Note: Descriptions are shown in the official language in which they were submitted.
2~ 7'~
PROPYLENE-BASED POLYMER, METHOD FOR ITS PRODUCTION,
COMPOSITION THEREOF, CATALYST COMPONENT FOR
POLYMERIZATION, AND METHOD FOR ITS PRODUCTION
Field of the Invention
The present invention relates to a propylene-based
polymer with excellent physical properties such as rigidity,
surface hardness, heat resistance, water vapor barrier ~
property, etc. which is suitable for use in automobiles,
consumer electric goods and packaging materials, to a method
for the production thereof,~to a composition containing it
and to a catalyst component for its polymerization and a
method for its production.
Backqround Art
Propylene-based polymers are generally inexpensive, and
exhibit characteristic properties including transparency,
mechanical strength, heat resistance, surface gloss, chemical
resistance, oil resistance, rigidity, flex clacking
resistance, etc., for which they thus have a wide range of
uses as industrial materials, food packaging materials,
cosmetic packaging materials, drug packaging materials, and
the like.
As mentioned above, propylene-based polymers exhibit
characteristics such as rigidity, impact resistance, etc.,
and are thus widely used in various production industries
including automobiles, consumer electric goods, miscellaneous
goods, and the like. Recently, producers are investigating
2i2'~
the prospect of making products thinner in order to render
them more light-weight and lower their cost, while increasing
the surface strength in order to prevent damage to the
surface thereof. That is, demand is increasing for
propylene-based polymers which have high rigidity, high
surface hardness and excellent impact resistance. Also,
demand has continued to increase for a higher level of
physical properties and workability, and particularly desired
are the maintaining of the rigidity and strength-at high
temperatures, durability, and the improvement of the
moldability of large-size moldings. - -
Regarding high rigidity and improved transparency andsurface gloss of propylene-based polymers, there have been
conventionally known methods which employ fillers such as Ia
and II~ group metal salts of monocarboxylic acids (for
example, sodium benzoate), III-IV group metal salts of
dicarboxylic acids (for example, adipic acid) and aliphatic
dicarboxylic acids (for example, aluminum adipate),
dibenzylidene sorbitol derivatives, talc and the like, as
nucleating agents (Japanese Examined Patent Publication
(KOKOKU) No. 39-1809, Japanese Unexamined Patènt Publication
(KOKAI) No. 60-139731, etc.), and methods which create a wide
distribution of the molecular weight of propylene-based
polymers (Japanese Unexamined Patent Publication (KOKAI)
Nos. 56-2307, 59-172507, and 62-195007, etc.).
However, although use of these nucleating agents results
in improvement in the aforementioned physical properties, it
212772i
cannot be said that they are necessarily sufficient for all
uses.
Consequently, it has been desired to obtain propylene-
based polymers suitable as materials for automobiles,
consumer electric goods and packaging materials, which have
excellent mechanical strength including impact resistance,
rigidity, etc. as well as surface hardness and heat
resistance, at the s-ame time lowering the density of the
products to render them more thin by reducing the~amount of
fillers such as talc and the like.
Furthermore, efforts are continuing to improve the
stereoregularity (isotacticity) of propylene-based polymers,
widen their molecular weight distribution, increase their
strength and durability which depend on the molecular weight
distribution, and improve the moldability in extrusion
molding, blow molding and the like.
Of these efforts, the development particularly of
catalysts with high activity and producing high isotacticity
are recently being ardently studied. All are catalyst
systems comprising a solid catalyst component containing
magnesium, titanium, a halogen and an electron-donating
compound as essential components, with an organoaluminum and
another electron-donating compound, and examples thereof are
disclosed in Japanese Unexamined Patent Publication (KOKAI)
Nos. 57-63310, 58-32604, 58-83006, 59-206408, 59-219311, 60-
130607, 61-209207, 61-211309, 62-72702, 62-104811, 62-11705,
63-199703, 63-264609, 1-126306, 1-311106, 3-62805, 3-70710,
Z~2772~
4-103604, 4-114009 and 4-202505.
The present inventors have also made recent disclosures
in this regard in Japanese Unexamined Patent Publication
(KOKAI) Nos. 4-43407, 4-149217, 4-178406, 4-180903, 4-185613,
4-198202, 4-198204, 5-9209 and 5-287019.
The propylene-based polymers disclosed in the preceding
-~ publications have a xylene-extraction insoluble portion of
-less than 99% and an isotactic pentad ratio (mmmm)~ of methyl
groups in the polypropylene of at most around 93-98%, as
measured by 13C nuclear magnetic resonance spectroscopy
(hereunder abbreviated to l3C-NMR), and thus there have been
limits to the improvement in the various physical properties
such as rigidity and heat resistance.
Disclosure of the Invention
The object of the present invention is to provide a
propylene-based polymer which has excellent rigidity, surface
hardness, heat resistance, transparency, surface gloss, water
vapor barrier property, etc., without any loss of the
original physical properties of propylene-based polymers,
which is suitable for use in automobiles, consumer electric
goods and packaging materials, and a method for its
production, as well as a composition containing it, a
catalyst component for its polymerization and a method for
the production thereof.
We the present inventors, as a result of a multitude of
research regarding methods of overcoming the above mentioned
problems, have discovered that the above mentioned problems
~Z Y'~
are overcome with a propylene-based polymer in which (1) the
xylene-extraction insoluble portion (XI) is 99.0 wt% or
greater, (2) the lsotactic pentad ratio (IP) is 98.0% or
greater as measured by l3C nuclear magnetic resonance
spectroscopy, (3) the isotactic average chain length (N) is
500 or greater, and (4) the total amount of each of the
fractions obtained by column separation of the xylene
insolubles~whose average chain length (Nf ) iS 800 or greater
accounts for 10 wt% or more of the entirety, and thus the
present invention has been completed.
Best Mode for Carr~inq Out the Invention
A concrete explanation of the characteristics of the
propylene-based polymer according to the present invention
will now be given.
(1) The xylene-extraction insoluble portion (XI) is the
percent by weight of the polymer which is insoluble in xylene
at 25C. Specifically, it is the percent by weight of the
polymer which is first dissolved in ortho-xylene at 135C and
then precipitated at 25C. The XI of the propylene-based
polymer of the present invention is 99.0~ or greater,
preferably 99.5% or greater, and more preferably 99.7% or
greater. If the XI is less than 99.0% then the polymer will
lack the desired rigidity, heat resistance, surface hardness,
surface gloss, transparency, water vapor barrier property,
etc.
(2) The isotactic pentad ratio of the polypropylene
molecular chain (hereunder sometimes abbreviated to IP) as
Z12772~
measured by l3C nuclear magnetic resonance spectroscopy is
determined according to the method of A. Zambelli,
Macromolecules, 6, 925, 1973. That is, it refers to the
isotacticity of the propylene-based polymer molecular chain
in pentad units, as measured using nuclear magnetic resonance
spectroscopy with isotopic carbon (l3C-NMR). The IP according
to the present invention is the measured value for the actual
polypropylene obtained by polymerization, and is not the
measured value for the polypropylene after the above
mentioned xylene extraction or other extraction, separation,
etc.
The classification of peaks was carried out based on the
revised edition of the above document, as described in
Macromolecules, 8, 687, 1975, and the IP was measured by the
proportion of the strength of the mmmm peaks out of the total
absorption peaks of the methyl carbons by l3C-NMR
spectroscopy.
The thus-measured IP of the propylene-based polymer must
be 98.0% or greater, because if it is lower than this value
the polymer will lack the desired rigidity, heat resistance,
surface hardness, surface gloss, transparency, water vapor
barrier property, etc. The IP of the propylene-based polymer
is preferably 98.5% or greater. A propylene-based polymer
with an IP of 99.0% or greater is particularly preferred.
(3) The isotactic average chain length (N) is the
isotactic average chain length of methyl groups in the
polypropylene molecule, and it may be calculated based on the
2~2772~
method reported by J.C. Randall (Polymer Sequence
Distribution, Academic Press, New York, 1977, chapter 2).
Specifically, the polypropylene is heated at a
temperature of 130C to dissolution in a mixed solvent of
1,2,4-trichlorobenzene/denterated benzene to make a polymer
concentration of 10 wt%.
This solution is placed in a glass sample tube having an
inside diameter of 10 mm~, and measured by 13C-NMR under the
same measuring conditions as for the isotactic pentad ratio
(IP) previously.
= We assume the definition of the 2-site model described
in "Shan-Nong Zhu, Xiao-Zhen Yang, Riichiro Chujo: Polymer
Journal, Vol.15, No.12, p.859-868, 1983", i.e. that two
active species are involved during polymerization. One of
the species is called catalyst-controlled polymerization and
the other is chain end-controlled polymerization. (Thé
details regarding catalyst-controlled polymerization and
chain end-controlled polymerization are described by Junji
Furukawa in Macromolecules: Essence and Topics 2,
"Macromolecular Synthesis", p.73, published by Kagaku Dojin,
KK., 1986).
