Note: Descriptions are shown in the official language in which they were submitted.
Cyclic compounds of trl~alent 3hoRphorus
The invention relate~ to noYel cyclic phosphoru~(III)
co~pound~ which act a~ bident te ligands to form complex
compounds with metals, i~ partIcular metalR of group 8 of
the Periodic Table of the Element~, and al~o to a process
for the preparation thereof. The comple~ compound~ are,
homogeneouRly di~olved, used as con~tituent~ o~ cata-
ly~t~, in particular hydroformylation ~ataly~t~.
Complex co~pounds which as the central ato~ ha~e a metal
of group 8 of ~he Periodic Table of the Elements a~d ~8
liga~ds have phosphorus(III) compounds, phosphine~ or
I phosphites a~d in addition, i desired, ~urther groups
capable of comple~ formatio~, ha~e in recent year~ gained
increasing importance a~ cataly~ts~ Thus the reaction,
practiced industrially on a large scale, of olefins with
~ynthesis gas to gi~e aldehydes (hydroformylation) is
carried out in the presence of cataly~t ~ystems compris- .!
ing cobalt and, in part~cular, rhodium and triphenyl-
pho~phine. Catalyst~ ba~ed on phosphine-containi~g
complex compounds ha~e also prove~l use~ul for the hydro-
genation of unsaturated, carbo~-carbon or carbon-oxyg~n
multiple bond~ with molecular hydrogen. In the case~ men-
io~ed, the ligand~ are usually pr~sent in exce~ othat ~he catalyst ~y~te~ comprises complex co~pound and
free ligand. In accordance with the ~olubility of the
catalyst~ in organic media, the reaction~ ara carried out
in homogeneou~ ~olution.
The known catalytic proces~e~ have proven very useful on
an industrial scale. Nevertheless, efforts are made to
~urther perEect the k~own proce~ses.
Thu~, attempt~ are made to increase the activity of the
catalyst~ by modification of the co~plex ligands and to
prolong their effecti~ene~s, 80 as to reduce the specific
catalyst re~uirement. In addition, e~orts are made to
develop matched liga~d systemis which solve indi~idual
problem~. An exampl~ ~hich ~a~ be ~e~tioned ii~ the
. ~
.
:
- 2 _
control of the regio~lecti~ity and tereo electivity of
chemical ~ynthe~es with the aim of obtaini~g pure
enantiom~rs ~rom prochiral ~tarting materials. Such
reactions are gai~ing ever greater importance, in par-
ticular in the ~ield o~ ~ine chemicals 2nd pharmaceuti-
cal~.
-
The influencing of th~ ster~osele~ti~ity is no longer, oronly insu~ficiently, po~sible usi~g monodentata ligand3.
There~ore, bide~tate ligands are predominantly used for
obtaining pure enantiomeric producta. Among these, the
ligand hitherto ac ieving importance have be~n predomi-
nantly diphosphines. Thus, for example for the 3elective
hydroformylation of ~,~-unsaturated esters such as
acrylic est:erR as prochiral compounds, u~e is made of
catalyst sy~tems ba~ed on cobalt/1,2-bi~(diphenyl-phos-
phi~o)ethane and rhodium/1,4-bi~(diphenylphosphino)-
buta~e. The reaction~ require energetic rePction con-
ditio~R, i.e. te~peratures o~ ~rom 120 to 150C and
pres~urea of ~rom 5 to 10 MPa. Furthermore, the catalyst
~yst~ms ha~e pro~en to be relati~ely unstable. Under
milder conditions uRing the rhodium catalyst, the
starting compounds are predominanl_ly hydrog~ated.
It is therefore an object of the i~Yentio~ to develop
phosphoru~ compoundR suitable as con~titueats of homoge-
25 ~eously ~oluble c~talyst 8y3temg, in the molecule o
¦ which compound~ ~here are present two phosphorus(III)
atom~ capable of complex fo~mation, in particular with
rhodium. The catalyst system~ ~hould pos~es~ high activi-
ty and regioselecti~ity and, for example, al~o make
po~sible stereospecific synthe~e~.
