Note: Descriptions are shown in the official language in which they were submitted.
2 1 2 9 1 2 1
o.z. 0050/43017
Mixtures of optically active cyclohexenone oxime ethers,
their preparation and intermediates therefor, and use
thereof as herbicides
De~cription
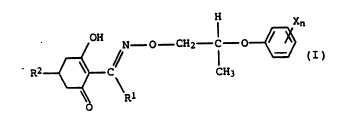
The present invention relate~ to novel mixture~
of optically active cyclohexenone oxime ethers, of R- or
S-configuration in the oxime ether moiety, of the general
formula I
~ N - O - CH~ ~ r
where the ~ub~tituent~ have the following meaning~:
Rl i~ a Cl-C~-alkyl group;
X i~ nitro, cyano, halogen, Cl-C,-alkyl or Cl-C,-halo-
alkyl;
n is 0 to 3 or 1 to 5 if all the Xs are halogen;
R' i~
a Cl-C,-alkoxy-Cl-C~-alkyl or Cl-C,-alkylthio-Cl-Cc-alkyl
group;
a C3-C7-cycloalkyl group or a C5-C7-cycloalkenyl
group, It being pos~ible if de~ired for these groups to
carry one to three ~ubstituent~ selected from a group
20. con~isting of Cl-C,~alkyl, Cl-C,-alkoxy, Cl-C,-alkylthio,
Cl-C~-haloalkyl, hydroxyl and halogen;
_ a 5-membered saturated heterocycle which contains
one or two oxygen and/or ~ulfur atom~ a~ heteroatoms and
which can, if de~ired, additionally carry one to thre~
substituent~ selected from a group consisting of
Cl-C~-alkyl, Cl-C~-alkoxy, Cl-C~-alkylthio and Cl-C~-halo-
alkyl;
a 6- or 7-membered ~aturated or mono- or diun-
~aturated heterocycle which contain~ one or two oxygen or
,
2129121
- 2 - O.Z. 0050/43017
sulfur atoms or an oxygen atom and a sulfur atom as
heteroatom~, :
it being po~ible if desired for the heterocycle
additionally to carry on~ to three ~ubstitu~nt~ ~elected
from a group consisting of hydroxyl, halogen, Cl-C~-alkyl,
C1-C,-alkoxy, C,-C,-alkylthio and Cl-C~-haloalkyl;
a 5-membered heteroaromatic, containing one to ~:
thre~ heteroatom~ select~d from a group co~si~ting of one
or two nitrog~n at~m~ and an oxygen or sulfur atom,
it b~ing possible fox the heteroaromatic if
desired additionally to carry one to three substituent~ `
selected from a group consisting of halogen, cyano,
Cl-C,-alkyl, Cl-C,-alkoxy, Cl-C,-alkylthio, Cl-C,-haloalkyl,
C2-C~-alkenyl, C~-C~-alkenyloxy and Cl-C~-alkoxy-
Cl-C,-alkyl;
a phenyl or pyridyl group, it being pos~ible for
these aromatic~ if de~ired additionally to carry one to
three ~ubstituen~ 3elected from a group con~isting of
halogen, nitro, cyano, Cl-C,-alkyl, Cl-C~-alkoxy,
Cl-C,-alkylthio~ C1-C,-haloalkyl, C3-C~-alkenyloxy,
C3-Cc-alkynyloxy a,~d an amino group -NR'Rb, where
R' is hydrogen, Cl-C,-alkyl, C3-Cc-alkenyl or
C3-Cc-alkynyl and
Rb i~ hydrog~n~ C1-C~-alkyl, C3-C6-alkenyl,
C3-C6-alkynyl, Cl-Cc-acyl or benzoyl which if de~ired ~:
can in turn carry one to three radicals selected
from a group con~i~ting of nitro, cyano, halogen,
Cl-C4-alkyl, Cl-C4-alkoxy, C,-C4-alkylthio and
Cl-C,-haloalkyl; :
_ and the agriculturally utili~able ~alts and
e~t¢rs of C~-C10-carboxylic acid~ and inorganic acid~ of
the compound~ I,
with the proviso that the mixture~ contain at
lea~t 75 mol% of i~omer~ of R-configuration in the oxLme
ether moiety.
The invention additionslly relates to a process
for preparing these compounds, their use as herbicid0~
2129121
- 3 - O.Z. 0050/43017
and herbicidal a~ent~ which co~tain these compound~ as
active substance~.
The invention furthermore relates to novel
mixtures of optically active hydroxylamine~, of R- or
S S-configuration, of the formula III
~N O- CH2 - C - O ~ r~I
CH3
where
i~ nitro, cyano, halogen, Cl-C~-alkyl or Cl-C,-halo-
alkyl and
n i~ O to 3 or 1 to 5 if all the X~ are halogen,
with the provi~o that the mixture~ contain at lea~t
75 mol~ of i~omsr~ of R-configuration, and a proceff~ for ~;
preparing the intermediate~
Herbicidally active cyclohexanedione~ of the
formul~
.
OH
~N - o - R~
RC
o .' ~
are already known fro~ the literature, where Rot Rd and R
have, inter alia~ th~ following meanings:
- US 4,440,566 ~R = ethyl or propyl; ~d Z benzyl; R-
_= 2-ethylthiopropyl);
- EP-A 238,021 and EP-A 125,094 (Rc = ethyl or propyl;
Rd = benzyl or but-2 enyl; R = a su~tituted
5-membered heteroaryl radical);
- EP-A 80,301 (Rc = ethyl or propyl; Rd 3 benzyl or
but-2-enyl; R - substituted phenyl);
- DE-A 3,838,309 (R ~ ethyl or propyl; Rd ~ a ~ub- :
~tituted 4-phenylbutylene Gr 4-phenylbutenylene
2129121
- 4 - O.Z. OOSO/43017
radical; R - a ~ubstituted S- to 7-membered hetero-
cycle);
- EP-A 456,112 (Rc ethyl or propyl; FP - a substitu-
ted 3-phenoxypropylene or 2-phenoxyethylene radical;
R - a ~ubstituted 5- to 7-membered hoterocycle).
The herbicidal propertie~ of the~e compound~, in
particular with re~pect to their selectivity for weeds in
graminaceous crop plants, however, may only give limited
~atisfaction.
Thus it was an ob~oct of the presont invontion to
provido novol mixturos of cyclohexonono oxim~ ethers
having improved solectivity for w~od~ in graminaceous
crops such a8 rice and m~izo.
Accordingly we have found t~at this object is
achievod by mixtur~ of optically active cycloh~xenone -
oxime othors I. Wo have additionally found herbicidal
compositions which contain those mixture~
Tho mixtures of optically active cycloh~xenone
oxime othors I are obtain~blo in variou~ ways, namely
prefor~bly in a manner known p~r se from already known
cyclohexenono~ of the formula II (DE-A 3,838,309,
EP-A 243,313, ~P-A 456,112) and the corresponding
mixture~ of optically active hydroxylamines of the
formula III (cf. EP-A 169,521): ~;
O H ~;
C ~ H~N O CH2 1--O ~ ~~~~
~ Rl CH3
_ rs II~
OH N - o CN2 C - O
C\ C~3
\~ Rl
~ 2129121
:..
- 5 - o.Z. 0050/43017
A suitable salt of the hydroxylamine III is
preferably used, in particular it~ hydrochloride, and the
reaction i~ carried out in the heterogeneous phase in an
inert sol~ent, for example in dimethyl sulfoxide, in an
alcohol ~uch as methanol, ethanol and isopropanol, in an
aromatic hydrocarbon such as benzene and toluene, in a
chlorinated hydrocarbon ~uch as chloroform and 1,2-di-
chloro~thane, in an aliphatic hydrocarbon ~uch as hexano
and cyclohexane, in an e~ter such as ethyl acetate or in
a~ ether ~uch as diethyl ether, dioxane and tetrahydro-
furan.
The reaction is conducted in the pre~ence of a
ba~e, an amount of b~se of about 0.5 to 2 molar equiva~
lents, bas~d on the amm~nium compound, normally being
~ufficient.
Suitable ba~es are, for example, carbonates,
hydrogencarbonate~, acetates, alkoxides or oxide~ of
alkali metals or alkaline earth metals, in particular
sodium hydroxide, potassium hydroxide, magnesium oxide or
calcium oxide. Organic bases such as pyridine and tert-
amines such as triethylamine are additionally suitable.
The reaction is preferably carried out in methanol using
sodium hydrogen carbonate as a base.
A variant of the process consi~ts in carrying out
the reaction without base with the free hydroxylamine
ba~es III, for example in the form of an aqueous 801u-
tion; depending on the solvent used for the compound II,
a ~ingle-phase or two-phase reaction mixture i~ obtained.
Suitable solvents for this variant are, for
example, alcohols such a~ methanol, ethanol, isopropanol
and cyclohexanol, aliphatic and aromatic, unchlorinated
or chlorinated hydrocarbon~ such as hexane, cyclohexane,
methylene chloride, toluene and 1,2-dichloroethane,
e~ters ~uch as ethyl acetate, nitrile~ ~uch a~ aceto-
nitrile and cyclic ethers ~uch as dioxane and tetrahydro-
furan.
