Note: Descriptions are shown in the official language in which they were submitted.
- 2128243
N:AL2742B - 1 - 1.18.1993
Proces~ for the ~G~Lion of soft gelatin cap~ule~
by a drip-feed proces~
The invention relates to a process for the
production of soft gelatin capsules by a drip-feed
process, the pasty or liquid filling material being
covered with a soft gelatin composition and being
introduced into a cooling bath to solidify the gelatin
composition.
The production of soft gelatin capsules i8
preferably carried out today by a stamping process, in
which the capsule wall is composed of and formed from two
gelatin halves which are stamped out of a gelatin ribbon.
Preferably, the Scherer process working according to the
rotary die method is used. In this process two endless
gelatin r;hhon~ run against two shaping rolls which are
adjacent and run against one another. While the gelatin
ribbons are pressed in the mold and thus form the capsule
halves, the flowable filling material reaches the result-
ing capsule via an exactly metering filling wedge.
Welding of the capsule halves, stamping out, a w~h;ng
process to free from adhering oil, passage through a
rotary drier and a final shelf-drying follow.
The rotary die method enables the production and
filling of the capsules in one working operation and
achieves hourly outputs of up to 100,000 pieces. A
typical characteristic of the Scherer capsules is the
central weld seam in the longit~l~;n~l direction. The
Scherer process possesses, inter alia, the following
disadvantages:
a) for preparation of the soft gelatin capsules only
gelatin can be employed whose quality can only be varied
within narrow limits. For example, the following specifi-
cations must be met: the types of gelatin must have gel
~trengths of on average 100-200 Bloom and visco~ity
values which remain heat-stable over a relatively long
period, because during the pouring of the gelatin r;hhon~
a uniform ribbon thickness must always be guaranteed. The
viscosities of the type~ of gelatin employed may thus
only fall by a maximum of 10-15% after several days'
21Z8Z4;~
-- 2
lo~in~ at 60~C.
b) The shaping rolls for forming the gelatin cap-
sules from the two gelatin ribbons must be very precisely
manufactured and work very accurately. They are therefore
expensive to produce and susceptible to trouble in
operation.
c) For the preparation of soft gelatin capsules, a
conditioning from 20 to 30% relative humidity at 22~C i8
necessary, as is inferred from the adsorption isotherm of
water in gelatin capsule material. All production and
pAc~ging areas must therefore be fully air-conditioned.
d) Apart from said high demands on the material and
the air-conditioning, the technique of soft gelatin
capsule production requires 80 much know-how that it is
only controlled by manufacturers specialized therein.
e) A further disadvantage consists in the fact that
the wastes from the gelatin r;hho~R remaining in the
stamping process, the so-called net wastes, can only be
reused to a small part (at most 5%) and therefore up to
60% of the gelatin composition employed has to be
disposed of. These net wastes are, for example,
contaminated with parting oils caused by production, and
with highly active substances as filling material,
contamination of the stamped net cannot be avoided. Such
net wastes are therefore to be treated as special waste.
Furthermore, these wastes frequently contain color
pigments which make recovery of the starting material
impossible.
f) As a result of the production process the fin-
ished capsule is affected by troublesome parting oilwhich has to be removed by effective lipoid solvents such
as tetrachloroethylene, methylene chloride etc. This
process step requires a particular technical and capital-
intensive outlay in order to avoid any contamination of
the soft gelatin capsuleR and the waste air with health-
damaging solvents. Additionally the treatment of soft
gelatin capsules cont~ning pharmaceutical substances
with such health-damaging solvents is also problematical
because the user of the soft gelatin capsule_ could ~hy
z~28;~43
-- 3
away from t~k;ng the soft gelatin capsules treated in
this way.
A further process for the production of soft
gelatin capsules according to the prior art is the drip-
feed and blowing process, also called the Globex processafter the developer. In this process the lipophilic
filling material is added dropwise from a nozzle, while
warm gelatin solution simultaneously flows from a tube
surrolln~;ng the nozzle like a jacket. During dropwise
addition to a cooling liquid of defined density (e.g.
liquid paraffin) the capsules assume spherical shape
owing to the interfacial tension and solidify. Oily
excipient materials are suitable as filling materials.
