
At the "on" time of the timer, two relays are closed
and emitters of T4-T6 are connected to pin ll's of IC15-
:
IC17. IC15-IC17 provide three-channel current outputs.
Three IC923 are employed to serve as current regulators.
IC923 is originally designed to be a voltage regulator with
an output current limit resistor R across pin 10 and pin 3.
W093/03790 PCT/US92/07221
31
The maximum current is set as 0-6/R- This feature is
adapted to form a current regulator. As soon as the condi-
'~ 10 tion (VoUt/RL)>Is is satisfied (where VOut is the output
voltage, RL load resistance and Is output voltage, RL load
¦ 15 resistance and Is output current preset), the output current
will be kept at the present level. R40, R45 and R50 are
maximum current limit resistance respectively. R41, R4~ and
~, 20 R51 are used to preset the desired current. Clg-C2l are
used to eliminate high frequency noise.
The output currents are monitored by a current meter A.
The switch Kl is used to select DC or pulse output. Other
circuits can be used in place thereof.
FIG. 13 is a schematic diagram of a device of this
invention. It shows a wristwatch-type device which houses
the iontotherapeutic device in the center in connection with
a belt-type battery package. The display unit, emergency
on/off switch, the input/output port, the interface cable to
~ a computer system, and the~sensor input port are also shown.
This device can~be comfortably worn by a patient during the
treatment. The weight of such devicè of thls invention will
ordinarily~be 5 oz. or less, prèferably 3 oz. or less.
FIG. 14 is a schematic diagram of a wrist-type ionto-
therapeutic device of this invention showing a connection
with a computer patient data and control system such as at a
clinical site or at a physician's office. The communication
between the iontotherapeutic device and a computer system
,
W093/03790 PCT/US92/07221
2 12 2 lS0 32
serves two purposes. It allows the commands and data
according to the physician's prescription to transfer to the
iontotherapeutic device via an interface cable. It also
allows the physician to read and assess the important data
of treatment using the device. By using telephone lines,
the communication can be to a remote site.
Various computers are satisfactory for use in the com-
puter system, including personal computers and larger compu-
ters. Various suitable programs can be used in the communi-
cation.
FIG. l5 is the schematic diagram of an iontotherapeutic
device of this invention using a belt or band to attach to
the subject being treated. Inside the belt there are bat-
tery elements connected in series. These batteries can be
either regular ones or rechargeable ones. The battery belt
can also be made into a shape of iewelry. The battery belt
; can be designed t~ house different numbers of battery ele-
.
~ents to power different treatment periods. The belt can be
made of suitable material such as plastic or leather mate-
rials or metals or combinations of materials. Its length
-
can be adjusted as needed.
FIG. 16 is a graph showing the time course for the
~ 50 reduction of the elevated blood glucose level (% change in
! B.G.L.) in the diabetic hairless rats as the result of
; transdermal delivery of insulin from the drug reservoir
electrode (containing 250 IU of insulin at pH 7.l) by Trans-
I dermal Periodic Iontotherapeutic System for 80 minutes and
W093/03790 PCT/US92/07221
33 2 1 2 21 50
the effect of current delivery mode. Keys: (0) direct
current made (2 mA), ( ) Square wave periodic mode (2 mA;
on/off = 4/1; Frequency = 2000 Hz).
FIG. 17 is a graph showing the time course for the
reduction of the elevated blood glucose level (% change in
B.G.L.) in the diabetic hairless rats as the result of
transdermal delivery of insulin from the pharmaceutical
reservoir electrode (containing 250 IU of insulin at pH 3.6)
by Transdermal Periodic Iontotherapeutic System with square
wave periodic mode (1 mA; on/off = 1/1; Frequency = 2000 Hz)
for 40 minutes.
FIG. 18 is a graph showing the effect of the frequency
generated by the Transdermal Periodic Iontotherapeutic Sys-
tem on the reduction of the elevated blood glucose level (%
change in B.G.L.) in the diabetic hairless rats. The fre-
.:~ ,
.
guency~of 2000 Hz produces a greater magnitude and a Ionger
duration of reduction than the 1000 Hz.
FIG. 19 is a~ graph showing the effect of the on/off
ratio in the~TransdermaI Periodic Iontotherapeutic System on
the reduction of the elevated b}ood glucose level (% change
~- in B.G.L.) in the diabetic~hairless rats. By regulating the
ratio, the magnitude and the duration of reduction in B.G.L.
~ 50
:~ ; in the diabetes can be controlled as desired.
FIG. 20 is a graph showing the effect~of the treatment
~ 55 duration by the Transdermal Periodic Iontotherapeutic System
; on the reduction of the elevated blood glucose level (%
, ~ .
W093~037~0 PCT/US92/07221
22 l~iO 34
change in B.G.L.) in the diabetic hairless rats. At pH 3.6,
which is lower than the isoelectric point of insulin (pH
5.3), with the dose current of l mA, on/off ratio of 8/l and
at a frequency of 2000 Hz, the treatment duration of 20-40
minutes appears to be equally effective.
FIG. 21 is a graph showing the effect of the treatment
duration by the Transdermal Periodic Iontotherapeutic System
on the reduction of the elevated blood glucose level (%
change in B.G.L.) in the diabetic hairless rats. AT pH 7.l,
which is higher than the isoelectric point of insulin (pH
5.3), with the dose current of l mA, on/off ratio of l/l and
at frequency of lO00 Hz, the treatment duration produces a
difference in the rate and the duration, but with equal
effectiveness.