The cyclohexenone II and the mixture of optically
2129121
- - 6 - o.Z. 0050/43017
active hydroxylamines III or their salts are expediently
employed in an approximstely ~toichiometric ratio, but in
some ca~es an exce~ of one or the other componente, up
to about 10 mol%, can be advantageous.
The reaction temperature i~ in general from 0C
to the boiling point of the reaction mixture, preferably
from 20 to 80C.
The reaction i8 complete after a few hour~. The
product can be i~olated in a customary manner, for
ex~mple by concentrating the mixture, partitioning the
residue in methylene chlorid~/water and distilling off
the solvent under reduced pre~sure.
Particular requirement~ with respect to the
pre~Qure do not have to be taken into account; in
general, the reaction is ther~fore carried out at at~
mospheric pressure or under the autogenous pressure of
the respective diluent.
On account of their acidic character, the opti~
cally active cyclohexenone oxime ethers I can form salts
of alkali metal or alkaline earth metal compounds, as
well a~ enol esters.
Alkali metal salts of the compound~ I can be
obtained by treating the 3-hydroxycyclohexenone compounds
with sodium hydroxide or alkoxide or potas~ium hydroxide
or alkoxide in agueous solution or in an organic solvent
_uch as methanol, ethanol, acetone and toluene.
Other metal salts such as manganeYe, copper,
zinc, iron, calcium, magnesium and barium salts can also
be prepared from the sodium salts in a cu_tomary manner,
a3 well as ammonium and pho~phonium Qalts by meanQ of
ammonia, or phosphonium, Qulfonium or sulfoxonium
hydroxidea.
The esters of the cQmpounds I are al~o obtainable
in a cu_tomary manner ( cf., for example, Organikum, VEB
DeutQcher Verlag der Wissen~chaften, Berlin, 17th edi-
tion, (1988) pp. 405-408).
The novel mLxture~ of optically active
2129121
- 7 - O.Z. 0050/43017
hydroxylamine~ III can be prepared via a number of known
proce~s ~tep~ starting from known precur~or~:
N x
/C~ t HO C~ - C O
D N - O~ C~3
C or.
O H Xn ~-
VI I ~ - o~2 - C O ~ .
CN
e
D 1~2~-CH2CH~-o~
~N--O--C~- C- 0
CH3
O .
Y~I
L - a leaving group, for example halogen such a~
chlorine, bromine or iodi~e, or CH3502-O-.
The optically active alkylati~g agent~ V (Tetra-
hedron Lett. 29, (1988) 5493; J. Org. Chem. 52 (198~
3587; EP-A 172 719; EP-A 230 379; US 4~841,079), but if
de~ired ai~o the optically active carbinol IV (Chem.
Pharm. Bull. 33, (1985) 1955) i~ preferably coupled by
the_Mit~unobu variant (Synthe~i~ 1, 1981; J. Med. Chem.
33, (1990) 187) with a cyclic hydroxyimide VI and the
prot~cted hydroxylamine derivative VII ob~ained in thi~
proce~ cleaved to give the fr~e optically actiYe
hydroxylamine III, for example u~ing 2-aminoethanol.
In the cyclic hydroxyLmide3 VI D i~, for example,
C2-C3-alkylene, C2-alkenylene or a 5- or 6-membered rlng
with or without a nitrogen atom, which can be ~aturated,
~ 21~9121
- 8 - O.Z. 005~/43017
partially unsaturated or aromatic, for ~xample phenylene,
pyridinylene, cyclopentylene, cyclohexylene or cyclo-
hexenylene.
For sxample, the following ~ub~tance~ are ~uit-
able:
N-ON ~ NLCH ~ N-O~
O O
.
~0 ¢ ' ~0
~
~0 .The reaction of the optically active alkylating
agent~ V with the hydroxyLmide3 VI i~ expediently carried
out in the pre~ence of a base. Suitable ba~e~ are prin-
cipally all tho~e which ar~ able to deprotonate the
hydroxyimide~ VI without atta~king the Lmide ~y~tem.
The~ ar~ particularly the ~o-called non-nucleophilic
ba~e~.
_ Examples which may be mentioned are inorganic
bases such a~ alkali metal and alkaline earth metal
carbonates, alkali metal and alkaline earth metal hydro-
gencarbonate~, organic ba~e~ ~uch a~ aliphatic, cyclo-
aliphatic and aromatic tertiary ~mine~ However, mixtures
of the~e base~ can al50 be u~ed.
Individual compounds which may be li~ted by way
of axample are the following base~: ~odium carbonate,
`~ 212~121
- - 9 - O.Z. 0050/43017
potassium carbonate, magnesium carbonate, calcium
carbonate, barium carbonate, the hydrogencarbonate~ of
these metals, trimethylamine, triethylamine, tributyl-
amine, ethyldiisopropylamin~, N,N-dimethylaniline,
4-lN,N-dimethylamino)pyridine, diazabicyclooctane,
diazabicycloundecane, N-methylpiperidine, 1,4-dimethyl-
piperazine, pyridine, quinoline, bipyridine and phenan-
throline. The inexp~nsive bases sodium carbonate and
potassium carbonate are preferred.
The base is generally added in equivalent amounts
up to an excess of 5 equivalents, based on the hydroxy-
imide. A greater excess is possible, but as a rule
conv~ys no additional advantages. The uso of a small
amount of ba~e i~ also pos~ibl~. ~owever, an amount of
base from 1 to 3 equivalents, in particular from 1 to 2
equivalents, based on the hydroxyimide VI, is preferably
employed.
The use of nucleophilic bas~s, for example alkali
metal and alkaline earth metal hydroxides, in particular
~odium hydroxide and potassium hydroxid~, is also pos-
~ibl~. In thi~ case, it is advantageous to employ the
base in equivalent amounts with re~pect to the hydroxy-
imide VI to prevent a nucleophilic attack of the hydroxyl
ions on the carboxyl function of the imide group.
The optically active alkylating agents V are
expediently reacted with the hydroxyimides VI in a
~olvent which is inert under the reaction conditions.
Advantageous ~olvents are, for exampls, polar aprotic
solvents such as dimethylformamide, N-methylpyrrolidone,
dimethyl sulfoxide, sulfolane and cyclic urea~. The
amount of solvent i~ generally not critical.
The reaction of the optically active alkylating
agents V with the hydroxyimides VI can al~o be carried
out u~ing phase tran~fer catalysis. In thi~ case, ~ol-
vents forming two phases with water, preferably chloro-
hydrocarbons, are employed.
Useful phase transfer catalysts are the
` 2129121
-- - 10 - O.Z. OOS0/43017
quaternary ammonium and phosphonium salts, polyethylene
qlycols, polyethylene glycol ethers and crown ethers
customarily used for ~uch purpose~, as are described, for
example, in Dehmlow et al., Phase Transfer Catalysi~
(1980), pp. 37-45 and pp. 86-93, Verlag Chemie, Weinheim.
The phase transfer catalysts are expediently
employed in amounts from 1 to 10% by volume, preferably
in amounts from 3 to 5% by volume, based on the volume of
the reaction mixture.
10The optically active alkylating agent~ V are
generally reacted with the hydroxyimide~ VI in a temper-
ature range from 0 to 140C, pr~ferably from 20 to 100C,
in pRrticular from 40 to 80C. A procedure i~ advantageo-
u~ly u~ed here in which the hydroxyimide VI is introduced
into the solvent together with the base and the alkyla-
ting agent V is metered into this solution. In thi~
procedure, it may prove convenient to add the hydroxy-
m~de at a lower temperature, for ex~mple at from 0 to
50C, and to heat the reaction mixture to the actual
reaction temperature only after this addition.
After reaction is complete, the cooled reaction
mixture is expediently treated with water, the hydroxyl-
amine derivatives VII formed precipitating a~ crystalline
~olid~ or as oil~. The hydroxylam;ne derivatives obtained
in this way can be further purified, if desired, by
recrystalliz~tion or by extraction.
The hydroxylamine derivative~ VII can be tem-
porarily stored or immediately converted into the opti-
cally active hydroxylam;nes III having a free amino
group.
This conversion can be carried out by proces~es
known per se, as ar~ described, for example, in
D~-A 3,615,973 and the papers cited therein. Preferably,
the proces~ as described in DE-A 3,615,973 is used,
according to which the optically active hydroxylamines
III were liberated by means of ethanolamine. The
liberation of the optically active hydroxylamines III
. .
2129121
.
- 11 - O.Z. 0050/43017
using other ba~es such as aqueous inorganic bases, with
amines, hydrazine~ or hydroxylamines or by means of
aqueous acids is also poQsible.
The optically active hydroxylamine~ III can be
i~olated from the reaction mixture~ obtained by the~e
proce~se~ by means of customary working-up method~, for
example by extraction or by crystallization. To increa~e
the tendency of these hydroxylamines III to crystallize,
it may often be beneficial to convert the~e into their
salts with inorganic acid or organic acids. To this end,
dilute solutions of these acid~ are generally reacted
with the hydroxylamine derivativeo, namely expediently in
equivalent amounts. Like the optically active hydroxyl-
amines III (baving a free amino gropp), the hydroxyl-
a _o~ium salts obtained can be directly processed to the
optically active cyclohexenone oxime athers of the
formula I or alternatively stored, if desired.
