Note: Descriptions are shown in the official language in which they were submitted.
~369~
PROCESS FOR PRODUCING MAGNESIUM SULFITE
HEXAHYDRATE IN A FLUE GAS DESULFURIZATION SYSTEM
Fleld of the Inventlon
The present lnvention relates to an improved flue
gas desulfurlzatlon system where calclum and magnesium
scrubblng components are used to remove sulfur dloxide from a
gaseous stream, and more speclflcally to such a system where
magneslum sulflte is produced and recovered.
Backqround on the Inventlon
The use of magneslum-enhanced lime scrubblng
processes to remove sulfur dioxlde from flue gases, such as
those from fossll fuel burnlng power plants, has been well
accepted commerclally. In such scrubblng processes, an
alkaline earth metal component, such as llme, wlth a
magneslum component, such as magneslum oxide or hydroxlde, ls
contacted with the flue gases ln a wet scrubber. Such
processes are generally descrlbed in US 3,919,393, US
3,919,394, US 3,914,378 and US 4,976,937. all of whlch are
asslgned to the assignee of the present lnventlon. As
descrlbed ln the latter patent, US 4,976,937, sulfur,
polysulfides or other thiosulfate (S2O3 ) precursors can be
added to the scrubblng ll~uor ln order to act as a free
radlcal scavenger so as to lnhlblt the oxldatlon or sulflte
produced ln the scrubblng process to sulfates.
74445-20
i ~
" . ~
The magnesium-~nh~nc~ lime scrubbing processes are
typically characterized by high levels of dissolved magnesium and
sulfites. The sulfite levels are increased by the suppression of
natural oxidation of sulfites to sulfates such as by the addition
of sulfur or other thiosulfate precursors. Sulfur reacts with
sulfites to produce thiosulfates. Such sulfite oxidation
inhibition shifts the scrubbing liquor chemistry from subsaturated
with respect to MgSO3 to a MgSO3 supersaturated mode.
Supersaturation for MgS03 may also be achieved without sulfur
addition if a high magnesium oxide or hydroxide level is provided.
The magnesium sulfite produced in a magnesium enhanced
lime scrubbing process for removal of sulfur dioxide from flue
gases is usually found as predominantly magnesium sulfite
trihydrate (MgSO3-3H20), with a minor amount of magnesium sulfite
hexahydrate (MgS03 6H20). The magnesium sulfite trihydrate is
usually formed as small crystals which are difficult to dewater and
can provide disposal problems. Magnesium sulfite hexahydrate, on
the other hand, is usually formed as large crystals and are more
easily separated and dewatered.
Ma~nesium sulfite does not exist, at present, as a
chemical commodity of any appreciable volume. The primary large
scale commercial application for magnesium sulfite is in the
magnesium bisulfite pulping process as practiced by the pulp and
paper industry. These are, however, in-situ, closed-loop processes
in which elemental sulfur is burned to produce sulfur dioxide gas
which is then absorbed by a magnesium hydroxide slurry in a
3 !) 9 ~1
countercurrent absorber to produce magnesium sulfite. At the
pulping digester pH of about 4.5, the sulfite is primarily in the
bisulfite form due to equilibrium reactions. Once the digestion
process is complete and the~paper pulp product and the by-product
magnesium lignosulfonate are removed, the "strong red liquor"
process slurry is directed to an evaporator where the magnesium
bisulfite is decomposed to sulfur dioxide which reports back to the
absorber, and magnesium oxide which is recovered and slaked to
magnesium hydroxide and returned to the absorber. There are losses
of both magnesium and sulfur dioxide in such processes, primarily
in the magnesium lignosulfonate by-product, but also in the sulfur
dioxide absorber, wastewater, and recovery furnaces. These losses
must be recovered by using make-up reagents, namely elemental
sulfur, magnesium oxide, and/or magnesium hydroxide.
These reagents are all expensive, prone to market price
fluctuations, and in addition, the processes required to
incorporate them into the bisulfite pulping process are energy
intensive and require careful control.
It is an object of the present invention to produce
magnesium sulfite in the hexahydrate form, from a magnesium-
enhanced lime scrubbing process, that may be used in bisulfite
pulping processes and other commercial processes.
SUMMARY OF THE lNv~NllON
The present process provides for the production of
magnesium hexahydrate in a wet scrubbing process for removing
~ 1 3 ~, ~ v~ !~
sulfur dioxide from a flue gas using a magnesium-enhanced lime. A
gaseous stream is contacted with an aqueous scrubbing medium
containing calcium and magnesium scrubbing components such that
sulfur dioxide in the gases is converted to magnesium sulfite. The
magnesium sulfite produced is primarily magnesium sulfite
trihydrate with a small amount of magnesium sulfite hexahydrate
present. As is conventional, a bleed stream or portion of the
magnesium sulfite-containing aqueous solution is discharged from
the wet scrubber.