For a more detailed description of the background for
the remaining FIGS., see the indicated Examples: FIG. 22
(Example ll); FIGS. 23A and 23B (Example 12); FIG. 24
- ~
(~EYample 14); F~G. 25 (~Bxample l5); FIGS. 26A and 26B
(Example 16); FIG. 27 (Example 17); FIG. 28 (Example 18);
FIG. 29 (Example~ l9); FIG. 30 (Example 20).
In carrying out the iontotherapeutic process for admin-
istering transdermally, sys~emically measured amounts of an
~ ionized pharmaceutical compound,~ it is first necessary to
- provide the pharmaceutical-containing unit dose in which the
pharmaceutical is in aqueous sol~ution. The pH of the
agueous solution is adjusted to an effective Ph either below
or above the pKa or the isoelectric point of the pharmaceu-
wO93/037g0 PCT/US92/07221
35 2 1 2 2I 50
s
tical. It is desirable to adjust the pH to an effective
level of about l pH unit above or below the pKa or isoelec-
tric point of the pharmaceutical, preferably to a~ effective
pH level of at least 1.5 or at least 2 pH units below or
above the pKa or isoelectric point of the pharmaceutical.
With particular pharmaceuticals, it is preferable to so
adjust the pH either below or above the pKa or isoelectric
point. For example, with regard to insulins, it is prefer-
able to adjust the pH below the pKa or isoelectric point,
such as to about 1.0 pH units or lower below, which for
co~,ercial insulins is about pH 5.3.
The formed unit dose is placed in the receptacle por-
tion provided in the pharmaceutical reservoir electrode, so
that the ionized pharmaceutical can be transdermally
,, -
absorbed~. If the unit dose form is a preformed self-con-
tained unit dose, it can be held in the receptacle portion
of the reservoir electrode by customary means such as clamp-
ing, snapping into position,~adhesive, or the like.
- ~ One~convenient form of the unit dose for the ionized
~` 45 pbarmaceutical solution is to disperse uniformly the agueous
solution of the ionized pharmaceutical in a polymeric
~atrix. The polymeric unit dose must be characterized by
being able to release the ionized pharmaceutical, when the
. ~
iontotherapeutic device is in operation, so that the ionized
pharmaceutical can be~absorbed transdermally. The unit dose
WOg3/03790 PCT/US92/0~221
5~ 21221~0 36
is in electrical contact with the skin of the subject being
treated when the iontotherapeutic device is in operation.
10For a description on making suitable unit dose in the
form of a polymeric matrix dosage unit, reference is made to
parent U.S. Application Serial No. 07/046,984, filed May 5,
1987, now U.S. Patent Application No. 5,042,975, which is
incorporated herein by reference.
20Additionally, descriptions are found in parent U.S.
¦ Application Ser. No. 07/~87,406, filed September 25, 1990,
25which is incorporated herein by reference.
The pharmaceuticals suitable for delivery by this poly-
mer disc can be the anti-diabetic drugs, such as insulins or
30sulfonyl ureas; the anti-diuretic peptide drugs, such as
vasopressin; the calcium-channel blocker-type anti-hyperten-
sive drugs, such as verapamil; the beta-blocker type anti-
hypertensive drugs, such as propranolol; narcotic analgesicdrugs, such as hydrocodone; non-steroidal anti-arthritic
: :
~40~drugs,~ such~as indomethacin: anti-bacterial antibiotics,
such as tetracyclines, penicillins and~cèphalosporins; anti-
~néoplostic~ drugs, such as methotrexate; and the peptide
hormones, such as luteinizing hormone-releasing hormone
(LNRN), oxytoxin, and the li~e.
50Pharmaceuticals suitable for use in the process of this
invention can be selected from the ~following or other
ionizable~pharmaceuticals which are capable of being trans-
dermally absorbed in the iontotherapeutic process, the fol-
lowing systemically-effective pharmaceuticals expected to be
: .
W093~03790 PCT/US92/07221
`` 37 2122150
s
capable of delivery by an iontotherapeutic device as
developed in this invention: Propranolol HCl, Ibuprofen,
Indomethacin HCl, Lorazepaml, Thioridazine Hcl, Tolazamide,
Doxycycline, Flurazepam, Minocycline, Disopyramide, Meto-
cloprimide HCl, Cephalothin sodium, Thiothixene, Vincris-
tine, Oxazepam, VAlproic acid, Temazepam, Hydralizine HCl,
Ampicillin sodium, Amantadine HCl, Acetohexamide, Haloperi-
dol, Doxepin, Cyclobenzaprine HCl, Sucralfate, Cephalaxin,
Cefazolin sodium, Ampicillin, Cefadroxil, Hydralizine HCl,
Reserpine and Hydrochlorthiazide, Clindamycin HCl, Carbeni-
¦ cillin disodium, Piroxicam, Fenoprofen calcium, Dialtiazem
HCl, ~hlorpropamide, Sulindac, Nefedipine, Cimetidine,
Naproxen, Piroxicam, Ranitidine HCl, Nadolal, Alprozolam,
Captopril, Triazolam, Chlordiazepoxide, Amitryptilline,
Dobutamide, Sulfamethoxazole, Trimethoprin, and the like.
The ionizable peptide pharmaceuticals used in the
processes and the unit doses of this invention and adminis-
tered by the devices of this invention are those which are
pha Daceutically effective and transdermally absorbable.
'45 - Desirably the peptides have at least five amino acid units
and more desirably at least nine amino acid units.
0 In operating the process, using for example a wrist-
- watch-type iontotherapeutic device such as provided by this
invention, the appropriate unit dose containing the pharma-
~55 ceutical required for the desired therapy is assembled in
the receptacle portion of the pharmaceutical reservoir elec-
W093/03790 PCT/USg2/07221
212215U 38
trode. Por example, if insulin is to be administered and
the pH of the insulin solution in the dose unit is pH 3.6,
insulin is a cationic and therefore the dosage unit is
assembled as a part of pharmaceutical reservoir electrode,
which is the anode. The desired waveform is selected and
preprogrammed, such as a square waveform. The pharmaceuti-
cal reservoir electrode used preferably is adapted to
receive a disposable unit dose, e.g., a polymeric matrix
unit dose, and to make electric contact with the skin of the
subject being treated. Such means is assembled in place.