The process gives seamless, round capsules at an hourly
output of up to 70,000 pieces. This drip-feed or blowing
process especially possesses the following disadvantages:
a) Only oily solutions can be processed as a filling
material.
b) The various process technology-related components,
such as oily filling, gelatin composition and cooled
precipitation baths (liquid paraffin) can only be suited
to one another with extreme difficulty, since it is a
question here of a three-phase system.
c) The residues of the precipitation bath (liquid
paraffin) adhering to the soft gelatin capsules must be
removed using a solvent. The same difficulties occur here
a~ in the rotary die or stamping process under f).
The processes for the preparation of soft gelatin
capsule~ known according to the prior art therefore
present technological and economical problems. The
complex process technology only allows the pharmaceutical
manufacturing companies to install and operate in-house
production plants for soft gelatin capsules with great
difficulty. Additional problems can occur as a result of
lack of knowledge about the properties of gelatin.
Problems furthermore occur in the purification of the
capsules from a&ering parting oil or cooling oil, for
which the disposal of the net wastes in the Scherer
process must still be coped with.
Z128~f~3
-- 4
The invention i8 therefore based on the object of
avoiding said difficulties occurring in the prior art in
the preparation of soft gelatin capsules, in particular
of making available a technologically and economically
relatively simple process which makes it possible for a
pharmaceutical manufacturing company to buy and operate
an in-house production plant for soft gelatin capsules
without great cost.
This object is achieved according to the inven-
tion in that deep-cooled liquids such as e.g. liquid
nitrogen are used as a cooling bath in a process of the
type mentioned at the beg; nn; n~.
The present invention in particular makes avail-
able a process for the preparation of soft gelatin
capsules by a drip-feed process, the filling material
being covered with a soft gelatin composition and
introduced into a cooling bath to solidify the gelatin
composition, which comprises using, for shaping, a
cooling bath which contains a deep-cooled liquid which
does not leave behind any biologically unacceptable or
harmful residue on the soft gelatin capsule.
Two-substance nozzles, for example concentric
double capillaries as are known from the Globex process
or from the "Spherex" capsule machine of the company
Freund, are employed here for the process according to
the invention. The warmed covering material (soft gelatin
composition) flows here into the outer capillary and the
filling material into the inner capillary. The cutting
off of the capsules can be controlled here, if necesRary,
timed in pulses or intermittently.
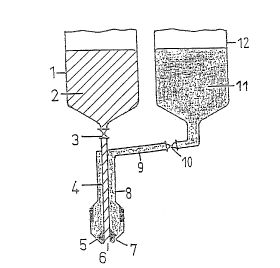
Fig. 1 shows: a cutaway view of a metering device
used for the process according to the invention.
A metering device with two-substance nozzles is
shown schematically in Fig. 1. The capsule wall material
11 is located in the heatable gelatin container 12. The
second contA; ner 1 contains the filling material 2. The
amount added can be controlled via the adjustable
metering devices 3 and 10. Covering material and filling
material flow via the supply pipes 9 and 4 into the
~ 2824 3
nozzle 7, where the gelatin solution is led via a jacket
pipe 8 such that it can cover the filling material
emerging from the inner nozzle head 6 by means of the
adjustable nozzle jacket 5. By means of an inlet regula-
tion which is intermittent or timed in pulses, theformation of capsules is effected.
The capsules are then introduced into an insu-
lated ;mmersion deep-freezer which is filled with a deep-
cooled liquid in a range from -70~C to -220~C, such as
e.g. liquid nitrogen. The actual shaping proces~ happens
here. As a result of the extreme temperature difference
between the warmed gelatin covering material and the
liquid gas, the latter evaporates immediately and sur-
rounds the capsule with a gas cushion which exerts a
uniform pressure on the capsule. The capsule therefore
assumes a spherical shape and solidifies.
The seamless capsules formed in this way are
conveyed out of the immersion bath by means of a conveyor
belt and after thawing are dried directly in a manner
known per se, i.e. without the purification steps using
organic solvents necessary in customary processes.