In accordance with the present invention, the aqueous
solution containing magnesium scrubbing components is maintained
such that a magnesium ion content of between about 5,000 - 12,000
parts per million (ppm) is present, a sulfite content of between
about 3,000 - 18,000 ppm is present, and a pH of between about 6.0
- 7.0 is present, so as to produce an aqueous scrubbing solution
supersaturated with respect to magnesium sulfite, which also
contains some suspended solids. A portion of the supersaturated
aqueous scrubbing solution is withdrawn from the scrubber and
suspended solids removed therefrom. After the suspended solids are
removed, the portion of supersaturated aqueous scrubbing solution
is treated, by lowering the temperature thereof, and optionally
adjusting the pH thereof above 7.0 to about 7.5. The temperature
of the portion of supersaturated aqueous scrubbing solution is
generally about 110 - 120~F (45 - 50~C) before treatment. The
treatment of the portion of supersaturated aqueous scrubbing
solution causes a conversion of magnesium sulfite trihydrate
2 1 3 ~
therein to magnesium sulfite hexahydrate, which magnesium sulfite
hexahydrate, so produced, crystallizes and is separated.
In an alternative embodiment of the present process, a
portion of the supersaturated aqueous scrubbing solution may be
passed directly to a cooler-crystallizer to convert magnesium
sulfite trihydrate to magnesium sulfite hexahydrate and the
suspended solids separated, along with the aqueous media, from the
magnesium sulfite hexahydrate produced.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will be further understood by reference to
the attached drawings showing an embodiment thereof, where:
Figure 1 is a schematic representation of a preferred
embodiment of the present process;
Figure 2 is a graphical representation showing a
predominance of magnesium sulfite hexahydrate form as a stable
species at lower temperatures vs the predominance of magnesium
sulfite trihydrate form at elevated temperatures in an aqueous
medium;
Figure 3 is a graphical representation of the pH effect
on the saturation value of magnesium sulfites relative to
bisulfites in an aqueous medium; and
2 ~ 3 3 ~ ~ ~
Figure 4 is a schematic representation of another
embodiment of the present process.
DE~ATT~n DESCRIPTION
The present process enables the recovery of magnesium
sulfite solids from a magnesium-enhanced lime scrubbing process for
removing sulfur dioxide from flue gases where the magnesium sulfite
resulting is in the form primarily as magnesium sulfite
hexahydrate.
Referring now to Figure 1, the process is illustrated,
showing a wet scrubbing unit 1 to which a sulfur dioxide-containing
gas is charged through line 3 and clean gas discharged through line
5. An aqueous scrubbing medium conta;n;ng calcium and magnesium
scrubbing components is charged to the wet scrubbing unit 1 through
line 7 and contacts the sulfur dioxide-containing gas to remove
sulfur dioxide therefrom. The aqueous scrubbing medium is recycled
through the wet scrubbing unit 1 by means of recycle line 9. The
aqueous scrubbing medium is a lime slurry containing magnesium ions
as described in the aforementioned US 3,919,393, US 3,919,394, US
3,914,378 and US 4,976,937. Also, as described in US 4,976,937, a
reductant, such as sulfur, a polysulfide, a thiosulfate, or a
thiosulfate precursor is added to the scrubbing liquor in the wet
scrubbing unit through line 11, so as to provide high levels of
dissolved magnesium and sulfites in the aqueous scrubbing liquor,
by suppressing oxidation of such sulfites to sulfates. Such
oxidation inhibition shifts the aqueous medium chemistry from
2 1 .3 ~, ~ 3 i-l
subsaturated with respect to magnesium sulfite to a supersaturated
mode. Either a thiosulfate compound or a thiosulfate precursor is
preferred as the reductant or oxidation inhibitor and is added in
an amount such as will preferably provide about 0.5 to 40
millimoles of thiosulfate per liter of aqueous medium contained in
the wet scrubber.
The aqueous scrubbing medium which is used to contact the
sulfur dioxide containing gas should be maintained so as to have a
magnesium ion content of between 5,000 - 12,000 ppm, preferably
about 6,000 - 8,500 ppm, a sulfite ion content of between 3,000 -
18,000 ppm, preferably between about 5,000 - 12,000 ppm, and a pH
of between 6.0 - 7Ø In order to obtain the magnesium ion and
sulfite ion content required, generally either a high magnesium
content lime would be required, or a reductant as mentioned
hereinbefore would need to be added to the scrubbing medium to
reduce oxidation of sulfites to sulfates.
