Note: Descriptions are shown in the official language in which they were submitted.
2127~
~THOD OF R13 MOVING TOXIC RESIN ACIDS ~D ~ Y ACIDS
FROM PULP AND PAPI~R EFlFLl~ENT AND OTlEilER STR~AMS
FIELD OlF I~NTION
This invention relates to the use of an aruonic material as a
S pretreatment step for improving the removal of resLll acids and fatty acids
from waste waters. More specifically this invention relates ~o the use of
albumin, gelatin, alginate or al~uc acid as a pretreatment step for improving
the removal of resin acids aIld fatty acids from waste waters, wherein the
alginate or alginic acid is preferred.
~A~K(j:RplJ~D ~ PRIC3R ART
Traditionally resin and fatty acids which naturally occur in various
forms during the manufacturing of pulp and paper products, provide
objectionable ~atures to these prbcesses and e~uent waters. Historically,
many ~hemical and mechanical methods have been used to remove, bind or
inactivate these compounds. Olle such example of a method ~or treating pulp
and paper waste water is found in U.S. Patent No. 4,738,750 which discloses
the use of a polyamine coagulant which coagulates lignins, degraded sugars
and other compounds which typically discolour the waste water. I~he
coagulation part;cles are increased in size by the addition of an acrylamide
polymer. The ilocculated material is then removed from the waste water.
The use of acrylamides in ef~uent flocculation is also dis~osed in U.S. Patent
No. 4,5~6,294.
U.S. Patent No. 4,089,780 discloses a method for removing colour
~om pulp and paper waste waters by fïrst treating the waste water with a
cationic water-soluble polyamine and thereafter adding a suitable organic
coagulating polymer.
The specific problem ~ removing toxic resin and fatty acids from
the waste water of a pulp and paper mLII has been addressed in Canadian
Patent Application 2,023,735. The process, of this patent application, utilizes
2~,7~
,... ..
-2-
a composition comprising a water-soluble non-ionic polyallylene ether
containing lower allyl groups in the alkyl chain and an ionic water-soluble
polymer. E~owever, the results achieved by this method show, at the very
best, a 37% removal of the resin acid after treatment. In order to be an
S effective waste water treatmen~ process, improved results over that shown in
the prior art must be ac~ieved.
SUP~IMA~Y OF~.INVENTIQN
l'hus according to the present invention there is provided a method
of removing toxic resin acids alld fatty acids from pulp and paper ef~luent and
other streams contadning resins a~ids and fat~ acids. According to the
metbod of the present invention, an ~nionic matenal is added to the waste
water pnor to cla~ cation with conventional cationic polymers. According
to tbe method of the present invention tbe anionic materlal is selected from
gelatin, albumin amd alginate, wherein the alginate is preferred.
In one embodiment of the present i2lvention there is provided a
method of purifying waste water containing resin acids and fatty acids
comprising adding an effective amount of an algLnate, in a metallis salt form
or acid form thereof, to the waste water and then adding a cationic polymer
to coagulate or flocculate the complexed alginate and resin acids and fatty
acids, and separate the resultant precipitate, thus reducing the concentration
of the resin and ~at~ acids in the waste waters.
This invention is also directed to a comiposition for treating waste
waters containing resin acids and fatty acids comprising an anionic material,
selected from the group consisting of gelatin, albumin, or an algiDiate, in a
metallic salt form or acid form thereof9 and a cationic polymer.
BRIEF ~ESGRIP~O~ OF THIE ~JRES
Figure 1 shows a schematic of a pilot plant snitable for the
treatment of was~e water according to the present invention.
2 ~ 2 ~
,
,. ~
I~ET~ILED DESCRlPrlON OF THE INVENTION
According to the present invention, there is provided a method of
improving the removal of resin acids and fatty acids frorn waste water. This
method comprises ~he addition of an anionic material to the waste water
S prior to treatment with conventional cationic polymers.