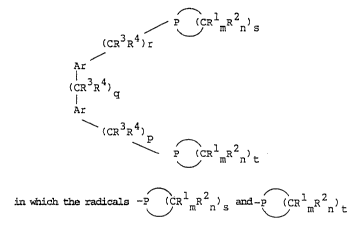
~he i~e~tion accordi~gly pro~ide no~el phosphorus
co~pound~ 3f the formula
.
l 2
/ p~__~CR mR n)s
(CR3R4)r
Ar ~:
(CR3~
: ::
Ar
\ ( 3R4)
~ P ~CRlmR2n~t
l 2 ~ :
In the for~Lula, the cyclic radicals -p ~CR mR n)s a~Ld
~ l 2
-P ~CR mR n)t are ~aturated or singly or multiply
unsaturated a~Ld ~i~e- or six-m~ered, R3 a~Ld R4 are
identical or different aLnd are hydro~en or the met~yl
radical, Rl and R2 are likewise ide~Ltical or diferer.Lt and
are hydrogen, an alkyl, araLlkyl or alkoxy radical in each
case ~Lavi~Lg ~rom 1 to 5 ca~bon atoms or are a halogen
atom; Rl and R2 can al~o be annelated benzene or naph-
thalene ring~. m and n are iden~ica~l or differ~nt and are
the nu~ber O or 1, ~ and k are ide~.tical or different and
are the ~umber 4 or 5. p, q, r are identical or differ~t
and are the number 0, 1, 2 or 3, Ar i~ finally a ~UbBti-
tuted or un~ubstituted aromatic radical haviny from 6 to ~ :
18 carbon atom~.
~re~err~d ligands are tho~e compou~ds whi~h corre~po~d to
the formula reproduced above a~d i~ which p a~d r are -::
each the number 1 an~ q i~ O or 1 and i~ which R3 a~d
are hydrog~n.
The aromatic radical denoted i~ the above ~ormula by Ar
~0 can be u~sub~tituted or ~ub~tituted and al~o be a multi~
cyclic or co~den~ed ri~g system. Preferably Ar i~ a `~: :
phe~ylene, ~aphthyle~e, biphenylene or bi~aphthyle~e
', :.
_ 4 _ 2 1 3 rl. ~ 7 3
radical, which in each ca~e can be unsubstituted or
E3UbRtitUted .
The P~III) atom~ pre~ent in the novel compound~ are
co~stituents of saturated or un~aturated, ~ive- or 8iX-
membered heterocyclic compound~. Pre~erence is gi~en toheterocycle which, be~ides the phoRphoru~ atom, further
comprise four carbon atoms, i . e. compounds which are
derived from sub~tituted or unRubstituted phospholaneR,
dihydropho~pholes and, in particular, phospholes. Accord-
10 ingly, 8 a~d t in the ~or~ula are preferi~bly 4 . Rl a~d R2are identical or di~erent and are preferably each a
~traight-chain or brznched alkyl radical having up t!o 5
carbon ato~s, the benzyl, pheuylethyl, tolyl, xylenyl or
mesityl radical. Rl and R2 cian also be annelated benzene
or naphthalene rings. The radicalR of corresponding
phosphorus-containing heterocycles are derived, for
example, Erom 2,3-ben%iophosphole~, 2,3,4,5-dibenzo-
phospholes and 2,3-indenophosphole.
The novel compou~d~ can be obtai~led in good yields in a
20 8imple way. A proven ~yntheais i8 the phosphorylation of
dihaloalkyl co~pou dR of the for~ula
/ ~CR3R )r lCR3R ~al
Ar
(CR3R4)
Ar
\ (~R3R4) l~R3R4Hal
in which p, ~, r, R3, R~ iand Ar are a~ defined aboYe iand
~al i~ a halogen ato~, pre~erably chlorine or bro~ine and
in particular brom1ne. The bromo compounds are obtained,
for exilmple, from the unhalogenated ba e ubstance~ by
br~mination with the aid of N-bromosuccinimide. -
Succe~Qful pho~ph~rylatio~ reagentB are alkali metal
compounds h ~ing a heterocyclic pho~phorus ianion. They
.