The other variables are selected and preprogrammed, such as
the frequency, the dose current and on/off ratio. The
device is attached to the subject being treated as by a band
attached to the device and adapted to be attached to and
detached from the subject. The switch of the device is
turned to "on" position and the device commences operation
of the iontotherapeutic process, which causes the ionized
pharmaceutical of reservoir electrode to be administered
transdermaIly and iontotherapeutically to provide a systemic
dosing.~ The particular waveform, mA,~ pharmaceutical reser-
voir electrode (i.e., cathode or anode), frequency, length
of treatment and other factors will be selected and prepro-
grammed depending upon the pharmaceutical being admin-
istered, the subject being treated and~others.
- Some pharmaceuticals, especially certain relatively low
¦ molecular weight pharmaceuticals, can be iontotherapeuti-
~ cally administered using either periodic DC mode or periodic
WOg3/03790 PCT/USg2/07Z21
39 212~15~
wave mode. For example, the periodic DC mode can be "on"
for about 0.5 to about 10 minutes, preferably about 1 to
about 5 minutes per hour. During the intervening period
during the hour, the device is in "off" position. The "on"
period can be more frequent or less frequent, if desired, to
provide effective treatment, such as one 'lon" period every
30 minutes or every ninth minute. In Example 5, it is shown
that hydrocodone can be administered following this general
procedure. The dose currents, the on/off ratios, the dosage
units and the devices described above can be used or adapted
to be used in the practice of the periodic DC mode process.
A few hours duration of treatment each day following
either procedure is ordinarily adequate, for example, 2 to
10 hours, depending upon factors such as the pharmaceutical,
the subject being treated, the iontotherapeutic factors
selected and the like.
.
~40
' :
~ .
:~
-~45
~, ' ' ,,
.
~ :
WOg3/03790 PCT/US92/0?221
21221~0
The following Examples are illustrative of the inven-
tion but are not intended to be limiting.
Example 1
An aqueous solution of insulin at concentration of 250
IU~ml is prepared by dissolving 96.9 mg (25.8 IU/mg) of pure
insulin in 10 ml of double-distilled, sterile water and
adjusted to pH 7.1 with 0.5N NaOH. Two ml of the insulin
solution so prepared is filled into a refillable dosage unit
having a microporous membrane as the drug-releasing surface.
This insulin-containing reservoir-type dosage unit is then
assembled as a part of the pharmaceutical reservoir elec-
trode and applied on the abdominal skin of 3 diabetic hair-
less rats with the transdermal periodic iontotherapeutic
system operating at 2 mA~with direct current mode or square-
wave periodic mode (on/off = 4/1; Frequency = 2000 Hz). The
résults on the reduction in blood glucose level are shown
and compared in FIG. 16.
~ 40
:~
Example 2
~45 ~ - An amount of~ 200 mg (25.8 IUjmg) of pure insulin is
dissolved in 10 ml of double-distilled, sterile water and
the pH is adjusted to 3.6 with 0.SN HCl. An amount of 200
- mg of hydroxypropylmethylcellulose is well dispersed in
another 10 ml of double-distilled sterile water using a
,,
magnetic stirrer with a stirring bar (5 cm in length) at a
rotation speed of 600 rpm. The temperature is controlled at
W093/03790 PCT/US92/07221
,.~ .
41 21 22I ~
about 80C. After the hydroxypropylmethylcellulose is dis-
persed homogeneously, the stirring is continued while the
mixture is cooled to about 40C.
The insulin solution prepared above is then added to
the dispersion of hydroxypropylmethylcellulose with inter-
mittent stirring to avoid any denature of insulin molecules,
using the same stirring mechanism as described above, at the
same stirring rate of 600 rpm for a period of two minutes.
The insulin/hydroxypropylmethylcellulose solution is then
placed in a refrigerator for congealing to occur. The insu-
lin-containing polymer matrix is cut into disc-shaped parts
with the appropriate dimensions, such as 2.5 cm in diameter
and 0.2 cm in thickness. The insulin-containing discs are
stored at 5C. The concentration of insulin in the discs is
about 250 IU/gm.
The insulin-containing polymeric matrix dosage forms
are removed as needed and assembled into the pharmaceutical
~ reservoir electrode. The pharmaceutical reservoir electrode
having the insulin-containing polymer unit dose form is the
r anode since the insulin molecules in the polymeric matrix
~- 45
dose units are cations at pH 3.6, which is lower than the
isoelectric point of insulin (pHiso = 5.3).
Application of thls insulin-containing polymeric matrix
unit dose is made onto the abdominal skin of 3 diabetic
hairless rats. The transdermal periodlc iontotherapeutic
~ system is then operated at 1 mA using an on/off ratio of
¦ 1/1, a frequency of 2000 Hz and a square wave mode, for 40
W093/03790 PCT/US92/0?221
21221~ 42
minutes. The result on the reduction in blood glucose level
is shown in FIG. 17.
Example 3
An aqueous solution of insulin at a concentration of
250 IU/ml is prepared by dissolving 133.8 mg (25.8 IU/mg) of
pure porcine insulin in 20 ml of citrate buffer at pH 3.6.
