Note: Descriptions are shown in the official language in which they were submitted.
W093/09781) PCT/U~92t~9656
2123611
TREATMENT OF MELANOMA ~IT~
ANTI~ENBE O~IGONUCLEOTIDE~ TO C-MYB PROTO-ONCOGENE
Field of the Tnvention
The invention relates to antisense
oligonucleotides to proto-oncogenes, in particular
antisense oligonucleotides to the c-mvb gene, and the use
of such oligonucleotides as antineoplastic agents.
Reference to Government Grant
The invention described herein was made in part
with government support under grant CA 54384 awarded by
National Institutes of Health. The government has
certain rights in the invention.
Backaround of the Invention
The proto-oncogene c-myb is the normal cellular
homologue of the avian myeloblastosis virus-transforming
gene v-myk. The c-mYb gene codes for a nuclear protein
expressed primarily in hematopoietic cells. It is a
proto-oncogene, that is, it codes for a protein which is
required for the survival of normal, non-tumor oells.
When the gene is altered in the appropriate manner, it
has the potential to become an oncogene. Oncogenes are
genes whose expression within a cell provides some
function in the transformation from normal to tumor cell.
The human c-myb gene ~as been isolated, cloned,
and sequenced~ Majello et al., Proc. Natl. Acad. Sci.
U.S.A. 83, 9636-9640 (1986). Antisense oligonucleotides
W O 93/09789 P ~ /US92/096~6
2 1 2 3 ~ 2 -
to human c-myb mRNA that is, oligonucleotides having a
nucleotide sequence complementary to the mRNA transcript
of the c-m~b gene, are disclosed in our allowed, co-
pending application Serial No. 427,659, filed October 27,
1989, and corresponding international patent application
WO90/05445, the entire disclosures of which are
incorporated herein by reference. C-myb antisense
oligonucleotides are disclosed therein as being useful
for the treatment of hematologic neoplasms, and for
immunosuppression.
Melanoma, also known as "malignant melanoma"
or "cutaneous melanoma", is a neoplasm of melanocytes
that has the potential for invasion and metastasis,
Melanocytes are melanosome-containing cells that
specialize in the biosynthesis and transport of melanin
pigment. Melanocytes reside in the skin at the basal
layer of the epidermis. Under a variety of stimuli, they
elaborate melanin pigment. Melanin synthesis occurs on
the melanosome, a well-defined intracellular organelle
within the melanosome.
At one time considered rare, the rate of
increase in the incidence of melanoma i5 greater than for
any other cancer, with the exception of bronchogenic
carcinoma. The incidence of melanoma is greatest among
Caucasians, and is influenced by ultraviolet light
exposure, and by geographical and occupational factors.
~he incidence of melanoma is increasing rapidly in the
United States and elsewhere, with an apparent doubling
every ten to seventeen years. Presently, melanoma
accounts for roughly one percent of cancers in the United
States, and about the same proportion of cancer deaths.
While it represents only about three percent of cutaneous
neoplasms, melanoma accounts for two thirds of all skin
cancer fatalities.
For the most part, melanoma first progr~s~s
through a radial growth phase at the site of the primary
lesion. This initial phase is characterized by little
~'l23~
wo93/o978s PCT/US92/09656
-- 3 --
or no competence to metastasize. Melanomas in this phase
are generally treatable by surgical procedures. In the
vertical growth phase, characterized by penetration into
deeper cutaneous tissues, a primary melanoma acquires
competence to metastasize. Surgery alone is ineffective
in treating the melanoma, once metastasis has occurred.
Chemotherapy, either alone or in combination
with surgery, has been utili~ed in the treatment of
melanoma. Dimethyltriazeno-imidazole (DTIC) is the most
active single agent for the treatment of metastatic
melanoma, with an overall objective response rate of only
21%. DTIC can cause disturbances in liver function. It
possesses modest hematologic toxicity.
Somewhat less effective in treating malignant
melanoma are the synthetic nitrosoureas, of which 1,3-
bis(2-chloroethyl)-1-nitrosourea (BCNU), 1-(2-
chloroethyl)-3-cyclohexyl-1-nitrourea tCCNU), methyl-CCNU
and chlorozotocin are best known. The nitrosoureas
display a somewhat more severe hematologic toxicity than
DTIC. Response rates range from 10% to 18%.
Unfortunately, there are no single agents or
combination regimens that induce a substantial number of
complete remissions in melanoma patients. There is an
urgent need for the development of more effective agents.5
summarY of the Invention
The invention provides a method for treating
melanoma. An effective amount of one or more c-myb
antisense oligonucleotides is administered to an
individual in need of such treatment. Each
oligonucleotide has a nucleotide sequence complementary
to at least a portion of the mRNA transcript of the human
c-mvb gene. The oligonucleotide is hybridizable to the
mRNA transcript. Preferably, the oligonucleotide is at
least a 12-mer oligonucleotide, that is, an oligomer
containing at least 12 nucleotide residues. In
particular, the oligomer is advantageou91y a 12-mer to
W093/09789 PCT/US92~09656
21~611 4
a 40-mer, preferably an oligodeoxynucleatide. While
oligonucleotides smaller than 12-mers may be utilized,
they are statistically more likely to hybridize with non-
targeted sequences, and for this reason may be less
specific. In addition, a single mismatch may destabilize
the hybrid. While oligonucleotides larger than 40-mers
may be utilized, uptake may be more difficult. Moreover,
partial matching of long sequences may lead to non-
specific hybridization, and non-specific effects.
Preferably, the oligonucleotide is a 15- to 3~-mer
oligodeoxynucleotide, more advantageously an 18- to 26-
mer.
While in principle oligonucleotides having a
sequence complementary to any region of the c-~y~ gene
find utility in the present invention, oligodeoxynucleo-
tides complementary to a portion of the c-myb mRNA trans-
cript including the translation initiation codon are
particularly preferred. Also preferred are
oligonucleotides complementary to a portian of the c-myb
~20 mRNA transcript lying within about 40 nucleotides
; upstream (the 5' direction) or about 40 nucleotides down-
stream (the 3' direction) from the translation initiation
codon.
The invention is also a method for purging bone
marrow of metastasized melanoma cells. Bone marrow
aspirated from a melanoma-inflicted individual is treated
with an effective amount of c-~y~ antisense
oligonucleotide, and the thus-treated cells are then
returned to the body of the afflicted individual. The
bone marrow purging technique may be utilized for an
autologous bone marrow rescue (transplantation), in
connection with a course of high dose chemotherapy.
The inYention is also a composition for the
treatment of melanoma comprising a pbarmaceutically
acceptable carrier and c-myb antisense oligonuclootide.
As used in the herein specification and
appended claims, unless otherwise indicated, the term
W O 93/09789 PC~/US92/09656
s ?l23~ll
"oligonucleotide" includes both oligomers of
ribonucleotides, i.e., oligoribonucleotides, and
oligomers of deoxyribonucleotides, i.e., oligo-
deoxyribonucleotides ~also re~erred to herein as "oligo-
deoxynucleotides"). Oligodeoxynucleotidesarepreferred.
As used herein, unless otherwise indicated, the
term "oligonucleotide" also includes oligomers which may
be large enough to be termed "polynucleotides".
