Note: Descriptions are shown in the official language in which they were submitted.
~~~0~~~
1
APPARATUS AND METHOD FOR PRODUCING A REFERENCE GAS
FIELD OF THE INVENTION
The present invention relates to an apparatus and a
method for generating a reference gas consisting of a
gaseous medium carrying a known concentration of vapors of
a volatile liquid substance.
BACKGROUND OF THE INVENTION
The generation of a reference gas having a known
concentration of a volatile liquid substance is very useful,
for instance, in the art of gas calibration, olfactometry
and medicine.
Gas calibrators are widely used by laboratories and
industries for the calibrations of sensors or other
analytical equipments. Such gas calibrators usually use a
permeation device, such as the one described in U.S. patent
No. 4,399,942 (Chand) issued on August 23, 1983.
Olfactometers are used for helping panelists to compare
and evaluate odor intensities. In fact, odors are difficult
to classify and to measure with automatic devices because
most odors are issued from complex chemical compositions.
The nuisance of an odor often results from a synergetic
effect between odor pollutants and the only practical method
for globally evaluating the odor nuisance is the use of a
human test panel along with an olfactometer.
For decades, the sensory technique for measuring odors
was the threshold method. This method typically involves the
sampling of the odor pollutants into hermetical bags and the
laboratory testing thereof by submitting the samples to
dilution with odorless air until at least half of the
members of a panel cease to perceive the odor pollutants in
the diluted sample. The relative intensity of the odor
pollutants are then deducted from the numbers of dilutions
~~~o~~~
2
of the original samples. The number of dilutions is the
figure used as a reference for quantifying the nuisance.
Because the threshold of the human nose is an extreme
of sensitivity, it varies from a person to another and also
for the same person subjected to different environmental
conditions. Therefore, the threshold technique is not very
accurate.
To achieve better results, a large number of panelists
is required through the use of statistical methods, thus
l0 requiring considerable time and resources to perform a
threshold analysis of a single mixture. one alternative way
for evaluating the odor nuisance is to provide air samples
and compare them with a reference gas carrying a known
concentration of a reference substance with a clearly
experienceable specific odor. In this technique, known as
suprathreshold odor referencing, dilutions of the reference
gas are achieved until both odors are believed to be
equivalent by a panelist. The concentration of the reference
gas is then recorded for comparing with the results of the
other panelists. The practices for referencing the odor
intensity in the suprathreshold region are well described in
standard ASTM E544-75, which was reapproved in 1988.
Like the olfactometer described in the standard ASTM
E544-75, the olfactometers found in the prior art are
generally made from standard laboratory parts. The parts
often contain glass and are heavy, fragile and energy
consuming. These early suprathreshold designs were either
using bottled reference gases, or were generating their own
reference gas by passing an odorless gaseous medium over the
3o surface of volatile liquid substance or by bubbling the
odorless gaseous medium into the liquid. An example of such
olfactometer is disclosed in Sweeten J. et al., Journal of
the Air Pollution Control Association, Volume 34, No. 12, pp
1208-1213, December 1984. The construction of an
olfactometer controlling a reference gas in an accurate and
reliable manner from standard components was requiring
3
performance compromises in lieu of complex and bulky
devices. In spite of all efforts, these devices generally
require analytical apparatuses for monitoring their proper
operation.
More recent olfactometers have reference gas generating
systems which are immersed into a liquid bath with a
controlled temperature for minimizing the effect of the
temperature of the gaseous medium on the partial pressure of
the volatile liquid substance. An example of such device is
disclosed in U.S. patent No. 4,934,386 (Walker et al.)
issued on June 19, 1990. However, the olfactometers with a
liquid bath are not suitable for fully portable
olfactometers.
Until now, the reference odor was provided to the
panelists through a plurality of sniffing ports having
respective concentrations and continuous flow of the
reference odor, or through a single sniffing port where the
reference odor is generally diluted by a factor of 2. A
reason for this is the lack of reliable reference gas
generators in which the concentration of the vapors of the
volatile reference substance is known and maintained. It is
not relevant to try to obtain precise dilution factors if
the reference gas cannot be controlled adequately. Things
become worse for portable equipment, as many of the standard
laboratory apparatus cannot be incorporated into
transportable equipment, be it glass parts, liquid baths, or
calibration and analytical instruments that were teamed with
most of the previous art olfactometers. The present
invention is believed to resolve that problem.