The optical purity of the intermediates III and
of the cyclohexenone oxime ether~ I depend~ on the
optical purity of the carbinols IV or alkylating agent~
V employed. According to the invention, carbinols IV
having the R-configuration or alkylating agent~ V having
the R-configuration are employed, namely with as high an
optical purity as possible, 80 that in the preparation of
the optically active hydroxylamines III and of the
opticalIy active cyclohexenone oxLme ether~ I, isomer
mixture~ are obtained in each ca~e who~e content of
i~omer~ having the R-configuration on the methyl-~ub-
~tituted C atom (in the oxime ether moiety) i~ at lea~t
75 mol%, in particular 90 to 100 mol%.
The optically active cyclohexenone oxim~ ether~
I can be obtained during the preparation a~ i~omer
mixture~, both E/Z isomer mixture~ (position of the oxime
ether moiety relative to R1) and diastereoi~omer mixture~
being po~ible. If desired, the isomer mixture~ can be
~eparated by the method~ cu~tomary for thi~ purpo~e, for
example by chromatography or by cry~tallization.
.-``` 212~121 :
- 12 - O.~ 0050/43017
The optically active cyclohexenone oxLm~ ethers
I can be written in ~everal tautomeric form~, which are
all included by the invention.
The collective terms used in th~ definition of
the ~ubstitu~nt~
- halogen,
-Cl-C~-alkyl, Cl-C,-alkoxy, Cl-C,-alkylthio, Cl-C,-halo- ~ :
alkyl,
- C2-C6-alkenyl, C2-C6-alkenyloxy,
- C3-C,-alkenyl, C3-C6-alkenyloxy, C3-C6-alkynyl,
C3-C,-alkynyloxy,
- Cl-C~-acyl ~,
are a ~hort way of writing an individual ligt of the ~ -
individual group memb~rs. All the al~yl, alkoxy, alkyl-
thio, haloalkyl, alkenyl, alkenylo~y, alkynyl and
alkynyloxy moieties can be straight-chain or branched.
The haloalkyl moieties can carry identical or different
halogen atoms.
The following term~ ~pecifically mean, for
example
- halogen: fluorine, chlorine, bromine and iodine;
- Cl-C~-alkyl: methyl, ethyl, n-propyl, l-methylethyl,
n-butyl, l-methylpropyl, 2-methylpropyl and l,1-di-
methylethyl;
- Cl-C~-alkoxy; methoxy, ethoxy, n-propoxy,
1-methylethoxy, n-butoxy, 1-methylpxopoxy, 2-methyl-
propoxy and l,l-dLmethylethoxy;
- C,-C~-alkylthio: methylthio, ethylthio 9 n-propylthio,
l-methylethylthio, n-butylthio, l-met~ylpropylthio,
_ 2-methylpropylthio and 1,1 dLmethylethylthio;
- Cl-C4-haloalkyl: fluoromethyl, difluoromethyl,
trifluoromethyl, chlorodifluoromethyl, dichloro-
fluoromethyl, trichloromethyl, l-fluoroethyl,
2-fluoroethyl, 2,2-difluoroethyl, 2,2,2-tri~luoro-
ethyl, 2-chloro-2,2-difluoroethyl, 2,2-dichloro-
2-fluoroethyl, 2,2,2-trichloroethyl and pentafluoro-
ethyl; ` ~;
212~121
- 13 - O.Z. 0050/43017
- C2-C~-alkenyl: ethenyl and C3-C6-alkenyl such a~
l-propenyl, 2-propenyl, l-methylethenyl, l-butenyl,
2-butenyl, 3-butenyl, l-methyl-l-propenyl, l-methyl-
2-propenyl, 2-methyl-1-propenyl, 2-methyl-
2-propenyl, l-pentenyl, 2-pentenyl, 3-pentenyl,
4-pentenyl, l-methyl-l-butenyl, 2-methyl-1-butenyl,
3-methyl-1-butenyl, 1-methyl-2-butenyl, 2-methyl-
2-butenyl, 3-methyl-2-butenyl, 1-methyl-3-butenyl,
2-methyl-3-butenyl, 3-methyl-3-butenyl,
1,1-dLmethyl-2-propenyl, 1,2-dimetbyl-1-propenyl,
1,2-dimethyl-2-propenyl, l-ethyl-l-propenyl,
l-ethyl-2-propenyl, l-hexenyl, 2-hexenyl, 3-hexenyl,
4-hexenyl, 5-hexenyl, l-methyl-l-pentenyl, 2-methyl-
1-pentenyl, 3-methyl-1-pent~nyl, 4-methyl-
1-pentenyl, 1-methyl-2-pentenyl, 2-methyl-
2-pentenyl, 3-methyl-2-pentenyl, 4-methyl-
2-pentenyl, 1-methyl-3-pentenyl, 2-methyl-
3-pentQnyl, 3-methyl-3-pentenyl, 4-methyl-
3-pentenyl, 1-methyl-4-pentenyl, 2-methyl-
4-pentenyl, 3-methyl-4-pentenyl, 4-methyl-
4-pentenyl, 1,1-dLmethyl-2-butenyl, 1,l-dLmethyl-
3-butenyl, 1,2-dLmethyl-l-butenyl, 1,2-dLmethyl-
2-butenyl, 1,2-dLmethyl-3-butenyl, 1,3-dimethyl-
1-butenyl, 1,3-dimethyl-2-butenyl, 1,3-dLmethyl-
3-butenyl, 2,2-dLmethyl-3-butenyl, 2,3-dimethyl-
l-butenyl, 2,3-dimethyl-2-butenyl, 2,3-dimethyl-
3-butenyl, 3,3-dLmethyl-l-butenyl, l-ethyl-
l-butenyl, l-ethyl-2-butenyl, 1-ethyl-3-butenyl,
2-ethyl-1-butenyl, 2-ethyl-2-butenyl, 2-ethyl-
_ 3-butenyl, 1,1,2-trimethyl-2-propenyl, l-ethyl-
1-methyl-2-propenyl,l-ethyl-2-methyl-1-propenyland
1-ethylo2-methyl-2-propenyl;
- C2-Cc-alkenyloxy: ethenyloxy and C3-C6-alkenyloxy
~uch a~ 2-propenyloxy, 2-butenyloxy, 3-butenyloxy,
1-methyl-2-propenyloxy, 2-methyl-2-propenyloxy,
2-pentenyloxy, 3-pentenyloxy, 4-pentenyloxy,
l-methyl-2-butenyloxy, 2-methyl-2-butenyloxy,
~.
2129121
- 14 - O.Z. 0050/43017
3-methyl-2-butenyloxy, 1-methyl-3-butenyloxy,
2-methyl-3-butenyloxy, 3-methyl-3-butenyloxy, ~ :
1,1-dimethyl-2-propenyloxy, 1,2-dimethyl-
2-propenyloxy, 1-ethyl-2-propenyloxy, 2-hexenyloxy,
3-hexenyloxy, 4-hexenyloxy, 5-hexenyloxy, l-methyl-
2-pentenyloxy, 2-methyl-2-pentenyloxy, 3-methyl-
2-pentenyloxy, 4-methyl-2-pentenyloxy, 1-methyl-
3-pentenyloxy, 2-methyl-3-pentenyloxy, 3-methyl-
3-pentenyloxy, 4-methyl-3-pentenyloxy, l-methyl-
4-pentenyloxy, 2-methyl-4-pentenyloxy, 3-methyl-
4-pentenyloxy,4-methyl-4-pentenyloxy,1,l-dimethyl-
2-butenyloxy, 1,2-dimethyl-2-butenyloxy,
1,2-dLmethyl-3-butenyloxy, 1,3-dLm~thyl-2-butenyl-
oxy, 1,3-dimethyl-3-butenyloxy, 2,2-dimethyl-
3-butenyloxy, 2,3-dim~thyl-2-butenyloxy,
2,3-dimethyl-3-butenyloxy; 1-ethyl-2-butenyloxy,
l-ethyl-3-butenyloxy,2-~thyl-2-butenyloxy,2-ethyl-
3-butenyloxy, 1,1,2-trimethyl-2-propenyloxy,
l-ethyl-l-methyl-2-propenyloxyandl-ethyl-2-methyl-
2-propenyloxy;
With re~pect to their herbicidal activity,
cyclohexenone~ of the formula I are preferred in whi~h
the variable~ have the following meaning~:
R~ Cl-C6-alkyl such as methyl, ethyl, n-propyl, n-butyl,
n-pentyl, n-hexyl, preferably ethyl and propyl;
X - nitro, cyano,
- halogen, preferab~y fluorine, chlori~ and
bromine; ~ -
- Cl-C4-alkyl, preferably methyl;
_ - C~-C~-haloalkyl, preferably difluoromethyl, tri- ~:
fluoromethyl, 2,2,2-trifluoroethyl and penta-
fluoroethyl; ~ :
halogen i~ particularly preferred; ~-
n 0 to 3 or 1 to 5 if all the Xa ar~ halogen; 0 to 3 ~::
i~ particularly preferred;
R2
- a Cl-C6-alkyl group such as methyl, ethyl, n-propyl,
2i2~
`
. - 15 - O.Z. 0~50/43017
1-methylethyl, n-butyl, l-methylpropyl, 2-methyl-
propyl and 1,1-dimethylethyl, n-pentyl,
l-methylbutyl, 2-methylbutyl, 3-methylbutyl, l,1-di-
methylpropyl, 1,2-dimethylpropyl, 2,2-dL~ethyl-
propyl, l-ethylpropyl, n-hexyl, l-methylpentyl,
2-methylpentyl, 3-methylpentyl, 4-methylpentyl,
1,1-dLmethylbutyl, 1,2-dimethylbutyl, 1,3-di-
methylbutyl, 2,2-dLmethylbutyl, 2,3-dLmethylbutyl,
3,3-~;methylbutyl, l-ethylbutyl, 2-ethylbutyl,
1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl,
1-ethyl-1-methylpropyl and 1-~thyl-2-methylpropyl,