The terms"oligonucleotide"and"oligodeoxynuc-
leotide" include not only oligomers and polymers of thecommon biologically significant nucleotides, i.e., the
nucleotides adenine ("A"), deoxyadenine ("dA"), guanine
("G"), deoxyguanine ("dG"), cytosine ("C"), deoxycytosine
. ("dC"), thymine ("T") and uracil ("U"), but also include
oligomers and polymers hybridizable to the c-myb mRNA
transcript which may contain other nucleotides.
Likewise, the terms "oligonucleotide" and
"oligodeoxynucleotide" includes oligomers and polymers
wherein one or more purine or pyrimidine moieties, sugar
moieties or internucleotide linkages is chemically
modified. The term "oligonucleotide" is thus understood
to also include oligomers which may properly be
designated as "oligonucleosides" because of modification
of the internucleotide phosphodiester bond. Such
modified oligonucleotides include, for example, the
methylphosphonate oligonucleosides, discussed below.
The term "phosphorothioate oligonucleotide"
means an oligonucleotide wherein one or more of the
internucleotide linkages is a phosphorothioate group,
0
--O -- P -- O~
W O 93/09789 PC~r/US92/09656
212~ 6
_
as opposed to the phosphodiester group
o
~o - P - 0~
which is characteristic of unmodified oligonucleotides.
By "methylphosphorate oligonucleoside" ismeant
an oligonuclèotide wherein one or more of the
internucleotide linkages s a methylphosphonate group,
ll
--O -- P -- O--
CH3
The term "downstream" when used in reference
to a direction along a nucleotide sequence means the
5'~3' direction. Similarly, the term "upstream" means
the 3'~5' direction.
The term "c-mYb mRNA transcript" means the
presently known mRNA transcript of the human c-mvb gene,
or any further transcripts which may be elucidated.
Brief Des¢ri~tion of the F~oures
Fig. 1 is a graph of the effect of c-myb sense
and antisense oligonucleotide on human melanoma cells
(CHP) n vitro. Cells were treated with ~i) no oligomer
("Control"), (ii) the indicated concentrations of an 18-
mer oligodeoxynucleotide complementary to codons 2-? of
the translated position of the c-~y~ mRNA transcript
("Antisense"), or (iii) the corresponding sense 18-mer
("Sense").
Fig. 2 is similar to Fig. 1, except that the
oligonucleotide treatment was extended for two days. The
oligonucleotide concentrations are cumulative of the two
day treatment period.
'
~093/09789 2 1 2 3 6 1 lPCT/~JS92/09656
-- 7 --
Fig. 3 is similar to Fig. 2, except that the
treatment was extended to five consecutive days. The
oligonucleotide concentrations are cumulative.
Fig. 4 is similar to Fig. 1, except that
another human melanoma cell line (SK MEL-37) was
substituted for the CHP cells.
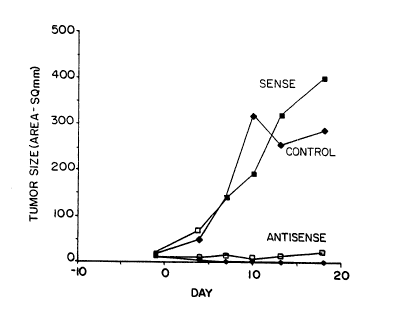
Fig. 5 is a plot of the effect of c-myb sense
and antisense oligomers on the growth of human melanoma
~SK MEL-37) cells transplanted into severe combined
immunodeficient mice. The mice received 100 ~g per day
for 7 days of (i) a 24-mer phosphorothioate
oligodeoxynucleotide complementary to codons 2-9 of the
translated portion of the c-myb mRNA transcript
("Antisense"; 2 mice), (ii) the corresponding sense 24-
lS mer phosphorothioate oligodeoxynucleotide ("Sense"; 1mouse) or ~iii) no oligomer ("Control"; 1 mouse).
Detailea De~criDtion of the Invention
It has now been discovered that the expression
of the human c-myb gene is important for the
proliferation of malignant melanoma. The role of this
proto-oncogene in cell proliferation is not restricted
to cells of hematopoietic ori~in, as previously thought.
The proliferation of malignant melanocytes, which are
neoplastic cells of epidermal and not hematologic origin,
is maintained by c-myb expression. Thus, the role of c-
mYb is more general than previously thought.
The putative DNA sequence complementary to the
mRNA transcript of the human c-~y~ gene has been reported
in Majello et al., Proc. Natl. Acad. Sci. U.S.A. 83,
9636-9640 (1986). Majello et ~1. further disclose the
predicted 640 amino acid sequence of the putative c-my~
protein. The initiation codon ATG appears at position
114, preceded by a 5'-untranslated region. The
termination codon TGA at position 2034 is followed by a
3'-untranslated region spanning about 1200 nucleotides,
WO 93/09789 PCI/US92/096~6
212361~ - 8 - ~
which is followed by a poly (A) tail of about 14 0
nucleotides.
The antisense oligonucleotides of the invention
may be synthesized by any of the known chemical oligonu-
cleotide synthesis methods. Such methods are generallydescribed, for example, in Winnacker, From Genes to
Clones: Introduction to Gene Technoloqv, VCH Verlagsges-
ellschaft mbH (Ibelgaufts trans. 1987). The antisense
oligonucleotides are most advantageously prepared by
utilizing any of the commercially available, automated
nucleic acid synthesizers. One such device, the Applied ~-
Biosystems 380B DNA Synthesizer, utilizes ~-cyanoethyl
phosphoramidite chemistry.
Since the complete nucleotide synthesis of DNA
complementary to the c-myb mRNA transcript is known, an-
tisense oligonucleotides hybridizable with any portion
of the mRNA transcript may be prepared by oligonucleotide
synthesis methods known to those skilled in the art.
While any length oliqonucleotide may be
utilized in the practice of the invention, sequences
shorter than 12 bases may be less specific in hybridizing
to the target c-mvb mRNA, may be more easily destroyed
by enzymatic digestion, and may be destabilized by
enzymatic digestion. Hence, oligonucleotides having 12
or more nucleotides are preferred.
Long sequences, particularly sequences longer
than about 40 nucleotides, may be somewhat less effective
in inhibiting c-myb translation because of decreased
uptake by the target cell. Thus, oligomers of 12-40
nucleotides are preferred, more preferably 15-30
nucleotides, most preferably 18-26 nucleotides. While
sequences of 18-21 nucleotides are most particularly
preferred, for unmodified oligonucleotides, slightly
longer chains of up to about 26 nucleotides, are
preferred for modified oligonucleot~des such as
phosphorothioate oligonucleotides, which hybridize less
strongly to mRNA than unmodified oligonucleotides. `
. .
W093/09789 PCT/US92/09656
9 ~12~61~
Oligonucleotides complementary to and
hybridizable with any portion of the c-myb mRNA
transcript are, in principle, effective for inhibiting
translation of the transcript, and capable of inducing
the effects herein described. It is believed that
translation is most effectively inhibited by blocking the
mRNA at a site at or near the initiation codon. Thus,
oligonucleotides complementary to the 5'-terminal region
of the c-mYb mRNA transcript are preferred. It is
believed that secondary or tertiary structure which might
interfere with hybridization is minimal in this region.
Moreover, it has been suggested that sequences that are
too distant in the 3'-direction from the initiation site
may be less effective in hybridizing the mRNA transcripts
because of a "read-through" phenomenon whereby the
ribosome is postulated to unravel the antisense/sense
duplex to permit translation of the message. See, e.g.,
Shakin, J. Biochemistry 261, 16018 tl986).
