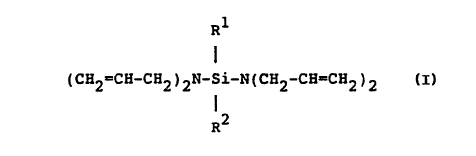Note: Descriptions are shown in the official language in which they were submitted.
2125959
-1-
BIS(DIALLYLAMINU) SILANES
Technical Field.
This invention relates to the use of
bis(diallylamino) silanes of the general formula
R1
(CH2=CH-CH2)2N-Si-N(CH2-CH=CH2)2
R2
where R1 and R2 are independently selected from
alkyl and aryl groups having 1 to 6 carbon atoms as
comonomers for lower olefins polymerized in highly
active Ziegler-Natta catalyst systems.
Background Art
In European Patent Publication 0423438,
Sivak et al propose the use of protected diallyl
amine monomers for copolymerization with ethylene,
propylene, and other lower alpha-olefins having up
to 8 carbon atoms. Protection of the otherwise
vulnerable amine group is provided by a silyl group
WO 94/10217 PCT/US93/10025
2125J5~!
-2-
having relatively bulky,substituents, such as lower
alkyl or phenyl groups. Diallyl amines are proposed
and several examples are given of silyl-protected
diallyl amines. However, bis-diallyl amines are not
contemplated.
Summary of Invention
I have invented new compounds of the
general formula
R1
(CH2=CH-CH2)2N-Si-N(CH2-CH=CH2)2
R2
where R1 and R2 are independently selected from
alkyl and aryl groups having 1 to 6 carbon atoms.
They may be used as cross-linking agents in polymers
and find special utility as comonomers for lower
olefins polymerized in highly active Ziegler-Natta
catalyst systems.
Detailed Description cf the Invention
My invention will be described with
respect to two paradigms, namely
bis(diallylamino)dimethylsilane and
bis(diallylamino)diphenylsilane.
WO 94/10217
- ~ ~ ~ PCT/US93/10025
-3-
EXAMPLE I
Bis(diallylaminoldimethylsilane
The equipment used for the synthesis of
bis(diallylamino)dimethylsilane was set up in the
following manner. A reflux condenser, mechanical
stirrer and 125 ml addition funnel were placed on a
2000 ml 3-necked, round-bottomed flask. An argon
inlet was connected to the top of the reflux
condenser and a heating mantle was placed on the
flask. The flask was flushed with argon (allowing
the argon to exit the setup through the top of the
addition funnel) until the atmosphere in the
glassware was assured of being inert.
Heptane (400 ml), triethylamine (136.62 g,
1.350 moles, 188 ml) and diallylamine (98.36 g,
1.012 moles, 125 ml) were charged into the reaction
flask. Dichlorodimethylsilane (43.55 g, 0.3375
moles, 41 ml) was placed in the addition funnel and
added to the reaction mixture in the flask over a
period of 70 minutes. The temperature of the
reaction mixture reached 37.5°C (as measured by a
thermocouple between the flask and heating mantle).
The reaction produces a large amount of
salts (triethylamine hydrochloride and diallylamine
hydrochloride) and twice during the reaction
additional heptane had to be added (200 ml portions
each time) in order to keep the slurry thinned to a
stirrable consistency.
WO 94/10217 PCT/US93/10025
2iy595~
-4-
After the addition of dichlorodimethyl-
silane was complete, the mixture was heated to
reflux for five hours. .The flask was then allowed
to cool to room temperature.
The salts were removed by filtration using
a buchner funnel covered with a latex dam to keep
exposure to the atmosphere to a minimum. the
product was isolated from the filtrate by
distillation. The heptane and excess amines were
removed at a vacuum of 40 mmHg with a temperature
range from ambient to 93°C. The product distilled
at 72.6-74.6°C at 1 mmHg.
61.2 g of bis(diallylamino)dimethylsilane
was isolated by this technique which corresponds to
a yield of 720 of theoretical after distillation.
EXAMPLE II
Bis(diallylamino)diphenylsilane
The glassware setup used for the
preparation of bis(diallylamino)diphenylsilane was
the same as the equipment used for the synthesis of
bis(diallylamino)dimethylsilane above except that a
1000 ml flask was used instead of a 2000 ml flask.
After the system was flushed with argon,
triethylamine (159.84 g, 1.580 moles, 220.2 ml),
diallylamine (84.48 g, 0.8699 moles, 107.3 ml) and
toluene (200 ml) were added to the reaction flask.
Dichlorodiphenylsilane (100.75 g, 0.3980 moles, 83.7
ml) was charged into the addition funnel and added
to the reaction mixture in the flask over a period
of 15 minutes, the temperature rose to 60°C.