Two ml of the insulin solution so prepared is filled into a
refillable dosage unit having a microporous membrane as the
drug-releasing surface. This insulin-containing reservoir-
type dosage unit is then assembled as a part of the pharma-
ceutical reservoir electrode of the iontotherapeutic device
and applied successively on the abdominal skin of 9 diabetic
hairless rats with the transdermal periodic iontotherapeutic
- system operating at 1 mA with square waveform mode to study
the effect of freguency, on/off ratio and treatment duration
on the reduction of blood glucose level. The results are
shown and compared, respectively in FIGS. 18, 19 and 20.
~ ~ :
Example 4
The same insulin solution is prepared~ in the same way
as in Example 1, except that a phosphate butter at pH 7.1 is
used to replace the double-distilled water. Two ml of the
insulin solution so prepared is filled into a refillable
dosage unit having a microporous membrane as the drug-
releasing surface. This unit dose is applied to 3 diabetic
hairless rats following the same operation procedures as in
::
r_W093/03790 PCT/USg2/07221
43 21221SO
s
Example 3 to study the effect of treatment duration on the
reduction of blood glucose level. The results are shown in
FIG. 21.
Example 5
A saturated solution of hydrocodone 9pKa = 8.56), a
narcotic analgesic drug, is prepared in citrate buffer at pH
4.0 and in phosphate buffer at pH 7.5. An aliquot of 3.5 ml
of this hydrocodone solution is filled into the reservoir
compartment, which is in contact with the stratum corneum
surface of the hairless rat abdominal skin, of each Valia-
Chien skin permeation cell with the receptor compartment
containing equal volume of a pH 7.4 buffered isotonic (drug-
free) saline solution. The transdermal periodic iontothera-
peutic system is then mounted with its electrodes immersing
in the skin permeation cell, one electrode in each of the
~ : -
two solution compartments. A current of 1 mA is applied for
2 min. periodically on the hour for 12 hours at either DC
: -
mode or periodic sguare wave mode (frequency, 2000 Hz;
on/off ratio, 1/1). The results are shown in Table I.
i 50
,
W093/037gO 2 1 2 2 1 ~ O PCT/~'S92/072~1
44
~ S
I Table I: Enhancement in Rate and Reduction in Time Lag
of the Skin Permeation Rate of Hydrocodone,
a Narcotic Analgesic Drug, by the
Transdermal Periodic Iontotherapeutic System
Skin Pe~meàtion Rate
(mcg/cm /hr + S.D.)
Mode pH 7.5 pH 4.0 Tlag (hrs)
Control 4.75 + 1.70 3.10 5.17
DC mode 7.61 ~ 2.74 37.5 0.72
-~- -
periodic wave mode7.01 + 1.16 59.4 0.90
Example 6
A saturated solution of methotrexate, an anti-neoplas-
tic drug, is prepared in double-distilled water and adjusted
to pH 8.0, which is higher than the pKa values of metho-
trexate (4.8 and 5.5). An aliguot of 3.5 ml of this metho-
trexate solution (2 mg/ml) is filled into the donor compart-
ment, which is in contact with the stratum corneum surface
of the hairless rat abdominal skin, of each Valia-Chien skin
permeation cell with the receptor compartment containing
egual volume of a pH 7.4 buffered isotonic (drug-free)
saline solution. The transdermal periodic iontotherapeutic
system is then mounted with its electrodes immersed in the
. skin permeation cell, one electrode in each of the two solu-
tion compartments. A DC current of 1 mA is applied for 10
minutes periodically on the hour for 5 hours with a fre-
S5 quency of 2000 Hz, a square wave form, and an on/off ratio
of 4/1. The results are illustrated in Table II:
W093/037gO PCT~US92/07221
~ 45 21 221 50
Table II: Enhancing Effect of Transdermal Periodic
Iontotherapeutic System (TPIS) on the Skin Permeation of
Methotrexate - An Anti-Neoplastic Drug
Time Cumulative Amount of Drug Absorbed (mcg/cm2)
(hrs) No TPIS With TPIS
1.33 0.0086 0.0820
2.33 0.0247 0.1373
3.33 0.0471 0.4223
4.16 0.0745 0.570s
5.16 0.1398 1.0835
Example 7
A saturated solution of propranolol (pKa - 9.45), a
beta-blocker type anti-hypertensi~e drug, is prepared in
citrate buffer at pH 3.68. The enhancing effect of the
transdermal periodic iontothérapeutic system is studied
under the same conditions as that outlined in Example 6.
The results are shown in Table III:
.
W093/037gO PCT/US92/07221
: 46
2 122 1S0
Table III: Enhancing Effect of Transdermal Periodic
Iontotherape7~}c System (TPIS) on the Skin Permeation
of Propranolol - An Anti-Hypertensive Beta-Blocker Drug
Time Cumulative Amount of Drug Absorbed (mcg/cm2)
(hrs) No TPIS With TPIS(2)
1;5 0.0691 0.5970
2.5 0.2615 1.1950
3.5 0.5845 3.3650
4 5 0.9955 5.2150
5 5 2.0800 9.0700
1) In the Valia-Chien skin permeation cell, a donor solu-
tion containing 13.3 mgfml of propranolol (pKa = 9.45)
at pH 3.68 was applied topically to hairless rat skinat 37C.
2) TPIS applied a DC current of lmA periodically at 10
min/hr, a frequency of 2000 Hz and an on/off ratio of
4/1.
~:
Example 8
;~ A saturated :solution of verapamil (pKa = 8.9), a cal-
~ci _ -channel blocker-type anti-hypertensive drug, is pre-
~ paréd~:~in :citrate~buffer at pH 3.68. The enhancing effect of
-~ 45 ~ ~ the:tra~nsdermal~ periodic iontotherapeutic system is studied
~- under the~ same conditi;ons as that ~outlined in Example 6.
.
The results are shown in Table IV.