The antisense oligonucleotide is preferably
directed to a site at or near the initiation codon for
protein synthesis. Oligonucleotides complementary to the
c-my~ mRNA, including the initiation codon (the first
codon at the 5' end of the translated portion of the c-
EY~ transcript, comprising nucleotides 114-116 of the
complete transcript) are preferred.
While antisense oligomers complementary to the
~'-terminal region of the c-mYb transcript are preferred,
particularly the region including the initiation codon,
it should be appreciated that usaful antisense oligomers
are not limited to those complementary to the sequenceæ
found in the translated portion (nucleotides 114 to 2031)
of the mRNA transcript, but also includes oligomers
complementary to nucleotide sequences contained in, or
exténding into, the 5'-and 3'-untranslated regions.
Oligomers whose complementarity extends into the 5'-
untranslated region o~ the c-myb transcript are believed
particularly effective in inhibiting c-_y~ translation.
WO 93/09789 PCI /US92/09656
212~611 10
Preferred oligonucleotidescomplementary tot~e
5'-untranslated region of the transcript include
molecules having a nucleotide sequence complementary to
a portion of the c-mvb mRNA transcript including the cap
nucleotide, that is, the nucleotide at the extreme 5'-end
of the transcript. The Sl nuclease assay procedure of
Molecular Clonina, 2nd edition (Sambrook et al., Eds.
1989), pages 7.66-7.70 (incorporated herein by reference)
was essentially followed to map the location of c-~yk cap
sites using mRNA isolated from the leukemic cell line
CCRF-CEM, which expresses high levels of c-~y~ mRNA. The
longest clearly visible band was located 90 base pairs
upstream of the published c-mvb cDNA (Majello ç~ al.,
Proc. ~atl. Acad. Sci. U.S.A., 83, 9536-9640 (1986)),
indicating the putative principle cap site. The position
of this site is in perfect agreement with the length of
the c-myb cDNA cloned from CCRF-CEN cells (Clarke et al.,
Mol. Cell. Biol., 8, 884-892 (1988)). Sl protection
~; assays also revealed faint bands in addition to the main
band corresponding to the cap site. These other bands
may represent rare or unstable c-Ey~ mRNA transcripts.
Multiple sites of transcription initiation are not
uncommon in genes such as c-myb which lack a perfect
TATAA box. The nucleotide sequence of the mRNA
transcript 5'-terminus beginning with the cap nucleotide
may be readily established, and antisense oligonuc-
leotides complementary and hybridizable thereto may be
prepared.
The following 40-mer oligodeoxynucleotide is
complementary to the c-mYb mRNA transcript beginning with
the initiation codon of the transcript and extending
downstream thereof (in the 3' direction): SEQ ID N0:1.
Smaller oligomers based upon the above
sequence, in particular, oligomers hybridizable to
segments of the c-mvb message containing the initiation
codon, may be utilized. Particularly pr~ferrQd are the
following 26- to 15-mers: ~
.
W0~3/09789 PCT/US92~09656
1l 2123611
SEQ ID NO:2,
SEQ ID NO:3,
SEQ ID NO:4,
SEQ ID NO:5,
SEQ ID NO:6,
SEQ ID NO:7,
SEQ I~ NO:8,
SEQ ID NO:9,
SEQ ID NO:10,
SEQ ID NO:ll,
SE~ ID N0:12 and
SEQ ID NO:13.
Oligodeoxynucleotides complementary to the c-
myb mRNA transcript beginning with the second codon of
the translated portion of the transcript (nucleotides
117-119 of the complete transcript) are another group of
preferred oligomers. Such oligomers include, for
example, the following 26- to 15-mers:
SEQ ID N0:14,
SE~ ID N0:15,
SEQ ID N0:16,
SEQ ID N0:17,
SEQ ID N0:18,
SEQ ID N0:19,
SEQ ID N0:20,
SEQ ID N0:21,
SEQ ID N0:22,
SEQ ID N0:23,
3Q SEQ ID N0:24 and
SEQ ID N0:25.
The oligonucleotide employed may represent an un-
modified or modified oligonucleotide. Thus, oligo-
nucleotides hybridizable to the c-~y~ mRNA transcript
finding utility according to the present invention
include not only oligomers of the biologically sig-
W O 93/09789 P ~ tUS92/09656
2 1 ~ 12 -
nificant native nucleotides, i.e., A, dA, G, dG, C, dC,
T and U, but also oligonucleotide species which have been
modified for improved stability and/or lipid solubility~
For example, it i5 known that enhanced lipid solubility
and/or resistance to nuclease digestion results by
substituting an alkyl group or alkoxy group for a phos-
phate oxygen in the internucleotide phosphodiester
linkage to form an alkylphosphonate oligonucleoside or
alkylphosphotriester oligonucleotide. Non-ionic
oligonucleotides such as these are characterized by
increased resistance to nuclease hydrolysis and/or
increased cellular uptake, while retaining the ability
to form stable complexes with complementary nucleic acid
sequences. The alkylphosphonates in particular, are
stable to nuclease cleavage and soluble in lipid. The
preparation of alkylphosphonate oligonucleosides is
disclosed in U.S. Patent 4,469,863.
Methylphosphonate oligomers can be prepared by a
variety of methods, both in solution and on insoluble
polymer supports (Agrawal and Riftina, Nucl. Acids Res.,
6, 3009-3024 (1979); Miller et al., Biochemistry, 18,
5134-5142 (1979), Miller et al., J. Biol. Chem., 255,
9659-9665 (1980); Miller et al., Nucl. Acids Res., 11,
5189-5204 (1983), Miller et al., Nucl. Acids Res., 11,
6225-6242 (1983), Miller et al., Biochemistry, 25, 5092-
5097 (1986); Engels and Jager, Anqew. Chem. Suppl. 912
(1982); Sinha et al., Tetrahedron Lett. 24, 877-880
(1983); Dorman et al, Tetrahedron, 40, 95~102 tl984);
Jager and Engels, Tetrahedron Lett., 25, 1437-1440
(1984); Noble et al., Nucl. Acids Res., 12, 3387-3404
~1984); Callahan et al., Proc. Natl. Acad. Sci. USA, 83,
1617-1621 (1986); Koziolkiewicz e~ hemica Scri~ta,
26, 251-260 (1986): Agrawal and Goodchild, Tetrahedron
Lett., 38, 3539-3542 (1987); Lesnikowski et ~1-.
Tetrahedron Lett., 28, 5535-5538 (1987); Sarin et al.,
Proc. Natl. Acad. Sci. USA, 85~ 7448-7451 ~1988)).
W093/09789 PCT/US92/09656
13 2123611
The most efficient procedure for preparation of
methylphosphonate oligonucleosides involves use of 5'-o-
d i m e t h o x y t r i t y l d e o x y n u c l e o s i d e - 3 ' - Q-
diisopropylmethylphosphoramidite intermediates, which are
similar to the methoxy or ~-cyanoethyl phosphoramidite
reagents used to prepare oligodeoxyribonucleotides. The
methylphosphonate oligomers can be prepared on controlled
pore glass polymer supports using anautomated DNA
synthesizer (Sarin et al., Proc. Natl. Acad. Sci. USA,
85, 7448-7451 (1988)).
Resistance to nuclease digestion may also be
achieved by modifying the internucleotide linkage at both
the 5' and 3' termini with phosphoroamidites according
to the procedure of Dagle et al., Nucl. Acids Res. 18,
4751-4757 (1990).