where the alkyl group is ~ubstituted by Ca-C,-alkoxy,
pr~f~rably methoxy, ethoxy, 1-methylethoxy or
1,1-dimethylethoxy, or by Cl-C~-alky~thio, preferably
methylthio or ethylthio, namely preferably in the
1-, 2- or 3-position;
2-ethylthiopropyl is very particularly preferred;
- a C3-C7-cycloalkyl group or a C5-C~-cycloalkenyl
group such a~ cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl, cyclopentenyl, cyclohexenyl
and cycloheptenyl, wherQ the~e group~ can be unsub-
~tituted or can carry one to three of the following
~ub~tituents~
Cl-C,-alkyl, Cl-C,-alkoxy, C~-C,-alkylthio and
Cl-C~-haloalkyl; 1-methylthio-1-cyclopropyl i5 very
particularly preferred;
- a 5-memb~red ~aturated h~terocycle ~uch as tetra-
hydrofuranyl, tetrahydrothienyl, dioxolanyl, di-
thiolanyl snd oxathiolanyl, in particular tetrahy-
_ drofuranyl, tetrahydrothienyl and dioxolanyl, where
the~e ring~ can be un~ub~tituted or ca~ carry one to
three ~ub~tituents selected from a group consisting
of Cl-C,-alkyl, Cl-C,-alkoxy, Cl-C4-alkylthio and
Ci-C,-haloalkyl;
35 - a 5-mem~ered heteroaromatic such a~ pyrrolyl,
pyrazolyl, Lmidazolyl, isoxazolyl, oxazolyl, iso-
thiazolyl, thiazolyl, furanyl and thienyl, in
2129121
. - 16 - o.Z. 0050/43017
particular isoxazolyl and furanyl, where th~ 5-
membered heteroaromatic can be unsubstituted or can
carry one to three substituents selected from a
group consisting of Cl-C~-alkyl, C,-C,-alkoxy,
Cl-C~-alkylthio, Cl-C,-haloalkyl and Cl-C,-alkoxy-
Cl-C,-alkyl sùch a~ methoxymethyl, 2-methoxyethyl,
2-methoxypropyl, 3-methoxypropyl, 2-methoxy-
l-methylethyl, ethoxymethyl, 2-ethoxyethyl,
2-ethoxypropyl, 3-ethoxypropyl, 2-ethoxy-1-methyl-
ethyl and l-ethoxy-l-methylethyl, preferably
methoxy~thyl and ethoxyethyl,
C2-C,-alkenyl such aB ethenyl and C3-C~-alkenyl,
preferably l-methyleth~n-l-yl,
C2-C~-alkenyloxy such as ~thenyloxy and C3-C~-al-
kenyloxy, in particular l-methylethen-l-yloxy,
- a 6- or 7-membered heterocycle which
a) can be ssturated, for example tetrahydropyran-
3-yl, tetrahydropyran-4-yl, tetrahydrothiopyran-
3-yl, tetr~hydrothiopyran-4-yl and dioxepan-5-yl,
b) can be mono- or diunsaturated, for example di-
hydropyran-3-yl, dihydropyran-4-yl, dihydrothio-
pyran-3-yl and dihydrothiopyran-4-yl,
where the heterocycles can be unsubstituted or can
carry one to three substituents ~elected from a
group con~i~ting sf hydroxyl, halogen, C,-C~-alkyl,
C~ alkoxy, Cl-C~-alkylthio and Cl-C4-haloalkyl;
tetrahydropyran-3-yl, tetrahydropyran-4-yl and
tetrahydrothiopyran-3-yl are very particularly
preferred;
- _ a phenyl or pyridyl group, both of which can be
unsubstituted or can carry one to three ~ubstituent~
~elected from a group con~i~ting of C1-C,-alkyl,
Cl-C~,-alkoxy, Cl-C~-alkylthio, Cl-C,-haloalkyl,
C3-C6-alkenyloxy, preferably 2-propenyloxy and
2-butenyloxy, C3-C~-alkynyloxy such as 2-propynyloxy,
2-butynyloxy, 3-butynyloxy, 1-methyl-2-propynyloxy,
2-pentynyloxy, 3-pentynyloxy, 4-pentynyloxy,
212912~
- 17 - o.Z. ~050/43017
l-methyl-3-butynyloxy, 2-methyl-3-butynyloxy,
l-methyl-2-butynyloxy, 1,1-dLmethyl-2-propynylox~,
l-ethyl-2-propynyloxy, 2-hexynyloxy, 3-hexynyloxy, ~ :~
4-hexynyloxy, 5-hexynylo~y, l-methyl-2-pentynyloxy, -:
1-methyl-3-pentynyloxy, 1-methyl-4-pentynyloxy,
2-methyl-3-pentynyloxy, 2-methyl-4-p~tynyloxy,
3-methyl-4-pentynyloxy, 4-methyl-2-pe~tynyloxy,
1,1-dLmethyl-2-butynyloxy, 1,1-dimethyl-3-butynyl-
oxy,l,2-dLmethyl-3-butynyloxy,2,2-dLmethyl-3-buty-
nyloxy, 1-~thyl-2-butynyloxy, 1-ethyl-3-butynyloxy,
2-ethyl-3-butynyloxy and l-ethyl-l-m~thyl-2-pro-
py~yloxy, prefer~bly 2-propynyloxy a~d 2-butynyloxy;
one of th~ three substituents on th~ phenyl or
pyridyl ring can al80 b~ an amino group -NR~Rb, wh~r~
R' i~ :
hydrogen,
Cl-C~-alkyl, preferably mQthyl or ~thyl, -~
C3-Cc-alkenyl, preferably 2-propenyl or 2-but~nyl,
C3-C~-alkynyl, preferably 2-propynyl or 2 butynyl,
and ~ :~
Rb i~
hydrogen,
Cl-C,-al~yl, preferably methyl or ethyl,
C3-C6-alkenyl, preferably 2-propenyl or 2-butenyl,
C3-C~-alkynyl, preferably 2-propynyl or 2 butynyl,
or i8
Cl-C6-acyl such a~ acetyl, propionylO butyryl,
2-methylpropionyl, n-pentanoyl, 2-methy~butyryl,
3 methylbutyryl, 2,2-dLmethylpropionyl, n-hexanoyl,
_ 2-methylpentanoyl, 3-methylpentanoyl~ 4-methyl-
pentanoyl,2,2-dLmethylbutyryl,2,3-dimethylbutyryl,
3,3-dLmethylbutyryl and 2-ethylbutyryl, pr~ferably
acety~ or propionyl,
or benzoyl which can be un~ub~tituted or can in turn
carry one to three radical~ selected from a group
con~i~ting of nitro, cyano, halogen, preferably
fluorine, chlorine and bromine, Cl-C~-alkyl,
2129121
- 18 - O.Z. 0050/43017
preferably methyl, Cl-C,-alkoxy, preferably methoxy
and ethoxy, Cl-C,-alkylthio, preferably methylthio,
and Cl-C~-haloalkyl, preferably tri~luoromethyl.
Suitable ~alt~ of the compound~ of the formula I
are agriculturally utilizable ~alts, for example alkali
metal ~alt~, in particular the ~odium or potas~ium ~alt,
alkaline earth metal ~alts, in particular the calcium,
magnesium or barium ~alt, the manganese, copper, zinc or
iron salt or the ammonium, phosphonium, sulfonium or
sulfoxonium salt~, for example ammonium salts, tetra-
alkyla _ onium salt~, benzyltrialkylammonium salts,
trialkylsulfonium salts or trialkylsulfoxonium salts.
Ester- of Cl-C1O-carboxylic acids are in par-
ticular undsrstood a- meaning C~-C,-al~ylcarboxylic acids
such as metbanecarboxylic acid (acetic acid), ethanecar-
boxylic acid (propionic acid), propanecarboxylic acid
(butyric acid), l-methylethanecarboxylic acid (isobutyric
acid), butanscar~oxylic acid, l-methylpropaneoarboxylic
acid, 2-methylpropanecarboxylic acid, l,l-d~methyl-
ethanecarboxylic acid, pentanecarboxylic acid, 1-meth-
ylbutanecarboxylic acid, 2-methylbutanecarboxylic acid,
3-methylbutanecarboxylic acid, 1,1-dimethylpropane-
carboxylic acid, 1,2-dimethylpropanecarboxylic acid,
2,2-dimethylpropanecarboxylic acid, l-ethylpropane-
carboxylic acid, benzoic acid and benzoic acids~ubstituted by halogen, hexanecarboxylic acid, l-methyl-
pentanecarboxylic acid, 2-methylpentanecarboxylic acid,
3-methylpentanecarboxylicacid,4-methylpentanecarboxylic
acid, l,l-dimethylbutanecarboxylic acid, 1,2-dimethyl-
butanecarboxylic acid, 1,3-dimethylbutanecarboxylic acid,
2,2-dimethylbutanecarboxylic acid, 2,3-dimethyl-
butanecarboxylic acid, 3,3-dimethylbutanecarboxylic acid,
l-ethylbutanecarboxylic acid, 2-ethylbutanecarboxylic
acid, 1,1,2-trimethylpropanecarboxylic acid, 1,2,2-tri-
methylpropanecarboxylic acid, l-ethyl-l-methylpropane-
carboxylic acid and l-ethyl-2-m~thylpropanecarboxylic
acid.