The present invention is based on the discovery that pre-treatment
of the waste water with an anionic material improves the removal of resin
acids cr fatty acids ~om the waste stream. Prior art treatments which
generally rely on the use of a ca~ionic polymer, either alone or together with
other compounds, show variable reported results. However, the average prior
art methods of rernoval of contaminants from the waste streams are wi~
the range of 3~60~o. According to one embodiment of the presen~ invention,
the addition of an alginate prior ~o clarification with conventional cationic
polymers impro~es the removal of resin acids up to 98~o and the removal of
fatty acids up to 94%.
This invention is particularly applicable to pulp and paper waste
water t~eatment, however, it can also be used for the treatmen~ of any waste
stream for the removal of resin acids and fatty acids.
The anionic material of the present invention is selected from the
group consisting of gelatin, albumin or alginate, wherein the alginates are
preferred. The alginates according to the present invention can be present
in one of its metallic salt forms or acid forms. ~ranous gelatins, albumins or
alginates of different molecular weights can be used according to the present
in~ertion. Representative examples of alginates wbich call be used according
to the present invention include alginic acid and sodium alginate, potassium
algina~e or magnesium alginate. The alginate is prepared for convenience a~
a 1% solution~ preferably a 1% sodium alginate solution. In order to ~ud the
affinity of the alginate for the resin acid or fatty acid material, in some cases
an acid was added to acidi~ the alg~nate. Any ~ype of acid would be
2~27~7
.....
-4-
appropriate, but for co~venience and for economic reasons, sulphuric acid
was used ~or this purpose. In one example of the invention, a 0.5 ml of
concentrated sulphuric acid was added per 100 ml of 1% solution of sodium
alginate to acidi~ the sodium alginate. Not to be bound by any particular
S theory, it is thought that the acidified al~nate in slightly hydrophobic and as
such has a greater afEinity for the hydrophobis resin and fatty acids.
A suitable dose of gelatin, album n or alginate, for the precipitation
of resin acids and fatty acids from waste waters, raIlges ~om 4 ppm to 250
ppm, preferably from 5 ppm to 50 ppm. In general the amount of anionic
material to be added to the was~e water is deterlI~ined empiricaLly for each
di~erent ~pe of waste stream to be treated. In general a dose of 50 ppm will
in most circumstances be adequate, but it is within the sldll of one in the art
to vary the concentration according to the need.
The anionic material is first added to the waste water to be treated
for a minimum contact time of 20 seconds before ~he addi~ion of a cationic
material. The resin acids-fatty acids/anionic material complex ;s quite stable
and thus can remained complexed, be~ore the addition of caffonic material
for long periods of time without adverse effect. A wide range of cationic
materials have been used in the prior art and are considered to be within the
scope of the present in~ention. For example, a number of cationic materials
are deiïned in U.S. Patent No. 3,3779274 (incorporated herein by reference).
Some examples of usable cationic materials include polyamines,
polyacrylamides, acrylamide co-polymers, diallyl dimethylammoniumchlonde,
cationic condensate polyrner sold under the tradename DEC 50 (Floerger).
The cationic polymers are used according to the present inveIltion
for ~he removal of the anionic material which is bound to the resin acids and
fatty acids. As with the anionic material the amount of cationic polymer
added to llocculate or precipitate the contaminating materials will vary
depending on the waste water to be ~reated. In the examples provided herein
2~2~i7
s-
the amount of polyacrylamide ranged from S ppm to 75 ppm, preferably
ranging from S ppm to 50 ppm. When a polyamine was used in conjunction
with the polyacrylamide the range of the polyamine was ~om 10 ppm to 300
ppm, preferably from 10 ppm ~o 100ppm.