The 2-site model may be categorized as follows:
~: Catalyst-controlled polymerization (enantiomorphic
process): probability of a D-body and an L-body
adding to the polymerization ends, that is, an
indication of the degree of disorder in an isotactic
component.
7~
~: Chain end-controlled polymerization (Bernoulli
process): Probability of forming a meso body by
addition of the same species as on the
polymerization end.~ -m~ r~
~: Proportion of ~ sites.
Homopolypropylene splits into 10 peaks of pentad units
due to the isotacticity of the methyl groups, but in order
- for the actual measured value to agree with the calculated
strength (area), ~, ~ and ~ are calculated by the least
square method, and based thereon the amounts Al-Alo of each of
- the pentad units are determined by the following equations.
Meso body Al = mmmm = ~(1-5~+5~2) + (l-~) a4
A2 = mmmr = ~(2~-6~2) + 2(1-~)~3(1-~)
A3 = rmmr = ~2 + (1_~)~2(1_~)2
A4 = mmrr = ~(2~-6~2) + 2(1-~)~2(1-~)2
A5 = mmrm = 2~2 + 2(1-~)~3(1-~)
A6 = rmrr = 2~2 + 2(1-~)~(1-a)3
Racemic A7 = rmrm = 2~2 + 2(1-~)~2(1-~)2
structure
A8 = rrrr = ~2 + 2(1-~) (1-~)4
A~ = mrrr = 2~2 + 2(1-~ )3
Alo= mrrm = ~(~_ 3~2 ) + ( ~ 2 ( 1 _~ ) 2
13 = (Y ( l--~)
Next, when the definitional equation for the average
chain length (N)
N = number of meso chains/number of meso units
2~277~
described in the aforementioned document by J.C. Randall is
applied to each of the pentad units A~-A7 calculated above, it
may be calculated by
N = Al+A2+A3+0-5(A4+ A5 +~ +~
o . 5 (A4 +A5 +A6 +A7 )
The N value according to the present invention is thcl~" ~,
measured value for the actual polypropylene obtained by
polymerization, and it is not the measured value of the
polypropylene after the above mentioned xylene extraction or
other extraction, separation, etc. N for the isotactic
propylene-based polymer of the present invention is 500 or
greater, preferably 700 or greater, and more preferably 800
or greater. If N is less than 500 then the polymer will lack
the desired rigidity, heat resistance, etc.
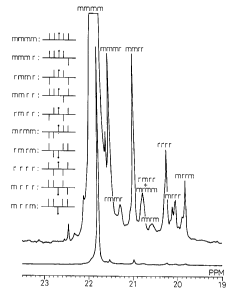
In general, the l3C-NMR signal of polypropylene gives
three major peaks for methylene, methine and methyl.
When the peak of the methyl regions is magnified, the
data shown in Fig. 1 is obtained, showing an asymmetrical
bond form of --mmmmrmmmm---, ...""", "",r. IIUIUIUIUU~ etc.
The crystallizable isotactic average chain length may be
considered as having a inverse relationship with the
asymmetrical bonds.
A larger number of asymmetrical bonds, i.e. more
racemic structures in between the mmmm structures, will
shorten the average chain length (N).
As mentioned above, the average chain length (N)
21~
calculated in this manner gives the length of the sequence of
the crystallizable isotactic structures, and thus it may be
understood that as this length becomes greater (fewer
asymmetrical bonds), the physical properties of the
propylene-based polymer, such as rigidity, heat resistance,
water vapor barrier property, etc. will improve.
(4) The average chain length of the fractions (Nf)
obtained by column separation of the xylene insolubles is
found by first dissolving the xylene-extraction insoluble
portion of the polypropylene obtained in (1) above in para-
xylene at a temperature of 130C, adding celite~thereto,
lowering the temperature to 30C at a temperature-lowering
rate of 10C/hour to deposit the celite, filling a column
therewith, raising the temperature from 70C to 130C by
2.5C at a time, separating off the fractions, and then
calculating the average chain length (N) of each of the
separated fractions by the aforementioned method as the
average chain length for each fraction (Nf).
In the propylene-based polymer according to the present
invention, the total amount of each of the fractions obtained
by column separation of the xylene insolubles whose average
chain length (Nf) is 800 or greater accounts for 10 wt% or
more of the entirety. It preferably accounts for 30 wt% or
more, and more preferably 50 wt% or more.
If the total amount of fractions with an average chain
length (Nf) of 800 or greater accounts for less than 10 wt%
of the entirety, then there will be less of the desired
2~7721
-
improving effect on the rigidity, surface hardness, heat
resistance and water vapor barrier property.
An explanation will now be given in regard to the method
of production of a propylene-based polymer according to the
present invention.
The propylene-based polymer according to the present
invention may be produced by the polymerization of propylene
using a polymerization catalyst which contains (A) a solid
catalyst component for polymerization having as essential
components a magnesium compound, titanium compound,
halogenous compound and a fir-st electron-donating compound,~
wherein the molar ratio of the first electron-donating
compound and the titanium atom content (D/T) incorporated
into the solid catalyst component for polymerization is such
that D/T > l; (B) an organoaluminum compound; and (C) a
second electron-donating compound.
Here, the magnesium compound may be exempli~ied by
magnesium halides such as magnesium dichloride, magnesium
dibromide and magnesium diiodide; alkoxy magnesiums such as
dimethoxy magnesium, diethoxy magnesium, dipropoxy magnesium,
dibutoxy magnesium and diphenoxy magnesium; carboxylates such
as magnesium laurate, magnesium stearate and magnesium
acetate; and alkyl magnesiums such as diethyl magnesium and
butylethyl magnesium. These various magnesium compounds may
be used either alone or with 2 or more thereof in
combination. Preferably, a magnesium halide or alkoxy
magnesium is used, or a magnesium halide is formed during
11
21~ 7'~'.~1.
formation of the catalyst. The above mentioned halogen is
most preferably chlorine.
The titanium compound may be exemplified by titanium
halides such as titanium tetrachloride, titanium trichloride,
titanium tetrabromide and titanium tetraiodide; alkoxy
titanium compounds such as tetramethoxy titanium, tetraethoxy
titanium, tetrapropoxy titanium, tetrabutoxy titanium and
tetraphenoxy titanium; and alkoxy titanium halides such as
ethoxy titanium chloride, butoxy titanium chloride, phenoxy
titanium chloride, dibutoxy titanium chloride and tributoxy
titanium;chloride, etc. These various titanium compounds may
be used either alone or with 2 or more thereof in
combination. Preferably, a halogenous tetravalent titanium
compound is used, and more preferably titanium tetrachloride.
The halogen in the halogenous compound is fluorine,
chlorine, bromine~or iodine, preferably chlorine, and
specific compounds as actual examples will depend on the
method of preparing the catalyst component; however, titanium
halides such as titanium tetrachloride, titanium
tetrabromide, etc.; silicon halides such as silicon
tetrachloride, silicon tetrabromide; and phosphorous halides
such as phosphorous trichloride and phosphorous pentachloride
may be given as examples, and depending on the method of
preparing the catalyst component a halogenated hydrocarbon,
halogen molecule or hydrohalogenic acid may also be used.
As the first electron-donating compound there may be
mentioned in general oxygen-containing compounds, nitrogen-
12
2t ~7~
containing compounds, phosphide-containing compounds, sulfur-
containing compounds, etc. Oxygen-containing compounds
include, for example, alcohols, ethers, esters, acid halides,
acid anhydrides, etc.
More specifically, there may be mentioned alcohols such
as methyl alcohol, ethyl alcohol, propyl alcohol, butyl
-alcohol, pentyl alcohol, hexyl alcohol, heptyl alcohol, octyl
alcohol, nonyl alcohol, decyl alcohol, 2-ethyl alcohol, oleyl .
alcohol, benzyl alcohol, phenylethyl alcohol, phenol, cresol,
ethyl phenol and naphthol;
ethers and diethers such as methyl ether, ethyl ether,
propyl ether, butyl ether, amyl ether, hexyl ether,
tetrahydrofuran, anisole and diphenyl ether;
esters such as ethyl acetate, ethyl chloracetate, ethyl
propionate, ethyl butyrate, ethyl acrylate, ethyl crotonate,
ethyl oleate, ethyl stearate, ethyl phenylacetate, methyl
benzoate, ethyl benzoate, propyl benzoate, butyl benzoate,
methyl toluate, ethyl toluate, propyl toluate, butyl toluate,
methyl ethylbenzoate, methyl anisate, ethyl anisate, methyl
ethoxybenzoate, ethyl ethoxybenzoate, ethyl cinnamate,
dimethyl phthalate, diethyl phthalate, dipropyl phthalate,
di-n-butyl phthalate, diisobutyl phthalate, dihexyl
phthalate, dioctyl phthalate, y-butyrolactone, ~-
valerolactone and ethylene carbonate;
acid chlorides such as acetyl chloride, benzoyl
chloride, toluic acid chloride and phthalic acid chloride;
and
13
21.~! Y7~
-
acid anhydrides such as maleic anhydride, phthalic
anhydride, etc.