.. ..
212912 ~
.
- 19 - O.Z. 0050/43017
PREPARATIO~ ~XAMPLES
(R)-2-~1-(2-(4-ehlorophenoxy)propyloxyimino]propyl]-
3-hydroxy-5-(1-methylthioeyelopropyl)-2-eyelohexen-1-one
A mixture of 1.0 g (3.9 mmol) of 3-hydroxy-
5-(1-methylthioeyelopropyl)-2-propionyl-2-eyclohexen-
l-one, 0.95 g (4.7 mmol) of (R)-0-~2-(4-ehlorophenoxy)-
propyllhydroxylamine and 80 ml of methanol was ~tirred
for 24 hours and then eoneentrated under r~dueed pres-
sure. The re~idue was taken up in tert-butyl methyl
ether, after whieh the ether pha~e was extraeted with 10~ ;
strength by weight ~odium hydroxide ~olution. The aqueous
phase was in turn ~xtraeted with tert-butyl methyl ether
and then aeidified with 10~ ~trength by weight hydroehlo-
rie aeid. It wa~ finally extraeted onee more with tert-
butyl methyl ether, after whieh the organie phase was
dried over sodium sulfate and eoneentrated under redueed
pressure. Yield: 59%; ta]~5 - - 13.1 (e 3 l.Q; in
methanol); --
~H-NMR (200 MHz, in CDCl3): ~ - 0.77 ppm (m,2~); 0.97 ppm
(m,2~); 1.10 ppm (t,3H); 1.35 ppm (d,3H); 1.60 ppm
(m,lH); 2.13 ppm (8,3~); 2.40 - 2.80 ppm (m,4H); 2.90 ppm
(q,2~); 4.20 ppm (m,2H); 4.6~ ppm (m,lH); 6.90 ppm ;~
(d,2H); 7.20 ppm (d,2H); 14.20 ppm (b~,lH).
PRECURSOR
(R)-O-t2-(4-~hlorophenoxy)propyllhydroxylamine -~
24.9 g (0.143 mol) of diethyl azodiearboxylat~
were ~lowly added dropwi~e to a solution of 23.3 g
(0.143 mol) of N-hydroxyphthalLmide, 33.3 g (0.127 mol)
of triphenylpho~phine and 23.7 g (0.127 mol) of (R)- -
2-(4-ehlorophenoxy)-1-propanol tCAS Reg. No. 92471-63-1,
Chem. Pharm. Bull. 33, (1985) 1955] in 300 ml of tetra-
hydrofuran. After a weakly exothermic reaetion, the
mixture wa~ ~tirred for about a furth~r 15 hours and then
concentrated. By ehromatography of the re~idue on ~iliea
gel (eyelohexane/ethyl acetate 1:1), 42 g of (R)-N-
t2-(4-ehlorophenoxy)propoxy]phthalimide were obtained.
This erude phthal;~;de produet was ~ubseguently
212gl~ 1
- 20 - O.z. 0050/43017
treated ~lowly with 100 ml of ethanolamine. After 5 hours
at 60C, the reaction mixture was poured into ice-water,
extracted with methylene chloride, and the combined
organic phase~ were washed with water, dried over ~odium
~ulfate and concentrated under reduced pre~sure. Yield:
81%; ta]25 ~ -20.8 (c ~ 1.0; in methanol).
H-NMR (360 M8z, in CDCl~ 1.25 ppm (d,3H); 3.75 ppm
(dd,lH); 3.85 ppm (dd,lH); 4.65 ppm (m,lH); 5.50 ppm
(b~,28); 6.90 ppm (d,2H); ?.20 ppm (d,2H). ~ -
In the following Tab}e I, further opt~cally -
active hydroxylamine~ III are shown which w~re prepared
or can be prepared in the ~ame way. The Table~ 2 to 8 ~ ~ -
contain optically active cyclohexenone oxime ether~ I
according to the invention. -
. ~
21 2 1 2 9 1 2 1 . Z . 0050/430l7
Table 1
H X
H2N 0--CH2 C 0~ III
` :.
CH3
' '
:
No. Xn Phys. data
~Rotational value ~a] D25;
c= 1.0,
in CH30H / lH-NMR lppm] )
~:;
1 . 01 2-Cl -34 . 1
1 . 02 3-Cl -23 . 2
1 . 03 4-Cl -2U . 8
20 1 . 04 2, 4-C12 -27 . 5
1. 05 2-CH3, 4-Cl -26 . 2
1. 06 2, 4-F2 -21. 5
1. 07 4-F -27 . 5
22 O.~. 0050/43017
212~121
Table 2
.
Cl "~ ,,
N CH2 C 0 ~
~ ~ I (Xn = 2-Cl)
R2 ~ C CH3
~ R
'. ~
No. R1 R2 Phys. data
~Rotational
value
[a] D25;
c = 1.0,
in CH30H/
lH-NMR ~ppm]/
m.p. [C])
`~
2.01 Ethyl (RS)-2H Tetrahydropyran-3-yl -42.0
2.02 Ethyl (R)-2H-Tetrahydropyran-3-yl
2.03 Ethyl (S)-2H-Tetrahydropyran-3-yl
2.Q4 Ethyl 2H-Tetrahydropyran-4-yl -37.2
2.05 Ethyl (RS)-2H-Tetrahydrothiopyran-3-yl -34.6
2.06 Ethyl ~R)-2H-Tetrahydrothiopyran-3-yl
2.07 Ethyl (S)-2H-Tetrahydrothiopyran-3-yl
-2.08 n-Propyl (RS~-2H-Tetrahydropyran-3-yl -33.6
2.09 n-Propyl (R)-2H-Tetrahydropyran-3-yl
2.10 n-Propyl (S)-2H-Tetrahydropyran-3-yl
2.11 n-Propyl 2H-Tetrahydropyran-4-yl -38.9
2.12 n-Propyl (RS)-2H-Tetrahydrothiopyran-3-yl -27.6
2.13 n~Propyl (R)-2H-Tetrahydrothiopyran-3-yl
2.14 n-Propyl (S~-2H-Tetrahydrothiopyran-3-yl
35 2.15 Ethyl Phenyl
2.16 Ethyl 2,4,6-Trimethylphenyl
2.17 Ethyl 4-(Prop-2-ynyloxy)phenyl
2.18 n-Propyl 4-Fluoro-3~nitrophenyl
2.19 n-Propyl (RS)-2-(Ethylthio)prop-1-yl
2.20 Ethyl 1-Methylthiocycloprop-1-yl
2.21 Ethyl 1,3-Dimethylpyrazol-5-yl
2.22 n-Propyl 3-Isopropyli~oxazol-5-yl
2.23 n-Propyl (RS)-Cyclohex-3-en-1-yl
23 O.Z. 005~/43017
212~121
.
Table 3
Cl
H
~ N CH2 C 0 ~ I (Xn = 3-Cl~ ;~
R2 ~ C CH3
\\ R~
' ~ ~'
No. Rl R2 Phys. data
(Rotational
value
[a] D25; ;,,,
c
in CH30~
1H-NMR [ppm]/
m.p. lC])
3.01 Ethyl (RS)-2H-Tetrahydropyran-3-yl -13.2
3.02 Ethyl (R)-2H-Tetrahydropyran-3-yl
3.03 Ethyl (S)-2H-Tetrahydropyran-3-yl
3.04 Ethyl 2H-Tetrahydropyran-4-yl -17.1
3.05 Ethyl (RS)-2H-Tetrahydrothiopyran-3-yl -11.8
3.06 Ethyl (R)-2H-Tetrahydrothiopyran-3-yl
3.07 Ethyl (S)-2H-Tetrahydrothiopyran-3-yl
3.08 n-Propyl (RS)-2H-Tetrahydropyran-3-yl -11.7
3~09 n-Propyl (R)-2H-Tetrahydropyran-3-yl
3.10 n-Propyl (S)-2H-Tetrahydropyran-3-yl
3~ 3.11 n-Propyl 2H-Tetrahydropyran-4-yl -12.5
3.12 n-Propyl (RS)-2H-Tetrahydrothiopyran-3 yl - 8~3
3.13 n-Propyl (R3-2H-Tetrahydrothiopyran-3-yl
3.14 n-Propyl (S)-2H-Tetrahydrothiopyran-3-yl
3.I5 Ethyl Phenyl -17.4
3.16 Ethyl 2,4,6-Trimethylphenyl -15.6
3.17 Ethyl 4-(Prop-2-inyloxy)phenyl -14.9
3.18 n-Propyl 4-Fluor-3-nitrophenyl -ll.g
3.19 n-Propyl (RS)-2-(Ethylthio)prop-1-yl -12.7
3.20 Ethyl 1-Methylthiocycloprop-l-yl -14.9
3.21 Ethyl 1,3-Dimethylpyrazol-5-yl
3.22 n-Propyl 3-Isopropylisoxazol-5-yl
3.23 n-Propyl (RS)-Cyclohex-3-en-1-yl -15.0
3.24 n-Propyl l-Methylthiocycloprop-1-yl -lS.1
24 O.Z. 0050/43017
212~121
~. .
