Note: Descriptions are shown in the official language in which they were submitted.
W093/11281 - 1 - 21 2 ~ 31 ~ PCT/GB92/021~7
ELEcTRoc~EMIcAL CELL.
This invention relstes to sn electrolytic cell. The
invention relates in psrticulsr to an electrolytic cell in
which ozone i8 to be produced.
Electrolytic cells are known in which water is
tlssociated into it6 respective elemental species, i.e. 2
and ~2 which are liberated at the snode and cathode
respectively. Under flppropriate conditions 03 is alsc
produced at the anode.
Shus in European Patent Specification No. 0 041 365
there is described an electrolytic process for the
production of ozone in which an electrol~te comprising an
4ueous 601ution of a very highly electronegative snion, for
esample the ~cids or salts of hesa-fluoro-anions, is
electrol~sed.
~ igh current efficiencies of up to 35~ for the
production of ozone have been reported. Current efficiency
(-lso ~nown as production efficiency of ozone) is a measure
of ctual ozone production relative to theoretical ozone
production for given inputs of electrical current, i.e. 35
current efficiency means under the conditions stated, the
2-3 gases evolved at the anode comprise 35~ (of the
theoretic~l 03 production) 0~ by weight and that 35~ of the
supplied current is utilised in the production of ozone.
Electrolytic cells are also known for the production of
ozone which comprise air cathodes ~nd in which the cathode
reaction which takes place is the reduction of ambient air
to water in an acidic elec~rol~te, the wster then being
oxidised at ~he anode to osygen and ozone. Consequently
since hydrogen is not producet at the cathode surface. the
cell voltage is Eubstantially reduced. Furthermore the
construction of the cell is less comples th~n cells which
comprise hydrogen evolving cathodes, for es4mple a separator
between the anode and cathode is not required since hydrogen
is not evolved at the cathode, and control of the oversll
3~ process is simpler, namely the need for periodic additions
of water is reduced.
- 2 _
Wo93/11281 2 i 2 ll 318 PCT/GB92~021~7
Thus, in US P~tent No.4 ,541, 989 there is described an
electrolytic cell for the production of ozone which
comprises a tubular snode F.tructure, the outer surface of
which functions ss sn anode within a tubular air cathode
structure, the inner surfsce of which functions ss ~
cathode. As disclosed in the aforementioned US Patent it is
desirsble in electrolytic cells for the production of ozone
thst the current density ~t the snode surface is grester
than that at the cathode surface, in psrticular that the
~ current density at the anode surface is at least about twice
that of the cathode surface in order that the power
consumption due to polari6ation losses at the cathode
surface may be reduced, thereby avoiding hydrogen evolutior
- and increasing air cathode lifetime.
~, Furthermore, there is a requirement in the electrolyt e
production of ozone thst the anode surface is cooled. Use of
a cooled anode surface substantially improves the current
efficienc~ of the process. It is known for example that
where the temperature of the anode is reduced from 25C to
0C, a fourfold improvement in the current efficiency may
be obtained. Furshermore, use of a cooled anode in
electrolytic cells for the production of ozone often
increases the lifetime of the anode.
The anode is typicnlly cooled by the flow of a
2S refrigerant fluid within the anode. It is therefore
important that the surface area of the anode is as large as
possible in order that effective heat e~change with the
electrol~e may take plsce thereby ensuring e~ficient
cooling of the anode. On the other hand a low anode surfsce
area relative to that of the cathode is desirable in order
that the anode current density may be high.
The concentric tubular arrangement as described in U5
P~tent 4, 541, 9B9 provides one way in w~ich the current
density may be greater at the anode than at the cathode
3S whilst still allowing efficient heat exchange.
However, this concentric tubular arrangement does
suffer from the dissdvantage tha~ the inter electrode gap,
WO93/11281 2 ~ 2 3 ~ 8 PCT~GB92/021~7
that is the distsnce between the outer surface of the ~node
and the inner surface of the cathode msy be
disadvantageously large in order to achieve the required low
surfsce area of the anode rel~tive to that of the cathode.