Phosphorothioate oligonucleotides contain a sulfur-
for-oxygen substitution in the internucleotide
phosphodiester bond. Phosphorothioate oligonucleotides
combine the properties of effective hybridization for
duplex formation with substantial nuclease resistance,
while retaining the water solubility of a charged
phosphate analogue. The charge is believed to confer the
property of cellular uptake via a receptor (Loke et al.,
Proc. Natl. Acad Sci. U.S.A. 86, 3474-3478 (1989)).
Phosphorothioate oligodeoxynucleotide are described
by LaPlanche, et al., Nucleic Acids Research 14, 9081
(1986) and by Stec et al., J. Am. Chem. Soc. 106, 6077
(lg84). The general synthetic method for phosphorothio-
ate oligonucleotides was modified by Stein et al., Nucl.
Acids Res., 16, 3209-3221 (1988), so that these compounds
may readily be synthesized on an automatic synthesizer
using the phosphoramidite approach.
Furthermore, recent advances in the production of
oligori~onucleotide analogues mean that other agents may
also be used for the purposes described here, e.g., 2'-0-
methylribonucleotides (Inove et al., ~ucleic Acids Res.
15, 6131 (1987) and chimeric oligonucleotides th,at are
W O 93/09789 PC~r/US92/09656
~ 3 6 1 ~ 14 _
composite RNA-DNA analogues (Inove et al., FEss Lett.
215, 327 (1987).
While inhibition of c-mvb mRNA translation is
possible utilizing either antisense oligoribonucleotides
or oligodeoxyribonucleotides, free oligoribonucleotides
are more susceptible to enzymatic attack by ribonucleases
than oligodeoxyribonucleotides. Hence,
oligodeoxyribonucleotides are preferred in the practice
of the present invention. Oligodeoxyribonucleotides are
further preferred because, upon hybridization with c-myb
mRNA, the resulting DNA-RNA hybrid duplex is a substrate
for RNase H, which specifically attacks the RNA portion
of DNA-RNA hybrid. Degradation of the mRNA strand of the
duplex releases the antisense oligodeoxynucleotide strand
for hybridization with additional c-myb messages.
In general, the antisense oligonucleotides used in
the method of the present invention will have a sequence
which is completely complementary to the target portion
of the c-myb message. Absolute complementarity is not
however required, particularly in larger oligomers.
Thus, reference herein to a "nucleotide sequence
complementary to at least a portion of the mRNA
transcript" of c-mYb does not necessarily mean a sequence
having 100% complementarity with the transcript. In gene-
ral, any oligonucleotide having sufficient com-
plementarity to form a stable duplex with c-mYb mRNA is
suitable. Stable duplex formation depends on the
sequence and length of the hybridizing oligonucleotide
and the degree of complementarity with the target region
of the c-myb message. Generally, the larger the
hybridizing oligomer, the more mismatches may be
tolerated. More than one mismatch probably will not be
tolerated for antisense oligomers of less than about 21
nucleotides. One skilled in the art may readily
determine the degree of mismatching which may be
tolerated between any given antisense oligomer and the
target c-mYb message sequence, based upon the melting
wo93/o978s PCT/US92/09656
2123611
point, and therefore the stability, of the resulti~g
duplex. Melting points of duplexes of a given base pair
composition can be readily determined from standard
texts, such as Molecular Cloninq: A Laboratory Manual,
(2nd edition, 1989), J. Sambrook et al., eds.
While oligonucleotides capable of stable hybridiza-
tion with any region of the c-myb message are within the
scope of the present invention, oligonucleotides
complementary to a region including the initiation codon
are believed particularly effective. Particularly
preferred are oligonucleotides hybridizable to a region
of the c-~y~ mRNA up to 40 nucleotides upstream (in the
5' direction) of the initiation codon or up to 40
nucleotides downstream (in the 3' direction) of that
codon.
For therapeutic use, the antisense oligonucleotides
may be combined with a pharmaceutical carrier, such as
a suitable liquid vehicle or excipient and an optional
auxiliary additive or additives. The liguid vehicles and
excipients are conventional and commercially available.
Illustrative thereof are distilled water, physiological
saline, aqueous solution of dextrose, and the like. The
c-~yb mRNA antisense oligonucleotides are preferably
administeredparenterally,mostpreferably intravenously.
The vehicle is designed accordingly. Alternatively,
oligonucleotide may be administered subcutaneously via
controlled release dosage forms.
The oligonucleotides may be conjugated to poly(L-
lysime) to increase cell penetration. Such conjugates
are described by Lemaitre et al., Proc. Natl. Acad. 5ci~
PSA, 84, 648-652 (1987). The procedure requires that the
3'-terminal nucleotide be a ribonucleotide. The
resulting aldehyde groups are then randomly coupled to
the epsilon-amino groups of lysine residues of poly(L-
lysine) by Schiff base formation, and then reduced withsodium cyanoborohydride. This procedure converts the 3'-
W093/09789 PCT/US92/096S6
2123611 16 -
terminal ribose ring into a morpholine structure
antisense oligomers.
In addition to administration with
conventional carriers, the antisense oligonucleotides may
be administered by a variety of specialized
oligonucleotide delivery techniques. For example,
oligonucleotides may be encapsulated in for therapeutic
delivery. The oligonucleotide, depending upon its
solubility, may be present both in the aqueous layer and
in the lipidic layer, or in what is generally termed a
liposomic suspension. The hydrophobic layer, generally
but not exclusively, comprises phospholipids such as
lecithin and sphingomyelin, steroids such as cholesterol,
ionic surfactants such as diacetylphosphate,
stearylamine, or phosphatidic acid, and/or other
materials of a hydrophobic nature. Oligonucleotides have
been successfully encapsulated in unilameller liposomes.
Reconstituted Sendai virus envelopes have been suc-
cessfully used to deliver RNA and DNA to cells. Arad et
al., Biochem. Biophy. Acta. 859, 88-94 (1986).
The c-mvb antisense oligonucleotides may be
administered de novo as the primary therapy.
Alternatively, the oligonucleotides may be administered
as an adjuvant following surgical removal of a melanoma
to patients who may be disease-free but at high risk of
recurrence.
A preferred method of administration of
oligonucleotide for treatment of melanoma comprises
either regional or systemic perfusion, as is appropriate.
According to a method of regional perfusion, the afferent
and efferent vessels supplying the extremity containing
the lesion are isolated and connected to a low-flow
perfusion pump in continuity with ~n oxygenator and a
heat exchanger. The iliac vessels may be used for
perfusion of the lower extremity. The axillary vessels
are cannulated high in the axilla for upper extremity
lesions. Oligonucleotide is added to the perfusion
W093/09789 2 1 2 ~ PCT/US92/09656
circuit, and the perfusion is continued for an
appropriate time period, e.g., one hour. Perfusion rates
of from 100 to 150 ml/minute may be employed for lower
extremity lesions, while half that rate should be
employed for upper extremity lesions. Systemic
heparinization may be used throughout the perfusion, and
reversed after the perfusion is complete. This isolation
perfusion technique permits administration of higher
doses of chemotherapeutic agent than would otherwise be
tolerated upon infusion into the arterial or venous sys-
temic circulation.