'., ~ " ",,,,,,~ , ;" ~ ~i, ,," ~
5 _ 2 ~ ~ 2 ~ 7 ~
are prepared by reaction of the het~rocycle~ phenylated
at the pho~phoru~ atom with an a:Lkali ~etal, preferably
lithium, ~odium or pota~sium, and i~ particular with a
sodium/pota~Ri~m alloy. The metal deri~ati~e, reacted
with the dihalo compound, gives th~ desired cyclic
phosphorus compounds.
The phosphorus compound~ of the invention act as biden-
tate ligands to form complex compounds with nu~erous
metal atomR. Among them, the complex compounds of rhodium
are of particular importance as highly active catalyst~
for th low-pressure hydroformylation of olefi~s to gi~e
aldehyde Tha reaction gi~2 ~ ~ery high proportion o~
~traight-chai~ compou~ds and only subordinate amounts of
branched-chain isomer~. Cataly~t components which ha~e
pro~en particularly useful are the compounds
2,2'-bis(3,4-dimethylpho3pholemethylene)-l,lr-biphenyl
and 2,2'-bis~3,4-dimethylphospholemethylene)-
1,1'-binaphthyl.
The hydroformylation of olefins usi.ng cataly~t sy~tems o~
rhodium and the no~el phosphorus(III) compound~ is
carried out at pressures of fro~ ~bout 0.1 to about
6 MPa, preferably from 0.5 to 3 MPa and in particular
from 1.5 to 2.0 MPa. The reaction t:emperature c n be from
about 20 to about 200C, the ra~ge ~rom about 50 to about
170C and in particulzr from about 80 to about 150C
being pr~ferred.
: ~:
The cataly~t is fed to the rPaction mixture preformed,
adYantageously dissol~ed in a sol~ent, or iB even formed
from the components in the mixture of the reactants under
the condition~ of the hydroformylatio~ reaction. Both in
the prior preparatio~ and in the oa~e of the in-~itu
preparation of the cataly~t, the rhodium can be used a~
metal in finely diYided fo~m or as a compound, for
ex~mple a~ th~ oxide~, the salt~ o~ i~org~nic or oryanic
acids or a~ carbo~yl.
Y~:., :.:: , : .: ::. .. : ~: i: ,"", . ",",,, ,"" ,. , .,.;, . ",,,},, ",,, . .j" , ,, : " , ., . : .. . .
- 6 ~ 32~73
The amounts of rhodium requirPd are very ~mall, aR little
¦ as about 1 x 10-6 mol of Rh per mole of olefi~ i8 ef~ec-
¦ ti~e. However, for economic rea~o~, increasing the
reaction rate, use i~ preferably made of ~rom about
5 1 ~ 10-2 to about 1 x 10-5 ~ol a~d in particular from
1 X 19-2 to 1 x lO-~ mol of Rh per mole o~ olefin. The
I molar ratio o~ rhodium to liga~d in the reaction ~ixture
i8 from about 1 to 1 up to 200, preferably ~rom about 1
to 2 up to 10 and in particular from about 1 to 2.5 up to
4.
~ynthesiR ga~ and olefin are continuously ~ed to the
rea~tor in the amount i~ which they are con3u~ed. The
molar ratio of carbo~ monoxide and hydrogen in the
synthesis ga~ ca~ vary within wide rangeR and can be from
about l to 10 up to about 10 to l, the type of the olefin
to be reacted being able to ha~e an influence on the
ratio to be ~elected. Preference i~ given to mixture~
which comprise carbon monoxide and h~drogen in a ratio of
~rom about 1 to 2 up to a~out 2 to 1 and in particular
20 from about 1 to 1.5 up to 1.5 to 1. Synthesis gas, based
on ole~in, is alway~ uaed in exoess; a molar excess of
~ynthe~i~ gaR (CO+E2), based on ole~in, of about 0.5 up
to about 20 iB usual, from about 1 up to about 10 is
pre~erred.
