Note: Descriptions are shown in the official language in which they were submitted.
2133284
-- 1 --
TITLB
Water-soluble Octacarboxy Substituted Tetra(2 3-
pyrazino~por~hyrazines, Pre~aration and Use thereof
FIBLD OF THB lNV~ lON
The present invention relates to
photoinactivators and more particularly to a novel
class of water soluble azaphathalocyanines (AzaPc's),
functionalized on the macrocyle with carboxy
substituents to yield octacarboxy substituted
tetra(2,3-pyrazino)porphyrazines (TPPA's). The
invention also relates to processes for their
preparation and the use thereof for the photodynamic
therapy of cancer and the inactivation of viruses or
bacteria in stored blood products.
DESCRIPTION OF THB lNV~N ~ ION
Background of the invention
Aza analogues of phthalocyanine (AzaPc's) are
photoinactivators with known applications, for example,
as photo-bleaching agents for textiles, control agents
to reduce the growth of microorganisms,
electrocatalysts for oxygen reduction, catalysts for
thiols and cumene autooxidation, materials for
electrochromic displays, and media for optical data
storage. Reference is made to GB 2,159,516 A, US
Patents 3,922,967, 4,033,718, 4,166,718 and 4,094,806.
213~284
-- 2
However, there remains a strong need for novel AzaPc's
with improved properties and over the prior art.
The potential efficiency of water soluble and
thermally stable AzaPc's in different oxidative
processes, such as oxidation of alkanes to alcohols and
conversion of alkenes to epoxides, is yet to be
discovered. Furthermore, these compounds are potential
catalysts in oxidation processes to remove organic
environmental pollutants. Accordingly, for many
important potential applications, water solubility
combined with the above capabilities are particularly
sought after properties.
In another field of art, phthalocyanines
(Pc's) and their various derivatives are beginning to
receive attention as photoinactivators in biological
systems. This is due to the ability of certain Pc
derivatives to efficiently inactivate cancer cells in
vivo and inactivate in vitro viruses or bacteria in
stored blood products. In this regard, water soluble
Pc's are receiving particular attention.
Most Pc derivatives, which have been
evaluated for photodynamic therapy of cancer to date,
carry substituents either on the benzene rings of the
macrocycle or on the central atom as axial ligands. In
contrast, the AzaPc's of the present invention, which
feature additional nitrogen atoms in the macrocycle,
have received very little attention as sensitizers for
photodynamic therapy. (TEUCHNER, K. et al.,
21332~
-- 3 --
Spectroscopic Properties of Potential Sensitizers for
the New Photodynamic Therapy Start Mechanisms via Two-
Step Excited Electronic State, (1993), Photochemistry
and Photobiology, Vol. 57, No. 3, 465).
It is also known that certain derivatives of
phthalocyanine (Pc), including AzaPc's, exhibit liquid
crystalline behaviour, in particular the formation of
so-called columnar discotic mesophases. This adds a
new dimension to their range of potential applications,
for example in electronic devices. (CAMMIDGE, Andrew
N. et al., Synthesis and Characterisation of some
1 4 8 11 15 18 22 25-Octa(alkoxymethyl)-
phthalocyanines: a New Series of Discotic Liquid
Crystals, J. CHEM. SOC. PERKIN TRANS. I, (1991) 3053).
Mesophase behaviour has been reported for metal-free
and metallated phthalocyanines substituted with eight
long chained alkyl, alkoxy or alkoxymethyl groups
either at peripheral or at the non-peripheral sites.
It is known that long chain substituted
tetrapyrazinoporphyrazine (TPPA) and its copper (II)
complex, exhibit columnar mesomorphism different from
their carbocyclic analogues. (OHTA, Kazuchika et al.,
Synthesis and Columnar MesomorPhism of
Octa(dodecyl)tetrapyrazinoporphyraxine and its
Copper(II) ComPlex, J. CHEM. SOC., CHEM. COMMUN.,(1989)
1611). The pyrazine derivatives are unique 7r-acceptors
whereas all long-chain substituted Pc derivatives are
~r-donor columnar mesogens.
2133284
- 4 -
Consequently, there is a strong need for the
development and new preparation routes of long-chain
substituted Aza-Pc's which can optionally feature
mesophase behaviour.