For e~ample, in a cell comprising an inner tubular anode
having an e~ternal radius of 2 cm and an outer tubular
cathode having an internal surface area twice that of the
anode, the internal radius of the cathode will be 4 cm. i.e.
the inter-electrode gap w$1i be 2 cm. This large
~ $nter-electrode gap leads to a high electr$csl resist~nce t~
the flow of current through the cell and an undesirable
increase in the voltage of the cell.
These sforementioned problems arise in particular with
this arrangement where the cathode ls an air cathode. In
addition to the aforementioned operational problems, ai:
cathodes are difficult to fabricate in 8 tubular form and
they lead to mechanical problems during manufacture of the
electrolytic cell, for e~ample sealing within the cell.
~ccording to a first aspect of the present invention
there is provided an electrol~t$c cell comprising first and
second electrode structures, at least the first electrode
structure having a surface area $n heat e~change relation
with the electrolyte and part only of said surface area
being electrolytically active.
2~ Typically, each of the first and ~econd electrode
strurtures has a surface area which is in heat transfer
relation with the elec~rolyte, a part only of said surface
area of the first electrode structure being electrolytically
active and the active surface area of said second electrode
structure being greater ~han that of the first electrode
structure.
In an electrolytic cell in which ozone is produced, the
first electrode structure is an anode structure and the
second electrote structure is a cashode structure, the
3~ electrolytically active surface area of the cathode
structure being greater than that of the anode structure.
WO~3/11281 PC~/GB92/021~7
~ 212~3~
Although the invention is described hereafter wls~
reference to an electrolytic cell in which ozone is to be
produced, we do not exclude its ~pplication tO generstior. of
products other than ozone.
The active surface areas of the anode ant cathode
structures will usually be of such an estent that in
operation the current density established at the surf~ce of
the anode structure is at lesst 20Z greater than that
established at the surface of the cathode structure.
Preferably the current density established at the
surface of the anode structure is at least 50~, especially
at least 802 8reater than that established at the surface of
the cathode structure.
The anode structure may have high surface area in heat
~ e~change relation with the electrolyte, thus faci1itatir.g
efficient heat exchange, yet only a part of that surface
area is electrolytically active thus ensuring re~atively
high anode current densities, relative to the cathode
current densit~.
By electrol~ticallr active anode sur~ace~ there is
mesnt that part of the anode surface at which the
electrolytic process takes plsce, that _s that part of the
anode surface with which the cathode surface has substan~ial
electrical interaction, i.e. current ~n the form of a flow
2S of ions through the electrol7te takes place between the
active anode surface and the active cathode surface.
In one form of the invention the ~node structure may be
cons~ructet from different ms~erials; for essmple one part
of the anode structure ma~ be construct2d from a suitable
anode material which presents said electrolytically active
ansde surface, and a second part may be constructed from a
material which is not capable of functioning as an
electrolyticslly active anode 6urface. In this case, the
ancde surface $ncludes both the surface which is constructed
3S from a suitable anode material and the surface which is not
so constructet, although the surface which functions as sn
WO93/11281 ~1 2 4 3 ~ ~ PCT/GB92/02157
active anode surface is only that surface which is
constructed from B suitable anode material.
~ owever, for simplicity of construction, we pre.er ~ha:
at least that part of the anode structure which has 5
surfsce sres esposed for contact with the electrolyte in
operation of the cell is made completely from a material
which is suitable for use as an anode snd thst a portion of
the esposed anode surface is prevented from functior._n6 as
n active anode curface by suppressing the elect-olvtic
interaction between that portion and the cathode structure,
for esample b~ providing means for masking that portion of
the ancde 6urface from the cathode surface.
~ ccording to a second aspect of the present invent'c..
there is provided an electrolytic cell comprising fi-s: and
1~ ,second electrode structures, part only of the surface sres
of at least the first electrode structure being
electrolytically active, and means for masking the remaining
part of the 6urface area of the first elec~rode structure in
such wa~ as to render said remaining part of the surface
area substantially electrolytically inactive.
The masking mesns msy comprise for es~mple, a coating,
cover, insert or any other suitable part, such tha~ therP i5
substantially no ozone producing electrol~tic interactior.
between the masked portion(s) of the anode surface and the
2S csthode surface.