The reactio~ can be carried out in the prese~ce o~
. solventa which are i~ert towards r~actants and product~
u~der the reactio~ conditions. The e ~ol~ents include,
~or example, a~omatic hyd~ocarbon ~uch as benze~e,
toluene and xyle~e, aliphatic hydrocarbons ~uch as
pentanea, kerosene and ~ineral oils, and alao alcohol~
etheris and e~ter~
Th~ hydrofiormylation iB carried out in conventio~al
reactor3, it can be carried out co~tinuou~ly or batch-
wi~e. I~ a continuoui~i reactio~ procedure, the product iB
co~ti~ually drawn off together with unreacted ~ynthe~is
ga~, separated from the ga~ phaRe by conde~sation and
:
.~ :'
: ~
' 7 _ ~13~7~
further treated conventionally, ~.g. purified ~y distil-
lation. The synthesi~ gas iB recirculated to-the reaction
zo~e. To keep the activity of the cataly~t sy~tem at a
uniformly high level, it can be ad~isable to draw cata-
lyRt of~ from the reactor ~rom time to time and toreplace it with fresh catalyst.
The ~o~el cla R of bide~tate ligands i~, together with
rhodium, ~uccessfully u~ed in the hydroformylation of
differe~t olefi~s. The e i~clude ~-olefin~ havi~g ~rom 2
to 20 carbon atoms, which ~an have substituent~ which are
inert under the reaction condition Examples of auitable
olefin~ are ethylene, propylene, 1-butene, 2~butene, ~lao
the pentenes, hexene~ and heptene~. The ~o~el lig~nd~ are
of particular importance becau~e of their stereospecific-
ity in the hydroformulation of prochiral olefins.
The following examples illustrate the in~entio~ withoutlimiting it to the embodime~t~ deRcribed in detail.
Example~
1.) Preparation of 2,2'-bis(3.4-dimethYlpho~phole-
methYlene)-l,1'-bi~hen~l
~ :
(a) 3,4-Dimethyl-l-Dhen~lphos~hole (according to A.
Breque et al., Sy~th., 1981, 12, 384)
:':
I~ a 1000 ml stirred flask, 44.7 g (250 mmol) of phenyl-
dichloropho~phine and 66.9 g (250 mmol) o~ phenyldibromo-
pho~phi~e are ~tirred together under ~itroge~ for 30minute~ at room temperature. With ice cooling, 42.0 g
(511 mmol) of di~ethylbutadie~e are the~ add~d to the
homogeneo~s ~olution and the mlxture i~ ~tirred for 24
hours with further cooli~g. The suspenRion obtained i
allowed to sta~d for a further 10 days at room tempera-
ture. During thi3 ti~ it i8 transformed com~letely into
a whit~, crystalli~e ~aB~ which i~ broken up in the flask
into very ~m~ll pieGes and i8 cover~d with 300 ml of
r~
~ ~39~73
hexane and 200 ml of dichloromethane.
By blowing i~ nitrogen ~or a period of 30 minute~, the
~u~pension i~ freed of exces~ dimethyl~utadie~e. With
~igorou~ ~tirring, a golution of 103 g (1094 mmol) of
2-methylpyridine in 100 ml of dichloromethane i9 added
dropwise at room temperature. In the cour3e of 24 hour~
there forms a mixture which can be ~eparat~d into two
liquid pha~es. A~t,er hydrolysis with 100 ml of 3 N
hydrochloric acid, the organic phase i8 separated off and
washed three times with 100 ml of water until ~eutral.