~:~, 50
", :
,'' :
:
~ 55
:~
::
~,,
~,
W093/03790 PCT!USg2/07221
47 21221~0
Table IV: Enhancing Effect of Transdermal Periodic
Iontotherapeutic System (TPIS) on the Skin Permeation
of Verapamill(l) - A Calcium-Channel Blocker-Type
A~tihypertensive Drug
Time Cumulative Amount of Drug Absorbed (mcg/cm2)
(hrs) No TPIS With TPIS
1.42 <0.0001 0.297
2.42 <0.0001 0.445
3.42 _ 0.695
4.17 - 0.973
5.17 <0,0001 1.g45
I
1) In the Valia-Chien skin permeation cell, a donor solu-
tion containing 23.95 mg/ml of verapamil (pKa = 8.9) at
pH 3.68 is applied topically to hairless rat skin at
370C-
¦ 2) TPIS applied a DC current of 1 mA periodically at 10
min/hr, a frequency of 2000 Hz and an on/off ratio of
3 4/1.
.,
Example 9
. A saturated solution of tetracycline HCl (pKa = 3.3,
7.8 and 9.7), an antibiotic drug, is prepared in phosphate
buffer at pH 9Ø The enhancing~effect of the transdermal
periodic iontotherapeutic system is investigated under the
same conditions as that outlined in Example 6. The results
are shown in Table V:
WO 93/037gO PCr/US92/07221
2122150` 48
Table V: Enhancing Effect of Transdermal Periodic
Iontotherapeutic System (TPIS) on the Skin Permeation
of Tetracycline Hcl(l) - A Calcium-Channel Blocker-Type
Time Cumulative Amount of Drug Absorbed (mcg/cm2)
(hrs) No TPIS With TPIS
1;25 0.0180 0.1765
2.25 o.o5so 0.2555
3.~5 0.0650 0.7815
4.25 0.1450 1.3235-
5.25 0.3040 3.5600 ..
1) In the Valia-Chien skin permeation cell, a donor solu-
tion containing 6.2 mg/ml of tetracycline HCl (pKa =
3.3, 7.8 and 9.7) at pH 9.0 is applied topically to
hairless rat skin at 370C.
2) TPIS applied a DC current of 1 mA periodically at 10
min/hr, a frequency of 2000 Hz, a square waveform and
an on/off ratio of 4/1.
1. ~
Example 10
A saturated solution of indomethacin (pKa = 4.5), a
non-steroidal anti-arthritic drug, is prepared in buffer
j solution at pH 2.5, which is 2 pN units below the pKa, and
at pH 5.5, which is one pH unit ~above the pKa, and at pH
4.5, the pKa. The enhancing effect of the transdermal
: periodic iontotherapeutic system is evaluated under the same
"'~ : ,
: conditions as that outlined in Example 6. The results are
shown in Table VI.
WOg3/03790 PCT/US92/07221
49 2122150 -
s
Table VI: Enhancing Effect of Transdermal Periodic
Iontotherapeutic System (TPIS) on the Skin Permeation
of Indomethacin - A Non-Steroidal Anti-Arthritic Drug
TPIS*Skin Permeation Rate (mcg/cm2/~r)
pH 2.5 pH 4.5 pH 5.5
No - - 1.47
Yes 0.76 0.44 6.30
*TPIS applied a DC current of 1.2 mA periodically at 5
min/hr, for 7 hours, with a frequency of 2000 Hz, a square
waveform and an on/off ratio of 2/1.
30Example 11
An aqueous buffer solution of vasopressin (50 mcg/ml
: containing 1.7 mcCi/ml H3-vasopressin) is prepared in
, ~ ,
citrate-phosphate buffer at pH 5Ø An aliquot of 3.5 ml of
this: vasopressin solution is filled into the refillable
::
dosage unit having a microporous membrane as the drug-
- : ~ releasing surface. ~The dosage unit is then assembled as a
-: ~ - part~of~the pharmaceutical reservoir electrode of the ionto-
- therapeutic device and m ~brane surface thereof is applied
to the stratum corneum sidè of hairless rat skin mounted in
:50 -the Valia-Chien skin permeation cell at 370C. Samples are
- ~ ,; - , ,~ -
withdrawn at regu}ar-intervals and radioactivity is measured
-:~ by scintillation counter to determine the amount of vaso-
pressin which has been transdermally absorbed.
-
WOg3/037gO PCT/US92/07221
21221~0
The results demonstrate that vasopressin permeates
through the hairless rat skin at constant, but slow rate for
30 hours (0.94 + 0.62 ng/cm2/hr) (FIG. 22).
When the skin is treated with transdermal periodic
iontophoretic system (TPIS) at current intensity of 0.5 and
lmA, frequency of 2 KHz, on/off ratio of 1/1, and at the
rate of 10 min. per 40 min. for 4 hours, the skin permeation
profiles are enhanced with rate increases from 0.94 (~0.62)
ng/cm2/hr (referred to as "passive diffusion" in FIG. 20) to
116.2 (+ 10.7) and 178.0 (+ 25) ng/cm2/hr, respectively.
After the treatment with transdermal periodic iontophoretic
system, referred to in following Table VII as "post-activa-
tion phase," the rate of skin permeation of vasopressin is
reduced to the basal rate of only 0.7 (+ 0.4) and 5.3 (+
0.5) ng/cm2/hr, respectively. The results of the experiment
are shown in FIG. 22 and in the following Table VII.