For systemic infusion, the oligonucleotides are
preferably delivered via a central venous catheter, which
is connected to an appropriate continuous infusion
device. Indwelling catheters provide long term access
to the intravenous circulation for freguent
administration of drugs over extended time periods. They
are generally surgically inserted into the external
cephalic or internal jugular vein under general or local
anesthesia. The subclavian vein is another common site
of catheterization. The infuser pump mày be external,
or may form part of an entirely implantable central
venous system such as the INFUSAPORT system available
from Infusaid Corp., Norwood, MA and the PORT-A-CATH
system available from Pharmacia Laboratories, Piscataway,
NJ. These devices are implanted into a subcutaneous
pocket under local anesthesia. A catheter, connected to
the pump injection port, is threaded through the
subclavian vein to the superior vena cava. The implant
contains a supply of oligonucleotide in a reservoir which
may be replenished as needed by injection of additional
drug from a hypodermic needle through a self-sealing
diaphragm in the re~ervoir. Completely implantable
infusers are preferred, as they are generally well
accepted by patients because of the convenience, ease of
maintenance and cosmetic advantage of such devices.
W093/09789 PCT/US92/09656
2123611
:
High dose chemotherapy has been coupled with
autologous bone marrow rescue (transplantation) in an
attempt to treat melanoma. While high dose chemotherapy
has resulted in significantly higher therapeutic
responses, patient survival is not generally prolonged:
Jones et al., Cancer Chemother. Pharmacol. 26, 155-6
(1990) (carboplatin, cyclophosphamide and BCNU); Lakhani
et al., Br. J. Cancer 61, 330-4 (1990) (melphalan);
Koeppler et al., Onkoloqie 12, 277-9 (1989) (melphalan
and BCNU); Thatcher et al., Cancer 63, 1296-302 (1989)
(DTIC); Wolff et al., J. Clin. Oncol. 7, 245-9 (1989)
(thiotepa); Shea et al., Arch. Dermatol. 124, 878-84
(1988) (cyclophosphamide, cisplatin and carmustine);
Tchekmedian et al., J. Clin. Oncol. 4, 1811-8 (1986)
(BCNU, melphalan, or both); Thomas et al., Oncoloav 43,
273-7 (1986) (BCNU and melphalan).
Melanoma, particularly in advanced stages, may be
substantially metastatic. Thus, one possible reason for
the lack of success of high dose chemotherapy and
autologous bone marrow purging in treating melanoma may
be a failure to properly purge the harvested marrow of
malignant cells which have metastasized to the bone
marrow. According to the present invention, c-mYb
antisense oligonucleotides may be used as bone marrow
purging agents for the in vitro cleansing of bone marrow
of melanoma cells in conjunction with high dose
conventional chemotherapy. While normal hematopoietic
cells are sensitive to c-mYb antisense, they are less
sensitive than malignant cells expressing c-~yk, as
taught in our copending patent application Serial No.
427,659 and corresponding international application
WO90/05445. This differential sensitivity makes possible
the use of c-my~ antisense oligonuclQotides in purging
bone marrow of neoplastic cells.
According to a method for bone marrow purging, bone
marrow is harvested from a donor by standard operating
room procedures from t~e iliac bones of the donor.
:
W093/09789 2 1 2 3 fi 1 I PCT/~'S92/0~656
19
Methods of aspirating bone marrow from donors are well-
known in the art. Examples of apparatus and processes
for aspirating bone marrow from do~ors are disclosed in
U.S. Patents 4,481,946 and 4,486,188, incorporated herein
by reference. Sufficient marrow is withdrawn so that the
recipient, who is either the donor (autologous
transplant) or another individual (allogeneic
transplant), may receive from about 4 x 10~ to about 8 x
10d processed marrow cells per kg of bodyweight. This
generally requires aspiration of about 750 to about 1000
ml of marrow. The aspirated marrow is filtered until a
single cell suspension, known to those skilled in the art
as a "buffy coat" preparation, is obtained. This
suspension of leukocytes is treated with c-myb antisense
lS oligonucleotides in a suitable carrier, advantageously
in a concentration of about 8 mg/ml. Alternatively, the
leucocyte suspension may be stored in liquid nitrogen
using standard procedures known to those skilled in the
art until purging is carried out. The purged marrow can
be stored frozen in liquid nitrogen until ready for use.
Methods of freezing bone marrow and biological substances
are disclosed, for example, in U.S. Patents 4,107,937 and
4,117,881.
Other methods of preparing bone marrow for treat-
ment with c-mYb antisense may be utilized, which methods
may result in even more purified preparations of hemato-
poietic cells than the aforesaid buffy coat preparation.
One or more growth factors may be added to the
aspirated marrow or buffy coat preparation to stimulate
growth of neoplasms, and thereby increase their sensi-
tivity to the toxicity of the c-myb antisense
oligonucleotides.
After treatment with the antisense oligonucleo-
tides, the cells to be transferred are washed with auto-
logous plasma or buffer to remove unincorporatedoligomer. The washed cells are then infused back into
the patient.
W093/09789 2 1 2 3 6 1 1 PCT/US92/09656
- 20 -
The c-mvb antisense oligonucleotides may b~
administered in a dosage effective for inhibiting the
proliferation of melanoma cells in the afflicted
individual, while maintaining the viability of normal
cells. Such amounts may vary depending on the nature and
extent of the neoplasm, the particular oligonucleotide
utilized, and other factors. The actual dosage admin-
istered may take into account the size and weight of the
patient, whether the nature of the treatment is prophy-
lactic or therapeutic in nature, the age, health and sexof the patient, the route of administration, whether the
treatment is regional or systemic, and other factors.
Inhibition of melanoma cell proliferation has been
observed at antisense concentrations of as low as 10
~g/ml. At 20 ~g/ml, inhibition was profound. Concen-
trations of from about 1 to about 100 ~g/ml may be
employed, preferably from about 10 ~g/ml to about 100
~g/ml, most preferably from about 20 ~g/ml to about 60
~g/ml. The patient should receive a sufficient daily
dosage of antisense oligonucleotide to achieve these
intercellular concentrations of drug. The daily dosage
may range from about 0.1 to 1,000 mg oligonucleotide per
day, preferably from about 10 to about 700 mg per day.
Greater or lesser amounts of oliqonucleotide may be
administered, as required. Those skilled in the art
should be readily able to derive appropriate dosages and
schedules of administration to suit the specific
circumstance and needs of the patient. Based upon the
in vivo study described herein, it is believed that a
course of treatment may advantageously comprise infusion
of the recommended daily dose of oligonucleotide for a
period of from about 3 to about 28 days, more preferably
from about 7 to about 10 days. Those skilled in tbe art
should readily be able to determine tbe optimal dosage
in ach case.
`~.
W093/09789 PCT/US92'09656
2123611
- 21 -
For an adult human being, a daily dose of about 3so
mg oligonucleotide is believed sufficient, to achieve an
effective intercellular concentration of 20 ~g.
For ex vivo antineoplastic application, such as,
for example, in bone marrow purging, the c-myb antisense
oligonucleotides may be administered in amounts effective
to kill neoplastic cells while maintaining the viability
of normal hematologic cells. Such amounts may vary
depending on the extent to which melanoma cells may have
metastasized to the bone marrow, the particular
oligonucleotide utilized, the relative sensitivity of t~e
neoplastic cells to the oligo-
nucleotide, and other factors. Concentrations from about10 to 200 ~g/ml per 105 cells may be employed, preferably
from about 40 to 150 ~g/ml per 105 cells. Supplemental
dosing of the same or lesser amounts of oligonucleotide
are advantageous to optimize the treatment. Thus, for
purging bone marrow containinq 2 x 107 cell per ml of
marrow volume, dosages of from about 2 to 40 mq antisense
per ml of marrow may be effectively utilized, preferably
from about 8 to 24 mg/ml. Greater or lesser amounts of
oligonucleotide may be employed.