Generally, in such wet scrubbing processes, the
temperature of the aqueous scrubbing medium and within the scrubber
is about 50~C. The aqueous scrubbing medium, after contact with
the sulfur dioxide will contain magnesium sulfite in the form of a
major amount of magnesium sulfite trihydrate and a minor amount of
magnesium sulfite hexahydrate and magnesium bisulfite. The aqueous
medium will thus comprise a solution of magnesium sulfite
containing some suspended solids, primarily CaS03 ~ ~ H20 and CaS04
~ ?~ H20.
~ ~ 3~3 .fj .'3 ~
-
According to an embodiment of the present invention, a
portion of the supersaturated aqueous scrubbing solution is
withdrawn from the wet scrubbing unit 1 through line 13 and fed to
a clarifier or thickener --15, where the suspended solids are
separated from the supersaturated aqueous scrubbing solution.
Thickened liquor, containing the suspended solids is ~;~ch~rged
from the thickener 15 through line 17, while the supersaturated
aqueous solution is passed from line 19 to a feed tank 21. The
suspended solids are removed so as to prevent scaling of later
components used in the process and to assure a high purity
magnesium sulfite hexahydrate production. From the feed tank 21,
the supersaturated aqueous solution is passed through line 23 to a
cooler 25 and then through line 27 to a crystallizer 29. In some
instances, the cooler 25 and crystallizer 29 could be a combined
piece of equipment.
By reference to Figure 2, it can be seen that the
relative saturation value of the sulfites, magnesium sulfite
trihydrate and magnesium sulfite hexahydrate, shift relative to the
temperature of a solution thereof. As shown, for example, at a pH
of 7.0 and at a temperature of about 50~C, the magnesium sulfite
trihydrate (line A) saturation will be about 1.3 or supersaturated,
while the magnesium sulfite hexahydrate saturation (line B) will be
about 0.7 or subsaturated. Upon cooling of the solution to about
25~C however, the magnesium sulfite hexahydrate saturation
increases to about 1.6, or supersaturated, while the magnesium
sulfite trihydrate saturation falls to about 0.7 or subsaturation.
Also, as shown, at a pH of 6.5 the saturation value of magnesium
sulfite trihydrate (line C) is about 1.0 at 50~C and about 0.6 at
25OC, while that of magnesium sulfite hexahydrate (line D) is about
0.5 at 50~C and about 1.4 at 25~C.
After cooling of the supersaturated aqueous scrubbing
solution, from the scrubber temperature of 45 - 50~C to a
temperature of between about 15 - 40~C and preferably to about 25~C
or below, in the cooler 25, the supersaturated aqueous solution
contains magnesium sulfite hexahydrate in stable form as the
predominant sulfite, and the solution is passed to the crystallizer
29, which may be any suitable commercially available crystallizer.
Upon crystallization of the magnesium sulfite hexahydrate from the
aqueous solution thereof, the aqueous medium and crystals are
discharged from the crystallizer 29 through line 31 to a dewatering
device 33. The water is separated in dewatering device 33 and
discharged through line 35 while the dewatered magnesium sulfite
hexahydrate crystals are passed through line 37 to a dryer 39 where
they are dried, with resultant water vapor discharged through line
41 while the dry magnesium sulfite hexahydrate crystals are passed
through line 43 to a collection device 45 as end product. The
drying of any surface moisture from the magnesium sulfite
hexahydrate crystals is important since, although such crystals are
stable, any entrained or surface moisture associated with the
crystals (MgS03 solution) will quickly oxidize to magnesium sulfate
(MgSO4) creating a white scale on surface of the otherwise clear
rhombic crystals of magnesium sulfite hexahydrate. Such sulfates
~ ~ 3 ~
.
could interfere with prospective uses of the product. The dried
magnesium sulfite hexahydrate crystals should be maintained in air-
tight containers for storage and shipping.
In an optional use of the magnesium sulfite hexahydrate,
the dewatered magnesium sulfite hexahydrate from line 37 could be
diverted through line 47 to a roaster 49 where the crystals can be
heated to produce magnesium oxide (MgO) as a salable product which
is discharged through line 51 and sulfur dioxide ( S~2 ) which is
discharged through line 53 for uses such as in the formation of
sulfuric acid.
In another embodiment of the process of the present
invention, the portion of supersaturated aqueous scrubbing solids,
after removal of the suspended solids in thickener 15 is passed
through line 19 to the feed tank 21 and the pH of the solution is
increased to a value of between 7.0 to 7.5 by an addition of an
alkaline reagent, such as lime, from a source 55 through line 57.