Table 4
H ~.
~ OH N O CH2 - I ~ Cl I (Xn = 4-Cl)
R2 ~ C CH3
Rl
O ~,
No. R1 R2 Phys. data
~Rot. value
~a]D25;
c = 1.0, .
in CH30H/
lH-NMR [ppm]/ ;~
. ~ m.p. [C])
4.01 Ethyl (RS)-2H-Tetrahydropyran-3-yl -11.9
4.02 Ethyl (R)-2H-Tetrahydropyran-3-yl
4.03 Ethyl (S)-2H-Tetrahydropyran-3-yl
4.04 Ethyl 2H-Tetrahydropyran-4-yl -12.4
4.05 Ethyl (RS)-2H-Tetrahydrothiopyran-3-yl -10.3
4.06 Ethyl (R)-2H-Tetrahydrothiopyran-3-yl
4.07 Ethyl ts)-2H-Tetrahydrothiopyran-3-yl
4.08 n-Propyl (RS)-2H-Tetrahydropyran-3-yl -10.8
4.09 n-Propyl (R)-2H-Tetrahydropyran-3-yl
4.10 n-Propyl (S)-2H-Tetrahydropyran-3-yl
4.11 n-Propyl 2a-Tetrahydropyran-4-yl - 8.9
4.12 n-Propyl (RS)-2H-Tetrahydrothiopyran-3-yl -12.1
4.13 n-Propyl (R)-2H-Tetrahydrothiopyran-3-yl
4.14 n-Propyl (S)-2H-Tetrahydrothiopyran-3-yl
4.15 Et~yl Phenyl -12.6
4.16 Ethyl 2,4,6-Trimethylphe~yl -13.0
4.17 Ethyl 4-(Prop-2-inyloxy)phenyl -13.0
4.18 n-Propyl 4-Fluor-3-nitrophenyl - 8.1
4.19 n-Propyl (RS)-2-(Ethylthio)prop-l-yl -11.5
4.20 Ethyl 1-Methylthiocycloprop-1-yl -13.1
4.21 Ethyl 1,3-Dimethylpyrazol-5-yl
4.22 n-Propyl 3-Isopropylisoxazol-5-yl
4.23 n-Propyl ~RS)-Cyclohex-3-en-1-yl
4.24 n-Propyl l-Ethylthiocycloprop-l-yl - 9.7
4.25 Ethyl l-Ethylthiocycloprop-l~yl -12.3
4.26 n-Propyl l-Propylthiocycloprop-l-yl - 9.1
4.27 Ethyl 1-Propylthiocycloprop-l-yl -12.6
:
O.Z. 0050/43017
`-`,` 212~121
Table 5
Cl
OH N O CH2 C O ~ Cl
R2 ~ C C~3 I (Xn - 2,4-Clz)
Rl ~
' "'~
No. Rl R2 Phys. data
(Rotational
value
la] D25;
lS ' c = 1.0, .
in CH30H/
1H-NMR [ppm]/
m.p. [C~
5.01 Ethyl (RS)-2H-Tetrahydropyran-3-yl -36.5
5.02 Ethyl (R)-2H-Tetrahydropyran-3-yl
5.03 Ethyl (S)-2H-Tetrahydropyran-3-yl
5.04 Ethyl 2H-Tetrahydropyran-4-yl -35.5
5.05 Ethyl (RS)-2H-Tetrahydrothiopyran-3-yl -25.3
5.06 Ethyl (R)-2H-Tetrahydrothiopyran-3-yl
5 . 0? . Ethyl (S)-2H-Tetrahydrothiopyran-3-yl
5.08 n-Propyl (RS)-2H-Tetrahydropyran-3-yl -32.5
5.09 n-Propyl (R)-2H-Tetrahydropyran-3-yl
5.10 n-Propyl (S)-2H-Tetrahydropyran-3-yl
5.11 n-Propyl 2H-Tetrahydropyran-4-yl -34.2
5.12 n-~ropyl (RS)-2H-Tetrahydrothiopyrar.-3-yl -22.2
5.13 n-Propyl (R)-2H-Tetrahydrothiopyran-3-yl
5.14 n-Propyl (S)-2~-Tetrahydrothiopyran-3-yl
5.*~ Ethyl Phenyl
5.16 Ethyl 2,4,6-Trimethylphenyl
5.17 Ethyl 4-(Prop-2-ynyloxy)phenyl
5.18 n-Propyl 4-Fluoro-3-nitrophenyl
5.19 n-Propyl (RS)-2-(Ethylthio)prop-l-yl
5.20 Ethyl l-Methylthiocycloprop-l-yl
5.21 Ethyl 1,3-Dimethylpyrazol-5-yl
5 . 22 n-Propyl 3-Isopropylisoxazol-5-yl
5.23 n-Propyl tRs)-cyclohex-3-en-l-yl
.
26 O . Z . 0050/43017 : ,
21 2 9 1 2 1
Table 6
CH3
N - 0 - C~2 - C - 0 ~ Cl
R1 I (Xn = 2-CH3, 4-Cl)
0
No. Rl R2 Phys. data
(Rotational
value
[a]D25;
c = 1.0,
in CH30H/
lH-NMR [ppm]~
m.p. ~C])
~~
6.01 Ethyl (RS)-2H-Tetrahydropyran-3-yl -26.5
6.02 Ethyl (R)-2H-Tetrahydropyran-3-yl
6.03 Ethyl (S)-2H-Tetrahydropyran-3-yl
6.04 Ethyl .H-Tetrahydropyran-4-yl -16.3
6.05 Ethyl (RS)-2H-Tetrahydrothiopyran-3-yl -17.1
6.06 Ethyl (R)-2H-Tetrahydrothiopyran-3-yl
6.07 Ethyl (S)-2H-Tetrahydrothiopyran-3-yl
6.08 n-Propyl (RS)-2H-Tetrahydropyran-3-yl -24.7
6.09 n-Propyl (R)-2H-Tetrahydropyran-3-yl
6.10 n-Propyl (S~-2H-Tetrahydropyran-3-yl
6.11 n-~ropyl 2H-Tetrahydropyran-4-yl -24.9
6.12 n-Propyl (RS)-2H-Tetrahydrothiopyran-3-yl -19.0
6.13 n-Propyl (R~-2H-Tetrahydrothiopyran-3-yl
6.1~ n-Propyl (S)-2H-Tetrahydrothiopyran~3-yl
35 6.15 Ethyl Phenyl
6.16 Ethyl 2,4,6-Trimethylphenyl
6.17 Ethyl 4-(Prop-2-ynyloxy)phenyl
6.18 n-Propyl 4-Fluoro-3-nitrophenyl
6.19 n-Propyl (RS)-2-(Ethylthio)prop-1-yl
6.20 Ethyl 1-Methylthiocycloprop-1-yl
6.21 Ethyl 1,3-Dimethylpyrazol-5-yl
6.22 n-Propyl 3-Isopropylisoxazol-5-yl
6.23 n-Propyl (RS)-Cyclohex-3-en-1-yl
~":" ' . '
27 O.Z. 0050/43017
` 212~121
.
Table 7 ~ ~
. ~
H \ ~:
N O C~2 C 0~ F :
R2 ~ C CH3
R1 I (Xn = 2~ 4-F2)
O
: ~ '
No. R1 R2 Phys. data ~ -
(Rotational
value
[~]D25~
c ~ 1.0,
in CH30H/
lH-NMR [ppm]/
m.p. [C])
-
7.01 Ethyl (RS)-2H-Tetrahydropyran-3-yl - 9.2
7.02 Ethyl (R)-2H-Tetrahydropyran-3-yl
7.03 Ethyl (S)-2H-Tetrahydropyran-3-yl
7.04 Ethyl 2H-Tetrahydropyran-4-yl -13.2
7.05 Ethyl (RS)-2H-Tetrahydrothiopyran-3-yl -10.5
7.06 Ethyl (R)-2H-Tetrahydrothiopyran-3-yl
7.07 Ethyl (S)-2H-Tetrahydrothiopyran-3-yl
7.08 n-Propyl (RS)-2H-Tetrahydropyran-3-yl - 6.6
7.09 n-Propyl (R)-2H-Tetrahydropyran-3-yl
7.10 n-Propyl (S)-2H--Tetrahydropyran-3-yl
7.11 n-Propyl 2~-Tetrahydropyran-4-yl -15.3
7.12 n-Propyl (RS)-2H-Tetrahydrothiopyran-3-yl -13.8
7.13 n-Propyl (R)-2H-Tetrahydrothiopyran-3-yl
7.14 n-Propyl (S)-2H-Tetrahydrothiopyran-3-yl
7.~ Ethyl Phenyl -15.3
7.16 Ethyl 2,4,6-Trimethylphenyl -17.3
7.17 Ethyl 4-~Prop-2-ynyloxy)phenyl -14.7
7.18 n--Propyl 4-Fluoro-3-nitrophenyl -11.3
7.19 n-Propyl (RS)-2-(Ethylthio)prop-l-yl -10.5
7.20 Ethyl 1-Methylthiocycloprop-l-yl -14.6
7.21 Ethyl 1,3-Dimethylpyrazol-5-yl
7.22 n-Propyl 3-Isopropylisoxazol-5-yl
7.23 n-Propyl (RS)-Cyclohex-3-en-1-yl -13,4
7.24 n-Propyl l-Methylthiocycloprop-1-yl - 8.9
.
