Note: Descriptions are shown in the official language in which they were submitted.
~V093/212192 ~ 3 3 ~9 0 PCT/US93/036$8 D. ~
1' `
ENDOTHELIN ANTAGO~ISTS II
BACKGROUND OF THE INVENTION
-;
The present invention relates to novel :~
antagonists of endothelin useful as pharmaceutical ::.
agents, to methods for their production, to
pharmaceutical compositions which include these
compounds and a pharmaceutically acceptable carrier, ~i:
and to pharmaceutical methods of treatment. More
particularly, the novel compounds of the present
invention are an~agonists of endothelin useful in :
treating elevated leveis of endothelin, acute and .
chronic renal failure, hypertension, myocardial
infarction, metabolic, endocrinological and
neurological disorders, congestive heart failure,
~ ~endotoxic shock, subarachnoid hemorrhage, arrhythmias,
; : :asthm~ preeclamp~ia, atherosclerotic disorders
including Raynaud's disease, re~tenosis, angina,
cancer,~ pul nary hypertension, ischemic disease,
gastri:c mucosal damage, hemorrhagic shock, ischemic
; bowel disease, and diabetes.
: .
Endothelin-l (ET-i), a potent vasoconstrictor, is
;2~ ~ a ~1 amino acid bicyclic peptide that was first
isolat@d from cultured porcine aortic endothelial
: cells. Endothelin-l, i8 one of a family of
structurally similar bicyclic peptide~ which include;
ET-2, ET-3, vasoactive intestinal contractor (VIC), :
~ and ~the sarafotoxinsl~SRTXs). T~q unique bicyclic
structure and corresponding arrangement of the
disulfide bridges of ET-l, which are the same for the -.
endothelins, VIC, and the sarafotoxins, ha~ led to :~
significant speculation as to the importance of the
resulting induced 3econdary structure to receptor
: binding and functional activity. ET-l analogues with
-~ incorrect disulfide pairings exhibit at least l00-fold
less vasoconstrictor activity. The flexible
: ' '
W093/21219 PCT/USg3/0365~
21~309(~
-2- ~;
C-terminal hexapeptide of ET-1 has been ~hown to be
important for binding to the ET receptor and
functional acti~ity in selected tissues.
Additionally, the C-terminal ~mino acid ~Trp-21) ~as a ``~
S critical role in binding and vasoconætrictor acti~ity,
since ET[1-20] exhibits approximately 1000-fold less
functional activity. -
Endothelin is involved in many human disease
states.
Several in vivo studies with ET antibodies ha~e
been reported in disea~e models. Left coronary artery
ligation and reperfusion to induce myocardial
infarction in th2 rat heart, caused a four- to
sevenfold increase in endogenous endothelin levels.
Administration of ET antibody was reported to reduce
the size of the infarction in a dose-dependent manner
(Watanabe, T., et al, "Endothelin in Myocardial
Infarction,~ ond.) 344:114 (~990)). Thus, ET
may be in~olved in the pathogenesis of congestive -~
heart failure and myocardial ischemia
~;Margulies, K.B., et al, "Increased Endothelin in
Experimental Heart FaiIure," Circulation 82:2226
~: : ( l 9 g O ) ) ~
Studies by Kon and colleagues using anti-ET
antibodies in an ischemic kidney model, to deactivate
endogenous ET, indicated the peptide's invol~ement in
acute renal ischemic injury ~Kon, V., et al,
"Glomerular Actions of Endothelin In Vivo, n J. Clin. ~"
Invest. 83:1762 tl989)). In isolated kidneys,
~ preexposed to specifiic a~tiendot;helin antibody and
then challenged with cyclosporine, the renal perfusate
flow and glomerular filtration rate increased, while . ~;~
renal resistance decreased as compared with isolated ~ `
kidneys preexpo~ed to a nonimmunized rabbit serum. -
The effecti~eness and pecificity of the anti-ET
antibody were confirmed by its capacity to pre~ent
renal deterioration caused by a single bolus dose
w093/21219 2 1 3 3 0 9 ~ PCT/~S93/0365~ ~
(150 pmol) of synthetic ET, but not by infuision of
angiotensin II, norepinephrine, or the thromboxane A2 ~:.
mimetic U-46619 in isolated kidneys ~Perico, N.,
et al, "Endothelin Mediate~ the Renal Vasoconstriction
Induced by Cyclosporine in the Rat,~ J. Am. Soc. .
Nephrol. l:76 ~l990)).
Others have reported inhibition of ET-l or ET-2-
induced va~oconstriction in rat isolated thoracic
aorta using a monoclonal antibody to ET-l (Koshi, T.,
et al, ~Inhibition of E~dothelin (ET)-l and ET-2- :
Induced Yasoconstriction by Anti-ET-l Monoclonal
Antibody, n Chem. Pharm. Bull., 39:1295 (l99l)).
Combined adr~inistration of ET-l and ET-l antibody
to rabbits showed significant inhibition of the blood `:
pre~sure (BP) and renal blood flow responses
(Miyamori, I., et al, Systemic and Regional Effects of
Endothelin in Rabbits: Effects of Endothelin
Antibody,~ Clin. Exp. Pharmacol. Physiol., 17:691
(1990)):-
: ~20~ Other investigators have reported that infusion
: of ET-specific antibodies into spontaneously :~
hypertensive rats ~SHR) decreased mean arterial
pres3ure (MAP), and increased glomerular filtration
rate and renal blood flow. In the control study with
.
~ normotensive Wistar-Ryoto rats (WRY) there were no
significant changes in these parameters (Ohno, A.
Effects of Endothelin-Specific Antibodies and
Endothelin in Spontaneously Hypertensive Rats,~
J. Tok~o Women~s Med. Coll., 6l:95l (l99l)).
~n additi~n,~elevated levels of endothelin have j -
been reported in several disease states (see Table I
below).
Burnett and co-workers recently demonstrated that
exogenous infusion of ET (2.5 ng/kg/mL) to
: 35 anestheti~ed dogs, producing a doubling of the :
circulating concentration, did have biological actions
: (Lerman, A., et al, ~Endothelin has Biological Actions
:
W093t2121s PCT~US93/03658_ ~
21330!~ j
-4-
at Pathophysiological Concentrations,~ Cirsulation
83:1808 (l99l)). Thus heart rate and cardiac output
decreased in association with increased renal and
~ystemic vascular resistances and antinatriuresis~ ~
These studie~ support a role for endothelin in the ;
regulation of cardiovascular, renal, and endocrine
function.
In the anesthetized dog with congestive heart
failure, a significant two- to threefold elevation of
circulating ET levels has been reported (Cavero, P.G.,
et al, n Endothelin in Experimental Congestive Heart
Failure in the Anesthetized Dog, n Am. J. PhYsiol.
259:F312 ~l990)), and studies in humans have shown
similar increases (Rodeheffer, R.J., et al,
n Circulating Plasma Endothelin Correlates With the
Severity of Congestive Heart Failure in Humans,~ ;
Am. J. Hypertension 4:9A (l99l)j. When ET was
chronically infused into male rats, to determine
; wheeher a ~long-term increase in circulating ET levels
would cause a sustained elevation in mean arterial
blood pressure, significant, su~tained, and dose-
dependent increases in mean arterial BP were observed.
Similar results were obserYed with ET-3 although
larger~doses were required (~ortenson, L.H., et al,
25~ ~ nChroni~: Hypertension Produced by Infu~ion of
Endothelin in Rats, n Hypertension, 15:729 (l990)3. ``
~; ~The distribution of the two cloned receptor ~`
- ~ subtypes, termed ETA and ETB, have been studied
extensively (Arai, H., et al, Nature 348:730 tl990),
Sakura~, T.~, et~ al, ature 348:732 (l990)!). The ET~
or Yascular smooth muscle receptor, i~ widely
distributed in cardiovascular tissues and in certain
regions of the brain (~in, H.Y., et al, Proc. Natl.
Acad. Sci. 88:3185 (l99l)). The ETB receptor,
originally cloned from rat lung, has been found in rat
cerebellum and in endothelial cells, although it is
not known if the ETB receptors are the same from these ~;
W093/21219 2 1 3 3 0 ~ O PCT/US93~036~8
.... ~ ' ' .
-5-
sources. The human ET receptor subtypes ha~e been
cloned and expre~sed (Sakamoto, A., et al, Biochem.
Biophys._Res. Chem. ~78:656 (1991), Hosoda, K., et al,
FE~S Lett. 2~7:23 (l991)). The ETA receptor clearly
mediates vasoconstriction and there have been a few
reports implicating the ETB receptor in the initial
vasodilatory response to ET (Takayanagi, R., et al,
FEBS Lett. 282:103 (1991)). However, recent data has
shown that the ETB receptor can also mediate
vasoconstriction in some tissue beds (Panek, R.L.,
et al, Biochem. Biophys. Res._Commun. 1~3(2):566
(1992)).
Comparison of the receptor affinities of the ETs
and SRTXs in rats and atri~ (ETA) or cerebellum and
hippocampus (ET9), indicate that SRTX-c is a selective
ETB ligand ~Williams, D.L., et al, Biochem. Biophys. -~
~es. Commun., 175:556 (1991)). A recent ~tudy showed
that selective ET8 agonists caused only vasodilation
in the rat aortic ring, possibly through the release
of EDRF from the endothelium (ibid~. Thus, reported ~-~
selective ETB agonists, for example, the linear analog ;~
ET[1,3,11,15-Ala] and truncated analogs ET16-21,
1,3,11,15-Ala], ETl8-21,11,15-Ala], and
N-Acetyl-ET[10-21,11,15-Ala~ caused vasorelaxation in
2S isoIated, endothelium-intact porcine pulmonary
arteries (Saeki, T., et al, Biochem. Bio~hys. Res. ~;~
Commun. 179:286 (1991)). However, some ET analogs are
poten~ vasocon~trictors in the rabbit pulmonary
artery, a tissue that appears to possess an ETB
y, nonselective type ~ofl receptor (ibid).
Plasma endothelin-l levels were dramatically
increa~ed in a patient with malignant
hemangioendothelioma (K. Nakagawa et al, Nip~on Hifuka ~`
Gakkai Zasshi, l990, 100, 1453-14S6).
The ~T receptor antagonist BQ-123 has been shown
to block ET-1 induced bronchoconstriction and tracheal
smooth muscle contraction in allergic sheep providing
WO93/2121~ 21~3og o PCT/US93/03658
-6-
evidence for expected efficacy in bronchopulmonary
diseases such as asthma (Noguchi, et al, Am Rev.
Respir. Dis., 1992, 145 (4 Part 2), A~58).
Circulating endothelin levels are elevated in
s women with preeclampsia and correlate closely with
serum uric acid levels and measures of renal
dysfunction. These observations indicate a role for
ET in renal constriction in preeclampsia (Clark B.A.,
et al, Am. J. Obstet. Gynecol., 1992, 166, 962-968).
Plasma immunoreactive endothelin-l concentrations
are elevated in patients with sepsiis and correlate
~ith the degree of illness and depression of cardiac
output (Pittett J., et al, Ann Surq., l99l, 213(3), -
262).
In addition the ET-l antagonist ~Q-123 has been
evaluated in a mouse model of endotoxic shock. This
ETA antagonist significantly increased the survival
rat~e~in~this model (Toshiaki M., et al, 20.12.90.
EP~0 4~36 l~as Al).
~ ~End~thelin is a potent agonist in the li~er -`-
eliciting both sustained vasoconstriction of the
hepatic vasculature and a significant increase in `~
hepatic glucose output (Gandhi C.B., et al, Journal of
iolo~ical~Chemistry, l990, 265~29), 17432). In ~,`
streptozotocin-diabetic rats there i~ an increased
sensiti~ity to endothelin-l (Tammesild P.J., et al,
Clin.~Ex~. PharmacoI. PhY~iiol , 1992, l9(4), 261). In
; addition increased levels of plasma ET-l have been
obser~ed in microalbuminuric insulin-dependent
ii diabete!s mellltuslpatients indicating a role for ET in
endocrine disorders such as diabetes (Collier A.,
et al, Diabetes Care, 992, 15(8), 1038). . `
` ETA antagonist re¢eptor blockade ha~i been found
to produce an antihypertensi~e effect in normal to low
renin modeiis of hypertenision with a time courise
similar to the inhibition of ET-l pre~sor responses ~-
(3asil M.~., et al, J. Hvpertension, 199~,
~' . .