The masking means may take any suitable shape and form
provided that it ~uppresses the electrolyt~c interaction
between the masked portion of the snode surface and the
cathode surface. The material used for effecting masking is
t~pic~ therefore an electr~cally in~ulating material. The
material should also be inert to the electrolyte, which may
be highly corroaive. Suitable materisls include inert
polymeric materials such as polyvinyl chloride or
polyfluorinated polymers, for esample
. 3~ pol~tetrafluoroethylene which are electrical insulators and
which h-ve resistance to osidising gases and escellent
resistance to highly acidic and corrosive solutions.
W093/11281 212 4 3 18 PCT/GB92/02157
The e~tent of the masking should be such as tO achieve
the desired current density difference between the active
anode surface and the cathode surface. Thus the unmasked
anode surface ares which functicns as a~ active anode
surface should be less than about 80~ of the area of the
cathode surface, preferably less than about 60~ of the
cathode surface.
The anode structure may have any suitable form, for
e~ample it may be in the form of a tube or or it may be a
planar anode. The electrol~tically sctive and inactive
surface areas of the anode structure may be distributed
around the periphery of the anode strueture. The anod~
structure may have an elongate configuration and the active
and inactive surfaces may estend longitudinally of the
,elongate anode structure.
The electrolytic cell preferably further comprises
means for circulating a coolant fluid in heat transfer
relation with the an~de structure in such a way that hest
exchange i8 ~ecured between the coolant a~d the electroly
through the active and inactive surface are~s of the anode
structure. A particularly preferred form of the anode by
which such coolant circulation may be achieved is a tubular
anode, the lumen of which provides a path for the flow of a
coolant fluid. The coolant fluid may flow through the lumen
of the anode or the lumen may be provided with a member
e~tending within the lumen, for esÆmple a hollow finger
through which the coolsnt fluid is caused to flow. The cold
finger may be constructed, for example, of copper.
The surfsce area of the tubular anode ~tructure which
is exposed for contact wi~h the electrolyte may be the outer
surface or periphery of the tubulsr structure, and the
electrolytically active and inactive parts of this surface
area may therefore be distributed peripherally around, and
estending longitudinally of, the tubular struc~ure.
In order that heat eschange may take place through the
inactive surfaces of the anode structure, the electrolytic
cell preferably comprises means for routing the electrolyte
WO93/11281 212 4 31~ PCT/GB92/021~7
along a path in which it is in he~t exchange relation Wit:.
substantially electrolytically inactive part of th~ surface
area of the anode structure. Where the ~node structure is of
elongate configuration, the routing means preferably
- provides at least one flow path e~tendinB longitudinally of
the ~node structure.
~ he active and inactive surfaces of the anode struc~ure
msy extend generally co-estensively with one another
longitudin~lly of the anode structure.
ln orter that ss little a6 possible electrolytic
interaction should take pl~ce between the cathode s~ructu-e
and the inactive anode surface~s), the cell preferably
comprises mesns for preventlng the flow of electrolyte
peripherally of the anode structure from a regior. where the
electrolyte is in commun~cation with an active surface of
' the anode structure to a region where the inactive surface
are~ is locsted.
The csthode structure may take any 6uitable form
although we generally prefer to use a plsnar or tubular
2~ structure. Where the csthode structure is in the form of a
tube, the inner surface of the tube may be the
electrolytically acti~e cathode 6urface and the radius of
the inner surface of the cathode structure may be reduced
compared to that hereinbefore described with reference to US
2~ Patent 4,541,989 thus reducing the inter elec~rode gap but
maintaining the differential current density between the
electrolytically active anode surface and the cathode
surface.
Preferabl~ however, the cathode structure is of a
planar configuration since ~e prefer to employ an air
c~thode (as described hereafter). Air cathodes of planar
configuration are readily manufactured and are easily
installed in the electrolytic cell. Furthermore, ~n
elec~rolytic cell comprising st least one tubuler anode snd
3~ at least one plsnar cathode allows both si~ple scaling of
the cell to much larger sizes BS hereinafter described, and
fscilitstes the schievement of the current density
WO93/11281 PCT/GB92/02157
212 i31g
difference between the active anode surface and the ca: ~de
surface defined according to the first aspect of the
invention.