The ~olution i~ dried o~er ~odium ~ulfate, filtered and
evaporated. Th~ remaining liquld i~ extracted with 300 ml
of hexane, the extract is filtered and evaporated. The
yellow oil thu~ ~btained requires no further purifica-
tion.
Yield: 71.17 g (378 mmol, 74.15% of theory).
31P-MMR (161.8 MHz, DC2C12): = -103 ppm (~, lP).
(b) 2,2'-Dimethvl-1~1'-bi~henvl (according to C.W.
~ohlpaint~r, dis~ertation 1990, Technische ~ni~eraitfit
Munchen)
In a 500 cm3 flask, 70 ml (0.6 mol) o~ 2-chlorotoluene
and 2 ml of 1,2-dibromoetha~e are added dropwise to
18.59 g (0.75 mol) Oc Mg tur~ing3 in 200 ~l of tetra-
hydrofuran over a period of 1 hour while stirrin~, the
mixture becoming gray and slightly war~. The mixture iB
subsequently heated for 18 hour~ under re~lux After
cooling, the ~u~pen~ion i~ filtered through gla~a wool
and added drop~Ji~e to a ~olution o~ 70 ml (0.6 mol) of
2-~hlorotolue~e and 3.0 g (4.5 mmol) of ci~-dichlorobi~-
(triphe~ylph~ophi~e)~ickel(II) in 250 ml of tetrah~dro-
furan over 2 hours at room temperature. After heating
under re~l~x for 20 hours, the liquid iÆ placed in a
000 C~3 ~eparating f~nnel a~d i~ 810wly cooled by care-
ful addition o~ 400 ml of toluene and 200 ml o~ a
~ 1 3 ~ ~ !7 3
~aturated, aqueouR ammonium chloride Rolution. The
organic phas2 i~ ~eparated off a~d succe~sively wa~hed
with 250 ml of 10% by weight ~trength ~Cl and three times
with 125 ml o~ water each time. The ~ol~ent i8 remo~ed in
an oil pump vacuum, a brownish yellow oil remaining.
Di~tillation at from 66.7 to 133.3 Pa gi~es the desired
product a~ a colorlesR liquid in a boiling point range of
75-77C~
Yield: 77.31 g (70.7% o~ theory).
GC/mas~ ~pectrum (AS 60, retention time 12.796 min): m/e
= 182 (55%, [(M)~.]), 167 (100%, ~(M-C~3)~.]), 152 (20%,
~(Cl2~0) .]), 1~8 (10%), 115 (12%)~ 83 ~18%), 76 (10%,
~(C6X~)~.])v 63 (15%), 39 (18%), 27 (12%).
(c) 2,2'-Bi3(bromomethylene)-1,1'-biphenyl (according to
W. Wenner, J. Org. Chem. 1952, 17, 523-528)
20.0 g (0.11 mol) of N-bromosuccinimide and 0.2 g of
azobisi~obutyronitrile are added to 7.1 g (0.039 mol) of
2,2'-bi~methyl-1,1'-biphe~yl in 15 ml of carbon tetsa-
chloride and heated under gentle re~lux. After 9 hour~,
the succinimide ia filtered off at room t~mperature via
a G3 glaRs ~rit and the r~ining liquid i8 evaposated at
40C in an oil pump vacuum to give a vi~cous, yellow oil.
By recrystallization from 15 ml of ligroin, the product
i~ obtai~ed in the for~ of fiae, white cryst?ls which are :
filtered o~f via a G4 glass fri~ and are dried in an oil
pump ~acuum.
Yield: 11.5 g (84% of theosy)
~elting point: 82C
: .