.
~`
W O 93/21219 2 1 3 3 0 9 0 PC~r/US93/03658 ~
--7-
10(Suppl 4), S49). The endothelins have been shown to
be arrhythmogenic, and to have positive chronotropic
and inotropic effects, thus ET receptor blockade would
be expected to be useful in arrhythmia and other ~
cardiovascular disorders (Han S.-P., et al, Life Sci.,
1990, 46, 767).
The widespread localization of the endothelins
and their receptors in the central nervous system and -
cerebrovascular circulation has been described -
(Nikolov R.K., et al, Druqs of Today, 1992, 28(5),
303-310). Intracerebroventricular administration of
ET-1 in rats has bee~ shown to evoke several
behavioral effects. The~e factor~ strongly suggest a
role for the ETs in neurological disorders. The
potent vasoconstrictor action of ETs on isolated
cerebral arteriolec suggests the importance of these
-~ peptides in the regu~lation of cerebrovascular tone. ~~
Increased~ET~levels have been reported in some CNS
disorders,~i.e., in the CSF of patients with
20 ~ ~subarachnoid hemorrhage and in the plasma of women ~-
w~ith~preecliampsia. Stimulation with ET-3 under
; conditions of;hypoglycemia have been shown to
accelerate the development of striatal damage as a
result of an influx of extracellular calcium.
Circulating or locally produced ET has been ~uggested
to~contribu~te to regulation of brain fluid balance
~through effects on the choroid plexus and CSF
production. ET-l induced lesion development in a new
model of local ischemia in the brain has been ;~
described.~ l !1 ' ii i` ". , ~:
Circulating and tissue endothelin
immunoreacti~ity is increased more than twofold in
patient~ with advanced atherosclerosi~ (A. ~erman, et
al, New Enqland J. Med., 1991, 325, 997-1001).
3~ Increased endothelin immunoreactivity has also been
associ~ted with Buerger'9 di~ea9e (~. Ranno, et al, J.
` Amer. Med. Assoc., 1990, 264, 2868) and Raynaud's -
~'
W093/21219 PCT/US93/03658
2 i 33090
-8-
phenomenon ~M.R. Zamora, et al, Lancet, 1990, 336,
1144-1147). Likewise, increased endothelin
concentrations were observed in hypercholesterolemic
rats (T. Horio, et al, Atherosclerosis, 1991, ~9,~239-
245).
An increase of circulating endothelin le~els was
observed in patients that underwent percutaneous
transluminal coronary angioplasty (PTCA) (A. Tahara,
et al, Metab. Clin. Exp., 1991, 40, 1235-1237, K.
Sanjay, et al, Circulation, 1991, 84(Suppl. 4), 726).
Increased plasma levels of endothelin have been
measured in rats (T.J. Stelzner, et al, Am. J.
Physiol., 1992, ~62, L614-~620) and individuals
(T. Miyauchi, et al, Jpn. J._Pharmacol., 1992, 58,
279P, D.J. Stewart, et al, Ann. Internal Medicine,
1991, 114 464-469) with pulmonary hypertension. -
-~ Elevated levels of endothelin have also been
measured in patients suffering from ischemic heart
disease~(M. Yasuda, et al, Amer. Heart J., 1990, 119
20 ~ ~801-806, ~S.G. Ray, et al, Br. Heart J., 1992, 67, 3~3- -`
386)~and either stable or unstable angina /J.T.
Stewart, et al, Br. Heart J., 1991, 66, 7-9).
Infusion of an endothelin antibody lh prior to
and lh after a 60 minute period of renal ischaemia
25~ resulted in changes in renal function versus control.
In addition, an increase in glomerular platelet-
activating factor was attributed to endothelin (A. ;~
~opez-Farre, et al, J. Physioloqy, l991, 444, 513-
522). In patients with chronic renal failure as well
; as in patientsion,regular hemodialysis tr,eatment mean
plasma endothelin levels were significantly increased
(F. Stockenhuber, et al, Clin. Sci. l~ond.), 1992, 82,
255-258). In addition it has been suggested that the
prolifarative effect of endothelin on mesangial cells
may bé a contrlbuting factor in chronic renal failure
(P.J. Schultz, J. Lab. Clin. Med., 1992, 119, 448-
449).
,
WO93/21219 2 1 3 3 o (3 o PCTIUS93/03658
Local intra-arterial administration of endothelin :~
has been shown to induce small intestinal mucosal
damage in rats in a dose-dependent manner (S. Mirua,
et al, Diqestion, 1991, 48, 163-172). Administration
S of endothelin-1 in the range o~ 50-500 pmol/kg into
the left gastric artery increa3ed the tissue type ;~
plasminogen activator release and platelet activating
formation, and induced gastric mucosal hemorrhagic
change in a dose dependent manner (I. Xurose, et al, .
Gut, 1992, 33, 868-871). Furthermore, it has been
shown that an anti-ET-1 antibody reduced ethanol-
induced vasoconstriction in a concentration-dependent -~
manner (E. Masud2, et al, Am. J. Physiol., 1992, 262,
G785-G790). Elevated endothelin levels have been
observed in patient~ suffering from Crohn's disease :~
and ulcerative colitis (S.H. Murch, et al, Lance.t,
1992, 339, 381-384)~
Recently the nonpeptide endothelin antagonist
RO 46-2005 has been reported to be effective in models .
of~acute renal ischemia and subarachnoid hemorrhage in ~-
~ rats~(3rd International Conference on Endothelin, `~:
- :~ Houston, Texas, February 1993). In addition, the ETA -
antagonist BQ^123 has been shown to prevent early
cerebral vasospasm following subarachnoid hemorrhage
(M. Clozel and H. Watanabe, Life Sci., 52:825-834
(1993)).
: .
~: ' ; ;.
,' ~
'
WO 93~21219 213 3 ~) 9 0 PCI/US93/03658
- 10-
TABLE I . Plasma Concentrations of ET- 1 in Humans
ET Plasma
C ndition NormalLevel~ Reported
Control( /mL)
Atherosclerosis 1.4 3.2 pmo~/L
Surgical operation 1.5 7.3
Buerger's disea~e 1.6 4.8
Takayasu's arteritis 1.6 5.3
Cardiogenic shock 0.3 3.7 :-
Congestive heart failure ~CHF) 9.7 20.4
Mild CHF 7.1 11.1 ~.
Se~ere CHF 7.1 13.8
Dilated cardio~yopathy 1.6 7.1
Preeclampsia 10.4 pmol/L 22.6 pmol/L
Pulmonary hypertension 1.45 3.S
Acute myocardial infarction 1.5 3.3
(~everal reports) 6.0 11.0
0.76 ~.9S :
0.50 3.8
Subarachnoid hemorrhage 0.4 2.2
Crohn's Disease 0-24 fmol/mg 4-6~ fmol/mg -~
Ulcerati~e coliti6 0-24 fmol/mg 20-50 fmol/mg --
Cold pres~or test 1.2 8.4
Raynaud'6 phenomenon 1.7 5.3 ~:
Raynaud'6/hand cooling 2.8 5.0
Hemodialysis c7 10.9
(several reports) 1.88 4.59 .~:
Chronic renal failure 1.88 10.1 :
Acute renal failure 1.5 10.4 ~:
~remia before he:modialy~i6 0.96 1.49
Uremia after hemodialysi6 0.96 2.19
Es~ential hypertension 18.5 33.9
Sep~i6 6yndrome 6.1 19.9
Postoperative cardiac 6.1 11.9
I~flammatory arthritide~ l.S 4.2 ~:
Malignant hemangioendothelioma 4.3 16.2
: 35 (after
removal)
,~ -
Rovero, P., et al, British Journal of
PharmacolQ~y 01, pages 232-236 (1990) disclosed
various analogs of the C-terminal hexapeptide of ET-l,
none of which wére reported;to bé antagonists of ET-1.
Doherty, A. M., et al, Ab~tract, Second
International Conference on Endothelin, Tsukuba,
Japan, December 9, 1990, and the published manuscript
~45 (J. Cardiovasc. Pharm. 17 (Suppl. 7), 1991,
pp. 559-561) disclosed various analog~ of the
C-terminal hexapeptide of ET-1, none of which
ex~libited any functional activity.
~.
~ 93/21219 2 1 3 ~ O 9 ~ PCT/US93/03658
- 1 1 - .
Copending United States Patent Appli~ation
Serial Number 07/995,480 discloses a ~eries of novel
antagonist~ of endothelin.
However, we have surprisingly and unexpected~y
found that a series of C-terminal hexapep~ide and
related analogs of ET-l are receptor antagoni~ts of -;~
endothelin. Additional data for the activity of this ;
series of peptides iY found in the followins -~
referenceQ (W.L. Cody, et al, J. Med. Chem., 1992, 35, ~;;
3301-3303., D.M. LaDouceur, et al, FASEB, lg92).
~ ~.
SUMMARY OF TEE INVENTION
Accordingly, the present irl~ention is a compound
of Formula I
AAl - AA2 - AA3 - AA4 - AA5 AA6 ~;
I
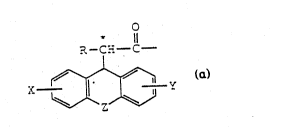
wherein AAl is ~-
' :'.
- R1 O
R-C-C -
`~ ZS X~ Y
wh~rein R is!,hydrogen~
alkyl,
alkenyl,
alkynyl,
, ; cycloalkyl,
~35 cycloalkylalkyl,
aryl,
heteroaryl,
fluorenylmethyl,
- ,- .
WO93/21219 ` 2 1 3 3 0 9 0 PCT/US93/0365~
, :.,
-12-
N R2, wherein R2 and R3 are each the same or
R3 different and each is
hydrogen,
alkyl, ~
alkenyl, .
alkynyl, .:
cycloalkyl, ~:
cycloalkylalkyl, ~-
aryl, :-
arylalkyl,
heteroaryl, or ~-~
fluorenylmethyl,
O ,~
-C-OR2, wherein R2 is as defined above,
-oR2, wherein R2 is as defined above,
O
-N-C-N-R3, wherein R2 and R3 are as defined
2 1 2 above,
O .
-C-C~R9)3, wherein R9 is F, Cl, ~r, or~
-CH2-OR2, wherein R2 is as defined above f '"
o ~.~
: 3 ~-
:~ -N-C-R , `.
~: 30 ¦Za
wherein R2a is hydrogen or alkyl and R3 i9 ag
defined above, ;
11 3
: -N-C-OR ,
2a
wherein R2a and R3 are as defined above
excluding R3 i8 hydrogen, or
-
O
-C-R2, wherein R2 is as defined above,
Rl is hydrogen or alkyl,
. ~''
~"~93/21219 2 `1 3 3~0 9 ~ PCT/VS93/03658 i
-13-
Z is :;'
-O-,
-S ()m~
wherein m is zero or an integer of~ -
1 or 2, -~
-N-, wherein R2 is as defined above,
12 :~
-(CH2)n-, wherein n is zero or an integer :~
of 1, 2, 3, or 4,
- ( CH2 ) n- CH=CH- ( CX2 ) n~
wherein n is as defined abo~e,
O ,~,
Il ,
-C-,
-CRl-, wherein R1 and R2 are as defined
IR2 above, or ~ ~:
~ ~ : R2 . ':~
: : 13
wherein R2 and R3 are each the same or
25 ~ different and each is as defined above,
X and Y are the same and substituted at the same
:~ : position on the aromatic ring and each may :
be one, two, three, or four sub6tituents
selected from the group consisting of
:30 : ~ hydrogen,
,, -: ~ :
: halogen,
:alkyl,
-CO2R2, wherein R2 is as defined above, ::
li ~ -CONR2,! whierein:R2 an`d R3 are'as defined ;
: ~ 35 13
~-- R abo~e,
NR2, wherein R2 and R3 are as defined
above, or
~ ~;
WO93/21219 213 3 O 9 ~ PCTIUS93/03658~;
-14- ;-::
nitro or ~:
O ~';,
R--C--C-- ~
S X--~--Y
wherein R, Z, X, a~d Y are as defined above; ~:
~
AA2 is
Rl O ,.