The present invention is described in greater
detail in the following non-limiting examples.
Ex~mple 1
Inhibition of CHP Melanoma Growth
By c-myb_Antisense Oliaonucleotide
CHP melanoma cells (Children's Hospital of
Philadelphia, Philadelphia, PA) were grown in RPMI
culture medium containing 2% oxalophosphate and 5% bovine
calf serum at 37C in 5% C02. Cells (5,000/ml) were
seeded in 200 ~1 volumes into individual wells of a 96
well Costar plate at day -3, and allowed to grow for
three days. At this time (day 0), the unmodif~ed
antisense 18-mer oligodeoxynucleotide (SEQ ID N0:22)
;~ complementary to codons 2-7 of t~e translated portion of
~,
W093~09789 rCT/US92/09656
2 123 6 1~ - 22 -
the c-myb mRNA, or the corresponding sense 18-mer (SEQ
ID NO:26), were added to the cultures at concentrations
of o, 10, 20, 50 and 100 ~g/ml for 1, 2 or 5 consecutive
days. On day 7, 100 ~1 of fresh medium was added to the
cultures. Cell viability/proliferation was determined
on day 8 utilizing a commercially available kit
(CELLTITER 96~ Promega, Madison, WI). The assay is based
on the ability of viable cells to convert a tetrazolium
salt into a blue formazan product which can be quantified
by measuring the absorbance at 570 nm with a conventional
microplate reader. The extent of absorbance at 570 nm
is proportional to the amount of formazan produced, and
thus the number of viable cells remaining. Accordingly,
10 ~1 of 3-(4,5-dimethylthiazol-2-yl)-2,5
diphenyltetrazolium bromide solution t5 mg/ml) was added
to 110 ~1 of cell suspension and incubated for 4 hours
at 37-C. 150 ~1 of acidified isopropanol (25 ml of
isopropanol plus 0.1 ml of 12 N HCl) was then added and
the solution mixed. The optical density was measured at
570 nm. Background from cell debris was eliminated by
subtracting a reference measurement at 630 nm.
The results of the viability assay are set forth
in Figures 1 (one-day treatment), 2 (two-day treatment)
and 3 (five-day treatment). The oligonucleotide
concentrations indicated in Figures 2 and 3 are
cumulative dosages for the two- and five-day treatments,
respectively. It may be appreciated from the figures
that treatment of the melanoma cells with antisense
oligomer resulted in sequence-specific killing. The
effect was most pronounced when the melanoma cells were
treated for one day with an antisense oligomer concen-
tration of at least 50 ~g/ml, or when the cells were
treated on consecutive days with as little as 20 ~g/ml
oligomer.
W O 93/09789PC~r/U~92/09656
- 23 2 1 2 3 6 1 1
ExamPle 2
Inhibition of SK MEL-37 Melanoma
By c-mvb Antisense Oliqonucleotide
5The effect of c-mYb antisense oligonucleotide on
another human melanoma line, SK MEL-37 (Sloan Kettering
Institute, New York, NY) was determined. Cells were
treated according to the procedure of Example 1 on day
0 with the same sense and antisense oligomers, in the
10 same concentrations. Cell viability was assayed on day
5. The results are shown in Figure 4.
Again, treatment of melanoma cells with a single
50 ~g/ml dose of antisense oligomer was sufficient to
induce substantial cell killing, in comparison to sense-
15 treated or untreated cells.
Example 3
Inhibition of SK MEL-37 Melanoma Growth
in vivo By c-myb Antisense Oligonucleotide
The effect of c-myb antisense oligonucleotide on
the growth of melanoma cells n vivo was investigated.
250,000 melanoma cells (SK NEL-37) were injected
subcutaneously into each of four severe combined
25 immunodeficient mice (C.B-17/LCRTAC-SCID DF from Fox
Chase Cancar Institute, Philadelphia PA) at day -21. The
tumors were allowed to grow to a size of approximately
5 mm in diameter. On day 1, ALZET constant infusion
pumps ~Alza Corporation, Palo Alto, CA) delivering a
30 total dosage of 700 ~g oli~omer over 7 days were
surgically implanted into the mice. The tumor area was
then monitored daily. One mouse received no oligomer.
Another mouse received a 24-mer "sense" phosphorothioate
oligonucleotide (corresponding to codons 2-~ of the
35 translated c-~yb mRNA). Two mice received a 24-mer anti-
sense phosphorothioate oligonucleotide having the
nucleotide sequence of SEQ ID NO: 16 (TATGCTGTGC CGGGGGT- r
CTTC GGGC), complementary to c-~yk mRNA codons 2-9. The
results are shown in Figure 5. Each curve represents one
W093/09789 PCT/US92/09656
212361~ - 24 -
animal. In the two antisense-treated animals, the tumor
size stayed the same or regressed. In contrast, the
tumors continued to grow in the sense-treated and control
animals.
The animals were sacrificed on day 19. The tumors
were removed and weighed. The weights of the tumors in
the control and sense-treated animals were 9.9 and 9.6
grams, respectively. The tumor weights in the two anti-
sense treated animals were only 0.1 and 0.2 grams.
The following non-limiting example illustrates one
methodology for purging bone marrow of metastatic
melanoma.
Exampl~ ~
Bone Marrow Purqinq with c-myb
Antisense Oliqonucleotide
Bone marrow is harvested from the iliac bones of
a donor under general anesthesia in an operating room
using standard techniques. Multiple aspirations are
taken into heparinized syringes. Suf f icient marrow is
withdrawn so that the marrow recipient will be able to
receive about 4 x 108 to about 8 x 10~ processed marrow
cells per kg of body weight. Thus, about 750 to 1000 ml
of marrow is withdrawn. The aspirated marrow is
transferred immediately into a transport medium (TC-l99,
Gibco, Grand Island, New York) containing 10,000 units
of preservative-free heparin per 100 ml of medium. The
aspirated marrow is filtered through three progressively
finer meshes until a single cell suspension results,
i.e., a suspension devoid of cellular aggregates, debris
and bone particles. The filtered marrow is then
processed further into an automated cell separator (e.g.,
Cobe 2991 Cell Processor) which prepares a "buffy coat"
product, (i.e., leukocytes devoid of red cells and
platelets). The buffy coat preparation is then placed
in a transfer pack for further processing and storage.
It may be stored until purging in liquid nitrogen using
standard procedures. Alternatively, purging can be
W093/09789 PCT/US92/096S6
- 25 21 2~ 61 1
carried out immediately, then the purged marrow may be
stored frozen in liquid nitrogen until it is ready for
transplantation.
The purging procedure may be carried out as
follows. Cells in the buffy coat preparation are
adjusted to a cell concentration of about 2 x 10~/ml in
TC-199 containing about 20% autologous plasma. C-~y~
antisense oligodeoxynucleotide, for example, in a
concentration of about 8 mg/ml, is added to the transfer
packs containing the cell suspension. Recombinant human
hematopoietic growth factors, e.g., rH IL-3 or rH GM-CSF,
may be added to the suspension to stimulate growth of
neoplasms and thereby increase their sensitivity c-mvb
antisense oligonucleotide toxicity. The transfer packs
are then placed in a 37-C waterbath and incubated for 18
- 24 hours with gentle shaking. The cells may then
either be frozen in liquid nitrogen or washed once at 4-C
in TC-l99 containing about 20% autologous plasma to
remove unincorporated oligomer. Washed cells are then
infused into the recipient. Care must be taken to work
under sterile conditions wherever possible and to
; maintain scrupulous aseptic techniques at all times.