SUMMARY OF THE INVENTION
The obj ect of the present invention is to provide an
apparatus for controlling the concentration of the vapors of
a volatile liquid substance in a reference gas with good
4
precision and a relative insensibility to the temperature or
the flow of a gaseous medium.
More particularly, the object of the present invention
is to provide an apparatus for generating a reference gas
consisting of a gaseous medium carrying a known
concentration of vapors of a volatile liquid substance, the
apparatus comprising:
an evaporator for mixing the gaseous medium and the
vapors into a mixed gas, the evaporator
l0 comprising:
- an absorbing material filled with the
volatile liquid substance; and
- a container for enclosing the absorbing
material, the container comprising a
gaseous medium inlet and a mixed gas
outlet;
a condenser for cooling the mixed gas to a given
temperature lower than the temperature of the
mixed gas in the container and condensing a part
of the vapors in the mixed gas, the condenser
comprising:
- a condensation tube having a first end
connected to the mixed gas outlet of the
container and a second end as a
reference gas outlet, the tube extending
above the container so that condensates
of the vapors fall by gravity towards
the container;
- cooling means for cooling at least a portion
of the tube;
- sensing means for obtaining a signal
proportional to the temperature of the
mixed gas at the reference gas outlet;
and
- means for controlling the cooling means and
maintaining the mixed gas at the
5
reference gas outlet at the given
temperature, the means for controlling
the cooling means being responsive to
the signal of the sensing means.
The object of the present invention is also to provide
a method for generating a reference gas consisting of a
gaseous medium carrying a known concentration of vapors of
a volatile liquid substance, the method comprising the steps
of
introducing the gaseous medium into a container
enclosing an absorbing material filled with the
volatile liquid substance for obtaining a mixed
gas;
cooling the mixed gas with a condenser to a given
temperature lower than the temperature of the
mixed gas in the container and condensing a part
of the vapors so that condensates of the vapors
fall by gravity towards the container;
obtaining a signal proportional to the temperature of
the mixed gas at an outlet of the condenser; and
controlling the cooling of the mixed gas in response to
the signal for maintaining the mixed gas at the
outlet at the given temperature.
The apparatus and the method according to the present
invention are particularly useful in suprathreshold odor
olfactometers, gas calibrators and in medicine for
evaluating the odor perception of a person.
A non restrictive description of a preferred embodiment
will now be given with reference to the appended drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
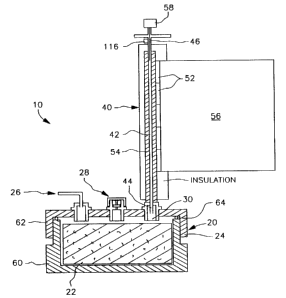
FIG. 1 is an elevational view of the apparatus
according to the present invention.
FIG. 2 is an exploded view of the container and the
leak-tight port shown in FIG. 1.
~~3U~~4
6
FIG. 3 is a partial enlarged view of the condensation
tube according to a preferred embodiment of the
present invention, showing the plurality of small
balls provided for improving the heat transfer;
FIG. 4 is a block diagram showing the means for
controlling the cooling means and maintaining the
mixed gas at the outlet at a given temperature,
according to the present invention.
FIG. 5 is a block diagram showing an implement that is
useful for the apparatus of FIG. 1.
DESCRIPTION OF A PREFERRED EMBODIMENT
Referring to FIG. 1, the apparatus 10 is provided for
producing a reference gas consisting of a gaseous medium
carrying a known concentration of vapors of a volatile
liquid substance. Such apparatus l0 may also be called a
"saturator".
The apparatus 10 comprises an evaporator 2o for mixing
the gaseous medium and the vapors, emanating from the
volatile liquid substance, into a mixed gas. The evaporator
20 comprises an absorbing material 22 enclosed in a
container 24. The container 24 is hermetical to the ambient
air and only the gaseous medium goes inside the container 24
through an inlet 26. The gaseous medium, such as nitrogen,
is provided in a compressed-gas cylinder or by a suitable
filtration equipment. In olfactometry, the gaseous medium
should be odorless and may be provided in a portable
compressed-gas cylinder in the case of portable
olfactometers if the sites where the tests are conducted are
not likely to provide a good source of odorless gas without
extensive filtration equipment. Some additional filtrations
of the gaseous medium prior to its insertion in the
apparatus l0 may be required for removing gas impurities and
dust. The gaseous medium may be any other gas or mixture of
gases suitable for the intended use of the apparatus lo,
~~~o~~d
with the additional requirement of achieving a good chemical
compatibility with both the volatile substance and the
materials used in the apparatus l0.