~ * 11 ~
--N--C~C---
Rl ( CH2 ) n
1 5 R4
`:
wherein R4 is
, ~
~ hydrogen, .1-
:~-: 20 ~ alkenyl, : `~;
alky~yl,
cycloalkyl,
aryl,
heteroaryl,
;25~ ~ ~ N R3b,
2b
wherein R2b and ~3b are each the
same or different and each i8
~ hydrogen,
~ . ....
lkyl,
cycloalkyl,
aryl, or
~ ,
heteroaryl,
-OR2b, wherein R2b i8 as defined above, -;`
- C - N- R3b,
~ 40 R2b
:,
: ~
:, ;~.
93/~1219 213309~0 PCr/U593/03658
- 1 5 -
wherein R2b and R3b are each
the same or different and .:
each i~ as def ined abotre f or
R2b 3.nd R3b,
O
-C-R2b, wherei~ R2~ is as defined
abo~e, .
NH ,
NH c N~ R2b wherein R2b is as defined -~
above, or ~.
O
- 15 -C-OR~, wherein R2b is as de~ined
abovei, and :;~
Rl and n axe as def ined above, or
- AA2 iS absent;
: ~ is
: ~ ; 20 ~ : Rl O
--N--C--C--
Rl ( CIH2 ) n
Rs ~i
25 i wherein R5 i~
: : hydrogen,
alkyl,
aryl,
heteroaryl,
, ,:
~-~`: 30~ : o
~ 2~
~ :35 wherein R2b and R3b are
: ; each the same or
different and each i9 as
~, - -, ,,
def ined above,
". ~ :
..
WO93~21219 PCT/US93/03658~ ^~
2133~9D
-16-
O '~
2b, wherein R2~ is as
defined above, or
0
-C-OR2b, wherein R2b is as
defined above, and
Rl and n are as defined above, or
AA3 is absent; ~::
AA4 and AA5 are each independently absent or each is
independently
Rl O ,~
C--C-- .
Rl ~ IH2) n
R6 `
wherein R6 is hydrogen,
alkyl,
20 ~ alkenyl,
alkynyl,
cycloalkyl, ;
aryl, or
heteroaryl, and
i2~5~ :: Rl and n are as defined above;
~iS
R ~,!'
N--C*-R
- ~ : :Rl: ( CH2 ) n r
R7 ;
wherein R7 is ~.
aryl or ~.
~;~- . heteroaryl, .
:
~: ', R8 i~3 '
, O :
-C-OR1, wherein Rl i~ as defined `-.
above,
~ :
"~O 93~21219213 3 0 9 0 PC~r/US93/03658
,, '' ;
-17-
-OR1, wherein Rl is as defined :.~
above, -
O
5-C-~-R1, wherein R1 is as defined
Il abo~e, or .
-CH2-ORl, wherein Rl is as defined
above, and
10Rl and n are as defined above;
stereochemi~try at C in A~ 2, AA3, A~4, or AA5 iS
D, L, or ~L and
3tereochemistry at C in AA6 is L; or a
pharmaceutically acceptable salt thereof.
. Ele~ated levels of endothelin ha~e been
postulated to be involved in a nu~ber of
pathophysiological states including diseases
20~ ~associated with:the cardiovascular system~às well as
~ari~ous metabolic and endoc~inological disorders. As
antagonists of endothelin, the compounds~of Formula I
are:~useful in~the treatment of hYpertension, :`.
myQcardial infarction, metabolic, endocrinological and
:neurolog:ical disorders, congestive heart~failure,
endot~oxic shock, subarachnoid hemorrhage, arrhythmias,
asthma, and chronic~and acute renal faiIure,
presclampsia, atherasclerotic di~orders including
-Raynaud'3~ diseas~e, re~tenosis, angina, cancer,
~ pulmonary~hypertension, ischemic disPase, ~astric
mucosal damage, hemorrhagic ~hock, i~chemic bowel `~
~; disease,-and d-~abetes,.~
A still further embodiment of the prqsent
nvention is a pharmaceutical composition for
35 ~. admini3tering an effe¢tive amount o a compound of `~
Formula I in unit dosage form in the treatment method~ :
me~tioned above.
FinaIly, the present in~ention i~ directed to
method~ for production of a compound of Formula I. ~.
'
WO93/21219 PCT/US93/03658,.~
2 :1 3 3 '0 ,~ O
-18- :~
DETAILED DESCRIPTION OF THE INVENTION :
In the compounds of Formula I, the term "alkyl"
means a straight or branched hydrocarbon radical ~ -~
having from 1 to 12 carbon atoms and includes, for
example, methyl, ethyl, n-propyl, isopropyl, n-butyl,
sec-butyl, isobutyl, tert-butyl, n-pentyl, n-hexyl,
n-heptyl, n-octyl, n-nonyl, n-decyl, undecyl, dodecyl,
and the like.
The term "alkenyl" means a straigh~ or branched
unsaturated hydrocarbon radical having from 2 to
12 carbon atoms alld includes, for example, ethenyl,
2-propenyl, 1-butenyl, 2-butenyl, 1-pentenyl,
2-pentenyl, 3-methyl-3-butenyl, 1-hexenyl, 2-hexenyl,
3-hexenyl, 3-heptenyl, 1-octenyl, 1-nonenyl,
1-decenyl, 1-undecenyl, l-dodecenyl, and the like.
The term "alkynyl" means a straight or branched
triple~bonded unsaturated hydrocarbon radical having
rom~2 to 12 carbon atoms and includes, for example,
e~hynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3-butynyl, .
1-pentynyl, 3-pentynyl, 1-hexynyl, 2-hexynyl,
3-hexynyl, 3-heptynyl, 1-octynyl, 2-octynyl, '~
1-nonynyl, 2-nonynyl, 3-nonynyl, 4-nonynyl, 1-decynyl,
2-decynyl, 2-undecynyl, 3-undecynyl, 3-dodecynyl, and
~ the like.
The term "cycloalkyl" means a saturated
hydrocarbon ring which contains from 3 to 12 carbon
atoms, for example,~ cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl, adamantyl, and the like.
30 ~i The term~ cyçloalkylalkyl n means a saturated
hydrocarbon ring attached to an alkyl group wherein
alkyl is as defined above. The saturated hydrocarbon
ring contains from 3 to 12 carbon atoms. ~xamples of
-~ such are cyclopropylmethyl, cyclopentylmethyl,
cyclohexylmethyl, adamantylmethyl and the like.
The terms "alkoxy n and "thioalkoxy n are O-alkyl
or S-alkyl as defined above for alkyl.
~: '
'
~ .
"'~93/21219 ~1 3 3 0 9 0 PCT/US93/03658 .
-l9- :
The term "aryl" means an aromatic radical which
is a phenyl group, a benzyl group, a naphthyl group, a
biphenyl group, a pyrenyl group, an anthracenyl group,
3,3-diphenylalanyl, lO,ll-dihydro-5H-dibenzo~a,d]~ ~
(cyclohepten-5-yl)glycyl, or a fluorenyl group and the :
like, unsubstituted or substituted by l to
4 substituents selected from alkyl as defined above,
alkoxy as defined above, thioalkoxy as defined above, ~.
hydroxy, thiol, nitro, halogen, amino, -NH-C-alkyl .
O
Il -
wherein alkyl is as defined above, -C-O-alkyl wherein
O ::
a}kyI is as defined above, -C-alkyl wherein alkyl is
: as defined above, or aryl.
The: term~-ar~yla1kyl"~means an aromatic radical
20 ~ a:ttac ~ d~to~an:~alkyl~radical~wherein aryl and alkyl ~-
aré~as~def;ined~above~for example benzyl,:
fluorenylmethyl~and~the like. . -`
The term~"heteroaryl" means a:heteroaromatic ~-
radical~which is 2-or 3-thienyl, 2- or 3-furanyl, 2-
25~ or 3-~pyrr~l l, 2-, 4-,:or 5-imidazolyI, 3-,~ 4-, or
`5~pyrazo}yl,:~2-~, 4-,::or 5-thiazolyl, 3-, 4-, or `~
5~-i ~ iazolyl,-~2-, 4-,~ or~5-~oxàzolyl, 3-, 4-, or '.`
isoxazolyl,;~:~3;-~or 5-1,2,4~-triazolyl, 4- or
~r~ 5~1~ 2~,~`3-eriazolyl,~ tetrazolyl, 2-, 3-,::or 4-pyridinyl,
30~ 3~ 4~ or~:~5~-:pyridazinyl, 2-pyrazinyl, 2-, 4-, or ~.
5-pyrimidinyl, 2-, 3-, 4-, 5-, 6-, 7-, or ;`
8-~quinolinyl,,/~ 3~ 4i- ,! 5-, 6-l, 7-, or
8-iqoquinolinyl, 2-, 3-, 4-, 5-, 6-, or 7-indolyl, 2-,
3-,~4~ 5-, 6-, or 7-benzo[b]thienyl, or 2-, 4-, 5-,
.~6-,~or 7-benzoxazolyl, 2-, 4-, 5-, 6-, or
7-benzimidazolyl, a-, 4-, 5-, 6-, or 7~benzothiazolyl,
uniubstituted~or substituted by 1 to 2 substituents
elected from alkyl as defined above, aryl as defined ;~.
~ .
WO93/21219 2 1 ~ 3 0 9 0 PCT/US93/036~_
-20-
above, alkoxy as defined abo~e, thioalkoxy as defined
above, hydroxy, thiol, ~
Il ~ , .
nitro, halogen, formyl, amino, -NH-C-alkyl wherein
O ~,
alkyl is as defined above, -C-O-alkyl wherein alkyl
O
' 11 ,''.'`
is as defined above, -C-alkyl wherein alkyl i~i as ~i:
de~ined above or phenyl.
The term "heterocycloalkyl" means 2- or ~`
3-tetrahydrothieno, 2- or 3-tetrahydrofurano, 2- or .-
3-pyrrolidino, 2-, 4-, or 5-thiazolidino, 2-, 4-, or -~
5-oxazolidino, 2-, 3-, or 4-piperidino, N-morpholinyl ~`
or N-thiamorpholinyl. :.
"Halogen" is fluorine, chlorine, bromine or ~-
iodine.
: The f~ollowing table:provides a lis~ of
a`bbreviations and definitions thereof used in the
pre~ént invention.
: : .
~.
~. .
, . . ..
.,::
,':
'
~93/21219 2 1 ~ ~ O 9 0 PCT/US93/03658
-21-
TA~LE
Abbreviation~ Amino Acid
Ala Alanine
Arg Arginine
Asn Asparaglne
Asp Aspartic acid -;~
Cy5 Cysteine ;
Glu Glutamic acid
Gln Glutamine ~,
Gly G3.ycine .
His Histidine -",
Ile Isoleucine
Leu Leucine ~`
. ~ .
Lys~ ysi~e
- Met ;Methionlne~ ~'
'Phe~ Phenylalanine
Pro~ Prollne
S~er:~ Serine ,,,
2Q Thr Threonine ~.
Trp Tryptophan~
Tyr Tyrosine
Val ~ ~ Valine ``i
25' : ~,Abbre~i~tion* Modified and Unusual Amino Acid
Bhg 10,11-Dihydro-5H-dibenzo[a,d]- ,~
~ " ,'" !~ cycljQhepten-5-yl,)glycine, or ;~
- ~-Amino-10,11-dihydro-5H-dibenzo-
[a,d]cycloheptene-5-acetic acid
, . Bip (Paraphenyl)phenylalanine :~
: ~ If the optical actiYity of the amino acid is other -:
'~ than ~S),~ the amino acid or abbreviation is
~: preceded by the appropriate configuration D(R) or
~, ' D~(RS).