According to a particular embodiment, the gelatin
shell also contains ph~r~-ceutical substance. Preferably
the gelatin shell contains a different pharmaceutical
substance from the filling material, which, for ex~mple,
can be utilized advantageously to avoid incompatibility
between ph~rr~ceutical substances.
Owing to the presence of a pharmaceutical sub-
stance in the shell, a sust~;ne~-release formulation
having an initial dose can also be developed, the initial
dose being located in the shell, in which filling
material the pharmaceutical substance is present in a
form having a delayed release.
Further preferred embo~ nts of the process according to
the invention are mentioned and cl~ in the dependent
claims.
An advantage of the process claimed compared with
the rotary die or stamping process consists in the fact
that gelatins having Bloom numbers of 50 to-300,
preferably 80 to 300, can be
2~28~43
-- 6
used.
Gelatins, fractionated gelatin or collagen are
used.
For the preparation of the soft gelatin composi-
tion, all known plasticizers, such as e.g. glycerol,sorbitol etc., can be employed in a range from 0.1 - 50%
(relative to the recipe material) and also all additive6
customary for capsule shells (colorants, opacifiers,
pigments, flavorings and sweeteners, preservatives etc.)
in a range from 0.1 - 30% (relative to the recipe
material).
The soft gelatin capsules can be enteric-coated
in a known manner with gastric juice-resistant substances
in a subsequent coating process.
According to the invention, substances from the
group of polyacrylic acids and their copolymers, poly-
methacrylic acids and their copolymers, cellulose acetate
phthalate, hydroxypropylmethylcellulose phthalate, azo-
crosslinked polymethacrylates, polyurethane/sugar
copolymers, a suitable sugar component in particular
being oilgomeric galactom~nnAnR or galactomannan deriva-
tives which are then crosslinked with aliphatic diiso-
cyanates, galactomannan derivatives such as ethyl- or
acetylgalactomannans, polysaccharides crosslinked with
adipic acid in the range from about 1 - 20%, relative to
the recipe material, can be incorporated directly into
the soft gelatin composition in dissolved form, e.g. in
the form of their water-soluble salts. Such a procedure
leads to a product which withstands the appropriate
procedures of the pharmacopoeias (GP, USP), e.g.
resistance to gastric juice is thereby achieved.
Controlled release can advantageously also be
effected by using as a filling material e.g. collagen
hydrolyzate or gelatins to which pharmaceutically accept-
able crossl;nk;ng agents, such as e.g. aldoses (xylose)or citral have been added which make possible a
controllable crossl;n~;ng of the filler matrix.
Sustained-release pharmaceutical forms with differing
release characteristics of the active compound can be
- 7 ~ 3
realised in this manner.
Filling materials which can be employed are
primarily fundamentally all pharmaceutical substances or
filler recipes enveloped in classically prepared soft
gelatin capsules. These are especially liquid filling
materials such as e.g. ethereal oils (pinene, myrtol,
peppermint oil etc.), oily substances such as vitamin E
or cod liver oil, garlic oil, omega-3-fatty acids,
evening primrose oil from Oenothera biennis, juniper oil,
hypericon oil, wheatgerm oil, lecithi_ etc. These
lipophilic substances can also be employed in the form of
microemulsions in an appropriate recipe composition.
Fur~he ~re, suitable filling materials are also
dispersions of phAr~ceutical substances in collagen
hydrolyzate or gelatin compo~itions.
Suitable particularly finely disperse pharma-
ceutical substance dispersions are also colloidally
disperse ph~ ceutical substance systems (nanosols)
whose properties and preparation are described in numer-
ous patent applications of ALFATEC-Pharma ~mhH.
The encapsulation process according to the
invention also permits the use of microcapsules or
coacervates.
The processing of ph~r~ceutical substances which
can be dissolved in the filling material (water-soluble
ph~rm~ceutical substances) can be realised according to
the invention by means of a filling material of collagen
hydrolyzates or gelatins which contains an addition of
plasticizer in the same concentration as the capsule
wall. In this manner, diffusion of the plasticizer
contained in the capsule wall into the filling material
can be avoided.
3S Plasticizers which can be used are those which
are selected from the group consisting of: glycerol,
propylene glycol, polyethylene glycol, triacetin,
sorbitol, sorbitan mixtures, and their mixtures.
