Note: Descriptions are shown in the official language in which they were submitted.
;;~;i - 2133~4
W093/20279 1 PCT/F193/00137
METHOD OF PRODUCING PULP
The present invention relates to a new method of
producing chemical pulp. The invention is an improvement
in solvent cooking methods (e.g. organic solvent diges-
tion), often referred to as "solvent pulping", being
generally as described in U.S. Patent No. 3,585,104.
Wood comprises cellulosic fibres with lignin both
inside the fibres and between the ~ibres, bon~;n~ the
- fibres together. In order to carefully separate the fibres
from each other, the lignin must be removed from between
the ~ibres, the removal usually being accomplished by
dissolving the lignin. Generally the cooking liquors
consist of sodium hydroxide (i.e. soda cook), sodium
hydroxide cont~;nin~ sodium sulphide (i.e. sulphate or
kraft cook, also called alkaline cook), or, for e~Ample,
sulphite ions (i.e. sulphite cook, also called acid
sulphite cook). Lignin can also be rc...o~d by some organic
solvents (hen~e the commercial name organosolv-process),
the best known of which are methanol and ethanol. Formic
acid is another proposed organic solvent.
Methanol and ethanol can be utilized as solvents in
both alkAl;~e and acid cooks. The advantage of acid cooks
~; is the simple recovery of the r,h~m;cals, as wood contains
-~ acids that make the cook acid, when only methanol or
ethanol are used as solvents. The disadvantage of acid
cooks is the poor quality of the produced pulp, because
the cellulosic fibres will, to some extent, degrade in
acid treatment. In alkaline cooks the quality of the
produced pulp will remain good, but the problem in
alk~l~ne cooks is the recovery of chemicals. Some alkali,
mostly a sodium-based alkali, will firstly have to be
~A~A into the cook and then recovered and used again. A
known ~lk~line organic solvent process is the "~rgano-
cell-~o~ess", presently hP;~g used in Germany, in which
~-o~ss the cook is a soda-methanol cook.
2sI3~ ~7 l (. '
W093/20279 PCT/F193/00137
According to the present invention, it has been
determined that during solvent pulping acids are formed in
the very beginning of the cook. The pH of the cook can be
kept in the desirable range (generally neutral) by
removing the acids from the beginning of the cook, i.e. ~ I
almost ;~me~;ately after they are formed. By keeping the
pH generally neutral, the pulp ~uality is enhanced, both
for continuous and batch cooking processes. This is
a~ lished, in general, by countercurrent flow during
continuous cooking, and in batch cooking by withdrawing
solvent from the batch digester after a predetermined time
from the start of the cook (and perhaps periodically
- thereafter), while the cook continues after the solvent
withdrawal, with new solvent added.
According to one aspect of the present invention
there is provided a method of producing cellulose pulp
from ~omm; nuted cellulosic fibrous material by organic
solvent pulping comprising the step of (a) controlling the
pH of the material during cooking so that it is generally
neutral by removing acids formed at the start of the cook
together with organic solvent used in the cook and by
adding alkali to the cellulosic fibrous material in an
amount sufficient to maintain the pH at the desired value.
This method may be practised utilizin~ a treatment vessel,
comprising further the further steps of: (b) Causing the
cellulosic fibrous material to flow in a first direction
into the treatment vessel, and to be ~al,loved from the
treatment vessel in the same, first, direction, whereby
the nePd~ amount of alkali is added to the material prior
to the treatment vessel; and (c) introducing an organic
solvent cont~;ning liquid stream to dissolve the lignin of
the cellulosic fibrous material into the treatment vessel
in a secon~ direction, opposite the first direction. Step
(a~ is practised by removing the acids formed during
pulping, together with spent organic solvent, from the
treatment vessel.
W093/20279 213 3 -~ 71 PCT/F193/00137
According to another aspect of the present invention,
there is provided a method of producing cellulosic pulp
from r,omm~ nuted cellulosic fibrous material by solvent
pulping comprising the steps of:
5causing the comm;nuted cellulosic fibrous material to
flow in a first stream in a first direction;
causing a stream of organic solvent to flow into
operative contact with the comminuted cellulosic fibrous
material countercurrent to the first stream, that is in a
lOsecond direction, opposite the first direction ~hroughout
the area of oparative contact between them, which causes
organic acids to be produced as the org~nic solvent
~ dissolves the lignin from the cellulosic fibrous material;
and
l5~e~ ving organic acids formed during solvent pulping
from intimate contact with the cellulosic fibrous material
along with the countercurrent flow of organic solvent.