-
~- 45
Il ~
~,: 50
WOg3/03~90 PCT/US92/07221
- 51 21221~0
Table VII: Effect of TPIS on Skin
Permeation Rate of Vasopressin
No TPIS 0.0 mA 9.12 (+1.06) 0.94 (+0.62)
With TPIS
a) Activation
phase(2) 0.5 mA <0.5 116.2(+0.4)
bj Post-Activation
phase 0.0 mA --- 0.7 (+0.4)
a) Activation
phase(2) 1.0 mA <0.5 178.0 (+2~.0)
b) Post-Activation
phase o.o mA --- 5 3 (-
l)In-vitro permeation across hairless rat skin mounted in
the Valia-Chien permeation cell.
2)Application of DC at on/off ratio of 1/1 and frequency of
2 KHz, by multi-channel TPIS unit (shown in FIG. 22 for 10
min. per 40 minute period, treatment repeated for six 40-
; ~minute cycles.
~40 Example 12
An~aqueous solùtion of ~insulin (5.3 IU/ml containing
~ 0.3 mcCi of Il25-insulin) is prepared and adjusted to pH 7.1
- 45
~-~ using naOH. An aliquot of 3.5 ml of this insulin solution
is filled into the refillàble dosage unit having a micro-
porous membrane as the drug-releasing surface. The dosage
. . .... . .
unit is then assembled as a part of the pharmaceutical
reservoir electrode of the iontotherapeutic device and mem-
brane surface thereof is applied to the stratum corneum side
of hairless rat skin mounted in the Valia-Chien skin permea-
:
W093/03790 PCT/US92/07221
21221~ 52
tion cell at 370C. Samples are withdrawn at regular time
intervals and radioactivity is measured by scintillation
counter to determine the amount of insulin whic~ has been
transdermally absorbed.
The results demonstrate that insulin permeates through
the hairless rat skin at constant, but at a slow rate for 48
hours (3.94 + 0.29 mcIU/cm2/hr) (FIG. 23A).
When the skin is treated with transdermal therapeutic
system (TIDD) at current intensity of lmA, frequency of O
Hz, on/off ratio of l/l, and at the rate of 5 min. per 60
min. for 7 hours, the skin permeation profiles are enhanced
with rate increased from 3.94 (+0.2~) mcIU/cm2/hr to 37.5
(+4.5) mcIU/cm2/hr. FIG. 23B shows comparison of insulin
permeation data in FIG. 23A using no iontotherapy (O) over a
7-hr. period with perméation data of same insulin solution
using TIDD iontotherapy.
Example 13
- .;, , , ~ . , ~ . .
An aqueous solution of insulin (5.3 IU/ml containing
45 ; 0.3 mcCi of I125-insu1in)~ i~ prepared and adjusted to pH
3.7, 5.2 or 7.1 using either~ HCl or naON solution. An
aliquot of 3.5 ml of this insulin solution is filled into
~the refillable dosàge unit having a microporous membrane as
;~ the drug-releasing surface. The~ dosage unit is then
assembled as a part of the pharmaceutical reservoir elec-
trode of the iontotherapeutic device and membrane surface
W O 93/03790 P(~r/US92/07221
53 2 I 2 2 1 5 0
thereof is applied to the stratum corneum side of hairless
rat skin mounted in the Valia-Chien skin permeation cell at
370C. Samples are withdrawn at regular time intervals and
radioactivity is measured by scintillation counter to deter-
mine the amount of insulin which has been transdermally
absorbed.
The results demonstrate that insulin permeates through
the hairless rat skin at constant, but at a slow rate fQr 48
hours, with permeability coefficient ranging from 6.50
(+4.2) to lO.02 (+l.94)x 10_7 cm/hr (Table VIII).
Permeabiity coefficient is the ratio of the steady state
rate of skin permeation of the pharmaceutical which is
transdermally absorbed/the concentration of~the pharmaceuti-
cal solution which is applied transdermally. ~The pharmaceu-
tical in this experiment is insulin.
~ 35
c ~ ~ When the skin is treated with transdermal therapeutic
..
~ ~ system (TIDD) ~at current intensity of lmA, frequency of 0
Hz, on/off~ ratiQ of l/l, and ~at the rate of 5 min. per 60
;~ ~ min.~for~7~ hours, the;skin permeation profiles are enhanced
with skin~permeability coefficient increased to a range from
~70.75 ~+8.56~x 10_7~to 24Z.59 ~(~18.43) x 10_7 cm/hr, which
show dependence on solutioh pH. The lower pH solution (pH
3.7) shows greater increase in TPIS-facilitated skin per-
~- meability.
,
: ~ :
: :
:
W O 93/03790 PC~r/US92/0~221
2122150 54
Table VIII: Skin Permeability Coefficient of Insulin
(Hairless Rats)
Donor Permeability Coefficient 1
10Solution (cm/hr~ SE) x 10 ( )
E~ No TIDD With TIDD
15. 3.7 6.50 (+1.4~) 242.59 (+18.43)
5.2 10.02 (+1.94) 120.07 (+22.86)
7.1 7.43 (+0.54) 70.76 (+8.56)
(1) Triplicate Determinations
25Example 14
An aqueous buffer solution of insulin (250 IU/ml) is
prepared in citrate-phosphate buffer at pH 3.68. An aliquot
of 2.5 ml of this insulin solution is filled into the
refillable dosage unit having a microporous membrane as the
drug-releasing surface. The dosage unit is then assembled
as a p~art of the pharmaceutical~ reservoir ~electrode of the
~ iontotherapeutic device and membrane surface thereof is
~applied~ to the skin at~abdominal region of~ 3 groups of
, -anesthetized, diabetic hai~rless~ rats. Blood samples are
vithdrawn~at regular time intervals and glucose levels are
measured by glucose analy'zer. The reduction in glucose
~ 50level from hyperglycemic state is the pharmacodynamic
-response to the insulin absorbed transdermally. The results
;-demonstrate that when the sk~in~is treated with transdermal
periodic iontophoretic system (TPIS) at current intensity of
1 mA, frequency of 2 KHz, on/off ratio of ~1/1, for 40 min.