WO93~21219 2 1 3 3 ~ 9 o PCT/US93/0365~
-22- ~;
Abbre~iation* Modified and Unusual Amino Acid
(cont) `~
Dip 3,3-Diphenylalanine
3Hyp 3-Hydroxyproline ;
4Hyp 4-Hydroxyproline ~
N-MePhe N-Methylphenylalanine '~;
N-MeAsp N-Methylaspartic acid
Nva Norvaline
Nle Norleucine .;
Orn ~ Ornithine ~
Abu 2-.Aminobutyric acid ,~.
Alg 2-Amino-4-pentenoic acid '~'!'~'~'`
(Allylglycine)
Arg(N02) NG-nitroarginine
Atm~ 2-Amino-3-(2-amino-5-
thiazole)propanoic acid ,~
Cpn~ : ~ 2-Amino-3-cyclopropanepropanoic acid
Cy~clopropylalanine)
15 ~ Chx ~ Cyolohexylalanine (Hexahydrophenyl-
;Emg ~ ~ 2-Amino-4, 5 ~RS) -epoxy-4-pentenoic
acid ;::
His~(Dnp) ;Nim- 2,4-Dinitrophenylhistidine
HomoGlu : 2-Aminoadipic acid
: HomoPhe : ~ 2-Amino-5-phenylpentanoic acid
Homophenylalanine)
Met(O) Methionine sulfoxide :
: Met (2) / ilMelthionine sulfone / l~ -
Nal 3-(1'-Naphthyl)alanine
: 2-Nal 3-(2'-Naphthyl)alanine
;,. .: ~,
:Nia 2-Amino-3-cyanopropanoic acid
. (Cyanoalanine)
: ~25 ~: ~ Pgl Phenylglycine
; P~y 2-Aminopentanoic acid (Propylglycine) ~
, ~ . ...
. .
- ' .
~093/21219 ~1 3 3 0 g o P~T/US93/03658
-23-
Abbrevi~tion* Modified and_Unusual Amino_Acid
(cont) .
Pha 2-Amino-6-(1-pyrrolo)-hexanoic acid
Pyr 2-Amino-3-(3-pyridyl)-propanoic acid `
(3-Pyridylalanine)
Tic 1,2,3,4-Tetrahydro-3- .
i~oquinolinecarboxylic acid ~-~
Tza 2~Amino-3-(4-thiazolyl)-propanoic
acid :.
:: Tyr(Ot-Bu) O-tertiary butyl-tyrosine !'i~
Tyr(OMe) O-Methyl-tyrosine
Tyr(OEt). O-Ethyl-tyrosine
Trp(For) Nin-Formyl-tryptophan
~- 10 Bheg 5H~-Di~enzo~a,d]cycloheptene glycine
Txg 9U-Thioxanthene glycine ~;
viation: ~Protectin~ Group
Acetyl~
15~ Ada~ l-Adamantyl~acetic acid
Adoc ~: Adamant~loxycarbonyl ~:
Bzl Benzyl ;~
MeBzl :4-Methylbenzyl
Z~ Benzylo~cycarbonyl
0.~ 2~-Br~-Z~ ortho-Bromobenzy}oxycarbonyl
2-Cl-Z :~ ortho~Chlorobenzyloxycarbo~yl
30m: ~ Benzyloxymethyl
t ' Boc i ~ , tertia ~ ~Bueyl~oxycarbonyl
TBS tertiary Butyldimethylsilyl
25~ ;~Dnp ~ 2,4-Dinltrophenyl
. For ; Formyl
Fmoc 9-Fluorenylmethyloxycarbonyl
~ N02- ~ Nitro
; : :: Tos 4-Toluenesulfonyl (to~yl)
.. . ~ ,
,
,: :
WO93/21219 ~1 3 3 0 .S O pcT/us93/036sa~ ~:
-24-
Abbreviation Protectina Group (cont)
Trt Triphenylmethyl (trityl)
Ada l-Adamantyl acetic acid
~z Benzylcarbonyl ~`:
tBu t-Butylcarbonyl ~
CF3CO Trifluoroacetyl ~-
Cxl Cyclohexylacetyl
Cxl(U) Cyclohexylurea
Et Propionyl
Pya 3-Pyridylacetyl
Me(U) Methylurea
Abbre~iat1on Solvents and Reagents
HO~c Acetic acid ~
CH3CN Acetonitrile ::
: DCM Dichloromethane
. .;
DCC N,N'-Dicyclohexylcarbodii~ide
DIEA N,N-Diisopropylethylamine
DMF Dimethylformamide ~-`
HCl Hydrochloric acid -
;: ~ HF Hydrofluoric acid
, ~
HOBt l-Hydroxybenzotriazole
KOH Potasqium hydroxide
TFA Trifluoroacetic acid
: 25 MBEA Resin Methylbenzhydrylamine resin
PAM Resin 4-(Oxymethyl)-phenylacetamidomethyl
.~ ~ resin ~
The compounds of Formula I are capable of further
forming both pharmaceutically acceptable acid addition
and/or base salts. All of these forms are within the
scope of the present invention.
WO93/2121g n go PCT/US93/03658
-25- :~
Pharmaceutically acceptable acid addition salts
of the compounds of Formula I include salts derived
from nonto~ic inorganic acid~ such as hydrochloric, ..
nitric, phosphoric, sulfuric, hydrobromic, hydriodic, :
hydrofluoric, phosphorous, and the like, as well as
the salts derived from nontoxic organic acids, such as ;~
aliphatic mono- and dicarboxylic acids, ~.
phenyl-substituted alkanoic acids, hydroxy alkanoic .~".
acids, alkanedioic acids, aromatic acids, aliphatic ;~
and aromatic sulfonic acids, etc. Such salts thus l~
include sulfate, pyrosulfate, bisulfate, sulfite, ~`
bisulfite, nitrate, phosphate, monohydrogenphosphate, .
dihydrogenphosphate, metaphosphate, pyrophosphate,
chloride, bromide, iodide, acetate, trifluoroacetate,
propionate, caprylate, isobutyrate, oxalate, malonate,
su:ccinate, suberate, sebacate, fumarate, maleate,
màndelate,:~benzoate, chlorobenzoate, methylbenzoate, ~:
dini~robenzoate, phthalate, benzenesulfonate,
:~ eoluenesulf~onate, phenylacetate, citrate, lactate, : mal~eate, tartrate,:methanesulfonate, and the like.
Also~contemplated:are salts of amino acids such as
arginate and the like and gluconate, galacturonate
: (s~ee,~for~e ampl:e, Berge, S. M., et al,
Phàrmaceutical Salt:s, n Journal of Pharmaceutical
~; : 25 ~ :Science, 66, pp. 1-19 (1977)). -
The:acid addition salts of said basic compounds
~ are~prepared by coneacting the free base form with a
;~: : :: suffic~ient amount of:the desired acid to produce the
~ ~ saIt in the conventional manner. Preferably a peptide
:~ 30 of Fonmula` I can be!con~erted to an acidi,c salt by I .treating with an aqueous solution of the desired acid,
-~- sùch that the re ulting pH is les~ than 4. The ;
; solution can be~::pa~sed through.a C18 cartridge to
~ absorb the peptide, washed with copious amounts of
~ 35 water, the peptide eluted with a polar organic solvent
~ such as, for example, methanol, acetonitrile, aqueous
, .. . . .
~ mixtures thereof, and the like, and isolated by `.
~ :,
,
W093/21219 PCT/US93/03658 ~'~
i~
-26-
concentrating under reduced pressure followed by
lyophilization. The free base form ~ay be regenerated
by contacting the salt form with a base and isolating
the free base in the conventional manner. The free
ba~e forms differ from their respective salt forms
somewhat in certain physical properties such as
solubility in polar solvents, but otherwise the salts
are equivalent to their respective free base for
purposes of the present invention~
Pharmaceutically acceptable base addition salts
are formed with metals or amines, such as alkali and
alkaline earth metals or organic amines. Examples of
metals used as cations are sodium, potassium,
magnesium, calcium, and the like. Examples of
suitable amines are N,N'-dibenzylethylenediamine,
chloroprocaine, choline, diethanolamine,
dicyclohexylamine, ethylenediamine, N-methylglucamine,
- and procaine (see, for example, Berge, S. M., et al.,
"Pharmaceutical Salts, n Journal of Pharmaceutical
Science;, 66, pp. l-l9 (1977)).
The base addition salts of said acidic compounds
are prepared by contacting the free acid form with a
sufficient amount of the desired base to produce the
; salt in the conventional manner. Preferably, a
peptide of Formula I can be converted to a ba~e salt
by treating with an aqueous solution of the desired
base, such that the resulting pH is greater than 9.
The solution can be passed through a Cl8 cartridge to
absorb the peptide, washed with copious amounts of
wate~r, the pept~ide eluted with a polar organic solvent
such as, for example, methanol, acetonitrile, aqueous
mixtures thereof, and the li~e, and isolated by
; concqntrating under reduced pressure followed by
lyophilization. The free acid form may be regenerated
by contacting the salt form with an acid and isolating
the free acid in the conventional manner. The free
acid forms differ from their re~pective qalt forms
~40 93/21219 ~l33~9 PC~r/US93/03658
-27-
somewhat in certain physical properties such as
solubility in polar solvents, but otherwise the salts
are equivalent to their respective free acid for
purposes of the present invention.
Certain of the compounds of the present invention
can exist in unsolvated forms as well as solvated
forms, including hydrated forms. In general, the
solvated forms, including hydrated forms, are ~-~
equivalent to unsolvated forms and are intended to be
encompassed within the scope of the present invention.
Certain of the compounds of the present invention
possess one or mo-e chiral centers and each center may -~
exist in the R(D) or S(L) configuration. The present
invention includes all enantiomeric and epimeric forms
as well as the appropriate mixtures thereof.
~ A preferred compound of Formula I is one wherein
iS: O ~ ~
~: : R - CH - C - ```
X - ~ - y
wherein R i9 -N-R2,
R3
wherein R2 and R3 are each the same
or different and each is
hydrogen,
alkyl,
alkenyl,
alkynyl,
cy~loalkyl,
cycloalkylalkyl,
~- - aryl,
- ~ arylalkyl,
~; 35 heteroaryl, or
fluorenylmethyl,
,:
~,:
WO93/21219 213 3 D 9 0 PCT/US93/0365~
-28-
O
-N-C-N-R3, wherein R2 and R3 are as ~:
1 2 1 2 defined above,
-C-C(R9)3, wherein R9 is F, Cl, Br,
or I,
1 0 l ~ ,'
-NH-C-R3, wherein R3 is as defined
above, or
O
11 3
-~H-C-OR ,
wherein R3 is as defined above
excluding R3 is hydrogen, :~
Z is - O-,
-20 -S ()m~ ':
wherein m is zero or an integer of ::~
l or 2, `:~-
N-, wherein R2 i9 as defined above,
- ~ 25 : 1 ~2 :
- ~CH2)n, wherein n is zero or an integer of
1, 2, 3, or 4, ~;
-(CH2)n-CHSCH-~CH2)n-, wherein n is as
defined above,
3Q ~ ~
~:.: : - C- :.
CH-, wherein Rl is hydrogen or alkyl,
O
R2
--C I : '
..