These first electron-donating compounds may be used
either alone or with 2 or more thereof in combination.
Preferably an ester, and more preferably a phthalic acid
ester, is used.
- Obviously, a single compound may contain 2 or more of
the four compounds including the magnesium compound, titanium
compound, halogen compound and first electron-donating
compound.
The amounts of the above mentioned components to be used
may be as desired so long as the effect of the present
invention is provided, but generally the following ranges are
preferred.
The amount of the titanium compound to be used may be at
a molar ratio in the range of 0.0001-1000, and preferably in
the range of 0.01-100, with respect to the amount of the
magnesium compound used. A halogen compound is used as
necessary, in which case it is used at a molar ratio in the
range of 0.01-1000, and preferably in the range of 0.1-100,
with respect to the amount of magnesium used, regardless of
whether or not a halogen is contained in the titanium
compound or magnesium compound. The amount of the first
electron-donating compound to be used is at a molar ratio in
the range of 0.001-10, and preferably in the range of 0.01-5,
with respect to the amount of the above mentioned magnesium
compound used.
14
- 21;27721.
The method of preparing the solid catalyst component to
be used according to the present invention may be any method
of preparing conventional solid catalyst components which are
obtained by contacting and reacting at once or in stages a
magnesium compound, a titanium compound and a first electron-
donating compound, and also if necessary a halogenous
compound as an auxiliary.
As specific examples of known preparation methods there
may be mentioned the following.
(1) A method in which a magnesium halide, and if
necessary a first electron-donating compound and a titanium
compound are contacted together.
(2) A method in which a solid component obtained by
contacting a magnesium halide, tetraalkoxy titanium and a
specific polymeric silicon compound, is contacted with a
halogenated titanium compound and/or a halogenated silicon
compound.
(3) A method in which a solid component obtained by
dissolution of a magnesium compound with tetraalkoxy titanium
and a first electron-donating compound, followed by
precipitation with a halide agent or halogenated titanium
compound, is contacted with a titanium compound.
(4) A method in which alumina or magnesia is treated
with a halogenated phosphorous compound and the product is
contacted with a magnesium halide, a first electron-donating
compound and a halogenated titanium compound.
(S) A method in which an organomagnesium compound,
2~'7'~
represented by Grignard reagents, is subjected to the action
of a reducing agent or halogenating agent, and then contacted
with a first electron-donating compound and a titanium
compound.
(6) A method in which an alkoxy magnesium compound is
contacted with a halogenating agent and/or a titanium
compound in the presence or absence of a first electron-
donating compound.
(7) A method in which a magnesium compound is dissolved
with tetraalkoxy titanium and treated with a polymeric
silicon compound, and then treated with a halogenated silicon
compound and an organometallic compound.
(8) A method in which a globular magnesium compound/
alcohol chelate is treated with a first electron-donating
compound and a halogenated titanium compound, etc.
Any of the above mentioned methods of preparing a solid
catalyst component may be employed for the production of a
propylene-based polymer according to the present invention,
but it must be a solid catalyst component for polymerization
wherein the molar ratio of the first electron-donating
compound and the titanium atom content (D/T) incorporated in
the solid catalyst component is at least such that D/T 2 1.
Here, it is more preferable if D/T ' 1.5.
If D/T < 1 it will be difficult to obtain the highly
isotactic propylene-based polymer of the present invention.
Thus, according to the present invention there is
provided a solid catalyst component for ~-olefin
16
Z~-,2'77~
polymerization whose essential components are a magnesium
compound, a titanium compound, a halogen compound and a first
electron-donating compound, wherein the molar ratio (D/T) of
the first electron-donating compound (D) and titanium (T)
contained in the solid catalyst component is such that D/T 2
1. This solid catalyst component was developed for the above
mentioned production of polypropylene with a high degree of
isotacticity, but it is also useful as a solid catalyst
component for common polymerization of propylene-based
polymers in general or of a-olefins other than propylene-
based polymers. Particularly, in order to obtain propylene-
based polymers with a high degree of isotacticity and the
demanded rigidity and heat resistance, D/T is preferably 1.5
or greater.
Also, in the case of solid catalyst components which do
not meet the above condition (D/T 2 1) when prepared by
conventional methods, they may be made to meet the above
conditions by further treatment as indicated below.
In such cases, the molar ratio of the first electron-
donating compound and titanium atom content in the solid
catalyst component prior to the further treatment for
modification (D/T)i and the molar ratio of the first
electron-donating compound and titanium atom content in the
modified catalyst component (D/T)m must have a relationship
such that (D/T)m/(D/T)i > 1, and it is more preferable if
(D/T)m/(D/T)i 2 2.
For example, a solid catalyst component prepared by any
17
212772~
of the above mentioned known methods, whose essential
components are magnesium, titanium, a halogen and a first
electron-donating compound may be further treated with a
first electron-donating compound and/or a halogenous compound
to increase D/T above its value prior to the treatment, and
thus modify the catalyst. The order and frequency of
treatment with the first electron-donating compound and
halogenous compound are not particularly restricted, but in
generally used methods of treating a solid catalyst
component, it is first treated with a first electron-donating
compound for incorporation and then treated and cleaned with
a halogenous compound, and then further washed with a
hydrocarbon.
The first electron-donating compound to be used for
modification of the catalyst component may be the same as or
different from the one used during the preparation of the
solid catalyst component prior to the further treatment. The
first electron-donating compound may be a single species or 2
or more used in combination. Preferred for use are esters,
and particularly phthalic acid esters.
The amount of the first electron-donating compound to be
used is in the range of 0.001-500 moles, and preferably in
the range of 0.01-50 moles, with respec~ to the titanium
atoms in the solid catalyst component.
If the amount of the first electron-donating compound is
extremely low, then it will be difficult to achieve the
relationship (D/T)m/(D/T)i ~ l, while if it is extremely high
18
the polymerization activity will be lowered, and thus neither
condition is desirable.
The halogenous compound to be used for modification of
the catalyst may be the same as or different from the one
used during the preparation of the solid catalyst component
prior to the further treatment. Of these, titanium halides,
silicon halides and halogenated hydrocarbons are preferred.
The halogenous compound may be a single species or 2 or more
used in combination.
The amount of the halogenous compound to be used is in
the range of a molar ratio of 0.1-10,000, preferably in the
range of a molar ratio of 1-3000, and more preferably in the
range of a molar ratio of 5-500, with respect to the titanium
atoms in the solid catalyst component. If the amount of the
halogenous compound is extremely low, then it will be
difficult to achieve the relationship (D/T)~/(D/T)L > l, while
if it is extremely high the polymerization activity will be
lowered and the amount of waste solution will increase, and
thus neither condition is desirable.
The treatment of the solid catalyst component with the
first electron-donating compound for its modification is
carried out at a temperature in the range of -30 to 150C,
and preferably 0 to 100C. Also, the treatment of the solid
catalyst component with the halogenous compound is carried
out at a temperature in the range of 0 to 200C, and
preferably 50 to 150C. Temperatures outside of this range
are not preferred since the polymerization activity will be
19
Z~7~
-
lowered as a result.
The treatment for modification of the solid catalyst
component using the first electron-donating compound and the
halogenous compound may normally be effected in a hydrocarbon
solvent. The hydrocarbon to be used here is preferably an
inert hydrocarbon, for example, an aliphatic hydrocarbon such
as pentane, hexane, heptane, octanel decane, etc. or an
aromatic hydrocarbon such as benzene, toluene, xylene, etc.
Any of these hydrocarbons may also be used as a solvent for
washing the solid catalyst component after its treatment with
the first electron-donating compound and the halogenous
compound.
The washing of the modified catalyst for olefin
polymerization using the above mentioned hydrocarbon, after
the unmodified solid catalyst component is treated with the
first electron-donating compound and washed with the
halogenous compound, is carried out at a temperature in the
range of 0-100C, and preferably 60-140C. Here, if the
washing temperature is extremely low then it will be
difficult to achieve the relationship (D/T)~/(D/T)i > 1, while
if it is extremely high the relationship (D/T)~/(D/T)i > 1
will be achieved but the polymerization activity will be
lowered, and thus neither condition is preferred.
If the solid catalyst component is not treated (washed)
with a halogenous compound after its treatment with a first
electron-donating compound, then the polymerization activity
will be greatly lowered and the effect of the present
21~ Y7~
invention will not be exhibited. The frequency of treatment
(washing) with the halogenous compound is not particularly
restricted, but for the effect of the present invention to be
adequately exhibited it should be carried out 2 to 4 times.
One washing will not adequately exhibit the effect of the
present invention, while too much washing will lower the
polymerization activity, and thus neither is desirable.