It is the primary object of the present invention to
20provide effective solvent pulping with a stable pH. This
and other aspects of the invention will become clear from
an inspection of the detailed description of the inven-
tion, and from the appenA~ claims.
25The method of producing pulp according to the
invention will become clear from the detailed description,
with reference made to the accompanying drawings, of
which:
30Figure l is a schemati.c of an exemplary embodiment
of a cook/extraction process according to thè invention;
Figure 2 is a schematic of another ~ rlary embodi-
ment of a cook/extraction process according to the
35invention;
W093/20279 ~33 ~ PCT/F193/00137
Figure 3 is a schematic of an exemplary embodiment of
the washing stage subsequent to the cook/extrac~ion
process;
Figure 4 is a schematic of an exemplary embodiment of
solvent recovery process according to the invention;
Figure 5 is a schematic of another exemplary embodi-
ment of a cook/extraction process according to the
invention;
Figure 6 and 7 illustrate the change of pH as
observed when eX~ining the cook/extraction process of
Figure 1,
. Figure 8 is a schematic diagram illustrating ap-
paratus for practising a method according to the present
invention;
~igure 9 a schematic of an exemplary embodiment of
solvent and alkali recovery process according to the
: invention; and
:~ Figure 10 is a possible proce~s alternative of an
application of the invention; and
Figure 11 is a possible process alternative of an
application of the invention on an industrial scale.
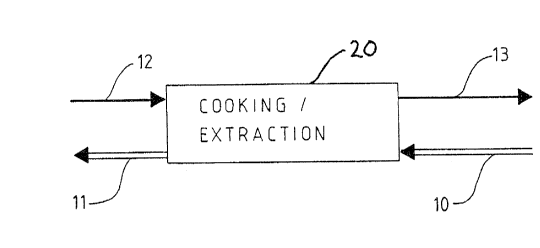
Figure 1 illustrates a schematic representation of
the principle of a countercurrent cook/extraction treat-
ment vessel/stage 20 according to the invention. The
number 10 refers to comminuted cellulosic fibrous material
(e.g. wood) being fed into the process and the number 11
refers to cellulosic fibres of the wood, produced by
dissolving and extracting lignin from the wood. The number
12 refers to the organic solvent being fed to the process
~ - 2133~7~
W093/20279 PCT/Fl93/00137
and the number 13 refers to the spent organic solvent
being exhausted from the process, with dissolved lignin
also present. The process of Fig. 1 was examined in
laboratory conditions with the surprising result shown in
Fig. 6.
In a normal organosolv cook of softwood the pH
followed the curve 100. As the acids in the wood are
dissolved the pH decreases in about 15 minutes to 3.6 -
3.5 and remains at this level fvr the rest of the cook. Ina organosolv cook of hardwood, the pH decreases to 3.8 -
4Ø This means that all acids either form or dissolve in
the initial phase of the cook. The poor fibre strength
produced is due to the acid conditions in the cook. The
low pH in the cook of softwood may also result in con-
densation of lignin which prevents dissolution of lignin.
In a countercurrent organosolv cook according to the
present invention the pH followed the curve 101. In the
initial stage of the extraction the pH was noticed to
decrease sharply, but then to quickly increase to almost
the original level of a~out 5.5. The reason for this must
be that the organic acids present in the wood are initial-
ly dissolved into the solvent, and as the process is a
countercurrent one, the acids are washed away with the
solvent during the later stages of the cook. The pH can be
maintained on an almost neu~ral level during the cook
without using alkali. The quality of the pulp is improved
because of reduced acidity during the la~er stages of the
cook.
In 1989, at the Solvent Pulping Conference, Quinde
gave a-lecture on what happens to the extractives of pine
in a methanol cook (80% methanol, 205 ~C). He concluded,
that 90% of resin acids and about one fourth of fatty
acids are dissolved during the first five minutes. This
supports the surprising result that the fall of the pH can
W093~20279 ~33~ ~ PCT/FI93/00137
be prevented by countercurrent flow of cooking liquor, as
shown in Fig. 6 (or by removin~ the formed acids shortly
after production in a ~atch digester). Obviously the
r~m~in;ng resin and fatty acids are removed due to the
countercurrent nature of the process. Thus, according to
the invention, the pH of the extraction process can be
controlled by removing the organic acids, formed during
the process, together with the always countercurrent flow
of solvent.