Where the cathodic structure is of plansr
configuration, we prefer that two of said cathode structu es
of planar configuration are present in the cell and that the
anode structure is located between, and in spaced relation
with, said cathode structures and that the anode struc~ure
has separste electrolytically active surfaces in confron~
l relation with the cathode structures. The anode structures
may then hsve electrolytically inactive surface areas
located between the said separate active surface areas. We
psrticulsrly prefer to employ a plurality of said anode
structures, each having electrolytically active and inac~ive
anode surface areas. The anode structures may be arrange~
between the two plsnar cathode structures in a direction
paralle} to said cathode structures.
~ccording to a third aspect of the present inventio&
there is provided sn electrolytic cell for the production of
ozone comprising at least one tubular structure the outer
surface of which functions as an anode surfsce and at least
one planar cath~de structure.
A cell in accordance with this third a6pect of the
invention may incorporate ~severally or collectively, or sny
combination ~hereof, as the conteYt admits~ those features
of said first and second aspects of the invention as
discussed hereinbefore.
In ~ preferred embodiment of this third aspect of the
invention, the cell comprises at lea~t two pla~ar cathotes
between which nre located one or more tubular anodes,
preferably at least two tubular anodes. The number of
tubular anodes which are employed depends 5t least to some
estent upon the desired rate of production of ozone from the
cell, the dimensions, in particular the length snd diameter
3S of the tubulsr anodes and the current density at the
electrolytically active anode ~urfaces during operation of
the cell. Thus, the cell msy comprise up to about 12 tubular
WO93/11281 212 ~ 3 l~ PCT/GB92/021~7
anodes where ozone production in the order of loo g~hour ie
desired. The ease with which the cell msy be scaled up in
orter to increase the rate of production of ozone, sim~lv b~
the provision of further tubular anodes between the air
cathodes and increasing the length of the ~node tubes and
area dimensions of the planar air cathode, is 8 substantial
advantage of this third aspect of the invention.
Furthermore, the cell may comprise more than two planar
cathode structures, with one or more tubular anode
~ structures arranged between each pair of cathode structures
such that the cell may comprise a row of tubular anodes
between each pair of opposingly faced planar cathodes.
Further, in the electrolytic cell accorting to this
third aspect of the invention, preferably onlv a portion of
~the outer surface of the tubular anode which is in heat
eschange relation with the electrolyte is electrolytically
ctive, and advantageously the cell comprises means m~sking
the remaining part of the surface area of the anode
~ structure in such a way as to render it substantially
- electrolytically inactive.
Active and inactive portions of the anode surfsce area
may be defined by providing in the cell a first boundary
means for bounding `eogether with the csthode structure and a
part only of the 6urface area of the anode structure a
2S chamber for enclosure of an electrol~te whereby said par~
only of the anode surface area constitutes an
electrolytically active surface area of the anode ~truc~ure
snd second boundary means for bounding together with a
further part or parts of the surface area of said anode
structure ae least one channel in fluid communication with
said chamber to provide for flow of electrolyte over said
further part(s~ of the surface area of the anode structure,
said further parts constituting a substantially
electrol~tically inac~ive surface area of the anode
. 35 structure. The first and second boundary means may furthe.
constitute a masking means as hereinbefore described, and
ma~, for e~ample be provided by an insulsting body or bodies
-- 10
WO93/11281 2 12 4 3 1 8 PCT/GB92/02157
extending between the anode surface and the cathode surf-~e.
The, or each insulating body msy be in the form of, for
e~smple, a wedge-shaped member or truncated cone, wlth its
narrow end in contact with and e~tending from the cathodo
surface to it6 wide end in contact with the outer surface of
one or more anode structures whereby to mask that portion of
the surface area of the anode structure which is not in
confronting relation with the cathode surfaces, from the
cathode 6urfaces.