:
,
,
WO 93/03~90 2 1 2 2 1 PCI/US92/07221
the blood glucose levels are reduced substantially. The
data show that the time course and the extent of reduction
in blood glucose levels in diabetic rats vary with the type
of waveform used (FIG. 24).
Example 15
An aqueous buffer solution of insulin (250 IU/ml) is
prepared in citrate-phosphate buffer at pH 3.68. An aliquot
of 2.5 ml of this insulin solution is filled into the
refillable dosage unit having a microporous membrane as the
drug-releasing surface. The dosage unit is then assembled
as a part of the pharmaceutical reservoir electrode of the
iontotherapeutic device and membrane surface thereof is
applied to the skin at abdominal region of 5 anesthetized,
diabetic hairless rats. Blood samples are withdrawn at
regular time intervals and glucose levels are measured by
. ~ . ,
glucose analyzer. The reduction in glucose level from
hyperglycemic state is the pharmacodynamic response to the
insulin absorbed transdermally. The results demonstrate
~ ~, . , . . ~ . ,
that when the skin is treated on Day 1 with transdermal
., , ~ :
periodic iontophoretic sys~tem (TPIS) with insulin in the
pharmaceutical reservoir electrode at current intensity of 1
mA, frequency of 2 ~KHz, square- waveform, on/off ratio of
:
1/1, for 40 min. the blood glucose levels are reduced sub-
stantially (FIG. 25A). On Day 3, the diabetic rats are
treated again with TPIS with no insulin in the pharmaceuti-
W093~03~90 2 1 2 2 1 5 0 PCT~US92~07221
56
cal reservoir electrode (placebo formulation), the blood
glucose is also reduced, indicating that part of the insulin
delivered transdermally on Day l forms a depot in the skin
tissue and can be triggered to be systemically absorbed on
Day 3 (FIG. 25B).
Example 16
An aqueous buffer solution of insulin (500 IU/ml) ;at pH
7.lO is used. An aliquot of 2.5 ml of this insulin solution
is filled into the refillable dosage unit having a micro-
porous membrane as the drug-releasing surface. The dosage
unit is then assembled as a part of the pharmaceutical
reservoir electrode of the iontotherapeutic device and mem-
brane surface thereof is applied to the skin at dorsal
region of 3 diabetic rabbits. Blood samples are withdrawn
at regular time intervals and analyzed for immunoreactive
. - , . .
insulin concentration by radioimmunoassay and for glucose
. .. . . ~
levels by glu¢ose analyzer. The reduction in glucose level
from hyperglycemic state is the pharmacodynamic response to
, ,~ ,. .,, .. : ,.. ~ . .
the insulin absorbed transdermally. ~The results demonstrate
that when the ~skin is ~treated with- transdermal periodic
; ~
iontophoretic system (TPIS~ at current intensity of l mA,
frequency of 2 KHz, on/off ratio of l/l, and`square waveform
- - , ~ ,- - , .. . . .
for 40 min. the plasma immunoreactive insulin concentration
increases rapidly and the blood glucose levels are reduced
substantially. The plasm;a insulin profile (FIG. 26A) as
well as the time course and the extent of reduction in blood
' W093/03790 PCT/US92/07221
' 57 21221SO
glucose levels (FIG. 26B) in diabetic rabbits are compared
with the results from the conventional subcutaneous adminis-
10tration of insulin. The data show that plasma insulin con-
centrations as well as blood glucose levels can be effec-
tively controlled using TPIS system of this invention. FIG.
24B shows that by using the TPIS system of this invention
the blood glucose level (B.G.L.) can be appropriately
reduced in a more controlled manner than by daily SC dosages
so as to prevent B.G.L. to fall below normal levels.
Example 17
An aq~eous buffer solution of insulin (500 IU/ml) at pH
7.10 is used. An aliquot of 2.5 ml of this insulin solution
is filled into the refillable dosage unit having a micro-
~- 3S porous membrane as the drug-releasing surface. $he dosage
unit is then assembled as a part of the pharmaceutical
reservoir electrode of the iontotherapeutic device and mem-
brane surface thereof is applied to the skin to the
abdominal skin of 2 ~groups of diabetic rabbits. Blood
samples are withdrawn at regular time intervals and analyzed
for immunoreactive insulin concentration by radioimmunoassay
and for glucose levels by glucose analyzer. The reduction
~- in glucose level from hyperglycemic state is the pharmaco-
dynamic response to the insulin absorbed transdermally.
The results demonstrate that when the skin is treated with
transdermal periodic iontophoretic system tTPIS) at current
W093/03~90 PCT/US92/07221
2122150 58
intensity of 1 mA, frequency of 2 XHz, on/off ratio of 1/1,
and square waveform for 40 min., the plasma immunoreactive
insulin concentration increases more rapidly and the blood
glucose levels are reduced more instantaneously than trans-
dermal iontophoretic delivery (TIDD) at current intensity of
4 mA for 80 min. (FIG. 27). The data in FIGS. 25A and B
show that the TPIS system of this invention provides both a
more rapid increase in plasma insulin concentration-after
administration and a more rapid reduction in blood glucose
levél than use of TIDD even though the corresponding current
intensity in the TIDD system is 4 tlmes as much (4 mA vs. 1
mA) and administration is 2 times as great (80 minutes vs.
40 minutes) as in the TPIS system.