Also, according to the present invention, the first
electron-donating compound may be a titanium compound
represented by the general formula TiXa-Yb (where X is a
halogen atom such as Cl, Br or I; a is 3 or 4; Y is an
electron-donating compound (l); and 0 ~ b ~ 3), and after the
treatment of the catalyst component for the incorporation,
washing is effected with a halogenous compound and again with
a hydrocarbon, so that the solid catalyst component is
improved to where D/T 2 1. Thus, when treatment of a solid
catalyst component is effected using a first electron-
donating compound, the frequency of treatment (washing) with
a halogenous compound according to the present invention must
generally be at least twice, as mentioned previously;
however, if TiXa-Yb is used, the effect of the present
invention will be adequately exhibited with a frequency of
treatment (washing) with the halogenous compound of 1-2
times. Furthermore, as mentioned later, since the amount of
the halogenous compound to be used may be reduced, it is
possible to largely reduce the amount of waste solution
discharged during washing of the modified solid catalyst
21
z~7~
component with the hydrocarbon.
TiXa (where X is a halogen atom such as Cl, Br or I and
a is 3 or 4) is generally known to readily form chelates with
electron-donating compounds, as described in, for example,
R.S.P. Coutts, P.C. Wailes, Advan. Organometal. Chem., 9,
135, 1970; Shinjikken Kagaku Koza, 4th edition, 17, Inorganic
Complexes/Chelated Complexes, Nihon Kagakukai Maruzen, 1991,
p.35; H.K. Kakkoen, J.Pursiainen, T.A. Pkkanen, M. Ahlgren,
E. Iiskola, J. Organomet. Chem., 453, 175, 1993; etc.
The X of TiXa-Yb is a halogen atom such as Cl, Br or I,
and of these Cl is preferred. The letter "a' is 3 or 4, and
preferably 4. Y (the first electron-donating compound) may
be selected from the ones mentioned earlier, and it may be
the same as or different from the one used during the
preparation of the unmodified solid catalyst component. When
preparing TiXa-Yb, the first electron-donating compound used
may be a single species or 2 or more in combination. Y is
preferably an ester, and more preferably a phthalic acid
ester. The molar ratio for the addition of Y to TiXa during
the preparation of TiXa-Yb is such that when the above
mentioned "a' is 3 then "b" of Y is 0 < b ~ 3, and when "a"
is 4, 0 < b S 2, and thus the number of electron-donating
groups of Y depends on the atomic valence of Ti. Most
preferably, a is 4 and b is 1.
The amount of TiXa-Yb to be used is at a molar ratio in
the range of 0.001-500, preferably a molar ratio in the range
of 0.01-50, and more preferably a molar ratio in the range of
22
21~77~1.
,
0.1-10, with respect to the titanium atoms in the solid
catalyst component prior to its modification. If the amount
of the TiXa-Yb used is extremely small, then it will be
difficult to achieve the relationship (D/T)m/(D/T)i > 1, while
if it is extremely large the polymerization activity will be
lowered, and thus neither condition is preferred.
The amount of the halogenous compound to be used may be
at a molar ratio in the range of 0.1 to 1000, preferably a
molar ratio in the range of 1 to SOO, more preferably at a
molar ratio in the range of 5 to 100, with respect to the
titanium atoms in the solid catalyst component.
Also, the selection of the halogenous compound may be
made in the same manner as described above.
Furthermore, the temperature for treatment of the solid
catalyst component with TiXa-Yb may be the same as the above
temperature for treatment with the first electron-donating
compound, and the temperature for washing of the solid
catalyst component with the halogenous compound may be the
same as mentioned above.
The treatment of the solid catalyst component with
TiXa-Yb and the washing with the halogenous compound is the
same as the treatment with the first electron-donating
compound and washing with the halogenous compound described
above.
There are no particular restrictions on the frequency of
treatment using TiXa-Yb and of washing with the halogenous
compound, but as mentioned above the effect of the present
23
- 2~ Y7~1.
invention will be adequately exhibited with a single or twice
washing by a halogenous compound after treatment with TiXa-Yb.
If washing is not effected with a halogenous compound, then
the outstanding properties according to the present invention
will not be obtained.
Prepolymerization
The modified solid catalyst component prepared in the
manner described above is used for the polymerization of
propylene by its combination with an organoaluminurn compound
and a second electron-donating compound, described below;
however, a small amount of the monomer may be prepolymerized
prior to the polymerization. This is usually about 0.01 -
1000 g per gram of the modified solid catalyst component
prepared in advance, and the temperature of prepolymerization
may be as desired between -30 and 80C. The
prepolymerization is usually carried out in the presence of
an organoaluminum compound and a second electron-donating
compound to be used in the polymerization described
hereunder. The prepolymerization may be generally carried
out in an inert hydrocarbon solvent, but it may also be
carried out in a liquid monomer, gaseous monomer, etc.
The monomer to be used for prepolymerization may be
propylene or, for example, an ~-olefin such as ethylene, 1-
butene, 3-methyl-1-butene, 3-methyl-1-pentene, 4-methyl-1-
pentene, ~,4-dimethyl-1-pentene, vinyl cyclopentane, vinyl
cyclohexane, etc.; styrene or a styrene derivative such as a-
methylstyrene, etc.; a diene such as butadiene, 1,9-
24
~1~7721
_
decadiene, etc.; or an allyltrialkylsilane. These monomersneed not be used alone, as two or more thereof may be used in
stages or in admixture. Hydrogen may be used as the
molecular weight modifier for the prepolymerization.
Propylene polymerization
The modified solid catalyst component mentioned above
may be used to polymerize a propylene-based polymer in the
presence of an organoaluminum compound and a second electron-
donating compound.
The organoaluminum compound to be used according to the
present invention may be exemplified by trialkylaluminum~
compounds such as trimethylaluminum, triethylaluminum,
tripropylaluminum, tributylaluminum, trihexylaluminum and
trioctylaluminum; alkylaluminum hydrides such as
dimethylaluminum hydride, diethylaluminum hydride and
dibutylaluminum hydride; alkylaluminum halides such as
dimethylaluminum chloride, diethylaluminum chloride,
diethylaluminum bromide and ethylaluminum sesquichloride;
alkylaluminum alkoxides such as diethylaluminum ethoxide and
diethylaluminum phenoxide; and aluminoxanes such as methyl
aluminoxane, ethyl aluminoxane and propyl aluminoxane. These
organoaluminum compounds may be used alone or with 2 or more
thereof in combination. Preferably, a trialkylaluminum
compound is used.
The second electron-donating compound to be used
according to the present invention may be the same as or
different from the first electron-donating compound, but as
21-~7~
-
representative examples thereof there may be mentioned
aromatic carboxylic acid esters, silicon compounds having an
Si-O-C or Si-N-C bond, acetal compounds, germanium compounds
having a Ge-O-C bond, and nitrogenous or oxygenous
heterocyclic compounds having an alkyl-substituted group.
Specific examples of these compounds include aromatic
carboxylic acid esters such as ethyl benzoate, ethyl p-
toluate and ethyl p-anisate; silicon compounds such as phenyl
trimethoxysilane, diphenyl methoxysilane, di-n-propyl
dimethoxysilane, di-i-propyl dimethoxysilane, di-t-butyl
dimethoxysilane, dicyclohexyl dimethoxysilane, dicyclopentyl
dimethoxysilane, cyclohexylmethyl dimethoxysilane, t-butyl
trimethoxysilane, cyclohexyl trimethoxysilane, thexyl
trimethoxysilane, tetramethoxysilane and tetraethoxysilane;
acetal compounds such as benzophenone dimethoxyacetal,
benzophenone diethoxyacetal, acetophenone dimethoxyacetal and
acetophenone diethoxyacetal; germanium compounds such as
diphenyldimethoxy germanium and phenyltriethoxy germanium;
and heterocyclic compounds such as 2,2,6,6-
tetramethylpiperidine and 2,2,6,6-tetramethylpyran.
These electron-donating compounds may be used alone or
with 2 or more thereof in combination. Preferably, a silicon
compound or acetal compound is used, and more preferably a
silicon compound having an Si-O-C bond is used.
The polymerization method for the production according
to the present invention is not particularly restricted and
may be a known one, including liquid phase polymerization
26
Z~2'Y'7~.
such as slurry polymerization or bulk polymerization, and
vapor phase polymerization. In addition, batch
polymerization, as well as method employing continuous
polymerization and batch polymerization may be applied. The
polymerization solvent to be used for slurry polymerization
is a saturated aliphatic or aromatic hydrocarbon such as
hexane, heptane, cyclohexane, toluene, etc., either alone or
as a mixture. The polymerization method in the production
according to the present invention may also be used for
multi-stage polymerization in 2 or more polymerization
reactors.