S
In general the process of the present invention can be used with
conventional waste water treatment processes and facilities already in place
such as primaIy sedimentation and filtration. If additional ~acilities are
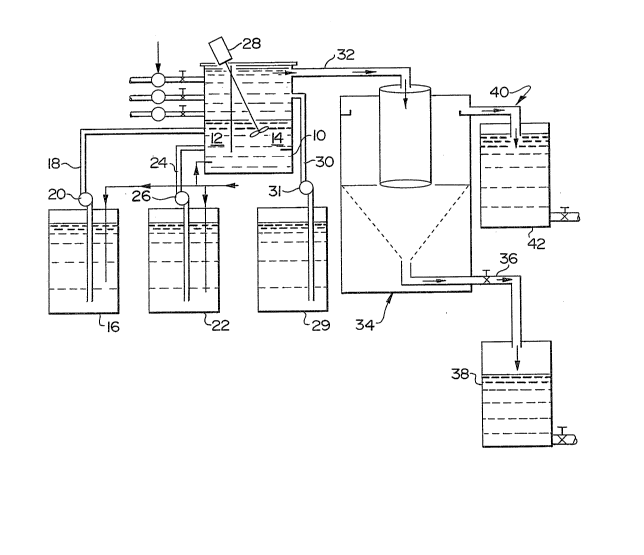
required, a treatment plant as depicted in Figare 1, could be used according
to the present invention.
In all of the examples described herein, sarnples of waste water
were taken ~om various pulp ~d paper mills a~d subjected ~o the treatment
according to the present iIlvention using ei~her a small scale laboratoIy
treatment facilit~, a pilot plant as shown in Figure 1 or a similar treatment
system. The primary mixing tank 10 comprises two zonesl zone 1, 12, and
zone 2, 14. The anionic material, ~or exaInple alginate is added to zone 1 of
the primary m~xing tank 10, from a first chemical feed tank 16, fed from the
~ank to the primary mi~g tank by conventional means, tbrough feed line 18
and pulping means 20. The effluent is pumped, by conventional means,
directly from the pulp and paper plant or other industrial plant or from a
storage holding facili~ into the primary mLxing tank 10 initiaUy into zone 1,
12. After an initial mixing of the effluent with the alginate, with a minimum
contact time of 20 seconds, cationic material is added to ilocculate and clari~
the effluent waste stream. If a low molecular weight cationic material such
as a polyamine is to be added to the prirnary mixer, it is added ~om a second
chemical feed tank 22, the low molecular sationic material being fed into
zone 1, 12, of the prima~y mL~ng taDk :10 by conventional ~eed lirles 24 and
pumping means 26. A high molecular weight cationic material, for example
polyacrylaII~ide, is then added to zone 2, 14, of the pnmary ~g tank 10
and communicated with the pretrea~ed ef~luent by way of a ~g means 2~.
A third chemical feed ta~k 29, containing the high molecular weight cationic
2~7~7
......
6-
material communicates with zone 2, 14, by conventional feed lines 30 and
pumping means 31. ~he contents of zone 1 and 2 are :in direc~
communication with each other as there is no separation between the two
~ones at the lower end of zone 2.
Following the polyacrylamide's coagulation of the waste materials
~om the efEluent, the treated material is removed from ~he p~ y mixi~g
tank by conventional means through feed line 32 and added to a clarifier
referred to generally by reference numeral 3~!. The clarifieF provides means
of separating the treated ei~luent ~om the coagulated material or sludge.
The trea~ed eflluent is held in the cla~i~ing tank for 60 to 120 miIlutes to
facilitate the se~ling of the coagulated material. This material is then
removed by conventional means through ~eed line 36 into a sludge tank 38.
Mechanical methods for removing the coagulated resi~s and fat~r acids could
include primary settling or iloatation such as sludge blan~cet clarification,
foam or froth ~oatation, dissolved or induced air floatation. l~e cli~ed
e~uent is ialso removed by conventional means through feed line 4~ to the
final effluent tanlc 42.
F~ow rate of the eflluent, in the pilot plant described above, is 6 to
8 gallons per minute.