The absorbing material 22 is filled with the volatile
liquid substance previously injected through a leak-tight
port 28 for preventing contamination with foreign matters
able to alter the substance. The absorbing material 22 is
preferably permeable to the gaseous medium for allowing a
good evaporation of the substance. However, complete
l0 saturation of the gaseous medium is not absolutely mandatory
when the mixed gas leaves the container 24 through the mixed
gas outlet 30. Of course, the absorbing material 22 and the
container 24 are made with materials not likely to alter the
properties to the substance. The ability of the absorbing
material 22 to become wet with the liquid substance is also
important when designing the apparatus l0.
As described in the standard ASTM E544-75, a very
suitable reference odorant is 1-butanol because of its
clearly experienceable specific odor. An example of a
suitable absorbing material 22 is a borosilicate wool. The
container 24 may be made of high-purity aluminum.
The mixed gas produced by the evaporator 20 is then
sent to the condenser 40 for cooling mixed gas to a given
temperature lower than the temperature of the mixed gas in
the container 24. The resulting effect of cooling the mixed
gas containing the gaseous medium saturated or almost
saturated with vapors of the substance is the condensation
of a part of the vapors inside the condenser, indicating
that the mixed gas is then completely saturated with the
3o substance. By knowing the mixed gas temperature at the
outlet of the condenser and since the mixed gas is
saturated, it is possible to determine with great precision
the concentration of the substance in ppm. This
concentration is a function of the partial vapor pressure of
the volatile substance, which itself is a function of the
temperature, and the total gas pressure at the evaporator
~l~p~~4~
outlet. Typically, the mixed gas is cooled at a temperature
about 0°C. Relationships between temperature and partial
vapor pressure of substances can be found in standard
chemical books, or can be experimentally established in
laboratory.
The condenser 40 comprises a condensation tube 42
having a first end 44 connected to the mixed gas outlet 30
and a second end 46 as the apparatus outlet. The tube 42
extends above the container 24 so that condensates of the
vapors that are generated therein fall by gravity towards
the container 24. The tube 42 is preferably vertical but it
may also be inclined. High-purity aluminum is an example of
a suitable material if the substance is 1-butanol.
Cooling means are provided for cooling at least a
portion of the tube 42 which, in return, cools the mixed gas
therein. The cooling means preferably comprises an array of
thermoelectric cells 52, which are solid state heat pumps
that utilize the Peltier effect. Another advantage of using
Peltier cells 52 is that they may be used as heaters during
2o cleaning process. Heating the condenser 40 helps removing
residues of the volatile liquid substance.
Preferably, a heat-conducting sleeve 54 is inserted
around the tube 42 and the Peltier cells 52 are aligned with
each other on one side of the sleeve 54. The sleeve 54
allows a more uniform distribution of the temperature
between the tube 42 and the cells 52. The other sides of the
sleeve 54 are insulated for improving the thermal stability
of the system and for preventing condensation of the ambient
humidity on the sleeve 54. A thermal paste, such the ones
used in power semiconductors, may be used between the tube
42 and the sleeve 54 for removing air and optimizing the
thermal transfer.
Heat transfer means, such as the cooling fins 56, may
be provided for dissipating the heat from the hot side of
the cells 52. The choice of a proper heat dissipating method
~~~o~~~
9
can be determined by the use of guidelines provided by the
thermoelectric cell and heat sink manufacturer's data books.
Because the temperature of the mixed gas at the outlet
46 has to be substantially equal to a given value, the
apparatus 10 is provided with sensing means for obtaining a
signal proportional to the temperature of the mixed gas at
the outlet 46, and means for controlling the cells 52 and
maintaining the mixed gas at the outlet 46 at the given
temperature. The means for controlling the cells 52 and the
sensing means form a feedback system so that the means for
controlling the cells 52 is responsive to the signal of the
sensing means as explained hereinafter. The sensing means
comprises a temperature probe 58 at the outlet 46.