BUMMARY OF THB lNv~N~ION
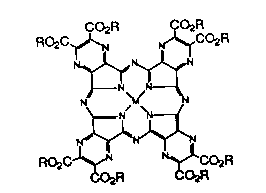
The invention provides a compound of the
formula
CO2R CO2R
RO2C~ CO2R
RO2C~N N~CO2R
CO2R ( I) CO2R
wherein
M is HH, Cu, VO, Co, Ni or Zn;
a d R is C2H5, n-C4H9, n-C6H13~ n~CsHt7 or ONa-
Also disclosed in a process for thepreparation of the novel compounds and uses therefor.
Other features and advantages of the
invention will become apparent to those of ordinary
skill in the art upon review of the following detailed
description, schematic diagrams and claims.
2133284
DBTAI~BD DB8CRIPTION OF T~E lNv~h.ION
The preparation of the
tetrapyrazinoporphyrazines (TPPA) of the present
invention will now be described in relation to a
preferred embodiment and for illustrative purposes
only. The tetrapyrazinoporphyrazines (TPPA) of the
present invention have the following formula 1-6:
CO2R CO2R
RO2C~, "~CO2R
\_N~ ~/
RO2C~ N~CO2R
~02R CO2R
1 M 2 HH 2 M z Cu 3 M = VO 4 M = Co 5 N = Ni
6 M = Zn
for 1-6a, R = C~g
b, R = n-C4H9
c, R = n-C6H13
d, R = n-C8H17
for 2-6e, R = ONa
2133284
A process for the preparation of the above
compounds is also included in the scope of the present
invention.
In order to construct the precursors of
AzaPc's la-d - 6a-d, it was surprisingly found that an
established protocol could be ingeniously adapted for
the preparation of 2,3-dicyano derivatives (LINSTEAD et
al. J. Chem. Soc. 1937, 911; ROTHKOPF, H.W. et al., G.
Chem. Ber. 1975, vol 108, 875; GALPERN, M.G. et al.,
I.G. Khim. Geterotsikl. Soedin. 1993, 58).:
NC$2 O~CO2R ~ ~,CO2R
NC H2 CO2R EIOH N~N~CO2R
5548%
7 8 9
for 8.9a, R = C2H5
b, R = n-C~H9
c, R = n-C6H13
d R = n-C8H~7
Scheme (I)
Condensation of diaminomaleodinitrile 7 with
dialkyldioxosuccinates 8a-d, in the presence of acetic
acid, gave o-dinitriles 9a-d in 55-68% yield.
_ 7 _ 213 328 4
Compounds 8a-d were prepared from disodium
dihydroxytartrate and an appropriate alcohol, using
Fox's method (Fox H.H., J. Org. Chem., 1947, 12, 535).
It is to be noted that compounds 8c-d are novel and are
included within the scope of the present invention as
key intermediates. Compounds 9a-d were purified
chromatographically on silica gel. Accordingly,
compounds 9a-d are precursors to the compounds of the
present invention.
PREPARATION OF H2TTPA la-d
Free base octaalkoxycarbonyl TPPA la-d were
synthesized from 9a-d by refluxing in ethanol
(buthanol) with 1,8-diazabicyclo t5.4.0] undec-7-ene
(DBU). Compounds la-d are soluble in chloroform. The
purification was accomplished by chromatography on
silica gel and recrystallization from tetrahydrofuran.
The yields of H2TPPA's vary from 12 to 27%. The
synthesis route is schematically illustrated as
follows:
DBU
9a-d > H2TppA(la-d)
EtOH(BuOH)12-27%
Scheme (II)
2133284
-- 8 --
PREPARATION OF THE METAL COMPLEXES OF TPPA
The metal complexes of octaalkoxycarbonyl
TPPA 2a-d - 6a-d were obtained by complexation of o-
dinitriles 9a-d with the appropriate metal salts in
quinoline. Reactions of 9a-d with metal salts at 160-
170 proceed unusually fast (ca. 5 min.) and further
heating results in a decrease in the yield of the metal
complexes. Lowering the temperature slows the reaction
down, but does not increase the product yield. Varying
the reaction conditions showed that formation of the
metal complexes requires the presence of basic solvents
to neutralize the liberated acid. Accordingly, the
possible synthesis routes can be schematically
illustrated as follows:
DBU
9a-d ---------> H2TPPA(la-d) Scheme (II)
EtOH(BuOH) 12-27%
I ~x.