The results from the experiments described in Fig. 6
can be further explained by means of ~ig. 7.
-
On region C of Fig. 7 the pH is below 4.5 whereby the15 quality of the pulp deteriorates. In the case of a normal
ethanol cook most of the time the pH is below this level
~'and this results in poor quality of pulp. This case is
represented by curve 104 in Fig. 7. However, when utiliz-
ing a countercurrent cooking procedure as according to the
invention, the pH remains below the minimum,desirable
level (line 105) ,for pH during the cook only a short
period of time. Since the time during which the pH is too
low is short, a pulp of good quality is produced. This is
represented by curve 103 in Fig. 7.
~-The pulp quality can be further improved by adding
alkali to the countercurrent cook at the beginning of the
cook. This case is shown in Fig. 7 by curve 102. The
,jalkali added ln the beginning increases the pH and acids
formed or dissolved neutralize the cook and the pH
decreases to an almost neutral value during the counter-
¢urrent cook. There is a maximum desirable pH, represented
by line 106, which is desirably not ex~se~ed (region A in
Fig. 7) because then the amount of alkali that has to be
recovered becomes so high that the recovery system becomes
expensive. By controlling the pH in this way within region
B, a pulp of good quality is produced.
- 21~3~74
WO93/2n?79 PCT/F193/00137
It can be said that it is most advantageous to keep
the pH of the solvent cookin~ process in the range of 4.5
- 12, but it can be momentarily allowed to decrease below
4.5 or increase above 12 at the beginning of the cook. The
minimum is re~che~ when no alkali is used and the m~X; mum
when alkali is used. Alkali can also be added in such an
amount as to maintain the pH of the cook between the
minimum and m2Y; ~l~m limits.
10A countercurrent extraction according to Fig. 2
ensures that the pH does not decrease at the initial
stages of the cook/extraction 20. An addition of alkali
- 14, for example CaO, Na3CO3 or NaOH, or a combination
thereof, to the wood material lO being fed into the cook,
neutralizes the initial stage of the cook and so prevents
the pH from falling. Thus the pH is prevented from
decrP~ ng to the level of about 3-4, where hydrolysis
would degrade the cellulosic fibres. The amount of acid
forming at the initial stages of the cook is 8-20 kg/ton
of pulp, and an amcunt of alkali corresponding to the
amount of acid is added, if the pH is to be kept out of
the acid range (e.g. generally nautral, for example a pH
of about 6.5 - 7.5). Due to the essentially completely
countercurrent nature of the cook there is some alkali
present at the initial stage~ of the cook 20, but it is
then w~h~ away and removed with the dissolved lignin in
stream 13.
When effecting countercurrent cooks in laboratory
conditions it was also discovered that a high ethanol or
methanol content (e.g. 70% or more) in the organic solvent
contAi n~ n~ liquid gives a better delignification result.
It has usually been presumed, that due to the water
included in the wood, the ethanol content could be 50% at
the most. When a countercurrent cook is effected, the
ethanol content can be substantially increased to ~ceed
50% of the organic solvent cont~ ng liquid by maintain-
W0~3/20279 PCT~Fl93/00137
21~3~7 ~ 8
ing a high ethanol content in the inlet streams 12 lFigs.1 and 2). According to the invention, the water is washed
away at the beginning of the cook and is removed with
streams 13. Thus the countercurrent cook gives a pos-
sibility of both controlling the pH and increasing thecontent of the organic solvent.
The residual solvent in the stream of fibres sub-
sequent to the extraction 20 must be washed away as shown
in Fig. 3. The stream ll of fibres is a mixture of organic
solvents and wood fibres. Because of the countercurrent
nature of the cook/extraction, it can be presumed that the
~ fibres are suspended in nearly pure solvent. In the
washing stage 21 the solvent is w~he~ away with washing
lS liquid 16, mostly water. The result is a wood fibre-water
suspension 15 and a solution 17 of water and solvent,
mostly a mixture of water and ethanol or methanol.
The solvent is recovered from stream 17 by a process
according to, for example, Fig. 4. The solution 17 of
- water and solvent is directed to distillation apparatus
22, where the water 18 and solvent 12 are separated. The
solvent 12 is directed to cook 20. As is shown in Fig. 4,
it is possible to effect a wash in 21 with water and still
maintain a high ethanol and/or methanol content in the
actual cook 20. The pulp, if any, still in the form of
chips prior to the wash, is fiberized prior to the wash to
optimize the wash. The organic solvent 12 preferably has
a substantially greater than 30% methanol and/or ethanol
content.