FIG. 2 shows an example of the construction of the
container 24. This container 24 comprises a hollow part 60
on which is screwed a lid 62. A gasket 64, made of an inert
material such as fluorocarbonate, seals the hollow part 60
to the lid 62. As aforesaid, the container 24 is made of a
material which is not likely to chemically react with the
2o volatile liquid substance. The interior of the container 24
is preferably polished for easying the cleaning thereof.
The lid 62 typically comprises three holes. The first
hole is the gaseous medium inlet 26, the second hole is for
receiving the leak-tight port 28, and the third hole is the
mixed gas outlet 30. The first hole and the third hole are
diametrically opposed so that the gaseous medium may go
through a maximum volume of contact. The position of the
second hole is less important since the volatile liquid
substance will soak the absorbing material 22 by
capillarity.
FIG. 2 also shows an example of construction of a leak-
tight port 28 through which the substance in a liquid state
is injectable by using a syringe (not shown). This leak-
tight port 28 is also important if the substance is likely
to chemically react with the oxygen in the ambient air. The
port 28 comprises an adaptor 70 screwable to the second hole
~t~o~~~
of the lid 62 and supporting a septum 72 penetrable with the
needle of the syringe (not shown), as used in
chromatography. A plug 74 with a funnel-shaped hole 75 is
set inside of the adaptor 70 for guiding the needle of the
5 syringe. Finally, a cap 76 is provided with a gasket 78 for
further sealing the port 28 and keeping it clean.
As shown in FIG. 3, the tube 42 may comprise means for
increasing the heat exchange contact surface, such as a
packed bed made of a plurality of chemically inert but heat
l0 conductive balls 80. The tube 42 is connected to the
container 24 by a Teflon~ fitting (not shown), which is
known in the art as being generally chemically inert and
thermally insulating.
FIG. 4 shows the block diagram representing the means
for controlling the cooling means and maintaining the mixed
gas at the outlet 46 at a given temperature. This means is
substantially a feedback system with a closed loop for
regulating the cells 52. The system comprises two entries,
the first being the given temperature at which the mixed gas
has to remain at the outlet 46 and the second being the
temperature read by the probe 58. The signal from the probe
58 is transformed by a signal conditioning module 90 into a
signal readable and subtractable with the signal
corresponding to the given temperature. The subtraction is
achieved by a subtraction module 92 and the resulting signal
gives a direct indication of the correction that has to be
done for maintaining the mixed gas at the outlet 46 at the
given temperature. The resulting signal is thus sent to a
calculation module 94 commanding the power supply 96 of the
cells 52. An apparatus built along the above described
guidelines will be able to achieve the function of
generating a known concentration of vapors of a volatile
substance with a good insensibility to the flow of the
carrier gas and to its incoming temperature. Its
predictability will also reduce the need to use continuous
gas monitoring instruments, and its relatively small size
11
and ruggedness allows its use in a portable instrument. The
components of the means for controlling the cells 52 may be
integrated to a computer 120 (FIG. 5) for the automation of
the whole process.
The apparatus 10 will require a given preheating time
before reaching the full operability. Given the low thermal
inertia of the condenser tube and its sleeve, the time
needed to reach the temperature setpoint can be achieved in
several minutes, which is magnitudes lower than setup times
l0 of liquid baths and temperature chambers. Furthermore, the
fast setup time of the apparatus 10 allows an additional way
to control the mixed gas concentration by varying the
temperature setpoint. The effect is that the concentration
of the vapors generated by the apparatus 10 can be changed
under user control while still keeping its features of
insensitiveness to carrier gas flow and temperature.
The present invention is also directed to a
corresponding method for producing a reference gas as
described hereinabove. The method comprises the steps of
2o introducing the gaseous medium into a container enclosing an
absorbing material filled with the volatile liquid substance
for obtaining a mixed gas. The mixed gas is then cooled with
a condenser to a given temperature lower than the
temperature of the mixed gas in the container and condensing
a part of the vapors of the substance so that condensates of
the vapors fall by gravity towards the container. Monitoring
the temperature of the mixed gas at the outlet of the
condenser allows control of the cooling means for
maintaining the mixed gas at the outlet of the condenser at
3o the given temperature.