The examples which follow illustrate ~ypical results for treatment
of waste waters ~sing the present invention and are not to be construed as
limiting. The waste water sar[lples were taken from local pulp and paper
plants. Standard techniques were used for the chemical analyses of the
treated and ~mtreated effluent.
Exampl,ç_l
The waste water sample was from the Bowater Paper Mac~ine and
Spill Tank. The sample was treated by first adding addified sodium alginate
at a dose of 50 ppm followed by the addition of polyacrylamide at 25 ppm.
2~2~7
. ~
7-
Chemical analysis of the fatty acids remaining in the ef~luent after treatment
showed a 94.4 percent reduction in fatt3! acids, when compared to the
untreated effluent, as shown below in Table 1. ~ . :
,
T~ble l
Fa~ cid an~ly~i~be~ore an.d a~ter emuent treatment
FattD AcidTreated Ef~uent IJn~ted E~luent
Palmitic Acid 50 655
Oleic Acid 175 2940
Linoleic Acid 360 6~0
~nolenic Acid 23 343
Totals 608 10780
Percent Fatty Acid Reduction 94.4%
- 2~27~7
-8-
Table 2 shows the results of the resin acid concentration in the
untreated eflluent and concentration of resin acids after treatment, according
to the present invention. The results demonstrate a 96~o reduction in the
total resin acid concentration. ~
;
Table 2
Res;n Acid Analysis be~or~ ~nd ~fter e~ ent tr~ent
Resin~cid Treated E~luent IJn~E~h~ent
PLmaric Acid 56 800
Sandaracopimaric 127 2610
Neoabietic 22 3810
Dehydroabietic S10 8660
Abietic 720 14430
Palustric 34 5010
Isopimaric 256 5480
Totals 1730 4080
Percent Resin Acid Reduction 96%
ExampiQ2
In this example, samples were ~aken ~om Canadian Key Fibres
Sewer number 1 and number 2 and treated according to ~e protocol
discussed above. In this example, alginate was added at a dose of 250 ppm
~ollowed by the addition of polyacrylamide at a dose of 10 ppm. R~sin acid
concentrations, before and after treatme~t, aTe shown below in Tables 3 and
4 for the samples obtained from sewer 1 and 2, respectively. ~e percent
reduction in fatty acid concentration was not determined in this Exarllple.
212 7 ~ ~ ~
~.` g
Table 3
Resill Acid Anal~is o~ untreated ~nd treated e~luent ~m
sewer # 1
lRe~in ~cidTreated Emuent Un~eated Emuellt
Pimaric Acid 6 142
Sandaracopimaric 8 123
Neoabietic 9 101
Dehydroabietic 80 3090
Abietic 55 1110
Palustric 16 223 -~
IsopLmanc 31 . 289
Totals 2û5 5080
Percellt Resin Acid Reduction 96%
~kl~
lResin ~i~ Analyæis Q~ntr~Ated an~ trentçd ~f~ent f~o~
~e~er # 2
Resin Acidl~ated Efnuent UlltreatedE~uellt
Pimaric Acid 4 121
Sandaracopimaric 5 91
Neoabietic 3 58
Dehydroabietic 55 1580 :
Abietic 26 750
Palustric 4 148 ~ :
Isopimaric 13 228
Totals 110 2980
Percent Resin Acid iE~eduction 96.3%
` ` 2~7~7
. -. ` . . ;
. .,` ;
Example 3
In this example, waste water was obtained from Bowater TMP
Pressate and Wash Water. Duplica~e samples were obtained. Each sample
was further sub-divided eight times and valying concentrations of alginate, 19
ppm or sn ppm, polyamine, 40 ppm or 100 ppm, and polyacrylamide, S0 ppm
or 75 ppm, were added. l~e percent reduction of fatty acids ranged from
62~o to 96%. The percent reduction of resin acids ranged from 68% to 98%.