Quinol ine
MTPPA 32-60~
2 M = Cu 3 M = VO 4 M = Co 5 M = Ni 6 M = Zn
for 2-6a, R = C2H5
b, R = n-C4H9
c, R = n-C6H13
d, R = n-C8H17
Scheme (III)
2133284
g
The compounds 2a-d - 6a-d were purified by silica gel
chromatography. The yields are good, 32-60~.
The compounds of the formula la-d - 6a-d,
symmetrically substituted with eight long chain
alkoxycarbonyl groups, may exhibit a discotic liquid
crystalline phase and could be used in electronic
devices.
Also included in the scope of the present
invention is a process for hydrolysis of the ester
groups of complexes 2a - 6a. Initial attempts to
hydrolyse the ethoxycarbonyl groups under either mild
or extreme acidic conditions failed. Hydrolysis did
proceed in alkaline aqueous solutions; however,
instability of the solubilized products made it
difficult to recover the octacarboxy derivatives. It
is possible to stop decomposition by quick
acidification to pH 2-3, resulting in precipitation of
the carboxylic products, however, in the procedure both
composition and yield of the product are unpredictable.
It was surprisingly discovered that
appropriate conditions could be obtained when using
very mild alkaline hydrolysis, have been established
for the preparation of these compounds. Since Pc-
carboxylic salts are insoluble in methanol, esters 2a -
6a ere treated with a mixture of 3 parts of NaOH-
saturated methanol an 1 part water resulting in
instantaneous dissolution of the complexes followed by
2133284
-- 10 --
precipitation of the salts 2e - 6e. The hydrolysis
method can be schematically illustrated as follows:
NaOH
2a - 6a --------> 2e - 6e 100%
S MeOH-H20
Scheme IV
The reaction was monitored by HPLC analysis of the
redissolved precipitate. Complexes 2e - 6e were
obtained as single isomeric compounds in quantitative
yields. They have Q-band maxima in the W -vis spectra
around 640 nm (in water). The sharp Q-band maxima are
indicative of substantial monomerization of the dye
molecules in solution. Furthermore, their extinction
coefficients are comparable to those of compounds 2a -
6a in organic solvents. Interestingly, in vivo thesedyes require light of 650-700 nm to promote tumour
regression in a mouse-tumour model, suggesting a
substantial red-shift of the Q-band due to association
with biomolecules. It is the in vivo red-shift that
renders this class of compounds of particular interest
for photodynamic therapy of medical conditions since
the 650-700 nm band provides a window with little
absorbance of endogenous biological molecules resulting
in optimal light penetration in tissues and biological
samples.
These derivatives constitute a novel class of
water soluble AzaPc's, functionalized on the macrocyle
- 2133284
with carboxy substituents. This is an alternative
class of photosensitizers, with a different pKa to
those currently available, for photodynamic therapy of
cancer and inactivation of viruses in stored blood
products.
EXPERIMENTAL:
The following examples illustrate but do not
limit the invention.
Example 1
5.6 Diethoxycarbonyl-2,3-dicyanopyrazine (9a):
A mixture of 7 (1.08g, 0.01 mol) and 8a (1.46
g, 0.01 mol) in glacial AeOH (10 mL), and EtOH (250 mL)
was stirred at 70-80C for 2 h. The solvent was
evaporated and the crude product was flash
chromatographed (silica gel, CHCl3) affording 1.42 g
(52%) of compound 9a.
Example 2
Tetra-2~3-(5~6-dibutoxycarbonylpyrazino)porPhyrazine
(lb):
5,6-Dibutoxycarbonyl-2,3-dicyanopyrazine 9b
(0.66 g, 2 mmol) was dissolved in n-butanol (25 mL).
The catalytic amount of 1.8-diazabicyclo[5.4.0] undec-
7-ene(DBU) was added and the mixture was refluxed for 4
2133284
- 12 -
h under the nitrogen atmosphere. The solvent was
removed under reduced pressure and the residue was
chromatographed (silica gel, CHCl3 - acetone) affording
compound lb (0.18 g, 27%).