The present invention may be embodied in other spe-
cific forms without departing from the spirit or essen-
tial attributes thereof and, accordingly, referenceshould be made to the appended claims, rather than to the
foregoing specification, as indicating the scope of the
invention.
All references cited herein with respect to
synthetic, preparative and analytical procedures are
incorporated by reference.
W093/09789 PCTiUS92/096~6
2123611 26 -
SEOUENCE LISTING
(1) GENERAL INFORNATION: ..
(i) APPLICANT: TEMPLE UNIVERSITY - OF THE
S COMMONWEALTH SYSTEM OF HIGHER EDUCATION
(a~ INVENTOR8: Gewirtz, Alan M.
Calabretta, Bruno
(ii) TITLE OF INVENTION: Treatment of Melanoma with
Antisense Oligonucleotides to c-~y~ Proto-oncogene.
(iii) NUMBER OF 8EQUENCE8: 26
(iv) CORR~8PONDENCE ADDRE88:
(A) ADDR~88B~: Temple University - of the Common-
wealth System of Higher Education
(B) 8TRBET: 406 University Services Building ~:
(C) CITY: Philadelphia
(D) BTATE: Pennsylvania :.
(E) QO~NTRY: U.S.A.
(F) ~IP: 19122
(v) COMPUTER READABLE FORH: `-
(A) MEDI~M TYPE: Diskette, 3.50 inch, 720 Kb
(B) COMP~TER: IBM PS/2
(C) OP~RATING 8Y8TEM: MS-DOS
(D) 80FTWARE: WordPerfect 5.1
tvi) CURRENT APPLICATION DATA:
: 25 (A) APPLICATION NUMBER:
(B) FILING DATE:
(C) CLAS8IFICATION:
(vii) PRIORITY APP~ICATION DATA:
(A) APPLICATION NUMBER: U.S. Application Serial
No. 792,999
(B) FILING DAT~: 15 November 1991
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAMES Monaco, Daniel A.
~B) R~I8~RATION N~NBER: 30,480
(C) ~FER~NCg/DOC~ET N~MBERs 60S6-159 PCT 1
(ix) T~COMNnNICATION INFORNATION:
; (A) TE~EP~ONE: (215) 568-8383
W O 93/09789 PC~r/US92/~9656
- 27 _ 2 1 2 ~ 6 1 1 :
(B) TELEFAX: (215) 568-5549
(C) TELEX: None
(2) INFORMATION FOR 8EQ ID NO:l:
(i) SEQUENCB CHARACTERISTICS:
(A) LENG~H: 40 Nucleotides
(B) TYPE: nucleic acid
(C) BTRANDEDNESS: single stranded
(D) TOPOLOGY: linear
(xi) 8EQ~ENCE DESCRIPTION: 8EQ ID NO:l:
CGTCACTGCT ATATATGCTG TGCCGGGGTC TTCGGGCCAT 40
(2) INFO~MATION FOR 8EQ ID NO:2:
(i) 8EQUENCE C~ARACTERI8TIC8:
(A) BENGTH: 26 Nucleotides
tB) TYPB: nucleic acid
(C) 8TRANDEDNE88: single stranded :~
(D) TOPOLOGY: linear
(xi) 8EQ~ENC~ DE8CRIPTION: 8EQ ID NO:2:
ATGCTGTGCC GGGGTCTTCG GGCCAT 26
(2) INFORNATION FOR 8EQ ID NO:3:
(i) 8EQUENCE CHARACTERISTIC8:
(A) ~ENGTH: 25 Nucleotides
(B) TYPB: nucleic acid
(C) 8TRANDEDNE88: single stranded
(D) TOPOLOGY: linear
(xi) 8EQUENC~ DESCRIPTION: SEQ ID NO:3:
TGCTGTGCCG GGGTCTTCGG GCCAT 25
(2) INFORMATION FOR 8EQ ID NO:4:
(i) 8BQUENCB CHARACTERI8TIC8~
(A) ~ENGT~: 24 Nucleotides
(B) TYP~s nucleic acid
(C) 8TRAND~DNE88: single stranded
(D) ~OPO~OGYs linear
(Xi) 8EQ~NCE DE8CRIPTION: 8EQ ID NO:4:
GCTGTGCCGG GGTCTTCGGG CCAT 24
Wo93/n97x9 PCT/US92/09656
2 123 6 li 28 -
(2) INFORMATION FOR SEQ ID NO:5:
(i) 8EQUENCE CHARACTERISTICS:
(A) LENGTH: 23 Nucleotides
(B) TYPE: nucleic acid
(C) 8TRANDEDNEg8: single stranded
(D) TOPOLOGY: linear
(xi) 8EQUENCE DESCRIPTION: SEQ ID NO:5:
- CTGTGCCGGG GTCTTCGGGC CAT 23 :
(2) INFO~NATION FOR 8EQ ID NO:6:
(i) 8EQUENCE CHARACTERISTIC8:
(A) LENGTH: 22 Nucleotides
(B) TYPE: nucleic acid
(C) 8TRANDEDNE88: single stranded
(D) TOPOLOGY: linear
(xi) 8EQUENCE DESCRIPTION: 8EQ ID NO:6:
TGTGCCGGGG TCTTCGGGCC AT 22
(2) INFORMATION FOR 8EQ ID NO:7:
(i) 8EQUENCB CHARACTERI8TIC8:
(A) LENGTH: 21 Nucleotides
(B) TYPE: nucleic acid
(C) 8TRANDEDNE8B: single stranded
(D) TOPOLOGY: linear
(xi) 8EQUENCE DESCRIPTION: SEQ ID NO:7:
GTGCCGGGGT CTTCGGGCCA T 21
(2) INFORMATION FOR 8EQ ID NO:8:
(i) 8EQ~ENCB CHARACTERI8TICS:
(A) L~NGTH: 20 Nucleotides
(B) TYPB: nucleic acid
(C) 8TRANDEDNE88s single stranded
(~) TOPO~O~Y: linear
(xi) 8EQ~BNC~ DB8CaIPTION: BBQ ID NO~8:
3~ TGCCGGGGTC TTCGGGCCAT 20
W093/09789 PCT/US92/09656
- 29 2I ~ ~ 6
(2) INFORMATION FOR SEQ ID NO:9:
(i) 8EQUENCE CHARACTERISTICS:
(A) LENGT~: 19 Nucleotides
(B) TYPE: nucleic acid
(C) 8TRANDEDNE88: sinqle stranded
(D) TOPOLOGY: linear
(xi) 8BQUBNCB DE8CRIPTION: 8EQ ID NO:9:
GCCGGGGTCT TCGGGCCAT 19
(2) INFORMATION FOR 8EQ ID NO:10:
(i) 8EQUBNCE CaARACTERI8TIC8:
(A) LENGTH: 18 Nucleotides
(B) TYPB: nucleic acid
(C) 8TRANDBDNE88: single stranded