The flr6t and 6econd boundary means, for e~amp'e one or
more insulating bodies may be provided as separate inserts
for provision within the cell. Conveniently however, the o-
e-ch insulating body is formed as part of a cell bod~
which the anode and cathode structures are supported. Thus
,,the cell body may be so configured that i~ mask~ ~he
surfaces of the anode which are not ir. con~-ont ng el&~_or.
with the cathode surfaces.
The acti~e anode surface will in this case be the
surface of the anode ad~acent the cathode surface and the
me-n di~tance between the acti~e anode surface and the
c~thode surface is preferably less than lOmm, more
prefe~ably less than 5mm and especially less than 4mm.
~s hereinbefore described it is desirable that as gre&.
an area of the anode structure as possible is in contact
2S with the electrolyte in order to achieve good heat exchange
efficiency between the electrolyte and the anode surface
whereby efficient cooling of the sctive anode surface ie
obtained. We therefore prefer that the masked portion(s) o-^
the anode surface is ne~ertheless e~posed tO the
electrol~te.
Where the masking me~ns is in the form of one or more
wedge-shaped in~ulating boties e~tending from the cathode
surface to the anode surface, the wedge may be shaped such
that the wedge onl~ comes into contact with a small area of
3S the anode surface to be masked. For e~ampie, the wedge may
be cut away to form circulation channels. at the portion of
the wedge which is adjacent the anode surface to be masked
WO93/11281 ~ 21~ 4 31~ PCT/GB~2/02157
so that electrolyte msy circulate over the ~node surfac~
between the masking wedge member and the anode surface.
Thus, even though the masked portions of the anode
structure pla7 no significant part in the electrolytic
interaction, the electrolyte may flow freely over
substantially 811 or a section of the masked and
electrolytically inactive portion of the anode structure,
providing an ~ncreased totsl surface srea for heat e~change
and thus cooling of the active anode surface.
~ ~ further advantage of the provision of, for example,
re-circulstion channels atjacent the masked and
elec~rolytically inactive anode portionts) is that a
recirculating flow of electrolyte may be achieved over the
active anode surface which serves to remove bubbles of
'gaseous products which may form on the active anode surface
and which may, if not removed, lead to an increase in the
cell voltage. This flow of electrolyte is achieved because
substa~tially no electrolysis takes place within the
recirculation channel~ so th~t the electrol~te $n the
channels tends to be unga6ified. whereas the formstion of
gaseous products of electrolysis takes place in the
electrolyte chambers thus protucing a gasified electrolyte.
A recirculating flow of electrolyte is there~y generated by
the density difference between the gasified and ungas;f,ed
2S electrolyte.
Fluid-tight seals should be maintained between the
electrol~te chambers and the re-circulation channelç in
order to prevent current leakage from electrolyte betweeh
~he aetive anote and cathode surfaces to the electrolyte
flowing within the recircula~ion ch~nnels.
~ccording to a fourth a~pect of the present invention
there is provided an electrolytic cell for the production of
ozone comprising an anode structure, n cathode structure and
a chamber for containing electrolyte within which
3S slectrolysis occurs and which further compri~es a~ least one
re-circulation channel in fluid communication with the
electrolyte but within which electrolysis does not occur.
WO93/11281 212 4 3 18 PCT/GB92/02157
The recirculation channels may be provided wit~ .e
cell itself or they may be pro~ided e~ternally of the ce.'.
As hereinbefore described we prefer to provide ~he
recirculation channels within the electrolytic cell and
especially adjacent the electrolytically inactive anode
surfsce.
Cell heat spaces which may serve as both disentrainment
areas for product gases and reservoirs for electrolyte may
be provided within the cell, into and from which elec:rolyte
~ from and into both the electrolyte chambers between .he
active anode and cathode surfaces, and recirculation
channels may flow, and from which product gases may be
collected.
~ir cathodes, which are commercially available
' components, are t~pically composed of
polytetrafluoroethylene-bonded-carbon containing sma'l
mounts of catalytic materials, for e~ample platinum.
