Note: Descriptions are shown in the official language in which they were submitted.
2128292
4801 U. S. P1
STEF~ILE FILLING SYSTEM
FIELD OF THE INVENT!ON
This application is a continuation-in-part of commonly assigned
pending U. S. patent application Serial No. 07/510,317, filed April 17,
1990 entitled "Method for Sterilizing an Enclosure with Noncondensing
Hydrogen Peroxide-Containing Gas." The benefit of the filing date of this
prior application is hereby claimed under 35 USC 120.
The present invention relates generally to a method and apparatus
for the sterila filling of medical solutions, and more particularly to a
method and apparatus that utilizes the on-line sterilization capability of
an access passageway to facilitate the subsaquent on-line sterile filling
with a sterile medical solution of a pre-sterilized empty container.
BACKGROUND OF THE INVENTION
Parenteral solutions, such as the medical solutions administered in
intravenous therapy for example, must be sterile when administered to
the patient, and therefore must be starile when packaged and stored.
These sterile solutions were originally packaged in glass bottles and more
recently in flexible containers such as plastic film bags. Flexible
containers are preferred over glass containers due to advantages such as
weight, ease of handling, disposability, and other considerations.
Conventionally the flexible container is fabricated and the solution
^'` 212g292
is sealed in the container. Then the container and solution is terminally
sterilized. The flexible container may be further packaged, shipped and
then stored until needed.
The flexible container is manufactured of a plastic film or other
.~. .
material that is suitably compatible with the medical solution so as to
minimize reaction with the solution or allow degradation and/or loss of
potency during manufacture and/or storage. Suitable plastic material
includes heat sealable PVC film for example.
A terminal sterilization method is selected so that the process is
compatible with both the material of the flexible container and the
medical solution therein. Suitable terminal sterilization methods include ~ -
radiation sterilization and steam autoclaving, for example.
Howover, an increasing number of parenteral administered solutions,
such as the new, biotechnically produced drugs, are not necessarily
compatible with any combination of the presently known flexible
containers and terminal sterilization processes currently in use. For
example, some therapeutic solutions packaged in flexible containers lose ;
their potency or change their composition if they are subjected to
terminal sterilization by the traditional energy or chemical sterilization
procedures. Energy sterilization processes include application of heat
such as by steam autociaving, or irradiation with Gamma, X-ray,
21~8292
microwave, plasma, for example. Chemical sterilization may include
sterilization by liquid or vapor hydrogen peroxide, ethylene oxide (ETO),
phenol, ethanol, or sodium hypochlorite for example.
The United States Food and Drug Administration (FDA) is currently
recommending that all medical and surgical products be sterilized to a
microbial survival probability of 10-6, an assurance that there is less than
one chance in one million that viable micro-organism, such as viruses,
bacteria, and spores, are present in the sterilized product.
For a general discussion of sterilization and techniques therefor,
see Jhe United States Phar~L~copeia XXII, ch. 1211, pp 1705 et seq., ~ ;;
Atkinson et al., Biochemical Engineering and Biotechnology Handbook, ~ -
Stockton Press, New York, N.Y. (1983), pp. 875-886, and Demain et al.,
M~.rlua~ustrial Microbio!oay and Biotechn~loqy, American Society for
Microbiology, Washington, D.C. (1986), pp. 345-362..
Thus there is a need for an alternative sterilization process that can
be economically, efficiently, and safely used on-line with current filling
processes.
There is also a need for a sterilizing and filling process that avoids
using expensive packaging materials, and costly manufacturing and/or
sterilization processes for large or small volume sterile packaging of
known and new drugs for parenteral administration that have unique and
2128292
sensitive characteristics, such as for example some of the biotechnically
produced solutions or drugs.
SUMMARY OF THE INVENTION
The present invention provides efficient on-line sterilization of an
access passageway to a container, including flexible, semi-rigid, or rigid
containers and allows for the subsequent sterile filling of the container
during an on-line process. The access passageway sterilization process
may be accomplished by any one of the variety of known sterilization
processes including gas sterilization, vapor sterilization, thermal - ~:
sterilization, radiation sterilization, plasma sterilization, and/or
chemical agent sterilization.
The present method has the advantage of achieving a high degree of
sterilization efficiency in a relatively short time, since it takes only
seconds to sterilize the small volume of the access passageway of a pre-
sterilized container. The on-line sterilization of the access passageway
can be performed at fluid filling line speeds, thus facilitating use in a
sterile filling process.