(D) TOPOLOGY: linear
(xi) 8BQ~BNCB DESCRIPTION: 8EQ ID NO:lO:
CCGGGGTCTT CGGGCCAT 18
. (2) INFO~MATION FOR 8BQ ID NO:ll:
(i) 8BQ~NCE CHARACTERI8TIC8:
:: (A) LENGT~: 17 Nucleotides
(B) TYPE: nucleic acid
(C) 8TRUNDEDNE88: single stranded
(D) TOPOLOGY: linear
(xi) 8EQUENCE DEæCRIPTION: 8EQ ID NO:ll:
CGGGGTCTTC GGGCCAT 17
(2) INFORNATION FOR 8EQ ID NO:12:
(i) 8EQ~ENC~ CHARACTERI8TICS:
(A) LENGTH: 16 Nucleotides
(B) TYPEs nucleic acid
tC) 8~RUNDEDNE88: sinqle strand~d
(D~ ~oPo~oays linear
(Xi) 8~Q~NCE D~8CRIPTIONs 8EQ ID NOs12:
GGGGTCTTCG GGCCAT 16
2) INFORNATION FOR 8~Q ID NO:13:
.~:
W093/097Xg PCT/US92/09656
212~.611 30 ~
(i) 8EQUENCE CHARACTERISTICS:
(A) LENGTH: 15 Nucleotides
(B) TYP~: nucleic acid
(C) 8TRANDEDNES8: single stranded
(D) TOPOLOGY: linear
(xi) 8EQUENCE DE8CRIPTION: SEQ ID NO:13:
GGGTCTTCGG GCCAT 15
; (2) INFORMATION FOR 8BQ ID NO:14:
(i) 8EQU~NCE CHARACTERI8TIC8:
(A) ~ENGTH: 26 Nucleotides
(B) TYP~: nucleic acid
; (C) 8TRANDEDNE88: single stranded
(D) TOPOLOGY: linear
(xi) 8EQ~ENCE DE8CRIPTION: 8EQ ID NO:14:
TATATGCTGT GCCGGGGTCT TCGGGC 26
(2) INFORMATION FOR 8EQ ID NO:15:
~:: (i) 8EQUENCE CHARACTBRI8TIC8:
(A) LENGTH: 25 Nucleotides
(B) TYeB nucleic acid
:
:~: (C) 8ThANDEDNE88: single stranded
: (D) TO~OLOGY: linear
(xi) 8BQUENCE DESCRIPTION: 8EQ ID NO:15:
ATATGCTGTG CCGGGGTCTT CGGGC 25
~2) INFORM~TION FOR 8EQ ID NO:16:
(i) 8EQUENCB CHaRACTERI8TIC8:
(A) L~NGT~: 24 Nucleotides
(B) TYPE: nucleic acid
(C) 8TRANDEDNE88s singlQ stranded
(D) TOPOLOGYs linear
(Xi) 8~Q~NCE DE8C~IP~ION: 8EQ lD NOs16:
: TATGCTGTGC CGGGGTCTTC GGGC 24
: 35
~ (2) INFOaMATION FOR 8EQ ID NO:17:
~, :
~ (i) 8EQ~ENCE CHARACTBRI8TIC8:
W O 93/097X9 PC~r/US92/096~6
3, 2 1 2~ 61 1
(A) LENGTH: 23 Nucleotides
(B) TYPE: nucleic acid
(C) 8TRANDEDNESS: single stranded
(D) TOPOLOGY: linear
(xi) 8EQVENCE DESCRIPTION: SEQ ID NO:17:
ATGCTGTGCC GGGGTCTTCG GGC 23
(2) INFORNATION FO% 8EQ ID NO:18:
(i) ~EQVENCE CHARACTERI8TICS:
(A) LENGTH: 22 Nucleotides
(B) TYPE: nucleic acid
(C) 8TRANDEDNE88: single stranded
(D) TOPO~OGY: linear
(xi) 8EQV~NCE DE8CRIPTION: ~EQ ID NO:18:
TGCTGTGCCG GGGTCTTCGG GC 22
(2) INFORMATION FOR 8EQ ID NO:19:
(i) 8EQVENCE CHARACTERI8TIC8:
(A) L~NGTH: 21 Nucleotides
~B) TYPB: nucleic acid
(C) 8TRANDEDNES8: single stranded
(D) TOPOLOGY: linear
txi) 8EQUENCE DESCRIPTION: SEQ ID NO:l9:
GCTGTGCCGG GGTCTTCGGG C 21
(2) INFORNATION FOR SEQ ID NO:20:
(i) 8EQ~ENCE CHARACTERI8TIC8:
(A) LENGT~: 20 Nucleotides
(B) TYPE: nucleic acid
(C) BT~ANDEDNE88: single stranded
(D) TOPO~OGY: linear
(xi) 8EQVENCE DE8CRIPTION: 8EQ ID NO:20:
CTGTGCCGGG GTCTTCGGGC 20
(2) INFORMATION FOR 8EQ ID NO:21:
(i) 8~Q~ENC~ C~ARACTERI8TIC8:
~: tA) LENGTH: 19 Nucleotides
W093/09789 PCT/US92/09656
'~ 1 2 ~ 32 -
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single stranded
(D) TOPOLOGY: linear
(xi) 8EQUENCE DESCRIPTION: SEQ ID NO:21:
TGTGCCGGGG TCTTCGGGC 19
(2) INFORNATION FOR ~EQ ID NO:22:
(i) 8EQUENC~ CHARACTERI8TIC8:
(A) LENGTH: 18 Nucleotides
(B) TYPE: nucleic acid
(C) 8TRANDEDNE8B: single stranded
(D) TOPOLOGY: linear
(xi) 8EQUENCE DE8CRIPTION: 8EQ ID NO:22:
GTGCCGGGGT CTTCGGGC 18
(2) INFORMATION FOR SEQ ID NO:23:
(i) 8EQUENCE CXARACTERISTICS:
(A) LENGTH: 17 Nucleotides
(B) TYPE: nucleic acid
(C) 8TRANDEDNE88: single stranded
(D) TOPO~OGY: linear
(xi) 8EQ~ENCE DE8CRIPTION: 8EQ ID NO:23:
TGCCGGGGTC TTCGGGC 17
(2) INFORMATION FOR SEQ ID NO:24:
(i) 8EQ~ENCE CH~RACTERISTIC8:
(A) LENGTH: 16 Nucleotides
~B) TYPE: nucleic acid
tc3 8TRANDEDNE88: single stranded
(D) TOPO~OGY: linear
(xi) 8BQUENCE DE8CRIPTION: 8EQ ID NO:24:
GCCGGGGTCT TCGGGC 16
(2) INFORMATION FOR 8EQ ID NO:25:
(i) 8EQ~ENC~ CXARACTERI8TIC8s
(A) LENGTH: 15 Nucleotides
(B) TYPE: nucleic acid
W O 93/09789 2 1 2 3 6 1 1 PC~r/US92/09656
- 33 -
(C) STRANDEDNESS: single stranded
(D) TOPOLOGY: linear
(xi) 8EQUENCE DESCRIPTION: SEQ ID NO:25:
CCGGGGTCTT CGGGC 15
(2) INFORNATION FOR 8EQ ID NO:26:
(i) 8EQUENCE CHARACTERI8TICS:
(A) LENGTH: 18 Nucleotides
(B) TYPE: nucleic acid
(C) 8TRANDEDNESS: single stranded
(D) TOPOLOGY: linear
(xi) SEQUENCB DESCRIPTION: 8EQ ID NO:26:
GCCCGAAGAC CCCGGCAC 18