~8 ~.Z. 0050/43017
-- ` 2 1 2 3 1 ? 1
Table 8
H
~ N O - CH2 - C - O ~ F
- R2 ~ C CH3
~ R1 I (Xn = 4-F)
O ' !:
No. Rl R2 Phys. data
(Rotational
value
~a]D25;
c - 1.0,
in CH30~/
lH-NMR [ppm]/
m.p. [Cl)
8.01 Ethyl tRS)-2H-Tetrahydropyran-3-yl -19.2
8.02 Ethyl (R)-2H-Tetrahydropyran-3-yl
8.03 Ethyl (S)-2H-Tetrahydropyran-3-yl
8.04 Ethyl 2H-Tetrahydropyran-4-yl ~19.1
8.05 Ethyl (RS)-2H-Tetrahydrothiopyran-3-yl -17.9
8.06 Ethyl (R)-2H-Tetrahydrothiopyran-3-yl
8.07 Ethyl (S)-2H-Tetrahydrothiopyran-3-yl
8.08 n-Propyl (RS)-2H-Tetrahydropyran-3-yl -18.3
8.09 n-Propyl (R)-2H-Tetrahydropyran-3-yl
8.10 n-Propyl (S)-2H-Tetrahydropyran-3-yl
8.11 n-Propyl 2H-Tetxahydropyran-4-yl -21.9
8.12 n-Pr.opyl (RS)-2H-Tetrahydrothiopyran-3-yl -17.9 ~:
8.13 n-Propyl (R)-2H-Tetrahydrothiopyran-3-yl
8.14 n-Propyl (S)-2H-Tetrahydrothiopyran-3-yl
8.~5 Ethyl Phenyl -18.3 :
8.16 Ethyl 2,4,6-Trimethylphenyl -19.3 :~
8.17 Ethyl 4-(Prop-2-ynyloxy)phenyl -20.3
8.18 n-Propyl 4-Fluoro-3-nitrophenyl -16.5 .
8.19 n-Propyl (RS)-2-(Ethylthio)prop-1 yl -14.5
8.20 Ethyl l-Methylthiocycloprop-l-yl ~19.1
8.21 Ethyl 1,3-Dimethylpyrazol-5-yl ~-.
8.22 n-Propyl 3-Isopropylisoxazol-5-yl `~
8.23 n-Propyl (RS)-Cyclohex-3-en-1-yl -20.5
8.24 n-Propyl 1-Methylthiocycloprop-l-yl -18.4
: .
29 O.Z. 0050/43017
212~121
The optically active cyclohexenone oxime ethers I are suita-
ble, both as isomer mixtures and in the form of the pure
isomers, as herbicides especially for combating pli~nts from
the Gramineae species. They are generally tolerated and are
thus selective in broadleaved crops and in monocotyledons
not belonging to the Gramineae. Some of the cyclohexenone
oxime ethers I according to the invention are also suitable
for selectively combating unwanted grasses in Gramineae.
The optically active cyclohexenone oxime ethers I, or
herbicidal agents containing them, may be applied for
instance in the form of directly sprayable solutions,
powders, su3pensions (including high-percentage aqueous,
oily or other suspensions), dispersions, em~lsions, oil
dispersions, pastes, dusts, broadcasting agents, or granules
by spraying, atomizing, dusting, broadcasting or watering.
The forms of application depend on the purpose for which the
agents are being used, but they should ensure as fine a -~
distribution of the active ingredients according to the
invention as possible.
The compounds I are generally suitable for the preparation
of solutions, emulsions, pastes and oil dispersions to be
sprayed direct. Suitable inert additives are mineral oil
fractions of medium to high boiling point, such as kerosene
or diesel oil, further coal-tar oils, and oils of vegetable
or animal origin, aliphatic, cyclic and aromatic hydrocar-
bons, e.g. toluene, xylene, paraffin, tetrahydronaphthalene,
alkylated naphthalenes or their derivatives, methanol,
ethanol, propanol, butanol, cyclohexanol, cyclohexanone,
chlorobenzene, isophorone or strongly polar solvents such as
N,N-dimethylformamide, dimethyl sulfoxide, N-methylpyrroli-
done or water.
Aqueous formulations ~ay be prepared from emulsion concen-
trates, dispersions, pastes, wettable powders or water-dis-
persible granules by adding water. To prepare emulsions,
pastes or oil dispersions the ingredients as such or dis-
solved in an oil or solvent may be homogenized in water by
means of wetting or dispersing agents, adherents or emulsi-
fie~s. ~owever, concentrates which are suitable for dilution
with water may also be prepared from active ingredient,
O.Z. 0050/43017
` ` 212~ 121
wetting agent, adherent, dispersing or emulsifying agent and
possibly solvent or oil.
., ~
Surfactants are: alkali metal, alkaline earth metal and
ammonium salts of aromatic sulfonic acids, e.g., ligninsul-
fonic acid, phenolsulfonic acid, naphthalenesulfonic acid
and dibutylnaphthalenesulfonic acid, and of fatty acids,
alkyl and alkylaryl sulfonates, and alkyl, lauryl ether and
fatty alcohol sulfates, and salts of sulfated hexadecanols,
heptadecanols, and octadecanols, salts of fatty alcohol
glycol ethers, condensation product~ of sulfonated naphtha-
lene and naphthalene derivative-~ with formaldehyde, con-
densation products of naphthalene or naphthalenesulfonic
acids with phenol and formaldehyde, polyoxyethylene octyl-
phenol ethers, ethoxylated isooctylphenol, ethoxylatedoctylphenol and ethoxylated nonylphenol, alkylphenol poly-
glycol ethers, tributylphenyl polyglycol ethers, alkylaryl
polyether alcohols, isotridecyl alcohol, fatty alcohol
ethylene oxide condensates, ethoxylated castor oil, polyox-
yethylene alkyl ethers, ethoxylated polyoxypropylene, lauryl
alcohol polyglycol ether acetate, sorbitol esters, lignin- -
sulfite waste liquors and methyl cellulose.
Powders, dusts and broadcasting agents may be prepared by :~
mixing or jointly grinding the active ingredients with a
solid carrier. -~
- - ~ .
Granules, e.g., coated, impregnated and homogeneous gran-
ules, may be prepared by bonding the active ingredients to
solid carriers. Solid carriers are mineral earths such as
silicic acids, silica gels, silicates, talc, kaolin, atta-
pulguQ clay, limestone, lime, chalk, bole, loess, clay,
dolomite, diatomaceous earth, calcium sulfate, magnesium ~ ~
surfate, magnesium oxide, ground plastics, fertilizers such -~-
as ammonium sulfate, ammonium phosphate, ammonium nitrate,
and ureas, and vegetable products such as grain meals, bark -
meal, wood meal and nutshell meal, cellulosic powders or
other solid carriers. -
~0 The formulations contain generally between 0.01 and 95, and
preferably between 0.5 and 90, % by weight of active ingre-
dient. The active ingredients are used in a purity of 90 to
: ''
3~ 1 2 9 1 2 1 z . 0050/430l7
100, and preferably 95 to lO0, % (according to the NMR
spectrum).
Examples of formulations are as follows:
I. A solution of 90 parts by weight of compound no. 2.05
and 10 parts by weight of N-methyl-a-pyrrolidone, which is
suitable for application in the form of very fine drops.
II. A mixture of 20 parts by weight of compound no. 2.12,
80 parts by weight of xylene, 10 parts by weight of the
adduct of 8 to 10 moles of ethylene oxide and 1 mole of
oleic acid-N-monoethanolamide, 5 parts by weight of the
calcium salt of dodecylbenzene~ulfonic acid, and 5 parts by
lS weight of the adduct of 40 moles of eth~lene oxide and
1 mole of castor oil. By finely dispersing the mixture in
100,000 parts by weight of water~ an aqueous dispersion
containing 0.02wt% of the active ingredient is obtained.
III. An aqueous dispersion of 20 parts by weight of com-
pound no. 4.20, 40 parts by weight of cyclohexanone, 30
parts by weight of isobutanol, 20 parts by weight of the
adduct of 40 moles of ethylene oxide and l mole of castor
oil. A mixture of this dispersion with 100,000 parts by
2S weight of water contains 0.02wt% of the active ingredient.
IV. An aqueous dispersion of 20 parts by weight of compound
no. 4.12, 25 parts by weight of cyclohexanone, 65 parts by
weight of a mineral oil fraction having a boilin~ point of ;~
from 210 to 280C, and 10 parts by weight of the adduct of -~
40 moles of ethylene oxide and 1 mole of sastor oil~ The
mixture of this dispersion with 100,000 parts by weight of
water contains 0.02wt% of the active ingredient.