The materials used as the snode surface in the
electrolytic cell of the present invention m~y be
conventional anode materials BS descrlbed more fully ir" for
example, Et~ropean Patent 0 041 365. The anode surface may be
constructed from platinum or lead dioxide, particularly leaa
dioxide in the beta crystalline form. ~owever, a special
form of carbon, specifically vitreous or glassy carbon is
2S particularly preferred for use as the anode surface ma~erial
s~nce it has a high oxygen overpotential and thus a h gh
efficiency for ozone production, it is 6table in strong acid
electrolytes and is ~table to oxidising cond~tions generated
in the cell. Furthermore, glass~ carbon is a material which
possesses poor electrical conductivit~ so that where
current is fed to the anode structure through a conducting
memeber provided adjacent only the electrolytically active
surfaces of the anode (as described hereafter), current
tends not to lesk from the electrolytically active anode
~S surface to the electrol~tically inactive anode surfaces.
The electrolyte used in the electrolytic cell is
typically a known electrolyte, for example, an aqueous
W093~11281 21~ 4 3 1 ~ PCT/GB92/02157
solution of ~ highly electronegative snion (~nd associ~ted
cation) such as are, for example, described in European
Patent O 041 365. The electronegative anion used is
preferably as electronegative as possible, ~nd more
preferably is a fluoro-ani~n. The fluoro-anion may be the
fluoro-anion of B Group V-B element of the Periodic Table,
for esample pho6phorous and arsenic which form hexa-fluoro
anions. Other related non-metallic elements such as Si and
Sb also form hesa-fluoro-anions. Other su~table
fluoro-anions ma~ be mentloned, inter alia P02F2-, HTiF~-,
NbF72-, TaF72~, NiF62-, ZrF62~, GeF62~, FeF62-. The
phosphorou6, ar6enic, boron and silicon fluoro-anions ~re
the preferred anions for addition to the squeous elec~-nly~
and in particular polyhalogenated boranes. We especial!y
refer to emplo~ the tetrafluoroborate in on.
The fluoro-anions may be added to the aqueous
electrolyte 601ution in the form of their respective acids
or as w-ter-601uble salts. Whereas the acid-form of the
fluoro-anions may be preferred because of their hi8he~
solubilitles in water, the fluoro-anion salts, for esample
60dium or potas~ium, offer the sdvantage that their aqueous
solutions have higher p~'s than do the solutions of their
- respective acid forms, and they therefore are less corrosiv~
towards the cathodes.
2S ~or current efficiency, it is desirable to increase the
fluoro-anion concentration in the electrolyte to its maximum
solubility since increasing the anion concentration
increases ozone current efficiency. ~owever, an increase in
the snion concentration also increase6 the corrosivity of
the electrolyte towards the cathodes. Suitable anion
concentrations may be readily determined by routine
experimentation.
The construction of the electrolytic cell may, apart
from its construction according to the various aspects of
3S the invention as hereinbefore tefined, follow conven~ional
tcchnolog~ taking into consiteration the corrosive nature of
the fluoro-anion electrolytes and the high osidising power
WO93/11281 212 4 318 PCT/GB92/021~7
of the ozone gases. Thus, the parts of the cell in conta~
with the corrosive electrolyte and o~idisin~ products of
electrolysis are preferably constructed Oc materisl~ whi~
are inert both to the highly corrosive electrolyte snd the
oxidising gases. The cell body may therefore be ccnstruct~d
from, or coated with, an inert material, for esample an
inert pol~meric material such 85 polyvinyl chloride or
polyfluorinated polymers, for example
polytetrafluoroethylene which have resistance to oxid$sing
gases and excellent resistance to highlv ac~d and corrosive
solutions.
Where the cathodic reaction taking place in the cell
produces h~drogen, the anode and csthode compartments o_ -he
cell should be separated such that the h~drogen evolved ~t
l~ ~'the cathode is not in fluid-flow contac~ with ~he gases
evolved st the snode. Such separators are well knowr. ~s the
art. Conventionally they are prepared from a perfluo.inPt~r
pol~meric cation exchsnge material, for example ~Nafion"
~rcgistered Trademark of E.I.Du Pont). Such a separator _
not needed in the preferred embodiments of the invention
where an air cathode is used and where hydrogen is no~
generated by the cathote process.