GC/ma~ spectrum ~AS60, retention time 17.273 min):
m/e = 340 (6%, [(~)~.J), 259 tl5%, C(M-Br)+.]), 179 (100%,
~M-2*Br)~.~), 152 (12%, ~(~12~8) ~
_ 10
El~mental a~aly~is: (C~ 2Br2; 340.07)
calc. C 49.45 ~ 3.55 Br 47.00,
fou~d C 49.20 ~ 3.58 Br 46.09
(d) (3,4-Dim~thvlphoRvholyl)sodium/pota~iu~
5 1.88 g (10 mmol) of 1-phenyl-3,4-dimethylphosphole in
40 ~1 of tetrahydro~uran are ~lowly admixed with 0.62 g
(20 mmol) of ~odium/pota~ium alloy in portio~s at room
temperature while stirring vigorouRly. After 2 hours,
exca~s metal alloy i8 filtered off via glas~ wool. The
dark brow~ suspen~io is cooled to -30C a~d a~mixed with
0.16 g (0.19 ml, 17 mmol) of 2-chloro-2-methylpropa~e.
Heating over a period of 1 hour leads to a dark red
~uspension which i~ procesYed further without isolation
of the product. Accordi~g to 3lP-NMR, the conversion i~
~uantitative.
3lP-{lH}-NMR (CDCl3): ~ = 55.40 ppm (8, lP), 52.36 ppm (8,
lP).
(e) 2,2' -~iY (3 ~ 4-dimethYl~!hospholemethYlene)-
l,l'-biphen~l
20 12.0 g (35 m~ol) of 2,2'-bi~(bromometh~l)-1,1'-biphenyl
in 30 ml of tetrahydrofuran are added dropwi~e to 15.8 g
(84 m~ol) of (3,4 dimethylpho~pholyl~sodium/pota~sium
~u~pended in 150 ml of tetrahydrofuran. After ~tirring
~or 12 hour~ the suspe~ion change~ in color ~rom dark
red to pale brown a~d the sol~ent i8 removed in an oil
pump varuu~. Th~ remaining brow~ re~idue is taken up in
toluene, with ~Br and NaBr precipitating. The ~uperna~
~ant, clear solution i8 e~aporated to a dark brown oil
and, ~y recry~tallization u~ing 10 ml of ethanol, the
product i~ obtained a~ a white, ~icrocry~talline powder.
Yield: 8.52 5 (27 ~mol~ 60% o~ theory)
Melti~g poi~t: 120~C
Mass spectrum ~Cl): m/~ = 402.2 (36.99%, ~ .]), 291.2
,_~
J ~ ~ 7 ..3
(14.87~, [(~-C~loP3~.]), 179.1 (4.14~, t(M-2~C~loP)~.]3,
154 (2.66%, [(M-2*C5~llP ~3
P-{l~}-NMR (CDCl3~: ~ = 3.99 (i, 2P)
l~_NMR (CDCl3): ~ - 0.89 (t, 6~, -C~3, 2J(~,H) = 7.4 Hz~,
1.19 (t, 6~, -C~3, 2J(~,H) = 7.4 ~z), 1.54 (dd, 2 ~,
-CH~E~ J(~,~) = 14.8 ~z, 2J(p,~) = 6.7~ ~z), 1.97 (d,
2 ~, -C~ , 2J(~,~) = 20.~ ~z), 3.55 (7, 2 ~, :C~-,
2J(P,H) = 6.78), 3.67 (t, 2 ~, :C'~-, 2J(P,H) = 6.78), 8.6
(m, 8 ~, aromatic)
~lemental analy~is: (C26~2aP2; 402.26)
calc. C 77.60 H 7.00 P 15.39
found C 77.08 H 7.05 P 15.68
2.) Preparation of 2,2'-bi~(3,4-dimeth~lphosphole-
methYlene)-l,l'-bina~hthyl
(a) 2,2'-Bi methyl-l,l'-binaphthyl
83.8 g (0.379 mol) of 1-brOInO-2-~ethylnaphthalene,
di~olved i~ 150 ml of ether and 150 ml of benze~e, are
alowly added dropwi~e at room te~perature to 10 g
(O.415 mol) of magneslum turni~gs (previously admixed
with a few dropR of 1,2-dibromoethane) ~uspe~dei~ in 30 ml
of ether. The dropwi~e addition of the halQg~nated
axomatic in c~rxied out over a period of 4 hour~, the
dropwise addition rate being Relected such that th~
reactio~ batch alway~ r~main~ ~t hand temperature.