Fig. 5 illustrates a process more developed than
heretofore described. The wood 30 introduced into the
process is heated at the beginning of the cook by feeding
steam 31 in the wood material in presteamer 23. Alkali 32
is at the same time introduced into the wood material to
increase the pH at the initial stage of the cook. A
~ - 2 1 3.~574
W093/20279 PCT/F193/00137
catalyst 33 can be introduced during either the pretreat-
ment 23 or at the beginning of the cook 20 to enhance the
dissolving of lignin. A co~mQnly used catalyst in acid
process is CaCl2. The Ca2~ ions are advantageous to the
process, but on the other hand the amount of acid is
detrimentally increased in the reaction ZCl- + 2H+ ->
2HCl. Organic bases, such as ~m;ne, can be used as
catalysts in acid, neutral and alkaline processes. Ethanol
or methanol 34 is added to make up for the losses of
solvent in ~he process.
The process has further been complemented by treat-
ment of the lignin-cont~;n;ng outlet liquid 36. Naturally,
the outlet liquids 13 of the-previous embodiments can also
be treated accordingly. The outlet stream 36 of solvent
from the cook 20 mostly contains solvent, polysaccharides,
lignin and water. The mixture 36 is directed to distilla-
- tion/evaporation stage 24, where the solvent, mostly
ethanol and/or methanol 37, is separatad from the water 38
and lignin and polys~c~-h~ride material 39. The solvent 37
can be returned to the cook 20 together with the solvent
12 from the distillation stage 22, while the water and '~!'
lignin material are directed to ~ombustion apparatus 25 or
other treatment device. One possibility is to separate the
lignin and to produce, for ~x~mple, vanillin from the
separated lignin. The added alkali 32, if any, is directed
to combustion apparatus 25 and can thereafter be reused,
or, if desired, ~s~..oved. Removal is typically practised if
the amount of the added alkali is small, e.g. 10-50 kg/ton
~f pulp.
In a laboratory test producing pulp of good quality,
CaCl2 was utilized as catalyst, the amount of which was 5-
50 g/l (that is about 5-50 grams of catalyst per litre of
material being treated). The temperature of the cook was
195 ~C and the duration of the cook 20 was 3 hours. Other
possible catalysts, besides Ca++, are Mg++ and Na+. For
W093/20279 ~3 j3 ~ 4 PCT/FI93/00137
the lignin extraction it is advantageous that the wood is
impregnated with alkali (14, 32), as ~his causes the wood
to swell and thereby improves the extraction of lignin.
Ethanol is probably a better solvent than methanol
for the practice of the inventionr but the deacetylation
that occurs in the wood produces some methanol. Thus there
is always some methanol present in the process, even if
the only fresh solvent 34 is ethanol.
The invention can be applied to numerous cooks, both
batch cooks and continuous cooks. The invention can, for
Px~ple, be utilized in continuous digesters with a
pretreatment zone or in continuous digesters with a
separate pretreatment vessel. When the invention is
practised with batch digesters, the acids produced at the
beglnning of the cook can be removed by withdrawing
solvent from the digester after a predetermined time (e.g.
about 5 - lO minutes, but dependent upon material being
cooked, the exact composition of the organic solvent,
etc.) - the produced acids being removed with the solvent
- and introducin~ at least some new solvent. While
normally not necessary since most of the acids are
produced within the first five minutes or so, the with-
drawal and replacement procedure can be practised periodi-
cally if desirable. The pH during batch cooking can
alternatively, or in addition, be maint~ne~ generally
neutral during the initial stages of the cook by adding
~ alkali to the material prior to or simultaneously with
introduction into the digester.
The invention may be further illustrated by the
following ~mples.
Example
. ~ t ~ .~- 7 d~ .
W093/2027~ PCT/F193~00137
A series of laboratory tests on softwood were
performed to study the method. In laboratory the or- Z
ganosolv cooking process was performed as follows:
Step l:
The chips were pretreated in a pretreatment liquor
consisting of NaOH dissolved in water. The pretreatment
time was about 30 minutes and the temperature 120 ~C. The
idea of the pretreatment step was to cause the fibre to
~O swell so as to enhance the subsequent ethanol extraction
step. Another reason for the pretreatment step was to
enable the add~tion of alkali to the chips to keep the pH
~ at a sufficiently high level during the rest of the cook.