Poorly water-soluble pharmaceutical substances
- 8 - 2 ~ 3
can be present in the filling material in known form,
e.g. solubilized.
Compared with the drip-feed or blowing process,
there is the advantage that not only oily but also pasty
and mobile solution~ can be processed as filling material
(oily suspensions of plant extracts, rutin,
beta-carotene, minerals, vit~min A, C and E combination~
etc.) or fillings which solidify (preferably soft gelatin
compositions) or are semisolid at room temperature.
Because of the shock-like ;~ersion deep-freezing of the
combination of f; 1 1; ng material and gelatin solution
enveloping this, the rheological properties and the
different densities of the three phases filling
material/gelatin solution/immersion bath do not need to
be particularly related to one another.
Further details on this point are contained in
the parallel international (PCT) applications listed in
the following. The contents of these parallel PCT appli-
cations, filed on the same date in the-German patent
office by the same inventors and applicants:
~rA~
2 ~ 3
W O 93/13754(CA 1,282,242)
W O 93/13757(CA 1,282,244)
W O 93/13753
and
W O 93/10768
W O 93/10762
W O93/10771
W O 93/10761
W O93/10760
W O 93/10769
W O 93/10770
W O 93/10766
W O 93/10767
W O 93/10764
Thus, in contrast to the Globex process, it is
possible as a result of the present invention to encap-
sulate, for example, recipes based on cold water-soluble
types of gelatin or gelatin as f;ll; ng material. Irres-
pective of the different densities of the three-phase
system, the spherical shape of the capsules is formed by
the immersion deep-freeze process. If e.g. identical
amounts of plasticizer are added to the covering and also
~o the gelatin-cont~in;ng filling material, a constant
residual moisture of the entire capsule is established on
drying.
The abovementioned "solid" fillings with hydro-
philic excipient materials in capsules offer the poss-
ibility of releasing the ph~r~ceutical substance with adelay. Thus, for example, using special types of gelatin
2128243
- 10 -
for filling recipes, which have a softening point above
37~C, a matrix for the pharmaceutical substance can be
formulated whose degradation takes place in a time-
controlled manner. Said "solid" fillings are furthermore
suitable to contain stable emulsions, pharmaceutical
substances dissolved in water or microcapsules.
In addition, additives, in particular polymeric
macromolecules for controlling pharmaceutical substance
release (controlled release) of the filling material can
be added to customary soft gelatin compositions:
for example, alginates, pectins, thermoreversible
alginate gels, agar-agar, albumins, casein, plant pro-
teins, gum arabic, xanthan, tragacanth, chitosan,
polyethylene glycol, natural and modified starches,
maltodextrin, methylcellulose, cellulose ether polysac-
charides, carboxymethylcelluloses, etherified carboxy-
methylcellulose, hydroxypropylcellulose, hydroxypropyl-
methylcellulose phthalate, cellulose acetate phthalate,
polyvinyl alcohol, polyvinylpyrrolidone, polyacrylic
acid, copolymers of methacrylic acid and methacrylic acid
esters, aldoses such as xylose, citral in varying propor-
tions and mixtures with one another can be used.
Furthermore, a controlled crossl;nk;ng of the
gelatin shell can be advantageously carried out for
retardation or controlled pharmaceutical substance
release from the gelatin capsules according to the
invention. For this purpose, in particular non-toxic
aldehydes such as e.g. xylose or other aldoses or citral
are suitable. After administration of such capsules
having crosslinked shells, a diffusion shell is formed in
the physiological medium. As a result of the degree of
crossl;n~;ng of the constituents of the capsule shell,
which can be affected e.g. via the amount of added
xylose, very different pharmaceutical release profiles
can be achieved.
A suitable deep-cooled liquid is any liquid in
which the gelatin composition instantly solidifies and
which leaves behind no harmful residues on or in the soft
gelatin capsule. Liquid nitrogen is particularly
2128243
preferred.