Example 18
An aqueous buffer solution of vasopressin (40 IU/ml) is
prepared in citrate-phosphate buffer at pH 5Ø Vasopressin
is an anti-diuretic pharmaceutical, which is used by
patients which have an excessive urine output. Vasopressin
caused a reduction of urine output and an increase in ion
content, such as sodium ion content. Ion content in the
urine is determined by using osmolarity measurement. An
aliquot of 3.5 ml of this vasopressin solution is filled
into the refillable dosage unit having al microporous mem-
. : ,
brane as the drug-releasing surface. The dosage unit is
then assembled as a part of the pharmaceutical reservoir
electrode of the iontotherapeutic device and membrane sur-
wO93/037gO PCT/US92/07221
~ 59 21221~0
face thereof is applied to the abdominal skin of 2 groups ofanesthetized rabbits. Blood samples are withdrawn and urine
samples are collected at regular time intervals and urine
osmolarity is measured by osmometer. The increases in
osmolarity from the basal level are the pharmacodynamic
responses to the vasopressin transdermally absorbed.
The results demonstrate that when the skin is treated
with transdermal periodic iontophoretic system (TPISj at
current density of 0.22 mA/cm2, frequency of 2 KHz, on/off
ratio of l/l, and square waveform for 40 min., the urine
osmolarity increases from the basal levels more rapidly and
substantially than with transdermal iontophoretic delivery
(TIDD) under the same experimental conditions (FIG. 28).
Example l9
An aqueous buffer solution of vasopressin (50 mcg/ml
containing l.7 mcCi/ml H3-vasopressin) is prepared in
~0 ,
citrate-phosphate buffer at pH 7.4 with varying ionic
strengths. An aliquot of 3.5 ml of this vasopressin solu-
tion is filled into the refillable dosage unit having a
microporous membrane as the drug-releasing surface. The
dosage unit is then assembled as a part of the pharmaceuti-
cal reservoir electrode of the iontotherapeutic device and
membrane surface thereof is applied to the stratum corneum
side of hairless rat skin mounted in the Valia-Chien skin
permeation cell at 370C. Samples are withdrawn at regular
,
W093/03790 PCT/US92/07221
s 2122150 60
time intervals and radioactivity is measured by scintilla-
tion counter to determine the amount of vasopressin which
has been transdermally absorbed. The results demonstrate
that vasopressin permeates through the hairless rat skin at
constant, but slow rate for 30 hours (1.32 +0.38 ng/cm2/hr).
I 15 When the skin is treated with transdermal periodic ionto-
¦ phoretic system (TPIS) at current intensity of 1 mA, fre-
quency of 2 KHz, on/off ratio of 1/1, and at the rate of 10
min. per 40 min. for 4 hours, the skin permeation profiles
are enhanced with rate increases from 1.32 (+0.38) ng/cm2/hr
(referred to as "passive diffusion") to the range of 65.9
(+13.1) to 632 (+65.C) ng/cm2/hr, depending upon the ionic
strength of vasopressin solution. The results of the
experiment are shown in the following Table IX.
Table IX: Effect of Ionic Strength on Skin
Permeation Rate of Vasopressin
Ionic Strenath Skin Permeation Ratel) Enhancement Factor
(ng/cm2/hr + SD)
0.488 65.9 (+ 13.1)49.9 (+ 18.0)
: i , .. ~
- 0.244 101.4 (+ 9.1) 76.8 (+ 6.9)
0.122 244.6 (+ 26.3)185.3 (+ 19.9)
0.061 632.6 (+ 65.0)472.8 (+ 59.0)
1)The rates determined in the activation phase with lag time
ranging from 0.48 (+ 0.21) to 0.86 (+ O.lS) hrs.
2)Compared to the skin permeation rate of vasopressin by
passive diffusion (1.32 ng/cm2/hr).
W093/03790 PCT/US92/07221
61 2 1 2 2l SO
s
The TPIS-facilitated skin permeation rate appears to be
dependent upon the ionic strength of drug solution. The
lower the ionic strength, the higher the rate of skin per-
meation and the greater the enhancement in skin permeability
lS (FIG. 29).
Example 20
An aqueous buffered solution of vasopressin (50 mcg/ml
containing l.7 mcCi/ml H3-vasopressin) is prepared in
citrate-phosphate buffer at pH 5.0 at ionic strength of
. 0.064. An aliquot of 3.5 ml of this vasopressin solution is
. filled into the refillable dosage unit having a microporous
membrane as the drug-releasing surface. The dosage uni.t is
then assembled as a part of the pharmaceutical reservoir
electrode of the iontotherapeutic device and membrane sur-
face thereof is applied to the stratum corneum side of hair-
less rat skin mounted in the Valia-Chien skin permeation
cell at 370C. Samples are withdrawn at regular time inter-
vals and radioactivity is measured by scintillation counter
to determine the amount of vasopressin which has been trans-
dermally absorbed.
The results demonstrate that vasopressin permeates
through the hairless rat skin at constant, but slow rate for
30 hours (0.98 ~ 0.26 ngjcm2~hr).
W093/037gO PCT/US92/07221
2122150 6?
When the skin is treated with transdermal periodic
iontophoretic system (TPIS) at current intensity of 0.3 mA
frequency of 16 KHz, on/off ratio of l/l, for 60 min., the
skin permeation profiles are enhanced with rate increases
from 0.98 (+ 0.26) ng/cm2/hr referred to as "passive dif-
fusion") to 757.3 (+ 53.2) ng/cm2/hr (FIG. 28), while the
duration of time lag is reduced from 9 hours down to 0.40 (~
0.06) hours). The data in FIG. 30 demonstrate the rever-
¦ sibility of skin permeability that in less than 2 hours
after the TPIS treatment, the skin permeability returns to
the rate before the TPIS treatment. Then, TPIS can be
applied again to facilitate the skin permeation of vaso-
pressin.
~; '
,
~ .