Sub~eque~tly, the reaction mixture iB heated at reflux
for another hour and is ~tirred fu~ther o~ernight at room
temperature. The suape~sio~ i~ added i~ portions ~rom a
dropping fun~el to 74 g (0.335 mol) o~ l-bromo-2-methyl-
naphthalene, dii~sol~ed in 250 ml of ether, and 2.5 g
(3.8 ~mol) o~ E(PPh3)2Ni~Cl2 (coupli~g catalyst). The
mi~ture i~ then heated for 24 hour~ under reflux, cooled
to roo~ t~perature and hydrolyzed with 200 ml oif water
a~d 200 ml of 20% by weight strength hydrochloric acid.
The orga~ic phas~ ~epariated ~rom the aqueous pha~e i~
/~
~2 - s~3~7~
w~shed two more time~ with 200 ml of water, i~ separated
again and i~ dried wi~h mag~eRium Rulfate. The organic
sol~e~ts are taken off in vacuo, the residue i~ distilled
via a Rmall Vigxeux colum~ at pressures ~ 1.3 Pa. ~nder
ths given conditionR, the desired compou~d goes over in
a ra~ge of 169-173~C as a green oil which i~ recrystal-
lized ~rom 10 ml of aceto~e at -18C.
Yield: 74.9 g (00265 mol, 69% of theory)
(b) 2,2'-BiRbromomethvl-l.l'-binaphthYl (according to W.
Wenner, J. Org. Chem. 1952, 17, 523-528)
28.2 g (0.1 mol) of 2,2'-dimethyl-1,1'-binaphthyl are
RuRpended together with 35.6 g (0.2 mol) of N-bromo-
Ruccinimide in 65 ml of carbon tetrachloride. 0.43 y of
azobisiRobutyronitrile i~ added in a number of portions
o~er 4 hour~ u~der reflux and the mixture i8 heated for
a urther 9 hours under reflu~. After filtering of the
~uccinimide and evaporating the filtrate, there remains
a yellow, ~iscous oil. It iR rec~r~tallized from 20 ml of
ligroin (light petroleum mixture~ and the desired white
~0 solid iB iltered off.
Yield: 35.55 g (0.08 mol, 80% of theory)
H-~MR (CDCl3): ~ = 8.0-9.0 (m, aromRtic, ~2 ~), 5.3 ppm
(8, C~2, 4~)
(C) 2, 2' -~iB (3,4-di~ethylpho~pholemethYlene)-
l,l'-binaphthyl
9.9 g (0.022 mol) of 2,2'-bisbromomethyl-1,1' ~inaphthyl
in 20 ~1 of tetrahydrofuran are added dropwise to a
suspen~io~ o~ 5.0 g (0.045 mol) of 3,4-di~ethylpho~pholyl
anion in 120 ~1 o~ tetrahydrofuran over 2 hourR. A~ter
39 ~tirriug for 18 hours at roo~ t~mperature, the sol~e~t i8
co~pletely rOEmo~ed and the whitish brown residue i8
extracted with BO ml of tolue~e i~ a Soxhlet apparatus.
~ubaequent e~aporation leads to a brown oil fsom which
.
:;. 13 ~ 7 3
the product ca~ be obtained a~ a light b~own ~olid by
recry~tallization u~ing 30 ml of n-hexane.
Yield: 5.46 g (0.01 mol, 4g% of theory)
31P-{lH}-NMR (CDCl3): ~ = -5.4 ppm (~, 2P)
Ma~ ~pectrum (Cl): m/e = 503.1 (5.6%, t(~)~.]), 3
(15.1% t(M~C~loP)'.]), 281~1, (15.4%, [(M-2*C~loP)~
154 (2.66%, [(M-2~C5H11P) ])-