As a result of the abovementioned shock deep-
freezing, an amorphous gelatin structure is formed during
the rapid, instantly forced transition of the gelatin
composition from the 801 state to the gel state. Such
gelatin structures are distinguished e.g. by a more rapid
dissolution time in comparison with conventional gelatin
capsules of identical gelatin quality.
Further deep-cooled liquids which may be suitable
for the process according to the invention are e.g.
liquid gases, e.g. argon or liquid air etc.
Thus using the process according to the invention
~oft gelatin capsules can be prepared which can be used
both as medicaments, as dietetic foodstuffs or in
cosmetics (e.g. bath oil-cont~;n;ng capsules or active
ingredient concentrate capsules).
Furthermore, in the preparation according to the
invention, the use of precision shaping roll~ which are
expensive and susceptible to trouble is unnecessary. The
technique is 80 simple that only a small outlay is
necessary in termR of apparatus and the process and the
nece~Rary know-how is substantially simpler to attain.
Large amounts of gelatin wastes are not obt~;ne~. The
expensive air-conditioning of the production areas can be
dispensed with. The gelatin capsule formed is seamless.
By arranging several production apparatuses in a row, the
hourly outputs can be increased almost a~ desired.
As a substantial advantage, it is l~nnecesRary in
the process according to the invention to clean the
finiRhed soft gelatin cap~ules from adhering oil (parting
oil between rolls and gelatin ribbons or liquid paraffin
from the precipitation bath), which is nece~sary in all
known processes according to the prior art. Therefore the
cost in terms of apparatus hereby necessary according to
the prior art can be saved. The problems which result in
the treatment of pharmaceutical substance-cont~;n;ng soft
gelatin cap~ules with health-impairing and ecologically
unacceptable solvents and in the removal of the solvents
from the waste air are completely inapplicable in the
2128243
- 12 -
proposed process. Moreover, the suitable deep-cooled
liquids employed such as e.g. liquid nitrogen are
chemically completely inert.
Example 1:
Recipe composition for the shell:
Gelatin 250 Bloom 2.5 parts
Glycerol (85% strength) 1 part
Water 6.5 parts
Recipe composition for the filling:
D,L-alpha-tocopherol acetate 1 part
Soybean oil 1 part
The gelatin granules are pre~wollen for 30
minutes and then dissolved at 60~C. Glycerol is added
with stirring. After degassing in vacuo, the composition
obtA;n~A is transferred to the storage contA;ner 11,
which is heated to 60~C, of the two-nozzle device
described in Fig. 1. The storage contA; ner 2, also heated
to 60~C, is filled with the solution of the tocopherol
acetate in soybean oil, warmed to the same temperature.
The gelatin solution is added via the jacket tube 8 such
that it can envelop, via the nozzle jacket 5, the filling
material emerging from the inner nozzle head 6. The
filling material is metered in via the supply tube 4.
Capsules are p;nche~ off at regular intervals with a dose
of 150 mg of tocopherol acetate per capsule and added
dropwise to the immersion bath filled with liquid nitro-
gen. They solidify immediately into the solid spherical
form and are co.~v~ed via a cG~e~or belt to a collecting
contA; ner . After thawing, they are dried in a known
manner to a residual moisture of 7 to 8%. (1-2 h dry
tumbler predyring, then dried in a recirculating air
drier to 7-8% residual moisture).
ExamPle 2:
Constituents of the capsule shell:
Gelatin 160 Bloom 30 parts
Glycerol 15 parts
Iron oxide 0.1 part
Water 54.9 parts
212824;~
- 13 -
The capsule shell material is prepared as in
Example 1, the iron oxide being suspended in the 801-
ution. For the filling material, the polyethylene glycol
4000 is mixed with polyethylene glycol 400 and warmed to
60~C. Aerosil and the active compound are suspended
therein. Both mixtures are filled into the respective
storage cont~;ners, which are heated to 60~C.
Constituents of the filling:
Polyethylene glycol 400 90 parts
Polyethylene glycol 4000 2 parts
Aerosil: 8 parts
25 mg of indometacin
The pharmaceutical sub~tance i8 homogeneou~ly
mixed with the excipient composition and ~haped analo-
gously to Example 1 in a liquid nitrogen bath to givecapsules cont~;n;ng 25 mg of indometacin.