V. A hammer-milled mixture of 80 parts by weight of com-
pound no. 4.19, 3 parts by weight of the sodium salt of
diisobutylnaphthalene-a-sulfonic acid, 10 parts by weight of
the sodium salt of a lignin-sulfonic acid obtained from a
sulfite waste liquor, and 7 parts by weight of powdered
silica gel. By finely dispersing the mixture in 20,000 parts
by weight of water, a spray liquor containing O.lwt~ of the
active ingredient is obtained.
-~2 ~.Z. 0050/43017
`: 212~121
VI. An intimate mixture of 3 parts by weight of compound
no. 6.01 and 97 parts by weight of particulate kaolin. The
dust contains 3wt% of the active ingredient.
VII. An intimate mixture of 30 parts by weight of compound
no. 6.05, 92 parts by weight of powdered silica gel and 8
parts by weight of paraffin oil sprayed onto the surface of
this silica gel. This formulation of the active ingredient
exhibits good adherence.
VIII. A stable aqueous dispersion of 40 parts by weight of
compound no. 6.11, 10 parts of the sodium salt of a phenol- -
sulfonic acid-urea-formaldehyde condensate, 2 parts of
silica gel and 48 parts of water, which dispersion can be
further diluted.
~ .:
IX. A stable oily dispersion of 20 parts by weight of
compound no. 6.12, 2 parts by weight of the calcium salt of
dodecylbenzenesulfonic acid, 8 parts by weight of a fatty
alcohol polyglycol ether, 2 parts by weight of the sodium
salt of a phenolsulfonic acid-urea-formaldehyde condensate
and 68 parts by weight of a paraffinic mineral oil.
X. A hammer-milled mixture of 1~ parts by weight of com-
25- pound no. 2.01, 4 parts by weight of the sodium salt of
diisobutylnaphthalene-a-sulfonic acid, 20 parts by weight of
the sodium salt of a lignin-sulfonic acid obtained from a ;
sulfite waste liquor, and 38 parts by weight of silica gel
and 38 parts by weight of kaolin. By finely disper~ing the
mixture in 10,000 parts by weight of water, a spray liquor
containing O.lwt~ of the active ingredient is obtained.
The active ingredients or the herbicidal agents containing
th~m may be applied pre- or postemergence. If certain crop
3S plants tolerate the active ingredients less well, applica-
tion techniques may be used in which the herbicidal agents
are sprayed from suitable equipment in such a manner that
the leaves of sensitive crop plants are if possible not
touched, and the agents reach the uncovered soil or the
leaves of unwanted plants growing beneath the crop plants
(post-directed, lay-by treatment).
33 O.Z. 0050/43017
` 212~121
The application rates depend on the objective to be
achieved, the time of the year, the plants to be combated
and their growth stage, and are from 0.001 to 3, preferably
0.01 to 1, kg of active ingredient per hectare.
In view of the numerous application methods possible, the
optically active cyclohexenone oxime ethers I or the agents
containing them may also be used in a further number of
crops for eliminating unwanted plants. Those which follow
are given by way of example:
_ . . . ~ .
Allium cepa onions
_ _ .
Ananas comosus pineapples
.
15 Arachis hypogaea peanuts (groundnuts)
Asparagus officinalis asparagus
_ _ .
Beta vulgaris spp. altissima sugarbeets
_
Beta vulgaris spp. rapa fodUer be~ts
Brassica napus var. napus rapeseed
Brassica napus var.napobrassica swedes
_ '
Brassica rapa var. silvestris
_ _ _ _ : : ~
Camellia sinensis tea plants
_ _ _ ~ .
Carthamus tinctorius safflower
_ _ _ ~
Carya illinoinensis pecan trees
, _ _ _
Citrus limon lemons
_ _ _ _ _ _ _
Citrus sinensis orange trees
_ _ _ . ~
Coffea arabica (Coffea canephora, coffee plants
30 Coffea liberica)
. _ ~T . ,, _ _
Cucumis sativus cucumbers
_ _ _
Cynodon dactylon Bermudagrass
~ .
Daucus carota carrots
.~ . . . _
Elais guineensis oil palms
_ _ _ _ _
Fragaria vesca strawberries
_ _ , , ~
Glycine max soybeans
_ , _ _ _ _ _ _
Gossypium hirsutum (Gossypium cotton
arboreum,
Gossypium herbaceum,
40 Gossypium vitifolium~
Helianthus annuus sunflowers
_ .
Hevea brasiliensis rubber plants
_
34 o.Z. 0050/43017
:-`` 212~l21
. ,
Hordeum vulgare barley
~ . 1 s ~ _ lus hops
Ipomoea batatas sweet potatoes
5 Juglans regia walnut trees
_
Lens culinaris lentils
Linum usitatissimum flax
Lycopersicon lycopersicum tomatoes
Malus spp. apple trees
` .: .
Manihot esculenta cassava
Medicago sativa alfalfa (lucerne) ~:~
.
Musa spp. banana piants
..................................... ............................ ... .................................... . ~:
Nicotiana tabacum (N. rustica) . tobacco :
.
lS Olea europaea . . olive trees
Oryza sativa rice
.
Phaseolus lunatus limabeans :: :
: : ~
Phaseolus vulgaris snapbeans, green be-
ans, dry beans
Picea abies Norway spruce , .-~
Pinus spp. pine trees -
~ ., .
Pisum sativum English peas : ~
~.
Prunus avium cherry trees ~ .
. : ~ .
~J Prunus persica peach trees .-~
. i.
Pyrus communis pear trees :-
.
Ribes sylvestre redcurrants
Ricinus communis castor-oil plants
_
30 Saccharum officinarum sugar cane
_
Secale cereale rye
Solanum tuberosum Irish potatoes ~ ~:
Sorghum bicolor (s. vulgare) sorghum
.
35 Theobroma cacao cacao plants .
Trifolium pratense red clover
Triticum aestivum wheat
Triticum durum wheat
Vicia faba tick beans
Vitis vinifera grapes .
Zea mays Indian corn, sweet
corn, maize
-
O.Z. 0050/43017
` 2129121
To increase the spectrum of action and to achieve synergis-
tic effects, the optically active cyclohexenone oxime ether~
I may be mixed and applied together with numerous represen-
tatives of other herbicidal or growth-regulating active
5 ingredient groups. Examples of suitable components are ;
diazines, 4H-3,1-benzoxazine derivatives, benzothiadiazi- ;~
nones, 2,6-dinitroanilines, N-phenylcarbamates, thiolcarba-
mates, halocarboxylic acids, triazines, amides, ureas,
diphenyl ethers, triazinones, uracils, benzofuran deriva-
tives, cyclohexane-1,3-dione derivatives bearing in the
2-position for example a carboxy or carbimino group, quino-
linecarboxylic acids, imidazolinones, sulfonamides, sulfony- -~
lureas, thetero)-aryloxyphenoxypropionic acids and salts,
e~ters, amides thereof, etc. --~
lS
It may also be useful to apply the compounds I, either alone -
or in combination with other herbicides, in admixture with
other crop protection agents, e.g., agents for combating
pests or phytopathogenic fungi or bacteria. Further interest
20 attaches to the miscibility with solutions of mineral salts `
used to remedy nutritional or trace element deficiencies.
Non-phytotoxic oils and oil concentrates may also be added. -~
Use examples -
The herbicidal action of the unsaturated cyclohexenone oxime
ethers of the formula I is demonstrated in greenhouse
experiments:
The vessels employed were plastic flowerpots filled with a
sandy loam containing about 3.0% humus. The seeds of the
test plants were sown separately, according to species.
For- the preemergence treatment, the active ingredients
emulsified or suspended in water were applied immediately
after the seeds had been sown, and sprayed through finely
distributing nozzles. The vessels were lightly sprinkler-ir-
rigated to induce germination and growth. Transparent
plastic covers were then placed on the vessels until the
plants had taken root. The cover ensured uniform germination
of the plants, insofar as this was not impaired by the
active ingredients.
36 O.Z. 0050/43017
`` 212~121
For the postemergence treatment, plants were used which had
been sown in the pots and grown there, or they were grown
separately as seedlings and transplanted to the pots a few
days before treatment. The plants were grown, depending on -~
5 growth form, to a height of 3 to 15 cm before being treated ~-
with the compounds, suspended or emulsified in water. The
application rates for postemergence treatment were 0.06 and
0.03 kg/ha. -
:: -
The pots were set up at temperatures, specific to this
species, of ~0 to 35C, or 10 to 25C. The experiments were
run for from 2 to 4 weeks. During this period the plants -- ;
were tended and their reactions to the various treatments ;
assessed.
The assessment scale was O to 100, 100 denoting nonemergence "~
or complete destruction of at least the visible plant parts,
and O denoting no damage or normal growth.
The plants used in the greenhouse experiments were Echinoch-
loa crus-galli, Oryza sativa, Setaria italica and Setaria
viridis.
Compounds 4.12 and 4.20, applied postemergence at a rate of
25 0.06 or 0.03 kg/ha, controlled unwanted grasses very well -~
and were simultaneously tolerated by rice as an example for ~`
a crop plant.