The anode and cathode structures are disposed within
the electrolytic cell with electrical leats lesding tO the
2S exterior of the cell. Electrical potential may be spplied tC
the anode by contact with part only of a surface of the
anode structure which corresponds to the electrolytically
active surface area of the anode structure, for example by
means of a conducting member, constructed from, for example,
copper, which is provided along the snode adjacent the
active anode surface in order that current is fed
predominantly to the active snode surface and not to tha~
anode surface which is masked from the cathode. The
conducting member is preferably provided on a surface of ~h~
~S anode which is not in direct contact with the electrolyte~
for example where the anode is in the form of a hollow tube
the conducting member may be provided within the lumen of
WO93/11281 - l5 - 2~ ~431 ~ PCT/GB92/021~7
the tube, in order that the conducting member is protectec
from the electrolyte.
The cell is sealed prior to use, and the cel' hesd
space is provided with suit~ble inlet ~nd outlet passages ;~
for water mske-up, where necessary, and the withdrawal of
the gases evolved from the cathode (where hydrogen is
produced) ~nd from the anode. Two discrete gas removal
systems ma~, where necessary, be used to keep the cathode
g~se~ separ~te fro~ the ~node gases. Nitrogen and/or air maY
be sdded, for e~ample pumped through. the gas h~ndling
system in order to entrain the evolved cathode ~nd anode
g~ses and carry them from the cell to the esterior where
they may be stored or utilised in the desired application.
The snode ~nd cathode structures sre connected by the
~-~aforementioned electrical leads, optionally through a
conducting member, to a source of power external to the
cell. Typically the cell is oper~ted at electrical
potentials in the order of 3-7 volts. The current densit~ e~
the snode surface m~y be in the range from ~bout a tenth o'
an ampere per 6quare centimetre of effective a~ode surface
up to about l.0 ampere per square centimetre of effec~ive
anode surface.
The invention is illustrated with reference to the
2~ sccompanying figures in which:
Figure 1 is ~ diagrammatic partly cut away view of an
electrolytic cell according to the invention,
Figure 2 is s view in section along the line A-A in figure
1,
Figure 3 is a view in section through the cell of Figure l
along the line B-B in figure 2,
- 3~
Figure 4 i6 a view in section through the cell of Figure l
slong the line C-C in figure 2,
- 16 _
WO93/11281 2 12 ~ 3 1 8 PCT~GB92/02157
Figure 5 is a view in section along the iine D-D in ~ igure
3, and
Figure 6 is a view in section along the line E-E in fi~ure
3.
Figure 7 is 8 view in section along the line F-F in figure
3.
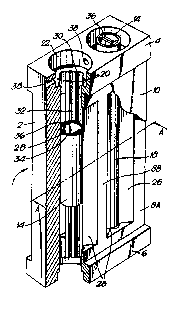
Referring to Figures 1 to 7, an electrolytic cell
suitable for tbe generation of ozone shown generally as 1 :~:
Figure 1 co~prises a main cell boty 2, havln~ top and ~ct;om
portions 4, 6 and spaced columns 8 extending there~etween~
~lthough three such columns 8 are present in the illust-~ted
~,,embodiment, a pair of end columns 8A and an intermediate
column 8B, there may be more or less according to the r.umbe-
of anode structures employed. A pair of air cathodes 10 wi~.
associated air chambers 12, and located between but spaced
from the air cathodes ~0, a psir of tubular glas~y carbon
anodes 14, are supported in the cell body. The colu~ns B,
which support the snodes and cathodes in spaced relatior.
~-ith one hnother, constitute means fcr bounding, togethe~
with active surfaces 16 of the air cathodes 10 and psrts
only of the outer surfaces of the anodes 18 which are in
2S confronting relstion with the active surfaces of the air
cathodes, electrolyte chambers 20. The electrolyte chambers
20 extend longitudinally from the top portion 4 to the
bottom portion 6 and at each end open into upper and lower
cell spaces, 22, 24 respectively ~see Figure 3) within the
top snd bottom portions 4 and 6, the arrangement being suc~.
that each cell space is in communication with a pair of
electrolyte chambers 20 on opposite 6ides of an anode 14.