Thc present invention is directed to a method and apparatus for the
on-line sterile filling of a container, including flexible, semi-rigid, or
rigid containers, with a sterile solution from an on-line filling
mechanism. The container has an open end and a hollow body portion for
~ 2128292
receiving the sterile solution. The container also includes an access
passageway tube having firs~ and second ends and a pierceable septum
sealing the passageway at an intermediate position within the tube. The
first open end of the access passageway tube is sealed to the open end of
the container so as to close the hollow body portion of the container. -
After the hollow body portion is closed, the container is sterilized, either
as a separate operation before the filling operation (i.e. pre-sterilized ;
off-line) or as a step in the filling operation (i.e. on-line). In either
situation, when the empty sterilized containers arrive on-line at the
filling station, the second end of the access passageway tube is isoiated
from the surrounding environment by axially advancing and sealably
seating the filling head to the second end of the access passageway tube.
The isolated end of the access passageway tube is then sterilized on-line
for a time period sufficient to reduce the viable micro-organism
population pr~sent to a predetermined acceptable level. Due to the small
volume of the accass passageway, this sterilization can be achieved at
on-line filling speeds. Then a filling nozzle is advanced from the filling
head through the isolated and sterilized end of the access passageway
tube. The nozzle pierces the septum of the tube and the sterilized hollow
body portion of the container is filled with the sterils solution. Before
the nozzle is withdrawrl from the septum, the filled container is sealed at
. . . . . . . . . . . . . ` . . , . . " ~ . . . . . .
;
6 2128292
a point on the container between the hollow body portion and the inserted
filling nozzle. The unneeded portion of the access passageway is then
detached and the sterile container containing sterile solution is ready for
further processing or use.
This invention, both as to its method of operation and apparatus,
will be best understood from the following description of spacific
embodiments when read in connection with the accompanyiny drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
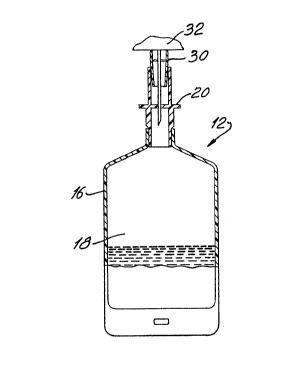
Figura 1 is a schematic representation of a flexible container having
an open end aligned but not attached to a separately fabricated access
passageway tube;
Figure 1A is an enlarged perspective view of the separately
fabricated access passageway tube of Figure 1;
Figure 2 is a schematic representation of the separately fabricated
access passageway tube s0alingly attached and closing the open end of the
flexible container;
Figure 2A is a schematic representation of an alternative separately
fabricated access passageway tube sealingly attached and closing the
open end of a rigid glass container;
Figure 2B is a schematic representation of an integral access
passageway fabricated with a semi-rigid container;
: .
212~292
Figure 3 is a schematic representation of a filling head mechanism
sealingly positioned at the exposed end of the access passageway tube;
Figure 3A is an enlarged view of the access passageway tube of
Figure 3; -~
Figure 3B is an enlarged view of the alternative access passageway
tube of Figure 2A sealingly contacted by an alternative filling head;
Figure 4 is a schematic representation of the access passageway
tube during sterilization by a representative on-line energy sterilization
process;
Figure 4A is an enlarged view of the access passageway tube of
Figure 4; ~:
Figure 4B is an enlarged view of the access passageway tube of
Figure 4 during sterilization by an alternative on-line chemical
sterilization process;
Figure 5 is a schematic representation of a filling nozzle piercing
the septum of the access passageway tube; :~
Figure 5A is an enlarged view of th~ access passageway tube of
Figure 5; ~ ~ ~
Figure 6 is a schematic representation of a seal produced at the neck ~ ~ :
portion of the filled flexible container prior to the filling nozzle being ~;
withdrawn from the septum;
212~292
8 ~ ~:
Figure 6A is an enlarged view of the access passageway tube of
Figure 6;
Figure 7 is a schematic representation of the access passageway
tube detached from the sealed neck portion of the fillecl flexible
container;
Figure 7A is an enlarged view of the access passageway tube of
Figure 7; and
Figure 8 is a schematic representation of the access passageway
tube after the filling head has been completely withdrawn from one end of
the tube and the filled and sealed flexible container is separated from the
other end.
DETA!~ED DESCRIPTIONS OF THE PREFERRED EMBODIMENTS
The sterile filling system for practicing the method of this
invention is schematically illustrated in Figures 1-8. A container, such as
a flexible plastic film bag 12 in Figure 1, or a fabricated paperboard
composite container, or a semi-rigid blow molded container, or a rigid
glass bottle, is manufactured with an open end 14. For discussion
purposes, the container 12 will be described as a flexible container
fabricated from two sheets of PVC plastic film, for example. However,
other flexible, semi-rigid and rigid container embodiments are also ~;
.................................................................................. ...
considered to be within the scope of this invention The two sheets of . ~
r~
:~ 212~292
g
plastic film are sealed together along the edges by any know sealing
technique, such as radio frequency (RF), ultrasonic or thermal welding.