The polymerization temperature is about -50C to 200C,
and preferably 20 to 150C, and the polymerization pressure
is from atmospheric pressure to 100 kg/cm2 G, and preferably
3-50 kg/cm2 G. Also, the molecular weight may be controlled
by the addition of an appropriate amount of hydrogen during
the polymerization.
In addition to homopolymerization of propylene in the
production according to the present invention, propylene may
be copolymerized with an ~-olefin represented by the general
formula R-CH=CH2 (where R is hydrogen or a hydrocarbon
residue of 1-20 carbon atoms, which may be branched).
Specific examples thereof include ethylene, l-butene, 3-
methyl-l-butene, 3-methyl-1-pentene, 4-methyl-1-pentene, 4,4-
dimethyl-l-pentene, vinyl cyclopentane, vinyl cyclohexane,
etc. Further examples include styrene and styrene
derivatives such as ~-methylstyrene, dienes such as
27
21~7~
butadiene, 1,5-hexadiene, 1,7-octadiene, 1,9-decadiene, etc.
and allyltrialkylsilanes. These monomers need not be used
alone, as two or more thereof may be used in admixture.
Of the propylene-based polymers according to the present
invention, propylene-ethylene block copolymers may be
produced by multi-stage polymerization in 2 or more
polymerization reactors, and here it is particularly
preferable to produce homopoLypropylene in the first stage.
In this case, if the homopolypropylene procured after
completion of the first stage polymerization is made to meet
the structural conditions for the present invention, then the
finally obtained copolymer will overcome the problems aimed
for by the present invention and will have the resulting
properties.
In addition, the propylene-based polymer obtained
according to the present invention may be made into a resin
composition with further improved crystallinity and high-
speed moldability by the addition of a publicly known
nucleating agent.
Examples of nucleating agents include fillers such as Ia
and IIa group metal salts of monocarboxylic acids (for
example, sodium benzoate), III-IV group metal salts of
dicarboxylic acids (adipic acid) and aliphatic dicarboxylic
acids (for example, aluminum p-t-butylbenzoate),
dibenzylidene sorbitol derivatives, talc and the like.
Particularly preferred are 1,3,2,4-dibenzylidene
sorbitol, 1,3,2,4-di-(p-methylbenzylidene) sorbitol, 1,3,2,4-
28
Z1277Z~.
-
di-(p-ethylbenzylidene) sorbitol, l,3,2,4-di-(p-
chlorbenzylidene) sorbitol, 1,3-p-chlorbenzylidene-2,4,-p-
methylbenzylidene sorbitol, sodium-bis-(4-t-butylphenyl)
phosphate, sodium-2,2-methylene-bis-(4,4-di-t-butylphenyl)
phosphate, sodium-2-2'-ethylidene-bis-(4,6-di-t-butylphenyl)
phosphate, etc, and inorganic fillers such as talk, calcium
carbonate.
The effect of the present invention will be quite
satisfactory if the amount of the nucleating agent used is in
a proportion in the range of at least 0.05-15 wt% in the
propylene-based polymer.
It is preferably added in an amount of 0.08-0.8 wt%, and
more preferably 0.1-0.5 wt%. However, since inorganic
compounds such as talc have less of a nucleating effect than
the nucleating agents listed above, they should be added in
an amount of 1-15 wt~, preferably 2-13 wt%, and more
preferably 5-10 wt%.
Other additives (for example, antioxidants,
weatherability stabilizers, antistatic agents, lubricating
agents, blocking inhibitors, anti-clouding agents, dyes,
pigments, oils, waxes, etc.) commonly used for thermoplastic
resins may be appropriately combined with the propylene-based
polymer or resin composition according to the present
invention, so long as the object of the present invention is
not impeded.
Examples of such additives include antioxidants such as
2,5-di-t-butylhydroxyquinone), 2,6-di-t-butyl-p-cresol, 4,4-
29
Z1~77~
thiobis-(6-t-butylphenol), 2,2-methylene-bis(4-methyl-6-t-
butylphenol), octadecyl-3-(3',5'-di-t-butyl-l'-hydroxyphenyl)
propionate and 4,4'-thiobis(6-butylphenol); ultraviolet
absorbers such as ethyl-2-cyano-3,3-diphenylacrylate, 2-(2'-
hydroxy-5-methylphenyl) benzotriazole and 2-hydroxy-4-
octoxybenzophenone; plasticizers such as dimethyl phthalate,
diethyl phthalate, wax, liquid paraffin and phosphate esters;
antistatic agents such as monostearate, sorbitan
monopalmitate, sulfated oleic acid, polyethylene oxide and
carbon wax; lubricating agents such as ethylene bis-
stearomide, butyl stearate, etc.; coloring agents such as
carbon black, phthalocyanine, quinacridone, indoline, azo-
based pigments, titanium oxide, iron oxide red, etc., and
fillers such as glass fiber, asbestos, mica, pallastonite,
calcium silicate, aluminum silicate, etc. In addition, many
other high molecular compounds may be blended in therewith so
long as the effect of the present invention is not impaired.
The melt index (MFR-JIS-7210, Table 1, condition 14) of
the propylene-based polymer according to the present
invention is not particularly restricted and may be selected
depending on the molding method and the intended use, but a
suitable range is usually 0.1-500 g/10 minutes.
The propylene-based polymer according to the present
invention may be molded into an injection molded body, form,
sheet, tube, bottle or the like using a publicly known fusion
molding method or compression molding method, and it may be
used alone or laminated with other materials.
2~77~1.
For example, such methods of lamination include so-
called dry laminate molding and sandwich lamination, wherein
a polyurethane, polyester or other based of dry laminate
adhesive is used to laminate another thermoplastic resin
layer onto the monolayer of the propylene-based polymer or
resin composition according to the present invention, or
alternatively, a co-extrusion lamination method, co-extrusion
method (feed-block method, multimanifold system), co- .
injection molding method or co-extrusion pipe molding method
may be employed.
The multi-layered laminated body obtained in this manner
may then be employed in a method which subjects it to a
reheating and drawing process using a vacuum molding machine,
compression molding machine or drawing blow machine, etc. or
the multi-layered laminated body or monolayered molding may
be subjected to a thermal drawing process using a single-
screw or twin-screw drawing machine.
Brief Description of the Drawinq
Fig. 1 is an example of a 13C-NMR spectral chart of the
methyl regions of homopolypropylene.
A more detailed description of the present invention
will now be provided with reference to the Examples.
Examples
The methods and equipment used to measure each of the
physical property values according to the present invention
are indicated below.
(l) Xylene insoluble portion (XI)
31
21~ Y'7~1.
A 2.5 g portion of the polymer was dissolved in ortho-
xylene (250 ml) at 135C, and the amount of the polymer (wt%)
which precipitated at 25C was determined as the xylene
insoluble portion (XI).
(2) Isotactic pentad ratio (mmmm)
The mmmm ratio is the isotactic ratio of pentad units of
methyl groups in the molecular chain of the propylene-based
polymer. The measurement was performed using a JNM-GSX400 .
(l3C nuclear resonance frequency of 100 MHz), product of Nihon
Denshi, KK. Each of the signals was classified according to
A. Zambelli et al., Macromolecules, 13, 267, 1980. The
measuring conditions were as follows.
Measurement mode: Proton decoupling method
Pulse width: 8.0 ~s
Pulse repetition time: 3.0 ~s
Integration frequency: 20,000 times
Solvent: Mixed solvent of 1,2,4-
trichlorobenzene/deuterated
benzene (75/25 vol%)
Internal standard: Hexamethyldisiloxane
Sample concentration: 300 mg/3.0 ml solvent
Measuring temperature: 120C
(3) Isotactic average chain length (N)
The isotactic average chain length (N) is calculated
based on the method reported by J.C. Randall (Polymer
Sequence Distribution, Academic Press, New York 1977, chapter
32
Z~27721.
2). Specifically, polypropylene is heated at 130C to
dissolution in a mixed solvent of 1,2,4-trichlorobenzene/
deuterated benzene, to a polymer concentration of 10 wt%.
This solution is placed in a glass sample tube having an
inside diameter of 10 mm~, and measured by l3C-NMR under the
same measuring conditions as above for the isotactic pentad
ratio (IP). Next, as explained previously, the average chain
length (N) may be calculated from the number of meso chains
and the number meso units according to the following
definition.
N = number of meso chains/number of meso units
(4) Column separation
The xylene-insoluble portion of the propylene-based
polymer is dissolved in para-xylene at a temperature of
130C, celite is added thereto, and the temperature is
lowered to 30C at a temperature-lowering rate of 10C/hour
to deposit the celite. The accretion is filled into a
column, the temperature is raised from 70C to 130C by 2.5C
at a time, and the fractions are separated off.
(5) Injection molding
A Toshiba Kikai, KK. IS-170FII (theoretical injection
volume 250 cm3) was used to prepare an Izod impact test
specimen, a flexural modulus test specimen, a heat distortion
temperature test specimen and a surface gloss test specimen
(flat pieces 15 cm x 11 cm of thickness 2 mm). These were
then allowed to stand for two full days in a constant
temperature room at 50% humidity and 23C, after which the
33
21Z77Z~.
physical properties thereof were measured.