WO 94/10217 ~ 1 ~ ~ 9 ~ ~ PCT/US93/10025
-5-
A large amount of solid precipitated from
the mixture. Toluene (100 ml) was added to the
flask through the addition funnel to rinse out the
remaining silane and make the reaction mixture more
stirrable and the mixture was heated to reflux for
two hours, then cooled to room temperature.
The salts were removed by filtration using
a sealed pressure filter under an inert atmosphere.
The product was isolated from the filtrate by
distillation. The heptane and excess amines were
removed at a vacuum of 40 mmHg with a temperature
range from ambient to 43°C. The product distilled
in a range from 160°/0.3 mmHg to 160°/0.1 mmHg.
101.5 g of bis(diallylamino)diphenylsilane
was isolated by this technique which corresponds to
a yield of 68$ of theoretical after distillation.
Similar preparations may be made for the
diethyl, dipropyl, dibutyl, dipentyl, dihexyl and
methylphenyl variants.
My new monomers may be incorporated into
chains of crystalline polypropylene and other lower
olefin polymers as described in the above-mentioned
Sivak et al patent. Thereafter, the silyl groups
may be removed by hydrolysis or alcoholysis
optionally promoted by acidic or basic catalysis and
the remaining copolymers, which may be mildly
cross-linked, will exhibit amine functionality or
ammonium functionality in the presence of acids.
My monomers may be homopolymerized using
Ziegler-Natta systems and/or copolymerized in
amounts to yield copolymers having ratios of lower
olefin to my monomer (m) of 0.1 mole ~ to 99.9
mole %.
WO 94/10217 PGT/US93/10025
-6-
Following are examples of propylene
copolymerizations with
bis(diallylamino)dimethylsilane ("BDMS") and
bis(diallylamino)diphenylsilane ("BDPS").
_General Copolymerization Procedure
Standard inert atmosphere techniques were
used to exclude moisture and oxygen throughout the
manipulations.
An apparatus consisting of a round bottom
flask fitted with a side arm, magnetic stirring bar
and a stopper was assembled hot from a drying oven
and was then either evacuated and refilled with
inert gas several times or (and) purged with inert
gas for at least 15 minutes. The flask was charged
with a given amount of solvent, heptane or toluene,
usually 125 mL. The solvents were freshly distilled
from sodium and triethyl-aluminum (TEA) over which
they had been refluxed for at least 18 hours under
an inert atmosphere.
At this point the inert gas atmosphere in
the flask was replaced with the gaseous comonomer by
a minimum of three cycles of evacuation and
refilling back to atmospheric pressure with the
comonomer. After the third cycle the solution was
stirred for at least ten minutes (usually longer) to
allow the solvent to become saturated with the
comonomer. Pressure was maintained at one
atmosphere via a bubbler.
Next was added an "external donor", which
was diphenyldimethoxysilane. Phenyltrimethoxysilane
may alternatively be used. Then the other comonomer
WO 94/10217 .-
2 ~ ~ ~ 9 5 ~ P~/US93/10025
_7_
was added. A given amount of alkyl aluminum
co-catalyst, which was in the form of a heptane
solution of about 25$ by weight (0.715 g/mL in
heptane), was also added to the flask.
The polymerization was initiated by the
addition of the transition metal containing
co-catalyst, which was a titanium tetrachloride on a
magnesium chloride support. At this point the flask
was lowered into a thermostated oil bath and
magnetic stirring was begun.
An excess of gaseous comonomer was passed
into the flask in order to replace any that was
consumed. Excess gaseous comonomer was allowed to
pass from the reaction vessel via a bubbler,
maintaining a pressure in the flask of one
atmosphere.
After a specified period of time the
reaction was quenched by the addition of acidified
alcohol (HC1 in iso-propanol, ethanol and/or
methanol). The quenched reaction slurry was
combined with the alcohol solution of volume at
least twice the original volume of the inert
reaction solvent. The resultant slurry was stirred
for at least 45 minutes and then filtered. This
treatment not only stopped the reaction, it
dissolved catalyst residues and removed silyl groups
and thus regenerated the amino groups.
If the filtration proceeded very slowly,
the slurry was combined with enough water to make
the filtration proceed at a convenient rate.
The polymer was resuspended in alcohol,
a stirred, filtered and vacuum dried overnight.
WO 94/10217 PCT/US93/10025
2~2~~59
_8_
Boiling heptane soluble content was
determined by standard methods.
Homopolymerization of propylene under
these conditions produces polypropylene with yields
in the range of 200-220 g polymer/g ,
titanium-containing catalyst. The extent of
reduction in polymer yield in the copolymerizations
relative to this homopolymer yield is used as a
rough guide to the utility of the comonomers in the
copolymerization systems.