'3
-~ 40 ~ wherein R2 and R3 are each the same or .
different and each is as defined above
and `
, ::
w093/21219 2133~) PCT/US93/036a8
-29- ::~
X and Y are the same and ~ubstituted at the same ;
position on the aromatic ring and each
substituent is selected from the group
consisting of .
hydrogen,
halogen, or -
alkyl;
AA2 is O
- NH - CH - C -
( IH2)n
R4 `
wherein R4 is hydrogen,
alkyl,
alkenyl,
alkynyl,
cycloalkyl,
aryl,
~- ~ 20 heteroaryl,
- N - R3b, . , . : '
R2b
wherein R2b and R3b are each
. .25 the same or different and
each i9
:: hydroge~, :
alkyl, :1
cycloalkyl, ~:
aryl, or ~-~
` I ! ; i ` ` hèteroaryl~
-OR2b, wherein R2b i9 as
defined above, ;:
3 -C-N-R3b, ;
::
.~
W O 93/~1219 2 1 3 3 PC~r/US93/03658
-30- ~:
wherein R2b and R3b are each
the same or different and
each i9 as defined above for
R2b and R3b
O
-C-R2b, wherein R2~ is as defined
above,
1~
-NH-C-NH-R2b, wherein R2b i~ as
defined above, or
O ,
-c-oR2b, wherein R2b is a~
defined above, and :~`
n is a9 defined a~ove or
AA2 is absent;
AA3 is
~20 : ~ - NH - CH - C -
t CIH2 ) n
RS `:
: wherein R5 is aryl, ``;heteroaryl, ~;
~ ~ o , ~.:
3 0 ~ - C - N- R3b, ``
2b
: wherein R2b and R3b are each the
same or different and teach i-q asl ' ~`
: 35 defined above,
~T-R2b, wherein R2b i8 as defined .
above, or
. ,.
'
WO93/21219 ~33~9~ PCT/US93/03658
-31- ~
o
-C-OR2b, wherein R2b is as defined
above, and
n is as defined above, or
AA3 is absent;
AA4 and AA5 are each independently absent or each is
O . .~
* 11
- NH - CH - C -
( IH2) n : ~
R6 ~;:
wherein R6 is hydrogen :
alkyl, .~.
alkenyl, -.
alkynyl,
cycloalkyl,
` aryl, or
~ ~ heteroaryl, and
n-is as defined above;
AA6 is ~.
-NH- CH-CO2H
25 ~ ~ tfH2)n
R7
` wherein R7 is aryl or
; 30 : heteroaryl, and
n is as defined above, or -~-
O
-NH-~CH-C--N-R1
; 35 ¦ ¦ wherein R7, Rl, and n are as
` (CH2)n Rl defined above,
: ~ 17
~ ` ,
W093/21219 2~ a9u PCT/US93/03658
-32-
stereochemistry at CH in AA1, AA2, AA3, AA4, or AA5 is
D~L, or D~, and -
* ~.
5stereochemistry at CH in AA6 is L; or a
pharmaceutically acceptable salt thereof.
A more preferred compound of Formula I is one
wherein AAl is .
* 11 , ~:
R-CH - C -
X~ Y '.'~
15wherein R is -N-R , :
wherein R2 and R3 are each the same
or different and each:is
20~ hydrogen,~ :
alkyl, ~ :
aryl, or
: fluorenylmethyl,
N-C-N-:R3, wherein R2 and R3 are
R2 R2 as~defined above,
C-C(R9)l,wherein R9 i9 F, Cl, Br, or I,
1l ~ ~
: ~ 3~ -NH-C-OR10, wherein R10 i9 hydrogen,
-~ ~ : : alkyl, aryl, or ~;;
. arylalkyl, excluding RlO
is hydrogen,
Z is O-,
: 40 -S~
WO g3/21219 2 l 3 3 0 ~ U PCr/US93io3658
-33-
-~CH2)n, wherein n is zero or an --
integer
of 1, 2, 3, or 4, or
- ~CH2)n-CH=CH- ~CH2)n'
wherein n is zero or an integer of
1 or 2 and
- X and Y are each the same and substituted at ~-
the same position on the aromatic ring
and each substituent is selected from
- the group con~isting of
hydrogen,
halogen, or
alkyl;
AA2 iC
l~i O
NH-CH-C- , ..
~ 1 2)n
,~
;~ : wherein R4 i~ hydrogen, ;:
: alkyl,
aryl,
heteroaryl, .
N-R3b , :
2b
wherein R2b and R3b are
: ~ ~ each the same or
different and each is
hydrogen or alkyl, ~-;
- ~ 35 -C-N-R3b '
R2b
.~ ~ - wherein R2b and R3b are ~:
each the same or
~ different and each is
hydrogen or alkyl, ~;
, ~ ;
:: , .
~ ' ~
h 1 ~ ~ U
W093/21219 PCT/US93fO3658
,
-34- : .
NH . -~,
-NH-C-NH-R2b wherein R2b i5 as ~;
defined above, ;~-~
or
O ~- ~
OR2b wherein R2b is as
defined above, and
n is an integer of l, 2, 3, or 4 or
AA2 is absent; ~.
~;A3 is o ~`
-NH-fH-C~
(CIH2)n
. R5 wherein R5 is `,~,!'.
20~ ~ : aryl,
heteroaryl,
N~R3b wherèin R3b i9
25 : ~ : hydrogen or :~
alkyl,
0 " -:
2b ~wherein R2 i9
~a ~ hydrogen or ~ `.
; alkyl, or
; Il oR2b~ wherein R2b is -
: ~ hydrogen or ::~
: n is an integer of l, 2, 3, or 4;
A~4 and AAs are each independently
O
40~ NH CH
(CH2)n ~ ;
~: 45 R :~
~: ~
: :
~, :
.
, ~ ..
WO93/21219 2l.3 ~ n g~ PCT/US93/036~8 ``':
-35-
wherein R6 is hydrogen, ;.
alkyl,
cycloalkyl, or .
aryl, and
S n is an integer of l, 2, 3, or 4; ,
AA6
*
- NH - fH - C02H
(fH2) n `- .
R7 -: .
wherein R7 is aryl or heteroaryl, and ~',.
n i8 zero or an integer of l, 2, 3,
: 15 or 4, or ...
O ~,
- NH - CH - C - - N- Rl ~ -
¦ wherein R7, Rl, and n are as
20 ~ ~2:'n~ defined above,~
R~
stereochemistry;at CH in:AA1, ~ , A~3, AA4, or AA5 is
2s~ ",D'~ t;
st:e:reochemistry:at CH in A~6~is L; or a
pharmaceutically:accepeable sale thereof.
pà~ticularly~ valuable~ are~
-30~ L-~B'hg-~eu-Asp:;Ile-Il,e-Trp; ~,
:D^~Bhg-Leu-Asp-I}e'-~Ile-Trp; ~
Ac-'L-Bhg~-~eu-Asp-Ile-Ile-Trp;
Ac-D~-Bhg-~eu-Asp-Ilè-Ile-Trp;
Ac-D-Bhg-Orn-Asp-Ile-Ile-Trp;
~ : 35 ~ ~c-D-~hg-~ys-Asp-Ile-Ile-Trp; i "
"~ : : Ac-D-Bhg-Asp-Asp-Ile-Ile-Trp;
; Ac-D-Bhg-Glu-Asp-Ile-Ile-Trp; ,:
' 'Ac-D-Bhg-Phe-Asp-Ile-Ile-Trp; - ~.''
Ac-D-Bhg-Arg-Asp-Ile-Ile-Trp;
~ ;4Q -~ Ac-D-Bhg-Asp-Ile-Ile-Trp; -~-
"~ Fmoc-D-Bhg-Leu-Asp-Ile-Ile~Trp;
~ : Fmoc-D-Bhg-Orn-Asp-Ile-Ile-Trp;
., ,~ . ~ . ;, .
WO93/21219 2 ~ ~ ~ O 9 ~ PCT~US93/~36~8
-36-
Fmoc-D-Bhg-Lys-Asp-Ile-Ile-Trp;
Fmoc-D-Bhg-Asp-Asp-Ile-Ile-Trp;
Fmoc-D-~hg-Glu-Asp-Ile-Ile-Trp; ~;
Fmoc-D-Bhg-Phe-Asp-Ile-Ile-Trp;
Fmoc-D-Bhg-Arg-Asp-Ile-Ile-Trp;
Fmoc-D-Bhg-Asp-Ile-Ile-Trp;
Ac-D-Bhg-Leu-Phe-Ile-Ile-Trp; ~'-
Ac-D-Bhg-Leu-Asn-Ile-Ile-Trp; -.
. Ac-D-Bhg-Leu-Glu-Ile-Ile-Trp;
Ac-D-Bhg-Leu-Gln-Ile-Ile-Trp;
Ac-D-Bhg-Leu-Tyr-Ile-Ile-Trp; ~.
Ac-D-Bhg-Leu-l-Nal-Ile-Ile-Trp;
Ac-D-Bhg-Leu-2-Nal-Ile-Ile-Trp;
Ac-D-Bhg-Leu-Trp-Ile-IIe-Trp;
Ac-D-Bhg-Leu-Asp-Val-Ile-Trp;
Ac-D-Bhg-Leu-Asp-Ile-Val-Trp; ~-~
Ac-D-Bhg-Leu-Asp-Chx-Ile-Trp;
Ac-D-Bhg-Leu-Asp-Ile-Chx-Trp;
Ac-D-Bhg-:Arg-Asp-Ile-Chx-Trp; . ~;
.
Ac-D-~hg-Lys-Asp-IIe-Chx-Trp;
Ac-D-Bhg-Orn-Asp-Ile-Chx-Trp;
Ac-D-Bhg-Asp-Asp-Ile-Chx-Trp;
Ac-D-Bhg-Glu-Asp-Ile-Chx-Trp; ~`
. Fmoc-D-Bhg-~eu-:Phe-Ile-Ile-Trp;
25 . Fmoc-D-Bhg-Leu-Asn-Ile-Ile-Trp;
Fmoc-D-Bhg-Leu-Glu-Ile-Ile-Trp;
Fmoc:-D~-Bhg-Leu-Gln-Ile-Ile-Trp;
- : Fmoc-D-Bhg-Leu- Tyr -Ile-Ile-Trp;
Fmoc-D-Bhg-Leu-Asp-Val-Ile-Trp;
30 . ,Fm",oc~-~-Bhg,~eu-~sp~,Ile-Val-Trp;
Fmoc-~D-Bhg-Leu-Asp-Chx-Ile-Trp;
Fmoc-~D-Bhg-Arg-ABp-chx-Ile-Trp;
' Fmoc-D-Bhg-Ly~-Asp-Chx-Ile-Trp;
Fmoc-D-Bhg-Orn-Asp-Chx-Ile-Trp; ,
35 .~ Fmoc-D-Bhg-Asp-Asp-Chx-Ile-Trp;
Fmoc-D-Bhg-Glu-Asp-Chx-Ile-Trp;
~: Fmoc-D-Bhg-Leu-Asp-Ile-Chx-Trp;
~:, : ':
WO93/21219 2 1 3 3 0 9 0 PCT/US93/036~8 ~
. ;
-37-
Fmoc-D-~hg-Arg-Asp-Ile-Chx-Trp;
Fmoc-D-Bhg-Lys-Asp-Ile-Chx-Trp; :~
Fmoc-D-Bhg-Orn-Asp-Ile-Chx-Trp;
Fmoc-D-Bhg-Asp-Asp-Ile-Chx-Trp;
Fmoc-D-~hg-Glu-Asp-Ile-Chx-Trp;
Ac-D-Bheg-Leu-Asp-Ile-Ile-Trp;
Ac-D-Bheg-Orn-Asp-Ile-Ile-Trp;
Ac-D-Bheg-Lys-Asp-Ile-Ile-Trp;
Ac-D-Bheg-Asp-Asp-Ile-Ile-Trp;
Ac-D-Bheg-Glu-Asp-Ile-Ile-Trp;
Ac-D-Bheg-Phe-Asp-Ile-Ile-Trp; :
Ac-D-Bheg-Arg-Asp-Ile-Ile-Trp;
Ac-D-Bheg-Asp-Ile-Ile-Trp; ;~
Fmoc-D-Bheg-Leu-Asp-Ile-Ile-Trp; :~
.