,
During the pretreatment step the amount of alkali was
varied between 0.25 to l.50 mol NaOH/l. It was found that
when the amount of alkali was below 0.25 mol NaOH/l the
delignification was insuffiaient and the amount of
unfiberized wood high. At alkali levels above l.OO mol
NaOH/l the yield loss in the cook became too high.
Probably the amount of alkali actually n~ depends on
type of wood used. For the tested Scandinavian spruce the
optimal alkali level was between 0.5 and l.O mol NaOH/l.
Step 2:
The chips were removed from the pretreatment liquor
by lifting the chips out. It was found that about 30 - 70
kg of NaOH per ton of pulp was entrained with the chips.
Alkali had also been consumed in the pretreatment
vessel. The consumption was 50 - 300 kg NaOH per ton of
pulp .
Step 3:
Subsequent to steps l and 2 the chips were taken to
a countercurrent ethanol extraction step. The duration of
this solvent extraction step was about 120 minutes and the
W093/20279 c~33~ 4 PCT/FI93~00137
12
temperature 185 ~C. Anthraquinone was added to the solvent
to improve delignification. The added amount of anthra-
quinone was between 0 and 1.0 mmol.
The strength of the ethanol solvent was varied
between 25% and 100%. The lowest residual lignin contents
were achieved when the ethanol content was between 40 and
70% in the liquid phase. When being tested for bleach-
ability and pulp properties, the pulp was found to have
pulp properties close to those of kraft pulp regarding
both bl~ ahility and strength.
~ Figure 8 ~hows an apparatus with which the process of
example 1 can be performed. The apparatus comprises a
conventional chips bin 201 connected by a conventional low
;~ pressure feeder 202 to a conventional horizontal steaming
vessel 203, which in turn is ~o~nected by a conventional
chute 203 to a conventional high pressure feeder 204. Wood
chips are first steamed and preheated and then taken
through the first pressure feeder 204 in line 205 to the
pretreatment vessel 206 in which the chips are treated in
an alkaline solution. The alkaline solution is introduced
into the syxLem in line 207. A eo~entional liquid/
material separator ~ys~em is provided at the top of the
vessel, with withdrawn liquid recirculated via line 208 to
the inlet high pressure port of the feeder 204. The alkali
can come from one or several of the following sources:
- waste water from alkaline bleAching stages, for ex-
ample, E or P stages:
- NaOH, either brought fresh to the mill or manufac-
tured at the mill by caustlcizing Na2CO3; and/or
- Na2CO3 if the alkalinity n~e~ is so low that only
part of the alkali must be NaOH.
W093/20279 2 13 3 ~ 7 4 PCT/Fl93tO0137
13
From the pretreatment vessel 206 the chips are taken
to the extraction zone 215 in the second pressure vessel
213 in which the pres ure is much higher than in pressure
vessel 206. The chips material passes via line 211 to the
top of the vessel 213 from a high pressure feeder 209 in
the bottom of the pretreatment vessel 206. A conventional
liquid/material separator system is provided at the top of
the vessal 213, with withdrawn liquid recirculated via
line 212 to the inlet high pressure port of the feeder
209. In order to control the temperature of the recir-
culating liquid so as to minimize the possible adverse
effects on the high pressure feeder 209, the liquid is
passed throu~h a heat axchanger 210.
The seconA vessel 213 preferably consists of two
zones: (1) zone 215 in which the chips are extracted with
: . ethanol/methanol; and (2) zone 216 in which the chips are
washed before being discharged from the vessel.
Filtrate from a subsequent washing or bleaching step
is used as w~s~ liquid. The washing liquid is intro-
duced into the bottom of the vessel 213 through line 218.
The pulp at the bottom of the vessel 213 is washed and
discharged into line 219.
: The ethanol and/or methanol is added at a point above
the w~sh1n~ zone 216 in line 217. The ethanol is intro-
duced at such .a strength and amount that optimal extrac-
tion ro~tions are achieved in the extraction zone 215.
If nececsary, water can be ssparated from the circulation
220 by distillation to control methanol/ethanol strength
in the extraction ~one 215.