Copolymerizations of Propylene with
Bis(diallylamino) dimethylsilane (BDMS)
Example III
The general copolymerization procedure
above was followed using the following quantities of
reagents and reaction conditions in the
polymerization.
Solvent heptane 100 mL
External Donor DPMS 75 NL
Comonomer BDMS 3.85 g
Cocatalyst triethylaluminum 4.3 mL (25 wt ~)
TiCl4/MgCl2 Catalyst 91 mg
Reaction Temperature 50°C
Reaction Time 2 hr
Alcohol Used in Work Up iso-propanol
The polymerization yielded 8.3 g of
polymer which is a yield of 91.2 g polymer/g
catalyst. This corresponds to about 40% of the
yield of a homopolymerization of propylene under
these conditions.
WO 94/10217 PCT/US93/10025
215959
_g_
Example IV
This polymerization was done using the
following quantities of reagents and reaction
conditions with the general copol ymerization
procedure from above.
Solvent heptane 75 mL
External Donor DPMS 225 NL
Comonomer BDMS 11.12 g
Cocatalyst triethylaluminum 12.9 mL (25 wt ~)
TiCl4/MgCl2 Catalyst 104 mg
Reaction Temperature 50C
Reaction Time 2 hr
Alcohol Used in Work Up iso-propanol
Polymer (5.8 g) was produced in this
polymerization which is a yield of 55.5 g polymer/g
catalyst which is approximately 25% of a comparable
homopolymerization.
Copolymerizations of Propylene with
Bis(diallylamino) diphenylsilane (BDPS)
Example V
The following quantities of reagents and
reaction conditions were used with the general
copolymerization procedure.
Solvent heptane 100 mL
External Donor DPMS 220 NL
Comonomer BDPS 18.19 g
Cocatalyst triethylaluminum 12.5 mL (25 wt
%)
TiCl4/MgCl2 Catalyst 137 mg
Reaction Temperature 50C
Reaction Time 2 hr
Alcohol Used in Work Up iso-propanol
WO 94/10217 PCT/US93/10025
~~2~g59
-10-
The polymerization yielded 15.4 g of polymer
which is a yield of 112.4 g polymer/g catalyst which is
in the range of 51-57$ of a comparable propylene
homopolymerization. 98.40 of a tested sample was
insoluble in boiling heptane.
Example VI
The following quantities of reagents and
reaction conditions were used with the general
copolymerization procedure.
Solvent heptane 50 mL
External Donor DPMS 400 NL
Comonomer BDPS 31.97 g
Cocatalyst triethylaluminum 23.5 mL (25 wt ~k)
TiCl4/MgCl2 Catalyst 117 mg
Reaction Temperature 50C
Reaction Time 2 hr
Alcohol Used in Work Up iso-propanol
Copolymer (17.0 g) was produced by this
copolymerization which corresponds to a yield of 145.3
g polymer/g catalyst. This is about 70~ of what is
produced in a comparable propylene homopolymerization.
The fraction insoluble in boiling heptane was 91.8 of
the tested sample.
Uptake of an Acid Dye by Polymer
Samples as a Test for Amine Incorporation
Example VII
A sample of propylene homopolymer was
prepared using the general polymerization conditions
given above and used as a blank in the dye uptake
experiments described below.
A stock solution of dye was prepared by
dissolving Acid Alizarin Blue BB dye (1.00 g) and
ammonium acetate (7.0 g) in water to obtain 80 grams of
solution.
4 10217
PCT/U593/10025
~~2~~5.~
-11-
Samples (200 mg) of the propylene homopolymer
and each of the four propylene/amine copolymers
produced iri Examples 1-4 above were placed in test
tubes and 10 mL of the stock dye solution was added.
The samples were sealed and shaken to assure dispersion
of the polymer powder in the dye and placed
in a 60°C water bath. The temperature of the bath was
increased to 100°C over a period of 30 minutes and held
at that temperature for 1 hour and then cooled to room
temperature.
The samples were filtered and rinsed
repeatedly with water until the rinsings were clear and
the color of the powder did not appear to change. The
rinsings were then continued with an equivalent amount
of water. The total amount of water used was about 500
ml. The samples were then suspended in boiling water
for about one minute and then filtered and this hot
wash was repeated two more times.
After the samples were dried, their color
intensities were compared and are described in Table 1
below.
WO 94/10217 PCT/US93/10025
-12-
Table 1
Dyed Propylene/Amine Copolymers
Sample Color
Propylene homopolymer very pale pink/
purple color
Example III slightly more intense
purple than propylene
homopolymer
Example IV pale purple
Example V pale purple
Example VI reasonably intense
purple/blue color