Fmoc-D-Bheg-Orn-Asp-Ile-Ile-Trp; `~-
; Fmoc-D-Bheg-Lys-Asp-Ile-Ile-Trp; ` ::~
: Fmoc-D-Bheg-Asp-Asp-Ile-Ile~-Trp;
: Fmoc-D-Bheg-Glu-Asp-Ile-Ile-Trp; ;~
Fmoc-D-Bheg-Phe-Asp-Ile-Ile-Trp;
20 ~ Fmoc-D-~heg-Arg-Asp-Ile-Ile-Trp;
: Fmoc-~-Bheg-Asp-Ile-Ile-Trp; -;
Ac-D-Bheg-~eu-Phe~-Ile-Ile-Trp;
Ac-D-Bheg-Leu-Asn-Ile-Ile-Trp;
Ac-D-Bheg-Beu-Glu-Ile-Ile-Trp;
25`~ Ac-D-~Bheg-~eu-Gln-I}e-Ile-Trp;
Ac~-D~-~Bheg-Leu-Tyr-Ile-Ile-Trp
- Ac-D-Bheg-Leu-l-Nal-Ile-Ile-Trp;
Ac-D-Bheg-~eu-2-Nal-Ile-Ile-Trp;
Ac-D-Bheg-Leu-Trp-Ile-Ile-Trp;
30 . ~ Ac-D-Bheg-Leu-As~-Va1-Ile-Trp;
~ : Ac-D-Bheg-Leu-Asp-Ile-Val-Trp;
s~ Ac-~-Bheg-~eu-A~p-Chx-Ile-Trp;
. Ac-D-Bhe~-~eu-Asp-Ile-Chx-Trp;
Ac-D-Bheg-Arg-A p-Ile-Chx-Trp;
35~ Ac-D-Bheg-Bys-Asp-Ile-Chx-Trp; ..
Ac-D-Bheg-Orn-Asp-Ile-Chx-Trp;
Ac-D-Bheg-Asp-Asp~Ile-Chx-Trp;
- - . ;, .
.
. ~
'.,:,`
WO93~21219 2 1 ~ 3 0 Q~ PCT/US93/03658
-38-
Ac-D-Bheg-Glu- Asp - I le-Chx-Trp;
Fmoc-D-Bheg-Leu-Phe-Ile-Ile-Trp;
Fmoc-D-Bheg-Leu-Asn-Ile-Ile-Trp;
Fmoc - D -Bheg-Leu-Glu-Ile-Ile-Trp;
Fmoc-D-Bheg-Leu-Gln-Ile-Ile-Trp;
Fmoc-D-Bheg-Leu-Tyr-Ile-Ile-Trp;
Fmoc-D-Bheg-Leu-Asp-Val-Ile-Trp;
Fmoc-D-Bheg-Leu-Asp-Ile-Val-Trp;
Fmoc-D-Bheg-Leu-Asp-Chx-Ile-Trp;
Fmoc-D-Bheg-Arg-Asp-Chx-Ile-Trp;
E~noc-D-Bheg-~ys-Asp-Chx-Ile-Trp;
hmoc-D-Bheg-Orn-Asp-Chx-Ile-Trp;
- Fmoc-D-Bheg-Asp-Asp-Chx-Ile-Trp;
Fmoc-D-Bheg-Glu-Asp-Chx-Ile-Trp;
Fmoc-D-Bheg-Leu-;Asp-Ile-Chx-Trp;
Fmoc-D-Bheg-Arg-Asp-Ile-Chx-Trp;
Fmoc-D-Bheg-Lys-Asp-I1e-Chx-Trp;
Fmoc-D-Bheg-Orn-Asp-Ile-Chx-Trp;
Fmoc-D-Bheg-Asp-Asp-Ile-Chx-Trp;
20~ Fmo~-D-Bheg-Glu-Asp-Ile-Chx-Trp;
Ac-D-Txg-Beu-Asp-Ile-Ile-Trp;
Ac-D-Txg-Orn-Asp-Ile-Ile-Trp;
` Ac-D-Txg-~ys-Asp-Ile-Ile-Trp;
~:~ Ac-~-Txg-Asp-Asp-Ile-Ile-Trp;
Ac-D-Txg-GIu-Asp:-Ile-Ile-Trp;
Ac-D-Txg-Phe-Asp-Ile-Ile-Trp;
Ac-D-~xg-~Arg-Asp-Ile-Ile-Trp;
,: ,
Ac-D--Txg-Asp-Ile-Ile-Trp; -~
Fmoc-D-Txg-Leu-Asp-Ile-Ile-Trp;
. ~ . Fmoc-D-Tx~-O~nT~sp,Ile-Ile-~Trp;
Fmoc-D-Txg- ~y9 -Asp-Ile-Ile-Trp;
Fmoc-D-Txg-Asp-Asp-Ile-Ile-Trp; ;:-
Fmoc-D-Txg-Glu-Asp-Ile-Ile-Trp;
- ~ Fmoc-D-Txg-Phe-Asp-Ile-Ile-Trp;
35~ Fmoc-D-Txg-Arg-Asp-Ile-Ile-Trp;
: Fmoc-D-Txg-Asp-Ile-Ile-Trp;
Ac-D-Txg-~eu-Phe-Ile-Ile-Trp;
~,
~,
W O 93/~1219 .P ~ /US93/03658
2,1 339 `
-39- ~:
Ac-D-Txg-Leu-Asn-Ile-Ile-Trp;
Ac-D-Txg-Leu-Glu-Ile-Ile-Trp;
Ac-D-Txg-Leu-Gln-Ile-Ile-Trp;
Ac-D-Txg-Leu-Tyr-Ile-Ile-Trp; ~ :
Ac-D-Txg-Leu-1-.Nal-Ile-Ile-Trp;
Ac-D-Txg-Leu-2-Nal-Ile-Ile-Trp; :
Ac D-Txg-Leu-Trp-Ile-Ile-Trp;
Ac-D-Txg-Leu-Asp-Val-Ile-Trp;
Ac-D-Txg-Leu-Asp-Ile-Val-Trp;
~c-D-Txg-Leu-Asp-Chx-Ile-Trp; ~;~
Ac-D-Txg-Leu-Asp-Ile-Chx-Trp;
Ac-D-Txg-Arg-Asp-Ile-Chx-Trp;
Ac-D-Txg-Lys~Asp-Ile-Chx-Trp; :~
Ac-D-Txg-Orn-Asp-Ile-Chx-Trp;
1~ Ac-D-Txg-Asp-Asp-Ile-Chx-Trp;
Ac-D-Txg-Glu-Asp-Ile-Chx-Trp;
Fmoc-D-Txg-Leu-Phe-Ile-Ile-Trp;
Fmoc-D-Txg-Leu-Asn~-Ile-Ile-Trp;
Fmoc-D-Txg-Leu-Glu-Ile-Ile-Trp;
Fmoc-D-Txg-Leu-Gln-Ile-Ile-Trp;
Fmoc-D-Txg-Leu-Tyr-Ile-Ile-Trp; ~:
Fmoc-D-Txg-Leu-Asp-Val-Ile-Trp;
; : Fmoc-D-Txg-Leu-Asp-Ile-Val-Trp;
- .
Fmoc-D-Txg-Leu-Asp-Chx-Ile-Trp;
Fmoc-D-Txg-Arg-~sp-Chx-Ile-Trp;
Fmoc-D-Txg-Lys-Asp-Chx-Ile-Trp;
Fmoc-D-Txg-Orn-Asp-Chx-Ile-Trp;
Fmoc-D-Txg-Asp-A p-Chx-Ile-Trp;
Fmoc-D-Txg-Glu-Asp-Chx-Ile-Trp;
Fmoc-D-Txg-Leu-Asp-Ile-Chx-Trp;
: Fmoc-D-Txg-Arg-Asp-Ile-Chx-Trp;
: Fmoc-D-Txg-~ys-Asp-Ile-Chx-Trp;
Fmoc-D-Txg-Orn-Asp-Ile-Chx-Trp;
:~ Fmoc-D-Txg-Asp-Asp-Ile-Chx-Trp;
Fmoc-D-Txg-Glu-Asp-Ile-Chx-Trp;
Et-D-Bhg-Leu-Asp-Ile-Ile-Trp;
Bz-D-Bhg-Leu-Asp-Ile-Ile-Trp;
W093/21219 2 1 3 3 0 9 0 PCT/US93/036~8 ,~
-40-
Pya-D-Bhg-Leu-Asp-Ile-Ile-Trp;
Cxl-D-Bhg-Leu-Asp-Ile-Ile-Trp;
Ada-D-Bhg-~eu-Asp-Ile-Ile-Trp;
Cxl(U)-D-Bhg-Leu-Asp-Ile-Ile-Trp;
Me(U)-D-Bhg-Leu-A~p-Ile-Ile-Trp;
tBu-D-Bhg-Leu-A~p-Ile-Ile~Trp;
CF3CO-D-Bhg-Leu-Asp-Ile-Ile-Trp; ~
Et-D-Bheg-Leu-Asp-Ile-Ile-Trp; ~,
Bz-D-Bheg-Leu-Asp-Ile-Ile-Trp;
Pya-D-Bheg-Leu-Asp-Ile-Ile-Trp; -~
Cxl-D-Bheg-Leu-Asp-Ile-Ile-Trp; -~
Ada-D-Bheg-Leu-Asp-Ile-Ile-Trp;
Cxl(U)-D-Bheg-Leu-Asp-Ile-Ile-Trp; -~
Me(U)-D-Bheg-Leu-Asp-Ile-Ile-Trp;
tBu-D-~heg-~eu-Asp-Ile-Ile-Trp;
- ~ CF3CO-D-Bheg-Leu-Asp-Ile-Ile-Trp;
Ac-D-Bhg-~eu-Asp;-Phe-Ile-Trp;
Ac-D-Bhg-Orn-Asp-Phe-Ile-Trp;
Ac-D~-Bhg-~ys-Asp-Phe-Ile-Trp; ;~-~
~ AG~D~-B ~-Asp-Asp-Phe-Ile-Trp;
Ac~-D-~hg-G}u- ~ -Phe-Ile-Trp;
Ac~-D~-Bhg-~Phe-Asp-Phe~-Ile-Trp;
Ac-~D-~hg-Arg-Asp-Phe-Ile-Trp;
Ac-D-~heg-heu-Asp-Phe-Ile-T~p;
~-; 25 ~ ;Ac-~D-Bheg-Orn-Asp-~Phe-I1e-T~; ;` Ac-~D-Bheg-Lys-A~p-Phe-Ile-Trp;
Ac-D-Bheg-Asp-Asp-Phe-Ile-Trp;
Ac-~D-~heg-Glu-Asp~-Phe-Ile-Trp;
Ac-D-Bheg-Phe-Asp-Phe-Ile-Trp; and
30 ~ Aci-D-~Bheg-~Argl-A~p,-PIhe-Ile-Trp;
or a pharmaceutically acceptable acid or base addition ~-
salt~thereof. ~ ~
The compounds~of Formula I are ~aluable
antagonists of endothelin. The tests employed ~"
35 ~ indi~cate that compounds of Fonmula I po~-Ress
endothel1n antagonist acti~ity. Thus, the compounds
of Pormula I were tested for their ability to inhibit
::
W O 93/212~9 ~ ~ 3 3 o ~ ~ PC~r/US93/03658
-41-
[125I] -ET-1([125I]-Endothelin-~) binding in a receptor
assay according to the following procedures~
E~nDOl~EhIN ~UECEPTOR BI~nDING ~k5SAY-A (ERBA-A)~ .:
I~rrACT CELL BI~nDING OF [125I]-ET-l `~`~
Materials and Terms Used:
Cell
The cells used were rabbit renal artery vascular
smooth muscle cells grown in a 48-well dish (1 cm2)
(confluent cells).
Growth Medla
The growth media was Dulbecco' 9 Modified
Eagles/Ham's F12 which contained 10~ fetal bovine
serum and antibiotics (penicillin/streptomycin/
fungizone).
As~ay Buffer -~
The~assay buffer was a medium 199 containing '
Hanks salts and 25 mM Hepes buffer (Gibco 380-23SOAJ~
supplemented~ with penicillin/streptomycin/fungizone
0.~5~ and~bovine ~erum albumin (1 mg/mL~
t125~ ET 1
Amersham radioiodinated endothelin~ 25I]-ET-1
was`used~at final concentration of 20,000 cpm/0.25 mL
(25 pM).: -~
~:: 30 . Protocol `~
First, add 0.5 mL warm assay buffer '(described ! '~
above) to the aspirated growth media and preincubate
for 2 to.3 hours in a 37C water bath (do not put back ;~
in the 5% carbon dioxide). Second, remove the assay i:
-~ ~:35:~ buffers, place the dish on ice, and add 150 ~L of cold :.
assay buffer described above to each well. Third, add
50~mL each of cold l125I]-ET-1 and competing ligand to -~
.,
'.