The extraction liquor is withdrawn from the extrac-
tor/digester 213 into a withdrawal conduit 214. The
withdrawn liquor cont~;~in~ the alkali used, the ethanol/-
W093/20279 - S~ 4 PCT/Fl93/00137
14
methanol used and the dissolved lignin, is taken to
recovery.
A simplified recovery system for solvent and alkali
is shown in Fig. 9. The extraction liquor 214 from Fig. 8
is treated in the following steps: (a) Ethanol/methanol
separation (250). The solvent is then reused in the
extraction vessel 213. (b) Evaporation (252) in which
water is separated. (c) Combustion (254) of lignin and
polysaccharides.
During combustion, a melt (255) consisting essential-
ly of Na2CO3 is produced. This melt is dissolved in water
and used as a Na2CO3 containing liquid in the pretreatment
vessel 206 or causticized to NaOH before using in the
pretreatment vessel 206. If the Na2CO3 amount is small it
does not have to be reused.
If the produced pulp is to be chlorine free, the pulp
can be ble~che~ with oxygen, ozone and peroxide. Fig. l0
illustrates a diagram of a process in whi~h the initial
bleaching is effected by oxygen and the actual final
ble~-h;ng by ozone and peroxide.
In the system of Fig. l0, the wood chips 300 that are
introduced into the process are fîrst heated by fe~
steam 301 to the wood material to a pretreatment vessel
323. Alkali 302 is at the same time introduced into the
wood material so that the chips are treated at a pH of ll
- 12. The alkali is obtained from the bleach plant
effluents, which contain 40 - 120 kg of NaOH/adt. If the
volume of the effluent is too big, the effluent has to be
evaporated in order to reduce the volume. Additional
alkali is brought if needed in the form of NaOH or Na2CO3.
After pretreatment 323 the chips 310 are introduced
to the extraction stage 320 where some methanol and/or
2~ ~3~ 74
W093/20279 PCT/Fl93/00137
ethanol 312' may be added in ~he beginning to control the
liquor- to - wood ratio, or to increase tha content of
methanol/ethanol in the beginning of the extraction
process 320.
After extraction the chips 311 are washed with the
bleach plant effluents 302'~ 303, 304, which may be acid
or alkali depending on how the ble-~h;n~ has done. If
possible, the acid filtrates 303 and 304 are used in the
washing 321 and the alkali filtrate 302 in chips pretreat-
ment 323. The washing liquid 317 con~aining solvent may
- have to be strengthened by distillation 322 before adding
methanol and/or ethanol 312 to the extraction stage 320.
The pulp 315 from the washing stage is introduced
into the bleach plant 330 in which the pulp is bleached in
the sequence OZP.
After ~he extraction stage 320 the waste liquor 306
is recovered, evaporated and combusted. If the alkali
amount is low, the Na2CO3 formed in the recovery process
is removed from the mill and fresh NaOH-is brought in.
Some of the Na2CO3 may be used in the pretreatment stage.
If the amount of alkali in the effluent stream is large it
is probably more prac~ical to causticize the formed Na2CO3
to NaOH and thus generate new NaOH for bleaching and pre-
treatment at the mill.
~Y?~I-le
In one successful test, the wood was pretreated with
a mixture of 75 % Na2CO3 and 25 % NaOH in amount that
corresponds about 200 kg Na/adt expressed as NaOH. This
means that about 50 kg~adt of NaOH was used and the rest
was Na2CO3.
The amount of NaOH needed in the bleaching sequence
OZEP is also about 50 kg/adt. Thus all alkali needed in
w093/20279 ~33~ 4 PCT/F193/00137
16
the pretre~tment of the chips in the form of NaOH is
received from the bleach plant effluent water. The rest of
the alkali can be used as Na2CO3 which is the form of the
Na when burning the residual liquor in a recovery boiler.
Thus, a mill does not need any causticizing plant,
but uses Na2CO3 from the combustion of spent liquor and
NaOH from bleach plant effluents.
Filtrates from the bleach plant can be used to
dissolve the Na2CO3 from the recovery boiler thus further
_ reducing the water effluent volume.
Fig. 11 also illustrates a schematic technical
dia~ram of a process in which the initial bleaching is
effected by oxygen and the actual final blearhing by ozone
- and peroxide.
While the invention has been described in connection
with what is presently considered to be the most practical
and preferred emho~;ment~ it is to be understood that the
invention is not to be limited to the disclosed embodi~
ment, but on the contrary, is intended to cover various
modifications and equivalent arrangements included within
the spirit and scope of the appended claims.