Four samples, run 1-8, run 2-8, run 1-6 and run 2-6 are described in more
detail. The dosage ~or these four samples was as follows. For run 1-8 and
run 2-8, alginate was added at a dose of S0 ppm, which was followed by
treatment vwith the low molecular weight cati~nic polymer polyamine at a
does of 100 ppm, which was then in turn followed by the addition of
polyacrylamide at a dose rate of 75 ppm. For run 1-6 and run 2-6, the
effluent was treated first with alginate at a dose of 19 ppm followed by
polyamine at 100 ppm and polyacrylarnide at 75 ppm. The resul~s from these
tests are shown in tables, which ~ollow. Table S shows the resin acid analysis
of the treated ef~uent and the untreated efrluent from run 1-6, demonstrating
a 93.3~o removal of resin acids.
Tahle S
l~esio Açid Analysjs Qf ~e~ted and untreated
from run 1-6
Resin Acid Treated Emuellt Untrea~ ent
Pimaric Acid 222 2960
Sanda~acopimaric 490 6360
Neoabietic 947 9540
Dehydroabietic 1210 8450
Abietic 19S0 46530 `
Palustric 140~ 170~0
Isopimaric 864 14740
_ _
Totals 7090 105670
Percent lResin Acid Reduction 93.3%
2~2,7~
-11-
Table 6 shows thP fat~ acid analysis of the untreated and treated
effluent of the same saTnple (run 1-6) demonstrating an 85.7% removal of
fatty acids.
T~ble 6
SFa~b Acid Anal~sis ~f tre~ted and untreated e~1uent
from r~n 1-6
Fae~ Acid Treated E~luent Unt~ Effluent
Palmitic Acid 70 450
Oleic Acid 217 1780
Iinoleic Acid 435 2780
Linolenic Acid 45 . 337
Totals 767 5350
Percent Fatt~ Acid Reduction 85.7%
Table 7 shows the resirl acid analysis of the uIltreated and treated
ei~luent ~om run 1~8 demonstrating a 97% reduction of total resin acids. ~ :
The corresponding fat~ acid analysis from this sample i5 shown in Table 8,
wherein the percentage of ~atty acid reduction was 95.85'o.
- 2~7~1~7
-12-
Tabl~ 7
Resin Aci~l Analysis of tre~ted and untr~ated effluent
Resin Acid ~eated E~fluentUntrea~edEDllueDlt
Pimaric Ac~d 94 2960
Sandalacopimaric 235 6360
Neoabietic 362 9540
Dehydroabietic 552 8450
Abietic 1~0 46530
PalustIic 548 17090
Isopimaric 4~ 14740
Totals 3210 105670
Percent Resin Acid Reduction 97%
~ .
lF~A~id ~alYsis Or tre~ed and un~nted Qfll~çnt
fr~m r~n 1~
Fatty Acid Treated Efl~uentUnheatedEflElue3llt
Palmitic Acid 24 450
Oleic Acid 66 1780
LinoleicAcid 127 2780 ~ :~
IinoleI~ic Acid 10 337
Totals 227 5350
Percent Fat~ Acid Reduction 9S.8~
Table 9 provides the results of the resin acid analysis of the treated
and un~reated ef~uent ~om run 2-6 and demoIIstrates a percent resini aciid
reduction of 9605~o.
:`~ 2~27~ ~
-13-
Table 9
Resin Acid Anal~ysis of treiated and lmtre~te~l e~luent
~rom r~la 2-6
~esin A~idTreated EmuentUnt~atedEi~iue~t
S Pirnaric Acid 98 3620
Sandaracopimaric 245 7590
Neoabietic 339 12æO
Dehydroabietic6B2 9710
Abietic 1740 40130
Palustric 38~ 19480
Isopimaric 438 18130
Totals 3930 110880
Percent Resin Acid Reduction 96.5%
Table 10 shows the corresponding fatty acid ianalysis, from run 2-6,
which demonstrates a 92% reduction ~n the treated effluellt according to the
present invention.