The columns have a wedge-like configuration snd hsve
inwardly directed surfaces 26, the surfaces of adjacent
columns converging from the cathode tO the anode at an angle
of convergence so as to define a longitudinally e~tending
active anode surface having an area half that of the cathode
W093/11281 _ 17 - 2 1 2 4 3 ~ ~ PCT/GB92/021~7
surface 16 which is bounded by the columns. The required
differential in active anode and cathode surface are~
thereby achieved provides in use the required current
ten6ity d~fferential between the acti~e anode and cathode
surfaces but allows a mean distance between the active and
cathode surfaces of less than 4mm.
The columns 8 mask the remainder of the anode surfaces
28 from the surfaces of the cathodes and are formed with
grooves or channels which constitute re-circulation channels
30 of curved, e.g. semi-circular profile, adjacent at least
a part 28~ of the in~ctive surfaces of the anodes 14. Each
channel 30 e~tends longitudinally from the top portion 4 to
the bottom port~on 6 and at each end opens into uppe- an~
lower cell ~paces 22, 24 (see figure 4) within the tcp n~
bottom portions 4, 6, the arrangement being such that eac~.
cavity 22, 24 is in communication with a pair o' channe;s 3G
a~sociated ~ith adjacent columns and a pair of elect,olyee
chambers 20 as described previously. Fluid tight seals are
maintained between the surface of the anodes and the columns
2~ Of the cell body by longitudinally e~tending resiliently
deformable seals 32 which prevent circumferential flow of
electrolyte sbout the periphery of the anodY from the
electrolyte cha~bers 20 to the re-circulation channels 3C
The lumen 34 of each anode is proYided with copper
2S conductors 36 adjacent the active surfaces of the anodes 1
through which electrical connection is made between each
electrolytically active surf2ce of the anodes 18 and the
source of electrical power. The anodes are constructed from
glass~ csrbon which posse~ses poor electrical conductivity
and which therefore serves to reduce the eendency for
current to leak to the inactive snode surfaces adjscent the
re-circulation channels 30. The lumen 34 also provides the
flow path for circulation of 8 coolant in heat transfer
relation with both the active and inactive 6urfaces of the
anode structures.
The sectional views of figures 3 to 7 show more clearly
the electrolyte flow around a single anode between a pair of
-- 18 -- `
WO 93tll281 2 I ~ '13 I 8 PCT/GB92/021S7
sir cathodes. Figure 3 shows the flow of electrolyte rro~
the cell head space 22 downwardly through the rec culati_
channels 30 which e~tend lengthwise of the anode structure
to the l~wer cell space 24 and figure 4 shows the flow of
electrolyte fr~m the lower cell space 24 upwardly throu~h
the electrol~te chambers 20, which also extend lengthwise Oc
the snode structure to the cell hesd space 22. Product gsses
are collected through gas outlet 38 provided from the cell
head space. Figures 5 to 7 show clearlY the provision wi~hir.
the cell of the cell head space 22 (Figure 5) and lower cel;
space 24 (figure 7), into and from which electrolyte frn~
both the recirculation cnannels snd the electrolyte chsmbers
flows.
In cperation of the cell, elec~rolyte is char~ed ~e :he
l~ ,cell and the electrodes lO and 14 are ccnnected tc a sour_e
of electrical power (not shown). Air is pumped th-ough
air chambers 12 by an air pump (not shown), and a coolant
fluid, for example a refrigerant, is caused to flow through
the anode lumen 34 from a refrigeration system (not shown)
externally of the cell. Ga~éous products of electrolysis
formed at the electrol~tically active anode surface 18 cause
the electrolyte to flow upwardly through the electrolyte
chambers 20 to the cell head space 22 where the gaseous
products are disentrained, the electroly~e thence flowing
2~ downw~rdly through the recirculation channels 30 adjacen.
the masked and electrolytically inacti~e anade surfaces ar
which no gsseous products of electrolysis are formed.
Product gases are collected ~ia the gas outlet 38.