`,
,
W093/21219 2 I ~ 3 o ,q o PCT/US93/03658
j,._
-42-
the solution ~at the same time if possible). Next,
place dish in a 37~C water bath for about 2 hours and
gently agitate the dish every 15 minutes. Discard the
radioactive incubation mixture in the sink and wash
wells 3 times with 1 mL of cold phosphate buffered
saline. Last, add 250 mL of 0.25 molar sodium
hydroxide, agitate for l hour on a rotator, and then
transfer the sodium hydroxide extract to gamma
counting tubes and count the radioactivity.
ENDOT~ELIN RECEPTOR BINDING ASSAY-B (ERBA-B)
[125I]-ET-l B~NDING IN RAT CEREBELLAR M}~BRANES
Materials and Terms Used:
Ti~Que Buffer
The tissue is made up o~ 20 mM tris(hydroxy- -~
methyl)aminome~thane hydrochloride ~Trizma) buffer,
2 mM ethylenediaminetetra acetate, l00 ~M --
phenylmethylsulf~onyl fluoride.
Tlssu-~Preparat~o~
First, thaw one aliquot of frozen rat cerebellar
membranes (2 mg protein in 0.5 mL). Next, add 0.5 mL
membrane aliquot to 4.5 mL cold tis~ue buffer,
; 2S polytron at 7,500 revolutions per mi~ute for `
l0 seconds. Fina1ly, dilute tissue suspension l/100
(0.l m~ suspension + 9.9 mL tissue buffer), polytron
again,~and pla~e ice. ~i
D~lutlq~ BUff~r
Medium 199 with Hank's salts plus 25 mM Hepes +
1 mg/mL bovine serum albumin.
-~ - - [l25I]-~T_l
-~ 35 - Amersham [l25I]-ET-l (aliquots of 2 x l06 cpm per
100 m~ aliquot of ~l25I]-ET-l with 5.2 mL dilution
W O 93/2121~ 21 3 3 n ~ ~ i PC~r/US93~03658
-43-
buffer, place on ice until use (final concentration
will be 20,000 cpm per tube, or 25 pM). `
Protocol ~ '~
Add 50 ~L each of cold ~125]-ET-1 and competing ''
ligand to tubes on ice. Mix in 150 ~L of tissue to ;
each tube, vortex briefly, then tap to force a}l
liquids to bottom (total assay volume - 250 ~L). Then'
place the tubes in a 37C water bath for 2 hours. ~
Add 2.5 mL cold wash buffer (50 mM Trizma buffer) ~'
to each tube, filter, and then wash tube with
additional 2.5 mL wash buffer and add to filter.
,i .
Finally, wash filters~ wlth an additional 2.5 mL of '~
cold wash buffer. ~'^
Count filters for radioactivity in gamma counter.
~.
IN VIIqUD~ nII8ITION Q~ ET-l STIMInL~TD lURAC~aIDONIC
ACID ~;iU~LE/USE~ IN ClnLTonUED RL~8BIT V~USC5ILiUR SMOOql~
20 ~ JnJSC~E~CEI~LS 8Y CCnDPqnn~DS OF FORDfCnL~ I
Antagonist activity is measured by the ability of
added~;co~ ounds to reduce endothelin-stimulated
ara ~ i~donic acid release~in cultured vascular smooth''`'
-25~ muscie~cel1s as arachidonic acid re}ease (AAR).
3H~ Arachidonic~Acid~Loading Media (LM) is
DME~F12~+~0.5~FCS x ~0.25~mCi/mL ~3H] arachidonic acid
( ~ rsham)~ Confluent monolayers of cultured rabbit
, , - ~ ;
renal artery vascular smooth muscle cells were
'~ 30~ ~ incuba~ed in ~Ot,5 ~ of the ~M ov~r 18 hou!rs, at 37C,
in 5% C02. The ~M was aspirated and the cells were
washed once with the assay buffer (Hank's BSS + lO mM
E~S + fa~tty acid-free BSA (1 mg/m~)), and incubated
for 5~mlnutes with 1 m~ of the prewarmed assay buffer.
35~ This~soiution was aspirated, followed by an additional
1 m~ of prewarmed a9say buffer, and further incubated '~
for another 5 minutes. A final 5-minute incubation
, - :
WO93/21219 2 1 3 3 0 9 ~ PCT/US93/03658_
-44-
was carried out in a similar manner. The same
procedure was repeated with the inclusion of lO ~L of
the te~t compound (l nM to l ~M) and lO ~L ET-l
(0.3 nM) and the incubation was extended for
30 minutes. This solution was then collected, lO ~L
of scintillation cocktail was added, and the amount of
[3H] arachidonic acid was determined in a li~uid
scintillation counter.
I~ VITRO ANTAGONISM OF ET-l STIMnLATED .-
VASOCONSTRICTION IN 'l~: RABBTT FEMORAL ARTERY (ETA) ~-
AND SARAFOTOXIN 6c `~
STIMnLATED VASO~ONSTRICTION IN T~E RABBIT P~LMONARY
ARTERY (ETB) -
~ -
Male New Zealand rabbits were killed by cervical
dislocation and exsanguination. Femoral and pulmonary
arteries~were~ isolated, cleaned of connecti~e tissue,
and cut into 4-mm rings. The endothelium was denuded
by placing the rings over hypodermic tubing (32 guage ~~
for femoral rings and 28 guage for pulmonary rings,
Small Parts, Inc, Miami, Florida) and gently rolling .
them.~ Denuded rings were mounted in 20 mL organ baths ~-
containing Krebs-bicarbonate buffer tcomposition in ~"
25~ mM: NaCl, 1}8.2; NaHC03, 24.8; KCl, 4.6; MgS04
7-H2O, 1.2; KH2P04, 1.2; Ca~l2-2H20; Ca-Na2 EDTA, ~-
0.026; dextrase, lO.0), that was maintained at 37C
and gassed continuously with 5% C2 in oxygen
IpH 7.4). Resting ten~ion was adju~ted to 3.0 g for
30 - femoralland 4.0 g pulm~nary arteries; the rings were -~
left for 90 minutes to equilibrate. Vascular rings
~ were tested for lack of functional endothelium (i.e., ,~
- lack of an endothelium-dependent relaxation response
,
to carbachol (l.0 ~M) in norepinephrine (0.03 ~M)
contracted rings. Agonist peptide~, ET-l (femoral),
and S6c (pulmonary), were cumulati~ely added at
lO-minute intervals. The ET antagoniQts were added
`
W093/21~19 2l.3 3 n ~ 0 PCT/US93/~3658
-45-
30 minutes prior to adding the agonist ancl pA 2 values
were calculated (Table I).
The data in Table I below show the endothelin
receptor binding and antagonist acti~ity of
representative compounds of Formula I.
: ':
'' ,;
, .:
'' `
,
~ ~ .
W O 93/212t9 ~ P ~ /US93/036~8
. ,,-`~ O '.
- 4 6 -
H E~ ~ 0
(~ . :.
X O O ~D ~0 O ~ O O .,
O ~_ O ~ O O O O '~
~U '¢ ~0 O O N O O O O O .
O H
'~'
_ ~1 ,~ n N Ul ~
o ~¢ ~ o ,~ o r ~1 o ~n o o - ~:
gJ ~ ~ o ~ ~ o o o ~ o o . .~
O H
~1 ,s,~
O _ ~ CD 11~ N If- ~
~ ~,~ ~ O U~ m o ~ o o ~
W~ O O ~ O O O O O O r ' :~
V~ ~ ~ ~ ~ ~ '~
~ : ' ~
~'
~ ' ,U ~ ''.. ',
- O ~
O I ~ t~ ' :-
~ U ID ..
m ~ ,, ,,
~H - ~ 1~ H H ~ l . .
~:! ~
~ ~ U ~ ~ t) ID .-.
O ~1 ~ ~ ~ :''
H O. ~ , , ~~ . ~ H ~ .`
- ~ i ~ : .
o o. ,~ ~ a. o. mD. a. D. ~:
H H ~ m ,~ ~:
~ 3 j j
E~ ~ o ~ ~
tn
! S~ C
tn cn I
c~ ~c s ~ a a a a n
. . ~
'~.
- ~D ~ Dr~a~ ~-
~ , ~
U~
W O 93/21219 2 1 3 3 ~ (3 ~ PC~r/US93/03658
.. . i`
-47-
General Method for Preparinq Compounds of Formula I
The compounds of Formula I may be prepared by
solid phase peptide synthesis on a peptide
synthesizer, for example, an Applied Biosystems 430A ~-
peptide synthesizer u~ing activated esters or
anhydrides of N-alpha-Boc protected amino acids, on `
PAM or MBHA resins. Additionally, the compounds of
Formula I may also be prepared by conventional
solution peptide ~ynthesis. Amino acid side chains
are protected as follows: Bzl(A~p, Glu, Ser),
2-Cl-Z~Lys), 2-Br-Z(Tyr), Bom(His), For(Trp), and
MeBzl(Cys). Each peptide resin (1.0 g) is cleaved -
with 9 mL of HF and 1 mL of ani~ole or p-cre~ol as a
scavenger (60 minutes, 0C). The peptide resin is
washed with cyclohexane, extracted with 30~ aqueous -`
HOAc, followed by glacial HOAc, concentrated under
redùced pressure, and lyophilized. (A peptide
conta~lning For(Trp) is dissolved in 0C, the pH is
;adjusted~to~12~.5 with lN ROH (2 minutes), neutralized
20 ~ with~glacial HOAc, desalted on Cl8 (as described
below)~, and lyophilized. The crude peptide is
puri~fied by preparative reversed phase high
performance liquid chromatography IRP-HPLC) on a C18
column ~(~.2 x 25.0 cm, 15.0 mL/min) with a linear-
25~ gradient of O.l~ TFA in water to O.l~ TFA in
; acetonitrile and~lyophilized. The homogeneity and
h composition of the resulting peptide i8 verified by
RP-HP~C, capillary~electrophoresis, thin layer
chromatography (T~C), proton nuclear magnetic
resonance spect,rom,etry ,(NMR~, an~ fast atom
bombardment mass spectrometry (FA~-MS).
~ ; The compounds of the present invention can be
- ~ ~ prepared and administered in a wide variety of oral
and parenteral dosage forms. Thus, the compounds of
the~pre~ent invention can be administered by
injection, that is, intravenously, intramuscularly,
~ ~ intracutaneously, subcutaneously, intraduodenally, or
:,
W093/21219 2 1 3 3 1~ 9 0 PCT/US93/03658 ~
-48- ;~
intraperitoneally. Also, the compounds of the present
invention can be administered by inhalation, for
example, intranasally. Additiona1ly, the compounds of
the present invention can be administered
transdermally. It will be obvious to tho~e skilled in
the art that the following dosage forms may comprise
as the active component, either a compound of
Formula I or a corresponding pharmaceutically ~
acceptable salt of a compound of Formula I. ;;
For preparing pharmaceutical compositions from
the compounds of the present invention,
pharmaceutically acceptable carriers can be either ~`
solid or liquid. Solid form preparations include
powders, tablets, pills, capsules, cachets,
suppositories, and dispersible granules. A solid -~
carrier can be one or more substances which may also -
act as diluents, fla~oring agents, binders,
preservatives, tablet-disintegrating agents, or an
encapsulating material. ` ~--
In powders, the carrier is a finely divided solid
which is in a mixture with the finely divided acti~e
componene. `~
- In tablets, the active component is mixed with ~;
the carrier ha~ing the necessary binding properties in ~`~
suitable proportions and compacted in the shape and
- size desired.