'
. . .~ . . .
2127 ~87
. .
- 14-
Table lO
Fatbt Acid Analysis of ~a~d ~nd ~ntr~t~.~luent
f~om run 2-6
Fat~ AcidTre~ted Ef~luent Unt~eat~ E~uent
Palmitic Acid 34 470
Oleic Acid 108 1540
Iinoleic Ac~d 200 2780
Linoleric Acid 22 300
Totals 3~ 5090
Percent Fat~ Acid Reduction 92.8~
Table 11 shows the res~n acid analysis of the untreated and treated
eilluent from run 2-8 demoIIstrating a 97.9% reduction of total resin acids.
15The co~responding fatty acid analysis from this sample is sh~wn in Table 12,
wherein the percentage of fa~ty acis1 reduction was 97~.
-rrable 11
R~in~Acid Analysi$ ~f tre~ nd untreatçd e~fluçnt
20i~om r~n 2~
Resin Acid'l'reated l~luent Untr~ted Emuent
PimaricAcid 18 3620
Sandaracopimanc 130 7590
Neoabietic 260 12æo
Dehydroabietic 371 9710 :
Abietic 888 40130
Palustric 386 19480
Isopimaric 249 18130
Totals 2340 110880
Percent Resin Acid Reduction 97.9%
2~27~7
.i :,.`:
-15-
T~b!e 12
F~t~ Acid AnallY~is of ~rea~ed and ~lntre~ed emuen~
~rom run 2-8
S lFat~ Acid Treated E~fluent Un~at~ El~l~t
Palmitic Acid 13 470
Oleic Acid 37 1540
Iinoleic Acid 96 2780
Linolenic Acid 12 300
Totals 158 5090
Percent Fatty Acid Reduction 97%
Ex~mple 4
The eflluent for this sample was the total mill effluent taken from
Canexel and the sample was treated according to the pilot plant, discussed
above and depicted in ~igure 1. In this example, alginate at a concentration
of 50 pprn was first added to the ef~uent after which polyamine was added
at a rate of 300 ppm followed by polyacrylamide at a rate of 47 ppm. This
dosage is considered to be a full dose and was used ~or the analysis in sample
2 and 3 shown in Table 13, which follows. A number modifications of the
concentration of chemicals used in the treatment of the Canexel mill ef~luent
were also performed. The modifications are as follows. In sample 4, no
2S polyac~ylamide was added. Sample 5 was treated with 50% dose nf the
acidffled sodium al~inate, i.e. 25 ppm instead of the 50 ppm. Sample 7 was
not treated with any al~ate and was trea~d with a 50% reduction in the
polyacrylamide. In sample 8, a 50% reduction oî algin~te was used. Th;s
sample therefore is similar to sample 5 noted above. Sample 9 used a 50%
reduction in alginate a~d in poly~ne.
2~27~
~ . .
- 1 6-
The results shou~ in Table 13 provide the amount of org~ic
materiial in the form of BOD (Biological O~ygen Demand), COD (Chemicial
~ygen Dema~d), DS (Dissolved Solids), SS (Suspended Solids), and TS
(To~ial Solids) and turbidity of ian untreated sample in comparison with the
sample treatments as described above. It is clear ~om Table 13 that a full
dose treatment of the compounds (alginate 50 ppm; polyamine 300 ppm; and
polyacrylamide 47 ppm), as shown in sample 2 and 3, provides ~or a 55-61%
reduction in BOD as compared to the untreated sample. These results also
are interesting in that sample 7, which does not contain any alginate iand only
the cationic polymers, shows 35% re:moval of BOD demonstrating an
improvement when alginate is used in a pretre~tment step.
2~27~8'~
. . .