(6) Izod impact strength (notching)
The measurement was performed according to JIS K7110.
The device used was a U-F impact tester, product of Uejima
Seisakusho, KK.
(7) Flexural modulus
The measurement was performed according to JIS K7203.
(8) Ethylene content
The calculation was made based on the 13C-NMR method
reported by C.J. Carman et al. (Macromolecules, 10, 537,
1977).
(9) MFR (Melt Flow Rate)
The measurement was made according to JIS K7210, Table
1, condition 14. The device used was a melt indexer, product
of Takara, KK.
(10) Heat distortion temperature
The measurement was made according to the JIS K7207B
method, using an HDT~VSPT tester, product of Toyo Seiki
Seisakusho, KK.
(11) Rockwell surface hardness
The samples to be measured were prepared with a press
molding machine at a temperature of 230C, and the
measurement was made using a Rockwell hardness meter, model
AR-10, product of Toyo Seiki Seisakusho, KK., according to
JIS K7202.
(12) Film molding
A 40 mm~ T-die film molding machine, product of Yoshii
34
~ yl~
Tekko, KK. was used to prepare a film of thickness 60 ~m,
under conditions of a die temperature of 230C, a cooling
temperature of 30C and a drawing rate of 10 m/min, and the
vapor permeability, haze and surface gloss were measured.
(13) Haze
The measurement was performed according to JIS K7105,
using a Haze meter, model HGM-2D, product of Suga Shikenki,
KK.
(14) Surface gloss
The measurement was performed according to JIS K7105,
using a gloss meter, model VG-lD, product of Nihon Denshoku
Kogyo, KK.
(15) Water Vapor permeability
The measurement was made according to ASTM-E96, using a
PERMATRAN W, product of Modern Controls, Inc., under
conditions of a temperature of 37.8C and 90% relative
humidity.
(16) Catalyst analysis
The unmodified solid catalyst components and the
modified solid catalyst components for olefin polymerization
were dissolved in dilute sulfuric acid, and the organic
portion was extracted with heptane. The Ti in the aqueous
layer was determined using an atomic absorption
spectrophotometer, model AA610S, product of Shimazu
Seisakusho, KK. The electron-donating compound in the
heptane layer was determined using a gas chromatograph 263-
50, product of Hitachi Seisakusho, KK.
Z~7721.
Example 1
(1) Preparation of unmodified solid catalyst component
~common method)
A 56.8 g (597 mmol) portion of anhydrous magnesium
dichloride was completely dissolved in 100 g (174 mmol) of
absolute ethanol, 500 ml of CP15N vaseline oil, product of
Idemizu Kosan, KK. and 500 ml of KF96 silicone oil, product
of Shinetsu Silicone, KK. in a nitrogen atmosphere at 120C.
The mixture was agitated for 3 minutes using a TK homomixer,
product of Tokushu-Kika Kogyo, KK., at 3000 rpm. It was then
transferred to 2 liters of anhydrous heptane while agitation
was maintained, without allowing the temperature to exceed
0C. The resulting white solid was washed with a sufficient
amount of anhydrous heptane and the substance was vacuum
dried at room temperature.
A 30 g portion of the resulting globular solid
MgCl2-2.5C2H5OH was suspended in 200 ml of anhydrous heptane.
While stirring at 0C, 500 ml (4.5 mol) of titanium
tetrachloride was added dropwise thereto over a period of one
hour. Heating was then initiated, and when the temperature
reached 40C, 4.96 g (17.8 mmol) of diisobutyl phthalate was
added thereto and the temperature was raised to 100C over a
period of about an hour. After conducting the reaction at
100C for 2 hours, the solid portion was collected by
filtration while still hot. Then, 500 ml (4.5 mol) of
titanium tetrachloride was suspended in the reaction product
and allowed to react therewith at 120C for one hour. After
36
21~77Zl.
completion of the reaction, the solid portion was again
collected by filtration while still hot, and washed 7 times
with 1.0 liter of hexane at 60C and 3 times with l.0 liter
of hexane at room temperature. The titanium content of the
resulting solid catalyst component was measured and found to
be 2.25 wt%. The component also contained 7.81 wt% of an
electron-donating compound (1).
t2) Preparation of modified solid catalyst component ..
A 20 g portion of the unmodified solid catalyst
component obtained above was suspended in 300 ml of toluene,
and was allowed to react for one hour with 2.78 g (10 mmol)
of diisobutyl phthalate at 25C. After completion of the
reaction, 100 ml (900 mmol) of titanium tetrachloride was
added thereto for reaction at 90C for one hour. After
completion of the reaction the solid portion was collected by
filtration while still hot, and then 300 ml of toluene and
100 ml (900 mmol) of titanium tetrachloride were suspended in
the reaction product and allowed to react therewith at 90C
for one hour. After completion of the reaction, the solid
portion was again collected by filtration while still hot,
and washed 7 times with 500 ml of toluene at 90C and 3 times
with 500 ml of hexane at room temperature. The titanium
content of the resulting solid catalyst component was
measured and found to be 1.01 wt%. The component also
contained 12.0 wt% of a first electron-donating compound. A
comparison of the results of analysis of the catalyst
components before and after their modification is provided in
37
~i~ 7'~
Table 1.
t3) Prepolymerization
Into a 3-liter autoclave were charged in a nitrogen
atmosphere 500 ml of n-heptane, 6.0 g (53 mmol) of
triethylaluminum, 0.39 g (17 mmol) of dicyclopentyl
dimethoxysilane and 10 g of the modified olefin
polymerization catalyst component obtained in (2) above, and
the mixture was-stirred for 5 minutes at a temperature of
about 0-5C. Propylene was then fed to the autoclave for
polymerization of 10 g of propylene per one gram of the
modified olefin polymerization catalyst component, for
prepolymerization for one hour at a temperature of about 0-
5C. The resulting prepolymerization solid catalyst
component was washed 3 times with 500 ml of n-heptane, and
was used for the following production of a propylene-based
polymer.
(4) Main polymerization
In a 60-liter stirrer-equipped autoclave were placed, in
a nitrogen atmosphere, 2.0 g of the prepolymerization solid
catalyst component prepared by the method described above,
11.4 g (lO0 mmol) of triethylaluminum and 6.84 g (30 mmol) of
dicyclopentyl dimethoxysilane, and then 18 kg of propylene
and hydrogen at 13,000 mol ppm with respect to the propylene
were fed thereto and the temperature was raised to 70C for
polymerization for one hour. After one hour the unreacted
propylene was removed to terminate polymerization. The
result was 6.56 kg of polypropylene~ or a polymerization
38
-
activity of 32.8 kg per gram of the solid catalyst component,
and the MFR of the polymer was 33.0 g/10 minutes. The
results of evaluation of the physical properties of the
polymer are given in Table 2.
Example 2
(1) Preparation of unmodi-fied solid~catalyst component -
-Conducted in the same~-manner as in Example 1. ;
(2) PreParation of TiCl4 r C~H4 ( CoOi C4Hg ! ~1
To a 1.0 liter solution of hexane containing 19 g (100
mmol) of titanium tetrachloride was added dropwise 27.8 g
(100 mmol) of diisobutyl phthalate: C6H4 ( COOiC4H9 ) 2, over a
period of about 30 minutes while maintaining a temperature of
0C. After the dropwise addition, the temperature was raised
to 40C for reaction for 30 minutes. After completion of the
reaction, the solid portion was collected and washed 3 times
with 500 ml of hexane to obtain the object substance.
(3) Preparation of modified olefin polymerization catalyst
component
A 20 g portion of the solid catalyst component obtained
in (1) above was suspended in 300 ml of toluene, and treated
for 1 hour with 5.2 g (11 mmol) of TiC14 [C6H4(COOi C4Hg)2] at
25C for incorporation. After completion of the
incorporation, the solid portion was collected by filtration
while still hot and was resuspended in 10 ml (90 mmol) of
titanium tetrachloride, stirred for one hour at 90C for
washing, and the solid portion collected by filtration while
39
2127'72~.
still hot, after which the reaction product was washed 5
times with 500 ml of toluene at 90C and 3 times with 500 ml
of hexane at room temperature. The titanium content of the
resulting solid catalyst component was measured and found to
be 0.91 wt%. The component also contained 10.6 wt% of a
first electron-donating compound. A comparison of the
results of analysis of the catalysts before and after their
modification is provided in Table 1.
r4) Prepolymerization
Into a 3-liter autoclave were charged in a nitrogen
atmosphere 500 ml of n-heptane, 6.0 g (53 mmol) of
triethylaluminum, 0.39 g (17 mmol) of dicyclopentyl
dimethoxysilane and 10 g of the modified olefin
polymerization catalyst component obtained in (3) above, and
the mixture was stirred for 5 minutes at a temperature of
about 0-5C. Propylene was then fed to the autoclave for
polymerization of 10 g of propylene for 1 g of the modified
olefin polymerization catalyst component, for
prepolymerization for one hour at a temperature of about 0-
5C. The resulting prepolymerization solid catalyst
component was washed 3 times with 500 ml of n-heptane, and
was used for the following production of a propylene-based
polymer.