Referring now to FIG. 5, there is shown a block diagram
of the construction of the apparatus l0 with an additional
implement 100. In the implement 100, the gaseous medium is
provided by two conduits 102 and 104. Both conduits 102 and
104 comprise a respective flow controller 106 and 108. Some
valves 110 are present for preventing leaks when the
12
apparatus 10 is not operated. Combined with the leak-tight
port 28, all the parts in contact with the reference vapors
are kept sealed. This allows the volatile liquid in the
container to be protected against degradation due to contact
with the oxygen from air. As a part of a portable equipment,
this feature allows to clean, fill the container and verify
the performance of the equipment prior to its on-site use.
No dismantling is necessary when the apparatus l0 is used
outside in less than perfect cleanliness conditions, thus
increasing the confidence in the results. In use, the.
conduit 102 will provide a source of gaseous medium that can
be mixed with the reference gas produced by the basic
components of the apparatus lo. By knowing the flow of the
reference gas and its concentration, it is possible to
dilute it with different amounts of gaseous medium in the
mixer 112 for obtaining different concentrations of gas at
the outlet 114, typically ranging, using 1-butanol for
instance, from 0,05 to a few hundreds ppm.
Alternatively, it is possible to vary the cooling
temperature of the Peltier cells 52 for varying the
concentration of the volatile substance.
In a suprathreshold olfactometry application, the
invention becomes especially useful as the flow of gas at
the outlet 114 has to remain constant while the vapor
concentration has to vary. The insensibility of the
apparatus to the flow of carrier gas allows this feature to
be implemented easily, making it a function of two equations
with two variables to determine the rate of the gases
flowing in the conduits 102 and 104. The equations for
calculating the concentrations at a constant flow are:
1 ~ Cout - Ccalc x Fcondenser ~ ~ Fcondenser + Fdilution
2 ~ F' condenser + Fdilution - Frequested
where: Copt is the requested concentration at the outlet
of the system.
2130~~~
13
is the concentration of the volatile liquid
substance in function of its partial vapor
pressure at the given temperature.
Fcondenser is the flow needed in conduit 104 for the
mixed gas in the condenser.
Fdilution is the flow needed in conduit 102 for
diluting the mixed gas.
Frequested ~-S the requested constant total flow.
Such function can be easily resolved by the computer
l0 120, which, when the temperature controlling means of the
apparatus l0 is also made by the same computer means, allows
for an automation of the instrument. The addition of a
pressure sensor 116, at the outlet 46, will give the
information needed to compensate for the back pressure
caused by the gas flowing through the conduits from the
outlet 46 up to the atmosphere and thus calculate a correct
vapor concentration value. This value is the one needed for
olfactometry purposes. The equation for compensating for the
back pressure in the conduits is:
( 3 ~ Cats = Ccalc x ( Patna ~ pcondenser
where : Cat, is the concentration of the volatile substance
in the mixed gas at the outlet 114;
C~al~ is the concentration of the volatile substance
as calculated in function of its partial vapor
pressure at the temperature of the condenser;
Pats is the ambient pressure as measured with
pressure sensor 118;
Pcondenser is the absolute pressure of the mixed gas
at the outlet.
As aforesaid, this feature can be easily automated
through the computer 120.
In conclusion, the present invention provides an
apparatus that is easily portable, thereby allowing on-site
odor evaluation where it is most desirable. The
stabilization time is very fast compared with other devices
in the art. The temperature of the mixed gas at the outlet
14
of the apparatus can be easily changed, thereby providing an
extensive range of concentrations. Moreover, the use of an
analytical instrument for monitoring the concentration of
the mixed gas is not mandatory because of the high precision
of the apparatus. Degradation of both the substance and
system components is minimum and the variation of the
concentration of the volatile liquid substance is
predictable and dependable.
In addition to gas calibration and olfactometry, the
to present invention may be used in medicine for precise
evaluation of the odor perception of a person.
Although a preferred embodiment of the invention has
been described in detail herein and illustrated in the
accompanying drawings, it is to be understood that the
invention is not limited to this precise embodiment and that
various changes and modifications may be effected therein
without departing from the scope or spirit of the invention .