Exam~le 3
Metal com~lexes of tetra-2 3-(5 6-dialkoxycarbonyl
pyrazino)porphyrazine (2a-d - 6a-d): General Procedure:
A mixture of o-dinitrile 9a-d, a metal salt
(in molar ratio 1:1, CuCl for 2a-d, VCl3 for 3a-d,
CoCl2 for 4a-d, NiCl2 for 5a-d, and Zn(OAc) 2- 2H20 for
6a-d), and freshly distilled quinoline (l mL per 100 mg
of o-dinitrile) was quickly heated to 170C. The
heating was discontinued after an intense greenish-blue
colour appeared (ca. 5 min.), and the resulting mixture
was allowed to cool. Metal complexes 2a-d - 6a-d were
extracted with CHCl3 or acetone. The solvent was
removed under reduced pressure and the residue was
chromatographed (silica gel, CHCl3 - acetone). The
yields of compounds 2a-d - 6a-d vary from 32% to 60%.
ExamPle 4
Metal complexes of tetra-2~3-(5~6-dicarboxYpyrazino)
por~hyrazine (2e - 6e); General procedure:
Complex 2a - 6a (120 mg) was added under
stirring to a mixture of NaOH-saturated MeOH (45 mL)
and water (15 mL). A precipitate appeared immediately.
The mixture was stirred for 30 min (reaction progress
213328~
- 13 -
was monitored by analytical HPLC), whereafter the solid
was filtered, washed with 25% aqueous MeOH and
dissolved in distilled water (30 mL). This solution
was neutralized to pH 6 - 7 with several drops of lN
HCl, filtered, and a little amount of EtOH was added to
precipitate the salt 2e -6e. The solid was
centrifuged, washed with 25% aqueous MeOH and dried for
4 h at 60C in vacuo (10 mm Hg). The yield of the
salts 2e - 6e was quantitative.
TEST EXPERIMENTS
Example 5
Tumour regression after PDT with 6e:
Photosensitizer. Zinc
octacarboxytetrapyrazino porphyrazine (6e) (MW 1113) in
sterile, physiological saline (0.154 M) at a
concentration of 0.12S mM.
Tumour model. Male Balb/c mice (6-7 weeks
old, Charles River Inc.) were inoculated in the right
hind thigh with 2 x 105 EMT-6 murine mammary tumour
cells suspended in 0.05 ml of Waymouth's medium (Gibco
Inc.). At 6 days postinoculation the tumors were 2-3
mm in diameter and this time point were selected for
therapeutic intervention.
Treatment protocol. Tumour bearing mice (n =
lO) were i.v. injected with 0.2 ml of 6e (formulated as
above), to give an administered dose of 1.0 ~mol/kg
(l.l mg/kg). Twenty-four hours after dye injection
2133284
- 14 -
tumors were exposed to an 8 mm circular red light beam
(400 J/cm2 at a fluence rate of 200 mW/cm2, 600-700 nm)
delivered by a 1000 W Xenon lamp, equipped with a 10 cm
water filter and LS700 and LL650 Corion~ filters.
Under these conditions no hyperthermia occurred during
illumination.
Tumour response. Complete tumour necrosis in
100% of treated animals were observed within 3 days
following PDT. When the same light dose was delivered
over a 600-650 nm band, partial tumour response
occurred, indicating the in vivo action spectrum of the
drug was red-shifted relative to its A~x = 640 nm in
homogenous solution. This is an important and
unexpected property of this drug since biological
samples including blood and most tissues, are more
transparent > 640 nm due to the absence of endogenous
chromophores at these wavelengths, thus facilitating
the penetration depth of therapeutic light. Another
unique observation with this drug following PDT, is the
absence of any noticeable response (redness, edema) to
the tissue/skin surrounding the tumour, even after
augmenting the administered drug dose from 1 to
lOmg/kg, indicative of the high tumour-selectivity of
the drug.
Accordingly, this invention also relates to
therapeutic compositions useful in the photodynamic
therapy of cancer in mammals, one active ingredient
therein being the tetrapyrazinoporphyrazines (TPPA) of
2133284
- 15 -
the present invention, described herein, together with
a pharmaceutically suitable diluent or carrier. Any
appropriate form of administration may be envisaged but
dosage is generally lower than for similar photodynamic
agents because of its high activity and because its
activity is strong at wavelengths where mammalian skin
is most transparent.
Although the invention has been described
above with respect with one specific form, it will be
evident to a person skilled in the art that it may be
modified and refined in various ways. It is therefore
wished to have it understood that the present invention
should not be limited in scope, except by the terms of
the following claims.