(5) Main polymerization
In a 60-liter stirrer-equipped autoclave were placed, in
a nitrogen atmosphere, 2.0 g of the prepolymerization solid
catalyst component prepared by the method described above,
- 2~Z7721.
11.4 g (100 mmol) of triethylaluminum and 6.84 g (30 mmol) of
dicyclopentyl dimethoxysilane, and then 18 kg of propylene
and hydrogen at 13,000 mol ppm with respect to the propylene
were fed thereto and the temperature was raised to 70C for
polymerization for one hour. After one hour the unreacted
propylene was removed to terminate polymerization. The
result was 6.64 kg of polypropylene, or a polymerization
activity of 34 kg per gram of the solid catalyst component,
and the MFR of the polymer was 34.2 g/10 minutes. The
results of evaluation of the physical properties of the
polymer are given in Table 2.
Comparison 1
In a 60-liter stirrer-equipped autoclave were placed, in
a nitrogen atmosphere, 6.0 g of AA-type titanium trichloride,
product of Toso Akuzo, KK. and 23.5 g (195 mmol) of
diethylaluminum chloride, and then 18 kg of propylene and
hydrogen at 8000 mol ppm with respect to the propylene were
fed thereto and the temperature was raised to 70C for
polymerization for one hour. After one hour the unreacted
propylene was removed to terminate polymerization. The
result was 6.23 kg of polypropylene, with an MFR of the
polymer of 32.2 g/10 minutes. The results of evaluation of
the physical properties of the polymer are given in Table 2.
Comparison 2
The prepolymerization and polymerization were conducted
41
~ ~!77~1
with the same methods and conditions as in Example 2, except
that the unmodified solid catalyst component prepared in
Example 1 (1) was used for the polymerization, and the
hydrogen was charged at 9300 mol ppm. The result was 6.88 kg
of polypropylene, with an MFR of the polymer of 33.0 g/10
minutes. The results of evaluation of the physical
properties of the polymer are given in Table 2.
Examples 3-5
Polypropylene was produced with the same methods and
conditions as in Example 2, except that the charging of
hydrogen was controlled during production of the propylene-
based polymers so that the MFRs of the resulting
polypropylene products were, respectively, 10.5 g/10 minutes,
2.7 g/10 minutes and 0.7 g/10 minutes. The results of
evaluation of the physical properties of the resulting
polymers are given in Table 2.
Comparison 3
A propylene-based polymer was produced with the same
methods and conditions as in Comparison 1, except that the
charging of hydrogen was controlled during production of the
propylene-based polymer so that the MFR of the resulting
polymer was 3.2 g/10 minutes. The results of evaluation of
the physical properties of the resulting polymer are given in
Table 2.
42
212~7721.
Example 6
(1) Preparation of unmodified solid catalyst component
In a nitrogen atmosphere, 47.6 g (500 mmol) of anhydrous
magnesium dichloride, 250 ml of decane and 234 ml (1.5 mol)
of 2-ethylhexyl alcohol were heated together at 130C for 2
hours for reaction to make a uniform solution, after which
11.1 g (75 mmol) of phthalic anhydride was added to the
solution which was further stirred at 130C for one hour to
dissolve the phthalic anhydride in the uniform solution.
After the resulting uniform solution was cooled to room
temperature, the entire amount thereof was added dropwise
over a period of an hour to 2.0 liters (18 mol) of titanium
tetrachloride which had been kept at -20C. After the
dropwise addition was completed, the temperature of the mixed
solution was raised to 110C over a period of 4 hours, and
when it reached 110C, 26.8 ml (125 mmol) of diisobutyl
phthalate was added thereto and the mixture was stirred for 2
hours at 110C for reaction. After completion of the
reaction, the solid portion was collected by filtration while
still hot, and then 2.0 liters (18 mol) of titanium
tetrachloride was suspended in the reaction product and
allowed to react therewith at 110C for 2 hours. After
completion of the reaction, the solid portion was again
collected by filtration while still hot, and washed 7 times
with 2.0 liters of decane at 110C and 3 times with 2.0
liters of hexane at room temperature, to obtain a solid
catalyst component. The results of analysis of the catalyst
43
Z~'~77~1.
are given in Table 1.
(2) PreParation of TiC14 r C~H~(COOi C4H9) 71
This was conducted as in (2) of Example 2.
(3) Preparation of modified olefin polymerization catalyst
component
A 40 g portion of the solid catalyst component obtained
in (1) above was suspended in 600 ml of toluene, and treated
for one hour with 10.3 g (22 mmol) of TiCl4 [C6H4(COOi C4H9)2]
obtained in (2) above at 90C for incorporation. After
completion of the incorporation, the solid portion was
collected by filtration while still hot and was resuspended
in 600 ml of toluene and 20 ml (180 mmol) of titanium
tetrachloride, stirred for one hour at 90C for washing, and
the solid portion collected by filtration while still hot,
after which the reaction product was washed 5 times with one
liter of toluene at 90C and 3 times with 1 liter of hexane
at room temperature, to obtain a modified olefin
polymerization catalyst component. The results of analysis
of the catalyst are given in Table 1.
(4) Prepolymerization
Into a 3-liter autoclave were charged in a nitrogen
atmosphere 500 ml of n-heptane, 6.0 g (0.053 mol) of
triethylaluminum, 4.15 g (0.017 mmol) of diphenyl
dimethoxysilane and 10 g of the improved olefin
polymerization catalyst component obtained in Example 2 (3)
above, and the mixture was stirred for 5 minutes at a
temperature of about 0-5C. Propylene was then fed to the
44
Z~Z~721.
autoclave for polymerization of 10 g of propylene per one
gram of the modified olefin polymerization catalyst
component, for prepolymerization for one hour at a
temperature of about 0-5C. The resulting prepolymerization
solid catalyst component was washed 3 times with 500 ml of n-
heptane, and was used for the following production of a
propylene-based polymer.
(5) Main propylene polymerization
In a 60-liter stirrer-equipped autoclave were placed, in
a nitrogen atmosphere, 200 mg of the prepolymerization solid
catalyst component prepared by the method described above,
11.4 g (100 mmol) of triethylaluminum and 7.32 g (30 mmol) of
diphenyl dimethoxysilane, and then 18 kg of propylene and
hydrogen at 5300 mol ppm with respect to the propylene were
fed thereto and the temperature was raised to 70C for
polymerization for one hour. After one hour the unreacted
propylene was removed to terminate polymerization. The
polymerization activity was 22.0 kg per gram of the solid
catalyst component. Also, the MFR of the resulting
polypropylene was 14.5 g/10 minutes. The results of
evaluation of the physical properties of the polymer are
given in Table 2.
Example 7
(1) Preparation of unmodified solid catalyst comPonent
A 50.0 g (440 mmol) portion of diethoxy magnesium and
15.3 g (55 mmol) of di-n-butyl phthalate were refluxed and
2~2772~1.
stirred in 250 ml of methylene chloride for one hour in a
nitrogen atmosphere. The resulting suspension was pressure-
fed to 2.0 liters (18 mol) of titanium tetrachloride, and the
temperature was raised to 110C for reaction for 2 hours.
After completion of the reaction, the precipitated solid was
reacted with 2.0 liters (18 mol) of titanium tetrachloride at
110C for 2 hours. After completion of this reaction, the
product was washed 3 times with 2.0 liters of n-decane at
110C and then with 2.0 liters of n-hexane at room
temperature, until chloride ion was no longer detected. It
was then dried under reduced pressure at 40C to obtain the
object solid catalyst component. The results of analysis of
the catalyst are given in Table 1.
(2) Preparation of TiCl4 r C~HL ( CoOi C4Hs ! 7 1
This was conducted as in (2) of Example 2.
(3) Preparation of modified olefin polymerization catalyst
component
A 40 g portion of the solid catalyst component obtained
in (1) above was suspended in 600 ml of toluene, and treated
for one hour with 10.3 g (22 mmol) of TiC14 [C6H4(COOi C4Hg)2]
obtained in (2) above at 90C for incorporation. After
completion of the incorporation, the solid portion was
collected by filtration while still hot and was resuspended
in 600 ml of toluene and 20 ml (180 mmol) of titanium
tetrachloride, stirred for one hour at 90C for washing, and
the solid portion collected by filtration while still hot,
after which the reaction product was washed 5 times with one
46
2127721.
liter of toluene at 90C and 3 times with 1 liter of hexane
at room temperature. The results of analysis of the catalyst
are given in Table 1.