The powders and tablets preferably contain f rom
fi~e or ten to about seventy percent of the acti~e
compound. Suitable carriers are magnesium carbonate,
magnesium s!teaF~te? ta,lc, ,sugar, lactose, pectin,
dextrin, starch, gelatin, tragacanth, methylcellulose,
~ sodium carboxymethylcellulose, a low melting wax,
`~- cocoa butter, and the like. The tenm "preparation" is
intended to include the f ormulation of the active
compound with encapsulating material as a carrier -~
~ providing a capsule in which the active component with
; or without other carriers, is surrounded by a carrier,
`:`
.;
W093/2l2l9 2 ~ 3 3 0 ~ ~ PCT/US93/03658 l~
-49-
which is thu~ in association with it. Similarly,
cachets and lozenges are included. Tablets, powders,
capsules, pills, cachets, and lozenges can be used as
solid dosage forms suitable for oral administratio~.
For preparing suppositories, a low melting wax,
such as a mixture of fatty acid glycerides or cocoa
butter, is first melted and the active component is
dispersed homogeneously therein, as by stirring. The
molten homogenous mixture is then poured into
convenient sized molds, allowed to cool, and thereby
to solidify.
Liquid form preparations include solutions,
suspensions, and emulsions, for example, water or
water propylene glycol solutions. For parenteral
injection liquid preparations can be formulated in
solution in aqueous polyethylene glycol solution.
Aqueous solutions suitable for oral use can be
prepared by dissol~ing the active component in water ~;
~and~adding suitable colorants, flavors, stabilizing
and thickening agents as desired.
Aqueous suspensions suitable for oral use can be
made by dispersing the finely divided active component
~- in water with viscous material, such as natural or
synthetic gums, resins, methylcellulose, sodium
carboxymethylcellulose, and other well-known
suspending agentq.
Also included are ~olid form preparations which
-~ are intended to be converted, shortly before use, to ~~
liquid form preparations for oral administration.
~i Such li~uid for~s incl~de~solutions, suspensions, and
emulsions. These preparations may contain, in -~
addition to the activa component, colorants, flavors,
stabilizers, buffers, artificial and natural
sweeteners, dispersants, thickeners, solubilizing
agents, and the like.
The pharmaceutical preparation is preferably in
unit dosage form. In such form the preparation is
W O 93/21219 2 l ~ 3 0 Q O PC~r/US93tO3658 `~
-50~
subdivided into unit do~es containing appropriate '`'-
quantities of the active component. The unit dosage
form can be a packaged preparation, the package `~
containing discrete quantities of preparation, such as `-
packeted tablets, capsules, and powders in vials or ''~'
ampoules. Also, the unit dosage form can be a
capsules, tablet, cachet, or lozenge itself, or it can `
be the appropriate number of any of these in packaged
form.
The quantity of active component in a unit dose
preparation may be varied or adjusted from 0.1 mg to ~'
100 mg preferably O.S mg to 100 mg according to the ;~
particular application and the potency of the active 'i~
component. The composition can, if desired, also `
lS contain other compatible therapeutic agents.
In therapeutic use as antagonist of endothelin,
the compounds utilized in the pharmaceutical method of ''~
this invention are a'dministered at the initial dosage
'of~ bout O.D1 mg~to~about 20 mg per kilogram daily. A '~
20 ~ da~ily~dose~range of about O.Ql mg to about 10 mg per '-
kilogram~is~preferred. The dosages, however, may be
varied depending upon the~ requirements of the patient, '~
the severity of the condition being treated, and the '~'compour,d being employed.~ Determination of the proper ~,
25~ dosage for~a particular situation is within the skill
of the art. Generally, treatment is initiated with
smaller dosages~which are less than the optimum dose
of the co~mpound. m ereafter, the dosage is increa~ed
by small increments until the optimum effect under the
~ circumstànces is reached. For convenience, the total '~
daily do~age may be divided and administered in
portions during the day, if desired. ''"
'The following nonlimiting examples illustrate the
inventors' preferred methods for preparing the
~ 35~ compounds of the invention. -~
,: ~ `.. '.'
; ~'.
`, ;;
WO93/21219 2 1 3 3 ~ 9 0 PCT/US93/03658
-51- `~
ExAMoeBE 1
Ac-D-Bhg-Leu-Asp-Ile-Ile-Trp
The linear hexapeptide is prepared by standard
solid phase synthetic peptide methodology utilizing a :
Boc/benzyl strategy (Stewart, J. M. and Young, J. D., -
Solid Phase Peptide Synthesis, Pierce Chemical Co., ~:
Rock~ord, IL, l984). All protected amino acids and ~.:
reagents are obtained from commercial ~ources with the
exception of N-~-Boc-DL-Bhg and are not further
purified. The protected peptide resin is prepared on :
an Applied Biosystems 430A Peptide Synthesizer, ~.
utilizing protocols supplied for a dicyclohexyl- ;
carbod~imide-mediated coupling scheme (Standard l.0,
Version 1.40). Starting with 0.710 g of
N~ oc-Trp-PAM resin (0.70 meq/g, 0.497 meq of
Boc-Trp(For) total) the protected peptide is prepared~
by the stepwise coupling of the following amino acids
(in order of addition~- N-~-Boc-Ile 0.5H20,
: -N-~-Boc-Ile 0.5H2O, N-~-Boc-Asp~BzI), N-a-Boc-Leu H2O,
: 20 and N-a-Boc-DL-Bhg. A typical cycle for the coupling :~
of an individual amino acid residue is illustrated
below (reproduced from the ABI manual):
All the single couple RV cycles conform to the
: following pattern:
25 : l) 33% TFA in DCM for 80 seconds
- 2) 50~ TFA in DCM for 18.5 minutes
3) Three DCM washes
~- 4) lO~ DIEA in DMF for 1 minute
5) lO~ DIEA in DMF for 1 minute ~;::-.
6) Five DMF washe~ . :-
7) Coupling period ! i .:
: 8) Fi~e DCM washes
After the coupling of N-~-Boc-DL-Bhg, the Boc
. group is removed with the end-NH2 cycle ~l.0l2 g).
The peptide is liberated from the solid support, ~:
and ~he carboxylate of aspartic acid deprotected by
W093/21219 213~ PCT/US93/03658
-52~
treatment with anhydrous hydrogen fluoride (9.0 mL),
anisole (O.5 mL), and dimethyl sulfide (O.5 mL)
(60 minutes, 0C). After removing the hydrogen
fluoride under a strei~m of nitrogen, the re~in is
washed with diethyl ether (3 x 30 mL) and extracted -~
with 20~ HOAc in water ~3 x 30 mL) and glacial HOAc
(2 x 30 mL). The aqueous extractions are combined, ~-
concentrated under reduced pressure, and lyophilized
(360 mg). The crude peptide is dissol~ed in 4.0 mL of
50~ TFA/H2O, filtered through a 0.4 L ~yringe filter,
and chromatographed on a Vydac 218TP l022 column
(2.2 x 25.0 cm, l~.0 m~/min, A: 0.1% TFA/H2O, ;~`
B: 0.l~ TFA/CH3CN, Gradient; 0% B for l0 minut~s, l0
to 40~ B over 120 minutes). Two individual fractions
are collected and combined based upon analysis by
analytical HPLC. The combined fractions are ~-
concentrated separately under reduced pressure
~l0 mL), diluted with~H2O (50 mL), and lyophilized
(40.0 mg/ea). Separation into the tWQ diastereomers
~20 (Isomers A and B) is effected under these conditions
tR~ Isomer A 15.63 min., Isomer B 16.79 min.). The
late~running peak fractions (Isomer B) are repurified -~
under the same experimental conditions with a gradient
of 30% to 50~ B over 120 minutes at lS mL/min to
afford purified product. ~Acetylation is carried out
with 20 mg of Isomer B in 90% acetic acid foIlowed by
addition of acetic anhydride (5 m~) and stirring
overnight. ~After evaporat~on and drying the product
Ac-D-Bhg-Leu-Asp-Ile-Ile-Trp is 99~ pure by HP~C.
~ ~Vydac 218 TP 101,22Icolymn (2.2 x 25.0 cm, 15.0 mL/min.
A: 0.1% TFA/CH3CN, Gradient 20% to 86% B over ;
22 min.)] tR - 18.66 minutes. The homogeneity and
structure of the resulting peptide is confinmed by
analytical HP~C. Proton Nuclear Magnetic Re~onance
Spectroscopy (Hl-NMR) and Fast Atom Bombardment Mass
Spectroscopy (FAB-MS), M~Na 972.0, M+2Na+ 995.9.
W093~21219 21 3 ~ ~ ~ O PCT/US93/03658 .~
-53- .
In a process analogous to Example l using the
appropriate amino acids, the corresponding compounds :
of Formula I are prepared as follows: ~:
. EXAMPhE 2
D-Bhg-Leu Asp-Ile-Ile-Trp; FAB-MS, M+l 907.4.
EXAMPLE 3 ~
L-Bhq-Leu-As~-Ile-Ile-Trp; FAB-MS, M+l 907.4. -
~ EXAMPLE 4 ~
Ac h-Bhq-Leu-Asp- le-Ile-Trp; FAB-MS, M+l 950Ø ~ .
EXAMPhE 5
Ac-D-Tx~-~eu-Asp-Ile-I1e-Trp; FA3-MS, M+Na 977Ø
EXAMPhE 6
Ac-D-Bheq-Leu-As~-Ile-Ile-Trp, FAB-MS, M+l 970.3. .~
20~ EXAMPhE 7
Ac-D-~hq-Orn-Asp-Ile-Ile-Trp; FAB-MS, M+l 951.2. .;
':,: ..,
EXAMPhE 8
Ac-D-3hq-Glu-Asp-Ile-Ile-T~p; FAB-MS, M+Na 988.8. ..
EXAMPLE 9 :~
Disodium~L~ of:Ac-D-3hg-~eu-Asp-Ile-Ile-Trp ~ ~
:A:~s~aturated~solution of sodium bicarbonate in ~.;.
~ water is prepared, diluted with water (l:lO), chilled ".
:~ 30 - l~to 0lC,, and lQ lm,L jofjt,he,j~o1ution! is added to :`.
approximately 50 mg of Ac-D-Bhg-~eu-Asp-Ile-Ile-Trp ~.
(Example l) wi-th stirring. The pH of the solution is
: greater than 9. After lO minutes, the solution is ;.
pa~sed:through a Cl8 cartridge, washed with water
35: ~ (100 mL), and the absorbed peptide is eluted with
methanol ~50 mL), concentrated under reduced pressure, ~:;
- . . .
~ ~ ,
:;
WO9~/21219 1 3 3 ~ ~ o PCT/US93/036~8 i~$r
.:
-54-
resuspended ln water (50 mL), and lyophilized (three
times) to give the title co~pound.
Ac-D-Bhg-Leu-Asp-Ile-Ile-Trp. disodium sal~; FAB-MS,
M+l 950.4, M+Na 972.l, M+2Na 994.3.
~
EXAMPLE lO ~-
Boc-Bhg
.~ ~
Bhg HCl (1.70 g, 5.43 mmol) is suspended in ;~
150 mL of p-dioxane:H20 (2:1) at room temperature. To
the stirred solution is added l.40 g (6.42 mmol) of
di-tert butyldicarbonate. The pH of the solution i5 :
adjusted to ~9.0 with lN NaOH and maintained at
between pH 9 and lO with aliquot additions of lN NaOH,
until the pH is constant~ The solution is
concentrated under reduced pressure to approximately ~
75 mL, overlain with ethyl acetate (50 imh), and -
acidified to approximately~pH 2.5~ with lO~ aqueous
HCl. The organic layer is~Yeparated, washed
successively with 10~ aqueous HCl (2 x 50 mL~, brine
~2 x 50 mL), H~20 (3 x 50 mL), and driled with MgS04.
The solution i9 ~iltered, concentrated under reduced
pressure, and the oil is recrystallized from ethyl
acetate:heptane (1.82 g). The white~solid is ,;
characterized by proton NMR, fast atom bombardment
mass spectrometry (M+1=368), and elemental analysis. i`-
: ~
: - `
'~
' '