;.-
o ~ oo ~- o ~`
W
:'
t~ t~ V' ~ ~ '
. ~; ~ ,
o
~ r~
o
~ o . ~ -
.
~ : ::
$ ~ * ~ 3 :~
~ o V) ~
2127~7
: '?`
:~` :```
.,j,~.
i~ i~
~D i'~
~ , ~
':i,.:
O
~ ~ ~ . ,' ~ .
o ~ o
!i~ :
~5
i~
i
i~
~ '.
o ~
~3
2~2~37
... ...
..... ~9
The results, of the analysis of resi~ acids and fatty acid, with sample
9, which uses a 50% dose of the alginate ~i.e. 25 ppm) and a 50~o dose of the
polyamine (i.e. 150 ppm), are shown in Tables 14 and 15, respectively. The
results are comparable with those discussed previously, wherein the resin acid
S is reduced by 98.4~o and the fatty acid is reducçd by 93.7%.
T~ble 14
Resin A~id An~lysis ~f~ ~r~ç~ ~n~l ~n~re~ted e~lu~nt
~-Ç~
l~e~in~cidll~ated l~ uent U~treat~lE:~luent
Pimaric AcidLess than 0.5 12
~andaracopimaric0.5 7.3
Neoabietic Less ~han 0.5 9.3
Dehydroabietic 5.1 266
Abietic 2.0 240
Palustric Less than 0.5 I~i~banO5
Isopimaric 1.6 125
Totals 10.7 660.1
Percent Resin Acid Reduction 98.4%
~ble 15
F~tty'~ A.nalysi$ ~ treated ~ n~reaç~1 e!i~lue
~rom C~ l Mill
F~t~cid Treated E~luent Untre&~ E~lu~t
Palmitic Acid 20 208
Oleic Acid 44 626
Iinoleic Acid 27 550
I~nolenic AciidLess than 0.5 73
Totals 91.5 1457
Percent Fatty Acid Reduction 93.7%
2~ ~,7~'7
.: ,;. ..
-20-
Ihe sample for this example was taken from the ef~uent from
Irving Paper Ltd. The sample was treated with the following ehemicals~
polyacrylamide, 10 ppm; polyamine, 5 ppm and gelatin 5 ppm. The results j,
are shown below ~n Table 16.
Table 16
AnalQ~is of tre~ted and untreated s~mple
Ulltreated Treated % R~
Suspended Solids (SS) 83.7 9 89 . .
BC)D 250 120 ~2
Dissolved Solids ~DS) 657 607 7.7 -
COD 625 382 39
Total Solids (TS) 717 ~06 15.S
~am~
In this example the sample was taken from the Bowaters Mersey
Paper Co. The sample was treated as follows: polyacrylamide, 20 ppm and
gelatin, 10 ppm. The results are showIl below in Table 17.
Tabl~ ~7
ntre.ated ~
Ulltreated Treated % Redllction ~ .
SS 186 3~ ~0
BOD 550 130 76.4
COD 1020 520 49
Example Z
~ this example the sample was tal~en from the Stora Forest
Indus~ies. The sample was treated as foll~ws: polyacrylamide, 15 ppm;
polyi~ine, 1S ppm andl gelati~, 10 ppm. The results ia~e shown below in :
Table 18.
2 1 2 7 ~ ~ 7
-2 1 -
Table 18
Anal~ ~ treated and untreated sample
IJnt~eated l~eatedl % Reduction
BOD 938 430 54
COD 2600 1240 53
SS ~7 10 90
DS 1836 1156 37
TS 2073 808.5 39
3[t is understood that the invention has been disclosed berein in
connection with certain examples and embodiments. However, such changes,
modifications or equivalents as can be used by those skilled in the art are
intended to be ~cluded. Accordingly, the disclosure is to be const ued as
exemplary, rather than limiting, and sueh changes within the principle of the
illvention as are obvious to one skilled in the art a~e intended to be included
with the scope of the claims.