The effect of an increase in pH on the saturation values of
magnesium sulfite is shown in Figure 3. If the pH of the
supersaturated aqueous solution is about 6.0, increase to about 7.0
may be sufficient to crystallize out magnesium sulfite hexahydrate,
or if the pH of the supersaturated aqueous solution is between
about 6.5 - 7.0, an increase to about 7.5 may be necessary to
assist in crystallizing out a sufficient amount of magnesium
sulfite hexahydrate.
The effect of pH on the percent sulfites, a
crystallizable moiety, as opposed to bisulfites which remain in
solution is shown in Figure 3. As shown, at a pH of about 5.0,
only about 8 percent of the sulfites in a solution will be in the
form of So3= ions, while the remainder will be HS03- ions, while
with an increase in pH to about 7.0, about 90% of the sulfites will
be in the form of S03= ions, while only about 10% will be in the
form of HS03- ions. Thus, increasing the pH of a sulfite ion
containing solution increases the sulfites present and thus would
provide an increased yield of magnesium sulfites in the present
process.
The aqueous media, after addition of a base to raise the
pH, is then passed through line 23 to cooler 25 and thence through
line 27 to crystallizer 29 for crystallization and subsequent
separation and drying of the magnesium sulfite hexahydrate
produced.
As an example of the present process, a magnesium-
containing lime scrubbing process was carried out using generally
the process described in US 3,919,393, with sulfur added to the
lime slurry according to the teachings of US 4,976,937 to provide
a thiosulfate content in the scrubbing liquor of about 2,000 ppm.
The scrubbing liquor was maintained so as to have a magnesium ion
content of between about 6,000 - 8,500 ppm and a sulfite ion
content of between about 8,000 - 16,000. A portion of the
supersaturated aqueous scrubbing solution was removed, clarified,
and cooled, with solids crystallizing from the solution. The
contents of the operating scrubber liquor and the cooled scrubbing
liquor were analyzed and the results were found to be:
f i 33694
Scrubber Crystalllzing
Operatlng Area
Temp ~C 50 20
pH 6.0 6.0
Calclum (mg/L) 88 88
Mg (mg/L) 6Z62 6262
Cl (mg/L) 6400 6400
SO4 (mg/L) 8506 8506
so3 (mg/L) 15412 15412
R.S. for MgSO3-3H2O1.21 0.69
R.S. for MgSO3-6H2O0.73 2.13
R.S. = 1 for solution to be saturated.
The value R.S. is used to ldentlfy the relative saturatlon of
the solutlon with a partlcular sulflte and, as shown, at the
20~C crystallizlng area the solutlon was very supersaturated
for MgSO3-6H2O whlch crystalllzed out from the solutlon. The
magneslum sulflte sollds that separated from the cooled
solutlon were determlned by analysls to be 96.08 percent by
welght MgSO3-6H2O and only 1.01 percent by welght MgSO3-3H2O.
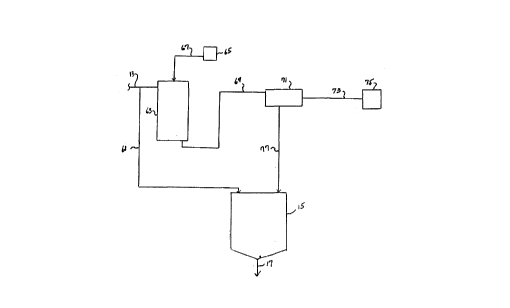
In the embodlment of the present process
lllustrated ln Flgure 4, a portlon of the supersaturated
aqueous scrubblng solutlon wlthdrawn from the wet scrubblng
unlt 1 through llne 13 ls divlded, with a portlon thereof
passing through line 61 to a clarlfler or thickener 15. The
other portlon ls passed by line 13 to a chlller-crystalllzer
unlt 63 to whlch llme ls added from a source 65 through llne
67. After coollng of the supersaturated aqueous solutlon and
crystalllzatlon of magneslum sulflte hexahydrate, the aqueous
media and crystals are passed through llne 69 to a separator
71, such as a hydroclone or screen washer. In
74445-20
"~
~133~9l~
the separator 71, the larger sized magnesium sulfite hexahydrate is
separated from the aqueous media and small sized suspended solids
and passed through line 73 to a collector 75. The aqueous solution
or media and suspended solids are then returned to the thickener 15
through line 77.
Thus, according to the present process, sulfur dioxide
can be removed from a flue gas by contact with a magnesium-enhanced
lime scrubbing medium with production and separation of magnesium
sulfite hexahydrate as a useful by-product.