The prepolymerization and the propylene polymerization
were conducted with the same methods and conditions as in
Example 6. The result was a polymerization activity of 21.1
kg per gram of the solid catalyst component. Also, the MFR
of the resulting polypropylene was 16.3 g/10 minutes. The .,
results of evaluation of the physical properties of the
polymer are given in Table 2.
47
2~27721.
E--
O
a
a--
_~
E
U7 ~ CO
E~ o o
~ . . .
, a
o
O ,I d~ O a~ o
~ E-~ ~ - - -
N 3~ o ~ ~
~ ~ _
~ ~ ~ Ed O
a~ - - -
c o ~ 3 ~o~
..
u~
~ ._
~-1 --O o u~
~,. ~ ....
a 0OOO
u~
~ o ._1 d C~ ~ U~ ~
~ ~, o E~ 3
._ .~, _
._ ~,
~ O
o ta.~ d~ CO ao _I co
a~....
3~ O ~
-
t'
N ~D t~
a~ a) a) a
~ 0 ~ ~ ~
,~ X X X X
E~
48
Table 2-1
Example or Xylene Isotactic Isotactic Weight MFR
Comparison extraction pentad ratio average proportion
insoluble (IP) mmmm chain length of fractions (g/10 min)
portion (XI) (%) (N) (Nf) with
(wt~) isotactic
average
chain length
N>800 (wt~)
Example 1 99.5 99. 5 816 80 33.0
Example 2 99. S 99.5 836 81 34.2
Comparison 1 98.6 97.7 225 <1 32.2
Comparison 2 98.8 98.9 326 7 33.0
Example 3 99.5 99.5 715 76 10.5
Example 4 99.5 99.4 651 72 2.7
Example 5 99.4 99.3 588 65 0.7
Example 6 99. 3 99.3 703 68 14.5
Example 7 99.3 99.4 765 70 16.3
Comparison 3 98.1 96.3 105 <1 3.2
Table 2-2
Example or Injection molding Film
Comparison
Izod impact Flexural Heat Rockwell Degree Degree Water vapor Haze
strength modulus distortion surface of of permeabi- (%)
(kg-cm/cm) (kg/cm2) temperature hardness surface surface lity
(C) (R scale) gloss gloss (g/cm2-day)
23C -20C (%) (~)
Example 1 1.7 -- 17900 130 104 86.0 120 5.6 2.4
Example 2 1.8 -- 18100 131 104 86.4 122 5.6 2.4
Comparison 2.0 -- 14600 119 93 83.2 46 7.9 23.3
Comparison 1.8 -- 16200 127 101 84.2 116 6.1 3.2
Example 3 2.0 -- 17900 130 103 85.1 117 5.5 2.8
Example 4 2.8 -- 17200 130 102 84.3 113 5.4 3.0
Example 5 4.4 -- 14200 110 96 83.9 -- -- --
Example 6 2.0 -- 17300 128 101 85.2 -- -- --
Example 7 2.0 -- 17500 128 101 85.3 -- -- --
Comparison 2.5 -- 12300 104 88 81.6 42 7.7 20.9
21~7.~L
Example 8
After propylene was polymerized (lst stage) in a 60-
liter stirrer-equipped autoclave in the same manner as in
Example 2, the liquid propylene was removed and a mixed gas
of ethylene/propylene = 40/60 (molar ratio) was fed at a rate
of 2.2 Nm3/hour and hydrogen at a rate of 20 NL/hour, at
75C, for copolymerization for 40 minutes (2nd stage). After
40 minutes the unreacted gas was removed to suspend the
polymerization. As a result 8.0 kg of a propylene/ethylene
block copolymer was obtained. The ethylene content as
determined by 13C-NMR was 9.7 wt%, and the MFR was 17.8 g/10
minutes. The results of evaluation of the physical
properties of the polymer are given in Table 3. In the
table, XI, IP and N shown are for the homopolypropylene
obtained after completion of the 1st stage polymerization.
Comparison 4
After propylene was polymerized in a 60-liter stirrer-
equipped autoclave in the same manner as in Comparison 1, the
liquid propylene was removed and a mixed gas of
ethylene/propylene = 40/60 (molar ratio) was fed at a rate of
2.2 Nm3/hour and hydrogen at a rate of 20 NL/hour, at 65C,
for copolymerization for 40 minutes. After 40 minutes the
unreacted gas was removed to suspend the polymerization. As
a result 7.7 kg of a propylene/ethylene block copolymer was
obtained. The ethylene content as determined by 13C-NMR was
9.6 wt%, and the MFR was 18.3 g/10 minutes. The results of
51
21Z7721.
_ evaluation of the physical properties of the polymer are
given in Table 3. In the table, XI, IP and N shown are for
the homopolypropylene obtained after completion of the 1st
stage polymerization.
Table 3-1
Example or Xylene Isotactic Isotactic Weight MFR
Comparison extraction pentad ratio average proportion
insoluble (IP) mmmm chain length of fractions (g/10 min)
portion (XI) (%) (N) (Nf) with
(wt%) isotactic
average
chain length
N>800 (wt%)
Example 8 99.5 99.5 830 79 17.8
Comparison 4 98.5 97.7 217 <1 18.3
w
Table 3-2
Example or Injection molding Film
Comparison
Izod impact Flexural Heat Rockwell Degree Degree Water vapor Haze
strength modulus distortion surface of of permeability (%)
(kg-cm/cm) (kg/cm2) tempera- hardness surface surface (g/cm2-day)
ture (R scale) gloss gloss
23C -20C (C) (Z) (~)
Example 8 6.4 3.9 14100 124 92 68.1 -- -- --
Comparison 5.5 3.3 12100 112 87 62.3 -- -- --
_Examples 9, 10 and Comparison 5 21~'y'~
As a propylene-based polymer composition there were
combined with a propylene-based polymer obtained according to
the present invention 0.05 wt% of di-t-butyl-p-cresol, 0.10
wt% of pentaerythrityl-tetrakis [3-(3,5-dibutyl-4-
hydroxyphenyl)] propionate and 0.10 wt% of calcium stearate,
using for the mixing a 20-liter supermixer (model SMV20),
product of Kawata Seisakusho Co., and a 30 mm~ twin-screw
extruder, product of Nakatani Kikai Co. was used to make
pellets. The following compounds were used as nucleating
agents, and the amounts were appropriately varied.
(Types of nucleating agents)
Nucleating agent A: Aluminum p-t-butylbenzoate
Nucleating agent B: Sodium 2,2-methylenebis (4,6-di-
tert-butylphenyl) phosphate
Table 4 lists the results of evaluation of the physical
properties of the compositions (Examples 9,10) prepared by
combining the above mentioned nucleating agents with the
polypropylene obtained in Example 2 and the composition
(Comparison 5) prepared by combining a nucleating agent with
the polypropylene obtained in Comparison 1.
Example 11 and Comparison 6
Table 4 also lists the results of evaluation of the
physical properties of the compositions obtained by combining
a nucleating agent with the propylene/ethylene block
copolymers obtained in Example 8 and Comparison 4, in the
same manner as in Example 9.
Table 4-1
Example or Xylene Isotactic Isotactic Weight MFR Type of
Comparison extraction pentad average proportion nucleatinginsoluble ratio chain of (g/10 agent and
portion (IP) mmmm length fractions min) content
(XI) (wt%) (%) (N) (Nf) with (wt%)
isotactic
average
chain
length
N>800 (wt%)
Example 9 99.5 99.5 836 81 34.2 A 0.2
Example 10 99.5 99.5 836 81 34.2 B 0.4
Comparison 5 98.8 98.9 326 7 32.2 B 0.4
Example 11 99.5 99.5 830 79 17.8 A 0.2
a~
Comparison 6 98.5 97.7 217 <1 18.3 A 0.2
Table 4-2
Example or Injection molding Film
Comparison
Izod impact Flexural Heat Rockwell Degree Degree Water vapor Haze
strength modulus distortion surface of of permeabilit (%)
(kg-cm/cm) (kg/cm2) temperatur hardness surface surface y
e (R scale) gloss gloss (g/cm2-day)
23C -20C (C) (~
Example 9 1.7 -- 22000 141 105 91.7 81 5.3 13.5
Example 10 1.9 -- 24500 148 109 93.8 84 5.1 11.6
Comparison 5 1.7 -- 19200 131 102 86.3 32 7.2 28.9
Example 11 6.2 3.8 16800 134 99 72.2 -- -- --
Comparison 6 5.3 3.0 14600 124 91 67.2 -- -- --
~I
Industrial Applicability 2~2 Y 7~i.
Since according to the present invention it is possible
to produce propylene-based polymers and compositions suitable
for automobiles, consumer electric goods and packaging, whose
physical properties including rigidity, surface hardness,
heat resistance, water vapor barrier property, etc. are more
excellent than those of the prior art, the present invention
has considerable industrial value.
58