The perimeter seal 16 extends along substantially the circumferential
perimeter of the flexible container, except for the opening 14, and defines
a hollow body portion 18. A hollow body portion is common to all
container embodiments of this invention, whether flexible, semi-rigid,
rigid or otherwise fabricated.
A separately manufactured access passageway tube 20 is fabricated
by molding or extruding a plastic material such as polyethylene or PVC,
for example. The access passageway tube 20 is adapted to fit and align
with the container opening 14. Alternatively, the access passageway may
be fabricated integrally with the container such as by known blow molding
or injection molding processes.
Referring now to Figure 1A, the access passageway tube 20 is
essentially a hollow tube, preferably cylindrical, although other shapes
such as an oval, square, diamond, or triangle are equally useable. In the
preferred embodiment, the access passageway tube 20 has two opposed
open ends, including a first end 22 and a second end 24. The tube also ~ ~
includes a pierceable septum 26 at an intermediate position within the ~ ~;
hollow passageway which completely divides and closes the passageway.
As shown in Figure 2, the access passageway tube 20 is permanently
2128292
attached and sealed to the open end 14 of the container. As shown in
Figures 2A, a slightly modified access passageway tube 21 is attached and
sealed to the outside open end of a glass bottle 23. As shown in Figure 2B
a further modified integral access passageway 25 is fabricated with a
semi-rigid blow molded container 27. All of the sealed, empty containers
may now be sterilized by any known suitable on-line or off-line
sterilizing process prior to being filled with an sterile solution.
Referring now to Figure 3 and 3A, a filling head 30 of the filling
mechanism 32 is axially moved into sealing contact with the second end
24 of the access passageway attached to the container.
The second end 24 of the access passageway 20 is sacured in an
airtight manner, and the closed volume within the sealed chamber 37 can
now be sterilized. A circumferential seal is formed at 36 when the distal,
tapered end of the nozzle mandrel 38 with the filling nozzle 34 therein
mates with or is removably received at the second end 24 of the access
passageway 20. The sealed chamber 37 of the access passageway 20 is
isolated from the environment to facilitate fast and efficient
sterilization. An alternative embodiment is shown in Figure 3B. ~:
Referring to Figures 4 and 4A, the access passageway is sterilized
on-line by passing through an energy sterilization unit such as shown
schematically by blocks 40 on both sides of the processing line.
212~292
1 1
Alternatively, as seen in Figure 4B, the access passageway can be
chemically sterilized with a vapor or liquid introduced into the sealed
chamber 37 by lumen 42 associated with the filling nozzle 34. Evacuation
and purging can also be accomplished through lumen 40 or additional
lumen as needed.
After the access passageway 20 is sterilized, the pierceable septum
26 of passageway is penetrated by the filling nozzle 24, as shown in
Figure 5 and 5A. The filling nozzle 34 provides fluid flow communication
with a sterile fluid source connected to the filling mechanism 32.
In Figure 4, the sterilization passageway is defined by the mandrel
38 in cooperation with the open end 24 when the distal, tapered end of the
mandrel is received therein. The tapered peripheral of mandrel 38 engages ::
:: :
the distal end portion of end 24 to provide a circumferential airtight seal
~. .
39.
Initially filling nozzle 34 is positioned above septum 26 while the
access passageway is sterilized. The access passageway 20 can be
sterilized, along with that portion of nozzle 34 that is situated within the ;
passageway, with subsequent evacuation and flush purge cycles as
required for the contemplated filling operation for the container 12.
Since the container has been pre-sterilized, when the filling nozzle
34 pierces septum 26, the sterile container 12 can be filled with the
::~` 2128292
1 2
desired sterile solution.
Thereafter, as can be seen in Figures 6 and 6A, the container is
resealed at seal 44 to insure continued sterility of the container and its
contents. After the container has been sealed, filling nozzle 34 and
mandrel 38 and the unneeded portion of the access passageway 20 are
separated from the container 12. Finally as seen in Figure 8, the nozzle
head is detached from the unneeded access passageway and positioned for
a subsequent sterilization and filling procedure.
Sealing of container 12 at seal 44 can be affected in any convenient
manner compatible with the container material and construction.
The present process is well suited for on-line filling operations of
off-line sterilized empty containers. This process is useful for the
sterilization of biological material which may degrade or loose potency if -~
the material is sterilized in a traditional terminal sterilization manner
after the container is filled.
The present process is also cost effective for the later on-line
sterile .filling of sterile empty containers previously sterilized by off-
line batch sterilization of the empty containers. ~-
The above discussion is int0nded by way of example only and is not
intended to limit the invention in any way except in the spirit and scope of
the appended claims.